1. Introduction
Following the results of the IMbrave150 trial [
1], the combination therapy of atezolizumab bevacizumab (Atez/Bev) has been identified as the most promising systemic treatment for advanced hepatocellular carcinoma (HCC). Atez is a programmed cell death ligand 1 (PD-L1) inhibitor, and Bev is an anti-vascular endothelial growth factor antibody. Although, the objective response rate (ORR) and disease control rate (DCR) of Atez/Bev are reportedly 30% and 74% [
2], respectively, there has been a need to develop a sensitive prognostic stratification tool in HCC patients treated with Atez/Bev. Previously proposed staging systems for HCC include the Barcelona Clinical Liver Cancer (BCLC) staging [
3], Cancer of the Liver Italian Program (CLIP ) score [
4], Japan Integrated Staging (JIS) [
5], and Albumin-Bilirubin-TNM of the Liver Cancer Study Group of Japan 6th edition (LCSGJ 6th) (ALBI-T) score [
6]. These systems consist of tumor burden and hepatic reserve function. On the other hand, systemic chemotherapies are performed in patients with good hepatic reserve function.
However, there are only a few reports on prognostic scores for HCC that reflect the malignant potential of the tumor and the tumor microenvironment (TME) [
7]. Immune checkpoint inhibitor efficacy is influenced by TME; therefore, a prognostic score reflecting TME is needed to predict the prognosis of HCC patients and the efficacy of Atez/Bev. In Japan, three tumor markers (TMs), namely, alpha-fetoprotein (AFP), fucosylated AFP (AFP-L3), and des gamma-carboxy prothrombin (DCP), can be used not only for surveillance of HCC but also for assessment of the malignant potential of HCC in Japan. These TMs have recently been reported to be associated with tumor growth [
8,
9] and TME [
10,
11,
12] in HCC. Although several studies have evaluated the association between number of positive TMs and prognosis [
13,
14,
15,
16,
17,
18,
19], to our knowledge, there have been no reports on such association in HCC patients treated with Atez/Bev.
The present study aimed to evaluate the ability of a previously proposed TM score involving AFP, AFP-L3, and DCP as TMs [
14,
15] in predicting prognosis and therapeutic efficacy in HCC patients administered Atez/Bev as first-line treatment.
2. Materials and Methods
2.1. Patients
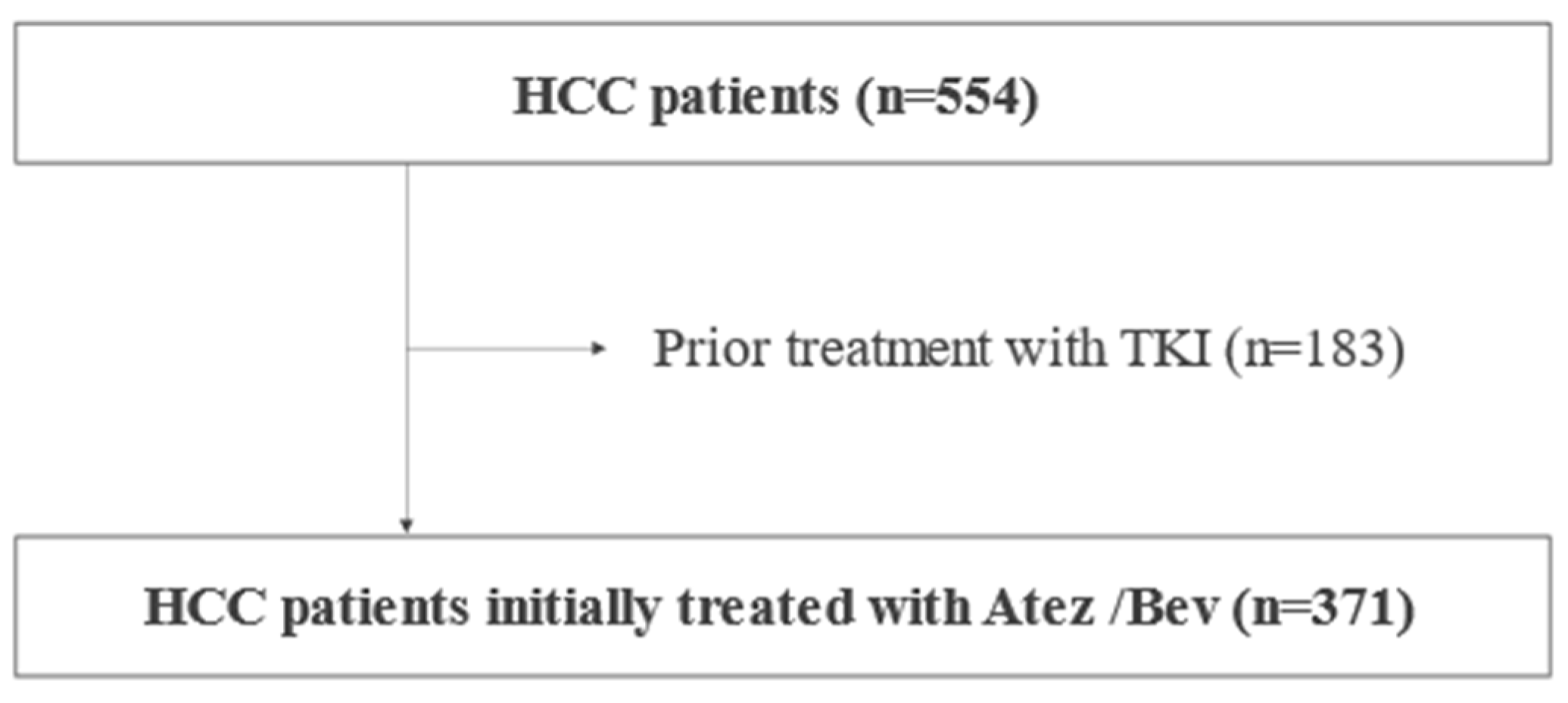
From September 2020 to December 2022, 749 Japanese HCC patients were treated with Atez/Bev at our institutions in Japan. After the exclusion of patients with a systemic pharmacotherapy history, or patients without clinical data regarding TMs, we analyzed the clinical characteristics and prognosis of 371 patients retrospectively (
Figure 1).
The decision to introduce Atez/Bev was made by the attending physician at each of the participating institutions. Treatment with intravenous Atez/Bev, which is composed of 1200 mg of atezolizumab plus bevacizumab at 15 mg/kg of body weight, was given every three weeks [
1] based on the guidelines provided by the manufacturer. Treatment was discontinued following observation of any unacceptable or serious adverse event (AE), or clinical tumor progression. Each patient underwent an upper gastrointestinal endoscopy examination for the surveillance of esophago-gastric varices (EGV) within six months of the introduction of Atez/Bev. When bleeding was detected or in cases with a high risk (EGV F2 or more, positive for red color sign), endoscopic treatment (variceal ligation, or injection sclerotherapy and ligation) was administered before starting Atez/Bev therapy.
