Submitted:
26 June 2023
Posted:
26 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
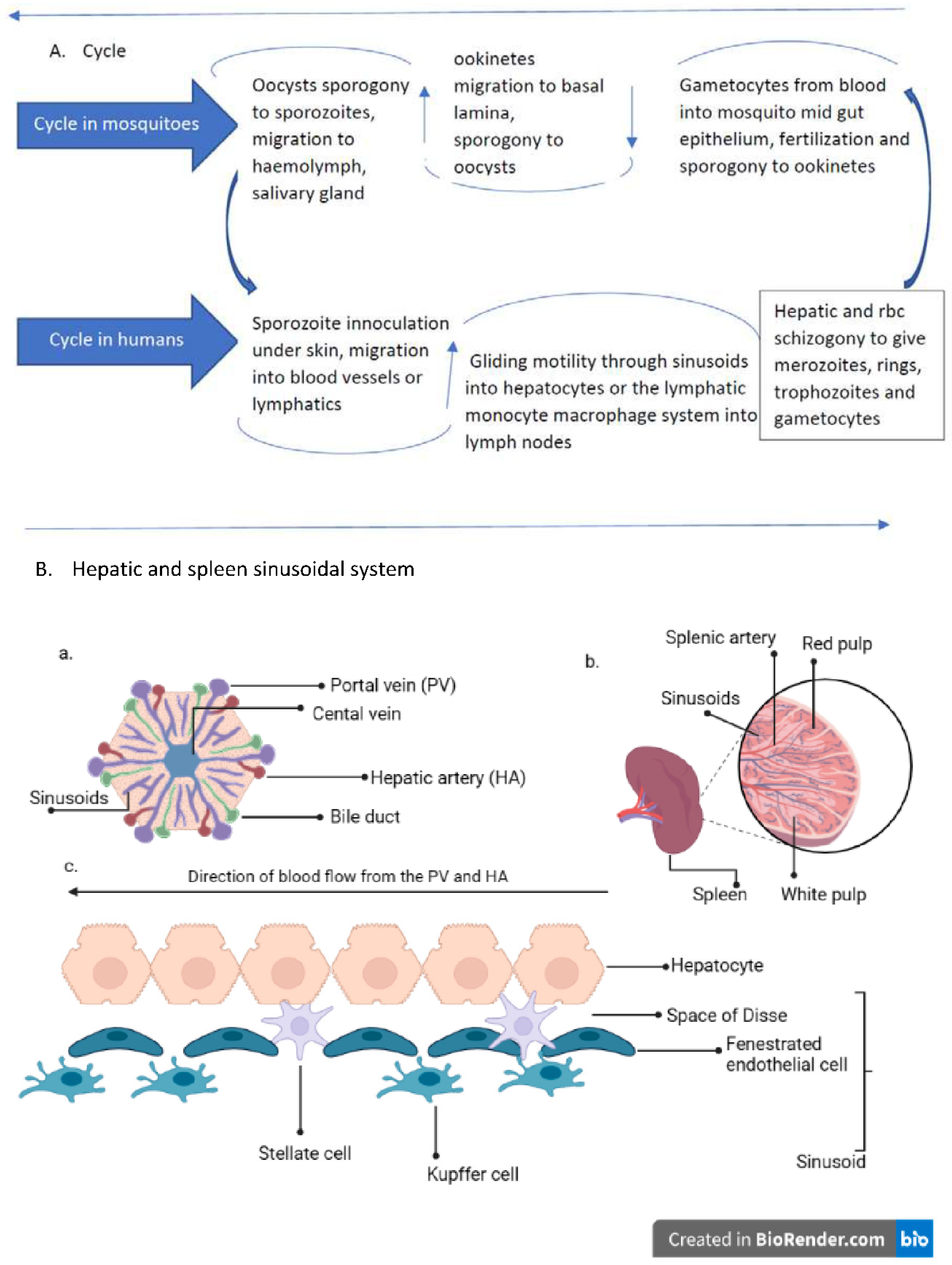
2. Human Plasmodium parasites life cycle and their recently found tissue niches
2.1. Sporozoite inoculation and invasion of tissues
2.2. Entry into extrahepatic tissues/cryptic sites
2.3. Development of asexual and sexual forms
2.4. The reticuloendothelial (RES)/mononuclear phagocyte system (MPS) in Plasmodium life cycle
2.5. RES/MPS as host sites of cryptic infections by Plasmodium parasites
2.6. Plasmodium vivax conundrum and strategic activities in an infection
3. Trends in malaria transmission in Botswana from the year 2008 - 2018
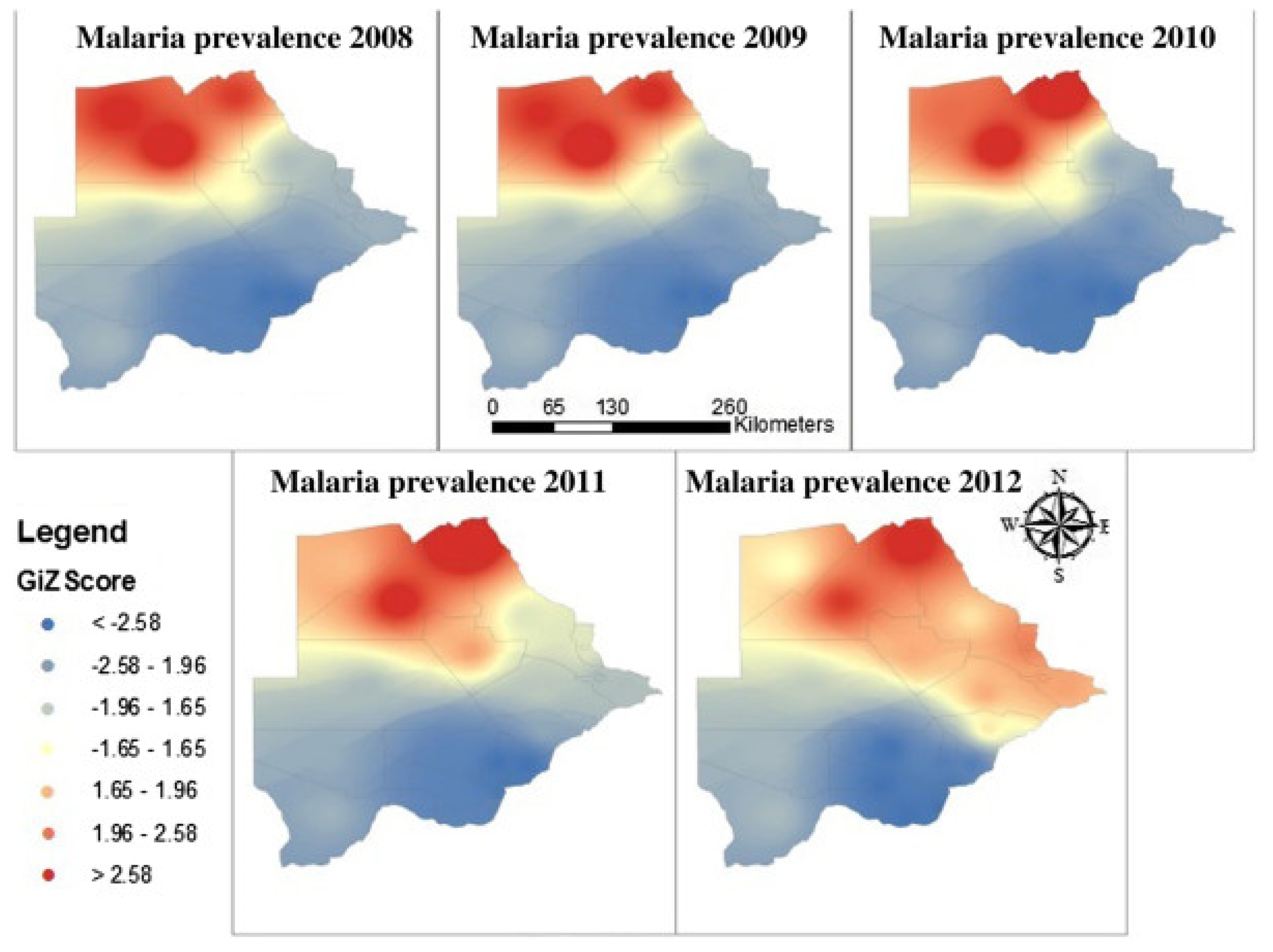
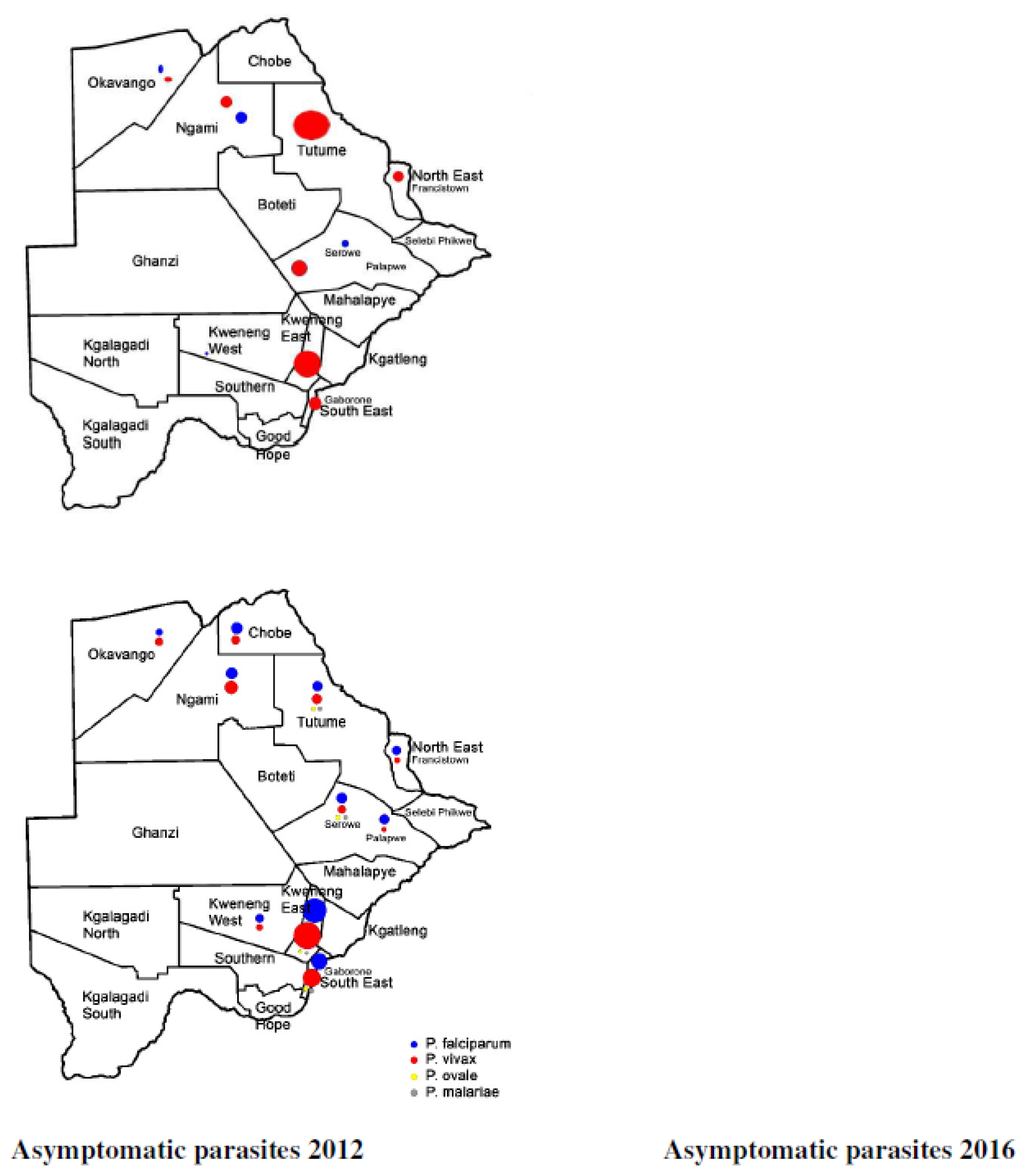
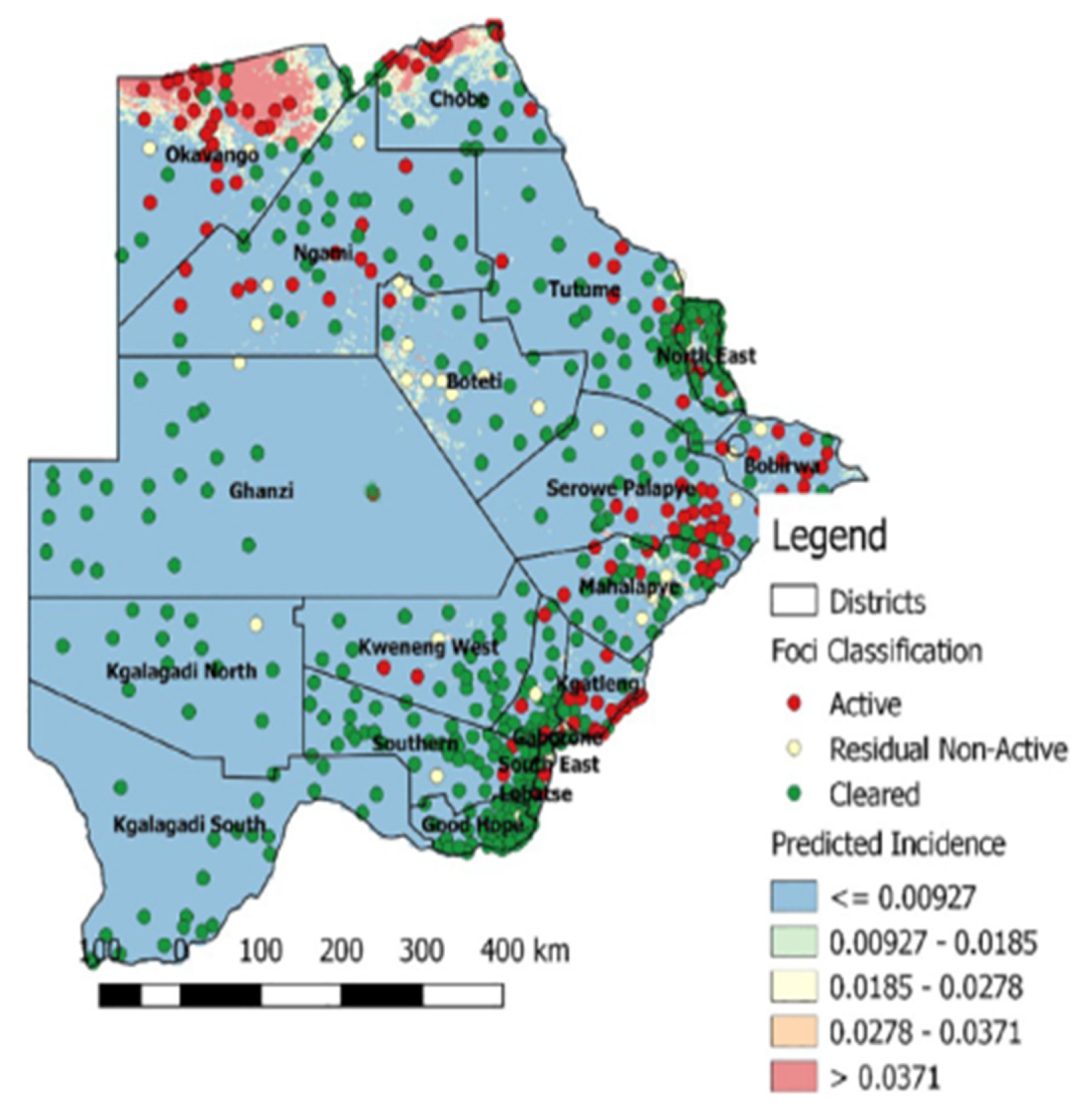
4. Discussion
5. Conclusion
Funding
Acknowledgments
Conflicts of Interest
References
- Feachem, R.G.A.; Chen, I.; Akbari, O.; Bertozzi-Villa, A.; Bhatt, S.; Binka, F.; Boni, M.F.; Buckee, C.; Dieleman, J.; Dondorp, A.; et al. Malaria eradication within a generation: Ambitious, achievable, and necessary. Lancet 2019, 394, 1056–1112. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, R.N.; Drakeley, C.; Djimde, A.A.; Hall, B.F.; Hay, S.I.; Hemingway, J.; Kaslow, D.C.; Noor, A.; Okumu, F.; Steketee, R.; et al. malERA: An updated research agenda for malaria elimination and eradication. PLoS Med. 2017, 14, e1002456. [Google Scholar] [CrossRef] [PubMed]
- malERA Refresh Consultative Panel on Tools for Malaria Elimination. malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication. PLoS Med. 2017, 14, e1002455. [Google Scholar] [CrossRef] [PubMed]
- Dowdle, W.R.; Cochi, S.L. The principles and feasibility of disease eradication. Vaccine 2011, 29 (Suppl. S4), D70–D73. [Google Scholar] [CrossRef]
- malERA Refresh Consultative Panel on Health Systems and Policy Research. malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication. PLoS Med. 2017, 14, e1002454. [Google Scholar] [CrossRef]
- malERA Refresh Consultative Panel on Characterising the Reservoir and Measuring Transmission. malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017, 14, e1002452. [Google Scholar] [CrossRef]
- Alegana, V.A.; Wright, J.; Bosco, C.; Okiro, E.A.; Atkinson, P.M.; Snow, R.W.; Tatem, A.J.; Noor, A.M. Malaria prevalence metrics in low- and middle-income countries: An assessment of precision in nationally-representative surveys. Malar J. 2017, 16, 475. [Google Scholar] [CrossRef]
- malERA Refresh Consultative Panel on Characterising the Reservoir and Measuring Transmission. malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017, 14, e1002452. [Google Scholar] [CrossRef]
- Quaye, I.K.; Aleksenko, L.; Oeuvray, C.; Yewhalaw, D.; Duah, N.; Gyan, B.; Haiyambo, D.H.; Dongho, G.B.D.; Torgby, R.A.; Amoah, L.; et al. The Pan African Vivax and Ovale Network (PAVON): Refocusing on Plasmodium vivax, ovale and asymptomatic malaria in sub-Saharan Africa. Parasitol. Int. 2021, 84, 102415. [Google Scholar] [CrossRef]
