1. Introduction
Brain malignancies are among the most dreaded forms of cancer because of their immediate impact on cognitive function, well-being, and unfavourable prognosis. These tumors exhibit irregular growth patterns and invade surrounding healthy brain tissue. The location and size of the tumor can lead to a variety of symptoms, including headaches, seizures, numbness, and difficulties with speech and vision. Glial cells, which provide support and protection to neurons, can transform into gliomas, the most prevalent type of primary malignant brain tumors [
1].
Gliomas comprise of various subtypes, including oligodendrogliomas, astrocytomas, and ependymomas, representing 24% of all primary brain and CNS malignancies worldwide. The incidence of gliomas differs depending on the histology; pilocytic astrocytomas being more prevalent in children and adolescents, low-grade oligodendrogliomas peaking between the ages of 30 and 40, and glioblastoma (GBM) rising in people in their 60s and 70s [
2]. A study on the prevalence and patterns of tumors in the brain and central nervous system revealed a considerable rise of 94.35 % in cases between 1990 and 2019 [
3]. A similar study found a 152.5 % increase in cases in North African and Middle Eastern countries over the same period [
4].
According to the World Health Organization (WHO) classification system, gliomas are classified into four types based on their histopathological characteristics, reflecting the tumor's aggressiveness and propensity for rapid growth and dissemination. Low-grade gliomas, which grow slowly, often have a better prognosis than high-grade gliomas. Such gliomas can originate from any glial cell and are categorized as either grade I or II, with examples including pilocytic astrocytoma and oligodendroglioma. Meanwhile, high-grade gliomas, often referred to as malignant gliomas, are a group of aggressive, rapidly proliferating tumors that fall into two classifications: grade III anaplastic gliomas and grade IV glioblastomas. Although the precise etiology of gliomas is unknown, numerous genetic abnormalities and mutations have been shown to contribute to the growth and development of the tumor. For example, mutations in the isocitrate dehydrogenase 1 and 2 (IDH1, IDH2 ) genes, which typically play essential roles in cellular metabolism, particularly in the citric acid cycle, are frequently observed in low-grade gliomas. In contrast, tumor protein 53 (TP53), phosphatase and tensin homolog (PTEN), and epidermal growth factor receptor (EGFR) gene mutations are frequently found in high-grade gliomas [
5]. Consequently, gliomas are challenging to diagnose and treat, and current treatments such as surgery, radiotherapy, and chemotherapy are frequently ineffective.
Recent studies suggest that cancer stem cells (CSCs) play a critical role in the development, progression, and recurrence of gliomas. CSCs are a subpopulation of tumor cells with self-renewal ability, can differentiate into various cell types, and are found to be resistant to conventional therapies, including chemotherapy, immunotherapy, and radiotherapy [
6]. A major attribution to the endowed properties of CSCs, also seen in gliomas, is the dysregulation of miRNAs. By modulating the expression of key genes and signaling pathways involved in the maintenance and differentiation of CSCs, abnormal miRNA can lead to the accumulation and expansion of CSCs, thereby promoting tumor growth and resistance to therapy [
7]. This provides hope for developing innovative cancer treatments based on an understanding of the molecular mechanisms of cancer.
MiRNAs are small in length, from 21-25 nucleotides, non-coding RNA molecules crucial in regulating gene expression. They are found in many organisms, including plants, animals, and viruses. They, through the binding to regions of messenger RNA (mRNA) molecules, exert post-translational regulation of genes. MiRNAs can regulate gene expression by binding to the specific areas of mRNAs, causing them to either degrade or inhibit their translation, ultimately preventing protein synthesis. Accordingly, they are associated with many biological processes, including development, differentiation, apoptosis, and immune responses [
8]. The dysregulated expression of miRNAs has been implicated in numerous diseases, including cancer, cardiovascular disease, and neurological disorders. They can be used as diagnostic and prognostic biomarkers for various diseases and as potential targets for therapeutic intervention.
MiRNAs are incapable of being translated into proteins; instead, they bind to the 3' untranslated region (UTR) of the target messenger RNA (mRNA), influencing its stability and/or translation. Approximately 33 percent of the human expression mRNAs involved in cell development, differentiation, and death are regulated by miRNAs.
Multiple miRNAs, including miR-21, miR-10b, miR-128, miR-34a, and others, are dysregulated in glioblastoma [
9]. These miRNAs regulate various pathways that contribute to the development and progression of glioblastomas, such as cell proliferation, invasion, and apoptosis. MiR-21, for example, has been shown to promote tumor growth and invasion by targeting various tumor suppressor genes when overexpressed in glioblastoma. By targeting the HOXD10 gene, miR-10b has also been shown to promote glioblastoma cell migration and invasion. The dysregulation of miRNAs in glioblastoma suggests that they could be potential targets for therapeutic targets. Strategies targeting specific miRNAs have been developed, such as miRNA replacement therapy and miRNA inhibition therapy, and are being investigated as potential treatments for glioblastoma.
Overall, the involvement of miRNAs in the advancement and growth of glioblastoma is significant, providing opportunities for novel therapeutic approaches. This review aims at providing an overview of the potential application of miRNA in determining the development and prognosis of GBM.
2. The role of miRNAs in gliomas
Numerous miRNAs participate in cancer development through cell cycle regulation, apoptosis, and DNA damage response pathways. As mentioned earlier, there is mounting evidence that various miRNA mutations may play a role in the advancement and growth of glioblastomas [
10]. Depending on whether they are under-expressed or over-expressed, microRNAs can have a dual effect on the cell cycle by both positively regulating survival pathways and disrupting them (
Table 1).
