1. Introduction
One of the main challenges faced by industries is to design effective products that generate less stress to the environment and make better use of natural resources. A major part of industrial crop residues is constituted by the biomass that remains after using the valuable part of the plants, which can generate pollution to the environment and harm to human health. However, plant residue is not only an environmental problem, it is also an economic issue as these materials still contain chemicals that could be useful raw materials for new products [
1]. The industrial use of all organs of a plant is not common, which means that a large part of the biomass obtained at harvest is discarded without generating any resources. Although there are examples of the use of these residues for the manufacture of new products, a large amount of biomass containing bioactive chemical constituents remains as industrial waste. In summary, the development of new products from residues would have three main advantages i) obtaining raw material at a very low cost, ii) no needs to increase culture costs, iii) increasing the efficiency of the use of plant biomass, which is in line with the sustainable use of natural resources [
2].
Curcuma longa Linn is a plant species cultivated mainly due to the properties of its rhizome, which is a highly appreciated spice with pharmacological properties [
3,
4]. While extracts from the rhizome of
C. longa are widely used in health and beauty products, including shampoos, body creams, and toothpastes, its leaves are considered a by-product with no industrial use. However, the leaves of
C. longa are also source of an essential oil rich in bioactive substances (mainly monoterpenes and monoterpene alcohols) that could be used as raw material for new value-added products [
5,
6,
7]. Essential oils are mixtures of bioactive volatile natural products that can be used for pest control [
8,
9]. The anti-insect action of an essential oil is associated to the co-evolution between plants and phytophagous insects, which has exerted an evolutionary pressure for millennia leading to the development of a diversified chemical arsenal in plants. Essential oils and their constituents (usually terpenoids or phenylpropanoids) have shown potential for controlling
T. castaneum, which is a secondary pest of stored grains that generates huge losses to the agroindustry [
9]. In addition to grain consuming, which reduces their commercial value,
T. castaneum is a nomadic insect capable of carrying parasites that increases the risk of spreading resistance genes [
10,
11].
The high toxicity presented by synthetic pesticides used to control
T. castaneum (mainly phosphine and methyl bromide) have led to the search of more sustainable and environmentally friendly ways to protect stored grains [
12,
13,
14]. Natural substances with anti-insect action are green alternatives for pest control. Despite the insecticidal activity of traditional pesticides, the repellent activity of some natural products allows preventive control without using high concentrations that would be necessary to kill the insects. In addition, the use of such substances meets the increasing desire of consumers for ecologically sustainable products [
15].
Although the potential of using essential oils as anti-insect agents is already well-reported, some difficulties (e.g. water solubility and prolonged stability) impair development of a final product. The essential oil of
C. longa leaves, for example, had its potential as an insecticide against
T. castaneum demonstrated 20 years ago [
16]. However, there were no advances to transform the oil into a raw material for a viable biotechnological product. In this context, the use of nanotechnology has been considered effective in overcoming the difficulties related to the development of bioproducts based on essential oils. Oil-in-water nano-emulsions are colloidal systems with size diameter below 300 nm in which the oil is homogeneously dispersed in water [
17]. Some advantages can be achieved by nano-emulsifying an essential oil, such as enhanced physical and chemical stability, protection against volatiles loss and improved bioactivity. These properties make nano-emulsification a promising strategy for the development of effective anti-insect agents.
The aim of the present study was to produce and evaluate the repellent action against T. castaneum of nano-emulsions containing the essential oil of C. longa leaves and its three main constituents.
2. Results and Discussion
2.1. Chemical Composition of the Essential Oil
Any biological activity is due to chemical and physical-chemical interactions between the active molecules and molecules present in the biological target. Thus, it is essential to know in depth the chemical composition of tested extracts. However, plants can vary their metabolism due to environmental factors and, therefore, increase or reduce the biosynthesis of certain metabolites. For this reason, in this work collections and extractions were carried out every quarter of the year in order to have a representative sample of the annual variation in the chemical composition of the essential oil of
Curcuma longa leaves. The complete chemical composition of the essential oils, including the representative mixture, is presented in
Table 1.
The average yield of the hydrodistillations was 0.27 ± 0.05% (w/w). The essential oils obtained were mainly composed of monoterpenes with smaller percentages of oxygenated monoterpenes, with α-phallandrene, p-cymene, 1,8-cineole and terpinolene being the most abundant. The oils were pooled in equal amounts, generating a representative mixture whose major constituents were p-cymene (26.0%), terpinolene (15.5%) and 1,8-cineole (15.1%). The results obtained corroborate data described in previous studies that suggest that there are differences between the essential oils of leaves and rhizomes of
C. longa. Although it is well described in the literature that rhizome oil is rich in turmerones (sesquiterpene ketones), such metabolites are not commonly found in leaf oil [
6,
27]. This indicates that extracting essential oil from leaves is not just a way to get more of the same oil from the rhizome, but rather a source of an oil with potentially different biological activity and mechanisms of action.
2.2. Preparation and Characterization of Nano-Emulsions
The nano-emulsification of oils in water can be achieved by several methodologies, including some with heating. The methodology to be chosen must consider the characteristics and limitations of the samples. As essential oils and their chemical constituents are volatile, in this work a series of nano-emulsions was prepared by a solvent-free and low-energy method without heating, in order to avoid losses by evaporation.
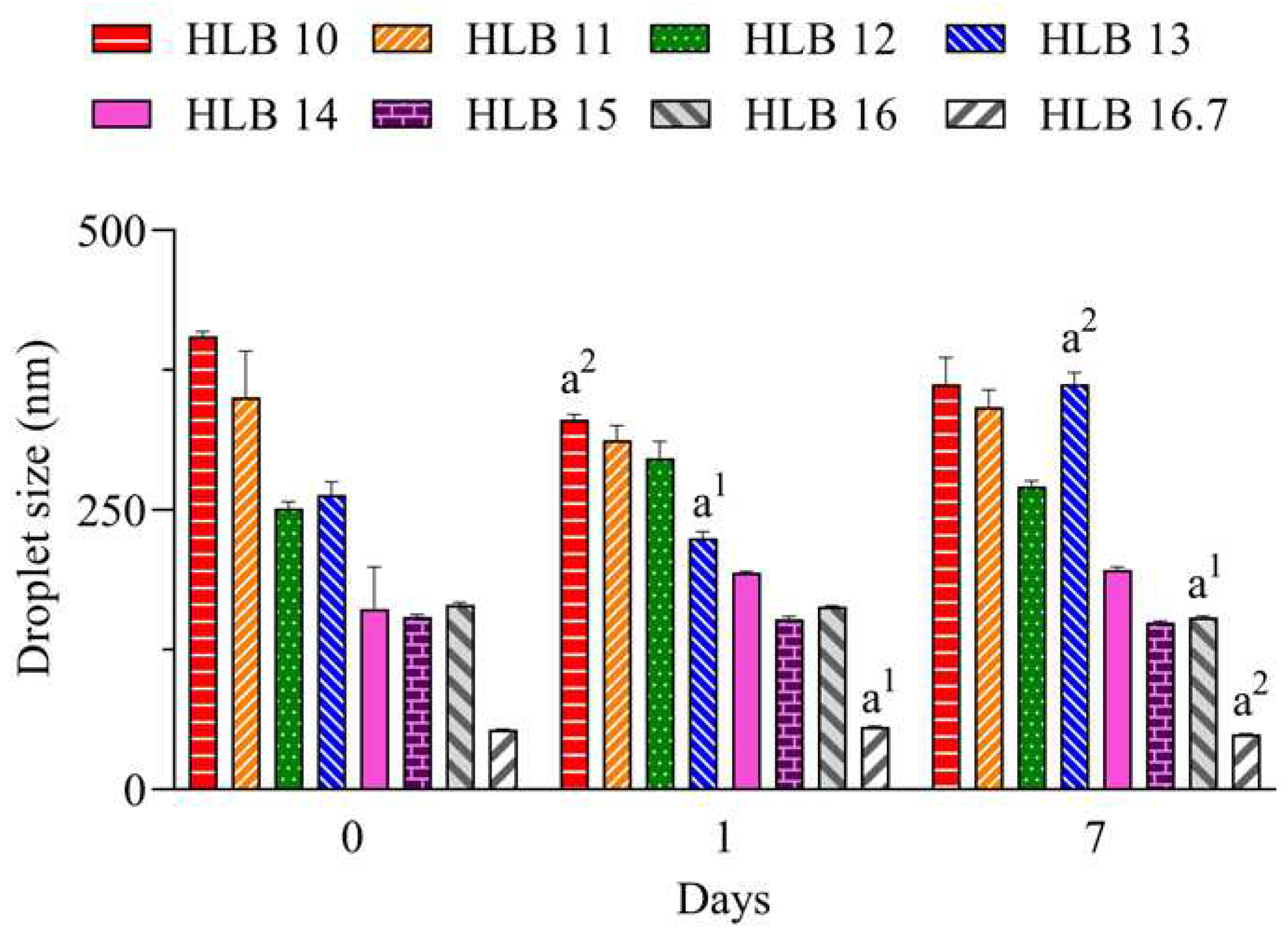
In
Figure 1 it can be seen that the nano-emulsions prepared with
C. longa essential oil and surfactants at HLB 14, 15, 16 and 16.7 presented droplet size below 200 nm during all evaluated period. At highest HLB value, which was reached solely with polysorbate 20, lower size (~ 55 nm) was observed. The hydrodynamic size is analyzed by dynamic light scattering based on the Brownian movement of droplets and overall a size between 20 and 300 nm can be used to define a nano-emulsion [
17].
