Submitted:
26 June 2023
Posted:
26 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
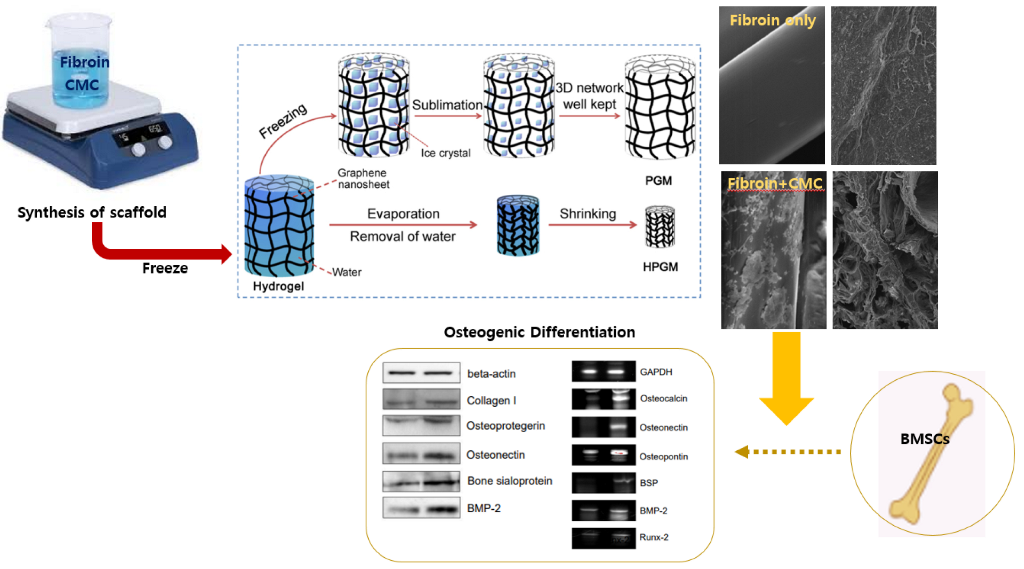
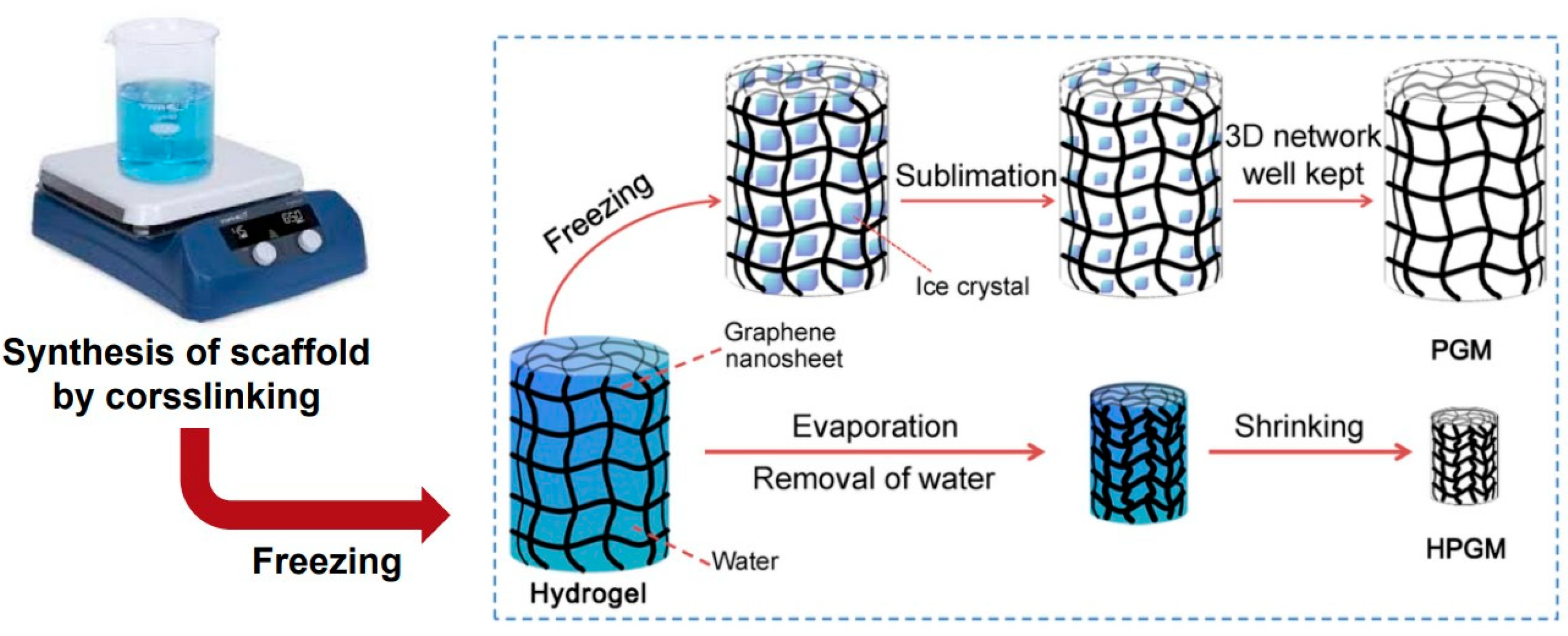
2.1. Fabrication of CMC Porous Scaffold Containing Silk Fibroin
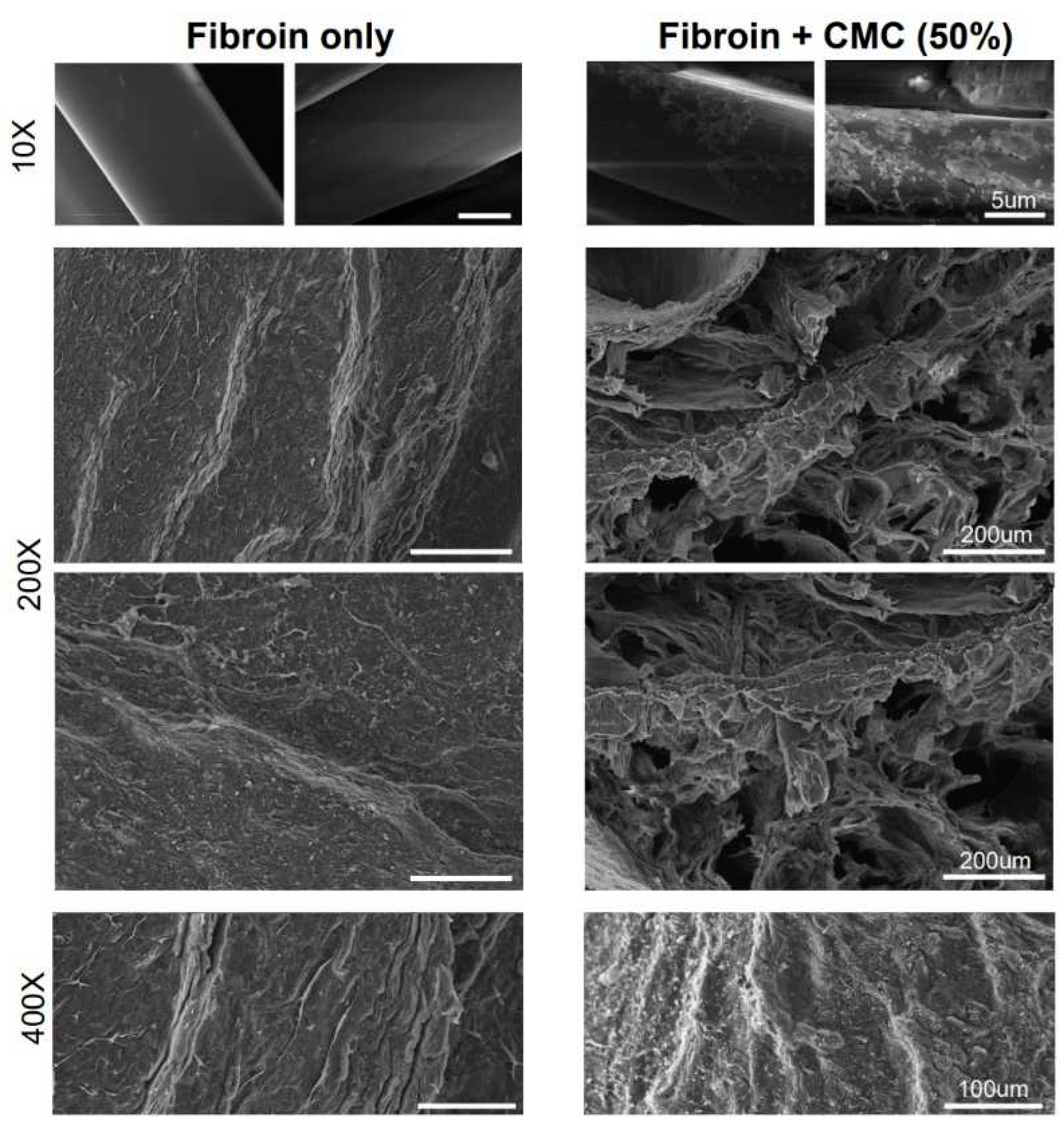
2.2. Surface Observation and Porosity Analysis of Scaffolds
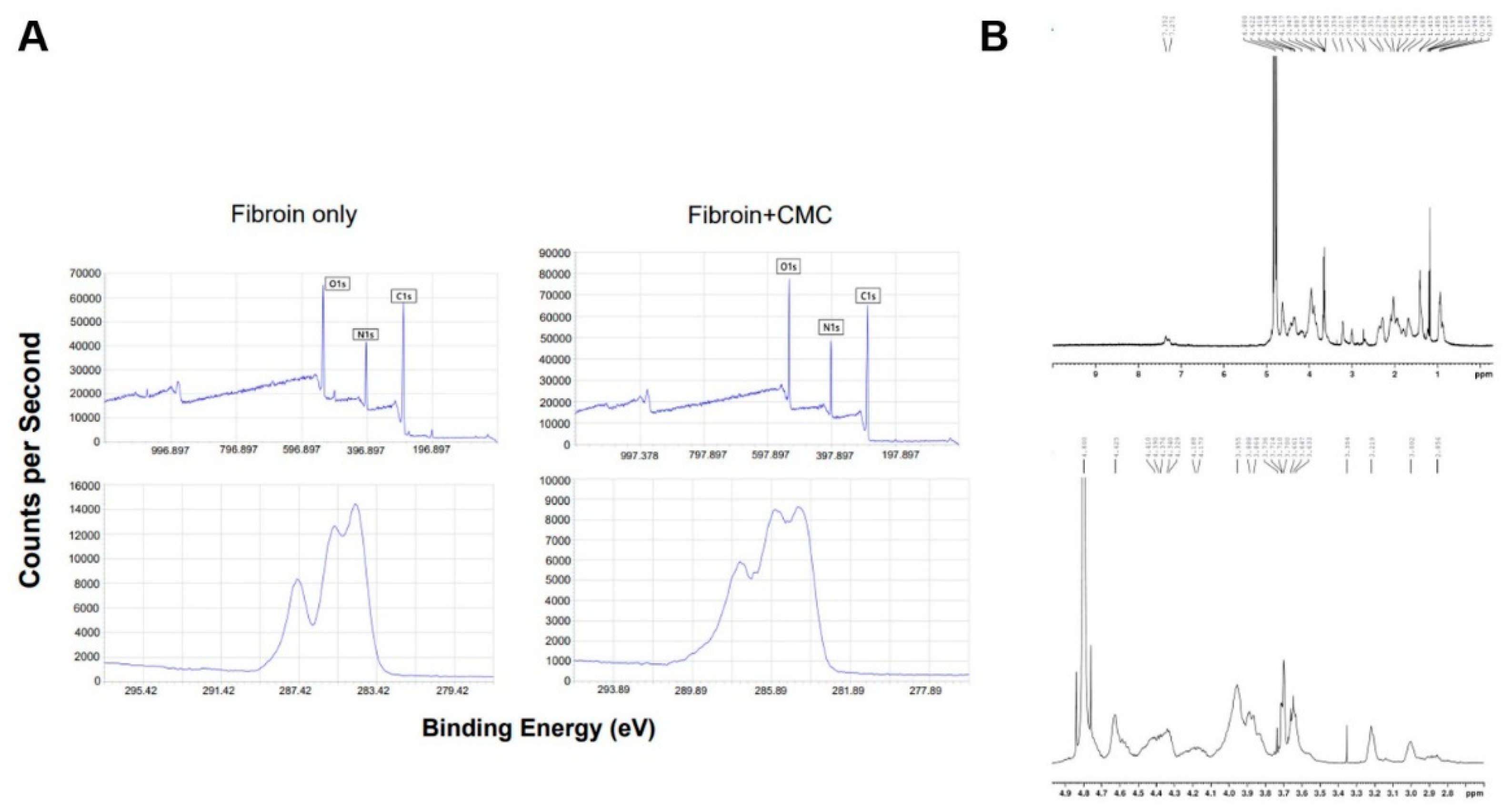
2.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.4. Structural Analysis Using H-NMR
2.5. Differential Scanning Calorimetry Analysis
2.6. Hydrophilicity Measurement
2.7. Water Absorption Rate Measurement
2.8. Isolation and Culture of BMSCs
2.9. MTT Assay
2.10. Alkaline Phosphatase Assay
2.11. Immunofluorescence Staining
2.12. Alizarin Red Staining
2.13. Western Blotting Analysis
3. Results
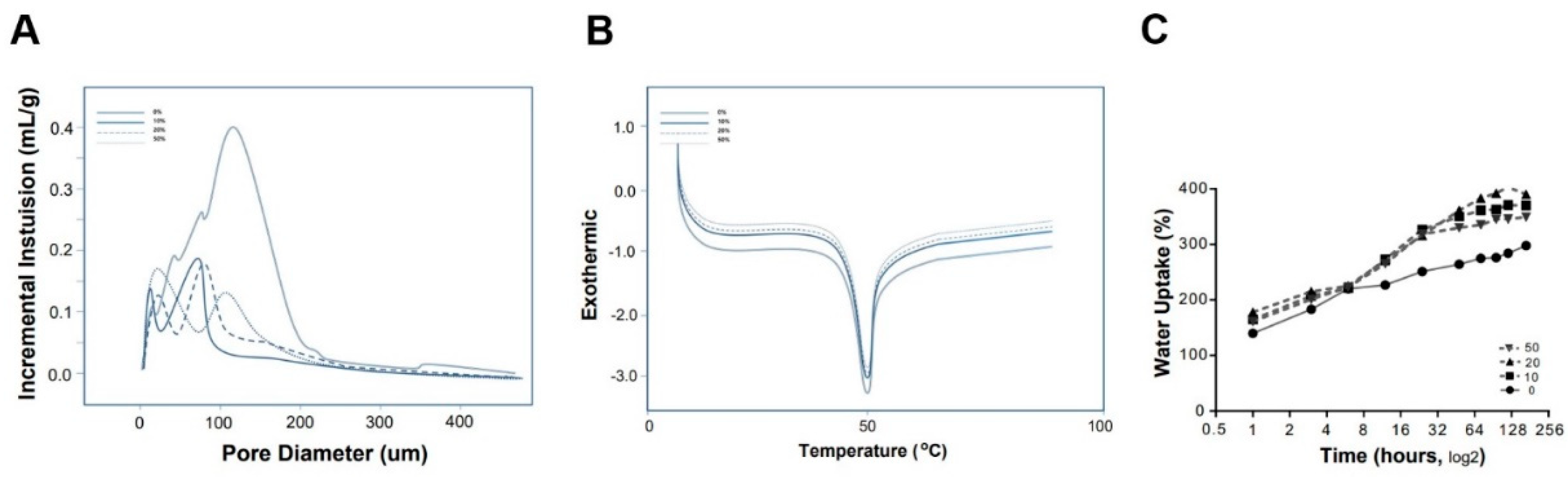
3.1. Fabrication of CMC Porous Scaffold and Analysis of the Scaffold Surface
3.2. Mechanical Analysis of the Fibroin/CMC
3.3. Properties of the Fibroin/CMC
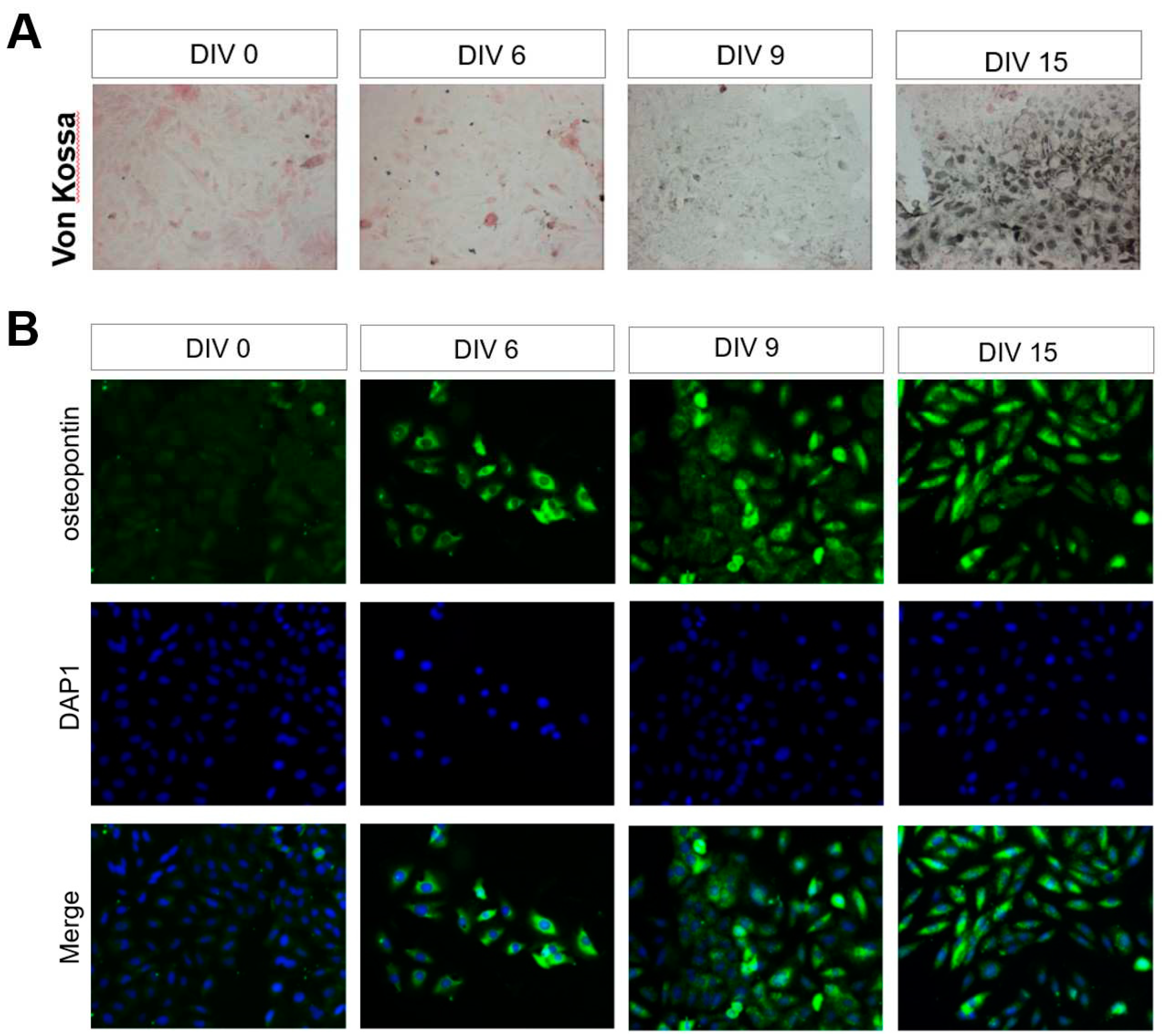
3.4. Identification of BMSCs under the Fibroin/CMC Scaffold
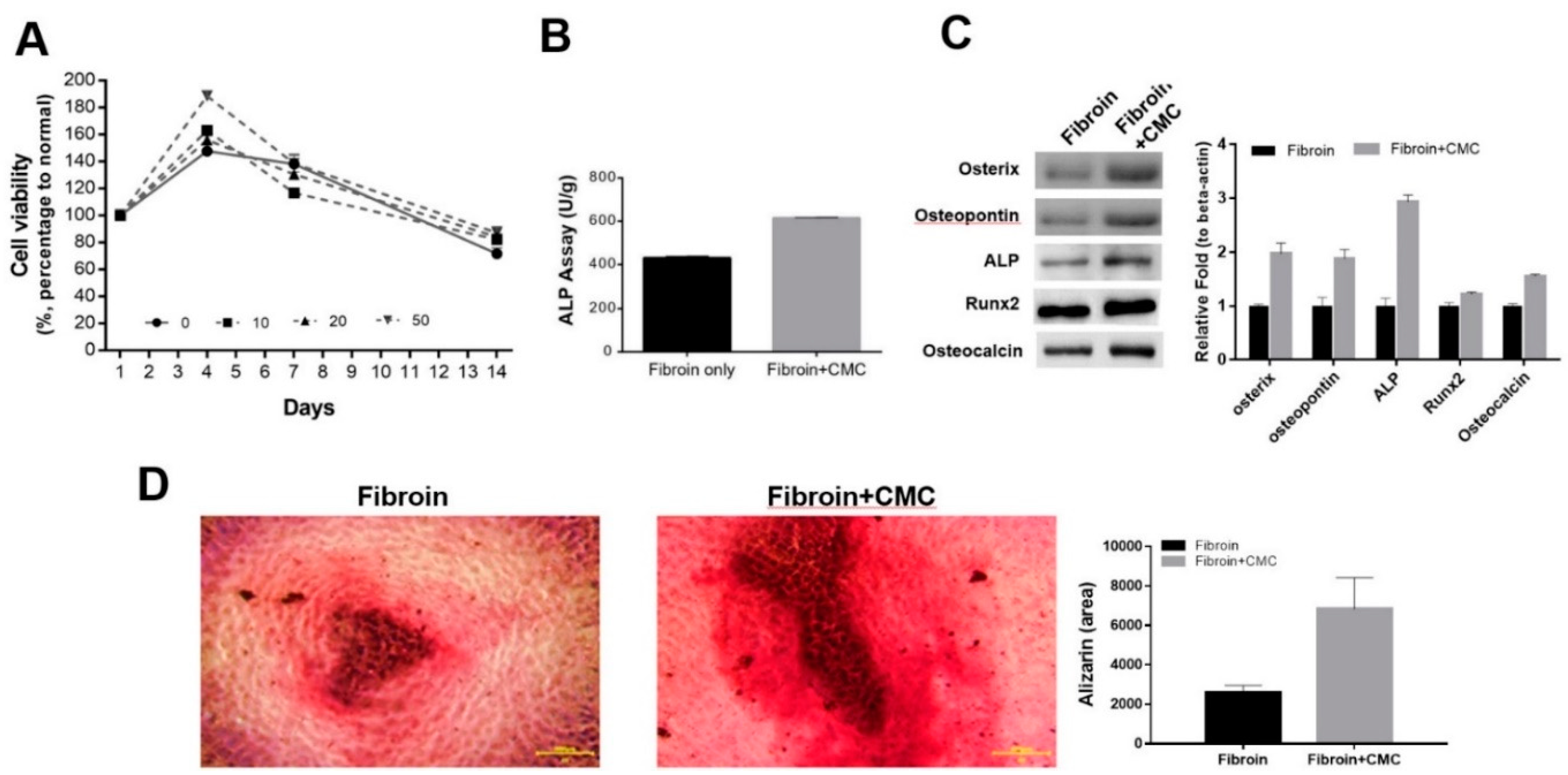
