Submitted:
23 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Bacterial cultures
2.2. Phage preparation
2.3. STEC biofilm disruption
2.3.1. In vitro biofilm disruption
2.3.2. STEC biofilm disruption on food-contact surfaces
2.3.2.1. Preparation of coupons
2.4. Statistical analysis
3. Results and Discussion
3.1. STEC biofilm disruption
3.1.1. In vitro STEC biofilm disruption


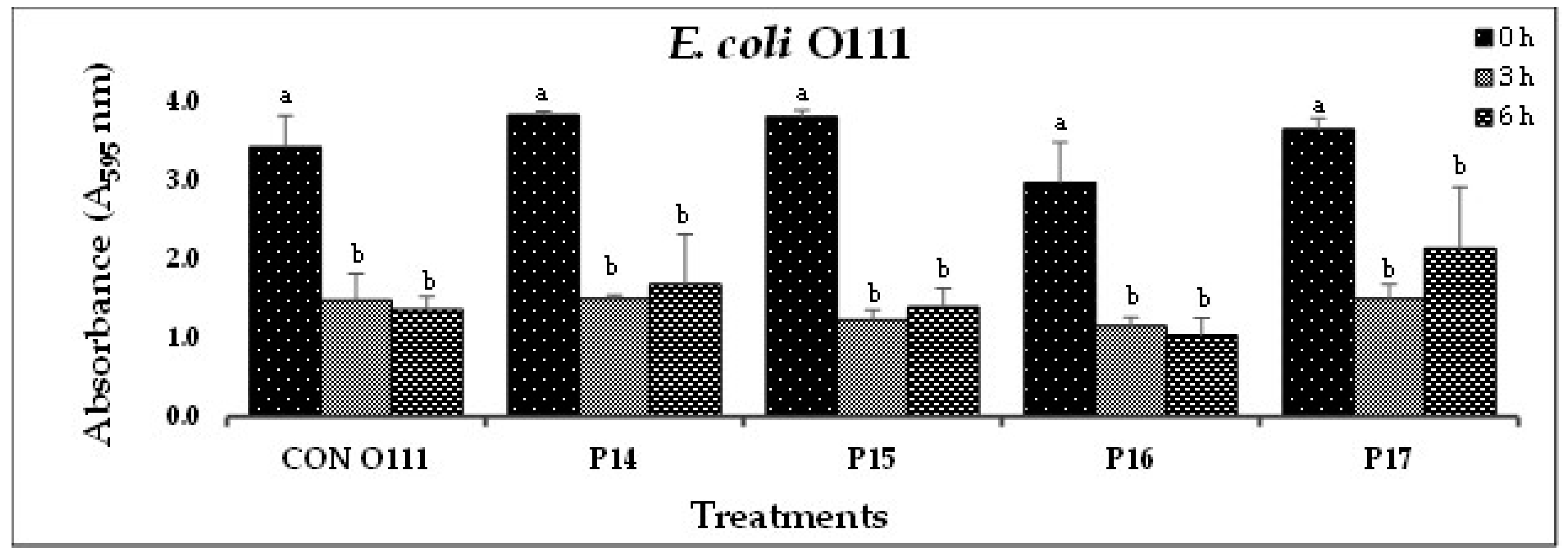

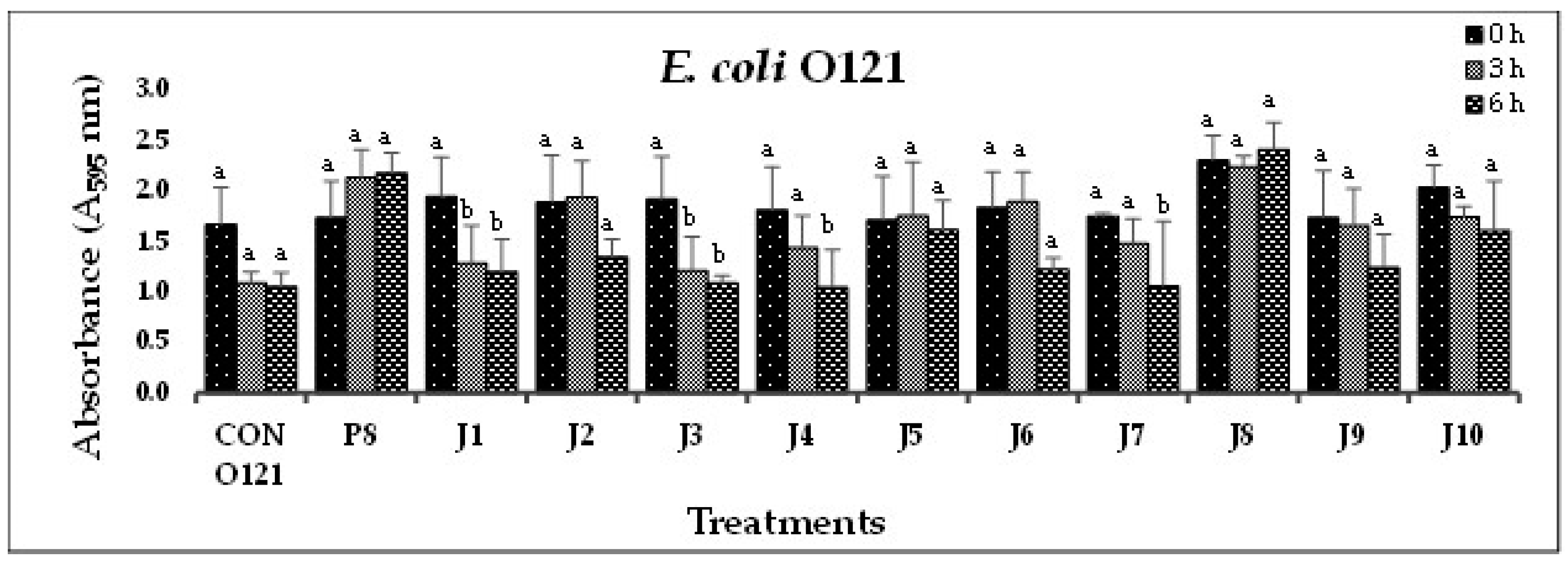
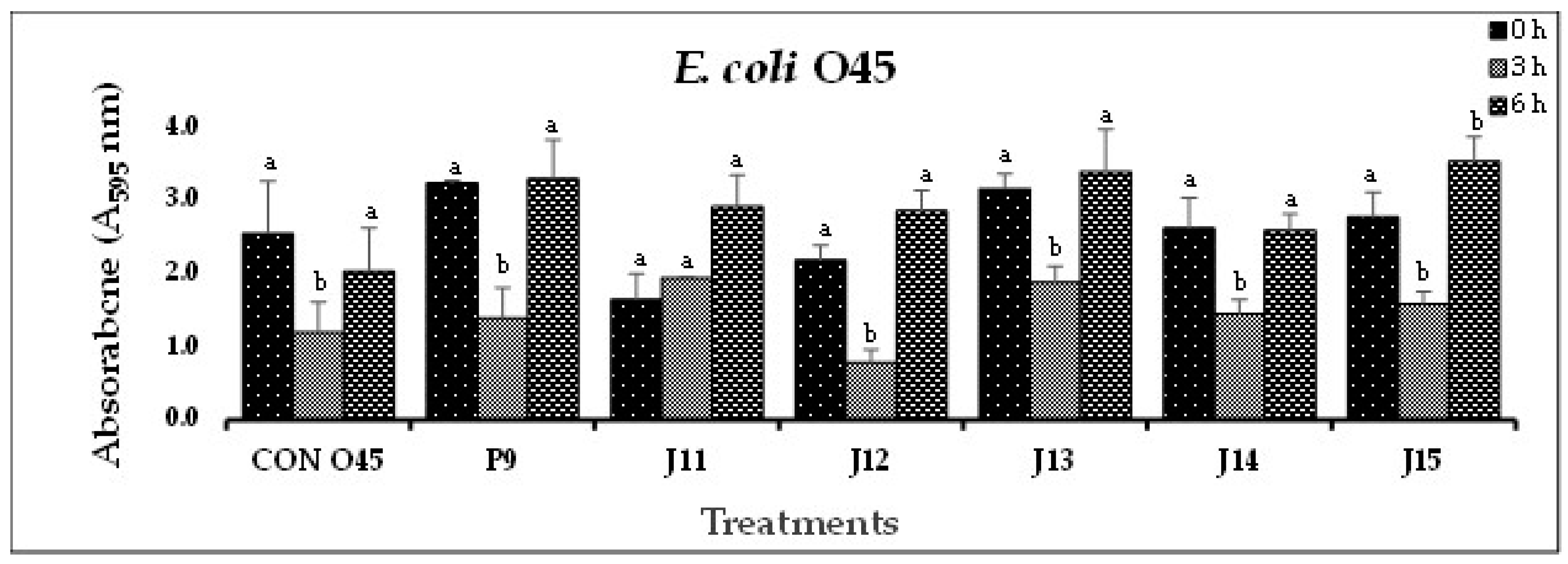
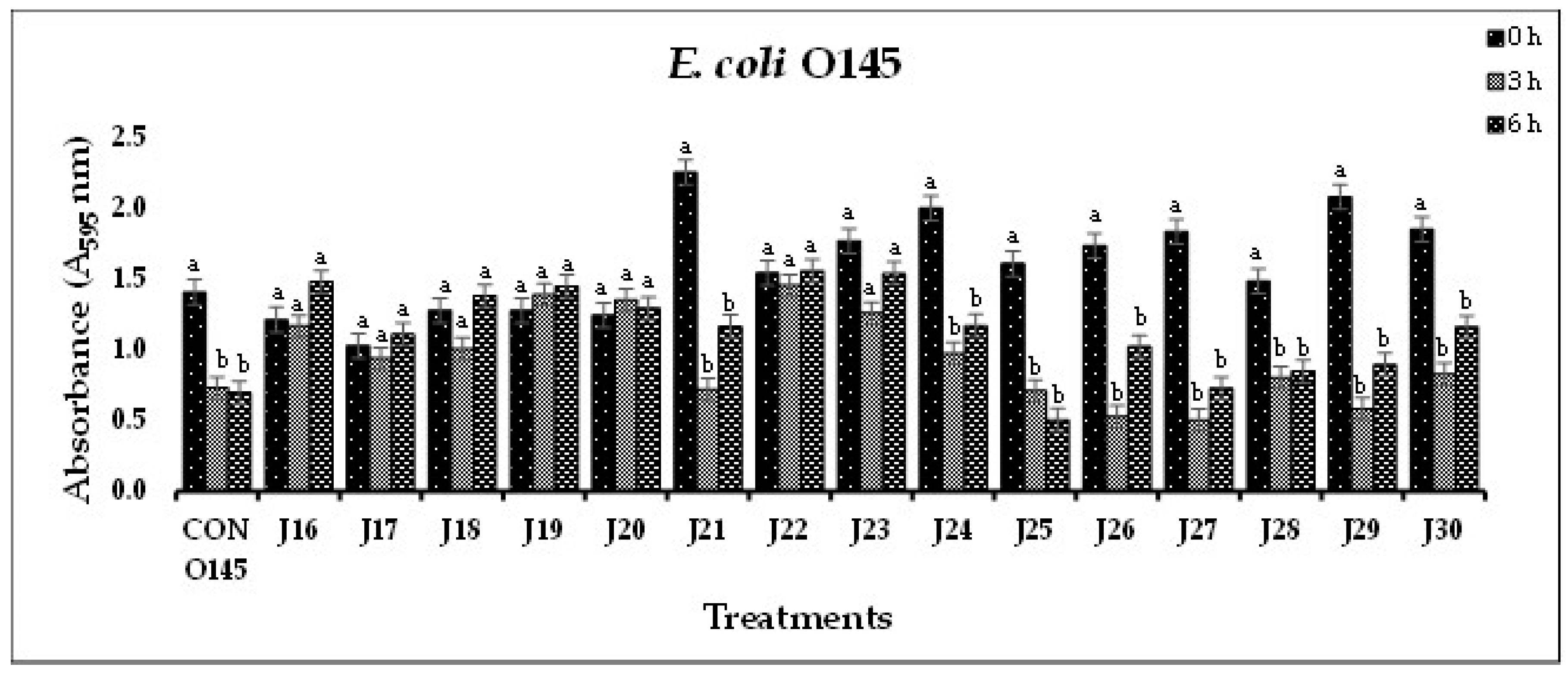
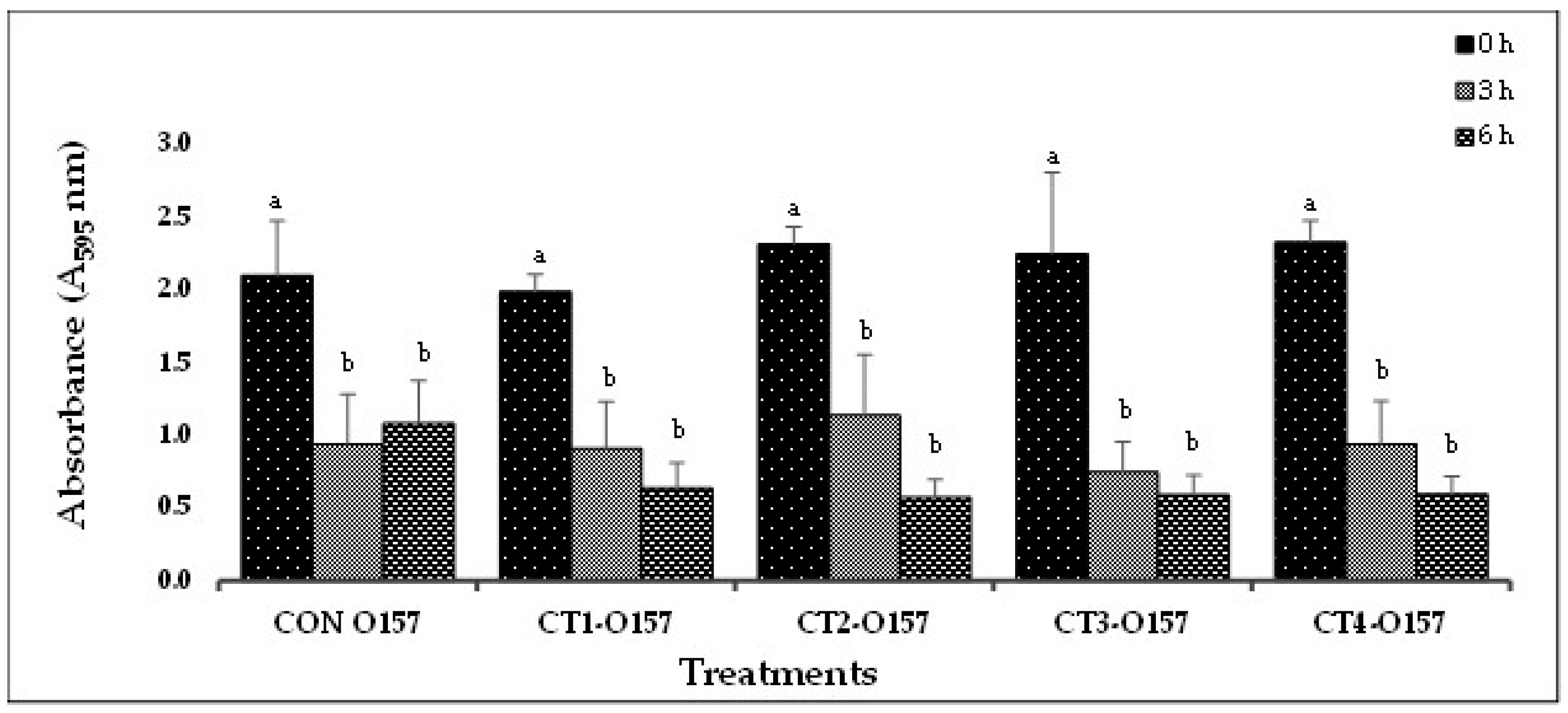
