1. Introduction
Heart failure (HF) is a complex cardiovascular condition associated with significant morbidity and mortality worldwide. It substantially burdens public health, with approximately 6 million people in the United States currently affected by HF.[
1] The prevalence of HF is expected to rise due to factors such as an aging population and the increasing prevalence of risk factors, including hypertension, diabetes, and obesity. Despite advancements in HF management, the 5-year mortality rate remains high, ranging from 30% to 50%.[
2]
Existing studies have demonstrated that the etiology of HF influences patient outcomes, with iCMP patients exhibiting higher mortality rates than niCMP patients.[
3] Interestingly, sex differences have also been observed in HF outcomes. Despite having higher hospitalization rates, women tend to exhibit better survival rates than men.[
4,
5] However, there is a paucity of research investigating sex-specific differences in mortality and predictors of death among individuals with HF, particularly those with ischemic (iCMP) and nonischemic (niCMP) cardiomyopathy.
Therefore, this study aims to comprehensively analyze mortality rates and identify predictors of death in women and men diagnosed with iCMP and niCMP. By examining sex-related differences in mortality and predictors of death, this study aims to provide valuable insights into the management and treatment of HF for both women and men.
2. Materials and Methods
This retrospective study was conducted on a cohort of 7,487 patients diagnosed with chronic HF at our Heart Institute from February 2017 to September 2020, and the research project was approved by the Research Ethics Committee.
The study included patients diagnosed with HF based on the Framingham criteria for HF diagnosis and echocardiographic measurements. Ischemic cardiomyopathy (iCMP) was diagnosed when there was more than 70% luminal reduction in at least one coronary artery with a significant area of myocardium depending on this artery flow, while nonischemic cardiomyopathy (niCMP), idiopathic and hypertensive CMP, was diagnosed in the presence of normal or nonobstructive coronary arteries. Echocardiographic data were collected from patients with both baseline and the closest echocardiogram available at the end of the study.
The study’s primary outcome was cardiovascular death, encompassing fatal myocardial infarction, stroke, or other cardiovascular causes of death. Mortality data were obtained from the patient’s medical records or through the individual registration status on the Federal Revenue's website. [
6]
Several clinical characteristics were analyzed, including age, the prevalence of comorbidities, the number of HF hospitalizations, and cardiac surgical interventions. The comorbidities examined were diabetes (defined as glycemia ≥126 mg/dL or glycated hemoglobin > 6.5% or under hypoglycemic drug), significant chronic kidney disease (CKD) (defined as creatinine ≥2 mg/dL), atrial fibrillation (AF), previous myocardial infarction (MI), and stroke. Cardiac surgical interventions assessed included percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), valve replacement, pacemaker implantation, cardiac resynchronization therapy (CRT), implantable cardioverter-defibrillators (ICD), and cardiac transplantation. Echocardiographic data included left ventricular ejection fraction (LVEF) and left ventricular diastolic diameters (LVDD) at baseline and the end of the study. HF was categorized based on LVEF as HF with reduced ejection fraction (HFrEF) when LVEF ≤40%, HF with mid-range ejection fraction (HFmrEF) when LVEF was between 41% and 49%, and HF with preserved ejection fraction (HFpEF) when LVEF ≥50%.
Statistical analysis
Statistical analysis involved presenting continuous variables as mean and standard deviation and categorical variables as frequencies and percentages. The normality of the data was assessed using the Kolmogorov-Smirnov test. Student's t-test and analysis of variance were employed to compare continuous variables between groups, while the chi-square test was used for categorical variables. A two-sided probability value of <0.05 was considered statistically significant. Multiple imputation was used to impute missing baseline and follow-up LVDD values. Multiple imputations used the MCMC method to deal with missing data. The imputed datasets were analyzed separately and combined to produce a single result, considering the uncertainty caused by missing data. The cumulative incidence of all-cause death was analyzed using the Kaplan-Meier (K-M) method with Šidák multiple-comparison adjustment. Cox proportional hazards models were utilized to identify variables independently associated with all-cause death. The chi-square score of the Cox proportional hazards model was used to determine the most robust predictors of all-cause death. The dependent variable in the Cox proportional hazards model was death, while the covariates included were those with p<0.1, such as age, sex, MI, diabetes, stroke, CKD, AF, baseline LVEF at echocardiogram, all coronary surgical interventions (PCI + CABG), and all devices implantation (pacemaker + cardiac resynchronization therapy + implantable cardioverter-defibrillator). The statistical analyses were performed using the SAS® Studio package (SAS Institute, Cary, NC).
3. Results
Table 1 shows the clinical characteristics and echocardiographic data for all 7,483 patients with HF and those with nonischemic and ischemic CMP studied during a mean follow-up period of 2.26 years.
In patients with HF and niCMP, men had a higher prevalence of idiopathic CMP [1,991 (40.7%) vs. 1,468 (30.1%); p=0.017], and women had more hypertensive CMP [687 (14.1%) vs. 737 (15.1%); p<0.001]. Diabetes, AF, MI, CKD, and stroke in all patients, AF, diabetes, CKD, and stroke in niCMP, and diabetes, MI, AF, CKD, and stroke in iCMP were the most prevalent comorbidities. The niCMP patients had more pacemaker implantation, ICD, CRT, and transplant (p<0.0001 for all). Baseline LVEF was higher in iCMP patients but did not change in the follow-up echocardiogram. In patients with niCMP, LVEF increased from baseline to the follow-up echocardiogram, from 41.9±15.6 to 44.8±15.0 (p<0.0001). Hospitalization and all causes of death were higher in iCMP patients.
