1. Introduction
According to the World Population Prospects [
1], in 2017, approximately 962 million people were older than 60; this number is projected to increase to 1.4 billion by 2030 and 2.1 billion by 2050. In addition, the population older than 80 is projected to increase from the current 143 million to 426 million by 2050. According to South Korea's "Population Trends and Projections of the World and Korea", the proportion of the world's population older than 65 will increase from 8.2% in 2015 to 17.6% in 2060; in South Korea, the proportion of the population older than 65 is expected to increase from 13.1% in 2015 to 40.1% by 2060 [
2]. The proportion of elderly people with limited mobility, frailty, and adverse outcomes (e.g., falls, fractures, loss of autonomy) is expected to increase correspondingly [
3].
Muscle health changes with age. Age-related loss of skeletal muscle mass and function (sarcopenia) is associated with frailty and increased risk (increasing by ~1%/year) and reaches approximately 50% by the age of 80, becoming a morbidity (chronic disease). Approximately 5-13% of people aged 60-70 years and 11-50% of people over 80 years old are affected by sarcopenia [
4,
5,
6]. Among adults over 60 years of age in the United States, the direct health care costs due to sarcopenia in 2000 were estimated at 18.5 billion US dollars [
7]. A study by Janssen et al. (2004) showed that a 10% decrease in the incidence of sarcopenia would reduce health care costs by 1.1 billion dollars annually. Therefore, the prevention and treatment of this condition is an urgent public health issue worldwide.
Sarcopenia was diagnosed based on the guidelines proposed by the European Working Group on Sarcopenia in the Elderly (EWGSOP) in 2010; these guidelines were updated in 2018 (EWGSOP2). The diagnosis of sarcopenia is based on three criteria: muscle strength, muscle quantity or quality, and physical performance [
8].
Although there are several methods for evaluating muscle mass, each has its own limitations. Computed tomography (CT) and magnetic resonance imaging (MRI) are the reference methods for measuring muscle mass [
9,
10]. Additionally, whole-body dual-energy X-ray absorptiometry (DXA) is a widely used first alternative in clinical settings. This method provides accurate and efficient measurements of whole-body and organ/tissue composition, but it is very expensive and cannot be used in most clinical or field settings [
11]. Sarcopenia can be assessed by a physical performance test, but this is challenging for elderly people with limited mobility. Therefore, clinical methods that can easily and quickly assess muscle mass and physical function are needed.
Bioimpedance analysis (BIA) is a noninvasive, easy-to-use, inexpensive, and portable method that has been used to assess body composition for decades. By using the impedance value obtained by BIA, participant height, weight, sex, etc., in a regression equation, lean body mass (LBM) and total body water (TBW) can be calculated [
12].
BIA does not directly measure TBW, body fat, or LBM. Instead, it measures electrical resistance (R) and reactance (Xc). Impedance (Z) is the sum of these two variables. R is negatively proportional to electrolytes in body water and living tissue. Xc is proportional to the capacitance of the cell membrane, which depends on the function, composition and structural integrity of the cell [
13,
14]. Traditionally, the BIA value is calculated as the square of participant height divided by the impedance. It is used to quantitatively infer body composition through a regression equation that includes empirical variables such as weight, sex, and height. Additionally, during the development of these predictive regression equations, it was discovered that several variables must be considered to ensure measurement accuracy. Specifically, body shape (approximated as 5 connected cylinders) and hydration level (the observed ratio of TBW to free fat mass [FFM]; 73%) must be taken into account, and the regression equations are based on the assumption that each body part has the same impedance coefficient (the body impedance ratio) [
15,
16].
Even though the estimation of body composition makes use of empirical variables (sex, race, age, etc.) in the BIA regression formula, by definition this formula is only suitable for use with the specific target groups for which it was developed, which limits its application and hinders generalization. Thus, BIA can only be used correctly after verifying its validity in a given instance [
17,
18].
In particular, unlike the general public, children and elderly individuals with poor eating habits, very poor nutritional status, or severe diseases or conditions (e.g., cancer, heart disease, thyroid disease, anorexia, or sarcopenia) have a different body composition that impairs BIA calculations [
19,
20]: Due to errors in bioelectrical variables generated during the process of estimating body composition, the values computed by the BIA regression equation are incorrect. To overcome this limitation, bioelectric variables can be directly used to evaluate body composition and changes in physiological status.
The bioelectric variables include resistance and induction resistance, the relationship between which indicates the physiological state of the human body. The variables directly measured by BIA are resistance and sense resistance. The phase angle (PhA) obtained by dividing Xc (obtained from BIA) by R can indicate the stable and pathological states of cells. For example, as PhA decreases, Xc decreases relative to R. Conversely, an increase in PhA indicates an increase in Xc versus R, which may reflect an improvement in biological cellular functions [
21,
22].
Recent studies have re-evaluated the use of PhA for assessing cell health, especially for detecting defects in cell membranes and cell function. Although accumulating evidence suggests that PhA can serve as a nutritional status [
23], disease prognosis [
24], or mortality risk indicator [
25], it remains uncertain whether it indicates muscle function or muscle quality.
Bioimpedance vector analysis (BIVA) is a BIA-derived method that utilizes raw impedance parameters to evaluate physiological states, facilitating the assessment of somatic cell mass and hydration without referring to predictive equations [
26]. This method has been used to assess many pathological conditions [
27] and is highly sensitive to changes in handgrip strength. However, validation studies that use BIVA to identify individuals with sarcopenia in the Korean population are currently lacking.
This study aims (1) to verify whether BIVA can support the clinical evaluation of sarcopenia in elderly individuals; and (2) to evaluate the relationships between PhA, physical performance, muscle mass, and other factors related to PhA.
2. Materials and Methods
2.1. Participants
The participants were recruited through local newspapers and social media advertisements. Participants who were registered at the public health centers of boroughs and volunteered to participate were also included. The study was approved by the Ethics Committee of the Korean National Sport University (No. 1263-201903-HR-010-02) and performed in accordance with the Declaration of Helsinki.
