Submitted:
26 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal use ethics
2.2. Histology
2.3. Senescent associated β-galactosidase (SA-β-Gal) staining
2.4. Immunofluorescent staining.
2.5. Brain cortex, heart, spleen tissue and bone marrow harvest
2.6. RNA isolation, cDNA synthesis and semi-quantitative and Quantitative -PCR (Q-PCR).
2.7. Statistical analysis: All data was analyzed with two group t test using Graphpad Prism 9. P<0.05 was considered statistically significant.
3. Results
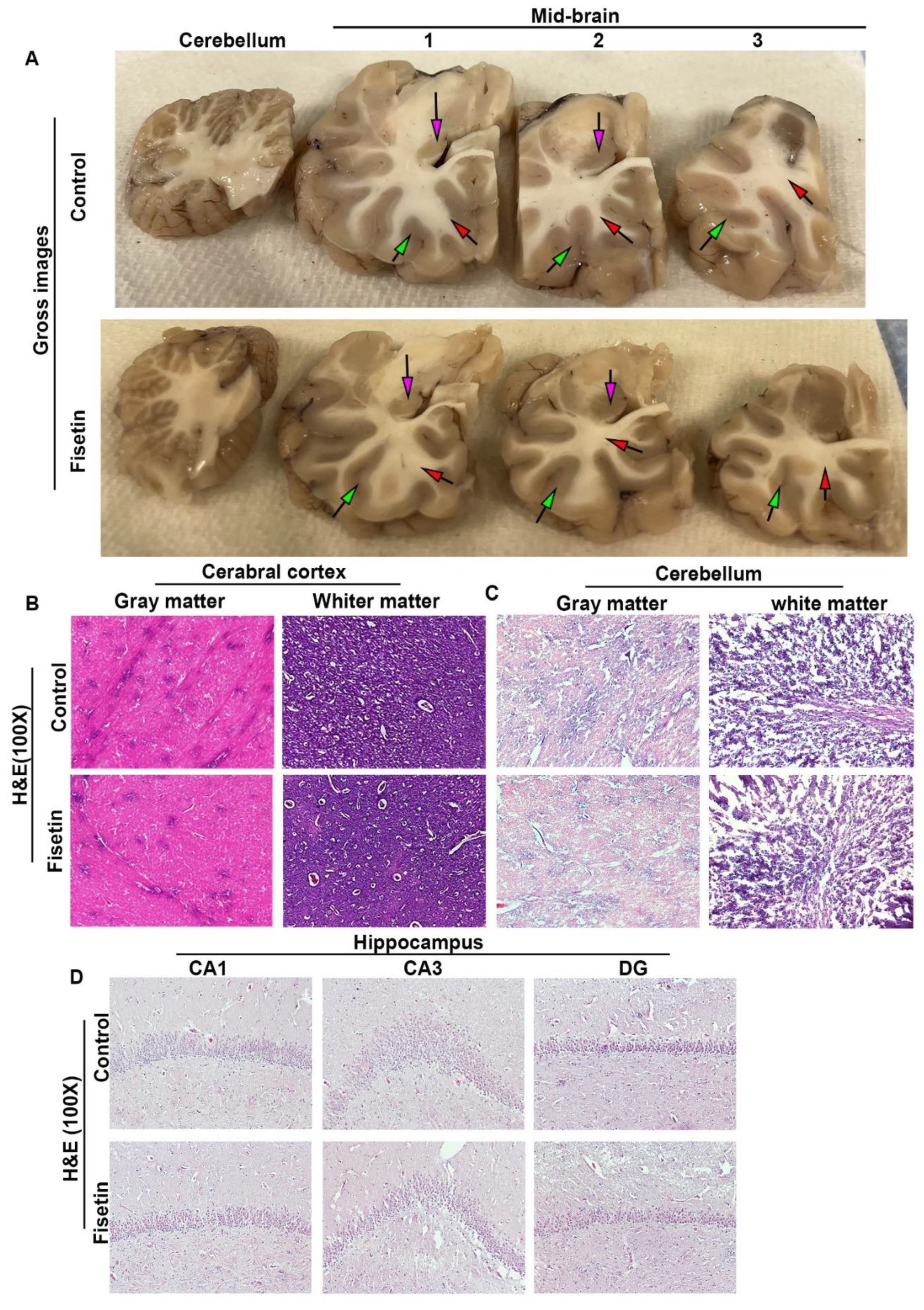
3.1. Fisetin treatment did not significantly affect sheep brain general morphology.
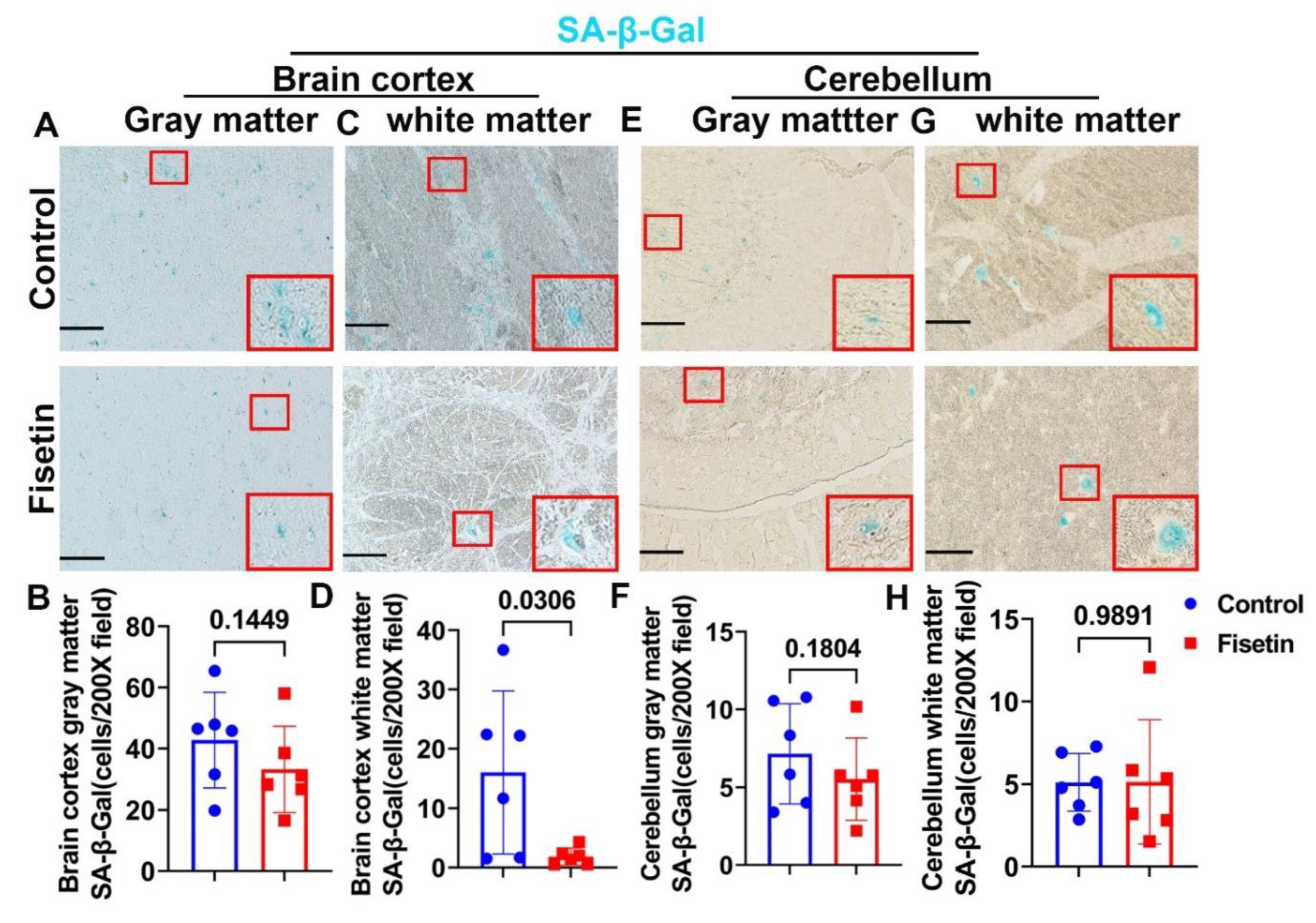
3.2. Fisetin treatment decreased SA- β-Gal positive cells.
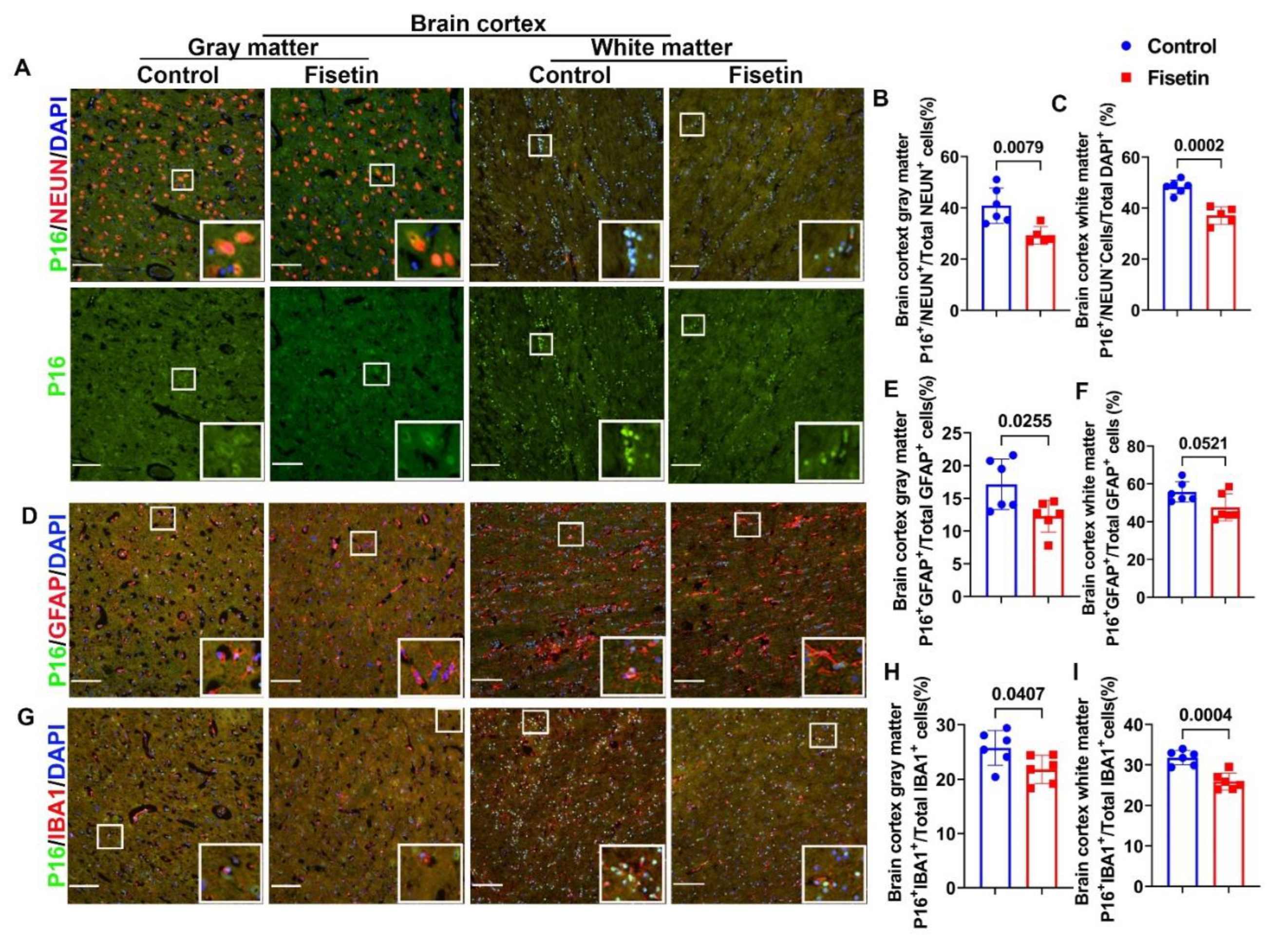
3.3. Effects of fisetin treatment on the cellular senescence of neurons, astrocytes, and microglial cells of cerebral cortex.