After receiving official approval, this study was conducted as a retrospective analysis of database records based on the Guidelines for Clinical Research issued by the Ministry of Health and Welfare of Japan. All procedures were conducted in accordance with the Declaration of Helsinki. Written informed consent was received from each of the enrolled patients.
2.2. Basal hepatic disease and assessment methods for hepatic function
Patients with positive anti-hepatitis C virus (HCV) test result were judged to have HCC due to HCV, and patients with positive hepatitis B virus (HBV) surface antigen (HBsAg) test result were judged to have HCC due to HBV. Patients with alcohol-induced HCC were defined as having a history of excessive alcohol consumption of > 60 g/day for five years or more. Patients not matching the above features were classified as others. Child-Pugh classification [
20], albumin-bilirubin (ALBI) grade, [
21] and modified ALBI (mALBI) grade, in which ALBI grade 2 was divided into two subgrades (2a and 2b) using an ALBI score of -2.27 as the cut-off value, [
22] were used to assess hepatic reserve function.
2.3. Diagnosis and therapeutic strategies for HCC
HCC was diagnosed based on hyperattenuation at the arterial phase or hypoattenuation at the portal phase, as determined using dynamic computed tomography (CT) or magnetic resonance imaging (MRI) with tumor staining on angiography. Tumor stage was evaluated using the BCLC stage. The treatment strategy for HCC in this study followed the
Clinical Practice Guidelines for Hepatocellular Carcinoma published by the Japan Society of Hepatology [
23].
2.4. Assessment of treatment response
Treatment response was assessed using dynamic CT or MRI, according to the Response Evaluation Criteria in Solid Tumors (RECIST ver 1.1) [
24]. The overall response rate (ORR) and disease control rate (DCR) were calculated based on the radiological response. ORR was defined as complete response or partial response of patients. DCR was defined as complete response, partial response, and stable disease of patients. Overall survival (OS) was defined as the time from the date of Atez/Bev treatment initiation to the date of death or last visit. Progression-free survival (PFS) was defined as the time from Atez/Bev treatment initiation to observation of clinical disease progression or death.
2.5. Methods for assessment of prognosis
The TM score consisted of AFP, AFP-L3, and DCP, and was measured before the start of Atez/Bev therapy. Elevations in the AFP (≥ 100 ng/ml), AFP-L3 (≥ 10%), and DCP (≥ 100 mAU/ml) values were defined as 1 point each, according to previous reports [
15,
16,
25]. A prognostic score was generated by summing up the number of positive TMs. All patients were graded according to the TM score from 0 to 3. TM score, BCLC stage, Japan Integrated Staging (JIS), and mALBI-T were used for the evaluation of prognosis, and their predictive abilities for OS and PFS were compared retrospectively. JIS was calculated using Child-Pugh class and TNM LCSGJ 6
th edition and had 6 grades from scores 0 to 5. mALBI-T was similarly calculated using mALBI grade, instead of Child-Pugh class, and the TNM LCSGJ 6
th edition and had 7 grades from scores 0 to 6.
2.6. Statistical analysis
Median values and interquartile range (IQR) were used to express continuous variables. Statistical analysis was performed using Student’s t-test, Fischer’s exact test, and Mann-Whitney’s U-test. After the introduction of Atez/Bev, PFS and OS were evaluated using the Kaplan-Meier method and log-rank test. Clinical prognostic factors for PFS and OS were analyzed using Cox-hazard analysis with age, gender (female), etiology (viral), mALBI grade ≥ 2b, BCLC stage, and TM score. Concordance index (c-index) was used for the evaluation of ability for stratification and prediction of prognosis by each method. The Holm’s method was used for multiple comparisons. A p-value of < 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using Easy R (EZR), ver. 1.56 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [
26].
3. Results
3.1. Characteristics of the HCC patients
The median age of the 371 HCC patients was 74 years, with 78.4% being men. About 80% of these patients showed an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, and their median body mass index (BMI) was 23.6 kg/m2 (IQR 21.2-26.1). The median observation period was 10.4 months. The basal liver diseases were viral hepatitis (n = 180, 48.5%), alcoholic liver disease (n = 76, 20.5%), and others (n = 115, 31.0%).
A Child-Pugh score of 5 was shown in 225 (60.6%) patients, a score of 6 in 103 (27.8%), and a score of 7 or more in 43 (11.6%). The median ALBI score was -2.47 (IQR -2.75- -2.14). mALBI grade 1 was shown in 144 (38.8%) patients, grade 2a in 93 (25.1%), grade 2b in 126 (34.0%), and grade 3 in 8 (2.2%). BCLC stage 0 was shown in 4 (1.1%) patients, stage A in 18 (4.9%), stage B in 142 (38.3%), stage C in 196 (52.8%), and stage D in 11 (3.0%) (
Table 1).
