1. Introduction
Microchimerism is a condition in which small numbers of cells from one individual are present in another, genetically different, individual's body [
1,
2]. Microchimerism is quite common. The most common form is fetomaternal microchimerism when cells from the developing fetus cross the placenta and enter the mother's bloodstream, or when a mother's cells cross into the developing fetus [
1,
2,
3,
4,
5]. It is important to note that the cells from the fetus or from the mother may persist in the other individual for decades [
3,
4]. Microchimerism can have both positive and negative effects. For example, fetal cells in the mother's body may play a role in tissue repair and immune function, while also potentially contributing to autoimmune diseases [
3,
4].
Microchimerism was also described in allotransplantation showing the presence of cells from organ transplant donors in the corresponding recipients, even after the organ was rejected [
6,
7,
8]. Microchimerism was observed in recipients after kidney allotransplantation, as well as in liver and lung recipients [
9]. Similar to the situation in pregnancy, donor cells in transplant recipients can cause graft-versus-host disease, and on the other hand they may contribute to a survival of the transplant with reduced pharmaceutical immunosuppression or even immunological tolerance [
10]. Microchimerism can also occur following allogeneic blood transfusion in patients, where donor cells have been detected decades after transfusion [
11,
12] as well as after stem cell treatment [
13]. Overall, microchimerism is an area of ongoing research and it is important to fully understand the implications and effects of this phenomenon on the human body.
Microchimerism was not only detected in recipients of allotransplants, but also in recipients of xenotransplants, both in non-human primates in preclinical trials [
14] as well as in humans in clinical trials such as extracorporeal splenic perfusion [
15], transplanting encapsulated pig islet cells into diabetic patients [
16] or others (for review see [
1]). For example, in a preclinical study of orthotopic pig heart transplantation, PERV sequences were detected in the blood samples in all eight transplanted baboons, and it was assumed that this was due to circulating cellular DNA from dead transplant cells or from circulating pig cells, e.g., microchimerism [
14]. Moreover, Western blot analysis showed no anti-PERV antibodies in the serum of the baboons, clearly demonstrating absence of an infection [
14].
Microchimerism is easier to detect in xenotransplantation than microchimerism in allotransplantation. Whereas in allotransplantation the absence or presence of the Y chromosome or differences in the HLA molecules were used for differentiation [
1], molecules of a different species can easily be detected. PCR methods may be used to detect cellular genes such as COX [
15]. However, since there are only two COX genes per cell, the number of pig cells in the recipient must be high enough to detect them. Since there are up to 60 PERV copies in a pig cell, the detection by PCR using highly conserved PERV polymerase (pol) primers is much more sensitive [
17]. However, this method does not allow to discriminate between microchimerism and potential PERV infection. A better approach is the use of short interspersed nuclear elements (SINE) which are present in pig cells with a copy number of more than 100,000. SINE are a group of interspersed repetitive sequences found in mammalian genomes, the non-LTR (long terminal repeats) retrotransposons. Non-LTR retrotransposons are divided into two groups primarily by their size; the SINE (in humans it is Alu) and long interspersed nuclear sequences (LINE, in humans L1). PRE-1 is the major SINE of the pig genome [
18]. The 233 bp PRE-1 sequence contains an RNA polymerase III split promoter (16-27 bp and 58-69 bp) as well as two short direct repeats (91-124 bp and 138-170bp) (Supplementary
Figure 1). The 3' termini of the elements consist of a poly A tail of variable length [
19]. It was estimated that there are 100,000 to 1 million copies per haploid genome [
18,
19]. Although none of these elements contains an open reading frame, they are transcribed in some pig tissues [
18]. PRE-1 sequences are unevenly distributed along the chromosomes as in the case of the human and mouse SINES. However, there is a difference, PRE-1 is localized on centromeric regions, but human and mouse SINES are not [
20]. Hypermethylation of the repetitive region PRE-1 was found to be associated with defective development and early abortion of cloned pigs [
21]. Since the sequence is specific for pigs [
18,
22,
23], it can be used to detect pig DNA and consequently pig cells with a thousand-fold higher sensitivity compared with cellular genes such as pGAPDH. This method was suitable for the detection of microcontamination of feed with animal materials [
19] and commercially purchased meat products [
23].
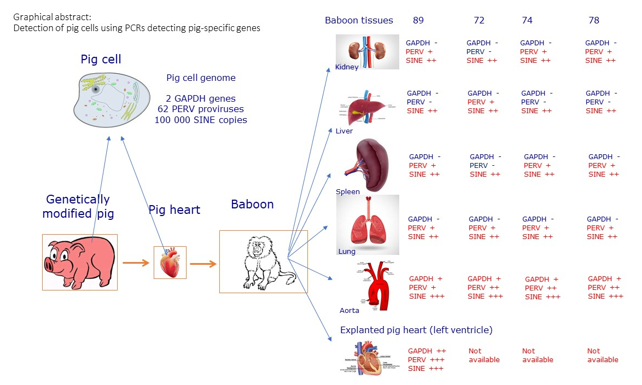
Here we analyzed different organs from four baboons, which received a heart from triple genetically modified pigs (GGTA1-KO, hCD46, hTBM) [
24] for the presence of PERV sequences, porcine GAPDH and SINE sequences. Furthermore, a RT real-time PCR was performed to detect PERV mRNA. Furthermore, we screened the explanted hearts for the presence of baboon cells.
