Submitted:
24 June 2023
Posted:
25 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Effects of Nicotinamide Riboside on Several Organs and Systems
2.1. Nicotinamide Riboside and the Nervous System
2.2. Nicotinamide Riboside and the Cardiovascular System
2.3. Nicotinamide Ribose and the Digestive System
2.4. Nicotinamide Riboside and the Urinary System
2.5. Nicotinamide Riboside and the Musculoskeletal System
3. Nicotinamide Riboside as a Tool to Mitigate Metabolic Disorders
3.1. Obesity
3.2. Nicotinamide Riboside and Diabetes
4. Nicotinamide Riboside for Healthy Aging and Longevity
4.1. Nicotinamide Riboside for Healthy Aging
4.2. Brain Aging, Cognitive Impairment and Neurodegenerative Diseases
4.3. Aging and Cancer
5. Safety and Bioavailability of Nicotinamide Riboside
5.1. Nicotinamide Riboside Safety
5.2. Nicotinamide Riboside Bioavailability
6. Borate-Stabilized Nicotinamide Riboside
6.1. Prebiotic Synthesis
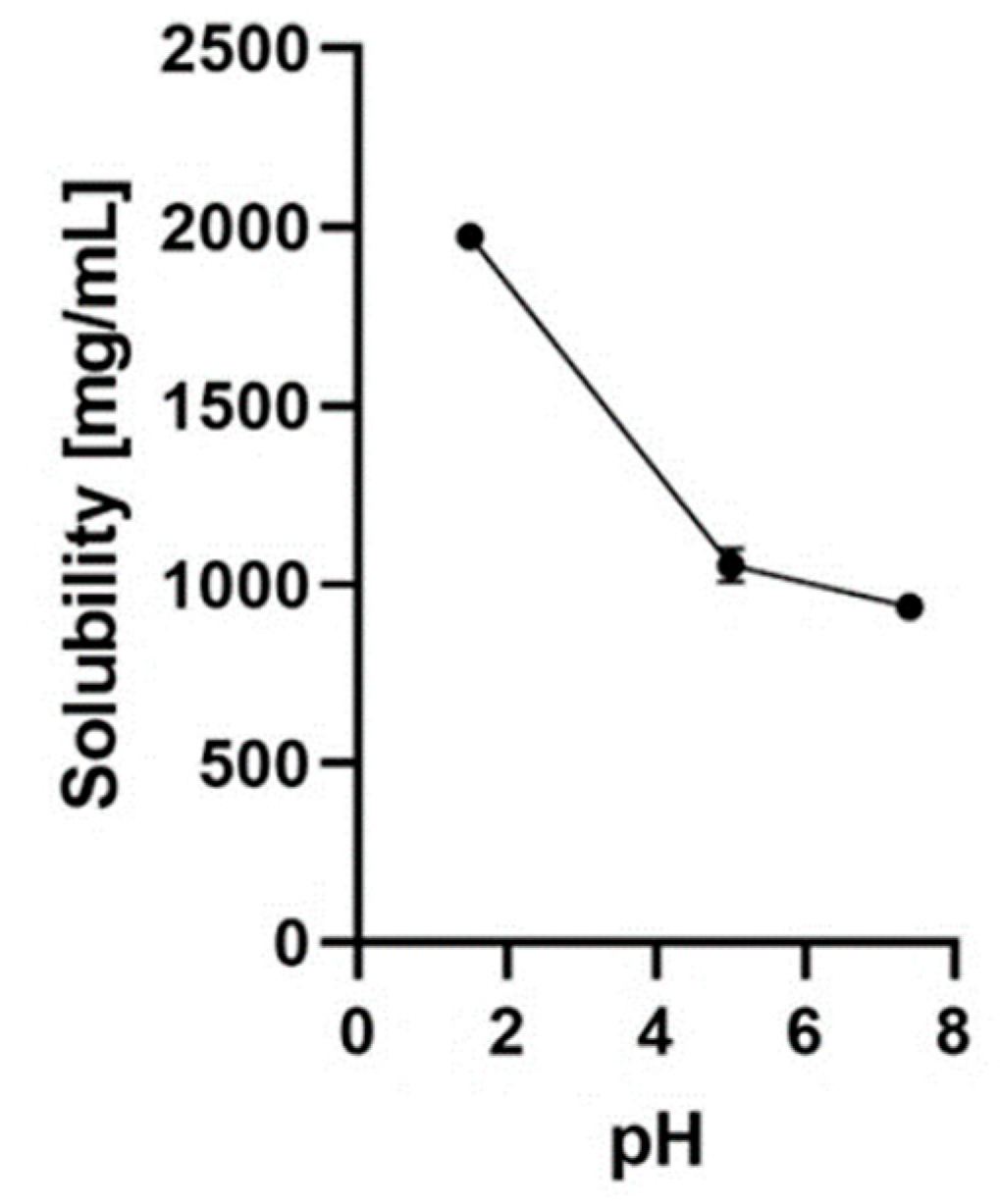
6.2. Solubility and Degradation Kinetics of Nicotinamide Riboside Borate
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Penberthy WT, Kirkland JB (2012) Chapter 19: Niacin. In: Erdman JW Jr, MacDonald IA, Zeisel SH (eds). Present knowledge in nutrition. 10th edition, International Life Sciences Institute (ILSI), Wiley–Blackwell, John Wiley & Sons, Ames, Iowa, USA, 293–306. [CrossRef]
- Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, Stødkilde-Jørgensen H, Møller N, Brenner C, Treebak JT, Jessen N (2018) A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr 108(2):343–353. [CrossRef]
- Conze D, Brenner C, Kruger CL (2019) Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep 9(1):9772. [CrossRef]
- Mehmel M, Jovanović N, Spitz U (2020) Nicotinamide riboside – the current state of research and therapeutic uses. Nutrients 12(6):1616. [CrossRef]
- Gilbert W (1986) Origin of life: the RNA world. Nature 319(6055):618. [CrossRef]
- White HB 3rd (1976) Coenzymes as fossils of an earlier metabolic state. J Mol Evol 7(2):101–104. [CrossRef]
- Chi Y, Sauve AA (2013) Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr Opin Clin Nutr Metab Care 16(6):657–661. [CrossRef]
- Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR (2018) Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun 9(1):1286. [CrossRef]
- Trammell SAJ, Yu L, Redpath P, Migaud ME, Brenner C (2016) Nicotinamide riboside is a major NAD+ precursor vitamin in cow milk. J Nutr 146(5):957–963. [CrossRef]
- Lee HJ, Yang SJ (2019) Supplementation with nicotinamide riboside reduces brain inflammation and improves cognitive function in diabetic mice. Int J Mol Sci 20(17):4196. [CrossRef]
- Bogan KL, Brenner C (2008) Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 28:115–130. [CrossRef]
- Belenky P, Stebbins R, Bogan KL, Evans CR, Brenner C (2011) Nrt1 and Tna1-independent export of NAD+ precursor vitamins promotes NAD+ homeostasis and allows engineering of vitamin production. PLoS One 6(5):e19710. [CrossRef]
- Bieganowski P, Brenner C (2004) Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss–Handler independent route to NAD+ in fungi and humans. Cell 117(4):495–502. [CrossRef]
- Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, Auwerx J, Yanes O, Brenner C, Cantó C (2016) NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun 7:13103. [CrossRef]
- Schmidt MS, Brenner C (2019) Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nat Metab 1(7):660–661. [CrossRef]
- Canto C (2022) NAD+ precursors: a questionable redundancy. Metabolites 12(7):630. [CrossRef]
- Cantó C, Auwerx J (2011) NAD+ as a signaling molecule modulating metabolism. Cold Spring Harb Symp Quant Biol 76:291–298. [CrossRef]
- Imai S, Guarente L (2014) NAD+ and sirtuins in aging and disease. Trends Cell Biol 24(8):464–471. [CrossRef]
- Zhang N, Sauve AA (2018) Regulatory effects of NAD+ metabolic pathways on sirtuin activity. Prog Mol Biol Transl Sci 154:71–104. [CrossRef]
- Okabe K, Yaku K, Tobe K, Nakagawa T (2019) Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci 26(1):34. [CrossRef]
- Buonvicino D, Ranieri G, Pittelli M, Lapucci A, Bragliola S, Chiarugi A (2021) SIRT1-dependent restoration of NAD+ homeostasis after increased extracellular NAD+ exposure. J Biol Chem 297(1):100855. [CrossRef]
- Carpi FM, Cortese M, Orsomando G, Polzonetti V, Vincenzetti S, Moreschini B, Coleman M, Magni G, Pucciarelli S (2018) Simultaneous quantification of nicotinamide mononucleotide and related pyridine compounds in mouse tissues by UHPLC–MS/MS. Sep Sci Plus 1(1):22–30. [CrossRef]
- Demarest TG, Babbar M, Okur MN, Dan X, Croteau DL, Fakouri NB, Mattson MP, Bohr VA (2019) NAD+ metabolism in aging and cancer. Annu Rev Cancer Biol 3:105–130. [CrossRef]
- Yang T, Chan NYK, Sauve AA (2007) Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. J Med Chem 50(26):6458–6461. [CrossRef]
- Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J (2012) The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15(6):838–847. [CrossRef]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM (2013) Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging 34(6):1581–1588. [CrossRef]
- Cantó C, Menzies KJ, Auwerx J (2015) NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22(1):31–53. [CrossRef]
- Conlon N, Ford D (2022) A systems-approach to NAD+ restoration. Biochem Pharmacol 198:114946. [CrossRef]
- Sharma C, Donu D, Cen Y (2022) Emerging role of nicotinamide riboside in health and diseases. Nutrients 14(19):3889. [CrossRef]
- Helman T, Braidy N (2023) Importance of NAD+ anabolism in metabolic, cardiovascular and neurodegenerative disorders. Drugs Aging 40(1):33–48. [CrossRef]
- Fang J, Wu H, Zhang J, Mao S, Shi H, Yu D, Chen Z, Su K, Xing Y, Dong H, Shi H (2022) A reduced form of nicotinamide riboside protects the cochlea against aminoglycoside-induced ototoxicity by SIRT1 activation. Biomed Pharmacother 150:113071. [CrossRef]
- She J, Sheng R, Qin ZH (2021) Pharmacology and potential implications of nicotinamide adenine dinucleotide precursors. Aging Dis 12(8):1879–1897. [CrossRef]
- Belenky P, Bogan KL, Brenner C (2007) NAD+ metabolism in health and disease. Trends Biochem Sci 32(1):12–19. Erratum in: Trends Biochem Sci, 2008, 33(1):1. [CrossRef]
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C (2007) Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell 129(3):473–484. [CrossRef]
- Yoshino J, Baur JA, Imai SI (2018) NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab 27(3):513–528. [CrossRef]
- Stock AJ, Ayyar S, Kashyap A, Wang Y, Yanai H, Starost MF, Tanaka-Yano M, Bodogai M, Sun C, Wang Y, Gong Y, Puligilla C, Fang EF, Bohr VA, Liu Y, Beerman I (2023) Boosting NAD ameliorates hematopoietic impairment linked to short telomeres in vivo. Geroscience Feb 24. [CrossRef]
- Hong G, Zheng D, Zhang L, Ni R, Wang G, Fan GC, Lu Z, Peng T (2018) Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radic Biol Med 123:125–137. [CrossRef]
- Wu J, Singh K, Lin A, Meadows AM, Wu K, Shing V, Bley M, Hassanzadeh S, Huffstutler RD, Schmidt MS, Blanco LP, Tian R, Brenner C, Pirooznia M, Kaplan MJ, Sack MN (2022) Boosting NAD+ blunts TLR4-induced type I IFN in control and systemic lupus erythematosus monocytes. J Clin Invest 132(5):e139828. [CrossRef]
- Zhang X, Tian B, Deng Q, Cao J, Ding X, Liu Q, Zhang Y, Ye C, Deng C, Qiu L, Guo C (2022) Nicotinamide riboside relieves the severity of experimental necrotizing enterocolitis by regulating endothelial function via eNOS deacetylation. Free Radic Biol Med 184:218–229. [CrossRef]
- Fan XK, Xu CQ, Cao KQ, Zhao GJ, Hong GL, Lu ZQ (2022) [Study on the protective effect and mechanism of nicotinamide riboside on lung injury in paraquat intoxicated mice]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi (Chin J Ind Hyg Occup Dis) 40(8):561–567. [CrossRef]
- Selli J, Vural Keles D, Keles ON, Celik M, Yetim Z (2023) Nicotinamide riboside preserves ovarian injury in experimental sepsis model in rats. Eurasian J Med Jan 17. [CrossRef]
