Submitted:
09 June 2023
Posted:
26 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
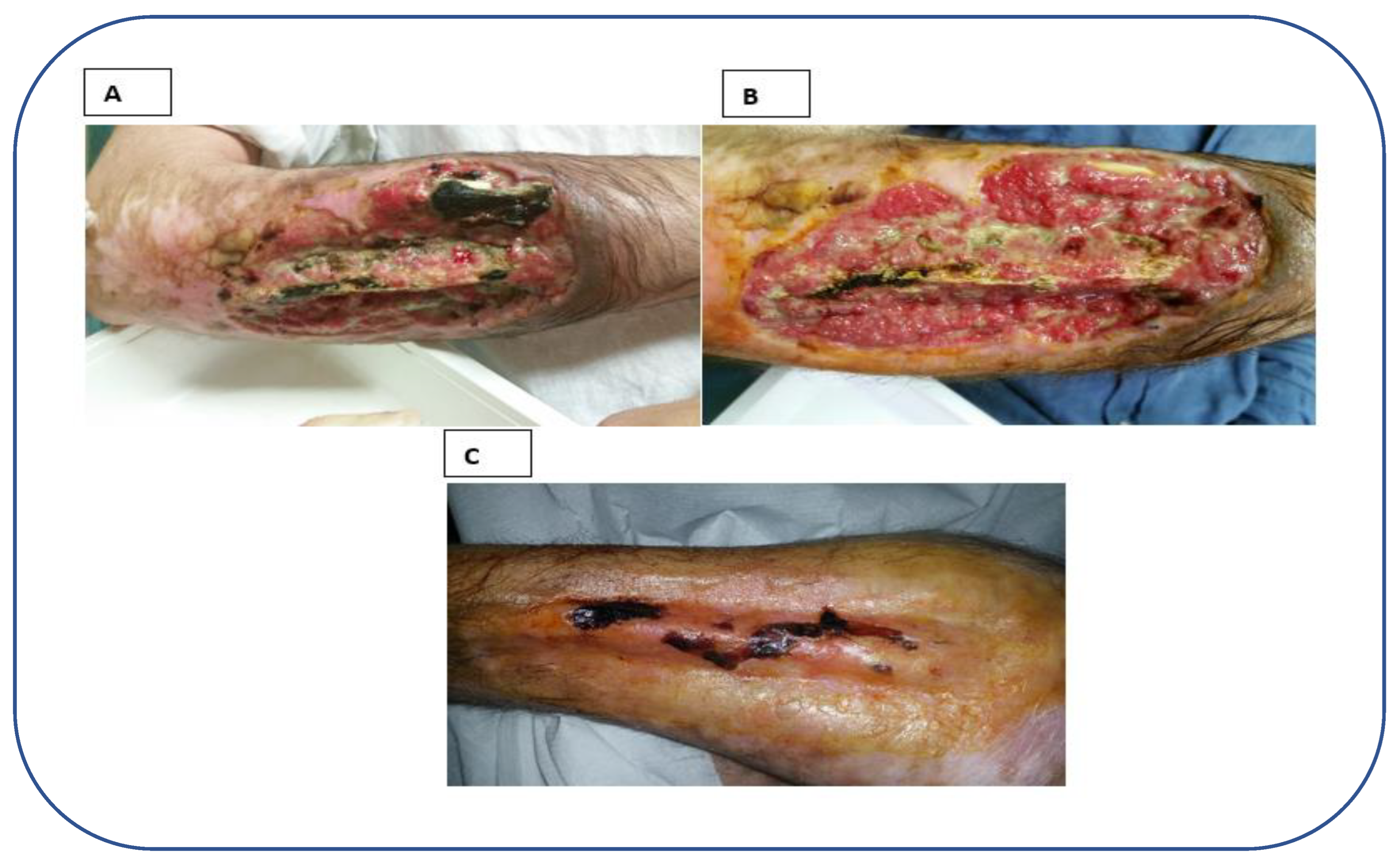
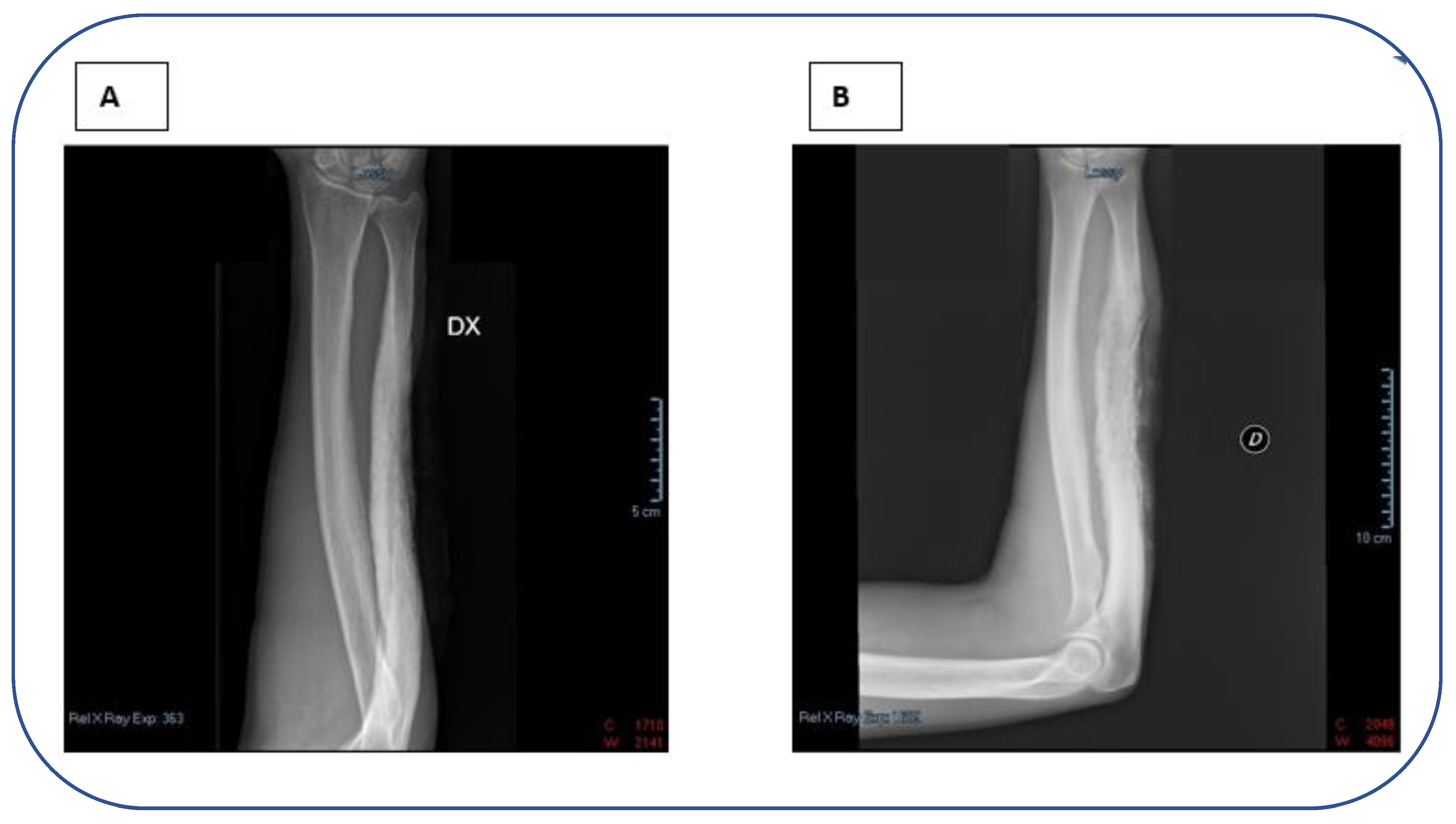
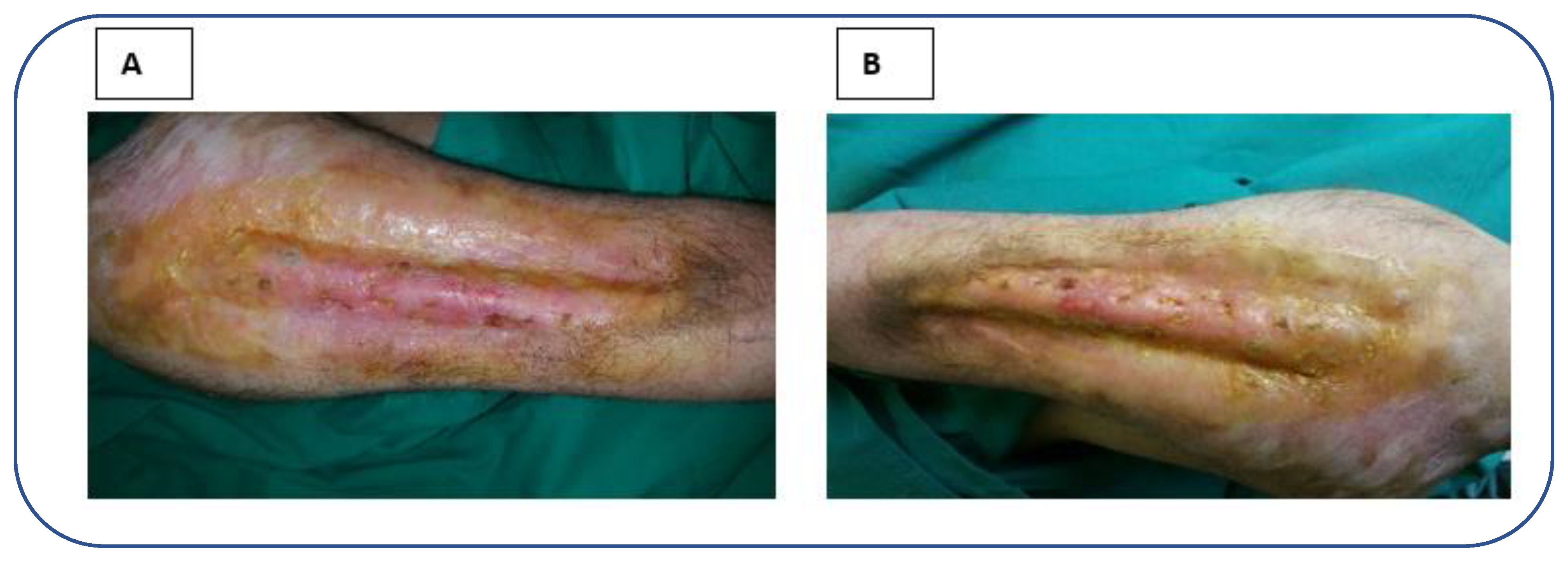
2. Case report
3. Discussion
References
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatologic Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef]
- Rapp, J.; Plackett, T.; Crane, J.; Lu, J.; Hardin, D.; Loos, P.; Kelly, R.; Hall, M.A.; Murray, C.; Keenan, S.; et al. Burn Wound Management in Prolonged Field Care Available online:. Available online: https://jts.amedd.army.mil/assets/docs/cpgs/Wound_Management_PFC_24_Jul_2017_ID62.pdf (accessed on Feb 4, 2023).
- Jiritano, F.; Serraino, G.F.; Rossi, M.; Dominijanni, A.; Brescia, A.; Renzulli, A. Ventricular assist device driveline infection: Treatment with platelet-rich plasma. Ann. Thorac. Surg. 2013, 96, e37–e38. [Google Scholar] [CrossRef]