2.1. MiRNA-21,221 and 222
One of the principal mechanisms of cancer cell survival is the avoidance of programmed cell death. This can be accomplished by dysregulating various miRNAs, including miRNA-21, miR-221, and miR-222 [
10]. MiRNA can exhibit either pro-apoptotic or anti-apoptotic characteristics. Those that target genes that promote apoptosis are frequently observed in glial tumors, and it is believed that miR-21, due to its anti-apoptotic nature, may have micro-oncogenic properties [
11]. In GBM cells and malignant brain tissues, miR-21 expression is significantly elevated. It modulates P53 and TGF-beta activity, consequently decreasing apoptosis [
12]. Furthermore, miR-21 can specifically suppress Fas ligand in tumor stem cells, inhibiting apoptosis [
13]. Predictably, gliomas demonstrated increased sensitivity to chemotherapy upon inhibition of miR-21 [
13]. Similarly, both miR-221 and miR-222 exhibit anti-apoptotic properties through their interaction with PUMA (p53 upregulated modulator of apoptosis), which typically controls apoptosis. In gliomas, an increased miR-221/222 level has been observed to decrease PUMA expression. This promotes the survival and growth of tumor cells [
14].
2.2. MiR-296 and 93:
Angiogenesis, a critical process for tumor survival, is affected by abnormal expression of these miRNAs.
In vitro studies have shown that excess VEGF, a proangiogenic factor, can enhance the endogenous production of miR-296 in human glial cells [
15]. In a feedback loop, miR-296 promotes the growth of new blood vessels by upregulating the expression of the vascular endothelial growth factor receptor 2 (VEGFR2) [
16]. The upregulation of miR-93 can suppress integrin-β8, a protein that participates in cell and cell-matrix interactions, consequently promoting angiogenesis. Studies have demonstrated that when human U87 glioblastoma cells are co-cultured with endothelial cells and miR-93 is overexpressed, there is a notable increase in both the proliferation and formation of tube-like structures by endothelial cells [
17].
2.3. MiRNA-138 and Mi RNA-490:
These miRNAs have tumor-suppressive properties and inhibit glioblastoma tumorigenicity [
18]. It was noted that there is an inverse relationship between the genetic expression of MiRNA-138 and the levels of CDK6, representing a crucial regulator of the G1 to S transition phase of the cell cycle that is usually increased in GBM [
19]. MiRNA-490 has been identified to function as a tumor suppressor in GBM, and glioma cell lines have significantly lower levels of this miRNA. It has been shown to inhibit HMGA2, an oncogenic protein, and TERF2, a protein responsible for telomere maintenance. MiRNA-138 and MiRNA-490 are downregulated in GBM, promoting tumorigenesis [
20].
2.4. MiRNA-128:
MiRNA-128 is one of the most frequently detected miRNAs in the brain. It is crucial for CNS cell development and maturation and neuronal maturation [
21]. It also regulates the migration of neurons in the cerebral cortex [
22]. In addition, it suppresses tumors by inhibiting the oncogenes PRC and BMI1, resulting in reduced proliferation of glioblastoma stem cells [
23]. Loss of MiRNA-128 occurs early in the formation of gliomas, leading to reduced differentiation and increased stemness, giving rise to the growth of more aggressive tumors [
24].
2.5. MiRNA-124 and MiRNA-137:
MiRNAs 124 and 137 are involved in determining the fate and differentiation of neurons. The growth factors EGF and PDGF have been shown to reduce the expression of these miRNAs [
24]. Upregulation of miRNA-124 promotes neuronal differentiation of progenitor cells, encourages the generation of neuroblasts and mature neurons, and decreases the invasiveness of GBM [
24,
25]. It also downregulates Sox9, which usually promotes the proliferation of precursor cells [
26]. MiRNA-124 is typically absent or underexpressed in GBM, resulting in tumor expansion and division of glioblastoma stem cells [
27]. Similarly, miRNA-137 plays a role in the maturation of immature neurons [
28]. It also inhibits the ability of glioblastoma stem cells to self-renew by suppressing LSD1 and TLX, which generally keep stem cells undifferentiated and capable of self-renewal [
29].
3. miRNAs and cancer-associated pathways.
In GBM, miRNAs act on pre-altered genetic pathways, such as the wingless/integrated (WNT) pathway, P53 pathway, and retinoblastoma (RB) pathway, leading to increased proliferation rate, decreased apoptosis, angiogenesis, de-differentiation, and metastasis [
