1. Introduction
In healthcare settings great attention is paid to the cleanliness, compliance to a range of routines, such as hand hygiene, is required to mitigate healthcare-associated infections [
1], surfaces, such as antimicrobial copper are utilized [
2]. While most surfaces, such as floors, tables and handles are easily sanitized by wiping with appropriate disinfectants, situation is different for textile surfaces. Textiles present porous surfaces prone to retaining moisture and possessing increased specific surface area to host microorganisms. In fact, in environments with controlled air quality, source of airborne particles carrying microorganisms may be traced to used textiles [
3]. Studies examining the survival times of individual pathogens show that the base material of textile is also an important factor, in fact survival of bacteria at room temperature was the longest, up to 206 days, on polyester [
2]. In fact, textile surfaces such as curtains and upholstery have been found to be contaminate over time in health-care settings and host pathogens up to 90 days [
5]. To mitigate these risks, textiles utilizing new biocidal treatments are developed by many researchers, utilising for example metallic nanoparticles, plasma treatments [
6], new organic biocidal substances [
7]. However new biocides have to comply with relevant regulations [
8], thus their application in near future is limited to prototypes.
In fact, the fiber-forming polymer itself may hinder the growth of bacteria and fungi, even the structure of the textile (woven, knitted, spunbond) can affect the suitability for use in health-care settings [
3]. Additionally active ingredients relying on metallic ions, such as zinc [
9] and silver [
10] have been demonstrated to be active towards antibiotic resistant bacteria. In light of the above facts, one may suggest that polypropylene fabric [
3] containing antimicrobial substances utilizing oligodynamic effect may bring a substantial reduction of both airborne particles and present living microorganisms. Possible difference between laboratory settings and real-life working environment points to a necessity to conduct a study of aforementioned hypothesis. During the investigation of standards for textiles in medical settings and textiles actually used in health care, authors found a private surgery clinic in Levoča district, Slovakia, willing to participate in a study proposed above, albeit under the condition of anonymity due to public perception of any possible findings. As active ingredients silver phosphate glass (SPG) and zinc pyrithione (ZP) were chosen as sources of silver and zinc ions, since they are intended for use in thermoplastics and possess valid registrations required for use in EU market. As for quantification of livig organisms on textile surfaces, dtermination of present adenosine triphosphate was suggested: All living organisms produce and utilize adenosine triphosphate (ATP) and standardized methods that use the detection and quantification of present ATP are used to confirm and quantify the sum of all living microorganisms on given surface [
11].
2. Materials and Methods
2.1. Materials
Isotactic polypropylene homopolymer with melt flow index of 25 g/10 min. in form of pellets was supplied by Slovnaft a.s., Bratislava, SK. Low-density polyethylene (LDPE) homopolymer wax in a powder form used as dispersing agent was produced by Honeywell, Morris Plains, NJ, USA. Pigments used to dope-dye polypropylene yarns: titanium dioxide, carbon black, PV-Echt-Rosa, copper phthalocyanine and phthalocyanine green G were purchased form NYLUS SK, Poprad, SK. Active antimicrobial ingredients silver phosphate glass and zinc pyrithione was supplied by SANITIZED AG, Burgdorf, CH.
Sets of ATP sampling tests UltraSnap including SystemSURE luminometer were purchased from Hygiena LLC, Camarrilo CA, USA. Envirocheck® Contact DC Disinfection Control contact plates, Tryptic soy agar contact plates, blood agar plates, glucose, tryptone, yeast extract and agar for agar plates were supplied by Merck KGaA, Darmstadt, DE.
2.2. Methods
2.2.1. Textile samples preparation.
Polypropylene (PP) yarns were produced in three steps: compounding, melt spinning and false-twist texturation. A masterbatch containing 5% by weight of zinc pyrithione (ZP) was produced by prepared by thoroughly mixing 500 grams of ZP and 250 grams of fine powdered polyethylene wax in Mixaco vertical container mixer (MIXACO Maschinenbau Dr. Herfeld GmbH & Co. KG, Neuenrade, DE) and subsequent addition of 9,25 kg of polypropylene. The resulting mixture was compounded on ZSK-25 twin screw compounder (Coperion GmbH, Stuttgart, DE). In a similar manner a masterbatch containing 6% silver phosphate glass (SPG) was prepared by compounding 600 g of SPG, 300 g o f polyethylene wax and 9,1 kg of PP. Mixtures of pigments were compounded the same way to provide masterbatches for dope dyeing for final yarn colours light-blue, gray-blue a and red. Of these masterbatches and PP pellets six mixtures were prepared: each of the three colours in two versions, one containing 2% by weight of 5% ZP masterbatch and one containing 2,5% by weight of 6% SPG masterbatch. In each case the content of pigment was 0,1 to 0,3% of pigment and 0,1% of ZP respectively 0,15% of SPG.
