Submitted:
21 June 2023
Posted:
25 June 2023
You are already at the latest version
Abstract
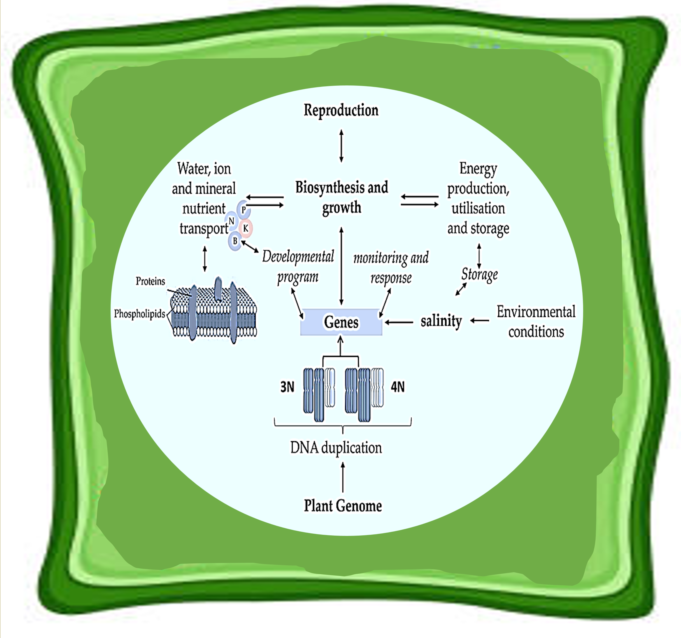
Keywords:
1. Introduction
2. Role of Autopolyploidy on Legume Response to Salinity Stress

3. Physiological and Molecular Status of Leguminous Polyploids
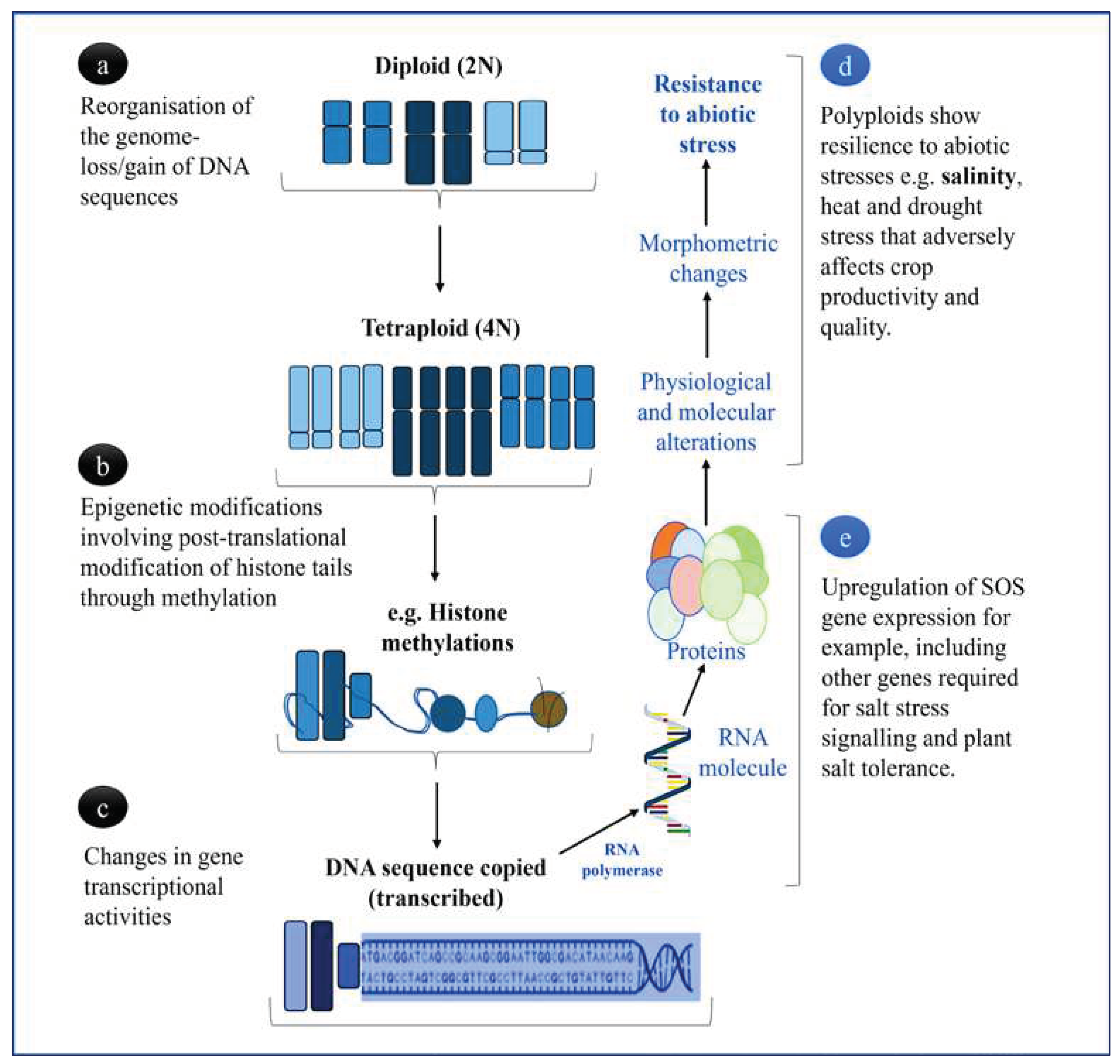
4. Ionic and Osmotic Stress Signalling Responses in Polyploidised Plants
5. Metabolic Changes of Legume Polyploids Exposed to Salinity Stress
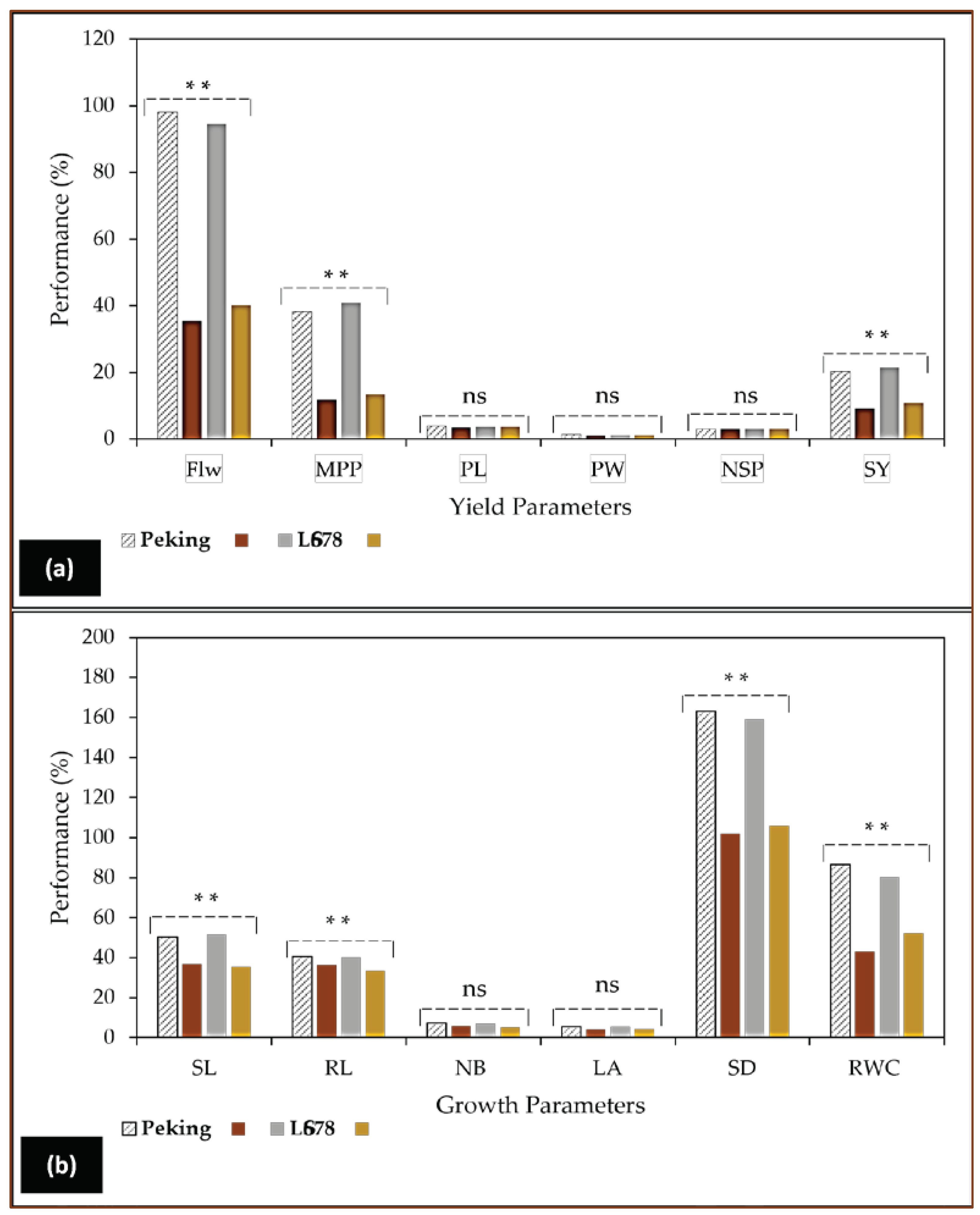
3. Gene Expression in Polyploids Under Salinity Conditions
| Common name | Species name | Gene family/ name | Function | Reference |
|---|---|---|---|---|
| Peanut | Arachis hypogeal L. | ABSCISIC ACID INSENSITITVE 4S (AhAB14s) | Gene downregulation enhanced survival rate, biomass accumulation and root/shoot ratio of seedlings | Luo et al. [74] |
| Chickpea | Cicer arietinum | CaRab-GTP (CaRabA2, CaRab-B, CabRab-C, CabRab-D, Cab-Rab-E, and CabRab-H | Regulate Na+ accumulation in leaves | Sweetman et al. [38] |
| Pigeon pea | Cajanus cajan L. | GAPDH, UBC and HSP90 | Housekeeping genes carrying out cellular maintenance through stable expression irrespective of internal/ external signals | Sinha et al. [75] |
| Soybean | Glycine max L. Merrill. | GmSALT3 and GmSALT18 | Regulate Na+ accumulation in leaf mesophylls via root-based mechanisms | Guo et al. [76] |
| Birdsfoot trefoil | Lotus japonicus | LjLTP10 | Gene preventing osmotic stress by managing water deficit through structural architecture such as cuticular composition. | Tapia et al. [77] |
| Barrel medic | Medicago truncatula | MtDef4.3 | Defense gene against infectious microbes, including rhizobia species. | Gao et al. [78] |
| Lupin | Lipinus angustifolius | Aldehyde dehydrogenase (ALDH) | Catalyse irreversible oxidation of aldehyde molecules to non-toxic carboxylic acids | Jimencz-Lopez [79] |
| Common bean | Phaseolus vulgaris | PvDREB | Circumvent salinity stress by mechanic and osmotic adjustment | Konzen et al. [80] |
| Barrel medic | M. truncatula | Nodule-specific Cysteine Rich (NCR) | Control nodule organogenesis and gene duplication. | Guefrachi et al. [81] |
| Cowpea | Vigna unguiculata ssp. sesquipedalis (asparagus bean) | Root-derived DEGs (GmsSOS1, SOS2 and OsNHX2) | Genes involved in redox reactions, enhancing antioxidant enzymes activity and reduces Na+ accumulation under salt stress | Pan et al. [82] |
| Mung bean | Vigna radiata L. Wilczek | VrKUP, VrHAK and VrKT | Regulate influx and efflux of K+ through K+ transporters and channels | Azzam et al. [3], Ragel et al. [83] |
| Red clover | Trifolium spp. (T. pratense L.) | NHX1 | Na+/H+ antiporter in plants, predominantly, Trifolium spp. | Delormel [84] |
| Pea | Pisum sativum | PsRPL3OE, PsChla/bBP and PsFDH | Regulate protein synthesis, photosynthesis, and long chain lipid synthesis in the mesophylls | Joshi et al. [85] |
| Wild peanut | Arachis duranensis | AdWRKYs (AdWRKY18, AdWRKY40, AdWRKY42, AdWRKY56 and AdWRKY64) | Genes regulating ABA accumulation, stomatal aperture, and root development | Zhang et al. [86] |
