1. Introduction
Breast cancer is a significant public health issue in the United States, with an estimated 339,250 new cases in 2022 alone. While the mortality rates of breast cancer have been consistently declining in the 21
st century, it still leads to tens of thousands of deaths annually with an estimated 43,250 in 2022 [
1]. The impact of breast cancer on individuals and society is substantial, affecting not only the physical health and well-being of those diagnosed but also their families, communities, and the healthcare system [
2,
3]. As such, understanding the risk factors, screening and diagnostic tools, and treatment options for breast cancer is critical to reducing its impact on public health.
Breast cancer can be classified into four subtypes based on molecular markers: Luminal A, Luminal B, HER2 Enriched, and Triple Negative, based on Estrogen Receptor (ER), Progesterone Receptor (PR) and HER2 (also known as ERBB2) expression [
4]. These subtypes have clinical implications as to the type of treatment selected and the overall outcomes [
5,
6,
7].
Galectins are a subfamily of lectins with a conserved carbohydrate recognition domain (CRD) capable of binding ß-galactoside glycoconjugates [
8]. There are three subgroups of galectins classified by their structure. Galectin-1 is in the prototypical group that also houses galectin-2, -5, -7, and -10 which are able to form dimers [
9]. The sole member of the second subgroup is the chimeric galectin-3 which is widely expressed in multiple organ systems including immune, epithelial, and neuronal tissues [
10]. The final subtype is tandem-repeat galectins with two CRDs that are more potent than previous groups regarding biologic response and include galactin-4, -8, -9, and -12 [
11]. These proteins bind to specific carbohydrate structures on the surface of cells, thereby regulating cell adhesion, migration, signaling, and death [
12,
13,
14,
15]. Galectins are expressed in a wide variety of tissues and cell types, and their functions are highly context-dependent [
16].
These biochemical functions have made galectins of interest in oncology. Galectin-1, -3, and -9 are the most extensively studied and are implicated in alterations in oncogenic pathways, apoptosis, T-cell immune response, and metastasis dependent upon specific galectin and glycoprotein interactions [
17]. Galectin-1, and -3 are both capable of binding to CD7 and CD45 and inducing apoptosis, with galectin-1 requiring CD7 and galectin-3 requiring CD45 [
18]. Elevated serum galectin-1 levels have been shown to decrease TH1 responses in nonmalignant cells, favoring TH2 cytokine profiles that promote tumor survival [
19]. Cancer metastasis is promoted by elevated galectin-3 levels interacting with MUC1, a transmembrane protein on most secretory epithelia, limiting its protective effects by increased interactions with CD44 and the ligands for e-selectins [
20]. Galectin-3 has also been shown to have oncogenic properties by interacting with RAS, its pathway, and BCL-2 [
21]. Galectin-9 has also demonstrated the ability to aid in avoidance cancer cell recognition through mediating TH-1 helper cell apoptosis [
22]. Therefore, there are multiple mechanisms by which galectins play a role in oncogenesis.
With these mechanisms in mind, there have been multiple studies examining galectin expression levels in various cancer tissues and sera of cancer patients using immunohistochemistry and enzyme-linked immunosorbent assays (ELISA) and recognizing their impact on oncological behavior [
23,
24,
25,
26,
27,
28,
29,
30,
31]. Galectins-1, -3, and -9 have been shown to be increased in the serum of breast cancer patients when compared to healthy controls [
32]. Targeting galectins have been demonstrated to have an impact on cancer progression [
25]. Trials have examined these mechanisms to identify potential therapeutic targets to treat multiple cancer types [
21,
33,
34,
35]
While a previous study has shown that different galectins mRNA expression can be increased or decreased in breast cancer and that their expression in the tumor tissue changes based on the breast cancer’s molecular subtype [
36], no study has shown how the corresponding circulating level of galectins changes based on molecular subtype. This study reports the serum concentrations of galectin-1, -3, and -9 in breast cancer patients organized by molecular subtype as well as a broad screening of other data points such as hormone receptor (HR) expression levels, and exposure to treatment. This provides better characterization of the changes in galectins in breast cancer by delivering a more nuanced understanding of the factors that shape their expression in the serum. This study joins efforts of others seeking for biomarker of diagnostic and prognostic potential in a family of proteins actively involved in oncological processes.
2. Materials and Methods
A heterogenous and random selection of a total of 137 breast cancer patient serum samples were collected from the Prisma Health Cancer Institute (PHCI) biorepository (Greenville, SC, USA). The sample collection years ranged from 2012–2022. Information about the nature of the PHCI and its standard operating procedures has been previously described [
37].
Patient information was collected from the PHCI database. The information included sex, ethnicity, race, principal diagnosis, TNM staging, overall staging, histology, primary versus metastatic sample, tissue site of resection, specimen age, specimen collection year, patient age at collection, patient smoking history, tissue exposure to oncological treatment, ER, PR, and HER2 IHC assay, and ER and PR percent expression on IHC staining.
The subtypes of the cancers were classified using the information of the ER, PR, and HER-2 IHC data using the following method: Luminal A (ER+/PR+, HER-2−), Luminal B (ER+/PR+, HER-2+), HER-2 Enriched (ER−, PR−, HER-2+), and Triple Negative (ER−, PR−, HER-2−). There was an absence of Ki-67 data and classification was based on methods previously described [
4].
Seventy-seven (56% of total) samples were classified as Luminal A (median age 59, min-max 19–89), 18 (13% of total) samples were classified as Luminal B (median age 52, min-max 34–74), 10 (7% of total) samples were classified as HER-2 Enriched (median age 60, min-max 44–77), and 32 (23% of total) samples were classified as Triple Negative (median age 55, min-max 30–80). The number of samples from stages I, II, III, and IV were 55, 51, 22, and 11, respectively.
