1. Introduction
Pulmonary oxygen toxicity (PO
2tox) results from prolonged exposure to a hyperoxic atmosphere, with the severity of symptoms increasing progressively with elevation of the inspired oxygen partial pressure (PiO
2) and the duration of exposure [
1]. Symptoms of PO
2tox include chest pain, tightness, cough, and substernal distress that may coincide with decreases in pulmonary function, specifically a reduction in forced vital capacity (FVC) and alveolar diffusion capacity (D
LCO) [
1,
2]. The toxic effects of oxygen are a concern for military and technical divers conducting prolonged multiday dives using oxygen rebreathers, and for patients undergoing hyperbaric oxygen therapy or aggressive oxygen therapy for respiratory insufficiency at normobaric pressure. While there are theoretical models that predict the expected level of pulmonary function deficit because of prolonged exposure to raised PiO
2 that are based upon the expected decline in FVC, there is considerable individual variation in susceptibility to a uniform degree of pulmonary oxygen poisoning [
3,
4,
5]. Currently, there are no methods to predict individual susceptibility to PO
2tox. Furthermore, a sensitive non-invasive biomarker that can detect changes in lung pathology at an early stage in the oxygen toxicity process has remained elusive.
Expired nitric oxide (F
ENO) measurements have been studied as an exhaled marker of airway inflammation in a variety of lung diseases including asthma, lung cancer, bacterial pneumonia, pulmonary fibrosis, and idiopathic pulmonary fibrosis [
6,
7]. Nitric oxide (NO) in expired air is derived from nitric oxide synthase (NOS) activity from various cellular sources including neutrophils, alveolar type-II cells, endothelial cells, and airway cells, as well as from non-enzymatic sources such as s-nitrosothiols and nitrite protonation [
6,
8]. All three types of NOS (neuronal [nNOS], inducible [iNOS], and endothelium [eNOS]) have been identified in the human lung [
9]. Endogenous NO in the lungs is thought to play an important role in host immune defenses by maintaining ciliary function, preventing the growth of bacteria and replication of viruses, modulating airway reactivity, facilitating surfactant production in the alveoli, and regulating inflammation and local blood flow in the lung [
7,
9].
The role of NO in the development or protection from O
2 toxicity has been investigated in animal studies by several investigators [
10,
11] to better understand the roles of oxidative and nitrosative stress on hyperoxia-induced cell damage and acute lung injury [
12,
13]. Garat
et al., [
10] found that the survival time of hyperoxic rats treated with the NOS inhibitor NG-Nitroarginine Methyl Ester (L-NAME) was reduced compared to a hyperoxic control group, suggesting a protective effect of endogenous NO during 100% O
2 breathing at normobaric pressure. Investigators at Duke University have shown that NO production may either exacerbate or mitigate the toxic effects of oxygen, depending on the NOS isoform that produces it [
11,
12]. These animal studies raise the intriguing possibility that individual variability of NO production in the lung may explain the large variability in individual susceptibility to PO
2tox. Thus, the aim of this study was to determine if F
ENO levels could provide an indication of PO
2tox susceptibility in humans.
3. Results
Baseline individual means and 2x the coefficient of variation (2 x SD/mean x100%) for F
ENO (at 50 ml/s expired flow rate) and the different pulmonary function tests (i.e., FVC, F
IVC, D
LCO) that were derived from the twice daily measurements (am and pm) taken on five consecutive days (n=20 data points per mean per subject for each pulmonary function test) are shown in the second column of
Table 1,
Table 2,
Table 3 and
Table 4. The remaining columns in each table show the percent change in that variable from each individual’s mean baseline level following the HBO exposures. In each of the tables the subject’s data are ordered from highest (top) to lowest (bottom) baseline F
ENO. Additional tables showing changes in D
LCO adjusted for Hb (D
LCO
adj), D
LCO adjusted for alveolar volume (D
LCO/VA), alveolar volume (V
A), and F
EV1 are presented in
Appendix A.
As shown in
Table 1 there was a threefold range (19 to 59 ppb) in the baseline F
ENO between subjects. Analysis of the F
ENO baseline data using all expired flow rates indicated a significant time of day effect, with F
ENO on average 10% lower in the afternoon compared to morning (p<0.001). There was, however, no difference detected between the pre-dive F
ENO taken on the morning before the six-hour dive and the baseline F
ENO (p=0.9994). Immediately following the six-hour oxygen exposure all eight subjects had significant decreases in F
ENO (i.e., values >2x CV less than their baseline) with the group mean change showing a 55% decrease (p<0.0001). By the morning after the dive, F
ENO levels had returned to normal in the majority of divers (6 out of 8).