- DiNicolantonio JJ, McCarty MF, O’Keefe JH (2022) Nutraceutical activation of Sirt1: a review. Open Heart 9(2):e002171. [CrossRef]
- Cheng YH, Zhao JH, Zong WF, Wei XJ, Xu Z, Yuan Y, Jiang YF, Luo X, Wang W, Qu WS (2022) Acute treatment with nicotinamide riboside chloride reduces hippocampal damage and preserves the cognitive function of mice with ischemic injury. Neurochem Res 47(8):2244–2253. [CrossRef]
- Lenherr N, Christodoulou J, Duley J, Dobritzsch D, Fairbanks L, Datta AN, Filges I, Gürtler N, Roelofsen J, van Kuilenburg ABP, Kemper C, West EE, Szinnai G, Huemer M (2021) Co-therapy with S-adenosylmethionine and nicotinamide riboside improves t-cell survival and function in Arts syndrome (PRPS1 deficiency). Mol Genet Metab Rep 26:100709. [CrossRef]
- Kodali M, Jankay T, Shetty AK, Reddy DS (2023) Pathophysiological basis and promise of experimental therapies for Gulf War Illness, a chronic neuropsychiatric syndrome in veterans. Psychopharmacology (Berl) 240(4):673–697. [CrossRef]
- Joshi U, Evans JE, Pearson A, Saltiel N, Cseresznye A, Darcey T, Ojo J, Keegan AP, Oberlin S, Mouzon B, Paris D, Klimas N, Sullivan K, Mullan M, Crawford F, Abdullah L (2020) Targeting sirtuin activity with nicotinamide riboside reduces neuroinflammation in a GWI mouse model. Neurotoxicology 79:84–94. [CrossRef]
- Jiang Y, Liu Y, Gao M, Xue M, Wang Z, Liang H (2020) Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct 11(1):378–391. [CrossRef]
- Vaur P, Brugg B, Mericskay M, Li Z, Schmidt MS, Vivien D, Orset C, Jacotot E, Brenner C, Duplus E (2017) Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J 31(12):5440–5452. [CrossRef]
- Kitaoka Y, Sase K, Tsukahara C, Fujita N, Arizono I, Takagi H (2020) Axonal protection by nicotinamide riboside via SIRT1-autophagy pathway in TNF-induced optic nerve degeneration. Mol Neurobiol 57(12):4952–4960. [CrossRef]
- Chen A, Kristiansen CK, Hong Y, Kianian A, Fang EF, Sullivan GJ, Wang J, Li X, Bindoff LA, Liang KX (2021) Nicotinamide riboside and Metformin ameliorate mitophagy defect in induced pluripotent stem cell-derived astrocytes with POLG mutations. Front Cell Dev Biol 9:737304. [CrossRef]
- Sun C, Seranova E, Cohen MA, Chipara M, Roberts J, Astuti D, Palhegyi AM, Acharjee A, Sedlackova L, Kataura T, Otten EG, Panda PK, Lara-Reyna S, Korsgen ME, Kauffman KJ, Huerta-Uribe A, Zatyka M, Silva LFSE, Torresi J, Zhang S, Hughes GW, Ward C, Kuechler ER, Cartwright D, Trushin S, Trushina E, Sahay G, Buganim Y, Lavery GG, Gsponer J, Anderson DG, Frickel EM, Rosenstock TR, Barrett T, Maddocks ODK, Tennant DA, Wang H, Jaenisch R, Korolchuk VI, Sarkar S (2023) NAD depletion mediates cytotoxicity in human neurons with autophagy deficiency. Cell Rep 42(5):112372. [CrossRef]
- Yang B, Dan X, Hou Y, Lee JH, Wechter N, Krishnamurthy S, Kimura R, Babbar M, Demarest T, McDevitt R, Zhang S, Zhang Y, Mattson MP, Croteau DL, Bohr VA (2021) NAD+ supplementation prevents STING-induced senescence in ataxia telangiectasia by improving mitophagy. Aging Cell 20(4):e13329. [CrossRef]
- Veenhuis SJG, van Os NJH, Janssen AJWM, van Gerven MHJC, Coene KLM, Engelke UFH, Wevers RA, Tinnevelt GH, Ter Heine R, van de Warrenburg BPC, Weemaes CMR, Roeleveld N, Willemsen MAAP (2021) Nicotinamide riboside improves ataxia scores and immunoglobulin levels in ataxia telangiectasia. Mov Disord 36(12):2951–2957. [CrossRef]
- Steinbruecker K, Tiefenthaler E, Schernthaler EM, Jungwirth J, Wortmann SB (2022) Nicotinamide riboside for ataxia telangiectasia: report of an early treated individual. Neuropediatrics Oct 12. [CrossRef]
- Gerasimenko M, Cherepanov SM, Furuhara K, Lopatina O, Salmina AB, Shabalova AA, Tsuji C, Yokoyama S, Ishihara K, Brenner C, Higashida H (2020) Nicotinamide riboside supplementation corrects deficits in oxytocin, sociability and anxiety of CD157 mutants in a mouse model of autism spectrum disorder. Sci Rep 10(1):10035. [CrossRef]
- Gerasimenko M, Higashida H (2023) Remission of social behavior impairment by oral administration of a precursor of NAD in CD157, but not in CD38, knockout mice. Front Immunol 14:1166609. [CrossRef]
- Harlan BA, Killoy KM, Pehar M, Liu L, Auwerx J, Vargas MR (2020) Evaluation of the NAD+ biosynthetic pathway in ALS patients and effect of modulating NAD+ levels in hSOD1-linked ALS mouse models. Exp Neurol 327:113219. [CrossRef]
- Zhou Q, Zhu L, Qiu W, Liu Y, Yang F, Chen W, Xu R (2020) Nicotinamide riboside enhances mitochondrial proteostasis and adult neurogenesis through activation of mitochondrial unfolded protein response signaling in the brain of ALS SOD1G93A mice. Int J Biol Sci 16(2):284–297. Erratum in: Int J Biol Sci, 2022, 18(5):2181-2183. [CrossRef]
- Obrador E, Salvador R, Marchio P, López-Blanch R, Jihad-Jebbar A, Rivera P, Vallés SL, Banacloche S, Alcácer J, Colomer N, Coronado JA, Alandes S, Drehmer E, Benlloch M, Estrela JM (2021) Nicotinamide riboside and pterostilbene cooperatively delay motor neuron failure in ALS SOD1G93A mice. Mol Neurobiol 58(4):1345–1371. [CrossRef]
- Freeberg KA, Udovich CAC, Martens CR, Seals DR, Craighead DH (2023) Dietary supplementation with NAD+-boosting compounds in humans: current knowledge and future directions. J Gerontol A Biol Sci Med Sci Apr 17:glad106. [CrossRef]
- Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR (2014) Activation of SIRT3 by the NAD⁺ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab 20(6):1059–1068. [CrossRef]
- Han S, Du Z, Liu K, Gong S (2020) Nicotinamide riboside protects noise-induced hearing loss by recovering the hair cell ribbon synapses. Neurosci Lett 725:134910. [CrossRef]
- Okur MN, Mao B, Kimura R, Haraczy S, Fitzgerald T, Edwards-Hollingsworth K, Tian J, Osmani W, Croteau DL, Kelley MW, Bohr VA (2020) Short-term NAD+ supplementation prevents hearing loss in mouse models of Cockayne syndrome. NPJ Aging Mech Dis 6:1. [CrossRef]
- Hamity MV, White SR, Walder RY, Schmidt MS, Brenner C, Hammond DL (2017) Nicotinamide riboside, a form of vitamin B3 and NAD+ precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain 158(5):962–972. [CrossRef]
- Hamity MV, Kolker SJ, Hegarty DM, Blum C, Langmack L, Aicher SA, Hammond DL (2022) Nicotinamide riboside alleviates corneal and somatic hypersensitivity induced by paclitaxel in male rats. Invest Ophthalmol Vis Sci 63(1):38. [CrossRef]
- Pîrvu AS, Andrei AM, Stănciulescu EC, Baniţă IM, Pisoschi CG, Jurja S, Ciuluvica R (2021) NAD+ metabolism and retinal degeneration (Review). Exp Ther Med 22(1):670. [CrossRef]
- Zhang X, Zhang N, Chrenek MA, Girardot PE, Wang J, Sellers JT, Geisert EE, Brenner C, Nickerson JM, Boatright JH, Li Y (2021) Systemic treatment with nicotinamide riboside is protective in two mouse models of retinal ganglion cell damage. Pharmaceutics 13(6):893. [CrossRef]
- Zhang X, Henneman NF, Girardot PE, Sellers JT, Chrenek MA, Li Y, Wang J, Brenner C, Nickerson JM, Boatright JH (2020) Systemic treatment with nicotinamide riboside is protective in a mouse model of light-induced retinal degeneration. Invest Ophthalmol Vis Sci 61(10):47. [CrossRef]
- Leung CKS, Ren ST, Chan PPM, Wan KHN, Kam AKW, Lai GWK, Chiu VSM, Ko MWL, Yiu CKF, Yu MCY (2022) Nicotinamide riboside as a neuroprotective therapy for glaucoma: study protocol for a randomized, double-blind, placebo-control trial. Trials 23(1):45. Erratum in: Trials, 2022, 23(1):134. [CrossRef]
- Silva SGN, Occhiutto ML, Costa VP (2023) The use of nicotinamide and nicotinamide riboside as an adjunct therapy in the treatment of glaucoma. Eur J Ophthalmol Mar 14:11206721231161101. [CrossRef]
- Zhou B, Zhao G, Zhu Y, Chen X, Zhang N, Yang J, Lin H (2021) Protective effects of nicotinamide riboside on H2O2-induced oxidative damage in lens epithelial cells. Curr Eye Res 46(7):961–970. [CrossRef]