Each of given mixtures was melt spun using an industrial melt spinning line with a single screw extruder (Research Institute for Man-Made Fibres, a.s., Svit, SK) with D = 45 mm and L/D = 44, at 230°C with melt throughput 151 g/min through two shafts, each with one gear metering pump supplying four spinnerets heated at 245°C, supplying each spinneret with 18,9 g/min of polymer melt, the take-up speed was 1450 m/min. Spun multifilament fibers were false-twist textured using Barmag AFK-6 draw texturizing machine (OC Oerlikon, Pfäffikon, CH) at texturation speed of 400 m/min. D/Y=1,55 and draw ratio of 1,545 connecting two ends by intermingling in air jet at 150 kPa resulting in yarns with linear density 167 ± 5 decitex.
A simple plain weave fabric was woven using pairs of PP yarns with same color: PP fibers containing SPG were used for warp beam with 26 ends per cm of warp width, PP fibers containing ZP were used for the weft, resulting in 22.1 threads/cm and fabrics having grammage 95 g/m2 ±5%.
2.2.2. Replacement of staff clothing and bedding in a small surgery department and frequency of measurements
Of the aforementioned fabrics, the textiles dyed red-purple were used for production of four pairs of women's tunics, two pairs of men's tunics were made from the green fabric, while light blue fabrics were used as bed sheets. The samples were then used the private surgery clinic by staff and on two beds, replacing the currently worn cotton-polyester blend clothing and bedding. Men's tunic to be worn by the attending physician and women's tunics to be worn during by nurse. The light-blue fabrics were used as bed sheets on the examining bed in room no.1 and surgical bed in room no.2. During examination and surgery another disposable polymer sheet was covering the bed sheet on both examining and surgical bed. Two rooms of the clinic in question were examined: Room no.1 is the doctor's office with area 32 m2 and high frequency of visits, approximately 35 patients each day with one physician and one nurse present. These persons were present in the room for 32,5 hours per week. In this room an ambient germicidal lamp is in operation outside business hours of the clinic. The doctor's office no.2 has area 9m2 and is used for minor surgery and preparation of tools with only one patient, physician and nurse present if used. The average weekly time of occupation of room no.2 is 22,5 hours with a daily surface (floor, tables) disinfection and cleaning routine.
Of the below mentioned samplings, for each method the first sampling numbered “0” was taken before replacing the usual staff clothing and bedding with presented polypropylene textiles with antimicrobial agents. Starting the next day, the staff clothing and beddings were replaced with abovementioned textiles, the measurement of air quality and textile viable microbe counts numbered as sampling 1 were made one week after the replacement, subsequent samplings numbered 2 and 3 were made with an interval of one week. Finally, after measurement 3 was taken the 4 samples – nurse's tunic, physician's tunic, bedding from examination bed and bedding from surgery bed - were each separately closed in a sealable plastic bag and left under ambient temperature for 24 hours. Subsequently new samplings with swabs for ATP and contact plates as described below in the text were taken, cultivated and evaluated. This measurement is listed as measurement 4 and shows the rate of survival of microbes from measurement no.3 without any additional bioburden.
2.2.3. Evaluation of impact on airborne particles, airborne microorganisms and viable microorganisms on textile samples.
Concentration of airborne particles was measured using TROTEC PC220 discrete particle counter (Trotec GmbH, Heinsberg, DE), in every measurement five separate particle count measurements in short succession were taken between 9:30 and 10:30 during clinic operation with the discrete particle counter in the corner of given room. For each size fraction of airborne particles, the counts were then averaged.