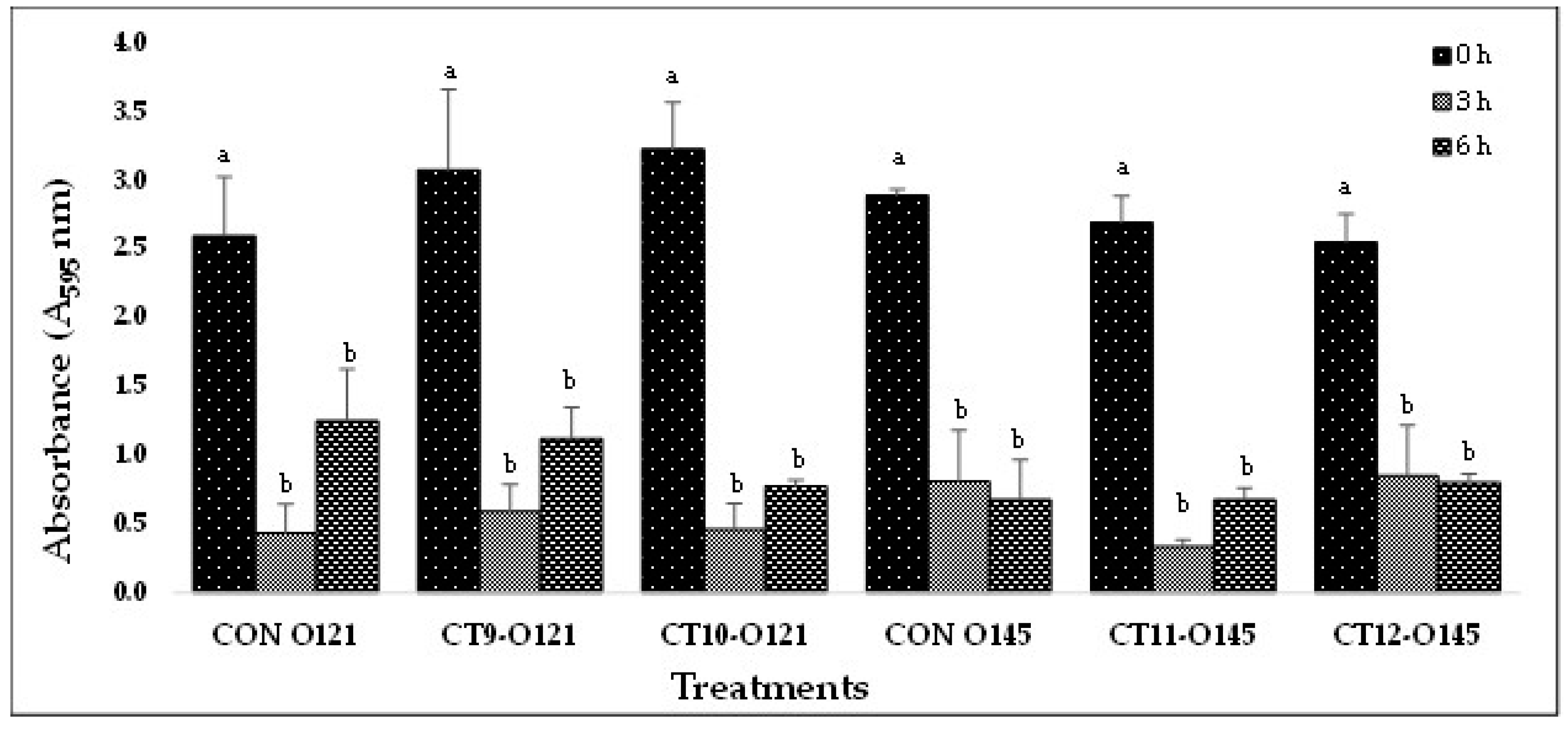
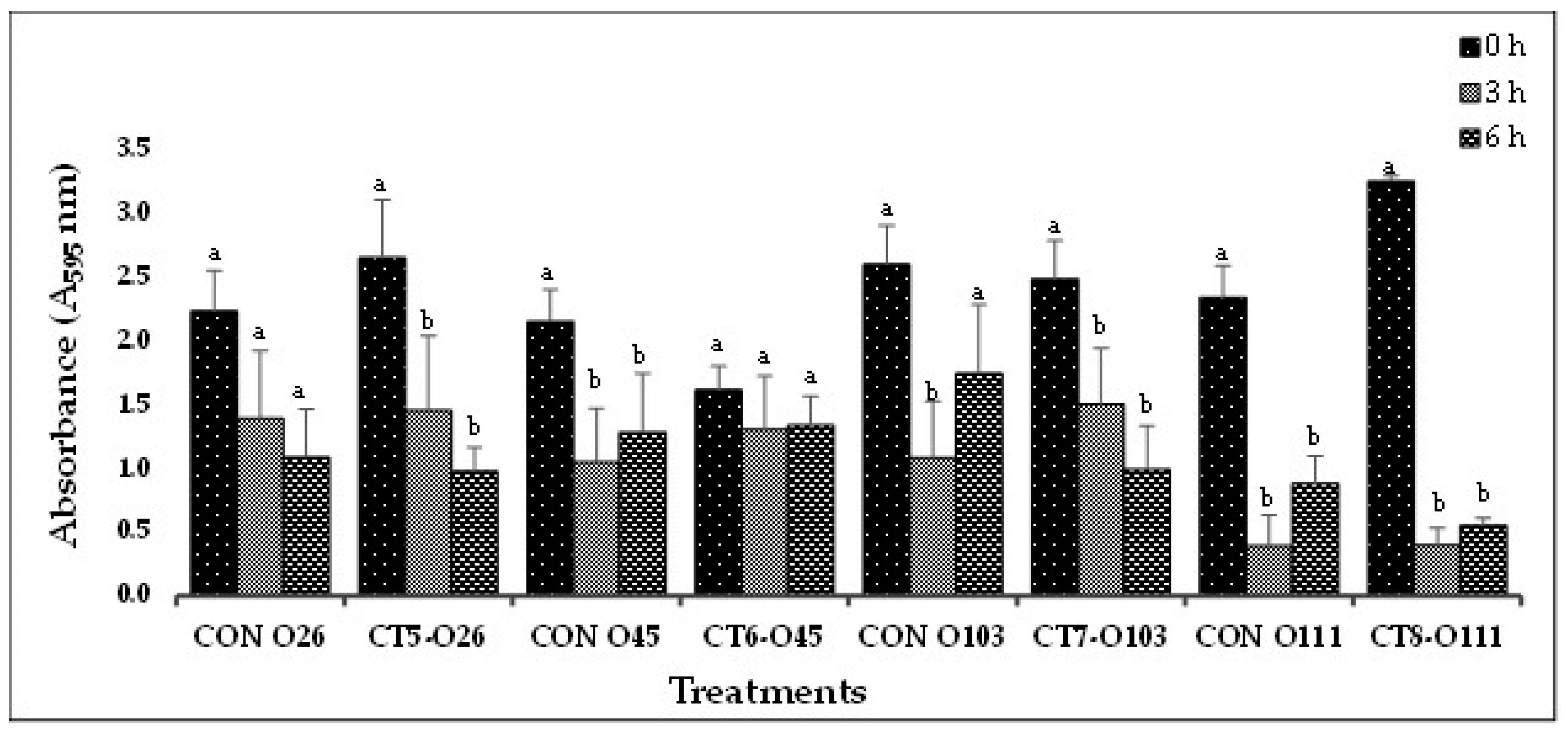
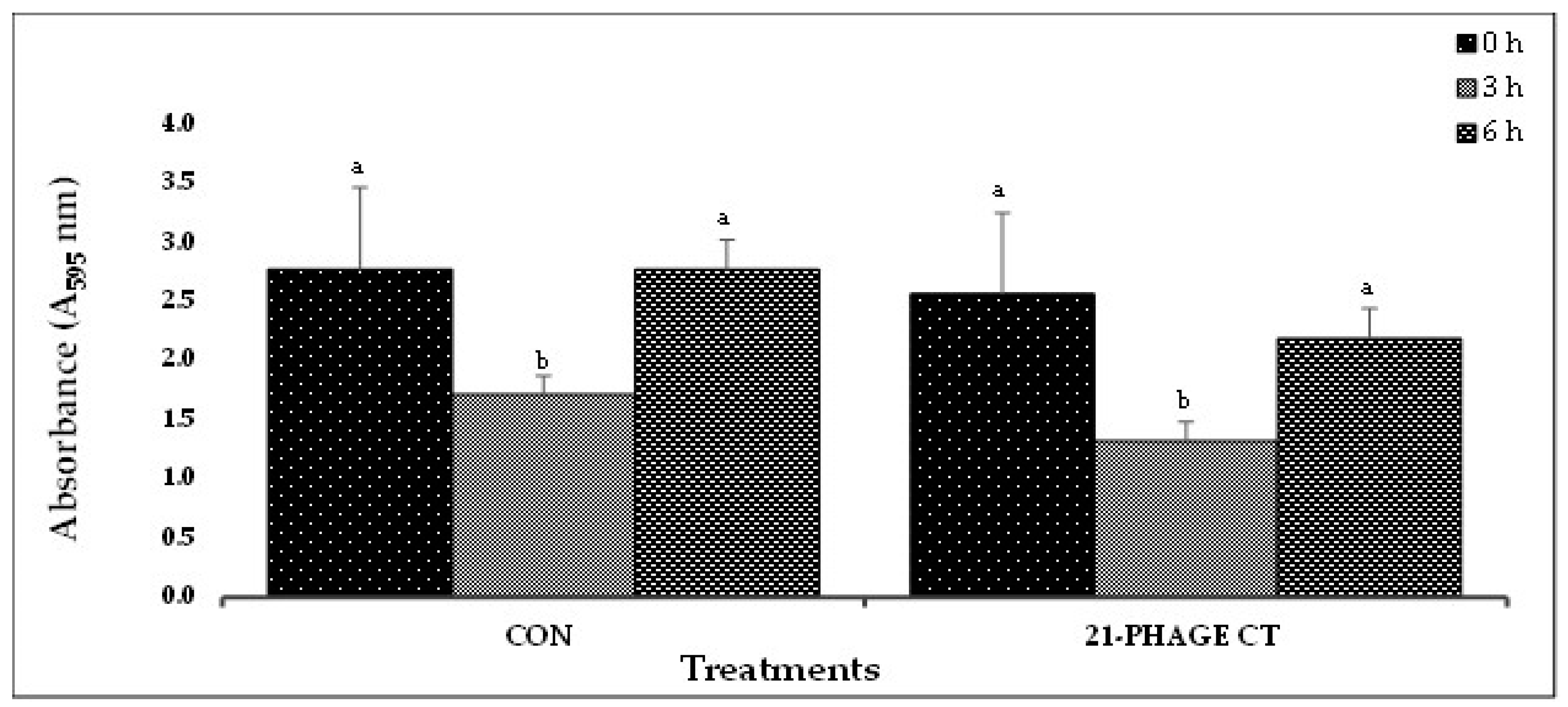
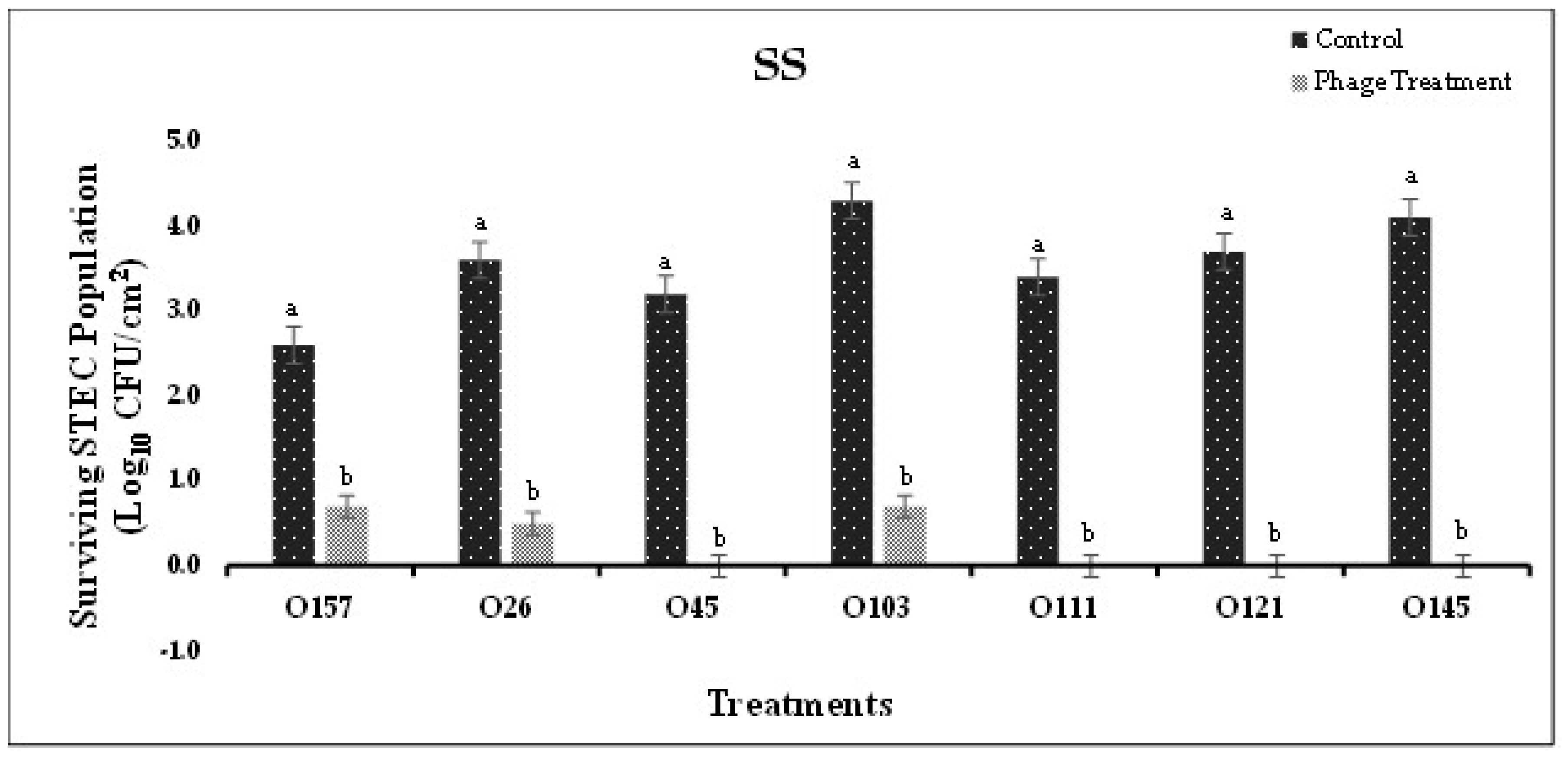
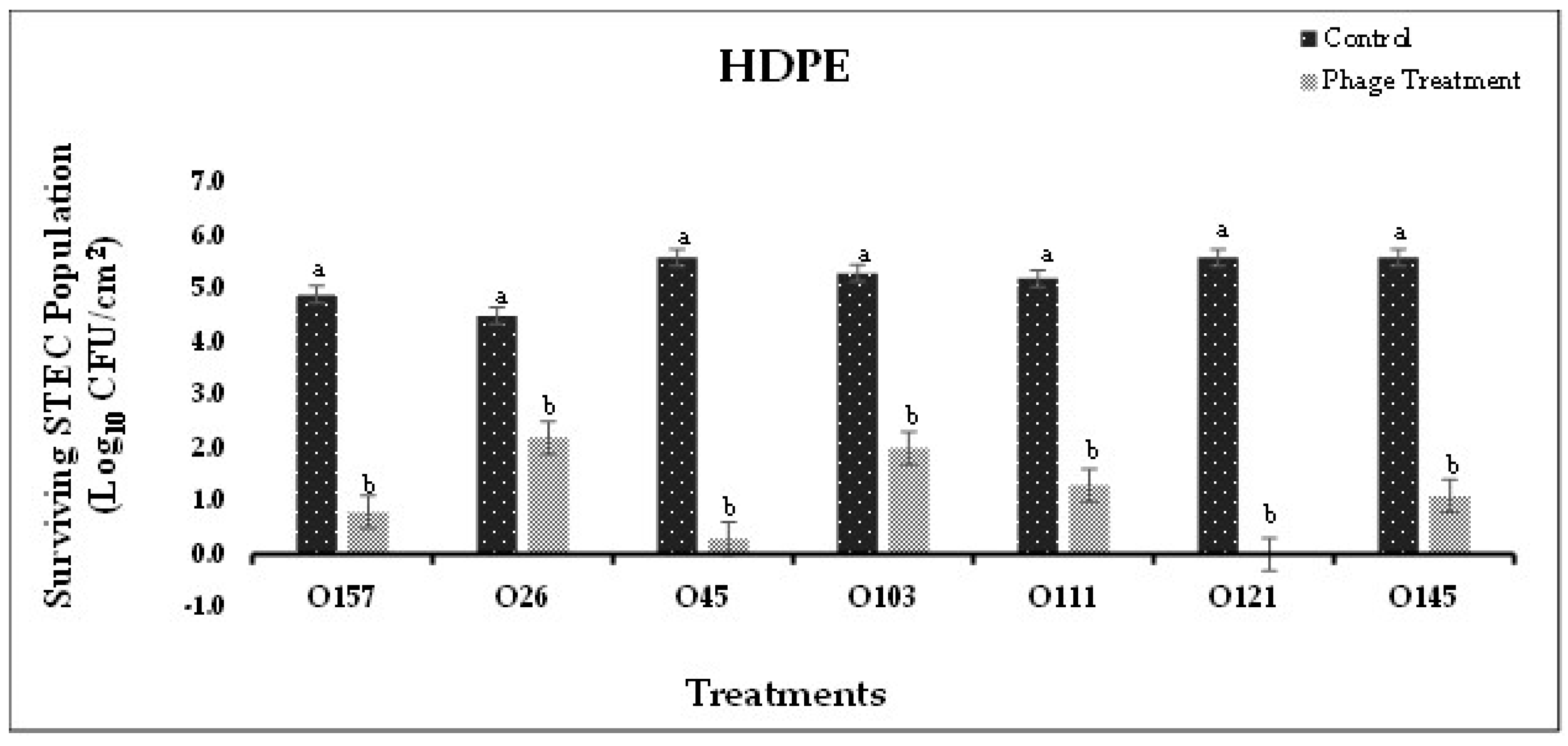
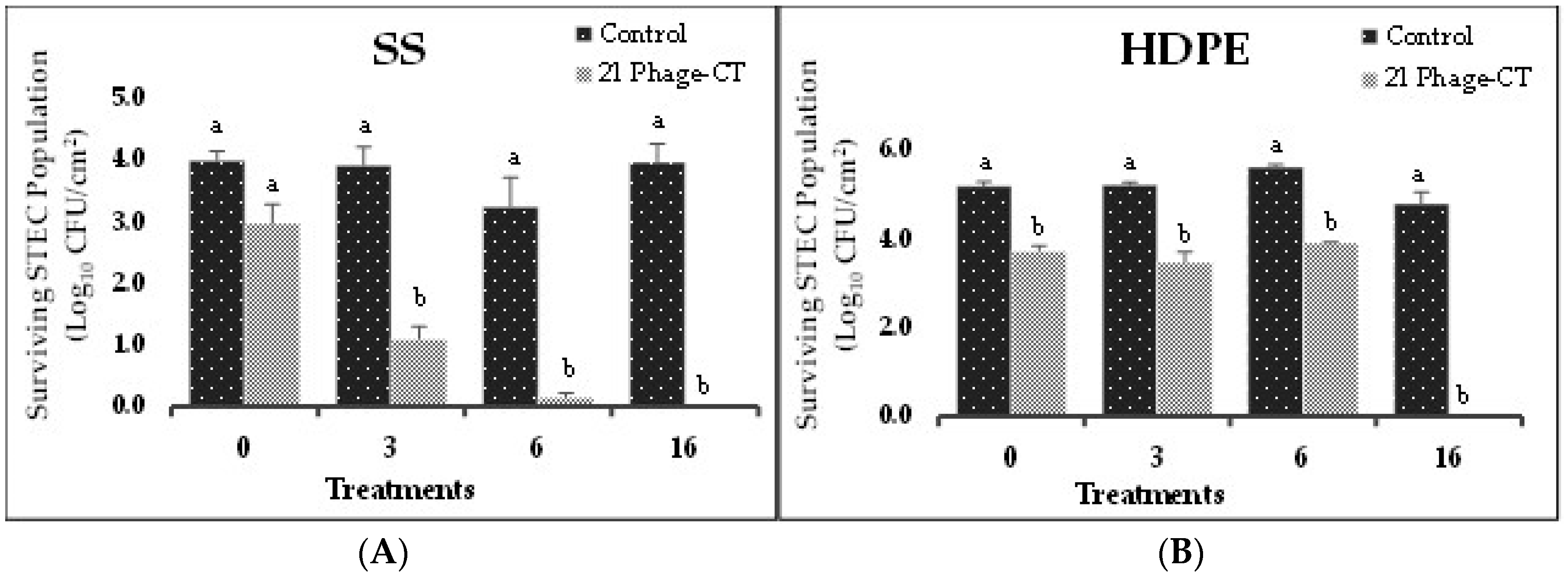
3.2. STEC biofilm disruption on food-contact surfaces
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; et al. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011, 17. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; et al. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983-2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for foodborne disease outbreaks-United States, 2009-2015. MMWR Surveill. Summ. 2018, 67, 1. [Google Scholar] [CrossRef]
- Tack, D.M.; Marder, E.P.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Hurd, S.; et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food-Foodborne Diseases Active Surveillance Network, 10 US sites, 2015-2018. Morb. Mortal. Wkly. Rep. 2019, 68, 369. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). National Shiga toxin-producing Escherichia coli (STEC) Surveillance Annual Report, 2020. Available online: https://www.cdc.gov/ecoli/surv2020/ (accessed on May 2023).