- Pang H, Jiang Y, Li J, Wang Y, Nie M, Xiao N, Wang S, Song Z, Ji F, Chang Y, Zheng Y, Yao K, Yao L, Li S, Li P, Song L, Lan X, Xu Z, Hu Z (2021) Aberrant NAD+ metabolism underlies Zika virus-induced microcephaly. Nat Metab 3(8):1109–1124. [CrossRef]
- Matasic DS, Brenner C, London B (2018) Emerging potential benefits of modulating NAD+ metabolism in cardiovascular disease. Am J Physiol Heart Circ Physiol 314(4):H839–H852. [CrossRef]
- Ahmad F, Tomar D, Aryal A C S, Elmoselhi AB, Thomas M, Elrod JW, Tilley DG, Force T (2020) Nicotinamide riboside kinase-2 alleviates ischemia-induced heart failure through P38 signaling. Biochim Biophys Acta Mol Basis Dis 1866(3):165609. [CrossRef]
- Blanco-Vaca F, Rotllan N, Canyelles M, Mauricio D, Escolà-Gil JC, Julve J (2022) NAD+-increasing strategies to improve cardiometabolic health? Front Endocrinol (Lausanne) 12:815565. [CrossRef]
- Liu Y, Huang Y, Xu C, An P, Luo Y, Jiao L, Luo J, Li Y (2022) Mitochondrial dysfunction and therapeutic perspectives in cardiovascular diseases. Int J Mol Sci 23(24):16053. [CrossRef]
- de Castro JM, Assumpção JAF, Stein DJ, Toledo RS, da Silva LS, Caumo W, Carraro CC, da Rosa Araujo AS, Torres ILS (2020) Nicotinamide riboside reduces cardiometabolic risk factors and modulates cardiac oxidative stress in obese Wistar rats under caloric restriction. Life Sci 263:118596. [CrossRef]
- Liu X, Zhang Y, Deng Y, Yang L, Ou W, Xie M, Ding L, Jiang C, Yu H, Li Q, Li T (2022a) Mitochondrial protein hyperacetylation underpins heart failure with preserved ejection fraction in mice. J Mol Cell Cardiol 165:76–85. [CrossRef]
- Ma S, Feng J, Lin X, Liu J, Tang Y, Nie S, Gong J, Wang L (2021) Nicotinamide riboside alleviates cardiac dysfunction and remodeling in pressure overload cardiac hypertrophy. Oxid Med Cell Longev 2021:5546867. [CrossRef]
- Zhang M, Weng H, Zheng J (2019) NAD+ repletion inhibits the endothelial-to-mesenchymal transition induced by TGF-β in endothelial cells through improving mitochondrial unfolded protein response. Int J Biochem Cell Biol 117:105635. [CrossRef]
- Pool L, Knops P, Manintveld OC, Brugts JJ, Theuns DAMJ, Brundel BJJM, de Groot NMS (2022) The HF-AF ENERGY Trial: nicotinamide riboside for the treatment of atrial fibrillation in heart failure patients. Cardiovasc Drugs Ther Oct 13. [CrossRef]
- Zhou B, Wang DD, Qiu Y, Airhart S, Liu Y, Stempien-Otero A, O’Brien KD, Tian R (2020) Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest 130(11):6054–6063. [CrossRef]
- Wang DD, Airhart SE, Zhou B, Shireman LM, Jiang S, Melendez Rodriguez C, Kirkpatrick JN, Shen DD, Tian R, O’Brien KD (2022) Safety and tolerability of nicotinamide riboside in heart failure with reduced ejection fraction. JACC Basic Transl Sci 7(12):1183-1196. [CrossRef]
- Oller J, Gabandé-Rodríguez E, Roldan-Montero R, Ruiz-Rodríguez MJ, Redondo JM, Martín-Ventura JL, Mittelbrunn M (2022) Rewiring vascular metabolism prevents sudden death due to aortic ruptures – brief report. Arterioscler Thromb Vasc Biol 42(4):462–469. [CrossRef]
- Shahzadi SK, Marzook H, Qaisar R, Ahmad F (2022) Nicotinamide riboside kinase-2 inhibits JNK pathway and limits dilated cardiomyopathy in mice with chronic pressure overload. Clin Sci (Lond) 136(2):181–196. [CrossRef]
- Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc J, Breton M, Decaux JF, Lavery GG, Baczkó I, Zoll J, Garnier A, Li Z, Brenner C, Mericskay M (2018) Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137(21):2256–2273. Erratum in: Circulation, 2018, 137(21):e690. [CrossRef]
- Zheng D, Zhang Y, Zheng M, Cao T, Wang G, Zhang L, Ni R, Brockman J, Zhong H, Fan GC, Peng T (2019) Nicotinamide riboside promotes autolysosome clearance in preventing doxorubicin-induced cardiotoxicity. Clin Sci (Lond) 133(13):1505–1521. [CrossRef]
- Podyacheva E, Toropova Y (2021) Nicotinamide riboside for the prevention and treatment of doxorubicin cardiomyopathy. Opportunities and prospects. Nutrients 13(10):3435. [CrossRef]
- Podyacheva E, N Yu N, V A V, Mukhametdinova D, Goncharova I, Zelinskaya I, Sviridov E, Martynov M, Osipova S, Toropova Y (2022) Intravenous nicotinamide riboside administration has a cardioprotective effect in chronic doxorubicin-induced cardiomyopathy. Int J Mol Sci 23(21):13096. [CrossRef]
- Marzook H, Gupta A, Tomar D, Saleh MA, Patil K, Semreen MH, Hamoudi R, Soares NC, Qaisar R, Ahmad F (2023) Nicotinamide riboside kinase-2 regulates metabolic adaptation in the ischemic heart. J Mol Med (Berl) 101(3):311–326. [CrossRef]
- Nie H, Zhang Y, Yu H, Xiao H, Li T, Yang Q (2021) Oral delivery of carrier-free dual-drug nanocrystal self-assembled microspheres improved NAD+ bioavailability and attenuated cardiac ischemia/reperfusion injury in mice. Drug Deliv 28(1):433–444. [CrossRef]
- Xiao Y, Phelp P, Wang Q, Bakker D, Nederlof R, Hollmann MW, Zuurbier CJ (2021) Cardioprotecive properties of known agents in rat ischemia-reperfusion model under clinically relevant conditions: only the NAD precursor nicotinamide riboside reduces infarct size in presence of Fentanyl, Midazolam and Cangrelor, but not Propofol. Front Cardiovasc Med 8:712478. [CrossRef]
- Toropova YG, Pechnikova NA, Zelinskaya IA, Zhuravsky SG, Kornyushin OV, Gonchar AI, Ivkin DY, Leonova YV, Karev VE, Karabak IA (2018) Nicotinamide riboside has protective effects in a rat model of mesenteric ischaemia–reperfusion. Int J Exp Pathol 99(6):304–311. [CrossRef]
- Mateuszuk Ł, Campagna R, Kutryb-Zając B, Kuś K, Słominska EM, Smolenski RT, Chlopicki S (2020) Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem Pharmacol 178:114019. [CrossRef]
- Mukherjee S, Chellappa K, Moffitt A, Ndungu J, Dellinger RW, Davis JG, Agarwal B, Baur JA (2017) Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology 65(2):616–630. Erratum in: Hepatology, 2017, 65(4):1427. [CrossRef]
- Dall M, Trammell SAJ, Asping M, Hassing AS, Agerholm M, Vienberg SG, Gillum MP, Larsen S, Treebak JT (2019) Mitochondrial function in liver cells is resistant to perturbations in NAD+ salvage capacity. J Biol Chem 294(36):13304–13326. [CrossRef]
- Lee HJ, Yang SJ (2019) Nicotinamide riboside regulates inflammation and mitochondrial markers in AML12 hepatocytes. Nutr Res Pract 13(1):3–10. [CrossRef]
- Fan R, Cui J, Ren F, Wang Q, Huang Y, Zhao B, Wei L, Qian X, Xiong X (2018) Overexpression of NRK1 ameliorates diet- and age-induced hepatic steatosis and insulin resistance. Biochem Biophys Res Commun 500(2):476–483. [CrossRef]
- Han X, Bao X, Lou Q, Xie X, Zhang M, Zhou S, Guo H, Jiang G, Shi Q (2019) Nicotinamide riboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ 7:e7568. [CrossRef]
- Bock KW (2020) Modulation of aryl hydrocarbon receptor (AHR) and the NAD+-consuming enzyme CD38: Searches of therapeutic options for nonalcoholic fatty liver disease (NAFLD). Biochem Pharmacol 175:113905. [CrossRef]
- Dall M, Hassing AS, Niu L, Nielsen TS, Ingerslev LR, Sulek K, Trammell SAJ, Gillum MP, Barrès R, Larsen S, Poulsen SS, Mann M, Ørskov C, Treebak JT (2021) Hepatocyte-specific perturbation of NAD+ biosynthetic pathways in mice induces reversible nonalcoholic steatohepatitis-like phenotypes. J Biol Chem 297(6):101388. [CrossRef]
- Quesada-Vázquez S, Bone C, Saha S, Triguero I, Colom-Pellicer M, Aragonès G, Hildebrand F, Del Bas JM, Caimari A, Beraza N, Escoté X (2022) Microbiota dysbiosis and gut barrier dysfunction associated with non-alcoholic fatty liver disease are modulated by a specific metabolic cofactors’ combination. Int J Mol Sci 23(22):13675. [CrossRef]
- Dellinger RW, Holmes HE, Hu-Seliger T, Butt RW, Harrison SA, Mozaffarian D, Chen O, Guarente L (2022) Nicotinamide riboside and pterostilbene reduces markers of hepatic inflammation in NAFLD: a double-blind, placebo-controlled clinical trial. Hepatology Sep 9. [CrossRef]
- Pham TX, Bae M, Kim MB, Lee Y, Hu S, Kang H, Park YK, Lee JY (2019) Nicotinamide riboside, an NAD+ precursor, attenuates the development of liver fibrosis in a diet-induced mouse model of liver fibrosis. Biochim Biophys Acta Mol Basis Dis 1865(9):2451–2463. [CrossRef]
