1. Introduction
Deep brain stimulation of the subthalamic nucleus (STN-DBS) has been a treatment for motor symptoms in patients with Parkinson’s disease (PD) for more than 30 years. Increased β-oscillatory activity was highlighted as a pathological activity within the basal ganglia of these patients, which decreased during voluntary movements or after dopaminergic medication [
1,
2]. Indeed, dopaminergic medication, as well as STN stimulation, induces changes in β-oscillations, which were found to be associated with improvements in motor symptoms [
3]. In addition, β-oscillations in the STN were shown to be principally located in the sensorimotor subdivision of the dorsolateral STN [
4].
The Lead-DBS imaging reconstruction toolbox has recently been made available to the scientific community (
www.lead-dbs.org) [
5]. It allows postoperative localization of the implanted cerebral DBS electrodes with different atlases, in particular the DISTAL atlas [
6] that defines population average-based functional subdivisions of the STN, an atlas with population-defined DBS targets [
7] , and an electrophysiological atlas for β- and α-oscillations [
8]. Nonetheless, few studies have attempted to compare these localization tools with real intraoperative electrophysiology. The concordance between microelectrode recordings and the DISTAL atlas in comparison to other anatomical atlases of the Lead-DBS toolbox has been verified [
9,
10].
A new directional electrode, the Vercise Cartesia electrode, has also recently been made available (Boston Scientific, Marlborough, MA, USA) for DBS in PD. This technology allows stimulation of segmented contacts in order to customize the stimulation for each patient [
11]. The definition of the DBS target in PD is currently still a matter of debate. Indeed, in addition to the stimulation of the STN per se, the stimulation of afferent or efferent tracts of the STN have also been proposed.
The primary objective of this study was to compare the location of β-oscillations proposed by the electrophysiological atlas implemented in the Lead-DBS software with the location of real intraoperative β-oscillations measured by using the Vercise Cartesia directional electrodes from the recordings of local field potentials (LFPs) of each of their eight contacts. The second objective was to analyse the efficacy of stimulated anatomical structures in improving motor symptoms.
2. Materials and Methods
2.1. Patients and Clinical Assessment
The targeting method and the DBS surgical procedure were the same as described earlier [
12]. During surgery, patients were awake while microelectrode recordings were taken and intraoperative macrostimulation was performed for determining the threshold of corticospinal tract excitation. The final trajectory was chosen when STN cells could be identified electrophysiologically and also depended on the clinical observation of corticospinal tract stimulation on the contralateral face and upper limb, with a stimulation threshold of ≥ 2.5 mA.
Intraoperative measurements of the LFPs were performed at the time of the study in 20 consecutive patients with PD who agreed to participate (7 female, 13 male; median age 61.5 years, percentiles: 25th, 51.2 years; 75th, 70.0 years). The contacts of the left hemisphere electrodes were numbered from E1 (the most ventral contact) to E8 (the most dorsal contact) and those of the right hemisphere electrodes from E9 to E16. The intermediate contacts were segmented in groups of three.
The motor outcome was assessed 1 year post-surgery. Improvements in scores on the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [
13] part III were assessed in an OFF-drug/ON-stimulation condition 1 year post-surgery (overnight withdrawal of dopaminergic treatment, routine evaluation in four conditions of medication and stimulation) versus an OFF-drug condition 1 month before surgery. The changes in motor scores were assessed as percentages of the preoperative MDS-UPDRS motor score. The lateralized scores included for the contralateral limbs the items 3.4, 3.5, 3.6, 3.7, and 3.8 for bradykinesia; for rigidity the item 3.3; and for tremor the items 3.15, 3.16, and 3.17.
The stimulated contacts at evaluation of motor outcome 1 year post-surgery were collected for analysis of the site of stimulation with its motor efficacy. The stimulated contacts were those identified during the clinical management of these patients in their first year of DBS.
2.2. β-Oscillation Measurements
The β-oscillations were computed from LFPs [0.5-3 kHz] recorded on each electrode contact, whatever was its location (no contact selection). LFPs were recorded in a monopolar configuration, with the extracranial skin flap as reference, using one of the sterilized and shielded wires of the Neurostar system (Neurostar, Tübingen, GE) connected to the skin flap through an alligator clip. The wires connecting the electrode contacts were also shielded up to their connection with the Mephisto amplifier (Neurostar, Tubingen, Germany). Thanks to this shielding and to the use of an extracranial reference, high quality β-oscillation measurements were obtained for every contact, eliminating the intrinsic contamination proper to the referencing of bipolar measurements or to intracerebral referecing. LFP recordings lasted at least 12s.
Postoperatively, for each contact, the spectrograms were computed by using fast Fourier transform on non-overlapping windows, achieving power spectral densities (PSD) with 1 Hz and 1 s resolution (MATLAB, MathWorks, USA). Epochs containing artefacts were identified by visual inspection and rejected. For each contact, the mean power of the β-oscillations was computed by averaging the successive PSDs of the spectrogram of the [13-31 Hz] frequency band [
12].
The differences in LFP power due to differences in the surface areas of the Vercise Cartesia contacts were not integrated into the analyses. Indeed, integrating these differences would have implied an increase in the power of non-directional contacts, which, due to larger surfaces, have a lower impedance in comparison with the directional contacts, as has been verified by others [
14]. To evaluate this difference, we measured a 21 Hz sinusoidal wave on electrode contacts bathed in a sodium chloride solution (0.9%). The LFPs were, as expected, less powerful on non-directional than on directional contacts (15%; Supplementary
Figure 1). We refer to the non-integration of that difference in the Discussion.
2.3. Postoperative Image Reconstruction
The DBS electrode trajectories were postoperatively reconstructed by using the Lead-DBS MATLAB toolbox (version 2.3.1;
https://www.lead-dbs.org/ [
5]). The default pipeline was applied: Advanced normalization tools (
https://stnava.github.io/ANTs/) allowed co-registration of brain images, combining the preoperative 3D T1-weighted (repetition time [TR] = 1930 ms, echo time [TE] = 2.36 ms, slice thickness 1 mm; referred to as “anat_t1” in the Lead-DBS toolbox), T2-weighted (TR = 2400 ms, TE = 225 ms, slice thickness 1 mm; referred to as “anat_t2” in the Lead-DBS toolbox), and FLAIR (TR = 5000 ms, TE = 386 ms, slice thickness 1 mm; referred to as “anat_t2star” in the Lead-DBS toolbox) MRIs (Skyra 3.0 T scanner, Siemens Medical Systems, Erlangen, Germany) with the postoperative CT scan performed the day after surgery (slice thickness between 0.6 and 1.25 mm, pixel spacing of 0.453/0.453; Somatom Definition Flash, Siemens Medical Systems). After co-registration, Lead-DBS performed an automatic correction for brain shifts [
15]. As applied in an earlier study [
10], then based on the preoperative volumes, the symmetric image normalization diffeomorphic mapping method [
16], was used to compute multispectral normalization to the ICBM 2009b nonlinear asymmetric space (Montreal Neurological Institute, MNI; [
17]). The unified segmentation method[
18] of Statistical Parametric Mapping software (SPM12;
https://fil.ion.ucl.ac.uk/spm/; [
19]) was applied when the previous approach was unsuccessful. The PaCER method was applied to pre-construct the DBS electrodes (without manual correction[
20]). The orientation of the electrode contacts was not corrected with the DiODe trechnic [
21].
The reconstructed images were segmented with the DISTAL atlas [
6], which brought out all the relevant subcortical structures, including the STN subdivisions. The anatomical structures in relation to each electrode contact were determined by superimposing an atlas that brought out subcortical structures, including the STN subdivisions (i.e. sensorimotor, associative, or limbic), the substantia nigra, the thalamus, the nucleus reticularis polaris (surrounding and enfolding the thalamus), and the zona incerta (ZI). This atlas was made from manual segmentations of a high-resolution brain template series (MNI, 152 template series), to which an atlas of histology and an atlas of structural connectivity were co-registered.
