1. Introduction
Multimorbidity is the coexistence of multiple health conditions potentially aggravating each other. A systematic review and meta-analysis of 126 studies showed that the global prevalence of multimorbidity is as high as 37.2%, and over half (51.0%) of worldwide population aged 60 years and older have multimorbid conditions [
1]. The prevalence of polypharmacy among patients of secondary-level hospital reaches 98%, with 5.1% having minor polypharmacy (two to three medications), 10% having moderate polypharmacy (four to five medications), and 83% having major polypharmacy (more than five medications) [
2]. Up to 17% of older adults in Germany have at least one potential drug-drug interaction (DDI) [
3]. Over half of nursing home residents (52.7%) are exposed to at least 1 DDI and 25.0% to more than one DDI [
4]. Prevalence of DDIs in palliative care ranges from 31 to 75% across various health care settings [
5]. In COVID-19 patients administered with ritonavir-containing therapy in the U.S., the weighted prevalence of major to contraindicated potential DDIs was 29.3%. Prevalence rates of DDIs among those 60 years and older with serious heart conditions, diabetes, and moderate chronic kidney disease were 60.2%, 63.4%, and 80.7%, respectively [
6].
Geriatric syndromes co-exist with acquired chronic diseases and contribute to multimorbidity. Multimorbidity predisposes a person to interactions between drugs administered for treatment of involved pathologies so that the resulting risk exceeds a simple summation of risks. Drug-drug interactions may lead to adverse drug reactions (ADRs), and medical error was reported to be the third leading cause of death in the U.S. [
7]. Polypharmacy is associated with increased emergency department transfer in older long-term care residents, with the strength of association increasing with the number of medications prescribed [
8].
One of the challenges facing healthcare today is the need of interdisciplinary team-based approach to management of patients with multiple health conditions. It is essential for a cardiologist to be aware of therapies their patients are prescribed by other medical specialists (neurologists, endocrinologists, rheumatologists, ophthalmologists, etc.) after onset of long-term treatment for cardiovascular disease. Another challenge is a lack of information on overall burden of DDIs in cardiovascular patients receiving medical help for multiple morbidities including diabetes, inflammatory conditions, etc. from multiple independent healthcare providers.
The present study aimed to assess the patterns of DDIs and polypharmacy in older patients with cardiovascular diseases based on electronic health records (EHRs) stored in health information system in 2019–2022.
2. Materials and Methods
2.1. Ethics
Observational cross-sectional analytical study was performed in accordance with the standards of Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the local Biomedical Ethics Committee (approval #230 from June 28, 2022) and registered at ClinicalTrials.gov (Identifier NCT05336565).
2.2. Inclusion Criteria
Inclusion criteria were the established diagnosis of cardiovascular disease, age of 75 years and older, and the presence of EHR in the regional health information system.
2.3. Sample Size Calculation
We assumed the prevalence of potentially serious DDIs in our cohort during the pandemic in a range from 17% to 81% [
3,
4,
5,
6]. We considered the acceptable margin of error of 5%, confidence level of 95%, and approximate population size of 20,000 with the latter roughly the third of population aged 75 years and older in the region. Taking into account assumed 18%-response distribution, we calculated sufficient sample size of 225 as follows: the sample size
n and margin of error E are given by
where N is the population size, r is the fraction of responses that we are interested in, and Z×(c/100) is the critical value for the confidence level c. Taking into account that the number of analyzed records (n = 704) exceeded the calculated sample size, we considered the sample size sufficient.
2.4. Sample Characteristics
The EHRs were obtained from the health information system implemented in 24 health care institutions of Tomsk and Tomsk Region. The EHRs covered period from 2019 to 2022. Probability serial nested sampling method was used for patient selection. Cardiovascular diagnosis of patients was established and/or verified by cardiologist. Unstructured text of 704 EHRs was analyzed. Patient sex was identified based on patient ID document presented for establishing the EHR.
2.5. Medication Lists and Sublists
The analyzed EHRs contained unstructured textual information on both medications taken by patients and medications prescribed to patients during medical care encounters. Two large medication lists were established, namely: prescribed medication list (P-List) and taken medication list (T-List) with 'P' and 'T' standing for prescribed and taken medications. These lists comprised medications taken by or prescribed to the entire study cohort to assess DDIs and polypharmacy burden at the population level. During the further analysis, the sublists of taken and prescribed medications were established based on patient sex and primary ICD class associated with medical care encounter. Combinations of taken or prescribed drugs associated with a given individual medical care encounters were also analyzed.
2.6. Polypharmacy, DDIs, and DDI Index
Prevalence rates of DDIs and polypharmacy were expressed as percentages. The use of five medications or more was considered polypharmacy. Pairwise DDIs were identified and classified into contraindicated, serious, requiring close monitoring, and minor using Medscape Drug Interaction Checker [
9].
Considering that individual records documented multiple DDIs classified into four different categories, we developed DDI index by introducing the following coefficients corresponding to drug impact categories: 1 (minor), 2 (monitor-closely), 3 (serious), and 4 (contraindicated). DDI index was calculated as the sum of relevant coefficients multiplied by number of corresponding DDIs as follows:
2.7. Statistics
Statistical processing of data was done using Microsoft Excel 2010 and STATISTICA 10 software. Figures were created using Microsoft Excel 2010, STATISTICA 10, and Adobe Illustrator. Normality of distribution of variables was checked by the Kolmogorov–Smirnov test and the Shapiro–Wilk test. Data are presented as percentages, absolute numbers, mean ± standard deviation, and median and interquartile range where appropriate. Significance of differences between non-normally distributed variables was assessed by Mann-Whitney U test. Significance of differences between normally distributed variables was assessed by Student’s t-test. Categorical variables were compared by Chi-Square test using 2 × 2 contingency tables. Values were considered statistically significant when p was < 0.05.
3. Results
3.1. EHRs
Out of 704 EHRs analyzed, 38.1% of records belonged to men, and 61.9% of records belonged to women. The records were created during ambulatory patient visits (n = 458), home visits by primary care physicians (n = 118), patient stays in hospital wards (n = 18), and hospital discharge procedures (discharge epicrisis records, n = 103) from January, 2019 to August, 2022. Information on prescribed drugs was present in 92.9% of EHRs; 51.7% of EHRs contained detailed information on drugs taken by patients.