- Yman, V.; Wandell, G.; Mutemi, D.D.; Miglar, A.; Asghar, M.; Hammar, U.; Karlsson, M.; Lind, I.; Nordfjell, C.; Rooth, I.; et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLoS Negl Trop. Dis. 2019, 13, e0007414. [Google Scholar] [CrossRef]
- Ruas, R.; Pinto, A.; Nuak, J.; Sarmento, A.; Abreu, C. Non-falciparum malaria imported mainly from Africa: A review from a Portuguese hospital. Malar J. 2017, 16, 298. [Google Scholar] [CrossRef]
- Naing, C.; Whittaker, M.A.; Nyunt Wai, V.; Mak, J.W. Is Plasmodium vivax malaria a severe malaria?: A systematic review and meta-analysis. PLoS Negl Trop. Dis. 2014, 8, e3071. [Google Scholar] [CrossRef]
- Moonen, B.; Cohen, J.M.; Snow, R.W.; Slutsker, L.; Drakeley, C.; Smith, D.L.; Abeyasinghe, R.R.; Rodriguez, M.H.; Maharaj, R.; Tanner, M.; et al. Operational strategies to achieve and maintain malaria elimination. Lancet 2010, 376, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Lover, A.A.; Baird, J.K.; Gosling, R.; Price, R.N. Malaria Elimination: Time to Target All Species. Am. J. Trop. Med. Hyg. 2018, 99, 17–23. [Google Scholar] [CrossRef]
- Frost, W.H. Some conceptions of epidemics in general by Wade Hampton Frost. Am. J. Epidemiol. 1976, 103, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Coosemans, M.; Wery, M.; Mouchet, J.; Carnevale, P. Transmission factors in malaria epidemiology and control in Africa. Mem. Inst. Oswaldo Cruz 1992, 87 (Suppl. S3), 385–391. [Google Scholar] [CrossRef]
- Sutherland, C.J.; Tanomsing, N.; Nolder, D.; Oguike, M.; Jennison, C.; Pukrittayakamee, S.; Dolecek, C.; Hien, T.T.; do Rosario, V.E.; Arez, A.P.; et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 2010, 201, 1544–1550. [Google Scholar] [CrossRef]
- Ponnudurai, T.; Lensen, A.H.; van Gemert, G.J.; Bensink, M.P.; Bolmer, M.; Meuwissen, J.H. Sporozoite load of mosquitoes infected with Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Formaglio, P.; Medvinsky, A.; Menard, R.; Amino, R. Imaging sporozoite cell traversal in the liver of mice. Methods Mol. Biol. 2013, 923, 401–410. [Google Scholar] [CrossRef]
- Amino, R.; Thiberge, S.; Shorte, S.; Frischknecht, F.; Menard, R. Quantitative imaging of Plasmodium sporozoites in the mammalian host. C. R. Biol. 2006, 329, 858–862. [Google Scholar] [CrossRef]
- Vanderberg, J.P. Development of infectivity by the Plasmodium berghei sporozoite. J. Parasitol. 1975, 61, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sidjanski, S.; Vanderberg, J.P. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am. J. Trop. Med. Hyg. 1997, 57, 426–429. [Google Scholar] [CrossRef]