The probabilistic volume containing β-oscillations by the Lead-DBS toolbox [
8] were applied (electrophysiological atlas of the STN activity, β-oscillations). At the time of developing this toolbox, the probabilistic location of β-oscillations was built from bipolar recordings of an externalized Medtronic electrode (quadripolar 3389 lead; 2 mm distance between centres of two adjacent contacts; Medtronic, Minnesota, USA). Power values (309; [7-35 Hz]) were collected over a group of 51 patients. The “power values were mapped onto subcortical anatomy of the brain in (MNI) space”. “Each datapoint was mapped to the Euclidean midpoint between the coordinates representing the two electrode contacts from which the signal was recorded” [
8]. At the time of developing this toolbox, the probabilistic location of the STN-DBS target was built from a group of 39 patients with PD by using the active contacts 1 year post-surgery.
2.4. Statistical Analyses
For each electrode, the contacts that presented significantly stronger β-oscillations were determined by comparing the PSDs of the β-oscillations of each contact with the PSDs of all eight electrode contacts. For this purpose, two-tailed Student t-tests were applied (MATLAB). The differences in β-oscillations across contacts were expressed as percentages of the mean PSDs of all eight electrode contacts.
Analysis of variance (ANOVA) on ranks was used to compare improvements in the MDS-UPDRS motor scores between groups of patients from the location of the stimulated contacts (Kruskal-Wallis, SigmaPlot; in the situation where the equal variance test failed P < 0.05).
3. Results
3.1. β-Oscillations
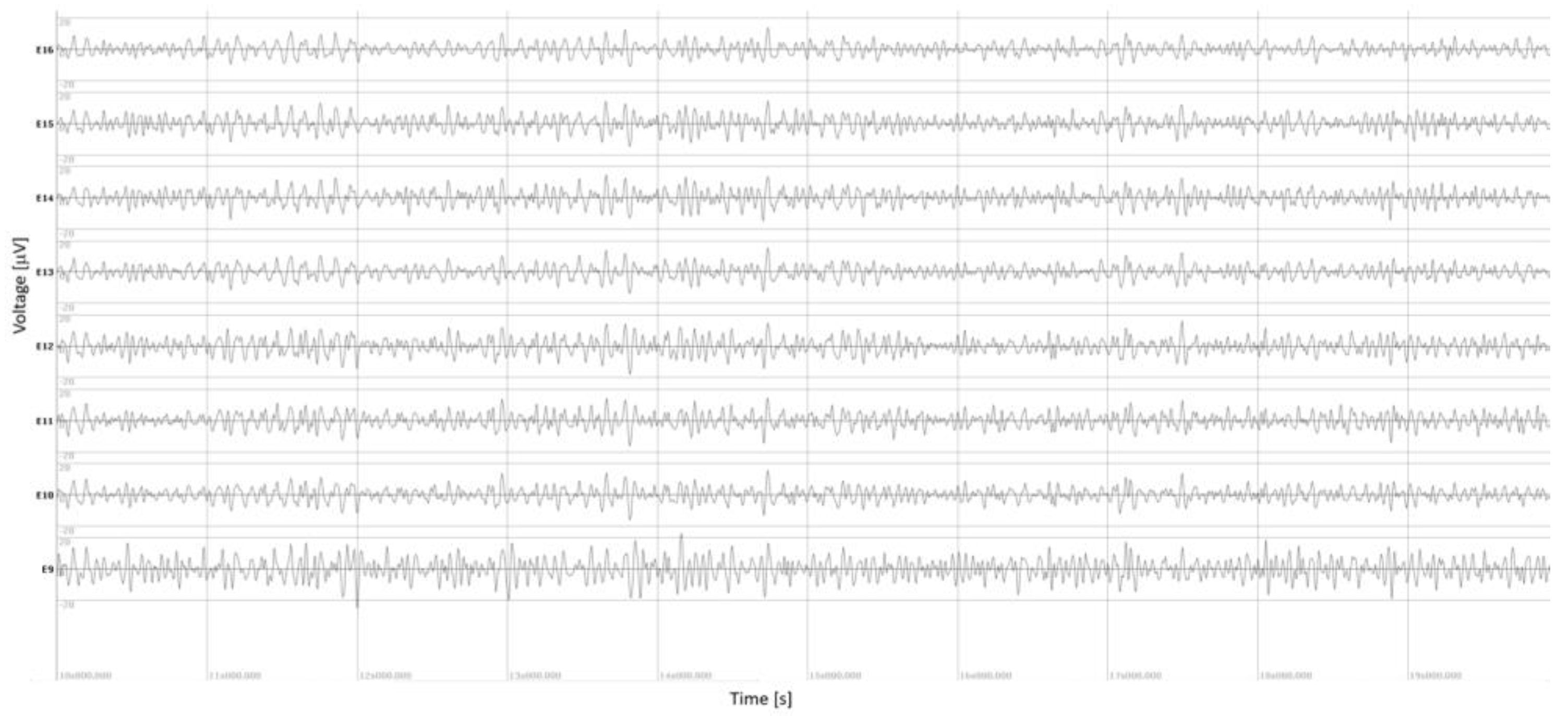
Intraoperative measurements of the LFPs were performed for 36 electrodes (both STNs for 16 patients, a single STN for 4 patients due to intraoperative time constraints). An example of the LFPs, recorded over all eight contacts of an electrode, is illustrated in
Figure 1 (here the right electrode of patient P4; note the high quality of LFPs obtained thanks to the shielding wires). The stronger β-oscillations measured on contact E9, bottom trace, are visible, even before performing statistics. This contact was found with stronger β-oscillations compared with those computed over all contacts of the electrode (130% stronger than the mean of β-oscillations measured on all eight contacts,
P < 1.10
-12).
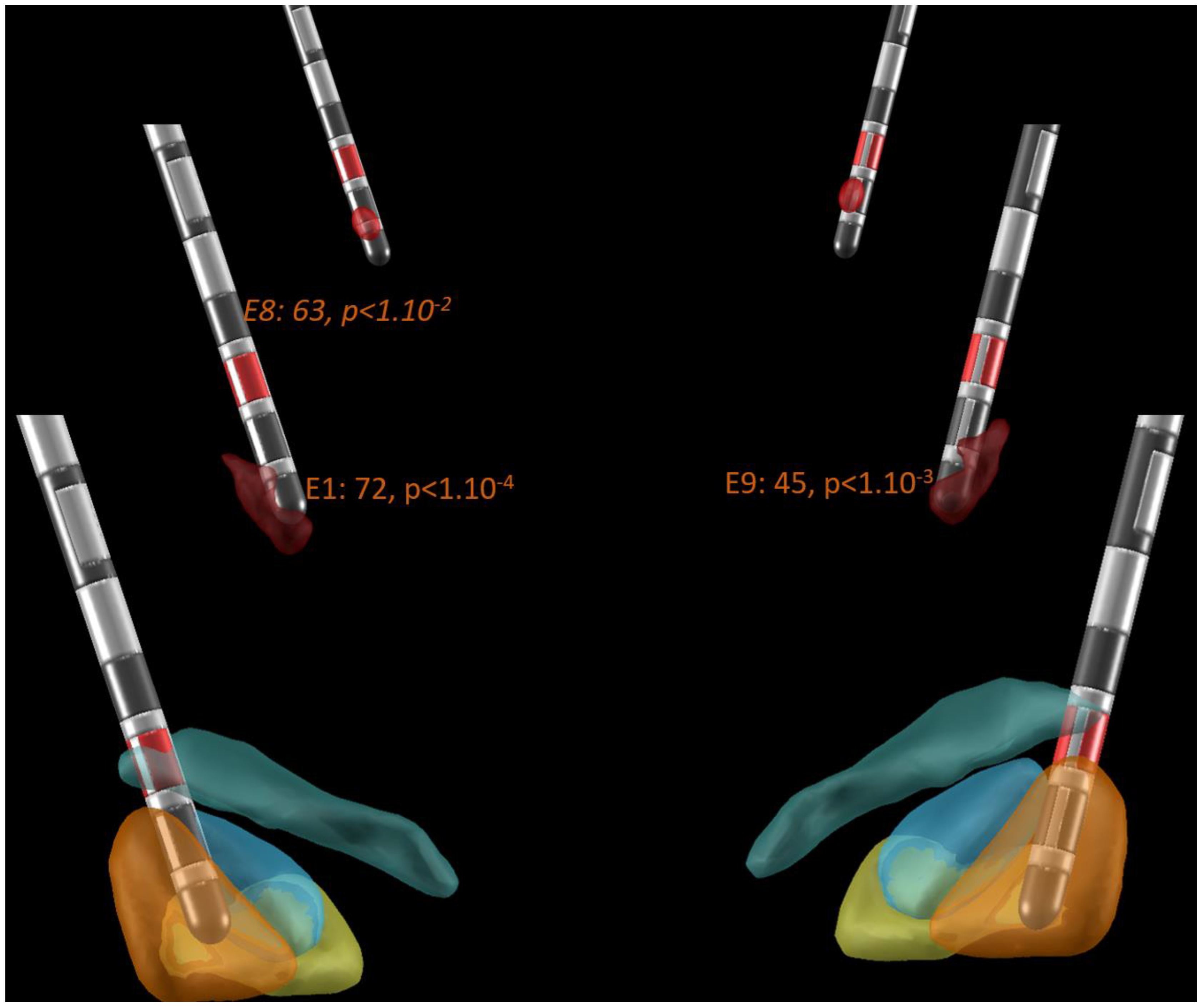
Figure 2 illustrates an example of the Lead-DBS electrode location with the probabilistic locations of β-oscillations (burgundy volume; [
8]), the STN (DISTAL atlas, sensorimotor: orange, limbic: yellow; associative: blue; [
6]), and the STN-DBS target (red volume; [
7]) in patient P16 in a posterior view. Note that the probabilistic locations of β-oscillations presented a banana-like shape in the dorsolateral sensorimotor STN with a larger volume ventrally. Here the most ventral contacts of the electrode of both STNs, E1 and E9, were found with significantly stronger β-oscillations than those computed over all contacts of the electrode (two-tailed Student t-tests: +72%,
P < 1.10
-4, and +45%
, P < 1.10
-3, respectively). In addition, the most dorsal contact of the left electrode, contact E8, also had stronger β-oscillations than those computed over all contacts of the electrode, but with a lower significance than that of the most ventral contact (
P < 1.10
-2 vs
P < 1.10
-4). This contact was located in the Campus Forelii (fields of Forel). The sites of stimulation are indicated with red contacts; here two contacts are dorsal to the site of measured and probabilistic β-oscillations in the sensorimotor subregion of the STN, and one contact is ventral to the site of measured β-oscillations in the Campus Forelii.
