Submitted:
25 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Chemical overview
3. Bioactivity overview
4. Derivatives
5. Antioxidant activity
- AOX-I (or primary AOX, or chain braking, or free radical scavenging activity):
- AOX-II (or secondary AOX, or preventing, or OIL behavior):
- AOX-III (or tertiary AOX, or fixing AOX, or repairing AOX):
- AOX-IV (or versatile AOX, or multifunctional AOX, or multipurpose AOX):
5.1. AOX-I chemical routes
5.3. Trends in activity
5.4. Structure-activity relationships
6. Perspectives
- -How much does the presence of redox metals modify the chemistry of the coffee components?
- -How effective are they as chelating agents?
- -Would they act as OH inactivating ligands?
- -Are any of them capable of repairing oxidatively damaged biological targets?
- -Which of them can be considered multifunctional antioxidants?
- -Are their derivatives safe enough to be used as medical drugs?
- -What are the metabolites of these derivatives, and what properties do they have?
- Nature gave us coffee. Revealing its chemical wonders is up to us.
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nolan, L. , The world's favorite beverage- coffee- and health. J. Herbs Spices Med. Plants 2001, 8, 119–159. [Google Scholar] [CrossRef]
- Muhie, S. H. , Strategies to improve the quantity and quality of export coffee in Ethiopia, a look at multiple opportunities. J. Agric.Food Res. 2022, 10, 100372. [Google Scholar] [CrossRef]
- Mussatto, S. I.; Machado, E. M. S.; Martins, S.; Teixeira, J. A. , Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioproc. Tech. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Elder, L. W. Staling vs. Rancidity in Roasted Coffee: Antioxygens Produced by Roasting. Ind. Eng. Chem. 1940, 32, 798–801. [Google Scholar] [CrossRef]
- Adhikari, R.; Shiwakoti, S.; Ko, J. Y.; Dhakal, B.; Park, S. H.; Choi, I. J.; Kim, H. J.; Oak, M. H. , Oxidative Stress in Calcific Aortic Valve Stenosis: Protective Role of Natural Antioxidants. Antioxidants 2022, 11, 1169. [Google Scholar] [CrossRef]
- Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. , Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. [Google Scholar] [CrossRef]
- Angwa, L. M.; Jiang, Y.; Pei, J.; Sun, D. , Antioxidant Phytochemicals for the Prevention of Fluoride-Induced Oxidative Stress and Apoptosis: a Review. Biol. Trace Elem. Res. 2022, 200, 1418–1441. [Google Scholar] [CrossRef] [PubMed]
- Baboo, K.; Chen, Z. Y.; Zhang, X. M. , Role of oxidative stress and antioxidant therapies in endometriosis. Reprod. Dev. Med. 2019, 3, 170–176. [Google Scholar] [CrossRef]
- Berríos-Cárcamo, P.; Quezada, M.; Quintanilla, M. E.; Morales, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y.; Ezquer, F. Oxidative stress and neuroinflammation as a pivot in drug abuse. A focus on the therapeutic potential of antioxidant and anti-inflammatory agents and biomolecules. Antioxidants 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Black, H. S. , A Synopsis of the Associations of Oxidative Stress, ROS, and Antioxidants with Diabetes Mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef]
- Bouyahya, A.; Menyiy, N. E.; Oumeslakht, L.; Allam, A. E.; Balahbib, A.; Rauf, A.; Muhammad, N.; Kuznetsova, E.; Derkho, M.; Thiruvengadam, M.; Shariati, M. A.; Omari, N. E. , Preclinical and clinical antioxidant effects of natural compounds against oxidative stress-induced epigenetic instability in tumor cells. Antioxidants 2021, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M. N.; Colone, M.; Gambioli, R.; Stringaro, A.; Unfer, V. , Oxidative stress and male fertility: Role of antioxidants and inositols. Antioxidants 2021, 10, 1283. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D. G.; Deng, W. , The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, N. J.; Zhang, L. J. , Oxidative stress in leukemia and antioxidant treatment. Chin. Med. J. 2021, 134, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G. A.; Nweke, F. N.; Nkeh-Chungag, B. N. , Free Radicals, Oxidative Stress-Related Diseases and Antioxidant Supplementation. Altern. Ther. Health Med. 2022, 28, 144–128. [Google Scholar]
- Forman, H. J.; Zhang, H. , Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A. R. Q.; Cunha, N.; Varela, E. L. P.; Brígido, H. P. C.; Vale, V. V.; Dolabela, M. F.; de Carvalho, E. P.; Percário, S. , Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy. Int. J. Mol. Sci. 2022, 23, 5949. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Lee, D.; Lee, S. H.; Kim, T. H. , Oxidative stress and antioxidant pathway in allergic rhinitis. Antioxidants 2021, 10, 1266. [Google Scholar] [CrossRef]
- Hou, T. Y.; Wu, S. B.; Kau, H. C.; Tsai, C. C. , The role of oxidative stress and therapeutic potential of antioxidants in graves’ ophthalmopathy. Biomedicines 2021, 9, 1871. [Google Scholar] [CrossRef]
- Hsueh, Y. J.; Chen, Y. N.; Tsao, Y. T.; Cheng, C. M.; Wu, W. C.; Chen, H. C. , The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef]
- Hu, X.; Dong, D.; Xia, M.; Yang, Y.; Wang, J.; Su, J.; Sun, L.; Yu, H. , Oxidative stress and antioxidant capacity: Development and prospects. New J. Chem. 2020, 44, 11405–11419. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D. H.; Kalhoro, M. S.; Rahu, B. A.; Sahito, R. G. A.; Yin, Y.; Yang, H.; Chughtai, M. I.; Tan, B. , The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediators Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Iakovou, E.; Kourti, M. , A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef]
- Kishimoto-Urata, M.; Urata, S.; Fujimoto, C.; Yamasoba, T. , Role of Oxidative Stress and Antioxidants in Acquired Inner Ear Disorders. Antioxidants 2022, 11, 1469. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D. S.; Bhardwaj, S.; Bhatia, S. K.; Verma, R.; Kumar, D. , Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saxena, J.; Srivastava, V. K.; Kaushik, S.; Singh, H.; Abo-El-Sooud, K.; Abdel-Daim, M. M.; Jyoti, A.; Saluja, R. , The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines 2022, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Y.; Wang, R.; Ren, F.; Wang, X. , Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines 2022, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Macvanin, M. T.; Gluvic, Z.; Zafirovic, S.; Gao, X.; Essack, M.; Isenovic, E. R. , The protective role of nutritional antioxidants against oxidative stress in thyroid disorders. Front. Endocrinol. (Lausanne) 2023, 13, 1092837. [Google Scholar] [CrossRef]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M. L. , Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M. C. , Oxidative stress and BPA toxicity: An antioxidant approach for male and female reproductive dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Meulmeester, F. L.; Luo, J.; Martens, L. G.; Mills, K.; van Heemst, D.; Noordam, R. , Antioxidant Supplementation in Oxidative Stress-Related Diseases: What Have We Learned from Studies on Alpha-Tocopherol? Antioxidants 2022, 11, 2322. [Google Scholar] [CrossRef]
- Nandi, S.; Ahmed, S.; Saxena, A. K. , Exploring the Role of Antioxidants to Combat Oxidative Stress in Malaria Parasites. Curr. Top. Med. Chem. 2022, 22, 2029–2044. [Google Scholar] [CrossRef]
- Nantachai, G.; Vasupanrajit, A.; Tunvirachaisakul, C.; Solmi, M.; Maes, M. , Oxidative stress and antioxidant defenses in mild cognitive impairment: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 79, 101639. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E. O.; Gerke-Duncan, M. B.; Holsinger, R. M. D. , Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Percário, S.; Da Silva Barbosa, A.; Varela, E. L. P.; Gomes, A. R. Q.; Ferreira, M. E. S.; De Nazaré Araújo Moreira, T.; Dolabela, M. F. , Oxidative Stress in Parkinson's Disease: Potential Benefits of Antioxidant Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2360872. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A. M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A. I. , Oxidative stress mitigation by antioxidants - An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Poznyak, A. V.; Grechko, A. V.; Orekhova, V. A.; Chegodaev, Y. S.; Wu, W. K.; Orekhov, A. N. , Oxidative stress and antioxidants in atherosclerosis development and treatment. Biology 2020, 9, 60. [Google Scholar] [CrossRef]
- Rajoka, M. S. R.; Thirumdas, R.; Mehwish, H. M.; Umair, M.; Khurshid, M.; Hayat, H. F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F. J.; Barba, F. J. , Role of food antioxidants in modulating gut microbial communities: Novel understandings in intestinal oxidative stress damage and their impact on host health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Gautam, P. , A Review on Antioxidants as Therapeutic in Use of Oxidative Stress and Neurodegenerative Disease. Int. J. Pharm. Qual. Assur. 2022, 13, 77–82. [Google Scholar]
- Rivas-Arancibia, S.; Hernández-Orozco, E.; Rodríguez-Martínez, E.; Valdés-Fuentes, M.; Cornejo-Trejo, V.; Pérez-Pacheco, N.; Dorado-Martínez, C.; Zequeida-Carmona, D.; Espinosa-Caleti, I. , Ozone Pollution, Oxidative Stress, Regulatory T Cells and Antioxidants. Antioxidants 2022, 11, 1553. [Google Scholar] [CrossRef]
- Shohag, S.; Akhter, S.; Islam, S.; Sarker, T.; Sifat, M. K.; Rahman, M. M.; Islam, M. R.; Sharma, R. , Perspectives on the Molecular Mediators of Oxidative Stress and Antioxidant Strategies in the Context of Neuroprotection and Neurolongevity: An Extensive Review. Oxid. Med. Cell. Longev. 2022, 2022, 7743705. [Google Scholar] [CrossRef]
- Taherkhani, S.; Valaei, K.; Arazi, H.; Suzuki, K. , An overview of physical exercise and antioxidant supplementation influences on skeletal muscle oxidative stress. Antioxidants 2021, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Shin, J. M.; Park, J.; Han, M.; Kim, T. H. , Oxidative Stress and Antioxidants in Chronic Rhinosinusitis with Nasal Polyps. Antioxidants 2023, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y. L.; Hsu, C. N. , Oxidative Stress-Induced Hypertension of Developmental Origins: Preventive Aspects of Antioxidant Therapy. Antioxidants 2022, 11, 511. [Google Scholar] [CrossRef]
- Theofanous, T.; Kourti, M. , Abrogating Oxidative Stress as a Therapeutic Strategy Against Parkinson’s Disease: A Mini Review of the Recent Advances on Natural Therapeutic Antioxidant and Neuroprotective Agents. Med. Chem. 2022, 18, 772–783. [Google Scholar] [CrossRef]
- Tsermpini, E. E.; Plemenitaš Ilješ, A.; Dolžan, V. , Alcohol-Induced Oxidative Stress and the Role of Antioxidants in Alcohol Use Disorder: A Systematic Review. Antioxidants 2022, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L. I. M.; Pierella, E.; Piccini, G. B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. , The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Vona, R.; Sposi, N. M.; Mattia, L.; Gambardella, L.; Straface, E.; Pietraforte, D. , Sickle cell disease: Role of oxidative stress and antioxidant therapy. Antioxidants 2021, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kang, P. M. , Oxidative stress and antioxidant treatments in cardiovascular diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. , The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef]
- Yang, N.; Guan, Q. W.; Chen, F. H.; Xia, Q. X.; Yin, X. X.; Zhou, H. H.; Mao, X. Y. , Antioxidants targeting mitochondrial oxidative stress: Promising neuroprotectants for epilepsy. Oxid. Med. Cell. Longev. 2020, 2020, 6687185. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M. S.; Quarta, A.; Marradi, M.; Ragusa, A. , Recent developments in the reduction of oxidative stress through antioxidant polymeric formulations. Pharmaceutics 2019, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Cioara, F. L.; Munteanu, M. A.; Brisc, M. C.; Uivarosan, D.; Brisc, C. , Current trends in neurodegeneration: Cross talks between oxidative stress, cell death, and inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Sinicropi, M. S.; Genchi, G. , Oxidative stress and neurodegeneration: the involvement of iron. BioMetals 2018, 31, 715–735. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. , Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef]
- Elfawy, H. A.; Das, B. , Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef]
- Espinós, C.; Galindo, M. I.; García-Gimeno, M. A.; Ibáñez-Cabellos, J. S.; Martínez-Rubio, D.; Millán, J. M.; Rodrigo, R.; Sanz, P.; Seco-Cervera, M.; Sevilla, T.; Tapia, A.; Pallardó, F. V. , Oxidative stress, a crossroad between rare diseases and neurodegeneration. Antioxidants 2020, 9, 313. [Google Scholar] [CrossRef]
- Franzoni, F.; Scarfò, G.; Guidotti, S.; Fusi, J.; Asomov, M.; Pruneti, C. , Oxidative Stress and Cognitive Decline: The Neuroprotective Role of Natural Antioxidants. Front. Neurosci. 2021, 15, 729757. [Google Scholar] [CrossRef]
- Gkekas, I.; Gioran, A.; Boziki, M. K.; Grigoriadis, N.; Chondrogianni, N.; Petrakis, S. , Oxidative stress and neurodegeneration: Interconnected processes in polyq diseases. Antioxidants 2021, 10, 1450. [Google Scholar] [CrossRef]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. , Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef]
- Hassan, W.; Noreen, H.; Rehman, S.; Kamal, M. A.; da Rocha, J. B. T. , Association of Oxidative Stress with Neurological Disorders. Curr. Neuropharmacol. 2022, 20, 1046–1072. [Google Scholar] [CrossRef]
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. , Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants 2023, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Lananna, B. V.; Musiek, E. S. , The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol. Dis. 2020, 139, 104832. [Google Scholar] [CrossRef] [PubMed]
- Mallet, M. L.; Hadjivassiliou, M.; Sarrigiannis, P. G.; Zis, P. , The Role of Oxidative Stress in Peripheral Neuropathy. J. Mol. Neurosci. 2020, 70, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Martínez Leo, E. E.; Segura Campos, M. R. , Systemic Oxidative Stress: A Key Point in Neurodegeneration — A Review. J. Nutr. Health Aging 2019, 23, 694–699. [Google Scholar] [CrossRef]
- Mendonca, H.; Carpi-Santos, R.; Da Costa Calaza, K.; Blanco Martinez, A. , Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: Galectin-3 participation. Neural Regen. Res. 2020, 15, 625–635. [Google Scholar]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimer’s Dis. 2021, 82, S109–S126. [Google Scholar] [CrossRef]
- Michalska, P.; León, R. , When it comes to an end: Oxidative stress crosstalk with protein aggregation and neuroinflammation induce neurodegeneration. Antioxidants 2020, 9, 740. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kanda, Y.; Sone, H.; Aoyama, H. , Oxidative Stress as a Common Key Event in Developmental Neurotoxicity. Oxid. Med. Cell. Longev. 2021, 2021, 6685204. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; Estrela, J. M.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S. L. , Oxidative stress, neuroinflammation and mitochondria in the pathophysiology of amyotrophic lateral sclerosis. Antioxidants 2020, 9, 901. [Google Scholar] [CrossRef]
- Pardillo-Díaz, R.; Pérez-García, P.; Castro, C.; Nunez-Abades, P.; Carrascal, L. , Oxidative Stress as a Potential Mechanism Underlying Membrane Hyperexcitability in Neurodegenerative Diseases. Antioxidants 2022, 11, 1511. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Júnior, H. J.; Landi, F.; Bernabei, R.; Marzetti, E. , Mitochondrial dysfunction, oxidative stress, and neuroinflammation: Intertwined roads to neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J. V.; Varrassi, G. , Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef]
- Rivas, F.; Poblete-Aro, C.; Pando, M. E.; Allel, M. J.; Fernandez, V.; Soto, A.; Nova, P.; Garcia-Diaz, D. Effects of Polyphenols in Aging and Neurodegeneration Associated with Oxidative Stress. Curr. Med, Chem, 2022, 29, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Morello, B.; Ahmadi, H.; Vohra, R.; Saruhanian, S.; Freude, K. K.; Hamann, S.; Kolko, M. , Oxidative stress in optic neuropathies. Antioxidants 2021, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rapposelli, S.; Sestito, S.; Herrera-Bravo, J.; Arancibia-Diaz, A.; Salazar, L. A.; Yeskaliyeva, B.; Beyatli, A.; Leyva-Gómez, G.; González-Contreras, C.; Gürer, E. S.; Martorell, M.; Calina, D. , Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation. J. Pers. Med. 2022, 12, 1515. [Google Scholar] [CrossRef]
- Sharma, S.; Advani, D.; Das, A.; Malhotra, N.; Khosla, A.; Arora, V.; Jha, A.; Yadav, M.; Ambasta, R. K.; Kumar, P. , Pharmacological intervention in oxidative stress as a therapeutic target in neurological disorders. J. Pharm. Pharmacol. 2022, 74, 461–484. [Google Scholar] [CrossRef]
- Sienes Bailo, P.; Llorente Martín, E.; Calmarza, P.; Montolio Breva, S.; Bravo Gómez, A.; Pozo Giráldez, A.; Sánchez-Pascuala Callau, J. J.; Vaquer Santamaría, J. M.; Dayaldasani Khialani, A.; Cerdá Micó, C.; Camps Andreu, J.; Sáez Tormo, G.; Fort Gallifa, I. , The role of oxidative stress in neurodegenerative diseases and potential antioxidant therapies. Adv. Lab. Med. 2022, 3, 342–350. [Google Scholar] [CrossRef]
- Simpson, D. S. A.; Oliver, P. L. , Ros generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. , Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Singh, E.; Devasahayam, G. , Neurodegeneration by oxidative stress: a review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020, 47, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Han, K.; Wang, Y.; Qu, R.; Liu, Y.; Wang, S.; Wang, Y.; An, Z.; Li, J.; Wu, H.; Wu, W. Microglial Activation and Oxidative Stress in PM2.5-Induced Neurodegenerative Disorders. Antioxidants 2022, 11, 1482. [Google Scholar] [CrossRef]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D. M.; Moya, P. R.; Piccart, E.; Hellings, N.; Eijnde, B. O.; Derave, W.; Schreiber, R.; Vanmierlo, T. , Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cellular and Molecular Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D. M.; Niculescu, A. G.; Lungu, I. I.; Radu, C. I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A. M.; Teleanu, R. I. , An Overview of Oxidative Stress, Neuroinflammation and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Volkert, M. R.; Crowley, D. J. , Preventing Neurodegeneration by Controlling Oxidative Stress: The Role of OXR1. Front. Neurosci. 2020, 14, 611904. [Google Scholar] [CrossRef]
- Aborode, A. T.; Pustake, M.; Awuah, W. A.; Alwerdani, M.; Shah, P.; Yarlagadda, R.; Ahmad, S.; Silva Correia, I. F.; Chandra, A.; Nansubuga, E. P.; Abdul-Rahman, T.; Mehta, A.; Ali, O.; Amaka, S. O.; Zuñiga, Y. M. H.; Shkodina, A. D.; Inya, O. C.; Shen, B.; Alexiou, A. , Targeting Oxidative Stress Mechanisms to Treat Alzheimer's and Parkinson's Disease: A Critical Review. Oxid. Med. Cell. Longev. 2022, 2022, 7934442. [Google Scholar] [CrossRef]
- Allan Butterfield, D.; Boyd-Kimball, D. , Mitochondrial oxidative and nitrosative stress and Alzheimer disease. Antioxidants 2020, 9, 818. [Google Scholar] [CrossRef]
- Anwar, M. M. , Oxidative stress-A direct bridge to central nervous system homeostatic dysfunction and Alzheimer's disease. Cell Biochem. Funct. 2022, 40, 17–27. [Google Scholar] [CrossRef]
- Beura, S. K.; Dhapola, R.; Panigrahi, A. R.; Yadav, P.; Reddy, D. H.; Singh, S. K. , Redefining oxidative stress in Alzheimer's disease: Targeting platelet reactive oxygen species for novel therapeutic options. Life Sci. 2022, 306, 120855. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Sharma, S. , Role of mitochondrial dysfunction, oxidative stress and autophagy in progression of Alzheimer's disease. J. Neurol. Sci. 2021, 421, 117253. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D. A.; Halliwell, B. , Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J. B.; Naiker, M.; Owoola, A. G.; Broszczak, D. A. , Oxidative stress in alzheimer's disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. , Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Adam, R. H. I.; Bansal, R.; Broersen, K. A review of oxidative stress products and related genes in early alzheimer's disease. J. Alzheimer’s Dis. 2021, 83, 977–1001. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Adam, R. H. I.; Broersen, K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer's Disease. J. Alzheimer’s Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C. W. , Emerging roles of oxidative stress in brain aging and Alzheimer's disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. , Chronic stress and oxidative stress as common factors of the pathogenesis of depression and alzheimer’s disease; the role of antioxidants in prevention and treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Kowalska, M.; Wize, K.; Prendecki, M.; Lianeri, M.; Kozubski, W.; Dorszewska, J. , Genetic variants and oxidative stress in alzheimer’s disease. Curr. Alzheimer Res. 2020, 17, 208–223. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. , Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Rummel, N. G.; Butterfield, D. A. , Altered Metabolism in Alzheimer Disease Brain: Role of Oxidative Stress. Antioxid. Redox Signal. 2022, 36, 1289–1305. [Google Scholar] [CrossRef]
- Shabbir, U.; Tyagi, A.; Elahi, F.; Aloo, S. O.; Oh, D. H. , The potential role of polyphenols in oxidative stress and inflammation induced by gut microbiota in alzheimer’s disease. Antioxidants 2021, 10, 1370. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S. R. , Linking oxidative stress and proteinopathy in alzheimer’s disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Alwasel, S. H.; Alhazza, I. M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. , Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Tzoneva, R. , Oxidative Stress and Aging as Risk Factors for Alzheimer’s Disease and Parkinson’s Disease: The Role of the Antioxidant Melatonin. Int. J. Mol. Sci. 2023, 24, 3022. [Google Scholar] [CrossRef]
- Zhao, Z. , Iron and oxidizing species in oxidative stress and Alzheimer's disease. Aging Med. 2019, 2, 82–87. [Google Scholar] [CrossRef]
- Chang, K. H.; Chen, C. M. , The role of oxidative stress in Parkinson’s disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef]
- Dionísio, P. A.; Amaral, J. D.; Rodrigues, C. M. P. , Oxidative stress and regulated cell death in Parkinson's disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. , Oxidative stress factors in Parkinson's disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Guo, J. D.; Zhao, X.; Li, Y.; Li, G. R.; Liu, X. L. , Damage to dopaminergic neurons by oxidative stress in Parkinson's disease (Review). Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- 1https://doi.org/Hassanzadeh, K.; Rahimmi, A. Oxidative stress and neuroinflammation in the story of Parkinson’s disease: Could targeting these pathways write a good ending? J. Cell. Physiol. 2018, 234, 23–32. [Google Scholar] [CrossRef]
- Hor, S. L.; Teoh, S. L.; Lim, W. L. , Plant polyphenols as neuroprotective agents in parkinson’s disease targeting oxidative stress. Curr. Drug Targets 2020, 21, 458–476. [Google Scholar] [CrossRef]
- Monzani, E.; Nicolis, S.; Dell'Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F. A.; Mosharov, E. V.; Sulzer, D.; Zecca, L.; Casella, L. , Dopamine, Oxidative Stress and Protein–Quinone Modifications in Parkinson's and Other Neurodegenerative Diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S. Y.; Shim, J. W. , Oxidative stress and cellular pathologies in Parkinson's disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. , Association between Heavy Metal Exposure and Parkinson’s Disease: A Review of the Mechanisms Related to Oxidative Stress. Antioxidants 2022, 11, 2467. [Google Scholar] [CrossRef]
- Rizor, A.; Pajarillo, E.; Johnson, J.; Aschner, M.; Lee, E. , Astrocytic oxidative/nitrosative stress contributes to parkinson’s disease pathogenesis: The dual role of reactive astrocytes. Antioxidants 2019, 8, 265. [Google Scholar] [CrossRef]
- Trist, B. G.; Hare, D. J.; Double, K. L. , Oxidative stress in the aging substantia nigra and the etiology of Parkinson's disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J. N. , Circadian rhythms, Neuroinflammation and Oxidative Stress in the Story of Parkinson's Disease. Cells 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. , Oxidative Stress in Parkinson's Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, N.; Pariante, C. M.; Zunszain, P. A. , Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G. H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A. A. H.; Iqbal, M. S.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. , Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A. N.; Patil, C. R. , Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Correia, A. S.; Cardoso, A.; Vale, N. , Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Gorlova, A.; Svirin, E.; Pavlov, D.; Cespuglio, R.; Proshin, A.; Schroeter, C. A.; Lesch, K. P.; Strekalova, T. , Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies. Int. J. Mol. Sci. 2023, 24, 915. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fernández, S.; Gurpegui, M.; Garrote-Rojas, D.; Gutiérrez-Rojas, L.; Carretero, M. D.; Correll, C. U. , Oxidative stress parameters and antioxidants in adults with unipolar or bipolar depression versus healthy controls: Systematic review and meta-analysis. J. Affect. Disord. 2022, 314, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A. L.; Maker, G. L.; Hood, S. D.; Drummond, P. D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef]
- Shao, A.; Lin, D.; Wang, L.; Tu, S.; Lenahan, C.; Zhang, J. , Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis. 2020, 11, 1537–1566. [Google Scholar] [CrossRef] [PubMed]
- Vaváková, M.; ɰuračková, Z.; Trebatická, J. , Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid. Med. Cell. Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Adamczyk-Sowa, M. , New Insights into the Role of Oxidative Stress Mechanisms in the Pathophysiology and Treatment of Multiple Sclerosis. Oxid. Med. Cell. Longev. 2016, 2016, 1973834. [Google Scholar] [CrossRef] [PubMed]
- Haider, L. , Inflammation, Iron, Energy Failure, and Oxidative Stress in the Pathogenesis of Multiple Sclerosis. Oxid. Med. Cell. Longev. 2015, 2015, 725370. [Google Scholar] [CrossRef] [PubMed]
- Hollen, C.; Neilson, L. E.; Barajas, R. F.; Greenhouse, I.; Spain, R. I. , Oxidative stress in multiple sclerosis—Emerging imaging techniques. Front. Neurol. 2023, 13, 1025659. [Google Scholar] [CrossRef]
- Ibitoye, R.; Kemp, K.; Rice, C.; Hares, K.; Scolding, N.; Wilkins, A. , Oxidative stress-related biomarkers in multiple sclerosis: A review. Biomark. Med. 2016, 10, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Ohl, K.; Tenbrock, K.; Kipp, M. , Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G. G.; Pacheco-Moisés, F. P.; Bitzer-Quintero, O. K.; Ramírez-Anguiano, A. C.; Flores-Alvarado, L. J.; Ramírez-Ramírez, V.; Macias-Islas, M. A.; Torres-Sánchez, E. D. , Immunology and oxidative stress in multiple sclerosis: Clinical and basic approach. Clin. Dev. Immunol. 2013, 2013, 708659. [Google Scholar] [CrossRef]
- Signorile, A.; Ferretta, A.; Ruggieri, M.; Paolicelli, D.; Lattanzio, P.; Trojano, M.; Rasmo, D. D. Mitochondria, oxidative stress, camp signalling and apoptosis: A crossroads in lymphocytes of multiple sclerosis, a possible role of nutraceutics. Antioxidants 2021, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tobore, T. O. , Oxidative/Nitroxidative Stress and Multiple Sclerosis. J. Mol. Neurosci. 2021, 71, 506–514. [Google Scholar] [CrossRef]
- Zha, Z.; Liu, S.; Liu, Y.; Li, C.; Wang, L. , Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis. Antioxidants 2022, 11, 1495. [Google Scholar] [CrossRef]
- Zhang, S. Y.; Gui, L. N.; Liu, Y. Y.; Shi, S.; Cheng, Y. , Oxidative Stress Marker Aberrations in Multiple Sclerosis: A Meta-Analysis Study. Front. Neurosci. 2020, 14, 823. [Google Scholar] [CrossRef]
- Akbari, A.; Majd, H. M.; Rahnama, R.; Heshmati, J.; Morvaridzadeh, M.; Agah, S.; Amini, S. M.; Masoodi, M. , Cross-talk between oxidative stress signaling and microRNA regulatory systems in carcinogenesis: Focused on gastrointestinal cancers. Biomed. Pharmacother. 2020, 131, 110729. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. , Oxidative stress and photodynamic therapy of skin cancers: Mechanisms, challenges and promising developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef]
- Andrisic, L.; Dudzik, D.; Barbas, C.; Milkovic, L.; Grune, T.; Zarkovic, N. , Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018, 14, 47–58. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N. K.; Jha, S. K.; Kesari, K. K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. , Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Basak, D.; Uddin, M. N.; Hancock, J. The role of oxidative stress and its counteractive utility in colorectal cancer (CRC). Cancers 2020, 12, 3336. [Google Scholar] [CrossRef]
- Bhatiya, M.; Pathak, S.; Banerjee, A. , Oxidative Stress and Cellular Senescence: The Key Tumor-promoting Factors in Colon Cancer and Beneficial Effects of Polyphenols in Colon Cancer Prevention. Curr. Cancer Ther. Rev. 2021, 17, 292–303. [Google Scholar] [CrossRef]
- Calaf, G. M.; Urzua, U.; Termini, L.; Aguayo, F. , Oxidative stress in female cancers. Oncotarget 2018, 9, 23824–23842. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A. W.; Tommasi, S.; Besaratinia, A. , Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N. M.; Sukocheva, O. A.; Madhunapantula, S. V.; Liu, J.; Sinelnikov, M. Y.; Nikolenko, V. N.; Bulygin, K. V.; Mikhaleva, L. M.; Reshetov, I. V.; Gu, Y.; Zhang, J.; Cao, Y.; Somasundaram, S. G.; Kirkland, C. E.; Fan, R.; Aliev, G. , Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Aranda-Rivera, A. K.; Ortega-Lozano, A. J.; Pedraza-Chaverri, J.; Mendoza-Hoffmann, F. , Lipid metabolism and oxidative stress in HPV-related cancers. Free Radic. Biol. Med. 2021, 172, 226–236. [Google Scholar] [CrossRef] [PubMed]
- D'Souza, L. C.; Mishra, S.; Chakraborty, A.; Shekher, A.; Sharma, A.; Gupta, S. C. , Oxidative Stress and Cancer Development: Are Noncoding RNAs the Missing Links? Antioxid. Redox Signal. 2020, 33, 1209–1229. [Google Scholar] [CrossRef]
- Ding, D. N.; Xie, L. Z.; Shen, Y.; Li, J.; Guo, Y.; Fu, Y.; Liu, F. Y.; Han, F. J. , Insights into the Role of Oxidative Stress in Ovarian Cancer. Oxid. Med. Cell. Longev. 2021, 2021, 8388258. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Soltani, A.; Hashemy, S. I. , Oxidative stress in cervical cancer pathogenesis and resistance to therapy. J. Cell. Biochem. 2019, 120, 6868–6877. [Google Scholar] [CrossRef]
- Ebrahimi, S. O.; Reiisi, S.; Shareef, S. , miRNAs, oxidative stress, and cancer: A comprehensive and updated review. J. Cell. Physiol. 2020, 235, 8812–8825. [Google Scholar] [CrossRef]
- García-Guede, Á.; Vera, O.; Ibáñez-de-Caceres, I. , When oxidative stress meets epigenetics: Implications in cancer development. Antioxidants 2020, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Gurer-Orhan, H.; Ince, E.; Konyar, D.; Saso, L.; Suzen, S. The role of oxidative stress modulators in breast cancer. Curr. Med, Chem, 2018, 25, 4084–4101. [Google Scholar] [CrossRef]
- Hayes, J. D.; Dinkova-Kostova, A. T.; Tew, K. D. , Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Jelic, M. D.; Mandic, A. D.; Maricic, S. M.; Srdjenovic, B. U. , Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E. V.; Gavriliuk, L. A.; Pokrovsky, V. S. , Oxidative Stress and Redox-Dependent Signaling in Prostate Cancer. Biochemistry (Moscow) 2022, 87, 413–424. [Google Scholar] [CrossRef]
- Katakwar, P.; Metgud, R.; Naik, S.; Mittal, R. , Oxidative stress marker in oral cancer: A review. J. Cancer Res. Ther. 2016, 12, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Y. , Oxidative stress and gender disparity in cancer. Free Radic. Res. 2022, 56, 90–105. [Google Scholar] [CrossRef]
- Klaunig, J. E. , Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H. Y. Reactive oxygen and nitrogen species in carcinogenesis: Implications of oxidative stress on the progression and development of several cancer types. Mini-Reviews Med. Chem. 2017, 17, 904–919. [Google Scholar] [CrossRef]
- Lee, D. Y.; Song, M. Y.; Kim, E. H. , Role of oxidative stress and nrf2/keap1 signaling in colorectal cancer: Mechanisms and therapeutic perspectives with phytochemicals. Antioxidants 2021, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhou, D.; Wang, Q.; Liu, W.; Yu, F.; Wu, F.; Chen, C. , Crosstalk of MicroRNAs and Oxidative Stress in the Pathogenesis of Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 2415324. [Google Scholar] [CrossRef]
- Mazzuferi, G.; Bacchetti, T.; Islam, M. O.; Ferretti, G. , High density lipoproteins and oxidative stress in breast cancer. Lipids Health Dis. 2021, 20, 143. [Google Scholar] [CrossRef]
- Mdkhana, B.; Goel, S.; Saleh, M. A.; Siddiqui, R.; Khan, N. A.; Elmoselhi, A. B. , Role of oxidative stress in angiogenesis and the therapeutic potential of antioxidants in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4677–4692. [Google Scholar]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. , Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef]
- Neganova, M.; Liu, J.; Aleksandrova, Y.; Klochkov, S.; Fan, R. , Therapeutic influence on important targets associated with chronic inflammation and oxidative stress in cancer treatment. Cancers 2021, 13, 6062. [Google Scholar] [CrossRef]
- Oh, B.; Figtree, G.; Costa, D.; Eade, T.; Hruby, G.; Lim, S.; Elfiky, A.; Martine, N.; Rosenthal, D.; Clarke, S.; Back, M. , Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Int. 2016, 4, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Saed, G. M.; Diamond, M. P.; Fletcher, N. M. , Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 2017, 145, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Saha, S. K.; Lee, S. B.; Won, J.; Choi, H. Y.; Kim, K.; Yang, G. M.; Dayem, A. A.; Cho, S. G. , Correlation between oxidative stress, nutrition, and cancer initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Khazaei, M. , Oxidative stress and cancer: The role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef]
- Sanati, M.; Afshari, A. R.; Kesharwani, P.; Sukhorukov, V. N.; Sahebkar, A. , Recent trends in the application of nanoparticles in cancer therapy: The involvement of oxidative stress. J. Control. Release 2022, 348, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J. P.; Chuang, Y. T.; Tang, J. Y.; Yang, K. H.; Chang, F. R.; Hou, M. F.; Yen, C. Y.; Chang, H. W. , The Impact of Oxidative Stress and AKT Pathway on Cancer Cell Functions and Its Application to Natural Products. Antioxidants 2022, 11, 1845. [Google Scholar] [CrossRef]
- Taucher, E.; Mykoliuk, I.; Fediuk, M.; Smolle-Juettner, F. M. , Autophagy, Oxidative Stress and Cancer Development. Cancers (Basel) 2022, 14, 1637. [Google Scholar] [CrossRef]
- Trošelj, K. G.; Tomljanović, M.; Jaganjac, M.; Matijević Glavan, T.; Čipak Gašparović, A.; Milković, L.; Borović Šunjić, S.; Buttari, B.; Profumo, E.; Saha, S.; Saso, L.; Žarković, N. , Oxidative Stress and Cancer Heterogeneity Orchestrate NRF2 Roles Relevant for Therapy Response. Molecules 2022, 27, 1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Ye, Y.; Xie, L.; Li, W. , Oxidative stress and liver cancer: Etiology and therapeutic targets. Oxid. Med. Cell. Longev. 2016, 2016, 7891574. [Google Scholar] [CrossRef]
- Wigner, P.; Grębowski, R.; Bijak, M.; Saluk-Bijak, J.; Szemraj, J. , The interplay between oxidative stress, inflammation and angiogenesis in bladder cancer development. Int. J. Mol. Sci. 2021, 22, 4483. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Q. , Cross-Talk between Oxidative Stress and m6A RNA Methylation in Cancer. Oxid. Med. Cell. Longev. 2021, 2021, 6545728. [Google Scholar] [CrossRef]
- Zahra, K. F.; Lefter, R.; Ali, A.; Abdellah, E. C.; Trus, C.; Ciobica, A.; Timofte, D. , The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxid. Med. Cell. Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Zhu, M.; Xu, W. , Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid. Med. Cell. Longev. 2016, 2016, 8589318. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, G.; Polimeni, A.; Ceccanti, M.; Caronti, B.; Di Certo, M. G.; Barbato, C.; Mattia, A.; Tarani, L.; Fiore, M. , Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145. [Google Scholar] [CrossRef]
- Liu, H. M.; Cheng, M. Y.; Xun, M. H.; Zhao, Z. W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. , Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Barreiro Arcos, M. L. Role of thyroid hormones-induced oxidative stress on cardiovascular physiology. Biochim. Biophys. Acta - Gen. Subj. 2022, 1866, 130239. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liu, Y.; Men, H.; Zheng, Y. , Protective Mechanism of Humanin Against Oxidative Stress in Aging-Related Cardiovascular Diseases. Front. Endocrinol. (Lausanne) 2021, 12, 683151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jin, Z. X.; Cai, J.; Li, R.; Deng, K. Q.; Ji, Y. X.; Lei, F.; Li, H. P.; Lu, Z.; Li, H. , Energy substrate metabolism and oxidative stress in metabolic cardiomyopathy. J. Mol. Med. 2022, 100, 1721–1739. [Google Scholar] [CrossRef]
- De Almeida, A. J. P. O.; De Almeida Rezende, M. S.; Dantas, S. H.; De Lima Silva, S.; De Oliveira, J. C. P. L.; De Lourdes Assunção Araújo De Azevedo, F.; Alves, R. M. F. R.; De Menezes, G. M. S.; Dos Santos, P. F.; Gonçalves, T. A. F.; Schini-Kerth, V. B.; De Medeiros, I. A. , Unveiling the Role of Inflammation and Oxidative Stress on Age-Related Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 1954398. [Google Scholar] [CrossRef]
- De Geest, B.; Mishra, M. , Role of Oxidative Stress in Diabetic Cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef]
- Dos Santos, J. L.; de Quadros, A. S.; Weschenfelder, C.; Garofallo, S. B.; Marcadenti, A. , Oxidative stress biomarkers, nut-related antioxidants, and cardiovascular disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef]
- Fabiani, I.; Aimo, A.; Grigoratos, C.; Castiglione, V.; Gentile, F.; Saccaro, L. F.; Arzilli, C.; Cardinale, D.; Passino, C.; Emdin, M. , Oxidative stress and inflammation: determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart Fail. Rev. 2021, 26, 881–890. [Google Scholar] [CrossRef]
- Farías, J. G.; Molina, V. M.; Carrasco, R. A.; Zepeda, A. B.; Figueroa, E.; Letelier, P.; Castillo, R. L. , Antioxidant therapeutic strategies for cardiovascular conditions associated with oxidative stress. Nutrients 2017, 9, 966. [Google Scholar] [CrossRef]
- Gaggini, M.; Ndreu, R.; Michelucci, E.; Rocchiccioli, S.; Vassalle, C. , Ceramides as Mediators of Oxidative Stress and Inflammation in Cardiometabolic Disease. Int. J. Mol. Sci. 2022, 23, 2719. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Shcherbik, N. , Effects of oxidative stress on protein translation: Implications for cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 2661. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The role of oxidative stress in cardiovascular aging and cardiovascular diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, V.; Djuric, D.; Pechanova, O.; Bolevich, S.; Tyagi, S. , Oxidative Stress and Cardiovascular Dysfunction: From Basic Science to Applied Investigations. Oxid. Med. Cell. Longev. 2020, 2020, 6985284. [Google Scholar] [CrossRef] [PubMed]
- Kander, M. C.; Cui, Y.; Liu, Z. , Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Kelly, F. J.; Fussell, J. C. , Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic. Biol. Med. 2017, 110, 345–367. [Google Scholar] [CrossRef]
- Lin, D.; Wang, L.; Yan, S.; Zhang, Q.; Zhang, J. H.; Shao, A. , The Role of Oxidative Stress in Common Risk Factors and Mechanisms of Cardio-Cerebrovascular Ischemia and Depression. Oxid. Med. Cell. Longev. 2019, 2019, 2491927. [Google Scholar] [CrossRef]
- Lüscher, T. F. , Ageing, inflammation, and oxidative stress: Final common pathways of cardiovascular disease. Eur. Heart J. 2015, 36, 3381–3383. [Google Scholar] [CrossRef]
- Mei, Y.; Thompson, M. D.; Cohen, R. A.; Tong, X. Autophagy and oxidative stress in cardiovascular diseases. Biochim. Biophys. Acta - Mol. Basis Dis. 2015, 1852, 243–251. [Google Scholar] [CrossRef]