- Roth, A.; Adapa, S.R.; Zhang, M.; Liao, X.; Saxena, V.; Goffe, R.; Li, S.; Ubalee, R.; Saggu, G.S.; Pala, Z.R.; et al. Unraveling the Plasmodium vivax sporozoite transcriptional journey from mosquito vector to human host. Sci. Rep. 2018, 8, 12183. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.M.; Pradel, G.; Vanderberg, J.P.; Hafalla, J.C.; Frevert, U.; Nussenzweig, R.S.; Nussenzweig, V.; Rodriguez, A. Migration of Plasmodium sporozoites through cells before infection. Science 2001, 291, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Loubens, M.; Marinach, C.; Paquereau, C.; Hamada, S.; Hoareau-Coudert, B.; Akbar, D.; Franetich, J.; Silvie, O. The claudin-like apicomplexan microneme protein is required for gliding motility and infectivity of Plasmodium sporozoites. PLoS Pathog. 2023, 19, e1011261. [Google Scholar] [CrossRef] [PubMed]
- Pradel, G.; Frevert, U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology 2001, 33, 1154–1165. [Google Scholar] [CrossRef]
- Frischknecht, F.; Matuschewski, K. Plasmodium Sporozoite Biology. Cold Spring Harb Perspect. Med. 2017, 7, a025478. [Google Scholar] [CrossRef]
- Frevert, U.; Usynin, I.; Baer, K.; Klotz, C. Nomadic or sessile: Can Kupffer cells function as portals for malaria sporozoites to the liver? Cell. Microbiol. 2006, 8, 1537–1546. [Google Scholar] [CrossRef]
- Wake, K. Cell-cell organization and functions of 'sinusoids' in liver microcirculation system. J. Electron. Microsc. (Tokyo) 1999, 48, 89–98. [Google Scholar] [CrossRef]
- Krettli, A.U.; Dantas, L.A. Which routes do Plasmodium sporozoites use for successful infections of vertebrates? Infect. Immun. 2000, 68, 3064–3065. [Google Scholar] [CrossRef]
- Baer, K.; Roosevelt, M.; Clarkson, A.B.J.; van Rooijen, N.; Schnieder, T.; Frevert, U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell. Microbiol. 2007, 9, 397–412. [Google Scholar] [CrossRef]
- Baer, K.; Klotz, C.; Kappe, S.H.I.; Schnieder, T.; Frevert, U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 2007, 3, e171. [Google Scholar] [CrossRef]
- Malleret, B.; Renia, L.; Russell, B. The unhealthy attraction of Plasmodium vivax to reticulocytes expressing transferrin receptor 1 (CD71). Int. J. Parasitol. 2017, 47, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Kho, S.; Qotrunnada, L.; Leonardo, L.; Andries, B.; Wardani, P.A.I.; Fricot, A.; Henry, B.; Hardy, D.; Margyaningsih, N.I.; Apriyanti, D.; et al. Hidden Biomass of Intact Malaria Parasites in the Human Spleen. N. Engl. J. Med. 2021, 384, 2067–2069. [Google Scholar] [CrossRef] [PubMed]
- Lepay, D.A.; Nathan, C.F.; Steinman, R.M.; Murray, H.W.; Cohn, Z.A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J. Exp. Med. 1985, 161, 1079–1096. [Google Scholar] [CrossRef]
- Nusrat, A.R.; Wright, S.D.; Aderem, A.A.; Steinman, R.M.; Cohn, Z.A. Properties of isolated red pulp macrophages from mouse spleen. J. Exp. Med. 1988, 168, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, K.; Hentzschel, F.; Valkiunas, G.; Marti, M. Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat. Rev. Microbiol. 2020, 18, 177–189. [Google Scholar] [CrossRef]
- Sturm, A.; Amino, R.; van de Sand, C.; Regen, T.; Retzlaff, S.; Rennenberg, A.; Krueger, A.; Pollok, J.; Menard, R.; Heussler, V.T. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 2006, 313, 1287–1290. [Google Scholar] [CrossRef]
