Submitted:
27 June 2023
Posted:
28 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Germplasm sourcing and field layout
2.2. Sample preparation
2.3. GC-MS identification and quantification of phytochemical constituents
3. Results
4. Discussion
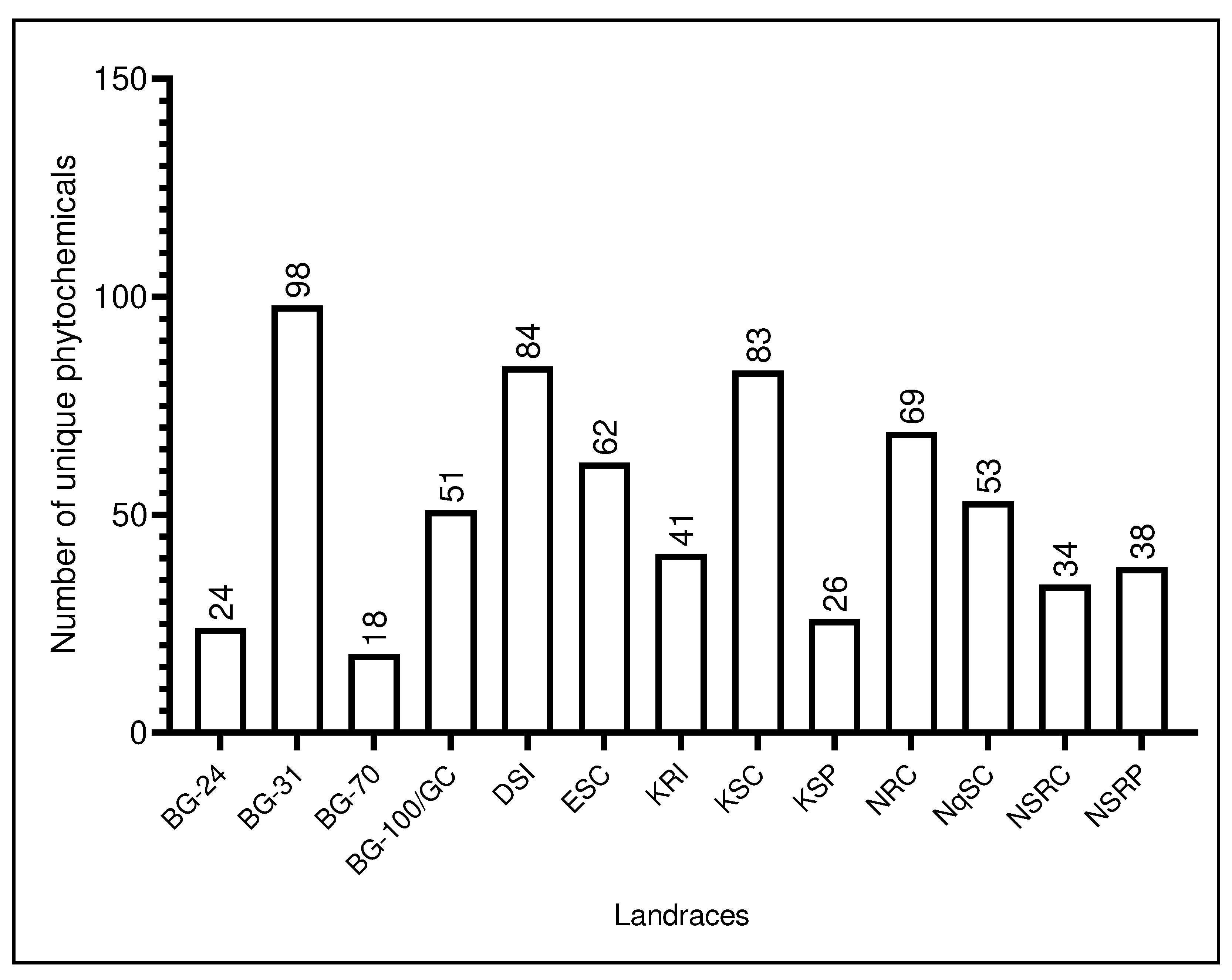
4.1. Phytochemicals unique to various L. siceraria landraces
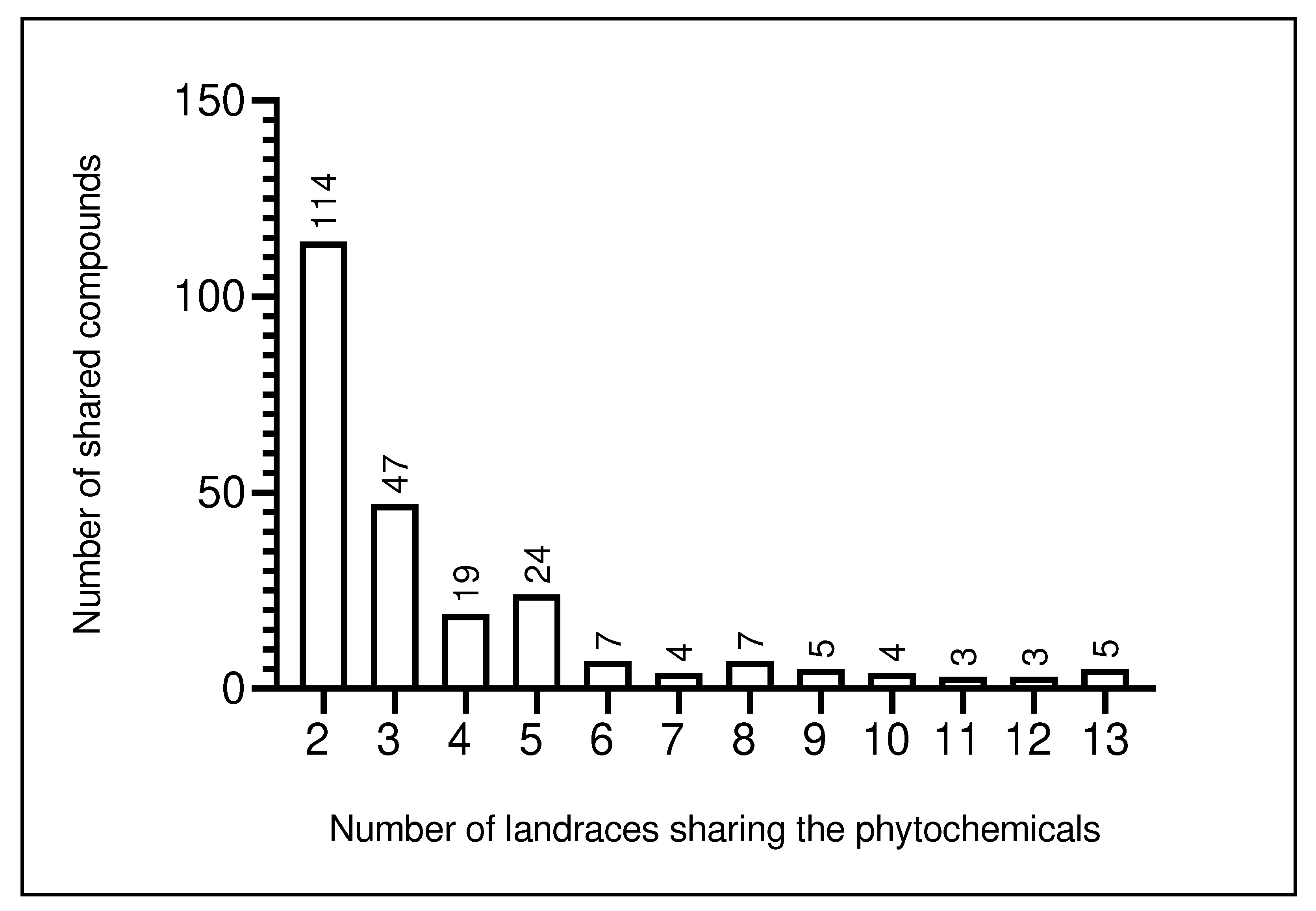
4.2. Phytochemicals shared among L. siceraria landraces
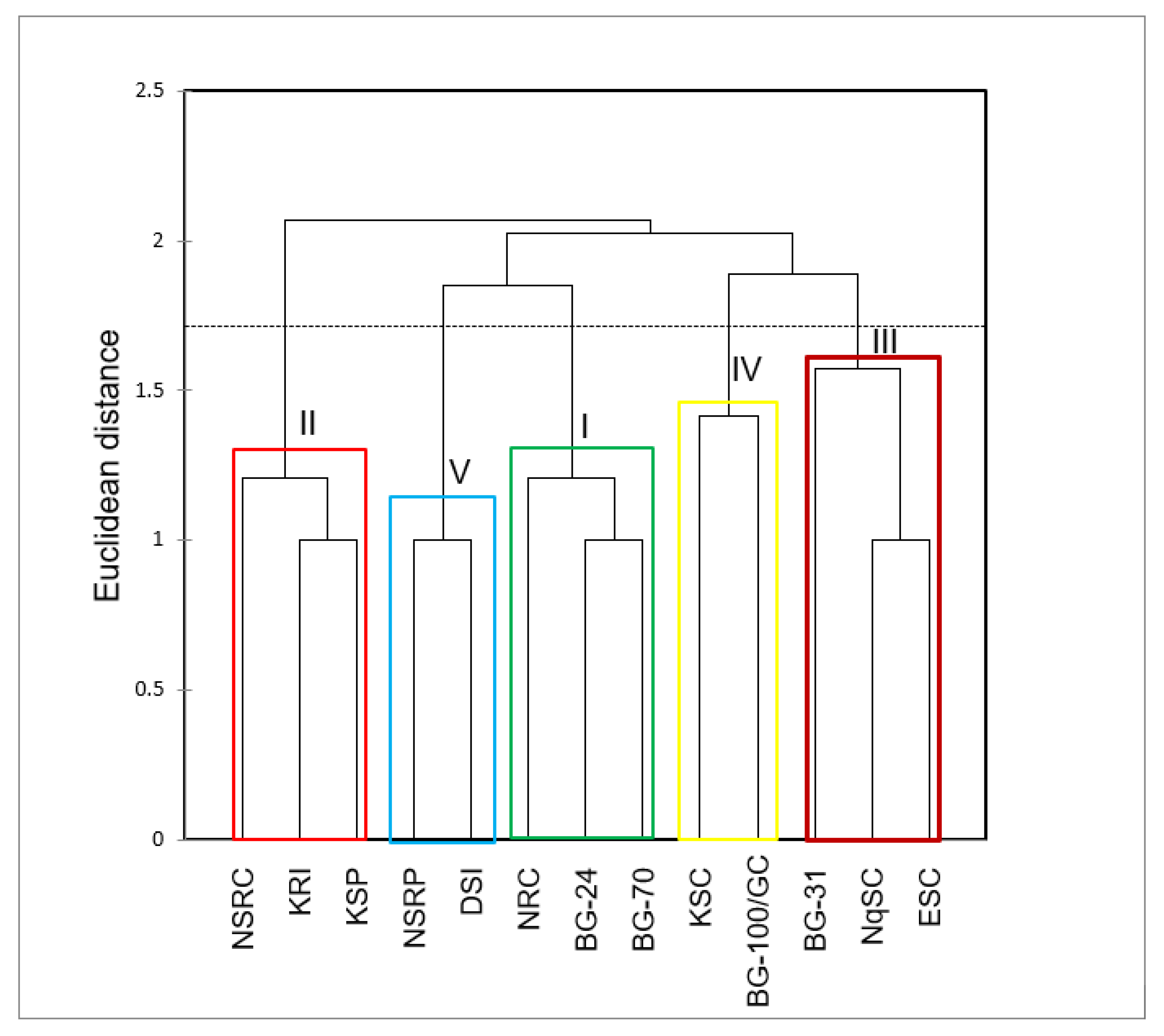
4.3. Agglomerative hierarchical cluster analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Saeed, M.; Khan, M.S.; Amir, K.; Bi, J.B.; Asif, M.; Madni, A.; Kamboh, A.A.; Manzoor, Z.; Younas, U.; Chao, S. Lagenaria siceraria fruit: A review of its phytochemistry, pharmacology, and promising traditional uses. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- de Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: current knowledge on food source, metabolism, and health effects. Crit. Rev. Food Sci. Nutr. 2021, 63, 1352–1389. [Google Scholar] [CrossRef]
- Chen, J.; Tan, J.; Duan, X.; Wang, Y.; Wen, J.; Li, W.; Li, Z.; Wang, G.; Xu, H. Plastidial engineering with coupled farnesyl diphosphate pool reconstitution and enhancement for sesquiterpene biosynthesis in tomato fruit. Metab. Eng. 2023, 77, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, J.; Najam-Us-Saqib, Q.; Anwar, T.; Khan, F.S.; Akram, M.; Munir, N.; Rebezov, M.; Shariati, M.A.; Thiruvengadam, M. Phytochemical Profile of Rock Jasmine (Androsace foliosa Duby ex Decne) by Using HPLC and GC–MS Analyses. Arab. J. Sci. Eng. 2021, 46, 5385–5392. [Google Scholar] [CrossRef]
- Nawade, B.; Yahyaa, M.; Reuveny, H.; Shaltiel-Harpaz, L.; Eisenbach, O.; Faigenboim, A.; Bar-Yaakov, I.; Holland, D.; Ibdah, M. Profiling of volatile terpenes from almond (Prunus dulcis) young fruits and characterization of seven terpene synthase genes. Plant Sci. 2019, 287, 110187. [Google Scholar] [CrossRef]
