1. Introduction
Replication of many viruses depends on the formation upon infection of cellular biomolecular condensates through a liquid-liquid phase separation (LLPS) mechanism, which biogenesis is driven by scaffold proteins [
1,
2,
3]. More specifically, viral RNA synthesis of human and bovine respiratory syncytial viruses (hRSV and bRSV, respectively) was shown to occur in cytoplasmic membrane-less viral factories, also termed inclusion bodies (IBs) [
4,
5]. RSV belongs to the
Mononegavirales order (
MNVs), which gathers single-stranded negative sense RNA viruses [
6]. The genomic RNA is tightly encapsidated by the viral nucleoprotein N, forming helical ribonucleocapsids (NCs), which are the template for both viral transcription and replication. Similarly to other
MNVs, RSV IBs concentrate all the elements required for viral RNA synthesis, i.e. the encapsidated genomic and antigenomic viral RNA, the viral polymerase L and its co-factor P, and the viral transcription factor M2-1. The N and P proteins are regarded as scaffold proteins for IBs’ formation, their expression being sufficient to induce LLPS and pseudo-IBs morphogenesis in cells and condensation
in vitro [
7] [
8]. Furthermore, for RSV the identification of sub-compartments within IBs called IBAGs (IBs associated granules), where viral mRNAs and the viral transcription factor M2-1 specifically concentrate, recently revealed that IBs are complex and present a high degree of organization [
4]. Given their critical role in the viral cycle, IBs represent targets of interest for the development of antivirals.
hRSV is the main cause of severe lower respiratory tract infection in children worldwide, and the leading cause of hospitalization of children under 6 months of age [
9]. Re-infections occur throughout life, leading to common cold in healthy adults, but to severe lower respiratory infections in immunocompromised and elderly people, with a burden comparable to influenza virus [
10].Despite the high impact of hRSV infections on public health, and although several vaccine candidates are currently in advanced stages of development [
11], the first vaccine to prevent RSV severe disease was approved by the Food and Drug Administration only on May 2023 and is restricted to elderly population (GSK’s Arexvy®) [
12]. The only specific treatment commercialized is a humanised monoclonal antibody directed against the fusion protein F responsible for viral entry (Palivizumab, Synagis). However, a poor benefit-cost ratio limits its use to prophylaxis of severe infection in pre-term and high-risk infants [
13]. Of note, a new prophylactic antibody targeting the pre-fusion form of F (Nirsevimab, Beyfortus®) has been very recently approved in the European Union for the prevention of RSV lower respiratory tract disease in newborns and infants during their first RSV season [
14]. Similarly, bRSV is a major cause of respiratory disease in young calves and responsible for large economic losses worldwide [
15]. Although commercial attenuated and inactivated bRSV vaccines are available, they remain poorly efficient to protect animals [
16,
17].
We have previously shown that cyclopamine (CPM), a plant alkaloid known to target the Hedgehog pathway, inhibits hRSV replication both in cell culture and
in vivo in a mouse model [
18,
19]. We have also recently demonstrated that CPM acts by hardening hRSV IBs. The R151K mutation in the M2-1 protein has been shown to induce resistance to CPM treatment. However, no direct interaction between M2-1 and CPM could be observed. Interestingly, we have also recently shown that CPM inhibits bRSV replication [
20]. The sequence alignement of orthopneumovirus M2-1 proteins highlights the conservation of M2-1 R151 residue suggesting that CPM could also be active against ovine RSV [
20]. Although CPM represents a new potential antiviral against RSV, its mechanism of action remains to be characterized to consider further improvements of the molecule.
RSV M2-1 is a 22 kDa protein that forms tetramers in solution [
21,
22]. It is composed of an N-terminal zinc-finger domain, a central oligomerisation domain, and a C-terminal globular domain (M2-1 core). The structure of hRSV M2-1 was obtained using X-ray crystallography [
23]. It displays a compact disk-shaped tetramer, referred to as a closed conformation. Later, it has been suggested that M2-1 might display conformational plasticity, by analogy with the M2-1 protein of the closely related human metapneumovirus (HMPV). The crystal structures of HMPV M2-1 indeed revealed M2-1 tetramer with a partially open conformation, where the core domain of one M2-1 protomer is projected to the outside of the disk in an open conformation, while the three other protomers adopt the same closed conformation as in the structure of RSV M2-1 [
24]. The hRSV M2-1 core domain interacts with both the P protein and viral mRNA, in a competitive manner [
21]. The recruitment of M2-1 into IBs depends on its interaction with P. The M2-1−P interaction is moreover required for dephosphorylation of M2-1 by the cellular phosphatase PP1 by a mechanism in which P serves as a scaffold protein that binds both M2-1 and PP1 [
25,
26]. M2-1 contains two phosphorylation sites, on residues S58 and S61. Dephosphorylation of M2-1 induces a switch of interaction from P to mRNA, leading to the co-localization of M2-1 with viral mRNA in IBAGs. Binding surfaces of P and RNA on M2-1 core domain, determined by Nuclear Magnetic Resonance (NMR), partly overlap [
27]. The binding surface of P was confirmed by the X-ray crystal structure of M2-1 in a closed conformation, in complex with a P peptide corresponding to the M2-1 binding motif (residues 95-110) of P [
28]. Furthermore, crystallographic data of hRSV M2-1 in complex with short RNA disclosed a dual RNA-binding mode [
29]. The M2-1 zinc finger domain contains an RNA binding site that recognizes an RNA base, that can be considered as specific. The M2-1 core domain binds to the RNA backbone, thus with no sequence specificity expected, although higher affinities were observed for some short RNAs, in particular A-rich sequences [
27]. Moreover M2-1 displays annealing of longer RNA with secondary structures, resulting in cooperative binding to two sites with increased affinity [
30]. These data highlight the complexity of M2-1 interactions with both P and RNA. Of note, M2-1 R151 residue, which induces resistance to CPM when mutated into a lysine, is critical for the interaction with RNA, and also involved in P-binding [
23,
27]. However, previous data suggested that CPM does not impair the interaction of M2-1 with RNA, nor that with P [
18].
In the present study, we aimed at shedding light on the mechanism of action of CPM. Using fluorescence recovery after photobleaching (FRAP) and hypotonic shock experiments, we first showed that CPM induces hardening of pseudo-IBs formed in cells upon transient expression of hRSV N and P only when M2-1 is co-expressed. Similar results were obtained in vitro by co-incubation of recombinant N, P, and M2-1 proteins. Study of M2-1 interaction with P and RNA using in vitro band shift assay by native gel electrophoresis, together with pull-down assays, revealed that CPM impairs the displacement by RNA of the M2-1−P interaction. Based on the structure of the M2-1−P complex, we generated mutations of the residue Y102 of P and showed that the mutation Y102L of P induces resistance to CPM, similarly to the R151K mutation of M2-1. Together, our results suggest that CPM acts on the M2-1−P interaction, and freezes M2-1 and P dynamics and induces IBs hardening.
