Submitted:
26 June 2023
Posted:
26 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Glitter particles characterization
2.2. Glitter dispersions’ preparation
2.3. Analysis of metals by Flame atomic absorption spectrometry (FAAS)
2.4. Embryonic development toxicity tests with sea-urchin embryos
2.5. Embryonic development toxicity tests with brown mussel embryos
2.6. Reference Substance Test
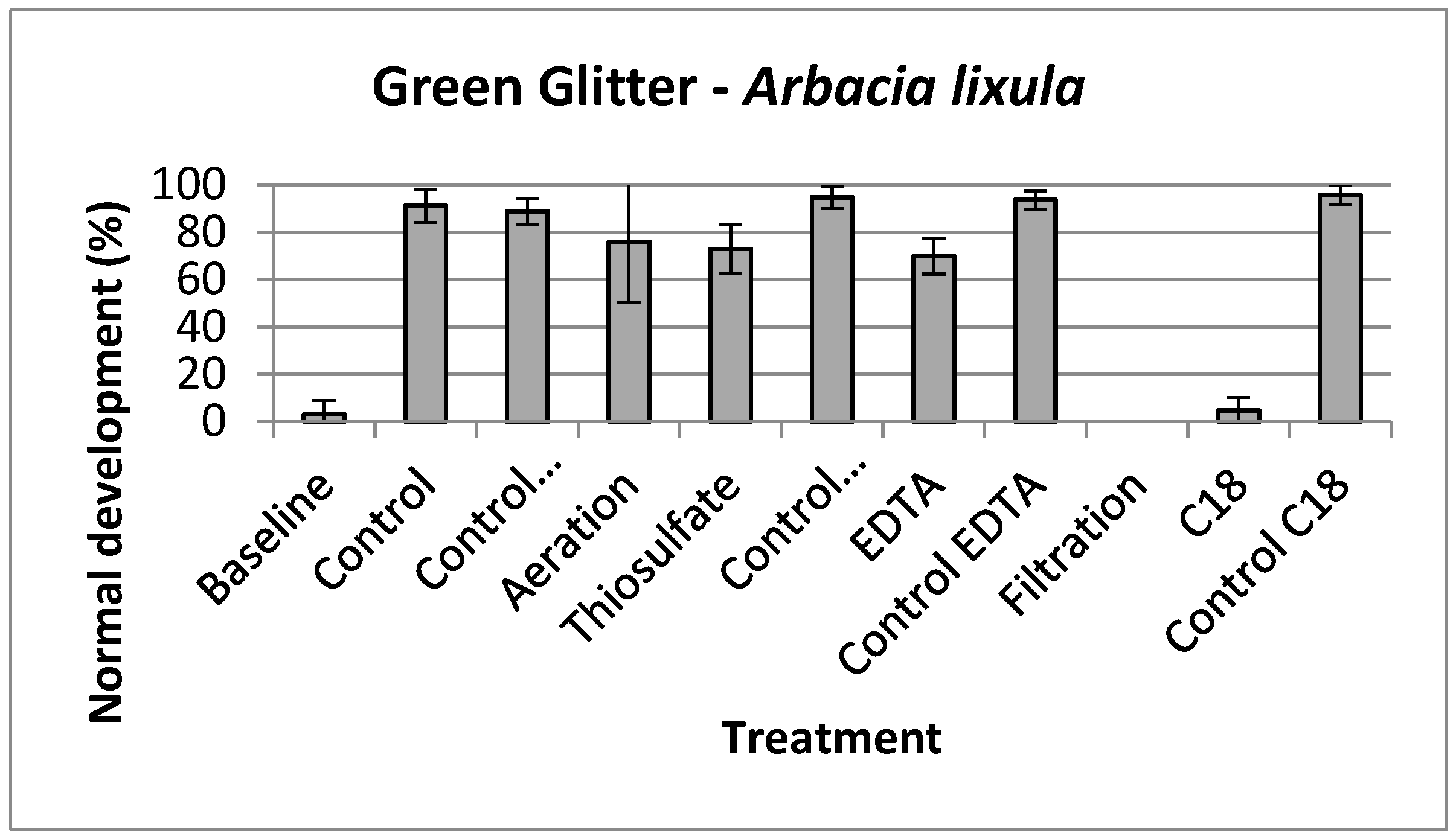
2.7. Toxicity Identification Evaluation (TIE)
2.8. Statistical analyses
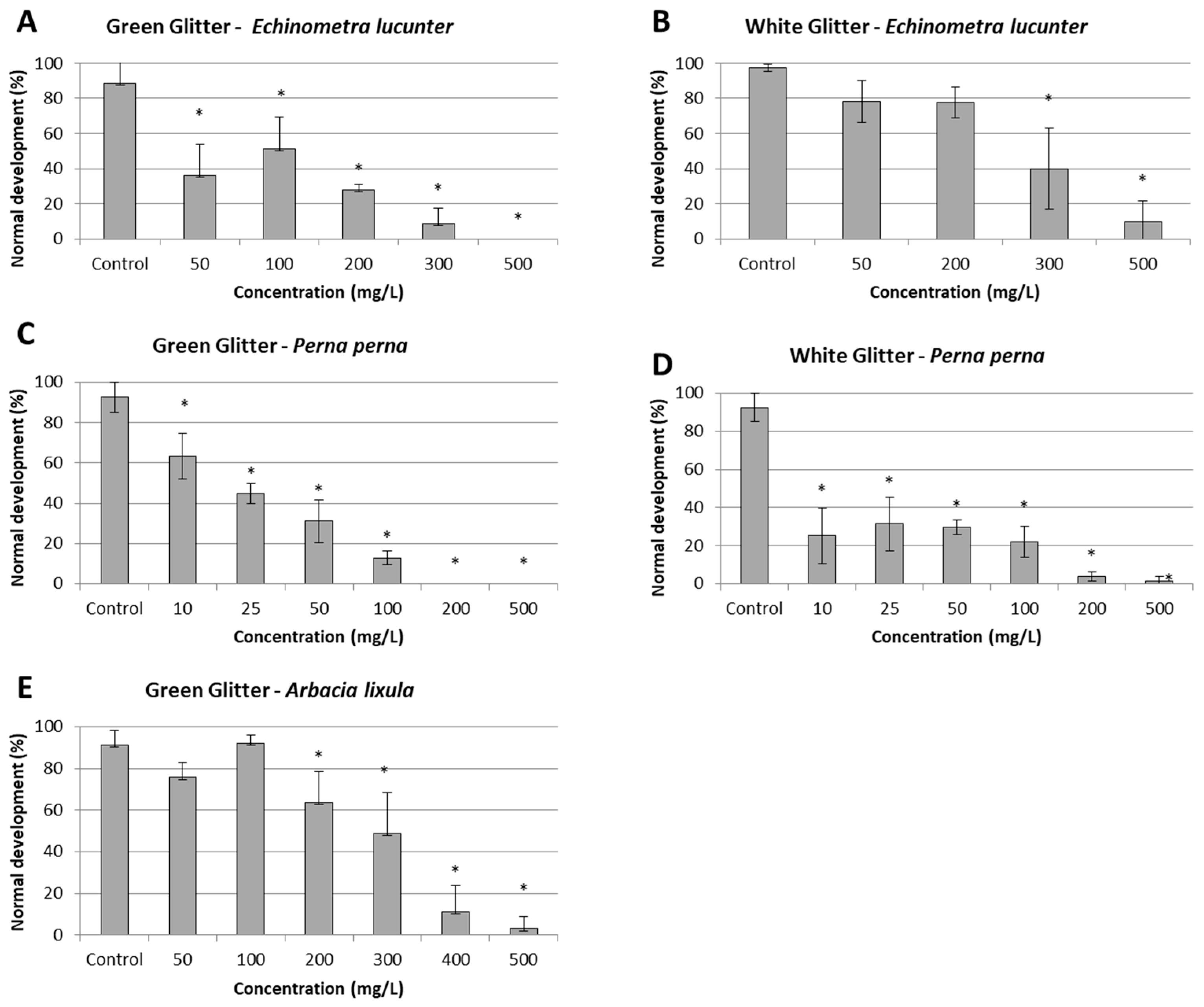
3. Results
3.1. Chemical analysis
3.2. Glitter Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez, G.; Santos, G.; Félix, M.; Hernández, H.; Rondón, C.. Good practices and trends in reverse logistics in the plastic products manufacturing industry. Procedia Manuf. 2019, 41, 367–374. [CrossRef]
- Pramanik, D.D.; Lei, S.; Kay, P.; Goycoolea, F.M. Investigating on the toxicity and bio-magnification potential of synthetic glitters on Artemia salina. Mar. Pollut. Bull., 2023, 190, 114828. [CrossRef]
- Suman, T.Y.; Jia, P.-P.; Li, W.-G.; Junaid, M.; Xin, G.-Y.; Pei, D.-S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard Mater, 2020, 400, 123220. [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.I. Microplastics in aquatic environments: occurrence, accumulation, and biological effects. Sci. Total Environ., 2019, 703, 134699. [CrossRef]
- Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T,L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms:What do we know and where should we focus our efforts in the future? Sci. Total Environ., 2018, 645, 1029-1039. [CrossRef]
- Frias, J.P.G.L., Nash, R. Microplastics: finding a consensus on the definition. Mar. Pollut. Bull., 2019, 138, 145-14. [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol., 2017, 1, 1–8. [CrossRef]
- Yurtsever, M. Glitters as a source of primary microplastics: an approach to environmental responsibility and ethics. J. Agric. Environ. Ethics, 2019a, 32(3), 459–478. [CrossRef]
- Besseling, E.; Foekema, E.M.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. The effect of microplastic on the uptake of chemicals by the lugworm Arenicola marina (L.) under environmentally relevant exposure conditions. Environ. Sci. Technol., 2017a, 51(15), 8795–8804. [CrossRef]
- Besseling, E.; Quik, J.T.; Sun, M.; Koelmans, A.A. Fate of nano-and microplastic in freshwater systems: A modeling study. Environ. Pollut., 2017b, 220, 540–548. [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; Van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int., 2017, 101, 133–142. [CrossRef]
- Rochman, C.M. 2018. Microplastics research - From sink to source. Science, 2018, 360(6384), 28–29. [CrossRef]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res., 2018, 137, 362–374. [CrossRef]
- Oberbeckmann, S.; Loeder, M.G.; Gerdts, G.; Osborn, A.M. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in northern European waters. FEMS Microbiol. Ecol., 2014, 90(2), 478–492. [CrossRef]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut., 2017, 230, 829–837. [CrossRef]
- Imhof, H.K.; Wiesheu, A.C.; Anger, P.M.; Niessner, R.; Ivleva, N.P.; Laforsch, C. Variation in plastic abundance at different lake beach zones - A case study. Sci Total Environ., 2018, 613, 530–553. [CrossRef]
- Yurtsever, M. Tiny, shiny, and colorful microplastics: Are regular glitters a significant source of microplastics? Mar. Pollut. Bull., 2019b, 146, 678-682. [CrossRef]
- Albanit, L.; Beverari, I.; Ribeiro, C.C.; Gimiliani, G.T.; Abessa, D.M.S. Toxicity of glitter to marine organisms: a baseline study with embryos of the sand-dollar Mellita quinquiesperforata. Int. Aquat. Res., 2023. [CrossRef]
- Rochman, C.S.; Kross, S.M.; Armstrong, J.B.; Bogan, M.T.; Darling, E.S.; Green, S.J.; Ashley, R.S.; Veríssimo, D. Scientific Evidence Supports a Ban on Microbeads. Environ. Sci. Technol., 2015, 49, 10759–10761. [CrossRef]
- Bhattacharya, P. 2016. A review of the impacts of microplastic beads used in cosmetics. Acta Biomed. Sci., 2016, 3(1), 47-52.