- Panda, P.; Verma, H. K.; Lakkakula, S.; Merchant, N.; Kadir, F.; Rahman, S.; Jeffree, M. S.; Lakkakula, B. V. K. S.; Rao, P. V. , Biomarkers of Oxidative Stress Tethered to Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 9154295. [Google Scholar] [CrossRef]
- Petek, T. H.; Petek, T.; Močnik, M.; Varda, N. M. , Systemic Inflammation, Oxidative Stress and Cardiovascular Health in Children and Adolescents: A Systematic Review. Antioxidants 2022, 11, 894. [Google Scholar] [CrossRef]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. , Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Peng, Y.; Lang, H.; Xinyong, C.; Zhiyi, Z.; Xiaocheng, W.; Hong, Z.; Liang, S. , Oxidative Stress in Radiation-Induced Cardiotoxicity. Oxid. Med. Cell. Longev. 2020, 2020, 3579143. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C. G.; López de Pablo, A. L.; Carmen González, M.; Arribas, S. M. , Implication of oxidative stress in fetal programming of cardiovascular disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Rotariu, D.; Babes, E. E.; Tit, D. M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A. F.; Vesa, C. M.; Behl, T.; Bungau, A. F.; Bungau, S. G. , Oxidative stress – Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. , Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A. E.; Pintus, G.; Eid, A. H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. - Landmark 2022, 27, 105. [Google Scholar] [CrossRef]
- Siti, H. N.; Kamisah, Y.; Kamsiah, J. , The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Jimenez, M. T. B.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; Daiber, A. , Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Sun, Y.; Rawish, E.; Nording, H. M.; Langer, H. F. , Inflammation in metabolic and cardiovascular disorders—role of oxidative stress. Life 2021, 11, 672. [Google Scholar] [CrossRef]
- Theofilis, P.; Vordoni, A.; Kalaitzidis, R. G. Oxidative Stress Management in Cardiorenal Diseases: Focus on Novel Antidiabetic Agents, Finerenone, and Melatonin. Life 2022, 12, 1663. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. , The Role of Oxidative Stress and Inflammation in Cardiovascular Aging. Biomed Res. Int. 2014, 2014, 615312. [Google Scholar] [CrossRef]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. , Oxidative Stress in Cell Death and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9030563. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, K.; Xing, W.; Dong, M.; Yi, M.; Zhang, H. , Role of Iron-Related Oxidative Stress and Mitochondrial Dysfunction in Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 5124553. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhu, Q. X.; Wu, X. L.; Tan, T. T.; Jiang, X. J. , Histone Methylation and Oxidative Stress in Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 6023710. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; Xiong, Y. , Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. J.; Yuan, S.; Zi, H.; Gu, J. M.; Fang, C.; Zeng, X. T. , Oxidative Stress Links Aging-Associated Cardiovascular Diseases and Prostatic Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 5896136. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, C. K.; Zhang, C. L.; Huang, Y. , Interplay between Oxidative Stress, Cyclooxygenases, and Prostanoids in Cardiovascular Diseases. Antioxid. Redox Signal. 2021, 34, 784–799. [Google Scholar] [CrossRef]
- Zhou, Y.; Murugan, D. D.; Khan, H.; Huang, Y.; Cheang, W. S. , Roles and therapeutic implications of endoplasmic reticulum stress and oxidative stress in cardiovascular diseases. Antioxidants 2021, 10, 1167. [Google Scholar] [CrossRef]
- Bin-Jumah, M. N.; Nadeem, M. S.; Gilani, S. J.; Mubeen, B.; Ullah, I.; Alzarea, S. I.; Ghoneim, M. M.; Alshehri, S.; Al-Abbasi, F. A.; Kazmi, I. , Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. , Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef]
- Cox, F. F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. , Protective Effects of Curcumin in Cardiovascular Diseases—Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Chlopicki, S. , Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Donia, T.; Khamis, A. , Management of oxidative stress and inflammation in cardiovascular diseases: mechanisms and challenges. Environ. Sci. Pollut. Res. 2021, 28, 34121–34153. [Google Scholar] [CrossRef] [PubMed]
- Gherghina, M. E.; Peride, I.; Tiglis, M.; Neagu, T. P.; Niculae, A.; Checherita, I. A. , Uric Acid and Oxidative Stress—Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dudley, S. C. , Magnesium, oxidative stress, inflammation, and cardiovascular disease. Antioxidants 2020, 9, 907. [Google Scholar] [CrossRef]
- Miller, M. R. , Oxidative stress and the cardiovascular effects of air pollution. Free Radic. Biol. Med. 2020, 151, 69–87. [Google Scholar] [CrossRef]
- Mozzini, C.; Setti, A.; Cicco, S.; Pagani, M. , The Most Severe Paradigm of Early Cardiovascular Disease: Hutchinson-Gilford Progeria. Focus on the Role of Oxidative Stress. Curr. Probl. Cardiol. 2022, 47, 100900. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, J.; Li, X.; Zhuang, J. , Intersection of the Ubiquitin–Proteasome System with Oxidative Stress in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12197. [Google Scholar] [CrossRef]
- Alu, S. N.; Los, E. A.; Ford, G. A.; Stone, W. L. , Oxidative Stress in Type 2 Diabetes: The Case for Future Pediatric Redoxomics Studies. Antioxidants 2022, 11, 1336. [Google Scholar] [CrossRef]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. , The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Bhatti, J. S.; Sehrawat, A.; Mishra, J.; Sidhu, I. S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G. K.; Reddy, P. H. , Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Darenskaya, M. A.; Kolesnikova, L. I.; Kolesnikov, S. I. , Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- David, J. A.; Rifkin, W. J.; Rabbani, P. S.; Ceradini, D. J. , The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N. D.; Dafoe, D. C.; Ichii, H. , The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; Rafieian-Kopaei, M. , Oxidative stress and antioxidants in diabetes mellitus. Asian Pac. J. Trop. Med. 2020, 13, 431–438. [Google Scholar]
- Gorini, F.; Sabatino, L.; Gaggini, M.; Chatzianagnostou, K.; Vassalle, C. , Oxidative stress biomarkers in the relationship between type 2 diabetes and air pollution. Antioxidants 2021, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. , The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O. M. , Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Lee, W. C.; Mokhtar, S. S.; Munisamy, S.; Yahaya, S.; Rasool, A. H. G. , Vitamin D status and oxidative stress in diabetes mellitus. Cell. Mol. Biol. 2018, 64, 60–69. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T. J.; Mikolajczyk, T. P. , Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Ly, L. D.; Xu, S.; Choi, S. K.; Ha, C. M.; Thoudam, T.; Cha, S. K.; Wiederkehr, A.; Wollheim, C. B.; Lee, I. K.; Park, K. S. , Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef]
- Qadir, M. M. F.; Klein, D.; Álvarez-Cubela, S.; Domínguez-Bendala, J.; Pastori, R. L. , The role of microRNAs in diabetes-related oxidative stress. Int. J. Mol. Sci. 2019, 20, 5423. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Riera, K. P.; Pérez-Severiano, F.; López-Meraz, M. L. , Oxidative stress: a common imbalance in diabetes and epilepsy. Metab. Brain Dis. 2023, 38, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. , Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J. S.; Nunes, S.; Rolo, A. P.; Reis, F.; Palmeira, C. M. , Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front. Physiol. 2019, 10, 1857. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Kumar, A.; Kumar, A. , Targeting oxidative stress through antioxidants in diabetes mellitus. J. Drug Target. 2018, 26, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Zhang, F.; Wang, R.; Ma, W.; Song, Y. , A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem. Biol. Interact. 2019, 310, 108665. [Google Scholar] [CrossRef] [PubMed]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. , The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. , Gallic acid and diabetes mellitus: Its association with oxidative stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E. C.; Huang, C.; Zhang, Y. , Oxidative stress and diabetes: antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Zorena, K.; Jaskulak, M.; Michalska, M.; Mrugacz, M.; Vandenbulcke, F. , Air Pollution, Oxidative Stress, and the Risk of Development of Type 1 Diabetes. Antioxidants 2022, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Lim, C. C.; Thurston, G. D. , Air Pollution, Oxidative Stress, and Diabetes: a Life Course Epidemiologic Perspective. Curr. Diab. Rep. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Bala, A.; Mondal, C.; Haldar, P. K.; Khandelwal, B. , Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: clinical efficacy of dietary antioxidants. Inflammopharmacology 2017, 25, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A. M.; Mani, V.; Vargas-De-la-cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; Stoicescu, M.; Radu, A. F.; Bungau, S. G. , Polyphenols targeting MAPK mediated oxidative stress and inflammation in rheumatoid arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef]
- Ferreira, H. B.; Melo, T.; Paiva, A.; Domingues, M. D. R. , Insights in the role of lipids, oxidative stress and inflammation in rheumatoid arthritis unveiled by new trends in lipidomic investigations. Antioxidants 2021, 10, 45. [Google Scholar] [CrossRef]
- Fonseca, L. J. S. D.; Nunes-Souza, V.; Goulart, M. O. F.; Rabelo, L. A. , Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold regarding Novel Biomarkers and Add-On Therapies. Oxid. Med. Cell. Longev. 2019, 2019, 7536805. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, A.; Bhatnagar, A. , Role of oxidative stress in pathophysiology of rheumatoid arthritis: insights into NRF2-KEAP1 signalling. Autoimmunity 2021, 54, 385–397. [Google Scholar] [CrossRef]
- Kunsch, C.; Sikorski, J. A.; Sundell, C. L. Oxidative stress and the use of antioxidants for the treatment of rheumatoid arthritis. Curr. Med, Chem,: Immunol. Endocr. Metab. Agents 2005, 5, 249–258. [Google Scholar] [CrossRef]
- López-Armada, M. J.; Fernández-Rodríguez, J. A.; Blanco, F. J. , Mitochondrial Dysfunction and Oxidative Stress in Rheumatoid Arthritis. Antioxidants 2022, 11, 1151. [Google Scholar] [CrossRef]
- Phull, A. R.; Nasir, B.; Haq, I. U.; Kim, S. J. , Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Quinonez-Flores, C. M.; Gonzalez-Chavez, S. A.; Del Rio Najera, D.; Pacheco-Tena, C. , Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. Biomed Res. Int. 2016, 2016, 6097417. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; John, P.; Bhatti, A. , Biogenic selenium nanoparticles: Potential solution to oxidative stress mediated inflammation in rheumatoid arthritis and associated complications. Nanomaterials 2021, 11, 2005. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Martínez-Nava, G. A.; Clavijo-Cornejo, D.; Fernández-Torres, J.; Sánchez-Sánchez, R. , Rheumatoid arthritis and oxidative stress, a review of a decade. Cell. Mol. Biol. 2022, 68, 174–184. [Google Scholar] [CrossRef]
- Amiri, M. , Oxidative stress and free radicals in liver and kidney diseases; an updated short-review. J. Nephropathol. 2018, 7, 127–131. [Google Scholar] [CrossRef]
- Andries, A.; Daenen, K.; Jouret, F.; Bammens, B.; Mekahli, D.; Van Schepdael, A. , Oxidative stress in autosomal dominant polycystic kidney disease: player and/or early predictor for disease progression? Pediatr. Nephrol. 2019, 34, 993–1008. [Google Scholar] [CrossRef]
- Aranda-Rivera, A. K.; Cruz-Gregorio, A.; Aparicio-Trejo, O. E.; Pedraza-Chaverri, J. , Mitochondrial redox signaling and oxidative stress in kidney diseases. Biomolecules 2021, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, G.; Leonardi, G.; Andreucci, M.; Bolignano, D. , Oxidative stress and kidney function: A brief update. Curr. Pharm. Des. 2018, 24, 4794–4799. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. , Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. , Oxidative stress in the pathogenesis and evolution of chronic kidney disease: Untangling ariadne’s thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P. G. , Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Lopez-Diaz, A. M.; Guerrero-Mauvecin, J.; Miguel, V.; Ramos, A. M.; Sanchez-Niño, M. D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A. B. , Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease. Antioxidants 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. , Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Ho, H. J.; Shirakawa, H. , Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2023, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K. , Role of oxidative stress in drug-induced kidney injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef]
- Hsu, C. N.; Tain, Y. L. , Developmental origins of kidney disease: Why oxidative stress matters? Antioxidants 2021, 10, 33. [Google Scholar] [CrossRef]
- Jha, J. C.; Banal, C.; Chow, B. S. M.; Cooper, M. E.; Jandeleit-Dahm, K. , Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef]
- Ling, X. C.; Kuo, K. L. , Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Lv, W.; Booz, G. W.; Fan, F.; Wang, Y.; Roman, R. J. , Oxidative stress and renal fibrosis: Recent insights for the development of novel therapeutic strategies. Front. Physiol. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kuragano, T.; Nanami, M.; Nagasawa, Y.; Hasuike, Y. , Misdistribution of iron and oxidative stress in chronic kidney disease. Free Radic. Biol. Med. 2019, 133, 248–253. [Google Scholar] [CrossRef]
- Ogura, Y.; Kitada, M.; Koya, D. , Sirtuins and renal oxidative stress. Antioxidants 2021, 10, 1198. [Google Scholar] [CrossRef]
- Ow, C. P. C.; Trask-Marino, A.; Betrie, A. H.; Evans, R. G.; May, C. N.; Lankadeva, Y. R. , Targeting oxidative stress in septic acute kidney injury: From theory to practice. J. Clin. Med. 2021, 10, 3798. [Google Scholar] [CrossRef] [PubMed]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. , Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms - Biomarkers - Interventions, and Future Perspectives. Oxid. Med. Cell. Longev. 2017, 2017, 6193694. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S. F.; Di Iorio, B. R.; Campiglia, P.; Heidland, A.; Marzocco, S. , Inflammation and oxidative stress in chronic kidney disease—potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef]
- Sakashita, M.; Tanaka, T.; Inagi, R. , Metabolic changes and oxidative stress in diabetic kidney disease. Antioxidants 2021, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Tamay-Cach, F.; Quintana-Pérez, J. C.; Trujillo-Ferrara, J. G.; Cuevas-Hernández, R. I.; Del Valle-Mondragón, L.; García-Trejo, E. M.; Arellano-Mendoza, M. G. , A review of the impact of oxidative stress and some antioxidant therapies on renal damage. Ren. Fail. 2016, 38, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Tejchman, K.; Kotfis, K.; Sieńko, J. , Biomarkers and mechanisms of oxidative stress—last 20 years of research with an emphasis on kidney damage and renal transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. , Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef]
- Tucker, P. S.; Dalbo, V. J.; Han, T.; Kingsley, M. I. , Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers 2013, 18, 103–115. [Google Scholar] [CrossRef]
- Uddin, M. J.; Kim, E. H.; Hannan, M. A.; Ha, H. , Pharmacotherapy against oxidative stress in chronic kidney disease: Promising small molecule natural products targeting nrf2-ho-1 signaling. Antioxidants 2021, 10, 258. [Google Scholar] [CrossRef]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N. K.; Kukreti, R.; Saso, L.; Rana, D. S.; Taneja, V.; Bhargava, V. , Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, R.; Wang, Z. , Contribution of Oxidative Stress to HIF-1-Mediated Profibrotic Changes during the Kidney Damage. Oxid. Med. Cell. Longev. 2021, 2021, 6114132. [Google Scholar] [CrossRef]
- Alsawaf, S.; Alnuaimi, F.; Afzal, S.; Thomas, R. M.; Chelakkot, A. L.; Ramadan, W. S.; Hodeify, R.; Matar, R.; Merheb, M.; Siddiqui, S. S.; Vazhappilly, C. G. , Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation. Biology 2022, 11, 1717. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Carro, B.; Cannata-Andía, J. B.; Mora-Fernández, C.; Navarro-González, J. F. , Klotho, Oxidative Stress, and Mitochondrial Damage in Kidney Disease. Antioxidants 2023, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Hojs, N. V.; Bevc, S.; Ekart, R.; Hojs, R. , Oxidative stress markers in chronic kidney disease with emphasis on diabetic nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y. L.; Hsu, C. N. , Perinatal Oxidative Stress and Kidney Health: Bridging the Gap between Animal Models and Clinical Reality. Antioxidants 2023, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M. A.; Lopes-Pacheco, M.; Rocco, P. R. M. , Oxidative Stress-Derived Mitochondrial Dysfunction in Chronic Obstructive Pulmonary Disease: A Concise Review. Oxid. Med. Cell. Longev. 2021, 2021, 6644002. [Google Scholar] [CrossRef]
- Bargagli, E.; Olivieri, C.; Bennett, D.; Prasse, A.; Muller-Quernheim, J.; Rottoli, P. , Oxidative stress in the pathogenesis of diffuse lung diseases: A review. Respir. Med. 2009, 103, 1245–1256. [Google Scholar] [CrossRef]
- Barnes, P. J. , Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Bast, A.; Weseler, A. R.; Haenen, G. R. M. M.; Den Hartog, G. J. M. , Oxidative stress and antioxidants in interstitial lung disease. Curr. Opin. Pulm. Med. 2010, 16, 516–520. [Google Scholar] [CrossRef]
- Cheresh, P.; Kim, S. J.; Tulasiram, S.; Kamp, D. W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta - Mol. Basis Dis. 2013, 1832, 1028–1040. [Google Scholar] [CrossRef]
- Dailah, H. G. , Therapeutic Potential of Small Molecules Targeting Oxidative Stress in the Treatment of Chronic Obstructive Pulmonary Disease (COPD): A Comprehensive Review. Molecules 2022, 27, 5542. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Maniscalco, M.; Balbi, B.; Ricciardolo, F. L. M. , Oxidative and nitrosative stress in the pathogenesis of obstructive lung diseases of increasing severity. Curr. Med. Chem. 2020, 27, 7149–7158. [Google Scholar] [CrossRef] [PubMed]
- Estornut, C.; Milara, J.; Bayarri, M. A.; Belhadj, N.; Cortijo, J. , Targeting Oxidative Stress as a Therapeutic Approach for Idiopathic Pulmonary Fibrosis. Front. Pharmacol. 2022, 12, 794997. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Carota, G.; Li Volti, G.; Giuffrè, M. , Biomarkers of Oxidative Stress for Neonatal Lung Disease. Front Pediatr. 2021, 9, 618867. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R. S.; Andrade, C. F. , Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef] [PubMed]
- Hecker, L. , Mechanisms and consequences of oxidative stress in lung disease: Therapeutic implications for an aging populace. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L642–L653. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Javad-Moosavi, S. A.; Reiter, R. J.; Yarahmadi, R.; Ghaznavi, H.; Mehrzadi, S. , Oxidative/nitrosative stress, autophagy and apoptosis as therapeutic targets of melatonin in idiopathic pulmonary fibrosis. Expert Opin. Ther. Targets 2018, 22, 1049–1061. [Google Scholar] [CrossRef]
- Kliment, C. R.; Oury, T. D. , Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic. Biol. Med. 2010, 49, 707–717. [Google Scholar] [CrossRef]
- Malaviya, R.; Laskin, J. D.; Laskin, D. L. , Oxidative stress-induced autophagy: Role in pulmonary toxicity. Toxicol. Appl. Pharmacol. 2014, 275, 145–151. [Google Scholar] [CrossRef]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A. E.; Zemskov, E. A.; Maltepe, E.; Fineman, J. R.; Wang, T.; Black, S. M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef]
- Ortiz-Quintero, B.; Martínez-Espinosa, I.; Pérez-Padilla, R. , Mechanisms of Lung Damage and Development of COPD Due to Household Biomass-Smoke Exposure: Inflammation, Oxidative Stress, MicroRNAs, and Gene Polymorphisms. Cells 2023, 12, 67. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Fois, A. G.; Collu, C.; Bandinu, A.; Zinellu, E.; Carru, C.; Pirina, P.; Mangoni, A. A.; Zinellu, A. , Oxidative stress-linked biomarkers in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Biomark. Med. 2018, 12, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- van der Vliet, A.; Janssen-Heininger, Y. M. W.; Anathy, V. , Oxidative stress in chronic lung disease: From mitochondrial dysfunction to dysregulated redox signaling. Mol. Aspects Med. 2018, 63, 59–69. [Google Scholar] [CrossRef]
- Zemskov, E. A.; Lu, Q.; Ornatowski, W.; Klinger, C. N.; Desai, A. A.; Maltepe, E.; Yuan, J. X. J.; Wang, T.; Fineman, J. R.; Black, S. M. , Biomechanical Forces and Oxidative Stress: Implications for Pulmonary Vascular Disease. Antioxid. Redox Signal. 2019, 31, 819–842. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Q.; Zheng, R. , The interplay between oxidative stress and autophagy in chronic obstructive pulmonary disease. Front. Physiol. 2022, 13, 1004275. [Google Scholar] [CrossRef]
- Zinellu, E.; Zinellu, A.; Fois, A. G.; Pau, M. C.; Scano, V.; Piras, B.; Carru, C.; Pirina, P. , Oxidative stress biomarkers in chronic obstructive pulmonary disease exacerbations: A systematic review. Antioxidants 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Digilio, F. A.; Galderisi, U.; Peluso, G. , The Emerging Role of Macrophages in Chronic Obstructive Pulmonary Disease: The Potential Impact of Oxidative Stress and Extracellular Vesicle on Macrophage Polarization and Function. Antioxidants 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z. , The pathophysiological role of mitochondrial oxidative stress in lung diseases. J. Transl. Med. 2017, 15, 207. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y. H.; Gou, X.; Li, F. Y.; Yang, X. Y. C.; Li, Y. M.; Chen, F. , Oxidative Stress and Antioxidative Therapy in Pulmonary Arterial Hypertension. Molecules 2022, 27, 3724. [Google Scholar] [CrossRef]
- Cejka, C.; Kubinova, S.; Cejkova, J. , The preventive and therapeutic effects of molecular hydrogen in ocular diseases and injuries where oxidative stress is involved. Free Radic. Res. 2019, 53, 237–247. [Google Scholar] [CrossRef]
- Dammak, A.; Huete-Toral, F.; Carpena-Torres, C.; Martin, A.; Pastrana, C.; Carracedo, G. , From oxidative stress to inflammation in the posterior ocular diseases: Diagnosis and treatment. Pharmaceutics 2021, 13, 1376. [Google Scholar] [CrossRef]
- Ivanov, I. V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. , Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef]
- Lemos, C. N.; Silva, L. E. C. M. D.; Faustino, J. F.; Fantucci, M. Z.; Murashima, A. D. A. B.; Adriano, L.; Alves, M.; Rocha, E. M. , Oxidative Stress in the Protection and Injury of the Lacrimal Gland and the Ocular Surface: are There Perspectives for Therapeutics? Front. Cell Dev. Biol. 2022, 10, 824726. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. , The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Perez-Garmendia, R.; Lopez De Eguileta Rodriguez, A.; Ramos-Martinez, I.; Zuñiga, N. M.; Gonzalez-Salinas, R.; Quiroz-Mercado, H.; Zenteno, E.; Hernández, E. R.; Hernández-Zimbrón, L. F. , Interplay between Oxidative Stress, Inflammation, and Amyloidosis in the Anterior Segment of the Eye; Its Pathological Implications. Oxid. Med. Cell. Longev. 2020, 2020, 6286105. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S. C.; Izzotti, A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog. Brain Res. 2008, 173, 385–407. [Google Scholar] [PubMed]
- Seen, S.; Tong, L. , Dry eye disease and oxidative stress. Acta Ophthalmol. (Copenh.) 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M. D.; Iyer, M.; Nair, A. P.; Venkatesan, D.; Mathavan, S.; Eruppakotte, N.; Kizhakkillach, S.; Chandran, M. K.; Roy, A.; Gopalakrishnan, A. V.; Vellingiri, B. , Oxidative stress and mitochondrial transfer: A new dimension towards ocular diseases. Genes Dis. 2022, 9, 610–637. [Google Scholar] [CrossRef]
- Tangvarasittichai, O.; Tangvarasittichai, S. , Oxidative stress, ocular disease and diabetes retinopathy. Curr. Pharm. Des. 2018, 24, 4726–4741. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M. , Oxidative stress and eye diseases. Jpn. J. Clin. Ophthalmol. 2011, 65, 1383–1393. [Google Scholar]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J. L.; Liew, G.; White, A. J. R. , Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef] [PubMed]
- Dammak, A.; Pastrana, C.; Martin-Gil, A.; Carpena-Torres, C.; Peral Cerda, A.; Simovart, M.; Alarma, P.; Huete-Toral, F.; Carracedo, G. , Oxidative Stress in the Anterior Ocular Diseases: Diagnostic and Treatment. Biomedicines 2023, 11, 292. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, H.; Chu, L.; Zhao, Y. , m6A Modification—Association with Oxidative Stress and Implications on Eye Diseases. Antioxidants 2023, 12, 510. [Google Scholar] [CrossRef] [PubMed]
- Shu, D. Y.; Chaudhary, S.; Cho, K. S.; Lennikov, A.; Miller, W. P.; Thorn, D. C.; Yang, M.; McKay, T. B. , Role of Oxidative Stress in Ocular Diseases: A Balancing Act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. , Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Bhatia, S.; Drake, D. M.; Miller, L.; Wells, P. G. , Oxidative stress and DNA damage in the mechanism of fetal alcohol spectrum disorders. Birth Defects Res. 2019, 111, 714–748. [Google Scholar] [CrossRef]
- Chiarello, D. I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C. M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta - Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Freire, V. A. F.; Melo, A. D. D.; Santos, H. D. L.; Barros-Pinheiro, M. , Evaluation of oxidative stress markers in subtypes of preeclampsia: A systematic review and meta-analysis. Placenta 2023, 132, 55–67. [Google Scholar] [CrossRef]
- Giussani, D. A.; Niu, Y.; Herrera, E. A.; Richter, H. G.; Camm, E. J.; Thakor, A. S.; Kane, A. D.; Hansell, J. A.; Brain, K. L.; Skeffington, K. L.; Itani, N.; Wooding, F. B. P.; Cross, C. M.; Allison, B. J. Heart disease link to fetal hypoxia and oxidative stress. Adv. Exp. Med. Biol. 2014, 814, 77–87. [Google Scholar]
- Godhamgaonkar, A. A.; Sundrani, D. P.; Joshi, S. R. , Role of maternal nutrition and oxidative stress in placental telomere attrition in women with preeclampsia. Hypertens. Pregnancy 2021, 40, 63–74. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. , Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A.; Sharma, R. K. , The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet. Gynecol. Surv. 2005, 60, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Guvendag Guven, E. S.; Karcaaltincaba, D.; Kandemir, O.; Kiykac, S.; Mentese, A. Cord blood oxidative stress markers correlate with umbilical artery pulsatility in fetal growth restriction. J. Matern.-Fetal Neonatal Med. 2013, 26, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Hung, T. H.; Burton, G. J. , Hypoxia and reoxygenation: A possible mechanism for placental oxidative stress in preeclampsia. Taiwan J. Obstet. Gynecol. 2006, 45, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Joo, E. H.; Kim, Y. R.; Kim, N.; Jung, J. E.; Han, S. H.; Cho, H. Y. , Effect of endogenic and exogenic oxidative stress triggers on adverse pregnancy outcomes: Preeclampsia, fetal growth restriction, gestational diabetes mellitus and preterm birth. Int. J. Mol. Sci. 2021, 22, 10122. [Google Scholar] [CrossRef]
- Marín, R.; Chiarello, D. I.; Abad, C.; Rojas, D.; Toledo, F.; Sobrevia, L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim. Biophys. Acta - Mol. Basis Dis. 2020, 1866, 165961. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Swiader, A.; Salvayre, R.; Guerby, P. , Oxidative stress, lipid peroxidation and premature placental senescence in preeclampsia. Arch. Biochem. Biophys. 2022, 730, 109416. [Google Scholar] [CrossRef]
- Perrone, S.; Tataranno, M. L.; Santacroce, A.; Bracciali, C.; Riccitelli, M.; Alagna, M. G.; Longini, M.; Belvisi, E.; Bazzini, F.; Buonocore, G. , Fetal Programming, Maternal Nutrition, and Oxidative Stress Hypothesis. J. Pediatr. Biochem. 2016, 6, 96–102. [Google Scholar]
- Perrone, S.; Tataranno, M. L.; Stazzoni, G.; Buonocore, G. Biomarkers of oxidative stress in fetal and neonatal diseases. J. Matern.-Fetal Neonatal Med. 2012, 25, 2575–2578. [Google Scholar] [CrossRef]
- Phoswa, W. N.; Khaliq, O. P. , The Role of Oxidative Stress in Hypertensive Disorders of Pregnancy (Preeclampsia, Gestational Hypertension) and Metabolic Disorder of Pregnancy (Gestational Diabetes Mellitus). Oxid. Med. Cell. Longev. 2021, 2021, 5581570. [Google Scholar] [CrossRef]
- San Juan-Reyes, S.; Gómez-Oliván, L. M.; Islas-Flores, H.; Dublán-García, O. , Oxidative stress in pregnancy complicated by preeclampsia. Arch. Biochem. Biophys. 2020, 681, 108255. [Google Scholar] [CrossRef]
- Siddiqui, I. A.; Jaleel, A.; Tamimi, W.; Al Kadri, H. M. F. , Role of oxidative stress in the pathogenesis of preeclampsia. Arch. Gynecol. Obstet. 2010, 282, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Taravati, A.; Tohidi, F. , Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J. Obstet. Gynecol. 2018, 57, 779–790. [Google Scholar] [CrossRef]
- Taysi, S.; Tascan, A. S.; Ugur, M. G.; Demir, M. Radicals, oxidative/nitrosative stress and preeclampsia. Mini-Rev. Med. Chem. 2019, 19, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M. B.; Ferreira, R. C.; Moura, F. A.; Bueno, N. B.; De Oliveira, A. C. M.; Goulart, M. O. F. , Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia. Oxid. Med. Cell. Longev. 2019, 2019, 8238727. [Google Scholar] [CrossRef] [PubMed]
- Teramo, K.; Piñeiro-Ramos, J. D. , Fetal chronic hypoxia and oxidative stress in diabetic pregnancy. Could fetal erythropoietin improve offspring outcomes? Free Radic. Biol. Med. 2019, 142, 32–37. [Google Scholar] [CrossRef]
- Thompson, L. P.; Al-Hasan, Y. , Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Tsai, S. Y. A.; Bendriem, R. M.; Lee, C. T. D. , The cellular basis of fetal endoplasmic reticulum stress and oxidative stress in drug-induced neurodevelopmental deficits. Neurobiol. Stress 2019, 10, 100145. [Google Scholar] [CrossRef]
- Jones, M. L.; Mark, P. J.; Mori, T. A.; Keelan, J. A.; Waddell, B. J. , Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol. Reprod. 2013, 88, 37. [Google Scholar] [CrossRef]
- Karowicz-Bilinska, A.; Kȩdziora-Kornatowska, K.; Bartosz, G. , Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic. Res. 2007, 41, 870–873. [Google Scholar] [CrossRef]
- Mundal, S. B.; Rakner, J. J.; Silva, G. B.; Gierman, L. M.; Austdal, M.; Basnet, P.; Elschot, M.; Bakke, S. S.; Ostrop, J.; Thomsen, L. C. V.; Moses, E. K.; Acharya, G.; Bjørge, L.; Iversen, A. C. , Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction. Int. J. Mol. Sci. 2022, 23, 1966. [Google Scholar] [CrossRef] [PubMed]
- Parraguez, V. H.; Sales, F.; Peralta, O.; Reyes, M. D.; Gonzalez-Bulnes, A. , Oxidative Stress and Fetal Growth Restriction Set Up Earlier in Undernourished Sheep Twin Pregnancies: Prevention with Antioxidant and Nutritional Supplementation. Antioxidants 2022, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Sales, F.; Peralta, O. A.; Narbona, E.; McCoard, S.; De los Reyes, M.; González-Bulnes, A.; Parraguez, V. H. , Hypoxia and oxidative stress are associated with reduced fetal growth in twin and undernourished sheep pregnancies. Animals 2018, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Schoots, M. H.; Bourgonje, M. F.; Bourgonje, A. R.; Prins, J. R.; van Hoorn, E. G. M.; Abdulle, A. E.; Muller Kobold, A. C.; van der Heide, M.; Hillebrands, J. L.; van Goor, H.; Gordijn, S. J. , Oxidative stress biomarkers in fetal growth restriction with and without preeclampsia. Placenta 2021, 115, 87–96. [Google Scholar] [CrossRef]
- Takagi, Y.; Nikaido, T.; Toki, T.; Kita, N.; Kanai, M.; Ashida, T.; Ohira, S.; Konishi, I. , Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004, 444, 49–55. [Google Scholar] [CrossRef]
- Barron, A.; McCarthy, C. M.; O’Keeffe, G. W. , Preeclampsia and Neurodevelopmental Outcomes: Potential Pathogenic Roles for Inflammation and Oxidative Stress? Mol. Neurobiol. 2021, 58, 2734–2756. [Google Scholar] [CrossRef]
- Han, C.; Huang, P.; Lyu, M.; Dong, J. , Oxidative stress and preeclampsia-associated prothrombotic state. Antioxidants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Haram, K.; Mortensen, J. H.; Myking, O.; Magann, E. F.; Morrison, J. C. , The role of oxidative stress, adhesion molecules and antioxidants in preeclampsia. Curr. Hypertens. Rev. 2019, 15, 105–112. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D. D. , Antioxidant property of coffee components: Assessment of methods that define mechanism of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Wang, J. Y.; Nemzer, B. , Antioxidant and antiradical activity of coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef]
- Aguiar, J.; Estevinho, B. N.; Santos, L. , Microencapsulation of natural antioxidants for food application – The specific case of coffee antioxidants – A review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Bothiraj, K. V.; Murugan, *!!! REPLACE !!!*; Vanitha, V. Green coffee bean seed and their role in antioxidant–a review. Int. J. Res. Pharm. Sci. 2020, 11, 233–240. [Google Scholar] [CrossRef]
- Hasballah, K.; Lestari, W.; Listiawan, M. Y.; Sofia, S. , Coffee by-products as the source of antioxidants: A systematic review. F1000Research 2022, 11, 220. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Ramírez, B.; Escobar, F. V.; del Castillo, M. D. , Antioxidant properties of high molecular weight compounds from coffee roasting and brewing byproducts. Bioact. Comp. Health Dis. 2019, 2, 48–63. [Google Scholar] [CrossRef]
- Agunbiade, H. O.; Fagbemi, T. N.; Aderinola, T. A. , Antioxidant properties of beverages from graded mixture of green/roasted coffee and hibiscus sabdariffa calyx flours. Appl. Food Res. 2022, 2, 100163. [Google Scholar] [CrossRef]
- Ahmed Ali, A. M.; Yagi, S.; Qahtan, A. A.; Alatar, A. A.; Angeloni, S.; Maggi, F.; Caprioli, G.; Abdel-Salam, E. M.; Sinan, K. I.; Zengin, G. , Evaluation of the chemical constituents, antioxidant and enzyme inhibitory activities of six Yemeni green coffee beans varieties. Food Biosci. 2022, 46, 101552. [Google Scholar] [CrossRef]
- AlAmri, O. D.; Albeltagy, R. S.; M. A. Akabawy, A.; Mahgoub, S.; Abdel-Mohsen, D. M.; Abdel Moneim, A. E.; Amin, H. K. Investigation of antioxidant and anti-inflammatory activities as well as the renal protective potential of green coffee extract in high fat-diet/streptozotocin-induced diabetes in male albino rats. J. Funct. Foods 2020, 71, 103996. [Google Scholar] [CrossRef]
- Alnsour, L.; Issa, R.; Awwad, S.; Albals, D.; Al-Momani, I. , Quantification of Total Phenols and Antioxidants in Coffee Samples of Different Origins and Evaluation of the Effect of Degree of Roasting on Their Levels. Molecules 2022, 27, 1591. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Perestrelo, R.; Câmara, J. S. , Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Angeloni, S.; Freschi, M.; Marrazzo, P.; Hrelia, S.; Beghelli, D.; Juan-García, A.; Juan, C.; Caprioli, G.; Sagratini, G.; Angeloni, C. , Antioxidant and Anti-Inflammatory Profiles of Spent Coffee Ground Extracts for the Treatment of Neurodegeneration. Oxid. Med. Cell. Longev. 2021, 2021, 6620913. [Google Scholar] [CrossRef]
- Anh-Dao, L. T.; Nhon-Duc, L.; Cong-Hau, N.; Thanh-Nho, N. , Variability of total polyphenol contents in ground coffee products and their antioxidant capacities through different reaction mechanisms. Biointerface Res. Appl. Chem. 2022, 12, 4857–4870. [Google Scholar]
- Aroufai, İ. A.; Sabuncu, M.; Dülger Altiner, D.; Sahan, Y. , Antioxidant properties and bioaccessibility of coffee beans and their coffee silverskin grown in different countries. J. Food Meas. Charact. 2022, 16, 1873–1888. [Google Scholar] [CrossRef]
- Bae, H. M.; Haile, M.; Kang, W. H. , Evaluation of antioxidant, organic acid, and volatile compounds in coffee pulp wine fermented with native yeasts isolated from coffee cherries. Food Sci. Technol. Int. 2022, 28, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.; Bulbarelli, A.; Lonati, E.; Cazzaniga, E.; Tassotti, M.; Mena, P.; Del Rio, D.; Palestini, P. , Study of the antioxidant effects of coffee phenolic metabolites on c6 glioma cells exposed to diesel exhaust particles. Antioxidants 2021, 10, 1169. [Google Scholar] [CrossRef]
- Castaldo, L.; Toriello, M.; Sessa, R.; Izzo, L.; Lombardi, S.; Narváez, A.; Ritieni, A.; Grosso, M. , Antioxidant and anti-inflammatory activity of coffee brew evaluated after simulated gastrointestinal digestion. Nutrients 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Oliva, A.; Uscanga-Sosa, D. P.; González-Rios, O.; Morales-Ramos, V. The use of coffee (Coffea arabica L.) pulp in the preparation of a beverage with antioxidant properties. Int. Food Res. J. 2022, 29, 274–282. [Google Scholar] [CrossRef]
- de Abreu Pinheiro, F.; Ferreira Elias, L.; de Jesus Filho, M.; Uliana Modolo, M.; Gomes Rocha, J. D. C.; Fumiere Lemos, M.; Scherer, R.; Soares Cardoso, W. , Arabica and Conilon coffee flowers: Bioactive compounds and antioxidant capacity under different processes. Food. Chem. 2021, 336, 127701. [Google Scholar] [CrossRef]
- Fu, X. P.; Shen, X. J.; Yin, X.; Zhang, Y. H.; Wang, X. F.; Han, Z. H.; Lin, Q.; Fan, J. P. , Antioxidant and pro-apoptosis activities of coffee husk (Coffea arabica) anthocyanins. Int. Food Res. J. 2021, 28, 1187–1195. [Google Scholar] [CrossRef]
- Garcia-Solis, S. E.; Perez-Perez, V.; Tapia-Maruri, D.; Villalobos-Castillejos, F.; Arenas-Ocampo, M. L.; Camacho-Diaz, B. H.; Alamilla-Beltran, L. , Microencapsulation of the green coffee waste extract with high antioxidant activity by spray-drying. J. Food Process. Preserv. 2022, 46, e16864. [Google Scholar] [CrossRef]
- Górecki, M.; Hallmann, E. , The antioxidant content of coffee and its in vitro activity as an effect of its production method and roasting and brewing time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef]
- Lemos, M. F.; de Andrade Salustriano, N.; de Souza Costa, M. M.; Lirio, K.; da Fonseca, A. F. A.; Pacheco, H. P.; Endringer, D. C.; Fronza, M.; Scherer, R. , Chlorogenic acid and caffeine contents and anti-inflammatory and antioxidant activities of green beans of conilon and arabica coffees harvested with different degrees of maturation. J. Saudi Chem. Soc. 2022, 26, 101467. [Google Scholar] [CrossRef]
- Lonati, E.; Carrozzini, T.; Bruni, I.; Mena, P.; Botto, L.; Cazzaniga, E.; Del Rio, D.; Labra, M.; Palestini, P.; Bulbarelli, A. , Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages. Molecules 2022, 27, 1049. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Latos-Brozio, M.; Kałuzna-Czaplińska, J.; Rosiak, A.; Chrzescijanska, E. , Antioxidant properties of green coffee extract. Forests 2020, 11, 557. [Google Scholar] [CrossRef]
- Montenegro, J.; dos Santos, L. S.; de Souza, R. G. G.; Lima, L. G. B.; Mattos, D. S.; Viana, B. P. P. B.; da Fonseca Bastos, A. C. S.; Muzzi, L.; Conte-Júnior, C. A.; Gimba, E. R. P.; Freitas-Silva, O.; Teodoro, A. J. , Bioactive compounds, antioxidant activity and antiproliferative effects in prostate cancer cells of green and roasted coffee extracts obtained by microwave-assisted extraction (MAE). Food Res. Int. 2021, 140, 110014. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Souza, M. A.; Paiva, P. G. D.; Silva, A. D.; Duarte, M. S. L.; Ribeiro, A. Q. , Coffee and Tea Group Contribute the Most to the Dietary Total Antioxidant Capacity of Older Adults: A Population Study in a Medium-Sized Brazilian City. J. Am. Coll. Nutr. 2021, 40, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Kalita, D.; Abshiru, N. , Quantification of major bioactive constituents, antioxidant activity, and enzyme inhibitory effects of whole coffee cherries (Coffea arabica) and their extracts. Molecules 2021, 26, 4306. [Google Scholar] [CrossRef]