2. Materials and Methods
2.1. Tissues
Tissues samples of skin, kidney, spleen, lung, aorta and peripheral blood mononuclear cells were taken from the transplanted baboons after euthanasia; in addition, tissue samples of the pericardium and the left ventricle were taken from the explanted pig heart All samples were frozen at -80
oC. Four animals were analyzed: baboon A with the animal number 17475, B (17493), C (17492), and D (17769). Animals B, C and D had been included in the study described in [
14], baboon B corresponds to animal O, baboon C corresponds to animal Q and baboon D corresponds to animal K. All four baboons received an orthotopic heart transplantation from a pig which was triple genetically modified: GGTA1-KO (knockout of the porcine GGTA1 gene which encodes for the α1,3-galactosyltransferase that synthesizes the Gal epitopes), hCD46 (expression of the human CD46, also called membrane cofactor protein, MCP) and hTBM (expression of the human thrombomodulin) [
24].
2.2. DNA and RNA Isolation
DNA and RNA were isolated from the tissues according to manufacturer´s instructions using the DNeasy Blood & Tissue kit and RNeasy kit (Qiagen, Hilden, Germany), respectively. DNA and RNA concentrations were determined using NanoDrop ND-1000 (Thermo Fisher Scientific Inc., Worcester, MA, USA) or Invitrogen Qubit 4 Fluorometer (Waltham, MA, USA).
2.3. PCR Methods
The PRE-1 PCR was performed using the primers described by [
23] (
Table 1) and the following conditions: 2 ng of DNA template,1X PCR buffer II (Applied Biosystems, Inc.), 0.2 mM dNTPs,1.5 mM MgCl
2, 1 unit Taq DNA polymerase. Each sample was subjected to an initial denaturation of 1 min at 95
oC, followed by 30 amplification cycles (95
oC for 30 s and 63
oC for 1 min). “Hot-start PCR’’ (automatic with AmpliTaq Gold) and an annealing/extension temperature of 63
oC or higher was critical to assay specificity. The PRE-1 PCR assay had a linear quantitation range of 10–0.00001 ng (0.01 pg), or 10
7, as shown by [
23].
The PERV pol PCR was performed using primers described by [
25] (
Table 1), the annealing temperature was changed to 62
oC to achieve a sensitivity of 10 copies. This PCR was run as a duplex PCR with primers and probes for the porcine glyceraldehyde 3-phosphate dehydrogenase (pGAPDH) and human GAPDH (hGAPDH), which also recognizes the baboon GAPDH (
Table 1).
2.4. Testing for PCMV/PRV; PCV3, CR Methods