Figure 3 shows the location of β-oscillations determined by Lead-DBS and the measured contacts with β-oscillations for the whole group of patients. For all patients (P), the yellow boxes indicate contacts (1 to 8 on left electrodes, L; 9 to 16 on right electrodes, R) found in or touching the probabilistic volume with β-oscillations by the Lead-DBS. The asterisks indicate contacts measured with significantly stronger β-oscillations than those computed over all contacts of the electrode (
** P < 0.01,
*** P < 0.001,
**** P < 0.0001). Supplementary Video 1 illustrates the same probabilistic locations and measured contacts with β-oscillations, together with their statistics, for anterior and posterior views (bottom left of figures).
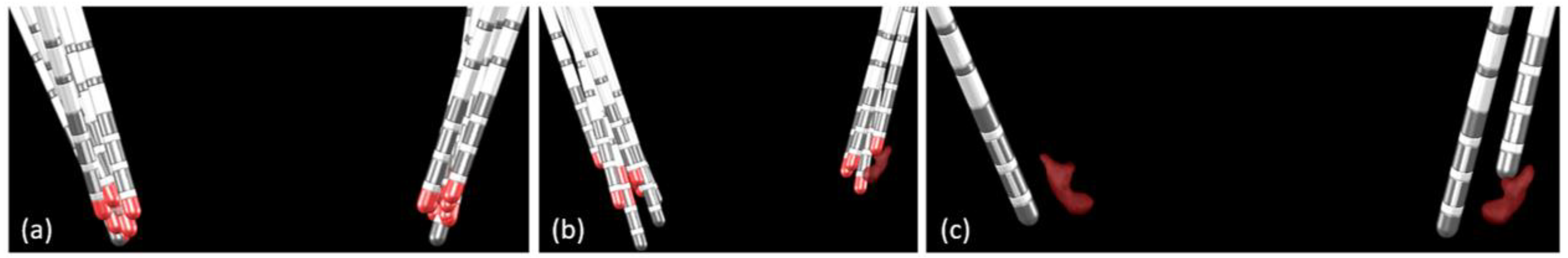
In 23 of the 36 measured electrodes, in 17 of the 20 patients, at least one contact was found in or touching the ventral subpart of the probabilistic volume with β-oscillations (
Figure 3, at least one yellow-filled cell per column;
Figure 4 (a)). In 20 of these 23 electrodes, at least one of the contacts found in or touching the ventral subpart of the probabilistic volume with β-oscillations was also measured with significantly stronger β-oscillations than those computed over all contacts of the electrode (
Figure 3, yellow-filled cells and black stars). In one of these 23 electrodes, the stronger β-oscillations were measured on a contact touching the dorsal subpart of the probabilistic volume with β-oscillations (patient P1, left electrode). In 2 of these 23 cases, the stronger β-oscillations were measured on a contact either dorsal or just ventral to the probabilistic volume (patients P6 and P8, left electrodes).
For 10 of the 36 measured electrodes, in 10 of the 20 patients, no contact was located in or touching the ventral subpart of the probabilistic volume with β-oscillations; however, at least one contact was measured with significantly stronger β-oscillations than those computed over all contacts of the electrode (
Figure 3, no yellow-filled cells per column, but black star(s);
Figure 4 (b)).
For 3 of the 36 measured electrodes, in 3 of the 20 patients, no contact was found in or touching the ventral subpart of the probabilistic volume with β-oscillations and no contact was measured with significantly stronger β-oscillations than those computed over all contacts of the electrode (
Figure 3, no yellow-filled cells and no black asterisks per column;
Figure 4 (c)). For one electrode (patient P6, right electrode), although the most ventral contact was still in contact with the dorsal part of the probabilistic volume with β-oscillations, no significantly stronger β-oscillations were found.
In addition, dorsal to the STN, β-oscillations were found on contacts located in or touching the Campus Forelii (fields of Forel), the ZI, or the internal capsule (IC) close to the Campus Forelii or ZI for 11 electrodes (
Figure 3, upper line, all but three of the cells with grey asterisks; Supplementary
Figure 2 (a)). In three medial electrodes, β-oscillations were found on contacts located in the thalamic ventral-oralis posterior (VLa) or ventral oralis anterior nucleus (
Figure 3, upper line, 3 of 14 cells with grey asterisks, Supplementary
Figure 2 (c); patients P3 left, P15 right, P6 left electrodes). For all three of these electrodes, the significance of the measured β-oscillations was low (
P < 0.01). For deeply inserted electrodes, β-oscillations were found on contacts located in the substantia nigra (
Figure 3, lower line, grey asterisks, Supplementary
Figure 2 (b); patients P8 left, P13 left, P1 left electrodes). For two of these three deep electrodes, the significance of the measured β-oscillations was low (
P < 0.01).
3.2. Electrode Location and Motor Outcome
The motor scores were not available for two patients (P1 and P9).