Counts of viable microorganisms in ambient air in observed rooms was determined using Aeroscop MAS-100 NT (Merck KGaA, Darmstadt, DE) with perforated air inlet of 300 orifices wide 0,6 mm, performing three samplings with 600 l of air taken during 15 minutes of air in each sampling: one sampling using agar plate for counting of yeasts and molds, blood agar plate and glucose-trypton-yeast extract agar plate (GTY-plate) for counting of airborne bacteria. After each sampling the agar plates were transported in cooling box (3-6°C) to certified laboratory for cultivation and analysis (Public Health Authority of the Slovak Republic, Poprad, SK). Plates from each sampling were cultivated 24 hours at corresponding temperatures (25°C for agar plates, 30°C for GTY-plates and 37°C for blood agar plates) and evaluated by a certified microbiological laboratory according to ISO 21527 for counts of yeasts and molds in the agar plates, and bacteria on the blood agar and GTY-plates.
As a quantification of total surviving microorganisms on studied surfaces was conducted using UltraSnap Surface ATP test swab with subsequent luminometric quantification of present ATP. In each measurement an area of 10x10 cm was according to provided user instructions. Used swab is then immersed in provided agent containing luciferase enzyme for 10 seconds and immediately evaluated by provided luminometer. The results are expressed in relative light units (LRU) and so provide a means of monitoring relative change of quantity of living organisms on monitored surface over time.
Measurement of counts of viable microorganisms on textiles was conducted by two separate methods: Envirocheck Contact DC Disinfection Control and 55 mm tryptic soy agar contact plates.
Envirocheck Contact DC Disinfection Control are flexible contact paddles with two sides containing CASO tryptic soy agar with different neutralizers and inhibitors, one for determination of yeasts and bacteria, the other side for counting molds. After swabbing a surface with an Envirocheck paddle, the paddle was transported together with other samples in a cooling box to certified laboratory, here the same day a 48-hour cultivation of said paddle at 37°C was initiated. After incubation density of microorganisms on measured paddle of 9,4cm2 was determined by comparation with model density chart supplied within the Envirocheck sets in limits of 103 to 107 for bacteria and yeasts and 102 – 105 for molds. In a similar manner the