PCR testing for PCMV/PRV, HEV, PLHV-1, -2 was performed as described in [
14], for PCV3 as described in [
26].
3. Results
3.1. Improvement of the Detection Methods
In order to detect PERV sequences, a real-time PCR was performed with primers and a probe binding to a highly conserved region in the PERV polymerase gene (pol) [
25]. The conditions were modified in such a way (see Material and methods) that 10 copies were detected (Supplementary
Table 1). The real-time PCR was performed as a duplex PCR detecting in parallel porcine glyceraldehyde 3-phosphate dehydrogenase (pGAPDH). The positive control was a gene block containing the PERV pol sequence between both primer binding sites as well as the target sequences of the real-time PCRs detecting
pGAPDH and human GAPDH (hGAPDH) [
29]. The sequences of the primer binding sites in hGAPDH are identical to the primer binding sites of baboon GAPDH.
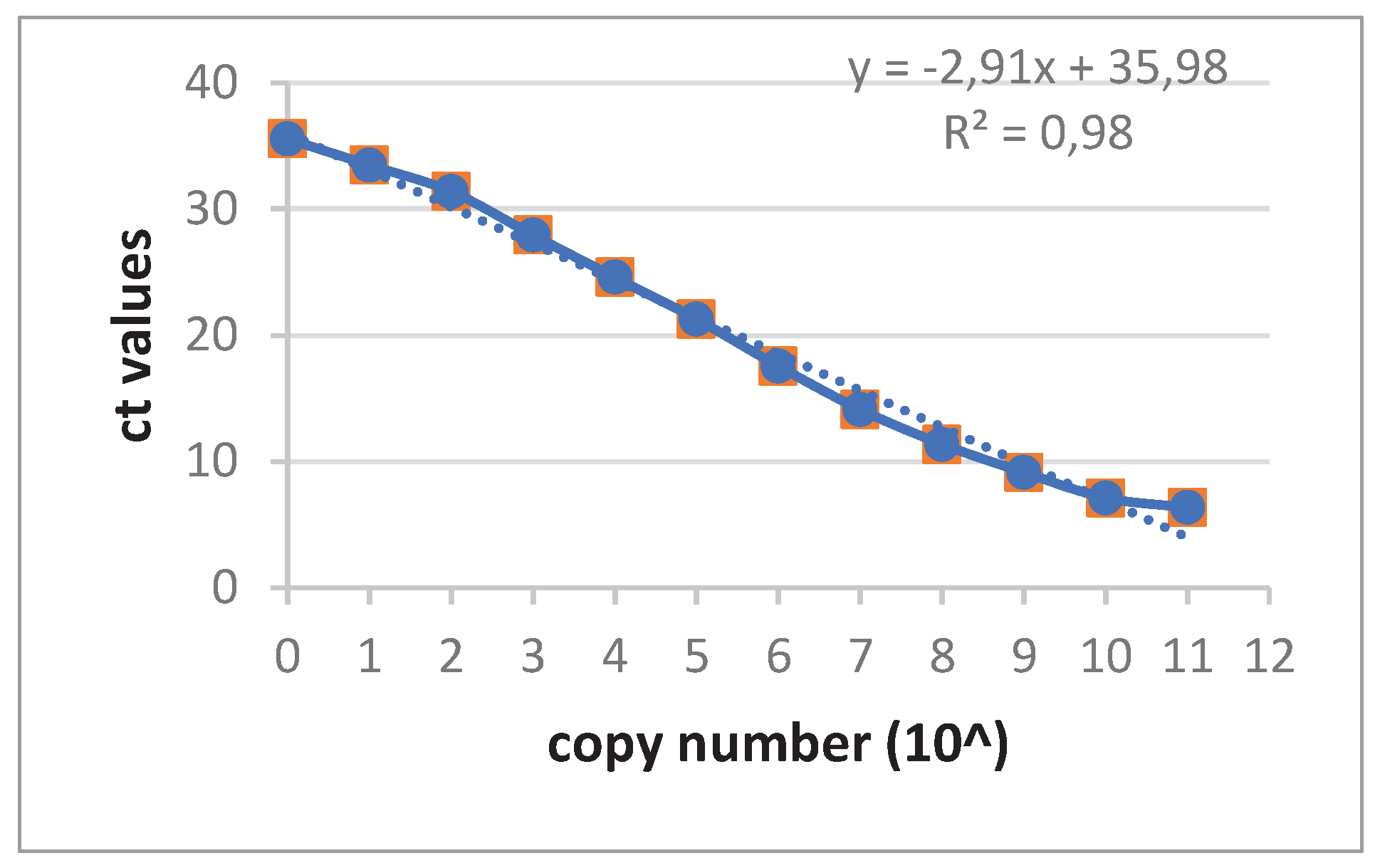
Although the conventional PCR detecting pig SINE sequences (PRE-1) worked well (not shown), a new real-time was developed with the same primers and in addition a specific probe (
Table 1). A dilution of the amplicon product of the PRE-1 PCR was used to obtain a standard curve for future determinations of the copy number (
Figure 1).
3.2. Detection of PERV Sequences in Baboon Tissues after Transplantation
A modified and highly sensitive PERV pol real-time PCR (see above) was used to confirm previous data that PERV sequences can be detected in the organs of baboons after transplantation of pig hearts [
14]. When tissues of baboon A were analyzed using this real-time PCR, PERV sequences were found in all organs with exception of the liver, with slightly different ct values, indicating different amounts of PERV sequences in different tissues (
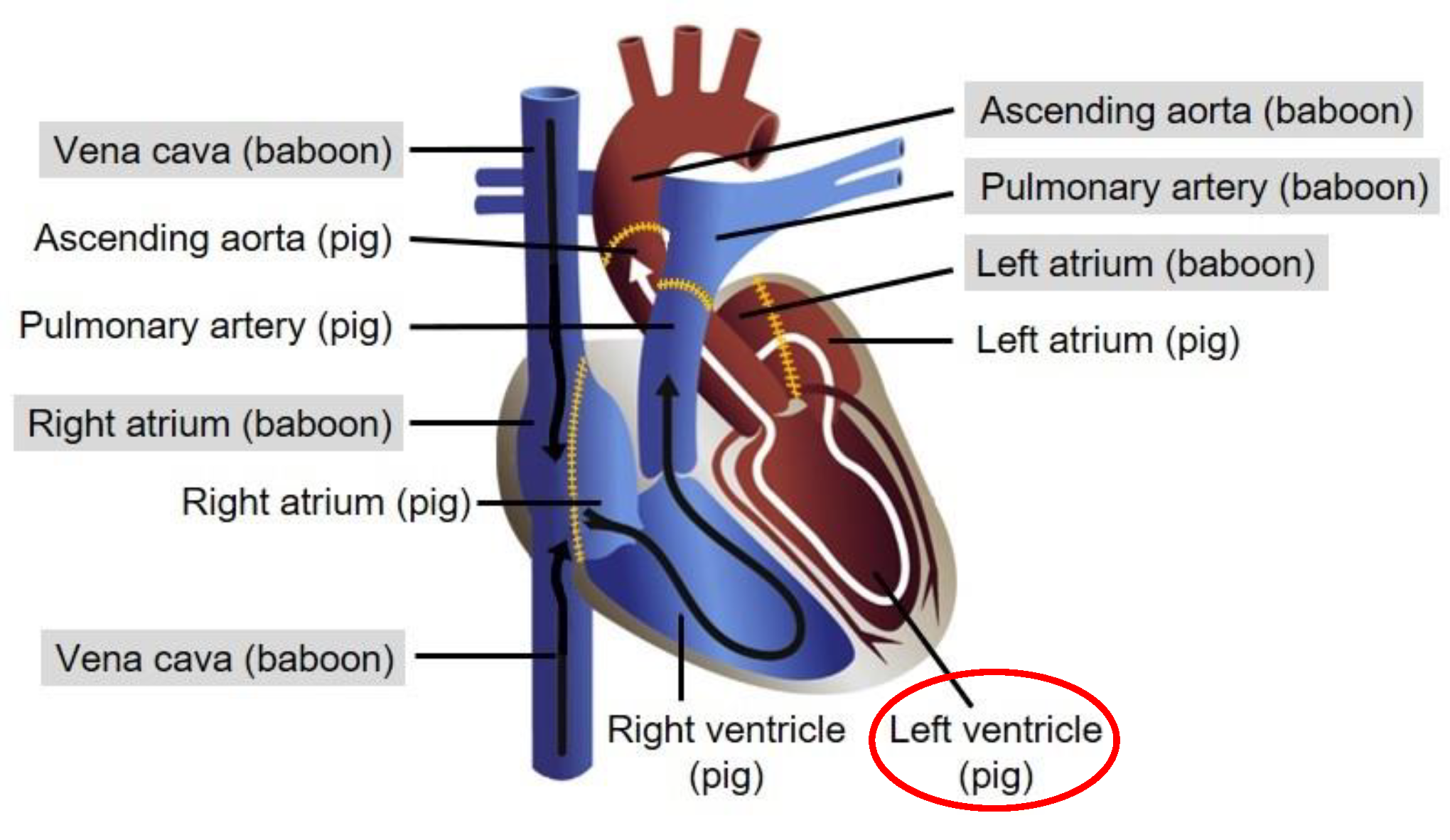
Table 2). The highest amount of PERV sequences in baboon tissues was found in the aorta. The pericardium was found to be a mixture of pig and baboon tissue, because nearly identical ct values of porcine GAPDH and baboon GAPDH were found. The left ventricle was mainly pig tissue (
Figure 2), indicated by the high amount of pGAPDH and PERV sequences, but there were also baboon cells present as detected by the human/baboon GAPDH PCR (see below). It is important to note that baboon A was negative for PCMV/PRV and PCV3 (
Table 2).
