Submitted:
26 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell culture
2.2. Reagents and antibodies
2.3. Plasmid construction
2.4. Luciferase Reporter Assay
2.5. RNA Extraction and Real-Time PCR (qRT-PCR)
2.6. RNA sequencing (RNA-seq)
2.7. Virus Infection in cell and mice
2.8. Mass Spectrometry
2.9. Coimmunoprecipitation and immunoblot assays
2.10. Statistical Analysis
3. Results
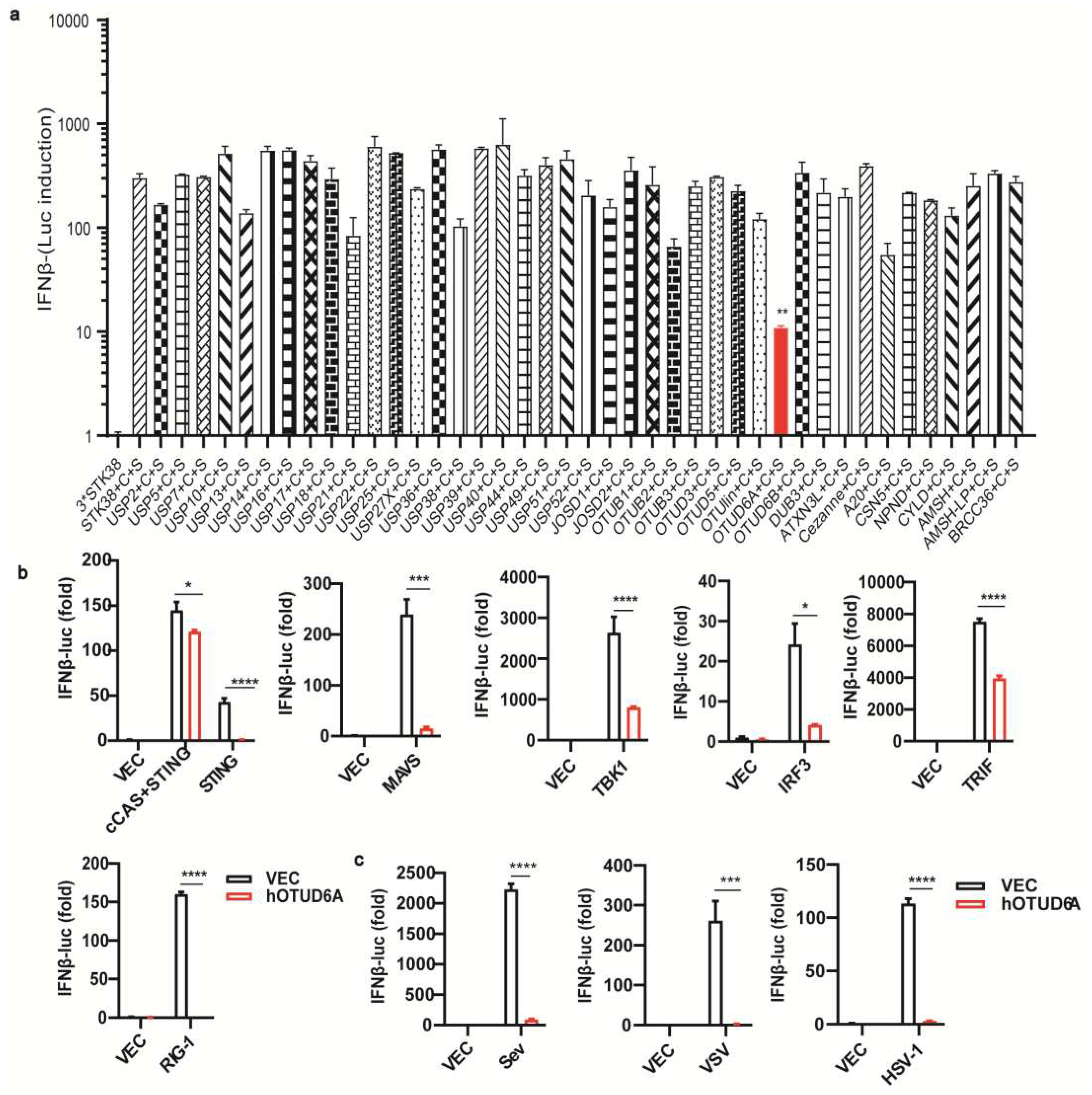
3.1. OTUD6A overexpression inhibits the production of type I IFN
3.2. OTUD6A deficiency enhances antiviral innate immunity in Vitro
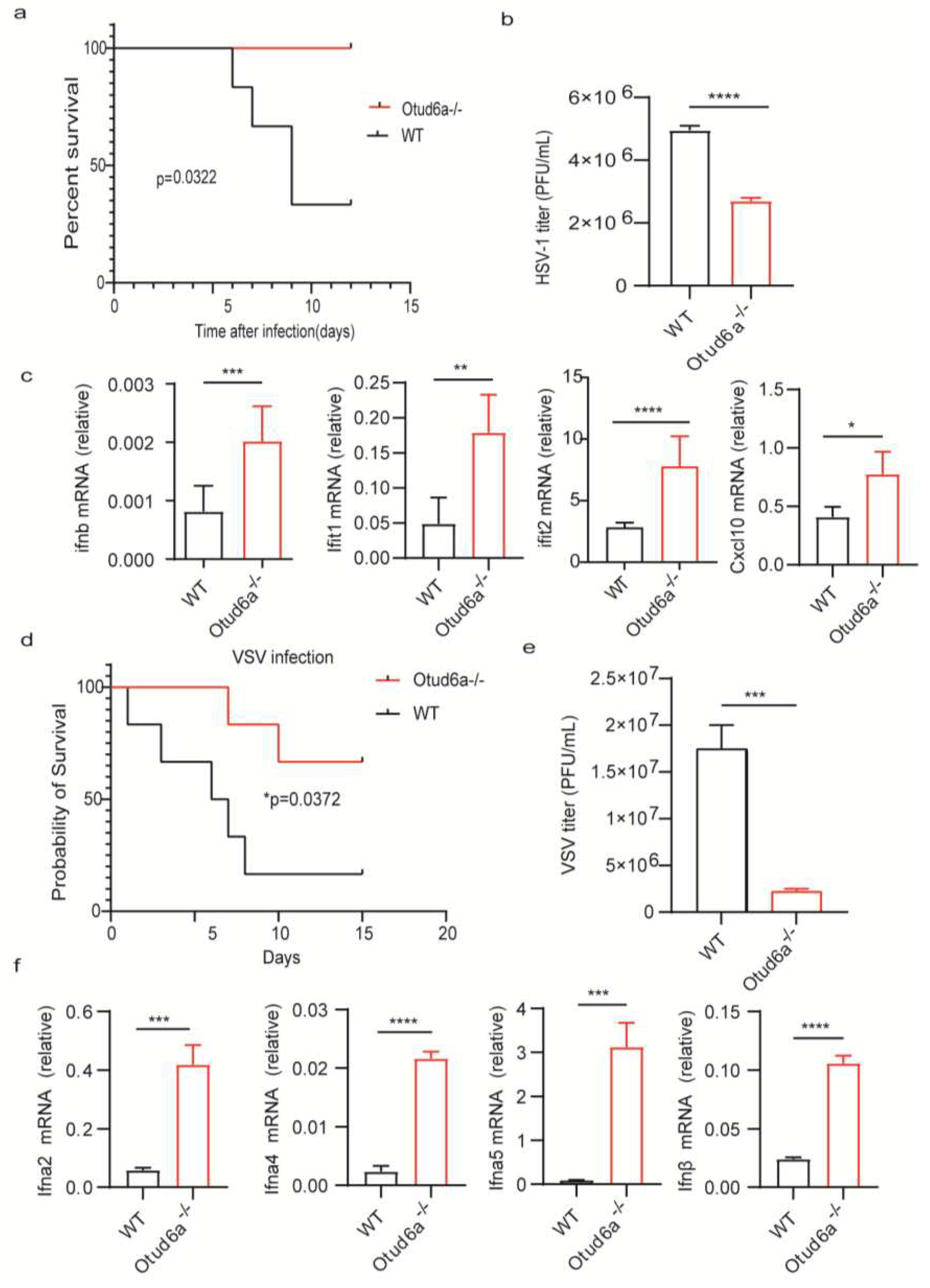
3.3. OTUD6A deficiency enhances antiviral innate immunity in Vivo
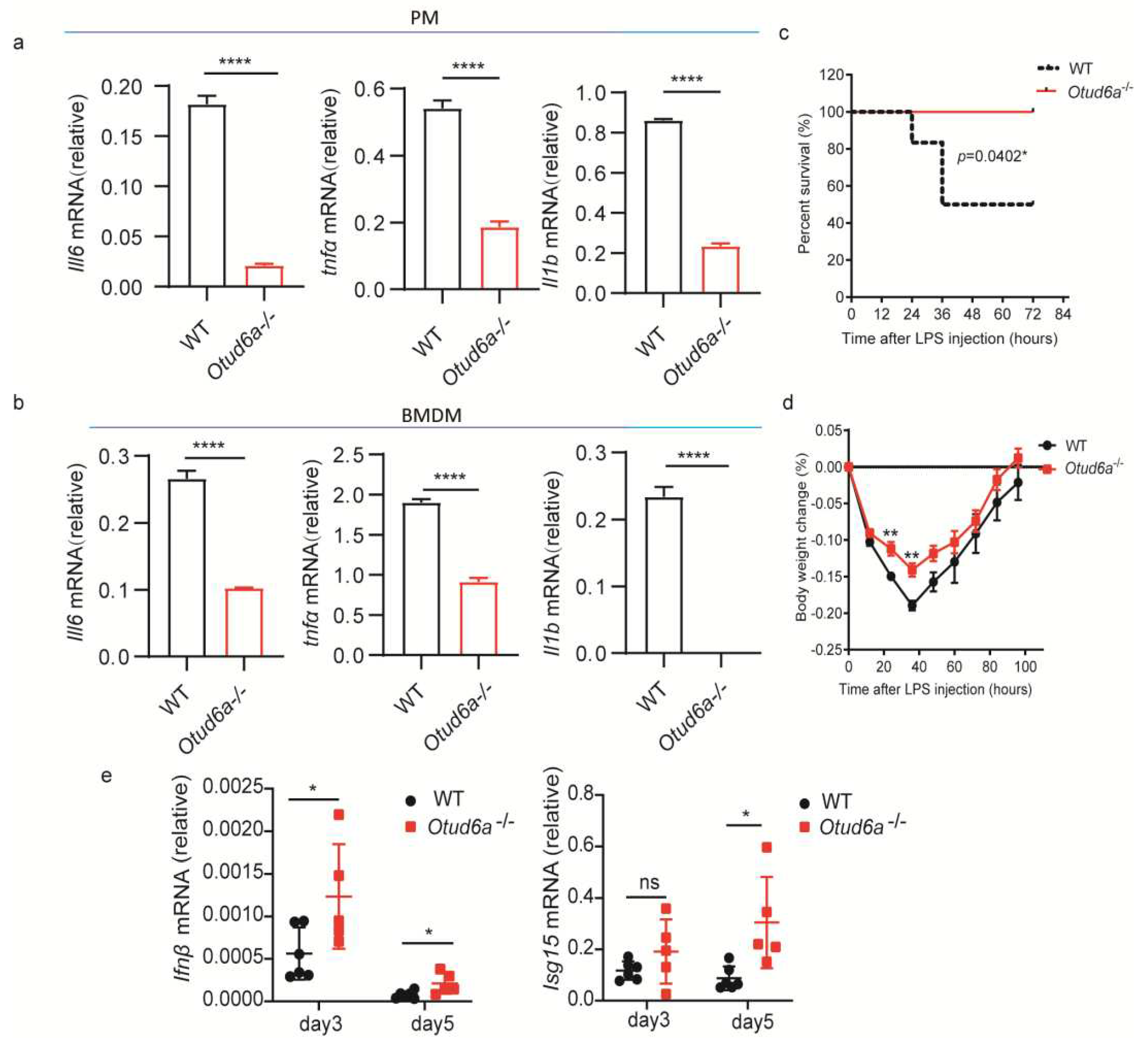
3.4. OTUD6A deficiency increases HSV-1 induced inflammatory responses
3.5. OTUD6A participates in the regulation of NF-κB mediated inflammation signaling pathway via UBC13.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ebstein, F.; Keller, M.; Paschen, A.; Walden, P.; Seeger, M.; Burger, E.; Kruger, E.; Schadendorf, D.; Kloetzel, P. M.; Seifert, U., Exposure to Melan-A/MART-126-35 tumor epitope specific CD8(+)T cells reveals immune escape by affecting the ubiquitin-proteasome system (UPS). Sci Rep 2016, 6, 25208. [CrossRef]
- Katayama, S.; Kukita, T.; Ishikawa, E.; Nakashima, S.; Masuda, S.; Kanda, T.; Akiyama, H.; Teshima, R.; Nakamura, S., Apple polyphenols suppress antigen presentation of ovalbumin by THP-1-derived dendritic cells. Food Chem 2013, 138, (2-3), 757-61. [CrossRef]
- Liu, J.; Guan, D.; Dong, M.; Yang, J.; Wei, H.; Liang, Q.; Song, L.; Xu, L.; Bai, J.; Liu, C.; Mao, J.; Zhang, Q.; Zhou, J.; Wu, X.; Wang, M.; Cong, Y. S., UFMylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nat Cell Biol 2020, 22, (9), 1056-1063. [CrossRef]
