Submitted:
27 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
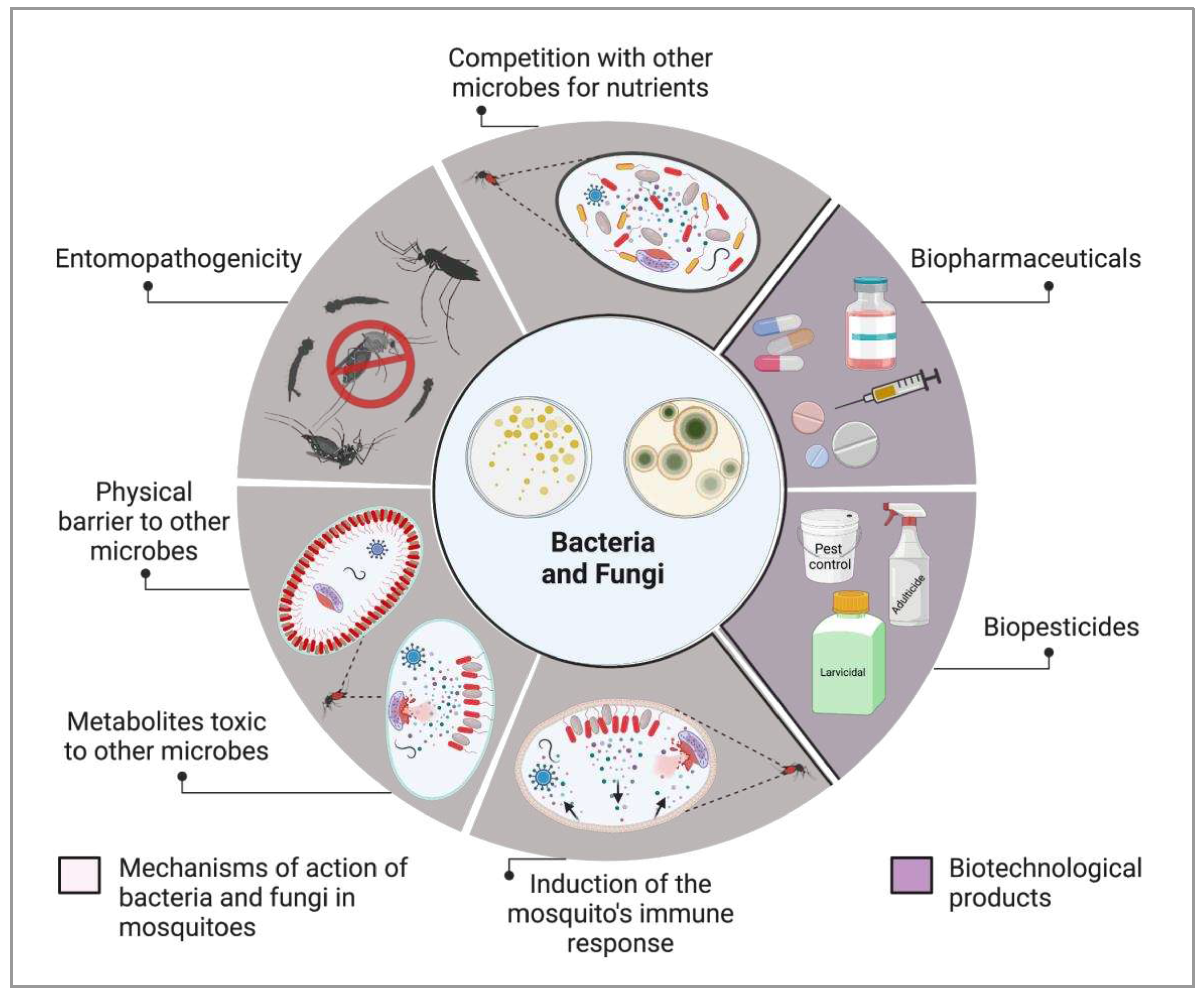
2. Bacteria for biological control of medically important mosquitoes
3. Fungi as vector mosquito biocontrol agents
| Fungus | Toxic formulation | Target mosquito genera | Refs | ||
|---|---|---|---|---|---|
| Aedes | Culex | Anopheles | |||
| Beauveria bassiana | Fungal suspensions | + | - | - | [142] |
| Surfaces treated with conidia | + | - | - | [145] | |
| Spores | + | - | - | [132] | |
| Oil-formulated spores | - | - | + | [146] | |
| Fungal suspensions | - | - | + | [149] | |
| Spores | - | - | + | [147] | |
| Fungal suspensions | + | + | - | [171] | |
| Metarhizium anisopliae | Conidial suspension | - | + | - | [172] |
| Fungal conidia | + | - | - | [173] | |
| Fungal suspensions | + | - | - | [142] | |
| Conidial suspension | + | + | - | [157] | |
| Oil formulation | - | + | + | [174] | |
| Secondary metabolites | + | + | + | [175] | |
| Aspergillus niger | Crude metabolites | + | + | + | [163] |
| Aspergillus flavus | Secondary metabolites | + | + | + | [164] |
| Suspensions of conidia | + | - | - | [166] | |
| Culture filtrates | - | + | - | [176] | |
| Aspergillus fumigatus | Secondary metabolites | + | + | + | [164] |
| Aspergillus parasiticus | Culture filtrates | - | + | - | [176] |
| Aspergillus tamarii | Endophytic Fungal Extracts | + | + | - | [165] |
| Aspergillus terreus | Mycelia (Ethyl acetate and methanol extracts) | + | + | + | [177] |
| Emodin compound | + | + | + | [178] | |
| Aspergillus nomius | Spores | + | - | - | [162] |
| Beauveria tenella | Blastospores suspensions | + | + | - | [179] |
| Cladophialophora bantiana | Secondary metabolites | + | + | - | [180] |
| Chrysosporium lobatum | Secondary metabolites | - | + | + | [181] |
| Chrysosporium tropicum | Secondary metabolites | + | + | + | [182] |
| Fusarium moniliforme | Isoquinoline type pigment | + | - | + | [183] |
| Fusarium oxysporum | Temephos + F. oxysporum extract | + | + | + | [184] |
| Fusarium vasinfectum | Culture filtrates | - | + | - | [176] |
| Isaria javanica | Conidial suspensions | + | - | - | [134] |
| Isaria cateniannulata | Conidial suspensions | + | - | - | [134] |
| Isaria tenuipes | Conidial suspensions | + | - | - | [167] |
| Isaria fumosorosea | Secondary metabolites | + | + | - | [168] |
| Paecilomyces sp. | Secondary metabolites | + | + | + | [131] |
| Penicillium daleae | Mycelium extract | + | + | - | [185] |
| Penicillium falicum | Culture filtrates | - | + | - | [176] |
| Penicillium marneffei | Spores | - | + | - | [186] |
| Penicillium sp. | Ethyl acetate extract | - | + | - | [187] |
| Ethyl acetate extract | + | + | - | [188] | |
| Pestalotiopsis virgulata | Ethyl acetate mycelia (EAM) extracts and liquid culture media (LCM) | + | - | + | [189] |
| Podospora sp. | Sterigmatocystin compound | - | - | + | [190] |
| Pycnoporus sanguineus | Ethyl acetate mycelia (EAM) extracts and liquid culture media (LCM) | + | - | + | [189] |
| Trichoderma asperellum | Methanolic extract | - | - | + | [169] |
| Trichoderma harzianum | Mycosynthesized silver nanoparticles (Ag NPs) | + | - | - | [191] |
| Trichoderma viride | Culture filtrates | - | + | - | [176] |
| Hyalodendriella sp. | EtOAc extract | + | - | - | [170] |
| Verticilluim lecanii | Spores | - | + | - | [186] |
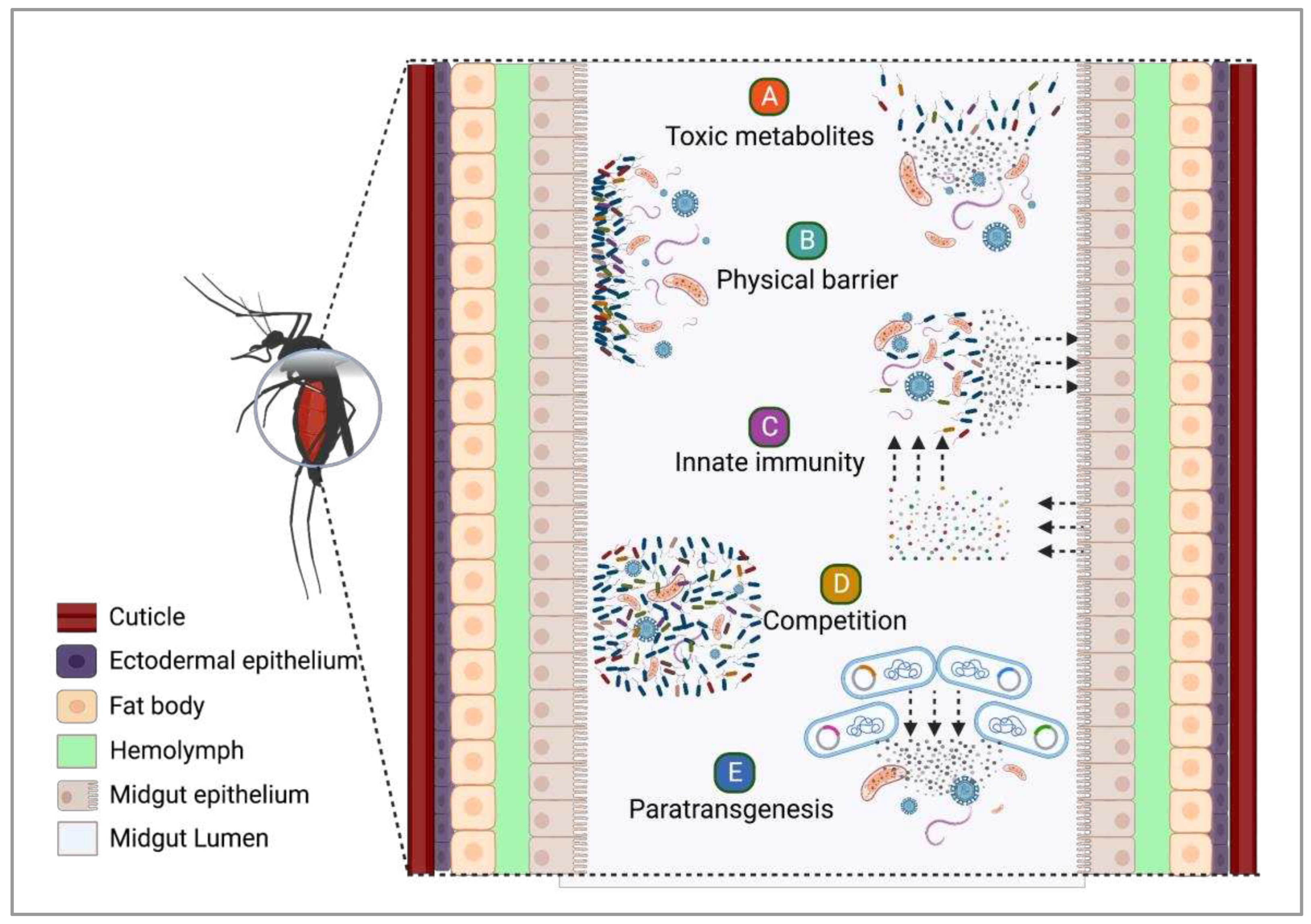
4. The role of insect-bacteria associations in vector competence
4.1. Symbiotic bacteria and their potential against infectious agents
4.2. Exploring the potential of fungi as anti-Plasmodium agents for malaria control
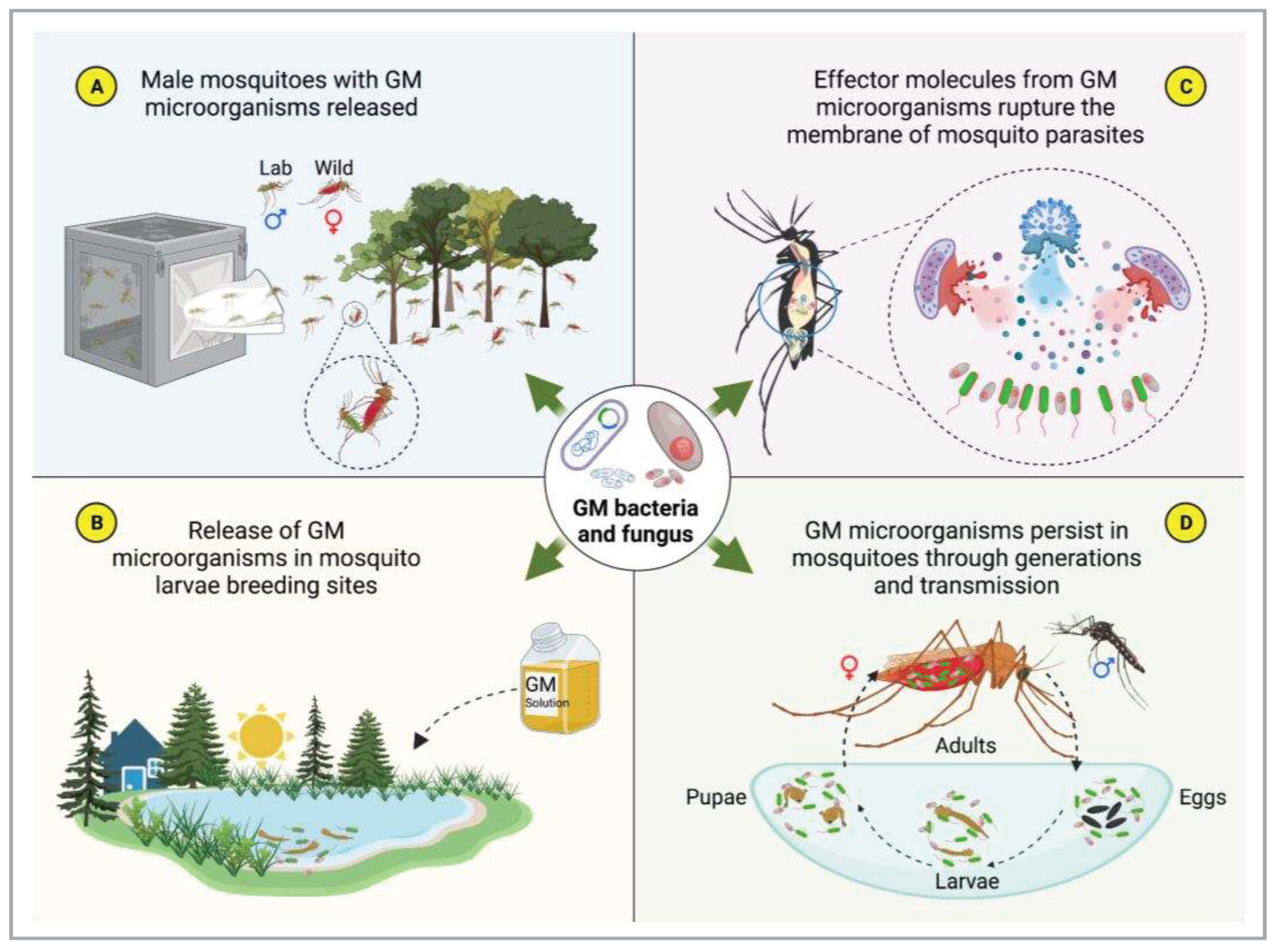
4.3. Symbiotic microorganisms and paratransgenesis
5. Roadmap for the development of microbe-based products for controlling mosquito borne diseases
6. Final considerations
Author Contributions
Funding
Conflicts of Interest
References
- Onen, O.; Aboh, A.A.; Mfam, A.N.; Akor, M.O.; Nweke, C.N.; Osuagwu, A.N. Microbial Diversity: Values and Roles in Ecosystems. Asian J Biol 2020, 9, 10–22. [Google Scholar]
- Rousk, J.; Bengtson, P. Microbial Regulation of Global Biogeochemical Cycles. Frontiers in Microbiology 2014, 5. [Google Scholar] [CrossRef]
- Rodríguez-Frías, F.; Quer, J.; Tabernero, D.; Cortese, M.F.; Garcia-Garcia, S.; Rando-Segura, A.; Pumarola, T. Microorganisms as Shapers of Human Civilization, from Pandemics to Even Our Genomes: Villains or Friends? A Historical Approach. Microorganisms 2021, 9, 2518. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Vlami, M.; De Souza, J.T. Antibiotic Production by Bacterial Biocontrol Agents. Antonie van leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Imasato, R.; Tomoda, H.; Ōmura, S. Yaequinolones, New Insecticidal Antibiotics Produced by Penicillium Sp. FKI-2140. The Journal of antibiotics 2006, 59, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Lactic Acid Bacteria: Their Applications in Foods. J. Bacteriol. Mycol 2018, 6, 89–94. [Google Scholar]
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for Future Foods. Journal of Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Torres-Guardado, R.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Microbial Interactions in Alcoholic Beverages. International Microbiology 2022, 25, 1–15. [Google Scholar] [CrossRef]
- Sevak, P.I.; Pushkar, B.K.; Kapadne, P.N. Lead Pollution and Bacterial Bioremediation: A Review. Environmental Chemistry Letters 2021, 19, 4463–4488. [Google Scholar] [CrossRef]
- Cowan, A.R.; Costanzo, C.M.; Benham, R.; Loveridge, E.J.; Moody, S.C. Fungal Bioremediation of Polyethylene: Challenges and Perspectives. Journal of Applied Microbiology 2022, 132, 78–89. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; García-Estévez, I.; Escribano-Bailón, M.T.; García-Fraile, P.; Rivas, R. Bacterial Fertilizers Based on Rhizobium Laguerreae and Bacillus Halotolerans Enhance Cichorium Endivia L. Phenolic Compound and Mineral Contents and Plant Development. Foods 2021, 10, 424. [Google Scholar] [CrossRef]
- Dunham, B. Microbial Pesticides: A Key Role in the Multinational Portfolio. New Ag International 2015, 32–36. [Google Scholar]
- Ruiu, L. Microbial Biopesticides in Agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef]
- Thongsripong, P.; Chandler, J.A.; Green, A.B.; Kittayapong, P.; Wilcox, B.A.; Kapan, D.D.; Bennett, S.N. Mosquito Vector-associated Microbiota: Metabarcoding Bacteria and Eukaryotic Symbionts across Habitat Types in Thailand Endemic for Dengue and Other Arthropod-borne Diseases. Ecology and evolution 2018, 8, 1352–1368. [Google Scholar] [CrossRef] [PubMed]
