Submitted:
27 June 2023
Posted:
28 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Immune cells in PsA pathogenesis
3. Key inflammatory cytokines in PsA
3.1. Role of TNF-α in PsA
3.2. Role of IL-23 in PsA
3.3. Role of IL-17 in PsA
3.4. Other cytokines or chemokines in PsA
4. Cytokine-targeted therapies in PsA
4.1. TNF-α inhibitors in PsA
4.2. IL-17 inhibitors in psoriatic arthritis
4.3. IL-23 inhibitors in PsA
4.4. JAKi in PsA
4.5. Other pharmacological treatments
5. Future perspectives and conclusion
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Rahman, P.; Ritchlin, C.; McInnes, I.B.; Elewaut, D.; Scher, J.U. Psoriatic arthritis from a mechanistic perspective. Nat. Rev. Rheumatol. 2022, 18, 311–325. [Google Scholar] [CrossRef] [PubMed]
- López-Medina, C.; Molto, A.; Sieper, J.; Duruöz, T.; Kiltz, U.; Elzorkany, B.; Hajjaj-Hassouni, N.; Burgos-Vargas, R.; Maldonado-Cocco, J.; Ziade, N.; et al. Prevalence and distribution of peripheral musculoskeletal manifestations in spondyloarthritis including psoriatic arthritis: results of the worldwide, cross-sectional ASAS-PerSpA study. RMD Open 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Tan, Y.; Yao, A.; Yang, X.; He, Y. Psoriasis to psoriatic arthritis: the application of proteomics technologies. Front. Med. (Lausanne) 2021, 8, 681172. [Google Scholar] [CrossRef] [PubMed]
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.; Stafford, L.; Bresnihan, B.; FitzGerald, O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatol. (Oxf. Engl.) 2003, 42, 1460–1468. [Google Scholar] [CrossRef]
- Haroon, M.; Gallagher, P.; FitzGerald, O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 1045–1050. [Google Scholar] [CrossRef]
- Queiro, R.; Morante, I.; Cabezas, I.; Acasuso, B. . HLA-B27 and psoriatic disease: a modern view of an old relationship. Rheumatol. (Oxf. Engl.) 2016, 55, 221–229. [Google Scholar] [CrossRef]
- Muto, M.; Date, Y.; Ichimiya, M.; Moriwaki, Y.; Mori, K.; Kamikawaji, N.; Kimura, A.; Sasazuki, T.; Asagami, C. Significance of antibodies to streptococcal M protein in psoriatic arthritis and their association with HLA-a*0207. Tissue Antigens 1996, 48, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Thorarensen, S.M.; Lu, N.; Ogdie, A.; Gelfand, J.M.; Choi, H.K.; Love, T.J. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis. 2017, 76, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Boix-Amorós, A.; Badri, M.H.; Manasson, J.; Blank, R.B.; Haberman, R.H.; Neimann, A.L.; Girija, P.V.; Jimenez Hernandez, A.; Heguy, A.; Koralov, S.B.; et al. Alterations in the cutaneous microbiome of patients with psoriasis and psoriatic arthritis reveal similarities between non-lesional and lesional skin. Ann. Rheum. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jadon, D.R.; Stober, C.; Pennington, S.R.; FitzGerald, O. Applying precision medicine to unmet clinical needs in psoriatic disease. Nat. Rev. Rheumatol. 2020, 16, 609–627. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J. Eur. Acad. Dermatol. Venereol. 2009, 23 suppl 1, 9–13. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic arthritis: pathogenesis and targeted therapies. Int. J. Mol. Sci. 2023, 24, 4901. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, O.; Winchester, R. Psoriatic arthritis: from pathogenesis to therapy. Arthritis Res. Ther. 2009, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Candia, L.; Marquez, J.; Hernandez, C.; Zea, A.H.; Espinoza, L.R. Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: a pathogenic role for innate immunity? J. Rheumatol. 2007, 34, 374–379. [Google Scholar]
- Jongbloed, S.L.; Lebre, M.C.; Fraser, A.R.; Gracie, J.A.; Sturrock, R.D.; Tak, P.P.; McInnes, I.B. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R15. [Google Scholar] [CrossRef]
- Lew, W.; Bowcock, A.M.; Krueger, J.G. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and "Type 1" inflammatory gene expression. Trends Immunol. 2004, 25, 295–305. [Google Scholar] [CrossRef]
- van Mens, L.J.J.; van de Sande, M.G.H.; Menegatti, S.; Chen, S.; Blijdorp, I.C.J.; de Jong, H.M.; Fluri, I.A.; Latuhihin, T.E.; van Kuijk, A.W.R.; Rogge, L.; et al. Brief report: interleukin-17 blockade with secukinumab in peripheral spondyloarthritis impacts synovial immunopathology without compromising systemic immune responses. Arthritis Rheumatol. 2018, 70, 1994–2002. [Google Scholar] [CrossRef]