- Ferens, W.A.; Hovde, C.J. Escherichia coli O157: H7: animal reservoir and sources of human infection. Foodborne Pathog. and Dis. 2011, 8, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Kintz, E. , Brainard, J.; Hooper, L.; Hunter, P. Transmission pathways for sporadic Shiga-toxin producing E. coli infections: A systematic review and meta-analysis. Int. J. Hyg. and Environ. Health. 2017, 220, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Marouani-Gadri, N.; Augier, G.; Carpentier, B. Characterization of bacterial strains isolated from a beef-processing plant following cleaning and disinfection-influence of isolated strains on biofilm formation by Sakaï and EDL 933 E. coli O157: H7. Int. J. Food Microbiol. 2009, 133, 62–67. [Google Scholar] [CrossRef]
- Silagyi, K.; Kim, S.-H.; Lo, Y.M.; Wei, C.-i. Production of biofilm and quorum sensing by Escherichia coli O157: H7 and its transfer from contact surfaces to meat, poultry, ready-to-eat deli, and produce products. Food Microbiol. 2009, 26, 514–519. [Google Scholar] [CrossRef]
- Wang, R.; Bono, J.L.; Kalchayanand, N.; Shackelford, S.; Harhay, D.M. Biofilm formation by Shiga toxin-producing Escherichia coli O157: H7 and Non-O157 strains and their tolerance to sanitizers commonly used in the food processing environment. J. Food Prot. 2012, 75, 1418–1428. [Google Scholar] [CrossRef]
- Gilbert, P.; McBain, A.J. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin. Microbiol. Rev. 2003, 16, 189–208. [Google Scholar] [CrossRef]
- Weerarathne, P.; Payne, J.; Saha, J.; Kountoupis, T.; Jadeja, R.; Jaroni, D. Evaluating the efficacy of sodium acid sulfate to reduce Escherichia coli O157:H7 and its biofilms on food-contact surfaces. LWT- Food Sci. Technol. 2021, 139, 110501. [Google Scholar] [CrossRef]
- Fouladkhah, A.; Geornaras, I.; Sofos, J.N. Biofilm Formation of O157 and Non-O157 Shiga toxin-producing Escherichia coli and multidrug-resistant and susceptible Salmonella Typhimurium and Newport and their inactivation by sanitizers. J. Food Sci. 2013, 78. [Google Scholar]
- Houari, A.; Di Martino, P. Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Lett. Appl. Microbiol. 2007, 45, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Uhlich, G.A.; Rogers, D.P.; Mosier, D.A. Escherichia coli serotype O157: H7 retention on solid surfaces and peroxide resistance is enhanced by dual-strain biofilm formation. Foodborne Pathog. and Dis. 2010, 7, 935–943. [Google Scholar] [CrossRef]
- Gilbert, P.; Allison, D.; McBain, A. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance. J. Appl. Microbiol. 2002, 92(s1). [Google Scholar] [CrossRef]
- Coates, A.; Hu, Y. Novel approaches to developing new antibiotics for bacterial infections. Br. J. Pharmacol. 2007, 152, 1147–1154. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G. Bacteriophage therapy. Antimicrob. Agents and Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Summers, W.C. Bacteriophage therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef]
- Ashelford, K.E.; Day, M.J.; Fry, J.C. Elevated abundance of bacteriophage infecting bacteria in soil. Appl. Environ. Microbiol. 2003, 69, 285–289. [Google Scholar] [CrossRef]
- Synnott, A.J.; Kuang, Y.; Kurimoto, M.; Yamamichi, K.; Iwano, H.; Tanji, Y. Isolation from sewage influent and characterization of novel Staphylococcus aureus bacteriophages with wide host ranges and potent lytic capabilities. Appl. Environ. Microbiol. 2009, 75, 4483–4490. [Google Scholar]
- Raya, R.R.; Varey, P.; Oot, R.A.; Dyen, M.R.; Callaway, T.R.; Edrington, T.S.; et al. Isolation and characterization of a new T-even bacteriophage, CEV1, and determination of its potential to reduce Escherichia coli O157: H7 levels in sheep. Appl. Environ. Microbiol. 2006, 72, 6405–6410. [Google Scholar] [CrossRef] [PubMed]
- Tanji, Y.; Shimada, T.; Fukudomi, H.; Miyanaga, K.; Nakai, Y.; Unno, H. Therapeutic use of phage cocktail for controlling Escherichia coli O157: H7 in gastrointestinal tract of mice. J. Biosci. and Bioeng. 2005, 100, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.A.; Sutherland, I.W.; Jones, M.V. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 1998, 144, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Sharma, M.; Millner, P.; Calaway, T.; Singh, M. Inactivation of Escherichia coli O157: H7 attached to spinach harvester blade using bacteriophage. Foodborne Pathog. Dis. 2011, 8, 541–546. [Google Scholar] [CrossRef]
- Sharma, M.; Ryu, J.H.; Beuchat, L. Inactivation of Escherichia coli O157: H7 in biofilm on stainless steel by treatment with an alkaline cleaner and a bacteriophage. J. Appl. Microbiol. 2005, 99, 449–459. [Google Scholar] [CrossRef]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Diez-Gonzalez, F. Reduction of Escherichia coli O157: H7 viability on hard surfaces by treatment with a bacteriophage mixture. Int. J. Food Microbiol. 2011, 145, 37–42. [Google Scholar] [CrossRef]
- Litt, P.K.; Jaroni, D. Isolation and physio-morphological characterization of Escherichia coli O157:H7-infecting bacteriophages recovered from beef cattle operations. Int. J. Microbiol. 2017, 7013236. [Google Scholar]
- Litt, P.K.; Saha, J.; and Jaroni, D. Characterization of bacteriophages targeting non-O157 Shiga toxigenic Escherichia coli. J. Food Prot. 2018, 81, 785–794. [Google Scholar] [CrossRef]
- Jayasundera, B.P.K. Identification of contamination sources and occurrence of shigatoxigenic E. coli on small-scale cow/calf operations in Oklahoma and Louisiana. Masters Thesis, Oklahoma State University, 2015. [Google Scholar]
- Kountoupis, T.; Litt, P.K.; Kakani, R.; Jaroni, D. Biofilm forming capabilities of Shiga-toxigenic Escherichia coli recovered from cow calf operations in Oklahoma and Louisiana. Poster session presentation at the annual meeting of International Association for Food Protection, Tampa, FL,USA, 2017.
- Vogeleer, P.; Tremblay, Y.D.; Jubelin, G.; Jacques, M.; Harel, J. Biofilm-forming abilities of Shiga toxin-producing Escherichia coli isolates associated with human infections. Appl. Environ. Microbiol. 2016, 82, 1448–1458. [Google Scholar] [CrossRef]
- Hood, S.K.; Zottola, E.A. Adherence to stainless steel by foodborne microorganisms during growth in model food systems. Internat. J. Food Microbiol. 1997, 37, 145–153. [Google Scholar] [CrossRef]
- Chan, T.; Lee, K.; Roulin, P. Escherichia coli biofilm development and bacteriophage T4 susceptibility. J. of Expt. Microbiol. Immunol. 2007, 11, 73–80. [Google Scholar]