soy contact plates were open at the site of measurement and the agar surface was pressed with a steady pressure for few seconds on investigated textile surface, immediately closed and stored and transported in the cooling box with other samples and brought to microbiological laboratory for cultivation and evaluation. Soy agar contact plates were cultivated for 48 hours at 37°C.
As a control for compliance with the necessary procedures and an indicator of sample contamination, in each measurement using agar plates one unused agar plate of each type was transported to the site and from the site in the same cooling box. Respective discovery of microbial growth would indicate contamination and such results would be ignored. All analyses of microbial cultivation were contracted to and conducted by accredited laboratory of Public Health Authority of the Slovak Republic in Poprad, Slovakia.
3. Results
3.2. Evaluation of airborne particles and, airborne microorganisms
On 4 occasions, one week apart electronic discrete particle counter (DPI) was set up for period of approximately 45 minutes, conducting five discrete measurements as described above
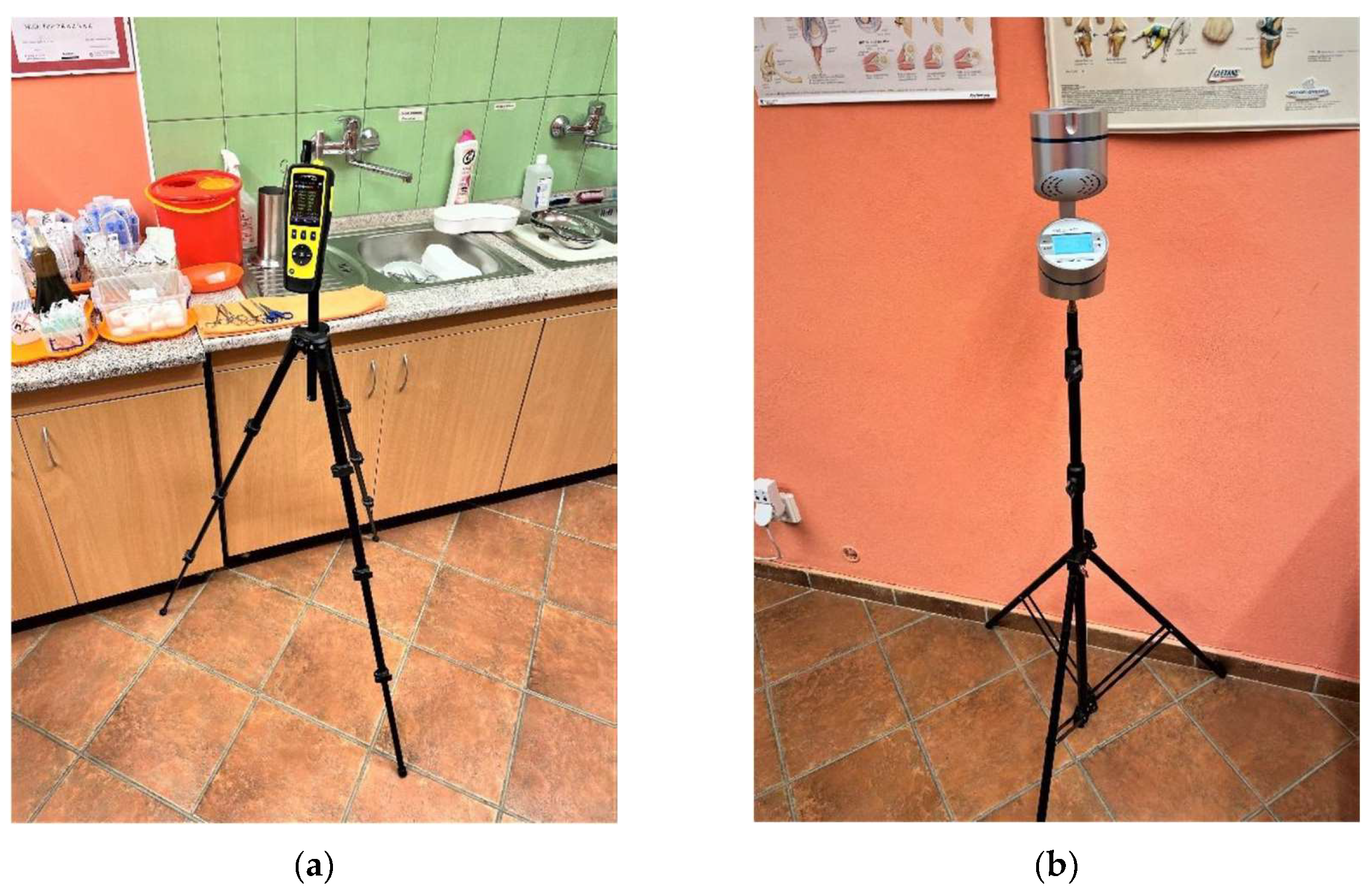
Figure 1.
Placement of the DPIs in investigated rooms (a) Placement of DPI in room no.1 - doctor's office with area 32 m2; (b) Placement of Aeroscop MAS-100 NT for airborne microorganism quantification in room no.1.
Figure 1.
Placement of the DPIs in investigated rooms (a) Placement of DPI in room no.1 - doctor's office with area 32 m2; (b) Placement of Aeroscop MAS-100 NT for airborne microorganism quantification in room no.1.
The observed values of found airborne particles per m3 of air in room no.1 are listed in
Table 1., values observed in room no.2 are listed in
Table 2, all listed values are averages of five independent readings in given day and location. Sampling numbered 0 is measurement of the initial conditions before replacement of bed sheets and staff apparel with ZP and SPG doper polypropylene fabric.
As described in paragraph 2.2.3. above, simultaneously with airborne discreet particle counting, three sampling of airborne microorganisms were conducted. Each sampling with different plate to distinguish different type of microorganisms with 600 of ambient air passing through the aeroscope for each plate. The results for all 4 sampling dates expressed in colony forming units per cubic meter of ambient air (CFU/m
3) are presented in
Table 3 and
Table 4.
