Submitted:
28 June 2023
Posted:
28 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Anterior ischemic optic neuropathies (AION) This category includes arteritic forms like giant cell arteritis (GCA), which has a pooled prevalence of approximately 51.74 in 100,000 for individuals over the age of 50 [15]. Non-arteritic forms have a reported prevalence of approximately 102.87 in 100,000 in the general population over the age of 40 in South Korea [16].
- Infiltrative optic neuropathies, such as leukemic optic neuropathy, which presents in approximately 16% and 18% of all chronic and acute leukemia cases, respectively [28].
2. Anatomy and Perfusion of the Visual Pathway
3. General Mechanisms of Nitro-Oxidative Stress in the Optic Nerve
3.1. Generation of Reactive Oxygen and Nitrogen Species
3.2. Oxidative Damage and Antioxidant Defense Systems
3.3. Oxidative Stress in Retinal Ganglion Cells
4. Oxidative Stress in Individual Optic Nerve Diseases
4.1. Glaucoma
4.1.1. General Aspects
4.1.2. Redox Parameters and Oxidative Stress Biomarkers in Glaucoma
4.1.3. Oxidative Stress in the Pathogenesis of Glaucoma
4.1.4. Interplay Between Mitochondrial and ER Stress in TMCs and RGCs
4.2. Therapeutic Perspectives in Glaucoma
4.2.1. Therapeutic Potential of Natural Compounds in Glaucoma
4.2.2. NOX-Inhibitors
4.2.3. Exploring Nrf-2 Activation for Antioxidant Therapy in Glaucoma
4.2.4. Rho Kinase Inhibitors in Glaucoma Treatment
4.2.5. ER-Stress Antagonists: Potential Antioxidant Drugs
4.2.6. Other Antioxidant Molecules
4.3. Leber’s hereditary optic neuropathy
4.3.1. General Aspects: Genetics, Clinical Presentation, and Current Therapeutic Options
- Subacute phase (<6 months): During this phase, patients commonly experience blurred vision and impaired color perception without pain, and their pupillary reflex remains unaffected [323]. Approximately 75% of cases initially experience visual loss in one eye, with the contralateral eye becoming affected within a few weeks [323,339]. Fundoscopy may reveal axonal loss in the papillomacular bundle and circumpapillary telangiectasias. Perimetry often demonstrates typical centrocaecal or central scotomas [323,339,340]. Optical coherence tomography (OCT) may show swelling of the peripapillary RNFL. Magnetic resonance imaging (MRI) is commonly performed for differential diagnosis [339].
- Dynamic phase (6–12 months): During this stage, fundoscopic signs such as telangiectasias and RNFL edema gradually regress [339].
- Chronic phase (>12 months): In the chronic phase, there is a further decline in visual acuity and visual field loss. Fundoscopic examinations may reveal optic nerve head atrophy, while OCT may indicate thinning of the RNFL [339].
4.3.2. Oxidative Stress in the Pathogenesis of Leber’s hereditary optic neuropathy
4.4. Therapeutic Perspectives in Leber’s hereditary optic neuropathy
4.5. Anterior Ischemic Optic Neuropathy
4.5.1. General Aspects: Prevalence, Clinical Presentation, and Current Therapies
4.5.2. Oxidative Stress in the Pathogenesis of Anterior Ischemic Optic Neuropathy
4.6. Therapeutic Perspectives in Anterior Ischemic Optic Neuropathy
4.6.1. Giant Cell Arteritis
4.6.2. Nonarteritic Anterior Ischemic Optic Neuropathy
4.7. Optic Neuritis
4.7.1. General aspects
4.7.2. Oxidative Stress in the Pathogenesis of Optic Neuritis
4.8. Therapeutic Perspectives in Optic Neuritis
5. Other Rare Optic Neuropathies
5.1. Traumatic optic neuropathies
5.1.1. General Characteristics
5.1.2. Pathogenesis of Traumatic Optic Neuropathies: Role of oxidative stress and Ca²⁺
5.1.3. Potential Antioxidants for Traumatic Optic Neuropathies
5.2. Compressive and Strecth Optic Neuropathies
5.2.1. General features
5.2.2. Oxidative Stress in The Pathogenesis of Dysthyroid Optic Neuropathy
5.2.3. Antioxidant Candidates in Dysthyroid Optic Neuropathy
5.3. Infiltrative Optic Neuropathies
5.4. Congenital Anomalies of the Optic Nerve
5.5. Nutritional and Toxic Optic Neuropathies
6. Conclusions and Future Directions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riordan-Eva, P. Clinical assessment of optic nerve disorders. Eye (Lond) 2004, 18, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Van Stavern, G.P.; Newman, N.J. Optic neuropathies. An overview. Ophthalmol Clin North Am 2001, 14, 61–71. [Google Scholar] [PubMed]
- Sanz-Morello, B.; Ahmadi, H.; Vohra, R.; Saruhanian, S.; Freude, K.K.; Hamann, S.; Kolko, M. Oxidative Stress in Optic Neuropathies. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Stingl, J.V.; Wagner, F.M.; Liebezeit, S.; Baumgartner, R.; Spät, H.; Schuster, A.K.; Prokosch, V.; Grehn, F.; Hoffmann, E.M. Long-Term Efficacy and Safety of Modified Canaloplasty Versus Trabeculectomy in Open-Angle Glaucoma. Life (Basel) 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, J.; Li, Y.; Jiang, B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci Rep 2021, 11, 13762. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [PubMed]