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull., 2017, 123, 122-126. [CrossRef]
- Piccardo, M.; Provenza, F.; Anselmi, S.; Renzi, M. Ecotoxicological assessment of “glitter” leachates in aquatic ecosystems: an integrated approach. Toxics, 2022, 10, 677. [CrossRef]
- Tagg, A.S.; Ivar do Sul, J.A. Is this your glitter ? An overlooked but potentially environmentally-valuable microplastic. Mar. Pollut. Bull., 2019, 146, 50–53. [CrossRef]
- Provenza, F.; Anselmi, S.; Specchiulli, A.; Piccardo, M.; Barceló, D.; Prearo, M.; Pastorino, P.; Renzi, M. Sparkling plastic: Effects of exposure to glitter on the Mediterranean mussel Mytilus galloprovincialis. Environ. Toxicol. Pharmacol., 2022, 96, 103994. [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A., Microplastics in cosmetics: environmental issues and needs for global bans. Environ. Toxicol. Pharmacol., 2019, 68, 75–79. [CrossRef]
- ECHA (European Chemicals Agency). Note on substance identification and the potential scope of a restriction on uses of ‘microplastics’. 2019. Available at: https://echa.europa.eu/ (Accessed May 19, 2023). 19 May.
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senthirajah, K.; Lundmark, A.; Rogers, Z.; SCB, S.; Evans, G.; Palanisami, T. Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant. Water Res., 2020, 173, 115549. [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat Geosci., 2018, 11(4), 251. [CrossRef]
- Harley-Nyang, D.; Memon, F.A.; Jones, N.; Galloway, T. Investigation and analysis of microplastics in sewage sludge and biosolids: A case study from one wastewater treatment works in the UK. Sci. Total Environ., 2022, 823, 153735. [CrossRef]
- Lusher, A.L.; Hurley, R.; Vogelsang, C.; Nizzetto, L.; Olsen, M. Mapping microplastics in sludge. NIVA-report 7215. 2017. https://hdl.handle.net/11250/2493527 (Accessed June 20, 2023).
- Rios Mendoza, L.M.; Balcer, M. Microplastics in freshwater environments: a review of quantification assessment. TrAC Trends Anal. Chem., 2019, 113:402–408. [CrossRef]
- Green, S.D.; Jefferson, M.; Boots, B.; Stone, L. (2021) All that glitter is litter? Ecological impacts of conventional versus biodegradable glitter in a freshwater habitat. J. Hazard Mater., 2021, 402, 124070. [CrossRef]
- USEPA (United States Environmental Protection Agency). Methods for Aquatic Toxicity Identification Evaluations: Phase I Toxicity Characterization Procedures (Second Edition). 1991. No. EPA/600/6-91/003, Duluth, MN, U.S. Environmental Protection Agency, Office of Water, Washington, DC.
- Camargo, J.B.D.A.; Araújo, G.S.; Cruz. A.C.F.; Fonseca, T.G.; Abessa, D.M.S. Use, development and improvement in the protocol of whole-sediment Toxicity Identification and Evaluation using benthic copepods. Mar. Pollut. Bull., 2015, 91(2): 511-517. [CrossRef]
- Anderson, B.S.; Hunt, J.W.; Phillips, B.M.; Thompson, B.; Lowe, S.; Taberski, K.; Carr, R.S. Patterns and trends in sediment toxicity in the San Francisco Estuary. Environ. Res., 2007, 105, 145–55. [CrossRef]
- Schubauer-Berigan, M.K.; Amato, J.R.; Ankley, G.T.; Baker, S.E.; Burkhard, L.P.; Dierkes, J.R.; Jenson, J.J.; Lukasewycz, M.T.; Norberg-King, T.J. The behavior and identification of toxic metals in complex mixtures: examples from effluent and sediment pore water toxicity identification evaluations. Arch. Environ. Contam. Toxicol., 1993, 24, 298–306. [CrossRef]
- Burgess, R.M.; Cantwell, M.G.; Pelletier, M.C.; Ho, K.T.; Serbst, J.R.; Cook, H.F.; Kuhn, A. Development of a toxicity identification evaluation procedure for characterizing metal toxicity in marine sediments. Environ. Toxicol. Chem., 2000, 19, 982–991. [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol., 1922, 30(5):377-392.