3. Discussion
The formation of cytoplasmic membrane-less organelles is a major characteristic of
MNVs infections. These structures, which have initially been considered as aggregates of dead-end products of viral infection, were recently shown to be viral factories, where replication and transcription of the viral genomes take place [
4]. Similarly to other cellular organelles such as stress granules or P bodies, the morphogenesis of these structures depends on a LLPS mechanism, driven by low-affinity interactions of multivalent proteins and/or proteins containing intrinsically disordered regions (IDRs), as well as RNA-protein interactions [
35]. In the case of hRSV, we have previously shown that the N protein associated to RNA and the P protein are both required for pseudo-IBs reconstitution in cells and
in vitro [
8,
33]. The tetrameric P protein with N- and C-terminal IDRs [
26,
34] has also a central role in the recruitment of the L polymerase, the transcription factor M2-1, as well as the cellular phosphatase PP1 to IBs [
25,
36].
IBs, in which viral RNA synthesis occurs, as well the protein-protein interactions sustaining their activity represent original targets for the development of new antiviral strategies. We have recently shown that CPM inhibits RSV replication by hardening IBs [
19]. However, although the R151K M2-1 mutation has been shown to induce resistance to CPM treatment, the exact molecular mechanism of CPM action remains unknown. Here, by studying pseudo-IBs properties in cells or
in vitro using FRAP, we first demonstrated that the activity of CPM on IBs relies on the presence of M2-1, and may also impact P mobility
in vitro, independently of the polymerase activity or the presence of cellular proteins. Our results also revealed that within pseudo-IBs formed in cells, the presence of M2-1 increases P mobility compared to the condition where only N and P are expressed. On the contrary, in a minimal system of
in vitro reconstituted pseudo-IBs, the addition of M2-1 did not affect P mobility. These observations suggest that transient interactions between P and its partners could be a key factor of IBs fluidity and dynamics.
Using
in vitro approaches, we then showed that CPM impairs formation of an M2-1−tRNA complex, when M2-1 is already in complex with P. This observation could explain previous results showing that M2-1 presents a diffuse localisation within IBs upon CPM treatment of infected cells, contrasting with its concentration into IBAGs together with viral mRNAs, as observed in untreated cells [
19]. Based on these results, we hypothesized that CPM could bind to M2-1−P complex. We thus thought to identify P residues that could interact with CPM. Analysis of the crystal structure of M2-1-P complex revealed that within the heterotetramer, the interaction between M2-1 and P slightly differs, in particular the orientation of the lateral chains of the residues R151 of M2-1 and Y102 of P. By investigating the impact of Y102F and Y102L substitutions on P, we observed that the P Y102L substitution induced a strong decrease in the polymerase activity but also viral resistance to CPM treatment. This result reveals the implication of this P residue in CPM activity. However, we were not able to validate the direct interaction of CPM with the M2-1−P complex using MST or NMR approaches with M2-1core domain and P peptide. We hypothesized that it could be explained by the poor water solubility of CPM, and a potential low affinity of CPM for the complex.
Our results also suggest that resistance to CPM does not depend on conformational changes of M2-1 and P binding sites. The direct implication of residues R151 of M2-1 and Y102 of P in a ternary complex with CPM thus remains to be determined. Interestingly, we recently showed that CPM also inhibited bRSV replication [
20]. Although the structure of bRSV M2-1-P complex is not available, the sequence alignement of the domains of M2-1 and P involved in the interaction shows that residues R151 of M2-1 and Y102 of P are conserved among orthopneumoviruses. During the course of this study, we probed the binding of CPM on the M2-1−P complex by crystallography. However, we did not succeed to observe CPM in crystals of M2-1 in complex with P peptide. To get additional insight, we thus performed docking experiments using the M2-1 tetramer, alone or in complex with a P
90-110 peptide (PDB accession code 6g0y). Many poses with M2-1 alone show CPM docking in the P-binding groove of M2-1, in the vicinity of R151 (
Figure 6A). Some pauses show CPM on the other side of the disk, close to where RNA was shown to bind in the crystal structure of the complex [
29]. Others show binding on the edge of the disk. With the M2-1−P heterotetramer, CPM predominantly docked next to the “specific RNA binding site” and on the edges of the disk formed by the tetramer (
Figure 6B). Overall this indicates that CPM might explore different binding sites, including binding sites of P and RNA, and therefore interfere with M2-1−RNA and M2-1−P complex formation.
Nevertheless, structural characterization of the mechanism of CPM action on the RSV M2-1−P complex would be necessary to allow a potential rational optimisation of this antiviral compound. Finally, this study clearly suggests that stabilisation of protein-protein interaction is a potent new approach to specifically modify LLPS dynamics and function. Given the central role of viral factories formed by LLPS upon MNVs infections and the numerous transient protein-protein interactions involved in their functionning, our data open new perspective to develop specific inhibitors against all these viruses.
4. Materials and methods
4.1. Plasmid constructs
All the viral sequences were derived from the hRSV strain Long, ATCC VR-26 (Genbank accession n° AY911262.1). Expression plasmids pCI-N, pCI-P and pCI-M2-1 were obtained by cloning mammalian codon optimized coding sequences of N, P and M2-1 into pCI (
Genbank accession n° U47119). The coding sequences were amplified by PCR (Phusion High-Fidelity DNA Polymerase, Thermofisher) using specific primers (sequences available upon request) and cloned into pCI by standart molecular biology procedures using NheI and EcoRI for N, MluI and XbaI for P, and MluI and NotI for M2-1. The BFP coding sequence was inserted into mammalian codon optimized coding sequence of P between residues 76 and 77 as described in (ref risso ballester 2021) to obtain the pCI-P-BFP expression vector. Plasmids for minigenome assay expressing the hRSV N, P, M2-1, and L proteins designated pN, pP, pM2-1 and pL, as well as the pM/Luc subgenomic minigenome expressing the firefly luciferase (Luc) reporter gene under the control of the M/SH gene start sequence were described previously [
21].
For bacterial expression of recombinant mCherry-N, N, P-BFP, P and M2-1 core proteins, previously described pET-mCherry-N, pET-N, pGEX-P-BFP, pGEX-P and pGEX-M2-1core plasmids were used [
8,
27,
37]. For expression of recombinant M2-1-mCherry fusion protein, the mCherry gene was amplified by PCR from the pmCherry vector (Clontech) and subcloned at the 3’ end of the M2-1 gene at SmaI-XhoI sites in pGEX-M2-1 plasmid [
27]. Point mutations were introduced in pP, pGEX-P, pM2-1 and pGEX-M2-1-mCherry by site-directed mutagenesis, using the Quikchange site-directed mutagenesis kit (Stratagene). Sequence analysis was carried out to check the integrity of all the constructs.
4.2. Cells
BSRT7/5 cells (BHK-21 cells that constitutively express the T7 RNA polymerase2) [
38] and HEp-2 cells (ATCC: CCL-23) were maintained respectively in DMEM and MEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), glutamine penicillin–streptomycin solution. Cells were grown in an incubator at 37 °C in 5% CO2. Transfection were performed with 2.5µL of Lipofectamine 2000 (Thermofisher) per 1 µg of DNA according to the manufacturer instructions.