The exclusion criteria were as follows: contraindications for BIA (such as neurological disease, musculoskeletal disorders, heart failure, renal disease, edema, cachexia, open wound, rash, cardiac pacemaker, or metal implant), hospitalization within the last 3 months and a history of limb amputation.
2.2. Anthropometric measurements
During all measurements, subjects were instructed to wear light clothing and to remove all metal objects, jewelry, and shoes. Measurements were taken on the same day after a fast of at least 4 hours. Body weight was measured in units of 0.5 kg (CAS DB-1, 106 South Korea), and height was measured in 1-mm units (SECA 274, 107 Germany).
2.3. Measurement of the bioelectrical impedance parameters
Quantum Desktop RJL-101 (RJL Systems, Clinton Twp, MI, USA), a 4-point electrode real-time BIA device, was used to assess the impedance parameters. Participants were asked to fast overnight, refrain from strenuous physical activity within 12 hours and avoid drinking alcohol within 24 hours prior to the measurement.
Adhesive-gel electrodes were placed at defined anatomical sites on the anterior of the dorsal surfaces of the right hand, wrist, ankle, and foot. For the hand and wrist, the proximal edge of the detecting (voltage) electrode was attached in such a manner that it formed an imaginary line that bisected the styloid process of the ulna and such that the proximal edge of the source (current) electrode bisected the metacarpophalangeal joint of the middle finger. For the ankle and foot, the proximal edge of the ankle-detecting electrode was attached in such a manner that it formed an imaginary line bisecting the medial malleolus, and the distal edge of the foot-source electrode was placed to form an imaginary line that passed through the metatarsophalangeal joints of the second and third toes [
28].
2.4. Definition of sarcopenia
Sarcopenia was diagnosed as low muscle strength and low muscle mass and/or low physical performance according to the EWGSOP2 guidelines [
9].
Appendicular skeletal muscle (ASM) was assessed by DXA. All centers used the same DXA model (Lunar DPX-L model, software version 3.4) for body composition measurements. The measurements were performed in medium scan mode with the subject lying in a supine position. The scanning time was approximately 20 min.
Grip strength was tested with a grip dynamometer. Muscle strength was assessed through handgrip testing with a Grip-D hand-held grip dynamometer (Takei, Niigata, Japan). The strength was recorded in kilograms (kg). The left and right hands were tested 3 times, and the highest values were used [
29].
Physical performance was assessed by asking participants to walk 6 meters at a comfortable pace. The test started when their first foot crossed the starting line and stopped when their second foot crossed the finish line [