Table 2 shows the clinical characteristics and echocardiographic data for women and men with HF from nonischemic and ischemic CMP.
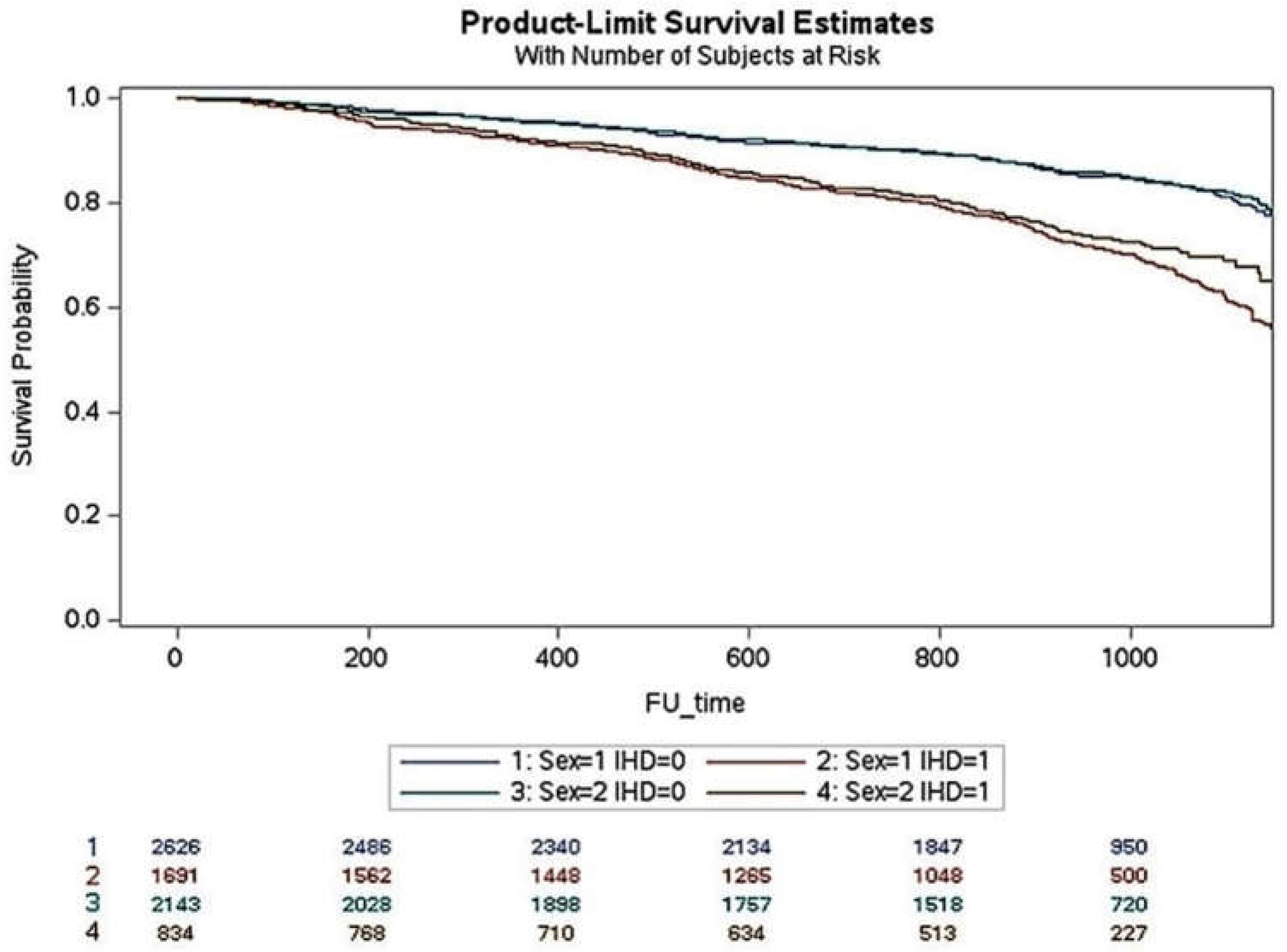
Women with niCMP were older and had a higher prevalence of HFpEF, diabetes, pacemaker, and CRT implantations. Women also had higher LVEF and lower LVDD in baseline and follow-up echocardiograms. In niCMP, the number of comorbidities, hospitalizations, and all causes of death were similar between women and men. Women with iCMP were older and had a higher prevalence of HFpEF. The number of comorbidities, coronary revascularization, pacemaker implantation, and hospitalizations were similar in women and men, but women had a lower incidence of all causes of death (24.5 vs. 29.8; p=0.004). Men had a higher prevalence of myocardial infarction, diabetes, CKD, AF, and HFrEF. Men received more ICD and CRT implantations. Over a 3-year follow-up period, the cumulative incidence of death in iCMP was higher in men than in women (p<0.001) but similar in niCMP (
Figure 1).
Multivariate analysis and predictors of all causes of death
Table 3,
Table 4 and
Table 5 show the Cox proportional hazards ratios and the chi-square score of the Cox proportional hazards model of all causes of death. Cox regression was adjusted for confounders such as age, sex, MI, diabetes, stroke, CKD, AF, baseline LVEF, myocardial revascularization (percutaneous coronary intervention and coronary artery bypass graft), device implantation (pacemaker, internal cardiac defibrillator, and cardiac resynchronization therapy), ischemic, idiopathic, and hypertensive cardiomyopathies.