11].
3.1. WNT pathway:
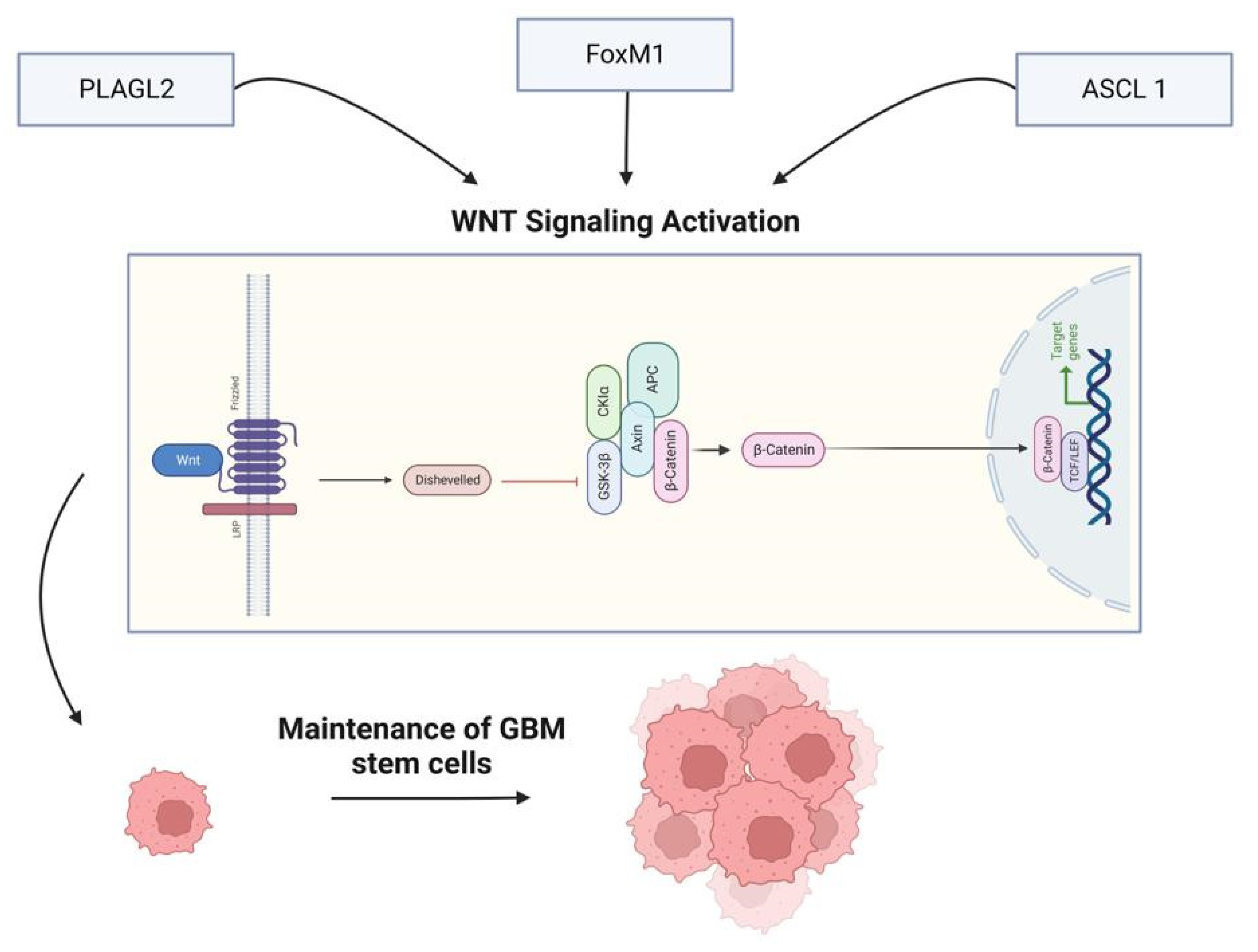
The WNT signaling pathway is vital in determining cell fate and regulating cell migration, cell polarity, and neural patterning. In addition to its normal function, the WNT pathway can also be activated abnormally in a variety of cancer, including melanoma, ovarian cancer, colorectal cancer, breast cancer, and prostate cancer. For example, the WNT pathway and the transcription factors associated with it have a significant impact on glioma growth and progression [
30]. Furthermore, activation of this pathway promotes the maintenance of GMB stem cells, and the resulting increase in self-renewal capacity is initiated by WNT signaling regulators such as PLAGL2, forkhead box protein M1 (FoxM1), and achaete-scute homolog (ASCL1) [
31]. Multiple WNT pathways have been identified, including a canonical WNT pathway and several non-canonical WNT pathways. The canonical WNT pathway is also known as the β-catenin-dependent WNT pathway because it involves the interaction of WNT proteins and β-catenin. However, non-canonical WNT pathways do not depend on β-catenin for their signaling; these pathways can be further classified into the Planar Cell Polarity (PCP) and WNT-Ca2+ pathways. The two pathways mentioned above are known to be linked with and involved in different cellular processes, but both, when abnormally induced, have been related to cancer development [
32].
Furthermore, the WNT pathway and miRNAs have a symbiotic relationship in cancer. The hyperactivation of the WNT pathway can be caused by miRNA dysregulation. MiRNA expression is monitored and controlled by the WNT signaling pathway [
33].
The binding of WNT ligands to various cell receptors, including LRP 5/6, frizzled receptor (FZD), protein tyrosine kinase 7 (PTK7), RAR-related orphan receptor (ROR), or receptor-like tyrosine kinase (RYK) activate both canonical and non-canonical WNT signaling pathways. To date, 19 WNT ligands have been identified, with specific ligands (WNT1, 2, 2B, 3, 3A, 7A, 7B, 9A, 9B, 10A, and 10B) primarily associated with the canonical pathway [
33]. On the other hand, WNT7A and WNT7B are mainly linked to the WNT/PCP pathway, while WNT5A and 5B are connected to the WNT/Ca2+ pathway, but they also play a role in the canonical pathway [
34]. In addition, research has indicated that ligands such as WNT1, 2, 2B, 3A, 5A, 6, 7A, and 7B are implicated in the progression of gliomas [
35].
MiR-122 targets WNT1 directly and inhibits the canonical WNT pathway and an increase in the levels of phosphorylated β-catenin, whereas other miRNAs, such as miR-133b, miR-362-3p, and miR-139-5p, target WNT1 indirectly and suppress the WNT/β-catenin pathway [
36]. In contrast, TRIM24 activates the WNT pathway and other signaling pathways in gliomas, such as EGFR AND STAT3 pathways. Furthermore, TRIM24 expression is negatively regulated by miR-138-2-3p, suppressing the protein levels of WNT1 and WNT3a. Moreover, lncRNA NCK1-AS1 acts as a "sponge" for miR-138-2-3p, activating the WNT pathway. This suggests that NCK1-AS1 binds to miR-138-2-3p, deterring it from interacting with its target genes and increasing WNT pathway activity [
37] (
Figure 1).
3.2. P53 Pathway
It was revealed that P53 is both a tumor suppressor and transcription factor; it is involved in many biological functions, including cell cycle arrest, genome stability, programmed cell death, regulation of cellular metabolism, and inhibition of the growth of new blood vessels [
34]. The P53 pathway comprises MDM2, ARF, CDKN2A, and P53, among which P53 is a critical regulator. This pathway is frequently abnormal in more than 50% of GBM patients [
38]. Possible P53 pathway changes include the loss of CDKN2A/ARK and the augmentation of MDM2 and MDM4. Furthermore, in gliomas, P53, MDM2, and ARF are frequently mutated, resulting in uncontrolled cell growth and compromised DNA repair. PTEN tightly regulates P53 through a negative feedback loop, denoting that an increase in PTEN expression can lead to a loss of P53 function, even if P53 is not mutated. Mutations in both genes are required for GBM progression and evasion of cell death [
12]. Moreover, the overexpression of particular miRNAs, such as miR-21 and miR-26, impacts this pathway and contributes to the development of GBM [
39].