4.3. Time-lapse microscopy and photobleaching experiments on pseudo-IBs
Live-cell imaging and FRAP experiments were realized using HEp-2 cells seeded in 15 or 4-wells Ibidi µ-Slide dishes with a polymer coverslip bottom and transfected with 0.22 µg pCI-P-BFP, 0.22 µg of pCI-N and 0.056 µg of pCI-M2-1 (WT or R151K mutant) for 24h. For FRAP experiments, imaging was performed using Leica SP8 inverted scanning confocal microscope with a 63× oil-immersion objective and a ×8 numerical zoom. Cells were maintained in a climate-controlled chamber (37 °C, 5% CO2) during imaging. FRAP acquisition was performed 1 h after addition of 10 μM CPM, or 0.5% DMSO. FRAP experiments were realized using the following settings: 8 s pre-bleach, 1 ms bleach and 60 s post-bleach at a frame rate of 1 image every 126 ms. Photobleaching of BFP was performed in a circular region at 100% laser intensity. Post-photobleaching fluorescence signals of the bleached region were quantified using the “ROI Intensity Evolution” tool of the Icy software [
39], in parallel of regions with identical dimensions in i) a non-photobleached pseudo-IBs located in the vicinity of the targeted pseudo-IB (controls for potential loss of fluorescence in the imaging field studied), ii) a background region. Normalization and averaging of the recovery curves were then performed using the easyFRAP web-based tool [
40]. For each experimental condition, two individual experiments were performed (n=2), during which 10 to 12 pseudo-IBs were analysed. Images from one
in cellulo FRAP replicate are shown in
Figure 1.
In vitro pseudo-IBs sizes were quantified using the “Spot detector” tool of the Icy software, incremented with a filter for >90% sphericity to discard artefactual values generated by the detection of two adjacent pseudo-IBs as a single entity.
For osmotic shock experiments, imaging was performed using an Olympus IX73 inverted microscope with a 63× oil-immersion objective and a ×2 numerical zoom. Hypotonic shocks were performed by incubating the cells in 10% MEM diluted in water (v/v) for 5 min. One image was acquired before hypotonic shock and the same cells were imaged every 1 min for 5 min during the shock. For each experimental data point, the hypotonic shock was applied to an entire well of a 15-well Ibidi µ-Slide dish, and one position was studied in order to keep the cell into focus. 7 to 10 acquisitions from 2 independent experiments were performed. Imaging fields of interest comprised 2 to 4 cells, and IBs present before and 5 min following the hypotonic shock were counted manually.
4.4. Photobleaching experiments on in vitro reconstituted pseudo-IBs.
As previously described [
8,
33],
in vitro pseudo-IBs were reconstituted in 10-20 µL droplets using mCherry-N and P-BFP proteins (3 and 12 µM, respectively), or N, P-BFP and mCherry-M2-1 (3, 12, and 12 µM, respectively) recombinant proteins in a 20 mM Tris/HCl pH 8.5, 150 mM NaCl, 10% molecular-crowding agent Ficoll buffer, supplemented with 150 µM CPM or 0.5% DMSO. Drops were incubated on 8-well Ibidi µ-Slide dishes for 30 min before imaging at room temperature, and imaged using a SP8 Leica scanning confocal microscope with a 63× oil-immersion objective and a ×4 numerical zoom.
FRAP experiments were realized using the following settings: 2 s pre-bleach, 1 ms bleach and 60 s post-bleach at a frame rate of 1 image every 1 or 1.476 s for N/P and N/P/M2-1 experiments, respectively (increase of time-interval was applied to overcome additional photobleaching during the post-bleach step of the experiments). Photobleaching of mCherry was performed in a circular region at 100% laser intensity.
In vitro FRAP data processing was identical to the
in vitro FRAP data processing described above. Images from one
in cellulo FRAP replicate are shown in
Figure 1.
4.5. Minigenome assay
BSRT7/5 cells at 90% confluence in 96-well dishes were transfected with a plasmid mixture containing 125 ng of pM/Luc, 125 ng of pN, 125 ng of pP, 62.5 ng of pL, and 31 ng of pM2-1 as well as 31 ng of pRSV-β-Gal (Promega) to normalize transfection efficiencies [
21]. Transfections were done in triplicate, and each independent transfection was performed three times. Cells were harvested 24 h post-transfection, then lyzed in luciferase lysis buffer (30 mM Tris pH 7.9, 10 mM MgCl
2, 1 mM DTT, 1% Triton X-100, and 15% glycerol). The luciferase activities were determined for each cell lysate with an Infinite 200 Pro (Tecan, Männedorf, Switzerland) and normalized based on β-galactosidase (β-Gal) expression.
4.6. Expression and purification of the recombinant proteins
The
Escherichia coli BL21 (DE3) bacteria strain (Novagen, Madison, WI) was transformed with the plasmids. Cultures were grown at 37 °C in 2xYT medium containing either 100 µg/ml of ampiciline (pGEX vectors) or 50 μg/ml kanamycin (pET vectors). After 8 h, an equal volume of 2xYT medium containing antibiotic was added to the cultures, and protein expression was induced by the addition of 80 μg/ml isopropyl β-d-1-thiogalactopyranoside (IPTG) overnight at 28 °C. Bacteria were then harvested by centrifugation. Purification of recombinant N, P, and M2-1core proteins has already been described ([
8,
27,
37].
For M2-1-mCherry (WT and R151K mutant) purification, pellets were resuspended in lysis buffer (20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.1% Triton X-100, 1 mg/ml lysozyme, and complete protease inhibitor cocktail (Roche)). After incubation on ice for 1h, the lysates were sonicated and benzonase (Novagen) (final concentration 5 U/ml) was added to the lysate, followed by incubation for 30 min at room temperature, before addition of NaCl up to a concentration of 1 M. After centrifugation at 10,000 g for 30 min at 4 °C, the lysates were incubated with Glutathione-Sepharose 4B beads (GE Healthcare) for 1 h at room temperature. Beads were washed three times with washing high-salt buffer (20 mM Tris–HCl, pH 7.4, 1 M NaCl) and three times with washing low-salt buffer (20 mM Tris–HCl, pH 7.4, 150 mM NaCl). To isolate GST-free M2-1-mCherry, beads were incubated with thrombin (Novagen). Purified proteins were then loaded on a Hi-Load 16/600 Superdex 200 column (Cytiva) and eluted in 20 mM Tris–HCl, pH 7.4, 150 mM NaCl. Finally, proteins were concentrated using a centrifugal concentrator with a MWCO of 100 kDa (Vivaspin turbo 4, Sartorius).
4.7. Band shift on native agarose gels.
Recombinant M2-1-mCherry (3 µM) and P proteins (30 µM) in 20 mM TrisHCl, pH 7.4,150 mM NaCl buffer were co-incubated for 1h at room temperature. CPM (675 µM) or equivalent volume of DMSO was then added to the samples before incubation 30 min at room temperature. tRNAs (10 µM) were then added, and samples incubated for 1h at room temperature. 50% sucrose loading buffer was added to the samples before loading on native 0.9% agarose gel stained with SYBR Safe (Invitrogen). The migration was performed in Tris–Glycine buffer during 6h at 80 V, before gel staining with amido black 10B.
4.8. Pulldown assay
GST-M2-1core proteins fixed on bead were incubated in the presence of purified recombinant P protein in PBS, in a final volume of 300 µl, in the presence of 400 µM CPM or DMSO (control condition). After 1 hour of incubation under agitation at 4°C beads were rinsed 2 times in 500 µl PBS and resuspended in the presence of 160 µg/ml of tRNA for 30 min, then washed 3 times in 500 µl PBS. 40 µl of Laemmli were added to beads, before boiling 5 min at 95°C for analysis by SDS-PAGE and Coomassie blue staining.
4.9. Docking of CPM onto M2-1
The structure of the CPM molecule (CAS 4449-51-8) was retrieved from PUBCHEM and converted into MOL2 format. Docking was performed on the Swiss-dock server (
http://www.swissdock.ch/docking#). Docking was performed on the M2-1 tetramer and on the M2-1−P protomer.