- Mascialino, B.; Leinonen, M.; Meier, T. Meta-analysis of the prevalence of Leber hereditary optic neuropathy mtDNA mutations in Europe. European Journal of Ophthalmology 2012, 22, 461–465. [Google Scholar] [CrossRef]
- Puomila, A.; Hämäläinen, P.; Kivioja, S.; Savontaus, M.L.; Koivumäki, S.; Huoponen, K.; Nikoskelainen, E. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur J Hum Genet 2007, 15, 1079–1089. [Google Scholar] [CrossRef]
- Rosenberg, T.; Nørby, S.; Schwartz, M.; Saillard, J.; Magalhães, P.J.; Leroy, D.; Kann, E.C.; Duno, M. Prevalence and Genetics of Leber Hereditary Optic Neuropathy in the Danish Population. Investigative Ophthalmology & Visual Science 2016, 57, 1370–1375. [Google Scholar] [CrossRef]
- Spruijt, L.; Kolbach, D.N.; de Coo, R.F.; Plomp, A.S.; Bauer, N.J.; Smeets, H.J.; de Die-Smulders, C.E. Influence of mutation type on clinical expression of Leber hereditary optic neuropathy. Am J Ophthalmol 2006, 141, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Griffiths, P.G.; Brown, D.T.; Howell, N.; Turnbull, D.M.; Chinnery, P.F. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet 2003, 72, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.C.; Davis, R.L.; Ravishankar, S.; Copty, J.; Kummerfeld, S.; Sue, C.M. Low disease risk and penetrance in Leber hereditary optic neuropathy. Am J Hum Genet 2023, 110, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Mackey, D.A.; Ong, J.S.; MacGregor, S.; Whiteman, D.C.; Craig, J.E.; Lopez Sanchez, M.I.G.; Kearns, L.S.; Staffieri, S.E.; Clarke, L.; McGuinness, M.B.; et al. Is the disease risk and penetrance in Leber hereditary optic neuropathy actually low? Am J Hum Genet 2023, 110, 170–176. [Google Scholar] [CrossRef]
- Li, K.J.; Semenov, D.; Turk, M.; Pope, J. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Research & Therapy 2021, 23, 82. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, K.A.; Oh, S.Y. Prevalence and incidence of non-arteritic anterior ischaemic optic neuropathy in South Korea: a nationwide population-based study. Br J Ophthalmol 2018, 102, 936–941. [Google Scholar] [CrossRef]
- Toosy, A.T.; Mason, D.F.; Miller, D.H. Optic neuritis. Lancet Neurol 2014, 13, 83–99. [Google Scholar] [CrossRef]
- Braithwaite, T.; Subramanian, A.; Petzold, A.; Galloway, J.; Adderley, N.J.; Mollan, S.P.; Plant, G.T.; Nirantharakumar, K.; Denniston, A.K. Trends in Optic Neuritis Incidence and Prevalence in the UK and Association With Systemic and Neurologic Disease. JAMA Neurol 2020, 77, 1514–1523. [Google Scholar] [CrossRef]
- Rodriguez, M.; Siva, A.; Cross, S.A.; O'Brien, P.C.; Kurland, L.T. Optic neuritis: a population-based study in Olmsted County, Minnesota. Neurology 1995, 45, 244–250. [Google Scholar] [CrossRef]
- Percy, A.K.; Nobrega, F.T.; Kurland, L.T. Optic neuritis and multiple sclerosis. An epidemiologic study. Arch Ophthalmol 1972, 87, 135–139. [Google Scholar] [CrossRef]
- Cockerham, G.C.; Goodrich, G.L.; Weichel, E.D.; Orcutt, J.C.; Rizzo, J.F.; Bower, K.S.; Schuchard, R.A. Eye and visual function in traumatic brain injury. J Rehabil Res Dev 2009, 46, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Arabi, A.; Ansari, I.; Shahraki, T.; Safi, S. A Systematic Literature Review on Traumatic Optic Neuropathy. J Ophthalmol 2021, 2021, 5553885. [Google Scholar] [CrossRef] [PubMed]
- Pirouzmand, F. Epidemiological trends of traumatic optic nerve injuries in the largest Canadian adult trauma center. J Craniofac Surg 2012, 23, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.R. Traumatic Optic Neuropathy. J Neurol Surg B Skull Base 2021, 82, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Blandford, A.D.; Zhang, D.; Chundury, R.V.; Perry, J.D. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol 2017, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Neigel, J.M.; Rootman, J.; Belkin, R.I.; Nugent, R.A.; Drance, S.M.; Beattie, C.W.; Spinelli, J.A. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology 1988, 95, 1515–1521. [Google Scholar] [CrossRef]
- Bartalena, L.; Piantanida, E.; Gallo, D.; Lai, A.; Tanda, M.L. Epidemiology, Natural History, Risk Factors, and Prevention of Graves’ Orbitopathy. Frontiers in Endocrinology 2020, 11. [Google Scholar] [CrossRef]
- Kincaid, M.C.; Green, W.R. Ocular and orbital involvement in leukemia. Surv Ophthalmol 1983, 27, 211–232. [Google Scholar] [CrossRef]