- da Silva Gonçalves, D.; Iturbe-Ormaetxe, I.; Martins-da-Silva, A.; Telleria, E.L.; Rocha, M.N.; Traub-Csekö, Y.M.; O’Neill, S.L.; Sant’Anna, M.R.V.; Moreira, L.A. Wolbachia Introduction into Lutzomyia Longipalpis (Diptera: Psychodidae) Cell Lines and Its Effects on Immune-Related Gene Expression and Interaction with Leishmania Infantum. Parasites & vectors 2019, 12, 1–13. [Google Scholar]
- Caragata, E.P.; Short, S.M. Vector Microbiota and Immunity: Modulating Arthropod Susceptibility to Vertebrate Pathogens. Current Opinion in Insect Science 2022, 100875. [Google Scholar] [CrossRef]
- Chavasse, D.C.; Yap, H.H.; Organization, W.H. Chemical Methods for the Control of Vectors and Pests of Public Health Importance; World Health Organization, 1997.
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl Trop Dis 2020, 14. [Google Scholar] [CrossRef]
- Rawlins, S.C.; Wan, J.O. Resistance in Some Caribbean Populations of Aedes Aegypti to Several Insecticides. Journal of the American Mosquito Control Association 1995, 11, 59–65. [Google Scholar] [PubMed]
- Nauen, R. Insecticide Resistance in Disease Vectors of Public Health Importance. Pest Management Science: formerly Pesticide Science 2007, 63, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Hamid, P.H.; Prastowo, J.; Ghiffari, A.; Taubert, A.; Hermosilla, C. Aedes Aegypti Resistance Development to Commonly Used Insecticides in Jakarta, Indonesia. PLoS One 2017, 12, e0189680. [Google Scholar] [CrossRef]
- Lopes, R.P.; Lima, J.B.P.; Martins, A.J. Insecticide Resistance in Culex Quinquefasciatus Say, 1823 in Brazil: A Review. Parasites & vectors 2019, 12, 1–12. [Google Scholar]
- Kaushal, J.; Khatri, M.; Arya, S.K. A Treatise on Organophosphate Pesticide Pollution: Current Strategies and Advancements in Their Environmental Degradation and Elimination. Ecotoxicology and Environmental Safety 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G. Current Issues in Organophosphate Toxicology. Clinica chimica acta 2006, 366, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.X.; Terry Jr, A.V. Neurotoxicity in Acute and Repeated Organophosphate Exposure. Toxicology 2018, 408, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Margalith, Y.; Ben-Dov, E. Biological Control by Bacillus Thuringiensis Subsp. Israelensis. Insect pest management: techniques for environmental protection 2000, 243–301. [Google Scholar]
- Polanczyk, R.A.; Garcia, M. de O.; Alves, S.B. Potencial de Bacillus Thuringiensis Israelensis Berliner No Controle de Aedes Aegypti. Revista de saúde pública 2003, 37, 813–816. [Google Scholar] [CrossRef]
- Boyce, R.; Lenhart, A.; Kroeger, A.; Velayudhan, R.; Roberts, B.; Horstick, O. Bacillus Thuringiensis Israelensis (B Ti) for the Control of Dengue Vectors: Systematic Literature Review. Tropical Medicine & International Health 2013, 18, 564–577. [Google Scholar]
- Balakrishnan, S.; Indira, K.; Srinivasan, M. RETRACTED ARTICLE: Mosquitocidal Properties of Bacillus Species Isolated from Mangroves of Vellar Estuary, Southeast Coast of India. Journal of parasitic diseases 2015, 39, 385–392. [Google Scholar] [CrossRef]
- Santana-Martinez, J.C.; Silva, J.J.; Dussan, J. Efficacy of Lysinibacillus Sphaericus against Mixed-Cultures of Field-Collected and Laboratory Larvae of Aedes Aegypti and Culex Quinquefasciatus. Bulletin of entomological research 2019, 109, 111–118. [Google Scholar] [CrossRef]
- Organization, W.H. Report of the Seventh WHOPES Working Group Meeting : WHO/HQ, Geneva, 2-4 December 2003 : Review of : Vectobac WG Permanet Gokilaht-S 5EC. 2004 .
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of Action of Bacillus Thuringiensis Cry and Cyt Toxins and Their Potential for Insect Control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Adang, M.J.; Crickmore, N.; Jurat-Fuentes, J.L. Diversity of Bacillus Thuringiensis Crystal Toxins and Mechanism of Action. In Advances in insect physiology; Elsevier, 2014; Vol. 47, pp. 39–87 ISBN 0065-2806.
- Berry, C.; O’Neil, S.; Ben-Dov, E.; Jones, A.F.; Murphy, L.; Quail, M.A.; Holden, M.T.; Harris, D.; Zaritsky, A.; Parkhill, J. Complete Sequence and Organization of PBtoxis, the Toxin-Coding Plasmid of Bacillus Thuringiensis Subsp. Israelensis. Applied and environmental microbiology 2002, 68, 5082–5095. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, L.; He, J.; Fu, K.; Li, X.; Jia, L.; Wang, R.; Zhang, W. Characterization of a Novel Bacillus Thuringiensis Toxin Active against Aedes Aegypti Larvae. Acta Tropica 2021, 223, 106088. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus Thuringiensis Toxins: An Overview of Their Biocidal Activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Broadwell, A.H.; Baumann, P. Sequence Analysis of the Mosquitocidal Toxin Genes Encoding 51.4-and 41.9-Kilodalton Proteins from Bacillus Sphaericus 2362 and 2297. Journal of Bacteriology 1988, 170, 2045–2050. [Google Scholar] [CrossRef]
- El-Bendary, M.; Priest, F.G.; Charles, J.-F.; Mitchell, W.J. Crystal Protein Synthesis Is Dependent on Early Sporulation Gene Expression in Bacillus Sphaericus. FEMS microbiology letters 2005, 252, 51–56. [Google Scholar] [CrossRef]
- Tangsongcharoen, C.; Chomanee, N.; Promdonkoy, B.; Boonserm, P. Lysinibacillus Sphaericus Binary Toxin Induces Apoptosis in Susceptible Culex Quinquefasciatus Larvae. Journal of invertebrate pathology 2015, 128, 57–63. [Google Scholar] [CrossRef]
- Liu, J.-W.; Porter, A.G.; Wee, B.Y.; Thanabalu, T. New Gene from Nine Bacillus Sphaericus Strains Encoding Highly Conserved 35.8-Kilodalton Mosquitocidal Toxins. Applied and Environmental Microbiology 1996, 62, 2174–2176. [Google Scholar] [CrossRef]
- Jones, G.W.; Nielsen-Leroux, C.; Yang, Y.; Yuan, Z.; Fiuza Dumas, V.; Monnerat, R.G.; Berry, C. A New Cry Toxin with a Unique Two-component Dependency from Bacillus Sphaericus. The FASEB Journal 2007, 21, 4112–4120. [Google Scholar] [CrossRef]
- Opota, O.; Gauthier, N.C.; Doye, A.; Berry, C.; Gounon, P.; Lemichez, E.; Pauron, D. Bacillus Sphaericus Binary Toxin Elicits Host Cell Autophagy as a Response to Intoxication. PLoS One 2011, 6, e14682. [Google Scholar] [CrossRef]
- Filha, M.H.N.L.S.; Berry, C.; Regis, L. Lysinibacillus Sphaericus: Toxins and Mode of Action, Applications for Mosquito Control and Resistance Management. In Advances in insect physiology; Elsevier, 2014; Vol. 47, pp. 89–176 ISBN 0065-2806.