- Jiang R, Zhou Y, Wang S, Pang N, Huang Y, Ye M, Wan T, Qiu Y, Pei L, Jiang X, Huang Y, Yang H, Ling W, Li X, Zhang Z, Yang L (2019) Nicotinamide riboside protects against liver fibrosis induced by CCl4 via regulating the acetylation of Smads signaling pathway. Life Sci 225:20–28. [CrossRef]
- Wang S, Wan T, Ye M, Qiu Y, Pei L, Jiang R, Pang N, Huang Y, Liang B, Ling W, Lin X, Zhang Z, Yang L (2018) Nicotinamide riboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol 17:89–98. [CrossRef]
- Yau WW, Chen GB, Zhou J, Francisco JC, Thimmukonda NK, Li S, Singh BK, Yen PM (2023) Nicotinamide riboside rescues dysregulated glycolysis and fatty acid β-oxidation in a human hepatic cell model of citrin deficiency. Hum Mol Genet 32(11):1922–1931. [CrossRef]
- Lee SR, Roh JY, Ryu J, Shin HJ, Hong EJ (2022) Activation of TCA cycle restrains virus-metabolic hijacking and viral replication in mouse hepatitis virus-infected cells. Cell Biosci 12(1):7. [CrossRef]
- Parikh SM (2019) Metabolic stress resistance in acute kidney injury: evidence for a PPAR-gamma-coactivator-1 alpha-nicotinamide adenine dinucleotide pathway. Nephron 143(3):184–187. [CrossRef]
- Simic P, Vela Parada XF, Parikh SM, Dellinger R, Guarente LP, Rhee EP (2020) Nicotinamide riboside with pterostilbene (NRPT) increases NAD+ in patients with acute kidney injury (AKI): a randomized, double-blind, placebo-controlled, stepwise safety study of escalating doses of NRPT in patients with AKI. BMC Nephrol 21(1):342. [CrossRef]
- Lin W, Wu X, Wen J, Fei Y, Wu J, Li X, Zhang Q, Dong Y, Xu T, Fan Y, Wang N (2021) Nicotinamide retains Klotho expression and ameliorates rhabdomyolysis-induced acute kidney injury. Nutrition 91–92:111376. [CrossRef]
- Piedrafita A, Balayssac S, Mayeur N, Gazut S, Grossac J, Buleon M, Alves M, Klein J, Minville V, Marcheix B, Schanstra JP, Faguer S (2021) The tryptophan pathway and nicotinamide supplementation in ischaemic acute kidney injury. Clin Kidney J 14(12):2490–2496. [CrossRef]
- Fontecha-Barriuso M, Lopez-Diaz AM, Carriazo S, Ortiz A, Sanz AB (2021) Nicotinamide and acute kidney injury. Clin Kidney J 14(12):2453–2462. [CrossRef]
- Pezzotta A, Perico L, Morigi M, Corna D, Locatelli M, Zoja C, Benigni A, Remuzzi G, Imberti B (2022) Low nephron number induced by maternal protein restriction is prevented by nicotinamide riboside supplementation depending on sirtuin 3 activation. Cells 11(20):3316. [CrossRef]
- Morevati M, Egstrand S, Nordholm A, Mace ML, Andersen CB, Salmani R, Olgaard K, Lewin E (2021) Effect of NAD+ boosting on kidney ischemia–reperfusion injury. PLoS One 16(6):e0252554. [CrossRef]
- Doke T, Mukherjee S, Mukhi D, Dhillon P, Abedini A, Davis JG, Chellappa K, Chen B, Baur JA, Susztak K (2023) NAD+ precursor supplementation prevents mtRNA/RIG-I-dependent inflammation during kidney injury. Nat Metab 5(3):414–430. [CrossRef]
- Ahmadi A, Begue G, Valencia AP, Norman JE, Lidgard B, Bennett BJ, Van Doren MP, Marcinek DJ, Fan S, Prince DK, Gamboa JL, Himmelfarb J, de Boer IH, Kestenbaum BR, Roshanravan B (2023) Randomized crossover clinical trial of coenzyme Q10 and nicotinamide riboside in chronic kidney disease. JCI Insight May 9:e167274. [CrossRef]
- Fletcher RS, Ratajczak J, Doig CL, Oakey LA, Callingham R, Da Silva Xavier G, Garten A, Elhassan YS, Redpath P, Migaud ME, Philp A, Brenner C, Canto C, Lavery GG (2017) Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol Metab 6(8):819–832. [CrossRef]
- Sonntag T, Ancel S, Karaz S, Cichosz P, Jacot G, Giner MP, Sanchez-Garcia JL, Pannérec A, Moco S, Sorrentino V, Cantó C, Feige JN (2022) Nicotinamide riboside kinases regulate skeletal muscle fiber-type specification and are rate-limiting for metabolic adaptations during regeneration. Front Cell Dev Biol 10:1049653. [CrossRef]
- Doig CL, Zielinska AE, Fletcher RS, Oakey LA, Elhassan YS, Garten A, Cartwright D, Heising S, Alsheri A, Watson DG, Prehn C, Adamski J, Tennant DA, Lavery GG (2020) Induction of the nicotinamide riboside kinase NAD+ salvage pathway in a model of sarcoplasmic reticulum dysfunction. Skelet Muscle 10(1):5. [CrossRef]
- Damgaard MV, Nielsen TS, Basse AL, Chubanava S, Trost K, Moritz T, Dellinger RW, Larsen S, Treebak JT (2022) Intravenous nicotinamide riboside elevates mouse skeletal muscle NAD+ without impacting respiratory capacity or insulin sensitivity. iScience 25(2):103863. [CrossRef]
- Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, Cartwright DM, Oakey L, Burley CV, Jenkinson N, Wilson M, Lucas SJE, Akerman I, Seabright A, Lai YC, Tennant DA, Nightingale P, Wallis GA, Manolopoulos KN, Brenner C, Philp A, Lavery GG (2019) Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep 28(7):1717–1728.e6. [CrossRef]
- Remie CME, Roumans KHM, Moonen MPB, Connell NJ, Havekes B, Mevenkamp J, Lindeboom L, de Wit VHW, van de Weijer T, Aarts SABM, Lutgens E, Schomakers BV, Elfrink HL, Zapata-Pérez R, Houtkooper RH, Auwerx J, Hoeks J, Schrauwen-Hinderling VB, Phielix E, Schrauwen P (2020) Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am J Clin Nutr 112(2):413–426. [CrossRef]
- Custodero C, Saini SK, Shin MJ, Jeon YK, Christou DD, McDermott MM, Leeuwenburgh C, Anton SD, Mankowski RT (2020) Nicotinamide riboside – a missing piece in the puzzle of exercise therapy for older adults? Exp Gerontol 137:110972. [CrossRef]
- Campelj D, Philp A (2022) NAD+ therapeutics and skeletal muscle adaptation to exercise in humans. Sports Med 52(Suppl 1):91–99. [CrossRef]
- Stocks B, Ashcroft SP, Joanisse S, Dansereau LC, Koay YC, Elhassan YS, Lavery GG, Quek LE, O’Sullivan JF, Philp AM, Wallis GA, Philp A (2021) Nicotinamide riboside supplementation does not alter whole-body or skeletal muscle metabolic responses to a single bout of endurance exercise. J Physiol 599(5):1513–1531. [CrossRef]
- Song M, Armenian SH, Bhandari R, Lee K, Ness K, Putt M, Lindenfeld L, Manoukian S, Wade K, Dedio A, Guzman T, Hampton I, Lin K, Baur J, McCormack S, Mostoufi-Moab S (2022) Exercise training and NR supplementation to improve muscle mass and fitness in adolescent and young adult hematopoietic cell transplant survivors: a randomized controlled trial {1}. BMC Cancer 22(1):795. [CrossRef]
- Kourtzidis IA, Stoupas AT, Gioris IS, Veskoukis AS, Margaritelis NV, Tsantarliotou M, Taitzoglou I, Vrabas IS, Paschalis V, Kyparos A, Nikolaidis MG (2016) The NAD+ precursor nicotinamide riboside decreases exercise performance in rats. J Int Soc Sports Nutr 13:32. [CrossRef]
- Kourtzidis IA, Dolopikou CF, Tsiftsis AN, Margaritelis NV, Theodorou AA, Zervos IA, Tsantarliotou MP, Veskoukis AS, Vrabas IS, Paschalis V, Kyparos A, Nikolaidis MG (2018) Nicotinamide riboside supplementation dysregulates redox and energy metabolism in rats: implications for exercise performance. Exp Physiol 103(10):1357–1366. [CrossRef]
- Deloux R, Tannous C, Ferry A, Li Z, Mericskay M (2018) Aged nicotinamide riboside kinase 2 deficient mice present an altered response to endurance exercise training. Front Physiol 9:1290. [CrossRef]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J (2016) NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352(6292):1436–1443. [CrossRef]
- Moon J, Kim HR, Shin MG (2018) Rejuvenating aged hematopoietic stem cells through improvement of mitochondrial function. Ann Lab Med 38(5):395–401. [CrossRef]
- Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, Suomalainen A (2014) Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med 6(6):721–731. [CrossRef]
- Schaefer PM, Huang J, Butic A, Perry C, Yardeni T, Tan W, Morrow R, Baur JA, Wallace DC (2022) Nicotinamide riboside alleviates exercise intolerance in ANT1-deficient mice. Mol Metab 64:101560. [CrossRef]