3.2. Prognosis of HCC patients treated with Atez/Bev
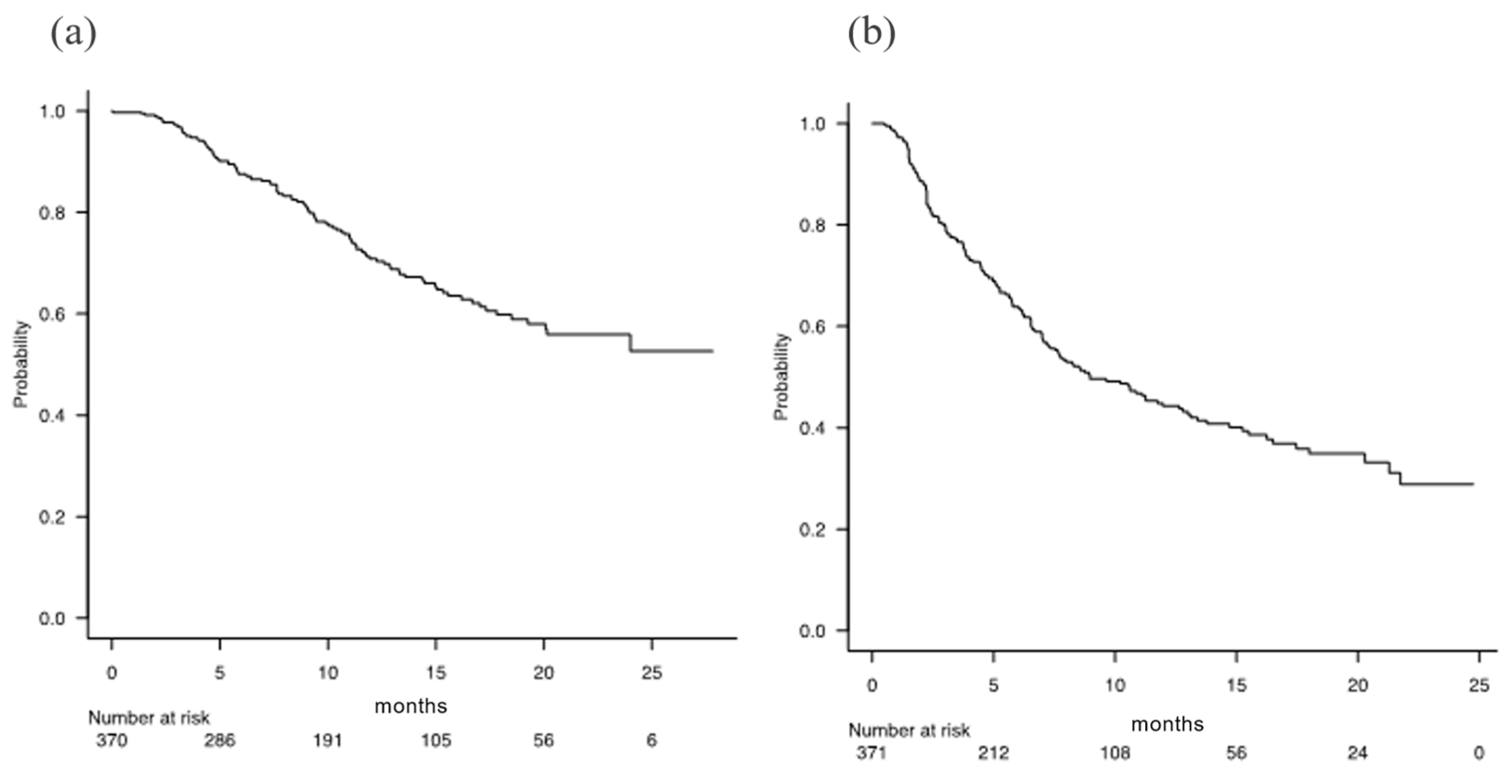
For all the 371 patients, the median OS was not applicable [NA] (95% CI 20.1-NA), and the median PFS was 8.9 months (95% CI 7.3-12.6) (
Figure 2). During the follow-up period, 106 patients (28.6%) died and 187 (50.4%) had invalid discontinuation of Atez/Bev. The best radiological responses using RECIST ver 1.1 were as follows: complete response (CR) in 17 (5.0%) patients, partial response (PR) in 87 (25.6%), stable disease (SD) in 172 (50.6%), and progressive disease (PD) in 64 (18.8%) (after exclusion of patients without imaging evaluation: n = 31).
3.3. Tumor marker score
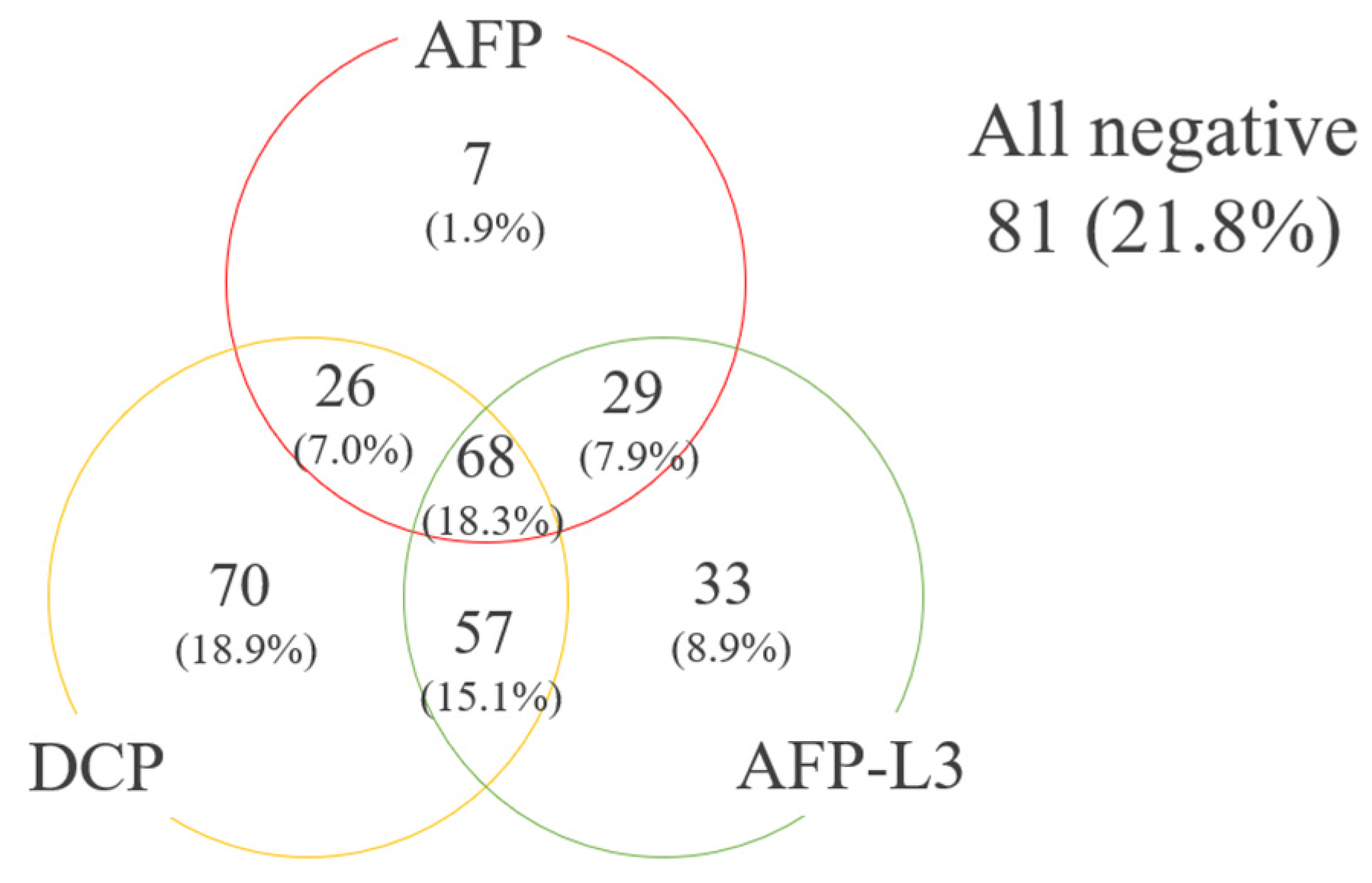
Among the 371 subjects, 130 (35.0%) showed positivity for AFP (≥ 100 ng/ml), 186 (50.1%) for AFP-L3 (≥ 10 %), and 220 (59.3%) for DCP (≥ 100 mAU/ml). A TM score of 0 was shown in 81 (21.8%) HCC patients, a score of 1 in 110 (29.6%), a score of 2 in 112 (29.9%), and a score of 3 in 68 (18.3%) (
Figure 3). Of the 110 with a TM score 1, 7 (6.4%) were AFP-positive.