The four subjects with the lowest baseline F
ENO and lowest post-dive F
ENO (subjects 2, 3, 4, and 9) had clinical symptoms of pulmonary O
2 toxicity and showed significant decreases in pulmonary function on one or more of the pulmonary function tests immediately following the six-hour exposure (see
Table 2,
Table 3 and
Table 4). The clinical symptoms reported included chest fullness/tightness, congestion, mild substernal burning, and tickling or cough on deep inhalation. Subjects 1, 5, 7, and 8, who had baseline F
ENO levels greater than the group mean of 34 ppb, showed no pulmonary function deficits or symptoms of pulmonary O
2 toxicity following the six-hour HBO exposure and thus conducted the eight-hour HBO exposure the following day. Immediately following the eight-hour dive three of these subjects had pulmonary function deficits (see
Table 2,
Table 3 and
Table 4) and all four subjects showed greater decreases in F
ENO than following their six-hour dive (mean ± SD F
ENO post-dive1 vs. post-dive 2 = 22.2 ± 3.4 ppb vs. 16.6 ± 2.7 ppb, respectively, n =4, p<0.01). Subject 5 had the highest baseline F
ENO and was the only subject who did not show symptoms of PO
2tox or a pulmonary function deficit following the HBO exposures.
During the three days following the dives, five subjects showed significant increases in F
ENO (see
Table 1)
. However, the timing of these increases and the duration of the elevated F
ENO was variable among the subjects. Consequently, the group analysis did not reveal any statistically significant change in the mean F
ENO from the pre-dive baseline during recovery days one (p= 0.8642), two (p=0.0579), or three (p=0.3358).
The pulmonary function test that demonstrated the greatest number of significant decrements following the oxygen exposures was D
LCO (see
Table 4). The three subjects with the lowest baseline F
ENO (subjects 4, 3, and 9) had the greatest relative decrements in D
LCO which persisted for one to three days post exposure. Subjects 1 and 8 also showed significant decreases in D
LCO during the recovery period. When D
LCO was corrected for V
A, all subjects except subject 5 showed significant decrements at some point during the recovery period (see
Table A2 in
Appendix A). Both D
LCO and D
LCO/VA showed a significant main effect of time (p<0.05 and p<0.01, respectively) that was predominantly due to lower values during the second day of recovery compared to baseline (see
Table 4 and
Table A2).