- Van Raemdonck, K.; Umar, S.; Palasiewicz, K.; Romay, B.; Volkov, S.; Arami, S.; Sweiss, N.; Shahrara, S. TLR7 endogenous ligands remodel glycolytic macrophages and trigger skin-to-joint crosstalk in psoriatic arthritis. Eur. J. Immunol. 2021, 51, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Lai, C.Y.; Yeh, D.W.; Liu, Y.L.; Su, Y.W.; Hsu, L.C.; Chang, C.H.; Catherine Jin, S.L.; Chuang, T.H. Involvement of M1 macrophage polarization in endosomal toll-like receptors activated psoriatic inflammation. Mediators Inflamm. 2018, 2018, 3523642. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, B.; Noordenbos, T.; Ambarus, C.; Krausz, S.; Cantaert, T.; Yeremenko, N.; Boumans, M.; Lutter, R.; Tak, P.P.; Baeten, D. Absence of a classically activated macrophage cytokine signature in peripheral spondylarthritis, including psoriatic arthritis. Arthritis Rheum. 2009, 60, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Nigrovic, P.A.; Lee, D.M. Mast cells in inflammatory arthritis. Arthritis Res. Ther. 2005, 7, 1–11. [Google Scholar] [CrossRef]
- Noordenbos, T.; Yeremenko, N.; Gofita, I.; van de Sande, M.; Tak, P.P.; Caňete, J.D.; Baeten, D. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 2012, 64, 99–109. [Google Scholar] [CrossRef]
- Chen, S.; Noordenbos, T.; Blijdorp, I.; van Mens, L.; Ambarus, C.A.; Vogels, E.; Te Velde, A.; Alsina, M.; Cañete, J.D.; Yeremenko, N.; et al. Histologic evidence that mast cells contribute to local tissue inflammation in peripheral spondyloarthritis by regulating interleukin-17A content. Rheumatol. (Oxf. Engl.) 2019, 58, 617–627. [Google Scholar] [CrossRef]
- Leijten, E.F.; van Kempen, T.S.; Boes, M.; Michels-van Amelsfort, J.M.; Hijnen, D.; Hartgring, S.A.; van Roon, J.A.; Wenink, M.H.; Radstake, T.R. Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid. Arthritis Rheumatol. 2015, 67, 2673–2678. [Google Scholar] [CrossRef]
- Soare, A.; Weber, S.; Maul, L.; Rauber, S.; Gheorghiu, A.M.; Luber, M.; Houssni, I.; Kleyer, A.; von Pickardt, G.; Gado, M.; et al. Cutting edge: homeostasis of innate lymphoid cells is imbalanced in psoriatic arthritis. J. Immunol. 2018, 200, 1249–1254. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Bogdanos, D.P. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun. Rev. 2017, 16, 10–15. [Google Scholar] [CrossRef]
- Tang, F.; Sally, B.; Ciszewski, C.; Abadie, V.; Curran, S.A.; Groh, V.; Fitzgerald, O.; Winchester, R.J.; Jabri, B. Interleukin 15 primes natural killer cells to kill via NKG2D and cPLA2 and this pathway is active in psoriatic arthritis. PLOS ONE 2013, 8, e76292. [Google Scholar] [CrossRef]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 968549. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; McInnes, I.B. Primed for inflammation: enthesis-resident T cells. Nat. Med. 2012, 18, 1018–1019. [Google Scholar] [CrossRef] [PubMed]
- Veale, D.J.; Ritchlin, C.; FitzGerald, O. Immunopathology of psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2005, 64 suppl 2, ii26–ii29. [Google Scholar] [CrossRef] [PubMed]
- Diani, M.; Altomare, G.; Reali, E. T cell responses in psoriasis and psoriatic arthritis. Autoimmun. Rev. 2015, 14, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, B.W.; Kavanaugh, A.; Reich, K. Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 2014, 141, 133–142. [Google Scholar] [CrossRef]
- Menon, B.; Gullick, N.J.; Walter, G.J.; Rajasekhar, M.; Garrood, T.; Evans, H.G.; Taams, L.S.; Kirkham, B.W. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014, 66, 1272–1281. [Google Scholar] [CrossRef]
- Penkava, F.; Velasco-Herrera, M.D.C.; Young, M.D.; Yager, N.; Nwosu, L.N.; Pratt, A.G.; Lara, A.L.; Guzzo, C.; Maroof, A.; Mamanova, L.; et al. Single-cell sequencing reveals clonal expansions of pro-inflammatory synovial CD8 T cells expressing tissue-homing receptors in psoriatic arthritis. Nat. Commun. 2020, 11, 4767. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, S.Y.; Kawakami, A.; Okada, A.; Koga, T.; Tamai, M.; Yamasaki, S.; Nakamura, H.; Origuchi, T.; Ida, H.; Eguchi, K. CD4+CD25(high)CD127(low/-) Treg cell frequency from peripheral blood correlates with disease activity in patients with rheumatoid arthritis. J. Rheumatol. 2011, 38, 2517–2521. [Google Scholar] [CrossRef]
- Yun, W.J.; Lee, D.W.; Chang, S.E.; Yoon, G.S.; Huh, J.R.; Won, C.H.; Lee, M.W.; Kim, S.E.; Kim, B.J.; Moon, K.C.; et al. Role of CD4CD25FOXP3 regulatory T cells in psoriasis. Ann. Dermatol. 2010, 22, 397–403. [Google Scholar] [CrossRef]