3.3. Retinoblastoma (RB) Pathway
Retinoblastoma (RB) is a phosphoprotein that suppresses the process of cell division during the G1-S phase, and its activity is controlled by cyclin-dependent kinases (CDKs) with their cyclin associates Cdk4 Cdk6 and Cyclin E-CDK2 [
40]. MiRNAs such as miR-124, miR-137, miR-34a, and miR-128 also regulate the RB pathway. MiR-124 and miR-137 downregulation is linked to gliomas, whereas their overexpression can increase cell cycle blockage. Furthermore, by targeting CDKs and transcription factors, these miRNAs can act as tumor suppressors, increasing cell cycle suppression and cell proliferation inhibition [
12].
3.3.1. MiRNAs as therapeutic targets for Gliomas:
MiRNAs can be expressed differently in normal and malignant tissues, with possible role in regulating the tumor development, invasion and metastasis. As a result, the aberrant level of expression of miRNAs can worsen the fate of gliomas. In addition, miRNAs are secreted in the cerebrospinal fluid (CSF) and bloodstream. They can freely migrate between normal and tumor cells, making them a potential diagnostic and prognostic biomarker for gliomas [
11].
One way to categorize miRNAs in cancer is based on their regulator function: oncogenic miRNAs (oncomiRs) (
Table 2) and tumor suppressor miRNAs (
Table 3). In miRNA-based therapy, either the inhibition or the mimic of defined miRNAs depending on their expression levels, is utilized. [
41].
Abbreviations:
Bmi-1 (B lymphoma Mo-MLV insertion region 1 homolog), EGFR (Epidermal growth factor receptor), Bmi-1 (B lymphoma Mo-MLV insertion region 1 homolog), SIRT1 (Sirtuin 1), p53 (Tumor protein p53), bcl-2 (B-cell lymphoma 2), TGF- β (Transforming growth factor beta), HOX (Homeobox), EGFR (Epidermal growth factor receptor).
The primary treatment for most central nervous system tumors involves maximally safe surgical resection, which enables accurate histopathological diagnosis, tumor genetic testing, and a reduction in tumor size. This is followed by radiotherapy with concurrent daily temozolomide (TMZ) and an additional six cycles of maintenance TMZ. The medial overall survival for radiotherapy + TMZ was 14.6 months, whereas patients who received radiotherapy had a median survival rate of 12.1 months [
42]. Unfortunately, when treating relapsed glioblastoma, the available options are not well established, and, despite several ongoing investigations, up-to-date none provided conclusive results to support any interventions that could extend overall survival.
Moreover, a considerable proportion of patients may not meet the criteria for second-line therapy. Therefore, some options include further surgical resection, reirradiation, or systemic treatments like lomustine or bevacizumab. [
43].
Zebrafish models for GBM are expected to improve diagnostic and therapeutic approaches, offering hope to GBM patients [
44].
Most studies on macrophages and microglia have found that the upregulation of many genes in GAMs, glioma-associated microglia, and macrophages, is inconsistent with support for GBM progression. Molecular targets offer M1 and M2 identifiers, the pro-inflammatory and anti-inflammatory macrophage subtypes, respectively, in a mixed manner. Despite this debate, some typical M2 options are available for therapeutic intervention. However, studies investigating macrophage polarization markers that encourage tumor growth and using macrophage-targeted therapies for GBM treatment have not yet been conducted in zebrafish models [
44].
Most tumors exhibit dysregulated miRNA expression patterns, and approximately 50% of miRNA-encoded human genes are located in genetic regions associated with cancer. These alterations often lead to a decrease in tumor suppressor genes or an increase in oncogenes, promoting tumor growth [
44]. The overexpression of miRNAs such as miR-7, miR-34a, miR-128, miR-124, miR-137, and miR-181 has been observed to impact GBM growth negatively. In contrast, miR-21, the first oncomiR to be studied, plays a crucial role in detrimental processes associated with GBM by targeting the genes mentioned previously and other genes involved in cell proliferation, survival, and drug resistance. MiRNA-21 is one of the many up-regulated miRNAs that have been identified. Likewise, other miRNAs that are also found to be upregulated in GBM and play pivotal roles in glioma genesis are the miR-17-92 cluster, miR-10b, miR-15b, miR-26a, miR-93, miR-148, miR-182, and miR-221/222 [
44]. Furthermore, by explicitly targeting vital proteins or enzymes involved in metabolic pathways such as glycolysis, oxidative phosphorylation, glutamine, glucose, and lipid metabolism, various miRNAs can facilitate the metabolic alterations common in GBM [
45]. For example, miR-106a and miR-143 target the GLUT-3 transporter and inhibit PKM2 expression, affecting glycolysis in GBM. This same outcome is achieved by targeting HK-2 and let-7a by miR-326, which also inhibits PKM2 expression. MiRNAs that disrupt the function of mitochondria and energetic homeostasis include Lrt-7, miR-16, and miR-23. Other miRNAs in GBM regulate mitochondrial proteins such as ATP5A1 and ATP5B. Given that GBM tumors are highly dependent on aerobic glycolysis, the ability of specific miRNAs to regulate oncogenes and tumor suppressor genes in RTK pathways and their secondary effector pathways is crucial for treating diseases [
46].
Despite the standard treatment for GBM, which includes surgery, radiotherapy, and TMZ, a subsequent recurrence of GBM is very common, and the average survival time is only 12 to 15 months because of the following problems:
High potential for invasion and proliferation, making it challenging to obliterate all tumors.
High mutational capability, which quickly promotes chemotherapeutic drug resistance, like TMZ.