- Wirth, M.C.; Berry, C.; Walton, W.E.; Federici, B.A. Mtx Toxins from Lysinibacillus Sphaericus Enhance Mosquitocidal Cry-Toxin Activity and Suppress Cry-Resistance in Culex Quinquefasciatus. Journal of invertebrate pathology 2014, 115, 62–67. [Google Scholar] [CrossRef]
- de Melo, J.V.; Jones, G.W.; Berry, C.; Vasconcelos, R.H.T.; de Oliveira, C.M.F.; Furtado, A.F.; Peixoto, C.A.; Silva-Filha, M.H.N.L. Cytopathological Effects of Bacillus Sphaericus Cry48Aa/Cry49Aa Toxin on Binary Toxin-Susceptible and-Resistant Culex Quinquefasciatus Larvae. Applied and environmental microbiology 2009, 75, 4782–4789. [Google Scholar] [CrossRef]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. Strategies for Culture of ‘Unculturable’Bacteria. FEMS microbiology letters 2010, 309, 1–7. [Google Scholar]
- Falqueto, S.A.; Pitaluga, B.F.; de Sousa, J.R.; Targanski, S.K.; Campos, M.G.; de Oliveira Mendes, T.A.; da Silva, G.F.; Silva, D.H.S.; Soares, M.A. Bacillus Spp. Metabolites Are Effective in Eradicating Aedes Aegypti (Diptera: Culicidae) Larvae with Low Toxicity to Non-Target Species. Journal of Invertebrate Pathology 2021, 179, 107525. [Google Scholar] [CrossRef] [PubMed]
- Katak, R.M.; Rocha, E.M.; Oliveira, J.C.; Muniz, V.A.; Oliveira, M.R.; Ferreira, F.A.; Silva, W.R.; Roque, R.A.; de Souza, A.Q.; Souza-Neto, J.A. Larvicidal Activities against Aedes Aegypti of Supernatant and Pellet Fractions from Cultured Bacillus Spp. Isolated from Amazonian Microenvironments. Tropical Medicine and Infectious Disease 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Susetyo, R.D.; Nafidiastri, F.A.; Zain, R.A.; Sari, R.P.; Geraldi, A. Potential Biocontrol Agent of Indigenous Bacillus Sp. EG6. 4: Molecular Identification, Larvicidal Toxicity, and Mechanism of Actions. Biodiversitas 2022, 23, 5431–5438. [Google Scholar]
- Maldonado, B.M.G.; Galan, W.L.J.; Rodriguez, P.C.; Quiroz, M.H. Evaluation of Polymer-Based Granular Formulations of Bacillus Thuringiensis Israelensis against Larval Aedes Aegypti in the Laboratory. J Am Mosq Control Assoc 2002, 18, 352–358. [Google Scholar]
- Armengol, G.; Hernandez, J.; Velez, J.G.; Orduz, S. Long-Lasting Effects of a Bacillus Thuringiensis Serovar Israelensis Experimental Tablet Formulation for Aedes Aegypti (Diptera: Culicidae) Control. Journal of Economic Entomology 2006, 99, 1590–1595. [Google Scholar] [CrossRef]
- de Araujo, A.P.; de Melo-Santos, M.A.V.; de Oliveira Carlos, S.; Rios, E.M.M.M.; Regis, L. Evaluation of an Experimental Product Based on Bacillus Thuringiensis Sorovar. Israelensis against Aedes Aegypti Larvae (Diptera: Culicidae). Biological Control 2007, 41, 339–347. [Google Scholar] [CrossRef]
- de Melo-Santos, M.A.V.; de Araújo, A.P.; Rios, E.M.M.; Regis, L. Long Lasting Persistence of Bacillus Thuringiensis Serovar. Israelensis Larvicidal Activity in Aedes Aegypti (Diptera: Culicidae) Breeding Places Is Associated to Bacteria Recycling. Biological Control 2009, 49, 186–191. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Rapley, L.P.; Benjamin, S. Bacillus Thuringiensis Var. Israelensis (Bti) Provides Residual Control of Aedes Aegypti in Small Containers. The American journal of tropical medicine and hygiene 2010, 82, 1053. [Google Scholar] [CrossRef]
- Kovendan, K.; Murugan, K.; Vincent, S.; Kamalakannan, S. Larvicidal Efficacy of Jatropha Curcas and Bacterial Insecticide, Bacillus Thuringiensis, against Lymphatic Filarial Vector, Culex Quinquefasciatus Say (Diptera: Culicidae). Parasitology research 2011, 109, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.E.-D.M.; Kawanna, M.A.; Bosly, H.A. Larvicidal Activity and Joint Action Toxicity of Certain Combating Agents on Culex Pipiens L. Mosquitoes. Annual Research & Review in Biology, 2013; 1055–1065. [Google Scholar]
- Bideshi, D.K.; Waldrop, G.; Fernandez-Luna, M.T.; Diaz-Mendoza, M.; Wirth, M.C.; Johnson, J.J.; Park, H.-W.; Federici, B.A. Intermolecular Interaction between Cry2Aa and Cyt1Aa and Its Effect on Larvicidal Activity against Culex Quinquefasciatus. Journal of microbiology and biotechnology 2013, 23, 1107–1115. [Google Scholar] [CrossRef]
- Ermolova, V.P.; Grishechkina, S.D.; Belousova, M.E.; Antonets, K.S.; Nizhnikov, A.A. Insecticidal Properties of Bacillus Thuringiensis Var. Israelensis. II. Comparative Morphological and Molecular Genetic Analysis of the Crystallogenic and Acrystallogenic Strains. Sel’skokhozyaistvennaya Biol. 2019, 54, 1281–1289. [Google Scholar] [CrossRef]
- Valtierra-de-Luis, D.; Villanueva, M.; Lai, L.; Williams, T.; Caballero, P. Potential of Cry10Aa and Cyt2Ba, Two Minority δ-Endotoxins Produced by Bacillus Thuringiensis Ser. Israelensis, for the Control of Aedes Aegypti Larvae. Toxins 2020, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.; da Costa, M.P.M.; de Mello Ferreira, I.L.; Lima, J.B.P. K-Carrageenan-Bacillus Thuringiensis Israelensis Hydrogels: A Promising Material to Combat Larvae of the Aedes Aegypti Mosquito. Carbohydrate Polymer Technologies and Applications 2021, 2, 100125. [Google Scholar] [CrossRef]
- Fernández-Chapa, D.; Luna-Olvera, H.A.; Ramirez-Villalobos, J.; Rojas-Verde, G.; Arévalo-Niño, K.; Galán-Wong, L.J. Viability and Reconstitution of Delta-Endotoxins from Bacillus Thuringiensis Var. Israelensis Extracts after Forty Years of Storage against Aedes Aegypti (Diptera: Culicidae). Egyptian Journal of Biological Pest Control 2021, 31, 1–7. [Google Scholar] [CrossRef]
- Gad, A.A.; Al-Dakhil, A.A. Efficacy of Bacillus Thuringiensis Israelensis (Bti) and Four Plant Extracts on the Mortality and Development of Culex Quinquefasciatus Say (Diptera: Cullicidae). Egyptian journal of biological pest control 2018, 28, 1–5. [Google Scholar] [CrossRef]
- Shililu, J.I.; Tewolde, G.M.; Brantly, E.; Githure, J.I.; Mbogo, C.M.; Beier, J.C.; Fusco, R.; Novak, R.J. Efficacy of Bacillus Thuringiensis Israelensis, Bacillus Sphaericus and Temephos for Managing Anopheles Larvae in Eritrea. Journal of the American Mosquito Control Association 2003, 19, 251–258. [Google Scholar]
- Pires, S.; Alves, J.; Dia, I.; Gomez, L.F. Susceptibility of Mosquito Vectors of the City of Praia, Cabo Verde, to Temephos and Bacillus Thuringiensis Var Israelensis. PLoS One 2020, 15, e0234242. [Google Scholar] [CrossRef]
- Derua, Y.A.; Kweka, E.J.; Kisinza, W.N.; Yan, G.; Githeko, A.K.; Mosha, F.W. The Effect of Coexistence between Larvae of Anopheles Gambiae and Culex Quinquefasciatus on Larvicidal Efficacy of Bacillus Thuringiensis Var. Israelensis. East Africa Science 2021, 3, 77–85. [Google Scholar] [CrossRef]
- Kroeger, A.; Horstick, O.; Riedl, C.; Kaiser, A.; Becker, N. The Potential for Malaria Control with the Biological Larvicide Bacillus Thuringiensis Israelensis (Bti) in Peru and Ecuador. Acta Tropica 1995, 60, 47–57. [Google Scholar] [CrossRef]
- Nartey, R.; Owusu-Dabo, E.; Kruppa, T.; Baffour-Awuah, S.; Annan, A.; Oppong, S.; Becker, N.; Obiri-Danso, K. Use of Bacillus Thuringiensis Var Israelensis as a Viable Option in an Integrated Malaria Vector Control Programme in the Kumasi Metropolis, Ghana. Parasites & vectors 2013, 6, 1–10. [Google Scholar]
- Dambach, P.; Louis, V.R.; Kaiser, A.; Ouedraogo, S.; Sié, A.; Sauerborn, R.; Becker, N. Efficacy of Bacillus Thuringiensis Var. Israelensis against Malaria Mosquitoes in Northwestern Burkina Faso. Parasites & vectors 2014, 7, 1–8. [Google Scholar]
- Demissew, A.; Balkew, M.; Girma, M. Larvicidal Activities of Chinaberry, Neem and Bacillus Thuringiensis Israelensis (Bti) to an Insecticide Resistant Population of Anopheles Arabiensis from Tolay, Southwest Ethiopia. Asian Pacific Journal of Tropical Biomedicine 2016, 6, 554–561. [Google Scholar] [CrossRef]
- Dambach, P.; Winkler, V.; Bärnighausen, T.; Traoré, I.; Ouedraogo, S.; Sié, A.; Sauerborn, R.; Becker, N.; Louis, V.R. Biological Larviciding against Malaria Vector Mosquitoes with Bacillus Thuringiensis Israelensis (Bti)–Long Term Observations and Assessment of Repeatability during an Additional Intervention Year of a Large-Scale Field Trial in Rural Burkina Faso. Global Health Action 2020, 13, 1829828. [Google Scholar] [CrossRef]
- Monnerat, R.G.; Dias, D.G.S.; Silva, S.F. da; Martins, E.S.; Berry, C.; Falcão, R.; Gomes, A.C.M.M.; Praça, L.B.; Soares, C.M.S. Screening of Bacillus Thuringiensis Strains Effective against Mosquitoes. Pesquisa Agropecuária Brasileira 2005, 40, 103–106. [Google Scholar] [CrossRef]
- Patil, C.D.; Patil, S.V.; Salunke, B.K.; Salunkhe, R.B. Insecticidal Potency of Bacterial Species Bacillus Thuringiensis SV2 and Serratia Nematodiphila SV6 against Larvae of Mosquito Species Aedes Aegypti, Anopheles Stephensi, and Culex Quinquefasciatus. Parasitology research 2012, 110, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Soares-da-Silva, J.; Pinheiro, V.C.S.; Litaiff-Abreu, E.; Polanczyk, R.A.; Tadei, W.P. Isolation of Bacillus Thuringiensis from the State of Amazonas, in Brazil, and Screening against Aedes Aegypti (Diptera, Culicidae). Revista Brasileira de Entomologia 2015, 59, 01–06. [Google Scholar] [CrossRef]
- Soares-da-Silva, J.; Queirós, S.G.; de Aguiar, J.S.; Viana, J.L.; dos RAV Neta, M.; da Silva, M.C.; Pinheiro, V.C.; Polanczyk, R.A.; Carvalho-Zilse, G.A.; Tadei, W.P. Molecular Characterization of the Gene Profile of Bacillus Thuringiensis Berliner Isolated from Brazilian Ecosystems and Showing Pathogenic Activity against Mosquito Larvae of Medical Importance. Acta tropica 2017, 176, 197–205. [Google Scholar] [CrossRef]
- Fatima, N.; Bibi, Z.; Rehman, A.; Bukhari, D.A. Biotoxicity Comparison of Bacillus Thuringiensis to Control Vector Borne Diseases against Mosquito Fauna. Saudi Journal of Biological Sciences 2023, 30, 103610. [Google Scholar] [CrossRef]
- López-Molina, S.; do Nascimento, N.A.; Silva-Filha, M.H.N.L.; Guerrero, A.; Sánchez, J.; Pacheco, S.; Gill, S.S.; Soberón, M.; Bravo, A. In Vivo Nanoscale Analysis of the Dynamic Synergistic Interaction of Bacillus Thuringiensis Cry11Aa and Cyt1Aa Toxins in Aedes Aegypti. PLoS Pathogens 2021, 17, e1009199. [Google Scholar] [CrossRef]
- Roy, M.; Chatterjee, S.; Dangar, T.K. Characterization and Mosquitocidal Potency of a Bacillus Thuringiensis Strain of Rice Field Soil of Burdwan, West Bengal, India. Microbial Pathogenesis 2021, 158, 105093. [Google Scholar] [CrossRef] [PubMed]
- Bernal, L.; Dussán, J. Synergistic Effect of Lysinibacillus Sphaericus and Glyphosate on Temephos-Resistant Larvae of Aedes Aegypti. Parasites & Vectors 2020, 13, 1–6. [Google Scholar]
- Almeida, J.; Mohanty, A.; Dharini, N.; Hoti, S.L.; Kerkar, S.; Kumar, A. A Report on Novel Mosquito Pathogenic Bacillus Spp. Isolated from a Beach in Goa, India. 2020.
