Introduction
Aging could be defined as a time-dependent functional decline that is strongly associated with cellular damage in critical organs. In animals, these time-dependent processes can manifest in several phenomena distributed in several tissues and organs. It has been attempted to summarize these phenomena as a collection of “Hallmarks of Aging” [
1,
2]. Hallmarks of aging were defined as phenomena that are observed in aged animals, and that their enhancement leads to accelerated aging, while their inhibition retard the aging process. It is well-documented that these phenomena are not independent of each other but connected in a complex network of interactions that are not fully resolved. Among the twelve identified Hallmarks of aging by Lopez-Ortin et al [
1,
2], “Mitochondrial Dysfunction” is, arguably, the most strongly connected with other Hallmarks of Aging. The most frequently observed manifestation of Mitochondrial Dysfunction in aging is the excess production of mitochondrial Reactive Oxygen Species, mROS [
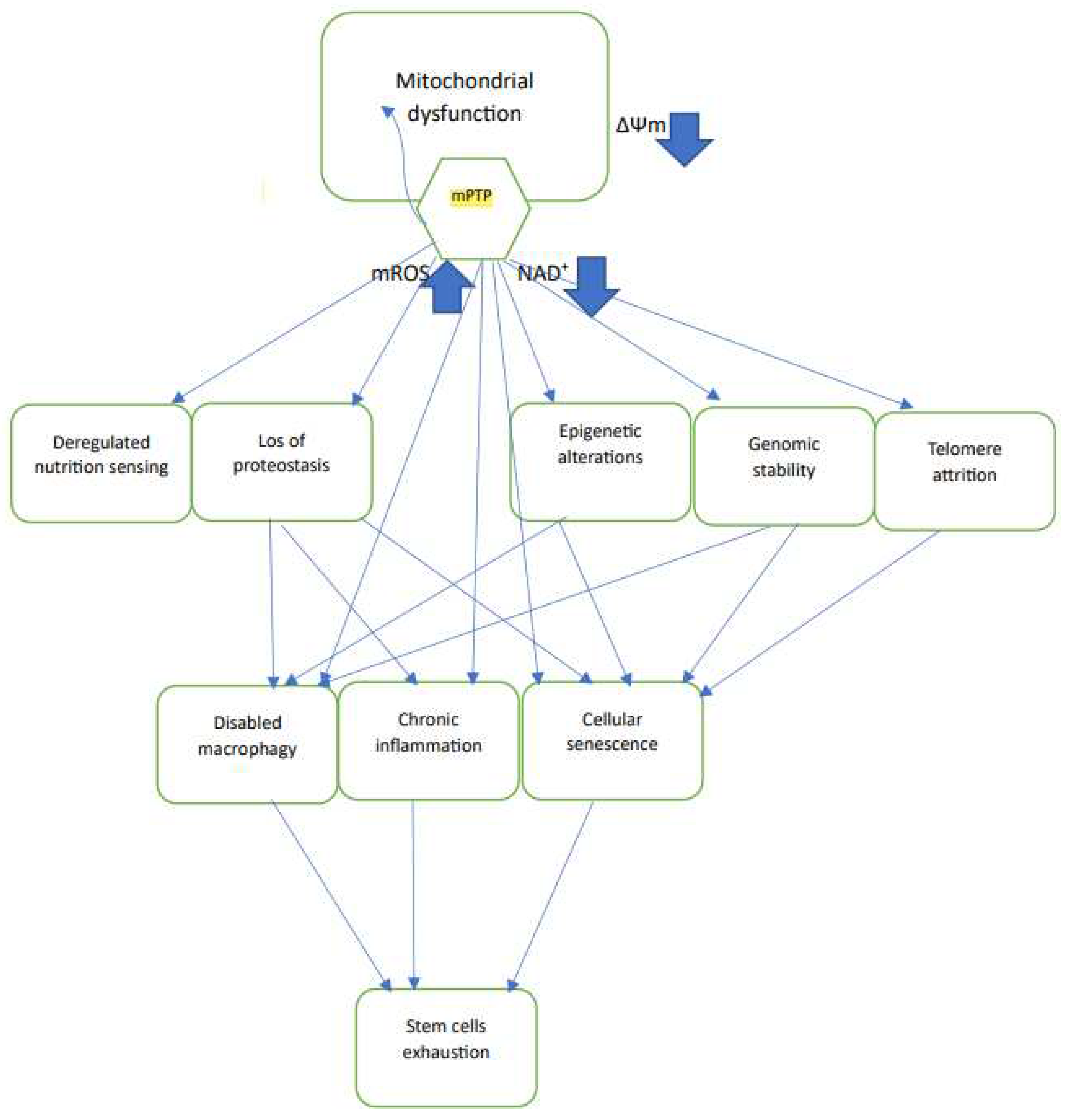
3] which negatively affects most of the other Hallmarks of Aging [
4] e.g., Genomic instability [
5], Telomere attrition [
6], Loss of proteostasis [
7], deregulated nutrient sensing [
8], cellular senescence [
9], inflammation [
10], stem cells exhaustion [
11], Loss of Epigenetic information [
12](
Figure 1). It was therefore suggested, early on, that excess mROS is the main driving force for aging, as postulated by the mitochondrial free radical theory of aging [
13,
14]. We have suggested previously that the principal pathway for the generation and delivery of excess mROS in aging cells, is the opening of the mitochondrial Permeability Transition Pore (mPTP) [
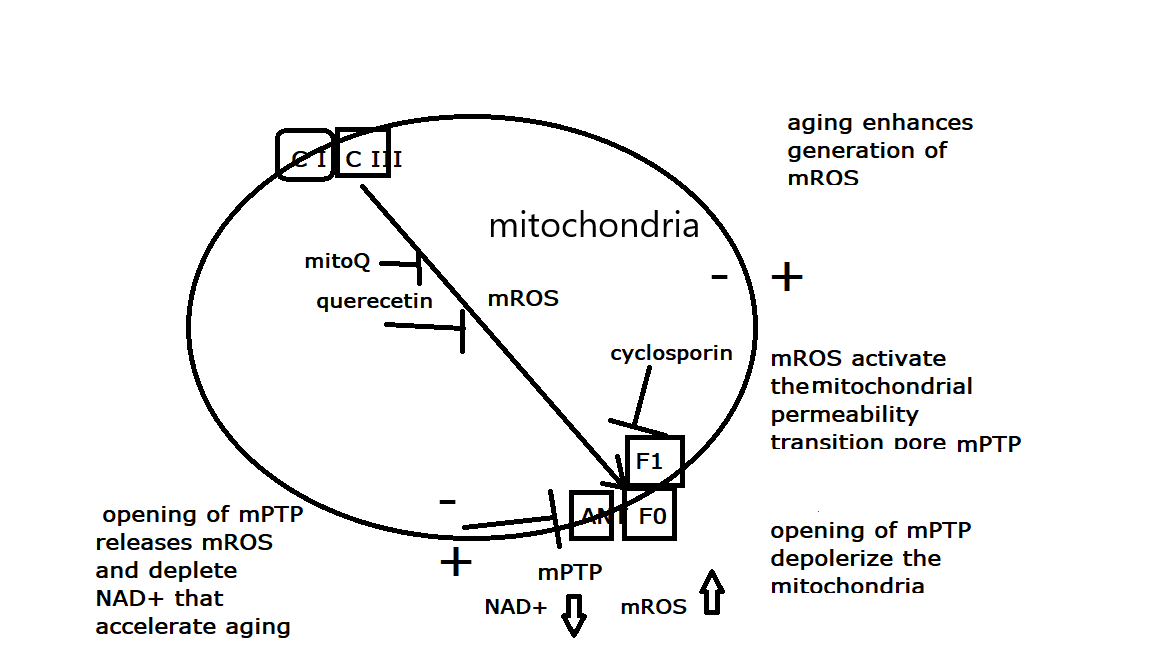
15,
16]. Since mPTP activation was shown to accelerate aging, and mPTP inhibition was shown to retard aging, enhanced activation of mPTP meets the definition of a “hallmark of Aging”.
The mPTP is a voltage-gated multi-components mega-channel, located in the inner mitochondrial membrane, composed mostly of ATP synthase (F1Fo) and the Adenine Nucleotide Translocator (ANT), that is activated by excess mROS production and/or calcium overloading (reviewed in [
17,
18]. In addition to releasing calcium, superoxide, hydrogen peroxide and other mROS, the open channel allows protons to flow into the matrix thus collapsing ∆Ψ
m and ∆pH inhibiting oxidative phosphorylation. Prolong opening of the channel also release mitochondrial substrates thus inhibiting electron transport and prevent the mitochondria from restoring ∆Ψ
m, and closing the channel, even after the release of the activating calcium and mROS. Another critical metabolite that is released from the matrix to the mitochondrial intermembrane space during channel opening is NAD+ which is then hydrolyzed by CD38 resulting in reduction of cellular NAD+ content [
19]. The reduction of cellular NAD+ content also negatively impacts most of the hallmarks of aging [
20] (
Figure 1).
Another often reported mitochondrial dysfunction in aging is the reduction in the magnitude of ∆Ψm (reviewed in [
21,
22,