- Wang, B.; Jie, Z. L.; Joo, D. H.; Ordureau, A.; Liu, P.; Gan, W. J.; Guo, J. P.; Zhang, J. F.; North, B. J.; Dai, X. P.; Cheng, X. H.; Bian, X. W.; Zhang, L. Q.; Harper, J. W.; Sun, S. C.; Wei, W. Y., TRAF2 and OTUD7B govern a ubiquitin-dependent switch that regulates mTORC2 signalling. Nature 2017, 545, (7654), 365-+. [CrossRef]
- Liu, J.; Zou, X.; Gotoh, T.; Brown, A. M.; Jiang, L.; Wisdom, E. L.; Kim, J. K.; Finkielstein, C. V., Distinct control of PERIOD2 degradation and circadian rhythms by the oncoprotein and ubiquitin ligase MDM2. Sci Signal 2018, 11, (556). [CrossRef]
- Chen, S.; Yang, J.; Yang, L.; Zhang, Y.; Zhou, L.; Liu, Q.; Duan, C.; Mieres, C. A.; Zhou, G.; Xu, G., Ubiquitin ligase TRAF2 attenuates the transcriptional activity of the core clock protein BMAL1 and affects the maximal Per1 mRNA level of the circadian clock in cells. FEBS J 2018, 285, (16), 2987-3001. [CrossRef]
- Jia, J. Y.; Bissa, B.; Brecht, L.; Allers, L.; Choi, S. W.; Gu, Y. X.; Zbinden, M.; Burge, M. R.; Timmins, G.; Hallows, K.; Behrends, C.; Deretic, V., AMPK is activated during lysosomal damage via a galectin-ubiquitin signal transduction system. Autophagy 2020, 16, (8), 1550-1552. [CrossRef]
- Hansen, F. M.; Tanzer, M. C.; Bruning, F.; Bludau, I.; Stafford, C.; Schulman, B. A.; Robles, M. S.; Karayel, O.; Mann, M., Data-independent acquisition method for ubiquitinome analysis reveals regulation of circadian biology. Nat Commun 2021, 12, (1), 254. [CrossRef]
- Murray, S. S.; Wong, A. W.; Yang, J.; Li, Y.; Putz, U.; Tan, S. S.; Howitt, J., Ubiquitin Regulation of Trk Receptor Trafficking and Degradation. Mol Neurobiol 2019, 56, (3), 1628-1636. [CrossRef]
- Murakami, T.; Felinski, E. A.; Antonetti, D. A., Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem 2009, 284, (31), 21036-46. [CrossRef]