30]. The 6-meter walk was performed 2 times, and the highest value was used.
Low muscle strength was defined as handgrip strength <28 kg for males and <18 kg for females. The criteria for low physical performance were 6-meter walk speed <1.0 m/s, and low muscle quantity was defined by DXA < 7.0 kg/m2 in males and <5.4 kg/m2 in females [
31].
2.5. Data processing and statistical analysis
Descriptive, univariate, and bivariate statistical analyses (mean, standard deviation [SD], and Pearson’s correlation coefficient) were performed with SPSS version 23.0 (IBM Corporation, Armonk NY, USA). BIVA included R and Xc values (Z vector). Raw values were normalized by subject height (R/H and Xc/H) and are presented in an R-Xc plot constructed using BIVA software (Piccoli A, Pastori G, available from apiccoli@unipd.it). The normal intervals of the reference population are expressed in percentiles (50th, 75th, and 95th) of the Gaussian bivariate probabilistic graph [
32]. Finally, we calculated the parameters required to generate the dimensionless R-Xc score graph for the Korean population using the formula described in an earlier study [
32]. A p value < 0.05 was considered statistically significant, and all tests were 2-tailed.
3. Results
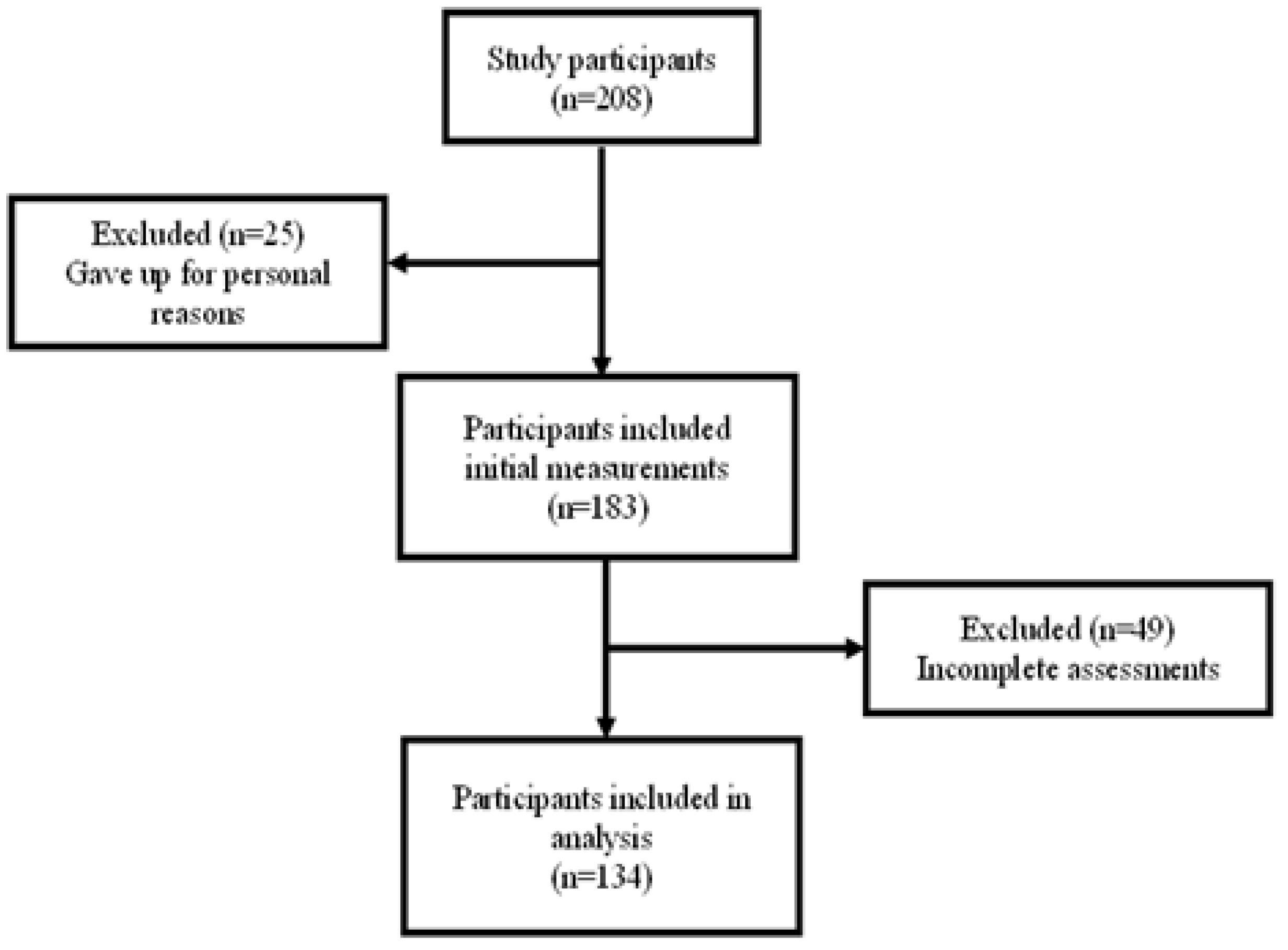
A total of 208 subjects were recruited for this study, but 25 subjects withdrew from the study due to personal reasons, so 183 subjects completed the beginning measurements. After excluding 49 subjects with incomplete data, the data of 134 subjects were finally processed and analyzed in this study (
Figure 1).
The 134 participants were 75 males and 59 females aged 69-91 (
Table 1). The bivariate 95% confidence ellipses of the mean vectors were calculated with the descriptive statistics reported in
Table 2 (i.e., the mean and SD of the vector components R/H and Xc/H and the correlation coefficient of the two components) as defined in BIVA. Males were significantly taller and heavier than females (p<0.001), and females had significantly higher R, R/H, and Xc/H than males (
p<0.001).
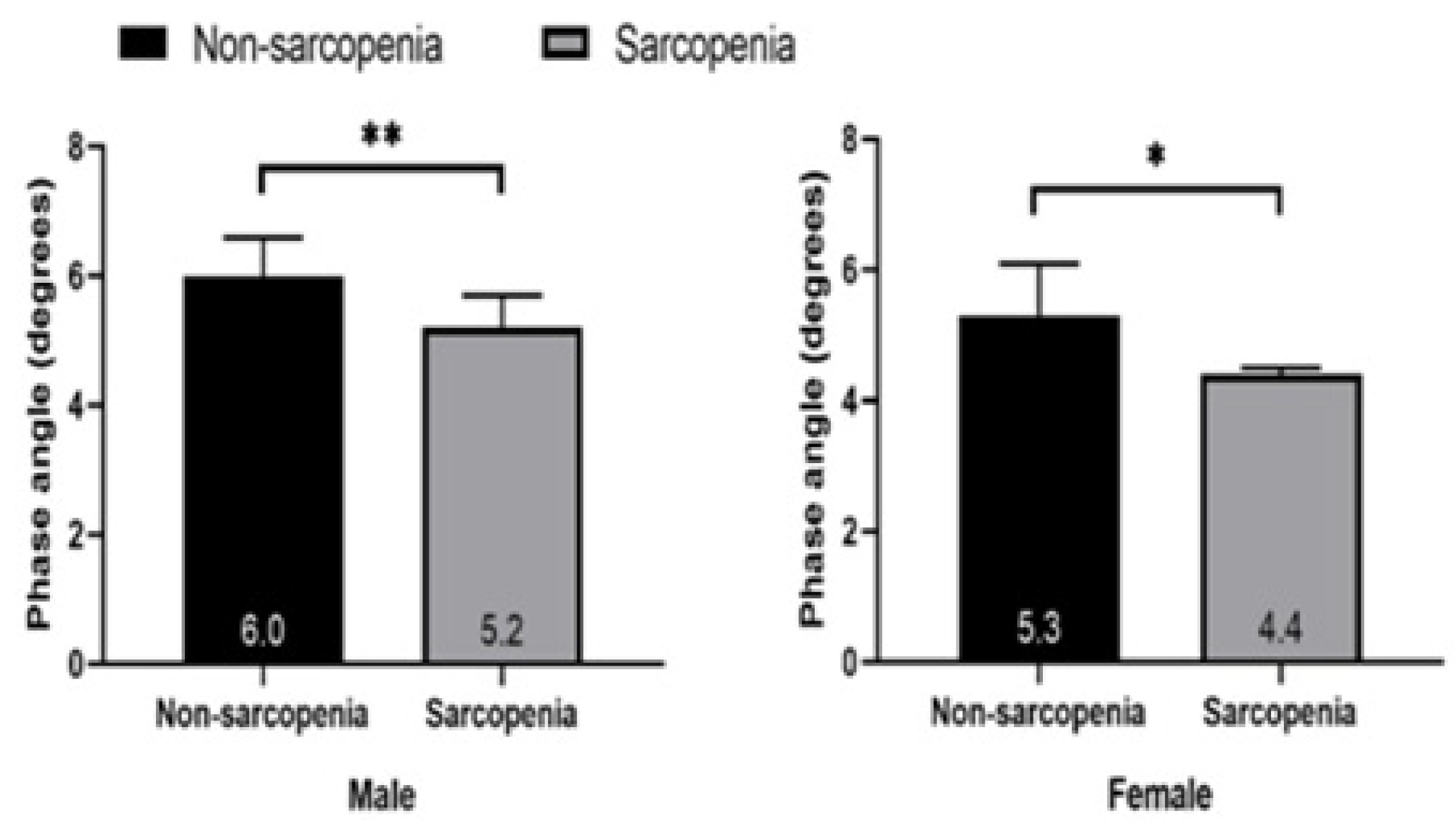
Table 2 shows that in the non-sarcopenia group, males had lower impedance (444.9 Ω in males and 536.7 Ω in females) and higher PhA than females (6.0° in males and 5.3° in females). In sarcopenia, males had lower impedance (513.7 in males and 595.9 in females) and higher PhA than females (5.2 in males and 4.4 in females). In men, the sarcopenia group (513.7 Ω) had higher impedance than the non-sarcopenia group (444.9 Ω) and a lower PhA (5.2±0.5°) than the non-sarcopenia group (6.0±0.6°). In women, the same trend was observed.