23]. It was recently suggested that the reduction of ∆Ψm in aged animal is a major cause of aging [
23,
24,
25,
26,
27] and this claim was supported by the demonstration that the optogenetic induced increase of ∆Ψm increased C. elegans lifespan [
25] and that calorie restriction, that increases C. elegans lifespan, restore ∆Ψ
m in aged animals [
26]. In animal cells ∆Ψ
m varies within relatively narrow range (130 – 180 mV) and many metabolic functions affect the magnitude of ∆Ψ
m. The ATP synthase drive the synthesis of ATP that is coupled to a flow of proton from the intermembrane space to the matrix thus reducing ∆Ψ
m. The transition from minimal rate of ATP synthesis (“state 4”) to maximal rate of ATP synthesis (“state 3”) is associated with 25-35 mV reduction of ∆Ψ
m. In addition, ∆Ψ
m depends on the electron transport substrate that drive the generation of ∆Ψ
m, the activity of electrogenic or proton-coupled transporters, and the activity of uncoupling proteins, all of which may reduce the magnitude of ∆Ψ
m [
28]. The upper limit of ∆Ψ
m is determined by the magnitude of the proton leak through the mitochondrial inner membrane. Since the proton leak increases exponentially with ∆Ψ
m [
29] it is not possible to significantly hyperpolarize the mitochondrial inner membrane. There is also a lower limit to the magnitude of ∆Ψ
m in viable mitochondria. Intermediate metabolism in the mitochondrial matrix depends on the electrogenic and proton-coupled transporters, and mROS signaling depend on ∆Ψ
m [
30]. In those circumstances that the electron transport system cannot generate a protonmotive force large enough to drive ATP synthesis, ATP generated by glycolysis can reverse ATP synthase and ATP hydrolysis by ATP synthase can increase ∆Ψ
m to a level that is sufficient to maintain functional mitochondria [
31]. If cellular ATP concentration is not sufficient to restore physiological level of ∆Ψ
m, the process of mitophagy will eliminate depolarized mitochondria [
16] and if a large number of mitochondria in the cell become depolarized, cell death by necrosis or apoptosis will follow [
32].
In this review I examined, critically, and in detail, the reported reduction of ∆Ψm in aged animals and conclude that the most reasonable explanation of most of these observations is the widely reported enhancement of the activation of mPTP in aging animals (reviewed in [
15,
16,
33,
34,
35,
36,
37]. In addition, I discuss the evidence that the lifespan extension of C. elegans obtained by the optogenetic increase of ∆Ψm [
25] most likely resulted from the predicted inhibition of the voltage-gated mPTP and that the restoration of ∆Ψ
m by calorie restriction in C. elegans [
26] also resulted from inhibition of mPTP.
The effect of aging on the magnitude of the mitochondrial membrane potential in animals
It is widely reported that the magnitude of ∆Ψ
m in old animals is lower than that of ∆Ψ
m in young animals (reviewed in [
21,
22,
23].
Table 1 summarizes representative studies of the effect of aging on ∆Ψ
m in different animals, different cell types and using different methods. To interpret these studies correctly it is necessary to examine the protocols of ∆Ψ
m measurements in these studies.