4. Discussion and Prospects
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Jager, I.; Borgonjen-van den Berg, K.J.; Giller, K.E.; Brouwer, I.D. Current and potential role of grain legumes on protein and micronutrient adequacy of the diet of rural Ghanaian infants and young children: using linear programming. Nutr J. 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mascher, M.; Abbo, S.; Jayakodi, M. Advancing grain legumes domestication and evolution studies with genomics. Plant Cell Physiol 2022, 63, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Koenen, E.J.M.; Ojeda, D.I.; Blakker, F.T.; Wieringa, J.J.; Kidner, C.; Hardy, O.J.; Pennington, R.T.; Herendeen, P.S.; Bruneau, A.; Hughes, C.E. The origin of the legumes is a complex paleopolyploid phylogenomic tangle closely associated with the Cretaceous-Paleogene (K-Pg) mass extinction event. Syst Biol 2021, 70, 508–526. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.C.; Richter, J.; Jobert, D.F.; Ficher, C. A dominance shift in arid savanna: An herbaceous legume outcompetes local C4 grasses. Ecol Evol 2018, 8, 6729–6787. [Google Scholar] [CrossRef] [PubMed]
- Xu-Ri, Dai, D.; Xu, X. The symbiotic nitrogen fixation by legumes in a legume-companion and a legume-dominant alpine steppe on the central Tibetan plateau. Ecol Res 2021, 36, 545–555. [CrossRef]
- Zhang, P.; Dumroese, R.K.; Pinto, J.R. Organic or inorganic nitrogen and rhizobia inoculation provide synergistic growth response of a leguminous forb and tree. Front Plant Sci 2019, 10, 1308. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediate superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hortic Res 2020, 7, 40. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.-K. Regulation of abscisic acid biosynthesis. Plant Physiol 2003, 133, 29–36. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unravelling salt stress signalling in plants. J Int Plant Biol 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Hassain, M.; Barthakar, S.; Paul, S.; Bharadwaj, N. ’ Migdadi, H.M.; Alghamdi, S.S.; Siddique, K.H.M. Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol Biochem 2017, 118, 199–217. [Google Scholar] [CrossRef]
- Pelé, A.; Rousseau-Gueutin, M.; Chèvre, A.-M. Speciation success of polyploid plants closely relates to the regulation of meiotic recombination. Front. Plant Sci. 2018, 9, 907. [Google Scholar] [CrossRef]
- Farhadi, N.; Panahandeh, J.; Motallebi-Azar, A.; Molchtarzadeh, S. Production of autotetaploid plants by in vitro chromosome engineering in Allium hirtifolium. Hort Plant J In Press. 2022, 1–13. [Google Scholar] [CrossRef]
- Meng, L.; Mohan, R.; Kwok, B.A.B.; Elofsson, M.; Sin, N.; Crews, C.M. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo anti-inflammatory activity. Proc Natl Acad Sci 1999, 96, 10403–10408. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Akutsu, M.; Okazaki, K. Mechanisms of action of nitrous oxide gas applied as a polyplodizing agent during meiosis in lilies. Sex Plant Reprod 2008, 22, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Chaparro, A.F.; Halder, S.; Trader, D.J. Synthesis and application of a clickable epoxomicin-based probe for proteasome activity analysis. Curr Prot 2022, 2, e490. [Google Scholar] [CrossRef]