- Sugiyama, H.; Gyulai, R.; Toichi, E.; Garaczi, E.; Shimada, S.; Stevens, S.R.; McCormick, T.S.; Cooper, K.D. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2005, 174, 164–173. [Google Scholar] [CrossRef]
- Schett, G.; McInnes, I.B.; Neurath, M.F. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 2021, 385, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Saddala, M.S.; Huang, H. Identification of novel inhibitors for TNFα, TNFR1 and TNFα-TNFR1 complex using pharmacophore-based approaches. J. Transl. Med. 2019, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Zamri, F.; de Vries, T.J. Use of TNF inhibitors in rheumatoid arthritis and implications for the periodontal status: for the benefit of both? Front. Immunol. 2020, 11, 591365. [Google Scholar] [CrossRef] [PubMed]
- de Vries, T.J.; El Bakkali, I.; Kamradt, T.; Schett, G.; Jansen, I.D.C.; D'Amelio, P. What are the peripheral blood determinants for increased osteoclast formation in the various inflammatory diseases associated with bone loss? Front. Immunol. 2019, 10, 505. [Google Scholar] [CrossRef]
- Kaaij, M.H.; van Tok, M.N.; Blijdorp, I.C.; Ambarus, C.A.; Stock, M.; Pots, D.; Knaup, V.L.; Armaka, M.; Christodoulou-Vafeiadou, E.; van Melsen, T.K.; et al. Transmembrane TNF drives osteoproliferative joint inflammation reminiscent of human spondyloarthritis. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Silvagni, E.; Missiroli, S.; Perrone, M.; Patergnani, S.; Boncompagni, C.; Bortoluzzi, A.; Govoni, M.; Giorgi, C.; Alivernini, S.; Pinton, P.; et al. From bed to bench and back: TNF-α, IL-23/IL-17A, and JAK-dependent inflammation in the pathogenesis of psoriatic synovitis. Front. Pharmacol. 2021, 12, 672515. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Haas-Smith, S.A.; Li, P.; Hicks, D.G.; Schwarz, E.M. Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J. Clin. Invest. 2003, 111, 821–831. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, H.L.; Kagami, S.; Phillips, K.G.; Kurtz, S.E.; Jacques, S.L.; Blauvelt, A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J. Immunol. 2011, 186, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, Z.; Gracey, E.; Venken, K.; Ritchlin, C.; Elewaut, D. Crossing the boundaries: il-23 and its role in linking inflammation of the skin, gut and joints. Rheumatol. (Oxf. Engl.) 2021, 60 Supplement 4, iv16–iv27. [Google Scholar] [CrossRef]
- Sherlock, J.P.; Cua, D.J. Interleukin-23 in perspective. Rheumatol. (Oxf. Engl.) 2021, 60 Supplement 4, iv1–iv3. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deshpande, M.; Grisotto, M.; Smaldini, P.; Garcia, R.; He, Z.; Gulko, P.S.; Lira, S.A.; Furtado, G.C. Skin expression of IL-23 drives the development of psoriasis and psoriatic arthritis in mice. Sci. Rep. 2020, 10, 8259. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.K.; Abria, C.; Mitra, A.; Raychaudhuri, S.P. Functional significance of MAIT cells in psoriatic arthritis. Cytokine 2020, 125, 154855. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Østergaard, M.; Wei, J.C.; Sieper, J.; Järvinen, P.; Tam, L.S.; Salvarani, C.; Kim, T.H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 2018, 77, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.; Kavanaugh, A. Psoriatic arthritis: current therapy and future approaches. Rheumatol. (Oxf. Engl.) 2015, 54, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Hayami, Y.; Naniwa, T.; Ueda, R. The Th17/IL-23 axis and natural immunity in psoriatic arthritis. Int. J. Rheumatol. 2012, 2012, 539683. [Google Scholar] [CrossRef]
- Tam, H.K.J.; Robinson, P.C.; Nash, P. Inhibiting IL-17A and IL-17F in rheumatic disease: therapeutics help to elucidate disease mechanisms. Curr. Rheumatol. Rep. 2022, 24, 310–320. [Google Scholar] [CrossRef]
- Iznardo, H.; Puig, L. Dual inhibition of IL-17A and IL-17F in psoriatic disease. Ther. Adv. Chronic Dis. 2021, 12, 20406223211037846. [Google Scholar] [CrossRef]
- Millar, N.L.; Akbar, M.; Campbell, A.L.; Reilly, J.H.; Kerr, S.C.; McLean, M.; Frleta-Gilchrist, M.; Fazzi, U.G.; Leach, W.J.; Rooney, B.P.; et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep. 2016, 6, 27149. [Google Scholar] [CrossRef]