3.5. The Beneficial Effect of CMC Scaffold on Osteogenic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghostine, S.; Carrion, C.; Souza, L.C.G.; Richard, P.; Bruneval, P.; Vilquin, J.-T.; Pouzet, B.; Schwartz, K.; Menasché, P.; Hagège, A.A. Long-Term Efficacy of Myoblast Transplantation on Regional Structure and Function After Myocardial Infarction. Circulation 2002, 106. [Google Scholar] [CrossRef]
- Gojo, S.; Kitamura, S.; Hatano, O.; Takakusu, A.; Hashimoto, K.; Kanegae, Y.; Saito, I. Transplantation of genetically marked cardiac muscle cells. J. Thorac. Cardiovasc. Surg. 1997, 113, 10–18. [Google Scholar] [CrossRef]
- Jain, M.; DerSimonian, H.; Brenner, D.A.; Ngoy, S.; Teller, P.; Edge, A.S.B.; Zawadzka, A.; Wetzel, K.; Sawyer, D.B.; Colucci, W.S.; et al. Cell Therapy Attenuates Deleterious Ventricular Remodeling and Improves Cardiac Performance After Myocardial Infarction. Circulation 2001, 103, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-K.; Jia, Z.-Q.; Weisel, R.D.; Mickle, D.A.; Zhang, J.; Mohabeer, M.K.; Rao, V.; Ivanov, J. Cardiomyocyte Transplantation Improves Heart Function. Ann. Thorac. Surg. 1996, 62, 654–661. [Google Scholar] [CrossRef]