Table 3 shows the Cox regression analysis results for the main predictors of death in all patients and women and men for all HF etiologies. CKD, diabetes, stroke, age, lower baseline LVEF, MI, device implantation, and revascularization were the main death predictors for all patients, women, and men. AF was a predictor of death for women and men.
Table 4 shows the Cox regression analysis results for the main predictors of death for niCMP in all patients and women, and men. CKD, diabetes, stroke, AF, age, lower baseline LVEF, device implantation, and idiopathic CMP were the main death predictors for all patients and men. CKD, diabetes, stroke, AF, age, and lower baseline LVEF were the main death predictors for women.
Table 5 shows the Cox regression analysis results for the main predictors of death in all patients and women and men for iCMP. CKD, stroke, diabetes, AF, MI, myocardial revascularization, age, and LVEF baseline, in all patients, stroke, CKD, diabetes, AF, myocardial revascularization, MI, and age, in women, and CKD, diabetes, stroke, AF, MI, age, LVEF baseline, were the main predictors of death. Myocardial revascularization was an independent predictor of death only in women.
4. Discussion
This study demonstrates a lower incidence of death in women with HF due to ischemic CMP than in men, while no significant sex-based differences were observed in nonischemic CMP. Women presented with different clinical characteristics, including older age, higher LVEF, and lower LVDD, compared to men in both ischemic and nonischemic CMP. However, sex was not an independent variable associated with all-cause mortality in either subgroup.
Our results were similar to that observed in previous studies of HF from ischemic and idiopathic CMP.[7-9] Analysis of two recent studies in patients with HFrEF showed that women were older and had a higher prevalence of obesity and hypertension. In these studies, women also had fewer comorbidities except hypertension and a lesser risk of hospitalization.[
10]
The lower incidence of death observed in women with HF due to ischemic cardiomyopathy (iCMP) aligns with previous studies that reported better survival rates in women than men with HF.[
11,
12] These findings may be attributed to several factors, including hormonal differences, cardiac remodeling patterns, and therapy response. Estrogen, for instance, has been associated with cardioprotective effects, including favorable effects on endothelial function, vasodilation, and antioxidant activity, which may contribute to better outcomes in women.[
13,
14] However, it should be noted that the role of hormones in HF outcomes is complex and multifactorial, and further research is warranted to understand the underlying mechanisms involved in premenopausal and the partial persistence of beneficial effects in postmenopausal.
Estrogen exerts a cardioprotective effect in HF by inhibiting sympathetic activity and the renin-angiotensin-aldosterone system, decreasing renin levels, angiotensin-converting enzyme activity, AT1 receptor density, aldosterone production, and increasing AT2 receptor density. Estrogen increases natriuretic peptides that intensify the renin-angiotensin-aldosterone system inhibition, promotes better endothelial response to injury, prevents left ventricular remodeling and diastolic function, and protects the coronary microvasculature.[
15,
16] Estrogens' protection at the cellular level is primarily done by increasing anti-oxidative defenses and maintaining mitochondrial integrity.[
17] These pathophysiological mechanisms of estrogens are probably partly responsible for the greater protection of myocardial cells observed in women. Estrogens' protection at the cellular level is primarily done by increasing anti-oxidative defenses and maintaining mitochondrial integrity.
In addition to the lower mortality rates, women in both iCMP and niCMP presented with distinct clinical characteristics compared to men. Women were older and had higher LVEF and lower LVDD than men in both subgroups. These differences may affect disease progression, outcomes, and treatment response. Older age has been associated with worse outcomes in HF, and the higher LVEF and lower LVDD values observed in women may reflect differences in cardiac remodeling, contractile function, and prognosis.[
18,
19].
However, despite the observed differences in clinical characteristics, sex was not found to be an independent variable associated with all-cause mortality in either iCMP or niCMP. These findings were similar to those previously observed in our chronic HF population,[
20] and suggest that other factors, such as atherosclerotic disease severity, comorbidities, genetic factors, or treatment strategies, may significantly influence HF outcomes. Likewise, HF severity, reflected by New York Heart Association (NYHA) functional class, biomarkers such as N-terminal pro-B-type natriuretic peptide,[
21] and echocardiographic parameters,[
22] is strongly associated with prognosis in HF. In our study, men had a higher prevalence of HFrEF than women, and previous studies showed a worse prognosis of HFrEF compared to HFmrEF and HFpEF.[
12,
23] Comorbidities, including older age, hypertension, diabetes, CKD, stroke, and AF, can further complicate HF management and influence mortality risk.[
24] The older age and the comparable number of comorbidities observed in women and men with niCMP and iCMP in our study may also explain why sex was not an independent variable for all causes of death. Genetic factors, such as specific gene polymorphisms or variations, may also contribute to individual variations in disease progression and response to treatment.[
25,
26] Nevertheless, genetic data were unavailable in our study. Treatment strategies can significantly impact HF outcomes, including optimal medical therapy, revascularization procedures, and device therapies.[
27] The utilization and adherence to these therapies may vary between sexes, potentially influencing outcomes. However, women and men had the same treatment for almost all treatment strategies in our study except for a higher ICD and CRT implantation in men with iCMP.
Future research should aim to identify and understand the interplay of these variables with sex-related factors to provide a more comprehensive understanding of HF outcomes. Large-scale prospective studies incorporating comprehensive clinical, genetic, and treatment data are needed to elucidate the complex interactions and identify potential targets for personalized interventions. Additionally, assessing long-term outcomes, including cardiovascular events, hospitalizations, and quality of life, would provide a more holistic view of the impact of sex and other factors on HF prognosis.