- Shepard, F.P. 1954. Nomenclature based on sand-silt-clay ratios. J. Sediment Res., 1954, 24(3): 151–158. [CrossRef]
- Rao, T.P.; Metilda, P.; Gladis, J.M. Overview of analytical methodologies for sea water analysis: Part I—Metals. Crit. Rev. Anal. Chem., 2005, 35(4), 247-288.
- ABNT (Associação Brasileira de Normas Técnicas). NBR 15350:2012. Ecotoxicologia aquática – Toxicidade crônica de curta duração – Método de Ensaio com ouriço-do-mar (Echinodermata Echinoidea). 2012, ABNT, São Paulo, 21p.
- ASTM (American Society for Testing and Materials). Standard guide for conducting laboratory soil toxicity or bioaccumulation tests with the sea urchin. ASTM E 1563-13. 2013.
- OECD (Organisation for Economic Co-operation and Development). Test No. 151: Early-life stage toxicity test with the sea urchin. OECD Guidelines for the Testing of Chemicals, 1992. Section 2: Effects on Biotic Systems.
- Benfenati, E., G. Marcomini, G. Losso, and F. Fanelli. The use of Arbacia lixula early embryo test for predicting mammalian acute toxicity of pesticides. Environ. Toxicol. Chem., 1997, 16, 585-590.
- Curiel, D.; Blasco, C.; Cuesta, A.M.; Martín-Díaz, M.J.; Bayona, J.M. Environmental risk assessment of industrial effluents: The sea urchin embryo bioassay as a tool for ecotoxicological evaluation. Chemosphere, 2007, 68: 411-418.
- Di Filippo, B.D.; Bocchetti, R.; Marcomini, A.; Fattorini, G. 2014. Effects of the heavy metals lead and cadmium on development and hatching of Arbacia lixula (Echinodermata: Echinoidea) embryos. Mar. Environ. Res., 2014, 97, 56-63.
- ABNT (Associação Brasileira de Normas Técnicas). Ecotoxicologia Aquática — Teste crônico de curta duração com embriões de bivalves (Mollusca — Bivalvae). NBR 16456; Rio de Janeiro, RJ, Brazil, 2016.
- Zaroni, L.P.; Abessa, D.M.S.; Lotufo, G.R.; Sousa, E.C.P.M.; Pinto, Y.A. Toxicity testing with embryos of marine mussels: protocol standardization for Perna perna (Linnaeus, 1758). Bull. Environ. Contam. Toxicol., 2005, 74, 793–800. [CrossRef]
- USEPA (United States Environmental Protection Agency). Sediment Toxicity Identification Evaluation (TIE) Phases I, II, and III Guidance Document (No. EPA/600/R-07/080), U.S. Environmental Protection Agency, Office of Water, Washington, DC. 2007.
- Ho, K.T.; Burgess, R.M. Marine sediment Toxicity Identification Evaluations (TIEs): history, principles, methods, and future research. In: Kassim, T.A., Barceló, D. (Eds.), Contaminated Sediments. The Handbook of Environmental Chemistry, vol. 5T. Springer Berlin Heidelberg, 2009, pp. 75–95.
- Hamilton, M.A.; Runo, R.C.; Thurton, R.V. Trimmed Spearman-Kärber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol., 1977, 11, 714–719; correction. Environ. Sci. Technol., 1978, 12, 417.
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package For Education And Data Analysis. Palaeontol. Electron., 2001, 1(4):1-9.
- StatSoft, Inc. STATISTICA for Windows, Version 6. StatSoft Inc. 2300. Tulsa. 2001.
- Brasil, República Federativa. Resolução CONAMA nº 357, 18 de março de 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Diário Oficial da União, 2005, 53, 58–63. www.mma.gov.br/port/conama/res/res05/res35705.pdf (accessed on March 28, 2020).