4.10. P peptide fold prediction
4.11. Microscale thermophoresis
Microscale thermophoresis is a technology that uses the motion of fluorescent molecules along a microscale temperature gradient to detect any changes in their hydration shell, which can be induced by the binding to a partner [
42,
43,
44,
45]}. A fixed concentration of
FITC-P95-112 (100 nM
) was incubated with increasing amounts of M2-1 core domain at room temperature for 15 min in PBS and 1% DMSO containing either 0 or 1 or 10 μM of CPM. The measurements were performed for 30 s using a Monolith NT.115 (NanoTemper Technologies GmbH, Munich, Germany) at 20°C (blue LED power at 80% and infrared laser power at medium). The data from two independent measurements were averaged and analysed using the temperature-jump phase and the standard fitting mode (derive
d from law of mass action) of the NTAnalysis software (Nanotemper technologies).
4.12. NMR
Preparation of
15N-labeled M2-1 core domain was reported before ([
27]. The same protocol was applied to wild-type and the R151K mutant. M2-1 core domain was in PBS pH 6.4 buffer supplemented with 1 mM DTT and 7.5% D
2O to lock the NMR spectrometer frequency. Measurements were carried out on a Bruker 700 MHz spectrometer equipped with a TXO cryoprobe. The temperature was set to 298 K.
1H-
15N correlation spectra were acquired using the BTROSY sequence. NMR data were processed with TopSpin 4.0 software (Bruker) and analysed with CcpNmr Analysis Assign 3.1 software [
46].
1H chemical shifts were referenced to DSS. Chemical shift assignment of the wild-type M2-1 core domain was reported previously [
47].
FITC-P95-112 peptide was purchased from Proteogenix. A stock solution was prepared by dissolving peptide in pure water, and by adjusting the pH to neutral by addition of 1 M NaOH. The final concentration was 1 mM and determined by UV-VIS, using ε(500nM) = 80.000 cm-1.M-1. Cyclopamine was dissolved in DMSO-d6 (Eurisotop) at 5 mg/mL, equivalent to 12 mM. Interaction experiments with FITC-P95-112 were performed using a constant protein concentration of 105 and 115 µM for WT and R151K mutant, respectively, and by adding small volumes of concentrated peptides, so that protein dilution remained negligible. Titration points were made with 0, 0.1, 0.25, 0.5, 1.0 and 1.5 molar equivalents of peptide.
Author Contributions
Conceptualization, M.G., M.-A. R.-W., C. S., D. M., and J.-F.E; methodology, M.G., M.-A. R.-W., C. S., D. M., J.R.-B. and C.D.; validation, M.G., M.-A. R.-W., C. S., D. M.; formal analysis, C.D., J.R.-B., D.M., J.F., C.-A. R.., C.S and M.G..; investigation, C.D., J.R.-B., D.M., J.F., C.-A. R.., C.S and M.G..; writing—original draft preparation, M.-A R.-W. and M.G.; writing—review and editing, M.G., M.-A. R.-W., D.M., C. S., and J.-F.E.; supervision, M.-A. R.-W. and M.G.; project administration, M.G.; funding acquisition, M.G., M.-A. R.-W., and C.S. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Hardening of pseudo IBs by CPM in cells requires the presence of M2-1 . (A-F) P-BFP mobility in biomolecular condensates was analysed by FRAP in HEp-2 cells transiently expressing N, P-BFP, ± M2-1 (B) or M2-1(R151K) (C), and treated with 10 µM CPM (blue) or DMSO (red) for one hour. (A-C) Representative images of time-lapse microscopy from FRAP experiments (left panels). Scale bars: 2 µm. Quantification of spontaneous re-distribution of fluorescence after photobleaching, corrected for background and bleaching during post-bleach imaging, and normalized to the post-bleach signals (right panels). Data are represented as mean ± SD of ≥ 20 FRAP events out of 2 independent experiments. (D) Hypotonic shock was applied on HEp-2 cells transiently expressing N, P-BFP ± M2-1 and treated with 10 µM CPM (blue) or DMSO (red) for one hour. Representative images of the impact of hypotonic shock on IBs (left panel). Proportions of IBs resisting a 5 min hypotonic shock are represented as mean percentages ± SD in the right panel (8 to 9 acquisitions from two independent experiments). Significance was tested using an unpaired t test followed by Welch correction; ***p < 0.001, ns = not significant. Scale bars: 10 µm.
Figure 1.
Hardening of pseudo IBs by CPM in cells requires the presence of M2-1 . (A-F) P-BFP mobility in biomolecular condensates was analysed by FRAP in HEp-2 cells transiently expressing N, P-BFP, ± M2-1 (B) or M2-1(R151K) (C), and treated with 10 µM CPM (blue) or DMSO (red) for one hour. (A-C) Representative images of time-lapse microscopy from FRAP experiments (left panels). Scale bars: 2 µm. Quantification of spontaneous re-distribution of fluorescence after photobleaching, corrected for background and bleaching during post-bleach imaging, and normalized to the post-bleach signals (right panels). Data are represented as mean ± SD of ≥ 20 FRAP events out of 2 independent experiments. (D) Hypotonic shock was applied on HEp-2 cells transiently expressing N, P-BFP ± M2-1 and treated with 10 µM CPM (blue) or DMSO (red) for one hour. Representative images of the impact of hypotonic shock on IBs (left panel). Proportions of IBs resisting a 5 min hypotonic shock are represented as mean percentages ± SD in the right panel (8 to 9 acquisitions from two independent experiments). Significance was tested using an unpaired t test followed by Welch correction; ***p < 0.001, ns = not significant. Scale bars: 10 µm.