The bivariate 95% confidence ellipses of mean vectors were calculated with the descriptive statistical measures reported in
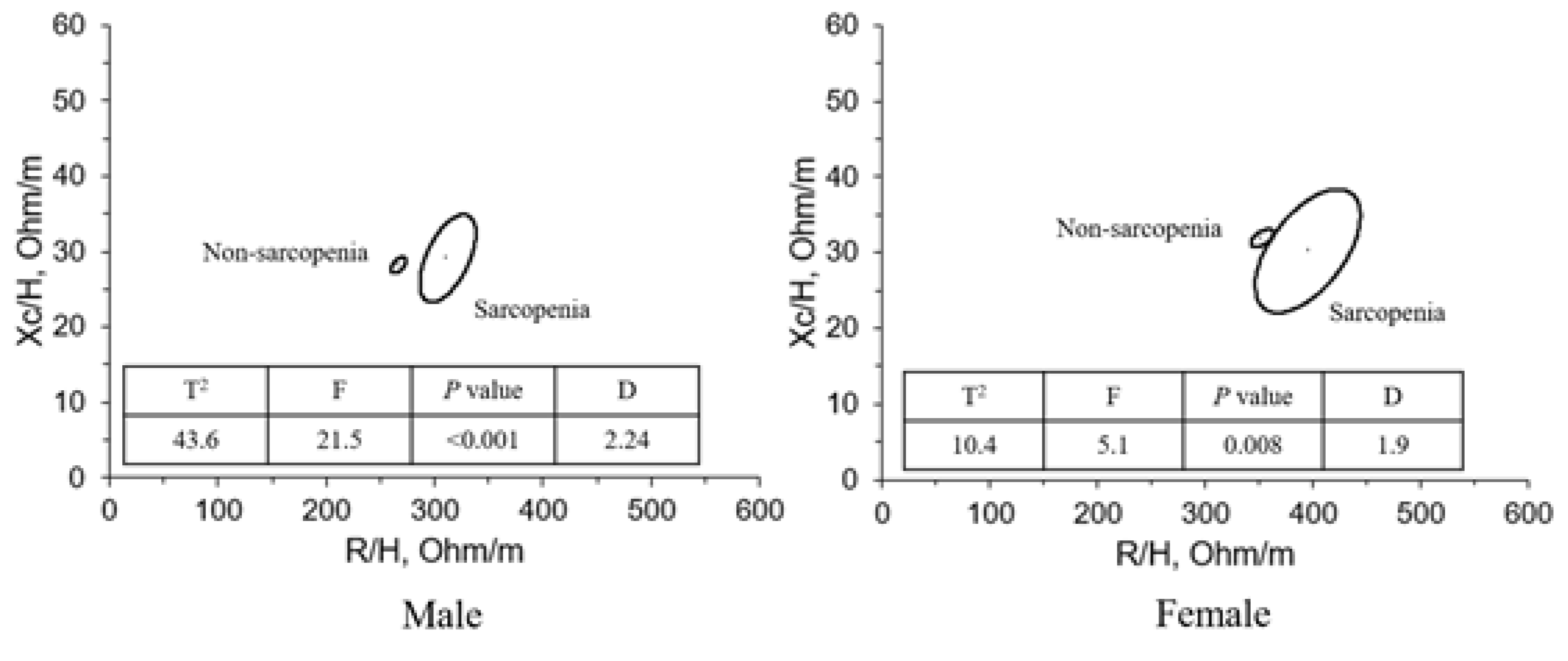
Table 3 (i.e., the mean and SD of vector components R/H and Xc/H and the correlation coefficient between the components) as defined through BIVA methods. The height-adjusted R significantly differed between the sarcopenic and nonsarcopenic groups, with the sarcopenic R/H significantly higher than the nonsarcopenic R/H. However, no significant differences in height-adjusted Xc were observed between the sarcopenic and nonsarcopenic groups, in men or in women. In addition, a significant difference in PhA between the sarcopenic and nonsarcopenic groups was observed, with the sarcopenic group having a lower PhA in males (
p<0.01) and females (
p<0.05).
Figure 2 shows the result of connecting the two centers of adjacent 95% confidence ellipses of males and of females. The spatial distribution of mean vectors on the R-Xc plane followed the same pattern in the two sexes. The sarcopenia group had longer impedance vectors in both males and females.