3.4. Overall survival and progression-free survival according to elevation of each tumor marker
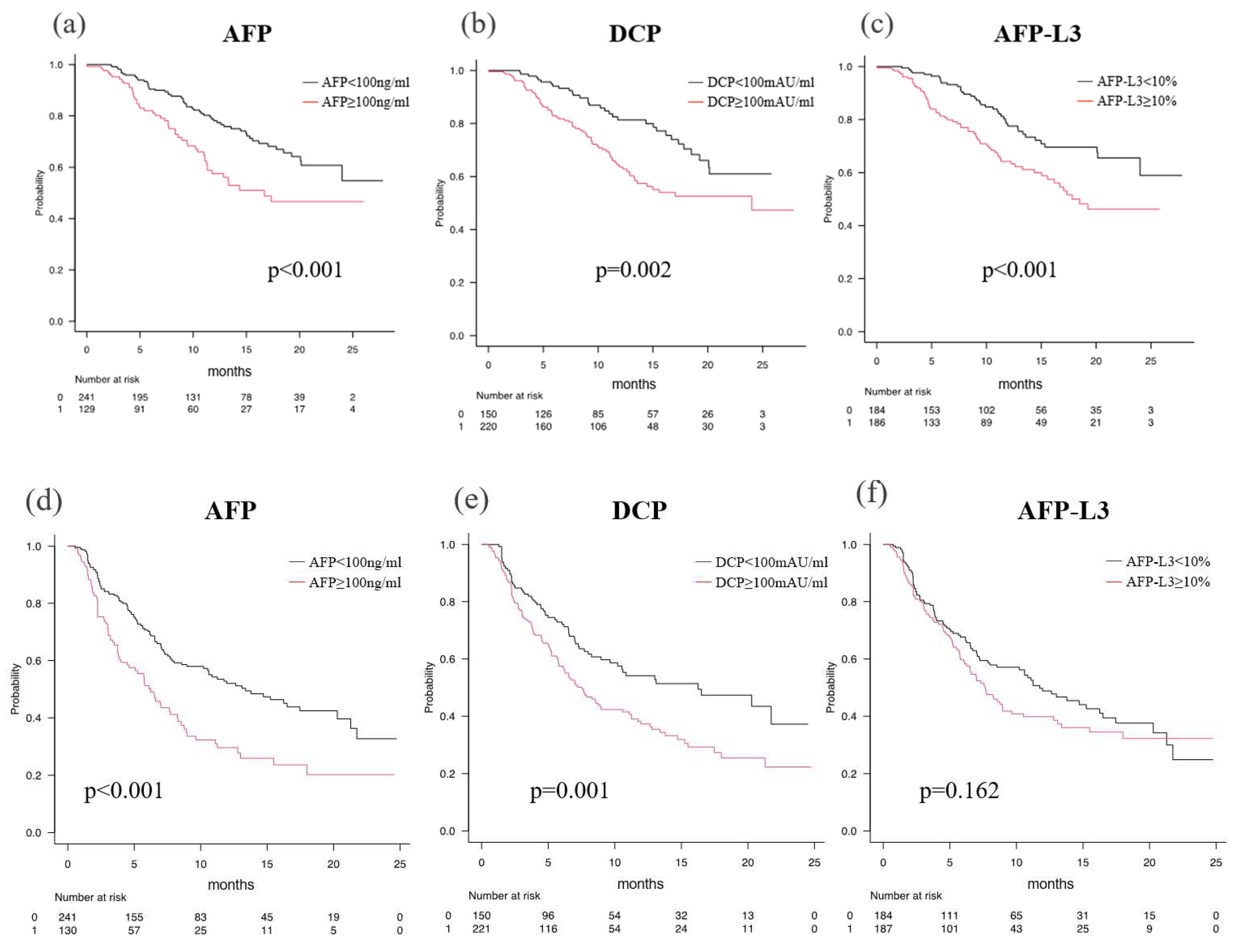
OS was better in patients without TM elevation than in patients with TM elevation according to the TM: the median OS (mOS) with AFP (< 100 ng/ml) was NA (95% CI: 24.0-NA), whereas the mOS with AFP (≥ 100 ng/ml) was 16.7 months (95% CI: 11.3-NA) (p < 0.001) (
Figure 4a). The mOS with AFP-L3 (< 10%) was NA (95% CI: 24.0-NA), whereas the mOS with AFP-L3 (≥ 10 %) was 17.8 months (95% CI: 15.0-NA) (p < 0.001) (
Figure 4b). The mOS with DCP (< 100 mAU/ml) was NA (95% CI: 20.1-NA), whereas the mOS with DCP (≥ 100 mAU/ml) was 24.0 months (95% CI: 13.5-NA) (p < 0.001) (
Figure 4c).
The approximate HRs of each TM for OS were as follows: AFP (≥ 100 ng/ml) (HR 1.890), AFP-L3 (≥ 10%) (HR 1.928), and DCP (≥ 100 mAU/ml) (HR 1.915) (
Table 2).
The median PFS (mPFS) was 13.4 months (95% CI: 10.2-20.3) for AFP (< 100 ng/ml), and 6.3 months (95% CI: 4.5-8.3) for AFP (≥ 100 ng/ml) (p < 0.001) (
Figure 4d). The mPFS was 16.3 months (95% CI: 9.6-NA) for DCP (< 100 mAU/ml) and 7.5 months (95% CI: 6.1-8.9) for DCP (≥ 100 mAU/ml) (
Figure 4e). AFP-L3 did not show a significant difference in PFS between patients without and with elevation [11.8 months (95% CI: 8.0-16.3) vs. 7.6 months (95% CI: 6.3-8.9)] (
Figure 4f). However, the HRs for PFS according to TM were nearly similar for AFP (≥ 100 ng/ml) and DCP (≥ 100 mAU/ml), but not for AFP-L3 (> 10%) (HRs: AFP 1.865, DCP 1.636, and AFP-L3 1.230) (
Table 2).