- Teh, C. E.; Lalaoui, N.; Jain, R.; Policheni, A. N.; Heinlein, M.; Alvarez-Diaz, S.; Sheridan, J. M.; Rieser, E.; Deuser, S.; Darding, M.; Koay, H. F.; Hu, Y.; Kupresanin, F.; O'Reilly, L. A.; Godfrey, D. I.; Smyth, G. K.; Bouillet, P.; Strasser, A.; Walczak, H.; Silke, J.; Gray, D. H., Linear ubiquitin chain assembly complex coordinates late thymic T-cell differentiation and regulatory T-cell homeostasis. Nat Commun 2016, 7, 13353. [CrossRef]
- Verheul, T. C. J.; Philipsen, S., A ubiquitin ligase toggles red cell differentiation. Blood 2021, 137, (2), 143-144. [CrossRef]
- Sun, H.; Zhang, Q.; Jing, Y. Y.; Zhang, M.; Wang, H. Y.; Cai, Z.; Liuyu, T.; Zhang, Z. D.; Xiong, T. C.; Wu, Y.; Zhu, Q. Y.; Yao, J.; Shu, H. B.; Lin, D.; Zhong, B., USP13 negatively regulates antiviral responses by deubiquitinating STING. Nat Commun 2017, 8, 15534. [CrossRef]
- Zhang, Z.; Fang, X.; Wu, X.; Ling, L.; Chu, F.; Li, J.; Wang, S.; Zang, J.; Zhang, B.; Ye, S.; Zhang, L.; Yang, B.; Lin, S.; Huang, H.; Wang, A.; Zhou, F., Acetylation-Dependent Deubiquitinase OTUD3 Controls MAVS Activation in Innate Antiviral Immunity. Mol Cell 2020, 79, (2), 304-319 e7. [CrossRef]
- Jiang, X.; Chen, Z. J., The role of ubiquitylation in immune defence and pathogen evasion. Nat Rev Immunol 2011, 12, (1), 35-48. [CrossRef]
- Mukhopadhyay, D.; Riezman, H., Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 2007, 315, (5809), 201-5. [CrossRef]
- Li, Y.; Xie, P.; Lu, L.; Wang, J.; Diao, L.; Liu, Z.; Guo, F.; He, Y.; Liu, Y.; Huang, Q.; Liang, H.; Li, D.; He, F., An integrated bioinformatics platform for investigating the human E3 ubiquitin ligase-substrate interaction network. Nat Commun 2017, 8, (1), 347. [CrossRef]