- Yahata, K.; Hart, M.N.; Davies, H.; Asada, M.; Wassmer, S.C.; Templeton, T.J.; Treeck, M.; Moon, R.W.; Kaneko, O. Gliding motility of Plasmodium merozoites. Proc. Natl. Acad. Sci. USA 2021, 118, e2114442118. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011, 10, 297. [Google Scholar] [CrossRef]
- Mancio-Silva, L.; Gural, N.; Real, E.; Wadsworth, M.H.; Butty, V.L.; March, S.; Nerurkar, N.; Hughes, T.K.; Roobsoong, W.; Fleming, H.E.; et al. A single-cell liver atlas of Plasmodium vivax infection. Cell. Host Microbe 2022, 30, 1048–1060.e5. [Google Scholar] [CrossRef] [PubMed]
- Hentzschel, F.; Gibbins, M.P.; Attipa, C.; Beraldi, D.; Moxon, C.A.; Otto, T.D.; Marti, M. Host cell maturation modulates parasite invasion and sexual differentiation in Plasmodium berghei. Sci. Adv. 2022, 8, eabm7348. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, C.; Joy, D.A. Host-Malaria Parasite Interactions and Impacts on Mutual Evolution. Front. Cell. Infect. Microbiol. 2020, 10, 587933. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.G. Malaria in Nyasaland: (Section of Tropical Diseases and Parasitology). Proc. R. Soc. Med. 1935, 28, 391–404. [Google Scholar] [PubMed]
- Hawking, F.; Wilson, M.E.; Gammage, K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1971, 65, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Bantuchai, S.; Imad, H.; Nguitragool, W. Plasmodium vivax gametocytes and transmission. Parasitol. Int. 2022, 87, 102497. [Google Scholar] [CrossRef]
- Enger, A.; Strand, O.A.; Ranheim, T.; Hellum, K.B. Exflagellation of microgametocytes in Plasmodium vivax malaria: A diagnostic conundrum. Med. Princ Pract. 2004, 13, 298–300. [Google Scholar] [CrossRef]
- Baton, L.A.; Ranford-Cartwright, L.C. Do malaria ookinete surface proteins P25 and P28 mediate parasite entry into mosquito midgut epithelial cells? Malar J. 2005, 4, 15. [Google Scholar] [CrossRef]
- Prudencio, M.; Rodriguez, A.; Mota, M.M. The silent path to thousands of merozoites: The Plasmodium liver stage. Nat. Rev. Microbiol. 2006, 4, 849–856. [Google Scholar] [CrossRef]
- Real, E.; Howick, V.M.; Dahalan, F.A.; Witmer, K.; Cudini, J.; Andradi-Brown, C.; Blight, J.; Davidson, M.S.; Dogga, S.K.; Reid, A.J.; et al. A single-cell atlas of Plasmodium falciparum transmission through the mosquito. Nat. Commun. 2021, 12, 3196. [Google Scholar] [CrossRef]
- Bray, R.S.; Garnham, P.C. The life-cycle of primate malaria parasites. Br. Med. Bull. 1982, 38, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Smalley, M.E.; Abdalla, S.; Brown, J. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Joice, R.; Nilsson, S.K.; Montgomery, J.; Dankwa, S.; Egan, E.; Morahan, B.; Seydel, K.B.; Bertuccini, L.; Alano, P.; Williamson, K.C.; et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci. Transl. Med. 2014, 6, 244re5. [Google Scholar] [CrossRef] [PubMed]