The determination of ∆Ψ
m in isolated mitochondrial is relatively simple. A permeable cation is allowed to equilibrate across the mitochondria inner membrane and reach electrochemical equilibrium; the concentrations of the cation in the mitochondrial matrix and the medium, at equilibrium, is determined, and ∆Ψ
m is calculated from the concentration ratio according to the Nernst equation: ∆Ψ
m = 60xlog([C]in/[C]out) [
38]. In most recent studies of ∆Ψ
m lipophilic, membrane permeable, cations are employed. In one method the distribution of radioactively labeled lipophilic cations such as TPP+, between the mitochondria and the medium, is determined after quick separation of mitochondria and the medium (e.g., filtration or centrifugation), and calculation of ∆Ψ
m from the ratio C
pelet/C
medium. Another method is the measurement of mitochondrial uptake of TPP+ from the medium, as measured by a TPP+ electrode immersed in the mitochondrial suspension, and calculation of ∆Ψ
m from the amount of TPP+ taken by a known volume of mitochondria. More frequently the uptake of lipophilic cationic fluorescent dyes is used to estimate the relative magnitude of ∆Ψ
m. Measuring ∆Ψ
m in isolated mitochondrial suspension with a fluorescent dye, in most routine measurements, use the quenching method which depends on the fact that at high matrix concentration the dye aggregates on the inner membrane surface and the fluorescence is quenched; the amount of accumulated dye is estimated from the residual fluorescence of the free dye that remains in the suspending medium . However, the aggregated dye is not free in the matrix solution and therefore an exact calculation of the magnitude ∆Ψ
m from the extent of quenching must be corrected for the aggregation or calibrated separately.
Most of the reported measurements of ∆Ψ
m in old animals were measured not in isolated mitochondria but in intact cells suspensions where ∆Ψ
m was estimated from the fluorescence of lipophilic ∆Ψ
m indicators that were accumulated by the cells(e.g. R-123, DioC
3(6), TMRE). This method is not as simple as the measurement of ∆Ψ
m in isolated mitochondria. In this method it is necessary to use very low dye concentrations to prevent aggregation and quenching in the mitochondrial matrix since that would saturate the fluorescence and the cell fluorescence would no longer be proportional to ∆Ψ
m. Other factors also needed to be considered, e.g., the mitochondrial content of the cells (which may be reduced in aging), the mitochondrial matrix volume, and the plasma membrane potential that drive the accumulation of the dye into the cell [
39,
40,
41]. The fluorescence maximum of the lipophilic cation JC-1 shifts from green fluorescence of the monomer to red fluorescence of the aggregated dye, and therefore ∆Ψ
m can be estimated from the ratio red/green fluorescence [
42]. However, a carefully selected concentration is required to ensure that the transition occurs over the range of concentration that is sensitive to high ∆Ψ
m. In this case too, if the concentration in the medium is too high all the mitochondria with significant ∆Ψ
m will have red fluorescence, regardless of the value of ∆Ψ
m, and only depolarized mitochondria will have green fluorescence.
Unfortunately, many of the studies of ∆Ψ
m in cells from old animals did not use the optimal dye concentrations and did not take into account all the factors that can influence the fluorescence, which render their interpretation more difficult. Most importantly, all the available studies either from cells or isolated mitochondria report the average ∆Ψ
m, but not values from single mitochondria, so it is quite possible, that the lower average values of ∆Ψ
m reported for cells or isolated mitochondria from old animals, represent a mixture of depolarized mitochondria and mitochondria with high ∆Ψ
m. [
43]. As discussed below there is not a single study that show that there is a normal distribution of ∆Ψ
m values in mitochondria from aged cell and that the lower ∆Ψ
m is not a mixture of fully polarized and depolarized mitochondria.
Kokoszka et al [
44] measured ∆Ψ
m in
isolated liver mitochondria from young and old mice. Using the TPP+ electrode they observed a small difference between mitochondria from young mice (180 mV) and old mice (170 mV). Kokoszka et al [
44] also reported inhibition of electron transport and increased oxidative stress. Most importantly mitochondria from old mice also exhibited enhanced activation of mPTP. It should be clear that since the measured ∆Ψ
m is an average of a large number of mitochondria, it is possible that ∆Ψ
m is not uniformly distributed. If that was the case a reduction of average ∆Ψ
m from 180 mV to 170 mV in mitochondria from old mice may indicates that ~35% of the mitochondria are depolarized and ~65% maintain the same ∆Ψ
m as young mice (180 mV). Therefore, it is possible to explain the small reduction in ∆Ψ
m in liver mitochondria from old mice as resulting from the aging induced enhanced activation of mPTP that resulted in a larger fraction of depolarized mitochondria. In the liver (or other organs) mitochondria with fully open mPTP will be cleared by mitophagy [
16] so this fraction should be normally small. However, aging inhibits mitophagy so in aged liver this fraction could be larger. Moreover, in isolated mitochondria, where mitophagy is absent, the fraction of depolarized mitochondria increases with time and only carefully selected isolation and incubation protocols retard this process somewhat.
The enhanced activation of mPTP in
isolated liver (and brain) mitochondria from old mice was confirmed independently by the measurement of the threshold for calcium induced calcium release [
45]. It should be pointed out that this common assay of mPTP activation does not really distinguish between mitochondria that are more sensitive to calcium induced activation of mPTP (the standard interpretation) and a mitochondrial preparation that already include depolarized mitochondria that cannot therefore accumulated calcium.
LaFranca et al [
46] measured ∆Ψ
m in isolated mitochondria from rat cortical and striatal neuron from young and old rats. Similar to Kokuszka et al [
44] they also used TPP+ electrode and reported relatively small reduction of average ∆Ψ
m , 10-15 mV, in neurons from old rat. Similarly, they also reported enhanced activation of the mPTP in cortical neurons from old rates. Very recently Gainutdinov et al [
47] also reported that brain mitochondria from old rats exhibit lower ∆Ψ
m, as measured by the quenching of safranine fluorescence, and that brain mitochondria from old rats also exhibited enhanced activation of mPTP.