Selected standardized method displays a range sufficient to reflect the effect of developed fabric on numbers of yeasts and molds, however the impact on counts
3.2. Quantification and identification of microorganisms present on textile surfaces
Simultaneously with aeroscopic sampling, swabs of clothing and bedding as described in paragraph 2.2.3. was conducted, all sampling sessions are numbered in accordance with measurement description in paragraph 2.2.2. All results of ATP swabs are listed in
Table 5,
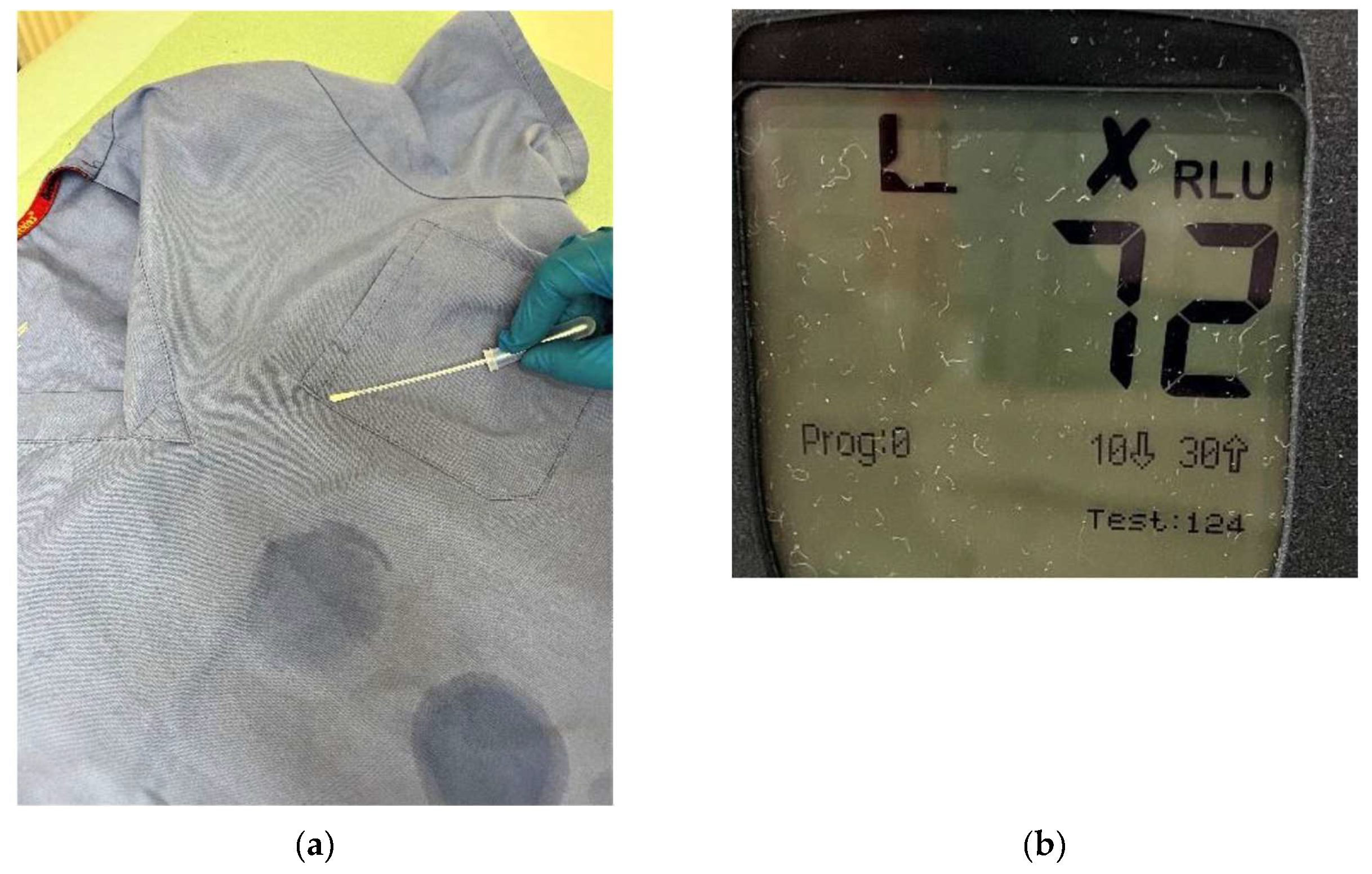
Figure 2.
Determination and quantification of present ATP on physicians tunic before replacement for PP fabric tunic(a) application of UltraSnap swab; (b) output of subsequent luminometric measurement.
Figure 2.
Determination and quantification of present ATP on physicians tunic before replacement for PP fabric tunic(a) application of UltraSnap swab; (b) output of subsequent luminometric measurement.
As illustrated in
Table 5, there is observable variability in the data, but reduction of living microorganisms is evident in all cases. For unambiguous quantification averages of samplings 1 to 3 for given surface can be compared to sampling 0, such comparation is summarized in
Table 6.
Further quantification of viable microorganisms is provided by the evaluation of Enviroceck contact plates, which allow estimation of order of magnitude of numbers of CFU per the area of given sampler, which is 9,4 cm2. Also, more resolution of numbers of molds vs. numbers of bacteria can be made. A summarization of results from the cultivation of Envirocheck contact plates at 37°C during 48 hours is presented in
Table 7 in in logarithmic form, where value of “0” means no yeasts and bacteria or molds were found to grow at given sample.