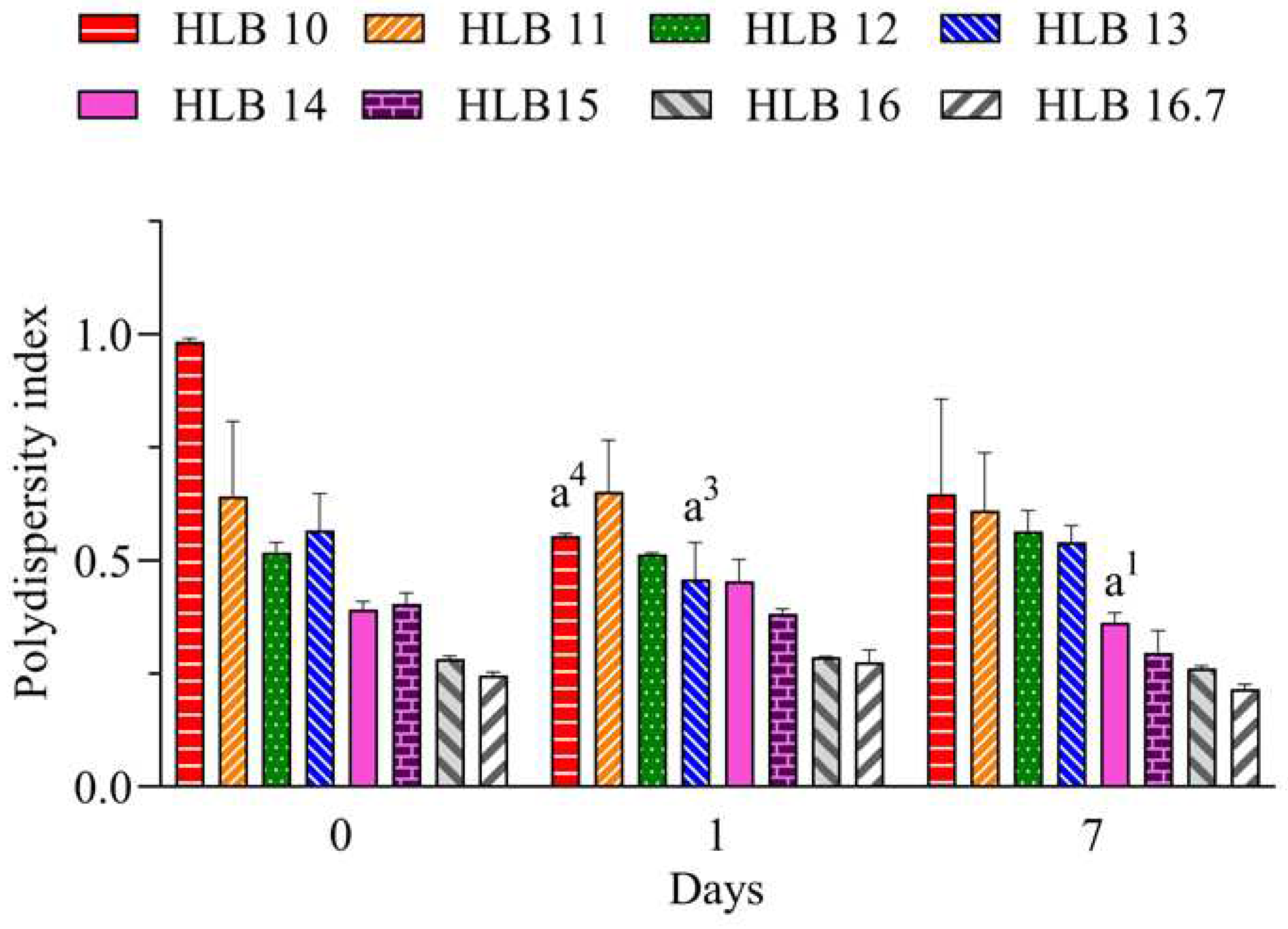
Polydispersity index (PdI) is related to the uniformity of droplets distribution. It can range from 0 to 1, where lower values (< 0.6) have a tendency for more monodisperse distribution and increasing values (> 0.7) have a tendency for polydisperse distribution, which is associated to large droplets or aggregated droplets [
28]. Values below 0.25 suggest a narrow distribution that also can contribute to a long-term stability of nano-emulsions [
29]. Therefore, considering the results for the nano-emulsions prepared with EO sowed in
Figure 2, lowest PdI values are attributable to those prepared at HLB 16.7. Therefore, considering the better performance of the system prepared only with polysorbate 20, it can be suggested that the required HLB (rHLB) of the essential oil is 16.7.