Study limitations
Our study has some limitations. It is a retrospective study in a specialized tertiary-care center where selection biases may occur, including patients with a more complex clinical picture. An adequate definition of symptoms is missing, especially the NYHA functional class of dyspnea and other variables associated with a worse prognosis, such as ventricular arrhythmia and a 6-min walk test. We were also unable to detail the cause of death adequately. Our analysis included cardiac and non-cardiac causes, including the deaths from COVID-19, which occurred between the pandemic months of March to September 2020. Finally, adequate information regarding drug treatment and dosages is also missing. However, our center advocates that the treatment of HF be as close as possible to current ‘Get With The Guidelines®’ care. Additionally, the study did not explore the potential impact of hormonal status, menopausal status, or hormone replacement therapy on HF outcomes, which could be relevant factors in sex-based differences.
5. Conclusions
In conclusion, while this study demonstrated a lower incidence of death in women with HF due to ischemic CMP and identified differences in clinical characteristics between sexes, sex was not found to be an independent variable associated with all-cause mortality in either ischemic CMP or nonischemic CMP. Factors like HF disease severity, comorbidities including coronary atherosclerotic severity, and treatment strategies likely contribute to the observed outcomes. Understanding these multifactorial influences is crucial for optimizing HF management and improving outcomes for both men and women with HF.
Author Contributions
Conceptualization: A.P.M.; methodology: A.P.M., A.C.P-B., C.H.C.; formal analysis, A.P.M., A.C.P-B.; investigation, A.P.M., A.C.P-B., C.H.C., S.D.A., N.K.N., L.A.M.C., E.A.B.; resources, A.P.M., A.C.P-B., C.H.C., S.D.A., N.K.N., L.A.M.C., E.A.B.; data curation, A.P.M., A.C.P-B., C.H.C., S.D.A.; writing—original draft preparation, A.P.M., A.C.P-B.; writing—review and editing, A.P.M., A.C.P-B., C.H.C., S.D.A., N.K.N., L.A.M.C., E.A.B; funding acquisition, A.P.M., E.A.B. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The research project was approved by the Research Ethics Committee (CAPpesq) of the Hospital das Clinicas da Faculdade de Medicina da Universidade de Sao Paulo (nº 4.436.791).
Informed Consent Statement
Patient consent was waived due to being a retrospective study, and patients cannot be identified.
Data Availability Statement
Data is unavailable due to privacy.
Acknowledgments
The patients' data were provided by José Antonio Ramos Neto and André Abreu of the Medical Information Unit of the Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Brazil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar]
- Braunwald, E. The war against heart failure: the Lancet lecture. Lancet 2015, 385, 812–824. [Google Scholar] [CrossRef]
- Halliday, B.P.; Gulati, A.; Ali, A.; et al. Sex- and age-based differences in the natural history and outcome of dilated cardiomyopathy. Eur J Heart Fail. 2018, 20, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of heart failure. Circ Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Cannatà, A.; Fabris, E.; Merlo, M.; et al. Sex Differences in the Long-term Prognosis of Dilated Cardiomyopathy. Can J Cardiol. 2020, 36, 37–44. [Google Scholar] [CrossRef]
- Brasil. Receita Federal. Brasília, 2022. Accessed April 11, 2022. https://servicos.receita.fazenda.gov.br/Servicos/CPF/ConsultaSituacao/ConsultaPublica.asp.
- O'Meara, E.; Clayton, T.; McEntegart, M.B.; et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007, 115, 3111–3120. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Krum, H.; Abraham, W.T.; et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med. 2016, 374, 1521–1532. [Google Scholar] [CrossRef]
- Dewan, P.; Rørth, R.; Jhund, P.S.; et al. Differential Impact of Heart Failure With Reduced Ejection Fraction on Men and Women. J Am Coll Cardiol. 2019, 73, 29–40. [Google Scholar] [CrossRef]
- Adams, K.F. Jr.; Fonarow, G.C.; Emerman, C.L.; et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005, 149, 209–216. [Google Scholar] [CrossRef]
- Fiechter, R.; Haider, A.; Bengs, S.; et al. Sex Differences in Long-Term Clinical Outcomes in Patients with Chronic Heart Failure. Cardiology. 2018, 140, 87–95. [Google Scholar]
- Meyer, M.R.; Barton, M. Estrogens and coronary artery disease: new clinical perspectives. Adv Pharmacol. 2016, 77, 307–360. [Google Scholar] [PubMed]
- Mahmoodzadeh, S.; Eder-Negrin, P.; Dworatzek, E.; et al. Sex differences in metabolic regulation of cardiovascular diseases. J Am Heart Assoc. 2019, 8, e011120. [Google Scholar]
- Piro, M.; Della Bona, R.; Abbate, A.; Biasucci, L.M.; Crea, F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol. 2010, 55, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Powell, B.S.; Dhaher, Y.Y.; Szleifer, I.G. Review of the Multiscale Effects of Female Sex Hormones on Matrix Metalloproteinase-Mediated Collagen Degradation. Crit Rev Biomed Eng. 2015, 43, 401–428. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; et al. Mitochondria: a central target for sex differences in pathologies. Clin Sci (Lond). 2017, 131, 803–822. [Google Scholar] [CrossRef]
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C. Jr.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003, 289, 194–202. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016, 37, 24–34. [Google Scholar]
- Mansur, A.P.; Del Carlo, C.H.; Gonçalinho, G.H.F.; et al. Sex Differences in Heart Failure Mortality with Preserved, Mildly Reduced and Reduced Ejection Fraction: A Retrospective, Single-Center, Large-Cohort Study. Int J Environ Res Public Health. 2022, 19, 16171. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004, 350, 655–663. [Google Scholar] [CrossRef]
- Kajimoto, K.; Minami, Y.; Otsubo, S.; Sato, N.; investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) Registry. Sex Differences in Left Ventricular Cavity Dilation and Outcomes in Acute Heart Failure Patients With Left Ventricular Systolic Dysfunction. Can J Cardiol. 2018, 34, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012, 33, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Olson, E.N. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007, 117, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, R.E.; Morales, A.; Siegfried, J.D. Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals. Genet Med. 2010, 12, 655–667. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; et al.; ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021, 42, 3599–3726. [Google Scholar]
Figure 1.