Thus, experiments with isolated mitochondria, either from mouse liver or rat brain suggest small reduction of average ∆Ψm in mitochondria from old animals (10-15 mV). Since this small reduction of ∆Ψm was, in all cases, associated with enhancement of the activation of mPTP it is possible that one explanation of the observed small reduction in the magnitude of ∆Ψm resulted from depolarization of a fraction of the mitochondria by mPTP opening, but only determination of ∆Ψm in individual mitochondria can test this explanation.
The earliest report that there is a reduction of ∆Ψ
m, when measured in intact hepatocytes from old rates, were from Bruce Ames laboratory. In their earliest paper Haggen et al [
48] used the accumulation of the radiolabeled cation TPP+ by hepatocytes to calculate ∆Ψ
m. They reported that in contrast to hepatocytes from young mice, that have a uniformly high ∆Ψ
m (154 mV), hepatocytes from old mice had much lower average ∆Ψ
m (101 mV) and could be fractionated into three fractions: a small fraction of cells with high ∆Ψ
m (same as young , 154 mV), a small fraction with intermediate ∆Ψ
m (93 mV), and a large fraction with low ∆Ψ
m (70 mV). It is quite possible, that even in the cell fraction with the low averaged ∆Ψ
m there is a mixture of mitochondria with high ∆Ψ
m and depolarized mitochondria. It is also possible that the density of mitochondria in hepatocytes from old rats is lower than that of hepatocytes from young rats. In most of their other experiments Haggen et al [
48] used the fluorescent potential indicator Rhodamine-123 and measured hepatocytes fluorescence by flow cytometry, a method that was also used in subsequent studies [
49,
50]. They reported >50% reduction in fluorescence intensity in hepatocytes from old mice compared to hepatocytes from young mice. The dye concentration used in these studies was very high (26 uM), orders of magnitude higher than the recommended dye concentrations needed to avoid aggregation and quenching. It was previously demonstrated that only when the external concentration of the dye is in the low nM range the fluorescence of the dye in the mitochondrial matrix is not quenched and the magnitude of ∆Ψ
m is proportional to the fluorescence intensity [
39,
40,
41]. Therefore, it is quite clear that in the experiments of Haggen et al [
48,
49,
50] the fluorescence intensity is saturated and therefore insensitive to the magnitude of ∆Ψ
m . However, since the dye will only accumulate in mitochondria that have a ∆Ψ
m >> 0 the fluorescence intensity is proportional to the number of mitochondria in the cells that are not depolarized and retain high ∆Ψ
m . If aging increases the number of mitochondria with activated mPTP, which are therefore depolarized, and also decreases mitochondrial density due to clearance of depolarized mitochondria by mitophagy, the fluorescence intensity will be lower in aged hepatocytes. Hagggen et al [
48,
49,
50] did not measured the mitochondrial density in hepatocytes from old and young rats. Therefore, these measurements suggest that in isolated hepatocytes from old mice ~50% of the mitochondria are either depolarized or were further cleared by mitophagy. Haggen et al [
48,
49,
50] reported other experiments with aged hepatocytes that support this interpretation: they showed that the reduction of ∆Ψ
m is associated with increased production of mROS which is known to be associated with enhanced activation of mPTP. Many of the studies of mitochondrial dysfunction in aging (and degenerative diseases) report the association of increased mROS production with reduced ∆Ψ
m [
51]. Since it is well established that, in general, mROS production increases exponentially with ∆Ψ
m [
52,
53,
54], the unusual association, observed in aging animals, of high mROS and low ∆Ψ
m most likely results from the enhanced activation of mPTP.
Cavazzoni et al [
55] measured ∆Ψ
m in hepatocytes from young and old rats with R-123 by flow cytometry, at relatively low concentration of R-123 (130 nM), which they claim is not saturating under their conditions. In contrast to Haggen et al [
48,
49,
50] they did not observe a significant difference of ∆Ψ
m between hepatocytes from young and old rats. However, the standard deviations in their measurements were very high ± 45 mV, obscuring, possibly, a small difference between young and old.
Rottenberg and Wu [
56] estimated the relative magnitude of ∆Ψ
m in intact lymphocytes from young and old mice. They use very low concentrations of the lipophilic dye DiOC
3(6), well below the saturating concentration [
39] and measured fluorescence by flow cytometry. They found that ∆Ψ
m was significantly lower in lymphocytes from old mice and that the fraction of cells with depolarized mitochondria is much larger in lymphocytes of old mice. In addition, the rate of respiration was inhibited in lymphocytes from old mice. Most significantly, cyclosporin, an inhibitor of mPTP, restored both the rate of respiration and ∆Ψ
m in lymphocytes from old mice thus proving that the lower ∆Ψ
m in lymphocytes from old mice is the result of enhanced activation of mPTP. Interestingly, the activation of mPTP in lymphocytes from old mice is largely confined to the older, memory T cells [
57], probably because most other types of lymphocytes have relatively short lifespan.
Sugrue et al [
58] measured ∆Ψ
m in senescent and non-senescent PC12 cells using confocal microscopy and the fluorescent probe CMTMR and reported that the fluorescence was lower in senescent cells. The measurement of ∆Ψ
m by CMTMR is controversial [
22] because, unlike the measurement of ∆Ψ
m with other cationic fluorescent probes, that is based upon equilibrium distribution of the free probe across the membrane, the cationic CMTMR, while accumulating in the matrix due to ∆Ψ
m is covalently bound in the matrix. Therefore, the amount of bound probe is not proportional to the magnitude of ∆Ψ
m, rather it would be proportional to the number of mitochondria that have significant ∆Ψ
m, because the probe would not be accumulate in mitochondria where ∆Ψ
m = 0. Therefore, the result of Surge et al [
58] should be interpreted that compared to non-senescent PC12 cells the number of mitochondria with ∆Ψ
m >>0 is smaller in senescent PC12 cells. Most importantly Sugrue et al [
58] also showed that the effect of aging on CMTMR fluorescence is the result of the enhanced activation of mPTP in senescent cells since cyclosporin restored CMTMR fluorescence to values similar to that of non-senescent PC12 cells.