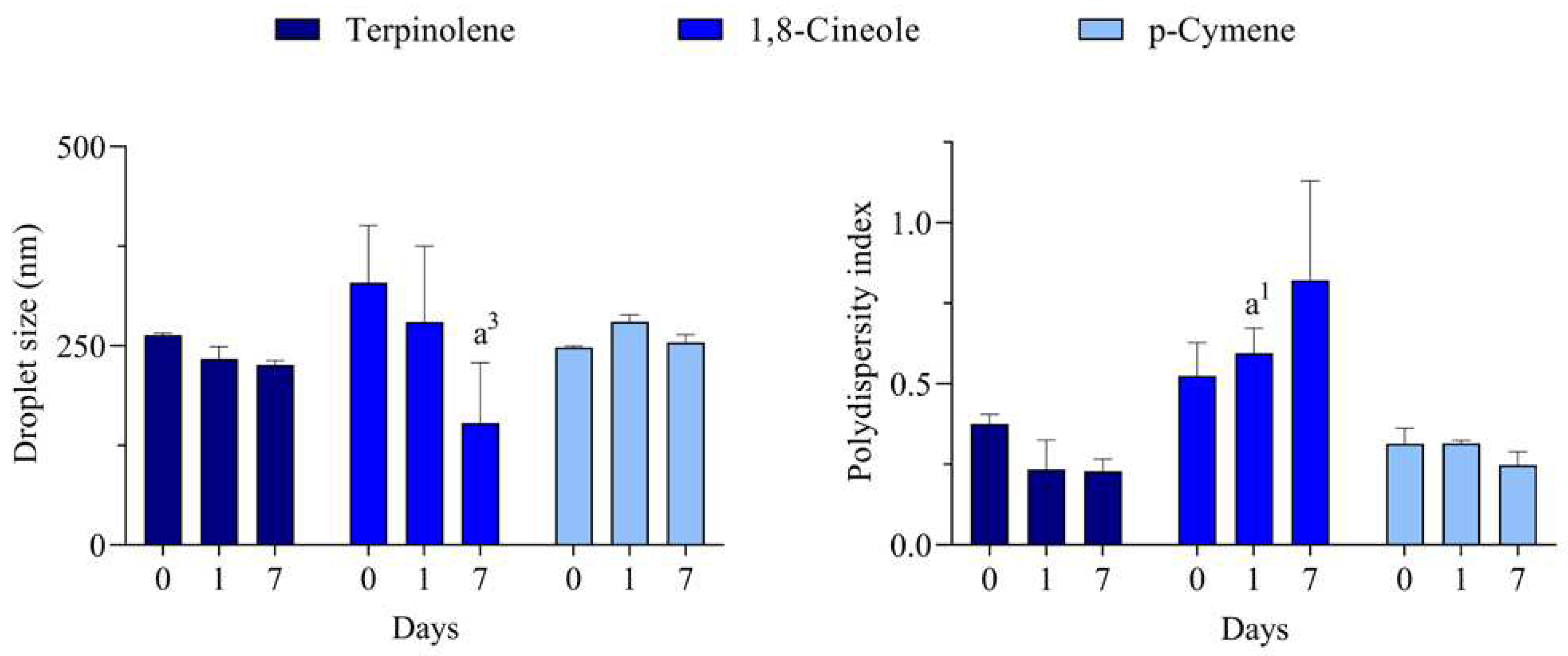
For all monoterpenes, the most transparent/translucent systems at all days were reached with polysorbate 80. An aspect more transparent was observed for terpinolene and p-cymene, while the 1,8-cineole is slightly more translucent. Due to the macroscopical evaluation, in which most of the system at the wide range of HLB were opaque, the DLS analysis was carried out only for the polysorbate 80-based systems and the results are presented in
Figure 3.
Unlike some processes performed under high energy input, the low energy emulsification method used in this work only generates nano-emulsions when the conditions are most appropriate. According to the HLB concept proposed by Griffin [
30], the best emulsions are formed when using a surfactant (or a mixture of them) in which the balance of the size and strength of the hydrophilic and lipophilic moieties of its molecule is the most suitable for emulsifying a given material. Therefore, it can be suggested that the rHLB of these monoterpenes is 15, which meets the HLB of the best surfactant used in the series.
Despite nano-emulsions are thermodynamically unstable systems due to unfavorable intermolecular interactions that occur in the oil/water interface, they can reach kinetic stability [
31]. A known mechanism for physical destabilization of nano-emulsions is the Ostwald ripening [
28]. This mechanism originates in the release of some partially soluble compounds by small droplets. These compounds pass through the external phase and are incorporated into large droplets in a continuous process that leads to complete destabilization. However, some complex oils have an alternative stabilizing mechanism, which is called Compositional Ripening. It was reported for essential oil-based nano-emulsions and relies on a tendency for maintenance of droplet composition [
32]. In this context, the presence of several compounds in the complex
C. longa essential oil may be contributing to the better system reached at HLB 16.5, since one would expect that a high HLB surfactant might also induce undesirable solubilization of compounds that might lead to Ostwald ripening. In the other hand, destabilization of nano-emulsions with pure terpenes would be triggered by this mechanism and therefore, enough stable systems could be only reach with polysorbate 80 (HLB 15), which slightly less hydrophilic than polysorbate 20 (HLB 16.7).
2.3. Stability of Essential Oil Nano-Emulsion
Although nano-emulsification increases the kinetic stability of oil-in-water mixtures, the system remains thermodynamically unstable with a tendency to break down over time. Therefore, it is important to verify the stability of the produced nano-emulsions.
A new nano-emulsion was prepared with the essential oil to evaluate its stability. As can be seen in
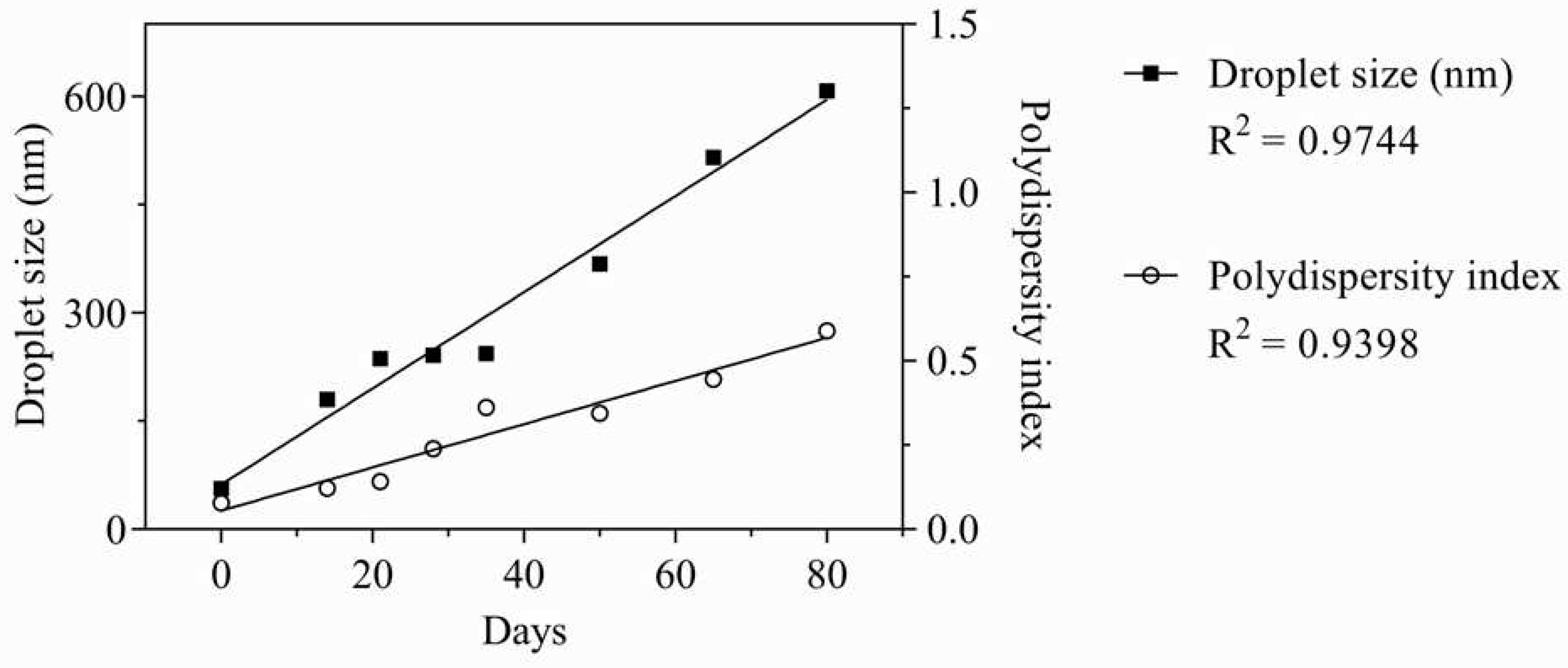
Table 2, the droplet size reduced significantly up to the day 21. After remaining stable, the droplets decreased again steadily from the day 50 until the end of the experiment on the day 80. The polydispersity index data corroborate the hypothesis that there was a growing disorganization of the nano-emulsion system. As with droplet size, the polydispersity index varied gradually during the experiment.