- Lv, Z.; Rickman, K. A.; Yuan, L.; Williams, K.; Selvam, S. P.; Woosley, A. N.; Howe, P. H.; Ogretmen, B.; Smogorzewska, A.; Olsen, S. K., S. pombe Uba1-Ubc15 Structure Reveals a Novel Regulatory Mechanism of Ubiquitin E2 Activity. Mol Cell 2017, 65, (4), 699-714 e6. [CrossRef]
- Hjerpe, R.; Bett, J. S.; Keuss, M. J.; Solovyova, A.; McWilliams, T. G.; Johnson, C.; Sahu, I.; Varghese, J.; Wood, N.; Wightman, M.; Osborne, G.; Bates, G. P.; Glickman, M. H.; Trost, M.; Knebel, A.; Marchesi, F.; Kurz, T., UBQLN2 Mediates Autophagy-Independent Protein Aggregate Clearance by the Proteasome. Cell 2016, 166, (4), 935-949. [CrossRef]
- Li, F.; Sun, Q.; Liu, K.; Zhang, L.; Lin, N.; You, K.; Liu, M.; Kon, N.; Tian, F.; Mao, Z.; Li, T.; Tong, T.; Qin, J.; Gu, W.; Li, D.; Zhao, W., OTUD5 cooperates with TRIM25 in transcriptional regulation and tumor progression via deubiquitination activity. Nat Commun 2020, 11, (1), 4184. [CrossRef]
- Wertz, I. E.; Newton, K.; Seshasayee, D.; Kusam, S.; Lam, C.; Zhang, J.; Popovych, N.; Helgason, E.; Schoeffler, A.; Jeet, S.; Ramamoorthi, N.; Kategaya, L.; Newman, R. J.; Horikawa, K.; Dugger, D.; Sandoval, W.; Mukund, S.; Zindal, A.; Martin, F.; Quan, C.; Tom, J.; Fairbrother, W. J.; Townsend, M.; Warming, S.; DeVoss, J.; Liu, J.; Dueber, E.; Caplazi, P.; Lee, W. P.; Goodnow, C. C.; Balazs, M.; Yu, K.; Kolumam, G.; Dixit, V. M., Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature 2015, 528, (7582), 370-5. [CrossRef]
- Wertz, I. E.; O'Rourke, K. M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D. L.; Ma, A.; Koonin, E. V.; Dixit, V. M., De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 2004, 430, (7000), 694-9. [CrossRef]
- Pickart, C. M.; Eddins, M. J., Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta 2004, 1695, (1-3), 55-72. [CrossRef]