- Reis, G.O.M.; Furtado, L.M.; Pereira, M.P.; Reis, E.L.; Imme, M.L.; Alves, T. Investigação da presença de micro plásticos em moluscos de cultivo de Santa Catarina. In: Anais do 9º Seminário de Ensino, Pesquisa, Extensão e Inovação do IFSC, 2023, Joinville. Proceedings... Campinas, Galoá, 2023. Available at: https://proceedings.science/sepei-2023/trabalhos/investigacao-da-presenca-de-micro-plasticos-em-moluscos-de-cultivo-de-santa-cata?lang=pt-br. (Accessed in Jun 22, 2023).
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Publ. Health, 2020, 17, 1212. [CrossRef]
- Capolupo, M.; Rafiq, A.; Coralli, I.; Alessandro, T.; Valbonesi, P.; Fabbri, D.; Fabbri, E. Bioplastic leachates characterization and impacts on early larval stages and adult mussel cellular, biochemical and physiological responses. Environ. Pollut., 2023, 319, 120951. [CrossRef]
- Brede, C.; Fjeldal, P.; Skjevrak, I.; Herikstad, H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam., 2003, 20, 684–689. [CrossRef]
- Hennesuse-Boxus, C.; Pacary, T. Emissions from plastics. Rapra Rev Rep., 2003, 14, 152.
- Kim, Y-J., Osako, M., Sakai, S-I. Leaching characteristics of polybrominated diphenyl ethers (PBDEs) from flame retardant plastics. Chemosphere, 2006, 65, 506–513. [CrossRef]
- Mutsuga, M.; Kawamura, Y.; Sugita-Konishi, Y.; Hara-Kudo, Y.; Takatori, K.; Tanamoto, K. Migration of formaldehyde and acetaldehyde into mineral water in polyethylene terephthalate (PET) bottles. Food Addit. Contam., 2006, 23, 212–218. [CrossRef]
- Fernandes, A.R.; Rose, M.; Charlton, C. 4-Nonylphenol (NP) in food contact materials: analytical methodology and occurrence. Food Addit. Contam., 2008, 25, 364–372. [CrossRef]
- Tønning, K.; Jacobsen, E.; Pedersen, E.; Nilsson, N.H. Phthalates in products that children are in direct contact with. Survey of chemical substances in consumer products, nº 109210. Danish Ministry of the Environment and EPA. 2010.
- Bila, D.M.; Dezotti, M. Desreguladores endócrinos no meio ambiente: efeitos e consequências. Quim. Nova, 2007, 30(3), 651. [CrossRef]
- García-Espiñeira, M.C.; Tejeda-Benítez, L.P.; Olivero-Verbel, J. Toxic Effects of Bisphenol A, Propyl Paraben, and Triclosan on Caenorhabditis elegans. Int J Environ Health Res., 2018, 15(4): 684. [CrossRef]
- Sarmah, R.; Kanta Bhagabati, S.; Dutta, R.; Nath, D.; Pokhrel, H.; Mudoi, L.P.; Sarmah, N.; Sarma, J.; Ahmed, A.M.; Nath, L.P; Ingtipi, L.; Kuotsu, K. Toxicity of a synthetic phenolic antioxidant, butyl hydroxytoluene (BHT), in vertebrate model zebrafish embryo (Danio rerio). Aquac. Res., 2020, 51(9). 3839-3846. [CrossRef]
- Yang, X.; Sun, Z.; Wang, W.; Zhou, Q.; Shi, G.; Wei, F.; Jiang, G. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total Environ., 2018, 643, 559–568. [CrossRef]
- Marquez-Sillero, I.; Aguilera-Herrador, E.; Cardenas, S.; Valcarcel, M. Determination of parabens in cosmetic products using multi-walled carbon nanotubes as solid phase extraction sorbent and corona charged aerosol detection system. J. Chromatogr. A, 2010, 1217(1), 1-6. [CrossRef]
- Carvalho, L.C.P.; Rola R.C.; da Silva Junior, F.M.; Martins, C.M.G. Toxicity and sublethal effects of methylparaben on zebrafish (Danio rerio) larvae and adults. Environ. Sci. Pollut. Res., 2021, 28, 45534-45544. [CrossRef]
- Li, H.; Zhang, S. Functions of vitellogenin in eggs. In: Kloc, M. (eds) Oocytes. Results and Problems in Cell Differentiation. Springer, Cham, 2017, pp.389-40. [CrossRef]
- Staples, C.A.; Murphy, S.R.; McLaughlin, J.E.; Leung, H.W.; Cascieri, T.C.; Farr, C.H. Determination of selected fate and aquatic toxicity characteristics of acrylic acid and a series of acrylic esters. Chemosphere, 2000, 40(1), 29-38. [CrossRef]
- Marchini, S.; Tosato, M.L.; Norberg-King, T.J.; Hammermeister, D.E.; Hoglund, M.D. Lethal and sublethal toxicity of benzene derivatives to the fathead minnow, using a short-term test. Environ. Toxicol. Chem., 1992, 11(2), 187–195. [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci, 2004, 275(1), 177-182.