- McGonagle, D.G.; McInnes, I.B.; Kirkham, B.W.; Sherlock, J.; Moots, R. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann. Rheum. Dis. 2019, 78, 1167–1178. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Schett, G. Effects of the IL-23–IL-17 pathway on bone in spondyloarthritis. Nat. Rev. Rheumatol. 2018, 14, 631–640. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Mease, P.J.; Gomez-Reino, J.J.; Adebajo, A.O.; Wollenhaupt, J.; Gladman, D.D.; Lespessailles, E.; Hall, S.; Hochfeld, M.; Hu, C.; et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann. Rheum. Dis. 2014, 73, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Anderson, J.K.; Magrey, M.; Merola, J.F.; Liu, Y.; Kishimoto, M.; Jeka, S.; Pacheco-Tena, C.; Wang, X.; Chen, L.; et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N. Engl. J. Med. 2021, 384, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, I.; Nakayamada, S.; Ueno, M.; Miyazaki, Y.; Iwata, S.; Kubo, S.; Sonomoto, K.; Anan, J.; Ohkubo, N.; Inoue, Y.; et al. Impact of serum interleukin-22 as a biomarker for the differential use of molecular targeted drugs in psoriatic arthritis: a retrospective study. Arthritis Res. Ther. 2022, 24, 86. [Google Scholar] [CrossRef]
- Kwon, O.C.; Park, M.C.; Kim, Y.G. Interleukin-32 as a biomarker in rheumatic diseases: A narrative review. Front. Immunol. 2023, 14, 1140373. [Google Scholar] [CrossRef]
- Al-Shobaili, H.A.; Farhan, J.; Zafar, U.; Rasheed, Z. Functional role of human interleukin-32 and nuclear transcription factor-kB in patients with psoriasis and psoriatic arthritis. Int. J. Health Sci. (Qassim) 2018, 12, 29–34. [Google Scholar]
- Dong, Y.; Zhong, J.; Dong, L. IL-33 in rheumatic diseases. Front. Med. (Lausanne) 2021, 8, 739489. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Bertino, L.; Di Salvo, E.; Papaianni, V.; Ventura-Spagnolo, E.; Gangemi, S. Possible roles of IL-33 in the innate-adaptive immune crosstalk of psoriasis pathogenesis. Mediators Inflamm. 2019, 2019, 7158014. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Rui, W.; Li, X.; Xuan, D.; Zheng, S.; Yu, Y.; Zhang, J.; Kong, N.; Zhu, X.; et al. New interleukins in psoriasis and psoriatic arthritis patients: the possible roles of interleukin-33 to Interleukin-38 in disease activities and bone erosions. Dermatology 2017, 233, 37–46. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Simon, D.; Nas, K.; Zaiss, M.M.; Luo, Y.; Zhao, Y.; Rech, J.; Schett, G. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Arthritis Res. Ther. 2020, 22, 26. [Google Scholar] [CrossRef]
- Abdelaal, N.H.; Elhefnawy, N.G.; Abdulmonem, S.R.; Sayed, S.; Saleh, N.A.; Saleh, M.A. Evaluation of the expression of the stromal cell-derived factor-1 alpha (CXCL 12) in psoriatic patients after treatment with methotrexate. J. Cosmet. Dermatol. 2020, 19, 253–258. [Google Scholar] [CrossRef]
- Yager, N.; Cole, S.; Lledo Lara, A.; Maroof, A.; Penkava, F.; Knight, J.C.; Bowness, P.; Al-Mossawi, H. Ex vivo mass cytometry analysis reveals a profound myeloid proinflammatory signature in psoriatic arthritis synovial fluid. Ann. Rheum. Dis. 2021, 80, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Abji, F.; Pollock, R.A.; Liang, K.; Chandran, V.; Gladman, D.D. Th17 gene expression in psoriatic arthritis synovial fluid and peripheral blood compared to osteoarthritis and cutaneous psoriasis. Clin. Exp. Rheumatol. 2018, 36, 486–489. [Google Scholar] [PubMed]
- Affandi, A.J.; Silva-Cardoso, S.C.; Garcia, S.; Leijten, E.F.A.; van Kempen, T.S.; Marut, W.; van Roon, J.A.G.; Radstake, T.R.D.J. CXCL4 is a novel inducer of human Th17 cells and correlates with IL-17 and IL-22 in psoriatic arthritis. Eur. J. Immunol. 2018, 48, 522–531. [Google Scholar] [CrossRef]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de Wit, M.d.; McInnes, I.; Dougados, M.; Primdahl, J.; McGonagle, D.G.; Aletaha, D.; Balanescu, A.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Soriano, E.R.; Corp, N.; Bertheussen, H.; Callis Duffin, K.; Campanholo, C.B.; Chau, J.; Eder, L.; Fernández-Ávila, D.G.; FitzGerald, O.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 2022, 18, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Sundanum, S.; Orr, C.; Veale, D. Targeted therapies in psoriatic arthritis-an update. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Mantravadi, S.; Ogdie, A.; Kraft, W.K. Tumor necrosis factor inhibitors in psoriatic arthritis. Expert Rev. Clin. Pharmacol. 2017, 10, 899–910. [Google Scholar] [CrossRef]
- Wendling, D.; Joshi, A.; Reilly, P.; Jalundhwala, Y.J.; Mittal, M.; Bao, Y. Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: an analysis of a large US claims database. Curr. Med. Res. Opin. 2014, 30, 2515–2521. [Google Scholar] [CrossRef]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J. Crohns Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef]