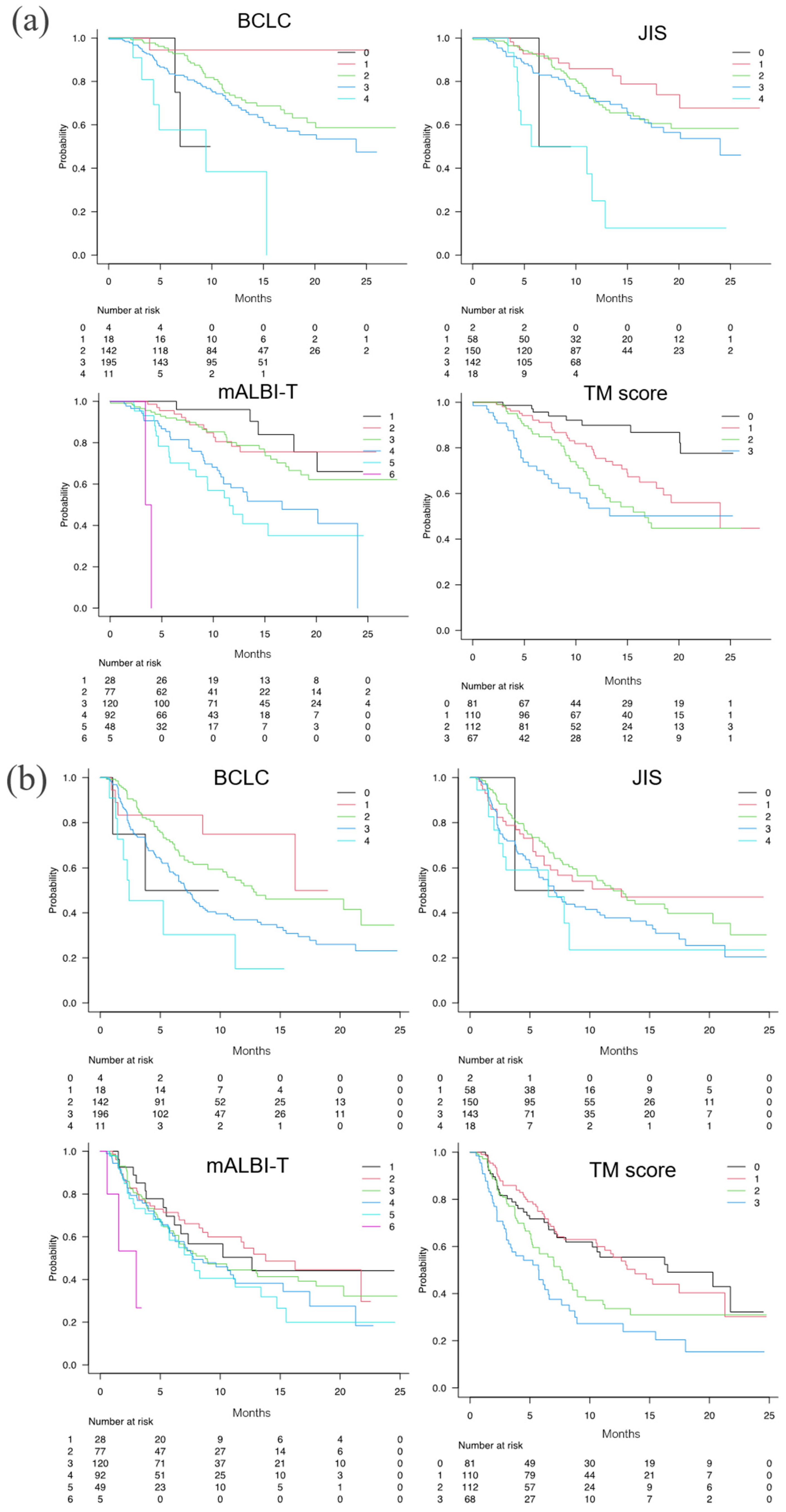
3.5. Predictive value according to TM score, BCLC stage, JIS, and mALBI-T score
The numbers of patients with a radiological response are shown in
Table 3. The ORR/DCR was 31.9%/80.6% for TM score 0, 29.5%/88.6% for score 1, 35.3%/79.4%for score 2, and 23.0%/7.21% for score 3, with no significant differences between the groups.
The mOS times for TM scores 0, 1, 2, and 3 were NA (95% CI NA-NA), 24.0 months (95% CI 17.8-NA), 16.7 months (95% CI 12.3-NA), and NA (95% CI 8.3-NA), respectively (p < 0.001, c-index 0.658) (
Figure 5a). The HR for death increased with increasing positive number of TMs (HR: TM score 1 = 2.525, TM score 2 = 3.796, TM score 3 = 4.977) (
Table 2). Although all the integrated scores were able to stratify the survival of patients, the c-index for TM score (0.658) was equal to those of the mALBI-T score (0.658) and superior to those of the BCLC stage (0.577) and JIS (0.586) in the present unresectable HCC patients treated with Atez/Bev.
The mPFS times for TM scores 0, 1, 2, and 3 were stratified as 16.5 months (95% CI 8.0-NA), 13.8 (95% CI 10.6-21.3), 7.7 months (95% CI 5.3-8.9), and 5.8 months (95% CI 3.0-7.6), respectively (p < 0.001; c-index 0.594) (
Figure 5b). The HR for PFS showed no significant difference between patients with a TM score of 0 and a TM score of 1, but the HR increased as the number of scores became more than a TM score of 2 (HR: TM score 2 = 1.595, score 3 = 2.345) (
Table 2). Although all the integrated scores were able to stratify the mPFS, the c-index of TM score (0.594) was superior to those of mALBI-T (0.547), BCLC stage (0.580), and JIS (0.554).