PERV sequences were also found in tissues of three other baboons (
Table 3). In the case of baboon B, PERV sequences were found in skin, liver, and lung but not in kidney and spleen, in baboon C only in liver and lung and in baboon D only in the skin. Detection of pGAPDH in the aorta showed that there are many pig cells despite the baboon origin of the tissue. However, we cannot exclude that tissue samples were taken from the site of anastomosis, i.e. where both porcine and baboon aorta are sutured together. The analyzed material from the pericardium was also a mixture of pig and baboon tissue. Baboon B was PCMV/PRV, HEV, PLHV-1, -2-, and -3 negative [
14], but PCV3 positive [
26]. Baboon C was PCMV/PRV and PCV3 positive, and baboon D was PCMV/PRV negative, but PLHV-1, -2 positive [
14,
26]. It is unknown whether the transmission of these viruses influenced the dissemination of pig cells.
3.3. Evidence for Microchimerism
Since we proposed based on previous results [
14] that the PERV sequences found in the baboon tissues are due to the presence of pig cells in the baboon tissues, e.g., due to microchimerism, a duplex real-time PCR was performed to detect a porcine cellular gene, pGAPDH. In none of the tissues of baboon A pGAPDH was detected with exception of the aorta indicating the presence of a high percentage of pig cells (
Table 2). This result is in agreement with the result of the PERVpol-specific real-time PCR, which also showed a high amount of PERV sequences in the aorta. The same was observed in the other three baboons (
Table 3).
The presence of PERV sequences in the tissues of the transplanted baboons indicated either infection of the recipient animal or the presence of pig cells, e.g., microchimerism. In the case of microchimerism the detection of PERV sequences in comparison with the results with porcine GAPDH can be explained by the higher copy number of PERV (up to 60) in the pig cell genome [
26], whereas pGAPDH has only two copies.
In order to answer the question whether it is an infection or microchimerism, another pig specific marker was used which has a high copy number in the pig genome compared with pGAPDH. SINE sequences were well suited for this purpose. SINE sequences were found in all the organs of baboon A (
Table 2). As expected, the amount of SINE sequences is very high (ct 6.54) in the left ventricle which is pig tissue (
Figure 2). The amount of SINE sequences is also high in the aorta, confirming the results with PERV and pGAPDH that numerous pig cells accumulated in this vessel. Similar results were obtained in the other three animals (
Table 3).
3.4. Baboon Cells in the Explanted Pig Heart
To analyze, whether baboon cells can be found in the transplanted pig heart, a real-time PCR detecting baboon/human GAPDH was performed. Indeed, baboon cells were found in high quantities in the left ventricle of the explanted pig heart from baboon A (
Table 2).
3.5. Absence of PERV Expression
To analyze whether PERV was expressed as mRNA in the baboon tissues with the highest prevalence of pig cells, RNA was isolated from the kidney, lung and spleen of baboon A and a RT real-time PCR was performed using PERVpol primers and the probe. In none of these tissues expression of PERV at the level of mRNA was observed (
Table 4).