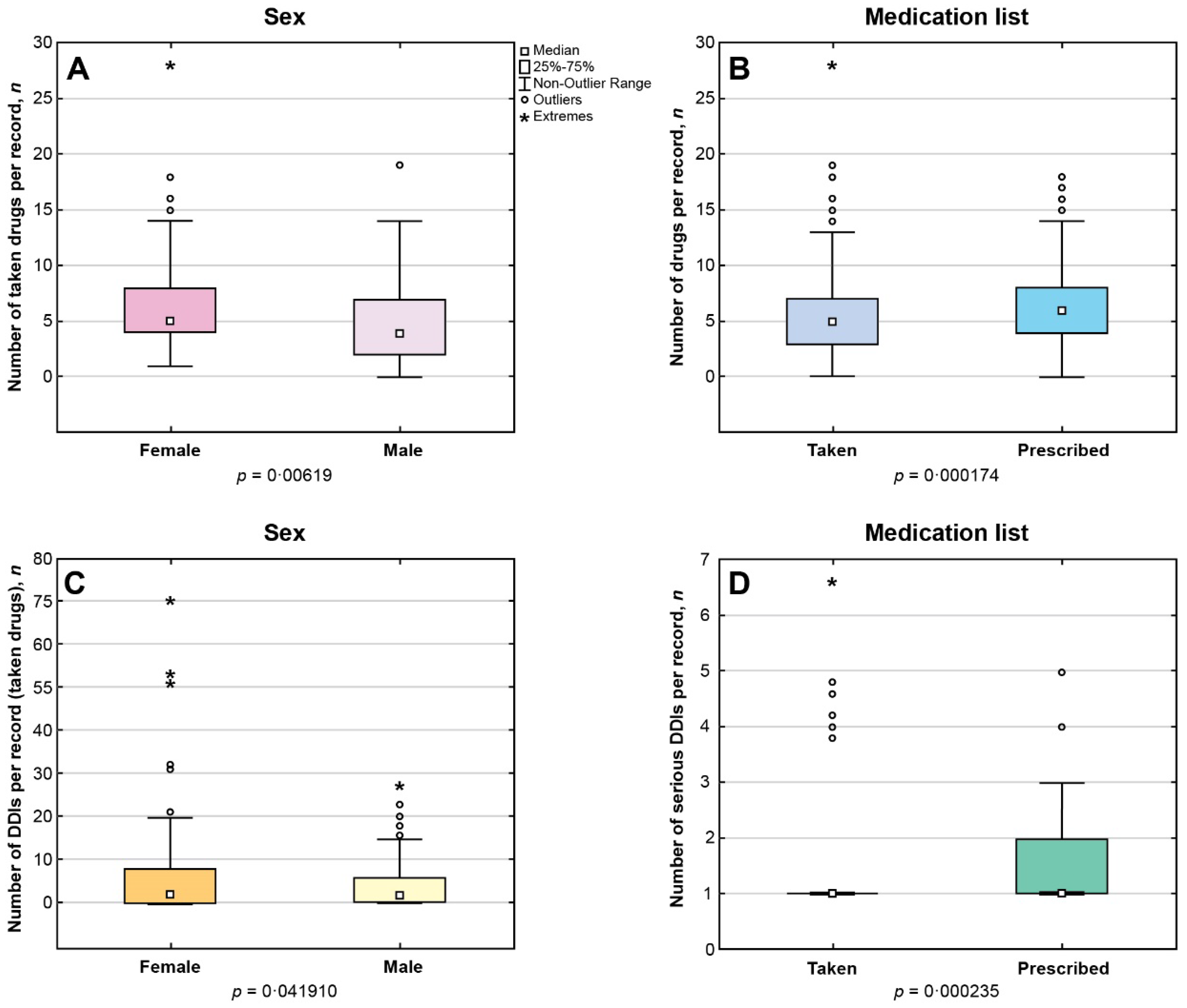
Among EHRs with documented information on pharmacotherapy, number of medications per record ranged from 1 to 28 for the taken drugs (Me = 5, IQR 3-7; n = 361) and from 1 to 18 for the prescribed drugs (Me = 6, IQR 4-8; n = 651),
p < 0.05. Female patients were taking significantly more drugs than men (
p < 0.05) (
Figure 1A). The number of medications per record in the P-List significantly exceeded the corresponding number in the T-List (
p < 0.05) (
Figure 1B).
Polypharmacy
In case of polypharmacy, median number of drugs prescribed to patient per record did not significantly differ from the median number of medications reported as 'taken': Me = 7, IQR 5-9 versus Me = 7, IQR 6-9 (p > 0.05). However, the prevalence of polypharmacy was significantly higher in the list of prescribed medications than in the list of taken medications (p < 0.05).
Polypharmacy occurred significantly more often in women than in men taking medications (p < 0.05). However, no sex-related differences were found in the rates of polypharmacy in the list of prescribed medications.