After the analysis of variance (ANOVA) described in
Table 2, the data were subjected to a subsequent analysis of linear regression to test the linearity between the physicochemical properties and time. Both droplet size and polydispersity index varied linearly with time (R
2 = 0.9744 and 0.9398, respectively), as shown in
Figure 4.
Due to their thermodynamic instability, it is already expected that the physicochemical properties of nano-emulsions change as a function of time. Thus, the greatest importance of a stability study of these products is to verify the time in which they maintain their physicochemical properties within the limits used to define a nano-emulsion.
Authors use different droplet size ranges to define whether an emulsion can be considered a nano-emulsion. According to the threshold value of 300 nm used in this work, the formulation can be considered a nano-emulsion up to the day 35. On the other hand, if the broader limit of 600 nm, adopted by some other authors, is used, the formulation would still be a nano-emulsion on the day 80. However, even with the gradual decay of its physicochemical properties, the nano-emulsion did not show visual signs of instability such as cremation or phase separation. This shows that the emulsion remained intact despite being outside the nanometric scale.
There is no consensus on the amount of time considered adequate for studies on the stability of nano-emulsions. Thus, there is great variation in the analysis times of the physicochemical properties of the nano-emulsions. While some studies track formulations for months, others publish only analysis results of freshly prepared emulsions. [
18,
33]. The data obtained in this work show good stability for this first nano-emulsion of the essential oil of
C. longa leaves.
2.4. Repellency Assay
Essential oils, as well as many other plant metabolites, are products of millenary co-evolution between plant species and their natural enemies [
34]. This is one of the reasons why essential oils and their constituents are known antimicrobial and anti-insect agents, acting as larvicide, insecticide or repellent against the latter [
8]. Volatile substances also have a great advantage over non-volatile metabolites, such as alkaloids and polyphenols: they can spread in the air and prevent damage to the plant, acting as protective agents. This property is very useful for the integrated management of pests, in which it is intended to avoid the degradation of plants of commercial interest causing the least possible damage to the environment and human populations. In this context, essential oils are good candidates for raw material for the development of biochemical biopesticides, which are defined by the United States Environmental Protection Agency (EPA) as naturally occurring substances that control pests by non-toxic mechanisms.
The oil from the leaves of
C. longa was chosen for this nanobiotechnological study because it has already been shown to be a lethal agent for adults and larvae of
T. castaneum [
16]. Logically, lethal agents can be good candidates for repellent agents if used at lower concentrations. However, essential oils are hydrophobic, which requires them to be dissolved in toxic organic solvents. Nano-emulsification is a strategy to produce oil-in-water mixtures with significant kinetic stability. Furthermore, nano-emulsions act as controlled release systems for bioactive compounds. For these reasons, the nano-emulsions of oil and its three main constituents had their repellent activities evaluated.
To classify the repellent activity, not only the repellency percentage value was used, but the statistical criterion previously described by Lima and coworkers, in which samples was only considered repellent when positive repellency percentage values are significantly different from the maximum value of those considered inactive (0.1%) [
18]. The results are described in
Table 3.
Although the repellent activity classification of some samples changed between 2 and 4 hours, there were no significant changes between the repellency values observed in the first and second readings (p > 0.05). This indicates that the nano-emulsions quickly reached maximum repellency and managed to keep it unchanged until the end of the experiment.
The essential oil showed repellent activity up to a concentration of 11 μg/cm
2, which is in accordance with those of samples considered significantly active in previous works [
36,
37]. These results can be explained by the chemical composition of the essential oil, which is rich in substances known to be active against
T. castaneum. The action of monoterpenes against insects is widely known [
38]. The three most abundant compounds in the essential oil, terpinolene, 1,8-cineole and p-cymene, have previously demonstrated repellent activity against
T. castaneum. In fact, as can be seen in
Table 1, approximately 71% of the representative mixture of the oil is composed of repellent compounds. However, this is the first time that nano-emulsions of terpinolene, 1,8-cineole and p-cymene were tested against this insect.
The p-cymene and terpinolene nano-emulsions showed repellent activity at the same concentrations as the essential oil. The 1,8-cineole nano-emulsion maintained its repellent activity even at the lowest tested concentration (1.1 μg/cm
2). The literature shows that 1,8-cineol is a monoterpenoid with high repellent activity, which was also verified for its nano-emulsion in this work [
22].
2.5. Ecotoxicity Assay
Pollution associated to pesticide accumulation is an emergent global problem [
39]. Even low-toxic products applied in low concentrations can be leached by rain and accumulate in aquatic environments, causing pollution and environmental imbalance. Ecotoxicity tests are useful tools to assess the effects of synthetic or natural pesticides that enter the environment. Algae, such as
Chlorella vulgaris, are important bioindicators as they form the basis of many aquatic food chains and are sensitive to the presence of pollutants in water [
40].
The ecotoxicity test was based on comparing the cell density of
C. vulgaris in the presence or absence of the essential oil nano-emulsion. The lowest active concentration of the nano-emulsion against
T. castaneum was chosen as the basis for the ecotoxicity test. However, as the repellency test takes place on a two-dimensional surface, a calculation was necessary to define a proportional concentration in the three-dimensional aquatic model of ecotoxicity. Considering that in the concentration of 11 μg/cm
2 there are 3.31 μg for each spatial dimension, the proportional three-dimensional concentration used was 36.5 μg/cm
3. The results obtained are described in
Table 4.
There were no significant differences between the cell densities in the groups on any of the days (p > 0.05). In addition, the comparison of the area under the curve values showed that there were no significant differences between the total amounts of cells found in both groups throughout the experiment. The results indicate that the nano-emulsion has low potential to cause environmental impacts to water ecosystems.
2.6. Molecular Docking
The bioactivity of compounds is ultimately the result of physicochemical interactions between their molecules with key molecules in target organisms. In silico molecular docking analysis allows the evaluation of ligand-receptor interactions, helping to understand the activity presented in in vivo or in vitro assays. Telomerase is a ribonucleoprotein responsible for replicating the ends of chromosomes and maintaining the genome in its integrity, basically consisting of a reverse transcriptase, or catalytic unit, and a non-coding RNA molecule [
41,
42,
43]. Its inhibition implies the shortening of the telomere sequence, which triggers several effects, such as senescence or apoptosis of several cells [
41].
The RMSD value obtained in the target validation step (PDB ID: 5CQG) was 0.678 Å. RMSD values below 2 Å are more accepted, since it indicates that a correct fit of the ligand occurred in a favorable spatial orientation in the protein [
44]. The origin coordinates obtained in validation for this target were: x = 22.08; y = 7.22; z = -31.75, at a radius it was 10 Å.
According to the coordinates obtained in the validation, the binding site was defined to start the docking with the molecules under study (terpinolene, 1,8-cineole and p- cymene). From this it was possible to evaluate the interactions, types of interactions, the distances of each one and the amino acid residues involved in the interactions and which atom or region each one is interacting with. The results are shown in
Table 5.