- Serra, R.; Buffone, G.; De Franciscis, A.; Mastrangelo, D.; Vitagliano, T.; Greco, M.; De Franciscis, S. Skin grafting followed by low-molecular-weight heparin long-term therapy in chronic venous leg ulcers. Ann. Vasc. Surg. 2012, 26, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Buffone, G.; Dominijanni, A.; Molinari, V.; Montemurro, R.; de Franciscis, S. Application of platelet-rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int. Wound J. 2013, 10, 612–615. [Google Scholar] [CrossRef]
- Serraino, G.F.; Dominijanni, A.; Jiritano, F.; Rossi, M.; Cuda, A.; Caroleo, S.; Brescia, A.; Renzulli, A. Platelet-rich plasma inside the sternotomy wound reduces the incidence of sternal wound infections. Int. Wound J. 2015, 12, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Rechichi, M.; Ferrise, M.; Romano, F.; Gallelli, L.; Toschi, V.; Dominijanni, A.; Meduri, A. Autologous platelet-rich plasma in the treatment of refractory corneal ulcers: A case report. Am. J. Ophthalmol. Case Reports 2020, 20, 100838. [Google Scholar] [CrossRef] [PubMed]
- Tsachiridi, M.; Galyfos, G.; Andreou, A.; Sianou, A.; Sigala, F.; Zografos, G.; Filis, K. Autologous platelet-rich plasma for nonhealing ulcers: A comparative study. Vasc. Spec. Int. 2019, 35, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yaltirik, M.; Koray, M.; Kocaelli, H.; Ofluoglu, D. Platelet-Rich Plasma in Trauma Patients Available online:. Available online: https://www.intechopen.com/chapters/63335 (accessed on Feb 4, 2023).