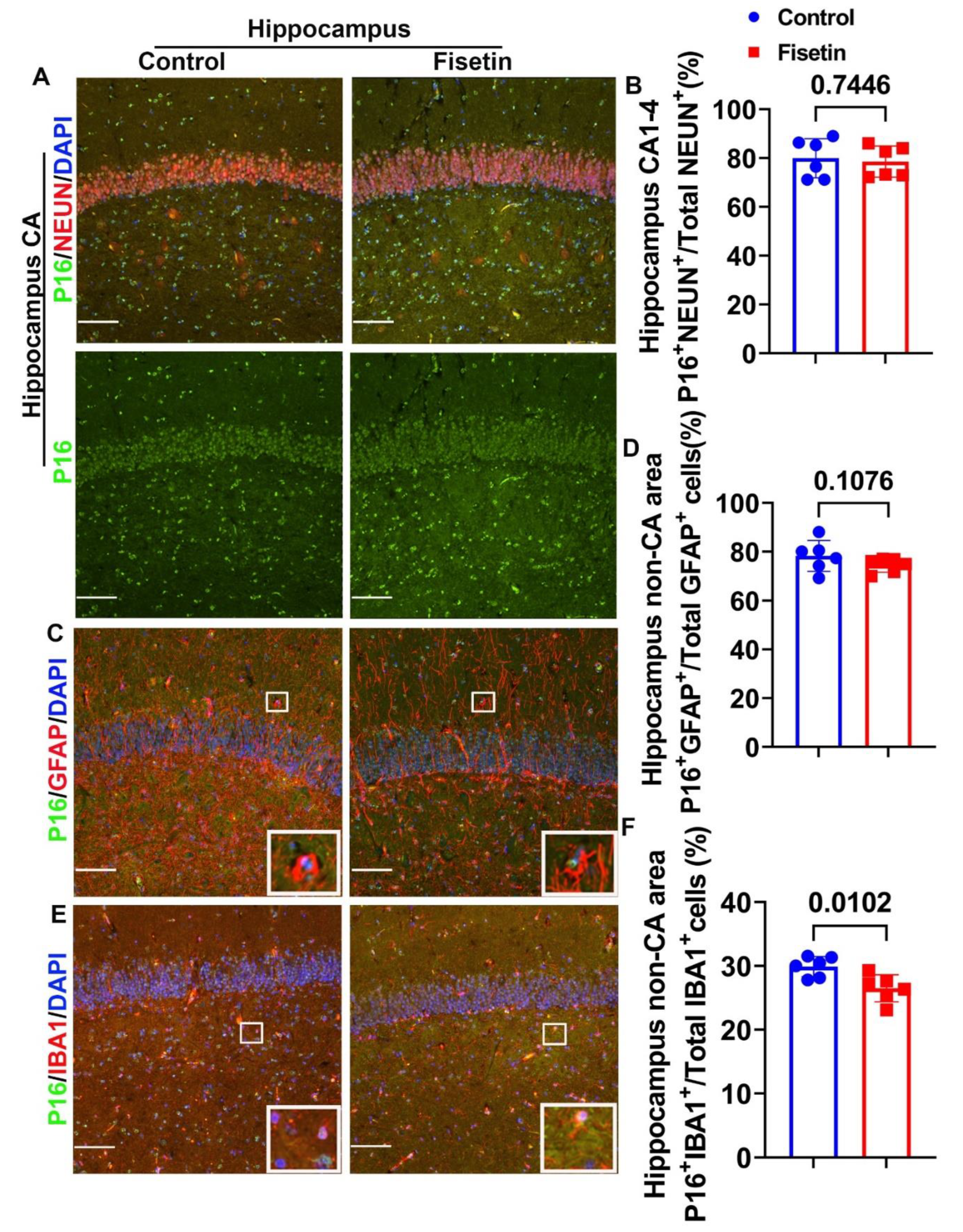
3.4. Effects of Fisetin treatments on cellular senescent of neurons, astrocytes, and microglial cells of hippocampus.
3.5. Effects of fisetin treatment on gene expression of sheep brain cortex.
3.6. Effect of fisetin treatment on gene expression of heart tissues.
3.7. Effects of Fisetin treatment on sheep spleen tissues gene expressions
3.8. Effects of fiestin treatment on gene expression of bone marrow nucleated cells.
Discussion
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.; Kim, B.K.; Park, S.K. Effects of Fisetin, a Plant-Derived Flavonoid, on Response to Oxidative Stress, Aging, and Age-Related Diseases in Caenorhabditis elegans. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef]
- Maher, P.; Akaishi, T.; Abe, K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci U S A 2006, 103, 16568–16573. [Google Scholar] [CrossRef]
- Maher, P. A comparison of the neurotrophic activities of the flavonoid fisetin and some of its derivatives. Free Radic Res 2006, 40, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch Biochem Biophys 2008, 476, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes Nutr 2009, 4, 297–307. [Google Scholar] [CrossRef]
- Ehren, J.L.; Maher, P. Concurrent regulation of the transcription factors Nrf2 and ATF4 mediates the enhancement of glutathione levels by the flavonoid fisetin. Biochem Pharmacol 2013, 85, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Currais, A.; Prior, M.; Dargusch, R.; Armando, A.; Ehren, J.; Schubert, D.; Quehenberger, O.; Maher, P. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer's disease transgenic mice. Aging Cell 2014, 13, 379–390. [Google Scholar] [CrossRef]
- Currais, A.; Farrokhi, C.; Dargusch, R.; Armando, A.; Quehenberger, O.; Schubert, D.; Maher, P. Fisetin Reduces the Impact of Aging on Behavior and Physiology in the Rapidly Aging SAMP8 Mouse. J Gerontol A Biol Sci Med Sci 2018, 73, 299–307. [Google Scholar] [CrossRef]
- Cho, I.; Song, H.O.; Cho, J.H. Flavonoids mitigate neurodegeneration in aged Caenorhabditis elegans by mitochondrial uncoupling. Food Sci Nutr 2020, 8, 6633–6642. [Google Scholar] [CrossRef]