The patient’s serum was used to determine the circulating galectin levels using an enzyme-linked immunosorbent assay (ELISA) as previously described [
32]. Of the 137 total samples in this study, 40 of the samples were previously used in the study by Blair et. al. (2021). Galectin-1, -3, and -9 concentrations were obtained using the ELISA kits from R&D Systems (Minneapolis, MN, USA).
All statistical analyses were performed using JMP® software by the SAS Institute (Cary, NC, USA). The distributions of each galectin were compared by the data of interest. Not all samples had data for every variable. Samples with missing data were excluded from analysis. Two-group comparisons were done by t-test. Multiple comparisons were performed with one-way ANOVA, with subsequent analysis using of all pairs using Student’s t-test if the ANOVA’s Probability > F was less than 0.05. Values of p less than 0.05 were considered statistically significant.
3. Results
3.1. Stage
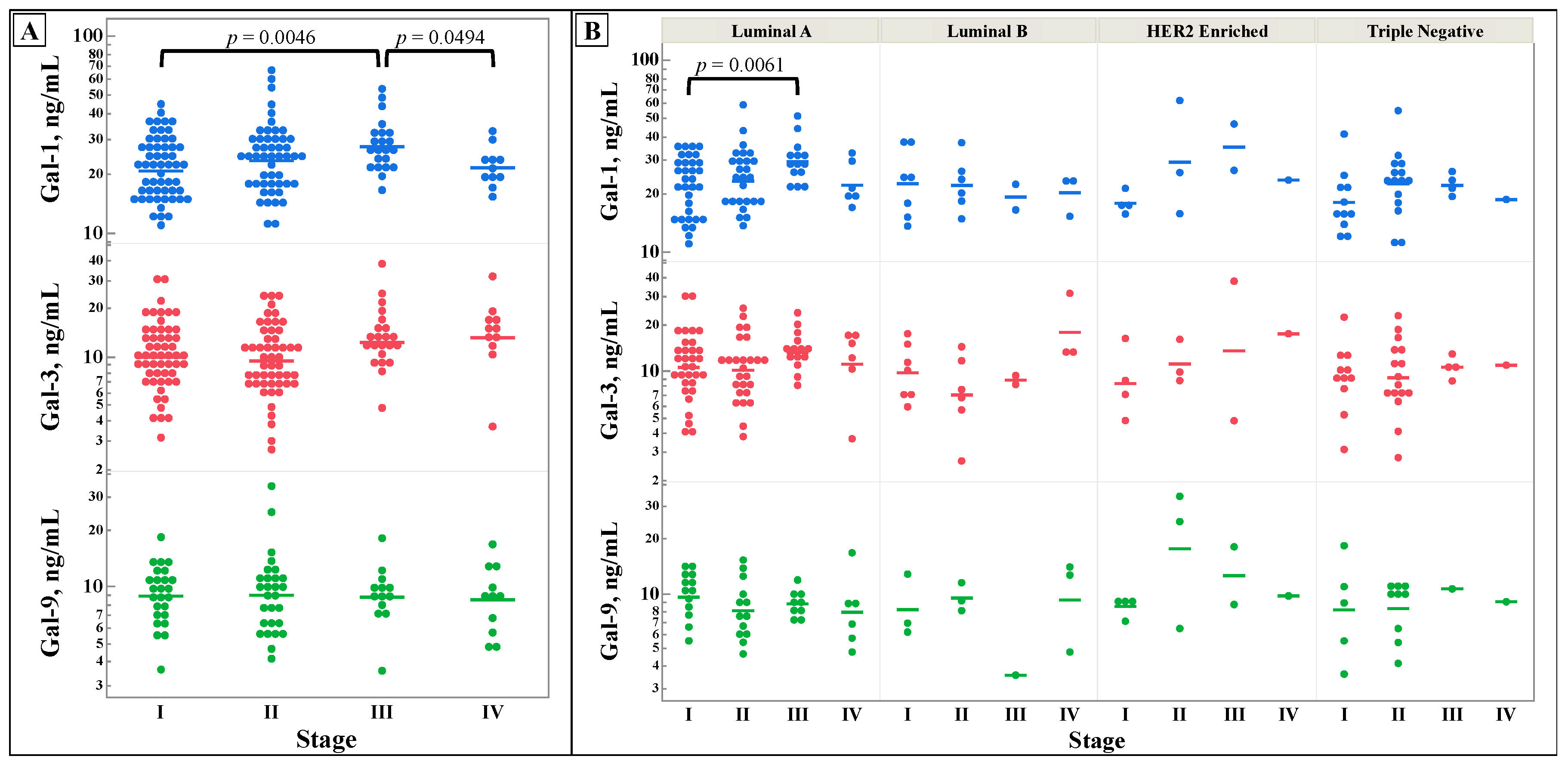
Figure 1 shows the differences in galectin levels between different stages of breast cancer. No significant difference was discovered for galectin-3 and -9, however, galectin-1 showed statistically significant differences between stage III and stage I and IV.
3.2. Molecular Data
Along with the molecular subtype data is the degree of hormone receptor expression in the tissue. As seen in
Figure 2, the level of hormone receptor expression does not correlate with galectin serum levels. While the specific level of hormone expression in breast cancer does not typically guide breast cancer treatment, its relationship with serum galectins is reported here for the benefit of future basic science investigations.
