Submitted:
27 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagle, D. V.; Zhao, H.; Baker, G. A. Deep eutectic solvents: sustainable media for nanoscale and functional materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef]
- Hansen, B. B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J. M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B. W.; Gurkan, B.; Maginn, E. J.; Ragauskas, A.; Dadmun, M.; Zawodzinski, T. A.; Baker, G. A.; Tuckerman, M. E.; Savinell, R. F.; Sangoro, J. R. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; and Royer, S.; Jérôme, F. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- LaRocca, M. M.; Baker, G. A.; Heitz, M. P. Assessing rotation and solvation dynamics in ethaline deep eutectic solvent and its solutions with methanol J. Chem. Phys. 2021, 155, 034505. [Google Scholar] [CrossRef]
- Smith, E. L.; Abbott, A. P.; Ryder, K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Sahu, S.; Banu, S.; Sahu, A. K.; Kumar, B.V.N. Phani; Mishra, A. K. ; Molecular-level insights into inherent heterogeneity of maline deep eutectic system. J. Mol. Liq. 2022, 350, 118478. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; De la Guardia, M.; Andruch, V.; Vilková, M. Deep Eutectic Solvents vs Ionic Liquids: Similarities and Differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity-benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Abbott, A. P.; Boothby, D.; Capper, G.; Davies, D. L.; Rasheed, R. K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Cruz, H.; Jordão, N.; Branco, L. C. Deep Eutectic Solvents (DESs) as Low-Cost and Green Electrolytes for Electrochromic Devices. Green Chem. 2017, 19, 1653–1658. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in Electrolytes for Rechargeable Li-Based Batteries and Beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Acree, W. E., Jr.; Wilkins, D. C.; Tucker, S. A.; Griffin, J. M.; Powell, J. R. Spectrochemical Investigations of Preferential Solvation. 2. Compatibility of Thermodynamic Models versus Spectrofluorometric Probe Methods for Tautomeric Solutes Dissolved in Binary Mixtures. J. Phys. Chem. 1994, 98, 2537–2544. [Google Scholar] [CrossRef]
- Rai, R.; Pandey, S. Solvatochromic probe response within ionic liquids and their equimolar mixtures with tetraethylene glycol. J. Phys. Chem. B 2014, 118, 11259–11270. [Google Scholar] [CrossRef]
- Nunes, R.; Nunes, N.; Elvas-Leitão, R.; Martins, F. Using Solvatochromic Probes to Investigate Intermolecular Interactions in 1,4-Dioxane/Methanol/Acetonitrile Solvent Mixtures. J. Mol. Liq. 2018, 266, 259–268. [Google Scholar] [CrossRef]