- De Niz, M.; Meibalan, E.; Mejia, P.; Ma, S.; Brancucci, N.M.B.; Agop-Nersesian, C.; Mandt, R.; Ngotho, P.; Hughes, K.R.; Waters, A.P.; et al. Plasmodium gametocytes display homing and vascular transmigration in the host bone marrow. Sci. Adv. 2018, 4, eaat3775. [Google Scholar] [CrossRef]
- Lee, R.S.; Waters, A.P.; Brewer, J.M. A cryptic cycle in haematopoietic niches promotes initiation of malaria transmission and evasion of chemotherapy. Nat. Commun. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Wake, K.; Kawai, Y.; Smedsrod, B. Re-evaluation of the reticulo-endothelial system. Ital. J. Anat. Embryol. 2001, 106, 261–269. [Google Scholar]
- van Furth, R.; Cohn, Z.A.; Hirsch, J.G.; Humphrey, J.H.; Spector, W.G.; Langevoort, H.L. Mononuclear phagocytic system: New classification of macrophages, monocytes and of their cell line. Bull. World Health Organ. 1972, 47, 651–658. [Google Scholar]
- Yona, S.; Gordon, S. From the Reticuloendothelial to Mononuclear Phagocyte System - The Unaccounted Years. Front. Immunol. 2015, 6, 328. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Guilliams, M.; De Kleer, I.; Henri, S.; Post, S.; Vanhoutte, L.; De Prijck, S.; Deswarte, K.; Malissen, B.; Hammad, H.; Lambrecht, B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013, 210, 1977–1992. [Google Scholar] [CrossRef]
- Okabe, Y.; Medzhitov, R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 2014, 157, 832–844. [Google Scholar] [CrossRef]
- Pradel, G.; Garapaty, S.; Frevert, U. Proteoglycans mediate malaria sporozoite targeting to the liver. Mol. Microbiol. 2002, 45, 637–651. [Google Scholar] [CrossRef]
- Hussain, T.; Linera-Gonzalez, J.; Beck, J.M.; Fierro, M.A.; Mair, G.R.; Smith, R.C.; Beck, J.R. The PTEX Pore Component EXP2 Is Important for Intrahepatic Development during the Plasmodium Liver Stage. mBio 2022, 13, e0309622-22. [Google Scholar] [CrossRef] [PubMed]
- Comazzetto, S.; Shen, B.; Morrison, S.J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell. 2021, 56, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Groom, A.C.; Schmidt, E.E.; MacDonald, I.C. Microcirculatory pathways and blood flow in spleen: New insights from washout kinetics, corrosion casts, and quantitative intravital videomicroscopy. Scanning Microsc. 1991, 5, 159–173, discussion 173-4. [Google Scholar] [PubMed]
- Steiniger, B.; Barth, P.; Hellinger, A. The perifollicular and marginal zones of the human splenic white pulp : Do fibroblasts guide lymphocyte immigration? Am. J. Pathol. 2001, 159, 501–512. [Google Scholar] [CrossRef]
- Buffet, P.A.; Safeukui, I.; Deplaine, G.; Brousse, V.; Prendki, V.; Thellier, M.; Turner, G.D.; Mercereau-Puijalon, O. The pathogenesis of Plasmodium falciparum malaria in humans: Insights from splenic physiology. Blood 2011, 117, 381–392. [Google Scholar] [CrossRef]
- Johnston, B.; Butcher, E.C. Chemokines in rapid leukocyte adhesion triggering and migration. Semin. Immunol. 2002, 14, 83–92. [Google Scholar] [CrossRef]