Most detailed description of viable microbes found on monitored textiles is provided by cultivation ana analysis of soy contact plates at 30°C, the summarization of numbers and identified organisms across individual measurements are presented in Tables 8 to 11 below
Table 8.
Quantification of present microbes in CFU/contact plate and identified organisms found on bedding in room no.1.
Table 8.
Quantification of present microbes in CFU/contact plate and identified organisms found on bedding in room no.1.
No. of
measurement |
CFU per contact plate |
identified organisms |
Conditions** |
| 0 |
60 |
aerobic sporulants, saprophytic staphylococci, micrococci, 2 CFU micromycetes |
21,9°C / 47,9% |
| 1 |
47 |
aerobic sporulants, saprophytic staphylococci, micrococci, bacillus cereus |
22,1°C / 49,3% |
| 2 |
22 |
aerobic sporulants, saprophytic staphylococci, micrococci |
22,3°C / 48,1% |
| 3 |
2 |
aerobic sporulants |
22,1°C / 47,7% |
| 4 |
3* |
saprophytic staphylococci |
21,8°C / 47,8% |
Table 9.
Quantification of present microbes in CFU/contact plate and identified organisms found on bedding in room no.2.
Table 9.
Quantification of present microbes in CFU/contact plate and identified organisms found on bedding in room no.2.
No. of
measurement |
CFU per contact plate |
identified organisms |
Conditions** |
| 0 |
96 |
aerobic sporulants, saprophytic staphylococci, micrococci |
21,9°C / 47,9% |
| 1 |
3* |
saprophytic staphylococci |
22,1°C / 49,3% |
| 2 |
5* |
micrococci |
22,3°C / 48,1% |
| 3 |
1* |
micrococci |
22,1°C / 47,7% |
| 4 |
14 |
aerobic sporulants, saprophytic staphylococci, micrococci |
21,8°C / 47,8% |
Table 10.
Quantification of present microbes in CFU/contact plate and identified organisms found nurse's tunic.
Table 10.
Quantification of present microbes in CFU/contact plate and identified organisms found nurse's tunic.
No. of
measurement |
CFU per contact plate |
identified organisms |
Conditions** |
| 0 |
96 |
aerobic sporulants, saprophytic staphylococci, micrococci |
21,9°C / 47,9% |
| 1 |
3* |
saprophytic staphylococci |
22,1°C / 49,3% |
| 2 |
5* |
micrococci |
22,3°C / 48,1% |
| 3 |
1* |
micrococci |
22,1°C / 47,7% |
| 4 |
14 |
aerobic sporulants, saprophytic staphylococci, micrococci |
21,8°C / 47,8% |
Table 11.
Quantification of present microbes in CFU/contact plate and identified organisms found nurse's tunic.
Table 11.
Quantification of present microbes in CFU/contact plate and identified organisms found nurse's tunic.
No. of
measurement |
CFU per contact plate |
identified organisms |
Conditions*** |
| 0 |
100** |
aerobic sporulants, saprophytic staphylococci, micrococci, 1 CFU micromycetes |
21,9°C / 47,9% |
| 1 |
100** |
aerobic sporulants, saprophytic staphylococci, micrococci, Streptococcus sp., 2 CFU micromycetes |
22,1°C / 49,3% |
| 2 |
100** |
aerobic sporulants, saprophytic staphylococci, micrococci |
22,3°C / 48,1% |
| 3 |
100** |
aerobic sporulants, saprophytic staphylococci, micrococci |
22,1°C / 47,7% |
| 4 |
30 |
aerobic sporulants, saprophytic staphylococci, micrococci, Acinetobacter sp., Pantoea sp., |
21,8°C / 47,8% |
5. Conclusions
Application of presented polypropylene textiles doped with silver phosphate glass and zinc pyrithione in real-life conditions in a doctor’s office exhibited reduction of count of bacteria and fungi surviving on their surface and proven to retain their antimicrobial properties over monitored period. Counting of discrete airborne particles showed increase in numbers of particles smaller than 0.5µm by almost approximately 50% in examination room, while a decrease of in numbers of particles of 10 µm approximately 1/3 is indicated in the data. for room no.1. Relative differences are even smaller for room no.2 with much les attending patients per day. The impact on observed dust particles in ambient air in observed rooms of the clinic is thus rather ambiguous and the authors conclude that expected reduction in airborne particles was not observed in this study. Regarding viable airborne microorganisms a clear reduction of yeasts and molds has been shown in room no.2, the surgery room with lower frequency of patient visits. However, this effect was not observed in room no.1, the doctor's office, with multiple times higher traffic of patients. Most promising are the results of total viable counts on studied textile surfaces: all used methods confirmed the reduction of viable microorganisms by at least 44% (doctors tunic) relative to measurement before the replacement of commonly used cotton and polyester based textiles with presented antimicrobial doped polypropylene. The relative reduction of viable microbes for both examination table and surgical table were at least 80% in all cases. Visible differences between the observed rooms can be attributed to numbers of passing patients and thus a higher bioburden in room no.1. This reduction was most pronounced in the final observation after 24 hours of isolation, which indicates that less than 8% of the original microorganisms survive on presented textiles after 24 hours without additional bioburden.