- Lapatto HAK, Kuusela M, Heikkinen A, Muniandy M, van der Kolk BW, Gopalakrishnan S, Pöllänen N, Sandvik M, Schmidt MS, Heinonen S, Saari S, Kuula J, Hakkarainen A, Tampio J, Saarinen T, Taskinen MR, Lundbom N, Groop PH, Tiirola M, Katajisto P, Lehtonen M, Brenner C, Kaprio J, Pekkala S, Ollikainen M, Pietiläinen KH, Pirinen E (2023) Nicotinamide riboside improves muscle mitochondrial biogenesis, satellite cell differentiation, and gut microbiota in a twin study. Sci Adv 9(2):eadd5163. [CrossRef]
- Perez-Sanchez C, Escudero-Contreras A, Cerdó T, Sánchez-Mendoza LM, Llamas-Urbano A, Arias-de la Rosa I, Pérez-Rodriguez M, Muñoz-Barrera L, Abalos-Aguilera MDC, Barbarroja N, Calvo J, Ortega-Castro R, Ruiz-Vilches D, Moreno JA, Burón MI, González-Reyes JA, Collantes-Estevez E, Lopez-Pedrera C, Villalba JM (2023) Preclinical characterization of pharmacological NAD+ boosting as a promising therapeutic approach in rheumatoid arthritis. Arthritis Rheumatol Apr 24. [CrossRef]
- Gonzalez JM, Jackson AR (2020) In ovo feeding of nicotinamide riboside affects broiler pectoralis major muscle development. Transl Anim Sci 4(3):txaa126. [CrossRef]
- Xu X, Jackson AR, Gonzalez JM (2021) The effects of in ovo nicotinamide riboside dose on broiler myogenesis. Poult Sci 100(3):100926. [CrossRef]
- Xu X, Alcocer HM, Gravely ME, Jackson AR, Gonzalez JM (2022) Effects of in ovo injection of nicotinamide riboside on high-yield broiler myogenesis. J Anim Sci 100(8):skac203. [CrossRef]
- Sambeat A, Ratajczak J, Joffraud M, Sanchez-Garcia JL, Giner MP, Valsesia A, Giroud-Gerbetant J, Valera-Alberni M, Cercillieux A, Boutant M, Kulkarni SS, Moco S, Canto C (2019) Endogenous nicotinamide riboside metabolism protects against diet-induced liver damage. Nat Commun 10(1):4291. [CrossRef]
- Igarashi M, Miura M, Williams E, Jaksch F, Kadowaki T, Yamauchi T, Guarente L (2019) NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell 18(3):e12935. [CrossRef]
- Cartwright DM, Oakey LA, Fletcher RS, Doig CL, Heising S, Larner DP, Nasteska D, Berry CE, Heaselgrave SR, Ludwig C, Hodson DJ, Lavery GG, Garten A (2021) Nicotinamide riboside has minimal impact on energy metabolism in mouse models of mild obesity. J Endocrinol 251(1):111–123. [CrossRef]
- Lozada-Fernández VV, deLeon O, Kellogg SL, Saravia FL, Hadiono MA, Atkinson SN, Grobe JL, Kirby JR (2022) Nicotinamide riboside-conditioned microbiota deflects high-fat diet-induced weight gain in mice. mSystems 7(1):e0023021. [CrossRef]
- Sauve AA (2022) Metabolic disease, NAD metabolism, nicotinamide riboside, and the gut microbiome: connecting the dots from the gut to physiology. mSystems 7(1):e0122321. [CrossRef]
- Kolba N, Zarei A, Cheng J, Agarwal N, Dadmohammadi Y, Khazdooz L, Abbaspourrad A, Tako E (2022) Alterations in intestinal brush border membrane functionality and bacterial populations following intra-amniotic administration (Gallus gallus) of nicotinamide riboside and its derivatives. Nutrients 14(15):3130. [CrossRef]
- Peluso AA, Lundgaard AT, Babaei P, Mousovich-Neto F, Rocha AL, Damgaard MV, Bak EG, Gnanasekaran T, Dollerup OL, Trammell SAJ, Nielsen TS, Kern T, Abild CB, Sulek K, Ma T, Gerhart-Hines Z, Gillum MP, Arumugam M, Ørskov C, McCloskey D, Jessen N, Herrgård MJ, Mori MAS, Treebak JT (2023) Oral supplementation of nicotinamide riboside alters intestinal microbial composition in rats and mice, but not humans. NPJ Aging 9(1):7. [CrossRef]
- Kim MB, Pham TX, vanLuling M, Kostour V, Kang H, Corvino O, Jang H, Odell W, Bae M, Park YK, Lee JY (2022) Nicotinamide riboside supplementation exerts an anti-obesity effect and prevents inflammation and fibrosis in white adipose tissue of female diet-induced obesity mice. J Nutr Biochem 107:109058. [CrossRef]
- Longo L, de Castro JM, Keingeski MB, Rampelotto PH, Stein DJ, Guerreiro GTS, de Souza VEG, Cerski CTS, Uribe-Cruz C, Torres ILS, Álvares-da-Silva MR (2023) Nicotinamide riboside and dietary restriction effects on gut microbiota and liver inflammatory and morphologic markers in cafeteria diet-induced obesity in rats. Nutrition 110:112019. [CrossRef]
- Yu X, Xue M, Liu Y, Zhou Z, Jiang Y, Sun T, Liang H (2020) Effect of nicotinamide riboside on lipid metabolism and gut microflora–bile acid axis in alcohol-exposed mice. Food Sci Nutr 9(1):429–440. [CrossRef]
- Crisol BM, Veiga CB, Lenhare L, Braga RR, Silva VRR, da Silva ASR, Cintra DE, Moura LP, Pauli JR, Ropelle ER (2018) Nicotinamide riboside induces a thermogenic response in lean mice. Life Sci 211:1–7. [CrossRef]
- Crisol BM, Veiga CB, Braga RR, Lenhare L, Baptista IL, Gaspar RC, Muñoz VR, Cordeiro AV, da Silva ASR, Cintra DE, Moura LP, Pauli JR, Ropelle ER (2020) NAD+ precursor increases aerobic performance in mice. Eur J Nutr 59(6):2427–2437. [CrossRef]
- Williams AS, Koves TR, Pettway YD, Draper JA, Slentz DH, Grimsrud PA, Ilkayeva OR, Muoio DM (2021) Nicotinamide riboside supplementation confers marginal metabolic benefits in obese mice without remodeling the muscle acetyl-proteome. iScience 25(1):103635. [CrossRef]
- Serrano A, Palou A, Bonet ML, Ribot J (2022) Nicotinamide riboside supplementation to suckling male mice improves lipid and energy metabolism in skeletal muscle and liver in adulthood. Nutrients 14(11):2259. [CrossRef]
- de Castro JM, Stein DJ, Medeiros HR, de Oliveira C, Torres ILS (2021) Nicotinamide riboside neutralizes hypothalamic inflammation and increases weight loss without altering muscle mass in obese rats under calorie restriction: a preliminary investigation. Front Nutr 8:648893. [CrossRef]
- Shi W, Hegeman MA, van Dartel DAM, Tang J, Suarez M, Swarts H, van der Hee B, Arola L, Keijer J (2017) Effects of a wide range of dietary nicotinamide riboside (NR) concentrations on metabolic flexibility and white adipose tissue (WAT) of mice fed a mildly obesogenic diet. Mol Nutr Food Res 61(8):1600878. [CrossRef]
- Shi W, Hegeman MA, Doncheva A, Bekkenkamp-Grovenstein M, de Boer VCJ, Keijer J (2019) High dose of dietary nicotinamide riboside induces glucose intolerance and white adipose tissue dysfunction in mice fed a mildly obesogenic diet. Nutrients 11(10):2439. [CrossRef]
- Shi W, Hegeman MA, Doncheva A, van der Stelt I, Bekkenkamp-Grovenstein M, van Schothorst EM, Brenner C, de Boer VCJ, Keijer J (2019) Transcriptional response of white adipose tissue to withdrawal of vitamin B3. Mol Nutr Food Res 63(13):e1801100. [CrossRef]
- Quesada-Vázquez S, Antolín A, Colom-Pellicer M, Aragonès G, Herrero L, Del Bas JM, Caimari A, Escoté X (2022) Reduction of obesity and insulin resistance through dual targeting of VAT and BAT by a novel combination of metabolic cofactors. Int J Mol Sci 23(23):14923. [CrossRef]
- Zhao H, Tian Y, Zuo Y, Zhang X, Gao Y, Wang P, Sun L, Zhang H, Liang H (2022) Nicotinamide riboside ameliorates high-fructose-induced lipid metabolism disorder in mice via improving FGF21 resistance in the liver and white adipose tissue. Food Funct 13(23):12400–12411. [CrossRef]
- Serrano A, Asnani-Kishnani M, Couturier C, Astier J, Palou A, Landrier JF, Ribot J, Bonet ML (2020) DNA methylation changes are associated with the programming of white adipose tissue browning features by resveratrol and nicotinamide riboside neonatal supplementations in mice. Nutrients 12(2):461. [CrossRef]
- Salic K, Gart E, Seidel F, Verschuren L, Caspers M, van Duyvenvoorde W, Wong KE, Keijer J, Bobeldijk-Pastorova I, Wielinga PY, Kleemann R (2019) Combined treatment with L-carnitine and nicotinamide riboside improves hepatic metabolism and attenuates obesity and liver steatosis. Int J Mol Sci 20(18):4359. [CrossRef]
- Nagy L, Rauch B, Szerafin T, Uray K, Tóth A, Bai P (2022) Nicotinamide-riboside shifts the differentiation of human primary white adipocytes to beige adipocytes impacting substrate preference and uncoupling respiration through SIRT1 activation and mitochondria-derived reactive species production. Front Cell Dev Biol 10:979330. [CrossRef]
- Nascimento EBM, Moonen MPB, Remie CME, Gariani K, Jörgensen JA, Schaart G, Hoeks J, Auwerx J, van Marken Lichtenbelt WD, Schrauwen P (2021) Nicotinamide riboside enhances in vitro beta-adrenergic brown adipose tissue activity in humans. J Clin Endocrinol Metab 106(5):1437–1447. [CrossRef]