- Müller-Ehmsen, J.; Whittaker, P.; Kloner, R.A.; Dow, J.S.; Sakoda, T.; Long, T.I.; Laird, P.W.; Kedes, L. Survival and Development of Neonatal Rat Cardiomyocytes Transplanted into Adult Myocardium. J. Mol. Cell. Cardiol. 2002, 34, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H. , et al. , Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation 1999, 100, 193–202. [Google Scholar] [CrossRef]
- Reinecke, H.; E Murry, C. Transmural Replacement of Myocardium after Skeletal Myoblast Grafting into the Heart. Cardiovasc. Pathol. 2000, 9, 337–344. [Google Scholar] [CrossRef]
- Sakai, T.; Li, R.-K.; Weisel, R.D.; Mickle, D.A.; Jia, Z.-Q.; Tomita, S.; Kim, E.-J.; Yau, T.M. Fetal cell transplantation: A comparison of three cell types. J. Thorac. Cardiovasc. Surg. 1999, 118, 715–725. [Google Scholar] [CrossRef]
- Scorsin, M. , et al. , Does transplantation of cardiomyocytes improve function of infarcted myocardium? Circulation 1997, 96, II-188-93. [Google Scholar]
- Liuyun, J.; Yubao, L.; Chengdong, X. Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J. Biomed. Sci. 2009, 16, 65–65. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, H.; Zhang, T.; Yao, Y.; Chen, Y.; Zhang, S.; Bai, B. Gene-modified BMSCs encapsulated with carboxymethyl cellulose facilitate osteogenesis in vitro and in vivo. J. Biomater. Appl. 2020, 35, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Methot, D.; Poppa, V.; Fujio, Y.; Walsh, K.; Murry, C.E. Cardiomyocyte Grafting for Cardiac Repair: Graft Cell Death and Anti-Death Strategies. J. Mol. Cell. Cardiol. 2001, 33, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Murry, C.E. Taking the Death Toll After Cardiomyocyte Grafting: A Reminder of the Importance of Quantitative Biology. J. Mol. Cell. Cardiol. 2002, 34, 251–253. [Google Scholar] [CrossRef]
- Leor, J. , et al. , Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation 2000, 102, III56-61. [Google Scholar]
- Kellar, R.S.; Landeen, L.K.; Shepherd, B.R.; Naughton, G.K.; Ratcliffe, A.; Williams, S.K. Scaffold-based three-dimensional human fibroblast culture provides a structural matrix that supports angiogenesis in infarcted heart tissue. Circulation 2001, 104, 2063–2068. [Google Scholar] [CrossRef]
- Thompson, W.D.; Smith, E.B.; Stirk, C.M.; Marshall, F.I.; Stout, A.J.; Kocchar, A. Angiogenic activity of fibrin degradation products is located in fibrin fragment E. J. Pathol. 1992, 168, 47–53. [Google Scholar] [CrossRef]
- Bootle-Wilbraham, C.; Tazzyman, S.; Thompson, W.; Stirk, C.; Lewis, C. Fibrin fragment E stimulates the proliferation, migration and differentiation of human microvascular endothelial cells in vitro. Angiogenesis 2001, 4, 269–275. [Google Scholar] [CrossRef]
- Naito, M.; Stirk, C.M.; Smith, E.B.; Thompson, W. Smooth Muscle Cell Outgrowth Stimulated by Fibrin Degradation Products: The Potential Role of Fibrin Fragment E in Restenosis and Atherogenesis. Thromb. Res. 2000, 98, 165–174. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Harvey, V.S.; Estrella, P.; Brown, L.F.; McDonagh, J.; Dvorak, A.M. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab. Investig. 1987, 57, 673–86. [Google Scholar]
- Christman, K.L.; Fok, H.H.; Sievers, R.E.; Fang, Q.; Lee, R.J.; Della Rocca, D.G.; Willenberg, B.J.; Qi, Y.; Simmons, C.S.; Rubiano, A.; et al. Fibrin Glue Alone and Skeletal Myoblasts in a Fibrin Scaffold Preserve Cardiac Function after Myocardial Infarction. Tissue Eng. 2004, 10, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sievers, R.E.; Schmiedl, U.; Wolfe, C.L.; Moseley, M.E.; Parmley, W.W.; Brasch, R.C.; Lipton, M.J. A model of acute regional myocardial ischemia and reperfusion in the rat. Magn. Reson. Med. 1989, 10, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, C.L.; E Moseley, M.; Wikstrom, M.G.; E Sievers, R.; Wendland, M.F.; Dupon, J.W.; E Finkbeiner, W.; Lipton, M.J.; Parmley, W.W.; Brasch, R.C.; et al. Assessment of myocardial salvage after ischemia and reperfusion using magnetic resonance imaging and spectroscopy. Circulation 1989, 80, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-Q.; Sun, Y.-P.; E Sievers, R.; Browne, A.E.; Pulukurthy, S.; Sudhir, K.; Lee, R.J.; Chou, T.M.; Chatterjee, K.; Parmley, W.W. Comparative effects of pretreatment with captopril and losartan on cardiovascular protection in a rat model of ischemia-reperfusion. J. Am. Coll. Cardiol. 2000, 35, 787–795. [Google Scholar] [CrossRef]
- Zhu, B.-Q.; Sun, Y.-P.; E Sievers, R.; Browne, A.E.; Lee, R.J.; Chatterjee, K.; Parmley, W.W. Effects of different durations of pretreatment with losartan on myocardial infarct size, endothelial function, and vascular endothelial growth factor. J. Renin-Angiotensin-Aldosterone Syst. 2001, 2, 129–133. [Google Scholar] [CrossRef]