3.3. DDIs
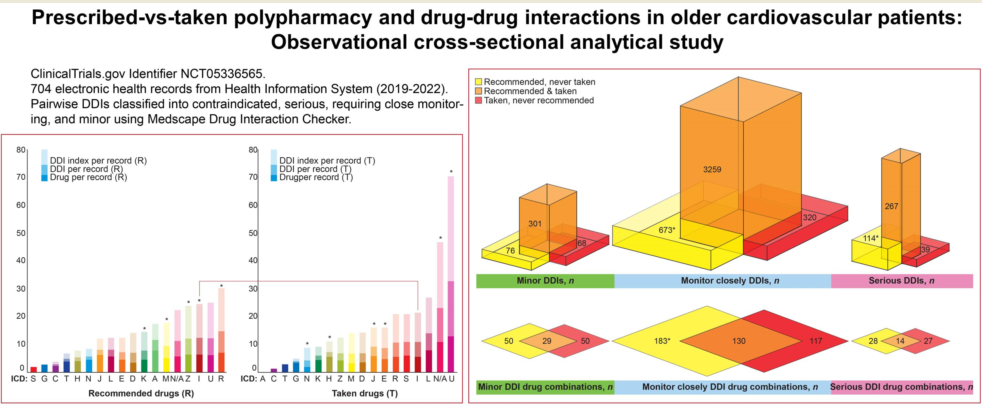
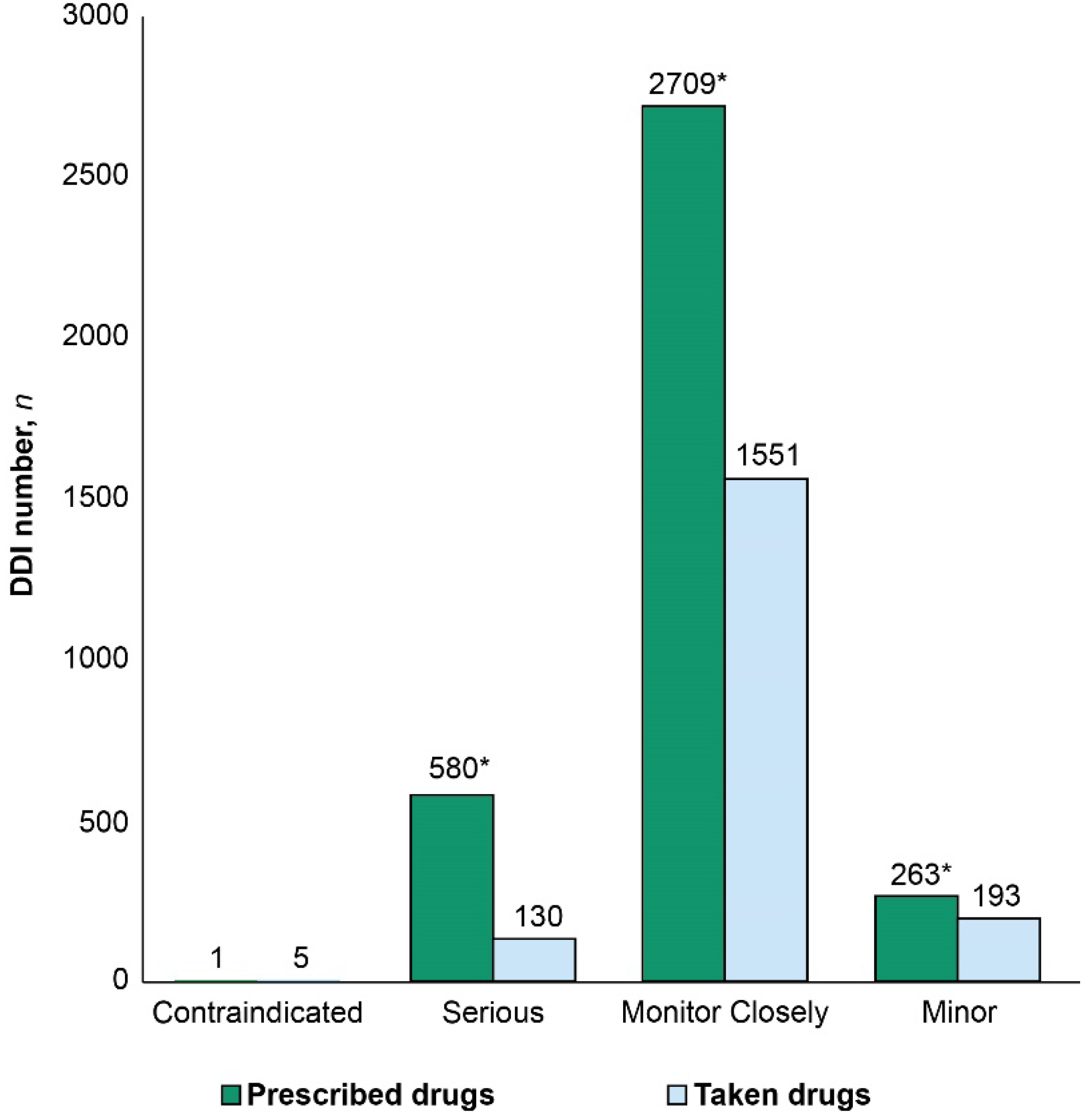
Number of DDIs per record ranged from 0 to 70 and from 0 to 39 for the taken and prescribed medications, respectively. The prevalence rates of DDIs were 73.5% and 68.5% in the T- and P-Lists, respectively. However, serious DDIs comprised 16% of drug interactions in the P-List and 7% of DDIs in the T-List (
p < 0.05). Median DDI numbers per record in the T- and P-Lists were Me = 2, IQR 0-7 versus Me = 3, IQR 0-7, respectively. Total number of DDIs was significantly higher in the P-List compared with the T-List (
p < 0.05) (
Figure 2).
In the T-List, we identified 365 pairwise drug combinations associated with DDIs, and the total number of DDI occurrences due to these combinations reached 1879. Among these, 249 drug combinations were associated with DDIs requiring close monitoring (n = 1551); 73 drug combinations were associated with minor DDIs (n = 193); 41 combinations were associated with serious DDIs (n = 130); and only two drug combinations were associated with contraindicated DDIs (n = 5).
In the P-List, we identified 439 drug combinations associated with DDIs, and these drug combinations resulted in more than seven-fold number of pairwise drug interactions (
n = 3261). Among these, 317 drug combinations were associated with DDIs requiring close monitoring (
n = 2709); 79 combinations were associated with minor DDIs (
n = 261); 42 combinations were associated with serious DDIs (
n = 290); and one combination was associated with contraindicated DDIs (
n = 2). Female sex was associated with a significantly higher median number of DDIs between taken drugs per record compared with male sex (
p < 0.05) (
Figure 1C). Significantly higher number of serious DDIs was identified in the P-List versus the T-List (
p < 0.05) (
Figure 1D).