- Nosal, B. M.; Sakaki, J. R.; Kim, D. O.; Chun, O. K. , Impact of coffee preparation on total phenolic content in brewed coffee extracts and their contribution to the body’s antioxidant status. Food Sci. Biotechnol. 2022, 31, 1081–1088. [Google Scholar] [CrossRef]
- Nzekoue, F. K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L. A.; Sagratini, G.; Vittori, S.; Caprioli, G. , Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-jakubik, A.; Socha, K.; Zujko, M. E. , Coffee infusions: Can they be a source of microelements with antioxidant properties? Antioxidants 2021, 10, 1709. [Google Scholar] [CrossRef]
- Pergolizzi, S.; D’Angelo, V.; Aragona, M.; Dugo, P.; Cacciola, F.; Capillo, G.; Dugo, G.; Lauriano, E. R. , Evaluation of antioxidant and anti-inflammatory activity of green coffee beans methanolic extract in rat skin. Nat. Prod. Res. 2020, 34, 1535–1541. [Google Scholar] [CrossRef]
- Šeremet, D.; Fabečić, P.; Cebin, A. V.; Jarić, A. M.; Pudić, R.; Komes, D. , Antioxidant and Sensory Assessment of Innovative Coffee Blends of Reduced Caffeine Content. Molecules 2022, 27, 448. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Nie, F.; Fang, H.; Liu, K.; Li, Z.; Li, X.; Chen, Y.; Chen, R.; Zheng, T.; Fan, J. , Comparison of chemical compositions, antioxidant activities, and acetylcholinesterase inhibitory activities between coffee flowers and leaves as potential novel foods. Food Sci. Nutr. 2022, 11, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Al-Shalabi, E.; Al-Bakri, A. G.; Zalloum, H.; Abu-Irmaileh, B.; Ibrahim, L. H.; Zeno, H. , Coffee Bean Polyphenols Can Form Biocompatible Template-free Antioxidant Nanoparticles with Various Sizes and Distinct Colors. ACS Omega 2021, 6, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Tomac, I.; Šeruga, M.; Labuda, J. , Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chem. 2020, 325, 126787. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; He, X.; Wu, L.; Yan, D.; Yan, S. , Chlorogenic Acid, the Main Antioxidant in Coffee, Reduces Radiation-Induced Apoptosis and DNA Damage via NF-E2-Related Factor 2 (Nrf2) Activation in Hepatocellular Carcinoma. Oxid. Med. Cell. Longev. 2022, 2022, 4566949. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Giannetti, I.; Dattilo, C.; Scaccini, C. , Coffee drinking influences plasma antioxidant capacity in humans. J. Agric. Food Chem. 2002, 50, 6211–6216. [Google Scholar] [CrossRef]
- Hoelzl, C.; Knasmüller, S.; Wagner, K.; Elbling, L.; Huber, W.; Kager, N.; Ferk, F.; Ehrlich, V.; Nersesyan, A.; Neubauer, O.; Desmarchelier, A.; Marin-Kuan, M.; Delatour, T.; Verguet, C.; Bezençon, C.; Besson, A.; Grathwohl, D.; Simic, T.; Kundi, M.; Schilter, B.; Cavin, C. , Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Molecular Nutrition and Food Res. 2010, 54, 1722–1733. [Google Scholar] [CrossRef]
- Hori, A.; Kasai, H.; Kawai, K.; Nanri, A.; Sato, M.; Ohta, M.; Mizoue, T. , Coffee intake is associated with lower levels of oxidative DNA damage and decreasing body iron storage in healthy women. Nutr. Cancer 2014, 66, 964–969. [Google Scholar] [CrossRef]
- Ahmad, S. S.; Khalid, M.; Kamal, M. A.; Younis, K. , Study of Nutraceuticals and Phytochemicals for the Management of Alzheimer's Disease: A Review. Curr. Neuropharmacol. 2021, 19, 1884–1895. [Google Scholar] [CrossRef]
- Bachheti, R. K.; Worku, L. A.; Gonfa, Y. H.; Zebeaman, M.; Deepti, *!!! REPLACE !!!*; Pandey, D. P.; Bachheti, A. Prevention and Treatment of Cardiovascular Diseases with Plant Phytochemicals: A Review. Evid. Based Complement. Alternat. Med. 2022, 2022, 5741198. [Google Scholar] [CrossRef]
- Bai, X.; Bian, Z.; Zhang, M. , Targeting the Nrf2 signaling pathway using phytochemical ingredients: A novel therapeutic road map to combat neurodegenerative diseases. Phytomedicine 2023, 109, 154582. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Aboulaghras, S.; El Menyiy, N.; El Omari, N.; Assaggaf, H.; Lee, L. H.; Montesano, D.; Gallo, M.; Zengin, G.; AlDhaheri, Y.; Bouyahya, A. , Phytochemical Compounds and Nanoparticles as Phytochemical Delivery Systems for Alzheimer’s Disease Management. Molecules 2022, 27, 9043. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Azam, S.; Cho, D. Y.; Su-Kim, I.; Choi, D. K. , Natural Phytochemicals as Novel Therapeutic Strategies to Prevent and Treat Parkinson's Disease: Current Knowledge and Future Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 6680935. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rana, T.; Sehgal, A.; Makeen, H. A.; Albratty, M.; Alhazmi, H. A.; Meraya, A. M.; Bhatia, S.; Sachdeva, M. Phytochemicals targeting nitric oxide signaling in neurodegenerative diseases. Nitric Oxide - Biol. Chem. 2023, 130, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Vesa, C. M.; Abid, A.; Behl, T.; Tit, D. M.; Purza, A. L.; Pasca, B.; Todan, L. M.; Endres, L. , Withaferin a—a promising phytochemical compound with multiple results in dermatological diseases. Molecules 2021, 26, 2407. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, Q.; Sun, P. , Natural phytochemicals that affect autophagy in the treatment of oral diseases and infections: A review. Front. Pharmacol. 2022, 13, 970596. [Google Scholar] [CrossRef]
- Dsouza, V. L.; Shivakumar, A. B.; Kulal, N.; Gangadharan, G.; Kumar, D.; Kabekkodu, S. P. , Phytochemical based Modulation of Endoplasmic Reticulum Stress in Alzheimer's Disease. Curr. Top. Med. Chem. 2022, 22, 1880–1896. [Google Scholar] [CrossRef]
- Eilam, Y.; Pintel, N.; Khattib, H.; Shagug, N.; Taha, R.; Avni, D. , Regulation of Cholesterol Metabolism by Phytochemicals Derived from Algae and Edible Mushrooms in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 13667. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D'Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; Tabolacci, C.; Jadeja, R. N. , Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef]
- Ganesan, K.; Quiles, J. L.; Daglia, M.; Xiao, J.; Xu, B. , Dietary phytochemicals modulate intestinal epithelial barrier dysfunction and autoimmune diseases. Food Front. 2021, 2, 357–382. [Google Scholar] [CrossRef]
- He, W. J.; Lv, C. H.; Chen, Z.; Shi, M.; Zeng, C. X.; Hou, D. X.; Qin, S. , The Regulatory Effect of Phytochemicals on Chronic Diseases by Targeting Nrf2-ARE Signaling Pathway. Antioxidants 2023, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Yanping, C.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z.; Fang, Y.; Junsong, X. , Phytochemicals and inflammatory bowel disease: a review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1321–1345. [Google Scholar] [CrossRef] [PubMed]
- Islam, S. U.; Ahmed, M. B.; Ahsan, H.; Lee, Y. S. , Recent molecular mechanisms and beneficial effects of phytochemicals and plant-based whole foods in reducing ldl-c and preventing cardiovascular disease. Antioxidants 2021, 10, 784. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H. J. , Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases. Life 2023, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L. F.; de Maio, M. C.; Minniti, G.; de Góes Corrêa, N.; Barbalho, S. M.; Quesada, K.; Guiguer, E. L.; Sloan, K. P.; Detregiachi, C. R. P.; Araújo, A. C.; de Alvares Goulart, R. , Effects of Medicinal Plants and Phytochemicals in Nrf2 Pathways during Inflammatory Bowel Diseases and Related Colorectal Cancer: A Comprehensive Review. Metabolites 2023, 13, 243. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Li, A.; Liu, R.; Yang, H.; Xia, X. , The Phytochemical Potential for Brain Disease Therapy and the Possible Nanodelivery Solutions for Brain Access. Front. Oncol. 2022, 12, 936054. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. , Disease-modifying treatment of Parkinson’s disease by phytochemicals: targeting multiple pathogenic factors. J. Neural Transm. 2022, 129, 737–753. [Google Scholar] [CrossRef]
- Nistor, M.; Pop, R.; Daescu, A.; Pintea, A.; Socaciu, C.; Rugina, D. , Anthocyanins as Key Phytochemicals Acting for the Prevention of Metabolic Diseases: An Overview. Molecules 2022, 27, 4254. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. A.; Rahman, M. H.; Biswas, P.; Hossain, M. S.; Islam, R.; Hannan, M. A.; Uddin, M. J.; Rhim, H. , Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer’s disease. Antioxidants 2021, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Naura, A. S. , Potential of phytochemicals as immune-regulatory compounds in atopic diseases: A review. Biochem. Pharmacol. 2020, 173, 113790. [Google Scholar] [CrossRef]
- Shirsath, N. R.; Goswami, A. K. , Natural phytochemicals and their therapeutic role in management of several diseases: A review. Curr. Tradit. Med. 2020, 6, 43–53. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, S. E.; Madiha, S.; Gaire, B. P.; Jin, M.; Yumnam, S.; Kim, S. Y. , Phytochemicals against TNFα-mediated neuroinflammatory diseases. Int. J. Mol. Sci. 2020, 21, 764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, S.; Li, Q.; Lang, W.; Li, W.; Jiang, X.; Wan, Z.; Chen, J.; Wang, H. , Epigallocatechin-3-gallate: A phytochemical as a promising drug candidate for the treatment of Parkinson’s disease. Front. Pharmacol. 2022, 13, 977521. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lu, B. , The effects of phytochemicals on circadian rhythm and related diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 882–892. [Google Scholar] [CrossRef]
- Yang, S. H.; Tao, G.; Yang, L.; Wu, X.; Liu, J. W.; Dagher, F.; Ou, S. Y.; Song, Y.; Huang, J. Q. , Dietary phytochemical and metabolic disease prevention: Focus on plant proteins. Front. Nutr. 2023, 10, 1089487. [Google Scholar] [CrossRef]
- Zahedipour, F.; Hosseini, S.; Henney, N.; Barreto, G.; Sahebkar, A. , Phytochemicals as inhibitors of tumor necrosis factor alpha and neuroinflammatory responses in neurodegenerative diseases. Neural Regen. Res. 2022, 17, 1675–1684. [Google Scholar]
- Bahadori, M. B.; Kirkan, B.; Sarikurkcu, C. Phenolic ingredients and therapeutic potential of Stachys cretica subsp. smyrnaea for the management of oxidative stress, Alzheimer's disease, hyperglycemia, and melasma. Ind. Crops Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Barakat, A. Z.; Bassuiny, R. I.; Abdel-Aty, A. M.; Mohamed, S. A. , Diabetic complications and oxidative stress: The role of phenolic-rich extracts of saw palmetto and date palm seeds. J. Food Biochem. 2020, 44, e13416. [Google Scholar] [CrossRef]
- Callcott, E. T.; Blanchard, C. L.; Oli, P.; Santhakumar, A. B. , Pigmented Rice-Derived Phenolic Compounds Reduce Biomarkers of Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, 1800840. [Google Scholar] [CrossRef]
- Carballeda Sangiao, N.; Chamorro, S.; de Pascual-Teresa, S.; Goya, L. , Aqueous extract of cocoa phenolic compounds protects differentiated neuroblastoma sh-sy5y cells from oxidative stress. Biomolecules 2021, 11, 1266. [Google Scholar] [CrossRef]
- Castaneda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J. R.; Galano, A. , Comprehensive Investigation of the Antioxidant and Pro-oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Carpi, S.; Sarcognato, S.; Zanotto, I.; Sayaf, K.; Colognesi, M.; Polini, B.; Digiacomo, M.; Macchia, M.; Nieri, P.; Carrara, M.; Cazzagon, N.; Russo, F. P.; Guido, M.; De Martin, S. , The phenolic compounds tyrosol and hydroxytyrosol counteract liver fibrogenesis via the transcriptional modulation of NADPH oxidases and oxidative stress-related miRNAs. Biomed. Pharmacother. 2023, 157, 114014. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, D. M.; Burrows, G. E.; Prenzler, P. D.; Hill, R. A. , Potential role of phenolic extracts of mentha in managing oxidative stress and alzheimer’s disease. Antioxidants 2020, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tong, C.; Zhou, J.; Gao, C.; Olatunji, O. J. , Protective Effects of Shorea roxburghii Phenolic Extract on Nephrotoxicity Induced by Cyclophosphamide: Impact on Oxidative Stress, Biochemical and Histopathological Alterations. Chem. Biodivers. 2022, 19, e202200053. [Google Scholar] [CrossRef] [PubMed]
- Katsinas, N.; Rodríguez-Rojo, S.; Enríquez-De-salamanca, A. , Olive pomace phenolic compounds and extracts can inhibit inflammatory-and oxidative-related diseases of human ocular surface epithelium. Antioxidants 2021, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, B. H.; Duchnik, E.; Marchlewicz, M. , Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Lion, Q.; Pichette, A.; Mihoub, M.; Mshvildadze, V.; Legault, J. , Phenolic extract from aralia nudicaulis L. rhizomes inhibits cellular oxidative stresses. Molecules 2021, 26, 4458. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; DaSilva, N. A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X. H.; Chen, H. D.; Seeram, N. P.; Ma, H. , Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]
- Oršolić, N.; Kunštić, M.; Kukolj, M.; Odeh, D.; Ančić, D. , Natural phenolic acid, product of the honey bee, for the control of oxidative stress, peritoneal angiogenesis, and tumor growth in mice. Molecules 2020, 25, 5583. [Google Scholar] [CrossRef]
- Ramli, I.; Posadino, A. M.; Zerizer, S.; Spissu, Y.; Barberis, A.; Djeghim, H.; Azara, E.; Bensouici, C.; Kabouche, Z.; Rebbas, K.; D'Hallewin, G.; Sechi, L. A.; Pintus, G. Low concentrations of Ambrosia maritima L. phenolic extract protect endothelial cells from oxidative cell death induced by H2O2 and sera from Crohn's disease patients. J. Ethnopharmacol. 2023, 300, 115722. [Google Scholar] [CrossRef]
- Ren, Y.; Sun-Waterhouse, D.; Ouyang, F.; Tan, X.; Li, D.; Xu, L.; Li, B.; Wang, Y.; Li, F. , Apple phenolic extracts ameliorate lead-induced cognitive impairment and depression- and anxiety-like behavior in mice by abating oxidative stress, inflammation and apoptosis via the miR-22-3p/SIRT1 axis. Food Funct. 2022, 13, 2647–2661. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Dua, T. K.; Das, S.; De Feo, V.; Dewanjee, S. , Wheat phenolics suppress doxorubicin-induced cardiotoxicity via inhibition of oxidative stress, MAP kinase activation, NF-κB pathway, PI3K/Akt/mTOR impairment, and cardiac apoptosis. Food Chem. Toxicol. 2019, 125, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Medina, A.; Redondo-Puente, M.; Dupak, R.; Bravo-Clemente, L.; Goya, L.; Sarriá, B. , Colonic Coffee Phenols Metabolites, Dihydrocaffeic, Dihydroferulic, and Hydroxyhippuric Acids Protect Hepatic Cells from TNF-α-Induced Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 1440. [Google Scholar] [CrossRef] [PubMed]
- Winarsi, H.; Septiana, A. T. , Improving oxidative stress and inflammation status of obese women with metabolic syndrome using phenolic-rich red kidney bean sprout milk yogurt. Int. Food Res. J. 2022, 29, 142–148. [Google Scholar] [CrossRef]
- Yener, I.; Kocakaya, S. O.; Ertas, A.; Erhan, B.; Kaplaner, E.; Oral, E. V.; Yilmaz-Ozden, T.; Yilmaz, M. A.; Ozturk, M.; Kolak, U. , Selective in vitro and in silico enzymes inhibitory activities of phenolic acids and flavonoids of food plants: Relations with oxidative stress. Food Chem. 2020, 327, 127045. [Google Scholar] [CrossRef]
- Nicoli, M. C.; Anese, M.; Manzocco, L.; Lerici, C. R. , Antioxidant properties of coffee brews in relation to the roasting degree. LWT 1997, 30, 292–297. [Google Scholar] [CrossRef]
- León-Carmona, J. R.; Alvarez-Idaboy, J. R.; Galano, A. , On the peroxyl scavenging activity of hydroxycinnamic acid derivatives: Mechanisms, kinetics, and importance of the acid-base equilibrium. Phys. Chem. Chem. Phys. 2012, 14, 12534–12543. [Google Scholar] [CrossRef]
- Baggio, J.; Lima, A.; Mancini Filho, J.; Fett, R. Identification of phenolic acids in coffee (Coffea Arabica L.) dust and its antioxidant activity. Ital. J. Food Sci. 2007, 19, 191–201. [Google Scholar]
- Beder-Belkhiri, W.; Zeghichi-Hamri, S.; Kadri, N.; Boulekbache-Makhlouf, L.; Cardoso, S.; Oukhmanou-Bensidhoum, S.; Madani, K. , Hydroxycinnamic acids profiling, in vitro evaluation of total phenolic compounds, caffeine and antioxidant properties of coffee imported, roasted and consumed in Algeria. Mediterr. J. Nutr. Metab. 2018, 11, 51–63. [Google Scholar] [CrossRef]
- Górnaś, P.; Dwiecki, K.; Siger, A.; Tomaszewska-Gras, J.; Michalak, M.; Polewski, K. , Contribution of phenolic acids isolated from green and roasted boiled-type coffee brews to total coffee antioxidant capacity. Eur. Food Res. Technol. 2016, 242, 641–653. [Google Scholar] [CrossRef]
- Saeed Alkaltham, M.; Musa Özcan, M.; Uslu, N.; Salamatullah, A. M.; Hayat, K. , Effect of microwave and oven roasting methods on total phenol, antioxidant activity, phenolic compounds, and fatty acid compositions of coffee beans. J. Food Process. Preserv. 2020, 44, e14874. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Morales, F. J. , Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J. Agric. Food Chem. 2005, 53, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, L. M.; Chávez-Quiroz, K.; Medina-Juárez, L. Á.; Gámez Meza, N. , Phenolic characterization, melanoidins, and antioxidant activity of some commercial coffees from Coffea arabica and Coffea canephora. J. Mex. Chem. Soc. 2012, 56, 430–435. [Google Scholar]
- Perrone, D.; Farah, A.; Donangelo, C. M. , Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef]
- Vignoli, J. A.; Bassoli, D. G.; Benassi, M. T. , Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food. Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Fuster, M. D.; Mitchell, A. E.; Ochi, H.; Shibamoto, T. , Antioxidative activities of heterocyclic compounds formed in brewed coffee. J. Agric. Food Chem. 2000, 48, 5600–5603. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, K.; Lee, K. G.; Ochi, H.; Shibamoto, T. , Antioxidative activity of heterocyclic compounds found in coffee volatiles produced by Maillard reaction. J. Agric. Food Chem. 2002, 50, 5480–5484. [Google Scholar] [CrossRef]
- Liu, Y.; Kitts, D. D. , Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Nebesny, E.; Budryn, G. , Antioxidative activity of green and roasted coffee beans as influenced by convection and microwave roasting methods and content of certain compounds. Eur. Food Res. Technol. 2003, 217, 157–163. [Google Scholar] [CrossRef]
- Cheong, M. W.; Tong, K. H.; Ong, J. J. M.; Liu, S. Q.; Curran, P.; Yu, B. , Volatile composition and antioxidant capacity of Arabica coffee. Food Res. Int. 2013, 51, 388–396. [Google Scholar] [CrossRef]
- Haile, M.; Bae, H. M.; Kang, W. H. , Comparison of the antioxidant activities and volatile compounds of coffee beans obtained using digestive bio-processing (elephant dung coffee) and commonly known processing methods. Antioxidants 2020, 9, 408. [Google Scholar] [CrossRef]
- Kang, D. E.; Lee, H. U.; Davaatseren, M.; Chung, M. S. , Comparison of acrylamide and furan concentrations, antioxidant activities, and volatile profiles in cold or hot brew coffees. Food Sci. Biotechnol. 2020, 29, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kulapichitr, F.; Borompichaichartkul, C.; Pratontep, S.; Lopetcharat, K.; Boonbumrung, S.; Suppavorasatit, I. , Differences in volatile compounds and antioxidant activity of ripe and unripe green coffee beans (Coffea arabica L. ‘Catimor’). Acta Hortic. 2017, 1179, 261–268. [Google Scholar] [CrossRef]
- Ludwig, I. A.; Sánchez, L.; De Peña, M. P.; Cid, C. , Contribution of volatile compounds to the antioxidant capacity of coffee. Food Res. Int. 2014, 61, 67–74. [Google Scholar] [CrossRef]
- Lin, S. Y.; Wang, L. H.; Lin, Y. H.; Han, Y. H.; Wang, Y. S.; Yeh, C. S. , Antioxidative Activity and Caffeine Content of Coffee from Different Preparation Methods. Taiwan. J. Agric. Chem. Food Sci. 2009, 47, 268–275. [Google Scholar]
- Stadler, R. H.; Fay, L. B. , Antioxidative Reactions of Caffeine: Formation of 8-Oxocaffeine (1,3,7-Trimethyluric Acid) in Coffee Subjected to Oxidative Stress. J. Agric. Food Chem. 1995, 43, 1332–1338. [Google Scholar] [CrossRef]
- Miłek, M.; Młodecki, Ł.; Dżugan, M. , Caffeine Content and Antioxidant Activity of Various Brews of Specialty Grade Coffee. Acta Sci. Pol. Technol. Aliment. 2021, 20, 179–188. [Google Scholar]
- León-Carmona, J. R.; Galano, A. , Is caffeine a good scavenger of oxygenated free radicals? J. Phys. Chem. B 2011, 115, 4538–4546. [Google Scholar] [CrossRef]
- Prior, R. L.; Wu, X.; Schaich, K. , Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Frankel, E. N.; Meyer, A. S. , The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J. R. , A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, N. A.; Zhu, F. , Coffee bean processing: Emerging methods and their effects on chemical, biological and sensory properties. Food. Chem. 2023, 412, 135489. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Salamatullah, A. M. , Relationship between the chemical composition and the biological functions of coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef] [PubMed]
- Hall, R. D.; Trevisan, F.; de Vos, R. C. H. , Coffee berry and green bean chemistry – Opportunities for improving cup quality and crop circularity. Food Res. Int. 2022, 151, 110825. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S. M.; Oliveira, M. B. P. P.; Alves, R. C. , Neuroprotective properties of coffee: An update. Trends Food Sci. Technol. 2021, 113, 167–179. [Google Scholar] [CrossRef]
- Kusumah, J.; Gonzalez de Mejia, E. , Coffee constituents with antiadipogenic and antidiabetic potentials: A narrative review. Food Chem. Toxicol. 2022, 161, 112821. [Google Scholar] [CrossRef]
- Munyendo, L. M.; Njoroge, D. M.; Owaga, E. E.; Mugendi, B. , Coffee phytochemicals and post-harvest handling—A complex and delicate balance. J. Food Compos. Anal. 2021, 102, 103995. [Google Scholar] [CrossRef]
- Pua, A.; Goh, R. M. V.; Huang, Y.; Tang, V. C. Y.; Ee, K. H.; Cornuz, M.; Liu, S. Q.; Lassabliere, B.; Yu, B. , Recent advances in analytical strategies for coffee volatile studies: Opportunities and challenges. Food. Chem. 2022, 388, 132971. [Google Scholar] [CrossRef]
- Van Dijk, A. E.; Olthof, M. R.; Meeuse, J. C.; Seebus, E.; Heine, R. J.; Van Dam, R. M. , Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef]
- Chen, X. , A review on coffee leaves: Phytochemicals, bioactivities and applications. Crit. Rev. Food Sci. Nutr. 2019, 59, 1008–1025. [Google Scholar] [CrossRef]
- Prakash, I.; R, S. S.; P, S. H.; Kumar, P.; Om, H.; Basavaraj, K.; Murthy, P. S. , Metabolomics and volatile fingerprint of yeast fermented robusta coffee: A value added coffee. LWT 2022, 154, 112717. [Google Scholar] [CrossRef]
- Sualeh, A.; Tolessa, K.; Mohammed, A. , Biochemical composition of green and roasted coffee beans and their association with coffee quality from different districts of southwest Ethiopia. Heliyon 2020, 6, e05812. [Google Scholar] [CrossRef]
- Campa, C.; Ballester, J. F.; Doulbeau, S.; Dussert, S.; Hamon, S.; Noirot, M. , Trigonelline and sucrose diversity in wild Coffea species. Food. Chem. 2004, 88, 39–43. [Google Scholar] [CrossRef]
- Campa, C.; Doulbeau, S.; Dussert, S.; Hamon, S.; Noirot, M. , Qualitative relationship between caffeine and chlorogenic acid contents among wild Coffea species. Food. Chem. 2005, 93, 135–139. [Google Scholar] [CrossRef]
- Dias, E. C.; Borém, F. M.; Pereira, R. G. F. A.; Guerreiro, M. C. , Amino acid profiles in unripe Arabica coffee fruits processed using wet and dry methods. Eur. Food Res. Technol. 2012, 234, 25–32. [Google Scholar] [CrossRef]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. , Characterization of fatty acid, amino acid and volatile compound compositions and bioactive components of seven coffee (Coffea robusta) cultivars grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef]
- Fitri; Laga, A. ; Dwyana, Z.; Tawali, A. B., Composition of amino acids and fatty acids on luwak coffee processing. Food Res. 2021, 5, 60–64. [Google Scholar] [CrossRef]
- Macheiner, L.; Schmidt, A.; Mayer, H. K. , Green coffee derived supplements and infusions as a source of polyamines and free amino acids. Eur. Food Res. Technol. 2021, 247, 85–99. [Google Scholar] [CrossRef]
- Esquivel, P.; Viñas, M.; Steingass, C. B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R. M.; Jiménez, V. M. Coffee (Coffea arabica L.) by-Products as a Source of Carotenoids and Phenolic Compounds—Evaluation of Varieties With Different Peel Color. Front. Sustain. Food Syst. 2020, 4, 590597. [Google Scholar] [CrossRef]
- Simkin, A. J.; Kuntz, M.; Moreau, H.; McCarthy, J. , Carotenoid profiling and the expression of carotenoid biosynthetic genes in developing coffee grain. Plant Physiol. Biochem. 2010, 48, 434–442. [Google Scholar] [CrossRef]
- Koshima, Y.; Kitamura, Y.; Islam, M. Z.; Kokawa, M. , Quantitative and qualitative evaluation of fatty acids in coffee oil and coffee residue. Food Sci. Technol. Res. 2020, 26, 545–552. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Peng, X.; Hu, G.; Qiu, M. , Identification of new diterpene esters from green Arabica coffee beans, and their platelet aggregation accelerating activities. Food. Chem. 2018, 263, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Liu, J. Q.; Peng, X. R.; Wan, L. S.; Zhou, L.; Zhang, T.; Qiu, M. H. , Characterization of diterpenoid glucosides in roasted puer coffee beans. J. Agric. Food Chem. 2014, 62, 2631–2637. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. , Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food. Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Peñuela-Martínez, A. E.; Zapata-Zapata, A. E.; Durango-Restrepo, D. L. Performance of different fermentation methods and the effect on coffee quality (coffea arabica l.). Coffee Sci. 2018, 13, 465–476. [Google Scholar] [CrossRef]
- Yeager, S. E.; Batali, M. E.; Guinard, J. X.; Ristenpart, W. D. , Acids in coffee: A review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2021, 63, 1010–1036. [Google Scholar] [CrossRef] [PubMed]
- Badmos, S.; Lee, S. H.; Kuhnert, N. , Comparison and quantification of chlorogenic acids for differentiation of green Robusta and Arabica coffee beans. Food Res. Int. 2019, 126, 108544. [Google Scholar] [CrossRef]
- Köseoglu Yilmaz, P.; Kolak, U. , SPE-HPLC Determination of Chlorogenic and Phenolic Acids in Coffee. J. Chromatogr. Sci. 2017, 55, 712–718. [Google Scholar] [CrossRef]
- Marmet, C.; Actis-Goretta, L.; Renouf, M.; Giuffrida, F. , Quantification of phenolic acids and their methylates, glucuronides, sulfates and lactones metabolites in human plasma by LC-MS/MS after oral ingestion of soluble coffee. J. Pharm. Biomed. Anal. 2014, 88, 617–625. [Google Scholar] [CrossRef]
- Risso, E. M.; Péres, R. G.; Amaya-Farfan, J. Determination of phenolic acids in coffee by micellar electrokinetic chromatography. Food. Chem. 2007, 105, 1578–1582. [Google Scholar] [CrossRef]
- Dos Santos, R. A.; Prado, M. A.; Pertierra, R. E.; Palacios, H. A. , Analysis of sugars and chlorogenic acid in coffee harvested at different ripening stages and after processing. Braz. J. Food Technol. 2018, 21, e2017163. [Google Scholar] [CrossRef]
- Pereira, P. V.; Bravim, D. G.; Grillo, R. P.; Bertoli, L. D.; Osório, V. M.; da Silva Oliveira, D.; da Cruz Pedrozo Miguel, M. G.; Schwan, R. F.; de Assis Silva, S.; Coelho, J. M.; Bernardes, P. C. , Microbial diversity and chemical characteristics of Coffea canephora grown in different environments and processed by dry method. World J. Microbiol. Biotechnol. 2021, 37, 51. [Google Scholar] [CrossRef] [PubMed]
- Chindapan, N.; Soydok, S.; Devahastin, S. , Roasting Kinetics and Chemical Composition Changes of Robusta Coffee Beans During Hot Air and Superheated Steam Roasting. J. Food Sci. 2019, 84, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effect of shading on yield, sugar content, phenolic acids and antioxidant property of coffee beans (Coffea Arabica L. cv. Catimor) harvested from north-eastern Thailand. J. Sci. Food Agric. 2012, 92, 1956–1963. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Lu, J.; Hu, G.; Qiu, M. , Ent-kaurane diterpenoids from the cherries of Coffea arabica. Fitoterapia 2019, 132, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Wan, L. S.; Peng, X. R.; Yu, M. Y.; Zhang, Z. R.; Zhou, L.; Li, Z. R.; Qiu, M. H. , Characterization of New Ent-kaurane Diterpenoids of Yunnan Arabica Coffee Beans. Nat. Prod. Bioprospect. 2016, 6, 217–223. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X. R.; Lu, J.; Hu, G. L.; Qiu, M. H. , New Dammarane Triterpenoids, Caffruones A–D, from the Cherries of Coffea arabica. Nat. Prod. Bioprospect. 2018, 8, 413–418. [Google Scholar] [CrossRef]
- Lang, R.; Fromme, T.; Beusch, A.; Lang, T.; Klingenspor, M.; Hofmann, T. , Raw coffee based dietary supplements contain carboxyatractyligenin derivatives inhibiting mitochondrial adenine-nucleotide-translocase. Food Chem. Toxicol. 2014, 70, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M. D. S. G.; Scholz, M. B. D. S.; Kitzberger, C. S. G.; Benassi, M. D. T. , Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food. Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Whitworth, M. B.; Cui, C.; Fisk, I. D. , Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef]
- Piccino, S.; Boulanger, R.; Descroix, F.; Sing, A. S. C. , Aromatic composition and potent odorants of the "specialty coffee" brew "Bourbon Pointu" correlated to its three trade classifications. Food Res. Int. 2014, 61, 264–271. [Google Scholar] [CrossRef]
- Angeloni, S.; Mustafa, A. M.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F. K.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E.; Caprioli, G. , Characterization of the aroma profile and main key odorants of espresso coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef] [PubMed]
- Hafsah, H.; Iriawati, I.; Syamsudin, T. S. , Dataset of volatile compounds from flowers and secondary metabolites from the skin pulp, green beans, and peaberry green beans of robusta coffee. Data Brief 2020, 29, 105219. [Google Scholar] [CrossRef]
- Kim, H. J.; Hong, D. L.; Yu, J. W.; Lee, S. M.; Lee, Y. B. , Identification of headspace volatile compounds of blended coffee and application to principal component analysis. Prev. Nutr. Food Sci. 2019, 24, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. G.; Shibamoto, T. Analysis of volatile components isolated from Hawaiian green coffee beans (Coffea arabica L.). Flavour Fragr. J. 2002, 17, 349–351. [Google Scholar] [CrossRef]
- Sarghini, F.; Fasano, E.; De Vivo, A.; Tricarico, M. C. , Influence of roasting process in six coffee Arabica cultivars: Analysis of volatile components profiles. Chem. Eng. Trans. 2019, 75, 295–300. [Google Scholar]
- Butt, M. S.; Sultan, M. T. , Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef]
- Colombo, R.; Papetti, A. , Decaffeinated coffee and its benefits on health: focus on systemic disorders. Crit. Rev. Food Sci. Nutr. 2021, 61, 2506–2522. [Google Scholar] [CrossRef]
- Dirks-Naylor, A. J. , The benefits of coffee on skeletal muscle. Life Sci. 2015, 143, 182–186. [Google Scholar] [CrossRef]
- Pourshahidi, L. K.; Navarini, L.; Petracco, M.; Strain, J. J. , A Comprehensive Overview of the Risks and Benefits of Coffee Consumption. Compr. Rev. Food Sci. Food Saf. 2016, 15, 671–684. [Google Scholar] [CrossRef]
- dos Santos, H. D.; Boffo, E. F. , Coffee beyond the cup: analytical techniques used in chemical composition research—a review. Eur. Food Res. Technol. 2021, 247, 749–775. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; El Ghoch, M.; Castellucci, B.; Chapela, S. P.; Carignano, M. D. L. A.; Laudisio, D.; Savastano, S.; Colao, A.; Muscogiuri, G. , Coffee consumption, health benefits and side effects: a narrative review and update for dietitians and nutritionists. Crit. Rev. Food Sci. Nutr. 2023, 63, 1238–1261. [Google Scholar] [CrossRef]
- Jeong, J. M.; Lee, K. I.; Kim, S. M. , Simultaneous determination of benzoic Acid, Caffeic acid and Chlorogenic acid in seeds of Eriobotrya japonica and their antibacterial Effect. J. Appl. Biol. Chem. 2014, 57, 89–93. [Google Scholar]
- Park, M. Y.; Kang, D. H. , Antibacterial Activity of Caffeic Acid Combined with UV-A Light against Escherichia coli O157:H7, Salmonella enterica Serovar Typhimurium, and Listeria monocytogenes. Appl. Environ. Microbiol. 2021, 87, e00631–21. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Soares, G.; Henriques, M. , Evaluation of antibacterial activity of caffeic acid encapsulated by β-cyclodextrins. J. Microencapsul. 2015, 32, 804–8. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kakino, Y.; Iwai, K.; Mochida, K.; Fujita, T. , In vitro antibacterial, antimutagenic and anti-influenza virus activity of caffeic acid phenethyl esters. Biocontrol Sci. 2005, 10, 155–161. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. , Antibacterial effect of caffeic acid phenethyl ester on cariogenic bacteria and streptococcus mutans biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251–20. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C. Y.; González-Rios, O.; Suárez-Quiroz, M. L.; González-Amaro, R. M.; Hernández-Estrada, Z. J.; Rayas-Duarte, P. , Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef] [PubMed]
- Elbestawy, M. K. M.; El-Sherbiny, G. M.; Moghannem, S. A. , Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M. F. R.; Ha, A. J. W.; Park, S. H.; Ha, S. D. , Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020, 91, 103500. [Google Scholar] [CrossRef]
- Bai, X.; Li, X.; Liu, X.; Xing, Z.; Su, R.; Wang, Y.; Xia, X.; Shi, C. , Antibacterial Effect of Eugenol on Shigella flexneri and Its Mechanism. Foods 2022, 11, 2565. [Google Scholar] [CrossRef]
- Bezerra, S. R.; Bezerra, A. H.; de Sousa Silveira, Z.; Macedo, N. S.; dos Santos Barbosa, C. R.; Muniz, D. F.; Sampaio dos Santos, J. F.; Melo Coutinho, H. D.; Bezerra da Cunha, F. A. , Antibacterial activity of eugenol on the IS-58 strain of Staphylococcus aureus resistant to tetracycline and toxicity in Drosophila melanogaster. Microb. Pathog. 2022, 164, 105456. [Google Scholar] [CrossRef] [PubMed]
- Devi, K. P.; Nisha, S. A.; Sakthivel, R.; Pandian, S. K. , Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, C.; Banks, L. A.; Hudaib, T. , Antifungal and antibacterial activities of eugenol and non-polar extract of Syzygium aromaticum L. J. Pharm. Sci. Res. 2018, 10, 337–339. [Google Scholar]
- Silva, J. C.; Silva Pereira, R. L.; Sampaio de Freitas, T.; Rocha, J. E.; Macedo, N. S.; de Fatima Alves Nonato, C.; Linhares, M. L.; Arruda Tavares, D. S.; Bezerra da Cunha, F. A.; Melo Coutinho, H. D.; Gonçalo de Lima, S.; Pereira-Junior, F. N.; Araújo Maia, F. P.; Pita Neto, I. C.; Galvão Rodrigues, F. F.; Garcia Santos, G. J. , Evaluation of antibacterial and toxicological activities of essential oil of Ocimum gratissimum L. and its major constituent eugenol. Food Biosci. 2022, 50, 102128. [Google Scholar] [CrossRef]
- Su, R.; Bai, X.; Liu, X.; Song, L.; Liu, X.; Zhan, X.; Guo, D.; Wang, Y.; Chang, Y.; Shi, C. , Antibacterial Mechanism of Eugenol Against Shigella sonnei and Its Antibacterial Application in Lettuce Juice. Foodborne Pathog. Dis. 2022, 19, 779–786. [Google Scholar] [CrossRef]
- Zhang, L. L.; Zhang, L. F.; Xu, J. G.; Hu, Q. P. , Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food Nutr. Res. 2017, 61, 1353356. [Google Scholar] [CrossRef]
- Amani, F.; Rezaei, A.; Kharazmi, M. S.; Jafari, S. M. , Loading ferulic acid into β-cyclodextrin nanosponges; antibacterial activity, controlled release and application in pomegranate juice as a copigment agent. Colloids Surf. Physicochem. Eng. Aspects 2022, 649, 129454. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M. J.; Simões, M. , Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. , Antibacterial properties of cinnamic and ferulic acids incorporated to starch and PLA monolayer and multilayer films. Food Control 2022, 136, 108878. [Google Scholar] [CrossRef]
- Tu, Q. B.; Shi, H. C.; Li, P.; Sheng, S.; Wu, F. A. , Antibacterial Activity of Ferulic Acid Ester against Ralstonia solanacearum and Its Synergy with Essential Oils. Sustainability 2022, 14, 16348. [Google Scholar] [CrossRef]
- Sung, W. S.; Jung, H. J.; Lee, I. S.; Kim, H. S.; Lee, D. G. , Antimicrobial effect of furaneol against human pathogenic bacteria and fungi. J. Microbiol. Biotechnol. 2006, 16, 349–354. [Google Scholar]
- Aulestia-Viera, P. V.; Gontijo, S. M. L.; Gomes, A. D. M.; Sinisterra, R. D.; Rocha, R. G.; Cortés, M. E.; dos Santos, M. F.; Borsatti, M. A. , Guaiacol/β-cyclodextrin for rapid healing of dry socket: antibacterial activity, cytotoxicity, and bone repair—an animal study. Oral Maxillofac. Surg. 2019, 23, 53–61. [Google Scholar] [CrossRef]
- Cooper, R. A. , Inhibition of biofilms by glucose oxidase, lactoperoxidase and guaiacol: The active antibacterial component in an enzyme alginogel. Int. Wound J. 2013, 10, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Chhibber, S.; Singh, V.; Harjai, K. , Guaiacol augments quorum quenching potential of ciprofloxacin against Pseudomonas aeruginosa. J. Appl. Microbiol. 2022, 133, 2235–2254. [Google Scholar] [CrossRef] [PubMed]
- Galvão, J. L. F. M.; Rosa, L. L. S.; Neto, H. D.; Silva, D. F.; Nóbrega, J. R.; Cordeiro, L. V.; de Figueiredo, P. T. R.; Andrade Júnior, F. P.; Filho, A. A. O.; Lima, E. O. , Antibacterial effect of isoeugenol against Pseudomonas aeruginosa. Braz. J. Pharm. Sci. 2022, 58, e20075. [Google Scholar] [CrossRef]
- Krogsgård Nielsen, C.; Kjems, J.; Mygind, T.; Snabe, T.; Schwarz, K.; Serfert, Y.; Meyer, R. L. , Antimicrobial effect of emulsion-encapsulated isoeugenol against biofilms of food pathogens and spoilage bacteria. Int. J. Food Microbiol. 2017, 242, 7–12. [Google Scholar] [CrossRef]
- Nielsen, C. K.; Subbiahdoss, G.; Zeng, G.; Salmi, Z.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R. L. , Antibacterial isoeugenol coating on stainless steel and polyethylene surfaces prevents biofilm growth. J. Appl. Microbiol. 2018, 124, 179–187. [Google Scholar] [CrossRef]
- Siva, S.; Li, C.; Cui, H.; Lin, L. , Encompassment of isoeugenol in 2-hydroxypropyl-β-cyclodextrin using ultrasonication: Characterization, antioxidant and antibacterial activities. J. Mol. Liq. 2019, 296, 111777. [Google Scholar] [CrossRef]
- Ajiboye, T. O.; Habibu, R. S.; Saidu, K.; Haliru, F. Z.; Ajiboye, H. O.; Aliyu, N. O.; Ibitoye, O. B.; Uwazie, J. N.; Muritala, H. F.; Bello, S. A.; Yusuf, I. I.; Mohammed, A. O. , Involvement of oxidative stress in protocatechuic acid-mediated bacterial lethality. Microbiol. Open 2017, 6, e00472. [Google Scholar] [CrossRef]
- Bernal-Mercado, A. T.; Vazquez-Armenta, F. J.; Tapia-Rodriguez, M. R.; Islas-Osuna, M. A.; Mata-Haro, V.; Gonzalez-Aguilar, G. A.; Lopez-Zavala, A. A.; Ayala-Zavala, J. F. , Comparison of single and combined use of catechin, protocatechuic, and vanillic acids as antioxidant and antibacterial agents against uropathogenic Escherichia coli at planktonic and biofilm levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef] [PubMed]
- Chao, C. Y.; Yin, M. C. , Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 2009, 6, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. S.; Tsao, S. M.; Yin, M. C. , In vitro antibacterial activity of roselle calyx and protocatechuic acid. Phytother. Res. 2005, 19, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D. S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I. C. F. R.; Janković, T.; Maksimović, Z. , Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef]
- Wu, M.; Tian, L.; Fu, J.; Liao, S.; Li, H.; Gai, Z.; Gong, G. , Antibacterial mechanism of Protocatechuic acid against Yersinia enterocolitica and its application in pork. Food Control 2022, 133, 108573. [Google Scholar] [CrossRef]
- Buathong, R.; Chamchumroon, V.; Schinnerl, J.; Bacher, M.; Santimaleeworagun, W.; Kraichak, E.; Vajrodaya, S. , Chemovariation and antibacterial activity of extracts and isolated compounds from species of Ixora and Greenea (Ixoroideae, Rubiaceae). PeerJ 2019, 2019, e6893. [Google Scholar] [CrossRef]
- Napiroon, T.; Bacher, M.; Balslev, H.; Tawaitakham, K.; Santimaleeworagun, W.; Vajrodaya, S. , Scopoletin from Lasianthus lucidus Blume (Rubiaceae): A potential antimicrobial against multidrug-resistant Pseudomonas aeruginosa. J. Appl. Pharm. Sci. 2018, 8, 1–6. [Google Scholar]
- De La Cruz-Sánchez, N. G.; Gómez-Rivera, A.; Alvarez-Fitz, P.; Ventura-Zapata, E.; Pérez-García, M. D.; Avilés-Flores, M.; Gutiérrez-Román, A. S.; González-Cortazar, M. , Antibacterial activity of Morinda citrifolia Linneo seeds against Methicillin-Resistant Staphylococcus spp. Microb. Pathog. 2019, 128, 347–353. [Google Scholar] [CrossRef]