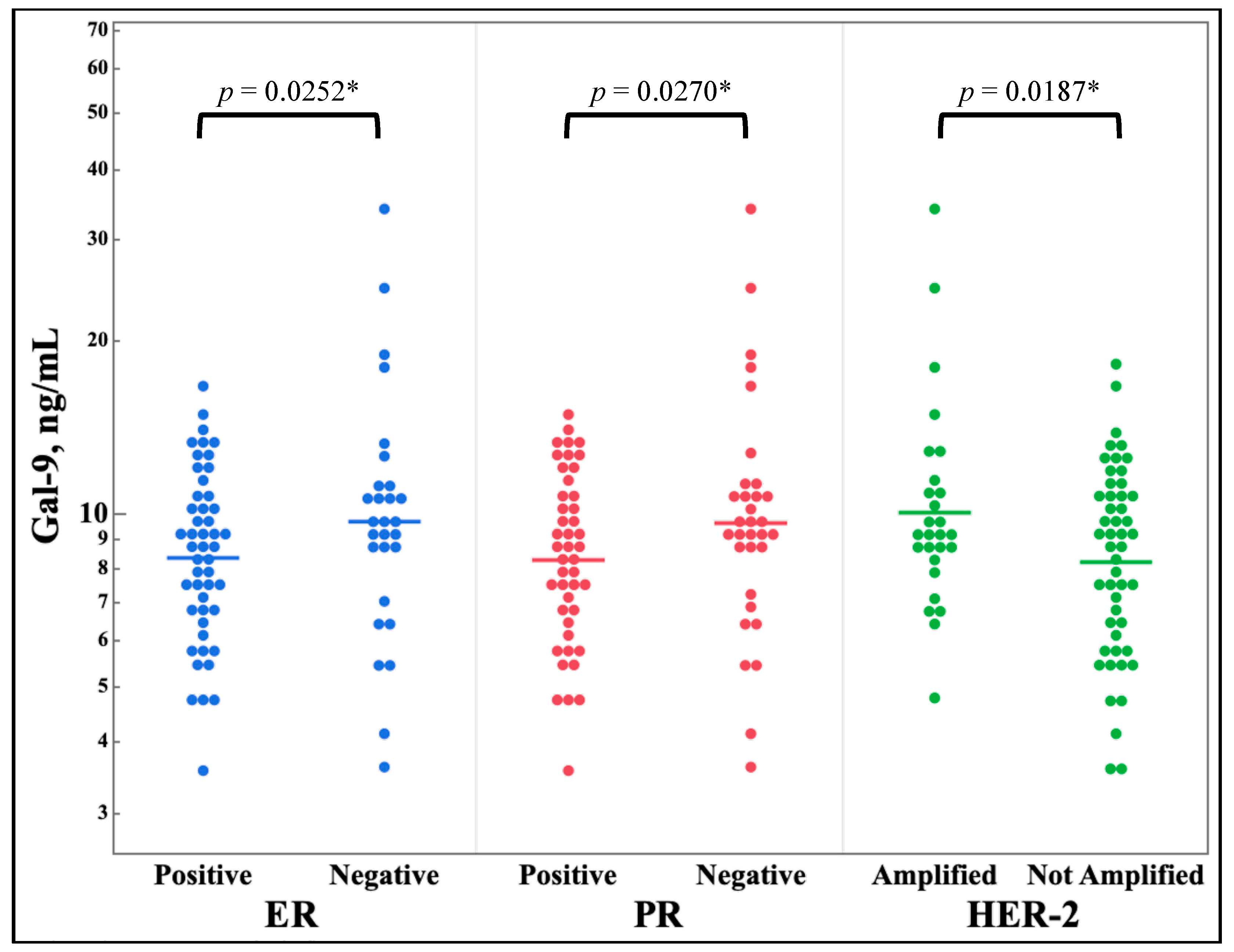
Figure 3 shows the differences in galectin levels between any expression versus non-expression of the estrogen receptor, the progesterone receptor, or HER-2. Galectin-9 showed a statistically significant decrease in serum levels for all three molecular markers.
3.3. Oncotype
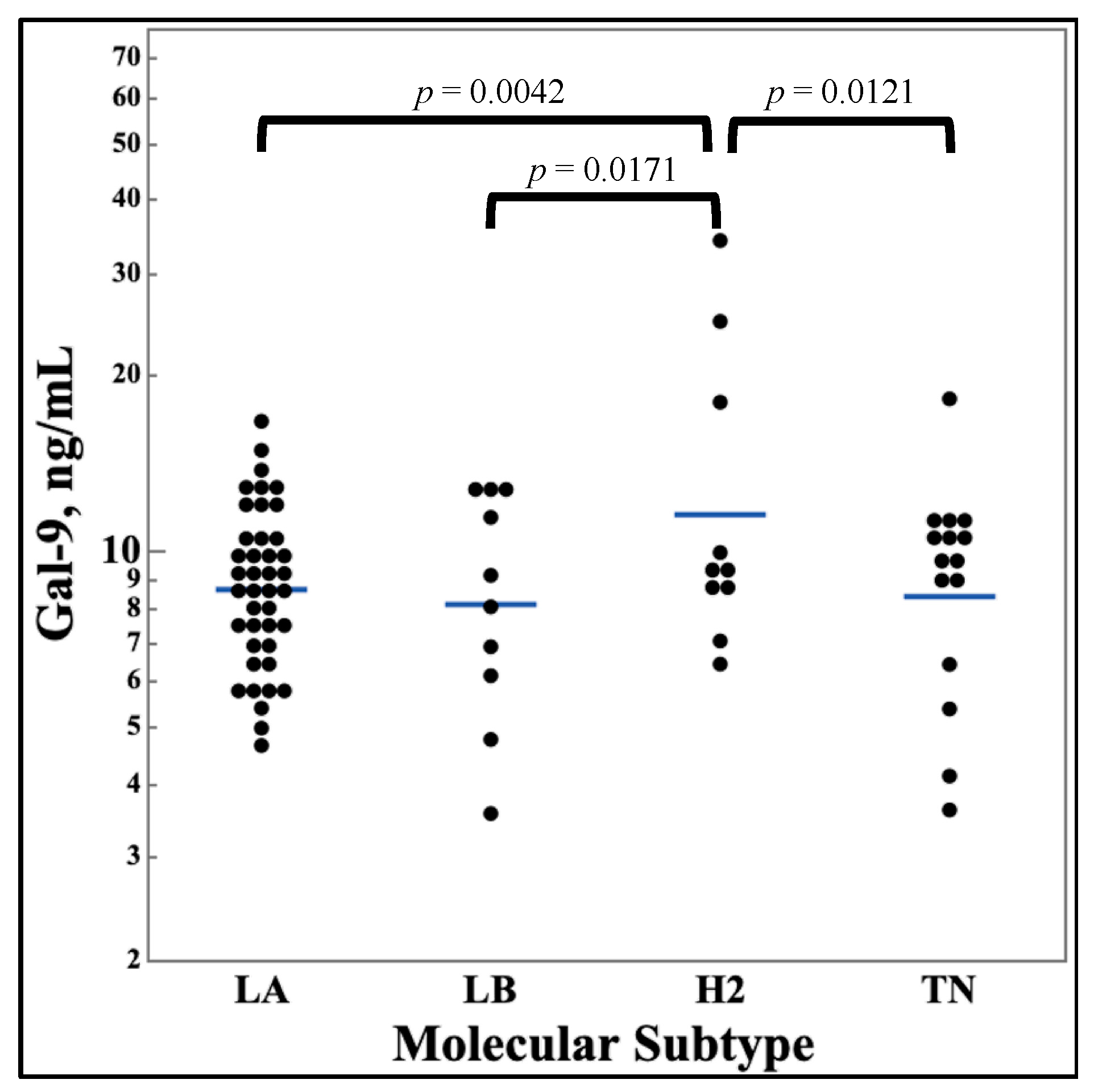
Figure 4 shows the differences in galectin levels between different molecular subtypes of breast cancer. No significant difference was discovered for galectin-1 and -3, however, galectin-9 showed a statistically significant increase for the HER2 Enriched subtype.