- Patel, L.; McNally, R.J.; Harrison, E.; Lloyd, I.C.; Clayton, P.E. Geographical distribution of optic nerve hypoplasia and septo-optic dysplasia in Northwest England. J Pediatr 2006, 148, 85–88. [Google Scholar] [CrossRef]
- Tear Fahnehjelm, K.; Dahl, S.; Martin, L.; Ek, U. Optic nerve hypoplasia in children and adolescents; prevalence, ocular characteristics and behavioural problems. Acta Ophthalmol 2014, 92, 563–570. [Google Scholar] [CrossRef]
- Jefferis, J.M.; Hickman, S.J. Treatment and Outcomes in Nutritional Optic Neuropathy. Curr Treat Options Neurol 2019, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Roda, M.; di Geronimo, N.; Pellegrini, M.; Schiavi, C. Nutritional Optic Neuropathies: State of the Art and Emerging Evidences. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A. Neuroprotection in Optic Neuropathy. Asia Pac J Ophthalmol (Phila) 2018, 7, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Casson, R.J.; Chidlow, G.; Wood, J.P.; Crowston, J.G.; Goldberg, I. Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol 2012, 40, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-Y.; Cringle, S.J.; Balaratnasingam, C.; Morgan, W.H.; Yu, P.K.; Su, E.-N. Retinal ganglion cells: Energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Progress in Retinal and Eye Research 2013, 36, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Gericke, A. Oxidative Stress and Vascular Dysfunction in the Retina: Therapeutic Strategies. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res 2006, 25, 490–513. [Google Scholar] [CrossRef]
- Mozaffarieh, M.; Flammer, J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol 2013, 13, 43–49. [Google Scholar] [CrossRef]
- Aslan, M.; Dogan, S.; Kucuksayan, E. Oxidative stress and potential applications of free radical scavengers in glaucoma. Redox Rep 2013, 18, 76–87. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol 2013, 13, 12–15. [Google Scholar] [CrossRef]
- Saccà, S.C.; Izzotti, A. Focus on molecular events in the anterior chamber leading to glaucoma. Cell Mol Life Sci 2014, 71, 2197–2218. [Google Scholar] [CrossRef] [PubMed]
- Langbøl, M.; Saruhanian, S.; Baskaran, T.; Tiedemann, D.; Mouhammad, Z.A.; Toft-Kehler, A.K.; Jun, B.; Vohra, R.; Bazan, N.G.; Kolko, M. Increased Antioxidant Capacity and Pro-Homeostatic Lipid Mediators in Ocular Hypertension—A Human Experimental Model. Journal of Clinical Medicine 2020, 9, 2979. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxidative Medicine and Cellular Longevity 2017, 2017, 9208489. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.Y.; Liu, P.K.; Wen, Y.T.; Quinn, P.M.J.; Levi, S.R.; Wang, N.K.; Tsai, R.K. Role of Oxidative Stress in Ocular Diseases Associated with Retinal Ganglion Cells Degeneration. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.P.; Sladen, P.E.; Yu-Wai-Man, P.; Cheetham, M.E. Induced Pluripotent Stem Cells for Inherited Optic Neuropathies-Disease Modeling and Therapeutic Development. J Neuroophthalmol 2022, 42, 35–44. [Google Scholar] [CrossRef]
- Freddi, T.d.A.L.; Ottaiano, A.C. The Optic Nerve: Anatomy and Pathology. Seminars in Ultrasound, CT and MRI 2022, 43, 378–388. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J.; Balaratnasingam, C.; Morgan, W.H.; Yu, P.K.; Su, E.N. Retinal ganglion cells: Energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog Retin Eye Res 2013, 36, 217–246. [Google Scholar] [CrossRef]
- De Moraes, C.G. Anatomy of the visual pathways. J Glaucoma 2013, 22 Suppl 5, S2–7. [Google Scholar] [CrossRef]
- Anderson, D.R. Vascular supply to the optic nerve of primates. Am J Ophthalmol 1970, 70, 341–351. [Google Scholar] [CrossRef]
- Linsenmeier, R.A.; Zhang, H.F. Retinal oxygen: from animals to humans. Prog Retin Eye Res 2017, 58, 115–151. [Google Scholar] [CrossRef]
- Arden, G.B.; Wolf, J.E.; Tsang, Y. Does dark adaptation exacerbate diabetic retinopathy?: Evidence and a linking hypothesis. Vision Research 1998, 38, 1723–1729. [Google Scholar] [CrossRef]
- Selhorst, J.B.; Chen, Y. The optic nerve. Semin Neurol 2009, 29, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Galetta, S.L. Chapter 1 - Anatomy and physiology of the afferent visual system. In Handbook of Clinical Neurology, Kennard, C., Leigh, R.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 102, pp. 3–19. [Google Scholar]
- Almazroa, A.; Burman, R.; Raahemifar, K.; Lakshminarayanan, V. Optic Disc and Optic Cup Segmentation Methodologies for Glaucoma Image Detection: A Survey. Journal of Ophthalmology 2015, 2015, 180972. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Mukherjee, R.; Dutta, K.; Sen, A. Deep learning based framework for automatic diagnosis of glaucoma based on analysis of focal notching in the optic nerve head. arXiv arXiv:2112.05748 2021.