- Das, J.; Singh, R.; Ladol, S.; Nayak, S.K.; Sharma, D. Fisetin prevents the aging-associated decline in relative spectral power of alpha, beta and linked MUA in the cortex and behavioral alterations. Exp Gerontol 2020, 138, 111006. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Y.; Wu, Q.; Yang, J.; Chen, J.; Zhong, S.; Eliezer, D.; Tan, Q.; Wu, C. Fisetin inhibits tau aggregation by interacting with the protein and preventing the formation of beta-strands. Int J Biol Macromol 2021, 178, 381–393. [Google Scholar] [CrossRef]
- Singh, S.; Garg, G.; Singh, A.K.; Bissoyi, A.; Rizvi, S.I. Fisetin, a potential caloric restriction mimetic, attenuates senescence biomarkers in rat erythrocytes. Biochem Cell Biol 2019, 97, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, A.; Ali, W.; Jo, M.H.; Park, J.; Ikram, M.; Kim, M.O. Fisetin Rescues the Mice Brains Against D-Galactose-Induced Oxidative Stress, Neuroinflammation and Memory Impairment. Front Pharmacol 2021, 12, 612078. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Noh, J.S.; Jung, Y.; Leem, S.H.; Hyun, J.W.; Chang, Y.C.; Kwon, T.K.; Kim, G.Y.; Lee, H.; Choi, Y.H. Fisetin Attenuated Oxidative Stress-Induced Cellular Damage in ARPE-19 Human Retinal Pigment Epithelial Cells Through Nrf2-Mediated Activation of Heme Oxygenase-1. Front Pharmacol 2022, 13, 927898. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Kim, D.H.; Kwon, C.Y.; Park, S.H.; Hong, S.H.; Bang, E.; Cheong, J.; Kim, G.Y.; Choi, Y.H. Fisetin Protects C2C12 Mouse Myoblasts from Oxidative Stress-Induced Cytotoxicity through Regulation of the Nrf2/HO-1 Signaling. J Microbiol Biotechnol 2023, 33, 591–599. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: from discovery to translation. J Intern Med 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Camell, C.D.; Yousefzadeh, M.J.; Zhu, Y.; Prata, L.; Huggins, M.A.; Pierson, M.; Zhang, L.; O'Kelly, R.D.; Pirtskhalava, T.; Xun, P.; et al. Senolytics reduce coronavirus-related mortality in old mice. Science 2021, 373. [Google Scholar] [CrossRef]