3.4. Treatment Status
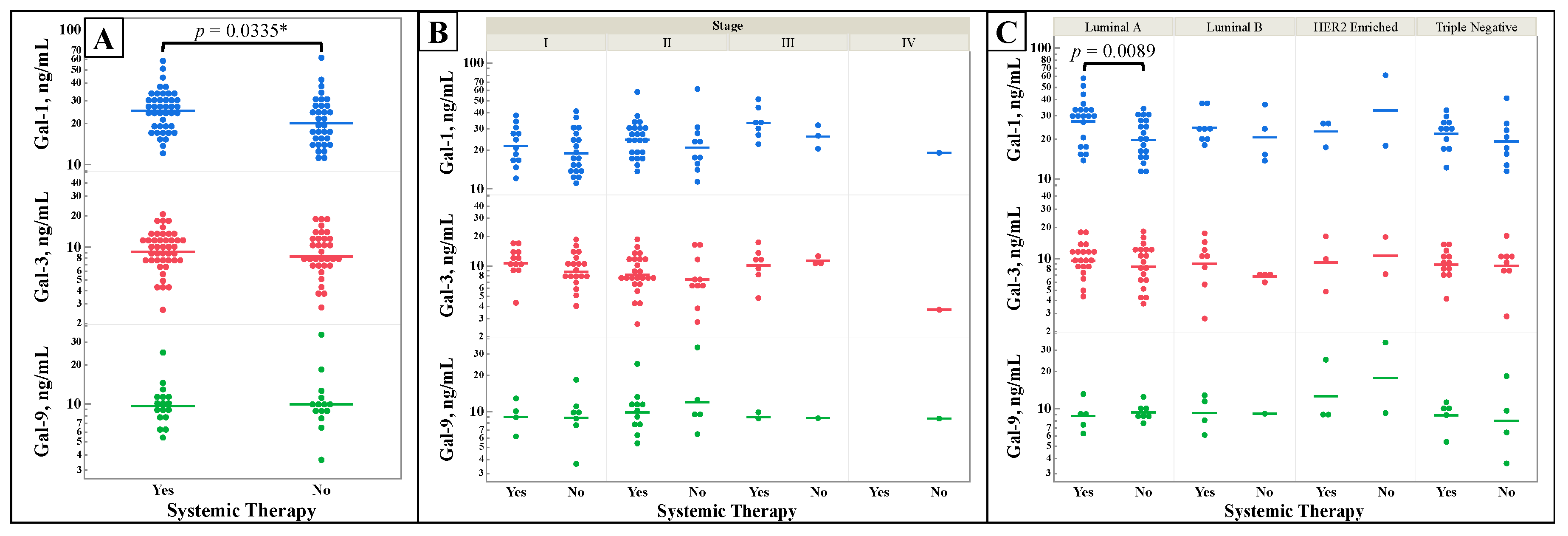
Figure 5 shows galectin levels by patients’ status of receiving either chemotherapy, hormonal therapy, or immunotherapy (Systemic Therapy) vs no therapy prior to serum collection. Some patients may have received neoadjuvant or adjuvant therapies depending on their time of diagnosis. Serum galectin-1 (
Figure 5A) was a statistically significantly increased overall in patients who had some form of systemic therapy when compared to serum that had not received any treatment. When breaking down galectin-1’s levels by molecular subtype (
Figure 5C) Luminal A again has a statistically significant increase in patients who received systemic therapy.
4. Discussion
4.1. Findings
There were no changes in galectin levels by stage, as seen in
Figure 1, except for galectin-1 in stage III when compared to stages I and IV. While galectin-1, -3, -9 have been established to be increased in the serum of breast cancer patients, there does not appear to be any appreciable pattern in these lectins by stage [
32]. This negative finding suggests that alterations in the levels of galectin-1, -3, -9 are not predictive of breast cancer stage. With higher stages of breast cancer conferring worse prognosis, the prognostic abilities of galectin-1,-3,-9 may be limited in breast cancer.
Figure 2 shows that there was no correlation between the specific serum galectin levels and hormone receptors’ specific level of expression in breast tissue. ER receptor binding has been shown to induce expression of galectin-3 in prostate cancer [
38], and galectin-1, -3, and -9 have been shown to be correlated with binary levels of HR expression in breast cancer tissue [
36]. Therefore, this negative finding of non-correlation with galectin serum levels is valuable as it also helps inform future studies and biomarker searches.