The life-table survival curves of women and men with chronic heart failure and nonischemic and ischemic cardiomyopathies. (sex=1: men; sex=2: women; IHD=0: nonischemic cardiomyopathy; IHD=1: ischemic cardiomyopathy).
Figure 1.
The life-table survival curves of women and men with chronic heart failure and nonischemic and ischemic cardiomyopathies. (sex=1: men; sex=2: women; IHD=0: nonischemic cardiomyopathy; IHD=1: ischemic cardiomyopathy).
Table 1.
Clinical characteristics and echocardiographic data in all patients with HF and those with nonischemic and ischemic CMP.
Table 1.
Clinical characteristics and echocardiographic data in all patients with HF and those with nonischemic and ischemic CMP.
| |
All patients |
niCMP |
iCMP |
p |
| |
N=7,483 |
N= 4,883 (65.2) |
N= 2,600 (34.8) |
|
| Age (Years) |
64.26±14.23 |
61.90±14.97 |
68.71±11.46 |
<0.001 |
| Female (%) |
3,066 (41.0) |
2,205 (45.2) |
861 (33.1) |
<0.001 |
| Comorbidities |
|
|
|
|
| Myocardial infarction (%) |
1,350(18.0) |
3(0.04) |
1,347(18.00) |
<0.001 |
| Diabetes (%) |
1,496(20.0) |
630(12.9) |
866(34.8) |
<0.001 |
| Chronic kidney disease (%) |
817(10.9) |
401(8.2) |
416(16.0) |
<0.001 |
| Stroke (%) |
317(4.2) |
152(3.1) |
165(6.4) |
<0.001 |
| Atrial Fibrillation (%) |
1,356(18.1) |
897(18.4) |
459(17.7) |
0.4439 |
| Number of Comorbidities (%) |
3,659 (48.9) |
1,636 (33.5) |
2,023 (77.8) |
<0.001 |
| N = 1 |
2,281(30.5) |
1,209(24.8) |
1,072(41.2) |
|
| N = 2 |
1,009(13.5) |
348(7.13) |
661(25.4) |
|
| N = 3 |
300(4.0) |
72(1.47) |
228(8.77) |
|
| N ≥ 4 |
69(0.9) |
7(0.14) |
62 (2.38) |
|
| Medication |
|
|
|
|
| ACE inhibitor or BRA |
4,482 (59.9) |
2,940 (60.2) |
1,549 (59.6) |
0.790 |
| Beta-blocker |
3,839 (51.3) |
2,471 (50.6) |
1,344 (51.7) |
0.610 |
| Spironolactone |
2,095 (28.0) |
1,323 (27.1) |
762 (29.3) |
0.128 |
| Diuretics |
2,499 (33.4) |
2,217 (45.4) |
1,199 (46.1) |
0.719 |
| Surgical intervention |
|
|
|
|
| Coronary artery bypass graft (%) |
741(9.9) |
0(0.00) |
741(28.5) |
<0.001 |
| Percutaneous coronary intervention (%) |
277(3.7) |
1(0.02) |
276(10.6) |
<0.001 |
| Pacemaker implantation (%) |
467(6.2) |
372(7.6) |
95(3.7) |
<0.001 |
| Implantable cardioverter-defibrillators (%) |
224(3.0) |
116(2.4) |
108(4.2) |
<0.001 |
| Cardiac resynchronization therapy (%) |
275(3.7) |
221(4.5) |
54(2.1) |
<0.001 |
| Transplant (%) |
175(2.3) |
141(2.9) |
34(1.3) |
<0.001 |
| Hospitalization (%) |
2,236(30.0) |
1,279(26.2) |
957(36.8) |
<0.001 |
| Echocardiogram |
|
|
|
|
| LVEF basal (%) |
43.01±15.39 |
41.87±15.55 |
45.14±14.86 |
<0.001 |
| LVEF final (%) |
44.87±14.82 |
44.76±14.99a
|
45.08±14.47 |
0.364 |
| LVDD basal (mm) |
58.22±9.57 |
59.22±9.88 |
56.31±8.66 |
<0.001 |
| LVDD final (mm) |
57.72±9.94 |
58.15±10.31b
|
56.90±9.15b
|
<0.001 |
| Type of heart failure |
|
|
|
|
| Reduced EF (%) |
3,359(44.9) |
2,383(48.8) |
976(37.5) |
<0.001 |
| Mildly reduced EF (%) |
1,436(19.2) |
896(18.4) |
540(20.8) |
0.0114 |
| Preserved EF (%) |
2,688(35.9) |
1,604(32.9) |
1,084(41.7) |
<0.001 |
| Death (%) |
1,475(19.5) |
745(15.3) |
730(28.1) |
<0.001 |
Table 2.
Clinical characteristics and echocardiographic data in women and men with HF and nonischemic and ischemic CMP.
Table 2.