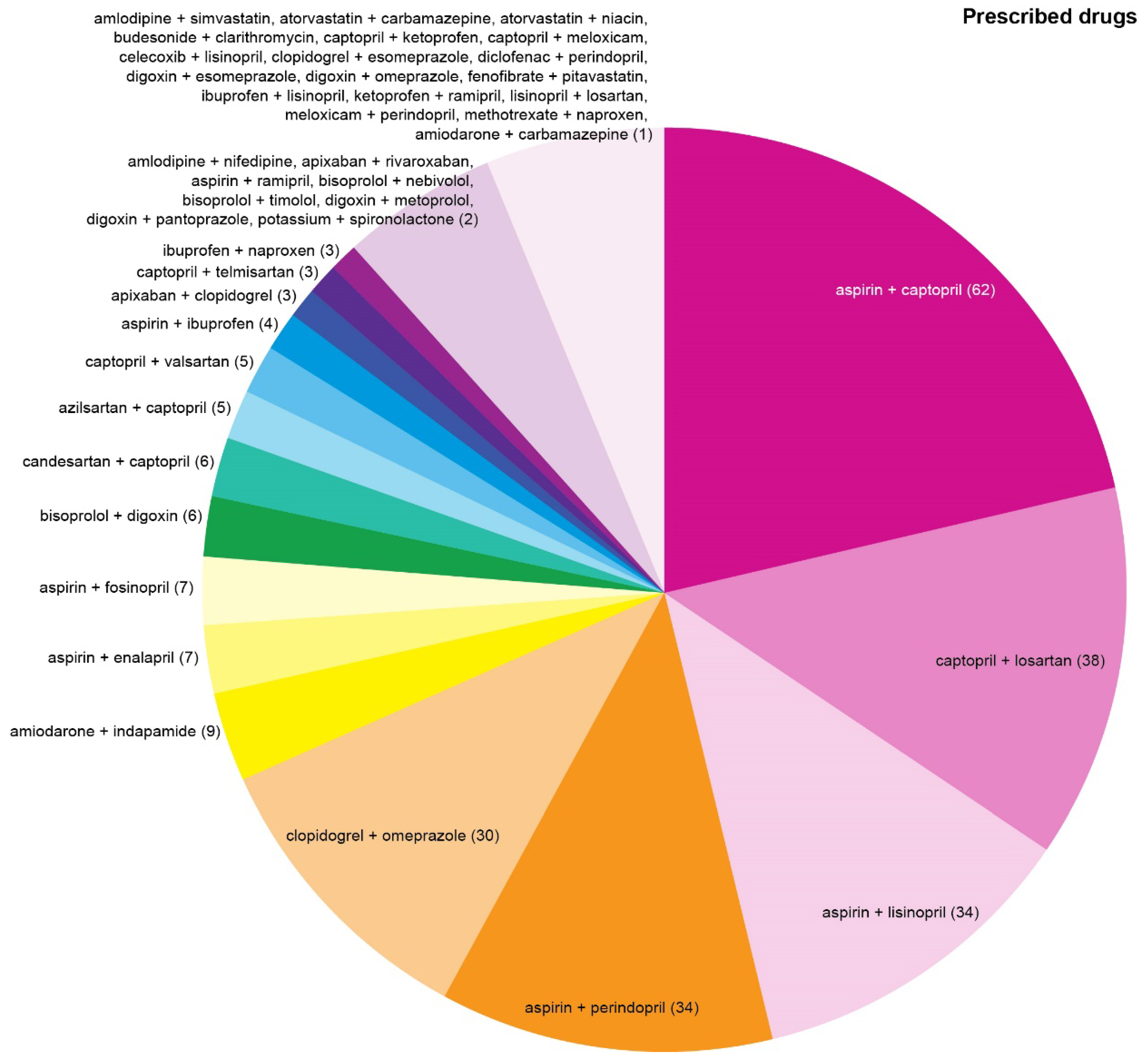
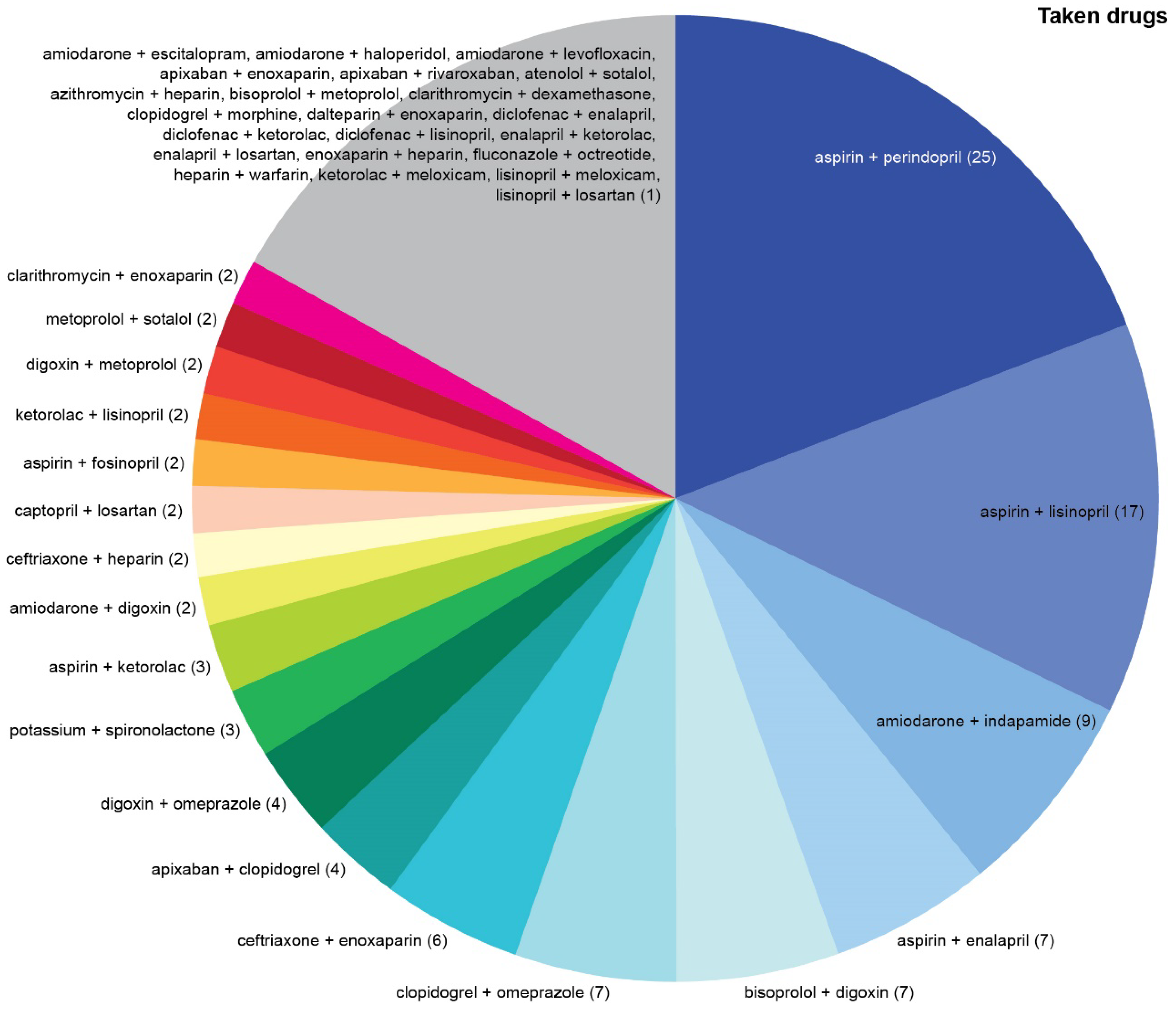
Top three most common drug combinations associated with serious/dangerous DDIs were aspirin + captopril, captopril + losartan, and aspirin + lisinopril in the P-List (
Figure 3) and aspirin + perindopril, aspirin + lisinopril, and amiodarone + indapamide in the T-List (
Figure 4).