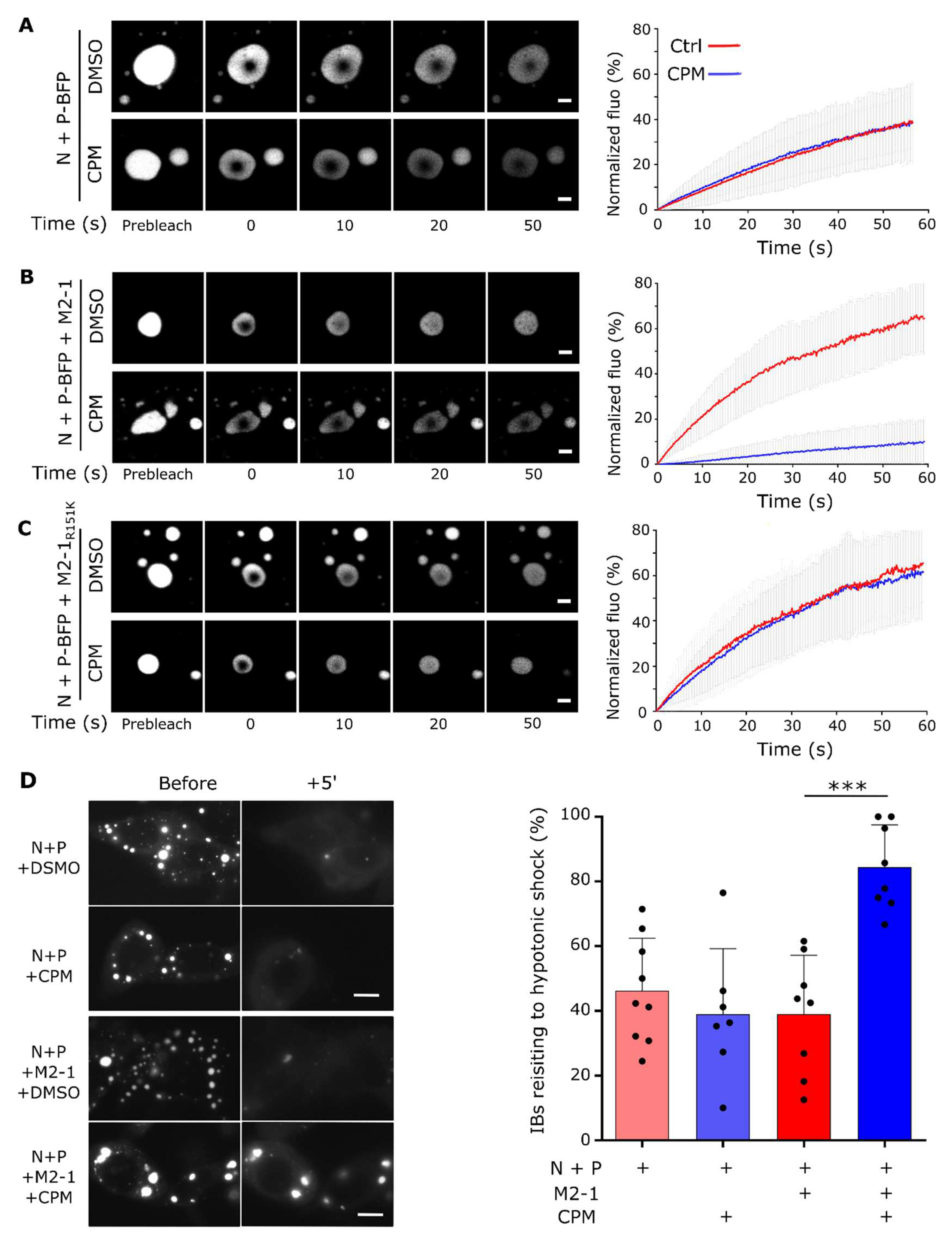
Figure 2.
Hardening of in vitro reconstituted pseudo-IBs by CPM depends on M2-1. (A) Recombinant GST-M2-1-mCherry proteins (WT and mutant R151K) were produced in E.coli. After GST cleavage, a gel filtration was performed to isolate M2-1-mCherry (left panel), (the arrow shows the peak of elution corresponding to M2-1-mCherry tetramer), and the purified proteins were analyzed by SDS-PAGE and Coomassie blue staining (right panel). (B-C) Recombinant mCherry-N and P-BFP (C) or N, P-BFP and M2-1-mCherry (D) proteins were co-incubated and phase separation was assessed using fluorescence microscopy. Scale bars: 10 µm. (D-F) mCherry-P (E, F, H, I) or M2-1-mCherry (G, J) mobility was analysed by FRAP in condensates following a 30 min incubation with 150 µM CPM (blue) or DMSO (red). Representative images of time-lapse microscopy from FRAP experiments on in vitro reconstituted pseudo-IBs (left panels). Scale bars 2 µm. Quantification of the spontaneous re-distribution of fluorescence of mCherry after photobleaching, corrected for background and bleaching during post-bleach imaging and normalized to the post-bleach signal (right panels). Data are represented as mean ± SD of ≥ 20 FRAP events out of 2 independent experiments.
Figure 2.
Hardening of in vitro reconstituted pseudo-IBs by CPM depends on M2-1. (A) Recombinant GST-M2-1-mCherry proteins (WT and mutant R151K) were produced in E.coli. After GST cleavage, a gel filtration was performed to isolate M2-1-mCherry (left panel), (the arrow shows the peak of elution corresponding to M2-1-mCherry tetramer), and the purified proteins were analyzed by SDS-PAGE and Coomassie blue staining (right panel). (B-C) Recombinant mCherry-N and