4. Discussion
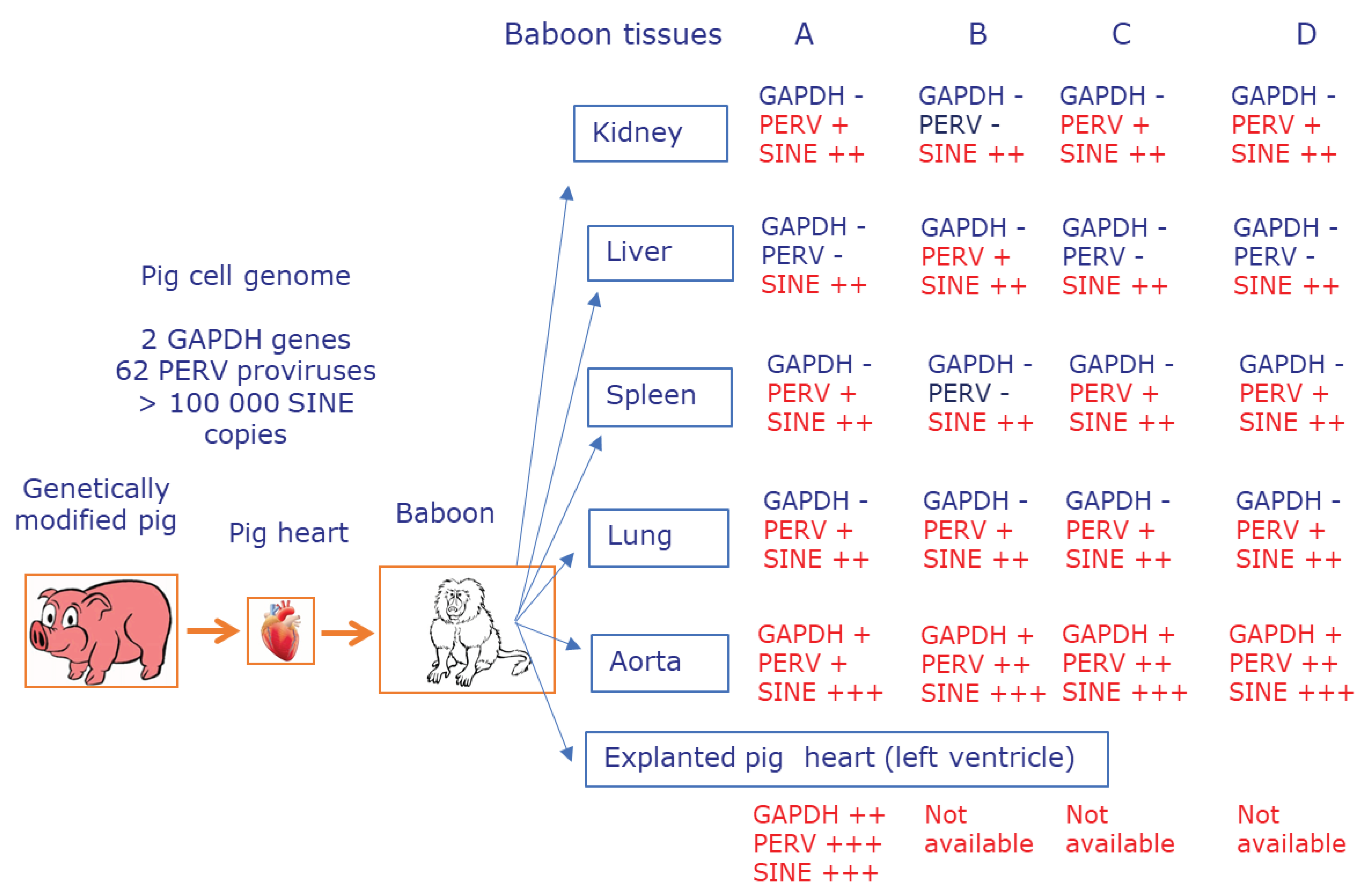
Here we demonstrated that after orthotopic transplantation of pig hearts into baboons, pig cells were found in nearly all of the analyzed organs of the recipients, which is called microchimerism (
Figure 3). We used different methods and confirmed with better methods previous results [
14] and demonstrated that xenotransplantation is like allotransplantation associated with microchimerism (
Table 2,
Table 3). The number of disseminated pig cells depended on the organ and the animal and it was relatively low because they could not be detected using a PCR detecting pig genes such as GAPDH, which are present twice in the pig genome. A higher sensitivity of detection of pig cells was achieved using a PCR detecting PERV sequences which are present up to 60 times in the pig genome [
30] (
Table 2,
Table 3). The pig cells were alive as shown by the detection of GAPDH mRNA (
Table 4). The presence of disseminated pig cells was also supported by previous studies showing disseminated cells expressing PCMV/PRV in different organs of the transplanted baboon [
31]. Since PCMV/PRV does not infect baboon cells, the detected cells should be pig cells.
That the highest amount of PERV sequences was found in the aorta seems logical. The baboon aorta is connected with the pig aorta and the entire blood stream is coming from the pig part of the aorta to the baboon part of the aorta (
Figure 2). However, we cannot exclude that tissue samples were taken from the site of anastomosis. The material collected as pericardium was not well defined. Based on the ct of pGAPDH and hGAPDH it represents a mixture of pig and baboon tissue. In contrast, the left ventricle is mainly pig tissue as demonstrated by the extremely high amount of PERV and pGAPDH sequences. Nevertheless, there are also baboon cells present as shown by the detection of hGAPDH (
Table 2). Whether this result is due to baboon blood cells circulating in the pig heart or whether there are also settled baboon endothelial and other cells is unknown.
Using a real-time PCR for SINE sequences in order to detect pig cells in the transplanted baboons created an extremely sensitive method for the detection of pig cells in recipients. This method will also be useful when screening for pig cells in human patients after xenotransplantation. It will also be useful for the discrimination between PERV infection and microchimerism.
However, the presence of pig genes, especially of SINE sequences, does not automatically mean, that there is no infection of recipient cells. Especially, if only a very few recipient´s cells are infected. A strategy which better discriminates between PERV infection and microchimerism is to study insertion of the virus into the cellular DNA and to determine whether the virus is integrated in pig DNA (pig cells, microchimerism) or in baboon DNA (infection). Such studies were performed in one preclinical trial: After transplantation of pig kidneys into rhesus macaques, PERV sequences were detected in the bladder of the animals [
32]. The authors could demonstrate that PERVs originated from porcine donor cells rather than an integrated provirus in the monkey chromosome. To determine PERV insertion into chromosomes, inverse PCR using PERV long terminal repeat (LTR) region-specific primers was conducted. The presence of pig cells in the monkey bladder after renal xenotransplantation was also demonstrated using specific-porcine mitochondrial DNA gene PCR [
32]. However, assuming that the integration into baboon DNA, e.g., infection, is a very rare event, the number of sequenced amplicons of the inverse PCR was much too low to exclude infection in some cells. This is the general disadvantage of this method.