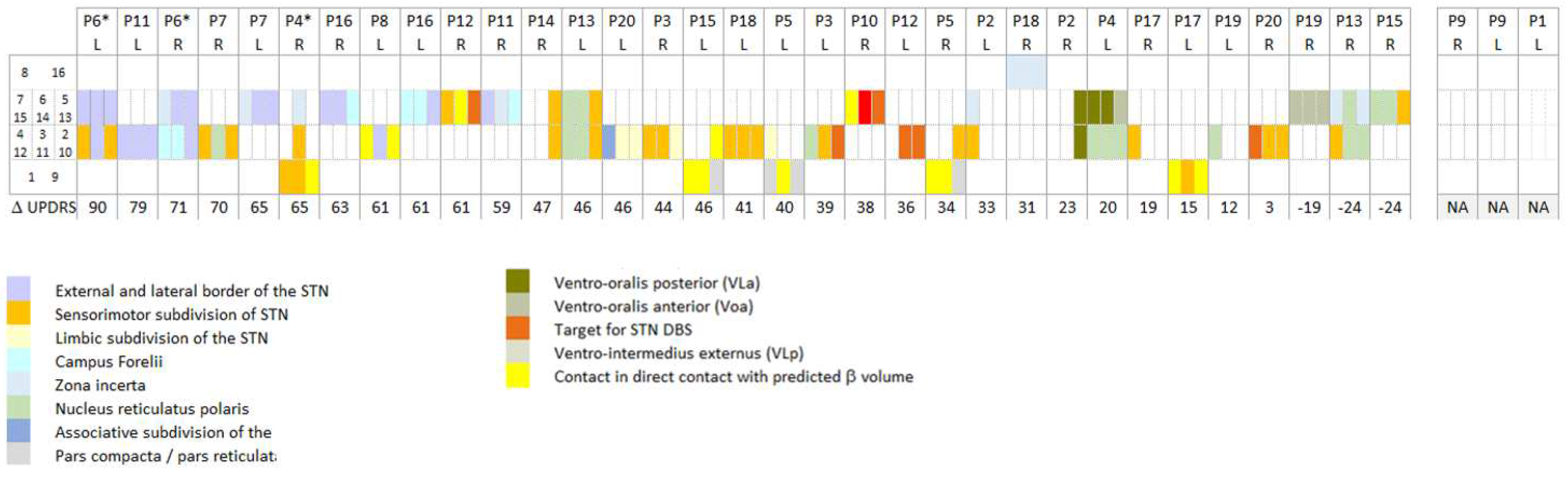
Figure 5 indicates the anatomical structures of the stimulated contacts as obtained with the Lead-DBS DISTAL atlas [
6] in 18 patients for 33 electrodes. The patients were ordered with decreasing lateralized MDS-UPDRS part III improvements of the contralateral hemibody, as stimulated 1 year post-surgery. Patients were then distributed in a post hoc analysis over three categories, from
Figure 5: if at least one stimulated contact was found external, dorsal and/or lateral to the STN (dorsal/lateral: ZI, campus de Forelii, IC), its associated improvement in the MDS-UPDRS score was attributed to the dorsal/lateral group (three right and five left electrodes in five patients). If stimulated electrode contacts were located in the STN, without any contact in the nucleus reticulatus polaris (NRP), the thalamus, or dorsal and/or lateral to the STN, the associated improvements in the MDS-UPDRS score were attributed to the STN category (eight right and seven left electrodes in 11 patients). If at least one stimulated contact was found in the NRP or in a thalamic nucleus, its associated improvement in the MDS-UPDRS score was attributed to the medial group (six right and four left electrodes in seven patients).
Figure 6 (a) illustrates the location of all electrodes for which at least one stimulated contact was found dorsal and/or lateral to the STN, i.e. the dorso/lateral group;
Figure 6 (b) illustrates the location of all electrodes of the STN category, or central group; and
Figure 6 (c) illustrates the location of all electrodes of the medial category.
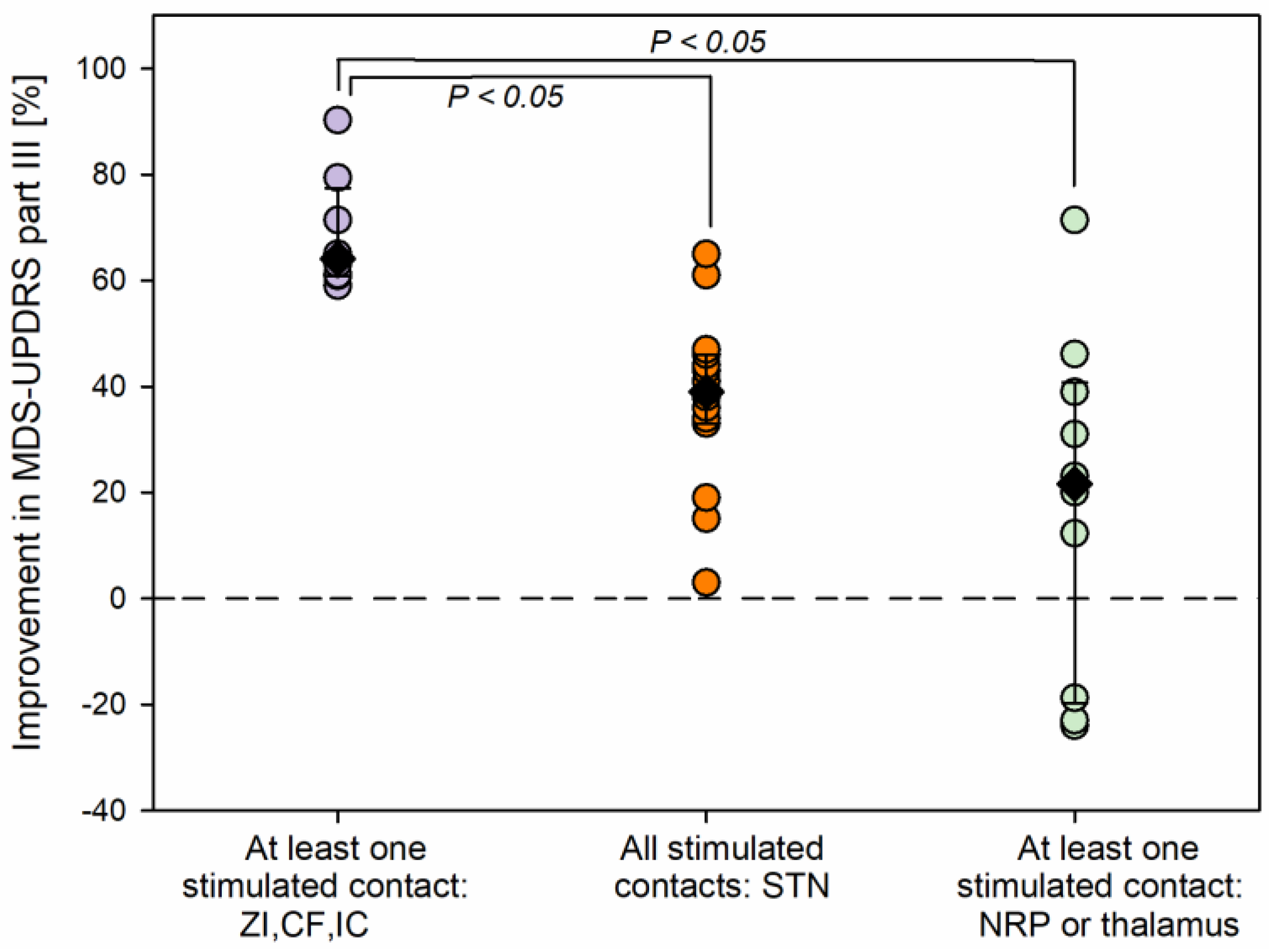
Figure 7 shows the improvements in MDS-UPDRS motor scores 1-year post-surgery for the three groups of patients. Statistically significant differences were found among the groups (Kruskal-Wallis one-way ANOVA on ranks,
H (2) = 15.2,
P < 0.001). The largest improvements were seen in patients for whom at least one stimulated contact was found dorsal and/or lateral to the STN (
Figure 7, purple circles, eight scores, median 64.1%, percentiles: 25
th, 60.9%; 75
th, 77.4%). Their scores were significantly higher than those in patients for whom contacts were found within the STN and with no stimulated contacts dorsal and/or lateral to the STN, or in the NRP, or in the thalamus (Multiple comparison procedure, Dunn’s method,
P < 0.05; orange circles, 15 scores: median 39.0%, percentiles: 25
th, 33.0%; 75
th, 46.0%) and higher than those in patients for whom at least a stimulated contact was found more medially, in the NRP or in a thalamic nucleus (Multiple comparison procedure, Dunn’s method,
P < 0.05; light green scores, 10 scores: median 21.5%, percentiles: 25
th, -19.8%; 75
th, 40.8%).
Among the group of patients with at least one stimulated contact located dorsal and/or lateral to the STN, patient P6 experienced a slightly husky voice, patients P11 and P4 a slight dysarthria, and patient P12 a temporary deviation of the lip towards the right (patients with asterisk,
Figure 5). No capsular side effect was observed for the other DBS patients in their daily life.
As described in
Figure 2 in the background and in Supplementary Video 3 (anterior view), the STN-DBS target, red volume [
7] is located just adjacent and dorsally to the ventral part of the probabilistic volume with β-oscillations. Hence, the present patient series does not confirm the STN-DBS target location as indicated by Lead-DBS. Instead, the study suggests the dorsal and/or lateral external border of the STN, but with the risk of capsular side effects.