- Wilczek, M. THE LAMINA CRIBROSA AND ITS NATURE. Br J Ophthalmol 1947, 31, 551–565. [Google Scholar] [CrossRef]
- Emery, J.M.; Landis, D.; Paton, D.; Boniuk, M.; Craig, J.M. The lamina cribrosa in normal and glaucomatous human eyes. Trans Am Acad Ophthalmol Otolaryngol 1974, 78, Op290–Op297. [Google Scholar]
- Quigley, H.A.; Hohman, R.M.; Addicks, E.M.; Massof, R.W.; Green, W.R. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol 1983, 95, 673–691. [Google Scholar] [CrossRef]
- Hayreh, S.S. Orbital vascular anatomy. Eye (Lond) 2006, 20, 1130–1144. [Google Scholar] [CrossRef]
- McCaa, C.S. The eye and visual nervous system: anatomy, physiology and toxicology. Environ Health Perspect 1982, 44, 1–8. [Google Scholar] [CrossRef]
- Juan, J.S.; Ana, I.R.; Rosa De, H.; Elena, S.-G.; Pilar, R.; José, A.F.-A.; Inés, L.-C.; Blanca, R.; Alberto, T.; José, M.R. Anatomy of the Human Optic Nerve: Structure and Function. In Optic Nerve; Felicia, M.F., Ed.; IntechOpen: Rijeka, 2018; Ch. 2. [Google Scholar] [CrossRef]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Cull, G.; Fortune, B.; Cioffi, G.A. Varicosities of intraretinal ganglion cell axons in human and nonhuman primates. Investigative ophthalmology & visual science 2003, 44, 2–9. [Google Scholar]
- Bristow, E.A.; Griffiths, P.G.; Andrews, R.M.; Johnson, M.A.; Turnbull, D.M. The Distribution of Mitochondrial Activity in Relation to Optic Nerve Structure. Archives of Ophthalmology 2002, 120, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Serasinghe, M.N.; Chipuk, J.E. Mitochondrial Fission in Human Diseases. Handb Exp Pharmacol 2017, 240, 159–188. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 2000, 29, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biology 2015, 13, 89. [Google Scholar] [CrossRef]
- Walsh, C.; Barrow, S.; Voronina, S.; Chvanov, M.; Petersen, O.H.; Tepikin, A. Modulation of calcium signalling by mitochondria. Biochim Biophys Acta 2009, 1787, 1374–1382. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol 2012, 4, a006783. [Google Scholar] [CrossRef]
- Elkholi, R.; Renault, T.T.; Serasinghe, M.N.; Chipuk, J.E. Putting the pieces together: How is the mitochondrial pathway of apoptosis regulated in cancer and chemotherapy? Cancer Metab 2014, 2, 16. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 2010, 35, 505–513. [Google Scholar] [CrossRef]
- Shu, D.Y.; Chaudhary, S.; Cho, K.-S.; Lennikov, A.; Miller, W.P.; Thorn, D.C.; Yang, M.; McKay, T.B. Role of Oxidative Stress in Ocular Diseases: A Balancing Act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J Physiol 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Taurone, S.; Ralli, M.; Artico, M.; Madia, V.N.; Scarpa, S.; Nottola, S.A.; Maconi, A.; Betti, M.; Familiari, P.; Nebbioso, M.; et al. Oxidative stress and visual system: a review. EXCLI J 2022, 21, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: regulation and function. Eur Heart J 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Kannenkeril, D.; Bosch, A.; Kolwelter, J.; Jung, S.; Striepe, K.; Ott, C.; Delles, C.; Schmieder, R.E. Dependency of flow-mediated vasodilatation from basal nitric oxide activity. Clin Physiol Funct Imaging 2021, 41, 310–316. [Google Scholar] [CrossRef]
- Tibballs, J. The role of nitric oxide (formerly endothelium-derived relaxing factor-EDRF) in vasodilatation and vasodilator therapy. Anaesth Intensive Care 1993, 21, 759–773. [Google Scholar] [CrossRef]
- Simonsen, U.; Rodriguez-Rodriguez, R.; Dalsgaard, T.; Buus, N.H.; Stankevicius, E. Novel approaches to improving endothelium-dependent nitric oxide-mediated vasodilatation. Pharmacol Rep 2009, 61, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br J Pharmacol 2019, 176, 212–227. [Google Scholar] [CrossRef]
- Rand, M.J.; Li, C.G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol 1995, 57, 659–682. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.R. Nitric oxide: a radical neurotransmitter in the central nervous system. Prog Neurobiol 1994, 42, 129–160. [Google Scholar] [CrossRef] [PubMed]
- Falak, N.; Imran, Q.M.; Hussain, A.; Yun, B.W. Transcription Factors as the "Blitzkrieg" of Plant Defense: A Pragmatic View of Nitric Oxide's Role in Gene Regulation. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Harari, O.; Liao, J.K. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr Pharm Des 2004, 10, 893–898. [Google Scholar] [CrossRef]
- Campbell, S.C.; Richardson, H.; Ferris, W.F.; Butler, C.S.; Macfarlane, W.M. Nitric oxide stimulates insulin gene transcription in pancreatic beta-cells. Biochem Biophys Res Commun 2007, 353, 1011–1016. [Google Scholar] [CrossRef]
- Gunnett, C.A.; Lund, D.D.; Chu, Y.; Brooks, R.M., 2nd; Faraci, F.M.; Heistad, D.D. NO-dependent vasorelaxation is impaired after gene transfer of inducible NO-synthase. Arterioscler Thromb Vasc Biol 2001, 21, 1281–1287. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Huie, R.E.; Padmaja, S. The reaction of no with superoxide. Free Radic Res Commun 1993, 18, 195–199. [Google Scholar] [CrossRef]
- Villamena, F.A. Chapter 2 - Chemistry of Reactive Species. In Reactive Species Detection in Biology; Villamena, F.A., Ed.; Elsevier: Boston, 2017; pp. 13–64. [Google Scholar] [CrossRef]