Conversely, the galectins serum levels were compared to the binary category of receptor expression or amplification.
Figure 3 shows that galectin-9 has decreased serum levels in samples with positive hormone receptor markers and an increased serum levels in HER2 amplified tissues. The relationship of ER status and galectin-9 has been examined in one study finding that galectin-9 expression did not correlate with ER status. However, there was a trend showing that ER positive breast cancers were more likely to be galectin-9 negative compared to ER negative breast cancer [
39]. This study finds the same relationship in the patient serum and highlights the importance of understanding the implications of galectin-9 levels in a breast cancer patient. For example, galectin-7 and -8 have shown relationships to breast cancer receptor status and have evidence suggesting potential uses as independent prognosticators for an impaired progression free survival [
40]. Interestingly, galectin-3 has not shown any relationship in this way [
41]. Unlike ER status, there have been no direct links between galectin-9 and HER2 specifically, but galectin-9 is an emerging biomarker for breast cancer invasiveness [
42,
43].
While these three galectins are known to be increased in breast cancer, and the differences between galectin expression in breast cancer tissue has been discussed [
36], the difference of serum galectins by molecular subtype has not been reported. As seen in figure 4, galectin-9 was observed to be higher in HER2 enriched breast cancer than the other three molecular subtypes while there were no differences in galectin-1 and -3 serum levels between the subtypes. This finding agrees with the previous finding regarding galectin-9’s decreased serum levels in samples with positive hormone receptor markers but an increase in serum levels in HER2 amplified tissues. Since HER2 Enriched breast cancer is defined based on amplified HER2 without positive HR, this seems to be an intuitive outcome. This finding should be further explored in studies specifically designed to investigate galectin-9’s relationship with HER2 enriched breast cancers compared to other the other molecular subtypes.
Finally, galectin-1 was found to be statistically significantly increased when cancerous tissues are exposed to cancer therapies other than resection or radiation. Therapies that samples could have received included chemotherapy, hormonal blockade, and immunotherapy. This represents the first demonstration of increasing galectin-1 expression in the setting of breast cancer treatments. Since the serum galectin levels are a picture of the whole body’s response, not just the cancer, this could increase in gal-1 could represent a rise based on an inflammatory response to the therapies used.
4.2. Limitations
This study has limitations based on the availability of samples for testing. The selection process for samples consisted of random sampling of the PHCI’s biorepository of breast cancer patients. This selection process can make analysis difficult as one is not guaranteed uniform comparison groups. However, it does provide an overview of the characteristics of a representative patient panel.
While it was interesting that galectin-1 increased in response to exposure to any chemotherapy, hormonal therapy, or immunotherapy it was not possible to retrieve information regarding the exposure of the tumor to specific therapeutic agents. Further delineation as to the response of serum galectins based on the specific chemotherapy, hormonal agents, or immunotherapy was not able to be determined. This area needs to be further investigated as the response of the galectin levels to specific therapies could provide clues as to the tumor response to therapy.
Low galectin-9 has been shown to have significantly decreased overall survival (OS) in triple negative breast cancer tumor cells with PDL-1 negativity [
44]. Therefore, the correlation between galectin levels and OS is of interest in oncology research. However, overall survival data was not available for our patient population.
5. Conclusions
These findings are broad and will serve to provide direction in future galectin research. Galectin-9’s increase in HER2 enriched breast cancers has the potential to be further fleshed out to provide a mechanistic understanding of the relationships, potential therapeutic targets, and use for indicators of disease. These findings are especially impactful in the context of the current interest in galectin-9 and its role in the tumor microenvironment regulating immune response to cancer via TIM-3 [
45,
46]. This study’s findings also provide evidence that future galectin investigations need to be cognizant of the relationship between the treatment status of the patient and level of circulating galectins. It is important to understand confounding variables such as patient treatment status that have the possibility of changing the inflammatory landscape in as these proteins often have intimate functions in both immune and inflammation processes.
Many studies have focused on measuring galectin expression levels in cancer tissue. While measuring a protein marker at the tissue level is important to understand their influence and impact in the development and progression of cancer, a complete understanding of these protein’s levels in the human serum is necessary to provide a complete picture. Effective and available biomarkers will rely on ease of access from the patient and ease of measurement. Serum galectin proteins lend themselves to these ends by being readily accessible and measurable by standard lab equipment.
As stated previously, the galectin-9 changes based on subtype provide an interesting area for future research and development of understanding. The mechanism of the increase in galectin-9 in HER2 enriched cancers and the relationship between serum levels and hormone and HER2 receptor expression could provide more insight to tumor biology.
Moving forward, it will be important to understand the changes in galectin levels before and after resection. As stated before, while serum galectins have the strength of being easily collected and measured, while providing possible insights into the tumor’s cancer biology, the fact that galectins are a part of a wide variety of biological processes provides some confounding factors. A way to better understand the relationship between serum galectin levels and the cancer biology would be to measure galectin levels pre and post resection of the tumor as it would provide better a more nuanced understanding of the mass’s contribution to the serum galectin load.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Summary of Serum Galectin Values by Cancer Patient and Sample Characteristic.
Author Contributions
Conceptualization, A.V.B.; methodology, A.V.B. and A.T.F.; validation, A.T.F..; formal analysis, A.T.F.; investigation, A.T.F., W.J.E. and A.V.B.; resources, A.V.B., J.C.M. and W.J.E.; data curation, A.T.F.; writing—original draft preparation, A.T.F. and H.D.S.; writing—review and editing, A.T.F., H.D.S., W.J.E. and A.V.B.; visualization, A.T.F.; supervision, A.V.B.; project administration, A.V.B.; funding acquisition, A.V.B., J.C.M. and W.J.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Prisma Health Cancer Institute Philanthropy Grant and Sargent Foundation. The APC was funded by the Biomedical Sciences Department at the University of South Carolina School of Medicine Greenville.