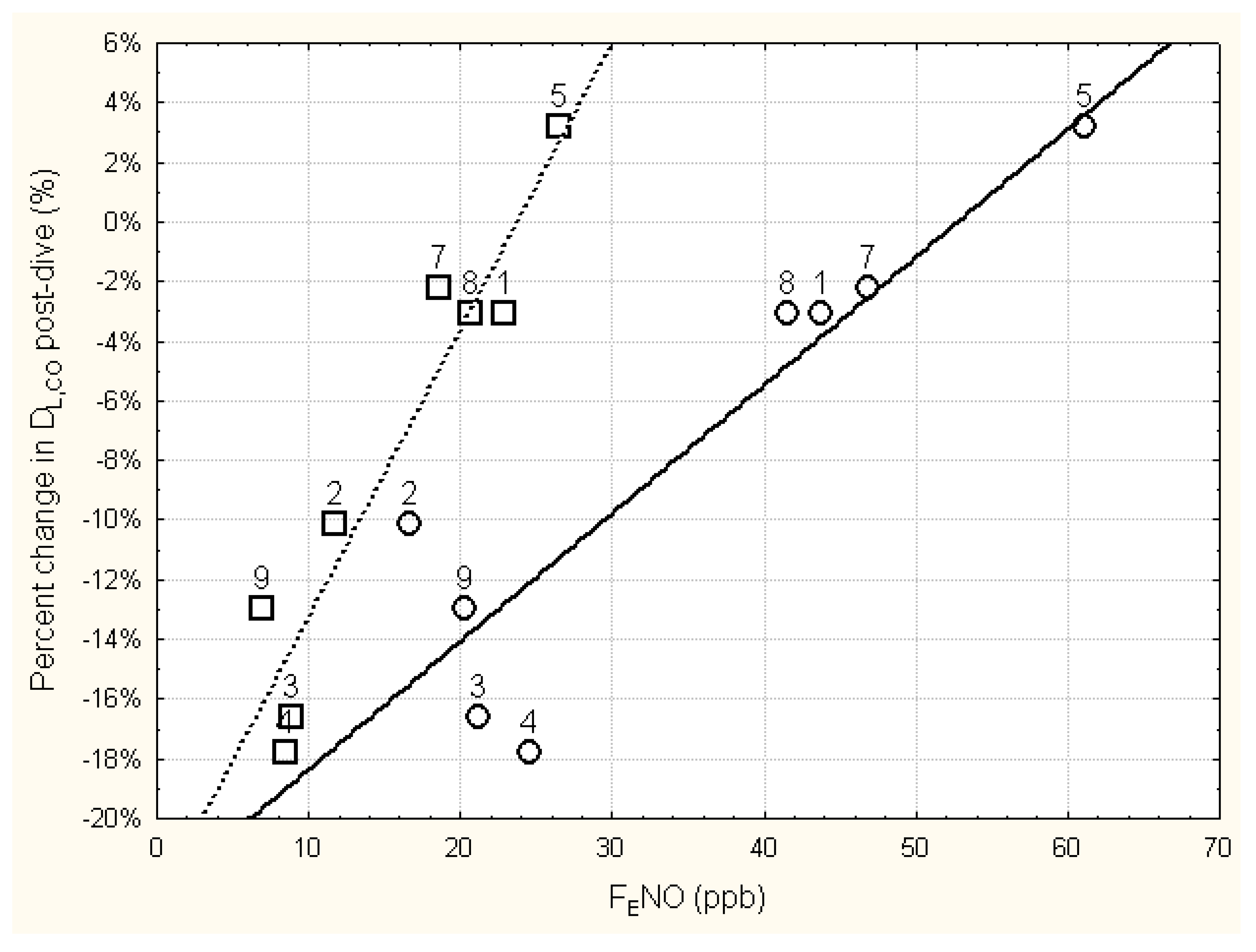
The relationship between the relative change in D
LCO immediately following the six-hour dive and the immediate pre- and post-dive levels of F
ENO is shown in
Figure 1. Regression analysis of these data found that the relative change in D
LCO immediately post-dive was significantly related to the immediate post-dive F
ENO (r=0.948, p<0.001), as well as to the pre-dive F
ENO (r=0.902, p<0.01). Using the mean baseline F
ENO in the regression analysis instead of the pre-dive F
ENO slightly improved the relationship (r=0.931, p<0.001).
While some subjects had significant decrements on the spirometry tests following the dives, the group mean relative changes on the spirometry tests averaged smaller than those found for DLCO. Immediately following the six-hour dive FIVC appeared to be more affected than FVC, however, neither FIVC or FVC showed a significant main effect of time following group analysis (p=0.0658 and p=0.2176, respectively).
The two-compartment model analysis of the FENO data showed a significant 58% decrease in J’awNO (mean ± SD, baseline vs. post-dive 1 = 1681 ± 747 pl/s vs. 709 ± 465 pl/s, p<0.001) with no change in CANO (mean ± SD baseline vs. post-dive 1 = 2.9 ± 1.2 ppb vs. 2.6 ± 0.6 ppb; p=0.995) immediately following dive 1. A comparison of pre and post exposure measurements for FENO and pulmonary function for the two subjects who conducted the surface control trials showed that all the dependent variables following the control exposure were within each individual’s normal daily variability (data not shown).
4. Discussion
Traditionally the “gold standard” for assessing PO
2tox has been to measure changes in pulmonary function using spirometry (i.e., FVC) or D
LCO. However, the sensitivity of these pulmonary function tests to assess PO
2tox susceptibility has been questioned [
21,
22], and more recent research has explored other components in exhaled breath as potential biomarkers of PO
2tox [
22,
23,
24,
25,
26,
27]. Since the initial discovery that NO was present in expired air [
28], F
ENO has been one of the most widely studied exhaled breath biomarkers of pulmonary health. F
ENO increases significantly in a variety of inflammatory airway diseases and is now commonly used to diagnose and phenotype asthmatics [
7]. It was thus originally hypothesized that F
ENO would increase following HBO exposure due to free oxygen radical initiation of inflammatory reactions in the lungs.