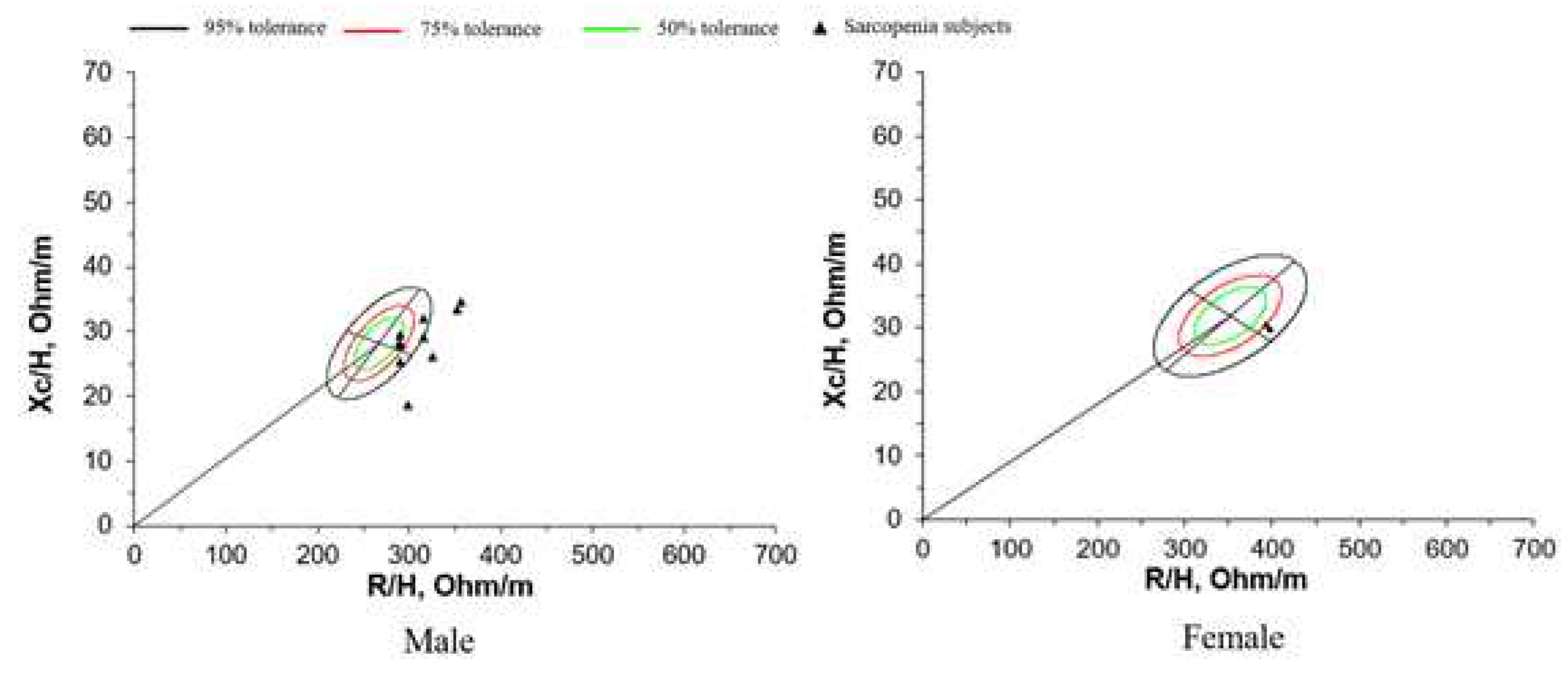
For each sex, the 50%, 75%, and 95% tolerance ellipses (i.e., the intervals in which a vector from an individual subject falls with a probability of 50%, 75%, and 95%, respectively) were calculated using the data reported in
Table 3 and are depicted as R-Xc graphs (
Figure 3). The size of the tolerance ellipses was determined by the variation of both vector components (larger ellipses were produced by groups with greater SD), and the elliptical shape was defined by the correlation coefficients between vector components.
With BIVA, intersubject variability in the impedance vector is represented with a bivariate normal distribution, i.e., a graph with elliptical probability regions (50%, 75%, and 95% tolerance ellipses) on the R–Xc plane, normalized by patient height (R/H, and Xc/H, in ohm/m). The vector position on the R-Xc graph is interpreted and ranked in the following two directions: (1) vector displacements parallel to the major axis of tolerance ellipses indicate progressive changes in soft tissue hydration; and (2) vectors lying on the left side and above the major axis or on the right side and below the major axis of tolerance ellipses indicate more or less soft tissue mass, respectively. : Resistance; Xc: reactance; :height[
46].
The study examined 134 individuals, 75 males and 59 females aged 69-91 years (
Table 4). Males had significantly greater height, weight, ASM, ASMI, muscle quality, and PhA than females (
p<0.001), and females had significantly higher R than males (
p<0.001).
The PhA of healthy elderly individuals was higher than that of sarcopenic elderly individuals in both males (6.0 ± 0.6° vs. 5.2 ±0.5°) and females (5.30 ± 0.8° vs. 4.4 ±0.1°).
Table 6 and
Table 7 show the correlation coefficients of PhA with age and ASM in males and females, respectively. PhA was correlated with age, ASM, HGS, and muscle quality in both males and females; in males, PhA was correlated with ASM/H2 and physical performance. These correlation coefficients in males were as follows: age (r=-0.275,
p<0.05), ASM (r=0.229,
p<0.05), ASM/Ht2 (r=0.352,
p<0.01), HGS (r=0.350,
p<0.01), physical performance (r=0.493,
p<0.001), and muscle quality (r=0.272,
p<0.05). In females, they were age (r=-0.389,
p<0.01), ASM (r=0.271,
p<0.05), HGS (r=0.640,
p<0.001), and muscle quality (r=0.524,
p<0.001).
PhA was a significant indicator of muscle strength in both males (β = 2.6; p < 0.01) and females (β = 3.4; p <.001) as well as muscle quality in both males (β = 0.07; p < 0.05) and females (β = 0.17; p < 0.001). In males, PhA was a significant indicator of physical performance (β = 0.3; p < 0.001).
The results of the multiple linear regression analyses are shown in
Table 9. In males, Model 1 included physical performance and PhA, the latter being the dependent variable; physical performance was positively associated with PhA. Model 2 included physical performance, ASM/H2 and PhA. Model 3 included physical performance, ASM/H2, muscle quality and PhA. All models (Models 1–3) had weak R2 values of 0.243, 0.303, and 0.347, respectively. In females, Model 1 included muscle quality and PhA, and Model 2 included muscle quality, ASM and PhA. Models 1 and 2 had weak R2 values of 0.274 and 0.439, respectively.