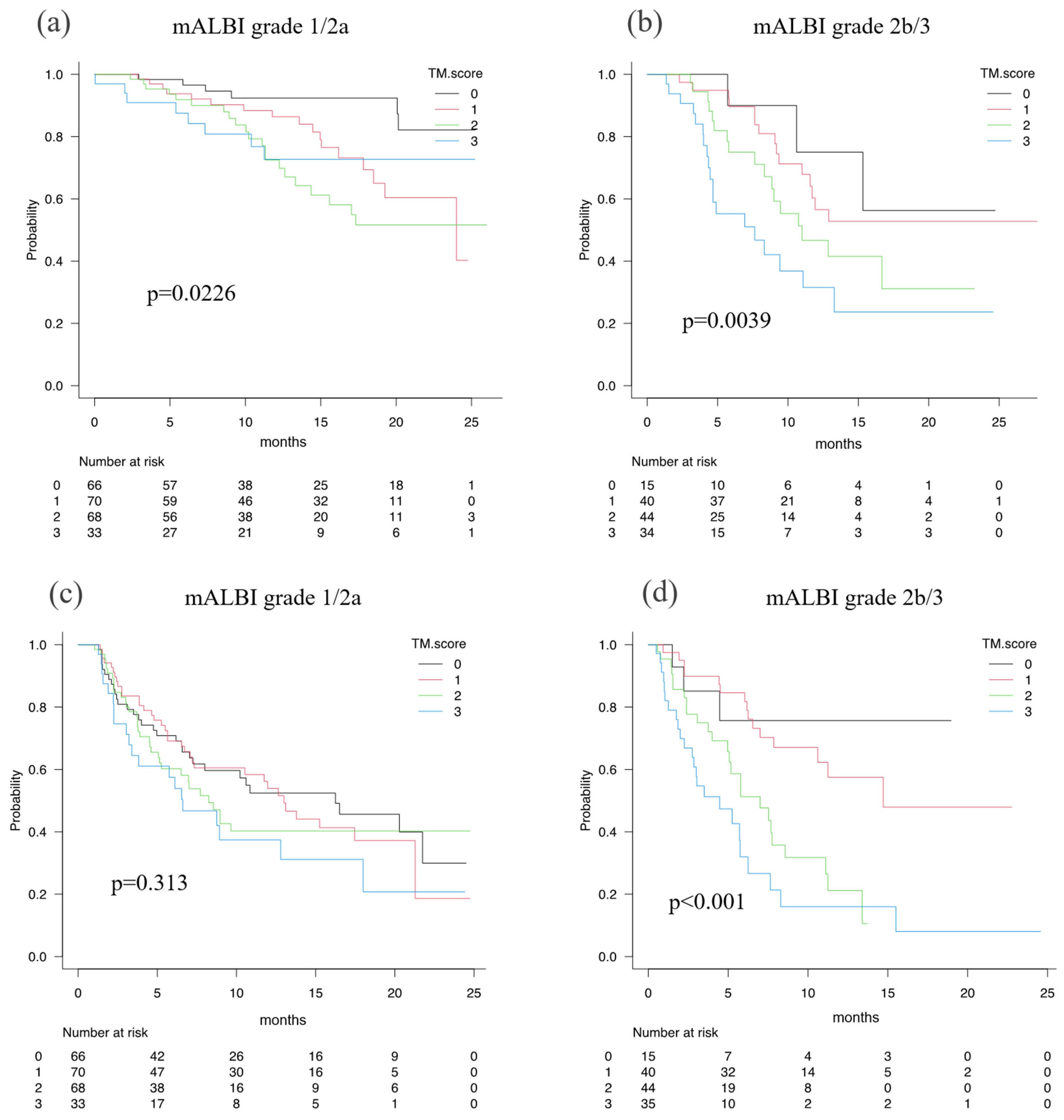
When the abilities of the TM score to predict the prognosis of HCC patients in terms of OS and PFS were examined according to mALBI 1/2a vs. mALBI 2b/3, OS was favorably stratified in the patients with mALBI 1/2a by TM score (p = 0.023, c-index 0.63), as well as in the patients with mALBI 2b/3 (p = 0.004, c-index 0.66). PFS was well stratified in the patients with mALBI 2b/3 (p < 0.001, c-index 0.67), but not in the patients with mALBI 1/2a (p = 0.313, c-index 0.55) (
Figure 6).
Examinations of the prognostic factors for death on Cox hazard univariate analysis showed that age (HR 1.035, 95% CI 1.011-1.060, p = 0.004), mALBI grade ≥ 2b (HR 2.804, 95% CI 1.908-4.120, p < 0.001), BCLC stage (HR 1.514, 95% CI 1.106-2.073, p = 0.009), and TM score (HR 1.586, 95% CI 1.307-1.924, p < 0.001) were significant prognostic factors. Furthermore, on multivariate analysis, TM score (HR 1.480, 95% CI 1.210-1.812, p < 0.001), mALBI grade ≥ 2b (HR 1.560, 95% CI 1.736-3.774, p < 0.001), and age (HR 1.037, 95% CI 1.013-1.061, p = 0.002) were also significant prognostic factors (
Table 4).
4. Discussion
In the present study, we found two key points. First, the present TM score, which is calculated based on the positive number of TMs, showed a favorable prognostic stratification ability for OS and PFS in HCC patients who received Atez/Bev therapy as a first-line systemic chemotherapy. Second, although the stratification ability of the TM score for OS was comparable to that of the mALBI-T score, the TM score was superior to all the other integrated scores in predicting PFS.
Previously, Ryu et al. [
13] described that the number of positive TMs correlates with the tumor size and prevalence of microvascular invasion, and is associated with a poorly differentiated pathological character of tumors. Kiriyama et al. [
14] showed that the recurrence rate in HCC patients with a triple-positive TM was the highest among HCC patients after radical resection. Ueno et al. [
17] reported that tumor recurrence after radiofrequency ablation is influenced by the number of positive TMs.
To the best of our knowledge, this is the first report to examine the prognostic stratification ability of the TM score in HCC patients treated with Atez/Bev. Several previous studies have shown that AFP, AFP-L3, and DCP can stratify the prognosis of HCC because AFP, AFP-L3, and DCP are considered to reflect the malignant potential of HCC [
8,
9,
10,
11,
12]. An elevated AFP has been shown to have an association with vascular invasion, poor differentiation, and the presence of satellite lesions in HCC [
27,
28,
29]. Moreover, AFP-L3 has been found to be associated with tumor burden (tumor number and tumor size) and the frequency of macrovascular invasion [
30,
31], whereas DCP has been found to be associated with tumor burden, occurrence of portal vein tumor invasion, and worse histological tumor grade [
32,
33,
34,
35]. Thus, it is thought reasonable to consider the TM score as a favorable prognostic predictive scoring system even for HCC patients treated with Atez/Bev. The HRs for OS and PFS became larger with increasing TM score. In addition, AFP and DCP have been reported to reflect not only tumor burden but also TME.
AFP is known to alter the proportion of CD4+/CD8+ T cells [
36] and to tilt TME toward immunosuppression by suppressing dendritic cells [
11] and NK cells [
10]. DCP has also been reported to be associated with CTNNB1 mutations [
37], and CTNNB1 mutations have been shown to be a factor for the poor response to the immune checkpoint inhibitor [
12,
38]. Therefore, it is plausible that the TM score can also predict PFS in HCC patients treated with Atez/Bev. The HR for PFS also increased with increasing positive number of TMs. Thus, the present TM score has the advantage of being easily used and is applicable to daily clinical practice.
The total staging system for HCC using the CLIP score [
4] includes AFP, BCLC staging [
3], JIS [
5], and ALBI-T score [
6] which basically indicate the tumor burden and hepatic reserve function. As the selection of therapeutic modalities depends on tumor burden and hepatic reserve function, these stagings are considered to be reasonable as a total staging system for predicting prognosis. On the other hand, systemic therapies for HCC including Atez/Bev are performed for unresectable HCC with a high tumor burden; however, these are recommended to be introduced in patients with better hepatic reserve function (Child-Pugh A). As a result, most of the patients who received Atez/Bev therapy usually have a good hepatic function. Therefore, the present TM score, which reflects tumor grade and TME, is considered to have a better prognostic value than the conventional integrated score, which consists of tumor progression and liver reserve.