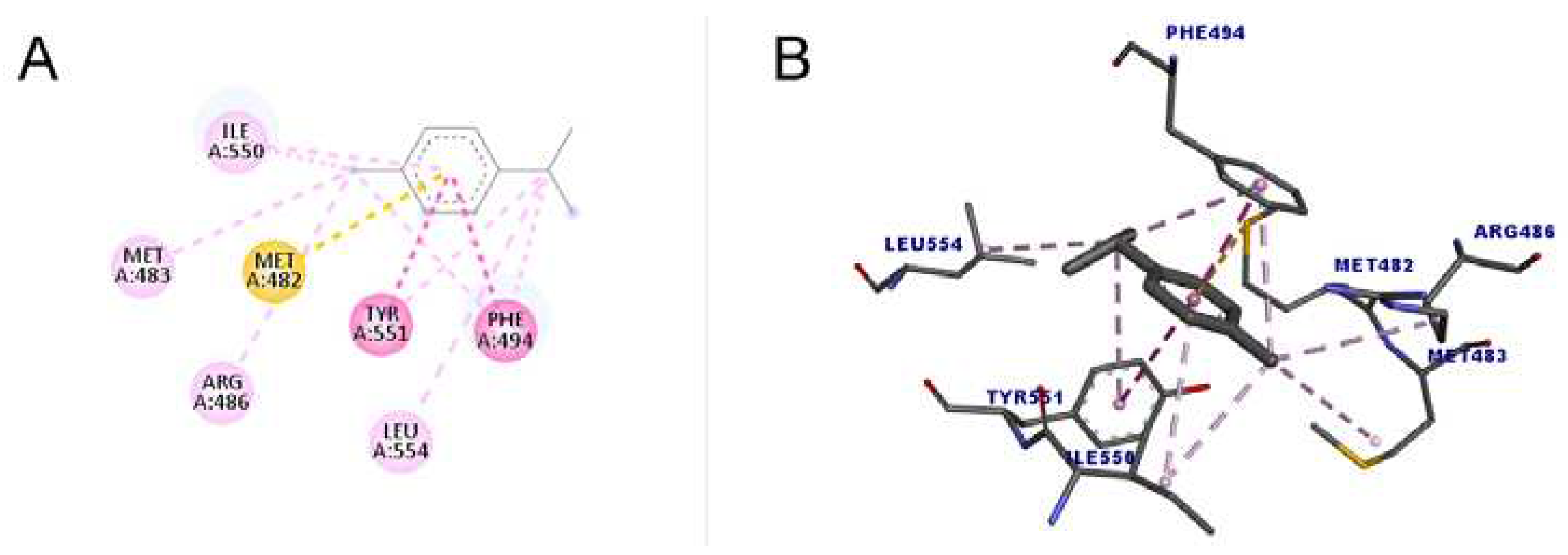
Figure 5 shows the docking between 1,8-cineole and the enzyme telomerase. A total of six interactions occurred, all of which were hydrophobic of the alkyl type, that is, interactions that occur between alkyl groups. It is noteworthy that of the six bonds, four were interactions with the amino acid LEU554. The docking Score value was 34.01.
As shown in
Figure 6, p-cymene performed better compared to 1,8-cineole both in number of interactions and in Score value (48.76). All interactions were hydrophobic: one pi-sulfur, one pi-pi stacked, one pi-pi t-shaped, four alkyl and four pi-alkyl, totaling eleven interactions. The pi-sulfur interaction occurred between the residue Met482 with the aromatic group of p-cymene, of order sp
2. The pi-pi connection stacked is a type of interaction that occurs between aromatic groups, containing a pi orbital, which was detected between the Phe494 residue and the ligand. As for the pi-pi T- shaped type, which occurred with Tyr551, it is an interaction that also occurs in cyclic systems, with double bonds, which form a specific angle of each ring, in a spatial configuration that has the T format [
45].
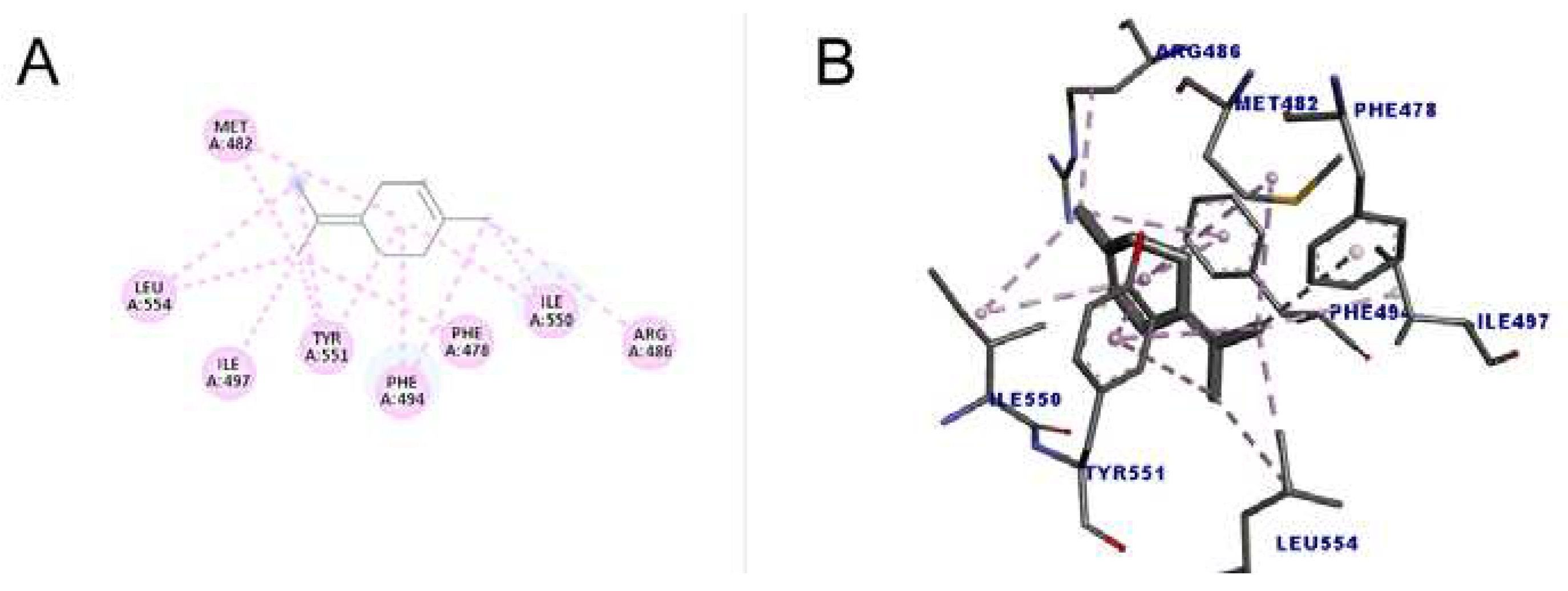
The molecule with the highest number of interactions and the highest Score (49.15) was terpinolene (1-methyl-4-propan-2-ylidenecyclohexene), which performed 14 interactions with the amino acid residues, of two types, alkyl and pi-alkyl interaction, indicating that due to these characteristics it may have a greater inhibitory action when compared to other substances. The results are shown in
Figure 7.
In a previous study that performed docking between telomerase obtained from
T. castaneum and a low molecular weight inhibitor (BIBR1532), it was possible to identify the region where the ligand is located and which inhibits telomerase activity [
46]. The result of this study demonstrated that the ligand was located in the RNA binding domain (TRBD). The presence of residues Phe478, Val491, Tyr551 and Leu554 in this region allowed greater stability in the orientation of the ligand around the site, from the formation of the called hydrophobic “pocket”. In addition to these amino acids, other residues line the inside of this pocket, such as Met482, Met483, lle497, Trp449, Ile550. Even though the BIBR1532 molecule did not induced a marked change in conformation, these interactions allowed a more favorable and firm position of the ligand in the site, causing an inhibitory action on the enzyme.