- A glimpse into the biosynthesis of Abdallah, I.I.; Quax, W.J. A glimpse into the biosynthesis of terpenoids. International conference on natural resources and life sciences. 2017, pp. 81–98. International conference on natural resources and life sciences. 2017, pp. 81–98. [CrossRef]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. 2021. [CrossRef]
- Deshpande, J.R.; A Choudhari, A.; Mishra, M.R.; Meghre, V.S.; Wadodkar, S.G.; Dorle, A.K. Beneficial effects of Lagenaria siceraria (Mol.) Standley fruit epicarp in animal models. Indian J. Exp. Biol. 2008, 46. [Google Scholar]
- Sosnowska, D.; Kajszczak, D.; Pods˛edek, A. The effect of different growth stages of black chokeberry fruits on phytonutrients, anti-lipase activity, and antioxidant capacity. Molecules. 2022, 27, 8031. [Google Scholar] [CrossRef]
- Ahmed, D.; Ejaz, N.; Saeed, R.; Dar, P. Cooking Effect on Anti-oxidative and Alpha-amylase Inhibitory Potential of Aqueous Extract of Lagenaria siceraria Fruit and its Nutritional Properties. Free. Radicals Antioxidants 2016, 6, 44–50. [Google Scholar] [CrossRef]
- Roopan, S.M.; Rajeswari, V.D.; Kalpana, V.N.; Elango, G. Biotechnology and pharmacological evaluation of Indian vegetable crop Lagenaria siceraria: an overview. Appl. Microbiol. Biotechnol. 2015, 100, 1153–1162. [Google Scholar] [CrossRef]
- Ileola, A.O.; Omodara, T.R.; Filua, O.D. Comparative Studies on the Effect of Boiling and Fermentation on Calabash Gourd Melon (Lagenaria siceraria) Seeds. Asian Food Sci. J. 2019, 1–12. [Google Scholar] [CrossRef]
- Wang, C.; Cao, J.; Hao, N.; Wu, T. Genetic and Molecular Regulation Mechanisms in the Formation and Development of Vegetable Fruit Shape. Appl. Sci. 2022, 12, 1514. [Google Scholar] [CrossRef]
- El-Rahman, A.A.; Mahmoud, A.Z.A.; Sayed, A.A.; El Latif, M.A.A. Physiochemical properties and phytochemical characteristics of bottle gourd (Lagenaria siceraria) seed oil. Egypt. J. Chem. 2021. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Odindo, A. Genetic diversity of bottle gourd (Lagenaria siceraria(Molina) Standl.) landraces of South Africa assessed by morphological traits and simple sequence repeat markers. South Afr. J. Plant Soil 2015, 33, 113–124. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.O.; Shimelis, H.A.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Drought tolerance of selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol. Biochem. 2017, 120, 75–87. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Ngwepe, R.M. Genetic resources of bottle gourd (Lagenaria siceraria (Molina) Standl.] and citron watermelon (Citrullus lanatus var. citroides (L.H. Bailey) Mansf. ex Greb.)- implications for genetic improvement, product development and commercialization: A review. South Afr. J. Bot. 2021, 145, 28–47. [Google Scholar] [CrossRef]
- Mkhize, P.; Mashilo, J.; Shimelis, H. Progress on Genetic Improvement and Analysis of Bottle Gourd [Lagenaria siceraria (Molina) Standl.] for Agronomic Traits, Nutrient Compositions, and Stress Tolerance: A Review. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Ntuli, NR.; Zobolo, AM. Effect of water stress on growth of colchicine induced polyploidy Coccinia palmata and Lagenaria sphaerica plants. Afr. J. Biotechnol. 2008, 7, 3548–3652. [Google Scholar]
- Nelson, LE.; Khama, OR.; Cedric, K. Improvement on grass establishment at a quarry rehabilitation site influenced by poultry manure in the subtropical South Africa. Afr. Crop Sci. J. 2014, 2, 98–107. [Google Scholar]
- 22. Naidoo, SIM.; Laurie, SM.; Odeny, DA.; Vorster, BJ.; Mphela, WM.; Greyling, MM.; Crampton, BG. Genetic analysis of yield and flesh colour in sweet potato. Afr. Crop Sci. J. 2016, 24, pp. 61–73. http://dx.doi.org/10.4314/acsj.v24i1.5.
- Buthelezi, L.G.; Mavengahama, S.; Sibiya, J.; Ntuli, N.R. Variation in Shoot, Peduncle and Fruit Growth of Lagenaria siceraria Landraces. Plants 2023, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Abishkar, K.; Pragya, A. Effect of various organic fertilizer on seedling health and vigour of different varieties of cucumber in rautahat condition. Malays. J. Sustain. Agric. 2020, 4, 81–85. [Google Scholar] [CrossRef]