- Firmansyah, A.; Winingsih, W.; Manobi, J. D. Y. , Review of scopoletin: Isolation, analysis process, and pharmacological activity. Biointerface Res. Appl. Chem. 2021, 11, 12006–12019. [Google Scholar]
- Mfonku, N. A.; Tadjong, A. T.; Kamsu, G. T.; Kodjio, N.; Ren, J.; Mbah, J. A.; Gatsing, D.; Zhan, J. Isolation and characterization of antisalmonellal anthraquinones and coumarins from Morinda lucida Benth. (Rubiaceae). Chem. Pap. 2021, 75, 2067–2073. [Google Scholar] [CrossRef]
- Naz, F.; Kumar, M.; Koley, T.; Sharma, P.; Haque, M. A.; Kapil, A.; Kumar, M.; Kaur, P.; Ethayathulla, A. S. , Screening of plant-based natural compounds as an inhibitor of FtsZ from Salmonella Typhi using the computational, biochemical and in vitro cell-based studies. Int. J. Biol. Macromol. 2022, 219, 428–437. [Google Scholar] [CrossRef]
- Qian, W.; Fu, Y.; Liu, M.; Wang, T.; Zhang, J.; Yang, M.; Sun, Z.; Li, X.; Li, Y. , In vitro antibacterial activity and mechanism of vanillic acid against carbapenem-resistant Enterobacter cloacae. Antibiotics 2019, 8, 220. [Google Scholar] [CrossRef]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. , Antibacterial Mechanism of Vanillic Acid on Physiological, Morphological, and Biofilm Properties of Carbapenem-Resistant Enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C. Z.; Sawadogo, R.; Yuan, J.; Zeng, J.; Xu, M.; Tan, T.; Yuan, C. S. , 4-Vinylguaiacol, an Active Metabolite of Ferulic Acid by Enteric Microbiota and Probiotics, Possesses Significant Activities against Drug-Resistant Human Colorectal Cancer Cells. ACS Omega 2021, 6, 4551–4561. [Google Scholar] [CrossRef] [PubMed]
- Sudhagar, S.; Sathya, S.; Anuradha, R.; Gokulapriya, G.; Geetharani, Y.; Lakshmi, B. S. , Inhibition of epidermal growth factor receptor by ferulic acid and 4-vinylguaiacol in human breast cancer cells. Biotechnol. Lett. 2018, 40, 257–262. [Google Scholar] [CrossRef]
- Cavin, C.; Holzhaeuser, D.; Scharf, G.; Constable, A.; Huber, W. W.; Schilter, B. , Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem. Toxicol. 2002, 40, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. , Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Izumi, K.; Natsagdorj, A.; Naito, R.; Marino, T.; Kadomoto, S.; Hiratsuka, K.; Shigehara, K.; Radono, Y.; Mizorami, A.; Narimoto, R.; Saito, Y.; Naragawa-Goto, R. , Coffee diterpenes, kahweol acetate and cafestol, synergistically induce apoptosis and inhibit the epithelial-mesenchymal transition of prostate cancer cells. Nishinihon J. Urol. 2019, 81, 364–371. [Google Scholar]
- Lee, K. A.; Chae, J. I.; Shim, J. H. , Natural diterpenes from coffee, cafestol and kahweol induce apoptosis through regulation of specificity protein 1 expression in human malignant pleural mesothelioma. J. Biomed. Sci. 2012, 19, 60. [Google Scholar] [CrossRef]
- Bovilla, V.; Anantharaju, P.; Dornadula, S.; Veeresh, P.; Kuruburu, M.; Bettada, V.; Ramkumar, K.; Madhunapantula, S. , Caffeic acid and protocatechuic acid modulate Nrf2 and inhibit Ehrlich ascites carcinomas in mice. Asian Pac. J. Trop. Biomed. 2021, 11, 244–253. [Google Scholar]
- Brautigan, D. L.; Gielata, M.; Heo, J.; Kubicka, E.; Wilkins, L. R. , Selective toxicity of caffeic acid in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2018, 505, 612–617. [Google Scholar] [CrossRef]
- Celińska-Janowicz, K.; Zarȩba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Pałka, J.; Miltyk, W. , Constituents of propolis: Chrysin, caffeic acid, p-coumaric acid, and ferulic acid induce PRODH/POX-dependent apoptosis in human tongue squamous cell carcinoma cell (CAL-27). Front. Pharmacol. 2018, 9, 336. [Google Scholar] [CrossRef]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. , Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Kocemba-Pilarczyk, K. A.; Majka, M. , Caffeic acid targets AMPK signaling and regulates tricarboxylic acid cycle anaplerosis while metformin downregulates HIF-1α-induced glycolytic enzymes in human cervical squamous cell carcinoma lines. Nutrients 2018, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Konieczny, P.; Majka, M. , Caffeic acid expands anti-tumor effect of metformin in human metastatic cervical carcinoma HTB-34 cells: Implications of AMPK activation and impairment of fatty acids de novo biosynthesis. Int. J. Mol. Sci. 2017, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Chang, K. S.; Tsui, K. H.; Hsu, S. Y.; Sung, H. C.; Lin, Y. H.; Hou, C. P.; Yang, P. S.; Chen, C. L.; Feng, T. H.; Juang, H. H. , The Antitumor Effect of Caffeic Acid Phenethyl Ester by Downregulating Mucosa-Associated Lymphoid Tissue 1 via AR/p53/NF-κB Signaling in Prostate Carcinoma Cells. Cancers (Basel) 2022, 14, 274. [Google Scholar] [CrossRef]
- Hou, C. P.; Tsui, K. H.; Chang, K. S.; Sung, H. C.; Hsu, S. Y.; Lin, Y. H.; Yang, P. S.; Chen, C. L.; Feng, T. H.; Juang, H. H. , Caffeic acid phenethyl ester inhibits the growth of bladder carcinoma cells by upregulating growth differentiation factor 15. Biomed. J. 2022, 45, 763–775. [Google Scholar] [CrossRef]
- Kapare, H.; Nagaraj, S.; Wakalkar, S.; Rathi, K. , Caffeic Acid Phenethyl Ester: A Potential Anticancer Bioactive Constituent of Propolis. Curr. Cancer Ther. Rev. 2022, 18, 181–192. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, G.; Wu, L.; Zhong, S.; Gao, X.; Tong, Y.; Cui, W.; Qin, Y.; Xu, W.; Xiao, X.; Zhang, Z.; Huang, G.; Zhou, X. , Caffeic acid phenethyl ester suppressed growth and metastasis of nasopharyngeal carcinoma cells by inactivating the NF-κB pathway. Drug Des. Devel. Ther. 2019, 13, 1335–1345. [Google Scholar] [CrossRef]
- Sung, H. C.; Chang, K. S.; Chen, S. T.; Hsu, S. Y.; Lin, Y. H.; Hou, C. P.; Feng, T. H.; Tsui, K. H.; Juang, H. H. , Metallothionein 2A with Antioxidant and Antitumor Activity Is Upregulated by Caffeic Acid Phenethyl Ester in Human Bladder Carcinoma Cells. Antioxidants 2022, 11, 1509. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. , Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M. L.; Al-Shabanah, O.; Hassan, Z. K.; Hafez, M. M. , Eugenol-induced autophagy and apoptosis in breast cancer cells via pi3k/akt/foxo3a pathway inhibition. Int. J. Mol. Sci. 2021, 22, 9243. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M. L.; Hafez, M. M.; Al-Hoshani, A.; Al-Shabanah, O. , Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Complement. Altern. Med. 2018, 18, 321. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharashi, L. A.; Bakheet, T.; AlHarbi, W. A.; Al-Moghrabi, N.; Aboussekhra, A. , Eugenol modulates genomic methylation and inactivates breast cancer-associated fibroblasts through E2F1-dependent downregulation of DNMT1/DNMT3A. Mol. Carcinog. 2021, 60, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D. P.; Militão, G. C. G.; De Morais, M. C.; De Sousa, D. P. , The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef]
- Choudhury, P.; Barua, A.; Roy, A.; Pattanayak, R.; Bhattacharyya, M.; Saha, P. , Eugenol restricts Cancer Stem Cell population by degradation of β-catenin via N-terminal Ser37 phosphorylation-an in vivo and in vitro experimental evaluation. Chem. Biol. Interact. 2020, 316, 108938. [Google Scholar] [CrossRef]
- Choudhury, P.; Barua, A.; Roy, A.; Pattanayak, R.; Bhattacharyya, M.; Saha, P. , Eugenol emerges as an elixir by targeting β-catenin, the central cancer stem cell regulator in lung carcinogenesis: An: In vivo and in vitro rationale. Food Funct. 2021, 12, 1063–1078. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Z.; Zeng, J.; Chen, L.; Wu, Q.; Mo, J.; Zhang, G.; Song, L.; Xu, W.; Zhang, S.; Guo, X. , Eugenol inhibits non-small cell lung cancer by repressing expression of NF-κB-regulated TRIM59. Phytother. Res. 2019, 33, 1562–1569. [Google Scholar] [CrossRef]
- Das, A.; Harshadha, K.; Dhinesh Kannan, S. K.; Hari Raj, K.; Jayaprakash, B. , Evaluation of therapeutic potential of Eugenol-A natural derivative of Syzygium aromaticum on cervical cancer. Asian Pac. J. Cancer Prev. 2018, 19, 1977–1985. [Google Scholar]
- Fangjun, L.; Zhijia, Y. , Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef]
- Fouad, M. A.; Sayed-Ahmed, M. M.; Huwait, E. A.; Hafez, H. F.; Osman, A. M. M. , Epigenetic immunomodulatory effect of eugenol and astaxanthin on doxorubicin cytotoxicity in hormonal positive breast Cancer cells. BMC Pharmacol. Toxicol. 2021, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Ghodousi-Dehnavi, E.; Hosseini, R. H.; Arjmand, M.; Nasri, S.; Zamani, Z. , A Metabolomic Investigation of Eugenol on Colorectal Cancer Cell Line HT-29 by Modifying the Expression of APC, p53, and KRAS Genes. Evid. Based Complement. Alternat. Med. 2021, 2021, 1448206. [Google Scholar] [CrossRef] [PubMed]
- Morsy, H. M.; Ahmed, O. M.; Zoheir, K. M. A.; Abdel-Moneim, A. , The anticarcinogenic effect of eugenol on lung cancer induced by diethylnitrosamine/2-acetylaminofluorene in Wistar rats: insight on the mechanisms of action. Apoptosis 2023. [Google Scholar] [CrossRef] [PubMed]
- Padhy, I.; Paul, P.; Sharma, T.; Banerjee, S.; Mondal, A. , Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement. Life 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Permatasari, H. K.; Effendi, A. B.; Qhabibi, F. R.; Fawwaz, F.; Dominique, A. , Eugenol isolated from Syzygium aromaticum inhibits HeLa cancer cell migration by altering epithelial-mesenchymal transition protein regulators. J. Appl. Pharm. Sci. 2021, 11, 49–53. [Google Scholar]
- Ranjitkar, S.; Zhang, D.; Sun, F.; Salman, S.; He, W.; Venkitanarayanan, K.; Tulman, E. R.; Tian, X. , Cytotoxic effects on cancerous and non-cancerous cells of trans-cinnamaldehyde, carvacrol, and eugenol. Sci. Rep. 2021, 11, 16281. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, W.; Bao, X.; Liu, X.; Yang, M.; Yin, C. , Eugenol modulates the NOD1-NF-κB signaling pathway via targeting NF-κB protein in triple-negative breast cancer cells. Front. Endocrinol. (Lausanne) 2023, 14, 1136067. [Google Scholar] [CrossRef]
- Shirazi, P. T.; Farjadian, S.; Dabbaghmanesh, M. H.; Jonaidi, H.; Alavianmehr, A.; Kalani, M.; Emadi, L. , Eugenol: A New Option in Combination Therapy with Sorafenib for the Treatment of Undifferentiated Thyroid Cancer. Iran J. Allergy Asthma Immunol. 2022, 21, 313–321. [Google Scholar]
- Kumar, N.; Kumar, S.; Abbat, S.; Nikhil, K.; Sondhi, S. M.; Bharatam, P. V.; Roy, P.; Pruthi, V. , Ferulic acid amide derivatives as anticancer and antioxidant agents: synthesis, thermal, biological and computational studies. Med. Chem. Res. 2016, 25, 1175–1192. [Google Scholar] [CrossRef]
- Ani, G.; Tanya, T. Y.; Reneta, T. Antitumor and apoptogenic effects of ferulic acid on cervical carcinoma cells. Res. J. Biotechnol. 2021, 16, 6–11. [Google Scholar]
- Bakholdina, L. A.; Markova, A. A.; Khlebnikov, A. I.; Sevodin, V. P. , Cytotoxicity of New Ferulic-Acid Derivatives on Human Colon Carcinoma (HCT116) Cells. Pharm. Chem. J. 2019, 53, 516–520. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Tang, J.; Wang, R. , Ferulic Acid Mitigates Growth and Invasion of Esophageal Squamous Cell Carcinoma through Inducing Ferroptotic Cell Death. Dis. Markers 2022, 2022, 4607966. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wu, H.; Fan, J.; Zhang, L.; Li, H.; Guo, H.; Yang, R.; Li, Z. , The Mixture of Ferulic Acid and P-Coumaric Acid Suppresses Colorectal Cancer through lncRNA 495810/PKM2 Mediated Aerobic Glycolysis. Int. J. Mol. Sci. 2022, 23, 12106. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, S. S.; Dantas, B. B.; Ribeiro-Filho, J.; Araújo, D. A. M.; Da Costa, J. G. M. , Chemical properties of caffeic and ferulic acids in biological system: Implications in cancer therapy. A review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Dodurga, Y.; Eroğlu, C.; Seçme, M.; Elmas, L.; Avcı, Ç. B.; Şatıroğlu-Tufan, N. L. , Anti-proliferative and anti-invasive effects of ferulic acid in TT medullary thyroid cancer cells interacting with URG4/URGCP. Tumor Biol. 2016, 37, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, R. I.; Nasr, M.; Rahsed, L. A.; Hamzawy, M. A. , Ferulic acid nanocapsules as a promising treatment modality for colorectal cancer: Preparation and in vitro/in vivo appraisal. Life Sci. 2022, 298, 120500. [Google Scholar] [CrossRef]
- ElKhazendar, M.; Chalak, J.; El-Huneidi, W.; Vinod, A.; Abdel-Rahman, W. M.; Abu-Gharbieh, E. , Antiproliferative and proapoptotic activities of ferulic acid in breast and liver cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 2571–2576. [Google Scholar]
- Eroğlu, C.; Seçme, M.; Bağcı, G.; Dodurga, Y. , Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumor Biol. 2015, 36, 9437–9446. [Google Scholar] [CrossRef]
- Fahrioğlu, U.; Dodurga, Y.; Elmas, L.; Seçme, M. , Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene 2016, 576, 476–482. [Google Scholar] [CrossRef]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu; Li, Q. ; Yang, S.; Zhang, Y.; Wang, Y., The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018, 18, 102. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A. K.; Loka, M.; Pandey, A. K.; Bishayee, A. , Ferulic acid-mediated modulation of apoptotic signaling pathways in cancer. Adv. Protein Chem. Struct. Biol. 2021, 125, 215–257. [Google Scholar] [PubMed]
- Luo, L.; Zhu, S.; Tong, Y.; Peng, S. , Ferulic acid induces apoptosis of HeLa and caski cervical carcinoma cells by down-regulating the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway. Med. Sci. Monit. 2020, 26, e920095–1. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. , Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F. , Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Shabani Varkani, M.; Clark, C. C. T.; Jafarnejad, S. , Quercetin as an anticancer agent: Focus on esophageal cancer. J. Food Biochem. 2020, 44, e13374. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F. I.; Abdollahi, M.; Nagulapalli Venkata, K. C.; Nabavi, S. M.; Bishayee, A. , Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Kubina, R.; Iriti, M.; Kabała-Dzik, A. , Anticancer potential of selected flavonols: Fisetin, kaempferol, and quercetin on head and neck cancers. Nutrients 2021, 13, 845. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I. A.; ur-Rehman, M.; Gilani, S. A.; Mehmood, Z.; Mubarak, M. S. , Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Mei, S.; Ma, H.; Chen, X. , Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021, 149, 111997. [Google Scholar] [CrossRef]
- Acquaviva, R.; Tomasello, B.; Di Giacomo, C.; Santangelo, R.; Mantia, A. L.; Naletova, I.; Sarpietro, M. G.; Castelli, F.; Malfa, G. A. , Protocatechuic acid, a simple plant secondary metabolite, induced apoptosis by promoting oxidative stress through ho-1 downregulation and p21 upregulation in colon cancer cells. Biomolecules 2021, 11, 1485. [Google Scholar] [CrossRef]
- Lin, H. H.; Chen, J. H.; Chou, F. P.; Wang, C. J. , Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, D. S.; Zimmerman, N. P.; Wang, L. S.; Ransom, B. W. S.; Carmella, S. G.; Kuo, C. T.; Siddiqui, J.; Chen, J. H.; Oshima, K.; Huang, Y. W.; Hecht, S. S.; Stoner, G. D. , Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tanaka, T.; Tanaka, M. , Potential Cancer Chemopreventive Activity of Protocatechuic Acid. J. Exp. Clin. Med. 2011, 3, 27–33. [Google Scholar] [CrossRef]
- Tsao, S. M.; Hsia, T. C.; Yin, M. C. , Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF- B pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Guo, Z.; Wang, Y.; Lei, J.; Yu, J. , Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy. Phytother. Res. 2018, 32, 2256–2263. [Google Scholar] [CrossRef]
- Yin, M. C.; Lin, C. C.; Wu, H. C.; Tsao, S. M.; Hsu, C. K. , Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: Potential mechanisms of action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Szaefer, H.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. , Tannic Acid: Specific Form of Tannins in Cancer Chemoprevention and Therapy-Old and New Applications. Curr. Pharmacol. Rep. 2020, 6, 28–37. [Google Scholar] [CrossRef]
- Bona, N. P.; Pedra, N. S.; Azambuja, J. H.; Soares, M. S. P.; Spohr, L.; Gelsleichter, N. E.; de M. Meine, B.; Sekine, F. G.; Mendonça, L. T.; de Oliveira, F. H.; Braganhol, E.; Spanevello, R. M.; da Silveira, E. F.; Stefanello, F. M. Tannic acid elicits selective antitumoral activity in vitro and inhibits cancer cell growth in a preclinical model of glioblastoma multiforme. Metab. Brain Dis. 2020, 35, 283–293. [Google Scholar] [CrossRef]
- Chariyarangsitham, W.; Krungchanuchat, S.; Khuemjun, P.; Pilapong, C. , Effect of advanced oxidation and amino acid addition on antioxidant capability, iron chelating property and anti-cancer activity of tannic acid. Arab. J. Chem. 2021, 14, 103312. [Google Scholar] [CrossRef]
- Chen, M. C.; Annseles Rajula, S.; Bharath Kumar, V.; Hsu, C. H.; Day, C. H.; Chen, R. J.; Wang, T. F.; Viswanadha, V. P.; Li, C. C.; Huang, C. Y. , Tannic acid attenuate AKT phosphorylation to inhibit UMUC3 bladder cancer cell proliferation. Mol. Cell. Biochem. 2022, 477, 2863–2869. [Google Scholar] [CrossRef]
- Hatami, E.; B Nagesh, P. K.; Sikander, M.; Dhasmana, A.; Chauhan, S. C.; Jaggi, M.; Yallapu, M. M. , Tannic Acid Exhibits Antiangiogenesis Activity in Nonsmall-Cell Lung Cancer Cells. ACS Omega 2022, 7, 23939–23949. [Google Scholar] [CrossRef]
- Nagesh, P. K. B.; Chowdhury, P.; Hatami, E.; Jain, S.; Dan, N.; Kashyap, V. K.; Chauhan, S. C.; Jaggi, M.; Yallapu, M. M. , Tannic acid inhibits lipid metabolism and induce ROS in prostate cancer cells. Sci. Rep. 2020, 10, 980. [Google Scholar] [CrossRef]
- Nagesh, P. K. B.; Hatami, E.; Chowdhury, P.; Kashyap, V. K.; Khan, S.; Hafeez, B. B.; Chauhan, S. C.; Jaggi, M.; Yallapu, M. M. , Tannic acid induces endoplasmic reticulum stress-mediated apoptosis in prostate cancer. Cancers (Basel) 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D. Y.; Kim, D. H.; Yoo, J. S.; Jo, E. S.; Rugamba, A.; Jang, K. J.; Yang, Y. M. , Tannic acid inhibits Non-small Cell Lung Cancer (NSCLC) stemness by inducing G0/G1 Cell cycle arrest and intrinsic apoptosis. Anticancer Res. 2020, 40, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ding, G. B.; Liu, W.; Fu, R.; Sajid, A.; Li, Z. , Tannic acid directly targets pyruvate kinase isoenzyme M2 to attenuate colon cancer cell proliferation. Food Funct. 2018, 9, 5547–5559. [Google Scholar] [CrossRef] [PubMed]
- Youness, R. A.; Kamel, R.; Elkasabgy, N. A.; Shao, P.; Farag, M. A. , Recent advances in tannic acid (gallotannin) anticancer activities and drug delivery systems for efficacy improvement; a comprehensive review. Molecules 2021, 25, 1486. [Google Scholar] [CrossRef]
- Cadoná, F. C.; Dantas, R. F.; de Mello, G. H.; Silva-Jr, F. P. , Natural products targeting into cancer hallmarks: An update on caffeine, theobromine, and (+)-catechin. Crit. Rev. Food Sci. Nutr. 2022, 62, 7222–7241. [Google Scholar] [CrossRef]
- Shojaei-Zarghani, S.; Rafraf, M.; Yari Khosroushahi, A.; Sheikh-Najafi, S. , Effectiveness of theobromine on inhibition of 1,2-dimethylhydrazine-induced rat colon cancer by suppression of the Akt/GSK3β/β-catenin signaling pathway. J. Funct. Foods 2020, 75, 104293. [Google Scholar] [CrossRef]
- Shojaei-Zarghani, S.; Yari Khosroushahi, A.; Rafraf, M. , Oncopreventive effects of theanine and theobromine on dimethylhydrazine-induced colon cancer model. Biomed. Pharmacother. 2021, 134, 111140. [Google Scholar] [CrossRef]
- De Souza Rosa, L.; Jordão, N. A.; Da Costa Pereira Soares, N.; De Mesquita, J. F.; Monteiro, M.; Teodoro, A. J. , Pharmacokinetic, antiproliferative and apoptotic effects of phenolic acids in human colon adenocarcinoma cells using in vitro and in silico approaches. Molecules 2018, 23, 2569. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, S.; Yang, S. , Vanillic acid suppresses HIF-1α expression via inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef]
- Velli, S. K.; Sundaram, J.; Murugan, M.; Balaraman, G.; Thiruvengadam, D. , Protective effect of vanillic acid against benzo(a)pyrene induced lung cancer in Swiss albino mice. J. Biochem. Mol. Toxicol. 2019, 33, e22382. [Google Scholar] [CrossRef]
- Wang, M.; Qi, Y.; Sun, Y. , Exploring the Antitumor Mechanisms of Zingiberis Rhizoma Combined with Coptidis Rhizoma Using a Network Pharmacology Approach. Biomed Res. Int. 2020, 2020, 8887982. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, X.; Zhu, Z.; Gong, Z.; Tang, W.; Hu, Y.; Cheng, C.; Wang, H.; Sarwar, A.; Chen, Y.; Liu, F.; Huo, J.; Wang, X.; Zhang, Y. , STING activation in macrophages by vanillic acid exhibits antineoplastic potential. Biochem. Pharmacol. 2023, 213, 115618. [Google Scholar] [CrossRef]
- Bezerra, D. P.; Soares, A. K. N.; De Sousa, D. P. , Overview of the role of vanillin on redox status and cancer development. Oxid. Med. Cell. Longev. 2016, 2016, 9734816. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Yazan, L. S.; Ismail, N.; Ismail, M. , Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009, 33, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Li, J. M.; Lee, Y. C.; Li, C. C.; Lo, H. Y.; Chen, F. Y.; Chen, Y. S.; Hsiang, C. Y.; Ho, T. Y. , Vanillin-Ameliorated Development of Azoxymethane/Dextran Sodium Sulfate-Induced Murine Colorectal Cancer: The Involvement of Proteasome/Nuclear Factor-κB/Mitogen-Activated Protein Kinase Pathways. J. Agric. Food Chem. 2018, 66, 5563–5573. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Kramb, J. P.; Suthiphongchai, T.; Surarit, R.; Srisomsap, C.; Dannhardt, G.; Svasti, J. , Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in Vivo. J. Agric. Food Chem. 2009, 57, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Kawasaki, N.; Choo, M. K.; Saitoh, Y.; Aozuka, Y.; Singhirunnusorn, P.; Ruchirawat, S.; Svasti, J.; Saiki, I. , Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur. J. Pharm. Sci. 2005, 25, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Naz, H.; Tarique, M.; Khan, P.; Luqman, S.; Ahamad, S.; Islam, A.; Ahmad, F.; Hassan, M. I. , Evidence of vanillin binding to CAMKIV explains the anti-cancer mechanism in human hepatic carcinoma and neuroblastoma cells. Mol. Cell. Biochem. 2018, 438, 35–45. [Google Scholar] [CrossRef]
- Ramadoss, D. P.; Sivalingam, N. , Vanillin extracted from Proso and Barnyard millets induce apoptotic cell death in HT-29 human colon cancer cell line. Nutr. Cancer 2020, 72, 1422–1437. [Google Scholar] [CrossRef]
- Srinual, S.; Chanvorachote, P.; Pongrakhananon, V. , Suppression of cancer stem-like phenotypes in NCI-H460 lung cancer cells by vanillin through an Akt-dependent pathway. Int. J. Oncol. 2017, 50, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.; Shamsi, A.; Queen, A.; Shahbaaz, M.; Khan, P.; Hussain, A.; Alajmi, M. F.; Rizwanul Haque, Q. M.; Imtaiyaz Hassan, M. , Targeting cyclin-dependent kinase 6 by vanillin inhibits proliferation of breast and lung cancer cells: Combined computational and biochemical studies. J. Cell. Biochem. 2021, 122, 897–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. C.; Chen, P. Y.; Hao, W. R.; Liu, Y. C.; Lyu, P. C.; Hong, H. J. , Cafestol Inhibits High-Glucose-Induced Cardiac Fibrosis in Cardiac Fibroblasts and Type 1-Like Diabetic Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 4503747. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, F. B.; Jeppesen, P. B.; Shokouh, P.; Laustsen, C.; Hermansen, K.; Gregersen, S. , Cafestol, a Bioactive Substance in Coffee, Has Antidiabetic Properties in KKAy Mice. J. Nat. Prod. 2017, 80, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O. M.; Adefegha, S. A.; Akinyemi, A. J.; Ademiluyi, A. O. , Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Chao, C. Y.; Mong, M. C.; Chan, K. C.; Yin, M. C. , Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jembrek, M. J. , Efficacy of Caffeic acid on diabetes and its complications in the mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef]
- Xu, W.; Luo, Q.; Wen, X.; Xiao, M.; Mei, Q. , Antioxidant and anti-diabetic effects of caffeic acid in a rat model of diabetes. Trop. J. Pharm. Res. 2020, 19, 1227–1232. [Google Scholar] [CrossRef]
- Yusuf, M.; Nasiruddin, M.; Sultana, N.; Badruddeen, *!!! REPLACE !!!*; Akhtar, J.; Khan, M. I.; Ahmad, M. Regulatory mechanism of caffeic acid on glucose metabolism in diabetes. Res. J. Pharm. Technol. 2019, 12, 4735–4740. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Muanprasat, C.; Pasachan, T.; Pongchaidecha, A.; Amornlerdpison, D.; Srimaroeng, C. , Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine 2019, 52, 187–197. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P. S. , The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef]
- Bagdas, D.; Etoz, B. C.; Gul, Z.; Ziyanok, S.; Inan, S.; Turacozen, O.; Gul, N. Y.; Topal, A.; Cinkilic, N.; Tas, S.; Ozyigit, M. O.; Gurun, M. S. , In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and cytotoxicity/genotoxicity profile. Food Chem. Toxicol. 2015, 81, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. , Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS One 2015, 10, e0120842. [Google Scholar] [CrossRef]
- Williamson, G. , Protection against developing type 2 diabetes by coffee consumption: Assessment of the role of chlorogenic acid and metabolites on glycaemic responses. Food Funct. 2020, 11, 4826–4833. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. , Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. (Lausanne) 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. , Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef] [PubMed]
- Topal, F. , Anticholinergic and antidiabetic effects of isoeugenol from clove (Eugenia caryophylata) oil. Int. J. Food Prop. 2019, 22, 583–592. [Google Scholar] [CrossRef]
- Park, J. E.; Kim, S. Y.; Han, J. S. , Scopoletin stimulates the secretion of insulin via a KATP channel-dependent pathway in INS-1 pancreatic β cells. J. Pharm. Pharmacol. 2022, 74, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Jang, J. H.; Park, J. E.; Han, J. S. , Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur. J. Pharmacol. 2018, 834, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Batra, G. K.; Anand, A.; Sharma, S.; Sharma, S.; Bhansali, S.; Patil, A. N. , Scopoletin Improves Glucose Homeostasis in the High-Fructose High-Fat Diet-Induced Diabetes Model in Wistar Rats. J. Med. Food 2023, 26, 270–274. [Google Scholar] [CrossRef]
- Liang, Y.; Zeng, X.; Guo, J.; Liu, H.; He, B.; Lai, R.; Zhu, Q.; Zheng, Z. , Scopoletin and umbelliferone from Cortex Mori as protective agents in high glucose-induced mesangial cell as in vitro model of diabetic glomerulosclerosis. Chin. J. Physiol. 2021, 64, 150–158. [Google Scholar] [PubMed]
- Verma, A.; Dewangan, P.; Kesharwani, D.; Kela, S. P. Hypoglycemic and hypolipidemic activity of scopoletin (coumarin derivative) in streptozotocin induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 79–83. [Google Scholar]
- Duangjai, A.; Nuengchamnong, N.; Suphrom, N.; Trisat, K.; Limpeanchob, N.; Saokaew, S. , Potential of coffee fruit extract and quinic acid on adipogenesis and lipolysis in 3T3-L1 adipocytes. Kobe J. Med. Sci. 2018, 64, E84–E92. [Google Scholar]
- AlEraky, D. M.; Abuohashish, H. M.; Gad, M. M.; Alshuyukh, M. H.; Bugshan, A. S.; Almulhim, K. S.; Mahmoud, M. M. , The Antifungal and Antibiofilm Activities of Caffeine against Candida albicans on Polymethyl Methacrylate Denture Base Material. Biomedicines 2022, 10, 2078. [Google Scholar] [CrossRef]
- Saracino, I. M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M. C.; Spisni, E. Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics 2022, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Biernasiuk, A.; Baj, T.; Malm, A. , Clove Essential Oil and Its Main Constituent, Eugenol, as Potential Natural Antifungals against Candida spp. Alone or in Combination with Other Antimycotics Due to Synergistic Interactions. Molecules 2023, 28, 215. [Google Scholar] [CrossRef]
- Cai, R.; Hu, M.; Zhang, Y.; Niu, C.; Yue, T.; Yuan, Y.; Wang, Z. , Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT 2019, 106, 50–56. [Google Scholar] [CrossRef]
- Gupta, P.; Mishra, P.; Mehra, L.; Rastogi, K.; Prasad, R.; Mittal, G.; Poluri, K. M. , Eugenol-acacia gum-based bifunctional nanofibers as a potent antifungal transdermal substitute. Nanomedicine 2021, 16, 2269–2289. [Google Scholar] [CrossRef]
- Hassanpour, P.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. , Antifungal activity of eugenol on Cryptococcus neoformans biological activity and Cxt1p gene expression. Curr. Med. Mycol. 2020, 6, 9–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Wu, X.; Jiang, M.; Jin, H.; Tao, K.; Hou, T. , Unraveling the polypharmacology of a natural antifungal product, eugenol, against Rhizoctonia solani. Pest Manag. Sci. 2021, 77, 3469–3483. [Google Scholar] [CrossRef]
- Goswami, L.; Gupta, L.; Paul, S.; Vermani, M.; Vijayaraghavan, P.; Bhattacharya, A. K. , Design and synthesis of eugenol/isoeugenol glycoconjugates and other analogues as antifungal agents against Aspergillus fumigatus. RSC Med. Chem. 2022, 13, 955–962. [Google Scholar] [CrossRef]
- Pinheiro, L. S.; Sousa, J. P.; Barreto, N. A.; Lima, A. L. A.; Dantas, T. B.; Pérez, A. L. A. L.; Menezes, C. P.; Abrantes, J. P.; Filho, A. A. O.; Lima, E. O. , Investigation of antifungal activity and mode of action of isoeugenol against strains of Cryptococcus neoformans. Lat. Am. J. Pharm. 2017, 36, 2220–2225. [Google Scholar]
- Cui, W.; Du, K. Y.; Ling, Y. X.; Yang, C. J. , Activity of eugenol derivatives against Fusarium graminearum Q1 strain and screening of isoeugenol mixtures. J. Plant Pathol. 2021, 103, 915–921. [Google Scholar] [CrossRef]
- Medeiros, D.; Oliveira-Júnior, J.; Nóbrega, J.; Cordeiro, L.; Jardim, J.; Souza, H.; Silva, G.; Athayde-Filho, P.; Barbosa-Filho, J.; Scotti, L.; Lima, E. , Isoeugenol and hybrid acetamides against candida albicans isolated from the oral cavity. Pharmaceuticals 2020, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D. J.; Stratford, M.; Gasson, M. J.; Narbad, A. , Structure-function analysis of the vanillin molecule and its antifungal properties. J. Agric. Food Chem. 2005, 53, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Xie, Y.; Zhong, Y. , o-Vanillin, a promising antifungal agent, inhibits Aspergillus flavus by disrupting the integrity of cell walls and cell membranes. Appl. Microbiol. Biotechnol. 2021, 105, 5147–5158. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Zhao, Y.; Xie, Y. , The antifungal activity of o-vanillin against Aspergillus flavus via disrupting ergosterol biosynthesis and promoting oxidative stress, and an RNA-seq analysis thereof. LWT 2022, 164, 113635. [Google Scholar] [CrossRef]
- Weng, W. T.; Kuo, P. C.; Brown, D. A.; Scofield, B. A.; Furnas, D.; Paraiso, H. C.; Wang, P. Y.; Yu, I. C.; Yen, J. H. , 4-Ethylguaiacol modulates neuroinflammation and Th1/Th17 differentiation to ameliorate disease severity in experimental autoimmune encephalomyelitis. J. Neuroinflammation 2021, 18, 110. [Google Scholar] [CrossRef]
- Weng, W. T.; Kuo, P. C.; Scofield, B. A.; Paraiso, H. C.; Brown, D. A.; Yu, I. C.; Yen, J. H. , 4-Ethylguaiacol Modulates Neuroinflammation and Promotes Heme Oxygenase-1 Expression to Ameliorate Brain Injury in Ischemic Stroke. Front. Immunol. 2022, 13, 887000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D. R.; Jiang, Y. S.; Sun, J. Y.; Li, H. H.; Luo, X. L.; Zhao, M. M. , Anti-inflammatory Mechanism Involved in 4-Ethylguaiacol-Mediated Inhibition of LPS-Induced Inflammation in THP-1 Cells. J. Agric. Food Chem. 2019, 67, 1230–1243. [Google Scholar] [CrossRef]
- Rahimi, M. R.; Semenova, E. A.; Larin, A. K.; Kulemin, N. A.; Generozov, E. V.; Łubkowska, B.; Ahmetov, I. I.; Golpasandi, H. , The ADORA2A TT Genotype Is Associated with Anti-Inflammatory Effects of Caffeine in Response to Resistance Exercise and Habitual Coffee Intake. Nutrients 2023, 15, 1634. [Google Scholar] [CrossRef]
- Mei, S.; Chen, X. , Combination of HPLC–orbitrap-MS/MS and network pharmacology to identify the anti-inflammatory phytochemicals in the coffee leaf extracts. Food Front. 2023. [Google Scholar] [CrossRef]
- Lu, R.; Wang, Y. G.; Qu, Y.; Wang, S. X.; Peng, C.; You, H.; Zhu, W.; Chen, A. , Dihydrocaffeic acid improves IL-1β-induced inflammation and cartilage degradation via inhibiting NF-κB and MAPK signalling pathways. Bone Joint Res. 2023, 12, 259–273. [Google Scholar] [CrossRef]
- Mateen, S.; Rehman, M. T.; Shahzad, S.; Naeem, S. S.; Faizy, A. F.; Khan, A. Q.; Khan, M. S.; Husain, F. M.; Moin, S. , Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur. J. Pharmacol. 2019, 852, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Barboza, J. N.; da Silva Maia Bezerra Filho, C.; Silva, R. O.; Medeiros, J. V. R.; de Sousa, D. P. , An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- De Araújo Lopes, A.; Da Fonseca, F. N.; Rocha, T. M.; De Freitas, L. B.; Araújo, E. V. O.; Wong, D. V. T.; Júnior, R. C. P. L.; Leal, L. K. A. M. , Eugenol as a promising molecule for the treatment of dermatitis: Antioxidant and anti-inflammatory activities and its nanoformulation. Oxid. Med. Cell. Longev. 2018, 2018, 8194849. [Google Scholar] [CrossRef] [PubMed]
- El-kady, A. M.; Ahmad, A. A.; Hassan, T. M.; El-Deek, H. E. M.; Fouad, S. S.; Althagfan, S. S. , Eugenol, a potential schistosomicidal agent with anti-inflammatory and antifibrotic effects against Schistosoma mansoni, induced liver pathology. Infect. Drug Resist. 2019, 12, 709–719. [Google Scholar] [CrossRef]
- Esmaeili, F.; Rajabnejhad, S.; Partoazar, A. R.; Mehr, S. E.; Faridi-Majidi, R.; Sahebgharani, M.; Syedmoradi, L.; Rajabnejhad, M. R.; Amani, A. , Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm. Dev. Technol. 2016, 21, 887–893. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Lu, Y.; Ma, C. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. Int. Immunopharmacol. 2015, 26, 264–271. [Google Scholar] [CrossRef]
- Mir, S. M.; Ravuri, H. G.; Pradhan, R. K.; Narra, S.; Kumar, J. M.; Kuncha, M.; Kanjilal, S.; Sistla, R. , Ferulic acid protects lipopolysaccharide-induced acute kidney injury by suppressing inflammatory events and upregulating antioxidant defenses in Balb/c mice. Biomed. Pharmacother. 2018, 100, 304–315. [Google Scholar] [CrossRef]
- Yin, Z. N.; Wu, W. J.; Sun, C. Z.; Liu, H. F.; Chen, W. B.; Zhan, Q. P.; Lei, Z. G.; Xin, X.; Ma, J. J.; Yao, K.; Min, T.; Zhang, M. M.; Wu, H. , Antioxidant and Anti-inflammatory Capacity of Ferulic Acid Released from Wheat Bran by Solid-state Fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [PubMed]
- Funakoshi-Tago, M.; Matsutaka, M.; Hokimoto, S.; Kobata, K.; Tago, K.; Tamura, H. , Coffee ingredients, hydroquinone, pyrocatechol, and 4-ethylcatechol exhibit anti-inflammatory activity through inhibiting NF-κB and activating Nrf2. J. Funct. Foods 2022, 90, 104980. [Google Scholar] [CrossRef]
- Abazari, M. F.; Nasiri, N.; Karizi, S. Z.; Nejati, F.; Haghiaminjan, H.; Norouzi, S.; Piri, P.; Estakhr, L.; Faradonbeh, D. R.; Kohandani, M.; Daliri, K.; Sanadgol, N.; Askari, H. An updated review of various medicinal applications of p-coumaric acid: From antioxidative and anti-inflammatory properties to effects on cell cycle and proliferation. Mini-Rev. Med. Chem. 2021, 21, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E. C. O.; Dos Santos, F. M.; Ribeiro, A. R. B.; De Souza, S. T.; Barreto, E.; Da Silva Fonseca, E. J. , Drug-induced anti-inflammatory response in A549 cells, as detected by Raman spectroscopy: A comparative analysis of the actions of dexamethasone and: p -coumaric acid. Analyst 2019, 144, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Rho, H. S.; Choi, K. , Anti-inflammatory Effects of a P-coumaric Acid and Kojic Acid Derivative in LPS-stimulated RAW264.7 Macrophage Cells. Biotechnol. Bioprocess Eng. 2019, 24, 653–657. [Google Scholar] [CrossRef]
- Moradi, M.; Farbood, Y.; Mard, S. A.; Dianat, M.; Goudarzi, G.; Khorsandi, L.; Seyedian, S. S. , p-Coumaric acid has pure anti-inflammatory characteristics against hepatopathy caused by ischemia-reperfusion in the liver and dust exposure. Iran. J. Basic Med. Sci. 2022, 26, 164–175. [Google Scholar]
- Pragasam, S. J.; Venkatesan, V.; Rasool, M. , Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Venkatesan, A.; Samy, J. V. R. A.; Balakrishnan, K.; Natesan, V.; Kim, S. J. , In vitro Antioxidant, Anti-inflammatory, Antimicrobial, and Antidiabetic Activities of Synthesized Chitosan-loaded p-Coumaric Acid Nanoparticles. Curr. Pharm. Biotechnol. 2023, 24, 1178–1194. [Google Scholar] [CrossRef]
- Allen, S.; Khattab, A.; Vassallo, M.; Kwan, J. , Inflammation and muscle weakness in COPD: Considering a renewed role for Theophylline? Curr. Respir. Med. Rev. 2018, 14, 35–41. [Google Scholar] [CrossRef]
- Bin, Y.; Xiao, Y.; Huang, D.; Ma, Z.; Liang, Y.; Bai, J.; Zhang, W.; Liang, Q.; Zhang, J.; Zhong, X.; He, Z. , Theophylline inhibits cigarette smoke-induced inflammation in skeletal muscle by upregulating hdac2 expression and decreasing nf-κb activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L197–L205. [Google Scholar] [CrossRef] [PubMed]
- Eid, N. S.; O’Hagan, A.; Bickel, S.; Morton, R.; Jacobson, S.; Myers, J. A. , Anti-inflammatory dosing of theophylline in the treatment of status asthmaticus in children. J. Asthma Allergy 2016, 9, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Talmon, M.; Massara, E.; Brunini, C.; Fresu, L. G. , Comparison of anti-inflammatory mechanisms between doxofylline and theophylline in human monocytes. Pulm. Pharmacol. Ther. 2019, 59, 101851. [Google Scholar] [CrossRef] [PubMed]
- Urbanova, A.; Kertys, M.; Simekova, M.; Mikolka, P.; Kosutova, P.; Mokra, D.; Mokry, J. , Bronchodilator and anti-inflammatory action of theophylline in a model of ovalbumin-induced allergic inflammation. Adv. Exp. Med. Biol. 2016, 935, 53–62. [Google Scholar] [PubMed]
- Bains, M.; Kaur, J.; Akhtar, A.; Kuhad, A.; Sah, S. P. , Anti-inflammatory effects of ellagic acid and vanillic acid against quinolinic acid-induced rat model of Huntington's disease by targeting IKK-NF-κB pathway. Eur. J. Pharmacol. 2022, 934, 175316. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Carvalho, T. T.; Hohmann, M. S. N.; Pinho-Ribeiro, F. A.; Fattori, V.; Manchope, M. F.; Zarpelon, A. C.; Baracat, M. M.; Georgetti, S. R.; Casagrande, R.; Verri, W. A. , Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; He, J. , Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef]
- Ibrahim, S. S.; Abd-allah, H. , “Spanlastic nanovesicles for enhanced ocular delivery of vanillic acid: design, in vitro characterization, and in vivo anti-inflammatory evaluation”. Int. J. Pharm. 2022, 625, 122068. [Google Scholar] [CrossRef] [PubMed]
- Kim, M. C.; Kim, S. J.; Kim, D. S.; Jeon, Y. D.; Park, S. J.; Lee, H. S.; Um, J. Y.; Hong, S. H. , Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Z.; Zhang, L.; Li, X.; Xu, B.; Xiao, Y.; Shi, X.; Zhang, H.; Liao, T.; Wang, P. , Vanillic Acid Reduces Pain-Related Behavior in Knee Osteoarthritis Rats Through the Inhibition of NLRP3 Inflammasome-Related Synovitis. Front. Pharmacol. 2021, 11, 599022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Y. , Vanillic acid alleviates lipopolysaccharides-induced endoplasmic reticulum stress and inflammation in human lung fibroblasts by `inhibiting MAPK and NF-κB pathways. Qual. Assur. Saf. Crop. Food 2022, 14, 55–63. [Google Scholar] [CrossRef]
- Ziadlou, R.; Barbero, A.; Martin, I.; Wang, X.; Qin, L.; Alini, M.; Grad, S. , Anti-inflammatory and chondroprotective effects of vanillic acid and epimedin C in human osteoarthritic chondrocytes. Biomolecules 2020, 10, 932. [Google Scholar] [CrossRef]
- Bezerra-Filho, C. S. M.; Barboza, J. N.; Souza, M. T. S.; Sabry, P.; Ismail, N. S. M.; de Sousa, D. P. Therapeutic potential of vanillin and its main metabolites to regulate the inflammatory response and oxidative stress. Mini-Rev. Med. Chem. 2019, 19, 1681–1693. [Google Scholar] [CrossRef]
- Cheng, H. M.; Chen, F. Y.; Li, C. C.; Lo, H. Y.; Liao, Y. F.; Ho, T. Y.; Hsiang, C. Y. , Oral Administration of Vanillin Improves Imiquimod-Induced Psoriatic Skin Inflammation in Mice. J. Agric. Food Chem. 2017, 65, 10233–10242. [Google Scholar] [CrossRef]