According to the results obtained in this work, it can be inferred that the tested monoterpenes are capable of interacting with amino acid residues similar to those of a known telomerase inhibitor (BIBR1532), which suggests that these structures may have potential inhibitory activity on the enzyme from T. castaneum.
3. Materials and Methods
3.1. Plant Material
Curcuma longa leaves were collected in the city of Macapá, state of Amapá, Brazil (0° 01’24’’N 51° 09’31’’W) in February, May, August and November 2020. A voucher specimen was used for the botanical identification and deposited in the herbarium of the Instituto de Pesquisas Científicas e Tecnológicas do Estado do Amapá (IEPA) under the registration code 019430.
3.2. Chemicals
Analytical grade acetone was purchased from Sigma-Aldrich (Brazil), terpinolene (≥95%, purity) and p-cymene (≥97% purity) were purchased from Merk (Brazil). 1,8-cineole (97% purity) was purchased from Quinarí (Brazil). Surfactants sorbitan monooleate, polysorbate 80, and polysorbate 20 were obtained from Sigma-Aldrich (Brazil). All other chemicals were obtained from Synth (Brazil).
3.3. Extraction and Determination of the Chemical Composition of the Essential Oil
The fresh leaves (700 g) were ground in a blender with distilled water (3 L) and transferred to a 5 L flask connected to a Clevenger-type apparatus containing a water-cooled receiver to avoid evaporation losses. After the extractions, the essential oils from different months were centrifuged, dried with anhydrous sodium sulfate and stored at -17 ± 1 °C. They were individually analyzed and used for the preparation of a representative mixture (25% of each oil) for nano-emulsion preparation and biological assay.
Qualitative gas chromatography (GC) analysis was performed by using a GC-MS-QP2010 gas chromatograph equipped with a ZB-5 capillary column (i.d.=0.25 mm, length 30 m, film thickness = 0.25 µm) and coupled to a mass spectrometer (MS) (Shimadzu, Barueri, SP, Brazil) using electron ionization at 70 eV (1 scan/s). The column temperature was programmed as follows: 60 °C for 5 min, and increased at 3 °C/min until 290 °C. Sample was diluted in chloroform (1:100, v/v), injected into the column and carried by helium at a flow rate of 1 mL/min (split injection ratio 1:40). The retention indices were calculated by the interpolation of each substance retention time and the retention time of a mixture of aliphatic hydrocarbons analyzed in the same conditions [
47]. The identification of the essential oil constituents was performed by comparison of their arithmetic indices and mass spectra with literature data [
26]. Quantitative analysis of the chemical constituents was performed by peak area normalization method using a GC coupled to flamed ionization detector (FID) (Shimadzu, Barueri, SP. Brazil). Conditions were the same of the GC-MS analysis with the exception of the carrier gas, which was hydrogen in the GC-FID.
3.4. Determination of Required Hydrophile-Lipophile Balance (rHLB) of C. longa Essential Oil and Its Major Constituents
A series of emulsions containing the representative mixture of the essential oils of C. longa and its main constituents terpinolene, 1,8-cineole and p-cymene were prepared at various HLB values (10 to 16.7) by using the non-ionic surfactants sorbitan monooleate (HLB 4.3), polysorbate 80 (HLB 15.0) or polysorbate 20 (HLB 16.7). The resulting HLB values were obtained by performing binary mixtures of surfactants in proportions determined by the equation rHLB = [(HLBA × mA) + (HLBB × mB)] / (mA + mB), in which HLBA and HLBB represent the HLB values of each surfactant. The mass (g) of each surfactant is represented by mA and mB.
The required hydrophile-lipophile balance (rHLB) value of each oil was determined as the HLB value capable of producing the nano-emulsion with the best macroscopic and physicochemical properties, remaining stable for 7 days.
3.5. Preparation of the Nano-Emulsions
The nano-emulsions were produced by a low-energy method. First, the essential oil or its major constituents were mixed with surfactant at specific rHLB, affording the oily phase (2.5%, w/w). Distilled water was added dropwise under continuous stirring until a final mass of 2.0 g.
3.6. Characterization of the Nano-Emulsions
The nano-emulsions had their droplet size distribution evaluated by dynamic light scattering (DLS) at 90° using the Nano ZS. (Malvern Instruments. Malvern. UK) equipped with a 10 mW “red” laser (X = 632.8 nm). Each nano-emulsion was analyzed immediately after preparation (day 0) and after 1 and 7 days of stored at room temperature. All measurements were performed in triplicate. Data was expressed as the mean ± standard deviation. Additionally, the essential oil nano-emulsion was submitted to a stability analysis being analyzed on days 0, 14, 21, 28, 35, 50, 65 and 80.
3.7. Insects
Adults of T. castaneum with about 7 days of age were used in the experiments. The insects were reared and maintained in the Arthropod Laboratory of the Federal University of Amapá with controlled temperature (28-30° C) and relative humidity (70-80%). The insects were reared in a growth chamber at 28 °C, 60% relative humidity, and fed wheat flour with 12-13% humidity mixed with yeast (10:1, w/w).
3.8. Repellency Assay
The repellency contact assay was carried out according to [
18]. Whatman filter papers with size diameter of 8.5 cm were sub-divided through a line into two equal sections identified as “test” and “control”.
Then, the filter papers were placed on Petri dishes and impregnated with the samples at the following concentrations: 176; 88; 44; 11 and 1.1 µg/cm
2 (n = 5). For the control, formulations were prepared under the same conditions as the nano-emulsions, but without the addition of essential oil or terpenes. After 10 min, twenty
T. castaneum were placed around the center of the plates, which were immediately covered with a fabric that allowed the exchange of vapors. After 2 h and 4 h the individuals at test section were counted and the percentage of repellency was calculated as follows:
Where: PR= percentage of repellency; C = number of insects in the control section; T = number of insects in the test section.
The repellent action of the five concentrations was classified according to the respective PR: class 0 (-0.1% to 0.1%), class I (0.1 to 20%), class II (20.1 to 40%), class III (40.1 to 60%), class I (60.1 to 80 %) or class V (80.1 to 100%) [
35]. Sample concentrations with mean PR value below -0.1 were considered attractive if statistically different from 0.1%, while those with mean PR above 0.1% were considered repellent if statistically different from -0.1%. Absence of statistically significant differences within these limit values were considered inactive.
3.9. Acute Toxicity against Chlorella vulgaris
Chlorella vulgaris was reared on the Laboratory of Algae Cultivation (Federal University of Amapá). Overall conditions of cultivation of the green algae were: aqueous solution of nitrogen/phosphorus/potassium (NPK, 15:05:05), constant photoperiod (2,000 Lux) and temperature of 24 ± 1 ºC.
The samples were solubilized in 50 mL of the medium at a concentration of 36.5 μg/cm3. The culture media of the control and test groups were prepared with the same cell density (approximately 30000 cells/mL). After 1, 2 and 3 days the cell densities were measured for the control (non-treated) and samples in quintuplicate.
The cells were counted in a Neubauer chamber coupled to an optical microscope Quimis® (BA410) with addition of lugol for better visualization. Cell growth inhibition was determined by comparing cell density values between test and control groups.
3.10. Molecular Docking
Terpinolene, 1,8-cineole and p-cymene ligands were optimized in the HyperChem software using the semi-empirical method of the RM1 type (Recife model 1) [
48]. The GOLD 2020.1 program (Genetic Optimization for Ligand Docking) was used to predict the best interactions between the ligand and the target [
49].
The crystal structure of the telomerase protein of the organism
T. castaneum was obtained from Protein Data Bank (PDB) database (PDB ID: 5CQG, 2.30 Å resolution, complexed with BIBR1532 inhibitor) [
46].