- Sbrana, I.; Sibio, A.D.; Lomi, A.; Scarcell, V. C-mitosis and numerical chromosome aberration analyses in human lymphocytes: 10 known or suspected spindle poisons. Mutat Res-Fund Mol M. 1993, 287, 57–70. [Google Scholar] [CrossRef]
- Giani, S.; Campanoni, P.; Breviario, D. A dual effect on protein synthesis and degradation modulates the tubulin level in rice cells treated with oryzalin. Planta 2002, 214, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Hafiz, I.A.; Silvestri, C. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef]
- Madani, H.; Escrich, A.; Hossani, B.; Sanchez-Munoz, R.; Khojasteh, A.P. Effect of polyploidy induction on natural metabolite production in medicinal plants. Biomolecules 2021, 11, 899. [Google Scholar] [CrossRef]
- Kozgunova, E.; Nishina, M.; Goshima, G. Kinetochore protein depletion underlies cytokinesis failure and somatic polyploidization to the moss Physcomitrella patens. eLife 2019, 8, e43652. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Wei, X.; Jahan, I.; Hasanuzzaman, M.; Sabuj, Z.H.; Zulfiqar, F.; Chen, J.; Iqbal, R.; Dastogeer, K.M.G.; Sohag, A.A.M.; Tonny, S.H.; Hamid, I.; Al-Ashkar, I.; Mirzapour, M.; El Sabagh, A.; Murata, Y. Exogenous nitric oxide promotes salinity tolerance in plants: A meta-analysis. Front Plant Sci 2022, 13, 957735. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Implication of nitric oxide under salinity stress: The possible interaction with other signaling molecules. J Plant Growth Regul. 2022, 41, 163–177. [Google Scholar] [CrossRef]
- Corneillie, S.; Storme, N.D.; Acker, R.; Fangel, J.U.; Bruyne, M.; Rycke, R.; Geelen, D.; Willats, W.G.T.; Vauholme, B.; Booerjan, W. Polyploidy affects plant growth and alters cell wall composition. Plant Physiol 2019, 179, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Forester, N.J.; Ashman, T-L. The direct effects of plant polyploidy on the legume-rhizobia mutualism. Ann Bot 2018, 121, 209–220. [Google Scholar] [CrossRef]
- Innes, L.A.; Denton, M.D.; Dundas, I.S.; Peck, D.M.; Humphries, A.W. The effect of ploidy number on vigor, productivity, and potential adaptation to climate change in annual Medicago species. Crop Sci. 2021, 61, 89–103. [Google Scholar] [CrossRef]
- Kanatas, P.; Gozoulis, I.; Travlos, I. Irrigation timing as a practice of effective weed management in established alfalfa (Medicago sativa L.) crop. Agron 2021, 11, 550. [Google Scholar] [CrossRef]
- Tossi, V.E.; Martínez Tosar, L.J.; Laino, L.E.; Iannicelli, J.; Regalado, J.J.; Escandón, A.S.; Baroli, I.; Causin, H.F.; Pitta-Álvarez, S.I. Impact of polyploidy on plant tolerance to abiotic and biotic stresses. Front Plant Sci 2022, 13, 869423. [Google Scholar] [CrossRef]
- Forrester, N.J.; Ashman, T.-L. Autopolyploidy alters nodule-level interactions in the legume-rhizobium mutualism. Am J Bot 2020, 107, 179–185. [Google Scholar] [CrossRef]