In the present study, the predictive ability of the TM score for PFS was different between HCC patients with mALBI grades 1/2a and 2b/3. This might suggest that the hepatic reserve function may affect TME. In fact, liver cirrhosis is a state of immunodeficiency due to the excessive secretion of inflammatory cytokines [
39], and a poorer hepatic reserve function, such as mALBI 2b/3, may worsen the microimmune environment and subsequently worsen TME as implied by elevated TMs. OS was significantly longer than PFS in HCC patients with mALBI 1/2a; however, the difference between PFS and OS was not significant for each score in HCC patients with mALBI 2b/3. These results were thought to be due to the therapeutic efficacy of postprogression treatments. To obtain improvement of prognosis in unresectable HCC, introducing systemic treatment is recommended in HCC patients with better condition and better hepatic reserve function as early as possible. Although transarterial catheter chemo embolization (TACE) has been performed as the initial recommended treatment for BCLC-B patients, the concept of TACE-refractory for switching to systemic treatment before a decline in the hepatic reserve function cause by repeated TACE has been reported recently [
40]. Previously, Hiraoka et al. [
15] reported that TACE-refractory was more likely in BCLC-B patients with a TM score of 2 or greater. Moreover, Kudo et al. proposed the concept of TACE-unsuitable, indicating the clinical features of resistance to TACE [
41]. The newest BCLC strategy [
42] recommends the introduction of systemic treatment prior to TACE in TACE-unsuitable BCLC-B patients. Early transition from TACE to systemic pharmacotherapy or prior systemic pharmacotherapy can lead to a better prognosis. Based on the present results, wherein HCC patients with a TM score of < 2 showed a very favorable prognosis when Atez/Bev was administered, Atez/Bev should be introduced to BCLC-B patients treated with TACE before reaching a TM score ≥ 2 if there is a poor response to treatment and TM values increase gradually.
This study has several limitations. First, although this is a multicenter study with a large cohort size, this study has a retrospective nature. Second, the observation period may not be sufficient. Prospective studies with longer observation periods are recommended to obtain a more definitive conclusion. Third, although AFP ≥ 100 ng/mL, AFP-L3 ≥ 10%, and DCP ≥ 100 mAU/mL were used in this study based on previous reports, more optimal cut-off values for this prognostic predicting system should be examined for patients with unresectable HCC treated with Atez/Bev.
5. Conclusions
The TM score involving AFP, AFP-L3, and DCP as TMs was useful in predicting prognosis and therapeutic efficacy in terms of OS and PFS in HCC patients administered Atez/Bev as a first-line treatment.
Author Contributions
Kazunari Tanaka, Atsushi Hiraoka and Takashi Kumada conceived the study, and participated in its design and coordination. Kazunari Tanaka, Atsushi Hiraoka, Toshifumi Tada, Masashi Hirooka, Kazuya Kariyama, Joji Tani, Masanori Atsukawa, Koichi Takaguchi, Ei Itobayashi, Shinya Fukunishi, Kunihiko Tsuji, Toru Ishikawa, Kazuto Tajiri, Hironori Ochi, Satoshi Yasuda, Hidenori Toyoda, Chikara Ogawa, Takashi Nishimura, Takeshi Hatanaka, Satoru Kakizaki, Noritomo Shimada, Kazuhito Kawata, Atsushi Naganuma, Hisashi Kosaka, Tomomitsu Matono, Yutaka Yata, Hideko Ohama, Fujimasa Tada, Kazuhiro Nouso, Asahiro Morishita, Akemi Tsutsui, Takuya Nagano, Norio Itokawa, Tomomi Okubo, Taeang Arai, Keisuke Yokohama, Hiroki Nishikawa, Michitaka Imai, Yohei Koizumi, Shinichiro Nakamura, Hiroko Iijima, Masaki Kaibori, Yoichi Hiasa, and Takashi Kumada performed data curation. Kazunari Tanaka performed statistical analyses and interpretation. Kazunari Tanaka, Kunihiko Tsuji and Atsushi Hiraoka drafted the text. All authors have read and approved the final version of the manuscript.
Institutional Review Board Statement
The entire study protocol was approved by the Institutional Ethics Committee of Ehime Prefectural Central Hospital (no. 30-66). After receiving the official approval, this study was conducted as a retrospective analysis of database records based on the Guidelines for Clinical Research issued by the Ministry of Health and Welfare of Japan. All procedures were performed in accordance with the declaration of Helsinki. The data were made anonymous before analysis to protect patient privacy. Written informed consent was obtained from all patients before treatment and this study received ethical approval for use of an opt-out methodology based on low risk to the participants.
Data Availability Statement
.Due to the nature of this research, the participants could not be contacted as to whether the findings could be shared publicly, thus supporting data, including datasets generated and/or analyzed for the current study, are not publicly available
Conflicts of Interest
Atsushi Hiraoka, MD, PhD: lecture fees from Chugai, Eli Lilly, AstraZeneca. Toshifumi Tada MD, PhD: lecture fees from AbbVie, Eisai, Chugai. Takeshi Hatanaka MD, PhD: lecture fees from Eisai. Other authors: No potential conflicts of interest to declare.
References
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2021, 76, 862–873. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in liver disease 1999, 19, 329–338. [Google Scholar] [CrossRef]
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998, 28, 751–755. [CrossRef]
- Kudo, M.; Chung, H.; Osaki, Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). Journal of gastroenterology 2003, 38, 207–215. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Michitaka, K.; Kudo, M. Newly Proposed ALBI Grade and ALBI-T Score as Tools for Assessment of Hepatic Function and Prognosis in Hepatocellular Carcinoma Patients. Liver cancer 2019, 8, 312–325. [Google Scholar] [CrossRef]
- Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, Himmelsbach V, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol 2022;76:353-363.