- Chronopoulos, P.; Manicam, C.; Zadeh, J.K.; Laspas, P.; Unkrig, J.C.; Göbel, M.L.; Musayeva, A.; Pfeiffer, N.; Oelze, M.; Daiber, A.; et al. Effects of Resveratrol on Vascular Function in Retinal Ischemia-Reperfusion Injury. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Gao, Y.; Song, M.; Cao, W.; Sun, X. Peroxynitrite is a novel risk factor and treatment target of glaucoma. Nitric Oxide 2020, 99, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cantó, A.; Olivar, T.; Romero, F.J.; Miranda, M. Nitrosative Stress in Retinal Pathologies: Review. Antioxidants (Basel) 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Roos, D. Chronic Granulomatous Disease. Methods Mol Biol 2019, 1982, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta Med Indones 2017, 49, 158–165. [Google Scholar]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Hsueh, Y.J.; Chen, Y.N.; Tsao, Y.T.; Cheng, C.M.; Wu, W.C.; Chen, H.C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat Rev Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr Atheroscler Rep 2017, 19, 42. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic Biol Med 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. Chemistry of ROS-mediated oxidation to the guanine base in DNA and its biological consequences. Int J Radiat Biol 2022, 98, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. Interplay of Guanine Oxidation and G-Quadruplex Folding in Gene Promoters. J Am Chem Soc 2020, 142, 1115–1136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, X.M.; Jiang, Z.S.; Wang, G.X.; Zhang, D.W. Protein tyrosine nitration in atherosclerotic endothelial dysfunction. Clin Chim Acta 2022, 529, 34–41. [Google Scholar] [CrossRef]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol 2008, 43, 24–33. [Google Scholar] [CrossRef]
- Sanz, A.; Caro, P.; Gómez, J.; Barja, G. Testing the vicious cycle theory of mitochondrial ROS production: effects of H2O2 and cumene hydroperoxide treatment on heart mitochondria. J Bioenerg Biomembr 2006, 38, 121–127. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Yuan, L.Q.; Wang, C.; Lu, D.F.; Zhao, X.D.; Tan, L.H.; Chen, X. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging (Albany NY) 2020, 12, 3662–3681. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell Mol Neurobiol 2015, 35, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol 2019, 11, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- MacMillan-Crow, L.A.; Thompson, J.A. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys 1999, 366, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Kapoor, R.; Felts, P.A. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 1999, 9, 69–92. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med Cell Longev 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Di Gioia, M.; Zanoni, I. Dooming Phagocyte Responses: Inflammatory Effects of Endogenous Oxidized Phospholipids. Front Endocrinol (Lausanne) 2021, 12, 626842. [Google Scholar] [CrossRef]
- Greig, F.H.; Kennedy, S.; Spickett, C.M. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation. Free Radic Biol Med 2012, 52, 266–280. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu Rev Biochem 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J Food Biochem 2020, 44, e13145. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 2016, 15, 71. [Google Scholar] [CrossRef]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol Appl Pharmacol 2015, 289, 361–370. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W.; Zukor, H.; Hascalovici, J.R.; Zeligman, D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 2009, 110, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Overview on Peroxiredoxin. Mol Cells 2016, 39, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic Biol Med 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res 2008, 52 Suppl 1, S128–138. [Google Scholar] [CrossRef]
- Engwa, G.A. Free Radicals and the Role of Plant Phytochemicals as Antioxidants Against Oxidative Stress-Related Diseases. Phytochemicals - Source of Antioxidants and Role in Disease Prevention, 2018. [Google Scholar]
- Yadav, A.; Mishra, P.C. Modeling the activity of glutathione as a hydroxyl radical scavenger considering its neutral non-zwitterionic form. J Mol Model 2013, 19, 767–777. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol Aspects Med 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Ascorbic acid as antioxidant. Vitam Horm 2023, 121, 247–270. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of α -lipoic acid. Molecular nutrition & food research 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Musayeva, A.; Unkrig, J.C.; Zhutdieva, M.B.; Manicam, C.; Ruan, Y.; Laspas, P.; Chronopoulos, P.; Göbel, M.L.; Pfeiffer, N.; Brochhausen, C.; et al. Betulinic Acid Protects from Ischemia-Reperfusion Injury in the Mouse Retina. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.A.; Di Polo, A. Mitochondrial dynamics, transport, and quality control: A bottleneck for retinal ganglion cell viability in optic neuropathies. Mitochondrion 2017, 36, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.S.; Gantner, M.L.; Smith, L.E.H. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog Retin Eye Res 2018, 64, 131–156. [Google Scholar] [CrossRef]
- Casson, R.J.; Chidlow, G.; Crowston, J.G.; Williams, P.A.; Wood, J.P.M. Retinal energy metabolism in health and glaucoma. Progress in Retinal and Eye Research 2021, 81, 100881. [Google Scholar] [CrossRef] [PubMed]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal Oxygen: Fundamental and Clinical Aspects. Archives of Ophthalmology 2003, 121, 547–557. [Google Scholar] [CrossRef]
- Cohen, L. Relationships between visual function and metabolism. Biochemistry of the Eye 1965, 36–50. [Google Scholar]