Author Contributions
Conceptualization, A.B.; yarn and textile preparation, M.K., B.B; investigation and measurements were contracted to Cleanliness Certification Center, s.r.o., Slovakia; writing—original draft preparation, T.Z, B.B.; writing—review and editing, M.K.; supervision, A.B.; project administration, T.Z.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was created thanks to support under the Operational programme Integrated Infrastructure for the project Centre for the Development of Textile Intelligence and Antimicrobial Technologies, ITMS code 313011AVF5, co-financed by European Regional Development Fund.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cummings, K. L., Anderson, D. J., Kaye, K. S. Hand hygiene noncompliance and the cost of hospital-acquired methicillin-resistant Staphylococcus aureus infection. Infect. Control Hosp. Epidemiol. 2010 31(4), pp. 357-364. [CrossRef]
- Kampf G. How long can nosocomial pathogens survive on textiles? A systematic review. GMS Hyg Infect Control. 2020 15:Doc10. [CrossRef]
- Ljungqvist, B., & Reinmüller, B. Some observations on protective efficacy of surgical clothing systems with additional clothing components concerning airborne bacteria-carrying particles measured during ongoing surgery. Eur. J. Parenter. Pharm. Sci. 2021 26(1). [CrossRef]
- Ullmann, C., Ljungqvist, B., & Reinmüller, B. Some aspects of protective efficacy of surgical clothing systems concerning airborne microorganisms based on results from measurements in a dispersal chamber and during surgical procedures Eur. J. Parenter. Pharm. Sci. 2017 22(2).
- Mitchell A, Spencer M, Edmiston C Jr. Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: a review of the literature. J Hosp Infect. 2015;90(4):285-292. [CrossRef]
- Botelho, C.M.; Fernandes, M.M.; Souza, J.M.; Dias, N.; Sousa, A.M.; Teixeira, J.A.; Fangueiro, R.; Zille,A. New Textile for Personal Protective Equipment—Plasma Chitosan/Silver Nanoparticles Nylon Fabric. Fibers 2021, 9, 3. [CrossRef]
- Gomes, L., Monteiro, P., Cotas, J., Gonçalves, A. M., Fernandes, C., Gonçalves, T., Pereira, L. Seaweeds’ pigments and phenolic compounds with antimicrobial potential. Biomol. Concepts 2022 13(1), 89-102. [CrossRef]
- Regulation on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006, 2008.
- Brayner, R., Ferrari-Iliou, R., Brivois, N., Djediat, S., Benedetti, M. F., Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6(4) 866–870. [CrossRef]
- Rajski, L., Juda, M., Los, A., Witun, E., Malm, A. Medical textiles with silver/nanosilver and their potential application for the prevention and control of healthcare-associated infections–mini-review. Curr. Issues Pharm. Med. Sci. 2019 32(2), 104-107,. [CrossRef]
- van Arkel, A., Willemsen, I., & Kluytmans, J. "The correlation between ATP measurement and microbial contamination of inanimate surfaces." Antimicrob. Resist. Infect. Control., 2021 10, 1-5. [CrossRef]
Table 1.
Distribution of dust particles according to size in individual samplings in room no.1, average values for each measurement.
Table 1.