- Catalán, J.; de Paz, J. L. G.; Reichardt, C. On the Molecular Structure and UV/vis Spectroscopic Properties of the Solvatochromic and Thermochromic Pyridinium-N-Phenolate Betaine Dye B30. J. Phys. Chem. A 2010, 114, 6226–6234. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Polarity of Ionic Liquids Determined Empirically by Means of Solvatochromic Pyridinium N-phenolate Betaine. Dyes. Green Chem. 2005, 7, 339–351. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Kamlet, M. J.; Abboud, J. L. M.; Abraham, M. H.; Taft, R. W. Linear Solvation Energy Relationships. 23. A Comprehensive Collection of the Solvatochromic Parameters, Pi*, Alpha, and Beta, and Some Methods for Simplifying the Generalized Solvatochromic Equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Kamlet, M. J.; Abboud, J. L.; Taft, R. W. The Solvatochromic Comparison Method. 6. The π* Scale of Solvent Polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Taft, R. W.; Kamlet, M. J. The Solvatochromic Comparison Method. 2. The α-Scale of Solvent Hydrogen-Bond Donor (HBD) Acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M. J.; Taft, R. W. The Solvatochromic Comparison Method. I. The ß-Scale of Solvent Hydrogen-Bond Acceptor (HBA) Basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Makris, D. P.; Lalas, S. Glycerol and Glycerol-Based Deep Eutectic Mixtures as Emerging Green Solvents for Polyphenol Extraction: The Evidence so Far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef] [PubMed]