3.11. Statistical Analysis
Analysis of variance (ANOVA) followed by Tukey’s test and linear regression were conducted using the Software GraphPad Prism 8.4.3 (San Diego, CA, USA). Differences were considered significant when p < 0.05.
4. Conclusions
The preparation of nano-emulsions containing the essential oil from leaves of Curcuma longa by methods with low energy input and without heating proved again to be a valid strategy to prepare stable and effective oil-in-water systems. The repellent nano-emulsions containing the essential oil of C. longa leaves proved to be a green alternative to synthetic pesticides, as it was safe against the bioindicator Chlorella vulgaris. Furthermore, the leaves of C. longa are by-products of obtaining the rhizome, which makes it an abundant material, with low cost and low environmental impact. The analysis of the chemical composition of the essential oil allowed the study of the isolated action of each of its major constituents. Terpinene, 1,8-cineole and p-cymene nano-emulsions were repellent at the same concentrations as the oil, with 1,8-cineole being active at an even lower concentration. The action of the major monoterpenes against T. castaneum may be, at least in part, related to their ability to interact with that insect's telomerase enzyme, especially terpinolene, which presented better results when compared to the other two compounds. The promising application in controlling T. castaneum by nano-emulsions from leaves of C. longa requires further detailed studies.
Author Contributions
Investigation: BF-R, MDNGJr, ACJS, HFS and FPM; Resources: JCTC and LR; Methodology: SMMF, CPF and LISH-M; Original Draft and supervision: CPF and GSB; Conceptualization, project administration, funding acquisition, formal analysis, writing - review & editing: RASC.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Programa Demanda Social, Programa de Desenvolvimento da Pós-Graduação (PDPG) e PROCAD-Amazônia. The APC was funded by the Federal University of Amapá.
Acknowledgments
The authors would like to thank Partal Periódicos Capes for providing access to the full texts of the articles used as reference in this work, and also the Fundação de Amparo à Pesquisa do Amapá (FAPEAP) for helping to raise funds from CAPES.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Banožić, M.; Babić, J.; Jokić, S. Recent Advances in Extraction of Bioactive Compounds from Tobacco Industrial Waste-a Review. Ind Crops Prod 2020, 144. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Karpichev, Y.; Pandey, A.; Chandra Kuhad, R.; Bhat, R.; Punia, R.; Aghbashlo, M.; Tabatabaei, M.; Gupta, V.K. Advancement in Valorization Technologies to Improve Utilization of Bio-Based Waste in Bioeconomy Context. Renewable and Sustainable Energy Reviews 2020, 131. [Google Scholar] [CrossRef]
- Jyotirmayee, B.; Mahalik, G. A Review on Selected Pharmacological Activities of Curcuma Longa L. Int J Food Prop 2022, 25. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma Longa Linn. in Relation to Its Major Active Constituent Curcumin. Front Pharmacol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Albaqami, J.J.; Hamdi, H.; Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Kuttithodi, A.M.; Famurewa, A.C.; Pathrose, B. Chemical Composition and Biological Activities of the Leaf Essential Oils of Curcuma Longa, Curcuma Aromatica and Curcuma Angustifolia. Antibiotics 2022, 11. [Google Scholar] [CrossRef]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Silva, M.A.N.; Deschamps, C.; Molento, M.B. Insecticide Activity of Curcuma Longa (Leaves) Essential Oil and Its Major Compound α-Phellandrene against Lucilia Cuprina Larvae (Diptera: Calliphoridae): Histological and Ultrastructural Biomarkers Assessment. Pestic Biochem Physiol 2019, 153. [Google Scholar] [CrossRef]
- Chaaban, A.; Gomes, E.N.; Richardi, V.S.; Martins, C.E.N.; Brum, J.S.; Navarro-Silva, M.A.; Deschamps, C.; Molento, M.B. Essential Oil from Curcuma Longa Leaves: Can an Overlooked by-Product from Turmeric Industry Be Effective for Myiasis Control? Ind Crops Prod 2019, 132. [Google Scholar] [CrossRef]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Kharazmi, M.S.; Rehman, A.; Jafari, S.M. Application of Essential Oils as Natural Biopesticides; Recent Advances. Crit Rev Food Sci Nutr 2023. [Google Scholar] [CrossRef]
- Karabörklü, S.; Ayvaz, A. A Comprehensive Review of Effective Essential Oil Components in Stored-Product Pest Management. Journal of Plant Diseases and Protection 2023. [Google Scholar] [CrossRef]
- Blaser, M.; Schmid-Hempel, P. Determinants of Virulence for the Parasite Nosema Whitei in Its Host Tribolium Castaneum. J Invertebr Pathol 2005. [Google Scholar] [CrossRef]
- Campbell, J.F.; Hagstrum, D.W. Patch Exploitation by Tribolium Castaneum: Movement Patterns, Distribution, and Oviposition. J Stored Prod Res 2002. [Google Scholar] [CrossRef]
- Lucas, D.; Mauguen, G.; Lesné, P.; Polard, E.; Jegaden, D. Exposure to Phosphine in Maritime Transport: A Real and Important Occupational Risk: A Report of Three Cases. Int Marit Health 2018, 69. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.H.; Koutros, S.; Lubin, J.H.; Coble, J.B.; Barone-Adesi, F.; Beane Freeman, L.E.; Sandler, D.P.; Hoppin, J.A.; Ma, X.; Zheng, T.; et al. Methyl Bromide Exposure and Cancer Risk in the Agricultural Health Study. Cancer Causes and Control 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, H.; Xie, J.; Wang, F.; Fan, S.; Yang, M.; Zheng, C.; Han, L.; Zhang, D. The Latest Research Progress on the Prevention of Storage Pests by Natural Products: Species, Mechanisms, and Sources of Inspiration. Arabian Journal of Chemistry 2022, 15. [Google Scholar] [CrossRef]
- Mfarrej, M.F.B.; Rara, F.M. Competitive, Sustainable Natural Pesticides. Acta Ecologica Sinica 2019. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Prajapati, V.; Verma, N.; Bahl, J.R.; Bansal, R.P.; Khanuja, S.P.; Kumar, S. Bioactivities of the Leaf Essential Oil of Curcuma Longa (Var. Ch-66) on Three Species of Stored-Product Beetles (Coleoptera). J Econ Entomol 2002, 95. [Google Scholar] [CrossRef]
- Sy, P.M.; Anton, N.; Idoux-Gillet, Y.; Dieng, S.M.; Messaddeq, N.; Ennahar, S.; Diarra, M.; Vandamme, T.F. Pickering Nano-Emulsion as a Nanocarrier for PH-Triggered Drug Release. Int J Pharm 2018, 549. [Google Scholar] [CrossRef]
- Lima, L.A.; Ferreira-Sá, P.S.; Garcia, M.D.N.; Pereira, V.L.P.; Carvalho, J.C.T.; Rocha, L.; Fernandes, C.P.; Souto, R.N.P.; Araújo, R.S.; Botas, G.; et al. Nano-Emulsions of the Essential Oil of Baccharis Reticularia and Its Constituents as Eco-Friendly Repellents against Tribolium Castaneum. Ind Crops Prod 2021, 162, 113282. [Google Scholar] [CrossRef]