The sarcopenia group had significantly lower PhA than the non-sarcopenia group in both
males (p<0.01) and females (p<0.05). The PhA of males was higher than that of females.
4. Discussion
This study used the AWGS criteria to diagnose sarcopenia in Korean elderly individuals, established BIVA reference values in 122 healthy Korean elderly people aged 69-91 years, and determined whether BIVA can be used to diagnose sarcopenia in individuals. In addition, correlations between PhA and physical performance, muscle quality, and other factors were determined.
Individuals with sarcopenia exhibited different bioelectrical properties than healthy older adults as found with BIVA. Specifically, sarcopenic individuals had lower PhAs and longer impedance vectors than nonsarcopenic individuals. This was consistent with the theoretical expectations of BIVA, since the mean vector of sarcopenia is in this region, with R-Xc corresponding to lean individuals [
26]. In general, a low PhA is associated with low somatic mass [
26] and a high extracellular water/intracellular water ratio [
33]. Additionally, Castillo-Martínez et al. [
34] found low PhA, low Xc/H, and high R/H values in cachectic individuals.
Norman et al. obtained similar impedance vector shifts in patients with lower grip strength. The authors interpreted this displacement as low cellular and muscle function and suggested that BIVA can be assessed instead of grip strength in certain patients [
35]. The same impedance vector shift was found in a study of elderly Italian individuals [
36].
The length of the BIVA vector represents the hydration of the soft tissue [
37]. Within the reference range, the sex-specific 75% tolerance ellipse indicates normal hydration; short vectors below this limit indicate overhydration, and long vectors above the 75% tolerance ellipse indicate underhydration [
38]. This explains why the majority of individual impedance patterns in sarcopenia patients appear outside the 75% tolerance ellipse.
This study found that females have lower PhAs than males. PhA was negatively correlated with age and positively correlated with ASM, ASMI (in males), HGS, physical performance (in males), and muscle quality. Furthermore, in older males, PhA was independently associated with ASMI, muscle quality (HGS/ASM), and physical function. In older females, PhA was independently associated with muscle mass and ASM. PhA in the sarcopenia group was significantly lower than that in the non-sarcopenia group.
Females have a lower PhA than males, and PhA decreases with age [
15,
39,
40]. PhA has been positively correlated with muscle mass [
41], HGS [
41], lower-extremity muscle function [
42], and physical performance [
43] (assessed by the individual's walking speed and ability to rise from a seated position).
Furthermore, PhA can be used to predict physical performance [
44]. A study of 1,009 Japanese community-dwelling elderly individuals demonstrated that PhA was moderately correlated with muscle strength and walking speed [
45]. Although these results are consistent with those in previous reports, we found that the strongest association between physical function and PhA was in older men, while the strongest association between HGS and PhA was observed in older women. One study demonstrated a weakly to moderately positive association between PhA and muscle quality (HGS/USM) in both men and women [
47]. The present study found that PhA and muscle quality (HGS/ASM) were moderately positively correlated in both males and females. Multiple linear regression analysis in this study showed that PhA was significantly associated with physical function, ASMI, and muscle mass in older men. Among older females, PhA was significantly associated with muscle mass and ASM.
Other studies have found that PhA is associated with high cell counts, good cell function, and membrane integrity [
27]. Loss of muscle mass results in a decrease in reactance, and a decrease in muscle mass causes a decrease in intracellular water, leading to an increase in impedance, which in turn causes a decrease in PhA. Increases in intramuscular fat and fibrous tissue lead to decreased muscle quality [
48]. Therefore, muscle quality and quantity were independently associated with PhA. Given that PhA measurements can determine muscle mass and muscle function, they may be an alternative to expensive methods for diagnosing sarcopenia.
Our findings can help to identify older adults who have begun to experience adverse health conditions, such as frailty, muscle loss, prolonged hospital stays, and reduced mobility, facilitating early prevention of sarcopenia to avoid later health issues. In the future, it is necessary to expand the sample size to further study the relationship between PhA and physical function in Korean female elderly individuals to verify whether PhA can be used as an explanatory factor for physical function in Korean female elderly individuals.
This study has several limitations. First, its sample size was relatively small. Since impedance values are affected by age, when establishing BIVA reference values, specific age-stratified population reference values (e.g., the age groups of 69-79 and 80-89) should be considered. Second, the participants in this study were elderly people who were able to live independently and who were relatively healthy. The prevalence of sarcopenia in older adults in this study was therefore lower than previously reported. Third, the scarcity of sarcopenia patients did not allow the establishment of a cutoff point for PhA. In the future, the study cohort should be expanded to obtain additional bioelectrical impedance data on elderly Korean individuals with sarcopenia. This would allow the determination of diagnostic reference value for impedance parameters in the sarcopenia population.
5. Conclusions
Sarcopenia imposes high costs on health services, severely lowers the quality of life of older adults, and is a current public health problem. It is important to quickly and easily identify sarcopenia and individuals at risk for sarcopenia so that prevention and treatment can be initiated. This study aimed to judge whether BIVA and PhA are suitable tools for the assessment and diagnosis of sarcopenia.
The results show that BIVA can detect changes in muscle mass in individuals with sarcopenia and is a practical method for assessing sarcopenia in the field. PhA is an indicator of muscle strength, muscle quality and, in males, physical performance. It is helpful in diagnosing sarcopenia in elderly individuals with reduced mobility. Compared with the diagnostic criteria of sarcopenia, BIA-derived BIVA and PhA are simpler, more convenient, and more economical in predicting and diagnosing sarcopenia.