- Nicolas, L.; Dossou-Yovo, J. Differential Effects of Bacillus Sphaericus Strain 2362 on Culex Quinquefasciatus and Its Competitor Culex Cinereus in West Africa. Medical and veterinary entomology 1987, 1, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Campos, J.; Cabrini, I.; Filho, C.; Hibi, S. Susceptibilidade de Populações de Culex Quinquefasciatus Say (Diptera: Culicidae) Sujeitas Ao Controle Com Bacillus Sphaericus Neide No Rio Pinheiros, São Paulo. BioAssay 2009, 2. [Google Scholar] [CrossRef]
- Lozano, L.C.; Ayala, J.A.; Dussán, J. Lysinibacillus Sphaericus S-Layer Protein Toxicity against Culex Quinquefasciatus. Biotechnology letters 2011, 33, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Hire, R.S.; Hadapad, A.B.; D’Souza, S.F.; Kumar, V. Interaction between Mosquito-Larvicidal Lysinibacillus Sphaericus Binary Toxin Components: Analysis of Complex Formation. Insect biochemistry and molecular biology 2013, 43, 1045–1054. [Google Scholar] [CrossRef]
- Iftikhar, S.; Riaz, M.A.; Majeed, M.Z.; Afzal, M.; Ali, A.; Saadia, M.; Ali, Z.; Ahmed, S. Isolation, Characterization and Larvicidal Potential of Indigenous Soil Inhabiting Bacteria against Larvae of Southern House Mosquito (Culex Quinquefasciatus Say). International Journal of Tropical Insect Science 2023, 1–11. [Google Scholar] [CrossRef]
- Allievi, M.C.; Palomino, M.M.; Prado Acosta, M.; Lanati, L.; Ruzal, S.M.; Sánchez-Rivas, C. Contribution of S-Layer Proteins to the Mosquitocidal Activity of Lysinibacillus Sphaericus. PLoS One 2014, 9, e111114. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; David, L.; Nazni, W.A.; Rozilawati, H.; Nurulhusna, H.; Afizah, A.N.; Rosilawati, R.; Roziah, A.; Teh, C.H.; Seleena, B. THERMALLY APPLIED LYSINIBACILLUS SPHAERICUS AND PYRETHROIDS AGAINST CULEX SITIENS WIEDEMANN AND CULEX QUINQUEFASCIATUS SAY IN MALAYSIA. Southeast Asian Journal of Tropical Medicine and Public Health 2016, 47, 747–758. [Google Scholar]
- Guo, Q.-Y.; Hu, X.-M.; Cai, Q.-X.; Yan, J.-P.; Yuan, Z.-M. Interaction of L Ysinibacillus Sphaericus Cry48Aa/Cry49Aa Toxin with Midgut Brush-border Membrane Fractions from C Ulex Quinquefasciatus Larvae. Insect Molecular Biology 2016, 25, 163–170. [Google Scholar] [CrossRef]
- Lozano, L.C.; Dussán, J. Synergistic Activity between S-Layer Protein and Spore–Crystal Preparations from Lysinibacillus Sphaericus against Culex Quinquefasciatus Larvae. Current microbiology 2017, 74, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Karch, S.; Asidi, N.; Manzambi, Z.M.; Salaun, J.J. Efficacy of Bacillus Sphaericus against the Malaria Vector Anopheles Gambiae and Other Mosquitoes in Swamps and Rice Fields in Zaire. Journal of the American Mosquito Control Association 1992, 8, 376–380. [Google Scholar]
- Rodrigues, I.B.; Tadei, W.P.; Dias, J.M.C. da S. Larvicidal Activity of Bacillus Sphaericus 2362 against Anopheles Nuneztovari, Anopheles Darlingi and Anopheles Braziliensis (Diptera, Culicidae). Revista do Instituto de Medicina Tropical de São Paulo 1999, 41, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Galardo, A.K.R.; Zimmerman, R.; Galardo, C.D. Larval Control of Anopheles (Nyssorhinchus) Darlingi Using Granular Formulation of Bacillus Sphaericus in Abandoned Gold-Miners Excavation Pools in the Brazilian Amazon Rainforest. Revista da Sociedade Brasileira de Medicina Tropical 2013, 46, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.A.; Adang, M.J.; Hua, G.; Rezende, T.M.T.; Rezende, A.M.; Shen, G.-M. Identification of Lysinibacillus Sphaericus Binary Toxin Binding Proteins in a Malarial Mosquito Cell Line by Proteomics: A Novel Approach towards Improving Mosquito Control. Journal of proteomics 2020, 227, 103918. [Google Scholar] [CrossRef] [PubMed]
- Dhayalan, A.; Kannupaiyan, J.; Govindasamy, B.; Pachiappan, P. Extraction and Characterization of Secondary Metabolites from the Soil Bacterium, Acidovorax Sp. SA5 and Evaluation of Their Larvicidal Activity Against Aedes Aegypti. International Journal of Environmental Research 2019, 13, 47–58. [Google Scholar] [CrossRef]
- Das, D.; Chatterjee, S.; Dangar, T.K. Characterization and Mosquitocidal Potential of the Soil Bacteria Aneurinibacillus Aneurinilyticus Isolated from Burdwan, West Bengal, India. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 2016, 86, 707–713. [Google Scholar] [CrossRef]
- Geetha, I.; Aruna, R.; Manonmani, A.M. Mosquitocidal Bacillus Amyloliquefaciens: Dynamics of Growth & Production of Novel Pupicidal Biosurfactant. The Indian journal of medical research 2014, 140, 427. [Google Scholar] [PubMed]
- Darriet, F.; Hougard, J.-M. An Isolate of Bacillus Circulans Toxic to Mosquito Larvae. Journal of the American Mosquito Control Association-Mosquito News 2002, 18, 65–67. [Google Scholar]
- Abinaya, M.; Vaseeharan, B.; Divya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Khaled, J.M.; Al-Anbr, M.N.; Benelli, G. Structural Characterization of Bacillus Licheniformis Dahb1 Exopolysaccharide—Antimicrobial Potential and Larvicidal Activity on Malaria and Zika Virus Mosquito Vectors. Environmental Science and Pollution Research 2018, 25, 18604–18619. [Google Scholar] [CrossRef]
- Favret, M.E.; Yousten, A.A. Insecticidal Activity of Bacillus Laterosporus. Journal of invertebrate pathology 1985, 45, 195–203. [Google Scholar] [CrossRef]
- Ruiu, L.; Floris, I.; Satta, A.; Ellar, D.J. Toxicity of a Brevibacillus Laterosporus Strain Lacking Parasporal Crystals against Musca Domestica and Aedes Aegypti. Biological Control 2007, 43, 136–143. [Google Scholar] [CrossRef]
- Zubasheva, M.V.; Ganushkina, L.A.; Smirnova, T.A.; Azizbekyan, R.R. Larvicidal Activity of Crystal-Forming Strains of Brevibacillus Laterosporus. Applied Biochemistry and Microbiology 2010, 46, 755–762. [Google Scholar] [CrossRef]
- Barbieri, G.; Ferrari, C.; Mamberti, S.; Gabrieli, P.; Castelli, M.; Sassera, D.; Ursino, E.; Scoffone, V.C.; Radaelli, G.; Clementi, E. Identification of a Novel Brevibacillus Laterosporus Strain With Insecticidal Activity Against Aedes Albopictus Larvae. Frontiers in microbiology 2021, 12, 624014. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Conti, B.; Hamze, R.; Muniz, E.R.; Fernandes, É.K.; Ruiu, L. Lethal and Sub-Lethal Activity of Brevibacillus Laterosporus on the Mosquito Aedes Albopictus and Side Effects on Non-Target Water-Dwelling Invertebrates. Journal of Invertebrate Pathology 2021, 184, 107645. [Google Scholar] [CrossRef]
- Das, K.; Mukherjee, A.K. Assessment of Mosquito Larvicidal Potency of Cyclic Lipopeptides Produced by Bacillus Subtilis Strains. Acta Tropica 2006, 97, 168–173. [Google Scholar] [CrossRef]
- Geetha, I.; Manonmani, A.M. Surfactin: A Novel Mosquitocidal Biosurfactant Produced by Bacillus Subtilis Ssp. Subtilis (VCRC B471) and Influence of Abiotic Factors on Its Pupicidal Efficacy. Letters in applied microbiology 2010, 51, 406–412. [Google Scholar] [CrossRef]
- Ramathilaga, A.; Murugesan, A.G.; Prabu, C.S. Biolarvicidal Activity of Peanibacillus Macerans and Bacillus Subtilis Isolated from the Dead Larvae against Aedes Aegypti-Vector for Chikungunya. Proceedings of the International Academy of Ecology and Environmental Sciences 2012, 2, 90. [Google Scholar]
- Geetha, I.; Paily, K.P.; Manonmani, A.M. Mosquito Adulticidal Activity of a Biosurfactant Produced by Bacillus Subtilis Subsp. Subtilis. Pest management science 2012, 68, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Parthipan, P.; Sarankumar, R.K.; Jaganathan, A.; Amuthavalli, P.; Babujanarthanam, R.; Rahman, P.K.; Murugan, K.; Higuchi, A.; Benelli, G.; Rajasekar, A. Biosurfactants Produced by Bacillus Subtilis A1 and Pseudomonas Stutzeri NA3 Reduce Longevity and Fecundity of Anopheles Stephensi and Show High Toxicity against Young Instars. Environmental Science and Pollution Research 2018, 25, 10471–10481. [Google Scholar] [CrossRef] [PubMed]
- Dahmana, H.; Sambou, M.; Raoult, D.; Fenollar, F.; Mediannikov, O. Biological Control of Aedes Albopictus: Obtained from the New Bacterial Candidates with Insecticidal Activity. Insects 2020, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.K.; Rath, A.; Pradhan, N.; Hazra, R.K.; Nayak, R.R.; Kanjilal, S. Cyclic Lipopeptide Biosurfactant from Bacillus Tequilensis Exhibits Multifarious Activity. 3 Biotech 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Short, S.M.; Bahia, A.C.; Saraiva, R.G.; Dong, Y.; Kang, S.; Tripathi, A.; Mlambo, G.; Dimopoulos, G. Chromobacterium Csp_P Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and in Vitro Anti-Pathogen Activities. PLoS pathogens 2014, 10, e1004398. [Google Scholar] [CrossRef] [PubMed]
- Short, S.M.; Van Tol, S.; MacLeod, H.J.; Dimopoulos, G. Hydrogen Cyanide Produced by the Soil Bacterium Chromobacterium Sp. Panama Contributes to Mortality in Anopheles Gambiae Mosquito Larvae. Scientific reports 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gnambani, E.J.; Bilgo, E.; Dabiré, R.K.; Belem, A.M.G.; Diabaté, A. Infection of the Malaria Vector Anopheles Coluzzii with the Entomopathogenic Bacteria Chromobacterium Anophelis Sp. Nov. IRSSSOUMB001 Reduces Larval Survival and Adult Reproductive Potential. Malaria Journal 2023, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J.; Harimuralikrishnaa, T.; Sivakumar, T.; Mahendran, S.; Ponmanickam, P.; Thangaraj, R.; Sevarkodiyone, S.; Alharbi, N.S.; Kadaikunnan, S.; Venkidasamy, B. Biogenic Synthesis of Silver Nanoparticles Using Pantoea Stewartii and Priestia Aryabhattai and Their Antimicrobial, Larvicidal, Histopathological, and Biotoxicity Potential. Bioengineering 2023, 10, 248. [Google Scholar] [CrossRef]
- Contreras, E.; Masuyer, G.; Qureshi, N.; Chawla, S.; Dhillon, H.S.; Lee, H.L.; Chen, J.; Stenmark, P.; Gill, S.S. A Neurotoxin That Specifically Targets Anopheles Mosquitoes. Nature communications 2019, 10, 2869. [Google Scholar] [CrossRef] [PubMed]
- Luiz Rosa da Silva, J.; Undurraga Schwalm, F.; Eugênio Silva, C.; da Costa, M.; Heermann, R.; Santos da Silva, O. Larvicidal and Growth-Inhibitory Activity of Entomopathogenic Bacteria Culture Fluids against Aedes Aegypti (Diptera: Culicidae). Journal of economic entomology 2017, 110, 378–385. [Google Scholar] [PubMed]
- da Silva, O.S.; Prado, G.R.; da Silva, J.L.R.; Silva, C.E.; da Costa, M.; Heermann, R. Oral Toxicity of Photorhabdus Luminescens and Xenorhabdus Nematophila (Enterobacteriaceae) against Aedes Aegypti (Diptera: Culicidae). Parasitology research 2013, 112, 2891–2896. [Google Scholar] [CrossRef]
- Vitta, A.; Thimpoo, P.; Meesil, W.; Yimthin, T.; Fukruksa, C.; Polseela, R.; Mangkit, B.; Tandhavanant, S.; Thanwisai, A. Larvicidal Activity of Xenorhabdus and Photorhabdus Bacteria against Aedes Aegypti and Aedes Albopictus. Asian Pacific Journal of Tropical Biomedicine 2018, 8, 31. [Google Scholar] [CrossRef]
- Patil, C.D.; Patil, S.V.; Salunke, B.K.; Salunkhe, R.B. Prodigiosin Produced by Serratia Marcescens NMCC46 as a Mosquito Larvicidal Agent against Aedes Aegypti and Anopheles Stephensi. Parasitology research 2011, 109, 1179–1187. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Borase, H.P.; Narkhede, C.P.; Salunke, B.K.; Patil, S.V. Mosquito Larvicidal and Pupaecidal Potential of Prodigiosin from Serratia Marcescens and Understanding Its Mechanism of Action. Pesticide biochemistry and physiology 2015, 123, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Heu, K.; Romoli, O.; Schönbeck, J.C.; Ajenoe, R.; Epelboin, Y.; Kircher, V.; Houël, E.; Estevez, Y.; Gendrin, M. The Effect of Secondary Metabolites Produced by Serratia Marcescens on Aedes Aegypti and Its Microbiota. Frontiers in Microbiology 2021, 12, 645701. [Google Scholar] [CrossRef]