- Wang, Y.; You, C.X.; Yang, K.; Wu, Y.; Chen, R.; Zhang, W.J.; Liu, Z.L.; Du, S.S.; Deng, Z.W.; Geng, Z.F.; et al. Bioactivity of Essential Oil of Zingiber Purpureum Rhizomes and Its Main Compounds against Two Stored Product Insects. J Econ Entomol 2015, 108. [Google Scholar] [CrossRef]
- Cao, J.Q.; Guo, S.S.; Wang, Y.; Pang, X.; Geng, Z.F.; Du, S.S. Toxicity and Repellency of Essential Oil from Evodia Lenticellata Huang Fruits and Its Major Monoterpenes against Three Stored-Product Insects. Ecotoxicol Environ Saf 2018, 160. [Google Scholar] [CrossRef]
- Saad, M.M.G.; El-Deeb, D.A.; Abdelgaleil, S.A.M. Insecticidal Potential and Repellent and Biochemical Effects of Phenylpropenes and Monoterpenes on the Red Flour Beetle, Tribolium Castaneum Herbst. Environmental Science and Pollution Research 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Li, Y.; Lei, C.L. Evaluation of Monoterpenes for the Control of Tribolium Castaneum (Herbst) and Sitophilus Zeamaise Motschulsky. Nat Prod Res 2009, 23. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Guo, S.S.; Zhang, W.J.; Geng, Z.F.; Deng, Z.W.; Du, S.S.; Zhang, J. Fumigant and Repellent Activities of Essential Oil Extracted from Artemisia Dubia and Its Main Compounds against Two Stored Product Pests. Nat Prod Res 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, J.L.; Xu, S.; Zhao, N.N.; Zhou, L.; Cheng, J.; Liu, Z.L. Evaluation of Repellency of Some Chinese Medicinal Herbs Essential Oils against Liposcelis Bostrychophila (Psocoptera: Liposcelidae) and Tribolium Castaneum (Coleoptera: Tenebrionidae). J Econ Entomol 2013, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Yang, K.; You, C.X.; Wang, Y.; Wang, C.F.; Wu, Y.; Geng, Z.F.; Su, Y.; Du, S.S.; Deng, Z.W. Bioactivity of Essential Oil from Artemisia Stolonifera (Maxim.) Komar. and Its Main Compounds against Two Stored-Product Insects. J Oleo Sci 2015, 64. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components By Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, Illinois, 2007; ISBN 1932633219. [Google Scholar]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma Longa l. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. a Review. Plants 2021, 10. [Google Scholar] [CrossRef]
- Karthik, P.; Ezhilarasi, P.N.; Anandharamakrishnan, C. Challenges Associated in Stability of Food Grade Nanoemulsions; 2017; Vol. 57, ISBN 9182125139. [Google Scholar]
- Sharma, S.; Loach, N.; Gupta, S.; Mohan, L. Phyto-Nanoemulsion: An Emerging Nano-Insecticidal Formulation. Environ Nanotechnol Monit Manag 2020, 14, 100331. [Google Scholar] [CrossRef]
- Griffin, C.W. Classification of Surface-Active Agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1. [Google Scholar]
- McClements, D.J.; Jafari, S.M. General Aspects of Nanoemulsions and Their Formulation; Elsevier Inc., 2018; ISBN 9780128118399. [Google Scholar]
- Rao, J.; McClements, D.J. Impact of Lemon Oil Composition on Formation and Stability of Model Food and Beverage Emulsions. Food Chem 2012, 134. [Google Scholar] [CrossRef]
- Botas, G. da S.; Cruz, R.A.S.; De Almeida, F.B.; Duarte, J.L.; Araújo, R.S.; Souto, R.N.P.; Ferreira, R.; Carvalho, J.C.T.; Santos, M.G.; Rocha, L.; et al. Baccharis Reticularia DC. and Limonene Nanoemulsions: Promising Larvicidal Agents for Aedes Aegypti (Diptera: Culicidae) Control. Molecules 2017, 22. [CrossRef]
- Rausher, M.D. Co-Evolution and Plant Resistance to Natural Enemies. Nature 2001. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.L.; Guy, R.H.; Speirs, R.D. Preliminary Evaluation of New Candidate Materials as Toxicants, Repellents, and Attractants Against Stored-Product Insects; U.S. Agricultural Research Service: Washington, DC, 1970. [Google Scholar]
- Sang, Y.L.; Wang, P.; Liu, J.Y.; Hao, Y.J.; Wang, X.L. Chemical Composition of Essential Oils from Three Rhododendron Species and Their Repellent, Insecticidal and Fumigant Activities. Chem Biodivers 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Zhang, J.W.; Zheng, Y.; Wang, D.; Wan, C.F.; Du, S.S. Insecticidal and Repellent Effects of the Essential Oils Extract from Zanthoxylum Myriacanthum against Three Storage Pests. Chem Biodivers 2023, 20. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Gad, H.A.; Ramadan, G.R.M.; El-Bakry, A.M.; El-Sabrout, A.M. Monoterpenes: Chemistry, Insecticidal Activity against Stored Product Insects and Modes of Action—a Review. Int J Pest Manag 2021. [Google Scholar] [CrossRef]
- Roy, D.N.; Goswami, R.; Pal, A. The Insect Repellents: A Silent Environmental Chemical Toxicant to the Health. Environ Toxicol Pharmacol 2017, 50, 91–102. [Google Scholar] [CrossRef]
- Silva, A.; Figueiredo, S.A.; Sales, M.G.; Delerue-Matos, C. Ecotoxicity Tests Using the Green Algae Chlorella Vulgaris-A Useful Tool in Hazardous Effluents Management. J Hazard Mater 2009, 167. [Google Scholar] [CrossRef]
- Noureini, S.K.; Kheirabadi, M.; Masoumi, F.; Khosrogerdi, F.; Zarei, Y.; Suárez-Rozas, C.; Salas-Norambuena, J.; Cassels, B.K. Telomerase Inhibition by a New Synthetic Derivative of the Aporphine Alkaloid Boldine. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, W.; Huang, W.; Plucinsky, B.; Garcia-Vazquez, N.; Robinson, N.J.; Schiemann, W.P.; Berdis, A.J.; Skordalakes, E.; Taylor, D.J. A Non-Natural Nucleotide Uses a Specific Pocket to Selectively Inhibit Telomerase Activity. PLoS Biol 2019, 17, 1–20. [Google Scholar] [CrossRef]
- Gillis, A.J.; Schuller, A.P.; Skordalakes, E. Structure of the Tribolium Castaneum Telomerase Catalytic Subunit TERT. Nature 2008, 455, 633–637. [Google Scholar] [CrossRef]
- Hevener, K.E.; Zhao, W.; Ball, D.M.; Babaoglu, K.; Qi, J.; White, S.W.; Lee, R.E. Validation of Molecular Docking Programs for Virtual Screening against Dihydropteroate Synthase. J Chem Inf Model 2009, 49, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, M.; Liu, J. π-π Stacking Interaction: A Nondestructive and Facile Means in Material Engineering for Bioapplications. Cryst Growth Des 2018, 18, 2765–2783. [Google Scholar] [CrossRef]
- Bryan, C.; Rice, C.; Hoffman, H.; Harkisheimer, M.; Sweeney, M.; Skordalakes, E. Structural Basis of Telomerase Inhibition by the Highly Specific BIBR1532. Structure 2015, 23, 1934–1942. [Google Scholar] [CrossRef]
- van Den Dool, H.; Dec. Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J Chromatogr A 1963. [CrossRef]
- Gutowska, I.; Machoy, Z.; Machaliński, B. The Role of Bivalent Metals in Hydroxyapatite Structures as Revealed by Molecular Modeling with the HyperChem Software. J Biomed Mater Res A 2005, 75, 788–793. [Google Scholar] [CrossRef]
- Ortega-Carrasco, E.; Lledós, A.; Maréchal, J.D. Assessing Protein-Ligand Docking for the Binding of Organometallic Compounds to Proteins. J Comput Chem 2014, 35, 192–198. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).