- Azzam, C.R.; Al-Taweel, S.K.; Abdel-Aziz, R.M.; Rabea, K.M.; Abou-Sreea, A.I.B.; Rady, M.M.; Ali, E.F. Salinity effects on gene expression, morphological and physio-biochemical responses of Stevia rebaudiana Bertoni in vitro. Plants 2021, 10, 820. [Google Scholar] [CrossRef]
- Razzaque, S.; Elias, S.M.; Haque, T.; Biswas, S.; Jewel, G.M.N.A.; Rahman, S.; Weng, X.; Ismail, A.M.; Juenger, T.E.; Seraj, Z.I. Gene expression analysis associated with salt stress in reciprocally crossed rice population. Sci Rep 2019, 9, 8249. [Google Scholar] [CrossRef] [PubMed]
- Slesak, I.; Libilc, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim Pol 2007, 54, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Nadeen, M.; Li, J.; Yahya, M.; Wang, M.; Ali, A.; Cheng, A.; Wang, X.; Ma, C. Grain legumes and fear of salt stress. Focus on mechanisms and management strategies. Int J Mol Sci 2019, 20, 799. [Google Scholar] [CrossRef]
- He, Y.; Fu, J.; Yu, C.; Wang, X.; Jiang, Q.; Hong, J.; Lu, K.; Xue, G.; Yan, C.; James, A.; Xu, L.; Chen, J.; Jiang, D. Increasing cyclic electron flow is related to Na+ sequestration into vacuoles for salt tolerance in soybean. J. Exp. Bot. 2015, 66, 6877–6889. [Google Scholar] [CrossRef]
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.; Luo, X.; Shuai, H.; Zhou, W.; Ding, J.; Du, J.; Liu, J.; Yang, F.; Wang, Q.; Liu, W.; Yong, T.; Wang, X.; Feng, Y.; Yang, W. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front Plant Sci 2017, 8, 1372. [Google Scholar] [CrossRef]
- Kang, Z.; Xiaowu, W.; Feng, C. Plant polyploidy: Origin, evolution, and its influence on crop domestication. Hort Plant J 2019, 5, 237–239. [Google Scholar] [CrossRef]
- Bouyer, D.; Geier, F.; Kragler, F.; Schnittger, A.; Pesch, M.; Wester, K.; Balkundle, R.; Timner, J.; Fleck, C.; Hulskamp, M. Two-dimensional patterning by a trapping depletion mechanism. The role of TTG1 and GL3 in Arabidopsis trichome formation. PLoS Biol 2008, 6, e141. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, C.; Khassanova, G.; Miller, T.K.; Booth, N.J.; Kurishbayev, A.; Jatayev, S.; Gupta, N.K.; Langridge, P.; Jenkins, C.L.D.; Soole, K.L.; Day, D.A.; Shavrukov, Y. Salt-induced expression of intracellular vesicle trafficking genes, CaRab-GTP, and their association with Na+ accumulation in leaves of chickpea (Cicer arietinum L.). BMC Plant Biol 2020, 20 (Suppl. 1), 183. [Google Scholar] [CrossRef] [PubMed]
- Kocira, A.; Staniak, M.; Tomaszewska, M.; Koprnas, R.; Cymerman, J.; Panasiewicz, K.; Lipinska, H. Legume cover crops as one of the elements of strategic weed management and soil quality improvement. A review. Agric 2020, 10, 394. [Google Scholar] [CrossRef]
- Magadlela, A.; Perz-Fernandez, A.; Kleinert, A.; Dreyer, L.L.; Valentine, A.J. Source of inorganic N affects the cost of growth in a legume tree species (Virginia divaricate) from the Mediterrean-type Fynbos ecosystem. J Plant Ecol 2016, 9, 752–761. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Berdugo-Cely, J.A.; Céron-Lasso, MdS.; Yockteng, R. Phenotypic and molecular analyses in diploid and tetraploid genotypes of Solanum tuberosum L. reveal promising genotypes and candidate genes associated with phenolic compounds, ascorbic acid contents, and antioxidant activity. Front Plant Sci 2023, 13, 1007104. [Google Scholar] [CrossRef]