Clinical characteristics and echocardiographic data in women and men with HF and nonischemic and ischemic CMP.
| |
niCMP N= 4,883 (65.2) |
iCMP N= 2,600 (34.8) |
| |
Men N=2,678 (54.8) |
Women N=2,205 (45.2) |
p |
Men N=1,736 (66.9) |
Women N=861 (33.1) |
p |
| Age (Years) |
60.18±14.24 |
64.0±15.5 |
<0.001 |
68.12±11.05 |
69.9±12.18 |
<0.001 |
| Comorbidities |
|
|
|
|
|
|
| Myocardial infarction (%) |
0(0.00) |
3(0.14) |
0.056 |
921(53.0) |
426(49.5) |
0.094 |
| Diabetes (%) |
306(11.4) |
324(14.7) |
<0.001 |
561(32.3) |
305(35.4) |
0.107 |
| Chronic kidney disease (%) |
245(9.2) |
156(7.1) |
0.009 |
308(17.7) |
108(12.5) |
<0.001 |
| Stroke (%) |
94(3.5) |
58(2.6) |
0.078 |
111(6.4) |
54(6.3) |
0.913 |
| Atrial fibrillation (%) |
539(20.1) |
358(16.2) |
<0.001 |
328(18.9) |
131(15.2) |
0.022 |
| Number of Comorbidities (%) |
916 (34.2) |
720 (32.7) |
0.334 |
1,362 (78.5) |
661 (76.8) |
0.126 |
| N = 1 |
669(25.0) |
540(24.5) |
|
705(40.5) |
367(42.6) |
|
| N = 2 |
203(7.6) |
145(6.6) |
|
449(25.8) |
212(24.6) |
|
| N = 3 |
42(1.6) |
30(1.4) |
|
159(9.1) |
69(8.0) |
|
| N ≥ 4 |
2(0.1) |
5(0.2) |
|
49(2.3) |
13(1.5) |
|
| Medication |
|
|
|
|
|
|
| ACE inhibitor or BRA |
1,649 (61.6) |
1,291 (58.5) |
0.283 |
1062 (61.2) |
487 (56.6) |
0.254 |
| Beta-blocker |
1,344 (50.2) |
1,127 (51.1) |
0.713 |
910 (52.4) |
434 (50.4) |
0.585 |
| Spironolactone |
744 (27.8) |
579 (26.3) |
0.366 |
519 (29.9) |
243 (28.2) |
0.514 |
| Diuretics |
1,245 (46.5) |
972 (44.1) |
0.302 |
783 (45.1) |
416 (48.3) |
0.350 |
| Surgical intervention |
|
|
|
|
|
|
| Coronary artery bypass graft (%) |
0(0) |
0(0) |
|
511(29.4) |
230(26.7) |
0.156 |
| Percutaneous coronary intervention (%) |
1(0.04) |
0(0) |
|
187(10.6) |
89(10.3) |
0.746 |
| Pacemaker implantation (%) |
162(6.1) |
210(9.5) |
<0.001 |
64(3.7) |
31(3.6) |
0.919 |
| Implantable cardioverter-defibrillators (%) |
74(2.8) |
42(1.9) |
0.050 |
88(5.1) |
20(2.3) |
0.001 |
| Cardiac resynchronization therapy (%) |
103(3.9) |
118(5.4) |
0.012 |
45(2.6) |
9(1.1) |
0.009 |
| Transplant (%) |
90(3.4) |
51(2.3) |
0.030 |
24(1.4) |
10(1.2) |
0.644 |
| Hospitalization (%) |
676(25.2) |
603(27.4) |
0.096 |
654(37.6) |
303(35.2) |
0.229 |
| Echocardiogram |
|
|
|
|
|
|
| LVEF basal (%) |
39.0±14.44 |
45.4±16.12 |
<0.001 |
43.3±14.48 |
48.8±14.95 |
<0.001 |
| LVEF final (%) |
42.14±14.64a
|
47.9±14.8a
|
<0.001 |
43.3±14.19 |
48.6±14.38 |
<0.001 |
| LVDD basal (mm) |
61.9±9.67 |
56.0±9.121 |
<0.001 |
57.8±8.42 |
53.2±8.30 |
<0.001 |
| LVDD final (mm) |
60.3±10.46b
|
54.8±9.51b
|
<0.001 |
58.3±8.90b
|
53.3±8.43c
|
<0.001 |
| Type of heart failure |
|
|
|
|
|
|
| Reduced EF (%) |
1,479(55.2) |
904(41.0) |
<0.001 |
719(41.4) |
257(29.8) |
<0.001 |
| Mildly reduced EF (%) |
543(20.3) |
353(16.0) |
<0.001 |
392(22.5) |
483(17.2) |
0.002 |
| Preserved EF (%) |
656(24.5) |
948(43.0) |
<0.001 |
628(36.1) |
456(53.0) |
<0.001 |
| Death (%) |
420(15.7) |
325(14.7) |
0.361 |
519(29.8) |
211(24.5) |
0.004 |
Table 3.
Cox regression analysis for all causes of death and the chi-square score of death predictors in all patients with heart failure adjusted for age, gender, myocardial infarction, diabetes, stroke, chronic kidney disease, atrial fibrillation, baseline left ventricular ejection fraction, myocardial revascularization (percutaneous coronary intervention and coronary artery bypass graft), device implantation (pacemaker, internal cardiac defibrillator, and cardiac resynchronization therapy), ischemic, idiopathic, and hypertensive cardiomyopathies.
Table 3.