- Bond, J.G.; Marina, C.F.; Williams, T. The Naturally Derived Insecticide Spinosad Is Highly Toxic to Aedes and Anopheles Mosquito Larvae. Medical and Veterinary Entomology 2004, 18, 50–56. [Google Scholar] [CrossRef]
- Darriet, F.; Duchon, S.; Hougard, J.M. Spinosad: A New Larvicide against Insecticide-Resistant Mosquito Larvae. Journal of the American Mosquito Control Association 2005, 21, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Romi, R.; Proietti, S.; Di Luca, M.; Cristofaro, M. Laboratory Evaluation of the Bioinsecticide Spinosad for Mosquito Control. Journal of the American Mosquito Control Association 2006, 22, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Antonio, G.E.; Sanchez, D.; Williams, T.; Marina, C.F. Paradoxical Effects of Sublethal Exposure to the Naturally Derived Insecticide Spinosad in the Dengue Vector Mosquito, Aedes Aegypti. Pest Management Science: formerly Pesticide Science 2009, 65, 323–326. [Google Scholar] [CrossRef]
- Thavara, U.; Tawatsin, A.; Asavadachanukorn, P.; Mulla, M.S. Field Evaluation in Thailand of Spinosad, a Larvicide Derived from Saccharopolyspora Spinosa (Actinomycetales) against Aedes Aegypti (L.) Larvae. Southeast Asian journal of tropical medicine and public health 2009, 40, 235. [Google Scholar]
- Jiang, Y.; Mulla, M.S. Laboratory and Field Evaluation of Spinosad, a Biorational Natural Product, against Larvae of Culex Mosquitoes. Journal of the American Mosquito Control Association 2009, 25, 456–466. [Google Scholar] [CrossRef]
- Aarthi, N.; Murugan, K. Larvicidal and Repellent Activity of Vetiveria Zizanioides L, Ocimum Basilicum Linn and the Microbial Pesticide Spinosad against Malarial Vector, Anopheles Stephensi Liston (Insecta: Diptera: Culicidae). Journal of Biopesticides 2010, 3, 199. [Google Scholar]
- Prabhu, K.; Murugan, K.; Nareshkumar, A.; Bragadeeswaran, S. Larvicidal and Pupicidal Activity of Spinosad against the Malarial Vector Anopheles Stephensi. Asian Pacific Journal of Tropical Medicine 2011, 4, 610–613. [Google Scholar] [CrossRef]
- Su, T.; Cheng, M.-L.; Thieme, J. Laboratory and Field Evaluation of Spinosad Formulation Natular T30 against Immature Culex Mosquitoes (Diptera: Culicidae). Journal of medical entomology 2014, 51, 837–844. [Google Scholar] [CrossRef]
- Sadanandane, C.; Gunasekaran, K.; Doss, P.S.B.; Jambulingam, P. Field Evaluation of the Biolarvicide, Spinosad 20 per Cent Emulsifiable Concentrate in Comparison to Its 12 per Cent Suspension Concentrate Formulation against Culex Quinquefasciatus, the Vector of Bancroftian Filariasis in India. The Indian Journal of Medical Research 2018, 147, 32. [Google Scholar] [CrossRef]
- Vijayan, V.; Balaraman, K. Metabolites of Fungi & Actinomycetes Active against Mosquito Larvae. The Indian journal of medical research 1991, 93, 115–117. [Google Scholar] [PubMed]
- Darbro, J.M.; Graham, R.I.; Kay, B.H.; Ryan, P.A.; Thomas, M.B. Evaluation of Entomopathogenic Fungi as Potential Biological Control Agents of the Dengue Mosquito, Aedes Aegypti (Diptera: Culicidae). Biocontrol science and technology 2011, 21, 1027–1047. [Google Scholar] [CrossRef]
- Blanford, S.; Jenkins, N.E.; Read, A.F.; Thomas, M.B. Evaluating the Lethal and Pre-Lethal Effects of a Range of Fungi against Adult Anopheles Stephensi Mosquitoes. Malaria journal 2012, 11, 1–10. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Muturi, E.J.; Dunlap, C.; Rooney, A.P. Strain-Specific Pathogenicity and Subversion of Phenoloxidase Activity in the Mosquito Aedes Aegypti by Members of the Fungal Entomopathogenic Genus Isaria. Scientific Reports 2018, 8, 9896. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Bedini, S.; Shivakumar, M.S. Isolation and Identification of Entomopathogenic Fungus from Eastern Ghats of South Indian Forest Soil and Their Efficacy as Biopesticide for Mosquito Control. Parasitology international 2020, 76, 102099. [Google Scholar] [CrossRef] [PubMed]
- Pathan, E.K.; Ghormade, V.; Tupe, S.G.; Deshpande, M.V. Insect Pathogenic Fungi and Their Applications: An Indian Perspective. Progress in Mycology: An Indian Perspective, 2021; 311–327. [Google Scholar]
- Renuka, S.; Vani H, C.; Alex, E. Entomopathogenic Fungi as a Potential Management Tool for the Control of Urban Malaria Vector, Anopheles Stephensi (Diptera: Culicidae). Journal of Fungi 2023, 9, 223. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Knols, B.G.; Samson, R.A.; Takken, W. Entomopathogenic Fungi for Mosquito Control: A Review. Journal of insect science 2004, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Kanzok, S.M.; Jacobs-Lorena, M. Entomopathogenic Fungi as Biological Insecticides to Control Malaria. Trends in parasitology 2006, 22, 49–51. [Google Scholar] [CrossRef]
- Fang, W.; Azimzadeh, P.; Leger, R.J.S. Strain Improvement of Fungal Insecticides for Controlling Insect Pests and Vector-Borne Diseases. Current opinion in microbiology 2012, 15, 232–238. [Google Scholar] [CrossRef]
- Cafarchia, C.; Pellegrino, R.; Romano, V.; Friuli, M.; Demitri, C.; Pombi, M.; Benelli, G.; Otranto, D. Delivery and Effectiveness of Entomopathogenic Fungi for Mosquito and Tick Control: Current Knowledge and Research Challenges. Acta Tropica 2022, 106627. [Google Scholar] [CrossRef]
- de Paula, A.R.; Brito, E.S.; Pereira, C.R.; Carrera, M.P.; Samuels, R.I. Susceptibility of Adult Aedes Aegypti (Diptera: Culicidae) to Infection by Metarhizium Anisopliae and Beauveria Bassiana: Prospects for Dengue Vector Control. Biocontrol Science and Technology 2008, 18, 1017–1025. [Google Scholar] [CrossRef]
- Buckner, E.A.; Williams, K.F.; Marsicano, A.L.; Latham, M.D.; Lesser, C.R. Evaluating the Vector Control Potential of the In2Care® Mosquito Trap against Aedes Aegypti and Aedes Albopictus under Semifield Conditions in Manatee County, Florida. Journal of the American Mosquito Control Association 2017, 33, 193–199. [Google Scholar] [CrossRef]
- Howard, A.F.; N’guessan, R.; Koenraadt, C.J.; Asidi, A.; Farenhorst, M.; Akogbéto, M.; Thomas, M.B.; Knols, B.G.; Takken, W. The Entomopathogenic Fungus Beauveria Bassiana Reduces Instantaneous Blood Feeding in Wild Multi-Insecticide-Resistant Culex Quinquefasciatus Mosquitoes in Benin, West Africa. Parasites & Vectors 2010, 3, 1–11. [Google Scholar]
- García-Munguía, A.M.; Garza-Hernández, J.A.; Rebollar-Tellez, E.A.; Rodríguez-Pérez, M.A.; Reyes-Villanueva, F. Transmission of Beauveria Bassiana from Male to Female Aedes Aegypti Mosquitoes. Parasites & vectors 2011, 4, 1–6. [Google Scholar]
- George, J.; Jenkins, N.E.; Blanford, S.; Thomas, M.B.; Baker, T.C. Malaria Mosquitoes Attracted by Fatal Fungus. PLoS One 2013, 8, e62632. [Google Scholar] [CrossRef] [PubMed]
- Valero-Jiménez, C.A.; van Kan, J.A.; Koenraadt, C.J.; Zwaan, B.J.; Schoustra, S.E. Experimental Evolution to Increase the Efficacy of the Entomopathogenic Fungus Beauveria Bassiana against Malaria Mosquitoes: Effects on Mycelial Growth and Virulence. Evolutionary applications 2017, 10, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, R.F.; Zafar, J.; Shakeel, M.; Zhang, Y.; Freed, S.; Xu, X.; Jin, F. Assessment of Lethal, Sublethal, and Transgenerational Effects of Beauveria Bassiana on the Demography of Aedes Albopictus (Culicidae: Diptera). Insects 2020, 11, 178. [Google Scholar] [CrossRef]
- Veys-Behbahani, R.; Sharififard, M.; Dinparast-Djadid, N.; Shamsi, J.; Fakoorziba, M.R. Laboratory Evolution of the Entomopathogenic Fungus Beauveria Bassiana against Anopheles Stephensi Larvae (Diptera: Culicidae). Asian Pacific Journal of Tropical Disease 2014, 4, S799–S802. [Google Scholar] [CrossRef]
- Bezalwar, P.; Gomashe, A.; Gulhane, P. Laboratory-Based Evaluation of the Potential of Beauveria Bassiana Crude Metabolites for Mosquito Larvae. Annihilation (IOSR-JPBS) 2014, 9, 15–20. [Google Scholar]
- Farida, B.; Sonia, H.; Hakima, M.-K.; Fatma, B.; Fatma, H. Histological Changes in the Larvae of the Domestic Mosquito Culex Pipiens Treated with the Entomopathogenic Fungus Beauveria Bassiana. Scientific Research and Essays 2018, 13, 1–10. [Google Scholar]
- US EPA, O. Biopesticide Active Ingredients Available online:. Available online: https://www.epa.gov/ingredients-used-pesticide-products/biopesticide-active-ingredients (accessed on 12 April 2023).
- ANVISA Listas de ingredientes ativos com uso autorizado e banidos no Brasil Available online:. Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2017/listas-de-ingredientes-ativos-com-uso-autorizado-e-banidos-no-brasil (accessed on 20 March 2023).
- Zimmermann, G. The Entomopathogenic Fungus Metarhizium Anisopliae and Its Potential as a Biocontrol Agent. Pesticide Science 1993, 37, 375–379. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Screen, S.; Bagga, S.; Hu, G.; St Leger, R.J. Expressed Sequence Tag (EST) Analysis of Two Subspecies of Metarhizium Anisopliae Reveals a Plethora of Secreted Proteins with Potential Activity in Insect Hosts. Microbiology 2003, 149, 239–247. [Google Scholar] [CrossRef]
- Schrank, A.; Vainstein, M.H. Metarhizium Anisopliae Enzymes and Toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.J.; Lee, J.Y.; Woo, R.M.; Shin, T.Y.; Gwak, W.S.; Woo, S.D. An Effective Entomopathogenic Fungus Metarhizium Anisopliae for the Simultaneous Control of Aedes Albopictus and Culex Pipiens Mosquito Adults. Journal of Asia-Pacific Entomology 2020, 23, 585–590. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Knols, B.G.; Takken, W. Infection of the Malaria Mosquito Anopheles Gambiae with the Entomopathogenic Fungus Metarhizium Anisopliae Reduces Blood Feeding and Fecundity. Journal of invertebrate pathology 2006, 91, 43–49. [Google Scholar] [CrossRef]
- Pereira, C.R.; de Paula, A.R.; Gomes, S.A.; Pedra Jr, P.C.O.; Samuels, R.I. The Potential of Metarhizium Anisopliae and Beauveria Bassiana Isolates for the Control of Aedes Aegypti (Diptera: Culicidae) Larvae. Biocontrol Science and Technology 2009, 19, 881–886. [Google Scholar] [CrossRef]
- Koodalingam, A.; Dayanidhi, M.K. Studies on Biochemical and Synergistic Effects of Immunosuppressive Concentration of Imidacloprid with Beauveria Bassiana and Metarhizium Anisopliae for Enhancement of Virulence against Vector Mosquito Culex Quinquefasciatus. Pesticide Biochemistry and Physiology 2021, 176, 104882. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.M.; Greenfield, B.P.; Greig, C.; Maffeis, T.G.; Taylor, J.W.; Piasecka, J.; Dudley, E.; Abdulla, A.; Dubovskiy, I.M.; Garrido-Jurado, I. Metarhizium Anisopliae Pathogenesis of Mosquito Larvae: A Verdict of Accidental Death. PloS one 2013, 8, e81686. [Google Scholar] [CrossRef]
- Jaber, S.; Mercier, A.; Knio, K.; Brun, S.; Kambris, Z. Isolation of Fungi from Dead Arthropods and Identification of a New Mosquito Natural Pathogen. Parasites & vectors 2016, 9, 1–10. [Google Scholar]
- Soni, N.; Prakash, S. Aspergillus Niger Metabolites Efficacies against the Mosquito Larval (Culex Quinquefasciatus, Anopheles Stephensi and Aedes Aegypti) Population after Column Chromatography. Am J Microbiol Res 2011, 2, 15–20. [Google Scholar]
- Balumahendhiran, K.; Vivekanandhan, P.; Shivakumar, M.S. Mosquito Control Potential of Secondary Metabolites Isolated from Aspergillus Flavus and Aspergillus Fumigatus. Biocatalysis and Agricultural Biotechnology 2019, 21, 101334. [Google Scholar] [CrossRef]