- Nakhaei-Nejad, M.; Hussain, A.M.; Zhang, Q.; Murray, A.G. Endothelial PI 3-kinase activity regulates lymphocyte diapedesis. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3608–H3616. [Google Scholar] [CrossRef]
- Hliscs, M.; Millet, C.; Dixon, M.W.; Siden-Kiamos, I.; McMillan, P.; Tilley, L. Organization and function of an actin cytoskeleton in Plasmodium falciparum gametocytes. Cell. Microbiol. 2015, 17, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Itkin, T.; Gur-Cohen, S.; Spencer, J.A.; Schajnovitz, A.; Ramasamy, S.K.; Kusumbe, A.P.; Ledergor, G.; Jung, Y.; Milo, I.; Poulos, M.G.; et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 2016, 532, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Angrisano, F.; Robinson, L.J. Plasmodium vivax - How hidden reservoirs hinder global malaria elimination. Parasitol. Int. 2022, 87, 102526. [Google Scholar] [CrossRef] [PubMed]
- Commons, R.J.; Simpson, J.A.; Watson, J.; White, N.J.; Price, R.N. Estimating the Proportion of Plasmodium vivax Recurrences Caused by Relapse: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2020. [Google Scholar] [CrossRef]
- Del Portillo, H.A.; Ferrer, M.; Brugat, T.; Martin-Jaular, L.; Langhorne, J.; Lacerda, M.V.G. The role of the spleen in malaria. Cell. Microbiol. 2012, 14, 343–355. [Google Scholar] [CrossRef]
- Lapp, S.A.; Korir-Morrison, C.; Jiang, J.; Bai, Y.; Corredor, V.; Galinski, M.R. Spleen-dependent regulation of antigenic variation in malaria parasites: Plasmodium knowlesi SICAvar expression profiles in splenic and asplenic hosts. PLoS ONE 2013, 8, e78014. [Google Scholar] [CrossRef]
- Betterman, K.L.; Harvey, N.L. The lymphatic vasculature: Development and role in shaping immunity. Immunol. Rev. 2016, 271, 276–292. [Google Scholar] [CrossRef]
- Landau, I.; Chabaud, A.G.; Vuong, P.N.; Deharo, E.; Gautret, P. Circulation in the lymphatic system and latency of Plasmodium merozoites. Preliminary note. Parasite 1995, 2, 185–186. [Google Scholar] [CrossRef]
- Iwakiri, Y. The lymphatic system: A new frontier in hepatology. Hepatology 2016, 64, 706–707. [Google Scholar] [CrossRef]
- Gray, E.E.; Cyster, J.G. Lymph node macrophages. J. Innate Immun. 2012, 4, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Giusiano, G. The Trojan Horse Model in Paracoccidioides: A Fantastic Pathway to Survive Infecting Human Cells. Front. Cell. Infect. Microbiol. 2021, 10, 605679. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.M.; Oghumu, S.; Gupta, G.; McGwire, B.S.; Drew, M.E.; Satoskar, A.R. Mechanisms of cellular invasion by intracellular parasites. Cell Mol. Life Sci. 2014, 71, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- Kronstad, J.W.; Hu, G.; Choi, J. The cAMP/Protein Kinase A Pathway and Virulence in Cryptococcus neoformans. Mycobiology 2011, 39, 143–150. [Google Scholar] [CrossRef]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef]