Institutional Review Board Statement
Ethical review and approval were waived for this study, which is nonhuman subject research and therefore does not require IRB approval. The study was approved by the Tissue Utilization Committee per the IRB-approved biorepository protocol and SOPs (IRB #Pro00069834).
Data Availability Statement
Acknowledgments
The authors would like to acknowledge the staff of the Prisma Health Cancer Institute Biorepository for assistance with sample acquisition, and Jonah Shealy and Alex Kesic for contributing to experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.; Leblanc, M.; Elgbeili, G.; Cordova, M.J.; Marin, M.F.; Brunet, A. The Mental Health Impacts of Receiving a Breast Cancer Diagnosis: A Meta-Analysis. Br J Cancer 2021, 125, 1582–1592. [Google Scholar] [CrossRef]
- Obeng-Gyasi, S.; Obeng-Gyasi, B.; Tarver, W. Breast Cancer Disparities and the Impact of Geography. Surg Oncol Clin N Am 2022, 31, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, L.; Sampson, M.; Stempel, M.; Jacks, L.M.; Patil, S.M.; King, T.; Morrow, M. Presenting Features of Breast Cancer Differ by Molecular Subtype. Ann Surg Oncol 2009, 16, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol Biomarkers Prev 2018, 27, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical Implications of the Intrinsic Molecular Subtypes of Breast Cancer. Breast 2015, 24 Suppl 2, S26–S35. [Google Scholar] [CrossRef]
- Raposo, C.D.; Canelas, A.B.; Barros, M.T. Human Lectins, Their Carbohydrate Affinities and Where to Find Them. Biomolecules 2021, 11, 1–22. [Google Scholar] [CrossRef]
- Houzelstein, D.; Gonçalves, I.R.; Fadden, A.J.; Sidhu, S.S.; Cooper, D.N.W.; Drickamer, K.; Leffler, H.; Poirier, F. Phylogenetic Analysis of the Vertebrate Galectin Family. Mol Biol Evol 2004, 21, 1177–1187. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a Novel Biomarker for Disease Diagnosis and a Target for Therapy (Review). Int J Mol Med 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Elola, M.T.; Croci, D.O.; Rabinovich, G.A. Integrating Structure and Function of “tandem-Repeat” Galectins. Front Biosci (Schol Ed) 2012, 4, 864–887. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Huergo, S.P.; Blidner, A.G.; Rabinovich, G.A. Galectins: Emerging Regulatory Checkpoints Linking Tumor Immunity and Angiogenesis. Curr Opin Immunol 2017, 45, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A. Turning “sweet” on Immunity: Galectin-Glycan Interactions in Immune Tolerance and Inflammation. Nat Rev Immunol 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.S.; Partridge, E.A.; Grigorian, A.; Silvescu, C.I.; Reinhold, V.N.; Demetriou, M.; Dennis, J.W. Complex N-Glycan Number and Degree of Branching Cooperate to Regulate Cell Proliferation and Differentiation. Cell 2007, 129, 123–134. [Google Scholar] [CrossRef]
- Raz, A.; Nakahara, S. Biological Modulation by Lectins and Their Ligands in Tumor Progression and Metastasis. Anticancer Agents Med Chem 2008, 8, 22–36. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a Glance. J Cell Sci 2018, 131. [Google Scholar] [CrossRef]
- Chou, F.C.; Chen, H.Y.; Kuo, C.C.; Sytwu, H.K. Role of Galectins in Tumors and in Clinical Immunotherapy. Int J Mol Sci 2018, 19, 430. [Google Scholar] [CrossRef]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.-T.; Baum, L.G. Galectin-3 and Galectin-1 Bind Distinct Cell Surface Glycoprotein Receptors to Induce T Cell Death. J Immunol 2006, 176, 778–789. [Google Scholar] [CrossRef]
- Cedeno-Laurent, F.; Watanabe, R.; Teague, J.E.; Kupper, T.S.; Clark, R.A.; Dimitroff, C.J. Galectin-1 Inhibits the Viability, Proliferation, and Th1 Cytokine Production of Nonmalignant T Cells in Patients with Leukemic Cutaneous T-Cell Lymphoma. Blood 2012, 119, 3534–3538. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, X.; Nash, G.B.; Stone, P.C.; Hilkens, J.; Rhodes, J.M.; Yu, L.G. Circulating Galectin-3 Promotes Metastasis by Modifying MUC1 Localization on Cancer Cell Surface. Cancer Res 2009, 69, 6799–6806. [Google Scholar] [CrossRef]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Klibi, J.; Niki, T.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Moulec, S.L.E.; Guigay, J.; Hirashima, M.; Guemira, F.; et al. Blood Diffusion and Th1-Suppressive Effects of Galectin-9-Containing Exosomes Released by Epstein-Barr Virus-Infected Nasopharyngeal Carcinoma Cells. Blood 2009, 113, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, M.A.; Mayoral, C.; Meneses, A.; Villalvazo, L.; Guzman, A.; Espinosa, B.; Ochoa, J.L.; Zenteno, E.; Guevara, J. Identification of Galectin-3 and Mucin-Type O-Glycans in Breast Cancer and Its Metastasis to Brain. Cancer Invest 2008, 26, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-L.; Hung, J.-Y.; Huang, S.-K.; Chou, S.-H.; Cheng, D.-E.; Jong, Y.-J.; Hung, C.-H.; Yang, C.-J.; Tsai, Y.-M.; Hsu, Y.-L.; et al. Lung Cancer-Derived Galectin-1 Mediates Dendritic Cell Anergy through Inhibitor of DNA Binding 3/IL-10 Signaling Pathway. J Immunol 2011, 186, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Méndez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting Galectin-1 Overcomes Breast Cancer-Associated Immunosuppression and Prevents Metastatic Disease. Cancer Res 2013, 73, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Moon, H.G.; Bok, I.C.; Jeong, C.Y.; Joo, Y.T.; Lee, Y.J.; Hong, S.C.; Choi, S.K.; Ha, W.S.; Jae, W.K.; et al. Galectin-1 Expression in Cancer-Associated Stromal Cells Correlates Tumor Invasiveness and Tumor Progression in Breast Cancer. Int J Cancer 2007, 120, 2331–2338. [Google Scholar] [CrossRef]