Regarding the intraoperative electrophysiological targeting of the STN, at the time of intraoperative stimulation, 9 of 40 electrodes (including electrodes for which β-oscillations were not recorded) were placed on trajectories added to those used for microelectrode recordings, whether or not recordings of the central and/or posterolateral microelectrodes showed typical STN cells. No microelectrode recordings were performed for these additional trajectories in order to avoid having to move the set of exploratory electrodes up and down again. In one of nine cases, a lateral electrode was inserted in addition to the exploratory central and posterolateral electrodes, contributing to the placement of the final DBS electrode lateral to the sensorimotor STN (patient P6, left electrode). In eight of nine cases, a medial or a posteromedial electrode was added in addition to the central and posterolateral trajectories. In six of these eight cases, the added electrode contributed to a deterioration in the targeting of the sensorimotor STN, the electrode being placed medially in the STN or medially to it (patient P7, right electrode; patient P4, left electrode; patients P13 and P19, both electrodes; Supplementary Video 2). On the other hand, the added trajectory contributed to an improvement in the targeting of the sensorimotor STN for two of eight electrodes (patient P5, left electrode; patient P10, left electrode; Supplementary Video 2). Thus, overall, six of nine added electrodes contributed to a deterioration in the targeting of the sensorimotor STN. In 3 of the other 31 STNs (patient P1, right electrode; patient P2, right electrode; patient P15, right electrode; Supplementary Video 2), the definitive electrode was located too medially, with no contact in the STN, where no trajectory was added at the time of stimulation.
4. Discussion
This study, based on high quality β-oscillation measurements from LFPs of the Vercise Cartesia directional electrode contacts, verifies for the first time the accuracy of the location of STN β-oscillations as indicated by the Lead-DBS MATLAB toolbox. In particular, the results validate the ventral part of the probabilistic banana-like shape in the dorsolateral sensorimotor STN in a different data set from a different centre, than that used for the establishment of the atlas.
In addition, the results of the study, including all electrode contacts, whatever were their location and without manual adjustment of electrode imaging reconstruction, in contrary to what can be found in the litteraure, are congruent with the existence of a very efficient stimulation area for reducing motor symptoms that is adjacent but external to the dorsolateral sensorimotor subregion of the STN. This stimulation area was more efficient than the area of the actual STN-DBS target for reducing motor symptoms. Nonetheless, lateral to the sensorimotor STN, the stimulation can induce corticospinal tract stimulation (i.e. capsular effects). The robustness of intraoperative stimulation during STN-DBS surgery was not supported by the present study, which instead supports the development of intraoperative imaging reconstruction tools for STN-DBS surgery.
4.1. β-Oscillations
The Lead-DBS toolbox reconstructed the location of STN β-oscillations [
8], as could be verified with intraoperative measurements. β-oscillations measured on the electrode contacts were confirmed to be located in the ventral part of the dorsolateral sensorimotor STN subdivision. The high agreement found between probabilistic and measured β-oscillations supports the use of the Lead-DBS toolbox to determine their location postoperatively. The ventral part of the banana-like shape probabilistic volume with β-oscillations was the site of maximum measured β-oscillations.
β-oscillations were computed from LFPs which was developed early in the STN-DBS field [
1], but here recorded in monopolar rather than bipolar mode. Indeed, using the shielded cables of the Neurostar electrophysiological system, we intraoperatively placed a neutral reference at the U-shaped skin flap, free of brain activity, allowing monopolar recordings while eliminating the intrinsic contamination proper to the referencing of bipolar measurements. Note that the technic of monopolar recordings, were here performed, without common average, nor intracranial referencing, nor excluding any contacts [
22]. The bipolar recordings used to build the electrophysiological atlas could have contributed to the banana-like shape of the β-oscillations in the probabilistic volume [
8].
Furthermore, if the difference in the surfaces of non-segmented contacts had been integrated, the spectral density would have to be increased by 15% for the most ventral contacts, 1 and 8. This would have again favoured assigning even stronger β-oscillations to the ventral banana-like shape of the probabilistic location of β-oscillations (e.g. patient P6, left STN, contact 1 in addition to or in place of contact 4). If this integration had been done, it would have been suspected that it was in order to support the findings of the present study. We have hence chosen to analyse the raw data without inclusion of that surface adaptation.
The β-oscillations found close to the Campus Forelii and close to the VLa could be due to the proximity of tracts involved in the pallido-subthalamic [
23], or cortico-subthalamic networks [
24] of the exaggerated β-oscillations in patients with PD.
4.2. Electrode Location and Motor Outcomes
The external dorsal and/or lateral borders of the sensorimotor STN do not correspond to the location of the STN-DBS target in the Lead-DBS toolbox. The toolbox suggests that the STN-DBS target would be close to the contacts located within the dorsal part of the banana-like shape of the β-oscillation volume, as illustrated in
Figure 2 [
7].
The patients in the study were the first to receive the Vercise Cartesia directional electrodes in our centre. The possibility cannot be excluded that the neurologists in charge of selecting which contacts to stimulate were biased by the motivation to use the segmented contacts. Whatever the methods applied for choosing the stimulation parameters 1-year post-surgery, the location of the electrodes, spanning from lateral to medial, offered the possibility to analyse the efficacy of stimulating the anatomical structures. Note that all contacts, either located in or not inside the STN, were included in the study, which is not always the case in the literature.
For improvement in rigidity in particular [
25] but not only, the external dorsal border of the sensorimotor STN can be preferred [
26]. The caudal field of Forel – or Campus of Forel and Campus Forelii – or the lenticular fasciculus could be involved in the efficacy of the stimulation of contacts dorsal to the sensorimotor STN. The lateral border of the STN has also been identified as an efficient stimulation site [
27,
28] as has the activation of the hyperdirect loop [
29,
30]. Several other tracts are located there, as again recently illustrated [
31]. Lateral to the STN is located the subthalamic fasciculus or pallidosubthalamic bundle, which “terminates in the lateral part of the STN”, as illustrated with 11.7 Tesla MRIs [
32]. The subthalamic fasciculus might also be an efficient site for motor improvement, in agreement with the excellent efficacy of globus pallidus externa stimulation [
10]. Nevertheless, the lateral border of the STN can induce capsular effects from corticospinal tract stimulation that can prevent the use of contacts. Access to segmented contacts, knowing its precise location, should become an advantage in this context. On the other hand, trajectories going through the NRP or from the Vla of the thalamus, i.e. more medial trajectories, did not result in efficient DBS targets for PD [
33].
4.3. Considerations for DBS Surgery
In other respects, the study suggests that intraoperative evaluation of corticospinal tract excitation failed to improve the efficacy of DBS [
12,
34]. The effects of corticospinal tract excitation can be confused with dyskinesia secondary to STN stimulation. Indeed, among the nine electrodes placed on added trajectories at the time of surgery based on intraoperative stimulation, only one contributed to getting closer to the lateral STN border; the others led to being closer to the medial STN border, which resulted in weak motor improvements. Note that because of the Ben-Gun array geometry with a distance of 2.8 mm between the posteromedial and posterolateral trajectories, and considering the size and shape of the STN, there was a very poor chance that an added medial trajectory could go through the STN when microelectrode recordings indicated STN cells on the central and posterolateral trajectories, for instance. Instead of intraoperative stimulation, the posterolateral STN could be targeted through the measurement of β-oscillations originating in the sensorimotor STN of the macro-contact of the explorative microelectrode in conjunction with microelectrode recordings.
As the Lead-DBS toolbox indicates contacts with β-oscillations, an imaging reconstruction suite as precise as it is, and its implemented anatomical atlases, should ideally be considered for intraoperative use with the acquired intraoperative 3D images.