- Lu, T.K.; Collins, J.J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. 2007, 104, 11197–11202. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.A.; Sutherland, I.W.; Clark, J.; Jones, M.V. Bacteriophage and associated polysaccharide depolymerases-novel tools for study of bacterial biofilms. J. Appl. Microbiol. 1998, 85, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Jiang, X. Application of bacteriophages to reduce biofilms formed by hydrogen sulfide producing bacteria on surfaces in a rendering plant. Can. J. Microbiol. 2015, 61, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Domka, J.; Lee, J.; Bansal, T.; Wood, T.K. Temporal gene-expression in Escherichia coli K-12 biofilms. Environ. Microbiol. 2007, 9, 332–346. [Google Scholar] [CrossRef]
- Schembri, M.A.; Kjaergaard, K.; Klemm, P. Global gene expression in Escherichia coli biofilms. Mol. Microbio. 2003, 48, 253–267. [Google Scholar] [CrossRef]
- Rabinovitch, A.; Aviram, I.; Zaritsky, A. Bacterial debris-an ecological mechanism for coexistence of bacteria and their viruses. J. Theor. Biol. 2003, 224, 377–383. [Google Scholar] [CrossRef]
- Sillankorva, S.; Neubauer, P.; Azeredo, J. Phage control of dual species biofilms of Pseudomonas fluorescens and Staphylococcus lentus. Biofouling 2010, 26, 567–575. [Google Scholar] [CrossRef]
- Meng, X.; Shi, Y.; Ji, W.; Meng, X.; Zhang, J.; Wang, H.; et al. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011, 77, 8272–8279. [Google Scholar] [CrossRef]
- Gong, C.; Heringa, S.; Singh, R.; Kim, J.; Jiang, X. Isolation and characterization of bacteriophages specific to hydrogen-sulfide-producing bacteria. Can. J. Microbiol. 2012, 59, 39–45. [Google Scholar] [CrossRef]
- Tremblay, Y.D.; Vogeleer, P.; Jacques, M.; Harel, J. High-throughput microfluidic method to study biofilm formation and host-pathogen interactions in pathogenic Escherichia coli. Appl. Environ. Microbiol. 2015, 81, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, L.D.; Bisha, B. Phage-based biocontrol strategies to reduce foodborne pathogens in foods. Bacteriophage 2011, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- O'Flynn, G.; Ross, R.; Fitzgerald, G.; Coffey, A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157: H7. Appl.Environmen. Microbiol. 2004, 70, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Kudva, I.T.; Hatfield, P.G.; Hovde, C.J. Characterization of Escherichia coli O157: H7 and other Shiga toxin-producing E. coli serotypes isolated from sheep. J. Clin. Microbiol. 1997, 35, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Dowsett, A.; Dennis, P.; Lee, J.; Keevil, C. Influence of plumbing materials on biofilm formation and growth of Legionella pneumophila in potable water systems. Appl. Environ. Microbiol. 1994, 60, 1842–1851. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Abuladze, T.; Li, M.; Menetrez, M.Y.; Dean, T.; Senecal, A.; Sulakvelidze, A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157: H7. Appl. Environ. Microbiol. 2008, 74, 6230–6238. [Google Scholar] [CrossRef]
- Hibma, A.M.; Jassim, S.A.; Griffiths, M.W. Infection and removal of L-forms of Listeria monocytogenes with bred bacteriophage. Int. J. Food Microbiol. 1997, 34, 197–207. [Google Scholar] [CrossRef]
- Soni, K.A.; Nannapaneni, R. Removal of Listeria monocytogenes biofilms with bacteriophage P100. J. Food Protect. 2010, 73, 1519–1524. [Google Scholar] [CrossRef]
- Briandet, R.; Lacroix-Gueu, P.; Renault, M.; Lecart, S.; Meylheuc, T.; Bidnenko, E.; et al. Fluorescence correlation spectroscopy to study diffusion and reaction of bacteriophages inside biofilms. Appl. Environ. Microbiol. 2008, 74, 2135–2143. [Google Scholar] [CrossRef]
- Triandafillu, K.; Balazs, D.; Aronsson, B.-O.; Descouts, P.; Quoc, P.T.; van Delden, C.; et al. Adhesion of Pseudomonas aeruginosa strains to untreated and oxygen-plasma treated poly (vinyl chloride)(PVC) from endotracheal intubation devices. Biomaterials 2003, 24, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Brocca, D.; Arvin, E.; Mosbaek, H. Identification of organic compounds migrating from polyethylene pipelines into drinking water. Water Res. 2002, 36, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, B.; Kowalski, D.; Rożej, A. Organic compounds migrating from plastic pipes into water. J. Water Supply: Res. Technol.-AQUA 2011, 60, 137–146. [Google Scholar] [CrossRef]
- Skjevrak, I.; Lund, V.; Ormerod, K.; Herikstad, H. Volatile organic compounds in natural biofilm in polyethylene pipes supplied with lake water and treated water from the distribution network. Water Res. 2005, 39, 4133–4141. [Google Scholar] [CrossRef] [PubMed]
| STEC | Bacterial Cocktail | Phage Cocktail Treatment (CT) | |
|---|---|---|---|
| O157 | ATCC 43895, WT: LF4, KF10 | CT1-O157 | P1, P2, P4, P6 |
| CT2-O157 | P1, P2, P5, P7 | ||
| CT3-O157 | P3, P5, P7 | ||
| CT4-O157 | P2, P3, P4, P7 | ||
| O26 | CDC 2003-3014, WT: BF8, QF6 | CT5-O26 | P10, P11, P12, P13 |
| O45 | CDC 2000-3039, WT: AF1, EF2 | CT6-O45 | P9, J12, J13, J15 |
| O103 | CDC 2006-3008, WT: AF10, GF6 | CT7-O103 | P19, P20, P21 |
| O111 | CDC 2010-3114, ATCC: 2440, 2180 | CT8-O111 | P14, P15, P16, P17 |
| O121 | CDC 2002-3211, ATCC 2219, 2203 | CT9-O121 | P8, J1, J4, J7 |
| CT10-O121 | P8, J3, J6, J9 | ||
| O145 | CDC 99-3311, ATCC 1652, 2208 | CT11-O145 | J21, J24, J26, J27 |
| CT12-O145 | J25, J28, J29, J30 | ||
| STEC | Bacterial Cocktail | Phage Cocktail |
|---|---|---|
| O157 | ATCC 43895, WT LF4 | P2, P6, P7 |
| O26 | CDC 2003-3014, WT QF6 | P11, P12, P13 |
| O45 | CDC 2000-3039, WT AF1 | P9, J12, J15 |
| O103 | CDC 2006-3008, WT AF10 | P19, P20, P21 |
| O111 | CDC 2010-3114, ATCC 2180 | P14, P15, P17 |
| O121 | CDC 2002-3211, ATCC 2219 | P8, J3, J7 |
| O145 | CDC 99-3311, ATCC 1652 | J18, J21, J29 |
| STEC | Bacterial Cocktail | Phage Cocktail |
|---|---|---|
| O157 | ATCC 43895, WT: LF4, KF10 | P3, P5, P7 |
| O26 | CDC 2003-3014, WT: BF8, QF6 | P10, P11, P12, P13 |
| O45 | CDC 2000-3039, WT: AF1, EF2 | P9, J12, J13, J15 |
| O103 | CDC 2006-3008, WT: AF10, GF6 | P19, P20, P21 |
| O111 | CDC 2010-3114, ATCC: 2440, 2180 | P14, P15, P16, P17 |
| O121 | CDC 2002-3211, ATCC: 2219, 2203 | P8, J3, J6, J9 |
| O145 | CDC 99-3311, ATCC: 2208, 1652 | J21, J24, J26, J27 |
| STEC | Individual Phage Treatment | Phage Cocktail Treatment (CT) |
|---|---|---|
| O157 | P4 | CT-2, CT-3 |
| O26 | P11 | CT-5 |
| O45 | P9 | CT-6 |
| O103 | P21 | CT-7 |
| O111 | P15 | CT-8 |
| O121 | J3 | CT-10 |
| O145 | J29 | CT-12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