Distribution of dust particles according to size in individual samplings in room no.1, average values for each measurement.
| |
Particle size |
|
| No. |
0.3µm |
0.5µm |
1.0µm |
2.5µm |
5.0µm |
10µm |
Conditions |
| 0 |
72613 |
22688 |
6119 |
1296 |
247 |
129 |
21,9°C / 47,9% |
| 1 |
109189 |
29491 |
5425 |
971 |
168 |
76 |
22,1°C / 49,3% |
| 2 |
137560 |
36561 |
6226 |
1080 |
165 |
83 |
22,3°C / 48,1% |
| 3 |
106454 |
29580 |
5923 |
1116 |
193 |
96 |
22,1°C / 47,7% |
Table 2.
Distribution of dust particles according to size in individual measurements in room no.2, average values for each measurement.
Table 2.
Distribution of dust particles according to size in individual measurements in room no.2, average values for each measurement.
| |
Particle size |
|
| No. |
0.3µm |
0.5µm |
1.0µm |
2.5µm |
5.0µm |
10µm |
Conditions |
| 0 |
90911 |
27366 |
6053 |
1055 |
150 |
83 |
21,9°C / 47,9% |
| 1 |
117653 |
32873 |
6273 |
1124 |
171 |
85 |
22,1°C / 49,3% |
| 2 |
130963 |
35359 |
6450 |
1133 |
157 |
84 |
22,3°C / 48,1% |
| 3 |
56692 |
16954 |
3766 |
661 |
93 |
49 |
22,1°C / 47,7% |
Table 3.
Analysis of cultivated aeroscope plates from room no.1, results for each sampling expressed in CFU/m3.
Table 3.
Analysis of cultivated aeroscope plates from room no.1, results for each sampling expressed in CFU/m3.
No. of
measurement |
Yeasts and molds |
Cultivated on blood medium |
Cultivated on GTK medium |
| 0 |
52 |
>535 |
>535 |
| 1 |
24 |
>535 |
>535 |
| 2 |
55 |
>535 |
>535 |
| 3 |
55 |
>535 |
>535 |
Table 4.
Analysis of cultivated aeroscope plates from room no.2, results for each sampling expressed in CFU/m3.
Table 4.
Analysis of cultivated aeroscope plates from room no.2, results for each sampling expressed in CFU/m3.
No. of
measurement |
Yeasts and molds |
Cultivated on blood medium |
Cultivated on GTK medium |
| 0 |
57 |
>535 |
>535 |
| 1 |
23 |
>535 |
>535 |
| 2 |
37 |
>535 |
>535 |
| 3 |
32 |
>535 |
>535 |
Table 5.
Quantification of present ATP on examined textiles in RLU.
Table 5.
Quantification of present ATP on examined textiles in RLU.
No.*
|
bedding in room no.1 |
bedding in room no.2 |
tunic,
nurse |
tunic, physician |
Conditions** |
| 0 |
400 |
106 |
67 |
72 |
21,9°C / 47,9% |
| 1 |
54 |
1 |
19 |
40 |
22,1°C / 49,3% |
| 2 |
16 |
3 |
21 |
14 |
22,3°C / 48,1% |
| 3 |
8 |
13 |
57 |
9 |
22,1°C / 47,7% |
Table 6.
Comparation of present ATP on examined textiles in RLU.
Table 6.
Comparation of present ATP on examined textiles in RLU.
Result
|
bedding in room no.1 |
bedding in room no.2 |
tunic,
nurse |
tunic, physician |
| 0 |
400 |
106 |
67 |
72 |
| Average 1-3 |
26 |
6 |
32 |
21 |
| Reduction |
-94% |
-94% |
-52% |
-71% |
Table 7.
Decimal logarithms of CFUs as indicated by Envirocheck contact plates after incubation.
Table 7.
Decimal logarithms of CFUs as indicated by Envirocheck contact plates after incubation.
| |
bedding in room no.1 |
bedding in room no.2 |
tunic - nurse |
tunic- physician |
| No. of sampling |
Yeasts and bacteria |
Molds |
Yeasts and bacteria |
Molds |
Yeasts and bacteria |
Molds |
Yeasts and bacteria |
Molds |
| 0 |
3 |
3 |
4 |
4 |
4 |
3 |
4 |
3 |
| 1 |
3 |
2 |
3 |
3 |
3 |
2 |
4 |
3 |
| 2 |
3 |
2 |
3 |
0 |
3 |
2 |
3 |
2 |
| 3 |
3 |
0 |
3 |
2 |
3 |
2 |
3 |
0 |
| 4 |
3 |
0 |
3 |
2 |
3 |
0 |
3 |
2 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).