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem., 2009, 18(1): 89-108. [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv., 2009, 27(1), 76-83. [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today, 2015, 10(3), 339-354. [CrossRef]
- Wang, X.; Bolan, N.; Tsang, D.C.W.; Sarkar, B.; Bradney, L.; Li, Y. A review of microplastics aggregation in aquatic environment: Influence factors, analytical methods, and environmental implications. J. Hazard. Mater., 2021, 402, 123496. [CrossRef]
- Singh, N.; Tiwari, E.; Khandelwal, N.; Darbha, G.K. Understanding the stability of nanoplastics in aqueous environments: effect of ionic strength, temperature, dissolved organic matter, clay, and heavy metals. Environ. Sci. Nano, 2019, 6, 2968-2976. [CrossRef]
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. J. Environ. Sci. Technol., 2018, 52, 1704-1724. [CrossRef]
- Fan, Z.; Zhuang, W.; Se, W.; Hao, F.; Degao, W. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: complex roles of pH, dissolved organic carbon and divalent cations. Chemosphere, 2019, 228, 195-203. [CrossRef]
- Choi, J.S.; Hong, S.H.; Park, J.W. Evaluation of microplastic toxicity in accordance with different sizes and exposure times in the marine copepod Tigriopus japonicas. Mar. Environ. Res., 2019, 153, 104838. [CrossRef]
- Koh, K.Y.; Chen, Z.; Lin, S.; Mohan, K.C.; Luo, X.; Chen, J.P. Leaching of organic matters and formation of disinfection by-product as a result of presence of microplastics in natural freshwaters. Chemosphere, 2022, 299, 134300. [CrossRef]
- Andrady, A.L.; Barnes, P.W.; Bornman, J.F.; Gouin, T.; Madronich, S.; White, C.C.; Zepp, R.G.; Jansen, M.A.K. Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation. Sci. Total Environ., 2022, 851 (Part 2), 158022. [CrossRef]
- No author. Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int. J. Toxicol., 2008, 27 Suppl 4: 1-82. [CrossRef]
| Element | Concentration (mg/L) | ||
|---|---|---|---|
| Glitter | Marine water | Effluents | |
| Ag | 0.01 | 0.005 | 0.1 |
| Zn | 0.014 | 0.09 | 5.0 |
| Mg | 0.25 | - | - |
| Ti | 0.001 | - | - |
| Mn | 0.008 | 0.1 | 1.0 |
| Fe | 0.018 | 0.3 | 15 |
| Ca | 0.27 | - | - |
| Glitter color | Arbacia lixula | Perna Perna | Echinometra lucunter | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 | NOEC | LOEC | EC50 | NOEC | LOEC | EC50 | NOEC | LOEC | |
| White | not analyzed | NC | <10 | 10 | 272.2 (261.5 – 282.9) | 200 | 300 | ||
| Green | 246.1 (235.8 – 256.4) | 100 | 200 | 23 (20.2 - 25.8) | < 10 | 10 | 105.9 (61.2 - 150.2) | < 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