Top three most common drug combinations associated with DDIs requiring close monitoring were aspirin + bisoprolol, aspirin + losartan, and aspirin + metoprolol in the P-List and aspirin + losartan, aspirin + bisoprolol, and bisoprolol + losartan in the T-List.
Nine drugs (digoxin, amiodarone, enalapril, metoprolol, enoxaparin, ceftriaxone, ketorolac, heparin, and sotalol) were associated with significantly higher DDI numbers in the T-List compared with the P-List (p < 0.05).
Only captopril and losartan were associated with significantly higher DDI numbers in the P-List relative to those in the T-List (p < 0.05), but the abundance of these DDIs contributed to significantly higher overall DDI burden among prescribed drugs.
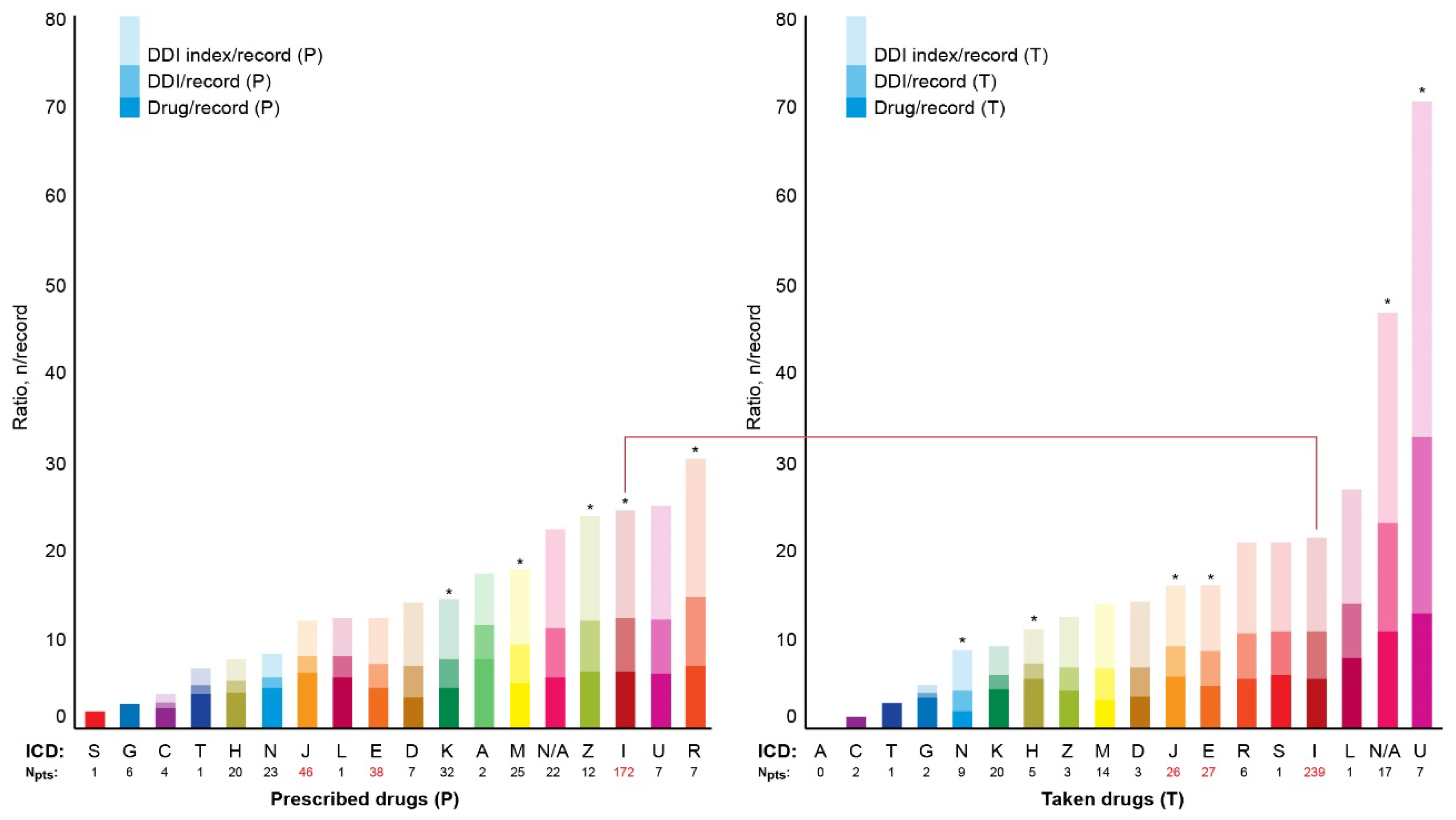
3.4. DDI index
Figure 5 shows median numbers of prescribed and taken drugs and the corresponding values of DDIs and DDI indexes per record depending on ICD class. Records without specified ICD class were marked 'N/A'. The DDI indexes ranged from 0 to 138. Top five DDI indexes were observed in the ICD classes R, U, I, Z, and N/A in the P-List and U, N/A, L, I, and S in the T-List (
Figure 5). The ICD class U was represented by code U07.1 (COVID-19, virus identified) in both the P-List and the T-List. The DDI indexes in the N/A category were top two in the P-List and top five in the T-List (
Figure 5).