- Mease, P.J.; Gladman, D.D.; Collier, D.H.; Ritchlin, C.T.; Helliwell, P.S.; Liu, L.; Kricorian, G.; Chung, J.B. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled Phase III trial. Arthritis Rheumatol. 2019, 71, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Schett, G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum. Dis. Clin. North Am. 2012, 38, 555–567. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Mease, P.J.; Kirkham, B.; Kavanaugh, A.; Ritchlin, C.T.; Rahman, P.; van der Heijde, D.; Landewé, R.; Conaghan, P.G.; Gottlieb, A.B.; et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Mease, P.J.; McInnes, I.B.; Rahman, P.; Ritchlin, C.T.; Blanco, R.; Dokoupilova, E.; Andersson, M.; Kajekar, R.; Mpofu, S.; et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res. Ther. 2018, 20, 47. [Google Scholar] [CrossRef]
- McInnes, I.B.; Behrens, F.; Mease, P.J.; Kavanaugh, A.; Ritchlin, C.; Nash, P.; Masmitja, J.G.; Goupille, P.; Korotaeva, T.; Gottlieb, A.B.; et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020, 395, 1496–1505. [Google Scholar] [CrossRef]
- Mease, P.J.; van der Heijde, D.; Ritchlin, C.T.; Okada, M.; Cuchacovich, R.S.; Shuler, C.L.; Lin, C.Y.; Braun, D.K.; Lee, C.H.; Gladman, D.D.; et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann. Rheum. Dis. 2017, 76, 79–87. [Google Scholar] [CrossRef]
- Nash, P.; Kirkham, B.; Okada, M.; Rahman, P.; Combe, B.; Burmester, G.R.; Adams, D.H.; Kerr, L.; Lee, C.; Shuler, C.L.; et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017, 389, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Smolen, J.S.; Behrens, F.; Nash, P.; Liu Leage, S.; Li, L.; Tahir, H.; Gooderham, M.; Krishnan, E.; Liu-Seifert, H.; et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann. Rheum. Dis. 2020, 79, 123–131. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Kavanaugh, A.; Merola, J.F.; Schett, G.; Scher, J.U.; Warren, R.B.; Gottlieb, A.B.; Assudani, D.; Bedford-Rice, K.; Coarse, J.; et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet 2020, 395, 427–440. [Google Scholar] [CrossRef]
- Mease, P.J.; Asahina, A.; Gladman, D.D.; Tanaka, Y.; Tillett, W.; Ink, B.; Assudani, D.; de la Loge, C.; Coarse, J.; Eells, J.; et al. Effect of bimekizumab on symptoms and impact of disease in patients with psoriatic arthritis over 3 years: results from BE ACTIVE. Rheumatol. (Oxf. Engl.) 2023, 62, 617–628. [Google Scholar] [CrossRef]
- Merola, J.F.; Landewé, R.; McInnes, I.B.; Mease, P.J.; Ritchlin, C.T.; Tanaka, Y.; Asahina, A.; Behrens, F.; Gladman, D.D.; Gossec, L.; et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023, 401, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Helliwell, P.S.; Hjuler, K.F.; Raymond, K.; McInnes, I. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann. Rheum. Dis. 2021, 80, 185–193. [Google Scholar] [CrossRef]
- Mease, P.J.; McInnes, I.B.; Tam, L.S.; Eaton, K.; Peterson, S.; Schubert, A.; Chakravarty, S.D.; Parackal, A.; Karyekar, C.S.; Nair, S.; et al. Comparative effectiveness of guselkumab in psoriatic arthritis: results from systematic literature review and network meta-analysis. Rheumatol. (Oxf. Engl.) 2021, 60, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sendid, B.; Jouault, T.; Poulain, D. Secukinumab failure in Crohn's disease: the yeast connection? Gut 2013, 62, 800–801. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, R.J.; Watad, A.; Fragkakis, E.M.; Dunsmuir, R.; Loughenbury, P.; Khan, A.; Millner, P.A.; Davison, A.; Marzo-Ortega, H.; Newton, D.; et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann. Rheum. Dis. 2019, 78, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, G.E.; Siebert, S. The role of IL-23 and the use of IL-23 inhibitors in psoriatic arthritis. Musculoskelet. Care 2022, 20, S12–S21. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Kavanaugh, A.; Gottlieb, A.B.; Puig, L.; Rahman, P.; Ritchlin, C.; Brodmerkel, C.; Li, S.; Wang, Y.; Mendelsohn, A.M.; et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013, 382, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; Ritchlin, C.; Rahman, P.; Puig, L.; Gottlieb, A.B.; Li, S.; Wang, Y.; Noonan, L.; Brodmerkel, C.; Song, M.; et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann. Rheum. Dis. 2014, 73, 1000–1006. [Google Scholar] [CrossRef]
- Kavanaugh, A.; Puig, L.; Gottlieb, A.B.; Ritchlin, C.; Li, S.; Wang, Y.; Mendelsohn, A.M.; Song, M.; Zhu, Y.; Rahman, P.; et al. Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: results from a randomized, placebo-controlled Phase III trial. Arthritis Care Res. (Hoboken) 2015, 67, 1739–1749. [Google Scholar] [CrossRef]