5. Conclusions
This study, based on β-oscillation measurements from monopolar LFPs of the Vercise Cartesia directional electrode contacts, verifies the accuracy of the location of β-oscillations as indicated by the Lead-DBS MATLAB toolbox. In particular, the results validate the ventral part of the probabilistic location in the dorsolateral sensorimotor STN. Moreover, the largest improvements of motor symptoms were found with DBS electrodes located on the dorsal and/or lateral external borders of the sensorimotor STN. The robustness of intraoperative stimulation during STN-DBS surgery was not, supported by the present study, which instead promotes the development of intraoperative imaging reconstruction tools for STN-DBS surgery.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Figure 1: Measurements of a 21 Hz sinusoidal wave on a Vercise Cartesia electrode.; Supplementary Figure 2: Illustration of other anatomical structures where β-oscillations were measured (posterior view, DISTAL atlas); Supplementary Video 1: Postoperative image reconstruction of the electrodes (anterior view; posterior view on the bottom left) with the predicted β-oscillations performed with Lead-DBS (yellow volume) and the statistics measured for contacts with stronger β-oscillations. Supplementary Video 2: Postoperative image reconstruction of the electrodes performed with Lead-DBS in all patients (anterior view) relative to subcortical structures using the DISTAL atlas. Supplementary Video 3: STN-DBS-predicted stimulation target according to the Lead-DBS target atlas.
Author Contributions
“Conceptualization, C.B.; methodology, C.B., R.T., A.K. and A.A.A..; software, R.T., A.K.; validation, V.F. and P.B.; formal analysis, C.B.; investigation, C.B., R.T., M.V.C., V.F., P.B., S.M.; resources, S.M. and P.B.; data curation, C.B.; writing—original draft preparation, C.B., A.A.A., R.T., F.V.; writing—review and editing, C.B., A.A.A., R.T., M.V.C., A.K., V.F., P.B., and S.M.; visualization, C.B., and A.A.A.; supervision, S.M., and P.B.; project administration, C.B.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Institutional Review Board Statement
This retrospective study was approved by our local Ethics Committee (CE N° 2020-02010). The ethical guidelines of the Declaration of Helsinki were applied.
Informed Consent Statement
All patients gave their approval either for general research or for the re-use of their data linked to DBS, by signing the dedicated forms.
Data Availability Statement
Apart from the open-access Lead-DBS toolbox (
www.lead-dbs.org), the MATLAB code used for analysing β-oscillations will be shared on request to C.B. The raw data will be shared, respecting the limitations of the University Hospitals of Geneva and in agreement with our local Ethics Committee, through the University of Geneva (
http://www.kheops.ch/).
Acknowledgments
We thank the patients who permitted their data to be included in the study. We also thank E. Tomkova-Chaoui, specialist nurse in our clinical unit for patients with movement disorders, and Dr sc. Eva Blondiaux, who proposed the use of a matrix to present all patient cases together.
Conflicts of Interest
“The authors declare no conflict of interest.”
References
- Brown, P.; A. Oliviero; P. Mazzone; A. Insola; P. Tonali and V. Di Lazzaro. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci, 2001. 21(3): p. 1033-8.
- Levy, R.; P. Ashby; W.D. Hutchison; A.E. Lang; A.M. Lozano and J.O. Dostrovsky. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain, 2002. 125(Pt 6): p. 1196-209.
- Brown, P.; P. Mazzone; A. Oliviero; M.G. Altibrandi; F. Pilato; P.A. Tonali and V. Di Lazzaro. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson's disease. Exp Neurol, 2004. 188(2): p. 480-90. [CrossRef]
- Zaidel, A.; A. Spivak; B. Grieb; H. Bergman and Z. Israel. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson's disease. Brain, 2010. 133(Pt 7): p. 2007-21. [CrossRef]
- Horn, A. and A.A. Kuhn. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage, 2015. 107: p. 127-135. [CrossRef]
- Ewert, S.; P. Plettig; N. Li; M.M. Chakravarty; D.L. Collins; T.M. Herrington; A.A. Kuhn and A. Horn. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage, 2018. 170: p. 271-282. [CrossRef]
- Horn, A.; A.A. Kuhn; A. Merkl; L. Shih; R. Alterman and M. Fox. Probabilistic conversion of neurosurgical DBS electrode coordinates into MNI space. Neuroimage, 2017. 150: p. 395-404. [CrossRef]
- Horn, A.; W.J. Neumann; K. Degen; G.H. Schneider and A.A. Kuhn. Toward an electrophysiological "sweet spot" for deep brain stimulation in the subthalamic nucleus. Hum Brain Mapp, 2017. 38(7): p. 3377-3390. [CrossRef]
- Nowacki, A.; T.A. Nguyen; G. Tinkhauser; K. Petermann; I. Debove; R. Wiest and C. Pollo. Accuracy of different three-dimensional subcortical human brain atlases for DBS -lead localisation. Neuroimage Clin, 2018. 20: p. 868-874. [CrossRef]
- Al Awadhi, A.; R. Tyrand; A. Horn; A. Kibleur; J. Vincentini; A. Zacharia; P.R. Burkhard; S. Momjian and C. Boex. Electrophysiological confrontation of Lead-DBS-based electrode localizations in patients with Parkinson's disease undergoing deep brain stimulation. Neuroimage Clin, 2022. 34: p. 102971. [CrossRef]
- Fernandez-Garcia, C.; G. Foffani; M. Dileone; M.J. Catalan-Alonso; M. Gonzalez-Hidalgo; J.A. Barcia and F. Alonso-Frech. Directional local field potential recordings for symptom-specific optimization of deep brain stimulation. Mov Disord, 2017. 32(4): p. 626-628. [CrossRef]
- Boex, C.; R. Tyrand; J. Horvath; V. Fleury; S. Sadri; M. Corniola; P.R. Burkhard and S. Momjian. What Is the Best Electrophysiologic Marker of the Outcome of Subthalamic Nucleus Stimulation in Parkinson Disease? World Neurosurg, 2018. 120: p. e1217-e1224. [CrossRef]
- Goetz, C.G.; B.C. Tilley; S.R. Shaftman; G.T. Stebbins; S. Fahn; P. Martinez-Martin; W. Poewe; C. Sampaio; M.B. Stern; R. Dodel; B. Dubois; R. Holloway; J. Jankovic; J. Kulisevsky; A.E. Lang; A. Lees; S. Leurgans; P.A. LeWitt; D. Nyenhuis; C.W. Olanow; O. Rascol; A. Schrag; J.A. Teresi; J.J. van Hilten; N. LaPelle and U.R.T.F. Movement Disorder Society. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord, 2008. 23(15): p. 2129-70. [CrossRef]
- Eleopra, R.; S. Rinaldo; G. Devigili; C. Lettieri; M. Mondani; S. D'Auria; M. Piacentino and M. Pilleri. Brain impedance variation of directional leads implanted in subthalamic nuclei of Parkinsonian patients. Clin Neurophysiol, 2019. 130(9): p. 1562-1569. [CrossRef]
- Schönecker, T., Kupsch, A., Kühn, A.A., Schneider, G.-H., Hoffmann, K.T.,. Automated optimization of subcortical cerebral MR imaging-atlas coregistration for improved postoperative electrode localization in deep brain stimulation. AJNR Am. J Neuroradiol., 2009. 30: p. 8. p: 30.
- Avants, B.B.; C.L. Epstein; M. Grossman and J.C. Gee. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal, 2008. 12(1): p. 26-41. [CrossRef]
- Fonov, V.; A.C. Evans; K. Botteron; C.R. Almli; R.C. McKinstry; D.L. Collins and G. Brain Development Cooperative. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage, 2011. 54(1): p. 313-27. [CrossRef]
- Ashburner, J. and K.J. Friston. Unified segmentation. Neuroimage, 2005. 26(3): p. 839-51. [CrossRef]
- Friston, K.J.; G. Tononi; G.N. Reeke, Jr.; O. Sporns and G.M. Edelman. Value-dependent selection in the brain: simulation in a synthetic neural model. Neuroscience, 1994. 59(2): p. 229-43.