- Costantini, E.; Sinjari, B.; Falasca, K.; Reale, M.; Caputi, S.; Jagarlapodii, S.; Murmura, G. , Assessment of the Vanillin Anti-Inflammatory and Regenerative Potentials in Inflamed Primary Human Gingival Fibroblast. Mediators Inflamm. 2021, 2021, 5562340. [Google Scholar] [CrossRef]
- Kim, M. E.; Na, J. Y.; Park, Y. D.; Lee, J. S. , Anti-Neuroinflammatory Effects of Vanillin Through the Regulation of Inflammatory Factors and NF-κB Signaling in LPS-Stimulated Microglia. Appl. Biochem. Biotechnol. 2019, 187, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.; Li, J.; Xu, C.; Jiang, W. , Vanillin Attenuates Cadmium-Induced Lung Injury Through Inhibition of Inflammation and Lung Barrier Dysfunction Through Activating AhR. Inflammation 2021, 44, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, D. F.; Zhang, X. Y.; Liu, D.; Xu, S. Y.; Chen, G. X.; Huang, B. X.; Ren, W. Z.; Wang, W.; Fu, S. P.; Liu, J. X. , Vanillin protects dopaminergic neurons against inflammation-mediated cell death by inhibiting ERK1/2, P38 and the NF-κB signaling pathway. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, Y.; Sun, J.; Li, H.; Huang, M.; Sun, X.; Zhao, M. , Elucidation of The Anti-Inflammatory Effect of Vanillin In Lps-Activated THP-1 Cells. J. Food Sci. 2019, 84, 1920–1928. [Google Scholar] [CrossRef]
- Jung, H. J.; Song, Y. S.; Lim, C. J.; Park, E. H. , Anti-angiogenic, anti-inflammatory and anti-nociceptive activities of vanillyl alcohol. Arch. Pharmacal Res. 2008, 31, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Cho, A. S.; Jeon, S. M.; Kim, M. J.; Yeo, J.; Seo, K. I.; Choi, M. S.; Lee, M. K. , Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zheng, S.; Sheng, Y.; Miao, T.; Xu, J.; Xu, W.; Huang, K.; Zhao, C. , Chlorogenic acid ameliorates obesity by preventing energy balance shift in high-fat diet induced obese mice. J. Sci. Food Agric. 2021, 101, 631–637. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Iqbal, M. S.; Srivastava, J. K. , Therapeutic promises of chlorogenic acid with special emphasis on its anti-obesity property. Curr. Mol. Pharmacol. 2020, 13, 7–16. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, K. L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. , Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Baek, J. H.; Kim, N. J.; Song, J. K.; Chun, K. H. , Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (AMPK). BMB Rep. 2017, 50, 566–571. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Park, C. S.; Park, Y. , Kahweol Reduces Food Intake of Caenorhabditis elegans. J. Agric. Food Chem. 2020, 68, 9683–9689. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. S.; Lee, S. G.; Kang, Y. J.; kwon, T. K.; Nam, J. O. , Kahweol inhibits adipogenesis of 3T3-L1 adipocytes through downregulation of PPARγ. Nat. Prod. Res. 2018, 32, 1216–1219. [Google Scholar] [CrossRef]
- Agunloye, O. M.; Oboh, G.; Ademiluyi, A. O.; Ademosun, A. O.; Akindahunsi, A. A.; Oyagbemi, A. A.; Omobowale, T. O.; Ajibade, T. O.; Adedapo, A. A. , Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Bıçakçı, N.; Karaboğa, İ.; Dökmeci, A. H.; Güzel, S.; Fidanol Erboğa, Z. , Cardioprotective effect of caffeic acid phenethyl ester on cardiac contusion following blunt chest trauma in rats. Biotechnic and Histochemistry 2019, 94, 442–448. [Google Scholar] [CrossRef]
- Kumaran, K. S.; Prince, P. S. M. , Protective effect of caffeic acid on cardiac markers and lipid peroxide metabolism in cardiotoxic rats: An in vivo and in vitro study. Metabolism 2010, 59, 1172–1180. [Google Scholar] [CrossRef]
- Salau, V. F.; Erukainure, O. L.; Islam, M. S. , Caffeic Acid Protects against Iron-Induced Cardiotoxicity by Suppressing Angiotensin-Converting Enzyme Activity and Modulating Lipid Spectrum, Gluconeogenesis and Nucleotide Hydrolyzing Enzyme Activities. Biol. Trace Elem. Res. 2021, 199, 1052–1061. [Google Scholar] [CrossRef]
- Silva, H.; Lopes, N. M. F. , Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef]
- Banitalebi, E.; Rahimi, A.; Faramarzi, M.; Mardaniyan Ghahfarrokhi, M. , The effects of elastic resistance band training and green coffee bean extract supplement on novel combined indices of cardiometabolic risk in obese women. Res. Pharm. Sci. 2019, 14, 414–423. [Google Scholar] [CrossRef]
- Caro-Gómez, E.; Sierra, J. A.; Escobar, J. S.; Álvarez-Quintero, R.; Naranjo, M.; Medina, S.; Velásquez-Mejía, E. P.; Tabares-Guevara, J. H.; Jaramillo, J. C.; León-Varela, Y. M.; Muñoz-Durango, K.; Ramírez-Pineda, J. R. , Green coffee extract improves cardiometabolic parameters and modulates gut microbiota in high-fat-diet-fed ApoE -/- mice. Nutrients 2019, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guzmán, O. J.; Álvarez, R.; Muñoz-Durango, K. , Changes in the plasma lipidome of healthy subjects after coffee consumption reveal potential cardiovascular benefits: A randomized controlled trial. Free Radic. Biol. Med. 2021, 176, 345–355. [Google Scholar] [CrossRef]
- Suzuki, A.; Nomura, T.; Jokura, H.; Kitamura, N.; Saiki, A.; Fujii, A. , Chlorogenic acid-enriched green coffee bean extract affects arterial stiffness assessed by the cardio-ankle vascular index in healthy men: a pilot study. Int. J. Food Sci. Nutr. 2019, 70, 901–908. [Google Scholar] [CrossRef]
- Baeza, G.; Bachmair, E. M.; Wood, S.; Mateos, R.; Bravo, L.; De Roos, B. , The colonic metabolites dihydrocaffeic acid and dihydroferulic acid are more effective inhibitors of in vitro platelet activation than their phenolic precursors. Food Funct. 2017, 8, 1333–1342. [Google Scholar] [CrossRef]
- Alam, M. A.; Sernia, C.; Brown, L. , Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J. Cardiovasc. Pharmacol. 2013, 61, 240–249. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; Song, M.; Fang, Z.; Zhang, L.; Coffie, J. W.; Zhang, L.; Ma, L.; Wang, Q.; Yang, W.; Fang, L.; Wang, S.; Gao, X.; Wang, H. , Ferulic acid protects cardiomyocytes from TNF-α/cycloheximide-induced apoptosis by regulating autophagy. Arch. Pharmacal Res. 2020, 43, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Monceaux, K.; Gressette, M.; Karoui, A.; Pires Da Silva, J.; Piquereau, J.; Ventura-Clapier, R.; Garnier, A.; Mericskay, M.; Lemaire, C. , Ferulic Acid, Pterostilbene, and Tyrosol Protect the Heart from ER-Stress-Induced Injury by Activating SIRT1-Dependent Deacetylation of eIF2α. Int. J. Mol. Sci. 2022, 23, 6628. [Google Scholar] [CrossRef] [PubMed]
- Neto-Neves, E. M.; Filho, C. D. S. M. B.; Dejani, N. N.; de Sousa, D. P. Ferulic acid and cardiovascular health: Therapeutic and preventive potential. Mini-Rev. Med. Chem. 2021, 21, 1625–1637. [Google Scholar] [CrossRef]
- Pandi, A.; Raghu, M. H.; Chandrashekar, N.; Kalappan, V. M. Cardioprotective effects of Ferulic acid against various drugs and toxic agents. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 92. [Google Scholar] [CrossRef]
- Salau, V. F.; Erukainure, O. L.; Olofinsan, K. A.; Msomi, N. Z.; Ijomone, O. K.; Islam, M. S. , Ferulic acid mitigates diabetic cardiomyopathy via modulation of metabolic abnormalities in cardiac tissues of diabetic rats. Fundam. Clin. Pharmacol. 2023, 37, 44–59. [Google Scholar] [CrossRef]
- Zhang, X. X.; Zhao, D. S.; Wang, J.; Zhou, H.; Wang, L.; Mao, J. L.; He, J. X. , The treatment of cardiovascular diseases: A review of ferulic acid and its derivatives. Pharmazie 2021, 76, 55–60. [Google Scholar]
- Xing, D.; Yoo, C.; Gonzalez, D.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Leonard, M.; Ko, J.; Faries, M.; Kephart, W.; Purpura, M.; Jäger, R.; Wells, S. D.; Sowinski, R.; Rasmussen, C. J.; Kreider, R. B. , Dose-response of paraxanthine on cognitive function: A double blind, placebo controlled, crossover trial. Nutrients 2021, 13, 4478. [Google Scholar] [CrossRef]
- Yoo, C.; Xing, D.; Gonzalez, D.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Leonard, M.; Ko, J.; Faries, M.; Kephart, W.; Purpura, M.; Jäger, R.; Wells, S. D.; Sowinski, R.; Rasmussen, C. J.; Kreider, R. B. , Acute paraxanthine ingestion improves cognition and short-term memory and helps sustain attention in a double-blind, placebo-controlled, crossover trial. Nutrients 2021, 13, 3980. [Google Scholar] [CrossRef]
- Choi, J. R.; Kim, J. H.; Lee, S.; Cho, E. J.; Kim, H. Y. , Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer's disease mouse model. Food Chem. Toxicol. 2020, 144, 111571. [Google Scholar] [CrossRef]
- Muley, M. M.; Thakare, V. N.; Patil, R. R.; Bafna, P. A.; Naik, S. R. , Amelioration of cognitive, motor and endogenous defense functions with silymarin, piracetam and protocatechuic acid in the cerebral global ischemic rat model. Life Sci. 2013, 93, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cui, T.; Xie, N.; Zhang, X.; Qian, Z.; Liu, J. , Protocatechuic acid improves cognitive deficits and attenuates amyloid deposits, inflammatory response in aged AβPP/PS1 double transgenic mice. Int. Immunopharmacol. 2014, 20, 276–281. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, X.; Lv, C.; Li, C.; Yu, Y.; Wang, X.; Han, F. , Protocatechuic acid ameliorates neurocognitive functions impairment induced by chronic intermittent hypoxia. Sci. Rep. 2015, 5, 14507. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ge, W.; Peng, C.; Guo, J.; Chen, N.; He, L. , Association between Dietary Theobromine and Cognitive Function in a Representative American Population: A Cross-Sectional Study. J. Prev. Alzheimers Dis. 2022, 9, 449–457. [Google Scholar] [CrossRef]
- Mendiola-Precoma, J.; Padilla, K.; Rodríguez-Cruz, A.; Berumen, L. C.; Miledi, R.; García-Alcocer, G. Theobromine-induced changes in A1 purinergic receptor gene expression and distribution in a rat brain Alzheimer's disease model. J. Alzheimer’s Dis. 2017, 55, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Singh, J. C. H.; Kakalij, R. M.; Kshirsagar, R. P.; Kumar, B. H.; Komakula, S. S. B.; Diwan, P. V. , Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharm. Biol. 2015, 53, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Ul Amin, F.; Shah, S. A.; Kim, M. O. , Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef]
- Al Asmari, A.; Al Shahrani, H.; Al Masri, N.; Al Faraidi, A.; Elfaki, I.; Arshaduddin, M. , Vanillin abrogates ethanol induced gastric injury in rats via modulation of gastric secretion, oxidative stress and inflammation. Toxicol. Rep. 2016, 3, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ciciliato, M. P.; de Souza, M. C.; Tarran, C. M.; de Castilho, A. L. T.; Vieira, A. J.; Rozza, A. L. , Anti-Inflammatory Effect of Vanillin Protects the Stomach against Ulcer Formation. Pharmaceutics 2022, 14, 755. [Google Scholar] [CrossRef]
- Mu, H. N.; Li, Q.; Fan, J. Y.; Pan, C. S.; Liu, Y. Y.; Yan, L.; Sun, K.; Hu, B. H.; Huang, D. D.; Zhao, X. R.; Chang, X.; Wang, C. S.; He, S. Y.; He, K.; Yang, B. X.; Han, J. Y. , Caffeic acid attenuates rat liver injury after transplantation involving PDIA3-dependent regulation of NADPH oxidase. Free Radic. Biol. Med. 2018, 129, 202–214. [Google Scholar] [CrossRef]
- Mu, H. N.; Zhou, Q.; Yang, R. Y.; Tang, W. Q.; Li, H. X.; Wang, S. M.; Li, J.; Chen, W. X.; Dong, J. , Caffeic acid prevents non-alcoholic fatty liver disease induced by a high-fat diet through gut microbiota modulation in mice. Food Res. Int. 2021, 143, 110240. [Google Scholar] [CrossRef]
- Pang, C.; Shi, L.; Sheng, Y.; Zheng, Z.; Wei, H.; Wang, Z.; Ji, L. , Caffeic acid attenuated acetaminophen-induced hepatotoxicity by inhibiting ERK1/2-mediated early growth response-1 transcriptional activation. Chem. Biol. Interact. 2016, 260, 186–195. [Google Scholar] [CrossRef]
- Pang, C.; Zheng, Z.; Shi, L.; Sheng, Y.; Wei, H.; Wang, Z.; Ji, L. , Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Biol. Med. 2016, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O. A. , About coffee, cappuccino and connective tissue growth factor-Or how to protect your liver!? Environ. Toxicol. Pharmacol. 2009, 28, 1. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O. A.; Lahme, B.; Siluschek, M.; Gressner, A. M. , Identification of paraxanthine as the most potent caffeine-derived inhibitor of connective tissue growth factor expression in liver parenchymal cells. Liver Int. 2009, 29, 886–897. [Google Scholar] [CrossRef]
- Klemmer, I.; Yagi, S.; Gressner, O. A. , Oral application of 1,7-dimethylxanthine (paraxanthine) attenuates the formation of experimental cholestatic liver fibrosis. Hepatol. Res. 2011, 41, 1094–1109. [Google Scholar] [CrossRef]
- Wei, D.; Wu, S.; Liu, J.; Zhang, X.; Guan, X.; Gao, L.; Xu, Z. , Theobromine ameliorates nonalcoholic fatty liver disease by regulating hepatic lipid metabolism via mtor signaling pathway in vivo and in vitro. Can. J. Physiol. Pharmacol. 2021, 99, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, H.; Driss, D.; Ben Amara, I.; Boudawara, O.; Boudawara, T.; Ellouz Chaabouni, S.; Mounir Zeghal, K.; Hakim, A. , Altered hepatic mRNA expression of immune response-associated DNA damage in mice liver induced by potassium bromate: Protective role of vanillin. Environ. Toxicol. 2016, 31, 1796–1807. [Google Scholar] [CrossRef]
- Ghanim, A. M. H.; Younis, N. S.; Metwaly, H. A. , Vanillin augments liver regeneration effectively in hioacetamide induced liver fibrosis rat model. Life Sci. 2021, 286, 120036. [Google Scholar] [CrossRef]
- Liang, J. A.; Wu, S. L.; Lo, H. Y.; Hsiang, C. Y.; Ho, T. Y. , Vanillin inhibits matrix metalloproteinase-9 expression through down-regulation of nuclear factor-κB signaling pathway in human hepatocellular carcinoma cells. Mol. Pharmacol. 2009, 75, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Makni, M.; Chtourou, Y.; Fetoui, H.; Garoui, E. M.; Boudawara, T.; Zeghal, N. , Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur. J. Pharmacol. 2011, 668, 133–139. [Google Scholar] [CrossRef]
- Abdelrahman, R. S.; El-Tanbouly, G. S. , Protocatechuic acid protects against thioacetamide-induced chronic liver injury and encephalopathy in mice via modulating mTOR, p53 and the IL-6/ IL-17/ IL-23 immunoinflammatory pathway. Toxicol. Appl. Pharmacol. 2022, 440, 115931. [Google Scholar] [CrossRef]
- Salama, A.; Elgohary, R.; Amin, M. M.; Elwahab, S. A. , Immunomodulatory effect of protocatechuic acid on cyclophosphamide induced brain injury in rat: Modulation of inflammosomes NLRP3 and SIRT1. Eur. J. Pharmacol. 2022, 932, 175217. [Google Scholar] [CrossRef]
- Adeyanju, A. A.; Molehin, O. R.; Asejeje, F. O.; Oyenuga, V.; Etokakpan, R. U. , Protocatechuic acid through modulation of signaling pathways and oxidative stress exerts protective effects in rat model of carbon tetrachloride-induced renal and reproductive toxicities. Comp. Clin. Pathol. 2022, 31, 465–474. [Google Scholar] [CrossRef]
- Kassab, R. B.; Theyab, A.; Al-Ghamdy, A. O.; Algahtani, M.; Mufti, A. H.; Alsharif, K. F.; Abdella, E. M.; Habotta, O. A.; Omran, M. M.; Lokman, M. S.; Bauomy, A. A.; Albrakati, A.; Baty, R. S.; Hassan, K. E.; Alshiekheid, M. A.; Abdel Moneim, A. E.; Elmasry, H. A. , Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ. Sci. Pollut. Res. 2022, 29, 12208–12221. [Google Scholar] [CrossRef]
- Lin, C. Y.; Tsai, S. J.; Huang, C. S.; Yin, M. C. , Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice. J. Agric. Food Chem. 2011, 59, 5117–5124. [Google Scholar] [CrossRef]
- Salama, A. A. A.; Elgohary, R.; Fahmy, M. I. , Protocatechuic acid ameliorates lipopolysaccharide-induced kidney damage in mice via downregulation of TLR-4-mediated IKBKB/NF-κB and MAPK/Erk signaling pathways. J. Appl. Toxicol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, N.; Park, J. Y.; Lee, S.; Cho, E. J.; Lee, S.; Kang, K. S.; Hwang, G. S.; Kim, S. N.; Kim, H. Y.; Shibamoto, T. , Protective effects of protocatechuic acid against cisplatin-induced renal damage in rats. J. Funct. Foods 2015, 19, 20–27. [Google Scholar] [CrossRef]
- Chattaraj, K. G.; Paul, S. , Inclusion of Theobromine Modifies Uric Acid Aggregation with Possible Changes in Melamine-Uric Acid Clusters Responsible for Kidney Stones. J. Phys. Chem. B 2019, 123, 10483–10504. [Google Scholar] [CrossRef]
- Hernandez, Y.; Costa-Bauza, A.; Calvó, P.; Benejam, J.; Sanchis, P.; Grases, F. Comparison of two dietary supplements for treatment of uric acid renal lithiasis: Citrate vs. citrate + theobromine. Nutrients 2020, 12, 2012. [Google Scholar] [CrossRef]
- Julià, F.; Costa-Bauza, A.; Berga, F.; Grases, F. , Effect of theobromine on dissolution of uric acid kidney stones. World J. Urol. 2022, 40, 2105–2111. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Silva, K. C.; Peixoto, E. B. M. I.; Borges, C. M.; de Faria, J. M. L.; de Faria, J. B. L. , Theobromine increases NAD+/Sirt-1 activity and protects the kidney under diabetic conditions. Am. J. Physiol. Ren. Physiol. 2015, 308, F209–F225. [Google Scholar] [CrossRef] [PubMed]
- Prediger, R. D. S. Effects of caffeine in Parkinson's disease: From neuroprotection to the management of motor and non-motor symptoms. J. Alzheimer’s Dis. 2010, 20, S205–S220. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G. W.; Cao, C. Caffeine and coffee as therapeutics against Alzheimer's disease. J. Alzheimer’s Dis. 2010, 20, S117–S126. [Google Scholar] [CrossRef]
- Chu, Y. F.; Chang, W. H.; Black, R. M.; Liu, J. R.; Sompol, P.; Chen, Y.; Wei, H.; Zhao, Q.; Cheng, I. H. , Crude caffeine reduces memory impairment and amyloid β1-42 levels in an Alzheimer's mouse model. Food Chem. 2012, 135, 2095–2102. [Google Scholar] [CrossRef]
- Di Martino, E.; Bocchetta, E.; Tsuji, S.; Mukai, T.; Harris, R. A.; Blomgren, K.; Ådén, U. , Defining a Time Window for Neuroprotection and Glia Modulation by Caffeine After Neonatal Hypoxia-Ischaemia. Mol. Neurobiol. 2020, 57, 2194–2205. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Strauß, E.; Schlör, A.; Sifringer, M.; Scheuer, T.; Bührer, C.; Schmitz, T. , Neuroprotection by caffeine in hyperoxia-induced neonatal brain injury. Int. J. Mol. Sci. 2017, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, M. R.; Emamghoreishi, M.; Amiri, A.; Keshavarz, M. , Neuroprotective effects of caffeine against beta-amyliod neurotoxicity: The involvement of glycogen synthase kinase-3β protein. Physiol. Pharmacol. (Iran) 2019, 23, 150–153. [Google Scholar]
- Ikram, M.; Park, T. J.; Ali, T.; Kim, M. O. , Antioxidant and neuroprotective effects of caffeine against Alzheimer’s and parkinson’s disease: Insight into the role of Nrf-2 and A2AR signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef]
- Karuppagounder, S. S.; Uthaythas, S.; Govindarajulu, M.; Ramesh, S.; Parameshwaran, K.; Dhanasekaran, M. , Caffeine, a natural methylxanthine nutraceutical, exerts dopaminergic neuroprotection. Neurochem. Int. 2021, 148, 105066. [Google Scholar] [CrossRef] [PubMed]
- Khadrawy, Y. A.; Salem, A. M.; El-Shamy, K. A.; Ahmed, E. K.; Fadl, N. N.; Hosny, E. N. , Neuroprotective and Therapeutic Effect of Caffeine on the Rat Model of Parkinson’s Disease Induced by Rotenone. J. Diet. Suppl. 2017, 14, 553–572. [Google Scholar] [CrossRef]
- Kolahdouzan, M.; Hamadeh, M. J. , The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Figueiredo, D.; Nascimento, A. A.; Cunha-Rodrigues, M. C.; Brito, R.; Calaza, K. C. Caffeine and Its Neuroprotective Role in Ischemic Events: A Mechanism Dependent on Adenosine Receptors. Cell. Mol. Neurobiol. 2022, 42, 1693–1725. [Google Scholar] [CrossRef]
- Xu, K.; Di Luca, D. G.; Orrú, M.; Xu, Y.; Chen, J. F.; Schwarzschild, M. A. , Neuroprotection by caffeine in the MPTP model of parkinson's disease and its dependence on adenosine A2A receptors. Neuroscience 2016, 322, 129–137. [Google Scholar] [CrossRef]
- Xu, K.; Xu, Y. H.; Chen, J. F.; Schwarzschild, M. A. , Neuroprotection by caffeine: Time course and role of its metabolites in the MPTP model of Parkinson's disease. Neuroscience 2010, 167, 475–481. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, J.; Park, H. J.; Park, E. S.; Oh, S.; Zheng, H.; Junn, E.; Voronkov, M.; Stock, J. B.; Mouradian, M. M. , Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson’s disease and DLB. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, E12053–E12062. [Google Scholar] [CrossRef] [PubMed]
- Yelanchezian, Y. M. M.; Waldvogel, H. J.; Faull, R. L. M.; Kwakowsky, A. , Neuroprotective Effect of Caffeine in Alzheimer’s Disease. Molecules 2022, 27, 3737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, L. , The Neuroprotective Effects of Moderate and Regular Caffeine Consumption in Alzheimer's Disease. Oxid. Med. Cell. Longev. 2021, 2021, 5568011. [Google Scholar] [CrossRef] [PubMed]
- Tan, E. K.; Chua, E.; Fook-Chong, S. M.; Teo, Y. Y.; Yuen, Y.; Tan, L.; Zhao, Y. , Association between caffeine intake and risk of Parkinson's disease among fast and slow metabolizers. Pharmacogenet. Genomics 2007, 17, 1001–1005. [Google Scholar] [CrossRef]
- Alves-Martinez, P.; Atienza-Navarro, I.; Vargas-Soria, M.; Carranza-Naval, M. J.; Infante-Garcia, C.; Benavente-Fernandez, I.; Del Marco, A.; Lubian-Lopez, S.; Garcia-Alloza, M. , Caffeine Restores Neuronal Damage and Inflammatory Response in a Model of Intraventricular Hemorrhage of the Preterm Newborn. Front. Cell Dev. Biol. 2022, 10, 908045. [Google Scholar] [CrossRef] [PubMed]
- Heise, J.; Schmitz, T.; Bührer, C.; Endesfelder, S. , Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat. Antioxidants 2023, 12, 295. [Google Scholar] [CrossRef]
- Kim, E.; Robinson, N. M.; Newman, B. M. , A Brewed Awakening: Neuropsychiatric Effects of Caffeine in Older Adults. Clin. Geriatr. Med. 2022, 38, 133–144. [Google Scholar] [CrossRef]
- Pohanka, M. Role of Caffeine in the Age-related Neurodegenerative Diseases: A Review. Mini-Rev. Med. Chem. 2022, 22, 2726–2735. [Google Scholar] [CrossRef]
- Ruggiero, M.; Calvello, R.; Porro, C.; Messina, G.; Cianciulli, A.; Panaro, M. A. , Neurodegenerative Diseases: Can Caffeine Be a Powerful Ally to Weaken Neuroinflammation? Int. J. Mol. Sci. 2022, 23, 12958. [Google Scholar] [CrossRef]
- Basu Mallik, S.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Dukie, S. A.; Grant, G.; Rao, C. M.; Arora, D. , Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016, 632, 218–223. [Google Scholar] [CrossRef]
- Saenno, R.; Dornlakorn, O.; Anosri, T.; Kaewngam, S.; Sirichoat, A.; Aranarochana, A.; Pannangrong, W.; Wigmore, P.; Welbat, J. U. , Caffeic Acid Alleviates Memory and Hippocampal Neurogenesis Deficits in Aging Rats induced by D-Galactose. Nutrients 2022, 14, 2169. [Google Scholar] [CrossRef] [PubMed]
- Zaitone, S. A.; Ahmed, E.; Elsherbiny, N. M.; Mehanna, E. T.; El-Kherbetawy, M. K.; ElSayed, M. H.; Alshareef, D. M.; Moustafa, Y. M. , Caffeic acid improves locomotor activity and lessens inflammatory burden in a mouse model of rotenone-induced nigral neurodegeneration: Relevance to Parkinson's disease therapy. Pharmacol. Rep. 2019, 71, 32–41. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D. K. , Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mukherjee, S.; Paliwal, P.; Singh, S. S.; Birla, H.; Singh, S. P.; Krishnamurthy, S.; Patnaik, R. Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn-Schmiedeberg's Arch. Pharmacol. 2019, 392, 1293–1309. [Google Scholar] [CrossRef]
- Kwon, S. H.; Lee, H. K.; Kim, J. A.; Hong, S. I.; Kim, H. C.; Jo, T. H.; Park, Y. I.; Lee, C. K.; Kim, Y. B.; Lee, S. Y.; Jang, C. G. , Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef]
- Liberato, J. L.; Rosa, M. N.; Miranda, M. C. R.; Lopes, J. L. C.; Lopes, N. P.; Gobbo-Neto, L.; Fontana, A. C. K.; Dos Santos, W. F. , Neuroprotective Properties of Chlorogenic Acid and 4,5-Caffeoylquinic Acid from Brazilian arnica (Lychnophora ericoides) after Acute Retinal Ischemia. Planta Med. 2023, 89, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, F.; Li, Z.; Mu, Y.; Yong, V. W.; Xue, M. , Neuroprotective Effects of Chlorogenic Acid in a Mouse Model of Intracerebral Hemorrhage Associated with Reduced Extracellular Matrix Metalloproteinase Inducer. Biomolecules 2022, 12, 1020. [Google Scholar] [CrossRef]
- Metwally, D. M.; Alajmi, R. A.; El-Khadragy, M. F.; Yehia, H. M.; Al-Megrin, W. A.; Akabawy, A. M. A.; Amin, H. K.; Abdel Moneim, A. E. , Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. J. Funct. Foods 2020, 75, 104202. [Google Scholar] [CrossRef]
- Rebai, O.; Belkhir, M.; Sanchez-Gomez, M. V.; Matute, C.; Fattouch, S.; Amri, M. , Differential Molecular Targets for Neuroprotective Effect of Chlorogenic Acid and its Related Compounds Against Glutamate Induced Excitotoxicity and Oxidative Stress in Rat Cortical Neurons. Neurochem. Res. 2017, 42, 3559–3572. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Soni, R.; Sharma, M.; Chatterjee, S.; Parihar, N.; Mukarram, M.; kale, R.; Sayyed, A. A.; Behera, S. K.; Khairnar, A. , Chlorogenic Acid: a Polyphenol from Coffee Rendered Neuroprotection Against Rotenone-Induced Parkinson’s Disease by GLP-1 Secretion. Mol. Neurobiol. 2022, 59, 6834–6856. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. S.; Rai, S. N.; Birla, H.; Zahra, W.; Rathore, A. S.; Dilnashin, H.; Singh, R.; Singh, S. P. , Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of da Neurons in a Parkinsonian Mouse Model. Oxid. Med. Cell. Longev. 2020, 2020, 6571484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, L.; Chen, B.; Fang, Y.; Lin, W.; Zhang, T.; Feng, X.; Tao, X.; Wu, Y.; Fu, X.; Lin, Z. , Chlorogenic acid exerts neuroprotective effect against hypoxia-ischemia brain injury in neonatal rats by activating Sirt1 to regulate the Nrf2-NF-κB signaling pathway. Cell Commun. Signal. 2022, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, B. J.; Bu, Y. , Protective effects of dihydrocaffeic acid, a coffee component metabolite, on a focal cerebral ischemia rat model. Molecules 2015, 20, 11930–11940. [Google Scholar] [CrossRef]
- Alharthy, K. M.; Balaha, M. F.; Devi, S.; Altharawi, A.; Yusufoglu, H. S.; Aldossari, R. M.; Alam, A.; Giacomo, V. D. , Ameliorative Effects of Isoeugenol and Eugenol against Impaired Nerve Function and Inflammatory and Oxidative Mediators in Diabetic Neuropathic Rats. Biomedicines 2023, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, Y.; Zhang, R.; Li, Y.; Yang, Z.; Yang, H. , Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. , Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Huang, R. , Ferulic acid: An extraordinarily neuroprotective phenolic acid with anti-depressive properties. Phytomedicine 2022, 105, 154355. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. , Ferulic acid, a phenolic compound with therapeutic effects in neuropsychiatric disorders, stimulates the production of nerve growth factor and endocannabinoids in rat brain. Physiol. Pharmacol. (Iran) 2017, 21, 279–294. [Google Scholar]
- Liu, G.; Nie, Y.; Huang, C.; Zhu, G.; Zhang, X.; Hu, C.; Li, Z.; Gao, Y.; Ma, Z. , Ferulic acid produces neuroprotection against radiation-induced neuroinflammation by affecting NLRP3 inflammasome activation. Int. J. Radiat. Biol. 2022, 98, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. M.; Shen, J. D.; Xu, L. P.; Li, H. B.; Li, Y. C.; Yi, L. T. , Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef]
- Long, T.; Wu, Q.; Wei, J.; Tang, Y.; He, Y. N.; He, C. L.; Chen, X.; Yu, L.; Yu, C. L.; Law, B. Y.; Wu, J. M.; Qin, D. L.; Wu, A. G.; Zhou, X. G. Ferulic Acid Exerts Neuroprotective Effects via Autophagy Induction in C. elegans and Cellular Models of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 3723567. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Javed, H.; Azimullah, S.; Khair, S. B. A.; Haque, M. E. , Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Devel. Ther. 2015, 9, 5499–55. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Arthur, R.; Upadhayay, S.; Kumar, P. , Ferulic acid ameliorates neurodegeneration via the Nrf2/ARE signalling pathway: A Review. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100190. [Google Scholar] [CrossRef]
- Thapliyal, S.; Singh, T.; Handu, S.; Bisht, M.; Kumari, P.; Arya, P.; Srivastava, P.; Gandham, R. , A Review on Potential Footprints of Ferulic Acid for Treatment of Neurological Disorders. Neurochem. Res. 2021, 46, 1043–1057. [Google Scholar] [CrossRef]
- Yin, C. L.; Lu, R. G.; Zhu, J. F.; Huang, H. M.; Liu, X.; Li, Q. F.; Mo, Y. Y.; Zhu, H. J.; Chin, B.; Wu, J. X.; Liu, X. W.; Cheng, B.; Ruan, J. X.; Liang, Y. H.; Song, H.; Guo, H. W.; Su, Z. H.; Zheng, H. , The study of neuroprotective effect of ferulic acid based on cell metabolomics. Eur. J. Pharmacol. 2019, 864, 172694. [Google Scholar] [CrossRef]
- Prasad, S. N. , Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: Behavioral and biochemical evidence. Neurochem. Res. 2013, 38, 330–345. [Google Scholar] [CrossRef]
- Costentin, J. , Main neurotropic and psychotropic effects of methylxanthines (caffeine, theophylline, theobromine, paraxanthine). PSN 2010, 8, 182–186. [Google Scholar] [CrossRef]
- Crotty, G. F.; Maciuca, R.; Macklin, E. A.; Wang, J.; Montalban, M.; Davis, S. S.; Alkabsh, J. I.; Bakshi, R.; Chen, X.; Ascherio, A.; Astarita, G.; Huntwork-Rodriguez, S.; Schwarzschild, M. A. , Association of caffeine and related analytes with resistance to Parkinson disease among LRRK2 mutation carriers: A metabolomic study. Neurology 2020, 95, e3428–e3437. [Google Scholar] [CrossRef]
- Guerreiro, S.; Toulorge, D.; Hirsch, E.; Marien, M.; Sokoloff, P.; Michel, P. P. , Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol. Pharmacol. 2008, 74, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Kinoshita, C.; Bhadhprasit, W.; Nakaki, T.; Aoyama, K. , A purine derivative, paraxanthine, promotes cysteine uptake for glutathione synthesis. J. Pharmacol. Sci. 2023, 151, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I. A.; Fasina, O. B.; Ayeni, M. F.; Ajayi, O. M.; Farombi, E. O. , Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem. Toxicol. 2019, 125, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Al Olayan, E. M.; Aloufi, A. S.; AlAmri, O. D.; El-Habit, O. H.; Abdel Moneim, A. E. , Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis. Sci. Total Environ. 2020, 723, 137969. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Álvarez-Fernández, M. A.; Cerezo, A. B.; Richard, T.; Troncoso, A. M.; Garcia-Parrilla, M. C. , Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-β and α-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef]
- Kale, S.; Sarode, L. P.; Kharat, A.; Ambulkar, S.; Prakash, A.; Sakharkar, A. J.; Ugale, R. R. , Protocatechuic Acid Prevents Early Hour Ischemic Reperfusion Brain Damage by Restoring Imbalance of Neuronal Cell Death and Survival Proteins. J. Stroke Cerebrovasc. Dis. 2021, 30, 105507. [Google Scholar] [CrossRef]
- Kangtao, *!!! REPLACE !!!*; Yangqian, *!!! REPLACE !!!*; Bais, S. Neuroprotective effect of protocatechuic acid through MAO-B inhibition in aluminium chloride induced dementia of alzheimer’s type in rats. Int. J. Pharmacol. 2018, 14, 879–888. [Google Scholar]
- Kho, A. R.; Choi, B. Y.; Lee, S. H.; Hong, D. K.; Lee, S. H.; Jeong, J. H.; Park, K. H.; Song, H. K.; Choi, H. C.; Suh, S. W. , Effects of protocatechuic acid (PCA) on global cerebral ischemia-induced hippocampal neuronal death. Int. J. Mol. Sci. 2018, 19, 1420. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. , Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: Review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 2019, 22, 72–82. [Google Scholar] [CrossRef]
- Lee, S. H.; Choi, B. Y.; Kho, A. R.; Jeong, J. H.; Hong, D. K.; Lee, S. H.; Lee, S. Y.; Lee, M. W.; Song, H. K.; Choi, H. C.; Suh, S. W. , Protective effects of protocatechuic acid on seizure-induced neuronal death. Int. J. Mol. Sci. 2018, 19, 187. [Google Scholar] [CrossRef]
- Lee, S. H.; Choi, B. Y.; Lee, S. H.; Kho, A. R.; Jeong, J. H.; Hong, D. K.; Suh, S. W. , Administration of protocatechuic acid reduces traumatic brain injury-induced neuronal death. Int. J. Mol. Sci. 2017, 18, 2510. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, T.; Lian, F.; Xu, T.; Yin, W.; Jiang, Y. , Anthocyanin-rich blueberry extracts and anthocyanin metabolite protocatechuic acid promote autophagy-lysosomal pathway and alleviate neurons damage in in vivo and in vitro models of Alzheimer's disease. Nutrition 2022, 93, 111473. [Google Scholar] [CrossRef] [PubMed]
- Mert, H.; Kerem, Ö.; Mıs, L.; Yıldırım, S.; Mert, N. , Effects of protocatechuic acid against cisplatin-induced neurotoxicity in rat brains: an experimental study. Int. J. Neurosci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Winter, A. N.; Brenner, M. C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D. A. , Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef]
- Zhang, H. N.; An, C. N.; Zhang, H. N.; Pu, X. P. , Protocatechuic acid inhibits neurotoxicity induced by MPTP in vivo. Neurosci. Lett. 2010, 474, 99–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Szeto, S. S. W.; Chong, C. M.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; Michael Siu, K. W.; Yuen Lee, S. M.; Chu, I. K. , Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef]
- Lee, M.; McGeer, E. G.; McGeer, P. L. , Quercetin, not caffeine, is a major neuroprotective component in coffee. Neurobiol. Aging 2016, 46, 113–123. [Google Scholar] [CrossRef]
- Alvarez-Arellano, L.; Salazar-García, M.; Corona, J. C. , Neuroprotective effects of Quercetin in pediatric neurological diseases. Molecules 2020, 25, 5597. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; D’Onofrio, G.; Nabavi, S. F.; Daglia, M.; Rastrelli, L.; Nabavi, S. M. , Neuroprotective effects of quercetin: From chemistry to medicine. CNS Neurol. Disord. Drug Targets 2016, 15, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I. U. H.; Bhat, R. , Quercetin: A bioactive compound imparting cardiovascular and neuroprotective benefits: Scope for exploring fresh produce, their wastes, and by-products. Biology 2021, 10, 586. [Google Scholar] [CrossRef]
- Chiang, M. C.; Tsai, T. Y.; Wang, C. J. , The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef] [PubMed]
- Costa, L. G.; Garrick, J. M.; Roquè, P. J.; Pellacani, C. , Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed]
- Fideles, S. O. M.; de Cássia Ortiz, A.; Buchaim, D. V.; de Souza Bastos Mazuqueli Pereira, E.; Parreira, M. J. B. M.; de Oliveira Rossi, J.; da Cunha, M. R.; de Souza, A. T.; Soares, W. C.; Buchaim, R. L. , Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System. Antioxidants 2023, 12, 149. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W. S.; Akkol, E. K. , Neuroprotective effects of quercetin in alzheimer’s disease. Biomolecules 2020, 10, 59. [Google Scholar] [CrossRef]
- Ossola, B.; Kääriäinen, T. M.; Männistö, P. T. , The multiple faces of quercetin in neuroprotection. Expert Opin. Drug Saf. 2009, 8, 397–409. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, J.; Yang, F.; Li, S.; Ma, W.; Chang, X.; Yang, L. , Neuroprotective Effects of Quercetin on Ischemic Stroke: A Literature Review. Front. Pharmacol. 2022, 13, 854249. [Google Scholar] [CrossRef]
- Luo, L.; Sun, T.; Yang, L.; Liu, A.; Liu, Q. Q.; Tian, Q. Q.; Wang, Y.; Zhao, M. G.; Yang, Q. , Scopoletin ameliorates anxiety-like behaviors in complete Freund's adjuvant-induced mouse model. Mol. Brain 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. H.; Ki, T. L.; Jae, H. Y.; Nam, I. B.; Dae, K. K. , Acetylcholinesterase inhibitors from the twigs of Vaccinium oldhami miquel. Arch. Pharmacal Res. 2004, 27, 53–56. [Google Scholar] [CrossRef]
- Gay, N. H.; Suwanjang, W.; Ruankham, W.; Songtawee, N.; Wongchitrat, P.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. , Butein, isoliquiritigenin, and scopoletin attenuate neurodegenerationviaantioxidant enzymes and SIRT1/ADAM10 signaling pathway. RSC Adv. 2020, 10, 16593–16606. [Google Scholar] [CrossRef]
- Kashyap, P.; Ram, H.; Shukla, S. D.; Kumar, S. , Scopoletin: Antiamyloidogenic, Anticholinesterase, and Neuroprotective Potential of a Natural Compound Present in Argyreia speciosa Roots by In Vitro and In Silico Study. Neurosci. Insights 2020, 15. [Google Scholar] [CrossRef]
- Pradhan, P.; Majhi, O.; Biswas, A.; Joshi, V. K.; Sinha, D. , Enhanced accumulation of reduced glutathione by Scopoletin improves survivability of dopaminergic neurons in Parkinson’s model. Cell Death Dis. 2020, 11, 739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, W.; Ge, C.; Li, X.; Sun, Z. , Scopoletin Attenuates Intracerebral Hemorrhage-Induced Brain Injury and Improves Neurological Performance in Rats. Neuroimmunomodulation 2021, 28, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ashafaq, M.; Tabassum, H.; Parvez, S. , Modulation of Behavioral Deficits and Neurodegeneration by Tannic Acid in Experimental Stroke Challenged Wistar Rats. Mol. Neurobiol. 2017, 54, 5941–5951. [Google Scholar] [CrossRef] [PubMed]
- Gerzson, M. F. B.; Bona, N. P.; Soares, M. S. P.; Teixeira, F. C.; Rahmeier, F. L.; Carvalho, F. B.; da Cruz Fernandes, M.; Onzi, G.; Lenz, G.; Gonçales, R. A.; Spanevello, R. M.; Stefanello, F. M. , Tannic Acid Ameliorates STZ-Induced Alzheimer’s Disease-Like Impairment of Memory, Neuroinflammation, Neuronal Death and Modulates Akt Expression. Neurotox. Res. 2020, 37, 1009–1017. [Google Scholar] [CrossRef]
- Hasanvand, A.; Hosseinzadeh, A.; Saeedavi, M.; Goudarzi, M.; Basir, Z.; Mehrzadi, S. , Neuroprotective effects of tannic acid against kainic acid-induced seizures in mice. Hum. Exp. Toxicol. 2022, 41. [Google Scholar] [CrossRef]
- Kim, S. W.; Kim, D. B.; Kim, H. S. , Neuroprotective effects of tannic acid in the postischemic brain via direct chelation of Zn2+. Anim. Cells Syst. 2022, 26, 183–191. [Google Scholar] [CrossRef]
- Salman, M.; Tabassum, H.; Parvez, S. , Tannic Acid Provides Neuroprotective Effects Against Traumatic Brain Injury Through the PGC-1α/Nrf2/HO-1 Pathway. Mol. Neurobiol. 2020, 57, 2870–2885. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yu, Z.; Qi, J. , Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced BV2 microglial cells via inhibition of NF-κB activation. Drug Dev. Res. 2019, 80, 262–268. [Google Scholar] [CrossRef]
- Bhat, J. A.; Gupta, S.; Kumar, M. , Neuroprotective effects of theobromine in transient global cerebral ischemia-reperfusion rat model. Biochem. Biophys. Res. Commun. 2021, 571, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J. A.; Kumar, M. , Neuroprotective Effects of Theobromine in permanent bilateral common carotid artery occlusion rat model of cerebral hypoperfusion. Metab. Brain Dis. 2022, 37, 1787–1801. [Google Scholar] [CrossRef]
- Shanahan, P.; O'Sullivan, J.; Tipton, K. F.; Kinsella, G. K.; Ryan, B. J.; Henehan, G. T. M. , Theobromine and related methylxanthines as inhibitors of Primary Amine Oxidase. J. Food Biochem. 2019, 43, e12697. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, C.; Janakiraman, U.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M. M.; Kalandar, A.; Khan, M. A. S.; Guillemin, G. J. , Vanillin Attenuated Behavioural Impairments, Neurochemical Deficts, Oxidative Stress and Apoptosis Against Rotenone Induced Rat Model of Parkinson’s Disease. Neurochem. Res. 2016, 41, 1899–19. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, C.; Manivasagam, T.; Nataraj, J.; Justin Thenmozhi, A.; Essa, M. M. , Neurosupportive Role of Vanillin, a Natural Phenolic Compound, on Rotenone Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 626028. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. , Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817. [Google Scholar] [CrossRef]
- Lan, X. B.; Wang, Q.; Yang, J. M.; Ma, L.; Zhang, W. J.; Zheng, P.; Sun, T.; Niu, J. G.; Liu, N.; Yu, J. Q. , Neuroprotective effect of Vanillin on hypoxic-ischemic brain damage in neonatal rats. Biomed. Pharmacother. 2019, 118, 109196. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Ghosh, B.; Ahmad, M. H.; Mondal, A. C. , Evaluation of Potential Neuroprotective Effects of Vanillin Against MPP+/MPTP-Induced Dysregulation of Dopaminergic Regulatory Mechanisms in SH-SY5Y Cells and a Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef]
- Huang, S. M.; Hsu, C. L.; Chuang, H. C.; Shih, P. H.; Wu, C. H.; Yen, G. C. , Inhibitory effect of vanillic acid on methylglyoxal-mediated glycation in apoptotic Neuro-2A cells. Neurotoxicology 2008, 29, 1016–1022. [Google Scholar] [CrossRef]