- Araujo, E.G.; Englbrecht, M.; Hoepken, S.; Finzel, S.; Kampylafka, E.; Kleyer, A.; Bayat, S.; Schoenau, V.; Hueber, A.; Rech, J.; et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin. Arthritis Rheum. 2019, 48, 632–637. [Google Scholar] [CrossRef]
- Gossec, L.; Siebert, S.; Bergmans, P.; de Vlam, K.; Gremese, E.; Joven-Ibáñez, B.; Korotaeva, T.V.; Lavie, F.; Noël, W.; Nurmohamed, M.T.; et al. Persistence and effectiveness of the IL-12/23 pathway inhibitor ustekinumab or tumour necrosis factor inhibitor treatment in patients with psoriatic arthritis: 1-year results from the real-world PsABio Study. Ann. Rheum. Dis. 2022, 81, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.B.; Strober, B.; Lebwohl, M.; Augustin, M.; Blauvelt, A.; Poulin, Y.; Papp, K.A.; Sofen, H.; Puig, L.; Foley, P.; et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018, 392, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, A.; Helliwell, P.S.; Boehncke, W.H.; Kollmeier, A.P.; Hsia, E.C.; Subramanian, R.A.; Xu, X.L.; Sheng, S.; Agarwal, P.; Zhou, B.; et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Rahman, P.; Gottlieb, A.B.; Kollmeier, A.P.; Hsia, E.C.; Xu, X.L.; Sheng, S.; Agarwal, P.; Zhou, B.; Zhuang, Y.; et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1126–1136. [Google Scholar] [CrossRef]
- Coates, L.C.; Gossec, L.; Theander, E.; Bergmans, P.; Neuhold, M.; Karyekar, C.S.; Shawi, M.; Noël, W.; Schett, G.; McInnes, I.B. Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: results through one year of a phase IIIb, randomised, controlled study (COSMOS). Ann. Rheum. Dis. 2022, 81, 359–369. [Google Scholar] [CrossRef]
- McInnes, I.B.; Rahman, P.; Gottlieb, A.B.; Hsia, E.C.; Kollmeier, A.P.; Xu, X.L.; Jiang, Y.; Sheng, S.; Shawi, M.; Chakravarty, S.D.; et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a Phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naive patients with active psoriatic arthritis. Arthritis Rheumatol. 2022, 74, 475–485. [Google Scholar] [CrossRef]
- Sweet, K.; Song, Q.; Loza, M.J.; McInnes, I.B.; Ma, K.; Leander, K.; Lakshminarayanan, V.; Franks, C.; Cooper, P.; Siebert, S. Guselkumab induces robust reduction in acute phase proteins and type 17 effector cytokines in active psoriatic arthritis: results from phase 3 trials. RMD Open 2021, 7. [Google Scholar] [CrossRef]
- Kristensen, L.E.; Keiserman, M.; Papp, K.; McCasland, L.; White, D.; Lu, W.; Wang, Z.; Soliman, A.M.; Eldred, A.; Barcomb, L.; et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann. Rheum. Dis. 2022, 81, 225–231. [Google Scholar] [CrossRef]
- Östör, A.; Van den Bosch, F.; Papp, K.; Asnal, C.; Blanco, R.; Aelion, J.; Alperovich, G.; Lu, W.; Wang, Z.; Soliman, A.M.; et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann. Rheum. Dis. 2022, 81, 351–358. [Google Scholar] [CrossRef]
- Mease, P.J.; Chohan, S.; Fructuoso, F.J.G.; Luggen, M.E.; Rahman, P.; Raychaudhuri, S.P.; Chou, R.C.; Mendelsohn, A.M.; Rozzo, S.J.; Gottlieb, A. Efficacy and safety of tildrakizumab in patients with active psoriatic arthritis: results of a randomised, double-blind, placebo-controlled, multiple-dose, 52-week phase IIb study. Ann. Rheum. Dis. 2021, 80, 1147–1157. [Google Scholar] [CrossRef]
- Mease, P.J.; Helliwell, P.S.; Gladman, D.D.; Poddubnyy, D.; Baraliakos, X.; Chakravarty, S.D.; Kollmeier, A.P.; Hsia, E.C.; Xu, X.L.; Sheng, S.; et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. The Lancet Rheumatol. 2021, 3, e715–e723. [Google Scholar] [CrossRef]
- Campanaro, F.; Batticciotto, A.; Zaffaroni, A.; Cappelli, A.; Donadini, M.P.; Squizzato, A. JAK inhibitors and psoriatic arthritis: A systematic review and meta-analysis. Autoimmun. Rev. 2021, 20, 102902. [Google Scholar] [CrossRef]
- Strand, V.; de Vlam, K.; Covarrubias-Cobos, J.A.; Mease, P.J.; Gladman, D.D.; Graham, D.; Wang, C.; Cappelleri, J.C.; Hendrikx, T.; Hsu, M.A. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from OPAL Broaden-a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open 2019, 5, e000806. [Google Scholar] [CrossRef]