- ANDERSON, B.; SALTZMAN, H.A. Retinal oxygen utilization measured by hyperbaric blackout. Archives of ophthalmology 1964, 72, 792–795. [Google Scholar] [CrossRef]
- Ames III, A. Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Canadian journal of physiology and pharmacology 1992, 70, S158–S164. [Google Scholar] [CrossRef]
- Birk, M.; Baum, E.; Zadeh, J.K.; Manicam, C.; Pfeiffer, N.; Patzak, A.; Helmstadter, J.; Steven, S.; Kuntic, M.; Daiber, A.; et al. Angiotensin II Induces Oxidative Stress and Endothelial Dysfunction in Mouse Ophthalmic Arteries via Involvement of AT1 Receptors and NOX2. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Gericke, A.; Mann, C.; Zadeh, J.K.; Musayeva, A.; Wolff, I.; Wang, M.; Pfeiffer, N.; Daiber, A.; Li, H.; Xia, N.; et al. Elevated Intraocular Pressure Causes Abnormal Reactivity of Mouse Retinal Arterioles. Oxid Med Cell Longev 2019, 2019, 9736047. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, H.; Xia, N.; Li, H.; van Beers, T.; Gericke, A.; Prokosch, V. Intraocular Pressure-Induced Endothelial Dysfunction of Retinal Blood Vessels Is Persistent, but Does Not Trigger Retinal Ganglion Cell Loss. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Zadeh, J.K.; Zhutdieva, M.B.; Laspas, P.; Yuksel, C.; Musayeva, A.; Pfeiffer, N.; Brochhausen, C.; Oelze, M.; Daiber, A.; Xia, N.; et al. Apolipoprotein E Deficiency Causes Endothelial Dysfunction in the Mouse Retina. Oxid Med Cell Longev 2019, 2019, 5181429. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, J.K.; Garcia-Bardon, A.; Hartmann, E.K.; Pfeiffer, N.; Omran, W.; Ludwig, M.; Patzak, A.; Xia, N.; Li, H.; Gericke, A. Short-Time Ocular Ischemia Induces Vascular Endothelial Dysfunction and Ganglion Cell Loss in the Pig Retina. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Scorziello, A.; Duchen, M.R. Three Distinct Mechanisms Generate Oxygen Free Radicals in Neurons and Contribute to Cell Death during Anoxia and Reoxygenation. The Journal of Neuroscience 2007, 27, 1129–1138. [Google Scholar] [CrossRef]
- Ono, T.; Tsuruta, R.; Fujita, M.; Aki, H.S.; Kutsuna, S.; Kawamura, Y.; Wakatsuki, J.; Aoki, T.; Kobayashi, C.; Kasaoka, S.; et al. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Research 2009, 1305, 158–167. [Google Scholar] [CrossRef]
- Dauth, A.; Breborowicz, A.; Ruan, Y.; Tang, Q.; Zadeh, J.K.; Bohm, E.W.; Pfeiffer, N.; Khedkar, P.H.; Patzak, A.; Vujacic-Mirski, K.; et al. Sulodexide Prevents Hyperglycemia-Induced Endothelial Dysfunction and Oxidative Stress in Porcine Retinal Arterioles. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Giurdanella, G.; Lazzara, F.; Caporarello, N.; Lupo, G.; Anfuso, C.D.; Eandi, C.M.; Leggio, G.M.; Drago, F.; Bucolo, C.; Salomone, S. Sulodexide prevents activation of the PLA2/COX-2/VEGF inflammatory pathway in human retinal endothelial cells by blocking the effect of AGE/RAGE. Biochem Pharmacol 2017, 142, 145–154. [Google Scholar] [CrossRef]
- Hein, T.W.; Xu, W.; Xu, X.; Kuo, L. Acute and Chronic Hyperglycemia Elicit JIP1/JNK-Mediated Endothelial Vasodilator Dysfunction of Retinal Arterioles. Invest Ophthalmol Vis Sci 2016, 57, 4333–4340. [Google Scholar] [CrossRef]
- Yan, S.D.; Schmidt, A.M.; Anderson, G.M.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem 1994, 269, 9889–9897. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci 2018, 193, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Li, M.; Song, J.; Li, Q.W.; Wang, J.; Di, S.; Tong, X.L.; Ni, Q. Luo Tong formula attenuates retinal inflammation in diabetic rats via inhibition of the p38MAPK/NF-κB pathway. Chin Med 2020, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, F.; Fidilio, A.; Platania, C.B.M.; Giurdanella, G.; Salomone, S.; Leggio, G.M.; Tarallo, V.; Cicatiello, V.; De Falco, S.; Eandi, C.M.; et al. Aflibercept regulates retinal inflammation elicited by high glucose via the PlGF/ERK pathway. Biochem Pharmacol 2019, 168, 341–351. [Google Scholar] [CrossRef]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol 2013, 2013, 343560. [Google Scholar] [CrossRef]
- Lee, S.R.; An, E.J.; Kim, J.; Bae, Y.S. Function of NADPH Oxidases in Diabetic Nephropathy and Development of Nox Inhibitors. Biomol Ther (Seoul) 2020, 28, 25–33. [Google Scholar] [CrossRef]
- Nakazawa, T.; Fukuchi, T. What is glaucomatous optic neuropathy? Jpn J Ophthalmol 2020, 64, 243–249. [Google Scholar] [CrossRef]
- Fortune, B.; Grzybowski, A. Glaucomatous or Non-glaucomatous Optic Neuropathy-It Is a Question? Am J Ophthalmol 2022, 234, A5–a7. [Google Scholar] [CrossRef]
- Burgoyne, C. The morphological difference between glaucoma and other optic neuropathies. J Neuroophthalmol 2015, 35 Suppl 1, S8–s21. [Google Scholar] [CrossRef]
- Anderson, D.R.; Patella, V.M. Automated static perimetry, 2nd ed.Mosby: Saint Louis (Mo.), 1999. [Google Scholar]
- Foster, P.J.; Buhrmann, R.; Quigley, H.A.; Johnson, G.J. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002, 86, 238–242. [Google Scholar] [CrossRef]
- McMonnies, C. Reactive oxygen species, oxidative stress, glaucoma and hyperbaric oxygen therapy. J Optom 2018, 11, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid Med Cell Longev 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Kroese, M.; Burton, H. Primary open angle glaucoma. The need for a consensus case definition. J Epidemiol Community Health 2003, 57, 752–754. [Google Scholar] [CrossRef]
- Khazaeni, B.; Khazaeni, L. Acute Closed Angle Glaucoma. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL), 2022.