- Demers, M.; Rose, A.A.N.; Grosset, A.A.; Biron-Pain, K.; Gaboury, L.; Siegel, P.M.; St-Pierre, Y. Overexpression of Galectin-7, a Myoepithelial Cell Marker, Enhances Spontaneous Metastasis of Breast Cancer Cells. Am J Pathol 2010, 176, 3023–3031. [Google Scholar] [CrossRef]
- Danguy, A.; Rorive, S.; Decaestecker, C.; Bronckart, Y.; Kaltner, H.; Hadari, Y.R.; Goren, R.; Zich, Y.; Petein, M.; Salmon, I.; et al. Immunohistochemical Profile of Galectin-8 Expression in Benign and Malignant Tumors of Epithelial, Mesenchymatous and Adipous Origins, and of the Nervous System. Histol Histopathol 2001, 16, 861–868. [Google Scholar] [CrossRef]
- Szöke, T.; Kayser, K.; Baumhäkel, J.D.; Trojan, I.; Furak, J.; Tiszlavicz, L.; Horvath, A.; Szluha, K.; Gabius, H.J.; Andre, S. Prognostic Significance of Endogenous Adhesion/Growth-Regulatory Lectins in Lung Cancer. Oncology 2005, 69, 167–174. [Google Scholar] [CrossRef]
- Barrow, H.; Guo, X.; Wandall, H.H.; Pedersen, J.W.; Fu, B.; Zhao, Q.; Chen, C.; Rhodes, J.M.; Yu, L.G. Serum Galectin-2, -4, and -8 Are Greatly Increased in Colon and Breast Cancer Patients and Promote Cancer Cell Adhesion to Blood Vascular Endothelium. Clin Cancer Res 2011, 17, 7035–7046. [Google Scholar] [CrossRef]
- Iurisci, I.; Tinari, N.; Natoli, C.; Angelucci, D.; Cianchetti, E.; Iacobelli, S. Concentrations of Galectin-3 in the Sera of Normal Controls and Cancer Patients. Clinical Cancer Research 2000, 6. [Google Scholar]
- Blair, B.B.; Funkhouser, A.T.; Goodwin, J.L.; Strigenz, A.M.; Chaballout, B.H.; Martin, J.C.; Arthur, C.M.; Funk, C.R.; Edenfield, W.J.; Blenda, A. V. Increased Circulating Levels of Galectin Proteins in Patients with Breast, Colon, and Lung Cancer. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as Modulators of Tumour Progression. Nat Rev Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Girotti, M.R.; Salatino, M.; Dalotto-Moreno, T.; Rabinovich, G.A. Sweetening the Hallmarks of Cancer: Galectins as Multifunctional Mediators of Tumor Progression. J Exp Med 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, L.; Soumoy, L.; Trelcat, A.; Verset, L.; Journe, F.; Saussez, S. New Treatment Strategy Targeting Galectin-1 against Thyroid Cancer. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Grosset, A.A.; Labrie, M.; Vladoiu, M.C.; Yousef, E.M.; Gaboury, L.; St-Pierre, Y. Galectin Signatures Contribute to the Heterogeneity of Breast Cancer and Provide New Prognostic Information and Therapeutic Targets. Oncotarget 2016, 7, 18183–18203. [Google Scholar] [CrossRef] [PubMed]
- Funkhouser, A.T.; Strigenz, A.M.; Blair, B.B.; Miller, A.P.; Shealy, J.C.; Ewing, J.A.; Martin, J.C.; Funk, C.R.; Edenfield, W.J.; Blenda, A. V. KIT Mutations Correlate with Higher Galectin Levels and Brain Metastasis in Breast and Non-Small Cell Lung Cancer. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Souza, D.S.; Macheroni, C.; Pereira, G.J.S.; Vicente, C.M.; Porto, C.S. Molecular Regulation of Prostate Cancer by Galectin-3 and Estrogen Receptor. Front Endocrinol (Lausanne) 2023, 14. [Google Scholar] [CrossRef]
- Irie, A.; Yamauchi, A.; Kontani, K.; Kihara, M.; Liu, D.; Shirato, Y.; Seki, M.; Nishi, N.; Nakamura, T.; Yokomise, H.; et al. Galectin-9 as a Prognostic Factor with Antimetastatic Potential in Breast Cancer. Clinical Cancer Research 2005, 11, 2962–2968. [Google Scholar] [CrossRef]
- Trebo, A.; Ditsch, N.; Kuhn, C.; Heidegger, H.H.; Zeder-Goess, C.; Kolben, T.; Czogalla, B.; Schmoeckel, E.; Mahner, S.; Jeschke, U.; et al. High Galectin-7 and Low Galectin-8 Expression and the Combination of Both Are Negative Prognosticators for Breast Cancer Patients. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Topcu, T.O.; Kavgaci, H.; Gunaldi, M.; Kocoglu, H.; Akyol, M.; Mentese, A.; Yaman, S.O.; Orem, A.; Ozdemir, F.; Aydin, F. The Clinical Importance of Serum Galectin-3 Levels in Breast Cancer Patients with and without Metastasis. J Cancer Res Ther 2018, 14, S583–S586. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.H.; Byun, K. Do; Park, E.H.; Lee, J.H.; Han, S.H. Association of Galectin 9 Expression with Immune Cell Infiltration, Programmed Cell Death Ligand-1 Expression, and Patient’s Clinical Outcome in Triple-Negative Breast Cancer. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Pally, D.; Banerjee, M.; Hussain, S.; Kumar, R. V.; Petersson, A.; Rosendal, E.; Gunnarsson, L.; Peterson, K.; Leffler, H.; Nilsson, U.J.; et al. Galectin-9 Signaling Drives Breast Cancer Invasion through Extracellular Matrix. ACS Chem Biol 2022, 17, 1376–1386. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Ishida, M.; Yanai, H.; Tsuta, K.; Sekimoto, M.; Sugie, T. Prognostic Significance of the Expression Levels of T-Cell Immunoglobulin Mucin-3 and Its Ligand Galectin-9 for Relapse-Free Survival in Triple-Negative Breast Cancer. Oncol Lett 2022, 23. [Google Scholar] [CrossRef]
- Yasinska, I.M.; Sakhnevych, S.S.; Pavlova, L.; Selnø, A.T.H.; Abeleira, A.M.T.; Benlaouer, O.; Silva, I.G.; Mosimann, M.; Varani, L.; Bardelli, M.; et al. The TiM-3-Galectin-9 Pathway and Its Regulatory Mechanisms in Human Breast Cancer. Front Immunol 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 Interacts with PD-1 and TIM-3 to Regulate T Cell Death and Is a Target for Cancer Immunotherapy. Nat Commun 2021, 12, 1–17. [Google Scholar] [CrossRef]
Figure 1.