- Dollerup OL, Trammell SAJ, Hartmann B, Holst JJ, Christensen B, Møller N, Gillum MP, Treebak JT, Jessen N (2019) Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J Clin Endocrinol Metab 104(11):5703–5714. [CrossRef]
- Dollerup OL, Chubanava S, Agerholm M, Søndergård SD, Altıntaş A, Møller AB, Høyer KF, Ringgaard S, Stødkilde-Jørgensen H, Lavery GG, Barrès R, Larsen S, Prats C, Jessen N, Treebak JT (2020) Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J Physiol 598(4):731–754. [CrossRef]
- Trammell SAJ, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C (2016) Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep 6:26933. [CrossRef]
- Chandrasekaran K, Najimi N, Sagi AR, Yarlagadda S, Salimian M, Arvas MI, Hedayat AF, Kevas Y, Kadakia A, Russell JW (2022) NAD+ precursors repair mitochondrial function in diabetes and prevent experimental diabetic neuropathy. Int J Mol Sci 23(9):4887. [CrossRef]
- Cercillieux A, Ratajczak J, Joffraud M, Sanchez-Garcia JL, Jacot G, Zollinger A, Métairon S, Giroud-Gerbetant J, Rumpler M, Ciarlo E, Valera-Alberni M, Sambeat A, Canto C (2022b) Nicotinamide riboside kinase 1 protects against diet and age-induced pancreatic β-cell failure. Mol Metab 66:101605. [CrossRef]
- Hu L, Guo Y, Song L, Wen H, Sun N, Wang Y, Qi B, Liang Q, Geng J, Liu X, Fu F, Li Y (2022) Nicotinamide riboside promotes Mfn2-mediated mitochondrial fusion in diabetic hearts through the SIRT1–PGC1α–PPARα pathway. Free Radic Biol Med 183:75–88. [CrossRef]
- Costa CJ, Cohen MW, Goldberg DC, Mellado W, Willis DE (2023) Nicotinamide riboside improves enteric neuropathy in streptozocin-induced diabetic rats through myenteric plexus neuroprotection. Dig Dis Sci Mar 15. [CrossRef]
- Lee HJ, Hong YS, Jun W, Yang SJ (2015) Nicotinamide riboside ameliorates hepatic metaflammation by modulating NLRP3 inflammasome in a rodent model of type 2 diabetes. J Med Food 18(11):1207–1213. [CrossRef]
- Wang ZH, Bao XG, Hu JJ, Shen SB, Xu GH, Wu YL (2021) Nicotinamide riboside enhances endothelial precursor cell function to promote refractory wound healing through mediating the Sirt1/AMPK pathway. Front Pharmacol 12:671563. [CrossRef]
- Lee SR, Jeong SH, Mukae M, Kim SY, Ko JW, Kwun HJ, Hong EJ (2023) Dietary supplementation with nicotinamide riboside improves fetal growth under hypoglycemia. J Nutr Biochem 116:109310. [CrossRef]
- Chu X, Raju RP (2022) Regulation of NAD+ metabolism in aging and disease. Metabolism 126:154923. [CrossRef]
- Ji LL, Yeo D (2022) Maintenance of NAD+ homeostasis in skeletal muscle during aging and exercise. Cells 11(4):710. [CrossRef]
- Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA (2013) Declining NAD+ induces a pseudohypoxic state disrupting nuclear–mitochondrial communication during aging. Cell 155(7):1624–1638. [CrossRef]
- Cercillieux A, Ciarlo E, Canto C (2022) Balancing NAD+ deficits with nicotinamide riboside: therapeutic possibilities and limitations. Cell Mol Life Sci 79(8):463. [CrossRef]
- Li W, Zhou Y, Pang N, Hu Q, Li Q, Sun Y, Ding Y, Gu Y, Xiao Y, Gao M, Ma S, Pan J, Fang EF, Zhang Z, Yang L (2022) NAD supplement alleviates intestinal barrier injury induced by ethanol via protecting epithelial mitochondrial function. Nutrients 15(1):174. [CrossRef]
- Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, Ragusa S, Stefanidis E, Ryu D, Rainer PY, Nikitin G, Giger S, Li TY, Semilietof A, Oggier A, Yersin Y, Tauzin L, Pirinen E, Cheng WC, Ratajczak J, Canto C, Ehrbar M, Sizzano F, Petrova TV, Vanhecke D, Zhang L, Romero P, Nahimana A, Cherix S, Duchosal MA, Ho PC, Deplancke B, Coukos G, Auwerx J, Lutolf MP, Naveiras O (2019) The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell 24(3):405–418.e7. [CrossRef]
- Zong L, Tanaka-Yano M, Park B, Yanai H, Turhan FT, Croteau DL, Tian J, Fang EF, Bohr VA, Beerman I (2021) NAD+ augmentation with nicotinamide riboside improves lymphoid potential of Atm-/- and old mice HSCs. NPJ Aging Mech Dis 7(1):25. [CrossRef]
- Sun X, Cao B, Naval-Sanchez M, Pham T, Sun YBY, Williams B, Heazlewood SY, Deshpande N, Li J, Kraus F, Rae J, Nguyen Q, Yari H, Schröder J, Heazlewood CK, Fulton M, Hatwell-Humble J, Das Gupta K, Kapetanovic R, Chen X, Sweet MJ, Parton RG, Ryan MT, Polo JM, Nefzger CM, Nilsson SK (2021) Nicotinamide riboside attenuates age-associated metabolic and functional changes in hematopoietic stem cells. Nat Commun 12(1):2665. [CrossRef]
- Yoshino J, Mills KF, Yoon MJ, Imai S (2011) Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14(4):528–536. [CrossRef]
- Zapata-Pérez R, Tammaro A, Schomakers BV, Scantlebery AML, Denis S, Elfrink HL, Giroud-Gerbetant J, Cantó C, López-Leonardo C, McIntyre RL, van Weeghel M, Sánchez-Ferrer Á, Houtkooper RH (2021) Reduced nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice. FASEB J 35(4):e21456. [CrossRef]
- Freeberg KA, Craighead DH, Martens CR, You Z, Chonchol M, Seals DR (2022) Nicotinamide riboside supplementation for treating elevated systolic blood pressure and arterial stiffness in midlife and older adults. Front Cardiovasc Med 9:881703. [CrossRef]
- Acklin S, Sadhukhan R, Du W, Patra M, Cholia R, Xia F (2022) Nicotinamide riboside alleviates cisplatin-induced peripheral neuropathy via SIRT2 activation. Neurooncol Adv 4(1):vdac101. [CrossRef]
- Mukherjee S, Mo J, Paolella LM, Perry CE, Toth J, Hugo MM, Chu Q, Tong Q, Chellappa K, Baur JA (2021) SIRT3 is required for liver regeneration but not for the beneficial effect of nicotinamide riboside. JCI Insight 6(7):e147193. [CrossRef]
- Zhang Q, Liu X, Li N, Zhang J, Yang J, Bu P (2018) Sirtuin 3 deficiency aggravates contrast-induced acute kidney injury. J Transl Med 16(1):313. Erratum in: J Transl Med, 2022, 20(1):46. [CrossRef]
- Kang H, Park YK, Lee JY (2021) Nicotinamide riboside, an NAD+ precursor, attenuates inflammation and oxidative stress by activating sirtuin 1 in alcohol-stimulated macrophages. Lab Invest 101(9):1225–1237. [CrossRef]
- Seldeen KL, Shahini A, Thiyagarajan R, Redae Y, Leiker M, Rajabian N, Dynka A, Andreadis ST, Troen BR (2021) Short-term nicotinamide riboside treatment improves muscle quality and function in mice and increases cellular energetics and differentiating capacity of myogenic progenitors. Nutrition 87–88:111189. [CrossRef]
- Jensen JB, Dollerup OL, Møller AB, Billeskov TB, Dalbram E, Chubanava S, Damgaard MV, Dellinger RW, Trošt K, Moritz T, Ringgaard S, Møller N, Treebak JT, Farup J, Jessen N (2022) A randomized placebo-controlled trial of nicotinamide riboside and pterostilbene supplementation in experimental muscle injury in elderly individuals. JCI Insight 7(19):e158314. [CrossRef]
- Dolopikou CF, Kourtzidis IA, Margaritelis NV, Vrabas IS, Koidou I, Kyparos A, Theodorou AA, Paschalis V, Nikolaidis MG (2020) Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: a double-blind cross-over study. Eur J Nutr 59(2):505–515. [CrossRef]
- Grezella C, Fernandez-Rebollo E, Franzen J, Ventura Ferreira MS, Beier F, Wagner W (2018) Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res Ther 9(1):108. [CrossRef]
- Delabie W, Maes W, Devloo R, Van den Hauwe MR, Vanhoorelbeke K, Compernolle V, Feys HB (2020) The senotherapeutic nicotinamide riboside raises platelet nicotinamide adenine dinucleotide levels but cannot prevent storage lesion. Transfusion 60(1):165–174. [CrossRef]
- Harrison DE, Strong R, Reifsnyder P, Kumar N, Fernandez E, Flurkey K, Javors MA, Lopez-Cruzan M, Macchiarini F, Nelson JF, Markewych A, Bitto A, Sindler AL, Cortopassi G, Kavanagh K, Leng L, Bucala R, Rosenthal N, Salmon A, Stearns TM, Bogue M, Miller RA (2021) 17-α-Estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell 20(5):e13328. Erratum in: Aging Cell, 2022, 21(11):e13672. [CrossRef]