- Khoshnam, S. E.; Sarkaki, A.; Rashno, M.; Farbood, Y. , Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sci. 2018, 211, 126–132. [Google Scholar] [CrossRef]
- Siddiqui, S.; Kamal, A.; Khan, F.; Jamali, K. S.; Saify, Z. S. , Gallic and vanillic acid suppress inflammation and promote myelination in an in vitro mouse model of neurodegeneration. Mol. Biol. Rep. 2019, 46, 997–1011. [Google Scholar] [CrossRef]
- Ullah, R.; Ikram, M.; Park, T. J.; Ahmad, R.; Saeed, K.; Alam, S. I.; Rehman, I. U.; Khan, A.; Khan, I.; Jo, M. G.; Kim, M. O. , Vanillic acid, a bioactive phenolic compound, counteracts lps-induced neurotoxicity by regulating c-jun n-terminal kinase in mouse brain. Int. J. Mol. Sci. 2021, 22, 361. [Google Scholar] [CrossRef]
- Kim, I. S.; Choi, D. K.; Jung, H. J. , Neuroprotective effects of vanillyl alcohol in gastrodia elata blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells. Molecules 2011, 16, 5349–5361. [Google Scholar] [CrossRef]
- Agunloye, O. M.; Oboh, G. , Hypercholesterolemia, angiotensin converting enzyme and ecto-enzymes of purinergic system: Ameliorative properties of caffeic and chlorogenic acid in hypercholesterolemic rats. J. Food Biochem. 2018, 42, e12604. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Oshiro, J.; Kim, K. H.; Park, Y. , Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A. F.; Ali, M.; Abdalla, M.; Ibrahim, I. M.; Elfiky, A. A. , Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef] [PubMed]
- Degotte, G.; Pirotte, B.; Frédérich, M.; Francotte, P. Potential of Caffeic Acid Derivatives as Antimalarial Leads. Lett. Drug Des. Discovery, 2022, 19, 823–836. [Google Scholar] [CrossRef]
- Ekeuku, S. O.; Pang, K. L.; Chin, K. Y. , Effects of caffeic acid and its derivatives on bone: A systematic review. Drug Des. Devel. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef]
- Elkamhawy, A.; Oh, N. K.; Gouda, N. A.; Abdellattif, M. H.; Alshammari, S. O.; Abourehab, M. A. S.; Alshammari, Q. A.; Belal, A.; Kim, M.; Al-Karmalawy, A. A.; Lee, K. , Novel Hybrid Indole-Based Caffeic Acid Amide Derivatives as Potent Free Radical Scavenging Agents: Rational Design, Synthesis, Spectroscopic Characterization, In Silico and In Vitro Investigations. Metabolites 2023, 13, 141. [Google Scholar] [CrossRef]
- Huang, C. W.; Lee, S. Y.; Wei, T. T.; Kuo, Y. H.; Wu, S. T.; Ku, H. C. , A novel caffeic acid derivative prevents renal remodeling after ischemia/reperfusion injury. Biomed. Pharmacother. 2021, 142, 112028. [Google Scholar] [CrossRef]
- Jöhrer, K.; Galarza Pérez, M.; Kircher, B.; Çiçek, S. S. , Flavones, Flavonols, Lignans, and Caffeic Acid Derivatives from Dracocephalum moldavica and Their In Vitro Effects on Multiple Myeloma and Acute Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 14219. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N. I.; Tabassum, N.; Kim, Y. M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Kim, C. K.; Yu, J.; Le, D.; Han, S.; Yu, S.; Lee, M. , Anti-inflammatory activity of caffeic acid derivatives from Ilex rotunda. Int. Immunopharmacol. 2023, 115, 109610. [Google Scholar] [CrossRef]
- Lee, S. Y.; Kuo, Y. H.; Du, C. X.; Huang, C. W.; Ku, H. C. , A novel caffeic acid derivative prevents angiotensin II-induced cardiac remodeling. Biomed. Pharmacother. 2023, 162, 114709. [Google Scholar] [CrossRef]
- Peng, X.; Wu, G.; Zhao, A.; Huang, K.; Chai, L.; Natarajan, B.; Yang, S.; Chen, H.; Lin, C. , Synthesis of novel caffeic acid derivatives and their protective effect against hydrogen peroxide induced oxidative stress via Nrf2 pathway. Life Sci. 2020, 247, 117439. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Y.; Liu, C. C.; Lee, S. C.; Kuo, Y. H.; Hsieh, T. J. , N-Octyl Caffeamide, a Caffeic Acid Amide Derivative, Prevents Progression of Diabetes and Hepatic Steatosis in High-Fat Diet Induced Obese Mice. Int. J. Mol. Sci. 2022, 23, 8948. [Google Scholar] [CrossRef]
- Zeng, Y. F.; Su, Y. L.; Liu, W. L.; Chen, H. G.; Zeng, S. G.; Zhou, H. B.; Chen, W. M.; Zheng, J. X.; Sun, P. H. , Design and synthesis of caffeic acid derivatives and evaluation of their inhibitory activity against Pseudomonas aeruginosa. Med. Chem. Res. 2022, 31, 177–194. [Google Scholar] [CrossRef]
- Abu-Hashem, A. A.; Hussein, H. A. R. , Synthesis and antitumor activity of new pyrimidine and caffeine derivatives. Lett. Drug Des. Discovery 2015, 12, 471–478. [Google Scholar] [CrossRef]
- Andrs, M.; Muthna, D.; Rezacova, M.; Seifrtova, M.; Siman, P.; Korabecny, J.; Benek, O.; Dolezal, R.; Soukup, O.; Jun, D.; Kuca, K. , Novel caffeine derivatives with antiproliferative activity. RSC Adv. 2016, 6, 32534–32539. [Google Scholar] [CrossRef]
- Boulaamane, Y.; Ibrahim, M. A. A.; Britel, M. R.; Maurady, A. , In silico studies of natural product-like caffeine derivatives as potential MAO-B inhibitors/AA2AR antagonists for the treatment of Parkinson's disease. J. Integr. Bioinform. 2022, 19. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Sierakowska, A.; Wandyszewska, N.; Warżajtis, B.; Rychlewska, U.; Wawrzyniak, R.; Mrówczyńska, L. , Antioxidant properties of thio-caffeine derivatives: Identification of the newly synthesized 8-[(pyrrolidin-1-ylcarbonothioyl)sulfanyl]caffeine as antioxidant and highly potent cytoprotective agent. Bioorg. Med. Chem. Lett. 2016, 26, 3994–3998. [Google Scholar] [CrossRef]
- Mitkov, J.; Kondeva-Burdina, M.; Zlatkov, A. , Synthesis and preliminary hepatotoxicity evaluation of new caffeine-8-(2-thio)-propanoic hydrazid-hydrazone derivatives. Pharmacia 2019, 66, 99–106. [Google Scholar] [CrossRef]
- Navid Soltani Rad, M.; Behrouz, S.; Zokaei, K.; Behrouz, M.; Ghanbariasad, A.; Zarenezhad, E. , Synthesis of some novel 8-(4-Alkylpiperazinyl) caffeine derivatives as potent anti-Leishmania agents. Bioorg. Chem. 2022, 128, 106062. [Google Scholar] [CrossRef]
- Reddy, A. B.; Hymavathi, R. V.; Swamy, G. N.; Sadiku, E. R. , Triazino-caffeine derivatives by intramolecular cyclization: Synthesis, characterization and antimicrobial studies. Lett. Org. Chem. 2018, 15, 540–545. [Google Scholar] [CrossRef]
- Reshetnikov, D. V.; Burova, L. G.; Rybalova, T. V.; Bondareva, E. A.; Patrushev, S. S.; Evstropov, A. N.; Shults, E. E. , Synthesis and Antibacterial Activity of Caffeine Derivatives Containing Amino-Acid Fragments. Chem. Nat. Compd. 2022, 58, 908–915. [Google Scholar] [CrossRef]
- Sierakowska, A.; Jasiewicz, B.; Piosik, Ł.; Mrówczyńska, L. , New C8-substituted caffeine derivatives as promising antioxidants and cytoprotective agents in human erythrocytes. Sci. Rep. 2023, 13, 1785. [Google Scholar] [CrossRef]
- Hwang, S. H.; Zuo, G.; Wang, Z.; Lim, S. S. , Novel aldose reductase inhibitory and antioxidant chlorogenic acid derivatives obtained by heat treatment of chlorogenic acid and amino acids. Food Chem. 2018, 266, 449–457. [Google Scholar] [CrossRef]
- Jo, H.; Zhou, Y.; Viji, M.; Choi, M.; Lim, J. Y.; Sim, J.; Rhee, J.; Kim, Y.; Seo, S. Y.; Kim, W. J.; Hong, J. T.; Lee, H.; Lee, K.; Jung, J. K. , Synthesis, biological evaluation, and metabolic stability of chlorogenic acid derivatives possessing thiazole as potent inhibitors of α-MSH-stimulated melanogenesis. Bioorg. Med. Chem. Lett. 2017, 27, 4854–4857. [Google Scholar] [CrossRef] [PubMed]
- Kataria, R.; Khatkar, A. , In-silico design, synthesis, ADMET studies and biological evaluation of novel derivatives of Chlorogenic acid against Urease protein and H. Pylori bacterium. BMC Chem. 2019, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mohsin, A.; Wang, Z.; Zhu, X.; Zhuang, Y.; Cao, L.; Guo, M.; Yin, Z. , Enhanced Biosynthesis of Chlorogenic Acid and Its Derivatives in Methyl-Jasmonate-Treated Gardenia jasminoides Cells: A Study on Metabolic and Transcriptional Responses of Cells. Front. Bioeng. Biotechnol. 2021, 8, 604957. [Google Scholar] [CrossRef]
- Mei, Y.; Pan, D.; Jiang, Y.; Zhang, W.; Yao, X.; Dai, Y.; Yu, Y.; Yao, X. , Target discovery of chlorogenic acid derivatives from the flower buds of Lonicera macranthoides and their MAO B inhibitory mechanism. Fitoterapia 2019, 134, 297–304. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. , The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Meng, X.; Chen, S.; Huang, D.; Xia, Y.; Zhu, S. , Antioxidant activities of chlorogenic acid derivatives with different acyl donor chain lengths and their stabilities during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 357, 129904. [Google Scholar] [CrossRef]
- Yang, J. Q.; Zeng, F. K.; Li, G.; Xu, T. S.; Shu, B. , Synthesis and bioactivity evaluation of novel chlorogenic acid derivatives containing amide group. Chin. Pharm. J. 2016, 51, 264–268. [Google Scholar]
- Abdou, A.; Elmakssoudi, A.; El Amrani, A.; JamalEddine, J.; Dakir, M. , Recent advances in chemical reactivity and biological activities of eugenol derivatives. Med. Chem. Res. 2021, 30, 1011–1030. [Google Scholar] [CrossRef]
- Abdou, A.; Idouaarame, S.; Salah, M.; Nor, N.; Zahm, S.; El Makssoudi, A.; Mazoir, N.; Benharref, A.; Dari, A.; Eddine, J. J.; Blaghen, M.; Dakir, M. , Phytochemical Study: Molecular Docking of Eugenol Derivatives as Antioxidant and Antimicrobial Agents. Lett. Org. Chem. 2022, 19, 774–783. [Google Scholar] [CrossRef]
- Alam, M. M. , Synthesis and anticancer activity of novel Eugenol derivatives against breast cancer cells. Nat. Prod. Res. 2023, 37, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Alam, M. M.; Elbehairi, S. E. I.; Shati, A. A.; Hussien, R. A.; Alfaifi, M. Y.; Malebari, A. M.; Asad, M.; Elhenawy, A. A.; Asiri, A. M.; Mahzari, A. M.; Alshehri, R. F.; Nazreen, S. , Design, synthesis and biological evaluation of new eugenol derivatives containing 1,3,4-oxadiazole as novel inhibitors of thymidylate synthase. New J. Chem. 2023, 47, 5021–5032. [Google Scholar] [CrossRef]
- Anjum, N. F.; Purohit, M. N.; Yogish Kumar, H.; Ramya, K.; Javid, S.; Salahuddin, M. D.; Prashantha Kumar, B. R. , Semisynthetic Derivatives of Eugenol and their Biological Properties: A Fleeting Look at the Promising Molecules. J. Biol. Active Prod. Nat. 2020, 10, 379–404. [Google Scholar] [CrossRef]
- Anjum, N. F.; Shanmugarajan, D.; Prashantha Kumar, B. R.; Faizan, S.; Durai, P.; Raju, R. M.; Javid, S.; Purohit, M. N. , Novel Derivatives of Eugenol as a New Class of PPARγ Agonists in Treating Inflammation: Design, Synthesis, SAR Analysis and In Vitro Anti-Inflammatory Activity. Molecules 2023, 28, 3899. [Google Scholar] [CrossRef]
- Anjum, N. F.; Shanmugarajan, D.; Shivaraju, V. K.; Faizan, S.; Naishima, N. L.; Prashantha Kumar, B. R.; Javid, S.; Purohit, M. N. , Novel derivatives of eugenol as potent anti-inflammatory agents via PPARγ agonism: rational design, synthesis, analysis, PPARγ protein binding assay and computational studies. RSC Adv. 2022, 12, 16966–16978. [Google Scholar] [CrossRef]
- Arianie, L.; Supriatna, M. I.; Kazal, N.; Widodo, N.; Warsito, W.; Iftitah, E. D. , Synthesis, In vitro, and In silico Studies of Methyl Eugenol Derivatives for Plasmodium falciparum Inhibitor. Trop. J. of Nat. Prod. Res. 2022, 6, 1446–1454. [Google Scholar]
- Dutra, J. A. P.; Maximino, S. C.; Gonçalves, R.; Morais, P. A. B.; de Lima Silva, W.; Rodrigues, R. P.; Neto, Á. C.; Júnior, V. L.; de Souza Borges, W.; Kitagawa, R. R. , Anti-Candida, docking studies, and in vitro metabolism-mediated cytotoxicity evaluation of Eugenol derivatives. Chem. Biol. Drug Des. 2023, 101, 350–363. [Google Scholar] [CrossRef]
- Elattar, E. M.; Galala, A. A.; Saad, H. E. A.; Badria, F. A. , Hyaluronidase Inhibitory Activity and In Silico Docking Study of New Eugenol 1,2,3-triazole Derivatives. Chem. Select 2022, 7, e202202194. [Google Scholar] [CrossRef]
- Fernandes, M. J. G.; Pereira, R. B.; Pereira, D. M.; Fortes, A. G.; Castanheira, E. M. S.; Gonçalves, M. S. T. , New eugenol derivatives with enhanced insecticidal activity. Int. J. Mol. Sci. 2020, 21, 9257. [Google Scholar] [CrossRef]
- Lima, Â. M. A.; de Paula, W. T.; Leite, I. C. H. L.; Gazolla, P. A. R.; de Abreu, L. M.; Fonseca, V. R.; Romão, W.; Lacerda, V.; de Queiroz, V. T.; Teixeira, R. R.; Costa, A. V. , Synthesis of Eugenol-Fluorinated Triazole Derivatives and Evaluation of Their Fungicidal Activity. J. Braz. Chem. Soc. 2022, 33, 1200–12. [Google Scholar] [CrossRef]
- Maurya, A. K.; Agarwal, K.; Gupta, A. C.; Saxena, A.; Nooreen, Z.; Tandon, S.; Ahmad, A.; Bawankule, D. U. , Synthesis of eugenol derivatives and its anti-inflammatory activity against skin inflammation. Nat. Prod. Res. 2020, 34, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Maximino, S. C.; Dutra, J. A. P.; Rodrigues, R. P.; Gonçalves, R. C. R.; Morais, P. A. B.; Ventura, J. A.; Schuenck, R. P.; Júnior, V. L.; Kitagawa, R. R.; Borges, W. S. , Synthesis of eugenol derivatives and evaluation of their antifungal activity against fusarium solani f. Sp. piperis. Curr. Pharm. Des. 2020, 26, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A. M.; da Silva, E. T.; Wardell, J. L.; de Souza, M. V. N. , Synthesis and First-Time Assessment of o-Eugenol Derivatives against Mycobacterium tuberculosis. Chem. Nat. Compd. 2020, 56, 633–638. [Google Scholar] [CrossRef]
- Muniz, D. F.; dos Santos Barbosa, C. R.; de Menezes, I. R. A.; de Sousa, E. O.; Pereira, R. L. S.; Júnior, J. T. C.; Pereira, P. S.; de Matos, Y. M. L. S.; da Costa, R. H. S.; de Morais Oliveira-Tintino, C. D.; Coutinho, H. D. M.; Filho, J. M. B.; Ribeiro de Sousa, G.; Filho, J. R.; Siqueira-Junior, J. P.; Tintino, S. R. , In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021, 337, 127776. [Google Scholar] [CrossRef]
- Nour, H.; Abdou, A.; Belaidi, S.; Jamal, J.; Elmakssoudi, A.; Dakir, M.; Chtita, S. , Discovery of promising cholinesterase inhibitors for Alzheimer's disease treatment through DFT, docking, and molecular dynamics studies of eugenol derivatives. J. Chin. Chem. Soc. 2022, 69, 1534–1551. [Google Scholar] [CrossRef]
- Nunes, D. O. S.; Vinturelle, R.; Martins, F. J.; dos Santos, T. F.; Valverde, A. L.; Ribeiro, C. M. R.; Castro, H. C.; Folly, E. , Biotechnological Potential of Eugenol and Thymol Derivatives Against Staphylococcus aureus from Bovine Mastitis. Curr. Microbiol. 2021, 78, 1846–1855. [Google Scholar] [CrossRef]
- Pelozo, M. F.; Lima, G. F. S.; Cordeiro, C. F.; Silva, L. S.; Caldas, I. S.; Carvalho, D. T.; Lavorato, S. N.; Hawkes, J. A.; Franco, L. L. , Synthesis of new hybrid derivatives from metronidazole and eugenol analogues as trypanocidal agents. J. Pharm. Pharm. Sci. 2021, 24, 421–434. [Google Scholar] [CrossRef]
- Teixeira, R. R.; Rodrigues Gazolla, P. A.; Borsodi, M. P. G.; Castro Ferreira, M. M.; Andreazza Costa, M. C.; Costa, A. V.; Cabral Abreu Grijó, B.; Rossi Bergmann, B.; Lima, W. P. , Eugenol derivatives with 1,2,3-triazole moieties: Oral treatment of cutaneous leishmaniasis and a quantitative structure-activity relationship model for their leishmanicidal activity. Exp. Parasitol. 2022, 238, 108269. [Google Scholar] [CrossRef]
- Wu, J.; Yin, W.; Zhang, Y.; Ye, H.; Li, Y.; Tian, J.; Huang, Z.; Zhang, Y. , Design and synthesis of the ring-opened derivative of 3-n-butylphthalide-ferulic acid-glucose trihybrids as potential anti-ischemic agents. Chin. Chem. Lett. 2020, 31, 1881–1886. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, Y. G.; Zheng, X. L.; Dang, Y. L.; Zhu, C. M.; Zhang, R. R.; Fu, Y. Y.; Zhou, T. Y.; Li, J. H. , Lipophilic ferulic acid derivatives protect PC12 cells against oxidative damage: Via modulating β-amyloid aggregation and activating Nrf2 enzymes. Food Funct. 2020, 11, 4707–4718. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, Z.; Lan, S.; Gan, X. , Design, synthesis, antiviral activity, and mechanisms of novel ferulic acid derivatives containing amide moiety. Bioorg. Chem. 2022, 128, 106054. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Y.; Sun, J.; Zheng, L. , Synthesis of mitochondria-targeted ferulic acid amide derivatives with antioxidant, anti-inflammatory activities and inducing mitophagy. Bioorg. Chem. 2022, 127, 106037. [Google Scholar] [CrossRef] [PubMed]
- Kolaj, I.; Wang, Y.; Ye, K.; Meek, A.; Liyanage, S. I.; Santos, C.; Weaver, D. F. , Ferulic acid amide derivatives with varying inhibition of amyloid-β oligomerization and fibrillization. Bioorg. Med. Chem. 2021, 43, 116247. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Peng, Q.; Liu, J.; Alolga, R. N.; Zhou, W. , A novel ferulic acid derivative attenuates myocardial cell hypoxia reoxygenation injury through a succinate dehydrogenase dependent antioxidant mechanism. Eur. J. Pharmacol. 2019, 856, 172417. [Google Scholar] [CrossRef]
- Montaser, A.; Huttunen, J.; Ibrahim, S. A.; Huttunen, K. M. , Astrocyte-Targeted Transporter-Utilizing Derivatives of Ferulic Acid Can Have Multifunctional Effects Ameliorating Inflammation and Oxidative Stress in the Brain. Oxid. Med. Cell. Longev. 2019, 2019, 3528148. [Google Scholar] [CrossRef] [PubMed]
- Khatkar, A.; Nanda, A.; Kumar, P.; Narasimhan, B. , Synthesis and antimicrobial evaluation of ferulic acid derivatives. Res. Chem. Intermed. 2015, 41, 299–309. [Google Scholar] [CrossRef]
- Drăgan, M.; Stan, C. D.; Iacob, A. T.; Dragostin, O. M.; Boancă, M.; Lupuşoru, C. E.; Zamfir, C. L.; Profire, L. , Biological evaluation of azetidine-2-one derivatives of ferulic acid as promising anti-inflammatory agents. Processes 2020, 8, 1401. [Google Scholar] [CrossRef]
- Drăgan, M.; Stan, C. D.; Iacob, A.; Profire, L. , Assessment of in vitro antioxidant and anti-inflammatory activities of new azetidin-2-one derivatives of ferulic acid. Farmacia 2016, 64, 717–721. [Google Scholar]
- Lan, J. S.; Zeng, R. F.; Jiang, X. Y.; Hou, J. W.; Liu, Y.; Hu, Z. H.; Li, H. X.; Li, Y.; Xie, S. S.; Ding, Y.; Zhang, T. , Design, synthesis and evaluation of novel ferulic acid derivatives as multi-target-directed ligands for the treatment of Alzheimer's disease. Bioorg. Chem. 2020, 94, 103413. [Google Scholar] [CrossRef]
- Gan, X.; Zhang, W.; Lan, S.; Hu, D. , Novel Cyclized Derivatives of Ferulic Acid as Potential Antiviral Agents through Activation of Photosynthesis. J. Agric. Food Chem. 2023, 71, 1369–1380. [Google Scholar] [CrossRef]
- Sang, Z.; Wang, K.; Han, X.; Cao, M.; Tan, Z.; Liu, W. , Design, Synthesis, and Evaluation of Novel Ferulic Acid Derivatives as Multi-Target-Directed Ligands for the Treatment of Alzheimer's Disease. ACS Chem. Neurosci. 2019, 10, 1008–1024. [Google Scholar] [CrossRef]
- Jung, J. S.; Yan, J. J.; Li, H. M.; Sultan, M. T.; Yu, J.; Lee, H. S.; Shin, K. J.; Song, D. K. , Protective effects of a dimeric derivative of ferulic acid in animal models of Alzheimer's disease. Eur. J. Pharmacol. 2016, 782, 30–34. [Google Scholar] [CrossRef]
- Yue, S. J.; Zhang, P. X.; Zhu, Y.; Li, N. G.; Chen, Y. Y.; Li, J. J.; Zhang, S.; Jin, R. Y.; Yan, H.; Shi, X. Q.; Tang, Y. P.; Duan, J. A. , A ferulic acid derivative FXS-3 inhibits proliferation and metastasis of human lung cancer A549 cells via positive JNK signaling pathway and negative ERK/p38, AKt/mTOR and MEK/ERK signaling pathways. Molecules 2019, 24, 2165. [Google Scholar] [CrossRef]
- Pinheiro, P. G.; Santiago, G. M. P.; da Silva, F. E. F.; de Araújo, A. C. J.; de Oliveira, C. R. T.; Freitas, P. R.; Rocha, J. E.; Neto, J. B. D. A.; da Silva, M. M. C.; Tintino, S. R.; Siyadatpanah, A.; Norouzi, R.; Dashti, S.; Wilairatana, P.; Coutinho, H. D. M.; da Costa, J. G. M. , Ferulic acid derivatives inhibiting Staphylococcus aureus tetK and MsrA efflux pumps. Biotechnol. Rep. 2022, 34, e00717. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.; Santiago, G.; Da Silva, F.; De Araujo, A.; De Oliveira, C.; Freitas, P.; Rocha, J.; De Araujo Neto, J.; Da Silva, M.; Tintino, S.; De Menezes, I.; Coutinho, H.; Da Costa, J. , Antibacterial activity and inhibition against Staphylococcus aureus NorA efflux pump by ferulic acid and its esterified derivatives. Asian Pac. J. Trop. Biomed. 2021, 11, 405–413. [Google Scholar]
- Kong, H.; Fu, X.; Chang, X.; Ding, Z.; Yu, Y.; Xu, H.; Wang, R.; Shan, Y.; Ding, S. , The ester derivatives of ferulic acid exhibit strong inhibitory effect on the growth of Alternaria alternata in vitro and in vivo. Postharvest Biol. Technol. 2023, 196, 112158. [Google Scholar] [CrossRef]
- Zhang, P. X.; Lin, H.; Qu, C.; Tang, Y. P.; Li, N. G.; Kai, J.; Shang, G.; Li, B.; Zhang, L.; Yan, H.; Liu, P.; Duan, J. A. , Design, synthesis, and in vitro antiplatelet aggregation activities of ferulic acid derivatives. J. Chem. 2015, 2015, 376527. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Tang, Y.; Li, B.; Liu, L.; Zhang, X.; Fu, H.; Duan, J. A. , Biological activity evaluation and structure-activity relationships analysis of ferulic acid and caffeic acid derivatives for anticancer. Bioorg. Med. Chem. Lett. 2012, 22, 6085–6088. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Huang, K.; Li, X.; Hao, X.; Stöckigt, J.; Zhao, Y. , Preparation of ferulic acid derivatives and evaluation of their xanthine oxidase inhibition activity. Nat. Prod. Res. 2007, 21, 196–202. [Google Scholar] [CrossRef]
- Serafim, T. L.; Carvalho, F. S.; Marques, M. P. M.; Calheiros, R.; Silva, T.; Garrido, J.; Milhazes, N.; Borges, F.; Roleira, F.; Silva, E. T.; Holy, J.; Oliveira, P. J. , Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 2011, 24, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O. S.; Atolani, O.; Banerjee, P.; Arolasafe, G.; Preissner, R.; Etukudoh, P.; Ibraheem, O. , Computational and experimental validation of antioxidant properties of synthesized bioactive ferulic acid derivatives. Int. J. Food Prop. 2018, 21, 101–113. [Google Scholar] [CrossRef]
- Kikugawa, M.; Tsutsuki, H.; Ida, T.; Nakajima, H.; Ihara, H.; Sakamoto, T. , Water-soluble ferulic acid derivatives improve amyloid-β-induced neuronal cell death and dysmnesia through inhibition of amyloid-β aggregation. Biosci. Biotechnol. Biochem. 2016, 80, 547–553A. [Google Scholar] [CrossRef]
- Malik, S. A.; Ali, K. F.; Dawood, A. H. , Synthesis, Characterization, and Preliminary Evaluation of Ferulic Acid Derivatives Containing Heterocyclic Moiety. J. Med. Chem. Sci. 2023, 6, 1444–1456. [Google Scholar]
- Machado, K. C.; Oliveira, G. L. S.; Islam, M. T.; Junior, A. L. G.; De Sousa, D. P.; Freitas, R. M. , Anticonvulsant and behavioral effects observed in mice following treatment with an ester derivative of ferulic acid: Isopentyl ferulate. Chem. Biol. Interact. 2015, 242, 273–279. [Google Scholar] [CrossRef]
- Bautista-Aguilera, O. M.; Alonso, J. M.; Catto, M.; Iriepa, I.; Knez, D.; Gobec, S.; Marco-Contelles, J. , N-Hydroxy-N-Propargylamide Derivatives of Ferulic Acid: Inhibitors of Cholinesterases and Monoamine Oxidases. Molecules 2022, 27, 7437. [Google Scholar] [CrossRef]
- Sang, Z.; Pan, W.; Wang, K.; Ma, Q.; Yu, L.; Yang, Y.; Bai, P.; Leng, C.; Xu, Q.; Li, X.; Tan, Z.; Liu, W. , Design, synthesis and evaluation of novel ferulic acid-O-alkylamine derivatives as potential multifunctional agents for the treatment of Alzheimer's disease. Eur. J. Med. Chem. 2017, 130, 379–392. [Google Scholar] [CrossRef]
- Cui, M. Y.; Xiao, M. W.; Xu, L. J.; Chen, Y.; Liu, A. L.; Ye, J.; Hu, A. X. , Bioassay of ferulic acid derivatives as influenza neuraminidase inhibitors. Arch. Pharm. 2020, 353, 1900174. [Google Scholar] [CrossRef]
- Adeyemi, O. S.; Awakan, O. J.; Atolani, O.; Iyeye, C. O.; Oweibo, O. O.; Adejumo, O. J.; Ibrahim, A.; Batiha, G. E. S. , New ferulic acid derivatives protect against carbon tetrachloride-induced liver injury in rats. Open Biochem. J. 2019, 13, 13–22. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, Z.; Liu, D.; Zeng, H.; Liang, J.; Hu, D.; Gan, X. , Ferulic acid derivatives with piperazine moiety as potential antiviral agents. Pest Manag. Sci. 2022, 78, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, C.; Emanuele, S.; Ferrante, F.; Celesia, A.; Giuliano, M.; Fiore, T. , Tributyltin(IV) ferulate, a novel synthetic ferulic acid derivative, induces autophagic cell death in colon cancer cells: From chemical synthesis to biochemical effects. J. Inorg. Biochem. 2020, 205, 110999. [Google Scholar] [CrossRef]
- Guzmán-López, E. G.; Reina, M.; Hernández-Ayala, L. F.; Galano, A. , Rational Design of Multifunctional Ferulic Acid Derivatives Aimed for Alzheime's and Parkinson's Diseases. Antioxidants 2023, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Basas-Jaumandreu, J.; López, J.; De Las Heras, F. X. C. , Resorcinol and m-guaiacol alkylated derivatives and asymmetrical secondary alcohols in the leaves from Tamarix canariensis. Phytochem. Lett. 2014, 10, 240–248. [Google Scholar] [CrossRef]
- Ordoudi, S. A.; Tsimidou, M. Z.; Vafiadis, A. P.; Bakalbassis, E. G. , Structure-DPPH• scavenging activity relationships: Parallel study of catechol and guaiacol acid derivatives. J. Agric. Food Chem. 2006, 54, 5763–5768. [Google Scholar] [CrossRef]
- Premkumar, J.; Sampath, P.; Sanjay, R.; Chandrakala, A.; Rajagopal, D. , Synthetic Guaiacol Derivatives as Promising Myeloperoxidase Inhibitors Targeting Atherosclerotic Cardiovascular Disease. ChemMedChem 2020, 15, 1187–1199. [Google Scholar] [CrossRef]
- Bhagat, S. D.; Mathur, R. K.; Siddhanta, N. N. , Synthesis of New Derivatives of Eugenol and Isoeugenol. J. Chem. Eng. Data 1982, 27, 209–210. [Google Scholar] [CrossRef]
- Nafie, M. S.; Elghazawy, N. H.; Owf, S. M.; Arafa, K.; Abdel-Rahman, M. A.; Arafa, R. K. , Control of ER-positive breast cancer by ERα expression inhibition, apoptosis induction, cell cycle arrest using semisynthetic isoeugenol derivatives. Chem. Biol. Interact. 2022, 351, 109753. [Google Scholar] [CrossRef] [PubMed]
- Shen, K. P.; Chang, W. T.; Lin, H. L.; Chu, L. W.; Chen, I. J.; Wu, B. N. , Structure-activity relationships of isoeugenol-based chlorophenylpiperazine derivatives on serotonergic/adrenergic receptor, platelet aggregation, and lipid peroxidation. Drug Dev. Res. 2010, 71, 285–293. [Google Scholar] [CrossRef]
- Zuhrufa, Z.; Julianto, T. S. , Molecular Interaction Analysis of COX-2 against Aryl Amino Alcohol Derivatives from Isoeugenol as Anti Breast Cancer using Molecular Docking. Bull. Chem. React. Eng. Catal. 2021, 16, 581–587. [Google Scholar] [CrossRef]
- Lu, W.; Wang, F.; Zhang, T.; Dong, J.; Gao, H.; Su, P.; Shi, Y.; Zhang, J. Search for novel histone deacetylase inhibitors. Part II: Design and synthesis of novel isoferulic acid derivatives. Bioorg. Med. Chem. 2014, 22, 2707–2713. [Google Scholar] [CrossRef] [PubMed]
- Merlani, M.; Barbakadze, V.; Amiranashvili, L.; Gogilashvili, L. , Synthesis of new dihydroxylated derivatives of ferulic and isoferulic acids. Bull. Georgian Natl. Acad. Sci. 2018, 12, 119–124. [Google Scholar]
- Ekowati, J.; Diyah, N. W.; Syahrani, A. , Synthesis and antiplatelet activities of some derivatives of p-coumaric acid. Chem. Chem. Technol. 2019, 13, 296–302. [Google Scholar] [CrossRef]
- Khatkar, A.; Nanda, A.; Kumar, P.; Narasimhan, B. , Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab. J. Chem. 2017, 10, S3804–S3815. [Google Scholar] [CrossRef]
- Lopes, S. P.; Castillo, Y. P.; Monteiro, M. L.; de Menezes, R. R. P. P. B.; Almeida, R. N.; Martins, A. M. C.; de Sousa, D. P. , Trypanocidal mechanism of action and in silico studies of p-coumaric acid derivatives. Int. J. Mol. Sci. 2019, 20, 5916. [Google Scholar] [CrossRef]
- Lopes, S. P.; Yepe, L. M.; Pérez-Castillo, Y.; Robledo, S. M.; De Sousa, D. P. , Alkyl and aryl derivatives based on p-coumaric acid modification and inhibitory action against leishmania braziliensis and plasmodium falciparum. Molecules 2020, 25, 3178. [Google Scholar] [CrossRef]
- Rodrigues, D. M.; Portapilla, G. B.; de Sicco, G. S.; da Silva, I. F. R.; de Albuquerque, S.; Bastos, J. K.; Campo, V. L. , Novel synthetic derivatives of cinnamic and p-coumaric acids with antiproliferative effect on breast MCF-7 tumor cells. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef]
- Shirai, A.; Kajiura, M.; Matsumura, K.; Omasa, T. , Improved photobactericidal activity of ultraviolet-a light in combination with isomerizable p-coumaric acid derivatives. Biocontrol Sci. 2015, 20, 231–238. [Google Scholar] [CrossRef]
- Reina, M.; Guzmán-López, E. G.; Romeo, I.; Marino, T.; Russo, N.; Galano, A. , Computationally designed: P -coumaric acid analogs: Searching for neuroprotective antioxidants. New J. Chem. 2021, 45, 14369–14380. [Google Scholar] [CrossRef]
- Cho, J.; Jung, H.; Kang, D. Y.; Sp, N.; Shin, W.; Lee, J.; Park, B. G.; Kang, Y. A.; Jang, K. J.; Bae, S. W. , The Skin-Whitening and Antioxidant Effects of Protocatechuic Acid (PCA) Derivatives in Melanoma and Fibroblast Cell Lines. Curr. Issues Mol. Biol. 2023, 45, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Lu, F. J.; Tseng, S. N.; Li, M. L.; Shih, S. R. , In vitro anti-influenza virus activity of synthetic humate analogues derived from protocatechuic acid. Arch. Virol. 2002, 147, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.; Martins, M.; Barreto, B.; Milhazes, N.; Garrido, E. M.; Silva, P.; Garrido, J.; Borges, F. , Structure-property-activity relationship of phenolic acids and derivatives. Protocatechuic acid alkyl esters. J. Agric. Food Chem. 2010, 58, 6986–6993. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Kawabata, J. , Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents. Tetrahedron 2005, 61, 8101–8108. [Google Scholar] [CrossRef]
- Sheng, G. H.; Zhou, Q. C.; Hu, X. M.; Wang, C. F.; Chen, X. F.; Xue, D.; Yan, K.; Ding, S. S.; Wang, J.; Du, Z. Y.; Liu, Z. H.; Zhang, C. Y.; Zhu, H. L. , Synthesis, structure, urease inhibitory, and cytotoxic activities of two complexes with protocatechuic acid derivative and phenanthroline. J. Coord. Chem. 2015, 68, 1571–1582. [Google Scholar] [CrossRef]
- Sheng, G. H.; Zhou, Q. C.; Sun, J.; Cheng, X. S.; Qian, S. S.; Zhang, C. Y.; You, Z. L.; Zhu, H. L. , Synthesis, structure, and urease inhibitory activities of three binuclear copper(II) complexes with protocatechuic acid derivative. J. Coord. Chem. 2014, 67, 1265–1278. [Google Scholar] [CrossRef]
- Wei, J.; Liu, K.; Du, C.; Zhou, Y.; Lin, C. A Novel Mannich Derivative of Protocatechuic Acid: Synthesis, Crystal Structure and Antioxidant Activity. Proc. Natl. Acad. Sci. India Sect. A - Phys. Sci. 2017, 87, 181–188. [Google Scholar] [CrossRef]
- Cai, X.; Yang, J.; Zhou, J.; Lu, W.; Hu, C.; Gu, Z.; Huo, J.; Wang, X.; Cao, P. , Synthesis and biological evaluation of scopoletin derivatives. Bioorg. Med. Chem. 2013, 21, 84–92. [Google Scholar] [CrossRef]
- Khunnawutmanotham, N.; Chimnoi, N.; Saparpakorn, P.; Techasakul, S. Synthesis and anti-acetylcholinesterase activity of scopoletin derivatives. Bioorg. Chem. 2016, 65, 137–145. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, P.; Wang, H.; Wei, Y.; Wang, C.; Hao, S. , Design and Synthesis of Scopoletin Sulfonate Derivatives as Potential Insecticidal Agents. Molecules 2023, 28, 530. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hua, J.; Zhou, J.; Zhang, H.; Zhu, H.; Cheng, Y.; Gust, R. , Synthesis and in vitro antitumor activity of novel scopoletin derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5008–5012. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhu, C.; Zhang, C.; Li, X.; Yu, Z.; Zhang, Z.; Shi, X. , Design, synthesis and biological evaluation of 3-aryl-7-hydroxy scopoletin derivatives as autophagy activators against tumorigenesis. Eur. J. Med. Chem. 2022, 244, 114805. [Google Scholar] [CrossRef]
- Luo, J.; Lai, T.; Guo, T.; Chen, F.; Zhang, L.; Ding, W.; Zhang, Y. , Synthesis and acaricidal activities of scopoletin phenolic ether derivatives: Qsar, molecular docking study and in silico Adme predictions. Molecules 2018, 23, 995. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, J.; Bao, N.; Guan, F.; Chen, L.; Sun, J. , Design, synthesis, and cytotoxic evaluation of novel scopoletin derivatives. Chem. Biol. Drug Des. 2018, 91, 641–646. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, L.; Sun, J. , Novel scopoletin derivatives kill cancer cells by inducing mitochondrial depolarization and apoptosis. Anticancer Agents Med. Chem. 2021, 21, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, N.; Chen, C.; Wang, Y.; Lei, Z.; Chen, L.; Sun, J. , Novel NO-releasing scopoletin derivatives induce cell death via mitochondrial apoptosis pathway and cell cycle arrest. Eur. J. Med. Chem. 2020, 200, 112386. [Google Scholar] [CrossRef]
- Yu, N.; Li, N.; Wang, K.; Deng, Q.; Lei, Z.; Sun, J.; Chen, L. , Design, synthesis and biological activity evaluation of novel scopoletin-NO donor derivatives against MCF-7 human breast cancer in vitro and in vivo. Eur. J. Med. Chem. 2021, 224, 113701. [Google Scholar] [CrossRef]
- Zhao, P.; Dou, Y.; Chen, L.; Li, L.; Wei, Z.; Yu, J.; Wu, X.; Dai, Y.; Xia, Y. , SC-III3, a novel scopoletin derivative, induces autophagy of human hepatoma HepG2 cells through AMPK/mTOR signaling pathway by acting on mitochondria. Fitoterapia 2015, 104, 31–40. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Wei, L.; Zheng, Y.; Zhang, H.; Wang, Y.; Cao, P.; Niu, A.; Wang, J.; Dai, Y. , Synthesis and antitumor activity of scopoletin derivatives. Lett. Drug Des. Discovery 2012, 9, 397–401. [Google Scholar] [CrossRef]
- Dahab, M. A.; Mahdy, H. A.; Elkady, H.; Taghour, M. S.; Elwan, A.; Elkady, M. A.; Elsakka, E. G. E.; Elkaeed, E. B.; Alsfouk, A. A.; Ibrahim, I. M.; Metwaly, A. M.; Eissa, I. H. , Semi-synthesized anticancer theobromine derivatives targeting VEGFR-2: in silico and in vitro evaluations. J. Biomol. Struct. Dyn. 2023. [Google Scholar] [CrossRef]
- Eissa, I. H.; G. Yousef, R.; Elkady, H.; Alsfouk, A. A.; Husein, D. Z.; Ibrahim, I. M.; El-Deeb, N.; Kenawy, A. M.; Eldehna, W. M.; Elkaeed, E. B.; Metwaly, A. M., New apoptotic anti-triple-negative breast cancer theobromine derivative inhibiting EGFRWT and EGFRT790M: in silico and in vitro evaluation. Mol. Divers. 2023. [Google Scholar] [CrossRef]
- Eissa, I. H.; Yousef, R. G.; Elkady, H.; Alsfouk, A. A.; Alsfouk, B. A.; Husein, D. Z.; Ibrahim, I. M.; Elkaeed, E. B.; Metwaly, A. M. , A New Anticancer Semisynthetic Theobromine Derivative Targeting EGFR Protein: CADDD Study. Life 2023, 13, 191. [Google Scholar] [CrossRef]
- Eissa, I. H.; Yousef, R. G.; Elkaeed, E. B.; Alsfouk, A. A.; Husein, D. Z.; Ibrahim, I. M.; Alesawy, M. S.; Elkady, H.; Metwaly, A. M. , Anticancer derivative of the natural alkaloid, theobromine, inhibiting EGFR protein: Computer-aided drug discovery approach. PLoS One 2023, 18, e0282586. [Google Scholar] [CrossRef] [PubMed]
- Elkaeed, E. B.; Yousef, R. G.; Elkady, H.; Alsfouk, A. A.; Husein, D. Z.; Ibrahim, I. M.; Metwaly, A. M.; Eissa, I. H. , New Anticancer Theobromine Derivative Targeting EGFRWT and EGFRT790M: Design, Semi-Synthesis, In Silico, and In Vitro Anticancer Studies. Molecules 2022, 27, 5859. [Google Scholar] [CrossRef]
- Georgieva, M.; Kondeva-Burdina, M.; Mitkov, J.; Tzankova, V.; Momekov, G.; Zlatkov, A. , Determination of the antiproliferative activity of new theobromine derivatives and evaluation of their in vitro hepatotoxic effects. Anticancer Agents Med. Chem. 2016, 16, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Pereira, R.; Pereira, M. P.; Serra, S. G.; Loesche, A.; Csuk, R.; Silvestre, S.; Costa, P. J.; Oliveira, M. C.; Xavier, N. M. , Furanosyl Nucleoside Analogues Embodying Triazole or Theobromine Units as Potential Lead Molecules for Alzheimer's Disease. Eur. J Org. Chem. 2018, 2018, 2667–2681. [Google Scholar] [CrossRef]
- Ivanchenko, D. G.; Romanenko, N. I.; Kornienko, V. I.; Polishchuk, N. N.; Sharapova, T. A. , Synthesis and Properties of 8-Substituted 1-(2-Oxopropyl)Theobromine Derivatives. Chem. Nat. Compd. 2019, 55, 509–512. [Google Scholar] [CrossRef]
- Xavier, N. M.; Sousa, E. C.; Pereira, M.; Loesche, A.; Serbian, I.; Csuk, R.; Oliveira, M. C. , Synthesis and biological evaluation of structurally varied 50-/60-isonucleosides and theobromine-containing n-isonucleosidyl derivatives. Pharmaceuticals 2019, 12, 103. [Google Scholar] [CrossRef]
- Alafeefy, A. M.; Alqasoumi, S. I.; Abdel Hamid, S. G.; El-Tahir, K. E. H.; Mohamed, M.; Zain, M. E.; Awaad, A. S. , Synthesis and hypoglycemic activity of some new theophylline derivatives. J. Enzyme Inhib. Med. Chem. 2014, 29, 443–448. [Google Scholar] [CrossRef]
- Aninye, I. O.; Berg, K. C.; Mollo, A. R.; Nordeen, S. K.; Wilson, E. M.; Shapiro, D. J. , 8-Alkylthio-6-thio-substituted theophylline analogues as selective noncompetitive progesterone receptor antagonists. Steroids 2012, 77, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Zhang, J.; Yang, S.; Lu, W.; Ding, L.; Zheng, Y.; Li, W. , Design, synthesis, biological evaluation, and molecular docking of 1,7-dibenzyl-substituted theophylline derivatives as novel BRD4-BD1-selective inhibitors. Med. Chem. Res. 2021, 30, 1453–1468. [Google Scholar] [CrossRef]
- Faghih, Z.; Emami, L.; Zomoridian, K.; Sabet, R.; Bargebid, R.; Mansourian, A.; Zeinali, B.; Rostami, Z.; Khabnadideh, S. , Aryloxy Alkyl Theophylline Derivatives as Antifungal Agents: Design, Synthesis, Biological Evaluation and Computational Studies. Chem. Select 2022, 7, e202201618. [Google Scholar] [CrossRef]
- Gopinatha, V. K.; Mantelingu, K.; Rangappa, K. S. , Synthesis and biological evaluation of theophylline acetohydrazide hydrazone derivatives as antituberculosis agents. J. Chin. Chem. Soc. 2020, 67, 1453–1461. [Google Scholar] [CrossRef]
- Hayallah, A. M.; Elgaher, W. A.; Salem, O. I.; Abdel Alim, A. A. M. , Design and synthesis of some new theophylline derivatives with bronchodilator and antibacterial activities. Arch. Pharmacal Res. 2011, 34, 3–21. [Google Scholar] [CrossRef]
- Hayallah, A. M.; Talhouni, A. A.; Abdel Alim, A. A. M. , Design and synthesis of new 8-anilide theophylline derivatives as bronchodilators and antibacterial agents. Arch. Pharmacal Res. 2012, 35, 1355–1368. [Google Scholar] [CrossRef]
- Hierrezuelo, J.; Manuel López-Romero, J.; Rico, R.; Brea, J.; Isabel Loza, M.; Cai, C.; Algarra, M. , Synthesis of theophylline derivatives and study of their activity as antagonists at adenosine receptors. Bioorg. Med. Chem. 2010, 18, 2081–2088. [Google Scholar] [CrossRef]
- Kiran, G.; Prasad, D. K.; Bakshi, V.; Gouthami, T. , In vitro anti-diabetic activity and molecular docking studies of theophylline containing acetylene derivatives. Biointerface Res. Appl. Chem. 2018, 8, 3618–3620. [Google Scholar]
- Managutti, P. B.; Mangasuli, S. N.; Malaganvi, S. S. , Synthesis, crystal structure, electronic structure, and anti-tubercular properties of two new coumarin derivatives bearing theophylline moiety. J. Mol. Struct. 2023, 1277, 134888. [Google Scholar] [CrossRef]
- Partyka, A.; Jarosz, J.; Wasik, A.; Jastrzębska-Więsek, M.; Zagórska, A.; Pawłowski, M.; Wesołowska, A. , Novel tricyclic[2,1-f]theophylline derivatives of LCAP with activity in mouse models of affective disorders. J. Pharm. Pharmacol. 2014, 66, 1755–1762. [Google Scholar] [CrossRef]
- Profire, L.; Şunel, V.; Lupaşcu, D.; Baican, M. C.; Bibire, N.; Vasile, C. , New theophylline derivatives with potential pharmacological activity. Farmacia 2010, 58, 170–176. [Google Scholar]
- Prohre, L.; Lupascu, D. A. N.; Sunel, V.; Bibire, N.; Vasile, C. , Synthesis and characterization of some new theophylline derivatives. Rev. Chim. 2010, 61, 553–556. [Google Scholar]
- Ruddarraju, R. R.; Kiran, G.; Murugulla, A. C.; Maroju, R.; Prasad, D. K.; Kumar, B. H.; Bakshi, V.; Reddy, N. S. , Design, synthesis and biological evaluation of theophylline containing variant acetylene derivatives as α-amylase inhibitors. Bioorg. Chem. 2019, 92, 103120. [Google Scholar] [CrossRef] [PubMed]
- Ruddarraju, R. R.; Murugulla, A. C.; Kotla, R.; Chandra Babu Tirumalasetty, M.; Wudayagiri, R.; Donthabakthuni, S.; Maroju, R.; Baburao, K.; Parasa, L. S. , Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of theophylline containing acetylenes and theophylline containing 1,2,3-triazoles with variant nucleoside derivatives. Eur. J. Med. Chem. 2016, 123, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Ruddarraju, R. R.; Murugulla, A. C.; Kotla, R.; Tirumalasetty, M. C. B.; Wudayagiri, R.; Donthabakthuni, S.; Maroju, R. , Design, synthesis, anticancer activity and docking studies of theophylline containing 1,2,3-triazoles with variant amide derivatives. MedChemComm 2017, 8, 176–183. [Google Scholar] [CrossRef]
- Saeedan, A. S.; Mohamed, M. A.; Soliman, G. A.; Alasiri, K. M.; Abdel-Kader, M. S.; Elnaggar, M. H. , Semisynthetic Theophylline Analogues as Potent Diuretics: An Integrated in vivo and Molecular Docking Study. Lat. Am. J. Pharm.y 2022, 41, 1408–1416. [Google Scholar]