4. Discussion
In our study, two primary drug lists (P-List and T-List with 'P' and 'T' standing for prescribed and taken medications) were established and analyzed to assess the prescription and intake patterns of medications documented in the electronic health records in the cohort of older cardiovascular patients. A sublist analysis allowed to assess the patterns of DDIs and polypragmasy at the group-based and individual levels.
There are many medical decision support systems available to assess potential DDIs while prescribing pharmacotherapy [
10,
11,
12,
13,
14]. Each of these systems has its advantages and disadvantages, and several systems may be used for in-depth assessment of a limited number of DDIs. We selected a single medical decision support system for DDI assessment. Medscape Drug Interaction Checker [
9] was chosen among other medical decision support systems because (i) it allowed to stratify the DDIs into four classes; (ii) it was user-friendly to operate; (iii) it provided information on underlying mechanisms of DDIs; and (iv) it was verified to be useful in assessing DDIs in cardiovascular and comorbid patients before [
15,
16,
17,
18,
19].
We analyzed DDIs on a pairwise basis because there are currently no commonly recognized resources allowing to assess higher-order DDIs though such techniques emerge and seem promising [
20]. Pairwise DDI identification allowed to provide the straightforward and comprehensible illustrations contributing to better understanding of DDI patterns. In our study, median DDI number per record often exceeded the corresponding number of drug combinations because pharmacokinetics and pharmacodynamics of one pairwise drug combination often involves more than one biotransformation pathway.
We developed easy-to-calculate DDI index to, at least partially, take into consideration the differential impact of DDI classes ranging from contraindicated to minor. More sophisticated scales may be developed by integrating quantitative systems pharmacology analysis with physiologically-based pharmacokinetic models. Multiscale modeling may predict potential pharmacodynamic DDIs, and, via clinical trial simulations, create testable hypotheses as to their potential clinical significance [
21]. It is essential to develop clinical decision support systems for data-driven prediction of ADRs triggered by DDIs [
22,
23]. However, it seems challenging to adequately measure the overlapping impact of DDIs, which is multifactorial and depends on genetic factors, ADR manifestation, and economic burden.
The frequency of occurrence of serious DDIs in our study was significantly higher in case of prescribed medications compared with that among taken drugs. The highest median DDI index in our study was observed in patients with COVID-19. Notably, in the study by [
31], clinically significant DDIs associated with comorbidities and polypharmacy among patients with COVID-19 were recorded more often upon admission and to a lesser degree during hospitalization suggesting that efforts of a medical team can successfully reduce risks associated with dangerous DDIs. It is essential that serious DDIs can hinder treatment response and complicate hospitalization in COVID-19 patients [
31].
Female patients in our study were taking significantly more drugs than men, and polypharmacy significantly more often occurred in women taking medications compared with men. The prevalence of polypharmacy was higher in case of prescribed than taken medications. Significant sex-related differences were observed in the polypharmacy rates, median number of drugs per record, and median number of DDIs per record in case of taken medications, and women scored over in all these parameters. No sex-related differences were found among prescribed medications. Female dominance in older age groups did not affect the observed sex-related differences in pharmacotherapy.
Sex-related differences found in our study agree with data of the large-scale analysis showing that women have a 60% increased risk of DDI and a 90% increased risk of DDI leading to major ADR as compared to men [
23]. Female sex and older age also contribute to non-adherence, in particular, to statins [
24]. We agree that potential effects of sex and gender on inappropriate prescribing and deprescribing remain poorly understood [
25]. Cognitive, behavioral, pharmacokinetic, and pharmacodynamic factors of adaptation underlying significantly higher scores in drug numbers, polypharmacy rates, and DDIs in women require further research.
Our study showed significant differences in the median numbers of serious DDIs per record (i.e. per single medical care encounter) between the prescribed and taken medications. The mismatch of serious, requiring close monitoring, and minor DDIs was also found between the large cohort-based lists of prescribed and taken medications. This observation may indirectly suggest suboptimal treatment compliance and/or non-adherence of patients to prescribed therapy. Considering significantly higher burden of serious DDIs among prescribed medications, the observed difference may be a sign of patient adaptation protecting them from exposure to serious DDIs.
Among the most commonly prescribed drug combinations associated with serious DDIs, the pair of 'aspirin + captopril' may be considered insignificant due to the use of low-dose aspirin in the majority of cases. Besides, captopril was often prescribed to be taken episodically when blood pressure remained high despite intake of other antihypertensives. It remains unclear whether the risk of taking this combination may be completely dismissed considering significant burden of polypharmacy and higher-order DDIs, which could potentially interfere with the pharmacokinetics of medications. Combination of 'aspirin + lisinopril', associated with serious DDIs, was among the most common in both the T- and the P-Lists. Other common drug combinations associated with serious DDIs differed between the R- and the T-Lists.
The Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology encourages implementing a multidisciplinary team approach and considering age-related changes in the pharmacokinetics and pharmacodynamics of cardiovascular drugs to address the issues of polypharmacy [
26]. The Working Group considers adherence to pharmacotherapy a key question. It is vital to thoroughly understand most common ADRs, practices of deprescribing [
26,
27], problems of omissions, and potentially inappropriate medications, which may require going beyond the guidelines and implementing binary or multicore team-based approach to care for vulnerable patients [
28].