More recently Durak and Turan [
59] reported that in cardiomyocytes from aged rats, ∆Ψ
m was significantly lower than that of ∆Ψ
m in cardiomyocytes from young rats. In their measurements they used the fluorescent probe JC-1 at very high, clearly saturating, concentration (4 uM). Therefore, their data must also be interpreted as resulting from an increased number of depolarized mitochondria in aged cardiomyocytes. Since they also reported increased production of mROS in aged cardiomyocytes, and that Liraglutide, a GLP-1R agonist, inhibits mROS production and restores ∆Ψ
m, their data are consistent with the interpretation that the increased depolarization of mitochondria in myocytes from old rats is the result of enhanced activation of mPTP.
Morris et al [
60] investigated mitochondrial metabolism in old and young Intestinal Stem Cells in Drosophila. They found that old Intestinal Stem Cells exhibit a distinct, Warburg-like, metabolic shift, similar to cancer cells, in which oxidative phosphorylation is inhibited and glycolysis is enhanced. This was largely the result of inhibition of calcium uptake by MCU which led to low activity of ETC resulting in inhibition of ATP synthesis. They reported lower membrane potential in old stem cells as measured by the fluorescence of TMRE (20 nM) from the mitochondria (labeled by mitoGFP). The average fluorescence intensity was ~20% lower in cells from old bees compared to young. While it is quite possible that such drastic metabolic shift is associated with lower ∆Ψ
m , the data shown report average cell fluorescence and it is not clear if the probe was equally distributed between the mitochondria in the cell. Morris et al [
60] also reported higher hydrogen peroxide production in old stem cells so it is likely that mPTP activity is also enhanced in these cells.
Mansell et al [
61] studies hematopoietic stem cells (HSC) from young and old mice. They measured ∆Ψ
m by flow cytometry using TMRM (100 nM + Verapamil, to inhibit probe efflux) and reported lower ∆Ψ
m in HSC from old mice. However, the HSC population form old mice exhibited two distinct fractions: a large fraction (~85%) with low ∆Ψ
m and a smaller fraction (~15%) with high ∆Ψ
m, like that of young cells. When separated by cell sorter the fraction with high ∆Ψ
m exhibit similar properties to young HSC whereas the HSC with low ∆Ψ
m exhibited the distinct profile of old HSC. Moreover, when HSC from old mice were treated with MitoQ, a strong mitochondria-specific antioxidant, the fraction of high ∆Ψ
m was greatly increased, largely reversing the effects of aging on HSC. Since MitoQ was shown to inhibit mPTP [
62], these finding are compatible with the interpretation that the reduction of ∆Ψ
m in HSC from old mice resulted from the enhanced activation of mPTP. Similarly, the antioxidant quercetin, which is also known to inhibit mPTP [
63] restored ∆Ψ
m in aged porcine oocytes [
64]
Perhaps the best evidence that the reduction of ∆Ψ
m in aged animals is the result of mPTP activation can be seen in the study of Zhang et al [
65]. They used JC-1 fluorescence to measure relative ∆Ψ
m in rat cardiomyocytes and reported a small reduction of ∆Ψ
m in cardiomyocytes from aged rats. They also reported increased mROS production, increased activation of mPTP, as well as enhanced frequency of mitoflashes (that indicate short opening of mPTP) in aged cardiomyocytes. They also showed that the tetra-peptide SS-31, which strongly associated with both ANT1 and ATP synthase (major components of the mPTP complex [
18]) inhibits many of the effects of aging on cardiomyocytes. In aged cardiomyocytes, SS-31 restored ∆Ψ
m, inhibited excess mROS production, inhibited mPTP activation, and inhibited excess activation of mitoflashes, providing strong evidence that the reduction of ∆Ψ
m in aged cardiomyocytes is the result of the enhanced activation of mPTP.
Mitochondrial membrane potential and aging in C. elegans
In recent years the worm C. elegans became an important system for studies of animal aging. The very short lifespan and the small number of cells of this simple organism enabled the relatively fast identification of many genes that either increase or decrease the worm lifespan, and the identification of many pharmacological agents that affect lifespan positively or negatively.
Brys et al [
66] studied the effect of the C. elegans daf-2 mutation on mitochondrial bioenergetics. They found that the daf-2 mutation, that encode the insulin/insulin growth factor-1-like receptor, and was shown to extend C. elegans lifespan, retard the deterioration of mitochondrial bioenergetic in aging worms. In particular, they showed that while ∆Ψ
m decline with age in wild type worms the daf-2 mutation increased ∆Ψ
m and largely retarded the decline of ∆Ψ
m with age. Byrs et al measured ∆Ψ
m in isolated mitochondria with the fluorescence dye DASPMI. They used high concentration (~6 uM) of DASPMI most likely in the quenching range. At day 1 the fluorescence ratio - control/+ FCCP (a measure of the magnitude of ∆Ψ
m) was 1.75. However, by day 6 this ratio was dramatically reduced to 1.20. In contrast, in the daf-2 mutant the ratio at day 6 was 1.60, not significantly different from the 1.75 ratio that was observed in wt at day 1. Because brys et al [
66]. use very high concentration of DASPMI it is unlikely that the fluorescence ratio is proportional to the magnitude of ∆Ψ
m. Most likely the lower ratio at old age indicates that there is an age-dependent increase in the number of depolarized mitochondria and that this effect of aging is inhibited in the daf-2 mutant. It has been shown previously that lifespan-extension of the daf-2 mutant is associated with a reduction of the level of VDAC1 which could lead to inhibition of mPTP [
67] and it is therefore possible that effect of the daf-2 mutation on ∆Ψ
m reflect the inhibition of mPTP in this mutant.