- Baskar, K.; Chinnasamy, R.; Pandy, K.; Venkatesan, M.; Sebastian, P.J.; Subban, M.; Thomas, A.; Kweka, E.J.; Devarajan, N. Larvicidal and Histopathology Effect of Endophytic Fungal Extracts of Aspergillus Tamarii against Aedes Aegypti and Culex Quinquefasciatus. Heliyon 2020, 6, e05331. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Karthi, S.; Chellappandian, M.; Ponsankar, A.; Thanigaivel, A.; Senthil-Nathan, S.; Chandramohan, D.; Ganesan, R. Aspergillus Flavus (Link) Toxins Reduces the Fitness of Dengue Vector Aedes Aegypti (Linn.) and Their Non-Target Toxicity against Aquatic Predator. Microbial pathogenesis 2019, 128, 281–287. [Google Scholar] [CrossRef]
- Karthi, S.; Vasantha-Srinivasan, P.; Ganesan, R.; Ramasamy, V.; Senthil-Nathan, S.; Khater, H.F.; Radhakrishnan, N.; Amala, K.; Kim, T.-J.; El-Sheikh, M.A. Target Activity of Isaria Tenuipes (Hypocreales: Clavicipitaceae) Fungal Strains against Dengue Vector Aedes Aegypti (Linn.) and Its Non-Target Activity against Aquatic Predators. Journal of fungi 2020, 6, 196. [Google Scholar] [CrossRef]
- Banu, A.N.; Balasubramanian, C. Optimization and Synthesis of Silver Nanoparticles Using Isaria Fumosorosea against Human Vector Mosquitoes. Parasitology research 2014, 113, 3843–3851. [Google Scholar] [CrossRef]
- Podder, D.; Ghosh, S.K. A New Application of Trichoderma Asperellum as an Anopheline Larvicide for Eco Friendly Management in Medical Science. Scientific reports 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Mao, Z.; Wang, W.; Su, R.; Gu, G.; Liu, Z.L.; Lai, D.; Zhou, L. Hyalodendrins A and B, New Decalin-Type Tetramic Acid Larvicides from the Endophytic Fungus Hyalodendriella Sp. Ponipodef12. Molecules 2019, 25, 114. [Google Scholar] [CrossRef]
- Lee, J.Y.; Woo, R.M.; Choi, C.J.; Shin, T.Y.; Gwak, W.S.; Woo, S.D. Beauveria Bassiana for the Simultaneous Control of Aedes Albopictus and Culex Pipiens Mosquito Adults Shows High Conidia Persistence and Productivity. AMB Express 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Alves, S.B.; Alves, L.F.A.; Lopes, R.B.; Pereira, R.M.; Vieira, S.A. Potential of Some Metarhizium Anisopliae Isolates for Control of Culex Quinquefasciatus (Dipt., Culicidae). Journal of Applied Entomology 2002, 126, 504–509. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Takken, W.; Knols, B.G. Infection of Adult Aedes Aegypti and Ae. Albopictus Mosquitoes with the Entomopathogenic Fungus Metarhizium Anisopliae. Acta tropica 2007, 102, 151–158. [Google Scholar] [CrossRef]
- Seye, F.; Ndiaye, M.; Faye, O.; Afoutou, J.M. Evaluation of Entomopathogenic Fungus Metarhizium Anisopliae Formulated with Suneem (Neem Oil) against Anopheles Gambiae Sl and Culex Quinquefasciatus Adults. Malaria Chemotherapy Cont Elim 2012, 1. [Google Scholar]
- Vivekanandhan, P.; Swathy, K.; Kalaimurugan, D.; Ramachandran, M.; Yuvaraj, A.; Kumar, A.N.; Manikandan, A.T.; Poovarasan, N.; Shivakumar, M.S.; Kweka, E.J. Larvicidal Toxicity of Metarhizium Anisopliae Metabolites against Three Mosquito Species and Non-Targeting Organisms. Plos one 2020, 15, e0232172. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Jebanesan, A.; Reetha, D. Larvicidal Effect of Extracellular Secondary Metabolites of Different Fungi against the Mosquito, Culex Quinquefasciatus Say. Tropical biomedicine 2005, 22, 1–3. [Google Scholar] [PubMed]
- Ragavendran, C.; Natarajan, D. Insecticidal Potency of Aspergillus Terreus against Larvae and Pupae of Three Mosquito Species Anopheles Stephensi, Culex Quinquefasciatus, and Aedes Aegypti. Environmental Science and Pollution Research 2015, 22, 17224–17237. [Google Scholar] [CrossRef]
- Chinnasamy, R.; Govindasamy, B.; Venkatesh, M.; Magudeeswaran, S.; Dhanarajan, A.; Devarajan, N.; Willie, P.; Perumal, V.; Mekchay, S.; Krutmuang, P. Bio-Efficacy of Insecticidal Molecule Emodin against Dengue, Filariasis, and Malaria Vectors. Environmental Science and Pollution Research 2023, 30, 61842–61862. [Google Scholar] [CrossRef] [PubMed]
- Pinnock, D.E.; Garcia, R.; Cubbin, C.M. Beauveria Tenella as a Control Agent for Mosquito Larvae. Journal of Invertebrate Pathology 1973, 22, 143–147. [Google Scholar] [CrossRef]
- Ragavendran, C.; Balasubramani, G.; Tijo, C.; Manigandan, V.; Kweka, E.J.; Karthika, P.; Sivasankar, P.; Thomas, A.; Natarajan, D.; Nakouti, I. Cladophialophora Bantiana Metabolites Are Efficient in the Larvicidal and Ovicidal Control of Aedes Aegypti, and Culex Quinquefasciatus and Have Low Toxicity in Zebrafish Embryo. Science of the Total Environment 2022, 852, 158502. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.S.; Prakash, S. Effects of Culture Media on Larvicidal Property of Secondary Metabolites of Mosquito Pathogenic Fungus Chrysosporium Lobatum (Moniliales: Moniliaceae). Acta tropica 2009, 109, 50–54. [Google Scholar] [CrossRef]
- Verma, P.; Prakash, S. Efficacy of Chrysosporium Tropicum Metabolite against Mixed Population of Adult Mosquito (Culex Quinquefasciatus, Anopheles Stephensii, and Aedes Aegypti) after Purification with Flash Chromatography. Parasitology research 2010, 107, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, F.S.; Palaniswamy, M.; Ravi, S.; Thangamani, A.; Pradeep, B.V. Larvicidal Activity of a Novel Isoquinoline Type Pigment from Fusarium Moniliforme KUMBF1201 against Aedes Aegypti and Anopheles Stephensi. Process Biochemistry 2015, 50, 1479–1486. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Karthi, S.; Shivakumar, M.S.; Benelli, G. Synergistic Effect of Entomopathogenic Fungus Fusarium Oxysporum Extract in Combination with Temephos against Three Major Mosquito Vectors. Pathogens and global health 2018, 112, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ragavendran, C.; Mariappan, T.; Natarajan, D. Larvicidal, Histopathological Efficacy of Penicillium Daleae against Larvae of Culex Quinquefasciatus and Aedes Aegypti plus Biotoxicity on Artemia Nauplii a Non-Target Aquatic Organism. Frontiers in pharmacology 2017, 8, 773. [Google Scholar] [CrossRef]
- Saady, R.H.; Mansoor, A.J. Laboratory Evaluation of the Entomopathogenic Fungi Penicillium Marneffei and Verticillium Lecanii against Culex Pipeins Molestus. Indian Journal of Forensic Medicine & Toxicology 2021, 15, 2126–2133. [Google Scholar]
- Arunthirumeni, M.; Vinitha, G.; Shivakumar, M.S. Antifeedant and Larvicidal Activity of Bioactive Compounds Isolated from Entomopathogenic Fungi Penicillium Sp. for the Control of Agricultural and Medically Important Insect Pest (Spodoptera Litura and Culex Quinquefasciatus). Parasitology International 2023, 92, 102688. [Google Scholar] [CrossRef]
- Ragavendran, C.; Manigandan, V.; Kamaraj, C.; Balasubramani, G.; Prakash, J.S.; Perumal, P.; Natarajan, D. Larvicidal, Histopathological, Antibacterial Activity of Indigenous Fungus Penicillium Sp. against Aedes Aegypti L and Culex Quinquefasciatus (Say)(Diptera: Culicidae) and Its Acetylcholinesterase Inhibition and Toxicity Assessment of Zebrafish (Danio Rerio). Frontiers in Microbiology 2019, 10, 427. [Google Scholar]
- Bücker, A.; Bücker, N.C.F.; Souza, A.Q.L. de; Gama, A.M. da; Rodrigues-Filho, E.; Costa, F.M. da; Nunez, C.V.; Tadei, W.P. Larvicidal Effects of Endophytic and Basidiomycete Fungus Extracts on Aedes and Anopheles Larvae (Diptera, Culicidae). Revista da Sociedade Brasileira de Medicina Tropical 2013, 46, 411–419. [Google Scholar] [CrossRef]
- Matasyoh, J.C.; Dittrich, B.; Schueffler, A.; Laatsch, H. Larvicidal Activity of Metabolites from the Endophytic Podospora Sp. against the Malaria Vector Anopheles Gambiae. Parasitology research 2011, 108, 561–566. [Google Scholar] [CrossRef]
- Sundaravadivelan, C.; Padmanabhan, M.N. Effect of Mycosynthesized Silver Nanoparticles from Filtrate of Trichoderma Harzianum against Larvae and Pupa of Dengue Vector Aedes Aegypti L. Environmental Science and Pollution Research 2014, 21, 4624–4633. [Google Scholar] [CrossRef]
- Dennison, N.J.; Jupatanakul, N.; Dimopoulos, G. The Mosquito Microbiota Influences Vector Competence for Human Pathogens. Current opinion in insect science 2014, 3, 6–13. [Google Scholar] [CrossRef]
- Carlson, J.S.; Short, S.M.; Angleró-Rodríguez, Y.I.; Dimopoulos, G. Larval Exposure to Bacteria Modulates Arbovirus Infection and Immune Gene Expression in Adult Aedes Aegypti. Developmental & Comparative Immunology 2020, 104, 103540. [Google Scholar]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito Microbiota and Implications for Disease Control. Trends in parasitology 2020, 36, 98–111. [Google Scholar] [CrossRef]
- Gabrieli, P.; Caccia, S.; Varotto-Boccazzi, I.; Arnoldi, I.; Barbieri, G.; Comandatore, F.; Epis, S. Mosquito Trilogy: Microbiota, Immunity and Pathogens, and Their Implications for the Control of Disease Transmission. Frontiers in microbiology 2021, 12, 630438. [Google Scholar] [CrossRef] [PubMed]
- Cansado-Utrilla, C.; Zhao, S.Y.; McCall, P.J.; Coon, K.L.; Hughes, G.L. The Microbiome and Mosquito Vectorial Capacity: Rich Potential for Discovery and Translation. Microbiome 2021, 9, 111. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Aksoy, S. Microbiota in Disease-Transmitting Vectors. Nature Reviews Microbiology 2023, 1–15. [Google Scholar] [CrossRef]
- Douglas, A.E. Lessons from Studying Insect Symbioses. Cell host & microbe 2011, 10, 359–367. [Google Scholar]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and Function of Bacterial Microbiota in the Mosquito Holobiont. Parasites & vectors 2013, 6, 1–12. [Google Scholar]
- Kumar, A.; Srivastava, P.; Sirisena, P.; Dubey, S.K.; Kumar, R.; Shrinet, J.; Sunil, S. Mosquito Innate Immunity. Insects 2018, 9, 95. [Google Scholar] [CrossRef]
- Ferreira, Q.R.; Lemos, F.F.B.; Moura, M.N.; Nascimento, J.O. de S.; Novaes, A.F.; Barcelos, I.S.; Fernandes, L.A.; Amaral, L.S. de B.; Barreto, F.K.; Melo, F.F. de Role of the Microbiome in Aedes Spp. Vector Competence: What Do We Know? Viruses 2023, 15, 779. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.A.; Dohna, H. zu; Nilsson, L.K.; Onorati, P.; Nakhleh, J.; Terenius, O.; Osta, M.A. The Environment and Species Affect Gut Bacteria Composition in Laboratory Co-Cultured Anopheles Gambiae and Aedes Albopictus Mosquitoes. Scientific Reports 2020, 10, 3352. [Google Scholar] [CrossRef]
- Mosquera, K.D.; Nilsson, L.K.J.; de Oliveira, M.R.; Rocha, E.M.; Marinotti, O.; Håkansson, S.; Tadei, W.P.; de Souza, A.Q.L.; Terenius, O. Comparative Assessment of the Bacterial Communities Associated with Anopheles Darlingi Immature Stages and Their Breeding Sites in the Brazilian Amazon. Parasites & Vectors 2023, 16, 156. [Google Scholar] [CrossRef]
- Santos, N.A.C. dos; Carvalho, V.R. de; Souza Neto, J.; Alonso, D.P.; Ribolla, P.E.M.; Medeiros, J.F.; Araujo, M. da S. Bacterial Microbiota from Lab-Reared and Field-Captured Anopheles Darlingi Midgut and Salivary Gland. Microorganisms 2023, 11, 1145. [Google Scholar] [CrossRef]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the Mosquito Midgut Microbiota in the Defense against Malaria Parasites. PLoS pathogens 2009, 5, e1000423. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Dong, Y.; Garver, L.S.; Sim, S.; Dimopoulos, G. Mosquito Immune Defenses against Plasmodium Infection. Developmental & Comparative Immunology 2010, 34, 387–395. [Google Scholar]
- Wang, Y.; Gilbreath III, T.M.; Kukutla, P.; Yan, G.; Xu, J. Dynamic Gut Microbiome across Life History of the Malaria Mosquito Anopheles Gambiae in Kenya. PloS one 2011, 6, e24767. [Google Scholar] [CrossRef] [PubMed]
- Gendrin, M.; Christophides, G.K. The Anopheles Mosquito Microbiota and Their Impact on Pathogen Transmission. In Anopheles mosquitoes-New insights into malaria vectors; IntechOpen, 2013 ISBN 953-51-1188-4.