- Arredondo, S.A.; Schepis, A.; Reynolds, L.; Kappe, S.H.I. Secretory Organelle Function in the Plasmodium Sporozoite. Trends Parasitol. 2021, 37, 651–663. [Google Scholar] [CrossRef]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.; Duraisingh, M.T. The Molecular Basis of Erythrocyte Invasion by Malaria Parasites. Cell. Host Microbe 2017, 22, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Motshoge, T.; Ababio, G.K.; Aleksenko, L.; Read, J.; Peloewetse, E.; Loeto, M.; Mosweunyane, T.; Moakofhi, K.; Ntebele, D.S.; Chihanga, S.; et al. Molecular evidence of high rates of asymptomatic P. vivax infection and very low P. falciparum malaria in Botswana. BMC Infect. Dis. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Motshoge, T.; Haiyambo, D.H.; Ayanful-Torgby, R.; Aleksenko, L.; Ntebela, D.; Malleret, B.; Renia, L.; Peloewetse, E.; Paganotti, G.M.; Quaye, I.K. Recent Molecular Assessment of Plasmodium vivax and Plasmodium falciparum Asymptomatic Infections in Botswana. Am. J. Trop. Med. Hyg. 2021. [Google Scholar] [CrossRef]
- Moonasar, D.; Maharaj, R.; Kunene, S.; Candrinho, B.; Saute, F.; Ntshalintshali, N.; Morris, N. Towards malaria elimination in the MOSASWA (Mozambique, South Africa and Swaziland) region. Malar J. 2016, 15, 419. [Google Scholar] [CrossRef]
- Chihanga, S.; Haque, U.; Chanda, E.; Mosweunyane, T.; Moakofhi, K.; Jibril, H.B.; Motlaleng, M.; Zhang, W.; Glass, G.E. Malaria elimination in Botswana, 2012-2014: Achievements and challenges. Parasit. Vectors 2016, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.C.; Mason, S.J.; Phindela, T.; Connor, S.J. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am. J. Trop. Med. Hyg. 2005, 73, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Moakofhi, K.; Mosweunyane, T.; Jibril, H.B.; Nkomo, B.; Motlaleng, M.; Ntebela, D.S.; Chanda, E.; Haque, U. Malaria control in Botswana, 2008-2012: The path towards elimination. Malar J. 2013, 12, 458. [Google Scholar] [CrossRef]
- Craig, M.H.; Sharp, B.L.; Mabaso, M.L.; Kleinschmidt, I. Developing a spatial-statistical model and map of historical malaria prevalence in Botswana using a staged variable selection procedure. Int. J. Health. Geogr. 2007, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Roucher, C.; Rogier, C.; Sokhna, C.; Tall, A.; Trape, J. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS ONE 2014, 9, e87169. [Google Scholar] [CrossRef]
- Thomson-Luque, R.; Bautista, J.M. Home Sweet Home: Plasmodium vivax-Infected Reticulocytes-The Younger the Better? Front. Cell. Infect. Microbiol. 2021, 11, 675156. [Google Scholar] [CrossRef]
- Bango, Z.A.; Tawe, L.; Muthoga, C.W.; Paganotti, G.M. Past and current biological factors affecting malaria in the low transmission setting of Botswana: A review. Infect. Genet. Evol. 2020, 85, 104458. [Google Scholar] [CrossRef]
- Moakofhi, K.; Edwards, J.K.; Motlaleng, M.; Namboze, J.; Butt, W.; Obopile, M.; Mosweunyane, T.; Manzi, M.; Takarinda, K.C.; Owiti, P. Advances in malaria elimination in Botswana: A dramatic shift to parasitological diagnosis, 2008-2014. Public. Health. Action 2018, 8, S34–S38. [Google Scholar] [CrossRef]