Genetic variations markedly increase or ameliorate the severity of potential DDIs and need to be considered when prescribing patients with polypharmacy. Currently, most guidelines on DDIs neither consider the potential effect of genetic polymorphisms in the strength of the interaction nor do they account for the complex interaction caused by the combination of DDI and DGI (drug-gene interactions) when there are multiple biotransformation pathways, referred to as DGGI (drug-gene-gene interactions) [
29]. The increasing availability of real-world drug outcome data linked to genetic technologies and resources is likely to enable the discovery of previously unrecognized, clinically important drug–drug–gene interactions to develop clinically useful models to reduce adverse DDIs and improve drug outcomes in the setting of increasing multimorbidity and polypharmacy [
29,
30].
We propose the following solutions to the problem of high DDI burden: (i) building better structure of EHRs; (ii) patient engagement into medication diaries and ADR documentation using specially built portals linked to EHRs [
32], (iii) identifying patients with clinically-significant polymorphisms of genes involved in drug metabolism [
29,
30], (iv) developing electronic decision-making support system for control over DDIs, and (v) interdisciplinary approach to team building [
28]. Data such as obtained in our study urge medical expert community to develop consensus guidelines for the pharmacotherapy of geriatric patients with multimorbidity.
5. Conclusions
High prevalence of serious DDIs and polypharmacy require implementation of deprescribing protocols in older cardiovascular patients. Control over DDIs and polypharmacy may contribute to better compliance and adherence by reducing potential ADRs. Further research is warranted to improve structure of EHRs, patient engagement in reporting ADRs using EHR-linked platform, patient clustering, genotyping, electronic decision support system development, and implementing interdisciplinary approach to medical team work to ensure safe and effective personalized care.
Author Contributions
Conceptualization, NDA; methodology, NDA, OMN, EVE, IAT, NPC, ASM, TAS, and NIR; validation, NDA; formal analysis, NDA, WYU, ASM, TAS, and NIR; investigation, NDA, EVE, and OMN; resources, NDA, EVE, OMN, ANR, SVP, and VAS; data curation, NDA; writing—original draft preparation, NDA and VAS; writing—review and editing, OMN, EVE, IAT, NPC, WYU, ASM, TAS, NIR, SVP, and ANR; figures, NDA; supervision, ANR, IAT, SVP, and VAS; project administration, NDA; funding acquisition, NDA. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation (project #22-15-00313).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, approved by the Institutional Biomedical Ethics Committee of Cardiology Research Institute (approval #230 from June 28, 2022), and registered at ClinicalTrials.gov (Identifier NCT05336565).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors thank the Russian Science Foundation for support (project #22-15-00313).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chowdhury, S.R.; Chandra Das, D.; Sunna, T.C.; Beyene, J.; Hossain, A. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. EClinicalMedicine 2023, 57, 101860. [Google Scholar] [CrossRef] [PubMed]
- Alhumaidi, R.M.; Bamagous, G.A.; Alsanosi, S.M.; Alqashqari, H.S.; Qadhi, R.S.; Alhindi, Y.Z.; Ayoub, N.; Falemban, A.H. Risk of Polypharmacy and Its Outcome in Terms of Drug Interaction in an Elderly Population: A Retrospective Cross-Sectional Study. J Clin Med 2023, 12, 3960. [Google Scholar] [CrossRef] [PubMed]
- Reinhild Haerig, T.; Krause, D.; Klaassen-Mielke, R.; Rudolf, H.; Trampisch, H.J.; Thuermann, P. Potentially inappropriate medication including drug-drug interaction and the risk of frequent falling, hospital admission, and death in older adults - results of a large cohort study (getABI). Front Pharmacol 2023, 14, 1062290. [Google Scholar] [CrossRef]
- Lion, S.; Evrard, P.; Foulon, V.; Spinewine, A. Drug-drug interactions in nursing home residents: analysis from the COME-ON trial. Age Ageing. 2023, 52, afac278. [Google Scholar] [CrossRef] [PubMed]
- Falconi, G.; Kashan, S. Drug Interactions in Palliative Care. 2022 Jun 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–.
- Igho-Osagie, E.; Brzozowski, K.; Jin, H.; Brown, J.; Williams, M.G.; Puenpatom, A. Prevalence of Potential Drug-drug Interactions With Ritonavir-containing COVID-19 Therapy in the United States: An Analysis of the National Health and Nutrition Examination Survey. Clin Ther 2023, 45, 390–399.e4. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Daniel, M. Medical error-the third leading cause of death in the US. BMJ 2016, 353, i2139. [Google Scholar] [CrossRef]
- Marincowitz, C.; Preston, L.; Cantrell, A.; Tonkins, M.; Sabir, L.; Mason, S. Factors associated with increased Emergency Department transfer in older long-term care residents: a systematic review. Lancet Healthy Longev 2022, 3, e437–e447. [Google Scholar] [CrossRef]
- Medscape. Drug Interaction Checker. https://reference.medscape.com/drug-interactionchecker; [accessed 26 June 2023].