- Nwiloh, B.I.; Uwakwe, A.A.; Akaninwor, J.O. Phytochemical screening and GC-FID analysis of ethanol extract of root bark of Salacia nitida L. Benth. J. Med. Plants Stud. 2016, 4, 283–287. [Google Scholar]
- Sabatino, L.; D'Anna, F.; Iapichino, G.; Moncada, A.; D'Anna, E.; De Pasquale, C. Interactive Effects of Genotype and Molybdenum Supply on Yield and Overall Fruit Quality of Tomato. Front. Plant Sci. 2019, 9, 1922. [Google Scholar] [CrossRef]
- Grimalt, M.; Sánchez-Rodríguez, L.; Hernández, F.; Legua, P.; Carbonell-Barrachina. A.; Almansa, M.S.; Amorós, A. Volatile Profile in Different Aerial Parts of Two Caper Cultivars (Capparis spinosa L.). J. Food Qual. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- 28. Taati, S.; Pilehvar, B.; Mirazadi, Z. Essential oil, phenol and flavonoid contents in leaves and fruits of Prunus scoparia (Spach) C.K. Schneid. populations. J. medicinal plants by- products. 2022, 2, pp. 201–209. [CrossRef]
- Sovia, E.; Anggraeny, D. Sugar Palm Fruits (Arenga pinnata) as Potential Analgesics and Anti-Inflammatory Agent. Mol. Cell. Biomed. Sci. 2019, 3, 107–14. [Google Scholar] [CrossRef]
- Drafor, G.; Duah, E.; Ankamah, N.A.; Kpene, G.E.; Mante, P.K. Investigating the Anticonvulsant Properties of Aqueous Ethanolic Extracts of the Leaves, Roots, and Fruits of Jatropha gossypifolia L. (Euphorbiaceae). Adv. Pharmacol. Pharm. Sci. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Asghar, A.; Aamir, M.N.; Sheikh, F.A.; Ahmad, N.; Elsherif, M.A.; Bukhari, S.N.A. Co-Combination of Pregabalin and Withania coagulans-Extract-Loaded Topical Gel Alleviates Allodynia and Hyperalgesia in the Chronic Sciatic Nerve Constriction Injury for Neuropathic Pain in Animal Model. Molecules 2022, 27, 4433. [Google Scholar] [CrossRef]