- Stavrakov, G.; Valcheva, V.; Voynikov, Y.; Philipova, I.; Atanasova, M.; Konstantinov, S.; Peikov, P.; Doytchinova, I. , Design, Synthesis, and Antimycobacterial Activity of Novel Theophylline-7-Acetic Acid Derivatives with Amino Acid Moieties. Chem. Biol. Drug Des. 2016, 87, 335–341. [Google Scholar] [CrossRef]
- Voynikov, Y.; Valcheva, V.; Momekov, G.; Peikov, P.; Stavrakov, G. , Theophylline-7-acetic acid derivatives with amino acids as anti-tuberculosis agents. Bioorg. Med. Chem. Lett. 2014, 24, 3043–3045. [Google Scholar] [CrossRef]
- Ye, J.; Mao, L.; Xie, L.; Zhang, R.; Liu, Y.; Peng, L.; Yang, J.; Li, Q.; Yuan, M. , Discovery of a Series of Theophylline Derivatives Containing 1,2,3-Triazole for Treatment of Non-Small Cell Lung Cancer. Front. Pharmacol. 2021, 12, 753676. [Google Scholar] [CrossRef]
- Yousaf, M.; Zahoor, A. F.; Faiz, S.; Javed, S.; Irfan, M. , Recent Synthetic Approaches Towards Biologically Potent Derivatives/Analogues of Theophylline. J. Heterocycl. Chem. 2018, 55, 2447–2479. [Google Scholar] [CrossRef]
- Zagórska, A.; Pawłowski, M.; Siwek, A.; Kazek, G.; Partyka, A.; Wróbel, D.; Jastrzȩbska-Wiȩsek, M.; Wesołowska, A. , Synthesis and pharmacological evaluation of novel tricyclic[2,1-f] theophylline derivatives. Arch. Pharm. 2013, 346, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Mukerjee, A.; Mishra, S. B. , Design, Synthesis and Molecular Docking of Vanillic Acid Derivatives as Amylolytic Enzyme Inhibitors. Pharm. Chem. J. 2021, 55, 427–435. [Google Scholar] [CrossRef]
- Ishimata, N.; Ito, H.; Tai, A. , Structure–activity relationships of vanillic acid ester analogs in inhibitory effect of antigen-mediated degranulation in rat basophilic leukemia RBL-2H3 cells. Bioorg. Med. Chem. Lett. 2016, 26, 3533–3536. [Google Scholar] [CrossRef] [PubMed]
- Satpute, M. S.; Gangan, V. D.; Shastri, I. , Synthesis and antibacterial activity of novel vanillic acid hybrid derivatives. Rasayan J. Chem. 2019, 12, 383–388. [Google Scholar] [CrossRef]
- Tang, J. F.; Lv, X. H.; Wang, X. L.; Sun, J.; Zhang, Y. B.; Yang, Y. S.; Gong, H. B.; Zhu, H. L. , Design, synthesis, biological evaluation and molecular modeling of novel 1,3,4-oxadiazole derivatives based on Vanillic acid as potential immunosuppressive agents. Bioorg. Med. Chem. 2012, 20, 4226–4236. [Google Scholar] [CrossRef]
- Tawfeeq, M. F.; Qassir, A. J. , Synthesis, characterization, and antibacterial evaluation of new vanillic acid derivatives. Iraqi J. Pharm. Sci. 2020, 29, 129–138. [Google Scholar] [CrossRef]
- Wodnicka, A.; Huzar, E. , Synthesis and photoprotective properties of new salicylic and vanillic acid derivatives. Curr. Chem. Lett. 2017, 6, 125–134. [Google Scholar] [CrossRef]
- Xu, B.; Xu, X.; Zhang, C.; Zhang, Y.; Wu, G. R.; Yan, M.; Jia, M.; Xie, T.; Jia, X.; Wang, P.; Lei, H. , Synthesis and protective effect of new ligustrazine-vanillic acid derivatives against CoCl2-induced neurotoxicity in differentiated PC12 cells. Chem. Cent. J. 2017, 11, 20. [Google Scholar] [CrossRef]
- Bender, O.; Celik, I.; Dogan, R.; Atalay, A.; Shoman, M. E.; Ali, T. F. S.; Beshr, E. A. M.; Mohamed, M.; Alaaeldin, E.; Shawky, A. M.; Awad, E. M.; Ahmed, A. F.; Younes, K. M.; Ansari, M.; Anwar, S. , Vanillin-Based Indolin-2-one Derivative Bearing a Pyridyl Moiety as a Promising Anti-Breast Cancer Agent via Anti-Estrogenic Activity. ACS Omega 2023, 8, 6968–6981. [Google Scholar] [CrossRef]
- Blaikie, L.; Kay, G.; Kong Thoo Lin, P. , Synthesis and in vitro evaluation of vanillin derivatives as multi-target therapeutics for the treatment of Alzheimer's disease. Bioorg. Med. Chem. Lett. 2020, 30, 127505. [Google Scholar] [CrossRef]
- Boiko, Y. A.; Nesterkina, M. V.; Shandra, A. A.; Kravchenko, I. A. , Analgesic and Anti-Inflammatory Activity of Vanillin Derivatives. Pharm. Chem. J. 2019, 53, 650–654. [Google Scholar] [CrossRef]
- da Silva Rodrigues, J. V.; Rodrigues Gazolla, P. A.; da Cruz Pereira, I.; Dias, R. S.; Poly da Silva, I. E.; Oliveira Prates, J. W.; de Souza Gomes, I.; de Azevedo Silveira, S.; Costa, A. V.; de Oliveira, F. M.; de Aguiar, A. R.; Canedo da Silva, C.; Teixeira, R. R.; de Paula, S. O. , Synthesis and virucide activity on zika virus of 1,2,3-triazole-containing vanillin derivatives. Antiviral Res. 2023, 212, 105578. [Google Scholar] [CrossRef]
- Ekowati, J.; Diyah, N. W.; Tejo, B. A.; Ahmed, S. , Chemoinformatics approach to design and develop vanillin analogs as COX-1 inhibitor. J. Public Health Afr. 2023, 14, 2517. [Google Scholar] [CrossRef]
- Freitas, C. S.; Santiago, S. S.; Lage, D. P.; Antinarelli, L. M. R.; Oliveira, F. M.; Vale, D. L.; Martins, V. T.; Magalhaes, L. N. D.; Bandeira, R. S.; Ramos, F. F.; Pereira, I. A. G.; de Jesus, M. M.; Ludolf, F.; Tavares, G. S. V.; Costa, A. V.; Ferreira, R. S.; Coimbra, E. S.; Teixeira, R. R.; Coelho, E. A. F. , In vitro evaluation of antileishmanial activity, mode of action and cellular response induced by vanillin synthetic derivatives against Leishmania species able to cause cutaneous and visceral leishmaniasis. Exp. Parasitol. 2023, 251, 108555. [Google Scholar] [CrossRef]
- Gao, J.; Qiu, S.; Liang, L.; Hao, Z.; Zhou, Q.; Wang, F.; Mou, J.; Lin, Q. , Design, synthesis, and biological evaluation of vanillin hydroxamic acid derivatives as novel peptide deformylase inhibitors. Curr. Comput. Aided Drug Des. 2018, 14, 95–101. [Google Scholar] [CrossRef]
- Gazolla, P. A. R.; de Aguiar, A. R.; Costa, M. C. A.; Oliveira, O. V.; Costa, A. V.; da Silva, C. M.; do Nascimento, C. J.; Junker, J.; Ferreira, R. S.; de Oliveira, F. M.; Vaz, B. G.; do Carmo, P. H. F.; Santos, D. A.; Ferreira, M. M. C.; Teixeira, R. R. , Synthesis of vanillin derivatives with 1,2,3-triazole fragments and evaluation of their fungicide and fungistatic activities. Arch. Pharm. 2023, 356, 2200653. [Google Scholar] [CrossRef]
- Gharai, P. K.; Khan, J.; Mallesh, R.; Garg, S.; Saha, A.; Ghosh, S.; Ghosh, S. , Vanillin Benzothiazole Derivative Reduces Cellular Reactive Oxygen Species and Detects Amyloid Fibrillar Aggregates in Alzheimer’s Disease Brain. ACS Chem. Neurosci. 2023, 14, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Gu, M. M.; Li, M.; Gao, D.; Liu, L. H.; Lang, Y.; Yang, S. M.; Ou, H.; Huang, B.; Zhou, P. K.; Shang, Z. F. , The vanillin derivative 6-bromine-5-hydroxy-4-methoxybenzaldehyde induces aberrant mitotic progression and enhances radio-sensitivity accompanying suppression the expression of PLK1 in esophageal squamous cell carcinoma. Toxicol. Appl. Pharmacol. 2018, 348, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Harismah, K.; Fazeli, F.; Zandi, H. , Structural analyses of vanillin derivative compounds and their molecular docking with mpro and rdrp enzymes of covid-19. Biointerface Res. Appl. Chem. 2022, 12, 1660–1669. [Google Scholar]
- He, H. W.; Wang, F. Y.; Zhang, D.; Chen, C. Y.; Xu, D.; Zhou, H.; Liu, X.; Xu, G. , Discovery of Novel α-Methylene-γ-Butyrolactone Derivatives Containing Vanillin Moieties as Antiviral and Antifungal Agents. J. Agric. Food Chem. 2022, 70, 10316–10325. [Google Scholar] [CrossRef]
- Illicachi, L. A.; Montalvo-Acosta, J. J.; Insuasty, A.; Quiroga, J.; Abonia, R.; Sortino, M.; Zacchino, S.; Insuasty, B. , Synthesis and DFT calculations of novel vanillin-chalcones and their 3-Aryl-5-(4-(2-(dimethylamino)- ethoxy)-3-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde derivatives as antifungal agents. Molecules 2017, 22, 1476. [Google Scholar] [CrossRef]
- Li, M.; Lang, Y.; Gu, M. M.; Shi, J.; Chen, B. P. C.; Yu, L.; Zhou, P. K.; Shang, Z. F. , Vanillin derivative VND3207 activates DNA-PKcs conferring protection against radiation-induced intestinal epithelial cells injury in vitro and in vivo. Toxicol. Appl. Pharmacol. 2020, 387, 114855. [Google Scholar] [CrossRef] [PubMed]
- Luković, J.; Mitrović, M.; Popović, S.; Milosavljević, Z.; Stanojević-Pirković, M.; An-Elković, M.; Zelen, I.; Šorak, M.; Muškinja, J.; Ratković, Z.; Nikolić, I. Antitumor effects of vanillin based chalcone analogs in vitro. Acta Pol. Pharm. - Drug Res. 2020, 77, 57–67. [Google Scholar]
- Ma, W.; Zhang, Q.; Li, X.; Ma, Y.; Liu, Y.; Hu, S.; Zhou, Z.; Zhang, R.; Du, K.; Syed, A.; Yao, X.; Chen, P. , IPM712, a vanillin derivative as potential antitumor agents, displays better antitumor activity in colorectal cancers cell lines. Eur. J. Pharm. Sci. 2020, 152, 105464. [Google Scholar] [CrossRef] [PubMed]
- Marton, A.; Kúsz, E.; Kolozsi, C.; Tubak, V.; Zagotto, G.; Buzás, K.; Quintieri, L.; Vizler, C. , Vanillin analogues o-vanillin and 2,4,6-trihydroxybenzaldehyde inhibit NFκB activation and suppress growth of A375 human melanoma. Anticancer Res. 2016, 36, 5743–5750. [Google Scholar] [CrossRef]
- Pagare, P. P.; Ghatge, M. S.; Musayev, F. N.; Deshpande, T. M.; Chen, Q.; Braxton, C.; Kim, S.; Venitz, J.; Zhang, Y.; Abdulmalik, O.; Safo, M. K. , Rational design of pyridyl derivatives of vanillin for the treatment of sickle cell disease. Bioorg. Med. Chem. 2018, 26, 2530–2538. [Google Scholar] [CrossRef]
- Sahoo, C. R.; Paidesetty, S. K.; Sarathbabu, S.; Dehury, B.; Senthil Kumar, N.; Padhy, R. N. , Molecular dynamics simulation, synthesis and topoisomerase inhibitory actions of vanillin derivatives: a systematic computational structural integument. J. Biomol. Struct. Dyn. 2022, 40, 11653–11663. [Google Scholar] [CrossRef]
- Scipioni, M.; Kay, G.; Megson, I.; Kong Thoo Lin, P. , Novel vanillin derivatives: Synthesis, anti-oxidant, DNA and cellular protection properties. Eur. J. Med. Chem. 2018, 143, 745–754. [Google Scholar] [CrossRef]
- Scipioni, M.; Kay, G.; Megson, I. L.; Kong Thoo Lin, P. , Synthesis of novel vanillin derivatives: Novel multi-targeted scaffold ligands against Alzheimer's disease. MedChemComm 2019, 10, 764–777. [Google Scholar] [CrossRef]
- Shanan, S. H.; Kadem, K. J. , The synthesis of some new heterocyclic compounds from vanillin derivatives. Int. J. Drug Deliv. Technol. 2021, 11, 376–378. [Google Scholar]
- Shastry, R. P.; Ghate, S. D.; Sukesh Kumar, B.; Srinath, B. S.; Kumar, V. , Vanillin derivative inhibits quorum sensing and biofilm formation in Pseudomonas aeruginosa: a study in a Caenorhabditis elegans infection model. Nat. Prod. Res. 2022, 36, 1610–1615. [Google Scholar] [CrossRef]
- Tokalı, F. S.; Şenol, H.; Katmerlikaya, T. G.; Dağ, A.; Şendil, K. , Novel thiosemicarbazone and thiazolidin-4-one derivatives containing vanillin core: Synthesis, characterization, and anticancer activity studies. J. Heterocycl. Chem. 2023, 60, 645–656. [Google Scholar] [CrossRef]
- Wu, Q.; Cai, H.; Yuan, T.; Li, S.; Gan, X.; Song, B. , Novel vanillin derivatives containing a 1,3,4-thiadiazole moiety as potential antibacterial agents. Bioorg. Med. Chem. Lett. 2020, 30, 127113. [Google Scholar] [CrossRef] [PubMed]
- Yuldasheva, N.; Acikyildiz, N.; Akyuz, M.; Yabo-Dambagi, L.; Aydin, T.; Cakir, A.; Kazaz, C. , The Synthesis of Schiff bases and new secondary amine derivatives of p-vanillin and evaluation of their neuroprotective, antidiabetic, antidepressant and antioxidant potentials. J. Mol. Struct. 2022, 1270, 133883. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Ji, R.; Zhang, D.; Ma, C.; Ma, W.; Ma, Y.; Jiang, X.; Du, K.; Zhang, R.; Chen, P. , Vanillin Derivatives Reverse Fusobacterium nucleatum-Induced Proliferation and Migration of Colorectal Cancer Through E-Cadherin/β-Catenin Pathway. Front. Pharmacol. 2022, 13, 841918. [Google Scholar] [CrossRef]
- Abou-Zied, H. A.; Youssif, B. G. M.; Mohamed, M. F. A.; Hayallah, A. M.; Abdel-Aziz, M. , EGFR inhibitors and apoptotic inducers: Design, synthesis, anticancer activity and docking studies of novel xanthine derivatives carrying chalcone moiety as hybrid molecules. Bioorg. Chem. 2019, 89, 102997. [Google Scholar] [CrossRef]
- Andonova, L.; Valkova, I.; Zheleva-Dimitrova, D.; Georgieva, M.; Momekov, G.; Zlatkov, A. , Synthesis of new N1 arylpiperazine substituted xanthine derivatives and evaluation of their antioxidant and cytotoxic effects. Anticancer Agents Med. Chem. 2019, 19, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Banga, A. R.; Sekhar, K. R.; Rayford, K. J.; Arun, A.; Odiase, P.; Garg, A. P.; Lima, M. F.; Nde, P. N.; Villalta, F.; Rachakonda, G. , Xanthine Analogs Suppress Trypanosoma cruzi Infection In Vitro Using PDEs as Targets. Microbiol. Res. (Pavia) 2022, 13, 721–739. [Google Scholar] [CrossRef]
- El-Kalyoubi, S.; Agili, F.; Zordok, W. A.; El-Sayed, A. S. A. , Synthesis, in silico prediction and in vitro evaluation of antimicrobial activity, dft calculation and theoretical investigation of novel xanthines and uracil containing imidazolone derivatives. Int. J. Mol. Sci. 2021, 22, 10979. [Google Scholar] [CrossRef]
- Gumber, D.; Yadav, D.; Yadav, R.; Kachler, S.; Klotz, K. N. , Bronchospasmolytic activity and adenosine receptor binding of some newer 1,3-dipropyl-8-phenyl substituted xanthine derivatives. Chem. Biol. Drug Des. 2020, 95, 600–609. [Google Scholar] [CrossRef]
- Hisham, M.; Youssif, B. G. M.; Osman, E. E. A.; Hayallah, A. M.; Abdel-Aziz, M. , Synthesis and biological evaluation of novel xanthine derivatives as potential apoptotic antitumor agents. Eur. J. Med. Chem. 2019, 176, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kapri, A.; Pant, S.; Gupta, N.; Nain, S. , Recent Advances in the Biological Significance of Xanthine and its Derivatives: A Review. Pharm. Chem. J. 2022, 56, 461–474. [Google Scholar] [CrossRef]
- Kasabova-Angelova, A.; Tzankova, D.; Mitkov, J.; Georgieva, M.; Tzankova, V.; Zlatkov, A.; Kondeva-Burdina, M. Xanthine derivatives as agents affecting non-dopaminergic neuroprotection in parkinson’s disease. Curr. Med, Chem, 2020, 27, 2021–2036. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C. H.; Zhang, B. H.; Huang, S. E.; Hsu, J. H.; Wang, Y. H.; Nguyen, T. T. N.; Lai, C. H.; Yeh, J. L. , Xanthine Derivative KMUP-1 Attenuates Experimental Periodontitis by Reducing Osteoclast Differentiation and Inflammation. Front. Pharmacol. 2022, 13, 821492. [Google Scholar] [CrossRef] [PubMed]
- Lai, C. H.; Chang, C. W.; Lee, F. T.; Kuo, C. H.; Hsu, J. H.; Liu, C. P.; Wu, H. L.; Yeh, J. L. , Targeting vascular smooth muscle cell dysfunction with xanthine derivative KMUP-3 inhibits abdominal aortic aneurysm in mice. Atherosclerosis 2020, 297, 16–24. [Google Scholar] [CrossRef]
- Lee, L. C.; Peng, Y. H.; Chang, H. H.; Hsu, T.; Lu, C. T.; Huang, C. H.; Hsueh, C. C.; Kung, F. C.; Kuo, C. C.; Jiaang, W. T.; Wu, S. Y. , Xanthine Derivatives Reveal an Allosteric Binding Site in Methylenetetrahydrofolate Dehydrogenase 2 (MTHFD2). J. Med. Chem. 2021, 64, 11288–11301. [Google Scholar] [CrossRef]
- Li, G.; Meng, B.; Yuan, B.; Huan, Y.; Zhou, T.; Jiang, Q.; Lei, L.; Sheng, L.; Wang, W.; Gong, N.; Lu, Y.; Ma, C.; Li, Y.; Shen, Z.; Huang, H. , The optimization of xanthine derivatives leading to HBK001 hydrochloride as a potent dual ligand targeting DPP-IV and GPR119. Eur. J. Med. Chem. 2020, 188, 112017. [Google Scholar] [CrossRef]
- Li, Q.; Meng, L.; Zhou, S.; Deng, X.; Wang, N.; Ji, Y.; Peng, Y.; Xing, J.; Yao, G. , Rapid generation of novel benzoic acid–based xanthine derivatives as highly potent, selective and long acting DPP-4 inhibitors: Scaffold-hopping and prodrug study. Eur. J. Med. Chem. 2019, 180, 509–523. [Google Scholar] [CrossRef]
- Lindemann, M.; Dukic-Stefanovic, S.; Hinz, S.; Deuther-Conrad, W.; Teodoro, R.; Juhl, C.; Steinbach, J.; Brust, P.; Müller, C. E.; Wenzel, B. , Synthesis of novel fluorinated xanthine derivatives with high adenosine a2b receptor binding affinity. Pharmaceuticals 2021, 14, 485. [Google Scholar] [CrossRef]
- Ma, Q. S.; Yao, Y.; Zheng, Y. C.; Feng, S.; Chang, J.; Yu, B.; Liu, H. M. , Ligand-based design, synthesis and biological evaluation of xanthine derivatives as LSD1/KDM1A inhibitors. Eur. J. Med. Chem. 2019, 162, 555–567. [Google Scholar] [CrossRef]
- Minard, A.; Bauer, C. C.; Chuntharpursat-Bon, E.; Pickles, I. B.; Wright, D. J.; Ludlow, M. J.; Burnham, M. P.; Warriner, S. L.; Beech, D. J.; Muraki, K.; Bon, R. S. , Potent, selective, and subunit-dependent activation of TRPC5 channels by a xanthine derivative. Br. J. Pharmacol. 2019, 176, 3924–3938. [Google Scholar] [CrossRef] [PubMed]
- Narsimha, S.; Battula, K. S.; Ravinder, M.; Reddy, Y. N.; Nagavelli, V. R. , Design, synthesis and biological evaluation of novel 1,2,3-triazole-based xanthine derivatives as DPP-4 inhibitors. J. Chem. Sci. 2020, 132, 59. [Google Scholar] [CrossRef]
- Pretze, M.; Neuber, C.; Kinski, E.; Belter, B.; Köckerling, M.; Caflisch, A.; Steinbach, J.; Pietzsch, J.; Mamat, C. , Synthesis, radiolabelling and initial biological characterisation of 18F-labelled xanthine derivatives for PET imaging of Eph receptors. Org. Biomol. Chem. 2020, 18, 3104–3116. [Google Scholar] [CrossRef]
- Qiao, M. Q.; Li, Y.; Yang, Y. X.; Pang, C. X.; Liu, Y. T.; Bian, C.; Wang, L.; Chen, X. F.; Hong, B. , Structure-activity relationship and biological evaluation of xanthine derivatives as PCSK9 inhibitors for the treatment of atherosclerosis. Eur. J. Med. Chem. 2023, 247, 115047. [Google Scholar] [CrossRef] [PubMed]
- Satish, M.; Sandhya, K.; Nitin, K.; Yashas Kiran, N.; Aleena, B.; Satish Kumar, A.; Guruprasad, K.; Rajakumara, E. , Computational, biochemical and ex vivo evaluation of xanthine derivatives against phosphodiesterases to enhance the sperm motility. J. Biomol. Struct. Dyn. 2022, 41, 5317–5327. [Google Scholar] [CrossRef]
- Shatokhin, S. S.; Tuskaev, V. A.; Gagieva, S. C.; Markova, A. A.; Pozdnyakov, D. I.; Denisov, G. L.; Melnikova, E. K.; Bulychev, B. M.; Oganesyan, E. T. , Synthesis, cytotoxicity and antioxidant activity of new 1,3-dimethyl-8-(chromon-3-yl)-xanthine derivatives containing 2,6-di-: Tert -butylphenol fragments. New J. Chem. 2022, 46, 621–631. [Google Scholar] [CrossRef]
- Singh, S.; Ojha, M.; Yadav, D.; Kachler, S.; Klotz, K. N.; Yadav, R. , Bronchospasmolytic and Adenosine Binding Activity of 8-(Proline / Pyra-zole)-Substituted Xanthine Derivatives. Curr. Drug Disc. Technol. 2021, 18, e22092020186181. [Google Scholar] [CrossRef]
- Specker, E.; Matthes, S.; Wesolowski, R.; Schütz, A.; Grohmann, M.; Alenina, N.; Pleimes, D.; Mallow, K.; Neuenschwander, M.; Gogolin, A.; Weise, M.; Pfeifer, J.; Ziebart, N.; Heinemann, U.; Von Kries, J. P.; Nazaré, M.; Bader, M. , Structure-Based Design of Xanthine-Benzimidazole Derivatives as Novel and Potent Tryptophan Hydroxylase Inhibitors. J. Med. Chem. 2022, 65, 11126–11149. [Google Scholar] [CrossRef]
- Tam, D. N. H.; Mostafa, E. M.; Tu, V. L.; Rashidy, A. I.; Matenoglou, E.; Kassem, M.; Soa, D. T.; Bayumi, A.; Emam, H. E. S.; Tran, L.; Dat, T. V.; Huy, N. T. , Efficacy of chalcone and xanthine derivatives on lipase inhibition: A systematic review. Chem. Biol. Drug Des. 2020, 95, 205–214. [Google Scholar] [CrossRef]
- Fujioka, K.; Shibamoto, T. Quantitation of volatiles and nonvolatile acids in an extract from coffee beverages: Correlation with antioxidant activity. J. Agric. Food Chem. 2006, 54, 6054–6058. [Google Scholar] [CrossRef]
- Li, H.; Lin, L.; Feng, Y.; Zhao, M.; Li, X.; Zhu, Q.; Xiao, Z. , Enrichment of antioxidants from soy sauce using macroporous resin and identification of 4-ethylguaiacol, catechol, daidzein, and 4-ethylphenol as key small molecule antioxidants in soy sauce. Food Chem. 2018, 240, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, J.; Sun, B.; Zhao, M.; Zheng, F.; Huang, M.; Sun, X.; Li, H. , Intracellular antioxidant effect of vanillin, 4-methylguaiacol and 4-ethylguaiacol: Three components in Chinese Baijiu. RSC Adv. 2017, 7, 46395–46405. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. , Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015, 6, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Shin, J. A.; Jeong, S. H.; Jia, C. H.; Hong, S. T.; Lee, K. T. , Comparison of antioxidant capacity of 4-vinylguaiacol with catechin and ferulic acid in oil-in-water emulsion. Food Sci. Biotechnol. 2019, 28, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Tańska, M.; Mikołajczak, N.; Konopka, I. Comparison of the effect of sinapic and ferulic acids derivatives (4-vinylsyringol vs. 4-vinylguaiacol) as antioxidants of rapeseed, flaxseed, and extra virgin olive oils. Food Chem. 2018, 240, 679–685. [Google Scholar] [CrossRef]
- Agunloye, O. M.; Oboh, G. , Caffeic acid and chlorogenic acid: Evaluation of antioxidant effect and inhibition of key enzymes linked with hypertension. J. Food Biochem. 2018, 42, e12541. [Google Scholar] [CrossRef]
- Coelho, V. R.; Vieira, C. G.; De Souza, L. P.; Moysés, F.; Basso, C.; Papke, D. K. M.; Pires, T. R.; Siqueira, I. R.; Picada, J. N.; Pereira, P. , Antiepileptogenic, antioxidant and genotoxic evaluation of rosmarinic acid and its metabolite caffeic acid in mice. Life Sci. 2015, 122, 65–71. [Google Scholar] [CrossRef]
- Fan, J.; Cai, X.; Feng, X.; Jiang, M.; Yu, X. , Studies on the antioxidant activity in vitro of caffeic acid. J. Chin. Inst. Food Sci. Technol. 2015, 15, 65–73. [Google Scholar]
- Genaro-Mattos, T. C.; Maurício, Â. Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. , Antioxidant activity of Caffeic acid against iron-induced free radical generation-A chemical approach. PLoS One 2015, 10, 0129963. [Google Scholar] [CrossRef]
- Jamali, N.; Mostafavi-Pour, Z.; Zal, F.; Kasraeian, M.; Poordast, T.; Nejabat, N. , Antioxidant ameliorative effect of caffeic acid on the ectopic endometrial cells separated from patients with endometriosis. Taiwan J. Obstet. Gynecol. 2021, 60, 216–220. [Google Scholar] [CrossRef]
- Kassa, T.; Whalin, J. G.; Richards, M. P.; Alayash, A. I. , Caffeic acid: an antioxidant with novel antisickling properties. FEBS Open Bio 2021, 11, 3293–3303. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M. , Determination of antioxidant activity of caffeic acid and p-coumaric acid by using electrochemical and spectrophotometric assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Mohammed, F. Z.; Al-Hussaini, A. S. E. D.; El-Shehabi, M. E. S. , Antidiabetic activity of caffeic acid and 18β-glycyrrhetinic acid and its relationship with the antioxidant property. Asian J. Pharm. Clin. Res. 2015, 8, 255–260. [Google Scholar]
- Purushothaman, A.; Babu, S. S.; Naroth, S.; Janardanan, D. , Antioxidant activity of caffeic acid: thermodynamic and kinetic aspects on the oxidative degradation pathway. Free Radic. Res. 2022, 56, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. , In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Sheng, X.; Zhu, Y.; Zhou, J.; Yan, L.; Du, G.; Liu, Z.; Chen, H. , Antioxidant Effects of Caffeic Acid Lead to Protection of Drosophila Intestinal Stem Cell Aging. Front. Cell Dev. Biol. 2021, 9, 735483. [Google Scholar] [CrossRef]
- Spagnol, C. M.; Assis, R. P.; Brunetti, I. L.; Isaac, V. L. B.; Salgado, H. R. N.; Corrêa, M. A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Uranga, J. G.; Podio, N. S.; Wunderlin, D. A.; Santiago, A. N. , Theoretical and Experimental Study of the Antioxidant Behaviors of 5-O-Caffeoylquinic, Quinic and Caffeic Acids Based on Electronic and Structural Properties. Chem. Select 2016, 1, 4113–4120. [Google Scholar] [CrossRef]
- Hosny, E. N.; Sawie, H. G.; Elhadidy, M. E.; Khadrawy, Y. A. , Evaluation of antioxidant and anti-inflammatory efficacy of caffeine in rat model of neurotoxicity. Nutr. Neurosci. 2019, 22, 789–796. [Google Scholar] [CrossRef]
- Ősz, B. E.; Jîtcă, G.; Ștefănescu, R. E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C. E. , Caffeine and Its Antioxidant Properties—It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- Ruiss, M.; Findl, O.; Kronschläger, M. , The human lens: An antioxidant-dependent tissue revealed by the role of caffeine. Ageing Res. Rev. 2022, 79, 101664. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A. J. S. C.; Gaspar, E. M.; Santos, P. M. P. , Mechanisms of potential antioxidant activity of caffeine. Radiat. Phys. Chem. 2020, 174, 108968. [Google Scholar] [CrossRef]
- Bai, D.; Liu, K.; He, X.; Tan, H.; Liu, Y.; Li, Y.; Zhang, Y.; Zhen, W.; Zhang, C.; Ma, Y. , Effect of Dietary Chlorogenic Acid on Growth Performance, Antioxidant Function, and Immune Response of Broiler Breeders under Immune Stress and Stocking Density Stress. Vet. Sci. 2022, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Bender, O.; Atalay, A. Polyphenol chlorogenic acid, antioxidant profile, and breast cancer. In Cancer: Oxidative Stress and Dietary Antioxidants; Academic Press: Ankara, Turkey, 2021; pp. 311–321. [Google Scholar]
- Carolina Chaves-Ulate, E.; Esquivel-Rodríguez, P. , Chlorogenic acids present in coffee: Antioxidant and antimicrobial capacity. Agron. Mesoam. 2019, 30, 299–311. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Zhao, N.; Yang, X.; Du, E.; Huang, S.; Guo, W.; Zhang, W.; Wei, J. , Effect of chlorogenic acid on intestinal inflammation, antioxidant status, and microbial community of young hens challenged with acute heat stress. Anim. Sci. J. 2021, 92, e13619. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. , Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I. N. E.; Ginting, C. N.; Nasution, S. L.; Suhartina, S.; Munshy, U. Z.; Rizal, R.; Widowati, W. Antioxidant and Anti-Inflammatory activity of Chlorogenic Acid on Lead-Induced Fibroblast Cells. J. Phys. Conf. Ser. 2019, 1374, 012006. [Google Scholar] [CrossRef]
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D. D. , Interactions between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chem. 2016, 213, 251–259. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. , Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Horticult. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Preetha Rani, M. R.; Anupama, N.; Sreelekshmi, M.; Raghu, K. G. , Chlorogenic acid attenuates glucotoxicity in H9c2 cells via inhibition of glycation and PKC α upregulation and safeguarding innate antioxidant status. Biomed. Pharmacother. 2018, 100, 467–477. [Google Scholar] [CrossRef]
- Xu, J. G.; Hu, Q. P.; Liu, Y. , Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, L.; Ruan, Z.; Mi, S.; Jiang, M.; Li, X.; Wu, X.; Deng, Z.; Yin, Y. , Chlorogenic acid ameliorates intestinal mitochondrial injury by increasing antioxidant effects and activity of respiratory complexes. Biosci. Biotechnol. Biochem. 2016, 80, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; De Paulis, T.; May, J. M. , Antioxidant effects of dihydrocaffeic acid in human EA.hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Moon, J. H.; Terao, J. , Antioxidant Activity of Caffeic Acid and Dihydrocaffeic Acid in Lard and Human Low-Density Lipoprotein. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Sørensen, A. D. M.; Petersen, L. K.; de Diego, S.; Nielsen, N. S.; Lue, B. M.; Yang, Z.; Xu, X.; Jacobsen, C. , The antioxidative effect of lipophilized rutin and dihydrocaffeic acid in fish oil enriched milk. Eur. J. Lipid Sci. Technol. 2012, 114, 434–445. [Google Scholar] [CrossRef]
- Adefegha, S. A.; Okeke, B. M.; Oboh, G. , Antioxidant properties of eugenol, butylated hydroxylanisole, and butylated hydroxyl toluene with key biomolecules relevant to Alzheimer’s diseases—In vitro. J. Food Biochem. 2021, 45, e13276. [Google Scholar] [CrossRef]
- Alminderej, F.; Bakari, S.; Almundarij, T. I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant activities of a new chemotype of piper cubeba L. Fruit essential oil (methyleugenol/eugenol): In silico molecular docking and admet studies. Plants 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R. V.; Sobral, P. J. A. , Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Candido Júnior, J. R.; Romeiro, L. A. S.; Marinho, E. S.; Monteiro, N. K. V.; de Lima-Neto, P. , Antioxidant activity of eugenol and its acetyl and nitroderivatives: the role of quinone intermediates—a DFT approach of DPPH test. J. Mol. Model. 2022, 28, 133. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F. N.; Yildirim, S.; Kandemir, F. M.; Aksu, E. H.; Guler, M. C.; Kiziltunc Ozmen, H.; Kucukler, S.; Eser, G. , The antiapoptotic and antioxidant effects of eugenol against cisplatin-induced testicular damage in the experimental model. Andrologia 2019, 51, e13353. [Google Scholar] [CrossRef]
- Ferreira, V. R. F.; Militani, I. A.; de Almeida, K. J.; Lunguinho, A. D. S.; Saczk, A. A.; Ionta, M.; da Silva, G. A. F.; Felix, F. S.; Nelson, D. L.; Cardoso, M. D. G. , Antioxidant and Cytotoxic Activity of Essential Oils and Their Principal Components: Spectrophotometric, Voltammetric, and Theoretical Investigation of the Chelating Effect of Eugenol and Carvacrol. ACS Food Sci. Technol. 2023, 3, 350–360. [Google Scholar] [CrossRef]
- Gülçin, I. , Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S. F.; Sadek, Z.; Edris, A. , Antioxidant and antimicrobial activities of clove bud essential oil and eugenol nanoparticles in alcohol-free microemulsion. J. Oleo Sci. 2012, 61, 641–648. [Google Scholar] [CrossRef]
- Hobani, Y. H.; Mohan, S.; Shaheen, E.; Abdelhaleem, A.; Faruque Ahmad, M.; Bhatia, S.; Abou-Elhamd, A. S. , Gastroprotective effect of low dose Eugenol in experimental rats against ethanol induced toxicity: Involvement of antiinflammatory and antioxidant mechanism. J. Ethnopharmacol. 2022, 289, 115055. [Google Scholar] [CrossRef]
- Horvathova, E.; Navarova, J.; Galova, E.; Sevcovicova, A.; Chodakova, L.; Snahnicanova, Z.; Melusova, M.; Kozics, K.; Slamenova, D. , Assessment of antioxidative, chelating, and DNA-Protective effects of selected essential oil components (Eugenol, Carvacrol, Thymol, Borneol, Eucalyptol) of plants and intact rosmarinus officinalis oil. J. Agric. Food Chem. 2014, 62, 6632–6639. [Google Scholar] [CrossRef] [PubMed]
- Mahboub, R.; Memmou, F. , Antioxidant activity and kinetics studies of eugenol and 6-bromoeugenol. Nat. Prod. Res. 2015, 29, 966–971. [Google Scholar] [CrossRef]
- Nam, H.; Kim, M. M. , Eugenol with antioxidant activity inhibits MMP-9 related to metastasis in human fibrosarcoma cells. Food Chem. Toxicol. 2013, 55, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Oroojan, A. A.; Chenani, N.; An'Aam, M. , Antioxidant Effects of Eugenol on Oxidative Stress Induced by Hydrogen Peroxide in Islets of Langerhans Isolated from Male Mouse. Int. J. Hepatol. 2020, 2020, 5890378. [Google Scholar] [CrossRef]
- Sharma, U. K.; Sharma, A. K.; Gupta, A.; Kumar, R.; Pandey, A.; Pandey, A. K. , Pharmacological activities of cinnamaldehyde and eugenol: Antioxidant, cytotoxic and anti-leishmanial studies. Cell. Mol. Biol. 2017, 63, 73–78. [Google Scholar] [CrossRef]
- Alam, M. A. , Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Dimitrić Marković, J. M.; Milenković, D.; Stepanić, V. , Antioxidative potential of ferulic acid phenoxyl radical. Phytochemistry 2020, 170, 112218. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, A.; Kharazmkia, A.; Mir, S.; Khorramabadi, R. M.; Darabi, S. , Ameliorative effect of ferulic acid on gentamicin-induced nephrotoxicity in a rat model; role of antioxidant effects. J. Renal Inj. Prev. 2018, 7, 73–77. [Google Scholar] [CrossRef]
- Hwang, H. J.; Lee, S. R.; Yoon, J. G.; Moon, H. R.; Zhang, J.; Park, E.; Yoon, S. I.; Cho, J. A. , Ferulic Acid as a Protective Antioxidant of Human Intestinal Epithelial Cells. Antioxidants 2022, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Dhiman, M.; Mantha, A. K. Ferulic acid: A natural antioxidant with application towards neuroprotection against Alzheimer's disease. In Funct. Foods Health Dis.; Springer: Singapore, 2018; pp. 575–586. [Google Scholar]
- Wang, Y.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Yu, J.; Chen, H.; He, J.; Luo, Y.; Zheng, P. , Dietary ferulic acid supplementation improves antioxidant capacity and lipid metabolism in weaned piglets. Nutrients 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. , Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zduńska-Pęciak, K.; Kołodziejczak, A.; Rotsztejn, H. , Two superior antioxidants: Ferulic acid and ascorbic acid in reducing signs of photoaging—A split-face comparative study. Dermatol. Ther. 2022, 35, e15254. [Google Scholar] [CrossRef]
- Azadfar, M.; Gao, A. H.; Chen, S. , Structural characterization of lignin: A potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015, 75, 58–66. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Y.; Shi, J.; Mohamed, S. R.; Xu, J.; Liu, X. , The Antioxidant Guaiacol Exerts Fungicidal Activity Against Fungal Growth and Deoxynivalenol Production in Fusarium graminearum. Front. Microbiol. 2021, 12, 762844. [Google Scholar] [CrossRef] [PubMed]
- Bortolomeazzi, R.; Sebastianutto, N.; Toniolo, R.; Pizzariello, A. , Comparative evaluation of the antioxidant capacity of smoke flavouring phenols by crocin bleaching inhibition, DPPH radical scavenging and oxidation potential. Food Chem. 2007, 100, 1481–1489. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, D. , Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [CrossRef]
- Gao, Q.; Li, Y.; Li, Y.; Zhang, Z.; Liang, Y. , Antioxidant and prooxidant activities of phenolic acids commonly existed in vegetablesand their relationship with structures. Food Sci. Technol. (Brazil) 2022, 42, e07622. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. , Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, S.; Li, F.; Zhang, B.; Qu, Y.; Sun, T.; Luo, T.; Li, D. , Investigation of antioxidant interactions between radix Astragali and Cimicifuga foetida and identification of synergistic antioxidant compounds. PLoS One 2014, 9, e87221. [Google Scholar] [CrossRef]
- Vieira, A. J. S. C.; Telo, J. P.; Pereira, H. F.; Patrocínio, P. F.; Dias, R. M. B. Antioxidant effect of naturally occurring xanthines on the oxidative damage of DNA bases. J. Chim. Phys. Phys.-Chim. Biol. 1999, 96, 116–123. [Google Scholar] [CrossRef]
- Boz, H. , p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Liu, X.; Ji, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. , p-Coumaric acid induces antioxidant capacity and defense responses of sweet cherry fruit to fungal pathogens. Postharvest Biol. Technol. 2020, 169, 111297. [Google Scholar] [CrossRef]
- Mozaffari Godarzi, S.; Valizade Gorji, A.; Gholizadeh, B.; Mard, S. A.; Mansouri, E. , Antioxidant effect of p-coumaric acid on interleukin 1-β and tumor necrosis factor-α in rats with renal ischemic reperfusion. Nefrologia 2020, 40, 311–319. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. , In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Zang, L. Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol. - Cell Physiol. 2000, 279, C954–C960. [Google Scholar] [CrossRef]
- Adefegha, S. A.; Oboh, G.; Ejakpovi, I. I.; Oyeleye, S. I. , Antioxidant and antidiabetic effects of gallic and protocatechuic acids: a structure–function perspective. Comp. Clin. Pathol. 2015, 24, 1579–1585. [Google Scholar] [CrossRef]
- El-Sonbaty, Y. A.; Suddek, G. M.; Megahed, N.; Gameil, N. M. , Protocatechuic acid exhibits hepatoprotective, vasculoprotective, antioxidant and insulin-like effects in dexamethasone-induced insulin-resistant rats. Biochimie 2019, 167, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Graton, M. E.; Ferreira, B. H. S. H.; Troiano, J. A.; Potje, S. R.; Vale, G. T.; Nakamune, A. C. M. S.; Tirapelli, C. R.; Miller, F. J.; Ximenes, V. F.; Antoniali, C. , Comparative study between apocynin and protocatechuic acid regarding antioxidant capacity and vascular effects. Front. Physiol. 2022, 13, 1047916. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, Q.; Ma, W.; Li, J.; Qu, L.; Wang, M. , Protocatechuic Acid Ameliorated Palmitic-Acid-Induced Oxidative Damage in Endothelial Cells through Activating Endogenous Antioxidant Enzymes via an Adenosine-Monophosphate-Activated-Protein-Kinase-Dependent Pathway. J. Agric. Food Chem. 2018, 66, 10400–10409. [Google Scholar] [CrossRef] [PubMed]
- Harini, R.; Pugalendi, K. V. , Antioxidant and antihyperlipidaemic activity of protocatechuic acid on streptozotocindiabetic rats. Redox Rep. 2010, 15, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hyogo, A.; Kobayashi, T.; del Saz, E. G.; Seguchi, H. , Antioxidant effects of protocatechuic acid, ferulic acid, and caffeic acid in human neutrophils using a fluorescent substance. Int J Morphol 2010, 28, 911–920. [Google Scholar] [CrossRef]
- Jiang, S. Q.; Chen, Z. L.; Zhang, S.; Ye, J. L.; Wang, Y. B. , Protective effects of protocatechuic acid on growth performance, intestinal barrier and antioxidant capacity in broilers challenged with lipopolysaccharide. Animal 2023, 17, 100693. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, D.; Chen, S. , Antioxidant activity and mechanism of protocatechuic acid in Vitro. Funct. Foods Health Dis. 2011, 1, 232–244. [Google Scholar] [CrossRef]
- Menezes, V. G.; Santos, J. M. S.; Macedo, T. J. S.; Lins, T. L. B. G.; Barberino, R. S.; Gouveia, B. B.; Bezerra, M. É. S.; Cavalcante, A. Y. P.; Queiroz, M. A. A.; Palheta, R. C.; Matos, M. H. T. , Use of protocatechuic acid as the sole antioxidant in the base medium for in vitro culture of ovine isolated secondary follicles. Reprod. Domest. Anim. 2017, 52, 890–898. [Google Scholar] [CrossRef]
- Safaeian, L.; Emami, R.; Hajhashemi, V.; Haghighatian, Z. , Antihypertensive and antioxidant effects of protocatechuic acid in deoxycorticosterone acetate-salt hypertensive rats. Biomed. Pharmacother. 2018, 100, 147–155. [Google Scholar] [CrossRef]
- Varì, R.; D'Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. , Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef]
- Yang, L.; Chen, X.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Zheng, P.; Chen, H.; Yan, H.; Huang, Z. , Effects of protocatechuic acid on antioxidant capacity, mitochondrial biogenesis and skeletal muscle fiber transformation. J. Nutr. Biochem. 2023, 116, 109327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. , Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid. Based Complement. Alternat. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, G. F.; Liu, X. Z.; An, L. J.; Guan, S. , Anti-ageing effects of protocatechuic acid from Alpinia on spleen and liver antioxidative system of senescent mice. Cell Biochem. Funct. 2011, 29, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Jamuna, S.; Karthika, K.; Paulsamy, S.; Thenmozhi, K.; Kathiravan, S.; Venkatesh, R. , Confertin and scopoletin from leaf and root extracts of Hypochaeris radicata have anti-inflammatory and antioxidant activities. Ind. Crops Prod. 2015, 70, 221–230. [Google Scholar] [CrossRef]
- Lee, H. I.; Lee, M. K. , Effects of scopoletin supplementation on insulin resistance and antioxidant defense system in chronic alcohol-fed rats. J. Korean Soc. Food Sci. Nutr. 2015, 44, 173–181. [Google Scholar] [CrossRef]
- Malik, A.; Kushnoor, A.; Saini, V.; Singhal, S.; Kumar, S.; Yadav, Y. C. , In vitro antioxidant properties of Scopoletin. J. Chem. Pharm. Res. 2011, 3, 659–665. [Google Scholar]
- Mogana, R.; Teng-Jin, K.; Wiart, C. , Anti-inflammatory, anticholinesterase, and antioxidant potential of scopoletin isolated from. Canarium patenti nervium Miq. (Burseraceae Kunth). Evid. Based Complement. Alternat. Med. 2013, 2013, 734824. [CrossRef]
- Panda, S.; Kar, A. , Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelos leaves in hyperthyroid rats. Phytother. Res. 2006, 20, 1103–1105. [Google Scholar] [CrossRef]
- Shaw, C. Y.; Chen, C. H.; Hsu, C. C.; Chen, C. C.; Tsai, Y. C. , Antioxidant properties of scopoletin isolated from Sinomonium acutum. Phytother. Res. 2003, 17, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Alawsy, T. T. J.; Al-Jumaili, E. F. Antioxidant activity of tannic acid purified from sumac seeds (Rhus coriaria L.): ItS scavenging effect on free radicaland active oxygen. Plant Arch. 2020, 20, 2901–2906. [Google Scholar]
- Daré, R. G.; Nakamura, C. V.; Ximenes, V. F.; Lautenschlager, S. O. S. , Tannic acid, a promising anti-photoaging agent: Evidences of its antioxidant and anti-wrinkle potentials, and its ability to prevent photodamage and MMP-1 expression in L929 fibroblasts exposed to UVB. Free Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef]
- Esmaiel, E. M.; Abo-Youssef, A. M.; Tohamy, M. A. , Antidiabetic and antioxidant effects of tannic acid and melatonin on streptozotocin induced diabetes in rats. Pak. J. Pharm. Sci. 2019, 32, 1453–1459. [Google Scholar]