Based on these results the best method to detect a real PERV infection is to demonstrate antibodies against PERV. The detection of antibodies is the easiest way to detect retrovirus infection and it is used for the diagnosis of human immunodeficiency viruses 1 and 2 (HIV-1, -2) and human T-cell lymphotropic viruses I and II (HTLV-I, -II) [
33], feline immunodeficiency virus (FIV) [
34], bovine leukemia virus (BLV) [
35,
36], and small ruminant lentiviruses (SRLVs) [
37]. Immunological assays such as Western blot assay or ELISA are easy to perform and the sera required for testing can be obtained easily. In the past numerous assays have been developed to detect PERV-specific antibodies, mainly Western blot assays and ELISAs using purified virus, recombinant viral proteins or peptides [
38,
39,
40]. Using these tests, in all cases no antibodies have been detected in animals and humans who had received pig cells or organs and in animals inoculated with high doses of PERV with and without pharmaceutical immunosuppression (for review see [
41,
42]), indicating that until now not a single PERV infection had been observed in vivo. The only exception was a limited PERV infection without evidence of replication but low antibody production in guinea pigs [
43]. The argument that detection of antibodies may be hampered by the fact that the recipients are immunosuppressed can be refuted by numerous publications showing that HIV-1 infected individuals produce a strong antibody response against the virus [
33] and by the fact that patients who received an allotransplant produced antibodies when vaccinated despite transplantation-associated immunosuppression [
44,
45,
46,
47].
Most importantly, the absence of PERV mRNA and genomic RNA in the cells in the baboon tissues (
Table 4) makes it unlikely that viral protein and viral particles will be produced in the pig cells. Consequently, no antiviral antibodies will be produced in the baboon. This was demonstrated by negative Western blot assays in the previous study [
14].
There is another important outcome from this study: We recently proposed to monitor xenotransplant tissue damage and rejection by the detection of cell-free pig DNA using integrated PERV sequences [
17]. This suggestion was based on the finding that free extracellular DNA is a good marker of rejection in allotransplantation and that the use of PERV sequences instead of pig cellular genes makes the method around 60 times more sensitive. Based on the results found here with the SINE sequences we propose now to use SINE sequences which will be a much more powerful approach due to the high copy number in the pig genome. This will enormously increase the sensitivity when screening for free extracellular DNA as marker of transplant rejection.
5. Conclusions
Pig cells have been detected in different tissues of baboons after transplantation of a pig heart. The highly sensitive detection method using primers and a probe for pig SINE is the most effective approach to detect pig cells and to exclude infection of the host.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Sequence and functional domains of PRE-1 (nucleotides 1-233) according Singer et al. [
18]. Forward and reverse primers and probe according Walker et al. [
23] were indicated, the differences in the sequence were shown in green. The sequence of the RNA polymerase III split promotor (nt 16-27 and nt 58-69) and the short direct repeats (nt 91-124, nt 138-170) as postulated by [
18] are underlined; Table S1: Determination of the effective annealing temperature and sensitivity of the PCR detecting SINE sequences (PER-1) in the pig genome.
Author Contributions
Conceptualization, J.D.; methodology, H.J., J.D. and L.K.; validation, H.J., J.D. and B.K., formal analysis, H.J., J.D. and L.K.; investigation, H.J., L.K., M.B., J-M.A. and M.L.; resources, M.M., J.R. and E.N.; data curation, H.J., J.D., M.B., J-M.A. and M.L; writing—original draft preparation, J.D.; writing—review and editing, H.J., L.K., M.B., M.L., B.K. and J.D.; visualization, M.B. and J.D.; supervision, B.R., B.K. and J.D.; project administration, B.R., B.K. and J.D.; funding acquisition, B.R., B.K. and J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deutsche Forschungsgemeinschaft, grant number TRR127.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the Government of Upper Bavaria (protocol code ROB-55.2-2532.Vet_02-14-184, date of approval: February 9th, 2015, and protocol code ROB-55.2-2532.Vet_02-19-158, date of approval: March 11th ,2020) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Denner, J. Microchimerism, PERV and Xenotransplantation. Viruses. 2023, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Bianchi DW, Khosrotehrani K, Way SS, MacKenzie TC, Bajema I, O'Donoghue K. Forever Connected: The Lifelong Biological Consequences of Fetomaternal and Maternofetal Microchimerism. Clin Chem. 2021, 67, 351–362. [Google Scholar] [CrossRef]
- Bianchi, D. W.; Zickwolf, G. K.; Weil, G. J.; Sylvester, S.; DeMaria, M. A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996, 93, 705–708. [Google Scholar] [CrossRef]
- Evans, Paul C. ; Lambert, Nathalie; Maloney, Sean; Furst, Dan E.; Moore, James M.; Nelson, J. Lee. Long-Term Fetal Microchimerism in Peripheral Blood Mononuclear Cell Subsets in Healthy Women and Women With Scleroderma. Blood. 1999, 93, 2033–2037. [Google Scholar]
- Chan WF, Gurnot C, Montine TJ, Sonnen JA, Guthrie KA, Nelson JL Male microchimerism in the human female brain. PLOS ONE. 2012, 7, e45592.
- Rao AS, Thomson AW, Shapiro R, Starzl TE. Chimerism after whole organ transplantation: its relationship to graft rejection and tolerance induction. Curr Opin Nephrol Hypertens. 1994, 3, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Starzl TE, Demetris AJ, Trucco M, Zeevi A, Ramos H, Terasaki P, Rudert WA, Kocova M, Ricordi C, Ildstad S, et al. Chimerism and donor-specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993, 55, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, Ramos H, Todo S, Tzakis A, Fung JJ, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993, 17, 1127–1152. [Google Scholar] [CrossRef]
- Rao AS, Starzl TE, Demetris AJ, Trucco M, Thomson A, Qian S, Murase N, Fung JJ.The two-way paradigm of transplantation immunology. Clin Immunol Immunopathol. 1996; 80(3 Pt 2), S46–S51.
- Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, McMichael J, Fung JJ, Starzl TE. Weaning of immunosuppression in liver transplant recipients. Transplantation 1997, 63, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bloch EM, Jackman RP, Lee TH, Busch MP. Transfusion-associated microchimerism: the hybrid within. Transfus Med Rev. 2013, 27, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Matsagos S, Verigou E, Kourakli A, Alexis S, Vrakas S, Argyropoulou C, Lazaris V, Spyropoulou P, Labropoulou V, Georgara N, Lykouresi M, Karakantza M, Alepi C, Symeonidis A. High Frequency of Post-Transfusion Microchimerism Among Multi-Transfused Beta-Thalassemic Patients. Front Med (Lausanne). 2022, 9, 845490. [Google Scholar]
- Nikbin B, Bonab MM, Talebian F. Microchimerism and stem cell transplantation in multiple sclerosis. Int Rev Neurobiol. 2007, 79, 173–202. [Google Scholar]
- Denner J, Längin M, Reichart B, Krüger L, Fiebig U, Mokelke M, Radan J, Mayr T, Milusev A, Luther F, Sorvillo N, Rieben R, Brenner P, Walz C, Wolf E, Roshani B, Stahl-Hennig C, Abicht JM. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci Rep. 2020, 10, 17531. [Google Scholar] [CrossRef]
- Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, Chapman LE, Lockey C, Onions D, Otto E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science. 1999, 285, 1236–1241. [Google Scholar]
- Wynyard, S.; Garkavenko, O.; Elliot, R. Multiplex high resolution melting assay for estimation of Porcine Endogenous Retrovirus (PERV) relative gene dosage in pigs and detection of PERV infection in xenograft recipients. J. Virol. Methods 2011, 175, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Detection of cell-free pig DNA using integrated PERV sequences to monitor xenotransplant tissue damage and rejection. Xenotransplantation. 2021, 28, e12688. [Google Scholar] [CrossRef]
- Singer, D.S. , Parent, L.J., Ehrlich, R. Identification and DNA sequence of an interspersed repetitive DNA element in the genome of the miniature swine. Nuc. Acids Res. 1987, 15, 2780. [Google Scholar] [CrossRef]
- Ellegren, H. Variable SINE 3' poly(A) sequences: an abundant class of genetic markers in the pig genome. Mamm Genome. 1993, 4, 429–434. [Google Scholar] [CrossRef]
- Yasue H, Takahashi H, Awata T, Popescu PC. Uneven-distribution of short interspersed repetitive sequence, PRE-1, on swine chromosomes. Cell Struct Funct. 1991, 16, 475–479. [Google Scholar] [CrossRef]
- Zhang X, Wang D, Han Y, Duan F, Lv Q, Li Z. Altered imprinted gene expression and methylation patterns in mid-gestation aborted cloned porcine fetuses and placentas. J Assist Reprod Genet. 2014, 31, 1511–1517. [Google Scholar] [CrossRef]
- Tajima K, Enishi O, Amari M, Mitsumori M, Kajikawa H, Kurihara M, Yanai S, Matsui H, Yasue H, Mitsuhashi T, Kawashima T, Matsumoto M. PCR detection of DNAs of animal origin in feed by primers based on sequences of short and long interspersed repetitive elements. Biosci Biotechnol Biochem. 2002, 66, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Walker JA, Hughes DA, Anders BA, Shewale J, Sinha SK, Batzer MA. Quantitative intra-short interspersed element PCR for species-specific DNA identification. Anal Biochem. 2003, 316, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, Dashkevich A, Baehr A, Egerer S, Bauer A, Mihalj M, Panelli A, Issl L, Ying J, Fresch AK, Buttgereit I, Mokelke M, Radan J, Werner F, Lutzmann I, Steen S, Sjöberg T, Paskevicius A, Qiuming L, Sfriso R, Rieben R, Dahlhoff M, Kessler B, Kemter E, Kurome M, Zakhartchenko V, Klett K, Hinkel R, Kupatt C, Falkenau A, Reu S, Ellgass R, Herzog R, Binder U, Wich G, Skerra A, Ayares D, Kind A, Schönmann U, Kaup FJ, Hagl C, Wolf E, Klymiuk N, Brenner P, Abicht JM. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018, 564, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Yang L, Güell M, Niu D, George H, Lesha E, Grishin D, Aach J, Shrock E, Xu W, Poci J, Cortazio R, Wilkinson RA, Fishman JA, Church G. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015, 350, 1101–1104. [Google Scholar] [CrossRef]
- Krüger L, Längin M, Reichart B, Fiebig U, Kristiansen Y, Prinz C, Kessler B, Egerer S, Wolf E, Abicht JM, Denner J. Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons. Viruses. 2019, 11, 650. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Hartl, R.T.; Groiss, S.; & Gemeiner, M.; & Gemeiner, M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J Immunol Methods 2005, 306, 16–27. [Google Scholar] [CrossRef]
- Behrendt R, Fiebig U, Norley S, Gürtler L, Kurth R, Denner J. A neutralization assay for HIV-2 based on measurement of provirus integration by duplex real-time PCR. J Virol Methods. 2009, 159, 40–46. [Google Scholar] [CrossRef]
- Halecker S, Metzger J, Strube C, Krabben L, Kaufer B, Denner J. Virological and Parasitological Characterization of Mini-LEWE Minipigs Using Improved Screening Methods and an Overview of Data on Various Minipig Breeds. Microorganisms. 2021, 9, 2617. [Google Scholar] [CrossRef]