- Ricci, I.; Valzano, M.; Ulissi, U.; Epis, S.; Cappelli, A.; Favia, G. Symbiotic Control of Mosquito Borne Disease. Pathogens and Global Health 2012, 106, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Eappen, A.G.; Smith, R.C.; Jacobs-Lorena, M. Enterobacter-Activated Mosquito Immune Responses to Plasmodium Involve Activation of SRPN6 in Anopheles Stephensi. Plos one 2013, 8, e62937. [Google Scholar] [CrossRef] [PubMed]
- Romoli, O.; Gendrin, M. The Tripartite Interactions between the Mosquito, Its Microbiota and Plasmodium. Parasites & vectors 2018, 11, 1–8. [Google Scholar]
- Shi, C.; Beller, L.; Wang, L.; Rosales Rosas, A.; De Coninck, L.; Héry, L.; Mousson, L.; Pagès, N.; Raes, J.; Delang, L. Bidirectional Interactions between Arboviruses and the Bacterial and Viral Microbiota in Aedes Aegypti and Culex Quinquefasciatus. MBio 2022, 13, e01021–22. [Google Scholar] [CrossRef] [PubMed]
- PuMPUNI, Charle. B.; Beier, M.S.; Nataro, J.P.; Guers, L.D.; Davis, J.R. Plasmodium Falciparum: Inhibition of Sporogonic Development in Anopheles Stephensi by Gram-Negative Bacteria. Experimental parasitology 1993, 77, 195–199. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural Microbe-Mediated Refractoriness to Plasmodium Infection in Anopheles Gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Dennison, N.J.; Saraiva, R.G.; Cirimotich, C.M.; Mlambo, G.; Mongodin, E.F.; Dimopoulos, G. Functional Genomic Analyses of Enterobacter, Anopheles and Plasmodium Reciprocal Interactions That Impact Vector Competence. Malaria journal 2016, 15, 1–15. [Google Scholar] [CrossRef]
- Bando, H.; Okado, K.; Guelbeogo, W.M.; Badolo, A.; Aonuma, H.; Nelson, B.; Fukumoto, S.; Xuan, X.; Sagnon, N.; Kanuka, H. Intra-Specific Diversity of Serratia Marcescens in Anopheles Mosquito Midgut Defines Plasmodium Transmission Capacity. Scientific reports 2013, 3, 1641. [Google Scholar] [CrossRef] [PubMed]
- Tchioffo, M.T.; Boissiere, A.; Churcher, T.S.; Abate, L.; Gimonneau, G.; Nsango, S.E.; Awono-Ambene, P.H.; Christen, R.; Berry, A.; Morlais, I. Modulation of Malaria Infection in Anopheles Gambiae Mosquitoes Exposed to Natural Midgut Bacteria. Plos one 2013, 8, e81663. [Google Scholar] [CrossRef]
- Bai, L.; Wang, L.; Vega-Rodríguez, J.; Wang, G.; Wang, S. A Gut Symbiotic Bacterium Serratia Marcescens Renders Mosquito Resistance to Plasmodium Infection through Activation of Mosquito Immune Responses. Frontiers in microbiology 2019, 10, 1580. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bai, L.; Jiang, Y.; Huang, W.; Wang, L.; Li, S.; Zhu, G.; Wang, D.; Huang, Z.; Li, X. A Natural Symbiotic Bacterium Drives Mosquito Refractoriness to Plasmodium Infection via Secretion of an Antimalarial Lipase. Nature microbiology 2021, 6, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Damiani, C.; Mancini, M.V.; Valzano, M.; Rossi, P.; Serrao, A.; Ricci, I.; Favia, G. Asaia Activates Immune Genes in Mosquito Eliciting an Anti-Plasmodium Response: Implications in Malaria Control. Frontiers in Genetics 2019, 10, 836. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Souza-Neto, J.; Torres Cosme, R.; Rovira, J.; Ortiz, A.; Pascale, J.M.; Dimopoulos, G. Reciprocal Tripartite Interactions between the Aedes Aegypti Midgut Microbiota, Innate Immune System and Dengue Virus Influences Vector Competence. PLoS neglected tropical diseases 2012, 6, e1561. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M. A Wolbachia Symbiont in Aedes Aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P. The w Mel Wolbachia Strain Blocks Dengue and Invades Caged Aedes Aegypti Populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The WMel Strain of Wolbachia Reduces Transmission of Zika Virus by Aedes Aegypti. Scientific reports 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L. Establishment of WMel Wolbachia in Aedes Aegypti Mosquitoes and Reduction of Local Dengue Transmission in Cairns and Surrounding Locations in Northern Queensland, Australia. Gates open research 2019, 3. [Google Scholar] [CrossRef]
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.; Arif, M.A.; Thohir, H.; NurSyamimi, H. Establishment of Wolbachia Strain WAlbB in Malaysian Populations of Aedes Aegypti for Dengue Control. Current biology 2019, 29, 4241–4248. [Google Scholar] [CrossRef]
- Fraser, J.E.; O’Donnell, T.B.; Duyvestyn, J.M.; O’Neill, S.L.; Simmons, C.P.; Flores, H.A. Novel Phenotype of Wolbachia Strain w Pip in Aedes Aegypti Challenges Assumptions on Mechanisms of Wolbachia-Mediated Dengue Virus Inhibition. PLoS Pathogens 2020, 16, e1008410. [Google Scholar] [CrossRef]
- Caragata, E.P.; Rancès, E.; Hedges, L.M.; Gofton, A.W.; Johnson, K.N.; O’Neill, S.L.; McGraw, E.A. Dietary Cholesterol Modulates Pathogen Blocking by Wolbachia. PLoS pathogens 2013, 9, e1003459. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, V.; Stainton, K.; Rainey, S.M.; Ant, T.H.; Dowle, A.A.; Larson, T.; Hester, S.; Charles, P.D.; Thomas, B.; Sinkins, S.P. Perturbed Cholesterol and Vesicular Trafficking Associated with Dengue Blocking in Wolbachia-Infected Aedes Aegypti Cells. Nature communications 2017, 8, 526. [Google Scholar] [CrossRef]
- Kambris, Z.; Cook, P.E.; Phuc, H.K.; Sinkins, S.P. Immune Activation by Life-Shortening Wolbachia and Reduced Filarial Competence in Mosquitoes. Science 2009, 326, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Rancès, E.; Ye, Y.H.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The Relative Importance of Innate Immune Priming in Wolbachia-Mediated Dengue Interference. PLoS pathogens 2012, 8, e1002548. [Google Scholar] [CrossRef] [PubMed]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. New England Journal of Medicine 2021, 384, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.B.; Riback, T.I.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A. Effectiveness of Wolbachia-Infected Mosquito Deployments in Reducing the Incidence of Dengue and Other Aedes-Borne Diseases in Niterói, Brazil: A Quasi-Experimental Study. PLoS neglected tropical diseases 2021, 15, e0009556. [Google Scholar] [CrossRef] [PubMed]
- Dodson, B.L.; Pujhari, S.; Brustolin, M.L.; Metz, H.C.; Rasgon, J.L. Variable Effects of Wolbachia on Alphavirus Infection in Aedes Aegypti. bioRxiv, 2023; 2023.01. 20.524939. [Google Scholar]
- Loreto, E.L.S.; Wallau, G.L. Risks of Wolbachia Mosquito Control. Science 2016, 351, 1273–1273. [Google Scholar] [CrossRef]
- Sanaei, E.; Charlat, S.; Engelstädter, J. Wolbachia Host Shifts: Routes, Mechanisms, Constraints and Evolutionary Consequences. Biological Reviews 2021, 96, 433–453. [Google Scholar] [CrossRef]
- Edenborough, K.M.; Flores, H.A.; Simmons, C.P.; Fraser, J.E. Using Wolbachia to Eliminate Dengue: Will the Virus Fight Back? Journal of virology 2021, 95, e02203–20. [Google Scholar] [CrossRef] [PubMed]
- Thi Hue Kien, D.; Edenborough, K.M.; da Silva Goncalves, D.; Thuy Vi, T.; Casagrande, E.; Thi Le Duyen, H.; Thi Long, V.; Thi Dui, L.; Thi Tuyet Nhu, V.; Thi Giang, N. Genome Evolution of Dengue Virus Serotype 1 under Selection by Wolbachia Pipientis in Aedes Aegypti Mosquitoes. Virus Evolution 2023, vead016. [Google Scholar] [CrossRef]
- Crawford, J.E.; Clarke, D.W.; Criswell, V.; Desnoyer, M.; Cornel, D.; Deegan, B.; Gong, K.; Hopkins, K.C.; Howell, P.; Hyde, J.S. Efficient Production of Male Wolbachia-Infected Aedes Aegypti Mosquitoes Enables Large-Scale Suppression of Wild Populations. Nature Biotechnology 2020, 38, 482–492. [Google Scholar] [CrossRef]
- Dobson, S.L.; Bordenstein, S.R.; Rose, R.I. Wolbachia Mosquito Control: Regulated. Science 2016, 352, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Toghueo, R.M.K.; Kemgne, E.A.M.; Eke, P.; Kanko, M.I.M.; Dize, D.; Sahal, D.; Boyom, F.F. Antiplasmodial Potential and GC-MS Fingerprint of Endophytic Fungal Extracts Derived from Cameroonian Annona Muricata. Journal of ethnopharmacology 2019, 235, 111–121. [Google Scholar] [CrossRef]
- Hayibor, K.; Kwain, S.; Osei, E.; Nartey, A.P.; Tetevi, G.M.; Owusu, K.B.-A.; Camas, M.; Camas, A.S.; Kyeremeh, K. Ghanaian Mangrove Wetland Endophytic Fungus, Penicillium Herquei Strain BRS2A-AR Produces (9Z, 11E)-13-Oxooctadeca-9, 11-Dienoic Acid with Activity against Trichomonas Mobilensis. International Journal of Biological and Chemical Sciences 2019, 13, 1918–1937. [Google Scholar] [CrossRef]
- Shi, Y.-N.; Pusch, S.; Shi, Y.-M.; Richter, C.; Maciá-Vicente, J.G.; Schwalbe, H.; Kaiser, M.; Opatz, T.; Bode, H.B. (±)-Alternarlactones A and B, Two Antiparasitic Alternariol-like Dimers from the Fungus Alternaria Alternata P1210 Isolated from the Halophyte Salicornia Sp. The Journal of organic chemistry 2019, 84, 11203–11209. [Google Scholar] [CrossRef]
- Cappelli, A.; Valzano, M.; Cecarini, V.; Bozic, J.; Rossi, P.; Mensah, P.; Amantini, C.; Favia, G.; Ricci, I. Killer Yeasts Exert Anti-Plasmodial Activities against the Malaria Parasite Plasmodium Berghei in the Vector Mosquito Anopheles Stephensi and in Mice. Parasites & vectors 2019, 12, 1–8. [Google Scholar]
- Niu, G.; Wang, B.; Zhang, G.; King, J.B.; Cichewicz, R.H.; Li, J. Targeting Mosquito FREP1 with a Fungal Metabolite Blocks Malaria Transmission. Scientific reports 2015, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Blanford, S.; Chan, B.H.; Jenkins, N.; Sim, D.; Turner, R.J.; Read, A.F.; Thomas, M.B. Fungal Pathogen Reduces Potential for Malaria Transmission. Science 2005, 308, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Heinig, R.L.; Thomas, M.B. Interactions between a Fungal Entomopathogen and Malaria Parasites within a Mosquito Vector. Malaria journal 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Vega-Rodríguez, J.; Ghosh, A.K.; Jacobs-Lorena, M.; Kang, A.; St. Leger, R.J. Development of Transgenic Fungi That Kill Human Malaria Parasites in Mosquitoes. Science 2011, 331, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Carballar-Lejarazú, R.; Rodriguez, M.H.; de la Cruz Hernández-Hernández, F.; Ramos-Castaneda, J.; Possani, L.D.; Zurita-Ortega, M.; Reynaud-Garza, E.; Hernández-Rivas, R.; Loukeris, T.; Lycett, G. Recombinant Scorpine: A Multifunctional Antimicrobial Peptide with Activity against Different Pathogens. Cellular and Molecular Life Sciences 2008, 65, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.B.; Marrelli, M.T. Paratransgenesis: A Promising New Strategy for Mosquito Vector Control. Parasites & vectors 2015, 8, 1–9. [Google Scholar]
- Wang, S.; Jacobs-Lorena, M. Paratransgenesis Applications: Fighting Malaria with Engineered Mosquito Symbiotic Bacteria. In Arthropod Vector: Controller of Disease Transmission, Volume 1; Elsevier, 2017; pp. 219–234.
- Ratcliffe, N.A.; Furtado Pacheco, J.P.; Dyson, P.; Castro, H.C.; Gonzalez, M.S.; Azambuja, P.; Mello, C.B. Overview of Paratransgenesis as a Strategy to Control Pathogen Transmission by Insect Vectors. Parasites & Vectors 2022, 15, 112. [Google Scholar]
- Wang, S.; Jacobs-Lorena, M. Transgenesis and Paratransgenesis for the Control of Malaria. In Mosquito Gene Drives and the Malaria Eradication Agenda; Jenny Stanford Publishing, 2023; pp. 21–37.