One of the first published papers on the effect of the hyperoxia on F
ENO reported that F
ENO increased with exposure to normobaric hyperoxic gas mixtures [
29]. However, the 10-minute normobaric oxygen exposures in this early study were unlikely to result in inflammation of the lungs. The results of the study by Schmetterer
et al., [
29] are in direct contrast with our finding of a marked acute reduction in F
ENO following prolonged HBO exposures. One potential reason for the disparate results is that at the time that Schmetterer
et al. [
29] performed their study, there was no standard method for measuring F
ENO. Since that time, it has become clear that F
ENO is highly dependent on the expired flow rate and thus the recommended guidelines for F
ENO measurements published by the ATS in 2005 [
20] have since standardized expired flow rates at 50 ml/s using a flow resistor that also prevents contamination of the F
ENO measurement from the high levels of NO found in the nasal cavity. Although we did observe significant increases in F
ENO during the recovery days in five subjects, which may be reflective of a delayed inflammatory reaction in the lungs, only one of these subjects (subject 9) exhibited consistent decrements in pulmonary function during all three recovery days that was concomitant with abnormally elevated F
ENO levels.
A second main finding from our study is that the duration of the HBO exposure affected the relative magnitude of the post dive decrease in F
ENO, with the eight-hour HBO dive resulting in significantly lower post dive F
ENO levels than the six-hour HBO exposure. This finding implies that the magnitude of the temporary F
ENO decrease following the HBO exposures may be dose dependent. Since conducting these pilot HBO dives in 2007, we have conducted a wide variety of dry human hyperoxic exposures with varying inspired oxygen partial pressures and exposure durations to determine if the F
ENO decreases found in the current study follow a predictable dose response relationship. Findings from these studies have been presented to the undersea and hyperbaric medical and research community at various scientific forums [
30,
31,
32] and were summarized in preliminary form in Fothergill and Weathersby [
33]. This study showed that the relative change in F
ENO following dry resting hyperoxic exposure follows an exponential decline that is tightly related to the hyperoxic dose of the preceding exposure [
33]. In the statistical model of the changes in F
ENO with varying HBO exposures, Fothergill & Weathersby [
33] used the following expression to define the hyperoxic dose of the HBO dives based upon the inspired partial pressure of the oxygen breathed (PiO
2) and the duration of the exposure:
Other investigators have also reported acute decreases in F
ENO levels following HBO exposures [
34,
35,
36,
37,
38,
39,
40]; However, the oxygen dose involved in these studies has rarely been great enough to induce changes in lung function or PO
2tox symptoms noticeable enough to determine if the F
ENO changes are related to PO
2tox susceptibility. Our study is therefore somewhat unique in that we were able to observe symptoms of PO
2tox and measure significant decreases in lung function in some of our subjects and relate them to the observed changes in F
ENO. Based upon our observations, we found that those individuals who had the lowest pre-dive F
ENO levels exhibited the lowest post-dive F
ENO levels and were most susceptible to PO
2tox.
This significant linear relationship between the pre-dive baseline levels of F
ENO and the relative decrease in D
LCO measured immediately post dive should be taken with caution when interpreting the effects of HBO exposure on PO
2tox susceptibility. In a more recent study in which healthy U.S. Navy trained divers were exposed to 6.5 h of 100% O
2 at 2.0 ATA [
27], one subject, who aborted the dive early due to severe PO
2tox symptoms, was found to have a 15% increase in D
LCO immediately post dive compared to his pre-dive base line [
41]. Concomitant with the increase in D
LCO was a 125% increase in total airway resistance and a 35% increase in proximal airway resistance (as measured using impulse oscillometery methodology) [
41]. We surmise that the elevated D
LCO post dive for this subject was an artifact caused by the increase in pulmonary resistance that resulted in a large negative interpulmonary pressure being generated during the fast inspiratory maneuver required to perform the D
LCO measurement. The negative interpulmonary pressure could result in increased blood volume entering the lung before the D
LCO breath hold maneuver, raising the potential sink for the inhaled carbon monoxide gas mixture and artifactually raising the D
LCO level. Therefore, we hypothesize that subjects who are particularly susceptible to PO
2tox might experience a narrowing of the airways, possibly due to loss of normal airway tone.
Acute changes in airway diameter can be evoked by increases in cholinergic nerve activity or withdrawal of nitrergic neural activity [
42]. Interestingly, noncholinergic neurotransmitters such as NO are thought to control human airway smooth muscle and normal airway tone via nitrergic parasympathetic nerves [
42]. Thus, factors that compromise normal nitrergic parasympathetic control of airway tone, such as reduced levels of NO, would act to cause narrowing of the airways. The acute post dive increase in airway resistance seen in the above PO
2tox case was concomitant with an extremely low post dive F
ENO of 3.5 ppb [
41]. This is consistent with a neurogenic PO
2tox response rather than an inflammatory reaction to the HBO exposure.