Treatment strategies aimed at targeting and reprogramming tumor-associated macrophages (TAMs) towards M1 anti-tumor macrophages are notably intriguing. These include approaches that alter the tumor microenvironment or inhibit angiogenesis. In experimental murine models, for instance, intertumoral delivery of IL-12 using a modified genetic virus, either alone or in conjunction with immune checkpoint inhibitors (ICIs), demonstrated promising outcomes. Several strategies for genetic, epigenetic, and metabolic remodeling of GBM tumors are being investigated. Radiotherapy, for example, showed anti-tumor efficacy when used alone or combined with histone deacetylase inhibitors and shRNAs against HDAC1 and SIRT1. MiRNA modification is an appealing target because it has the potential to either kill cancer cells or reprogramme the TME. [
44].
4. Potential challenges associated with targeting miRNAs in gliomas:
While targeting miRNAs appears to be a significant breakthrough in glioma therapy, various challenges hinder its clinical application from becoming a reality in the near future. A notable characteristic of miRNAs is their ability to affect a vast network of proteins by targeting multiple mRNAs, while numerous miRNAs can influence a single mRNA. This can be explained by the degeneracy of miRNA target recognition; the binding site of a miRNA is only partially complementary to the target mRNA, allowing some mismatch and resulting in low specificity of miRNAs [
47]. Simultaneously, competition may emerge between miRNAs and other factors for binding sites on specific mRNAs. As a result, targeting a single miRNA could have unintended consequences to the point where other pathways might counteract the desired therapeutic effect [
48].
Furthermore, as a result of alternative splicing, different 3' untranslated regions (UTRs) with different miRNA binding sites may exist, allowing a single miRNA to target many gene isoforms, resulting in divergent regulation [
49]. To demonstrate how the diversity of 3' UTRs impedes miRNA therapy, it was shown that several genes involved in fundamental physiological functions resist miRNA control due to a lack of miRNA binding sites to their short 3' UTRs [
50]. In addition, miRNA diversifies even more during embryonic development due to the extension of 3' UTRs via alternative polyadenylation [
51].
MiRNAs' ability to target multiple mRNAs can lead to challenges in both delivering miRNA therapies and directing them to specific targets, especially under stressful conditions like those found in gliomas. Moreover, miRNAs may potentially relocate across different intracellular compartments, complicating therapy delivery [
52].
Gliomas possess highly heterogeneous microenvironments with varying physiological properties, such as blood flow, throughout the tumor. This can result in inconsistent treatment outcomes due to the altered availability and potency of miRNA therapies [
53]. This heterogeneity can ultimately cause gliomas to develop resistance to miRNA-targeting agents, limiting their effectiveness. Furthermore, gliomas' diverse genomic and phenotypic properties underly the complexity of identifying defined miRNAs consistently dysregulated across various glioma types.
Crosstalk among signaling pathways in gliomas also complicates the identification of specific miRNAs driving tumor growth. For instance, gliomas' commonly activated PI3K/AKT and MAPK/ERK pathways promote cell survival, proliferation, and migration. However, their interaction makes it challenging to pinpoint specific miRNAs as therapy targets and contributes to gliomas becoming more resistant [
54]. This highlights the need to understand the complex interplay between different pathways in developing effective treatment options.
Lastly, clinical trials for miRNA-based therapeutics have not yet been conducted due to prolonged and challenging regulatory clearances and substantial investment requirements.
5. Conclusion:
Dysregulated miRNA expression has been connected to tumor progression and unfavourable prognosis in glioma patients. This review highlights the numerous studies pinpointing specific miRNAs associated with glioma progression and prognosis. For instance, miR-21, miR-10b, and miR-221/222 have been identified as upregulated in glioma tissues, correlating with tumor aggressiveness and poor patient survival. Conversely, miR-7, miR-128, and miR-124 have been observed as downregulated in glioma tissues, relating to improved prognosis. Despite this, specific miRNAs can serve as diagnostic markers for glioma, and preclinical studies indicate that miRNA-targeting therapeutic interventions hold promise. Nevertheless, certain miRNAs could potentially act as diagnostic indicators for glioma, and preliminary studies imply that therapeutic strategies targeting miRNAs show potential.
Author Contributions
O.T., M.M., A.A., A.A., S.A., and R.A. were all involved in writing the main manuscript; O.T. wrote the abstract and was responsible for compiling and organizing the work for publishing; A.A. and A.A. prepared
Table 1 and
Figure 1; S.A. and R.A. prepared
Table 2 and
Table 3; C.M. reviewed and edited the paper; S.P. conceptualized, supervised, edited, and revised the initial and final draft; All authors read and approved the final manuscript.
Funding
Qatar National Research Fund, Grant ID: UREP29-044-3-013.