Dingley et al [
68] determined ∆Ψ
m in whole worms by including 1 uM TMRE in the growth medium. Before fluorescence measurement the worms were washed to clear the dye from the intestine and the TMRE fluorescence was measured from the terminal Pharyngal bulb where the density of mitochondria is high. They compared TMRE fluorescence in wild type (N2) with that of the gas-1 mutant. The gas-1 mutant serves as model for accelerated aging since it carries a mutation in a complex I peptide that increases mROS production. The gas-1 mutant exhibits increased oxidative stress and shorter lifespan relative to N2. TMRE fluorescence was decrease by 63% in the gas-1 mutant suggesting much lower ∆Ψ
m. However, Dingley et al [
68] also measured separately mitochondrial density with mitotracker green PM and showed that the density of the mitochondria is reduced by 48% in the gas-1 mutant. Thus, the reduction of TMRE fluorescence resulted largely from the reduction of mitochondrial density. If we correct for the reduction of mitochondrial density, the fluorescence from mitochondria in the gas-1 mutation is ~15% lower than that of N2. Dingley et al [
68] incubated the worms for 24 hours with 1 uM TMRE, which, even after washing the worms, is expected to be well into the quenching range. Experiments with isolated C. elegans mitochondria show that even with 0.3 uM TMRE in the medium the dye in the mitochondria is largely quenched [
69], whereas it was necessary to use only 20 nM of TMRE in mitochondrial suspension to avoid aggregation and quenching [
70]. Therefore, the 25% reduction of fluorescence in Dingley et al experiments suggests that ~25% of the mitochondria in the gas-1 mutant are depolarized. It was previously shown by flow cytometry of isolated C. elegans mitochondria that under any condition a fraction of the mitochondria is depolarized [
43]. It is therefore quite likely that enhancement of mPTP by the excess mROS generated by the gas-1 mutant increases the fraction of depolarized mitochondria. This may also explain the reduction in the mitochondrial density since depolarized mitochondria are expected to be cleared by mitophagy [
16], and cells with high number of depolarized mitochondria may proceed to apoptosis [
32]. More recently Kwon et al [
71] found that the reduction of TMRE fluorescence in the gas-1 mutant can be accounted for entirely by the reduction of mitochondrial density.
Lemire et al [
72] also studied ∆Ψ
m in C. elegans mutants that affect C. elegans lifespan. They used the lipophilic cation diSC
3(3) at high concentration (4 uM) to measure ∆Ψ
m in several strains that extend C. elegans lifespan (i.e., daf-2, age-1, clk-1, isp-1 and eat-2). At high concentration diSC
3(3) aggregates in the mitochondria and the fluorescence maximum shift to the red. Lemire et al determined the fluorescence maximum of diS-C
3(3), in whole worms at the larval stage (L4), of the various life-extending strains and found that at the larval stage ∆Ψ
m, as estimated from the fluorescence maximum shift, is lower in all the life-extending strains compared to that of the wild type (N2). These results appear to contradict the results of Bry et al [
66] but it should be emphasized that these measurements were done with the whole larva and not with mitochondria isolated from the adult worms; there are significant differences between the properties of adult and larval mitochondria [
43]. Moreover, disruption of mitochondrial function during development was shown to increase lifespan [
73].
Cho et al [
74] studied the effect of flavonoids on neurodegeneration in aging C. elegans. They found that flavonoids mitigate neurodegeneration in aging C. elegans and that this effect was associated with an early reduction of ∆Ψ
m. They measured ∆Ψ
m with TMRE, at 100 nM, in whole worm at the larval stage (L4). The effect of flavonoids on ∆Ψ
m was comparable to the weak uncoupler DNP and suggest that this uncoupling effect enhance mitophagy which protect the worms against neurodegeneration. Uncouplers are known activators of the mPTP [
75] and mPTP activation enhances mitophagy [
16]. Thus, activating mPTP at an early age could lead to activation of protection mechanisms such as mitophagy or the mitochondria unfolded protein response, UPRmt (see below). Cho et al [
74] also report that ∆Ψ
m was much lower in the adult worms than in the larval state regardless of the flavonoids but do not present the evidence.
Dilberger et al [
76] studied the effects of paraquat on C. elegans longevity, mROS production and ∆Ψ
m. Paraquat is known to induce excess mROS production, similar to aging, and can therefore be considered a pharmacological model of aging in C. elegans. Indeed, Dilberger et al [
76] showed that paraquat, depending on the concentration greatly reduced C. elegans lifespan. This was associated with greatly increased mROS production, mitochondrial fragmentation and greatly reduced ∆Ψ
m . Dilberger et al [
76] measured ∆Ψ
m in isolated mitochondria from the fluorescence quenching of R123; paraquat (5 mM) reduce ∆Ψ
m by ~50%. In addition, they showed that paraquat increased mitochondrial fragmentation. Paraquat is known to induce the activation of mPTP [
77] which is known to increase mitochondrial fragmentation in C. elegans [
67], therefore the reduction of ∆Ψ
m by paraquat and the resulting reduction in lifespan can be attributed to the activation of mPTP.
Since Paraquat induces the mitochondrial Unfolded Protein Response, UPRmt, in C. elegans [
24,
78] the results of Dilberger et al [
76] suggest that the activation of mPTP is sufficient to activate UPRmt. Berry et al [
24] also showed that reduction of ∆Ψ
m by the uncoupler FCCP is sufficient to activate UPRmt in C. elegans. However, FCCP is known to activate mPTP [
75] and Angeli et al [
79] showed that genetic or pharmacological inhibition of mPTP inhibits UPRmt. Moreover, it was recently shown that two signals from the mitochondria to the cytosol induce UPRmt: excess mROS, and accumulation of mitochondrial protein precursors [
80]; excess mROS is released by the activation of mPTP, and the depolarization of mitochondria resulting from the activation of mPTP will inhibit the uptake of mitochondrial protein precursors, providing further support to the conclusion that activation of UPRmt depends in the activation of mPTP.
Interestingly, in their study Berry et al [
24] measured ∆Ψ
m from TMRE fluorescence from the whole body of the worms after incubating the worms for 24 hours with 100nM TMRE. It appears, however, that at this high TMRE concentration the dye in the mitochondria matrix is largely aggregated and largely quenched. This can be deduced from the parallel titrations of the effect of FCCP on TMRE fluorescence, and the activation of UPRmt. In the very wide range of FCCP concentrations from 10 pM to 10 uM, UPRmt was activated only at a narrow range around 10 nM, presumably resulting in a small reduction in ∆Ψ
m. However, the titration of TMRE fluorescence over the entire range of FCCP concentration did not show the expected linear titration curve. Already at very low concentration, 10 pM there was ~ 30% reduction in TMRE fluorescence but increasing concentrations even up to 3 uM did not reduce the fluorescence further. Only at 25uM, presumably when the mitochondria become completely depolarized, the fluorescence was significantly reduced (~80%). Thus, TMRE fluorescence values at 10 pM FCCP and 1 nM FCCP, which were insufficient to activate UPRmt, and the TMRE fluorescence value at 10 nM FCCP, that was sufficient to activate UPRmt, were all the same, demonstrating that at this concentration of TMRE the fluorescence magnitude over nearly the entire range of ∆Ψ
m values is not sensitive to the magnitude of ∆Ψ
m, and only when the mitochondria is depolarized the TMRE fluorescence is reduced as expected.