- Elsallabi, O.; Patruno, A.; Pesce, M.; Cataldi, A.; Carradori, S.; Gallorini, M. Fisetin as a Senotherapeutic Agent: Biopharmaceutical Properties and Crosstalk between Cell Senescence and Neuroprotection. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Liu, L.; Yue, X.; Sun, Z.; Hambright, W.S.; Feng, Q.; Cui, Y.; Huard, J.; Robbins, P.D.; Wang, Z.; Mu, X. Senolytic elimination of senescent macrophages restores muscle stem cell function in severely dystrophic muscle. Aging (Albany NY) 2022, 14, 7650–7661. [Google Scholar] [CrossRef]
- Liu, L.; Yue, X.; Sun, Z.; Hambright, W.S.; Wei, J.; Li, Y.; Matre, P.; Cui, Y.; Wang, Z.; Rodney, G.; et al. Reduction of senescent fibro-adipogenic progenitors in progeria-aged muscle by senolytics rescues the function of muscle stem cells. J Cachexia Sarcopenia Muscle 2022, 13, 3137–3148. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.; Nelson, A.L.; Goff, A.; Billings, J.; Kloser, H.; Huard, C.; Mitchell, J.; Hambright, W.S.; Ravuri, S.; Huard, J. Fisetin Attenuates Cellular Senescence Accumulation During Culture Expansion of Human Adipose-Derived Stem Cells. Stem Cells 2023. [Google Scholar] [CrossRef]
- Li, S.; Livingston, M.J.; Ma, Z.; Hu, X.; Wen, L.; Ding, H.F.; Zhou, D.; Dong, Z. Tubular cell senescence promotes maladaptive kidney repair and chronic kidney disease after cisplatin nephrotoxicity. JCI Insight 2023, 8. [Google Scholar] [CrossRef]
- Ju, H.Y.; Kim, J.; Han, S.J. The flavonoid fisetin ameliorates renal fibrosis by inhibiting SMAD3 phosphorylation, oxidative damage, and inflammation in ureteral obstructed kidney in mice. Kidney Res Clin Pract 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Sivalingam, K.; Shibu, M.A.; Peramaiyan, R.; Day, C.H.; Shen, C.Y.; Lai, C.H.; Chen, R.J.; Viswanadha, V.P.; Chen, Y.F.; et al. Protective effect of Fisetin against angiotensin II-induced apoptosis by activation of IGF-IR-PI3K-Akt signaling in H9c2 cells and spontaneous hypertension rats. Phytomedicine 2019, 57, 1–8. [Google Scholar] [CrossRef]