- Bhuvaneswari, G.; Thirugnanasampandan, R.; Gogulramnath, M. Effect of colchicine induced tetraploidy on morphology, cytology, essential oil composition, gene expression and antioxidant activity of Citrus limon (L.) Osbeck. Physiol Mol Biol Plants 2020, 26, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.C.; Youn, B.; Zhao, Y.; Santhamma, B.; Coates, R.M.; Croteau, R.B.; Kang, C.H. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc Natl Acad Sci USA 2007, 104, 5360–5365. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Miao, L.; Hou, Q.; Darwish, D.B.; Alrdahe, S.S.; Ali, A.; Benedito, V.A.; Tadege, M.; Wang, X.B.; Zhao, J. Overexpression of terpenoid biosynthesis genes from garden sage (Salvia officinalis) modulates rhizobia interaction and nodulation in soybean. Front Plant Sci 2021, 12, 783269. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From central to specialized metabolism: an overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front Plant Sci 2019, 10, 835. [Google Scholar] [CrossRef]
- Kalia, V.C.; Gong, C.; Patel, S.K.S.; Lee, J.-K. Regulation of plant mineral nutrition by signal molecules. Microorganisms 2021, 9, 774. [Google Scholar] [CrossRef]
- Jekabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Romanovs, M.; Levinsh, G. Effects of salinity on growth, ion accumulation and mineral nutrition of different accessions of a crop wild relative legume species, Trifolium fragiferum. Plants 2022, 11, 797. [Google Scholar] [CrossRef]
- Devkar, V.; Thirumalaikumar, V.P.; Xue, G.-P.; Vallarino, J.G.; Turechova, V.; Strnad, M.; Fernie, A.R.; Hoefgen, R.; Mueller-Roeber, B.; Balazadeh, S. Multifaceted regulatory function of tomato SITAFI in the response to salinity stress. New Physiol 2019, 225, 1681–1698. [Google Scholar] [CrossRef]
- Amin, I.; Rasool, S.; Mir, M.A.; Wani, W.; Masoodi, K.Z. Ion homeostasis for salinity tolerance in plants: A molecular approach. Physiol Plant 2021, 171, 578–594. [Google Scholar] [CrossRef]
- Tu, Y.; Jiang, A.; Gan, L.; Hossan, M.; Zhang, J.; Peng, B.; Xiong, Y.; Song, Z.; Cui, D.; Xu, W.; Zhang, J.; He, Y. Genome duplication improves rice root resistance to salt stress. Rice (NY) 2014, 7, 15. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, L.; Zhang, H.; Liu, B. Evolution of physiological responses to salt stress in hexaploidy wheat. PNAS 2014, 111, 11882–11887. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Hortic 2017, 3, 30. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.-Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; Lee, I.-J. Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; Lee, I-J. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front Microbiol 2017, 8, 686. [Google Scholar] [CrossRef]
- Mangena, P. Role of benzyladenine seed priming on growth and physiological and biochemical response of soybean plants grown under high salinity stress conditions. Int J Agron 2020, 8847098. [Google Scholar] [CrossRef]