- Myeku, N.; Clelland, C. L.; Emrani, S.; Kukushkin, N. V.; Yu, W. H.; Goldberg, A. L.; Duff, K. E., Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med 2016, 22, (1), 46-53. [CrossRef]
- Collins, G. A.; Goldberg, A. L., The Logic of the 26S Proteasome. Cell 2017, 169, (5), 792-806. [CrossRef]
- Bhoj, V. G.; Chen, Z. J., Ubiquitylation in innate and adaptive immunity. Nature 2009, 458, (7237), 430-7. [CrossRef]
- 27. Yoshida, Y.; Saeki, Y.; Murakami, A.; Kawawaki, J.; Tsuchiya, H.; Yoshihara, H.; Shindo, M.; Tanaka, K., A comprehensive method for detecting ubiquitinated substrates using TR-TUBE. Proc Natl Acad Sci U S A 2015, 112, (15), 4630-5. [CrossRef]
- Cheng, J.; Guo, J.; North, B. J.; Wang, B.; Cui, C. P.; Li, H.; Tao, K.; Zhang, L.; Wei, W., Functional analysis of deubiquitylating enzymes in tumorigenesis and development. Biochim Biophys Acta Rev Cancer 2019, 1872, (2), 188312. [CrossRef]
- Mevissen, T. E.; Hospenthal, M. K.; Geurink, P. P.; Elliott, P. R.; Akutsu, M.; Arnaudo, N.; Ekkebus, R.; Kulathu, Y.; Wauer, T.; El Oualid, F.; Freund, S. M.; Ovaa, H.; Komander, D., OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 2013, 154, (1), 169-84. [CrossRef]
- Yao, F.; Zhou, Z.; Kim, J.; Hang, Q.; Xiao, Z.; Ton, B. N.; Chang, L.; Liu, N.; Zeng, L.; Wang, W.; Wang, Y.; Zhang, P.; Hu, X.; Su, X.; Liang, H.; Sun, Y.; Ma, L., SKP2- and OTUD1-regulated non-proteolytic ubiquitination of YAP promotes YAP nuclear localization and activity. Nat Commun 2018, 9, (1), 2269. [CrossRef]
- Zhang, Z.; Wang, D.; Wang, P.; Zhao, Y.; You, F., OTUD1 Negatively Regulates Type I IFN Induction by Disrupting Noncanonical Ubiquitination of IRF3. J Immunol 2020, 204, (7), 1904-1918. [CrossRef]