- Vonbach, P.; Dubied, A.; Krähenbühl, S.; Beer, J.H. Evaluation of frequently used drug interaction screening programs. Pharm World Sci 2008, 30, 367–374. [Google Scholar] [CrossRef]
- Marcath, L.A.; Xi, J.; Hoylman, E.K.; Kidwell, K.M.; Kraft, S.L.; Hertz, D.L. Comparison of Nine Tools for Screening Drug-Drug Interactions of Oral Oncolytics. J Oncol Pract 2018, 14, e368–e374. [Google Scholar] [CrossRef]
- Hecker, M.; Frahm, N.; Bachmann, P.; Debus, J.L.; Haker, M.C.; Mashhadiakbar, P.; Langhorst, S.E.; Baldt, J.; Streckenbach, B.; Heidler, F.; Zettl, U.K. Screening for severe drug-drug interactions in patients with multiple sclerosis: A comparison of three drug interaction databases. Front Pharmacol 2022, 13, 946351. [Google Scholar] [CrossRef]
- Amkreutz, J.; Koch, A.; Buendgens, L.; Trautwein, C.; Eisert, A. Clinical decision support systems differ in their ability to identify clinically relevant drug interactions of immunosuppressants in kidney transplant patients. J Clin Pharm Ther 2017, 42, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Kheshti, R.; Aalipour, M.; Namazi, S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract 2016, 5, 257–263. [Google Scholar] [CrossRef]
- Das, B.; Ramasubbu, S.K.; Agnihotri, A.; Kumar, B.; Rawat, V.S. Leading 20 drug-drug interactions, polypharmacy, and analysis of the nature of risk factors due to QT interval prolonging drug use and potentially inappropriate psychotropic use in elderly psychiatry outpatients. Ther Adv Cardiovasc Dis 2021, 15, 17539447211058892. [Google Scholar] [CrossRef]
- Roca, B.; Roca, M. Assessment of Drug Interactions with Online Electronic Checkers in Multi-Pathological Patients. Pharmacology 2022, 107, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Şen, S.; Karahan, E.; Büyükulaş, C.; Polat, Y.O.; Üresin, A.Y. Colchicine for cardiovascular therapy: A drug interaction perspective and a safety meta-analysis. Anatol J Cardiol 2021, 25, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.A.; Kedir, A.; Kahaliw, W. Survey on Polypharmacy and Drug-Drug Interactions Among Elderly People with Cardiovascular Diseases at Yekatit 12 Hospital, Addis Ababa, Ethiopia. Integr Pharm Res Pract 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Jain, S.; Jain, P.; Sharma, K.; Saraswat, P. A Prospective Analysis of Drug Interactions in Patients of Intensive Cardiac Care Unit. J Clin Diagn Res 2017, 11, FC01–FC04. [Google Scholar] [CrossRef]
- Yao, X.; Tsang, T.; Sun, Q.; Quinney, S.; Zhang, P.; Ning, X.; Li, L.; Shen, L. Mining and visualizing high-order directional drug interaction effects using the FAERS database. BMC Med Inform Decis Mak 2020, 20, 50. [Google Scholar] [CrossRef]
- Alrowais, F.; Alotaibi, S.S.; Hilal, A.M.; Marzouk, R.; Mohsen, H.; Osman, A.E.; Alneil, A.A.; Eldesouki, M.I. Clinical Decision Support Systems to Predict Drug-Drug Interaction Using Multilabel Long Short-Term Memory with an Autoencoder. Int J Environ Res Public Health 2023, 20, 2696. [Google Scholar] [CrossRef]
- Yu, L.; Xu, Z.; Cheng, M.; Lin, W.; Qiu, W.; Xiao, X. MSEDDI: Multi-Scale Embedding for Predicting Drug-Drug Interaction Events. Int J Mol Sci 2023, 24, 4500. [Google Scholar] [CrossRef]
- Brattig Correia, R.; de Araújo Kohler, L.P.; Mattos, M.M.; Rocha, L.M. City-wide electronic health records reveal gender and age biases in administration of known drug-drug interactions. NPJ Digit Med 2019, 2, 74. [Google Scholar] [CrossRef] [PubMed]
- Ingersgaard, M.V.; Helms Andersen, T.; Norgaard, O.; Grabowski, D.; Olesen, K. Reasons for Nonadherence to Statins - A Systematic Review of Reviews. Patient Prefer Adherence 2020, 14, 675–691. [Google Scholar] [CrossRef]
- Rochon, P.A.; Petrovic, M.; Cherubini, A.; Onder, G.; O'Mahony, D.; Sternberg, S.A.; Stall, N.M.; Gurwitz, J.H. Polypharmacy, inappropriate prescribing, and deprescribing in older people: through a sex and gender lens. Lancet Healthy Longev 2021, 2, e290–e300. [Google Scholar] [CrossRef]
- Tamargo, J.; Kjeldsen, K.P.; Delpón, E.; Semb, A.G.; Cerbai, E.; Dobrev, D.; Savarese, G.; Sulzgruber, P.; Rosano, G.; Borghi, C.; Wassman, S.; Torp-Pedersen, C.T.; Agewall, S.; Drexel, H.; Baumgartner, I.; Lewis, B.; Ceconi, C.; Kaski, J.C.; Niessner, A. Facing the challenge of polypharmacy when prescribing for older people with cardiovascular disease. A review by the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur Heart J Cardiovasc Pharmacother 2022, 8, 406–419. [Google Scholar] [CrossRef]
- Anfinogenova, N.D.; Trubacheva, I.A.; Popov, S.V.; Efimova, E.V.; Ussov, W.Y. Trends and concerns of potentially inappropriate medication use in patients with cardiovascular diseases. Expert Opin Drug Saf 2021, 20, 1191–1206. [Google Scholar] [CrossRef]
- Anfinogenova, Y.; Grakova, E.V.; Shvedova, M.; Kopieva, K.V.; Teplyakov, A.T.; Popov, S.V. Interdisciplinary approach to compensation of hypoglycemia in diabetic patients with chronic heart failure. Heart Fail Rev 2018, 23, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Roll, S.C. The Influence of Pharmacogenetics on the Clinical Relevance of Pharmacokinetic Drug-Drug Interactions: Drug-Gene, Drug-Gene-Gene and Drug-Drug-Gene Interactions. Pharmaceuticals (Basel) 2021, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Malki, M.A.; Pearson, E.R. Drug-drug-gene interactions and adverse drug reactions. Pharmacogenomics J 2020, 20, 355–366. [Google Scholar] [CrossRef]
- Spanakis, M.; Ioannou, P.; Tzalis, S.; Papakosta, V.; Patelarou, E.; Tzanakis, N.; Patelarou, A.; Kofteridis, D.P. Drug-Drug Interactions among Patients Hospitalized with COVID-19 in Greece. J Clin Med 2022, 11, 7172. [Google Scholar] [CrossRef]
- Lyles, C.R.; Nelson, E.C.; Frampton, S.; Dykes, P.C.; Cemballi, A.G.; Sarkar, U. Using Electronic Health Record Portals to Improve Patient Engagement: Research Priorities and Best Practices. Ann Intern Med 2020, 172, S123–S129. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).