- Flores-Sánchez, B.C.; Tatham, A.J. Acute angle closure glaucoma. Br J Hosp Med (Lond) 2019, 80, C174–c179. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.L.; Tham, C.C. Normal-tension glaucoma: Current concepts and approaches-A review. Clin Exp Ophthalmol 2022, 50, 247–259. [Google Scholar] [CrossRef]
- Patel, K.; Patel, S. Angle-closure glaucoma. Disease-a-Month 2014, 60, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Tawfik, M.A.; Waisbourd, M.; Katz, L.J. Primary angle-closure glaucoma: an update. Acta Ophthalmol 2016, 94, 217–225. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Quigley, H.A. Number of people with glaucoma worldwide. Br J Ophthalmol 1996, 80, 389–393. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: a review. Jama 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Thomas, R.; Li, S.Z.; Wang, N.L. Development of angle closure and associated risk factors: The Handan eye study. Acta Ophthalmologica 2022, 100, e253–e261. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The Diagnosis and Treatment of Glaucoma. Dtsch Arztebl Int 2020, 117, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.V.; Ervin, A.M.; Friedman, D.S.; Jampel, H.D.; Hawkins, B.S.; Vollenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013, 158, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.-P.; Stefánsson, E. The impact of ocular blood flow in glaucoma. Progress in Retinal and Eye Research 2002, 21, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.C.; Roberts, M.D.; Burgoyne, C.F. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci 2008, 85, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Prum, B.E., Jr.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W., Jr.; Lim, M.C.; Williams, R.D. Primary Open-Angle Glaucoma Preferred Practice Pattern(®) Guidelines. Ophthalmology 2016, 123, P41–p111. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.P.; Yuan, H.H.; Zhu, X.; Cui, Y.Y.; Li, H.; Feng, X.M.; Qiu, Y.; Chen, H.Z.; Zhou, W. Activation of muscarinic receptors protects against retinal neurons damage and optic nerve degeneration in vitro and in vivo models. CNS Neurosci Ther 2014, 20, 227–236. [Google Scholar] [CrossRef]
- Li, T.; Lindsley, K.; Rouse, B.; Hong, H.; Shi, Q.; Friedman, D.S.; Wormald, R.; Dickersin, K. Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis. Ophthalmology 2016, 123, 129–140. [Google Scholar] [CrossRef]
- Rouland, J.F.; Berdeaux, G.; Lafuma, A. The economic burden of glaucoma and ocular hypertension: implications for patient management: a review. Drugs Aging 2005, 22, 315–321. [Google Scholar] [CrossRef]
- Dirani, M.; Crowston, J.G.; Taylor, P.S.; Moore, P.T.; Rogers, S.; Pezzullo, M.L.; Keeffe, J.E.; Taylor, H.R. Economic impact of primary open-angle glaucoma in Australia. Clin Exp Ophthalmol 2011, 39, 623–632. [Google Scholar] [CrossRef]
- McGinley, P.; Ansari, E.; Sandhu, H.; Dixon, T. The cost burden of falls in people with glaucoma in National Health Service Hospital Trusts in the UK. J Med Econ 2020, 23, 106–112. [Google Scholar] [CrossRef]
- Shih, V.; Parekh, M.; Multani, J.K.; McGuiness, C.B.; Chen, C.-C.; Campbell, J.H.; Miller-Ellis, E.; Olivier, M.M.G. Clinical and Economic Burden of Glaucoma by Disease Severity: A United States Claims-Based Analysis. Ophthalmology Glaucoma 2021, 4, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Wolfram, C.; Breitscheidel, L.; Shlaen, M.; Verboven, Y.; Pfeiffer, N. Direct cost and predictive factors for treatment in patients with ocular hypertension or early, moderate and advanced primary open-angle glaucoma: the CoGIS study in Germany. Graefes Arch Clin Exp Ophthalmol 2013, 251, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Lee, P.P.; Goldberg, I.; Kotak, S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol 2011, 152, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, K.; Dada, R.; Dada, T. Oxidative DNA damage and reduced expression of DNA repair genes: Role in primary open angle glaucoma (POAG). Ophthalmic Genetics 2017, 38, 446–450. [Google Scholar] [CrossRef]
- Izzotti, A.; Saccà, S.C.; Longobardi, M.; Cartiglia, C. Mitochondrial Damage in the Trabecular Meshwork of Patients With Glaucoma. Archives of Ophthalmology 2010, 128, 724–730. [Google Scholar] [CrossRef]
- Saccà, S.C.; Pascotto, A.; Camicione, P.; Capris, P.; Izzotti, A. Oxidative DNA Damage in the Human Trabecular Meshwork: Clinical Correlation in Patients With Primary Open-Angle Glaucoma. Archives of Ophthalmology 2005, 123, 458–463. [Google Scholar] [CrossRef]
- Nucci, C.; Di Pierro, D.; Varesi, C.; Ciuffoletti, E.; Russo, R.; Gentile, R.; Cedrone, C.; Pinazo Duran, M.D.; Coletta, M.; Mancino, R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol Vis 2013, 19, 1841–1846. [Google Scholar]
- Zanon-Moreno, V.; Garcia-Medina, J.J.; Gallego-Pinazo, R.; Vinuesa-Silva, I.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Antioxidant Status Modifications by Topical Administration of Dorzolamide in Primary Open-Angle Glaucoma. European Journal of Ophthalmology 2009, 19, 565–571. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Lerner, S.F.; Brunzini, R.; Evelson, P.A.; Llesuy, S.F. Oxidative stress markers in aqueous humor of glaucoma patients. American Journal of Ophthalmology 2004, 137, 62–69. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.A.; Mousa, A.; Osman, E.A.; Al-Obeidan, S.A. Decreased total antioxidants in patients with primary open angle glaucoma. Curr Eye Res 2013, 38, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Himori, N.; Kunikata, H.; Yamazaki, M.; Shiga, Y.; Omodaka, K.; Takahashi, H.; Nakazawa, T. Age- and sex-dependency of the association between systemic antioxidant potential and glaucomatous damage. Sci Rep 2017, 7, 8032. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci Rep 2016, 6, 25792. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Correlation between Systemic Oxidative Stress and Intraocular Pressure Level. PLOS ONE 2015, 10, e0133582. [Google Scholar] [CrossRef]