- Wiener, R.; DiBello, A. T.; Lombardi, P. M.; Guzzo, C. M.; Zhang, X.; Matunis, M. J.; Wolberger, C., E2 ubiquitin-conjugating enzymes regulate the deubiquitinating activity of OTUB1. Nat Struct Mol Biol 2013, 20, (9), 1033-9. [CrossRef]
- Wiener, R.; Zhang, X.; Wang, T.; Wolberger, C., The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature 2012, 483, (7391), 618-22. [CrossRef]
- Nakada, S.; Tai, I.; Panier, S.; Al-Hakim, A.; Iemura, S.; Juang, Y. C.; O'Donnell, L.; Kumakubo, A.; Munro, M.; Sicheri, F.; Gingras, A. C.; Natsume, T.; Suda, T.; Durocher, D., Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature 2010, 466, (7309), 941-6. [CrossRef]
- Shi, L.; Liu, J.; Peng, Y.; Zhang, J.; Dai, X.; Zhang, S.; Wang, Y.; Liu, J.; Long, J., Deubiquitinase OTUD6A promotes proliferation of cancer cells via regulating Drp1 stability and mitochondrial fission. Mol Oncol 2020, 14, (12), 3169-3183. [CrossRef]
- Zhao, Y.; Huang, X.; Zhu, D.; Wei, M.; Luo, J.; Yu, S.; Tian, Y.; Zheng, X., Deubiquitinase OTUD6A promotes breast cancer progression by increasing TopBP1 stability and rendering tumor cells resistant to DNA-damaging therapy. Cell Death Differ 2022, 29, (12), 2531-2544. [CrossRef]
- Fu, X.; Zhao, J.; Yu, G.; Zhang, X.; Sun, J.; Li, L.; Yin, J.; Niu, Y.; Ren, S.; Zhu, Y.; Xu, B.; Huang, L., OTUD6A promotes prostate tumorigenesis via deubiquitinating Brg1 and AR. Commun Biol 2022, 5, (1), 182. [CrossRef]
- Peng, Y.; Liu, J.; Wang, Z.; Cui, C.; Zhang, T.; Zhang, S.; Gao, P.; Hou, Z.; Liu, H.; Guo, J.; Zhang, J.; Wen, Y.; Wei, W.; Zhang, L.; Liu, J.; Long, J., Prostate-specific oncogene OTUD6A promotes prostatic tumorigenesis via deubiquitinating and stabilizing c-Myc. Cell Death Differ 2022, 29, (9), 1730-1743. [CrossRef]
- Park, S. H.; Jung, E. H.; Kim, G. Y.; Kim, B. C.; Lim, J. H.; Woo, C. H., Itch E3 ubiquitin ligase positively regulates TGF-beta signaling to EMT via Smad7 ubiquitination. Mol Cells 2015, 38, (1), 20-5. [CrossRef]