Author Contributions
Conceptualization, S.T., F.L.J., and C.-H.K. ; methodology, S.T., S-H E., J-Y. L., J.H.C., and C.-H.K. ; Formal analysis, S.T., F.L.J., and C.-H.K. ; Wright-original draft preparation, S.T., F.L.J., S.T., S-H E., J-Y. L., J.H.C., and C.-H.K. ; Writing-review and editing, S.T., F.L.J., S.T., S-H E., J-Y. L., J.H.C., and and C.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the MSIT(Ministry of Science and ICT), Korea, under the ICAN(ICT Challenge and Advanced Network of HRD) program(IITP-2023-2020-0-01832) supervised by the IITP(Institute of Information & Communication Technology Planning & Evaluation.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institution Review Board of Korean Nation Sport University (No. 1263-201903-HR-010-02).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in this study.
Data Availability Statement
The datasets generated during and/or analyzed during the study will be available from the corresponding author on reasonable request.
Acknowledgments
This research was funded by Soonchunhyang University. We would like to thank American Journal Experts (
www.aje.com) for English Language editing. The authors have no conflicts of interest to disclose.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations Secretariat, E.S.A. World population prospects: the 2017 revision Key Findings and Advance Tables (2017).
- Statistics Korea. Population Trends and Projections of the World and Korea. 2015.
- Sadighi Akha, AA. Aging and the immune system: An overview. J Immunol Methods. 2018;463:21-26. [CrossRef]
- Alfonso, J. Cruz-Jentoft, Francesco Landi, Stéphane M. Schneider, Clemente Zúñiga, Hidenori Arai, Yves Boirie, Liang-Kung Chen, Roger A. Fielding, Finbarr C. Martin, Jean-Pierre Michel, Cornel Sieber, Jeffrey R. Stout, Stephanie A. Studenski, Bruno Vellas, Jean Woo, Mauro Zamboni, Tommy Cederholm, Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS), Age and Ageing, Volume 43, Issue 6, November 2014, Pages 748–759. [CrossRef]
- Pérez-Zepeda, M.U. , Gutiérrez-Robledo, L.M. & Arango-Lopera, V.E. Sarcopenia prevalence. Osteoporos Int 24, 797 (2013). [CrossRef]
- von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129-133. [CrossRef]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80-85. [CrossRef]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis [published correction appears in Age Ageing. 2019 Jul 1;48(4):601]. Age Ageing. 2019;48(1):16-31. [CrossRef]
- Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography: A Systematic Review. J Gerontol A Biol Sci Med Sci. 2019;74(10):1671-1678. [CrossRef]
- Loenneke JP, Dankel SJ, Bell ZW, Spitz RW, Abe T, Yasuda T. Ultrasound and MRI measured changes in muscle mass gives different estimates but similar conclusions: a Bayesian approach. Eur J Clin Nutr. 2019;73(8):1203-1205. [CrossRef]
- Toombs RJ, Ducher G, Shepherd JA, De Souza MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring). 2012;20(1):30-39. [CrossRef]
- Jeon KC, Kim SY, Jiang FL, et al. Prediction Equations of the Multifrequency Standing and Supine Bioimpedance for Appendicular Skeletal Muscle Mass in Korean Older People. Int J Environ Res Public Health. 2020;17(16):5847. Published 2020 Aug 12. [CrossRef]
- Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. 2016;103(3):712-716. [CrossRef]
- Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care. 2017;20(5):330-339. [CrossRef]
- Barbosa-Silva MC, Barros AJ. Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care. 2005;8(3):311-317. [CrossRef]
- Organ LW, Bradham GB, Gore DT, Lozier SL. Segmental bioelectrical impedance analysis: theory and application of a new technique. J Appl Physiol (1985). 1994;77(1):98-112. [CrossRef]
- Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am J Clin Nutr. 1996;64(3 Suppl):524S-532S. [CrossRef]
- Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430-1453. [CrossRef]
- Marra M, Sammarco R, De Lorenzo A, et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol Imaging. 2019;2019:3548284. Published 2019 May 29. [CrossRef]
- Sheean P, Gonzalez MC, Prado CM, McKeever L, Hall AM, Braunschweig CA. American Society for Parenteral and Enteral Nutrition Clinical Guidelines: The Validity of Body Composition Assessment in Clinical Populations. JPEN J Parenter Enteral Nutr. 2020;44(1):12-43. [CrossRef]
- Roche S, Lara-Pompa NE, Macdonald S, et al. Bioelectric impedance vector analysis (BIVA) in hospitalised children; predictors and associations with clinical outcomes. Eur J Clin Nutr. 2019;73(10):1431-1440. [CrossRef]
- Piccoli A, Nigrelli S, Caberlotto A, et al. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am J Clin Nutr. 1995;61(2):269-270. [CrossRef]
- Zhang G, Huo X, Wu C, Zhang C, Duan Z. A bioelectrical impedance phase angle measuring system for assessment of nutritional status. Biomed Mater Eng. 2014;24(6):3657-3664. [CrossRef]
- Stobäus N, Pirlich M, Valentini L, Schulzke JD, Norman K. Determinants of bioelectrical phase angle in disease. Br J Nutr. 2012;107(8):1217-1220. [CrossRef]
- Norman K, Wirth R, Neubauer M, Eckardt R, Stobäus N. The bioimpedance phase angle predicts low muscle strength, impaired quality of life, and increased mortality in old patients with cancer. J Am Med Dir Assoc. 2015;16(2):173.e17-173.e1.73E22. [CrossRef]
- Piccoli, A. , Rossi, B., Pillon, L., & Bucciante, G. (1994). A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney international, 46(2), 534–539. [CrossRef]
- Norman, K. , Stobäus, N., Pirlich, M., & Bosy-Westphal, A. (2012). Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clinical nutrition (Edinburgh, Scotland), 31(6), 854–861. [CrossRef]
- Jiang, F., Tang, S., Eom, J. J., Song, K. H., Kim, H., Chung, S., & Kim, C. H. (2022). Accuracy of Estimated Bioimpedance Parameters with Octapolar Segmental Bioimpedance Analysis. Sensors (Basel, Switzerland), 22(7), 2681. [CrossRef]
- Legrand, D. , Vaes, B., Matheï, C., Adriaensen, W., Van Pottelbergh, G., & Degryse, J. M. (2014). Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. Journal of the American Geriatrics Society, 62(6), 1030–1038. [CrossRef]
- Cesari, M. , Kritchevsky, S. B., Newman, A. B., Simonsick, E. M., Harris, T. B., Penninx, B. W., Brach, J. S., Tylavsky, F. A., Satterfield, S., Bauer, D. C., Rubin, S. M., Visser, M., Pahor, M., & Health, Aging and Body Composition Study (2009). Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. Journal of the American Geriatrics Society, 57(2), 251–259. [CrossRef]
- Chen, L. K. , Woo, J., Assantachai, P., Auyeung, T. W., Chou, M. Y., Iijima, K., Jang, H. C., Kang, L., Kim, M., Kim, S., Kojima, T., Kuzuya, M., Lee, J., Lee, S. Y., Lee, W. J., Lee, Y., Liang, C. K., Lim, J. Y., Lim, W. S., Peng, L. N., … Arai, H. (2020). Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. Journal of the American Medical Directors Association, 21(3), 300–307.e2. [CrossRef]
- Piccoli, A., Nigrelli, S., Caberlotto, A., Bottazzo, S., Rossi, B., Pillon, L., & Maggiore, Q. (1995). Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. The American journal of clinical nutrition, 61(2), 269–270. [CrossRef]
- Chertow GM, Lowrie EG, Wilmore DW, et al. Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J Am Soc Nephrol. 1995;6:75–81.