- Dong, D. C.; Winnik, M. A. The Py scale of solvent polarities. Can. J. Chem. 1984, 62, 2560–2565. [Google Scholar] [CrossRef]
- Pandey, S.; Baker, S.N.; Pandey, S.; Baker, G. A. Fluorescent Probe Studies of Polarity and Solvation within Room Temperature Ionic Liquids: A Review. J. Fluoresc. 2012, 22, 1313–1343. [Google Scholar] [CrossRef] [PubMed]
- Street, K. W., Jr.; Acree, W. E., Jr. Experimental Artifacts and Determination of Accurate Py Values. Analyst 1986, 111, 1197–1201. [Google Scholar] [CrossRef]
- Street, K. W., Jr.; Acree, W. E., Jr.; Fetzer, J. C.; Shetty, P. H.; Poole, C. F. Polycyclic Aromatic Hydrocarbon Solute Probes. Part V: Fluorescence Spectra of Pyrene, Ovalene, Coronene, and Benzo[ghi]perylene Dissolved in Liquid Alkylammonium Thiocyanate Organic Salts. Appl. Spectrosc. 1989, 43, 1149–1153. [Google Scholar] [CrossRef]
- Tucker, S. A.; Cretella, L. E.; Waris, R.; Street, K. W., Jr.; Acree, W. E., Jr.; Fetzer, J. C. Polycyclic Aromatic Hydrocarbon Solute Probes. Part VI: Effect of Dissolved Oxygen and Halogenated Solvents on the Emission Spectra of Select Probe Molecules. Appl. Spectrosc. 1990, 44, 269–273. [Google Scholar] [CrossRef]