- Sithole, N.; Modi, A.T. Responses of selected bottle gourd [Lagenaria siceraria(Molina Standly)] landraces to water stress. Acta Agric. Scand. Sect. B — Soil Plant Sci. 2015, 65, 350–356. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, Y.; Huang, B.; Hu, X.; Tang, Y.; Xu, X.; Wu, M.; Gong, Z.; Luo, Y.; Gong, M.; et al. Control of fruit softening and Ascorbic acid accumulation by manipulation of SlIMP3 in tomato. Plant Biotechnol. J. 2022, 20, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Nawade, B.; Yahyaa, M.; Reuveny, H.; Shaltiel-Harpaz, L.; Eisenbach, O.; Faigenboim, A.; Bar-Yaakov, I.; Holland, D.; Ibdah, M. Profiling of volatile terpenes from almond (Prunus dulcis) young fruits and characterization of seven terpene synthase genes. Plant Sci. 2019, 287, 110187. [Google Scholar] [CrossRef] [PubMed]
- Hodgkison, R.; Ayasse, M.; Häberlein, C.; Schulz, S.; Zubaid, A.; Mustapha, W.A.W.; Kunz, T.H.; Kalko, E.K.V. Fruit bats and bat fruits: the evolution of fruit scent in relation to the foraging behaviour of bats in the New and Old World tropics. Funct. Ecol. 2013, 27, 1075–1084. [Google Scholar] [CrossRef]
- Al-Bahadily, D.H.C.; Shari, F.H.; Najm, M.A.A.; Al-Salman, H.N.K. Antimicrobial activity of the compound 2-Piperidinone, N-[4-Bromo-n-butyl]- extracted from pomegranate peels. Asian J. Pharm. 2019, 13, 46–53. [Google Scholar] [CrossRef]
- Madhavan, S.A.; Priyadharshini, R.; Sripriya, R. In-vitro pharmacological and Gc-Ms analysis of bioactive compounds presents in Bytteneria herbaceae. Zool. Entomol. Lett. 2021, 1, pp. 20–26 https://wwwzoologicaljournalcom/. [Google Scholar]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Tapfuma, K.I.; Nchabeleng, E.K.; Adebo, O.A.; Hussan, R.; Williams, R.D.; Ravuluvulu, A.B.; Ndinteh, D.T.; Gan, R.-Y.; Habimana, O.; Niemann, N.; et al. Antibacterial activity and gas chromatography mass spectrometry (GC–MS)-based metabolite profiles of Celtis africana and its endophytic extracts. Ind. Crop. Prod. 2020, 157, 112933. [Google Scholar] [CrossRef]