- Castillo-Martínez L, Colín-Ramírez E, Orea-Tejeda A, et al. Cachexia assessed by bioimpedance vector analysis as a prognostic indicator in chronic stable heart failure patients. Nutrition. 2012;28(9):886–891.
- Norman K, Pirlich M, Sorensen J, et al. Bioimpedance vector analysis as a measure of muscle function. Clin Nutr. 2009;28(1):78–82.
- Marini, E. , Buffa, R., Saragat, B., Coin, A., Toffanello, E. D., Berton, L., Manzato, E., & Sergi, G. (2012). The potential of classic and specific bioelectrical impedance vector analysis for the assessment of sarcopenia and sarcopenic obesity. Clinical interventions in aging, 7, 585–591. [CrossRef]
- Piccoli, A. , Rossi, B., Pillon, L., & Bucciante, G. (1994). A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney international, 46(2), 534–539. [CrossRef]
- Piccoli, A. , Brunani, A., Savia, G., Pillon, L., Favaro, E., Berselli, M. E., & Cavagnini, F. (1998). Discriminating between body fat and fluid changes in the obese adult using bioimpedance vector analysis. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity, 22(2), 97–104. [CrossRef]
- Bosy-Westphal, A. , Danielzik, S., Dörhöfer, R. P., Later, W., Wiese, S., & Müller, M. J. (2006). Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN. Journal of parenteral and enteral nutrition, 30(4), 309–316. [CrossRef]
- Brunani, A., Perna, S., Soranna, D., Rondanelli, M., Zambon, A., Bertoli, S., Vinci, C., Capodaglio, P., Lukaski, H., & Cancello, R. (2021). Body composition assessment using bioelectrical impedance analysis (BIA) in a wide cohort of patients affected with mild to severe obesity. Clinical nutrition (Edinburgh, Scotland), 40(6), 3973–3981. 6. [CrossRef]
- Basile, C., Della-Morte, D., Cacciatore, F., Gargiulo, G., Galizia, G., Roselli, M., Curcio, F., Bonaduce, D., & Abete, P. (2014). Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Experimental gerontology, 58, 43–46. [CrossRef]
- Yamada, Y. , Buehring, B., Krueger, D., Anderson, R. M., Schoeller, D. A., & Binkley, N. (2017). Electrical Properties Assessed by Bioelectrical Impedance Spectroscopy as Biomarkers of Age-related Loss of Skeletal Muscle Quantity and Quality. The journals of gerontology. Series A, Biological sciences and medical sciences, 72(9), 1180–1186. [CrossRef]
- Tomeleri, C. M. , Cavalcante, E. F., Antunes, M., Nabuco, H., de Souza, M. F., Teixeira, D. C., Gobbo, L. A., Silva, A. M., & Cyrino, E. S. (2019). Phase Angle Is Moderately Associated With Muscle Quality and Functional Capacity, Independent of Age and Body Composition in Older Women. Journal of geriatric physical therapy (2001), 42(4), 281–286. [CrossRef]
- Mullie, L. , Obrand, A., Bendayan, M., Trnkus, A., Ouimet, M. C., Moss, E., Chen-Tournoux, A., Rudski, L. G., & Afilalo, J. (2018). Phase Angle as a Biomarker for Frailty and Postoperative Mortality: The BICS Study. Journal of the American Heart Association, 7(17), e008721. [CrossRef]
- Yamada, M. , Kimura, Y., Ishiyama, D., Nishio, N., Otobe, Y., Tanaka, T., Ohji, S., Koyama, S., Sato, A., Suzuki, M., Ogawa, H., Ichikawa, T., Ito, D., & Arai, H. (2019). Phase Angle Is a Useful indicator for Muscle Function in Older Adults. The journal of nutrition, health & aging, 23(3), 251–255. [CrossRef]
- Piccoli, A., Codognotto, M., Piasentin, P., & Naso, A. (2014). Combined evaluation of nutrition and hydration in dialysis patients with bioelectrical impedance vector analysis (BIVA). Clinical nutrition (Edinburgh, Scotland), 33(4), 673–677. [CrossRef]
- Akamatsu, Y., Kusakabe, T., Arai, H., Yamamoto, Y., Nakao, K., Ikeue, K., Ishihara, Y., Tagami, T., Yasoda, A., Ishii, K., & Satoh-Asahara, N. (2022). Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. Journal of cachexia, sarcopenia and muscle, 13(1), 180–189. [CrossRef]
- McGregor, R. A., Cameron-Smith, D., & Poppitt, S. D. (2014). It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longevity & healthspan, 3(1), 9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).