- Abbade, L.P.F.; Ferreira, R.S.; Dos Santos, L.D.; Barraviera, B. Chronic venous ulcers: A review on treatment with fibrin sealant and prognostic advances using proteomic strategies. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Paolino, F.M.; Rizzo, B.A.; Russo, A.; Southworth, S.; Serra, R.; Gallelli, L. The use of growth factors, CD34+ cells and fibrin for the management of chronic venous ulcers. Int. Wound J. 2016, 13, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Marston, W.A.; Snyder, R.J.; Lee, T.D.; Cargill, D.I.; Slade, H.B. Spray-applied cell therapy with human allogeneic fi broblasts and keratinocytes for the treatment of chronic venous leg ulcers: A phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2012, 380, 977–985. [Google Scholar] [CrossRef]
- Asadi, M.; Alamdari, D.H.; Rahimi, H.R.; Aliakbarian, M.; Jangjoo, A.; Abdollahi, A.; Bahar, M.M.; Azadmand, A.; Forghani, N.; Sadegh, M.N.; et al. Treatment of life-threatening wounds with a combination of allogenic platelet-rich plasma, fibrin glue and collagen matrix, and a literature review. Exp. Ther. Med. 2014, 8, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.A.N.; Vieira, L.M.; Barraviera, B.; Barraviera, S.R.C.S. Treatment of venous ulcers with fibrin sealant derived from snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 226–229. [Google Scholar] [CrossRef]
- Abbade, L.; Barraviera, S.R.C.S.; Silvares, M.R.; Seabra Ferreira Junior, R.; Carneiro, M.T.R.; Medolago, N.B.M.; Barraviera, B. A new fibrin sealant derived from snake venom candidate to treat chronic venous ulcers. J. Am. Acad. Dermatol. 2015, 72, AB271. [Google Scholar] [CrossRef]
- Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; Lima, A.B.B. d. C.O.; Haddad, G.R.; Gatti, M.A.N.; Medolago, N.B.; Rigotto Carneiro, M.T.; dos Santos, L.D.; Ferreira, R.S.; et al. Treatment of Chronic Venous Ulcers With Heterologous Fibrin Sealant: A Phase I/II Clinical Trial. Front. Immunol. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Mohammadi, M.H.; Molavi, B.; Mohammadi, S.; Nikbakht, M.; Mohammadi, A.M.; Mostafaei, S.; Norooznezhad, A.H.; Ghorbani Abdegah, A.; Ghavamzadeh, A. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A single-arm clinical trial. Transfus. Apher. Sci. 2017, 56, 160–164. [Google Scholar] [CrossRef]
- Suthar, M.; Gupta, S.; Bukhari, S.; Ponemone, V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J. Biomed. Sci. 2017, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Alonso, N.; Lobato, I.; Hernández, I.; Sebastian, K.S.; Rodríguez, B.; March, A.G.; Perez-Salvador, A.; Arce, V.; Garcia-Alvarez, A.; Gomez-Fernandez, M.C.; et al. Autologous platelet-rich plasma in the treatment of venous leg ulcers in primary care: A randomised controlled, pilot study. J. Wound Care 2018, 27, S20–S24. [Google Scholar] [CrossRef]
- Nolan, G.S.; Smith, O.J.; Heavey, S.; Jell, G.; Mosahebi, A. Histological analysis of fat grafting with platelet-rich plasma for diabetic foot ulcers—A randomised controlled trial. Int. Wound J. 2022, 19, 389–398. [Google Scholar] [CrossRef]
- Meznerics, F.A.; Fehérvári, P.; Dembrovszky, F.; Kovács, K.D.; Kemény, L.V.; Csupor, D.; Hegyi, P.; Bánvölgyi, A. Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Saad Setta, H.; Elshahat, A.; Elsherbiny, K.; Massoud, K.; Safe, I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: A comparative study. Int. Wound J. 2011, 8, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kazakos, K.; Lyras, D.N.; Verettas, D.; Tilkeridis, K.; Tryfonidis, M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury 2009, 40, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Moneib, H.A.; Youssef, S.S.; Aly, D.G.; Rizk, M.A.; Abdelhakeem, Y.I. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: A comparative study. J. Cosmet. Dermatol. 2018, 17, 495–501. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