Comparison of serum galectin-1, -3, and -9 levels, as determined by ELISA, between stages of breast cancer (A), and broken down by molecular subtype (B). Multiple comparisons were performed with one-way ANOVA, with subsequent analysis using of all pairs using Student’s t-test if the ANOVA’s probability > F was less than 0.05. N for all, Luminal A, Luminal B, HER2 Enriched, and Triple Negative are 138, 79, 18, 10, and 31 respectively.
Figure 1.
Comparison of serum galectin-1, -3, and -9 levels, as determined by ELISA, between stages of breast cancer (A), and broken down by molecular subtype (B). Multiple comparisons were performed with one-way ANOVA, with subsequent analysis using of all pairs using Student’s t-test if the ANOVA’s probability > F was less than 0.05. N for all, Luminal A, Luminal B, HER2 Enriched, and Triple Negative are 138, 79, 18, 10, and 31 respectively.
Figure 2.
Graph of serum galectin-1, -3, and -9 levels, determined by ELISA, by level of estrogen and progesterone receptor expression in breast cancer via immunohistochemical stain. N = 97 Blue, Gal-1; Red, Gal-3; Green, Gal-9; ER, Estrogen Receptor; PR, Progesterone Receptor; IHC, Immunohistochemistry.
Figure 2.
Graph of serum galectin-1, -3, and -9 levels, determined by ELISA, by level of estrogen and progesterone receptor expression in breast cancer via immunohistochemical stain. N = 97 Blue, Gal-1; Red, Gal-3; Green, Gal-9; ER, Estrogen Receptor; PR, Progesterone Receptor; IHC, Immunohistochemistry.
Figure 3.
Comparison of serum galectin-9 levels of breast cancer patients, as determined by ELISA, by binary expression of estrogen and progesterone receptor and HER-2 amplification determined by immunohistochemical stain. n = 77. *One-tail t-test.
Figure 3.
Comparison of serum galectin-9 levels of breast cancer patients, as determined by ELISA, by binary expression of estrogen and progesterone receptor and HER-2 amplification determined by immunohistochemical stain. n = 77. *One-tail t-test.
Figure 4.
Comparison of serum galectin-9 levels between molecular subtypes of breast cancer. Galectin-9 levels were measured from serum samples of breast cancer patients by ELISA assays. Patients were classified by molecular subtype of breast cancer. Multi-pair analysis of galectin-9 by subtype were done with Student’s t-test. n = 77. LA, Luminal A; LB, Luminal B; H2, HER2 Enriched; TN, Triple Negative.
Figure 4.
Comparison of serum galectin-9 levels between molecular subtypes of breast cancer. Galectin-9 levels were measured from serum samples of breast cancer patients by ELISA assays. Patients were classified by molecular subtype of breast cancer. Multi-pair analysis of galectin-9 by subtype were done with Student’s t-test. n = 77. LA, Luminal A; LB, Luminal B; H2, HER2 Enriched; TN, Triple Negative.
Figure 5.
Comparison of galectin-1, -3, and -9 levels by patient exposure to immunological, chemotherapy, or hormone therapy, “Systemic Therapy”. Galectin levels were measured from serum samples of breast cancer patients by ELISA assays. Patients were classified by status of receiving any form of immunological, chemotherapy, or hormone therapy. The serum levels of galectins of systemic therapy exposed versus non-exposed patients were compared alone (A), by stage (B), and by molecular subtype (C). Two-group comparisons were done by t-test. Multiple comparisons were performed with one-way ANOVA, with subsequent analysis using of all pairs using Student’s t-test if the ANOVA’s probability > F was less than 0.05. n = 76. *One-tail t-test.
Figure 5.
Comparison of galectin-1, -3, and -9 levels by patient exposure to immunological, chemotherapy, or hormone therapy, “Systemic Therapy”. Galectin levels were measured from serum samples of breast cancer patients by ELISA assays. Patients were classified by status of receiving any form of immunological, chemotherapy, or hormone therapy. The serum levels of galectins of systemic therapy exposed versus non-exposed patients were compared alone (A), by stage (B), and by molecular subtype (C). Two-group comparisons were done by t-test. Multiple comparisons were performed with one-way ANOVA, with subsequent analysis using of all pairs using Student’s t-test if the ANOVA’s probability > F was less than 0.05. n = 76. *One-tail t-test.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).