- Amirjani, M.R. Effect of salinity stress on growth, mineral composition, proline content, antioxidant enzymes of soybean. Amer J Plant Physiol, 2010, 5, 350–360. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Datta, S.; Gupta, P.; Tah, J. Analysis of genetic parameters on mutant populations of mungbean (Vigna radiata L.) after ethyl methane sulphonate treatment. Not Sci Biol 2010, 4, 137–143. [Google Scholar] [CrossRef]
- Goyal, S.; Wani, M.R.; Raina, A.; Laskar, R.A.; Khan, S. Phenotypic diversity in mutagenized population of urdbean (Vigna mungo (L.) Hepper). Heliyon 2021, 7, e06356. [Google Scholar] [CrossRef] [PubMed]
- Laskar, R.A.; Laskar, A.A.; Raina, A.; Khan, S.; Younus, H. Induced mutation analysis with biochemical and molecular characterization of high yielding lentil mutant lines. Int J Biol Macromol 2018, 109, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Corneillie, S.; De Storme, N.D.; van Acker, R.; Fangel, J.U.; De Bryne, M.; De Rycke, R.; Geelen, D.; Willats, W.G.T.; Vanholme, B.; Boerjan, W. Polyploidy affects plant growth and alters cell wall composition. Plant Physiol 2019, 179, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Brisibe, E.A.; Ekanem, N.G. Ploidy level and nucleotide variations in inflorescence dichotomous cultivars of plantain (Musa spp. AAB genome). BMC Genomics 2019, 20, 713. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.-Y.; Dilkes, B.; Luo, H.; Douglas, A.; Yakubova, E.; Lahner, B.; Salt, D.E. Polyploids exhibit higher potassium uptake and salinity tolerance in Arabidopsis. Sci 2013, 341, 658–659. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu Rev Plant Biol 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front Plant Sci 2019, 10, 80. [Google Scholar] [CrossRef]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-dependent protein kinase: Hubs in plant stress signaling and development. Plant Physiol 2013, 163, 523–530. [Google Scholar] [CrossRef]
- Almadanim, M.C.; Concalves, N.M.; Rosa, M.T.G.; Alexandre, B.M.; Cordeiro, A.M.; Rodriques, M.; Saibo, N.J.M.; Soares, C.M.; Romao, C.V.; Oliveira, M.M.; Abreu, I.A. The rice cold-responsive calcium-dependent protein kinase OsCPK17 is regulated by alternative splicing and post-translational modifications. Biochim et Biophysic Acta Mol Cell Res 2018, 1865, 231–246. [Google Scholar] [CrossRef]
- Demirkol, G. PopW. improves salt stress tolerance of red clover (Trifolium pratense L.) via activating phytohormones and salinity related genes. Biologia 2023, 78, 979–991. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Sun, J.; Cao, Q.; Tang, Z.; Liu, M.; Xu, T.; Ma, D.L.Z.; Sun, J. Root-zone-specific sensitivity of K+- and Ca2+-permeable chemicals to H2O2 determines ion homeostasis in salinized diploid and hexaploidy Ipomoea trifida. J Exp Bot 2019, 70, 1389–1405. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front Plant Sci 2019, 10, 800. [Google Scholar] [CrossRef]