P-BFP (C) or N, P-BFP and M2-1-mCherry (D) proteins were co-incubated and phase separation was assessed using fluorescence microscopy. Scale bars: 10 µm. (D-F) mCherry-P (E, F, H, I) or M2-1-mCherry (G, J) mobility was analysed by FRAP in condensates following a 30 min incubation with 150 µM CPM (blue) or DMSO (red). Representative images of time-lapse microscopy from FRAP experiments on in vitro reconstituted pseudo-IBs (left panels). Scale bars 2 µm. Quantification of the spontaneous re-distribution of fluorescence of mCherry after photobleaching, corrected for background and bleaching during post-bleach imaging and normalized to the post-bleach signal (right panels). Data are represented as mean ± SD of ≥ 20 FRAP events out of 2 independent experiments.
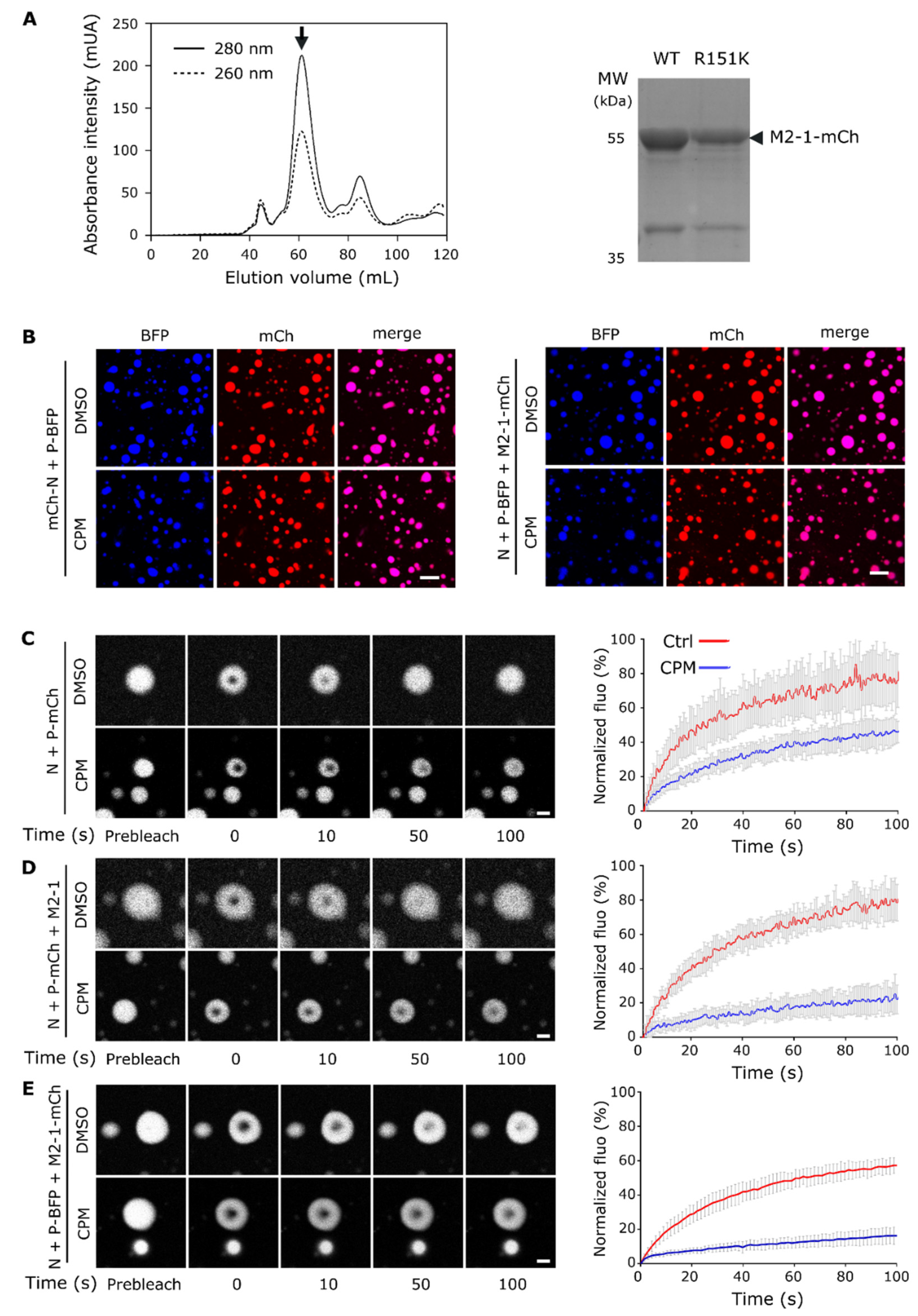
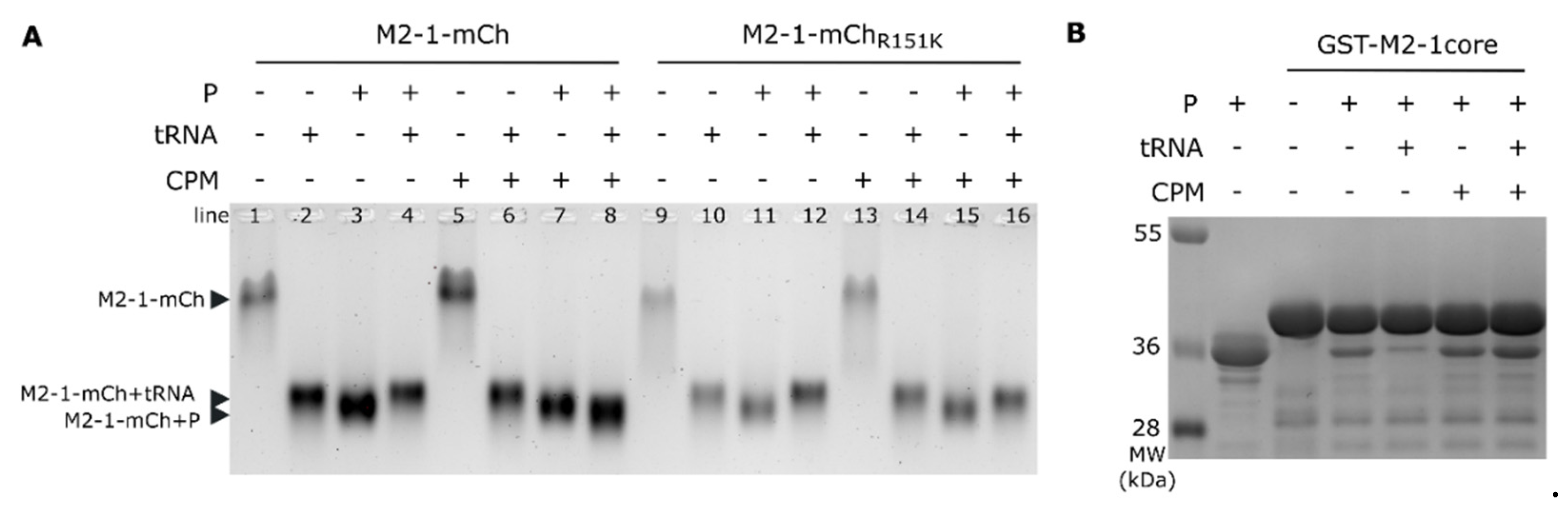
Figure 3.
CPM affects competition between P and tRNA for M2-1 binding in vitro. (A) Analysis of M2-1-mCherry migration alone or incubated in the presence of tRNA or P, in the absence or presence of CPM, by native polyacrylamide gel electrophoresis. M2-1-mCherry was observed using UV. (B) Pulldown of P by GST-M2-1core in the absence or the presence of CPM and/or tRNA; analyzed by SDS-PAGE and Coomassie blue staining.
Figure 3.
CPM affects competition between P and tRNA for M2-1 binding in vitro. (A) Analysis of M2-1-mCherry migration alone or incubated in the presence of tRNA or P, in the absence or presence of CPM, by native polyacrylamide gel electrophoresis. M2-1-mCherry was observed using UV. (B) Pulldown of P by GST-M2-1core in the absence or the presence of CPM and/or tRNA; analyzed by SDS-PAGE and Coomassie blue staining.
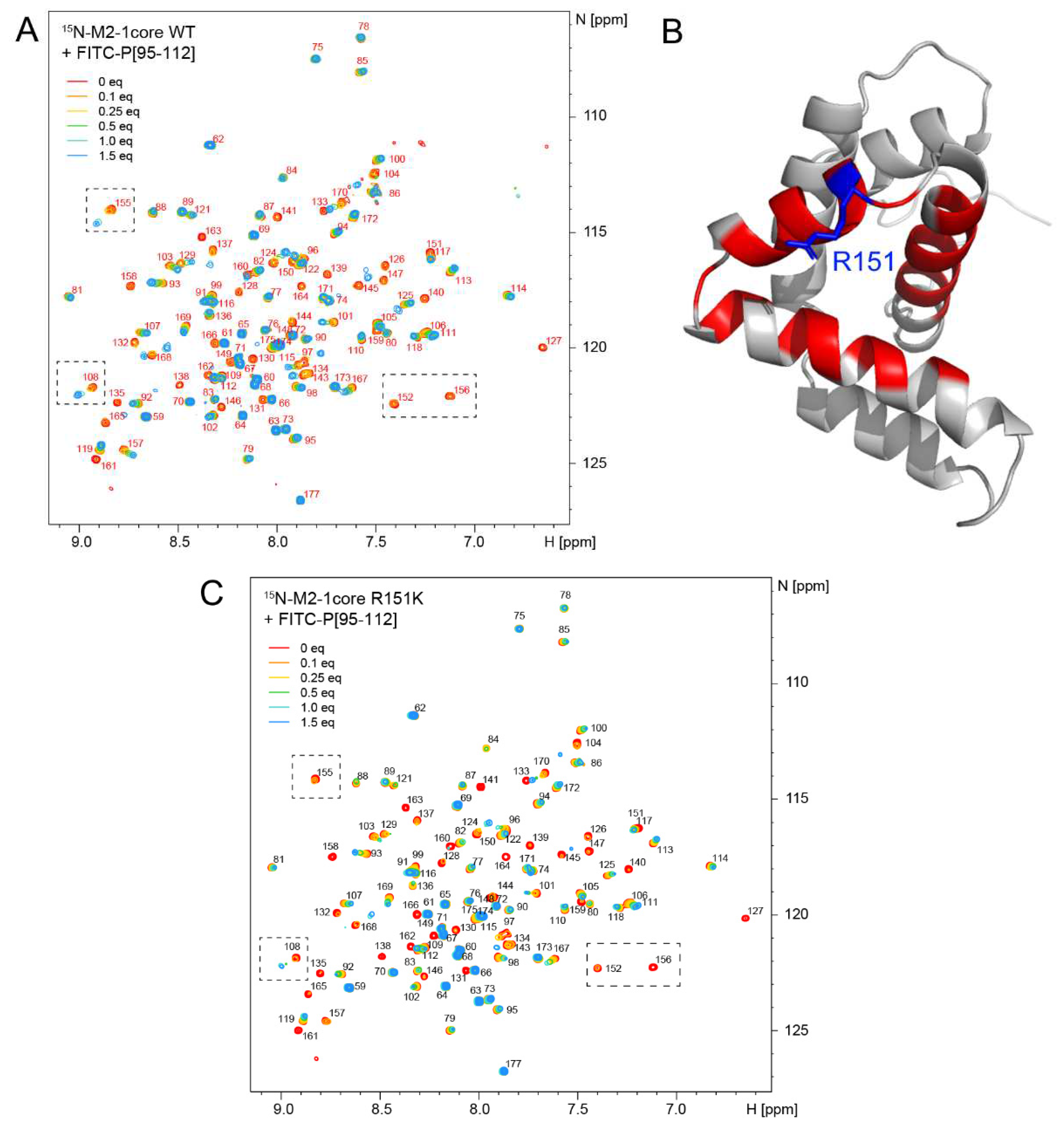
Figure 4.