References
- Gousias, K., Theocharous, T., & Simon, M. (2022). Mechanisms of Cell Cycle Arrest and Apoptosis in Glioblastoma. Biomedicines, 10(3), 564. https://doi.org/10.3390/biomedicines10030564. [CrossRef]
- Davis, M. E. (2018). Epidemiology and overview of gliomas. Seminars in Oncology Nursing, 34(5), 420–429. [CrossRef]
- Fan, Y. , Zhang, X., Gao, C., Jiang, S., Wu, H., Liu, Z., & Dou, T. (2022). Burden and trends of brain and central nervous system cancer from 1990 to 2019 at the Global, regional, and Country Levels. Archives of Public Health, 80(1). [CrossRef]
- Mohammadi, E. , Moghaddam, S. S., Azadnajafabad, S., Maroufi, S. F., Rashidi, M. M., Naderian, M., Jafari, A., Sharifi, G., Ghasemi, E., Rezaei, N., Malekpour, M. R., Kompani, F., Rezaei, N., Larijani, B., & Farzadfar, F. (2023). Epidemiology of Brain and Other Central Nervous System Cancers in the North Africa and Middle East Region: A Systematic Analysis of the Global Burden of Disease Study 1990-2019. World Neurosurgery, 171, 796-819. [CrossRef]
- Yan, H. , Parsons, D. W., Jin, G., McLendon, R., Rasheed, B. A., Yuan, W., Kos, I., Batinic-Haberle, I., Jones, S., Riggins, G. J., Friedman, H., Friedman, A., Reardon, D., Herndon, J., Kinzler, K. W., Velculescu, V. E., Vogelstein, B., & Bigner, D. D. (2009). IDH1 and IDH2 mutations in gliomas. The New England Journal of Medicine, 360(8), 765-773. [CrossRef]
- Tomei, S. , Ibnaof, O., Ravindran, S., Ferrone, S., & Maccalli, C. (2021). Cancer stem cells are possible key players in regulating anti-tumor immune responses: The role of immunomodulating molecules and microRNAs. Cancers (Basel), 13(7), 1674. [CrossRef]
- Tomei, S., Volontè, A., Ravindran, S., Mazzoleni, S., Wang, E., Galli, R., & Maccalli, C. (2021). MicroRNA expression profile distinguishes glioblastoma stem cells from differentiated tumor cells. Journal of Personalized Medicine, 11(4), 264. [CrossRef]
- Rezaei, O. , Honarmand, K., Nateghinia, S., Taheri, M., & Ghafouri-Fard, S. (2020). miRNA signature in glioblastoma: Potential biomarkers and therapeutic targets. Experimental and Molecular Pathology, 117, 104550. [CrossRef]
- Buruiană, A. , Florian, Ș. I., Florian, A. I., Timiș, T.-L., Mihu, C. M., Miclăuș, M., Oșan, S., Hrapșa, I., Cataniciu, R. C., Farcaș, M., & Șușman, S. (2020). The roles of MIRNA in glioblastoma tumor cell communication: Diplomatic and aggressive negotiations. International Journal of Molecular Sciences, 21(6), 1950. [CrossRef]
- Beylerli, O. , Gareev, I., Sufianov, A., Ilyasova, T., & Zhang, F. (2022). The role of microrna in the pathogenesis of glial brain tumors. Non-Coding RNA Research, 7(2), 71–76. [CrossRef]
- Mafi, A. , Rahmati, A., Babaei Aghdam, Z., Salami, R., Salami, M., Vakili, O., & Aghadavod, E. (2022). Recent insights into the microRNA-dependent modulation of gliomas from pathogenesis to diagnosis and treatment. Cell Molecular Biology Letters, 27(1), 65. [CrossRef]
- Sati, I. S., & Parhar, I. (2021). MicroRNAs regulate cell cycle and cell death pathways in glioblastoma. International Journal of Molecular Sciences, 22(24), 13550. [CrossRef]
- Gaur, A. B. , Holbeck, S. L., Colburn, N. H., & Israel, M. A. (2011). Downregulation of Pdcd4 by miR-21 facilitates glioblastoma proliferation in vivo. Neuro-Oncology, 13(6), 580–590. [CrossRef]
- Chen, L. , Zhang, J., Han, L., Zhang, A., Zhang, C., Zheng, Y.,... Kang, C. (2012). Downregulation of miR-221/222 sensitizes glioma cells to temozolomide by regulating apoptosis independently of p53 status. Oncology Reports, 27, 854-860. [CrossRef]
- Cheng, W. , Ren, X., Zhang, C., Han, S., & Wu, A. (2017). Expression and prognostic value of microRNAs in lower-grade glioma depends on IDH1/2 status. Journal of Neuro-Oncology, 132, 207–218. [CrossRef]
- Würdinger, T. , Tannous, B. A., Saydam, O., Skog, J., Grau, S., Soutschek, J., Weissleder, R., Breakefield, X. O., & Krichevsky, A. M. (2008). miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell, 14(5), 382-393. [CrossRef]
- Fang, L. , Deng, Z., Shatseva, T., Yang, J., Peng, C., Du, W. W., Yee, A. J., Ang, L. C., He, C., Shan, S. W., & Yang, B. B. (2011). MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-β8. Oncogene, 30, 806–821. [CrossRef]
- Sadeghipour, N. , Kumar, S. U., Massoud, T. F., & Paulmurugan, R. (2022). A rationally identified panel of microRNAs targets multiple oncogenic pathways to enhance chemotherapeutic effects in glioblastoma models. Scientific Reports, 12(1). [CrossRef]
- Qiu, S. , Huang, D., Yin, D., Li, F., Li, X., Kung, H.-fu, & Peng, Y. (2013). Suppression of tumorigenicity by microRNA-138 through inhibition of EZH2-CDK4/6-PRB-E2F1 signal loop in glioblastoma multiforme. Biochimica Et Biophysica Acta (BBA) - Molecular Basis of Disease, 1832(10), 1697–1707. [CrossRef]
- Vinchure, O. S. , Whittemore, K., Kushwah, D., Blasco, M. A., & Kulshreshtha, R. (2021). miR-490 suppresses telomere maintenance program and associated hallmarks in glioblastoma. Cellular and Molecular Life Sciences, 78(5), 2299–2314. [CrossRef]