- Belenky PA, Moga TG, Brenner C (2008) Saccharomyces cerevisiae YOR071C encodes the high affinity nicotinamide riboside transporter Nrt1. J Biol Chem 283(13):8075–8079. [CrossRef]
- Orlandi I, Alberghina L, Vai M (2020) Nicotinamide, nicotinamide riboside and nicotinic acid-emerging roles in replicative and chronological aging in yeast. Biomolecules 10(4):604. [CrossRef]
- Odoh CK, Guo X, Arnone JT, Wang X, Zhao ZK (2022) The role of NAD and NAD precursors on longevity and lifespan modulation in the budding yeast, Saccharomyces cerevisiae. Biogerontology 23(2):169–199. [CrossRef]
- Belenky P, Christensen KC, Gazzaniga F, Pletnev AA, Brenner C (2009) Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. Quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism. J Biol Chem 284(1):158–164. Erratum in: J Biol Chem, 2009, 284(12):8208. [CrossRef]
- Braidy N, Poljak A, Grant R, Jayasena T, Mansour H, Chan-Ling T, Guillemin GJ, Smythe G, Sachdev P (2014) Mapping NAD+ metabolism in the brain of ageing Wistar rats: potential targets for influencing brain senescence. Biogerontology 15(2):177–198. [CrossRef]
- Verdin E (2015) NAD⁺ in aging, metabolism, and neurodegeneration. Science 350(6265):1208–1213. [CrossRef]
- Reiten OK, Wilvang MA, Mitchell SJ, Hu Z, Fang EF (2021) Preclinical and clinical evidence of NAD+ precursors in health, disease, and ageing. Mech Ageing Dev 199:111567. [CrossRef]
- Braidy N, Liu Y (2020) Can nicotinamide riboside protect against cognitive impairment? Curr Opin Clin Nutr Metab Care 23(6):413–420. [CrossRef]
- Birkisdóttir MB, van Galen I, Brandt RMC, Barnhoorn S, van Vliet N, van Dijk C, Nagarajah B, Imholz S, van Oostrom CT, Reiling E, Gyenis Á, Mastroberardino PG, Jaarsma D, van Steeg H, Hoeijmakers JHJ, Dollé MET, Vermeij WP (2022) The use of progeroid DNA repair-deficient mice for assessing anti-aging compounds, illustrating the benefits of nicotinamide riboside. Front Aging 3:1005322. Erratum in: Front Aging, 2022, 3:1086552. [CrossRef]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, Stevnsner TV, Mattson MP, Bohr VA (2018) NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A 115(8):E1876–E1885. [CrossRef]
- Xie X, Gao Y, Zeng M, Wang Y, Wei TF, Lu YB, Zhang WP (2019) Nicotinamide ribose ameliorates cognitive impairment of aged and Alzheimer’s disease model mice. Metab Brain Dis 34(1):353–366. [CrossRef]
- Larrick JW, Mendelsohn AR (2021) Modulation of cGAS-STING pathway by nicotinamide riboside in Alzheimer’s disease. Rejuvenation Res 24(5):397–402. [CrossRef]
- Roboon J, Hattori T, Ishii H, Takarada-Iemata M, Nguyen DT, Heer CD, O’Meally D, Brenner C, Yamamoto Y, Okamoto H, Higashida H, Hori O (2021) Inhibition of CD38 and supplementation of nicotinamide riboside ameliorate lipopolysaccharide-induced microglial and astrocytic neuroinflammation by increasing NAD. J Neurochem 158(2):311–327. [CrossRef]
- Chu X, Hou Y, Meng Q, Croteau DL, Wei Y, De S, Becker KG, Bohr VA (2022) Nicotinamide adenine dinucleotide supplementation drives gut microbiota variation in Alzheimer’s mouse model. Front Aging Neurosci 14:993615. [CrossRef]
- Ryu WI, Shen M, Lee Y, Healy RA, Bormann MK, Cohen BM, Sonntag KC (2022) Nicotinamide riboside and caffeine partially restore diminished NAD availability but not altered energy metabolism in Alzheimer’s disease. Aging Cell 21(7):e13658. [CrossRef]
- Yulug B, Altay O, Li X, Hanoglu L, Cankaya S, Lam S, Velioglu HA, Yang H, Coskun E, Idil E, Nogaylar R, Ozsimsek A, Bayram C, Bolat I, Oner S, Tozlu OO, Arslan ME, Hacimuftuoglu A, Yildirim S, Arif M, Shoaie S, Zhang C, Nielsen J, Turkez H, Borén J, Uhlén M, Mardinoglu A (2023) Combined metabolic activators improve cognitive functions in Alzheimer’s disease patients: a randomised, double-blinded, placebo-controlled phase-II trial. Transl Neurodegener 12(1):4. [CrossRef]
- Li CC, Chen WX, Wang J, Xia M, Jia ZC, Guo C, Tang XQ, Li MX, Yin Y, Liu X, Feng H (2020) Nicotinamide riboside rescues angiotensin II-induced cerebral small vessel disease in mice. CNS Neurosci Ther 26(4):438–447. [CrossRef]
- Schöndorf DC, Ivanyuk D, Baden P, Sanchez-Martinez A, De Cicco S, Yu C, Giunta I, Schwarz LK, Di Napoli G, Panagiotakopoulou V, Nestel S, Keatinge M, Pruszak J, Bandmann O, Heimrich B, Gasser T, Whitworth AJ, Deleidi M (2018) The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep 23(10):2976–2988. [CrossRef]
- Brakedal B, Dölle C, Riemer F, Ma Y, Nido GS, Skeie GO, Craven AR, Schwarzlmüller T, Brekke N, Diab J, Sverkeli L, Skjeie V, Varhaug K, Tysnes OB, Peng S, Haugarvoll K, Ziegler M, Grüner R, Eidelberg D, Tzoulis C (2022) The NADPARK study: a randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab 34(3):396–407.e6. [CrossRef]
- Vreones M, Mustapic M, Moaddel R, Pucha KA, Lovett J, Seals DR, Kapogiannis D, Martens CR (2023) Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin. Aging Cell 22(1):e13754. [CrossRef]
- Gaare JJ, Dölle C, Brakedal B, Brügger K, Haugarvoll K, Nido GS, Tzoulis C (2023) Nicotinamide riboside supplementation is not associated with altered methylation homeostasis in Parkinson’s disease. iScience 26(3):106278. [CrossRef]
- Pang N, Hu Q, Zhou Y, Xiao Y, Li W, Ding Y, Chen Y, Ye M, Pei L, Li Q, Gu Y, Sun Y, Fang EF, Chen M, Zhang Z, Yang L (2023) Nicotinamide adenine dinucleotide precursor suppresses hepatocellular cancer progression in mice. Nutrients 15(6):1447. [CrossRef]
- Park JM, Han YM, Lee HJ, Park YJ, Hahm KB (2021) Nicotinamide riboside vitamin B3 mitigated C26 adenocarcinoma-induced cancer cachexia. Front Pharmacol 12:665493. [CrossRef]
- Hamity MV, White SR, Blum C, Gibson-Corley KN, Hammond DL (2020) Nicotinamide riboside relieves Paclitaxel-induced peripheral neuropathy and enhances suppression of tumor growth in tumor-bearing rats. Pain 161(10):2364–2375. [CrossRef]
- Kahn B, Borrelli M, Libby T (2022) A narrative review of nicotinamide adenine dinucleotide (NAD)+ intermediates nicotinamide riboside and nicotinamide mononucleotide for keratinocyte carcinoma risk reduction. J Drugs Dermatol 21(10):1129–1132. [CrossRef]
- Conze DB, Crespo-Barreto J, Kruger CL (2016) Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum Exp Toxicol 35(11):1149–1160. Erratum in: Hum Exp Toxicol, 2018, 37(4):448. [CrossRef]
- Dellinger RW, Santos SR, Morris M, Evans M, Alminana D, Guarente L, Marcotulli E (2017) Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis 3:17. Erratum in: NPJ Aging Mech Dis, 2018, 4:8. [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck D, Castenmiller J, de Henauw S, Hirsch-Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Engel KH, Frenzel T, Heinonen M, Marchelli R, Neuhäuser-Berthold M, Pöting A, Poulsen M, Sanz Y, Schlatter JR, van Loveren Agnès de Sesmaisons-Lecarré H, Germini A, Knutsen HK (2019) Safety of nicotinamide riboside chloride as a novel food pursuant to Regulation (EU) 2015/2283 and bioavailability of nicotinamide from this source, in the context of Directive 2002/46/EC. EFSA J 17(8):e05775. [CrossRef]
- Marinescu AG, Chen J, Holmes HE, Guarente L, Mendes O, Morris M, Dellinger RW (2020) Safety assessment of high-purity, synthetic nicotinamide riboside (NR-E) in a 90-day repeated dose oral toxicity study, with a 28-day recovery arm. Int J Toxicol 39(4):307–320. Erratum in: Int J Toxicol, 2022, 41(1):47. [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Prieto Maradona M, Marchelli R, Neuhäuser-Berthold M, Poulsen M, Schlatter JR, van Loveren H, Albert O, de Sesmaisons Lecarré A, Knutsen HK (2021) Extension of use of nicotinamide riboside chloride as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J 19(11):e06843. [CrossRef]
- Sun P, Qie S, Pan B (2022) Nicotinamide riboside will play an important role in anti-aging therapy in humans, especially in the face skin anti-aging treatment. Aesthetic Plast Surg 46(Suppl 1):192–194. [CrossRef]