Cox regression analysis for all causes of death and the chi-square score of death predictors in all patients with heart failure adjusted for age, gender, myocardial infarction, diabetes, stroke, chronic kidney disease, atrial fibrillation, baseline left ventricular ejection fraction, myocardial revascularization (percutaneous coronary intervention and coronary artery bypass graft), device implantation (pacemaker, internal cardiac defibrillator, and cardiac resynchronization therapy), ischemic, idiopathic, and hypertensive cardiomyopathies.
| |
Variable |
Hazard ratio |
95% Confidence Limits |
Variable |
Score Chi-Square |
p |
| All patients |
CKD |
3.24 |
2.89 |
3.63 |
CKD |
976.46 |
<0.001 |
| |
Stroke |
2.62 |
2.25 |
3.05 |
Diabetes |
251.47 |
<0.001 |
| |
Diabetes |
2.22 |
1.98 |
2.48 |
Stroke |
224.30 |
<0.001 |
| |
MI |
1.42 |
1.25 |
1.61 |
Age |
74.90 |
<0.001 |
| |
Device |
1.31 |
1.11 |
1.54 |
LVEF1 |
73.43 |
<0.001 |
| |
Revascularization |
1.19 |
1.03 |
1.38 |
MI |
33.01 |
<0.001 |
| |
Idiopathic CMP |
1.15 |
1.00 |
1.32 |
Device |
8.69 |
0.003 |
| |
Age |
1.02 |
1.02 |
1.03 |
Revascularization |
4.49 |
0.034 |
| |
LVEF baseline |
0.99 |
0.98 |
0.99 |
Idiopathic |
3.88 |
0.049 |
| |
|
|
|
|
|
|
|
| Women |
CKD |
3.54 |
2.90 |
4.30 |
CKD |
446.85 |
<0.001 |
| |
Stroke |
3.07 |
2.37 |
3.99 |
Diabetes |
119.93 |
<0.001 |
| |
Diabetes |
2.57 |
2.15 |
3.07 |
Stroke |
106.32 |
<0.001 |
| |
AF |
2.02 |
1.66 |
2.45 |
AF |
65.22 |
<0.001 |
| |
Revascularization |
1.30 |
1.02 |
1.66 |
Age |
39.31 |
<0.001 |
| |
MI |
1.25 |
1.00 |
1.55 |
LVEF baseline |
13.93 |
<0.001 |
| |
Age |
1.03 |
1.02 |
1.03 |
Revascularization |
8.10 |
0.004 |
| |
LVEF baseline |
0.99 |
0.98 |
0.99 |
MI |
3.92 |
0.048 |
| |
|
|
|
|
|
|
|
| Men |
CKD |
2.92 |
2.54 |
3.36 |
CKD |
557.33 |
<0.001 |
| |
Stroke |
2.24 |
1.85 |
2.71 |
Stroke |
136.14 |
<0.001 |
| |
Diabetes |
1.92 |
1.67 |
2.20 |
Diabetes |
123.55 |
<0.001 |
| |
AF |
1.91 |
1.67 |
2.20 |
AF |
83.88 |
<0.001 |
| |
MI |
1.44 |
1.24 |
1.66 |
LVEF baseline |
52.02 |
<0.001 |
| |
Device |
1.34 |
1.09 |
1.64 |
Age |
43.16 |
<0.001 |
| |
Revascularization |
1.26 |
1.06 |
1.50 |
MI |
34.55 |
<0.001 |
| |
Age |
1.02 |
1.01 |
1.02 |
Device |
5.77 |
0.016 |
| |
LVEF baseline |
0.98 |
0.97 |
0.98 |
Revascularization |
4.87 |
0.027 |
Table 4.
Cox regression analysis for all causes of death and the chi-square score of death predictors in patients with heart failure from nonischemic cardiomyopathy adjusted for age, gender, myocardial infarction, diabetes, stroke, chronic kidney disease, atrial fibrillation, baseline left ventricular ejection fraction, myocardial revascularization (percutaneous coronary intervention and coronary artery bypass graft), device implantation (pacemaker, internal cardiac defibrillator, and cardiac resynchronization therapy), ischemic, idiopathic, and hypertensive cardiomyopathies.
Table 4.
Cox regression analysis for all causes of death and the chi-square score of death predictors in patients with heart failure from nonischemic cardiomyopathy adjusted for age, gender, myocardial infarction, diabetes, stroke, chronic kidney disease, atrial fibrillation, baseline left ventricular ejection fraction, myocardial revascularization (percutaneous coronary intervention and coronary artery bypass graft), device implantation (pacemaker, internal cardiac defibrillator, and cardiac resynchronization therapy), ischemic, idiopathic, and hypertensive cardiomyopathies.