Berry et al [
69] targeted a fungal light-driven proton pump to the mitochondria of C. elegans to selectively increase the mitochondrial protonmotive force. They call this optogenetic tool mtON. They Used TMRE at high quenching concentration (300 nM) to measure ∆Ψ
m in isolated mitochondria from the fluorescence quenching of the suspending medium. They showed that in isolated non-respiring mitochondria applying light and all trans retinal (ATR), the catalytic center of the light-driven proton pump, resulted in the generation of ∆Ψ
m that was collapsed by the uncoupler CCCP. Using the pH indicator BCECF they also showed that mtON generated a pH gradient as expected. The ∆Ψ
m that was generated by the substrate succinate (in the presence of rotenone, “state 2”) was not significantly different from that generated by mtON at maximal illumination. However, applying both succinate and mtON did not increase ∆Ψ
m above the value that is generated by either succinate or mtON alone suggesting that the magnitude of ∆Ψ
m in state 2 is limited by the membrane proton permeability. Berry et al [
69] also showed that mtON alone (without succinate) was able to generate ATP, similar to ATP generated by succinate oxidation. In “state 3” (i.e., succinate + ADP) mtON inhibited respiration by 50%, presumably because mtON can increase ∆Ψ
m in state 3 (however, Berry et al did not measure ∆Ψ
m in “state 3”). Electron transport inhibitors (i.e., rotenone, antimycin A and azide) kill most C. elegans worms within hours, presumably because they collapse ∆Ψ
m, and mtON can significantly increase worms survival in the presence of electron transport inhibitors. Finally, Berry et al showed that turning on mtON in the gas-1 mutant significantly increased motility that was otherwise greatly reduced in the mutant, compared to wt. Since the reported reduction of ∆Ψ
m in the gas-1 mutant [
68] undoubtedly resulted from the enhancement of the activation of mPTP by the excess mROS produced by the gas-1 mutation (see above) the restoration of motility to the gas-1 mutant by mtON could be attributed to inhibition of the voltage-gated mPTP by the ∆Ψ
m that is generated by mtON.
mtON should be an effective inhibitor of mPTP not only because high ∆Ψm decrease the probability of opening of the voltage-gated mPTP but also, and perhaps more importantly, because it enables the channel to close quickly, after releasing the excess calcium and mROS that activated the channel opening. Normally, the electron transport proton pumps cannot easily restore ∆Ψm to close the channel because the respiratory substrates are also released by the channel and electron transport is inhibited. But the mtON-generated ∆Ψm is not dependent on electron transport and can therefore close the channel quickly after a relatively short opening.
Very recently Berry et al [
25] showed that the optogentic generation of ∆Ψ
m, (mtON) in C. elegans during the entire adult lifespan increases C. elegans lifespan. They also showed that both 4 day old and 10 days old worms increased thrashing in liquid (but no in solids) when mtON is activated, demonstrating that muscle activity, that normally deteriorate in old worms can be partially rescued by ∆Ψ
m generated by mtON. In this study they estimated ∆Ψ
m not in isolated mitochondria but from whole worm incubated with TMRE (100 nM). The TMRE fluorescence was collected from the head or the pharyngal bulbs that have high concentration of mitochondria. They show that compared to TMRE fluorescence from the worms at day 1, TMRE fluorescence is greatly reduced with aging with ~75% reduction in day 4 and ~50% reduction in day 10. Berry et al measured mitochondrial density at day 4 but not at day 1 so the reduction in TMRE fluorescence is most likely, partially at least, due to decrease mitochondrial density and partially due to decreased ∆Ψ
m (cf. [
68], [
71]). In a separate experiment, with 4 days old worms Berry et al [
25] showed the turning mtON greatly increase median TMRE fluorescence. In this experiment they did measure, separately, mitochondrial density, with mitotracker, and normalized the TMRE fluorescence to the mitochondrial density.
As discussed above at 100 nM TMRE the probe is aggregated and quenched in the mitochondrial matrix so it is not sensitive to small changes in ∆Ψ
m, and the large reduction in fluorescence in aging worms is most likely due to an increased fraction of depolarized mitochondria and/or mitophagy cleared depolarized mitochondria. If the average ∆Ψ
m in mitochondria of aged worm was 50-75% lower than that of young worms, as claimed by Berry et al, it is not expected that uncoupler like FCCP will stimulate respiration very much in old worms since the enhancement of respiration by uncoupler results from the collapse of the protonmotive force. However, the fact that Berry et al observed no difference in the effect of FCCP on respiration between young and old worms suggests that there was no difference in ∆Ψ
m of respiring mitochondria in old and young cells, and the lower TMRE fluorescence result from lower mitochondria density and/or a larger fraction of depolarized mitochondria that do not respire. The explanation, that the low ∆Ψ
m that is observed in old worms resulted from enhanced activation of mPTP, and that that mtON increase lifespan and healthspan by inhibiting mPTP, is compatible with all the observations reported by Berry et al [
25].