The M2-1−P complex displays motions at the µs-ms time scale. (A) Binding of the FITC-P95-112 peptide to 15N-labeled M2-1core domain was followed by 2D NMR (1H frequency 700 MHz, temperature 298 K). Amide signals are annotated with the corresponding residue number. Increasing amounts (0.1 to 1.5 molar equivalents) of FITC-P95-112 peptide were added to 100 µM WT M2-1core and a 1H-15N BTROSY spectrum was acquired at each titrating point. Saturation was reached at a peptide:protein molar ratio of 1.5:1. (B) The signals of nearly all residues belonging to the P-binding site remain broad at the last titration point. They are mapped in red on the structure of M2-1core (PDB 2L9J). (C) Titration of 15N-labeled M2-1core R151K mutant by 0.1 to 1.5 molar equivalents FITC-P95-112 peptide was followed by measuring 1H-15N BTROSY spectra.
Figure 4.
The M2-1−P complex displays motions at the µs-ms time scale. (A) Binding of the FITC-P95-112 peptide to 15N-labeled M2-1core domain was followed by 2D NMR (1H frequency 700 MHz, temperature 298 K). Amide signals are annotated with the corresponding residue number. Increasing amounts (0.1 to 1.5 molar equivalents) of FITC-P95-112 peptide were added to 100 µM WT M2-1core and a 1H-15N BTROSY spectrum was acquired at each titrating point. Saturation was reached at a peptide:protein molar ratio of 1.5:1. (B) The signals of nearly all residues belonging to the P-binding site remain broad at the last titration point. They are mapped in red on the structure of M2-1core (PDB 2L9J). (C) Titration of 15N-labeled M2-1core R151K mutant by 0.1 to 1.5 molar equivalents FITC-P95-112 peptide was followed by measuring 1H-15N BTROSY spectra.
Figure 5.
Role of the residue Y102 of P protein in CPM antiviral activity. (A) Close-up view of the RSV M2-1−P binding site (PDB 6g0y). The four M2-1 protomers were structurally aligned. The M2-1 core domain is shown in gray ribbon, and the bound P90-110 peptide in green. The side chains of P and M2-1 residues that were previously shown to be critical for RSV replication in vitro by alanine scanning are shown in sticks. The cation-pi interaction between M2-1 R151 (blue) and P Y102 (yellow) is indicated with broken line. (B) Polymerase activity in the presence of mutated P and CPM. BSRT7/5 cells were transfected with plasmids encoding the N, P, L and M2-1 proteins and the M/Luc subgenomic minireplicon together with pCMV β-gal for transfection standardization. P mutants expressed instead of the corresponding wild type P are indicated below the histogram. Luciferase activity, reflecting viral RNA synthesis, was measured 24 h after transfection, normalized to the β-galactosidase activity, and expressed as percentage of the WT proteins activity. The mean value ± SD from 3 independent experiments performed in triplicate or quadruplicate are shown. Nested One way ANOVA followed by two-sided Dunn's multiple comparison tests against untreated group (ns= not significant, **** p< 0.0001; * p<0.05). Western blot analysis showing efficient expression of P mutant proteins in BSRT7/5 cells for one representative experiment is shown. (C) Analysis of M2-1-mCherry migration alone or incubated in the presence of tRNA or P mutants, in the absence or presence of CPM, by native polyacrylamide gel electrophoresis. M2-1-mCherry was observed using UV.
Figure 5.
Role of the residue Y102 of P protein in CPM antiviral activity. (A) Close-up view of the RSV M2-1−P binding site (PDB 6g0y). The four M2-1 protomers were structurally aligned. The M2-1 core domain is shown in gray ribbon, and the bound P90-110 peptide in green. The side chains of P and M2-1 residues that were previously shown to be critical for RSV replication in vitro by alanine scanning are shown in sticks. The cation-pi interaction between M2-1 R151 (blue) and P Y102 (yellow) is indicated with broken line. (B) Polymerase activity in the presence of mutated P and CPM. BSRT7/5 cells were transfected with plasmids encoding the N, P, L and M2-1 proteins and the M/Luc subgenomic minireplicon together with pCMV β-gal for transfection standardization. P mutants expressed instead of the corresponding wild type P are indicated below the histogram. Luciferase activity, reflecting viral RNA synthesis, was measured 24 h after transfection, normalized to the β-galactosidase activity, and expressed as percentage of the WT proteins activity. The mean value ± SD from 3 independent experiments performed in triplicate or quadruplicate are shown. Nested One way ANOVA followed by two-sided Dunn's multiple comparison tests against untreated group (ns= not significant, **** p< 0.0001; * p<0.05). Western blot analysis showing efficient expression of P mutant proteins in BSRT7/5 cells for one representative experiment is shown. (C) Analysis of M2-1-mCherry migration alone or incubated in the presence of tRNA or P mutants, in the absence or presence of CPM, by native polyacrylamide gel electrophoresis. M2-1-mCherry was observed using UV.