- Dhingra, D.; Pandey, A.; Pandey, S. Pyrene Fluorescence To Probe a Lithium Chloride-Added (Choline Chloride + Urea) Deep Eutectic Solvent. J. Phys. Chem. B 2019, 123, 3103––3111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Anjali; Dhingra, D.; Yadav, A.; Pandey, S. Effect of lithium salt on fluorescence quenching in glycerol: a comparison with ionic liquid/deep eutectic solvent. Phys. Chem. Chem. Phys. 2022, 24, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Kadyan, A.; Pandey, S. Florescence Quenching within Lithium Salt-Added Ionic Liquid. J. Phys. Chem. B 2018, 122, 5106 –5113. [Google Scholar] [CrossRef]
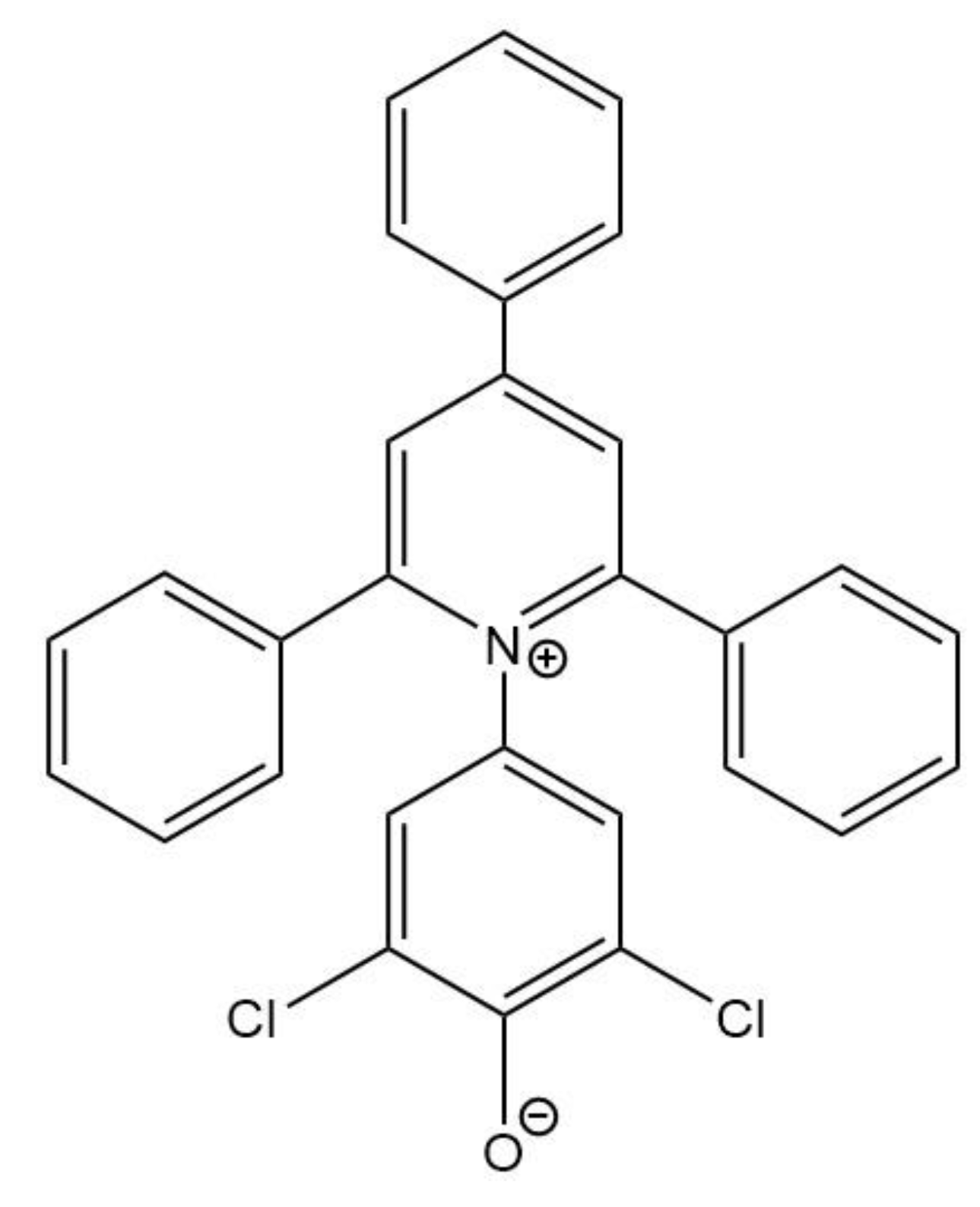
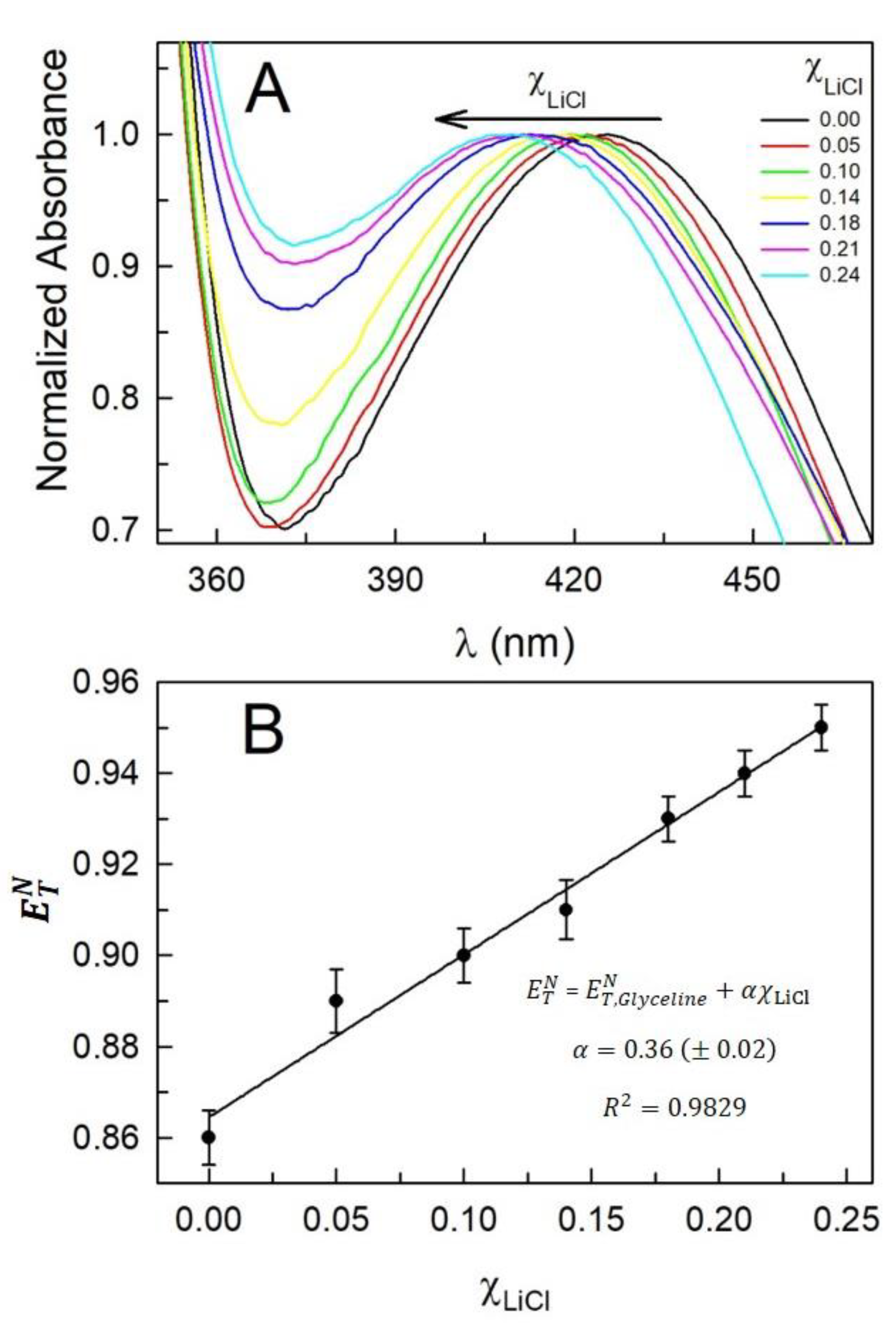
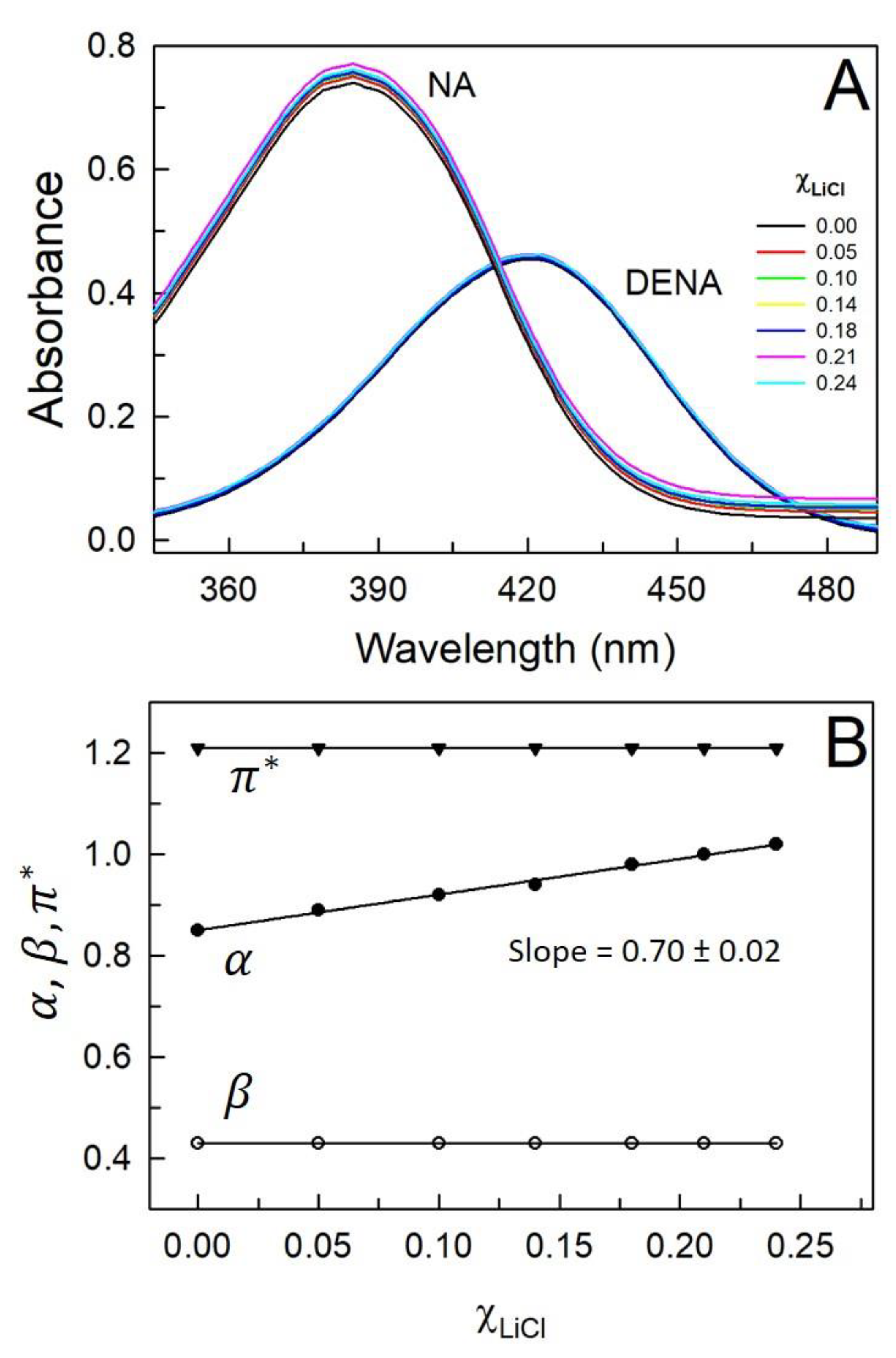
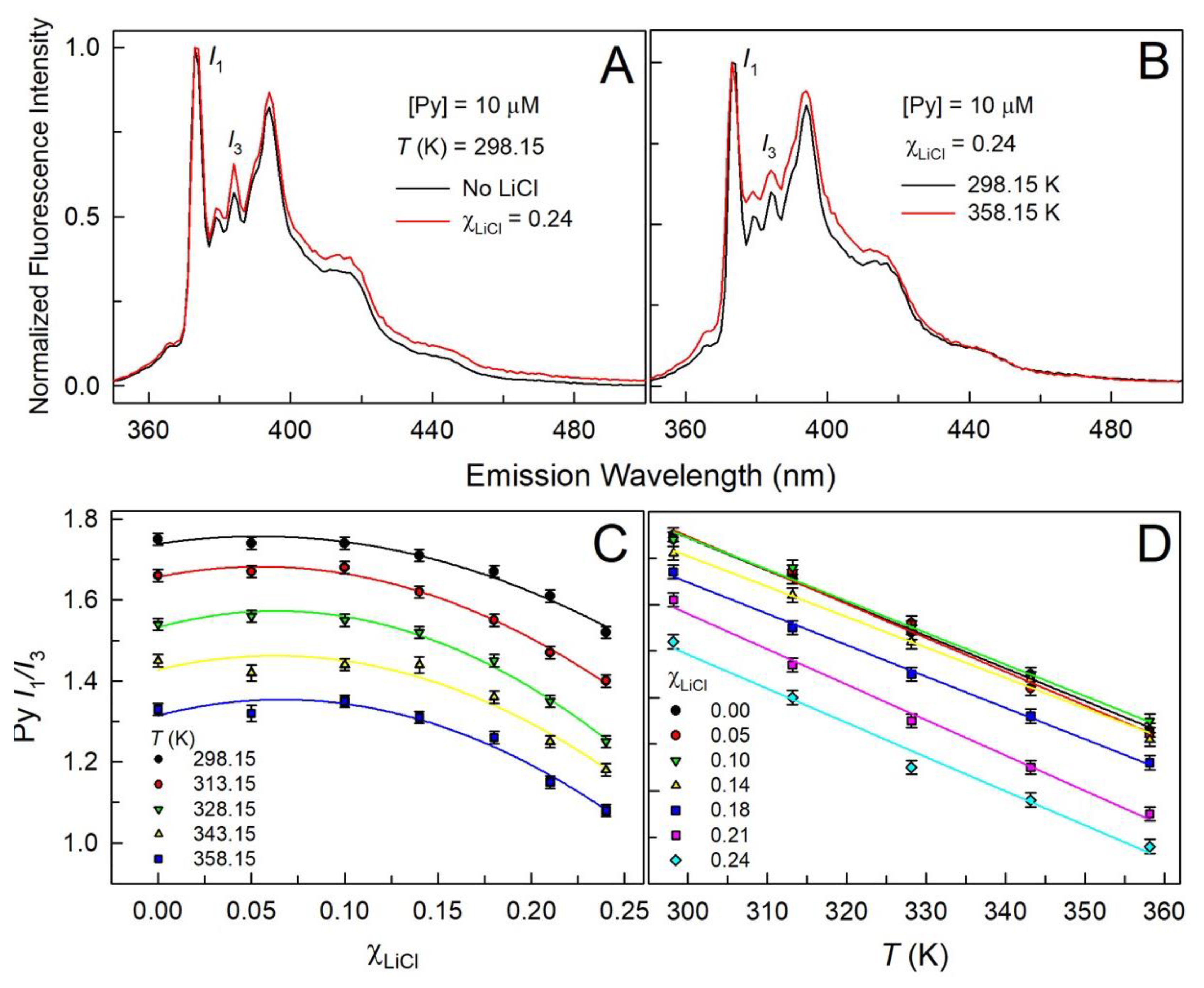
| (mol.kg-1) |
(nm) |
(nm) |
(nm) |
β | α | |||
|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.00 | 426 | 0.86 | 422 | 1.21 | 385 | 0.43 | 0.85 |
| 0.5 | 0.05 | 422 | 0.89 | 422 | 1.21 | 385 | 0.43 | 0.89 |
| 1.0 | 0.10 | 419 | 0.90 | 422 | 1.21 | 385 | 0.43 | 0.92 |
| 1.5 | 0.14 | 417 | 0.91 | 422 | 1.21 | 385 | 0.43 | 0.94 |
| 2.0 | 0.18 | 413 | 0.93 | 422 | 1.21 | 385 | 0.43 | 0.98 |
| 2.5 | 0.21 | 411 | 0.94 | 422 | 1.21 | 385 | 0.43 | 1.00 |
| 3.0 | 0.24 | 409 | 0.95 | 422 | 1.21 | 385 | 0.43 | 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