| |
Variables |
Hazard ratio |
95% Confidence Limits |
Variables |
Score Chi-Square |
p |
| All patients |
CKD |
3.59 |
3.03 |
4.24 |
CKD |
608.58 |
<0.001 |
| |
Stroke |
3.00 |
2.39 |
3.77 |
AF |
149.38 |
<0.001 |
| |
Diabetes |
2.66 |
2.25 |
3.14 |
Diabetes |
136.74 |
<0.001 |
| |
AF |
2.27 |
1.94 |
2.66 |
Stroke |
93.62 |
<0.001 |
| |
Device |
1.41 |
1.14 |
1.75 |
LVEF basal |
26.90 |
<0.001 |
| |
Idiopathic |
1.28 |
1.09 |
1.52 |
Age |
35.18 |
<0.001 |
| |
Age |
1.02 |
1.01 |
1.02 |
Device |
11.82 |
0.001 |
| |
LVEF basal |
0.98 |
0.98 |
0.99 |
Idiopathic |
8.53 |
0.004 |
| |
|
|
|
|
|
|
|
| Women |
CKD |
3.82 |
2.94 |
4.96 |
CKD |
301.64 |
<0.001 |
| |
Diabetes |
2.89 |
2.29 |
3.64 |
Diabetes |
86.44 |
<0.001 |
| |
Stroke |
2.39 |
1.63 |
3.52 |
AF |
49.24 |
<0.001 |
| |
AF |
2.00 |
1.56 |
2.57 |
Stroke |
20.37 |
<0.001 |
| |
Age |
1.02 |
1.02 |
1.03 |
Age |
18.78 |
<0.001 |
| |
LVEF basal |
0.99 |
0.98 |
0.99 |
LVEF basal |
13.44 |
<0.001 |
| |
|
|
|
|
|
|
|
| Men |
CKD |
3.75 |
3.01 |
4.68 |
CKD |
325.64 |
<0.001 |
| |
Stroke |
3.70 |
2.78 |
4.92 |
Stroke |
116.60 |
<0.001 |
| |
AF |
2.60 |
2.12 |
3.19 |
AF |
95.47 |
<0.001 |
| |
Diabetes |
2.31 |
1.83 |
2.93 |
Diabetes |
37.23 |
<0.001 |
| |
Device |
1.52 |
1.13 |
2.05 |
LVEF basal |
29.34 |
<0.001 |
| |
Idiopathic |
1.50 |
1.18 |
1.90 |
Device |
14.15 |
<0.001 |
| |
Age |
1.01 |
1.00 |
1.02 |
Idiopathic |
8.20 |
0.004 |
| |
LVEF basal |
0.98 |
0.97 |
0.99 |
Age |
8.63 |
0.003 |
Table 5.
Cox regression analysis for all causes of death and the chi-square score of death predictors in patients with heart failure from ischemic cardiomyopathy adjusted for age, gender, myocardial infarction, diabetes, stroke, chronic kidney disease, atrial fibrillation, baseline left ventricular ejection fraction, myocardial revascularization (percutaneous coronary intervention and coronary artery bypass graft), device implantation (pacemaker, internal cardiac defibrillator, and cardiac resynchronization therapy), ischemic, idiopathic, and hypertensive cardiomyopathies.
Table 5.
Cox regression analysis for all causes of death and the chi-square score of death predictors in patients with heart failure from ischemic cardiomyopathy adjusted for age, gender, myocardial infarction, diabetes, stroke, chronic kidney disease, atrial fibrillation, baseline left ventricular ejection fraction, myocardial revascularization (percutaneous coronary intervention and coronary artery bypass graft), device implantation (pacemaker, internal cardiac defibrillator, and cardiac resynchronization therapy), ischemic, idiopathic, and hypertensive cardiomyopathies.
| |
Variables |
Hazard ratio |
95% Confidence Limits |
Variables |
Score Chi-Square |
p |
| All patients |
CKD |
2.53 |
2.16 |
2.95 |
CKD |
286.12 |
<0.001 |
| |
Stroke |
2.09 |
1.71 |
2.57 |
Stroke |
85.80 |
<0.001 |
| |
Diabetes |
1.93 |
1.66 |
2.24 |
Diabetes |
73.63 |
<0.001 |
| |
AF |
1.58 |
1.34 |
1.86 |
AF |
35.05 |
<0.001 |
| |
MI |
1.45 |
1.24 |
1.68 |
LVEF baseline |
24.25 |
<0.001 |
| |
Revascularization |
1.18 |
1.01 |
1.37 |
Age |
39.57 |
<0.001 |
| |
Age |
1.02 |
1.02 |
1.03 |
MI |
22.10 |
<0.001 |
| |
LVEF baseline |
0.98 |
0.98 |
0.99 |
Revascularization |
4.61 |
0.032 |
| |
|
|
|
|
|
|
|
| Women |
Stroke |
3.91 |
2.72 |
5.62 |
CKD |
116.26 |
<0.001 |
| |
CKD |
3.39 |
2.51 |
4.58 |
Stroke |
61.25 |
<0.001 |
| |
Diabetes |
2.27 |
1.71 |
3.01 |
Diabetes |
27.92 |
<0.001 |
| |
AF |
1.93 |
1.40 |
2.66 |
AF |
26.06 |
<0.001 |
| |
Revascularization |
1.38 |
1.05 |
1.83 |
Age |
16.72 |
<0.001 |
| |
MI |
1.35 |
1.01 |
1.80 |
Revascularization |
4.58 |
0.032 |
| |
Age |
1.03 |
1.01 |
1.04 |
MI |
4.26 |
0.039 |
| |
|
|
|
|
|
|
|
| Men |
CKD |
2.41 |
2.00 |
2.89 |
CKD |
175.89 |
<0.001 |
| |
Diabetes |
1.87 |
1.56 |
2.23 |
Diabetes |
45.81 |
<0.001 |
| |
Stroke |
1.80 |
1.40 |
2.30 |
Stroke |
40.28 |
<0.001 |
| |
AF |
1.48 |
1.22 |
1.80 |
LVEF baseline |
29.06 |
<0.001 |
| |
MI |
1.45 |
1.21 |
1.74 |
Age |
26.61 |
<0.001 |
| |
Age |
1.02 |
1.01 |
1.03 |
MI |
18.16 |
<0.001 |
| |
LVEF baseline |
0.98 |
0.97 |
0.99 |
AF |
15.71 |
<0.001 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).