- Saenz, F.E.; Arevalo-Cortes, A.; Valenzuela, G.; Vallejo, A.F.; Castellanos, A.; Poveda-Loayza, A.C.; Gutierrez, J.B.; Alvarez, A.; Yan, Y.H.; Benavides, Y.; et al. Malaria epidemiology in low-endemicity areas of the northern coast of Ecuador: High prevalence of asymptomatic infections. Malar J. 2017, 16, 300. [Google Scholar] [CrossRef]
- Lover, A.A.; Dantzer, E.; Hongvanthong, B.; Chindavongsa, K.; Welty, S.; Reza, T.; Khim, N.; Menard, D.; Bennett, A. Prevalence and risk factors for asymptomatic malaria and genotyping of glucose 6-phosphate (G6PD) deficiencies in a vivax-predominant setting, Lao PDR: Implications for sub-national elimination goals. Malar J. 2018, 17, 218. [Google Scholar] [CrossRef]
- Niang, M.; Diop, F.; Niang, O.; Sadio, B.D.; Sow, A.; Faye, O.; Diallo, M.; Sall, A.A.; Perraut, R.; Toure-Balde, A. Unexpected high circulation of Plasmodium vivax in asymptomatic children from Kedougou, southeastern Senegal. Malar J. 2017, 16, 497. [Google Scholar] [CrossRef] [PubMed]
- Obadia, T.; Nekkab, N.; Robinson, L.J.; Drakeley, C.; Mueller, I.; White, M.T. Developing sero-diagnostic tests to facilitate Plasmodium vivax Serological Test-and-Treat approaches: Modeling the balance between public health impact and overtreatment. BMC Med. 2022, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Russell, D.G. The interaction of Leishmania species with macrophages. Adv. Parasitol. 1992, 31, 175–254. [Google Scholar] [CrossRef]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Lafuse, W.P.; Story, R.; Mahylis, J.; Gupta, G.; Varikuti, S.; Steinkamp, H.; Oghumu, S.; Satoskar, A.R. Leishmania donovani infection induces anemia in hamsters by differentially altering erythropoiesis in bone marrow and spleen. PLoS ONE 2013, 8, e59509. [Google Scholar] [CrossRef] [PubMed]
- Miralles, G.D.; Stoeckle, M.Y.; McDermott, D.F.; Finkelman, F.D.; Murray, H.W. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect. Immun. 1994, 62, 1058–1063. [Google Scholar] [CrossRef]
- Engwerda, C.R.; Kaye, P.M. Organ-specific immune responses associated with infectious disease. Immunol. Today 2000, 21, 73–78. [Google Scholar] [CrossRef]
- Requena, J.M.; Soto, M.; Doria, M.D.; Alonso, C. Immune and clinical parameters associated with Leishmania infantum infection in the golden hamster model. Vet. Immunol. Immunopathol. 2000, 76, 269–281. [Google Scholar] [CrossRef]
- Liu, Y.; Pop, R.; Sadegh, C.; Brugnara, C.; Haase, V.H.; Socolovsky, M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood 2006, 108, 123–133. [Google Scholar] [CrossRef]
- Arevalo-Herrera, M.; Lopez-Perez, M.; Medina, L.; Moreno, A.; Gutierrez, J.B.; Herrera, S. Clinical profile of Plasmodium falciparum and Plasmodium vivax infections in low and unstable malaria transmission settings of Colombia. Malar J. 2015, 14, 154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