- Gui, T.-Y.; Gao, D-H.; Ding, H-C.; Yan, X.-H. Identification of Respiratory Burst Oxidase Homolog (Rboh) family genes from Pyropia yezoensis and their correlation with archeospore release. Front Plant Sci 2022, 13, 929299. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Becana, M. Molecular responses of legumes to abiotic stress: Post-translational modifications of proteins and redox signaling. J Exp Bot 2021, 72, 5876–5892. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wan, Q.; Zhang, K.; Zhang, X.; Guo, R.; Wang, C.; Zheng, C.; Liu, F.; Ding, Z.; Wan, Y. AhABI4s negatively regulate salt-stress response in peanut. Front Plant Sci 2021, 12, 741641. [Google Scholar] [CrossRef]
- Sinha, P.; Saxena, R.K.; Singh, V.K.; Krishnamurthy, L.; Varshney, R.K. Selection and validation of housekeeping genes as reference for gene expression studies in pigeonpea (Cajanus cajan) under heat and salt stress conditions. Front Plant Sci 2015, 6, 1071. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, J.; Liu, Y.; Yu, L.; Chang, R.; Guan, R.; Qiu, L. Identification of a novel salt tolerance-related locus in wild soybean (Glycine soja Sieb. & Zucc.). Front Plant Sci 2021, 12, 791175. [Google Scholar] [CrossRef]
- Tapia, G.; Morales-Quintana, L.; Parra, C.; Berbel, A.; Alcorta, M. Study of nsLTPs in Lotus japonicus genome reveal a specific epidermal cell member (LjLTP10) regulated by drought stress in aerial organs with a putative role in cutin formation. Plant Mol Biol 2013, 8, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Selee, B.; Schnabel, E.L.; Poehlman, W.L.; Chavan, S.A.; Frugoli, J.A.; Feltus, F.A. time series transcriptome analysis in Medicago truncatula shoot and root tissue during early nodulation. Front Plant Sci 2022, 13, 861639. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, J.C. Narrow-leafed lupin (Lupinus angustifolius L.) functional identification and characterization of the aldehyde dehydrogenase (ALDH) gene superfamily. Plant Gene 2016, 6, 67–76. [Google Scholar] [CrossRef]
- Konzen, E.R.; Recchia, G.H.; Cassieri, F.; Caldas, D.G.G.; yTeran, J.C.B.M.; Gepts, P.; Tsai, S.M. DREB genes from common bean (Phaseolus vulgaris L.) show broad to specific abiotic stress responses and distinct levels of nucleotide diversity. Int J Genomics 2019, 9520642. [Google Scholar] [CrossRef]
- Guefrachi, I.; Nagymihaly, M.; Pislariu, C.I.; Van de Velde, W.; Ratet, P.; Mars, M.; Udvardi, M.K.; Kondorosi, E.; Mergaert, P.; Alunni, B. Extreme specificity of NCR gene expression in Medicago truncatula. BMC Genomics 2014, 15, 712. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yu, X.; Shao, J.; Liu, Z.; Gao, T.; Zheng, Y.; Zeng, C.; Liang, C.; Chen, C. Transcriptomic profiling and analysis of differentially expressed genes in asparagus bean (Vigna unguiculata ssp. sesquipedalis) under salt stress. PLoS ONE 2019, 14, e0219799. [Google Scholar] [CrossRef] [PubMed]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front Plant Sci 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Delormel, T.Y.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Physiol 2019, 224, 585–604. [Google Scholar] [CrossRef]