- Ito TK, Sato T, Hakamata A, Onoda Y, Sato S, Yamazaki F, Horikawa M, Takahashi Y, Kitamoto T, Suzuki M, Uchida S, Odagiri K, Setou M (2020) A nonrandomized study of single oral supplementation within the daily tolerable upper level of nicotinamide affects blood nicotinamide and NAD+ levels in healthy subjects. Transl Med Aging 4:45–54. [CrossRef]
- Ito TK, Sato T, Takanashi Y, Tamannaa Z, Kitamoto T, Odagiri K, Setou M (2021) A single oral supplementation of nicotinamide within the daily tolerable upper level increases blood NAD+ levels in healthy subjects. Transl Med Aging 5:43–51. [CrossRef]
- Turner J, Licollari A, Mihalcea E, Tan A (2021) Safety evaluation for Restorin® NMN, a NAD+ precursor. Front Pharmacol 12:749727. [CrossRef]
- Okabe K, Yaku K, Uchida Y, Fukamizu Y, Sato T, Sakurai T, Tobe K, Nakagawa T (2022) Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Front Nutr 9:868640. [CrossRef]
- Pencina K, Lavu S, Dos Santos M, Beleva YM, Cheng M, Livingston D, Bhasin S (2022) MIB-626, an oral formulation of a microcrystalline unique polymorph of β-nicotinamide mononucleotide, increases circulating nicotinamide adenine dinucleotide and its metabolome in middle-aged and older adults. J Gerontol A Biol Sci Med Sci Feb 19:glac049. [CrossRef]
- Trammell SAJ, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C (2016) Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7:12948. [CrossRef]
- Airhart SE, Shireman LM, Risler LJ, Anderson GD, Nagana Gowda GA, Raftery D, Tian R, Shen DD, O’Brien KD (2017) An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One 12(12):e0186459. [CrossRef]
- Campbell MTD, Jones DS, Andrews GP, Li S (2019) Understanding the physicochemical properties and degradation kinetics of nicotinamide riboside, a promising vitamin B3 nutritional supplement. Food Nutr Res 63:3419. [CrossRef]
- Yaku K, Palikhe S, Izumi H, Yoshida T, Hikosaka K, Hayat F, Karim M, Iqbal T, Nitta Y, Sato A, Migaud ME, Ishihara K, Mori H, Nakagawa T (2021) BST1 regulates nicotinamide riboside metabolism via its glycohydrolase and base-exchange activities. Nat Commun 12(1):6767. [CrossRef]
- Chellappa K, McReynolds MR, Lu W, Zeng X, Makarov M, Hayat F, Mukherjee S, Bhat YR, Lingala SR, Shima RT, Descamps HC, Cox T, Ji L, Jankowski C, Chu Q, Davidson SM, Thaiss CA, Migaud ME, Rabinowitz JD, Baur JA (2022) NAD precursors cycle between host tissues and the gut microbiome. Cell Metab 34(12):1947–1959.e5. [CrossRef]
- Preugschat F, Carter LH, Boros EE, Porter DJ, Stewart EL, Shewchuk LM (2014) A pre-steady state and steady state kinetic analysis of the N-ribosyl hydrolase activity of hCD157. Arch Biochem Biophys 564:156–163. [CrossRef]
- Kropotov A, Kulikova V, Nerinovski K, Yakimov A, Svetlova M, Solovjeva L, Sudnitsyna J, Migaud ME, Khodorkovskiy M, Ziegler M, Nikiforov A (2021) Equilibrative nucleoside transporters mediate the import of nicotinamide riboside and nicotinic acid riboside into human cells. Int J Mol Sci 22(3):1391. [CrossRef]
- Kropotov A, Kulikova V, Solovjeva L, Yakimov A, Nerinovski K, Svetlova M, Sudnitsyna J, Plusnina A, Antipova M, Khodorkovskiy M, Migaud ME, Gambaryan S, Ziegler M, Nikiforov A (2022) Purine nucleoside phosphorylase controls nicotinamide riboside metabolism in mammalian cells. J Biol Chem 298(12):102615. [CrossRef]
- Kulikova V, Shabalin K, Nerinovski K, Dölle C, Niere M, Yakimov A, Redpath P, Khodorkovskiy M, Migaud ME, Ziegler M, Nikiforov A (2015) Generation, release, and uptake of the NAD precursor nicotinic acid riboside by human cells. J Biol Chem 290(45):27124–27137. [CrossRef]
- Grant R, Berg J, Mestayer R, Braidy N, Bennett J, Broom S, Watson J (2019) A pilot study investigating changes in the human plasma and urine NAD+ metabolome during a 6 hour intravenous infusion of NAD. Front Aging Neurosci 11:257. [CrossRef]
- Zarei A, Khazdooz L, Madarshahian S, Enayati M, Mosleh I, Lin T, Yan B, Ufheil G, Wooster TJ, Abbaspourrad A (2021) Synthesis, stability, and bioavailability of nicotinamide riboside trioleate chloride. Nutrients 14(1):113. [CrossRef]
- Yang Y, Mohammed FS, Zhang N, Sauve AA (2019) Dihydronicotinamide riboside is a potent NAD+ concentration enhancer in vitro and in vivo. J Biol Chem 294(23):9295–9307. [CrossRef]
- Giroud-Gerbetant J, Joffraud M, Giner MP, Cercillieux A, Bartova S, Makarov MV, Zapata-Pérez R, Sánchez-García JL, Houtkooper RH, Migaud ME, Moco S, Canto C (2019) A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Mol Metab 30:192–202. [CrossRef]
- Ciarlo E, Joffraud M, Hayat F, Giner MP, Giroud-Gerbetant J, Sanchez-Garcia JL, Rumpler M, Moco S, Migaud ME, Cantó C (2022) Nicotinamide riboside and dihydronicotinic acid riboside synergistically increase intracellular NAD+ by generating dihydronicotinamide riboside. Nutrients 14(13):2752. [CrossRef]
- Makarov MV, Migaud ME (2019) Syntheses and chemical properties of β-nicotinamide riboside and its analogues and derivatives. Beilstein J Org Chem 15:401–430. [CrossRef]
- Neamţu AS, Biţă A, Scorei IR, Rău G, Bejenaru LE, Bejenaru C, Rogoveanu O, Oancea CN, Radu A, Pisoschi CG, Neamţu J, Mogoşanu GD (2020) Simultaneous quantitation of nicotinamide riboside and nicotinamide in dietary supplements via HPTLC–UV with confirmation by online HPTLC–ESI–MS. Acta Chromatogr 32(2):128–133. [CrossRef]
- Kim HJ, Benner SA (2017) Prebiotic stereoselective synthesis of purine and noncanonical pyrimidine nucleotide from nucleobases and phosphorylated carbohydrates. Proc Natl Acad Sci U S A 114(43):11315–11320. [CrossRef]
- Kim HJ, Benner SA (2018) A direct prebiotic synthesis of nicotinamide nucleotide. Chemistry 24(3):581–584. [CrossRef]
- Kim HJ, Benner SA, Scorei IR (2018) Synthesis and stabilization of nicotinamide ribose and its derivatives. United. States Patent and Trademark Office (USPTO), Provisional.
- Kim HJ, Furukawa Y, Kakegawa T, Bita A, Scorei R, Benner SA (2016) Evaporite borate-containing mineral ensembles make phosphate available and regiospecifically phosphorylate ribonucleosides: borate as a multifaceted problem solver in prebiotic chemistry. Angew Chemie Int Ed Engl 55(51):15816–15820. [CrossRef]
- Scorei R (2012) Is boron a prebiotic element? A mini-review of the essentiality of boron for the appearance of life on Earth. Orig Life Evol Biosph 42(1):3–17. [CrossRef]
- Nielsen FH (2018) Boron in aging and longevity. In: Malavolta M, Mocchegiani E (eds). Trace elements and minerals in health and longevity. Book Series “Healthy Ageing and Longevity (HAL)”, vol. 8, Springer, Cham, Switzerland, 163–177. [CrossRef]
- Hunter JM, Nemzer BV, Rangavajla N, Biţă A, Rogoveanu OC, Neamţu J, Scorei IR, Bejenaru LE, Rău G, Bejenaru C, Mogoşanu GD (2019) The fructoborates: part of a family of naturally occurring sugar–borate complexes – biochemistry, physiology, and impact on human health: a review. Biol Trace Elem Res 188(1):11–25. [CrossRef]
- Biţă A, Scorei IR, Bălşeanu TA, Ciocîlteu MV, Bejenaru C, Radu A, Bejenaru LE, Rău G, Mogoşanu GD, Neamţu J, Benner SA (2022) New insights into boron essentiality in humans and animals. Int J Mol Sci 23(16):9147. [CrossRef]
- Biţă A, Scorei IR, Rangavajla N, Bejenaru LE, Rău G, Bejenaru C, Ciocîlteu MV, Dincă L, Neamţu J, Bunaciu A, Rogoveanu OC, Pop MI, Mogoşanu GD (2023) Diester chlorogenoborate complex: a new naturally occurring boron-containing compound. Inorganics 11(3):112. [CrossRef]
- Mitruţ I, Scorei IR, Manolea HO, Biţă A, Mogoantă L, Neamţu J, Bejenaru LE, Ciocîlteu MV, Bejenaru C, Rău G, Mogoşanu GD (2022) Boron-containing compounds in Dentistry: a narrative review. Rom J Morphol Embryol 63(3):477–483. [CrossRef]
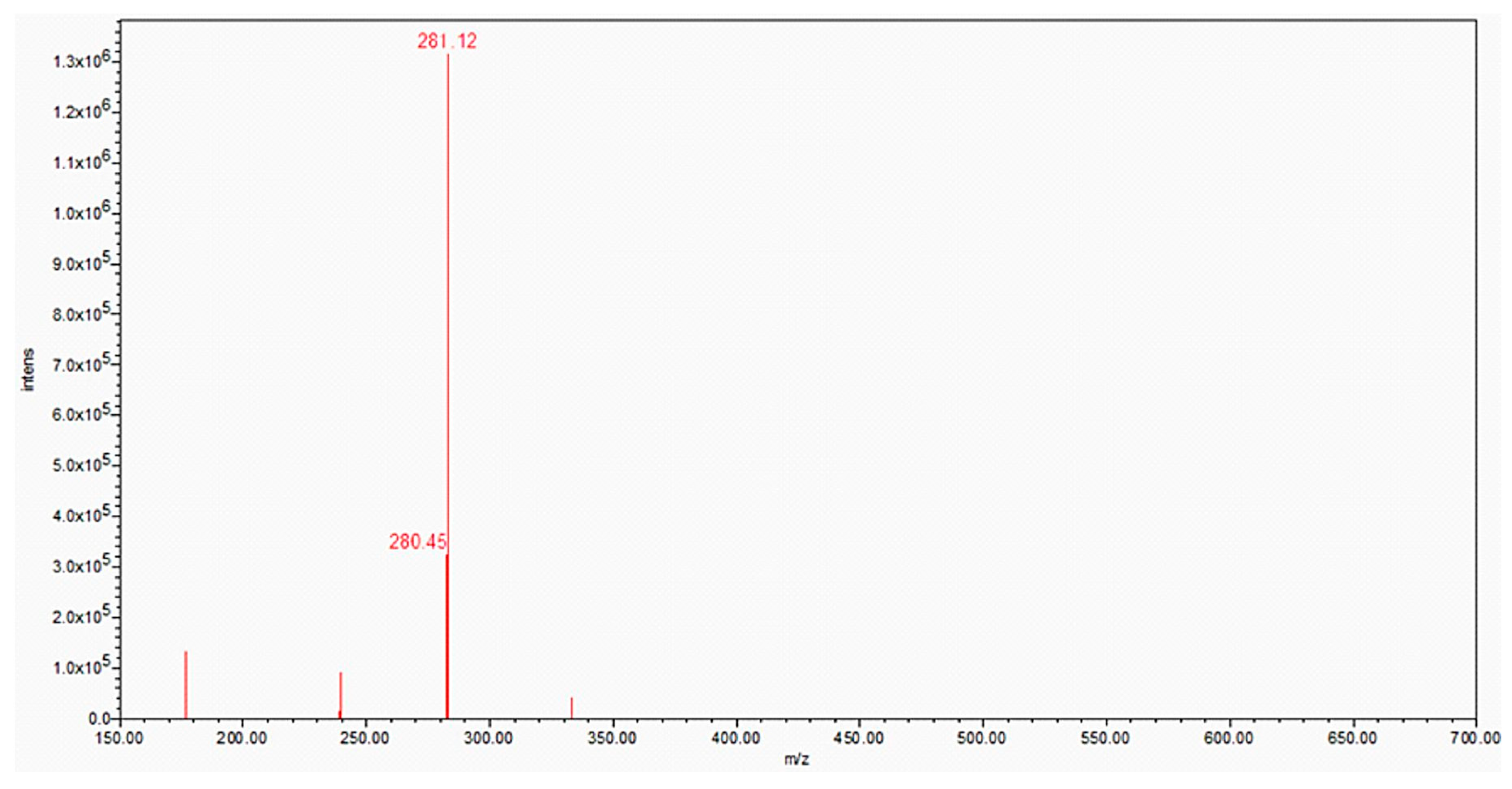
- Neamţu AS, Biţă A, Scorei IR, Mogoşanu GD, Pisoschi CG (2021) Solubility, stability and degradation kinetics of nicotinamide riboside borate, a vitamin B3 derivative, in physiological solutions. Fiziologia (Physiology) 31(2):33–42. Available online: http://revista_fiziologia.umft.ro/archives/Fiziologia_2021_31_2102_Final.pdf.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