- Husch, A.; V.P. M; P. Gemmar; J. Goncalves and F. Hertel. PaCER - A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation. Neuroimage Clin, 2018. 17: p. 80-89. [CrossRef]
- Dembek, T.A.; A. Hellerbach; H. Jergas; M. Eichner; J. Wirths; H.S. Dafsari; M.T. Barbe; S. Hunsche; V. Visser-Vandewalle and H. Treuer. DiODe v2: Unambiguous and Fully-Automated Detection of Directional DBS Lead Orientation. Brain Sci, 2021. 11(11). [CrossRef]
- Averna, A.; I. Debove; A. Nowacki; K. Peterman; B. Duchet; M. Sousa; E. Bernasconi; L. Alva; M.L. Lachenmayer; M. Schuepbach; C. Pollo; P. Krack; T.K. Nguyen and G. Tinkhauser. Spectral Topography of the Subthalamic Nucleus to Inform Next-Generation Deep Brain Stimulation. Mov Disord, 2023. [CrossRef]
- Mallet, N.; A. Pogosyan; L.F. Marton; J.P. Bolam; P. Brown and P.J. Magill. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci, 2008. 28(52): p. 14245-58. [CrossRef]
- Gulberti, A.; W. Hamel; C. Buhmann; K. Boelmans; S. Zittel; C. Gerloff; M. Westphal; A.K. Engel; T.R. Schneider and C.K. Moll. Subthalamic deep brain stimulation improves auditory sensory gating deficit in Parkinson's disease. Clin Neurophysiol, 2015. 126(3): p. 565-74. [CrossRef]
- Akram, H.; S.N. Sotiropoulos; S. Jbabdi; D. Georgiev; P. Mahlknecht; J. Hyam; T. Foltynie; P. Limousin; E. De Vita; M. Jahanshahi; M. Hariz; J. Ashburner; T. Behrens and L. Zrinzo. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson's disease. Neuroimage, 2017. 158: p. 332-345. [CrossRef]
- Maks, C.B.; C.R. Butson; B.L. Walter; J.L. Vitek and C.C. McIntyre. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatry, 2009. 80(6): p. 659-66. [CrossRef]
- Elias, G.J.B.; A. Boutet; S.E. Joel; J. Germann; D. Gwun; C. Neudorfer; R.M. Gramer; M. Algarni; V. Paramanandam; S. Prasad; M.E. Beyn; A. Horn; R. Madhavan; M. Ranjan; C.S. Lozano; A.A. Kuhn; J. Ashe; W. Kucharczyk; R.P. Munhoz; P. Giacobbe; S.H. Kennedy; D.B. Woodside; S.K. Kalia; A. Fasano; M. Hodaie and A.M. Lozano. Probabilistic Mapping of Deep Brain Stimulation: Insights from 15 Years of Therapy. Ann Neurol, 2021. 89(3): p. 426-443. [CrossRef]
- Garcia-Garcia, D.; J. Guridi; J.B. Toledo; M. Alegre; J.A. Obeso and M.C. Rodriguez-Oroz. Stimulation sites in the subthalamic nucleus and clinical improvement in Parkinson's disease: a new approach for active contact localization. J Neurosurg, 2016. 125(5): p. 1068-1079. [CrossRef]
- Chen, Y.; S. Ge; Y. Li; N. Li; J. Wang; X. Wang; J. Li; J. Jing; M. Su; Z. Zheng; T. Luo; C. Qiu and X. Wang. Role of the Cortico-Subthalamic Hyperdirect Pathway in Deep Brain Stimulation for the Treatment of Parkinson Disease: A Diffusion Tensor Imaging Study. World Neurosurg, 2018. 114: p. e1079-e1085. [CrossRef]
- Peeters, J.; A. Boogers; T. Van Bogaert; T.A. Dembek; R. Gransier; J. Wouters; W. Vandenberghe; P. De Vloo; B. Nuttin and M. Mc Laughlin. Towards biomarker-based optimization of deep brain stimulation in Parkinson's disease patients. Front Neurosci, 2022. 16: p. 1091781. [CrossRef]
- Avecillas-Chasin, J.M.; T.A. Hurwitz; N.M. Bogod and C.R. Honey. An Analysis of Clinical Outcome and Tractography following Bilateral Anterior Capsulotomy for Depression. Stereotact Funct Neurosurg, 2019. 97(5-6): p. 369-380. [CrossRef]
- Oishi, K.; S. Mori; J.C. Troncoso and F.A. Lenz. Mapping tracts in the human subthalamic area by 11.7T ex vivo diffusion tensor imaging. Brain Struct Funct, 2020. 225(4): p. 1293-1312. [CrossRef]
- Chen, C.C.; A. Pogosyan; L.U. Zrinzo; S. Tisch; P. Limousin; K. Ashkan; T. Yousry; M.I. Hariz and P. Brown. Intra-operative recordings of local field potentials can help localize the subthalamic nucleus in Parkinson's disease surgery. Exp Neurol, 2006. 198(1): p. 214-21. [CrossRef]
- Houeto, J.L.; M.L. Welter; P.B. Bejjani; S. Tezenas du Montcel; A.M. Bonnet; V. Mesnage; S. Navarro; B. Pidoux; D. Dormont; P. Cornu and Y. Agid. Subthalamic stimulation in Parkinson disease: intraoperative predictive factors. Arch Neurol, 2003. 60(5): p. 690-4. [CrossRef]
Figure 1.
Example of local field potentials (LFPs) recorded intraoperatively on an electrode in patient P4 (right electrode). Intraoperative LFPs recorded on all eight contacts of the electrodes (band pass filtering [13-31 Hz]). E9, right most ventral contact, bottom trace; E16, right most dorsal contact, top trace; directional contacts, in between. For this electrode, stronger β-oscillations were measured on contact E9 (+130%, P < 1.10-12).
Figure 1.
Example of local field potentials (LFPs) recorded intraoperatively on an electrode in patient P4 (right electrode). Intraoperative LFPs recorded on all eight contacts of the electrodes (band pass filtering [13-31 Hz]). E9, right most ventral contact, bottom trace; E16, right most dorsal contact, top trace; directional contacts, in between. For this electrode, stronger β-oscillations were measured on contact E9 (+130%, P < 1.10-12).
Figure 2.
Postoperative image reconstruction of the electrodes performed with Lead-DBS in patient P16 (posterior view). Foreground: Reconstructed location of subthalamic nucleus sensorimotor (orange), limbic (yellow), and associative (blue) subregions; Campus Forelii (grey green, DISTAL atlas), with stimulated contacts shown in red on all three planes. Second plane: Probabilistic location of β-oscillations (burgundy volume) with statistics of measured β-oscillations. If significantly stronger, the percentage of the difference in power of the β-oscillations of a contact, in comparison to the power of the β-oscillations of all eight electrode contacts, is indicated with the significance of the difference (E1, left most ventral contact; E9, right most ventral contact E8, left most dorsal contact; directional contacts, in between). Background: STN-DBS probabilistic stimulation target according to the DBS target atlas (red volume in background).
Figure 2.
Postoperative image reconstruction of the electrodes performed with Lead-DBS in patient P16 (posterior view). Foreground: Reconstructed location of subthalamic nucleus sensorimotor (orange), limbic (yellow), and associative (blue) subregions; Campus Forelii (grey green, DISTAL atlas), with stimulated contacts shown in red on all three planes. Second plane: Probabilistic location of β-oscillations (burgundy volume) with statistics of measured β-oscillations. If significantly stronger, the percentage of the difference in power of the β-oscillations of a contact, in comparison to the power of the β-oscillations of all eight electrode contacts, is indicated with the significance of the difference (E1, left most ventral contact; E9, right most ventral contact E8, left most dorsal contact; directional contacts, in between). Background: STN-DBS probabilistic stimulation target according to the DBS target atlas (red volume in background).
Figure 3.
Graphical representation of contacts found in, or touching, the probabilistic volume with β-oscillations by Lead-DBS. Filled yellow boxes are contacts in or touching the ventral subpart of the probabilistic volume with β-oscillations; unfilled yellow boxes are contacts in or touching the dorsal subpart of the probabilistic volume with β-oscillations by the Lead-DBS toolbox with contacts measured with stronger β-oscillations (
**P < 0.01;
***P < 0.001,
****P < 0.0001; grey asterisks for contacts found outside the subthalamic nucleus, refer to Supplementary
Figure 2). For all patients (P, in column), for left (L) and right (R) electrodes, the contacts of the electrodes are represented in rows: contacts E1 (left) and E9 (right), most ventral contacts; contacts E8 (left) and E16 (right), most dorsal contacts; directional contacts, in between. Electrodes are ordered with decreasing improvements in lateralized MDS-UPDRS part III scores of the contralateral hemibody, as stimulated 1 year post-surgery (NA, not available for two patients).
Figure 3.