- Yan, L.; Jia, Q.; Cao, H.; Chen, C.; Xing, S.; Huang, Y.; Shen, D. Fisetin ameliorates atherosclerosis by regulating PCSK9 and LOX-1 in apoE(-/-) mice. Exp Ther Med 2021, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Sung, J.Y.; Kang, Y.J.; Choi, H.C. Fisetin alleviates cellular senescence through PTEN mediated inhibition of PKCdelta-NOX1 pathway in vascular smooth muscle cells. Arch Gerontol Geriatr 2023, 108, 104927. [Google Scholar] [CrossRef]
- Gao, X.; Usas, A.; Proto, J.D.; Lu, A.; Cummins, J.H.; Proctor, A.; Chen, C.W.; Huard, J. Role of donor and host cells in muscle-derived stem cell-mediated bone repair: differentiation vs. paracrine effects. FASEB J 2014, 28, 3792–3809. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res 2012, 40, e115. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.O.; Zhu, Y.; Weigand, B.M.; Tripathi, U.; Burns, T.C.; Tchkonia, T.; Kirkland, J.L. Cellular senescence in aging and age-related diseases: Implications for neurodegenerative diseases. Int Rev Neurobiol 2020, 155, 203–234. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol 2020, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tchkonia, T.; Jiang, J.; Kirkland, J.L.; Sun, Y. Targeting Senescent Cells for a Healthier Aging: Challenges and Opportunities. Adv Sci (Weinh) 2020, 7, 2002611. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 2015, 112, E6301–E6310. [Google Scholar] [CrossRef]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; Johnson, K.O.; Stout, M.B.; Giorgadze, N.; et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 2015, 4, e12997. [Google Scholar] [CrossRef]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Maher, P.A. Using Plants as a Source of Potential Therapeutics for the Treatment of Alzheimer's Disease. Yale J Biol Med 2020, 93, 365–373. [Google Scholar]
- Maher, P. Preventing and Treating Neurological Disorders with the Flavonol Fisetin. Brain Plast 2021, 6, 155–166. [Google Scholar] [CrossRef]