- Smith, K.; Gunaratnam, L.; Morley, M.; Franovic, A.; Mekhail, K.; Lee, S., Silencing of epidermal growth factor receptor suppresses hypoxia-inducible factor-2-driven VHL-/- renal cancer. Cancer Res 2005, 65, (12), 5221-30. [CrossRef]
- You, F.; Wang, P.; Yang, L.; Yang, G.; Zhao, Y. O.; Qian, F.; Walker, W.; Sutton, R.; Montgomery, R.; Lin, R.; Iwasaki, A.; Fikrig, E., ELF4 is critical for induction of type I interferon and the host antiviral response. Nat Immunol 2013, 14, (12), 1237-46. [CrossRef]
- Liu, X.; Fang, Y.; Lv, X.; Hu, C.; Chen, G.; Zhang, L.; Jin, B.; Huang, L.; Luo, W.; Liang, G.; Wang, Y., Deubiquitinase OTUD6A in macrophages promotes intestinal inflammation and colitis via deubiquitination of NLRP3. Cell Death Differ 2023. [CrossRef]
- Chang, J. H.; Xiao, Y.; Hu, H.; Jin, J.; Yu, J.; Zhou, X.; Wu, X.; Johnson, H. M.; Akira, S.; Pasparakis, M.; Cheng, X.; Sun, S. C., Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector-like T cells. Nat Immunol 2012, 13, (5), 481-90. [CrossRef]
- Ni, J.; Guan, C.; Liu, H.; Huang, X.; Yue, J.; Xiang, H.; Jiang, Z.; Tao, Y.; Cao, W.; Liu, J.; Wang, Z.; Wang, Y.; Wu, X., Ubc13 Promotes K63-Linked Polyubiquitination of NLRP3 to Activate Inflammasome. J Immunol 2021, 206, (10), 2376-2385. [CrossRef]
- Hu, L.; Xu, J.; Xie, X.; Zhou, Y.; Tao, P.; Li, H.; Han, X.; Wang, C.; Liu, J.; Xu, P.; Neculai, D.; Xia, Z., Oligomerization-primed coiled-coil domain interaction with Ubc13 confers processivity to TRAF6 ubiquitin ligase activity. Nat Commun 2017, 8, (1), 814. [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O'Garra, A., Type I interferons in infectious disease. Nat Rev Immunol 2015, 15, (2), 87-103. [CrossRef]