- Suzuki, M.; Shiraha, H.; Fujikawa, T.; Takaoka, N.; Ueda, N.; Nakanishi, Y.; Koike, K.; Takaki, A.; Shiratori, Y. Des-gamma-carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinoma. J Biol Chem 2005, 280, 6409–6415. [Google Scholar] [CrossRef]
- Yue, P.; Gao, Z.H.; Xue, X.; Cui, S.X.; Zhao, C.R.; Yuan, Y.; Yin, Z.; Inagaki, Y.; Kokudo, N.; Tang, W.; et al. Des-γ-carboxyl prothrombin induces matrix metalloproteinase activity in hepatocellular carcinoma cells by involving the ERK1/2 MAPK signalling pathway. Eur J Cancer 2011, 47, 1115–1124. [Google Scholar] [CrossRef]
- Guerra, N.; Tan, Y.X.; Joncker, N.T.; Choy, A.; Gallardo, F.; Xiong, N.; Knoblaugh, S.; Cado, D.; Greenberg, N.M.; Raulet, D.H. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008, 28, 571–580. [Google Scholar] [CrossRef]
- Pardee, A.D.; Shi, J.; Butterfield, L.H. Tumor-derived α-fetoprotein impairs the differentiation and T cell stimulatory activity of human dendritic cells. J Immunol 2014, 193, 5723–5732. [Google Scholar] [CrossRef]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov 2019, 9, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.; Takami, Y.; Wada, Y.; Tateishi, M.; Matsushima, H.; Mikagi, K.; Saitsu, H. Double- and Triple-Positive Tumor Markers Predict Early Recurrence and Poor Survival in Patients with Hepatocellular Carcinoma within the Milan Criteria and Child-Pugh Class A. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2017, 21, 957–966. [Google Scholar] [CrossRef]
- Kiriyama, S.; Uchiyama, K.; Ueno, M.; Ozawa, S.; Hayami, S.; Tani, M.; Yamaue, H. Triple positive tumor markers for hepatocellular carcinoma are useful predictors of poor survival. Annals of surgery 2011, 254, 984–991. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M.; et al. Prediction of Prognosis of Intermediate-Stage HCC Patients: Validation of the Tumor Marker Score in a Nationwide Database in Japan. Liver cancer 2019, 8, 403–411. [Google Scholar] [CrossRef]
- Hiraoka, A.; Ishimaru, Y.; Kawasaki, H.; Aibiki, T.; Okudaira, T.; Toshimori, A.; Kawamura, T.; Yamago, H.; Nakahara, H.; Suga, Y.; et al. Tumor Markers AFP, AFP-L3, and DCP in Hepatocellular Carcinoma Refractory to Transcatheter Arterial Chemoembolization. Oncology 2015, 89, 167–174. [Google Scholar] [CrossRef]
- Ueno, M.; Hayami, S.; Shigekawa, Y.; Kawai, M.; Hirono, S.; Okada, K.; Tamai, H.; Shingaki, N.; Mori, Y.; Ichinose, M.; et al. Prognostic impact of surgery and radiofrequency ablation on single nodular HCC ⩽5 cm: Cohort study based on serum HCC markers. J Hepatol 2015, 63, 1352–1359. [Google Scholar] [CrossRef]
- Toyoda, H.; Kumada, T.; Tada, T.; Sone, Y.; Kaneoka, Y.; Maeda, A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer 2015, 4, 126–136. [Google Scholar] [CrossRef]
- Toyoda, H.; Kumada, T.; Osaki, Y.; Oka, H.; Urano, F.; Kudo, M.; Matsunaga, T. Staging Hepatocellular Carcinoma by a Novel Scoring System (BALAD Score) Based on Serum Markers. Clin. Gastroenterol. Hepatol. 2006, 4, 1528–1536. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. The British journal of surgery 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O'Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M.; et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017, 6, 325–336. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Seymour, L.; Litière, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1 - Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur J Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Kariyama, K.; Toyoda, H.; Yasuda, S.; Tsuji, K.; Hatanaka, T.; Kakizaki, S.; Naganuma, A.; Ishikawa, T.; et al. Simple Scoring System for Predicting TACE Unsuitable among Intermediate-Stage Hepatocellular Carcinoma Patients in the Multiple Systemic Treatment Era. Oncology 2022, 100, 65–73. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone marrow transplantation 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Peng, S.Y.; Chen, W.J.; Lai, P.L.; Jeng, Y.M.; Sheu, J.C.; Hsu, H.C. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer 2004, 112, 44–50. [Google Scholar] [CrossRef]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hosaka, T.; Ikeda, K.; Seko, Y.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, F.; Suzuki, Y.; Saitoh, S.; et al. Highly sensitive AFP-L3% assay is useful for predicting recurrence of hepatocellular carcinoma after curative treatment pre- and postoperatively. Hepatol Res 2011, 41, 1036–1045. [Google Scholar] [CrossRef]
- Farinati, F.; Marino, D.; De Giorgio, M.; Baldan, A.; Cantarini, M.; Cursaro, C.; Rapaccini, G.; Del Poggio, P.; Di Nolfo, M.A.; Benvegnù, L.; et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006, 101, 524–532. [Google Scholar] [CrossRef]
- Imamura, H.; Matsuyama, Y.; Miyagawa, Y.; Ishida, K.; Shimada, R.; Miyagawa, S.; Makuuchi, M.; Kawasaki, S. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg 1999, 86, 1032–1038. [Google Scholar] [CrossRef]
- Fujiyama, S.; Tanaka, M.; Maeda, S.; Ashihara, H.; Hirata, R.; Tomita, K. Tumor markers in early diagnosis, follow-up and management of patients with hepatocellular carcinoma. Oncology 2002, 62 Suppl 1, 57–63. [Google Scholar] [CrossRef]
- Shimada, M.; Takenaka, K.; Fujiwara, Y.; Gion, T.; Kajiyama, K.; Maeda, T.; Shirabe, K.; Sugimachi, K. Des-gamma-carboxy prothrombin and alpha-fetoprotein positive status as a new prognostic indicator after hepatic resection for hepatocellular carcinoma. Cancer 1996, 78, 2094–2100. [Google Scholar] [CrossRef]
- Miyaaki, H.; Nakashima, O.; Kurogi, M.; Eguchi, K.; Kojiro, M. Lens culinaris agglutinin-reactive alpha-fetoprotein and protein induced by vitamin K absence II are potential indicators of a poor prognosis: a histopathological study of surgically resected hepatocellular carcinoma. J Gastroenterol 2007, 42, 962–968. [Google Scholar] [CrossRef]
- Gao, Q.; Qiu, S.J.; Fan, J.; Zhou, J.; Wang, X.Y.; Xiao, Y.S.; Xu, Y.; Li, Y.W.; Tang, Z.Y. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007, 25, 2586–2593. [Google Scholar] [CrossRef]
- Ahn, K.S.; O'Brien, D.R.; Kim, Y.H.; Kim, T.S.; Yamada, H.; Park, J.W.; Park, S.J.; Kim, S.H.; Zhang, C.; Li, H.; et al. Associations of Serum Tumor Biomarkers with Integrated Genomic and Clinical Characteristics of Hepatocellular Carcinoma. Liver Cancer 2021, 10, 593–605. [Google Scholar] [CrossRef]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef]
- Bonnel, A.R.; Bunchorntavakul, C.; Reddy, K.R. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2011, 9, 727–738. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Kadoya, M.; Okusaka, T.; Miyayama, S.; Yamakado, K.; Tsuchiya, K.; Ueshima, K.; Hiraoka, A.; et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014, 87 Suppl 1, 22–31. [Google Scholar] [CrossRef]
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).