- Adlakha, Y. K. , & Saini, N. (2014). Brain Micrornas and insights into biological functions and therapeutic potential of brain enriched MIRNA-128. Molecular Cancer, 13(1), 33. [CrossRef]
- Franzoni, E. , Booker, S. A., Parthasarathy, S., Rehfeld, F., Grosser, S., Srivatsa, S., Fuchs, H. R., Tarabykin, V., Vida, I., & Wulczyn, F. G. (2015). Mir-128 regulates neuronal migration, outgrowth and intrinsic excitability via the intellectual disability gene PHF6. eLife, 4. [CrossRef]
- Peruzzi, P. , Bronisz, A., Nowicki, M. O., Wang, Y., Ogawa, D., Price, R., Nakano, I., Kwon, C.-H., Hayes, J., Lawler, S. E., Ostrowski, M. C., Chiocca, E. A., & Godlewski, J. (2013). MicroRNA-128 coordinately targets polycomb repressor complexes in Glioma Stem Cells. Neuro-Oncology, 15(9), 1212–1224. [CrossRef]
- Reséndiz-Castillo, L. J. , Minjarez, B., Reza-Zaldívar, E. E., Hernández-Sapiéns, M. A., Gutiérrez-Mercado, Y. K., & Canales-Aguirre, A. A. (2022). The effects of altered neurogenic microRNA levels and their involvement in the aggressiveness of periventricular glioblastoma. Neurologia (English Edition), 37(9), 781-793. [CrossRef]
- Yang, J. , Zhang, X., Chen, X., Wang, L., & Yang, G. (2017). Exosome mediated delivery of Mir-124 promotes neurogenesis after ischemia. Molecular Therapy - Nucleic Acids, 7, 278–287. [CrossRef]
- Olde Loohuis, N. F., Kos, A., Martens, G. J., Van Bokhoven, H., Nadif Kasri, N., & Aschrafi, A. (2011). MicroRNA networks direct neuronal development and plasticity. Cellular and Molecular Life Sciences, 69(1), 89–102. [CrossRef]
- Ye, X., Wei, W., Zhang, Z., He, C., Yang, R., Zhang, J., Wu, Z., Huang, Q., & Jiang, Q. (2017). Identification of microRNAs associated with glioma diagnosis and prognosis. Oncotarget, 8(16), 26394-26403. [CrossRef]
- Mahmoudi, E. , & Cairns, M. J. (2017). Mir-137: An important player in neural development and Neoplastic Transformation. Molecular Psychiatry, 22(1), 44–55. [CrossRef]
- Sun, G. Q. , Ye, P., Murai, K., Lang, M.-F., Li, S., Zhang, H., Li, W., Fu, C., Yin, J., Wang, A., Ma, X., & Shi, Y. (2011). miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nature Communications, 2(1). [CrossRef]
- Koni, M. , Pinnarò, V., & Brizzi, M. F. (2020). The Wnt signalling pathway: A tailored target in cancer. International Journal of Molecular Sciences, 21(20), 7697. [CrossRef]
- Lee, Y., Lee, J.-K., Ahn, S. H., Lee, J., & Nam, D.-H. (2016). Wnt signaling in glioblastoma and therapeutic opportunities. Laboratory Investigation, 96(2), 137-150. [CrossRef]
- Lecarpentier, Y. , Schussler, O., Hébert, J.-L., & Vallée, A. (2019). Multiple targets of the canonical Wnt/β-catenin signaling in cancers. Frontiers in Oncology, 9, 1248. [CrossRef]
- Tabnak, P. , Mafakheri, A., Haji Emsailpoor, Z., Kazemi, T., & Shekari, N. (2021). Regulatory interplay between microRNAs and Wnt pathway in glioma. Biomedicine & Pharmacotherapy, 143, 112187. [CrossRef]
- Zhang, Y. , Dube, C., Gibert, M., Cruickshanks, N., Wang, B., Coughlan, M., Yang, Y., Setiady, I., Deveau, C., Saoud, K., Grello, C., Oxford, M., Yuan, F., & Abounader, R. (2018). The P53 pathway in glioblastoma. Cancers, 10(9), 297. [CrossRef]
- Griveau, A. , Seano, G., Shelton, S. J., et al. (2018). A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell, 33(5), 874-889.e7. [CrossRef]
- Wang, G. , Zhao, Y., & Zheng, Y. (2014). miR-122/Wnt/β-catenin regulatory circuitry sustains glioma progression. Tumor Biology, 35(9), 8565–8572. [CrossRef]
- Tabnak, P. , Mafakheri, A., Haji Emsailpoor, Z., Kazemi, T., & Shekari, N. (2021). Regulatory interplay between microRNAs and Wnt pathway in glioma. Biomedicine & Pharmacotherapy, 143, 112187. [CrossRef]
- Wang, T.-J. , Huang, M.-S., Hong, C.-Y., Tse, V., Silverberg, G. D., & Hsiao, M. (2001). Comparisons of tumor suppressor p53, p21, and p16 gene therapy effects on glioblastoma tumorigenicity in situ. Biochemical and Biophysical Research Communications, 287(1), 173–180. [CrossRef]
- Aloizou, A.-M. , Pateraki, G., Siokas, V., Mentis, A.-F. A., Liampas, I., Lazopoulos, G., Kovatsi, L., Mitsias, P. D., Bogdanos, D. P., Paterakis, K., & Dardiotis, E. (2020). The role of mirna-21 in gliomas: Hope for a novel therapeutic intervention? Toxicology Reports, 7, 1514–1530. [CrossRef]
- Ezhevsky, S. A. , Ho, A., Becker-Hapak, M., Davis, P. K., & Dowdy, S. F. (2001). Differential regulation of retinoblastoma tumor suppressor protein by G1 cyclin-dependent kinase complexes in vivo. Molecular and Cellular Biology, 21(14), 4773–4784. [CrossRef]
- 41. Chen M, Medarova Z, Moore A. (2021). Role of microRNAs in glioblastoma. Oncotarget, 12(17), 1707-1723. [CrossRef]
- Stupp, R., Mason, W. P., & van den Bent, M. J. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. Oncology Times, 27(9), 15–16. [CrossRef]
- Tan, A. C. , Ashley, D. M., López, G. Y., Malinzak, M., Friedman, H. S., & Khasraw, M. (2020). Management of glioblastoma: State of the art and future directions. CA: A Cancer Journal for Clinicians, 70(4), 299–312. [CrossRef]