- Medzhitov, R., Origin and physiological roles of inflammation. Nature 2008, 454, (7203), 428-35. [CrossRef]
- Damgaard, R. B.; Walker, J. A.; Marco-Casanova, P.; Morgan, N. V.; Titheradge, H. L.; Elliott, P. R.; McHale, D.; Maher, E. R.; McKenzie, A. N. J.; Komander, D., The Deubiquitinase OTULIN Is an Essential Negative Regulator of Inflammation and Autoimmunity. Cell 2016, 166, (5), 1215-1230 e20. [CrossRef]
- Panda, S.; Nilsson, J. A.; Gekara, N. O., Deubiquitinase MYSM1 Regulates Innate Immunity through Inactivation of TRAF3 and TRAF6 Complexes. Immunity 2015, 43, (4), 647-59. [CrossRef]
- Wang, X. M.; Yang, C.; Zhao, Y.; Xu, Z. G.; Yang, W.; Wang, P.; Lin, D.; Xiong, B.; Fang, J. Y.; Dong, C.; Zhong, B., The deubiquitinase USP25 supports colonic inflammation and bacterial infection and promotes colorectal cancer. Nat Cancer 2020, 1, (8), 811-825. [CrossRef]
- Li, S.; Zheng, H.; Mao, A. P.; Zhong, B.; Li, Y.; Liu, Y.; Gao, Y.; Ran, Y.; Tien, P.; Shu, H. B., Regulation of virus-triggered signaling by OTUB1- and OTUB2-mediated deubiquitination of TRAF3 and TRAF6. J Biol Chem 2010, 285, (7), 4291-7. [CrossRef]
- Yamamoto, M.; Okamoto, T.; Takeda, K.; Sato, S.; Sanjo, H.; Uematsu, S.; Saitoh, T.; Yamamoto, N.; Sakurai, H.; Ishii, K. J.; Yamaoka, S.; Kawai, T.; Matsuura, Y.; Takeuchi, O.; Akira, S., Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat Immunol 2006, 7, (9), 962-70. [CrossRef]
- Wu, B.; Qiang, L.; Zhang, Y.; Fu, Y.; Zhao, M.; Lei, Z.; Lu, Z.; Wei, Y. G.; Dai, H.; Ge, Y.; Liu, M.; Zhou, X.; Peng, Z.; Li, H.; Cui, C. P.; Wang, J.; Zheng, H.; Liu, C. H.; Zhang, L., The deubiquitinase OTUD1 inhibits colonic inflammation by suppressing RIPK1-mediated NF-kappaB signaling. Cell Mol Immunol 2022, 19, (2), 276-289. [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