- Fiebig U, Fischer K, Bähr A, Runge C, Schnieke A, Wolf E, Denner J. Porcine endogenous retroviruses: Quantification of the copy number in cell lines, pig breeds, and organs. Xenotransplantation. 2018, 25, e12445. [Google Scholar] [CrossRef]
- Fiebig U, Abicht JM, Mayr T, Längin M, Bähr A, Guethoff S, Falkenau A, Wolf E, Reichart B, Shibahara T, Denner J. Distribution of Porcine Cytomegalovirus in Infected Donor Pigs and in Baboon Recipients of Pig Heart Transplantation. Viruses. 2018, 10, 66. [Google Scholar] [CrossRef]
- Heo, Y.; Cho, Y.; Oh, K.B.; Park, K.H.; Cho, H.; Choi, H.; Kim, M.; Yun, I.J.; Lee, H.J.; Kim, Y.B. Detection of Pig Cells Harboring Porcine Endogenous Retroviruses in Non-Human Primate Bladder After Renal Xenotransplantation. Viruses 2019, 11, 801. [Google Scholar] [CrossRef]
- Schochetman G, Epstein JS, Zuck TF. Serodiagnosis of infection with the AIDS virus and other human retroviruses. Annu Rev Microbiol. 1989, 4, :629–659. [Google Scholar]
- Westman ME, Coggins SJ, van Dorsselaer M, Norris JM, Squires RA, Thompson M, Malik R. Feline immunodeficiency virus (FIV) infection in domestic pet cats in Australia and New Zealand: Guidelines for diagnosis, prevention and management. Aust Vet J. 2022, 100, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Evermann JF, Jackson MK. Laboratory diagnostic tests for retroviral infections in dairy and beef cattle. Vet Clin North Am Food Anim Pract. 1997, 13, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Mammerickx M, Portetelle D, Burny A. The diagnosis of enzootic bovine leukosis. Comp Immunol Microbiol Infect Dis. 1985;8(3-4):305-9Duvigneau, J.C.; Hartl, R.T.; Groiss, S.; & Gemeiner, M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J Immunol Methods 2005, 306, 16–27. [Google Scholar]
- Kalogianni AI, Stavropoulos I, Chaintoutis SC, Bossis I, Gelasakis AI. Serological, Molecular and Culture-Based Diagnosis of Lentiviral Infections in Small Ruminants. Viruses. 2021, 13, 1711. [Google Scholar] [CrossRef]
- Galbraith DN, Kelly HT, Dyke A, Reid G, Haworth C, Beekman J, Shepherd A, Smith KT. Design and validation of immunological tests for the detection of Porcine endogenous retrovirus in biological materials. J Virol Methods. 2000, 90, 115–124. [Google Scholar] [CrossRef]
- Matthews AL, Brown J, Switzer W, Folks TM, Heneine W, Sandstrom PA. Development and validation of a Western immunoblot assay for detection of antibodies to porcine endogenous retrovirus. Transplantation. 1999, 67, 939–943. [Google Scholar] [CrossRef]
- Tacke SJ, Bodusch K, Berg A, Denner J. Sensitive and specific immunological detection methods for porcine endogenous retroviruses applicable to experimental and clinical xenotransplantation. Xenotransplantation. 2001, 8, 125–135. [Google Scholar]
- Denner J, Tönjes RR. Infection barriers to successful xenotransplantation focusing on porcine endogenous retroviruses. Clin Microbiol Rev. 2012, 25, 318–343. [Google Scholar] [CrossRef]
- Denner, J. Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology. 2018, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Argaw T, Colon-Moran W, Wilson CA. Limited infection without evidence of replication by porcine endogenous retrovirus in guinea pigs. Limited infection without evidence of replication by porcine endogenous retrovirus in guinea pigs. J Gen Virol. 2004, 85(Pt 1), 15–19. [Google Scholar]
- Lopez A, Mariette X, Bachelez H, Belot A, Bonnotte B, Hachulla E, Lahfa M, Lortholary O, Loulergue P, Paul S, Roblin X, Sibilia J, Blum M, Danese S, Bonovas S, Peyrin-Biroulet L. Vaccination recommendations for the adult immunosuppressed patient: A systematic review and comprehensive field synopsis. J Autoimmun. 2017, 80, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Giannella M, Righi E, Pascale R, Rinaldi M, Caroccia N, Gamberini C, Palacios-Baena ZR, Caponcello G, Morelli MC, Tamè M, Busutti M, Comai G, Potena L, Salvaterra E, Feltrin G, Cillo U, Gerosa G, Cananzi M, Piano S, Benetti E, Burra P, Loy M, Furian L, Zaza G, Onorati F, Carraro A, Gastaldon F, Nordio M, Kumar-Singh S, Abedini M, Boffetta P, Rodríguez-Baño J, Lazzarotto T, Viale P, Tacconelli E, On Behalf Of The Orchestra Study Group Workpackage. Evaluation of the Kinetics of Antibody Response to COVID-19 Vaccine in Solid Organ Transplant Recipients: The Prospective Multicenter ORCHESTRA Cohort. Microorganisms. 2022, 10, 1021. [Google Scholar] [CrossRef]
- Danziger-Isakov L, Kumar D; AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Vaccination in solid organ transplantation. Am J Transplant. 2013, 13 Suppl 4, 311–317. [Google Scholar]
- Pittet LF, Posfay-Barbe KM. Immunization in transplantation: review of the recent literature. Curr Opin Organ Transplant. 2013, 18, 543–548. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).