- Chen, J.; Tan, J.; Duan, X.; Wang, Y.; Wen, J.; Li, W.; Li, Z.; Wang, G.; Xu, H. Plastidial engineering with coupled farnesyl diphosphate pool reconstitution and enhancement for sesquiterpene biosynthesis in tomato fruit. Metab. Eng. 2023, 77, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, H.; Ahmed, D.; Qamar, M.T. Study of Phytochemicals ofMelilotus indicusand Alpha-Amylase and Lipase Inhibitory Activities of Its Methanolic Extract and Fractions in Different Solvents. ChemistrySelect 2019, 4, 7679–7685. [Google Scholar] [CrossRef]
- Rasool, F.; Sharma, D.; Anand, P.S.; Magani, S.; Tantravahi, S. Evaluation of the Anticancer Properties of Geranyl Isovalerate, an Active Ingredient of Argyreia nervosa Extract in Colorectal Cancer Cells. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A.; Thukral, A.K.; Bhardwaj, R. Amino acid profiling of the leaves of plants in the vicinity of river Beas, India. J. Chem. Pharm. 2015, 7, 504–510. [Google Scholar]
- Hasan, M.; Rima, R. Genetic engineering to improve essential and conditionally essential amino acids in maize: transporter engineering as a reference. Transgenic Res. 2021, 30, 207–220. [Google Scholar] [CrossRef]
- Odia, A.; Esezobor, O.Z. Therapeutic Uses of Amino Acids. 2017. [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Hampel, D.; Swatski, A.; Mosandl, A.; Wüst, M. Biosynthesis of Monoterpenes and Norisoprenoids in Raspberry Fruits (Rubus idaeus L.): The Role of Cytosolic Mevalonate and Plastidial Methylerythritol Phosphate Pathway. J. Agric. Food Chem. 2007, 55, 9296–9304. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Castillo, S.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest Treatment with Oxalic Acid Improves Postharvest Storage of Lemon Fruit by Stimulation of the Antioxidant System and Phenolic Content. Antioxidants 2021, 10, 963. [Google Scholar] [CrossRef]
- Suzuki, J.; Ootaka, A.; Onoue, S.; Onoue, M. Synthesis and acaricidal activity of phenylpiperazine derivatives. J. Pestic. Sci. 2021, 46, 189–197. [Google Scholar] [CrossRef]
- Maamoun, A.A.; El-Akkad, R.H.; Farag, M.A. Mapping metabolome changes in Luffa aegyptiaca Mill fruits at different maturation stages via MS-based metabolomics and chemometrics. J. Adv. Res. 2019, 29, 179–189. [Google Scholar] [CrossRef] [PubMed]