Although our study was not designed to elucidate the underlying mechanisms responsible for the reduction in F
ENO with HBO exposure, our results are consistent with the observation from previous animal work [
10,
43] that suggests that endogenous levels of NO may serve to protect the lung from hyperoxic lung injury [
43]. Based upon our current findings, we suspect that once F
ENO levels fall below a critical level the antioxidant defense and other processes in the lung that depend on NO become overwhelmed by the hyperoxic stress, resulting in changes in lung function and symptoms of PO
2tox. However, as discussed in a review paper by Lui
et al., [
44] the role of the various NOS isoforms in the generation of NO in the face of hyperoxic stress and the impact of NO in the pathogenesis of acute lung injury is still under debate.
Several studies have attempted to ascertain the underlying mechanisms responsible for the decrease in F
ENO with HBO exposures [
45,
46,
47]. The common thesis of these studies centers around the hypothesis that the decrease in F
ENO with hyperoxic exposures is due to decreased enzymatic generation of NO due to oxidation of tetrahydrobiopterin (BH
4), which is an essential cofactor required for NO production by NOS [
48]. Fismen
et al. [
45] found that increased O
2 concentrations reduced BH
4 levels in human endothelial cells in a dose-dependent manner without directly affecting the NOS enzyme. Similarly, Hesthammer
et al. [
46] reported that BH
4 levels in human umbilical vein endothelial cells (HUVEC) decreased in a dose dependent manner. Although the latter study found that HUVEC NO production was also decreased following a 40 kPa O
2 exposure, a further decrease in HUVEC NO production was not observed when the oxygen exposure was increased to 60 kPa. In a follow up study by Hesthammer
et al., [
47] in which BH
4 was measured in venous blood samples of subjects exposed to 100% oxygen for 90 minutes at atmospheric pressure, both F
ENO and BH
4 significantly decreased when measured 10 minutes after the exposure. Although oxidation of BH
4 levels and its subsequent uncoupling/inhibitory effects on NOS on NO production appear to be a plausible reason for the reduced F
ENO with hyperoxic exposures, other mechanisms including the reaction of oxygen or superoxide radicals with NO to form peroxynitrite likely also contribute to the reduced F
ENO.
4.1. Study Strengths and Limitations
To our knowledge, this is the first study that combined measurements of FENO with traditional measures of pulmonary function in healthy divers to assess PO2tox susceptibility following provocative HBO exposures that resulted in significant decrements in lung function. While the current study involved a small number of subjects, the study design incorporated multiple baseline and recovery measurements of FENO and pulmonary function to provide a robust indication of daily inter-individual variation and accurately define when these dependent variables fell significantly outside of the individual’s normal range, following the HBO exposure. The results clearly showed a wide individual variability in pulmonary function changes resulting from the HBO exposures, with half our subject population showing minimal changes in lung function following the six-hour dive and the other half showing significant decreases that were more than two standard deviations below their normal day-to-day range. While this experimental design allowed us to analyze individual susceptibility to PO2tox, the small n approach leaves group statistical analysis susceptible to type II errors from the large variability in individual responses to the HBO stress. However, given our primary aim, we felt the small n approach was ethically more defensible as a pilot study on individual PO2tox susceptibility than a larger n study with limited individual pre-dive data but a higher power to detect group level changes in pulmonary function post dive.
An additional limitation of the current study is that only two subjects completed a control (normobaric air) condition and that the study design was unblinded. This may have led to experimenter and subject bias regarding the expectation of pulmonary function decrements and PO
2tox symptoms following the HBO exposures. While performing the pulmonary function measurements in accordance with the ATS recommendations [
15,
16,
17,
18] will help to reduce this potential bias, most of the spirometry measurements are dependent upon the individual performing a maximal inspiratory and/or expiratory effort to determine if pulmonary function is affected by the HBO exposure. In contrast, measurements of F
ENO are conducted at a fixed expired flow rate and do not require a maximum effort by the subject. F
ENO may thus offer an alternative or complementary assessment of pulmonary hyperoxic stress that is less prone to the subject’s effort than traditional spirometry measurements. While we acknowledged that there are many sources of NO in the lungs that can contribute to F
ENO, and that the underlying mechanistic role of NO in hyperoxic acute lung injury is still controversial, F
ENO may provide a useful noninvasive marker of the hyperbaric oxidative stress response of the lungs, and lead to new insights into individual susceptibility to PO
2tox.