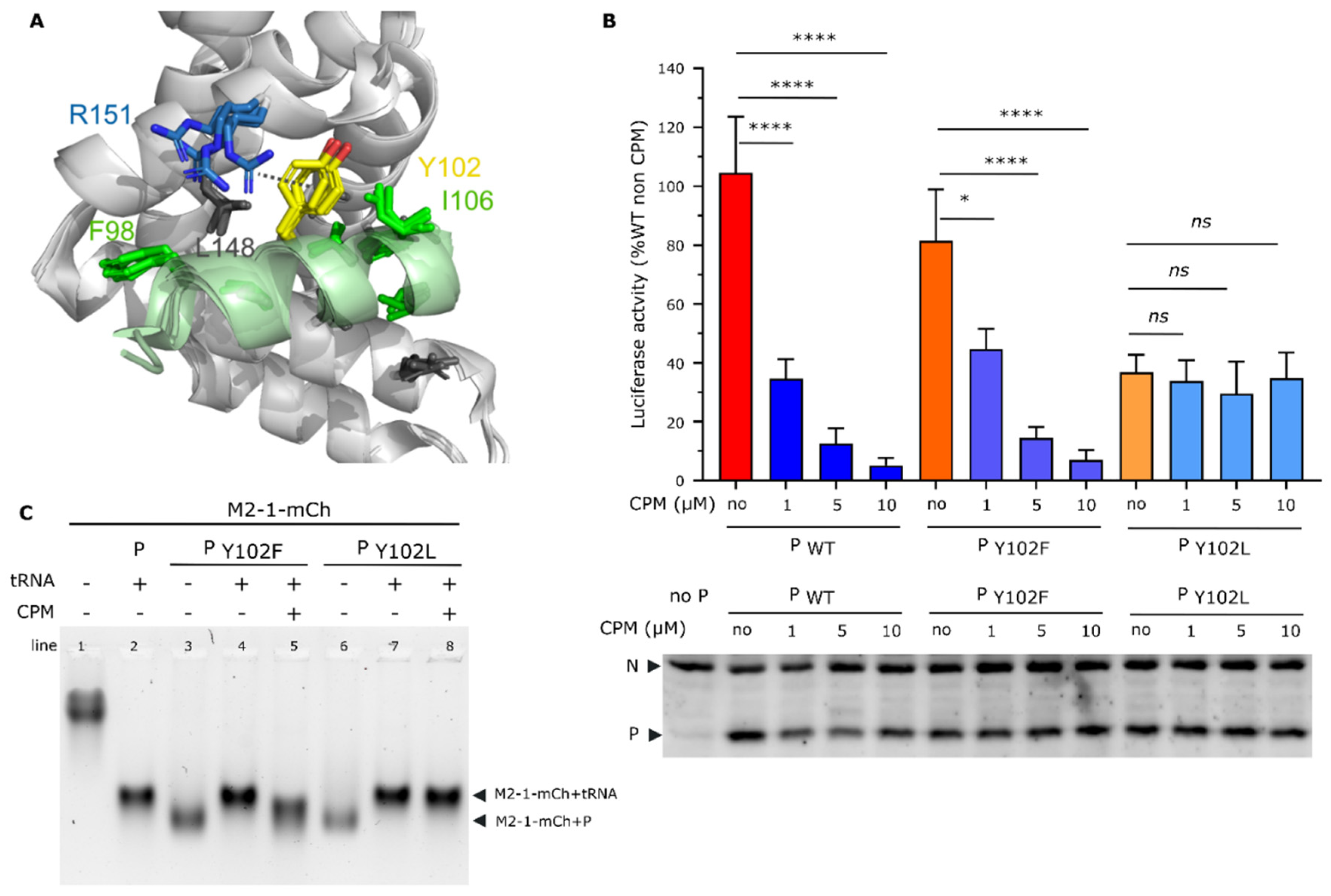
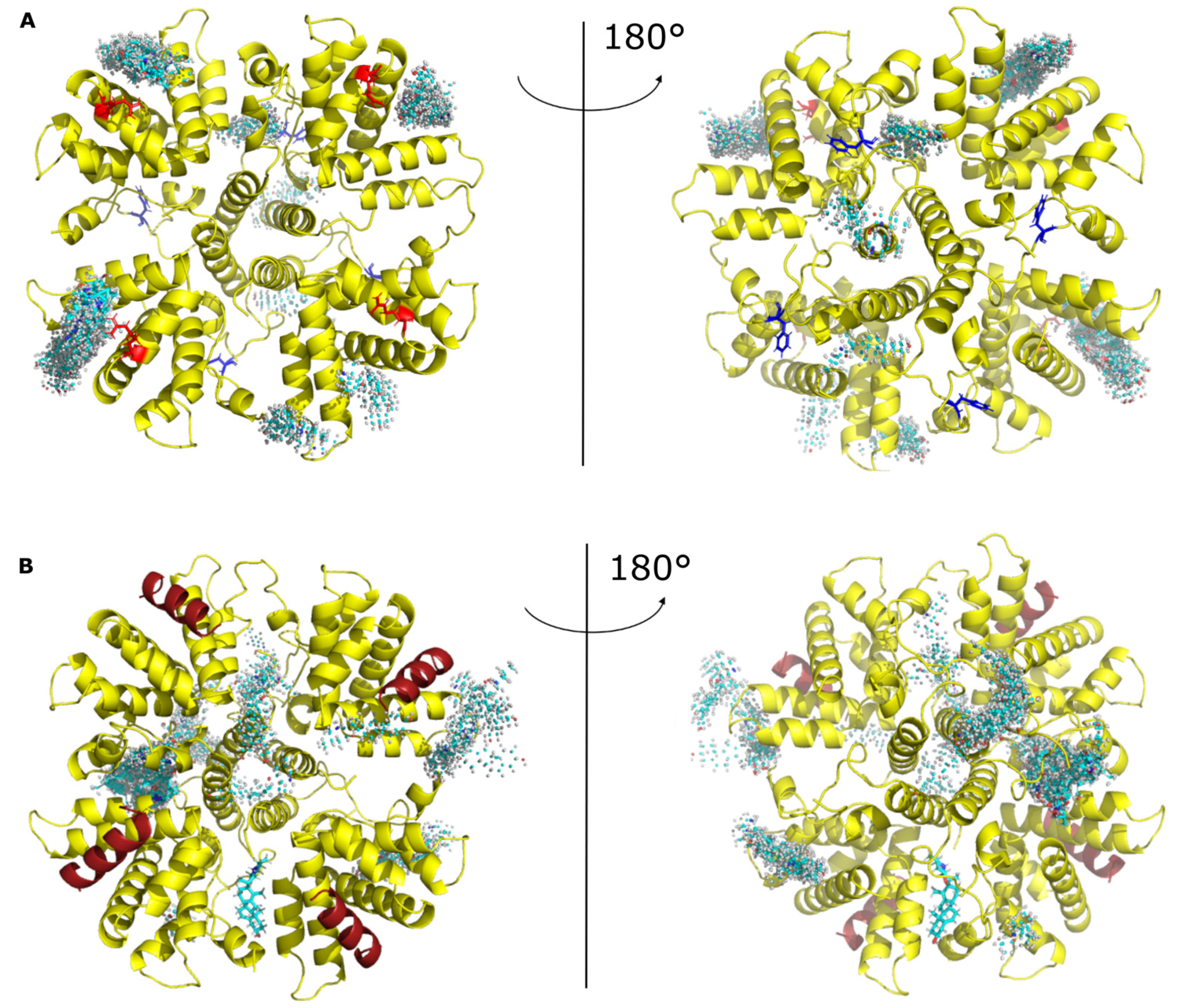
Figure 6.
CPM explores different binding sites on M2-1. Docking experiments were performed using (A) the M2-1 tetramer without peptide as well as (B) the M2-1 tetramer in complex with the P95-110 peptide (PDB 6g0y). Two views are shown for each, rotated by 180°. M2-1 is in yellow cartoon, and the R151 residue in red sticks. The P peptide is in brick red cartoon. CPM carbon atoms are in cyan sticks and spheres.
Figure 6.
CPM explores different binding sites on M2-1. Docking experiments were performed using (A) the M2-1 tetramer without peptide as well as (B) the M2-1 tetramer in complex with the P95-110 peptide (PDB 6g0y). Two views are shown for each, rotated by 180°. M2-1 is in yellow cartoon, and the R151 residue in red sticks. The P peptide is in brick red cartoon. CPM carbon atoms are in cyan sticks and spheres.