- Strand, V.; de Vlam, K.; Covarrubias-Cobos, J.A.; Mease, P.J.; Gladman, D.D.; Chen, L.; Kudlacz, E.; Wu, J.; Cappelleri, J.C.; Hendrikx, T.; et al. Effect of tofacitinib on patient-reported outcomes in patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors in the phase III, randomised controlled trial: OPAL Beyond. RMD Open 2019, 5, e000808. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Coates, L.C.; Kivitz, A.J.; Mease, P.J.; Gladman, D.D.; Covarrubias-Cobos, J.A.; FitzGerald, O.; Fleishaker, D.; Wang, C.; Wu, J.; et al. Safety and efficacy of tofacitinib in patients with active psoriatic arthritis: interim analysis of OPAL balance, an open-label, long-term extension study. Rheumatol. Ther. 2020, 7, 553–580. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Lertratanakul, A.; Papp, K.A.; van den Bosch, F.E.; Tsuji, S.; Dokoupilova, E.; Keiserman, M.W.; Bu, X.; Chen, L.; McCaskill, R.M.; et al. Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 56-week data from the randomized controlled Phase 3 SELECT-PsA 2 study. Rheumatol. Ther. 2021, 8, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Muensterman, E.; Engelhardt, B.; Gopalakrishnan, S.; Anderson, J.K.; Mohamed, M.F. Upadacitinib pharmacokinetics and exposure-response analyses of efficacy and safety in psoriatic arthritis patients – Analyses of phase III clinical trials. Clin. Transl. Sci. 2022, 15, 267–278. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Kato, K.; Magrey, M.; Merola, J.F.; Kishimoto, M.; Pacheco-Tena, C.; Haaland, D.; Chen, L.; Duan, Y.; Zueger, P.; et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Lertratanakul, A.; Anderson, J.K.; Papp, K.; Van den Bosch, F.; Tsuji, S.; Dokoupilova, E.; Keiserman, M.; Wang, X.; Zhong, S.; et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021, 80, 312–320. [Google Scholar] [CrossRef]
- van der Heijde, D.; Baraliakos, X.; Gensler, L.S.; Maksymowych, W.P.; Tseluyko, V.; Nadashkevich, O.; Abi-Saab, W.; Tasset, C.; Meuleners, L.; Besuyen, R.; et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018, 392, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, W.J.G.; Dolhain, R.J.E.M.; Ritter, T.E.; Watkins, T.R.; Arterburn, S.J.; Dekkers, G.; Gillen, A.; Tonussi, C.; Gilles, L.; Oortwijn, A.; et al. MANTA and MANTA-RAy: rationale and design of trials evaluating effects of filgotinib on semen parameters in patients with inflammatory diseases. Adv. Ther. 2022, 39, 3403–3422. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Costa, L.; Triggianese, P.; Maione, F.; Bertolini, N.; Vastarella, M.; Chimenti, M.S.; Tasso, M. Recent developments for new investigational JAK inhibitors in psoriatic arthritis. Expert Opin. Investig. Drugs 2023, 1–11. [Google Scholar] [CrossRef]
- Mease, P.J.; Deodhar, A.A.; van der Heijde, D.; Behrens, F.; Kivitz, A.J.; Neal, J.; Kim, J.; Singhal, S.; Nowak, M.; Banerjee, S. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 815–822. [Google Scholar] [CrossRef]
- Mease, P.; Helliwell, P.; Silwinska-Stanczyk, P.; Miakisz, M.; Ostor, A.; Peeva, E.; Vincent, M.S.; Sun, Q.; Sikirica, V.; Winnette, R.; et al. Efficacy and safety of tyrosine kinase 2/Janus kinase 1 inhibitor brepocitinib for active psoriatic arthritis: A Phase IIb randomized controlled trial. Arthritis Rheumatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Myerson, G.E.; Fleischmann, R.M.; Lioté, F.; Díaz-González, F.; Van den Bosch, F.; Marzo-Ortega, H.; Feist, E.; Shah, K.; Hu, C.; et al. A Phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J. Rheumatol. 2016, 43, 1724–1734. [Google Scholar] [CrossRef]
- Edwards, C.J.; Blanco, F.J.; Crowley, J.; Birbara, C.A.; Jaworski, J.; Aelion, J.; Stevens, R.M.; Vessey, A.; Zhan, X.; Bird, P. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann. Rheum. Dis. 2016, 75, 1065–1073. [Google Scholar] [CrossRef]
- Wells, A.F.; Edwards, C.J.; Kivitz, A.J.; Bird, P.; Nguyen, D.; Paris, M.; Teng, L.; Aelion, J.A. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford) 2018, 57, 1253–1263. [Google Scholar] [CrossRef]
- Orr, C.; Veale, D.J. Is there a need for new agents with novel mechanisms of action in psoriatic arthritis? Ann. Rheum. Dis. 2014, 73, 951–953. [Google Scholar] [CrossRef]
- Mease, P.J.; Genovese, M.C.; Weinblatt, M.E.; Peloso, P.M.; Chen, K.; Othman, A.A.; Li, Y.; Mansikka, H.T.; Khatri, A.; Wishart, N.; et al. Phase II study of ABT-122, a tumor necrosis factor- and Interleukin-17A-Targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 2018, 70, 1778–1789. [Google Scholar] [CrossRef]