| Prov | LR |
Area | Fruit color | Fruit texture | Fruit shape | Seed type | Seed color | Seed texture | Seed size | Seed line | Seed shape |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KZN | KSP | Khangelani | Pale green | Smooth | Pear | Asiatica | Brown | Leathery | Large | Present | Slightly oblong to rectangular |
| KZN | KSC | Khangelani | Pale green | Smooth | Curvilinear | Asiatica | Brown | Leathery | Large | Present | Slightly oblong to rectangular |
| KZN | KRI | Khangelani | Green | Rough | Isodiametric | Siceraria | Dark brown | Leathery | Large | Present | Slightly oblong to rectangular |
| KZN | NRC | Ndumo | Dark green | Rough | Cylindrical | Siceraria | Creamy brown | Smooth | Small | Absent | Oblong |
| KZN | NSRC | Nquthu | Green | Semi-rough | Curvilinear | Intermediate | Brown | Leathery | Medium | Present | Slightly oblong |
| KZN | NSRP | Nquthu | Pale green | Semi-rough | Pear | Intermediate | Brown | Leathery | Medium | Present | Slightly oblong |
| KZN | NqSC | Nquthu | Pale green | Smooth | Semi-Curvilinear | Asiatica | Light brown | Leathery | Medium | Present | Slightly oblong |
| KZN | DSI | Dundee | Dark green | Smooth | Isodiametric | Siceraria | Dark brown | Smooth | Large | Present | Oblong |
| KZN | ESC | Emkhandlwini | Pale green | Smooth | Curvilinear | Asiatica | Light brown | Leathery | Medium | Present | Slightly oblong |
| LP | BG-24 | Go-Phasa | Pale green | Corrugate | Cavate | Siceraria | Dark brown | Smooth | Small | Absent | Oblong |
| LP | BG-31 | Kgohloane | Dark green | Smooth | Cavate | Intermediate | Brown | Leathery | Large | Present | Slightly oblong to rectangular |
| LP | BG-70 | Go-Phasa | Pale green | Smooth | Pyriform | Asiatica | Light brown | Leathery | Large | Present | Slightly oblong to rectangular |
| LP | BG-100/GC | Kgohloane | Pale green | Semi-rough | Cylindrical | Asiatica | Light brown | Leathery | Medium | Present | Slightly oblong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