In a more recent report, Berry et al [
26] investigated the relationship between dietary restriction (DR), that extend lifespan in C. elegans, and ∆Ψ
m. Here they show again that aging, from day 1 to day 4 is associated with a ~50% reduction in TMRE fluorescence under normal dietary conditions, while when on DR the reduction of TMRE fluorescence is much smaller, ~20%. In this study they also measured TMRE fluorescence from the pharyngal bulb of whole worms, but in this case, they incubated the worms with 1 uM TMRE compared to 100 nM in their previous study. They do not explain this change of protocol, but it is clear that under these new conditions TMRE fluorescence from the mitochondrial matrix is in full quenching mode and the fluorescence cannot be proportional to the magnitude of ∆Ψ
m (see discussion above). Therefore, in this study as well, the reduction of TMRE fluorescence in aged cells could indicate either a reduction in the density of mitochondria or an increase in the number of depolarized mitochondria, most likely both. Berry et al [
26] also show several genetic and pharmacological manipulations that modulate the effect of DR on longevity and TMRE fluorescence. The uncoupler FCCP Inhibited the effect of DR both on longevity and TMRE fluorescence as expected. The mutant eat -2, a genetic model of DR, increased TMRE fluorescence at day 4 and extends lifespan, and this effect was also inhibited by FCCP. a deletion of the uncoupling protein, unc-p, a deletion of the adenine Nucleotide translocators ant-1,2, and the deletion of IF1 protein (ATP synthase inhibitor) mal-2, all raised TMRE fluorescence at day 1 but only ant-1,2 and mal-2 inhibited the reduction of TMRE fluorescence at day-4 and extended lifespan. While the authors attempt to explain all these effects as resulting from modulations of the bioenergetic functions of mitochondria, it is clear that all of these effects can be attributed directly to activation or inhibition of mPTP: it was shown that life extension by the eat-2 mutant depend on the inhibition of mPTP [
67], and that FCCP is an activator of mPTP [
75]. The Adenine nuclear translocators 1 and 2 are components of mPTP [
18] and their deletion will inhibit mPTP [
67]. The protein IF1 interacts with ATP synthase, another component of mPTP [
18], and was shown to activate mPTP [
81], therefore, the deletion of mal-2 is expected to inhibit mPTP. Thus, all the genetic and pharmacological effects on ∆Ψ
m that are described by Berry et al [
26] can be attributed to activation or inhibition of mPTP. Finally, cyclosporin, an inhibitor of mPTP was shown to extend lifespan in C. elegans [
82], and it was also shown that mPTP is inhibited in DR in mammals [
83,
84,
85].
Conclusions
Mitochondria dysfunction is a well-established “hallmark of Aging” which strongly interacts with most other “hallmarks of aging”. However, there are various manifestations of mitochondrial dysfunction in aging which raises two questions: what are the causes of the mitochondrial dysfunctions that are associated with aging, and what are the consequences, for health and longevity for each of the aging associated mitochondrial dysfunctions. As discussed in detail in this review one of the most frequently reported mitochondrial dysfunction in aging is the reduction of ∆Ψ
m that is observed in many types of aging cells; but the cause of this effect is still not established. It was suggested previously that this may be the result of oxidative damage to mitochondrial phospholipids such as cardiolipin [
48]. Such damage could increase proton permeability and thus reduced ∆Ψ
m [
15]. However, there is no evidence that mitochondria of old cells are uncoupled. Alternatively, it was suggested that the lower ∆Ψ
m in aging is a result of aging-specific metabolic state that impair bioenergetic functions and that this leads to disease and shorten lifespan [
23,
24,
25,
26,
27]. However, it was not specified what exactly is this metabolic state and how does aging leads to this state.
Here we offer a new interpretation: the lower ∆Ψ
m that is observed in aging cells results from the enhanced activation of the mPTP which increase the fraction of depolarized mitochondria. This interpretation is compatible with all the evidence reviewed here and is strongly supported by many of the reviewed studies. Several studies demonstrated that inhibition of mPTP restore ∆Ψ
m in aging cells (
Table 1). Most of the studies that reported aging-associated reduction of ∆Ψ
m were conducted with whole cells, tissues or even intact animals (e.g., worms). However, most of these studies employed high concentrations of fluorescent ∆Ψ
m indicators in which the fluorescence is in the quenching mode and the fluorescence is not proportional to the magnitude of ∆Ψ
m. Therefore, the fluorescence is only proportional to the number of mitochondria with significant ∆Ψ
m. Any reduction in probe fluorescence in aging cells, under these assay conditions, simply indicate loss of mitochondria with ∆Ψ
m either due to depolarization or elimination of depolarized mitochondria by mitophagy, both of which could be the result of mPTP activation. Only a few studies measured mitochondria density, with appropriate probe, and these suggest that aging is associated with both reduction of mitochondrial density and increase fraction of depolarized mitochondria. Many of the studies that reported lower ∆Ψ
m in aged cells also reported enhanced activation of mPTP (table 1), which support this interpretation of the results. Of all the studies of the reduction of ∆Ψ
m in whole aging cells, only the study of Morris et al 2020, that reported a 20% lower average ∆Ψ
m in aging Intestinal Stem Cells from Drosophila, used a valid protocol for ∆Ψ
m measurement in whole cells, with a low, non-quenching, TMRE concentration (20 nM), and were the fluorescence was collected directly from within the labeled mitochondria. However, Morris et al showed that the old Intestinal Stem Cells in Drosophila were in a very distinct metabolic state with largely inhibited mitochondrial calcium transporter, MCU, which could explain the lower ∆Ψ
m. This state was not reported in any other aging cells study and does not appear to represent a general feature of mitochondrial dysfunction in aging cells.
Several experiments with isolated mitochondria from tissues of aged animals reported small reduction (~10%) of average ∆Ψ
m. While the protocols of these experiments are more reliable, these results are also compatible with the suggestion that in aging cells there is a larger fraction of depolarized mitochondria. Whether this is the case or not can only be decided by measuring ∆Ψ
m of individual mitochondria (cf. [
43]). If there was really a significant reduction of average ∆Ψ
m in aging cells it is expected that there would be a reduction of mROS production and increased uncoupling, but this is not observed in aging cells – it is widely reported that mROS production is increased in aged cells and that the coupling is not impaired.
The conclusion that the reduction of ∆Ψ
m in aging cells results from mitochondrial depolarization by the enhanced activation of mPTP in aging cells provide a straightforward explanation to all the reported effects of ∆Ψ
m on aging. Enhanced activation of mPTP leads to excess release of mROS and destruction of NAD+ and this leads to many of the reported effects of aging (
Figure 1), to age-dependent degenerative diseases, and eventually to cell death by necrosis or apoptosis. On the other hand, a limited activation of mPTP, particularly at an early age, initiates protective mechanisms such as UPRmt, mitophagy and mitochondria biogenesis. Inhibition of mPTP activation by dietary restriction, drugs or artificially produced ∆Ψ
m, retards the process of aging, inhibits degenerative diseases, and increases longevity.