- Reimunde, P. , Pensado-López, A., Carreira Crende, M., Lombao Iglesias, V., Sánchez, L., Torrecilla-Parra, M., Ramírez, C. M., Anfray, C., & Torres Andón, F. (2021). Cellular and molecular mechanisms underlying glioblastoma and zebrafish models for the discovery of new treatments. Cancers, 13(5), 1087. [CrossRef]
- Dong, Z., & Cui, H. (2019). Epigenetic modulation of metabolism in glioblastoma. Seminars in Cancer Biology, 57, 45–51. [CrossRef]
- Fernández-de Frutos, M. , Galán-Chilet, I., Goedeke, L., Kim, B., Pardo-Marqués, V., Pérez-García, A., Herrero, J. I., Fernández-Hernando, C., Kim, J., & Ramírez, C. M. (2019). MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor Substrate 2, Insulin Receptor, Insulin-Degrading Enzyme, and Liver X Receptor Pathway. Molecular Cell Biology, 39(22). [CrossRef]
- Bagga, S. , Bracht, J., Hunter, S., Massirer, K., Holtz, J., Eachus, R., & Pasquinelli, A. E. (2005). Regulation by let-7 and lin-4 mirnas results in target mrna degradation. Cell, 122(4), 553–563. [CrossRef]
- Correia de Sousa, M. , Gjorgjieva, M., Dolicka, D., Sobolewski, C., & Foti, M. (2019). Deciphering miRNAs' Action through miRNA Editing. International Journal of Molecular Sciences, 20(24), 6249. [CrossRef]
- Wu, D. , Khan, F. A., Huo, L., Sun, F., & Huang, C. (2022). Alternative splicing and MicroRNA: Epigenetic mystique in male reproduction. RNA Biology, 19(1), 162-175. [CrossRef]
- Stark, A. , Brennecke, J., Bushati, N., Russell, R. B., & Cohen, S. M. (2005). Animal microRNAs confer robustness to gene expression and have a significant impact on 3'UTR evolution. Cell, 123(6), 1133-1146. [CrossRef]
- Ji, Z. , Lee, J. Y., Pan, Z., Jiang, B., & Tian, B. (2009). Progressive lengthening of 3' untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic development. Proceedings of the National Academy of Sciences, 106(17), 7028-7033. [CrossRef]
- Turunen, T. A. , Roberts, T. C., Laitinen, P., Väänänen, M.-A., Korhonen, P., Malm, T., Ylä-Herttuala, S., & Turunen, M. P. (2019). Changes in nuclear and cytoplasmic microRNA distribution in response to hypoxic stress. Scientific Reports, 9(1). [CrossRef]
- Nicholson, J. G., & Fine, H. A. (2021). Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discovery, 11(3), 575-590. [CrossRef]
- Zhou, J. , Du, T., Li, B., Rong, Y., Verkhratsky, A., & Peng, L. (2015). Crosstalk Between MAPK/ERK and PI3K/AKT Signal Pathways During Brain Ischemia/Reperfusion. ASN Neuro, 7(5), 1759091415602463. [CrossRef]
- Mizoguchi, M., Guan, Y., Yoshimoto, K., Hata, N., Amano, T., Nakamizo, A., & Sasaki, T. (2013). Clinical implications of microRNAs in human glioblastoma. Frontiers in Oncology, 3, 19. [CrossRef]
- Guessous, F. , Alvarado-Velez, M., Marcinkiewicz, L., Zhang, Y., Kim, J., Heister, S., Kefas, B., Godlewski, J., Schiff, D., Purow, B., & Abounader, R. (2013). Oncogenic effects of miR-10b in glioblastoma stem cells. Journal of Neuro-Oncology, 112(2), 153–163. [CrossRef]
- Shan, Z. N. , Tian, R., Zhang, M., Gui, Z. H., Wu, J., Ding, M., Zhou, X. F., & He, J. (2016). miR128-1 inhibits the growth of glioblastoma multiforme and glioma stem-like cells via targeting BMI1 and E2F3. Oncotarget, 7(48), 78813-78826. [CrossRef]
- Ma, R. , Yan, W., Zhang, G., Lv, H., Liu, Z., Fang, F., Zhang, W., Zhang, J., Tao, T., You, Y., Jiang, T., & Kang, X. (2012). Upregulation of miR-196b confers a poor prognosis in glioblastoma patients via inducing a proliferative phenotype. PLoS One, 7(6), e38096. [CrossRef]
- Xie, T. , Liu, P., Chen, L., Chen, Z., Luo, Y., Chen, X., Feng, Y., & Luo, X. (2015). MicroRNA-15a down-regulation is associated with adverse prognosis in human glioma. Clinical and Translational Oncology, 17(7), 504-510. [CrossRef]
- Liu, Z. , Liu, Y., Li, L., Xu, Z., Bi, B., Wang, Y., & Li, J. Y. (2014). MiR-7-5p is frequently downregulated in glioblastoma microvasculature and inhibits vascular endothelial cell proliferation by targeting RAF1. Tumour Biology, 35(10), 10177-10184. [CrossRef]
- Kefas, B. , Godlewski, J., Comeau, L., Li, Y., Abounader, R., Hawkinson, M., Lee, J., Fine, H., Chiocca, E. A., Lawler, S., & Purow, B. (2008). microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Research, 68(10), 3566-3572. [CrossRef]
- Luan, S. , Sun, L., & Huang, F. (2010). MicroRNA-34a: A novel tumor suppressor in p53-mutant glioma cell line U251. Archives of Medical Research, 41(2), 67-74. [CrossRef]
- Rathod, S. S. , Rani, S. B., Khan, M., Muzumdar, D., & Shiras, A. (2014). Tumor suppressive miRNA-34a suppresses cell proliferation and tumor growth of glioma stem cells by targeting Akt and Wnt signaling pathways. FEBS Open Bio, 4, 485-495. [CrossRef]
- Fan, Y. N. , Meley, D., Pizer, B., & Sée, V. (2014). Mir-34a mimics are potential therapeutic agents for p53-mutated and chemo-resistant brain tumor cells. PLoS One, 9(9). [CrossRef]
- Bhaskaran V, Nowicki MO, Idriss M, Jimenez MA, Lugli G, Hayes JL, Mahmoud AB, Zane RE, Passaro C, Ligon KL, Haas-Kogan D, Bronisz A, Godlewski J, Lawler SE, Chiocca EA, Peruzzi P. (2019). The functional synergism of microRNA clustering provides therapeutically relevant epigenetic interference in glioblastoma. Nature Communications, 10(1), 442. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).