- A Rando, T.; Blau, H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994, 125, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, M.C.; Maclean, D.; Maroko, P.R. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am. J. Pathol. 1978, 90, 57–70. [Google Scholar] [PubMed]
- Zahedi, M.; Parham, A.; Dehghani, H.; Mehrjerdi, H.K. Equine bone marrow-derived mesenchymal stem cells: optimization of cell density in primary culture. Stem Cell Investig. 2018, 5, 31–31. [Google Scholar] [CrossRef]
- Kloner, R.A.; Dow, J.; Chung, G.; Kedes, L.H. Intramyocardial injection of DNA encoding vascular endothelial growth factor in a myocardial infarction model. J. Thromb. Thrombolysis 2000, 10, 285–289. [Google Scholar] [CrossRef]
- Li, W.; Tanaka, K.; Chiba, Y.; Kimura, T.; Morioka, K.; Uesaka, T.; Ihaya, A.; Sasaki, M.; Tsuda, T.; Yamada, N. Role of MMPs and plasminogen activators in angiogenesis after transmyocardial laser revascularization in dogs. Am. J. Physiol. Circ. Physiol. 2003, 284, H23–H30. [Google Scholar] [CrossRef]
- Havenith, M.G.; Visser, R.; Schendel, J.M.C.S.-V.; Bosman, F.T. Muscle fiber typing in routinely processed skeletal muscle with monoclonal antibodies. Histochem. 1990, 93, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, L.E.; Hemo, I.; Keshet, E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998, 125, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Dai, W.; Shao, Z. X-ray photoelectron spectroscopic and Raman analysis of silk fibroin–Cu(II) films. Biopolymers 2006, 82, 144–151. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Rippon, A.J.; Farrell, D.H. Alternative Adhesion Sites in Human Fibrinogen for Vascular Endothelial Cells. Biochemistry 1996, 35, 4169–4175. [Google Scholar] [CrossRef]
- Vailhé, B.; Ronot, X.; Tracqui, P.; Usson, Y.; Tranqui, L. In vitro angiogenesis is modulated by the mechanical properties of fibrin gels and is related to alpha(v)beta3 integrin localization. . 1998, 33. [Google Scholar]
- Sahni, A.; Odrljin, T.; Francis, C.W. Binding of Basic Fibroblast Growth Factor to Fibrinogen and Fibrin. J. Biol. Chem. 1998, 273, 7554–7559. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