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H. Y. , Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Karakurt, S.; Adali, O. , Tannic acid inhibits proliferation, migration, invasion of prostate cancer and modulates drug metabolizing and antioxidant enzymes. Anticancer Agents Med. Chem. 2016, 16, 781–789. [Google Scholar] [CrossRef]
- Lou, W.; Chen, Y.; Ma, H.; Liang, G.; Liu, B. , Antioxidant and α-amylase inhibitory activities of tannic acid. J. Food Sci. Technol. 2018, 55, 3640–3646. [Google Scholar] [CrossRef]
- Türkan, F.; Taslimi, P.; Saltan, F. Z. , Tannic acid as a natural antioxidant compound: Discovery of a potent metabolic enzyme inhibitor for a new therapeutic approach in diabetes and Alzheimer's disease. J. Biochem. Mol. Toxicol. 2019, 33, e22340. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Liu, S.; Zhuang, Y.; Yang, H.; Li, Y.; Chen, S.; Wang, L.; Yin, L.; Yao, Y.; He, S. , Tannic acid modulates intestinal barrier functions associated with intestinal morphology, antioxidative activity, and intestinal tight junction in a diquat-induced mouse model. RSC Adv. 2019, 9, 31988–31998. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Wang, L.; Yin, L.; Yang, H.; Chen, C.; Zheng, Q.; He, S. Tannic acid attenuates intestinal oxidative damage by improving antioxidant capacity and intestinal barrier in weaned piglets and IPEC-J2 cells. Front. Nutr. 2022, 9, 1012207. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Chen, J.; Guo, S.; Wang, S.; Liu, Z.; Zheng, L.; Qi, Y.; Xu, P.; Li, L.; Zhang, Z.; Ding, B. , Effects of tannic acid on growth performance, relative organ weight, antioxidative status, and intestinal histomorphology in broilers exposed to aflatoxin B1. Front. Vet. Sci. 2022, 9, 1037046. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Sun, X.; Dai, X.; Gu, C.; Gu, M.; Wang, A.; Wei, W.; Yang, S. , Effects of Tannic Acid on Antioxidant Activity and Ovarian Development in Adolescent and Adult Female Brandt’s Voles. Reprod. Sci. 2021, 28, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Hadi, N.; Khan, N. U.; Hadi, S. M. , Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 2003, 9, BR325–BR330. [Google Scholar] [PubMed]
- Wu, F.; Liu, R.; Shen, X.; Xu, H.; Sheng, L. Study on the interaction and antioxidant activity of theophylline and theobromine with SOD by spectra and calculation. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2019, 215, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Ekin, S.; Yildirim, S.; Akkoyun, M. B.; Gok, H. N.; Arihan, O.; Oto, G.; Akkoyun, T.; Basbugan, Y.; Aslan, S. , Theophylline attenuates bleomycin-induced oxidative stress in rats: The role of IL-6, NF-κB, and antioxidant enzymes. Braz. J. Pharm. Sci. 2022, 58, e20804. [Google Scholar] [CrossRef]
- Santos, P. M. P.; Silva, S. A. G.; Justino, G. C.; Vieira, A. J. S. C. , Demethylation of theophylline (1,3-dimethylxanthine) to 1-methylxanthine: The first step of an antioxidising cascade. Redox Rep. 2010, 15, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prahalathan, P.; Raja, B. , Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in L-NAME-induced hypertensive rats: A dose-dependence study. Redox Rep. 2011, 16, 208–215. [Google Scholar] [CrossRef]
- Salau, V. F.; Erukainure, O. L.; Ibeji, C. U.; Olasehinde, T. A.; Koorbanally, N. A.; Islam, M. S. , Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Jamali, Z.; Bayrami, D.; Salimi, A. , Vanillic acid alleviates methamphetamine-induced mitochondrial toxicity in cardiac mitochondria via antioxidant activity and inhibition of MPT Pore opening: an in-vitro study. BMC Pharmacol. Toxicol. 2023, 24, 33. [Google Scholar] [CrossRef]
- Stanely Mainzen Prince, P.; Rajakumar, S.; Dhanasekar, K. , Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur. J. Pharmacol. 2011, 668, 233–240. [Google Scholar] [CrossRef]
- Vinothiya, K.; Ashokkumar, N. , Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed. Pharmacother. 2017, 87, 640–652. [Google Scholar] [CrossRef]
- Gonzalez-Baro, A. C.; Izquierdo, D.; Heras, A.; Colina, A. , UV/Vis spectroelectrochemistry of o-vanillin: Study of the antioxidant properties. J. Electroanal. Chem. 2020, 859, 113844. [Google Scholar] [CrossRef]
- Kamat, J. P.; Ghosh, A.; Devasagayam, T. P. A. , Vanillin as an antioxidant in rat liver mitochondria: Inhibition of protein oxidation and lipid peroxidation induced by photosensitization. Mol. Cell. Biochem. 2000, 209, 47–53. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta - Gen. Subj. 2011, 1810, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Fauziah, N.; Herdiman, H.; Afni, M.; Afifah, E.; Kusuma, H. S. W.; Nufus, H.; Arumwardana, S.; Rihibiha, D. D. , Antioxidant and anti aging assays of Oryza sativa extracts, vanillin and coumaric acid. J. Nat. Remedies 2016, 16, 88–99. [Google Scholar] [CrossRef]
- Xiong, S.; Li, R.; Ye, S.; Ni, P.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.; Zhang, X. , Vanillin enhances the antibacterial and antioxidant properties of polyvinyl alcohol-chitosan hydrogel dressings. Int. J. Biol. Macromol. 2022, 220, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Yazama, F. , Antioxidant properties of ethyl vanillin in vitro and in vivo. Biosci. Biotechnol. Biochem. 2011, 75, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Groborz, K.; Wołoszynowska, M.; Ratusz, K.; Białecka-florjańczyk, E.; Fabiszewska, A. , Enzymatic synthesis of lipophilic esters of phenolic compounds, evaluation of their antioxidant activity and effect on the oxidative stability of selected oils. Biomolecules 2021, 11, 314. [Google Scholar] [CrossRef]
- Bielski, B. H. J.; Arudi, R. L.; Sutherland, M. W. , A study of the reactivity of HO2/O2 - with unsaturated fatty acids. J. Biological. Chem. 1983, 258, 4759–4761. [Google Scholar] [CrossRef]
- Terpinc, P.; Abramovič, H. , A kinetic approach for evaluation of the antioxidant activity of selected phenolic acids. Food Chem. 2010, 121, 366–371. [Google Scholar] [CrossRef]
- Sies, H. , Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Masuda, T.; Yamada, K.; Maekawa, T.; Takeda, Y.; Yamaguchi, H. , Antioxidant mechanism studies on ferulic acid: Identification of oxidative coupling products from methyl ferulate and linoleate. J. Agric. Food Chem. 2006, 54, 6069–6074. [Google Scholar] [CrossRef]
- Masuda, T.; Yamada, K.; Maekawa, T.; Takeda, Y.; Yamaguchi, H. , Antioxidant mechanism studies on ferulic acid: Isolation and structure identification of the main antioxidation product from methyl ferulate. Food Sci. Technol. Res. 2006, 12, 173–177. [Google Scholar] [CrossRef]
- Miche, H.; Brumas, V.; Berthon, G. Copper(II) interactions with nonsteroidal antiinflammatory agents. II. Anthranilic acid as a potential OH-inactivating ligand. J. Inorg. Biochem. 1997, 68, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, S.; Bouchaut, M.; Brumas, V.; Berthon, G. Copper-ligand interactions and physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic. Res. 2000, 32, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Steenken, S.; Jovanovic, S. V. , How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J. R. , On the evolution of one-electron-oxidized deoxyguanosine in damaged DNA under physiological conditions: A DFT and ONIOM study on proton transfer and equilibrium. Phys. Chem. Chem. Phys. 2012, 14, 12476–12484. [Google Scholar] [CrossRef] [PubMed]
- Tronche, C.; Goodman, B. K.; Greenberg, M. M. , DNA damage induced via independent generation of the radical resulting from formal hydrogen atom abstraction from the C1'-position of a nucleotide. Chem. Biol. 1998, 5, 263–271. [Google Scholar] [CrossRef]
- Pogozelski, W. K.; Tullius, T. D. , Oxidative Strand Scission of Nucleic Acids: Routes Initiated by Hydrogen Abstraction from the Sugar Moiety. Chem. Rev. 1998, 98, 1089–1108. [Google Scholar] [CrossRef]
- Dedon, P. C. , The Chemical Toxicology of 2-Deoxyribose Oxidation in DNA. Chem. Res. Toxicol. 2007, 21, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cui, Y.; Niedernhofer, L. J.; Wang, Y. , Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage. Chem. Res. Toxicol. 2016, 29, 2008–2039. [Google Scholar] [CrossRef]
- Pérez-González, A.; Castañeda-Arriaga, R.; Álvarez-Idaboy, J. R.; Reiter, R. J.; Galano, A. , Melatonin and its metabolites as chemical agents capable of directly repairing oxidized DNA. J. Pineal Res. 2019, 66, e12539. [Google Scholar] [CrossRef]
- Galano, A.; Francisco-Marquez, M. , Reactions of OOH radical with β-carotene, lycopene, and torulene: Hydrogen atom transfer and adduct formation mechanisms. J. Phys. Chem. B 2009, 113, 11338–11345. [Google Scholar] [CrossRef]
- Mortensen, A. , Scavenging of benzylperoxyl radicals by carotenoids. Free Radic. Res. 2002, 36, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D. C.; McClure, T. D. , Antioxidant reactions of β-carotene: Identification of carotenoid-radical adducts. Chem. Res. Toxicol. 1996, 9, 8–11. [Google Scholar] [CrossRef]
- Joshi, R.; Gangabhagirathi, R.; Venu, S.; Adhikari, S.; Mukherjee, T. , Antioxidant activity and free radical scavenging reactions of gentisic acid: In-vitro and pulse radiolysis studies. Free Radic. Res. 2012, 46, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S. B.; Kamat, J. P.; Naik, D. B. , Antioxidant activity and free radical scavenging reactions of hydroxybenzyl alcohols. Biochemical and pulse radiolysis studies. Chem. Biol. Interact. 2009, 182, 119–127. [Google Scholar] [CrossRef]
- Hata, K.; Lin, M.; Katsumura, Y.; Muroya, Y.; Fu, H.; Yamashita, S.; Nakagawa, H. Pulse radiolysis study on free radical scavenger edaravone(3-methyl-1-phenyl-2-pyrazolin-5-one).2: A comparative study on edaravone derivatives. J. Radiat. Res. 2011, 52, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Galano, A. , OH radical scavenging activity of edaravone: Mechanism and kinetics. J. Phys. Chem. B 2011, 115, 1306–1314. [Google Scholar] [CrossRef]
- Galano, A. , On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 2011, 13, 7178–7188. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D. X.; Reiter, R. J. , On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J Pineal Res 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D. X.; Reiter, R. J. , Cyclic 3-hydroxymelatonin, a key metabolite enhancing the peroxyl radical scavenging activity of melatonin. RSC Adv. 2014, 4, 5220–5227. [Google Scholar] [CrossRef]
- Tamba, M.; Torreggiani, A. , Hydroxyl radical scavenging by carnosine and Cu(II)-carnosine complexes: A pulse-radiolysis and spectroscopic study. Int. J. Radiat. Biol. 1999, 75, 1177–1188. [Google Scholar]
- Sakurai, K.; Sasabe, H.; Koga, T.; Konishi, T. , Mechanism of hydroxyl radical scavenging by rebamipide: Identification of mono-hydroxylated rebamipide as as a major reaction product. Free Radic. Res. 2004, 38, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A. , The role of electron-transfer and H-atom donation on the superb antioxidant activity and free radical reaction of curcumin. Food Chem. 2012, 135, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Touriño, S.; Lizárraga, D.; Carreras, A.; Lorenzo, S.; Ugartondo, V.; Mitjans, M.; Vinardell, M. P.; Julía, L.; Cascante, M.; Torres, J. L. , Highly galloylated tannin fractions from witch hazel (Hamamelis virginiana) bark: Electron transfer capacity, in vitro antioxidant activity, and effects on skin-related cells. Chem. Res. Toxicol. 2008, 21, 696–704. [Google Scholar] [CrossRef]
- Pérez-González, A.; Galano, A. , On the outstanding antioxidant capacity of edaravone derivatives through single electron transfer reactions. J. Phys. Chem. B 2012, 116, 1180–1188. [Google Scholar] [CrossRef]
- Nakanishi, I.; Shimada, T.; Ohkubo, K.; Manda, S.; Shimizu, T.; Urano, S.; Okuda, H.; Miyata, N.; Ozawa, T.; Anzai, K.; Fukuzumi, S.; Ikota, N.; Fukuhara, K. , Involvement of electron transfer in the radical-scavenging reaction of resveratrol. Chem. Lett. 2007, 36, 1276–1277. [Google Scholar] [CrossRef]
- Nakanishi, I.; Ohkubo, K.; Miyazaki, K.; Hakamata, W.; Urano, S.; Ozawa, T.; Okuda, H.; Fukuzumi, S.; Ikota, N.; Fukuhara, K. , A Planar Catechin Analogue Having a More Negative Oxidation Potential than (+)-Catechin as an Electron Transfer Antioxidant against a Peroxyl Radical. Chem. Res. Toxicol. 2004, 17, 26–31. [Google Scholar] [CrossRef]
- Hill, T. J.; Land, E. J.; McGarvey, D. J.; Schalch, W.; Tinkler, J. H.; Truscott, T. G. , Interactions between carotenoids and the CCl3O2 • radical. J. Am. Chem. Soc. 1995, 117, 8322–8326. [Google Scholar] [CrossRef]
- Packer, J. E.; Mahood, J. S.; Mora-Arellano, V. O.; Slater, T. F.; Willson, R. L.; Wolfenden, B. S. , Free radicals and singlet oxygen scavengers: Reaction of a peroxy-radical with β-carotene, diphenyl furan and 1,4-diazobicyclo(2,2,2)-octane. Biochem. Biophys. Res. Commun. 1981, 98, 901–906. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L. H.; Sampson, J.; Rice-Evans, C.; Everett, S. A. , Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997, 418, 91–97. [Google Scholar] [CrossRef]
- Everett, S. A.; Kundu, S. C.; Maddix, S.; Willson, R. L. , Mechanisms of free-radical scavenging by the nutritional antioxidant β-carotene. Biochem. Soc. Trans. 1995, 23, 230S. [Google Scholar] [CrossRef]
- Cao, L.; Yu, H.; Shao, S.; Wang, S.; Guo, Y. , Evaluating the antioxidant capacity of polyphenols with an off-on fluorescence probe and the mechanism study. Anal. Methods 2014, 6, 7149–7153. [Google Scholar] [CrossRef]
- Mikulski, D.; Eder, K.; Molski, M. , Quantum-chemical study on relationship between structure and antioxidant properties of hepatoprotective compounds occurring in Cynara scolymus and Silybum marianum. J. Theor. Comput. Chem. 2014, 13, 1450004. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A. M.; Castro-Arredondo, S. I.; Balandrán-Quintana, R. R. , Computational study of the structure-free radical scavenging relationship of procyanidins. Food Chem. 2014, 161, 155–161. [Google Scholar] [CrossRef]
- Wang, G.; Xue, Y.; An, L.; Zheng, Y.; Dou, Y.; Zhang, L.; Liu, Y. , Theoretical study on the structural and antioxidant properties of some recently synthesised 2,4,5-trimethoxy chalcones. Food Chem. 2014, 171, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Praveena, R.; Sadasivam, K.; Deepha, V.; Sivakumar, R. , Antioxidant potential of orientin: A combined experimental and DFT approach. J. Mol. Struct. 2014, 1061, 114–123. [Google Scholar] [CrossRef]
- Galano, A.; Martínez, A. , Capsaicin, a tasty free radical scavenger: Mechanism of action and kinetics. J. Phys. Chem. B. 2012, 116, 1200–1208. [Google Scholar] [CrossRef]
- Martínez, A.; Galano, A.; Vargas, R. , Free radical scavenger properties of α-mangostin: Thermodynamics and kinetics of HAT and RAF mechanisms. J. Phys. Chem. B 2011, 115, 12591–12598. [Google Scholar] [CrossRef]
- Dimitrić Marković, J. M.; Milenković, D.; Amić, D.; Mojović, M.; Pašti, I.; Marković, Z. S. , The preferred radical scavenging mechanisms of fisetin and baicalein towards oxygen-centred radicals in polar protic and polar aprotic solvents. RSC Adv. 2014, 4, 32228–32236. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Zhang, L.; Qian, Y.; Yu, D.; Gong, X.; Liu, Y. , A theoretical study of the structure-radical scavenging activity of hydroxychalcones. Comput. Theor. Chem. 2012, 982, 74–83. [Google Scholar] [CrossRef]
- Mazzone, G.; Toscano, M.; Russo, N. , Density functional predictions of antioxidant activity and UV spectral features of nasutin A, isonasutin, ellagic acid, and one of its possible derivatives. J. Agric. Food Chem. 2013, 61, 9650–9657. [Google Scholar] [CrossRef]
- Castañeda-Arriaga, R.; Alvarez-Idaboy, J. R. , Lipoic acid and dihydrolipoic acid. A comprehensive theoretical study of their antioxidant activity supported by available experimental kinetic data. J. Chem. Inf. Model. 2014, 54, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Alvarez-Idaboy, J. R. , Glutathione: Mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011, 1, 1763–1771. [Google Scholar] [CrossRef]
- Farmanzadeh, D.; Najafi, M. , On the antioxidant activity of the tryptophan derivatives. Bull. Chem. Soc. Jpn. 2013, 86, 1041–1050. [Google Scholar] [CrossRef]
- Galano, A. , Mechanism and kinetics of the hydroxyl and hydroperoxyl radical scavenging activity of N-acetylcysteine amide. Theor. Chem. Acc. 2011, 130, 51–60. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K. U. , Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef]
- Galano, A.; Álvarez-Diduk, R.; Ramírez-Silva, M. T.; Alarcón-Ángeles, G.; Rojas-Hernández, A. , Role of the reacting free radicals on the antioxidant mechanism of curcumin. Chem. Phys. 2009, 363, 13–23. [Google Scholar] [CrossRef]
- Medina, M. E.; Galano, A.; Alvarez-Idaboy, J. R. , Theoretical study on the peroxyl radicals scavenging activity of esculetin and its regeneration in aqueous solution. Phys. Chem. Chem. Phys. 2014, 16, 1197–1207. [Google Scholar] [CrossRef]
- Jeremić, S.; Filipović, N.; Peulić, A.; Marković, Z. , Thermodynamical aspect of radical scavenging activity of alizarin and alizarin red S. Theoretical comparative study. Comput. Theor. Chem. 2014, 1047, 15–21. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. , Density functional theory study of the structure-antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Marković, Z.; Crossed D Signorović, J.; Dimitrić Marković, J. M.; Živić, M.; Amić, D. , Investigation of the radical scavenging potency of hydroxybenzoic acids and their carboxylate anions. Monatsh. Chem. 2014, 145, 953–962. [Google Scholar] [CrossRef]
- Pérez-González, A.; Galano, A.; Alvarez-Idaboy, J. R. , Dihydroxybenzoic acids as free radical scavengers: Mechanisms, kinetics, and trends in activity. New J. Chem. 2014, 38, 2639–2652. [Google Scholar] [CrossRef]
- Urbaniak, A.; Szelag, M.; Molski, M. , Theoretical investigation of stereochemistry and solvent influence on antioxidant activity of ferulic acid. Comput. Theor. Chem. 2013, 1012, 33–40. [Google Scholar] [CrossRef]
- Fifen, J. J.; Nsangou, M.; Dhaouadi, Z.; Motapon, O.; Jaidane, N. , Solvent effects on the antioxidant activity of 3,4-dihydroxyphenylpyruvic acid: DFT and TD-DFT studies. Comput. Theor. Chem. 2011, 966, 232–243. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J. R.; Russo, N. , Antioxidant activity of trans -resveratrol toward hydroxyl and hydroperoxyl radicals: A quantum chemical and computational kinetics study. J. Org. Chem. 2012, 77, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Benayahoum, A.; Amira-Guebailia, H.; Houache, O. , Homolytic and heterolytic O-H bond cleavage in trans-resveratrol and some phenantrene analogs: A theoretical study. Comput. Theor. Chem. 2014, 1037, 1–9. [Google Scholar] [CrossRef]
- Medina, M. E.; Iuga, C.; Álvarez-Idaboy, J. R. , Antioxidant activity of fraxetin and its regeneration in aqueous media. A density functional theory study. RSC Adv. 2014, 4, 52920–52932. [Google Scholar] [CrossRef]
- Cordova-Gomez, M.; Galano, A.; Alvarez-Idaboy, J. R. , Piceatannol, a better peroxyl radical scavenger than resveratrol. RSC Adv. 2013, 3, 20209–20218. [Google Scholar] [CrossRef]
- Marković, Z.; Milenković, D.; Orović, J.; Dimitrić Marković, J. M.; Stepanić, V.; Lučić, B.; Amić, D. , Free radical scavenging activity of morin 2′-O- phenoxide anion. Food Chem. 2012, 135, 2070–2077. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, L.; Li, Y.; Yu, D.; Zheng, Y.; An, L.; Gong, X.; Liu, Y. , A DFT study on the structure and radical scavenging activity of newly synthesized hydroxychalcones. J. Phys. Org. Chem. 2013, 26, 240–248. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; Zhang, L.; Wu, W.; Yu, D.; Liu, Y. , Theoretical study on the antioxidant properties of 2′- hydroxychalcones: H-atom vs. electron transfer mechanism. J. Mol. Model. 2013, 19, 3851–3862. [Google Scholar] [CrossRef]
- Qian, Y. P.; Shang, Y. J.; Teng, Q. F.; Chang, J.; Fan, G. J.; Wei, X.; Li, R. R.; Li, H. P.; Yao, X. J.; Dai, F.; Zhou, B. , Hydroxychalcones as potent antioxidants: Structure-activity relationship analysis and mechanism considerations. Food Chem. 2011, 126, 241–248. [Google Scholar] [CrossRef]
- Martínez, A.; Hernández-Marin, E.; Galano, A. , Xanthones as antioxidants: A theoretical study on the thermodynamics and kinetics of the single electron transfer mechanism. Food Funct. 2012, 3, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Kuzmicz, R.; Pawlowski, T. S.; Litwinienko, G. , Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J. L.; Olivier, Y.; Trouillas, P. , Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Dimitrić Marković, J. M.; Milenković, D.; Amić, D.; Popović-Bijelić, A.; Mojović, M.; Pašti, I. A.; Marković, Z. S. , Energy requirements of the reactions of kaempferol and selected radical species in different media: towards the prediction of the possible radical scavenging mechanisms. Struct. Chem. 2014, 25, 1795–1804. [Google Scholar] [CrossRef]
- Dorović, J.; Marković, J. M. D.; Stepanić, V.; Begović, N.; Amić, D.; Marković, Z. , Influence of different free radicals on scavenging potency of gallic acid. J. Mol. Model. 2014, 20, 2345. [Google Scholar] [CrossRef]
- Alberto, M. E.; Russo, N.; Grand, A.; Galano, A. , A physicochemical examination of the free radical scavenging activity of Trolox: Mechanism, kinetics and influence of the environment. Phys. Chem. Chem. Phys. 2013, 15, 4642–4650. [Google Scholar] [CrossRef]
- Lengyel, J.; Rimarčík, J.; Vagánek, A.; Klein, E. , On the radical scavenging activity of isoflavones: Thermodynamics of O-H bond cleavage. Phys. Chem. Chem. Phys. 2013, 15, 10895–10903. [Google Scholar] [CrossRef]
- Senthil kumar, K.; Kumaresan, R. , A DFT study on the structural, electronic properties and radical scavenging mechanisms of calycosin, glycitein, pratensein and prunetin. Comput. Theor. Chem. 2012, 985, 14–22. [Google Scholar] [CrossRef]
- Marković, Z. S.; Dimitrić Marković, J. M.; Milenković, D.; Filipović, N. , Mechanistic study of the structure-activity relationship for the free radical scavenging activity of baicalein. J. Mol. Model. 2011, 17, 2575–2584. [Google Scholar] [CrossRef]
- Jeremić, S. R.; Šehović, S. F.; Manojlović, N. T.; Marković, Z. S. , Antioxidant and free radical scavenging activity of purpurin. Monatsh. Chem. 2012, 143, 427–435. [Google Scholar] [CrossRef]
- Marković, Z. S.; Marković, S.; Dimitrić Marković, J. M.; Milenković, D. , Structure and reactivity of baicalein radical cation. Int. J. Quantum Chem. 2012, 112, 2009–2017. [Google Scholar] [CrossRef]
- Focsan, A. L.; Pan, S.; Kispert, L. D. , Electrochemical study of astaxanthin and astaxanthin n-octanoic monoester and diester: Tendency to form radicals. J. Phys. Chem. B 2014, 118, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Amić, D.; Milenković, D.; Dimitrić-Marković, J. M.; Marković, S. , Examination of the chemical behavior of the quercetin radical cation towards some bases. Phys. Chem. Chem. Phys. 2013, 15, 7370–7378. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Kawashima, T.; Ohkubo, K.; Kanazawa, H.; Inami, K.; Mochizuki, M.; Fukuhara, K.; Okuda, H.; Ozawa, T.; Itoh, S.; Fukuzumi, S.; Ikota, N. , Electron-transfer mechanism in radical-scavenging reactions by a vitamin E model in a protic medium. Org. Biomol. Chem. 2005, 3, 626–629. [Google Scholar] [CrossRef]
- Ouchi, A.; Nagaoka, S. I.; Abe, K.; Mukai, K. , Kinetic study of the aroxyl radical-scavenging reaction of α-tocopherol in methanol solution: Notable effect of the alkali and alkaline earth metal salts on the reaction rates. J. Phys. Chem. B 2009, 113, 13322–13331. [Google Scholar] [CrossRef]
- Galano, A.; Francisco Marquez, M.; Pérez-González, A. Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef]
- Medina, M. E.; Iuga, C.; Alvarez-Idaboy, J. R. , Antioxidant activity of propyl gallate in aqueous and lipid media: A theoretical study. Phys. Chem. Chem. Phys. 2013, 15, 13137–13146. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G. F.; Cabrini, L.; Zambonin, L.; Landi, L. , Solvent and pH effects on the antioxidant activity of caffeic and other phenolic acids. J. Agric. Food Chem. 2006, 54, 2932–2937. [Google Scholar] [CrossRef]
- Marino, T.; Galano, A.; Russo, N. , Radical Scavenging Ability of Gallic Acid toward OH and OOH Radicals. Reaction Mechanism and Rate Constants from the Density Functional Theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef]
- Galano, A.; León-Carmona, J. R.; Alvarez-Idaboy, J. R. , Influence of the environment on the protective effects of guaiacol derivatives against oxidative stress: Mechanisms, kinetics, and relative antioxidant activity. J. Phys. Chem. B 2012, 116, 7129–7137. [Google Scholar] [CrossRef] [PubMed]
- León-Carmona, J. R.; Galano, A. , Free radical scavenging activity of caffeine's metabolites. Int. J. Quantum Chem. 2012, 112, 3472–3478. [Google Scholar] [CrossRef]
- Galano, A.; Pérez-González, A. , On the free radical scavenging mechanism of protocatechuic acid, regeneration of the catechol group in aqueous solution. Theor. Chem. Acc. 2012, 131, 1265. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K. U. Abnormal Solvent Effects on Hydrogen Atom Abstractions. 1. The Reactions of Phenols with 2,2-Diphenyl-1-picrylhydrazyl (dpph•) in Alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K. U. , Abnormal Solvent Effects on Hydrogen Atom Abstraction. 3. Novel Kinetics in Sequential Proton Loss Electron Transfer Chemistry. J. Org. Chem. 2005, 70, 8982–8990. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K. U. , Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef] [PubMed]
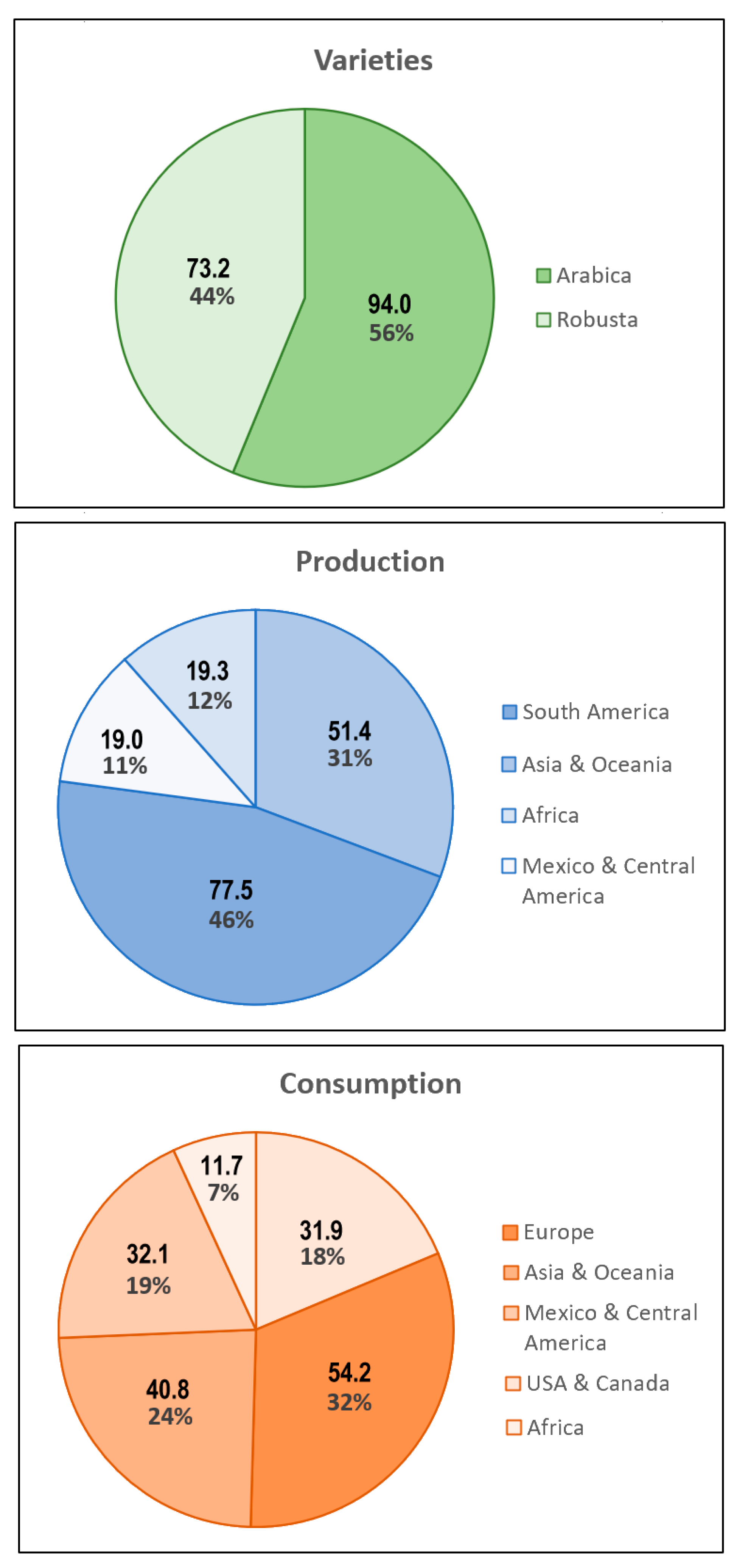

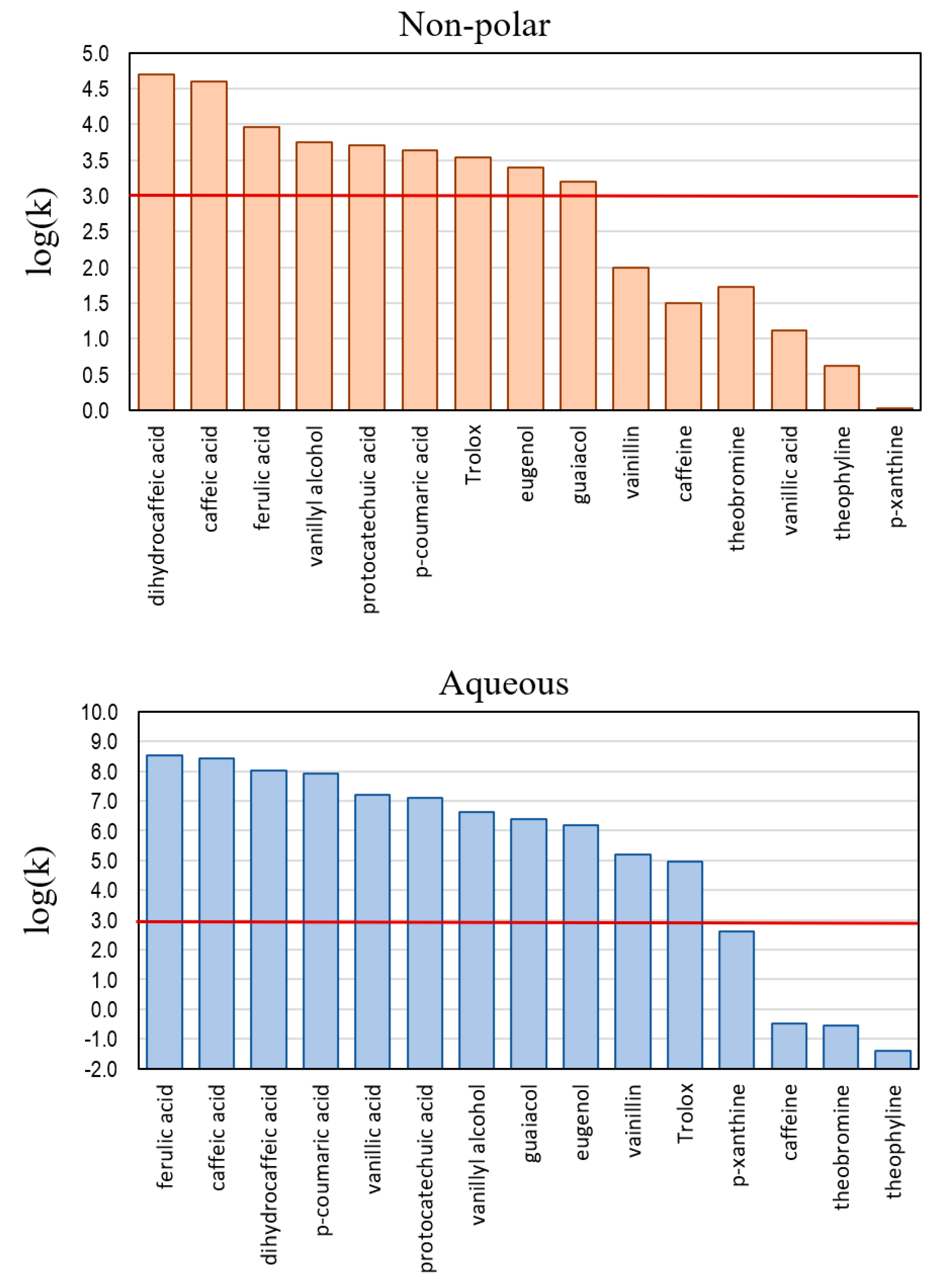
| Family | Compounds | Ref. |
|---|---|---|
| Alkaloids | Caffeine, theobromine, theophyline, trigonelline, nicotinic acid, pyrrolomorpholine spiroketal alkaloids. | [489,490,491,492,493,494] |
| Amino acids | Alanine, asparagine, leucine, glycine, aspartic acid, histidine, phenylalanine, serine, lysine, valine | [495,496,497,498] |
| Carotenoids | β-carotene, α-carotene, violaxanthin, neoxanthin. | [499,500] |
| Fatty acids | Linoleic, palmitic, oleic, stearic, arachic, docosanoic, tetracosanoic, eicosenoic, myristic acid, linolenic acids. | [496,497,501] |
| Flavonoids | Quercetin, catechin, epicatechin, picatechin gallate, kaempferol, luteolin, fisetin, rutin, myricetin, quercitrin, pigenin. | [502,503] |
| Organic acids | Acetic, citric, lactic, quinic, oxalic, malic, succinic, formic, tartaric acids. | [491,504,505,506] |
| Phenolic acids | Caffeic, chlorogenic, cinnamic, p-hydroxybenzoic acid, ferulic, p-coumaric, vanillic, benzoic, protocatechuic, gentosic, cinapic, caftaric acids. | [483,507,508,509,510] |
| Sugars | Sucrose, fructose, glucose, arabinose. | [511,512,513,514] |
| Terpenes | Ursolic acid, caffruones, caffruenols, tricalysiolides, caffarolides, bengalensol, mascarosides, villanovane, atractyligenin, cafestol, kahweol, dehydrocafestol, cafestal. | [490,502,503,515,516,517,518,519] |
| Volatiles | aldehydes, esters, ketones, furans, thiophenes, pyrazine, pyrroles, thiazoles, olefins, alcohols, oxazoles, pyridine, geosmin. | [520,521,522,523,524,525,526] |
| Benefits | Key components | Ref. |
|---|---|---|
| Antibacterial | caffeic acid caffeic acid phenethyl ester chlorogenic acids eugenol ferulic acid furaneol guaiacol isoeugenol protocatechuic acid scopoletin vanillic acid |
[533,534,535] [536,537] [538] [539,540,541,542,543,544,545,546,547] [548,549,550,551] [552] [553,554,555] [547,556,557,558,559] [560,561,562,563,564,565] [566,567,568,569,570,571] [561,572,573] |
| Anticarcinogenic | 4-vinylguaiacol cafestol and kahweol caffeic acid caffeic acid phenethyl ester chlorogenic acids eugenol ferulic acid quercetin mangiferin protocatechuic acid tannic acid theobromine vanillic acid vanillin |
[574,575] [576,577,578,579] [580,581,582,583,584,585] [586,587,588,589,590] [538,591] [592,593,594,595,596,597,598,599,600,601,602,603,604,605,606,607,608] [609,610,611,612,613,614,615,616,617,618,619,620,621,622,623] [624,625,626,627,628] [629] [630,631,632,633,634,635,636] [637,638,639,640,641,642,643,644,645,646] [647,648,649] [650,651,652,653,654] [655,656,657,658,659,660,661,662,663] |
| Antidiabetic | cafestol caffeic acid caffeol chlorogenic acids isoeugenol scopoletin trigonelline |
[664,665] [666,667,668,669,670] [483,671] [666,672,673,674,675,676,677] [678] [679,680,681,682,683] [684] |
| Antifungal | caffeine eugenol furaneol isoeugenol vanillin |
[685] [686,687,688,689,690,691] [552] [692,693,694,695] [696,697,698] |
| Antiinflamatory effects | 4-ethylguaiacol caffeine dicaffeoylquinic acids dihydrocaffeic acid eugenol ferulic acid flavonoids mangiferin phenolic acids and pyrocatechol p-coumaric acid rutin theophylline vanillic acid vanillin vanillyl alcohol |
[699,700,701] [702] [703] [704] [539,705,706,707,708,709,710] [711,712] [629] [703] [629] [713] [714,715,716,717,718,719] [703] [720,721,722,723,724] [725,726,727,728,729,730,731,732] [733,734,735,736,737,738,739] [740] |
| Antiobesity | chlorogenic acids kahweol |
[672,741,742,743,744] [745,746,747] |
| Cardioprotection | caffeic acid chlorogenic acids dihydrocaffeic acid ferulic acid |
[748,749,750,751,752] [591,753,754,755,756] [757] [758,759,760,761,762,763,764] |
| Cognitive enhancement | paraxanthine protocatechuic acid theobromine vanillic acid |
[765,766] [767,768,769,770] [771,772] [773,774] |
| Gastroprotection | chlorogenic acids vanillin |
[591] [775,776] |
| Hepatoprotection | caffeic acid chlorogenic acids dihydrocaffeic acid paraxanthine theobromine vanillin |
[777,778,779,780] [591] [453] [781,782,783] [784] [785,786,787,788] |
| Immunoregulation | p-coumaric acid protocatechuic acid |
[718] [789,790] |
| Kidney protection | protocatechuic acid theobromine |
[791,792,793,794,795] [796,797,798,799] |
| Neuroprotection | caffeine caffeic acid chlorogenic acids dihydrocaffeic acid eugenol ferulic acid isoeugenol paraxanthine protocatechuic acid quercetin scopoletin tannic acid theobromine trigonelline lipid-Lower vanillic acid vanillyl alcohol |
[800,801,802,803,804,805,806,807,808,809,810,811,812,813,814,815,816,817,818,819,820,821] [483,822,823,824] [483,825,826,827,828,829,830,831,832,833,834] [835] [836] [837,838,839,840,841,842,843,844,845,846,847] [836,848] [816,849,850,851,852] [853,854,855,856,857,858,859,860,861,862,863,864,865,866] [624,867,868,869,870,871,872,873,874,875,876] [877,878,879,880,881,882] [883,884,885,886,887,888] [889,890,891] [483] [736,892,893,894,895,896] [897,898,899,900] [901] |
| Lipid-Lowering Effects | caffeic acid chlorogenic acids |
[902] [591,671,902,903] |
| Parent molecule | Ref. |
|---|---|
| caffeic acid | [752,904,905,906,907,908,909,910,911,912,913,914,915] |
| caffeine | [916,917,918,919,920,921,922,923,924] |
| chlorogenic acid | [925,926,927,928,929,930,931,932] |
| eugenol | [933,934,935,936,937,938,939,940,941,942,943,944,945,946,947,948,949,950,951,952] |
| ferulic acid | [609,611,953,954,955,956,957,958,959,960,961,962,963,964,965,966,967,968,969,970,971,972,973,974,975,976,977,978,979,980,981,982,983,984,985] |
| guaiacol | [986,987,988] |
| isoeugenol | [694,989,990,991,992] |
| isoferulic acid | [993,994] |
| p-coumaric acid | [716,995–1001] |
| protocatechuic acid | [1002–1008] |
| scopoletin | [683,1009–1020] |
| theobromine | [1021–1029] |
| theophylline | [1030–1051] |
| vanillic acid | [1052–1058] |
| vanillin | [1059–1085] |
| xanthine | [1086–1109] |
| Common name | Structure | IUPAC name | Ref. |
|---|---|---|---|
| 4-ethylguaiacol |  |
4-ethyl-2-methoxyphenol | [1110–1112] |
| 4-vinylguaiacol | 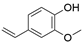 |
4-ethenyl-2-methoxyphenol | [1113–1115] |
| caffeic acid | 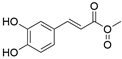 |
(E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | [669,748,1116–1128] |
| caffeine | 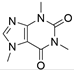 |
1,3,7-trimethylpurine-2,6-dione | [806,1129–1132] |
| chlorogenic acid | 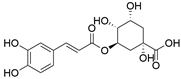 |
(1S,3R,4R,5R)-3-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,4,5-trihydroxycyclohexane-1-carboxylic acid | [404,405,748,1116,1133–1143] |
| dihydrocaffeic acid | 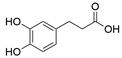 |
3-(3,4-dihydroxyphenyl)propanoic acid | [1144–1146] |
| eugenol |  |
2-methoxy-4-prop-2-enylphenol | [706,707,934,1147–1160] |
| ferulic acid | 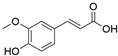 |
(E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid | [711,712,837,1114,1161–1168] |
| guaiacol | 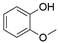 |
2-methoxyphenol | [1169,1170] |
| isoeugenol | 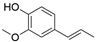 |
2-methoxy-4-[(E)-prop-1-enyl]phenol | [547,559,1171] |
| isoferulic acid | 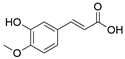 |
(E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoic acid | [1172–1175] |
| paraxanthine | 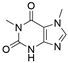 |
1,7-dimethyl-3H-purine-2,6-dione | [1176] |
| p-coumaric acid | 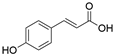 |
(E)-3-(4-hydroxyphenyl)prop-2-enoic acid | [714,1122,1177–1181] |
| protocatechuic acid |  |
3,4-dihydroxybenzoic acid | [561,1182–1195] |
| scopoletin | 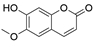 |
7-hydroxy-6-methoxychromen-2-one | [1196–1201] |
| tannic acid | 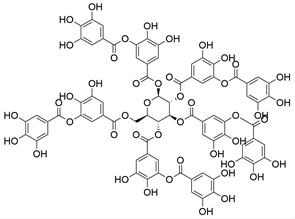 |
[2,3-dihydroxy-5-[[(2R,3R,4S,5R,6S)-3,4,5,6-tetrakis[[3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyl) oxybenzoyl]oxy]oxan-2-yl]methoxycarbonyl]phenyl] 3,4,5-trihydroxybenzoate | [1202–1212] |
| theobromine | 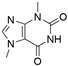 |
3,7-dimethylpurine-2,6-dione | [1213,1214] |
| theophylline | 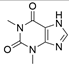 |
1,3-dimethyl-7H-purine-2,6-dione | [1214–1216] |
| vanillic acid |  |
4-hydroxy-3-methoxybenzoic acid | [1217–1221] |
| vanillin | 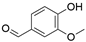 |
4-hydroxy-3-methoxybenzaldehyde | [788,1112,1218,1222–1226] |
| vanillyl alcohol | 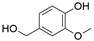 |
4-(hydroxymethyl)-2-methoxyphenol | [1227,1228] |
| xanthine | 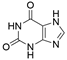 |
3,7-dihydropurine-2,6-dione | [1176,1213] |
| Single Step Mechanisms | |
|---|---|
|
Radical Adduct Formation (RAF) HnAntiox + •R → [HnAntiox-R]• Relevant for antioxidants with multiple bonds and electrophilic free radicals. Viable in polar and non polar media. |
Examples: Carotenoids + •OOH,[1243] or benzylperoxyl [1244] or alkyl, alkoxyl, and alkylperoxyl radicals. [1245] •OH scavenging activity of caffeine, [478] gentisic acid, [1246] hydroxybenzyl alcohols, [1247] edaravone,[1248,1249] melatonin,[1250] and its metabolites,[1251,1252] carnosine, [1253] and rebamipide. [1254] |
|
Single Electron Transfer (SET) HnAntiox + •R → HnAntiox +• + R- Relevant for electrophilic free radicals and antioxidants that are good electron donors. Viable in polar media. |
Examples: For antioxidants curcumin,[1255] and highly galloylated tannin fractions.[1256] Edaravone derivatives + •OH, •OCCl3 and CH3COO•.[1257] Resveratrol with oxygen radical.[1258] Catechin analogues with ROO•. [1259] Carotenoids with CCl3OO• [1260,1261] and •NO2 [1262,1263]. |
|
Formal Hydrogen Atom Transfer (f-HAT) HnAntiox + •R → Hn-1Antiox• + HR Relevant for antioxidants with labile H atoms. Viable in polar and non polar media. |
Examples: Polyphenols, [1264] chlorogenic acids, [1265] procyanidins, [1266] chalcones,[1267] cynarine, [1265] orientin, [1268] capsaicin, [1269] silybin, [1265] α-mangostin, [1270] fisetin, [1271] hydroxychalcones, [1272] baicalein, [1271] ellagic acid, [1273] Lipoic acids, [1274] glutathione, [1275] tryptophan, [1276], N-acetylcystein amide.[1277] |
| Multiple Step Mechanisms | |
|
Sequential Proton Loss Electron Transfer (SPLET) HnAntiox → Hn-1Antiox- + H+ Hn-1Antiox- + •R → Hn-1Antiox• + R- Relevant for antioxidants with acid protons. Viable in polar and protic solvents. |
Examples: Curcumin, [1278,1279] esculetin, [1280] alizarin, [1281] deoxybenzoins,[1282] hydroxybenzoic acids, [1283–1286] resveratrol, [1287,1288] fraxetin, [1289] piceatannol, [1290] morin, [1291] hydroxychalcones, [1292–1294] xanthones, [1295] flavonoids, [1296] quercetin, [1297] kaempferol, [1298] gallic acid, [1299] Trolox, [1300] isoflavonoids, [1301,1302] baicalein, [1303] purpurin.[1304] |
|
Sequential Electron Proton Transfer (SEPT) HnAntiox + •R → Hn-1Antiox•+ + R− Hn-1Antiox•+ → Hn-1Antiox• + H+ Relevant for antioxidants that are good electron donors. Viable in polar and protic solvents. |
Examples: Baicalein, [1305] astaxanthin, [1306] quercetin, in the presence of bases that have HOMO energies lower than that of the SOMO of its radical cation.[1307] DPPH and galvinoxyl radical scavenging activity of vitamin E models. [1308] The theroxyl radical-scavenging process of α-tocopherol.[1309] |
|
Sequential Proton Loss Hydrogen Atom Transfer (SPLHAT) HnAntiox → Hn-1Antiox− + H+ Hn-1Antiox− + •R → Hn-2Antiox•− + HR Relevant for antioxidants with acid protons and labile H atoms. Viable in polar and protic solvents. |
Examples: α-mangostin, [1270] ellagic acid, [1310] propyl gallate, [1311] caffeic and other phenolic acids. [1312] Esculetin + •OOCH3 and •OOCHCH2 radicals. [1280] Gallic acid + •OH. [1313] |
| Component | k (M-1 s-1, at 298 K) | Ref. |
|---|---|---|
| caffeic acid | 3.93E+04 | [457] |
| caffeine | 3.19E+01 | [478] |
| dihydrocaffeic acid | 4.95E+04 | [457] |
| eugenol | 2.49E+03 | [1314] |
| ferulic acid | 9.13E+03 | [457] |
| guaiacol | 1.55E+03 | [1314] |
| p-coumaric acid | 4.35E+03 | [457] |
| paraxanthine | 1.05E+00 | [1315] |
| protocatechuic acid | 5.14E+03 | [1316] |
| theobromine | 5.34E+01 | [1315] |
| theophyline | 4.21E+00 | [1315] |
| Trolox | 3.40E+03 | [1300] |
| vainillin | 9.75E+01 | [1314] |
| vanillic acid | 1.29E+01 | [1314] |
| vanillyl alcohol | 5.67E+03 | [1314] |
| Component | k (M-1 s-1, at 298 K, pH=7.4) | Ref. |
|---|---|---|
| caffeic acid | 2.69E+08 | [457] |
| caffeine | 3.29E-01 | [478] |
| dihydrocaffeic acid | 1.04E+08 | [457] |
| eugenol | 1.55E+06 | [1314] |
| ferulic acid | 3.36E+08 | [457] |
| guaiacol | 2.38E+06 | [1314] |
| p-coumaric acid | 8.51E+07 | [457] |
| paraxanthine | 4.18E+02 | [1315] |
| protocatechuic acid | 1.26E+07 | [1316] |
| theobromine | 2.76E-01 | [1315] |
| theophyline | 3.86E-02 | [1315] |
| Trolox | 8.96E+04 | [1300] |
| vainillin | 1.54E+05 | [1314] |
| vanillic acid | 1.65E+07 | [1314] |
| vanillyl alcohol | 4.12E+06 | [1314] |
| Component | Mechanism | Site | Ref. |
|---|---|---|---|
| caffeic acid | f-HAT | 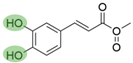 |
[457] |
| caffeine | RAF |  |
[478] |
| dihydrocaffeic acid | f-HAT | 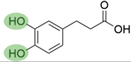 |
[457] |
| eugenol | f-HAT | 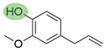 |
[1314] |
| ferulic acid | f-HAT |  |
[457] |
| guaiacol | f-HAT |  |
[1314] |
| p-coumaric acid | f-HAT | 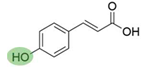 |
[457] |
| paraxanthine | RAF |  |
[1315] |
| protocatechuic acid | f-HAT | 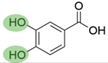 |
[1316] |
| theobromine | RAF |  |
[1315] |
| theophyline | RAF | 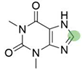 |
[1315] |
| vainillin | f-HAT |  |
[1314] |
| vanillic acid | f-HAT | 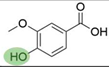 |
[1314] |
| vanillyl alcohol | f-HAT | 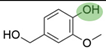 |
[1314] |
| Component | Mechanism | Site or species | Ref. | |
|---|---|---|---|---|
| caffeic acid | SPLET | phenolate anion | [457] | |
| caffeine | RAF | 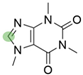 |
[478] | |
| dihydrocaffeic acid | SPLET | phenolate anion | [457] | |
| eugenol | SPLET | phenolate anion | [1314] | |
| ferulic acid | SPLET | phenolate anion | [457] | |
| guaiacol | SPLET | phenolate anion | [1314] | |
| p-coumaric acid | SPLET | phenolate anion | [457] | |
| paraxanthine | RAF | 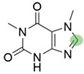 |
[1315] | |
| protocatechuic acid | SPLET | phenolate anion | [1316] | |
| theobromine | RAF | 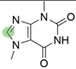 |
[1315] | |
| theophyline | RAF | 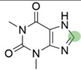 |
[1315] | |
| vainillin | SPLET | phenolate anion | [1314] | |
| vanillic acid | SPLET | phenolate anion | [1314] | |
| vanillyl alcohol | SPLET | phenolate anion | [1314] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