- Joshi, A.; Dang, H.Q.; Vaid, N.; Tuteja, N. Isolation of high salinity stress tolerant gene from Pisum sativum by random overexpression in Escherichia coli and their functional validation. Plant Signal Behav 2009, 4, 400–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, P.; Xiong, F.; Zhang, X.; Song, H. WRKY Genes improve drought tolerance in Arachis duranensis. Front Plant Sci 2022, 13, 910408. [Google Scholar] [CrossRef]
- Mergaert, P.; Kereszt, A.; Kondorosi, E. Gene expression in nitrogen-fixing symbiotic nodule cells in Medicago truncatula and other nodulating plants. Plant Cell 2020, 32, 42–68. [Google Scholar] [CrossRef]
- Garg, R.; Jain, M. Transcriptome analyses in legumes: A resource for functional genomics. Plant Genomics 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Sprent, J.I.; Ardley, J.; James, E.K. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytol. 2017, 215, 40–56. [Google Scholar] [CrossRef]
- Trujillo, D.I.; Silverstein, K.A.; Young, N.D. Nodule-specific plat domain proteins are expanded in the Medicago lineage and required for nodulation. New Phytol 2019, 222, 1538–1550. [Google Scholar] [CrossRef]
- Poehlman, W.L.; Schnabel, E.L.; Chavan, S.A.; Frugoli, J.A.; Feltus, F.A. Identifying temporally regulated root nodulation biomarkers using time series gene co-expression network analysis. Front Plant Sci 2019, 10, 1409. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sun, W.; Yang, G.; Sun, G. WRKY transcription factors in legumes. BMC Plant Biol 2018, 18, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int J Mol Sci 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Tyerman, S.D. Nonselective cation channels. Multiple functions and commonalities. Plant Physiol 2002, 128, 327–328. [Google Scholar] [CrossRef]
- Taffouo, V.D.; Kouamou, J.K.; Ngalangue, L.M.T.; Ndjeudji, B.A.N.; Akoa, A. Effects of salinity stress on growth, ions partitioning and yield of some cowpea (Vigna unguiculata L. Walp.) cultivars. Int J Bot 2009, 5, 135–143. [Google Scholar] [CrossRef]
- HanumanthaRao, B.; Nair, R.M.; Nayyar, H. Salinity and high temperature tolerance in mungbean [Vigna radiata (L.) Wilczek] from a physiological perspective. Front Plant Sci 2016, 7, 957. [Google Scholar] [CrossRef]
- Kurz, M. Compatible solute influence on nucleic acids: Many questions but few answers. Saline Syst 2008, 4, 6. [Google Scholar] [CrossRef]
- Koning, R.; Kiekens, R.; Toili, M.E.M.; Angenon, G. Identification and expression analysis of the genes involved in the raffinose family oligosaccharides pathways of Phaseolus vulgaris and Glycine max. Plants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Y.; Liu, J.H. Comparative transcriptome analysis reveals synergistic and disparate defense pathways in the leaves and roots of trifoliate orange (Poncirus trifoliata) autotetraploids with enhanced salt tolerance. Hortic Res 2020, 7, 88. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J Int Plant Biol 2018, 60, 796–804. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