- Ravula, A.R.; Teegala, S.B.; Kalakotla, S.; Pasangulapati, J.P.; Perumal, V.; Boyina, H.K. Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: An updated review. Eur J Pharmacol 2021, 910, 174492. [Google Scholar] [CrossRef]
- Gruendler, R.; Hippe, B.; Sendula Jengic, V.; Peterlin, B.; Haslberger, A.G. Nutraceutical Approaches of Autophagy and Neuroinflammation in Alzheimer's Disease: A Systematic Review. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Beausejour, C.M.; Krtolica, A.; Galimi, F.; Narita, M.; Lowe, S.W.; Yaswen, P.; Campisi, J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 2003, 22, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.; Gonzales, M.; Garbarino, V.; Kautz, T.; Palavicini, J.; Lopez-Cruzan, M.; Dehkordi, S.K.; Mathews, J.; Zare, H.; Xu, P.; et al. Senolytic therapy to modulate the progression of Alzheimer's Disease (SToMP-AD) - Outcomes from the first clinical trial of senolytic therapy for Alzheimer's disease. Res Sq 2023. [Google Scholar] [CrossRef]
- Antignano, I.; Liu, Y.; Offermann, N.; Capasso, M. Aging microglia. Cell Mol Life Sci 2023, 80, 126. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.Y.; McNeely, T.L.; Baker, D.J. Untangling senescent and damage-associated microglia in the aging and diseased brain. FEBS J 2023, 290, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.Y.; Chang, P.C.; Shen, Y.C.; Lin, C.; Tsai, C.F.; Chen, J.H.; Yeh, W.L.; Wu, L.H.; Lin, H.Y.; Liu, Y.S.; et al. Regulatory effects of fisetin on microglial activation. Molecules 2014, 19, 8820–8839. [Google Scholar] [CrossRef]
- Chen, C.; Yao, L.; Cui, J.; Liu, B. Fisetin Protects against Intracerebral Hemorrhage-Induced Neuroinflammation in Aged Mice. Cerebrovasc Dis 2018, 45, 154–161. [Google Scholar] [CrossRef]
- Cordaro, M.; D'Amico, R.; Fusco, R.; Peritore, A.F.; Genovese, T.; Interdonato, L.; Franco, G.; Arangia, A.; Gugliandolo, E.; Crupi, R.; et al. Discovering the Effects of Fisetin on NF-kappaB/NLRP-3/NRF-2 Molecular Pathways in a Mouse Model of Vascular Dementia Induced by Repeated Bilateral Carotid Occlusion. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Maher, P. Modulation of the Neuroprotective and Anti-inflammatory Activities of the Flavonol Fisetin by the Transition Metals Iron and Copper. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Liu, C.; Xue, R.; Wang, Y.; Sun, Y.; Liang, Z.; Fan, W.; Jiang, J.; Zhao, J.; Su, Q.; et al. Fisetin inhibits cardiac hypertrophy by suppressing oxidative stress. J Nutr Biochem 2018, 62, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Athapaththu, A.; Choi, Y.H.; Park, C.; Jin, C.Y.; Kang, C.H.; Lee, M.H.; Kim, G.Y. Fisetin Inhibits NLRP3 Inflammasome by Suppressing TLR4/MD2-Mediated Mitochondrial ROS Production. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
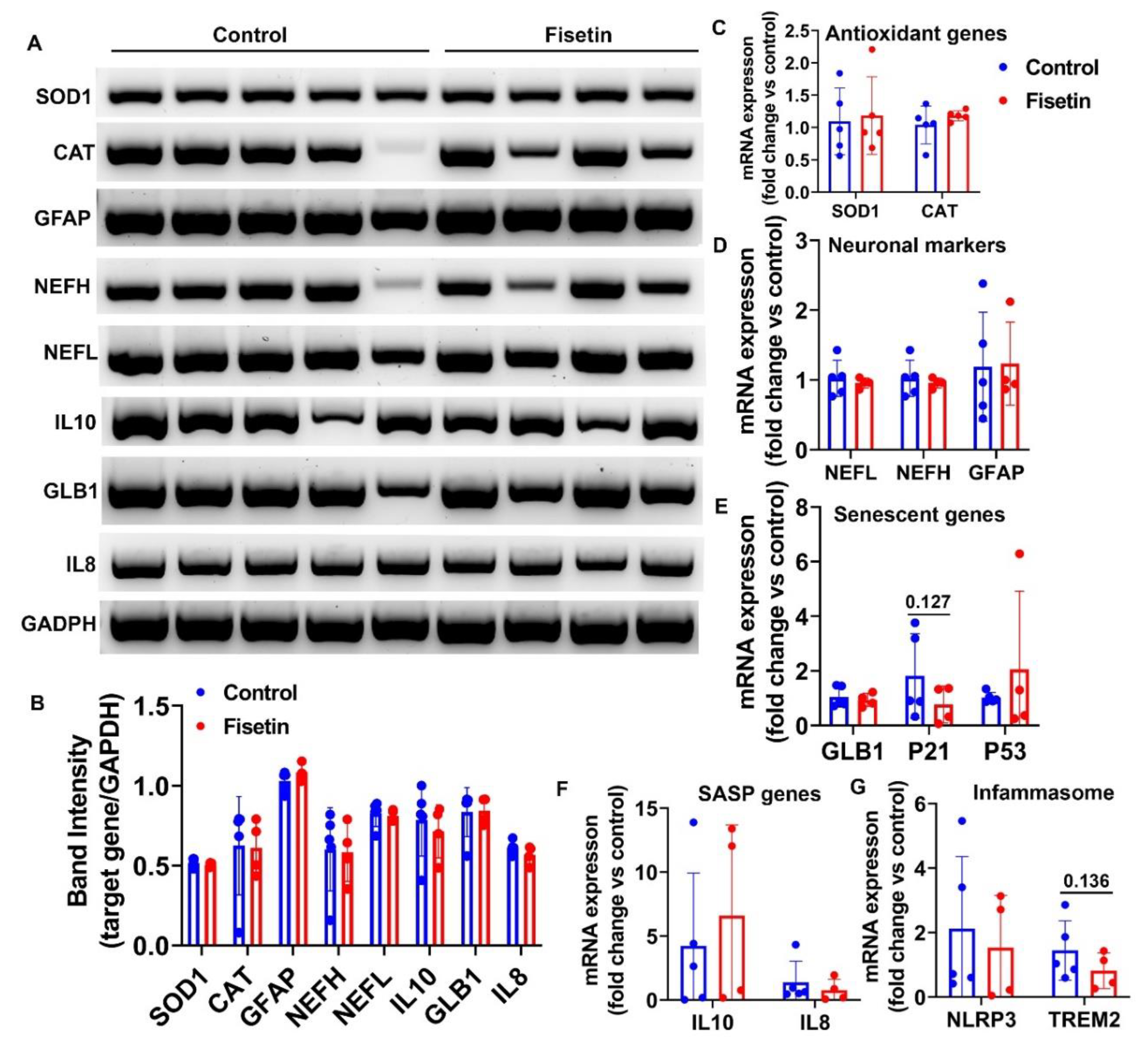
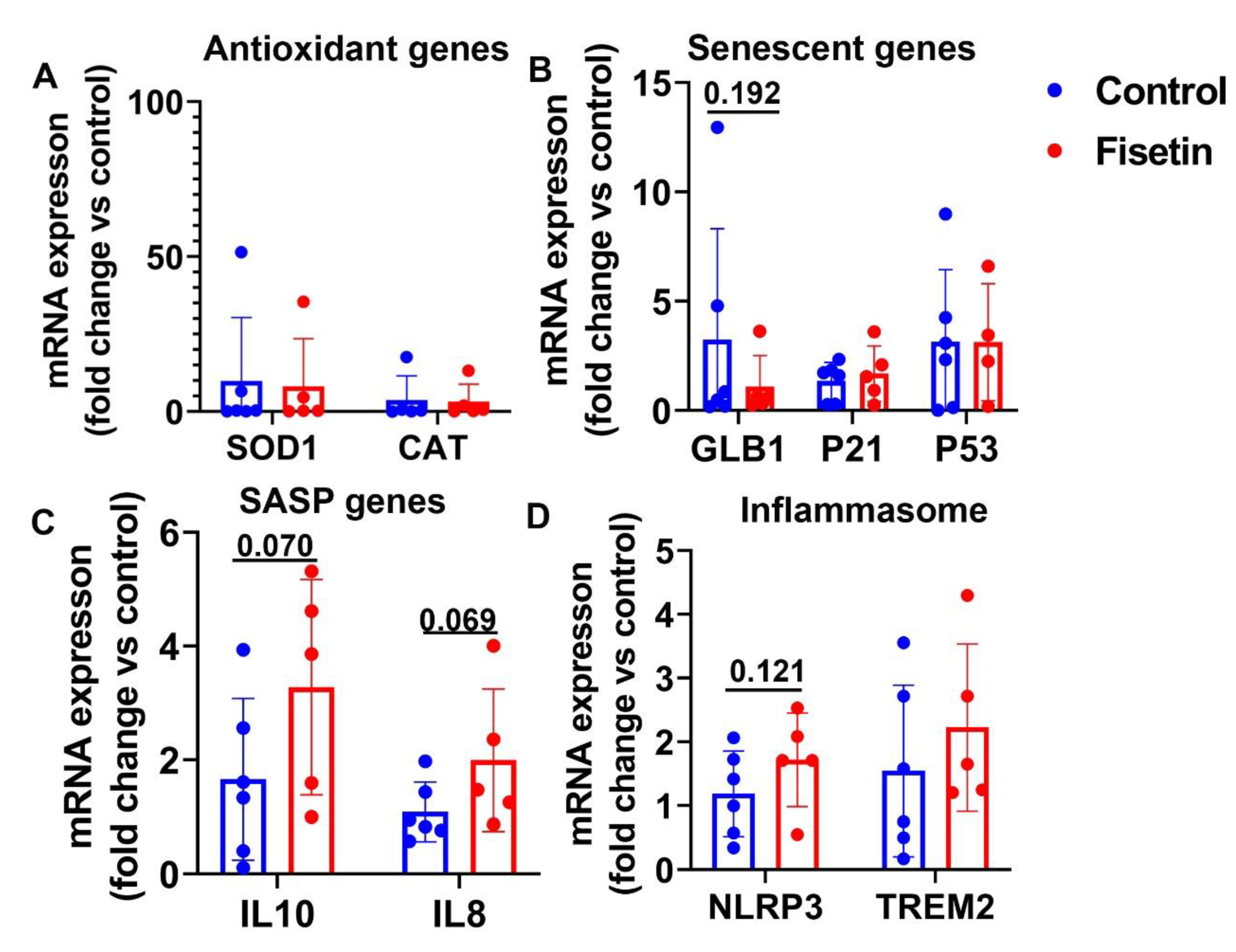
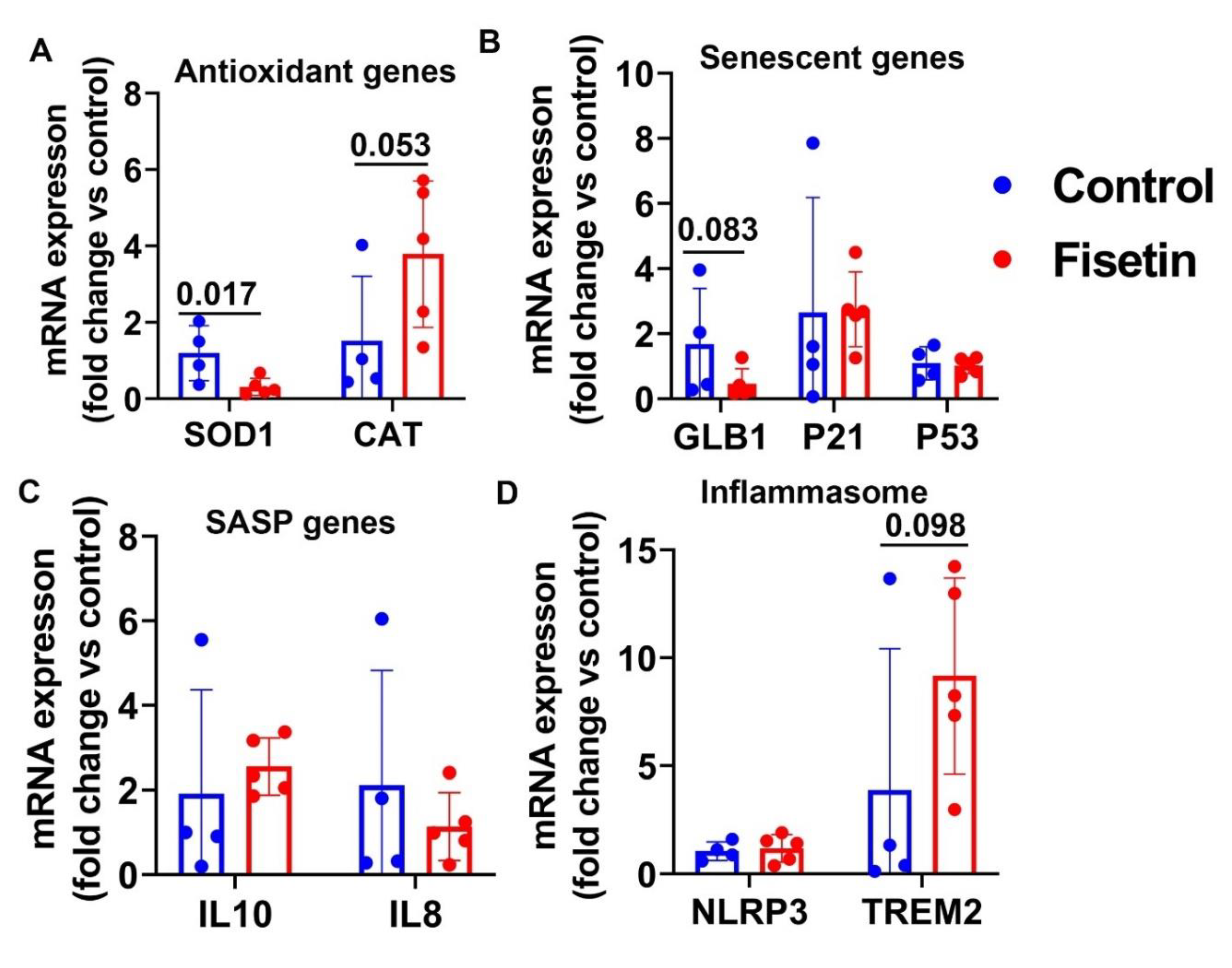
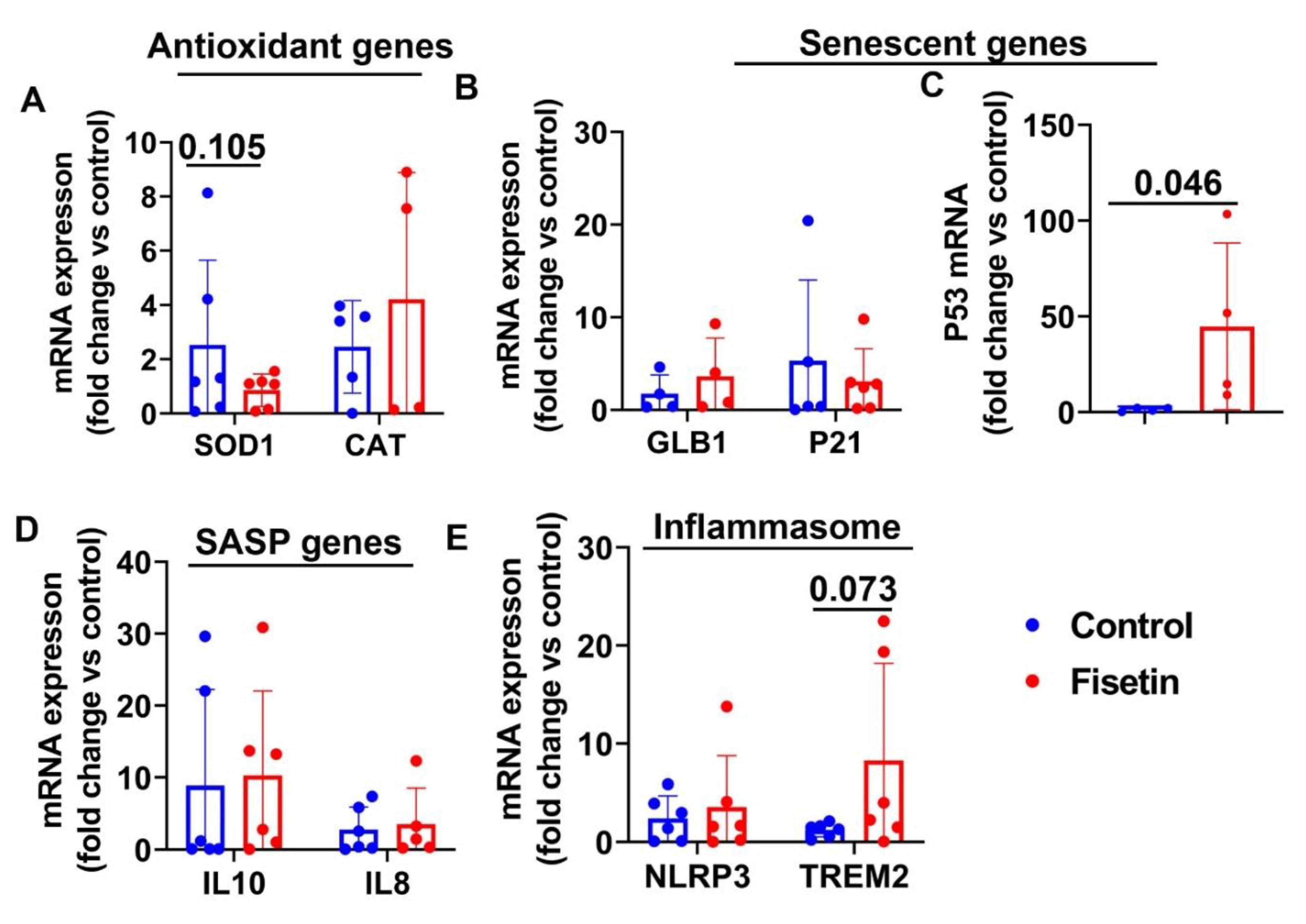
| Accession # | Gene ID | Forward primer(5’-3’) | Reverse primer(5’-3’) | Product size(bp) |
|---|---|---|---|---|
| XM_018065254.1 | Goat GFAP | caggatctgctcaacgtcaa | atctccacggtcttcaccac | 197 |
| XM_015102219.3 | Sheep GLB1 | agtccccctacctacgcact | ggtcgaagtcaccaggatgt | 213 |
| NM_001145185.2 | Sheep SOD1 | ggttccacgtccatcagttt | tttgtcagccttcacattgc | 143 |
| XM_005690077.3 | Goat CAT | ctcgtccaggatgtggtttt | gagcctgattctccagcaac | 215 |
| XM_042234806.1 | Sheep NEFH | acatcgcatcctaccaggag | cagcgatctcaatgtccaga | 135 |
| XM_015093090.3 | Sheep NEFL | aagcgcatagacagcctgat | ctctcggtcagcacagtgaa | 242 |
| HM043737.1 | Sheep GAPDH | acagtcaaggcagagaacgg | ggttcacgcccatcacaaac | 235 |
| X78306.1 | Sheep IL8 | tcgatgccaatgcataaaaa | ttggggtctaagcacacctc | 147 |
| NM_001009327.1 | Sheep IL10 | tgttgacccagtctctgctg | ttcacgtgctccttgatgtc | 136 |
| FJ943992.1 | Sheep P21 | gagagcgatggaacttcgac | agtggtcctcctgagacgtg | 186 |
| FJ855223.1 | Sheep P53 | cctgctcccgtactcagaag | ctggcagaacagcttgttga | 247 |
| XM_042250404.1 | Sheep NLRP3 | ctgtgcacacggtggtattc | ctctgagtcccaaggctcac | 157 |
| XM_004018807.5 | Sheep TREM2 | agcctttcggaagaggagag | agctggtaacctgggttgtg | 179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