Graphical representation of contacts found in, or touching, the probabilistic volume with β-oscillations by Lead-DBS. Filled yellow boxes are contacts in or touching the ventral subpart of the probabilistic volume with β-oscillations; unfilled yellow boxes are contacts in or touching the dorsal subpart of the probabilistic volume with β-oscillations by the Lead-DBS toolbox with contacts measured with stronger β-oscillations (
**P < 0.01;
***P < 0.001,
****P < 0.0001; grey asterisks for contacts found outside the subthalamic nucleus, refer to Supplementary
Figure 2). For all patients (P, in column), for left (L) and right (R) electrodes, the contacts of the electrodes are represented in rows: contacts E1 (left) and E9 (right), most ventral contacts; contacts E8 (left) and E16 (right), most dorsal contacts; directional contacts, in between. Electrodes are ordered with decreasing improvements in lateralized MDS-UPDRS part III scores of the contralateral hemibody, as stimulated 1 year post-surgery (NA, not available for two patients).
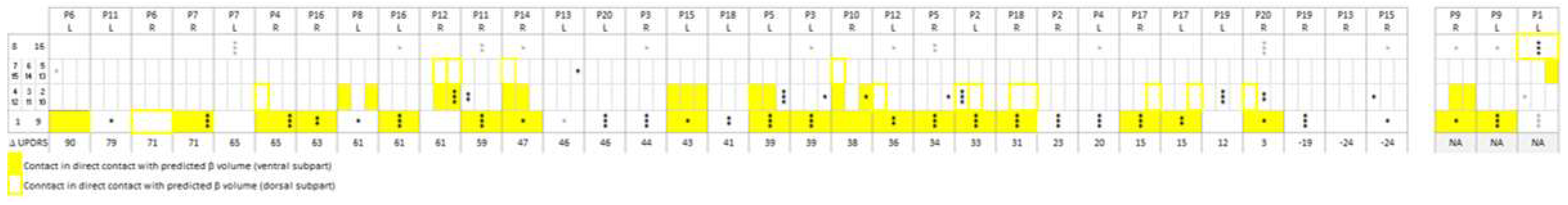
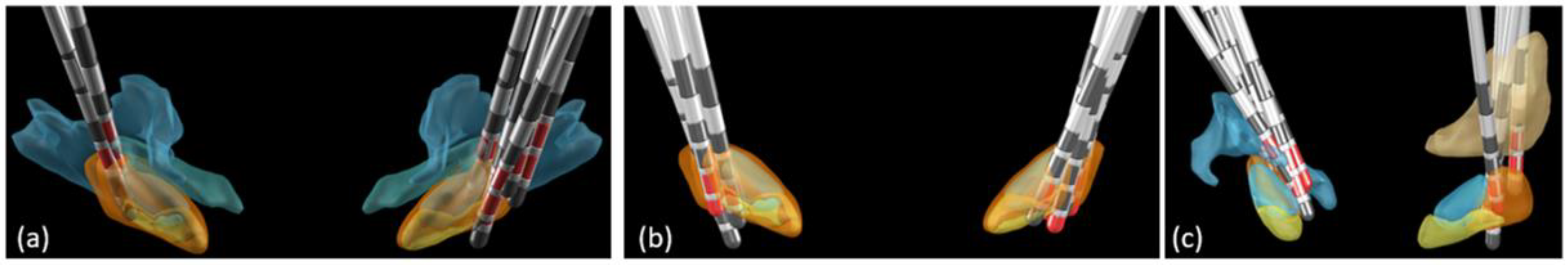
Figure 4.
Postoperative image reconstruction of the electrodes, with the probabilistic β-oscillations performed with Lead-DBS (posterior view). Contacts measured with significantly stronger β-oscillations than those computed over all electrode contacts (red contacts) were found for either (a) electrodes with at least one contact in or touching the ventral subpart of the probabilistic volume with β-oscillations, or (b) electrodes not in or touching the probabilistic volume with β-oscillations. (c): electrodes not found with β-oscillations and nor in nor adjacent to the probabilistic ventral part of the β-oscillations.
Figure 4.
Postoperative image reconstruction of the electrodes, with the probabilistic β-oscillations performed with Lead-DBS (posterior view). Contacts measured with significantly stronger β-oscillations than those computed over all electrode contacts (red contacts) were found for either (a) electrodes with at least one contact in or touching the ventral subpart of the probabilistic volume with β-oscillations, or (b) electrodes not in or touching the probabilistic volume with β-oscillations. (c): electrodes not found with β-oscillations and nor in nor adjacent to the probabilistic ventral part of the β-oscillations.
Figure 5.
Graphical representation of the anatomical structures where stimulated contacts were located. Anatomical structures were those found by applying the Lead-DBS DISTAL atlas. The stimulated contacts are those stimulated 1 year post-surgery at the time of the evaluation of the lateralized MDS-UPDRS part III scores, reported in the last row of the matrix. For all patients (P), for left (L) and right (R) electrodes, the contacts of the electrodes are represented in rows: contacts 1 (left) and 9 (right), most ventral contacts; contacts 8 (left) and 16 (right), most dorsal contacts; directional contacts, in between. Electrodes are ordered with decreasing motor improvements (bottom row; NA, not available for two patients). *Patients with capsular side effects.
Figure 5.
Graphical representation of the anatomical structures where stimulated contacts were located. Anatomical structures were those found by applying the Lead-DBS DISTAL atlas. The stimulated contacts are those stimulated 1 year post-surgery at the time of the evaluation of the lateralized MDS-UPDRS part III scores, reported in the last row of the matrix. For all patients (P), for left (L) and right (R) electrodes, the contacts of the electrodes are represented in rows: contacts 1 (left) and 9 (right), most ventral contacts; contacts 8 (left) and 16 (right), most dorsal contacts; directional contacts, in between. Electrodes are ordered with decreasing motor improvements (bottom row; NA, not available for two patients). *Patients with capsular side effects.
Figure 6.
Postoperative image reconstruction of the electrodes (anterior view) with the stimulated contacts (red contacts). Anatomical structures were those found by applying the Lead-DBS DISAL atlas. The stimulated contacts are those stimulated 1 year post-surgery at the time of the evaluation of the lateralized MDS-UPDRS part III scores: (a) For electrodes with at least one stimulated contact in the zona incerta (ZI), the campus Forelii (CF) or the internal capsule (IC); (b) for electrodes with stimulated contacts in the subthalamic nucleus without any contact in the ZI, in the CF, in the IC, in the nucleus reticulatus polaris (NRP), or in the thalamus; (c) for electrodes with at least one stimulated electrode contact in the NRP or in the thalamus.
Figure 6.
Postoperative image reconstruction of the electrodes (anterior view) with the stimulated contacts (red contacts). Anatomical structures were those found by applying the Lead-DBS DISAL atlas. The stimulated contacts are those stimulated 1 year post-surgery at the time of the evaluation of the lateralized MDS-UPDRS part III scores: (a) For electrodes with at least one stimulated contact in the zona incerta (ZI), the campus Forelii (CF) or the internal capsule (IC); (b) for electrodes with stimulated contacts in the subthalamic nucleus without any contact in the ZI, in the CF, in the IC, in the nucleus reticulatus polaris (NRP), or in the thalamus; (c) for electrodes with at least one stimulated electrode contact in the NRP or in the thalamus.
Figure 7.
Comparison in improvements in the MDS-UPDRS part III scores of the contralateral hemibody, as stimulated 1 year post-surgery for different anatomical structures. Purple symbols: at least one stimulated contact in the external dorsal/lateral borders of the STN (in the zona incerta, ZI, or campus Forelii, CF, or internal capsule, CI; orange symbols: stimulated contacts in the STN without any stimulated contact in the ZI, CF or IC, nucleus reticularis polaris (NRP) or thalamus; green symbols: patients with at least one stimulated electrode contact in the NRP or in the thalamus.
Figure 7.
Comparison in improvements in the MDS-UPDRS part III scores of the contralateral hemibody, as stimulated 1 year post-surgery for different anatomical structures. Purple symbols: at least one stimulated contact in the external dorsal/lateral borders of the STN (in the zona incerta, ZI, or campus Forelii, CF, or internal capsule, CI; orange symbols: stimulated contacts in the STN without any stimulated contact in the ZI, CF or IC, nucleus reticularis polaris (NRP) or thalamus; green symbols: patients with at least one stimulated electrode contact in the NRP or in the thalamus.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).