- Sun, S.; Ding, Z.; Yang, X.; Zhao, X.; Zhao, M.; Gao, L.; Chen, Q.; Xie, S.; Liu, A.; Yin, S.; et al. Nanobody: A small antibody with big implications for tumor therapeutic strategy. Int. J. Nanomedicine 2021, 16, 2337–2356. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Bakulev, A.L.; Kokhan, M.M.; Samtsov, A.V.; Khairutdinov, V.R.; Morozova, M.A.; Zolkin, N.A.; Kuryshev, I.V.; Petrov, A.N.; Artemeva, A.V.; et al. Efficacy and safety of netakimab, A novel anti-IL-17 monoclonal antibody, in patients with moderate to severe plaque psoriasis. Results of A 54-week randomized double-blind placebo-controlled PLANETA clinical trial. Dermatol. Ther. (Heidelb) 2021, 11, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Weinberg, M.A.; Morris, A.; Reich, K. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: a multicentre, randomised, placebo-controlled, phase 2b study. Lancet 2021, 397, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
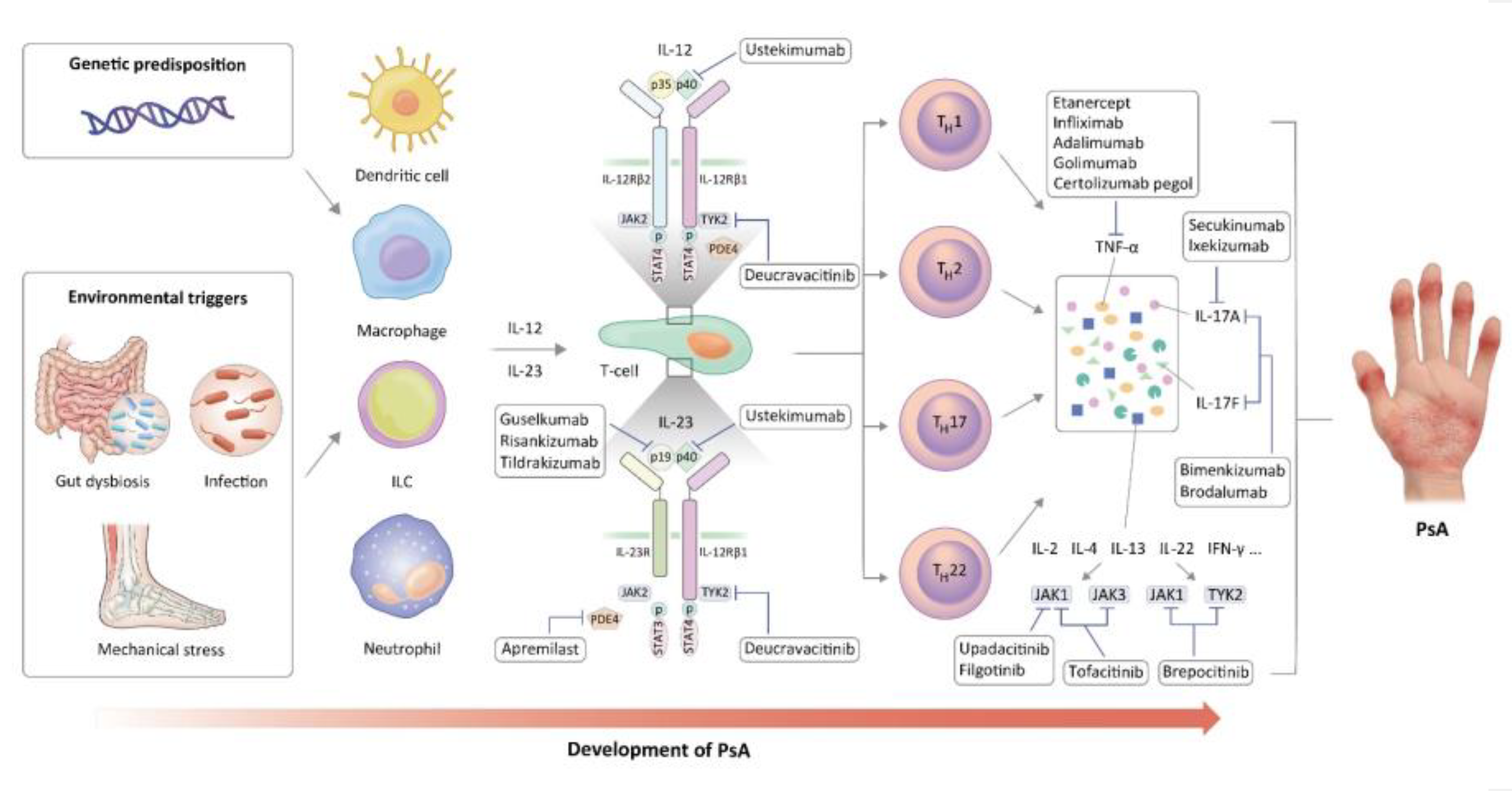
| Cytokine | Main source | Function in PsA |
|---|---|---|
| TNF-α | Macrophages, T cells, NK cells, mast cells | Elevation in the levels of cytokines, chemokines, matrix metalloproteinases, and adhesion molecules from various immune cells Induction of osteoclasts to promote the degradation of cartilage and bone |
| IL-23 | Dendritic cells, macrophages | Promotion of Th17 cell differentiation and GM-CSF production |
| IL-12 | Dendritic cells, macrophages | Promotion of Th1 cell differentiation through STAT4 |
| IL-17A/F | Th17 cells, NK cells, type 3 innate lymphoid cells | Stimulation of fibroblast-like synoviocytes, chondrocytes, and osteoclasts Elevation in the levels of proinflammatory cytokines and matrix metalloproteinases Induction of neutrophil recruitment |
| IL-21 | T cells, NK cells | Promotion of T cell differentiation (Specifically, into Th17 cells) |
| IL-22 | T cells, innate lymphoid cells | Activation of fibroblast-like synoviocytes; Promotion of osteoclast and bone degradation |
| IL-32 | NK cells, T cells, monocytes, epithelial cells | Potentiation of inflammation through the activation of NF-κB signaling and promotion of osteoclast differentiation |
| IL-33 | Macrophages, dendritic cells, mast cells, epithelial cells | Activation of Th1/Th17-mediated inflammation |
| IFN γ | Th1 cells, type 1 innate lymphoid cells, NK cells | Activation of macrophage and T-cell Promotion of RANKL secretion |
| GM-CSF | Macrophages, T cells, synovial fibroblasts | Recruitment of various immune cells |
| IL-9 | Th9 cells | Promotion of Th17-associated inflammation and IL-17A production |
| IL-6 | Macrophages, T cells, endothelial cells | Stimulation of STAT3 signaling to increase the production of proinflammatory cytokines |
| IL-15 | Macrophages | Promotion of T cell proliferation, natural killer cell activation, and production of IFN-γ, TNF-α, and IL-17 |
| IL-1α | Macrophages, dendritic cells | Induction of IL-17, IL-21 and IL-22 expression by γδT cells in combination with IL-23 |
| b/tsDMARDs | Target | Dose and administration route | FDA or EMA approval |
|---|---|---|---|
| Etanercept | TNF-α | 50 mg weekly, SC | FDA, EMA |
| Infliximab | TNF-α | 5 mg/kg at weeks 0, 2, and 6; every 8 weeks thereafter, IV | FDA, EMA |
| Adalimumab | TNF-α | 40 mg every 2 weeks, SC | FDA, EMA |
| Golimumab | TNF-α | 50 mg weekly, SC | FDA, EMA |
| Certolizumab pegol | TNF-α | 200 mg every 2 weeks or 400 mg every 4 weeks, SC | FDA, EMA |
| Ustekinumab | p40 subunit of IL-12 and IL-23 | (BW<100 kg) 45 mg/kg at weeks 0, 4, and 12; every 12 weeks thereafter, SC (BW>100kg) 90 mg/kg at weeks 0, 4, and 12; every 12 weeks thereafter, SC |
FDA, EMA |
| Guselkumab | p19 subunit of IL-23 | 100 mg at weeks 0 and 4; every 8 weeks thereafter, SC | FDA, EMA |
| Risankizumab | p19 subunit of IL-23 | 150 mg at weeks 0 and 4; every 12 weeks thereafter, SC | FDA, EMA |
| Tildrakizumab | p19 subunit of IL-23 | 100 mg at weeks 0 and 4; every 12 weeks thereafter, SC | - |
| Secukinumab | IL-17A | 150 mg weekly for 4 weeks; monthly thereafter, SC | FDA, EMA |
| Ixekizumab | IL-17A | 160 mg at weeks 0 and 80 mg at weeks 2, 4, 6, 8, 10, and 12; 80 mg every 4 weeks thereafter, SC | FDA, EMA |
| Bimekizumab | IL-17A/F | 320 mg every 4 weeks for 16 weeks, every 8 weeks thereafter, SC | EMA |
| Brodalumab | IL-17 receptor | 210 mg at weeks 0, 1, and 2; every 2 weeks thereafter, SC | - |
| Tofacitinib | JAK1/3 | 5 mg twice daily, orally | FDA, EMA |
| Upadacitinib | JAK1 | 15 mg once daily, orally | FDA, EMA |
| Filgotinib | JAK1 | 200 mg once daily, orally | - |
| Deucravacitinib | TYK2 | 6 mg once daily, orally | - |
| Brepocitinib | JAK1/TYK2 | 30 mg or 60 mg once daily, orally | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