- Huang, W.; Wang, S.; Jacobs-Lorena, M. Use of Microbiota to Fight Mosquito-Borne Disease. Frontiers in genetics 2020, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ioka, D.; Matsuoka, H.; Endo, H.; Ishii, A. Bacteria Expressing Single-Chain Immunotoxin Inhibit Malaria Parasite Development in Mosquitoes. Molecular and biochemical parasitology 2001, 113, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ghosh, A.K.; Bongio, N.; Stebbings, K.A.; Lampe, D.J.; Jacobs-Lorena, M. Fighting Malaria with Engineered Symbiotic Bacteria from Vector Mosquitoes. Proceedings of the National Academy of Sciences 2012, 109, 12734–12739. [Google Scholar] [CrossRef]
- Wang, S.; Dos-Santos, A.L.; Huang, W.; Liu, K.C.; Oshaghi, M.A.; Wei, G.; Agre, P.; Jacobs-Lorena, M. Driving Mosquito Refractoriness to Plasmodium Falciparum with Engineered Symbiotic Bacteria. Science 2017, 357, 1399–1402. [Google Scholar] [CrossRef]
- Villegas, L.M.; Pimenta, P.F.P. Metagenomics, Paratransgenesis and the Anopheles Microbiome: A Portrait of the Geographical Distribution of the Anopheline Microbiota Based on a Meta-Analysis of Reported Taxa. Memórias do Instituto Oswaldo Cruz 2014, 109, 672–684. [Google Scholar] [CrossRef]
- Bongio, N.J.; Lampe, D.J. Inhibition of Plasmodium Berghei Development in Mosquitoes by Effector Proteins Secreted from Asaia Sp. Bacteria Using a Novel Native Secretion Signal. PLoS One 2015, 10, e0143541. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.V.; Spaccapelo, R.; Damiani, C.; Accoti, A.; Tallarita, M.; Petraglia, E.; Rossi, P.; Cappelli, A.; Capone, A.; Peruzzi, G. Paratransgenesis to Control Malaria Vectors: A Semi-Field Pilot Study. Parasites & vectors 2016, 9, 1–9. [Google Scholar]
- Raharimalala, F.N.; Boukraa, S.; Bawin, T.; Boyer, S.; Francis, F. Molecular Detection of Six (Endo-) Symbiotic Bacteria in Belgian Mosquitoes: First Step towards the Selection of Appropriate Paratransgenesis Candidates. Parasitology research 2016, 115, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Marinotti, O.; Serrão, D.M.; Correa, L.V.; Katak, R. de M.; de Oliveira, J.C.; Muniz, V.A.; de Oliveira, M.R.; do Nascimento Neto, J.F.; Pessoa, M.C.F. Culturable Bacteria Associated with Anopheles Darlingi and Their Paratransgenesis Potential. Malaria journal 2021, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tzschaschel, B.D.; Guzmán, C.A.; Timmis, K.N.; Lorenzo, V. de An Escherichia Coli Hemolysin Transport System-Based Vector for the Export of Polypeptides: Export of Shiga-like Toxin IIeB Subunit by Salmonella Typhimurium AroA. Nature biotechnology 1996, 14, 765–769. [Google Scholar] [CrossRef]
- Riehle, M.A.; Moreira, C.K.; Lampe, D.; Lauzon, C.; Jacobs-Lorena, M. Using Bacteria to Express and Display Anti-Plasmodium Molecules in the Mosquito Midgut. International journal for parasitology 2007, 37, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Ricci, I.; Marzorati, M.; Negri, I.; Alma, A.; Sacchi, L.; Bandi, C.; Daffonchio, D. Bacteria of the Genus Asaia: A Potential Paratransgenic Weapon against Malaria. Transgenesis and the management of vector-borne disease 2008, 49–59.
- Dehghan, H.; Mosa-Kazemi, S.H.; Yakhchali, B.; Maleki-Ravasan, N.; Vatandoost, H.; Oshaghi, M.A. Evaluation of Anti-Malaria Potency of Wild and Genetically Modified Enterobacter Cloacae Expressing Effector Proteins in Anopheles Stephensi. Parasites & Vectors 2022, 15, 63. [Google Scholar]
- Ward, T.W.; Jenkins, M.S.; Afanasiev, B.N.; Edwards, M.; Duda, B.A.; Suchman, E.; Jacobs-Lorena, M.; Beaty, B.J.; Carlson, J.O. Aedes Aegypti Transducing Densovirus Pathogenesis and Expression in Aedes Aegypti and Anopheles Gambiae Larvae. Insect molecular biology 2001, 10, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Suchman, E.; Buchatsky, L. Densoviruses for Control and Genetic Manipulation of Mosquitoes. Advances in virus research 2006, 68, 361–392. [Google Scholar]
- Ren, X.; Hoiczyk, E.; Rasgon, J.L. Viral Paratransgenesis in the Malaria Vector Anopheles Gambiae. PLoS pathogens 2008, 4, e1000135. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Rasgon, J.L. Densonucleosis Viruses (‘Densoviruses’) for Mosquito and Pathogen Control. Current opinion in insect science 2018, 28, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, J.L. Using Infections to Fight Infections: Paratransgenic Fungi Can Block Malaria Transmission in Mosquitoes. Future microbiology 2011, 6, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2022/1438 of 31 August 2022 Amending Annex II to Regulation (EC) No 1107/2009 of the European Parliament and of the Council as Regards Specific Criteria for the Approval of Active Substances That Are Micro-Organisms (Text with EEA Relevance); 2022; Vol. 227, 31 August.
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What Can We Learn from Commercial Insecticides? Efficacy, Toxicity, Environmental Impacts, and Future Developments. Environmental Pollution 2022, 118983. [Google Scholar] [CrossRef]
- Whitford, F.; Pike, D.; Burroughs, F.; Hanger, G.; Johnson, B.; Brassard, D.; Blessing, A. The Pesticide Marketplace, Discovering and Developing New Products. PPP-71. 2006. Available online: http://www. ppp.purdue. edu/Pubs/ppp-71.pdf.
- Roadmappers, T.I. A Roadmap for the Development of Ivermectin as a Complementary Malaria Vector Control Tool. The American journal of tropical medicine and hygiene 2020, 102, 3. [Google Scholar] [CrossRef]
- Koul, O. Biopesticides: Commercial Opportunities and Challenges. Development and Commercialization of Biopesticides 2023, 1–23. [Google Scholar]
- Deshayes, C.; Siegwart, M.; Pauron, D.; Froger, J.-A.; Lapied, B.; Apaire-Marchais, V. Microbial Pest Control Agents: Are They a Specific and Safe Tool for Insect Pest Management? Current medicinal chemistry 2017, 24, 2959–2973. [Google Scholar] [CrossRef] [PubMed]
- Beech, C.; Rose, N.; Dass, B. Regulation of Transgenic Insects. In Transgenic Insects: Techniques and Applications; CABI GB, 2022; pp. 493–517.
| Bacterium | Toxic formulation | Target mosquito genera | Refs | ||
|---|---|---|---|---|---|
| Aedes | Culex | Anopheles | |||
| Bacillus thuringiensis var. israelensis (Bti) | Extract (spores and crystals) | + | - | - | [50] |
| Sporulated culture powder (Tablet formulation XL-47) | + | - | - | [51] | |
| Spores and crystals tablet | + | - | - | [52] | |
| Spores and crystals tablet | + | - | - | [53] | |
| VectoBac WG | + | - | - | [54] | |
| Formulated product | - | + | - | [55] | |
| Binary mixtures (Bti plus Deltamethrin) | - | + | - | [56] | |
| Cry2Aa and Cyt1Aa crystals | - | + | - | [57] | |
| Crystallogenic variants. | + | + | - | [58] | |
| Two recombinant proteins (Cry10Aa and Cyt2Ba) | + | - | - | [59] | |
| Xpp81Aa toxin combined with Cry2Aa and Cry4Aa | + | - | - | [35] | |
| Kappa-carrageenan and Vectobac 12 AS hydrogels | + | - | - | [60] | |
| Bti extracts | + | - | - | [61] | |
| Vectobac® AS | - | + | - | [62] | |
| Granular formulation (Vectobac G) | - | - | + | [63] | |
| Dispersible granule (strain AM65-52) | + | + | + | [64] | |
| Bti strain Becker Microbial Products (BMP) | - | + | + | [65] | |
| Bti product VECTOBAC TP® sprayed | - | - | + | [66] | |
| Bti Water Dispersible Granular (WDG) formulation | - | - | + | [67] | |
| Water dispersible granule (Bti strain AM65-52 formulation, VectoBac® WDG) | - | - | + | [68] | |
| Water-dispersible granule Bti VectoBac (WDG) | - | - | + | [69] | |
| Bti VectoBac® WG, AM65-52 strain | - | - | + | [70] | |
| Bacillus thuringiensis (Other strains) | Total and lyophilised culture | + | + | - | [71] |
| Bacterial cultures | + | + | + | [72] | |
| Bacterial suspensions (spores and crystals) | + | - | - | [73] | |
| Spores | + | + | + | [74,75] | |
| Parasporal crystalline inclusion bodies | + | - | - | [35] | |
| Culture supernatant | + | - | + | [29] | |
| Synergistic interaction (Purified Cry11Aa and Cyt1Aa Toxins) | + | - | - | [76] | |
| Synergistic action of the Cry and Cyt proteins | - | - | + | [77] | |
| Lysinibacillus sphaericus (Lbs) | Culture supernatant | + | - | + | [29] |
| Spores and vegetative cells | + | + | - | [30] | |
| Cell suspension plus glyphosate | + | - | - | [78] | |
| Spore crystals (lyophilized powder) | + | + | + | [79] | |
| Spores | - | + | - | [80] | |
| Granular formulation (Vectobac G) | - | + | + | [63] | |
| Vectolex G | - | + | - | [81] | |
| S-layer protein | - | + | - | [82] | |
| Purified BinA and BinB proteins | - | + | + | [83,84] | |
| Spore-crystals and purified S-layers protein | - | + | + | [85] | |
| Synergy of Mtx and Cry proteins | - | + | - | [44] | |
| Purified BinA and BinB proteins | - | + | - | [39] | |
| VectoLex® WG plus Pyrethroid Resigen® | - | + | - | [86] | |
| Cry48Aa and Cry49Aa proteins combined | - | + | - | [87] | |
| Synergistic interaction (S-Layer and spores/crystals) | - | + | - | [88] | |
| VectoLex (ABG-6185) | - | - | + | [89] | |
| Suspension (Lyophilized bacteria) | - | - | + | [90] | |
| VectoLex® CG | - | - | + | [91] | |
| Bin toxin proteins | - | - | + | [92] | |
| Acidovorax sp. | Cell-Free Supernatant | + | - | - | [93] |
| Aneurinibacillus aneurinilyticus | Bacterial suspension | + | + | + | [94] |
| Bacillus amyloliquefaciens | Biosurfactant | + | + | + | [95] |
| Bacillus cereus | Culture supernatant | + | - | + | [29] |
| Bacillus circulans | Spores | + | + | + | [96] |
| Brevibacillus halotolerans | Supernatant and pellet fractions of bacterial cultures | + | - | - | [48] |
| Bacillus licheniformis | Dahb1 exopolysaccharide (Bl-EPS) | + | - | + | [97] |
| Brevibacillus laterosporus | Suspension of sporulated cells | + | - | + | [98] |
| Spore and the canoe-shaped parasporal body (CSPB) structure | + | - | - | [99] | |
| Purified protein crystals | + | - | + | [100] | |
| Pellets (cells and spores) | + | - | - | [101] | |
| Spores | + | - | - | [102] | |
| Bacillus paranthracis | Pellets (cells) | + | - | - | [47] |
| Bacillus safensis | Supernatant and pellet fractions of bacterial cultures | + | - | - | [48] |
| Pellets (cells) | + | - | - | [47] | |
| Bacillus subtilis | Culture supernatant | + | - | + | [29] |
| Crude cyclic lipopeptides (CLPs) | - | + | - | [103] | |
| Crude surfactin | - | - | + | [104] | |
| Bacterial biomass | + | - | - | [105] | |
| Biosurfactants | - | - | + | [106,107] | |
| Bacillus megaterium | Bacterial culture | + | - | - | [48] |
| Bacillus nealsonii | Secondary metabolites | + | - | - | [108] |
| Bacillus tequilensis | Cyclic Lipopeptide Biosurfactant | - | - | + | [109] |
| Bacillus velezensis | Bacterial culture | + | - | - | [48] |
| Pellets (cells) | + | - | - | [47] | |
| Chromobacterium sp. | Hydrogen cyanide | + | - | + | [110,111] |
| Chromobacterium anophelis | Bacterial suspension | - | - | + | [112] |
| Pantoea stewartii | Silver nanoparticles | + | + | + | [113] |
| Paraclostridium bifermentans | Clostridial neurotoxin | - | - | + | [114] |
| Peanibacillus macerans | Bacterial biomass | + | - | - | [105] |
| Photorhabdus luminescens | Secondary metabolites (Culture fluids) | + | - | - | [115] |
| Secondary metabolites | + | - | - | [116] | |
| Photorhabdus luminescens subsp. akhurstii | Bacterial cell suspension | + | - | - | [117] |
| Pseudomonas sp. | Bacterial cell suspension | - | + | - | [84] |
| Priestia aryabhattai | Silver nanoparticles | + | + | + | [113] |
| Serratia marcescens | Prodigiosin | + | - | + | [118,119] |
| Bacterial suspension | + | - | - | [120] | |
| Serratia nematodiphila | Bacterial cultures | + | + | + | [72] |
| Saccharopolyspora spinosa | Spinosad (Tracer®) | + | - | + | [121] |
| Spinosad formulation | + | + | + | [122] | |
| Spinosad-based product (Laser®) | + | + | + | [123] | |
| Spinosad | + | - | - | [124] | |
| Spinosad - Tablet (DT) and granules (GR) | + | - | - | [125] | |
| Spinosad powder | - | + | - | [126] | |
| Spinosad formulation | - | - | + | [127,128] | |
| Natular T-30 formulation | - | + | - | [129] | |
| Formulation Emulsifiable Concentrate | - | + | - | [130] | |
| Streptomyces sp. | Secondary metabolites | + | - | - | [108,131] |
| Xenorhabdus indica | Bacterial cell suspension | + | - | - | [117] |
| Xenorhabdus nematophila | Secondary metabolites | + | - | - | [116] |
| Secondary metabolites (Culture fluids) | + | - | - | [115] | |
| Xenorhabdus stockiae | Bacterial cell suspension | + | - | - | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