- Benoist d'Azy, C.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PLoS One 2016, 11, e0166915. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Cao, W.; Sun, X. The Association of Oxidative Stress Status with Open-Angle Glaucoma and Exfoliation Glaucoma: A Systematic Review and Meta-Analysis. Journal of Ophthalmology 2019, 2019, 1803619. [Google Scholar] [CrossRef]
- Aboobakar, I.F.; Allingham, R.R. Genetics of exfoliation syndrome and glaucoma. Int Ophthalmol Clin 2014, 54, 43–56. [Google Scholar] [CrossRef]
- Ritch, R.; Schlötzer-Schrehardt, U. Exfoliation syndrome. Surv Ophthalmol 2001, 45, 265–315. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Hsu, W.-M.; Chou, C.-K.; Chen, S.-J.; Peng, C.-H.; Chi, C.-W.; Ho, L.L.-T.; Liu, J.-H.; Chiou, S.-H. Significant Variation of the Elevated Nitric Oxide Levels in Aqueous Humor from Patients with Different Types of Glaucoma. Ophthalmologica 2002, 216, 346–350. [Google Scholar] [CrossRef]
- Fernández-Durango, R.; Fernández-Martínez, A.; García-Feijoo, J.n.; Castillo, A.; de la Casa, J.M.n.; García-Bueno, B.; Pérez-Nievas, B.G.; Fernández-Cruz, A.; Leza, J.C. Expression of Nitrotyrosine and Oxidative Consequences in the Trabecular Meshwork of Patients with Primary Open-Angle Glaucoma. Investigative Ophthalmology & Visual Science 2008, 49, 2506–2511. [Google Scholar] [CrossRef]
- Llobet, A.; Gasull, X.; Gual, A. Understanding trabecular meshwork physiology: a key to the control of intraocular pressure? News Physiol Sci 2003, 18, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Lerner, S.F.; Brunzini, R.; Evelson, P.A.; Llesuy, S.F. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol 2004, 137, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Javadzadeh, A.; Rashtchizadeh, N.; Moharrery, M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis 2011, 17, 41–46. [Google Scholar]
- Saccà, S.C.; Izzotti, A.; Rossi, P.; Traverso, C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res 2007, 84, 389–399. [Google Scholar] [CrossRef]
- Hogg, P.; Calthorpe, M.; Batterbury, M.; Grierson, I. Aqueous humor stimulates the migration of human trabecular meshwork cells in vitro. Invest Ophthalmol Vis Sci 2000, 41, 1091–1098. [Google Scholar] [PubMed]
- Zhou, L.; Li, Y.; Yue, B.Y. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol 1999, 180, 182–189. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Current Opinion in Pharmacology 2013, 13, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Wax, M.B. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci 2000, 20, 8693–8700. [Google Scholar] [CrossRef]
- Tezel, G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res 2008, 173, 409–421. [Google Scholar] [CrossRef]
- Tezel, G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog Retin Eye Res 2022, 87, 100998. [Google Scholar] [CrossRef]
- Ju, W.K.; Kim, K.Y.; Lindsey, J.D.; Angert, M.; Duong-Polk, K.X.; Scott, R.T.; Kim, J.J.; Kukhmazov, I.; Ellisman, M.H.; Perkins, G.A.; et al. Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Invest Ophthalmol Vis Sci 2008, 49, 4903–4911. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Fourth, A.P.O.R.I.C.W.G. The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci 2009, 50, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Levkovitch-Verbin, H.; Valenta, D.; Baumrind, L.; Pease, M.E.; Quigley, H.A. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest Ophthalmol Vis Sci 2002, 43, 2236–2243. [Google Scholar] [PubMed]
- Mantzaris, M.D.; Bellou, S.; Skiada, V.; Kitsati, N.; Fotsis, T.; Galaris, D. Intracellular labile iron determines H2O2-induced apoptotic signaling via sustained activation of ASK1/JNK-p38 axis. Free Radical Biology and Medicine 2016, 97, 454–465. [Google Scholar] [CrossRef]
- Harada, C.; Nakamura, K.; Namekata, K.; Okumura, A.; Mitamura, Y.; Iizuka, Y.; Kashiwagi, K.; Yoshida, K.; Ohno, S.; Matsuzawa, A.; et al. Role of apoptosis signal-regulating kinase 1 in stress-induced neural cell apoptosis in vivo. Am J Pathol 2006, 168, 261–269. [Google Scholar] [CrossRef]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biology 2016, 10, 233–242. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Chang, Y.-C.; Chen, P.-H.; Li, C.-Y.; Wu, W.-C.; Kao, Y.-H. MicroRNA-100 Mediates Hydrogen Peroxide-Induced Apoptosis of Human Retinal Pigment Epithelium ARPE-19 Cells. Pharmaceuticals 2021, 14, 314. [Google Scholar] [CrossRef]
- The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol 1998, 126, 498–505. [Google Scholar] [CrossRef]
- Killer, H.E.; Pircher, A. Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye (Lond) 2018, 32, 924–930. [Google Scholar] [CrossRef]
- Stroman, G.A.; Stewart, W.C.; Golnik, K.C.; Curé, J.K.; Olinger, R.E. Magnetic resonance imaging in patients with low-tension glaucoma. Arch Ophthalmol 1995, 113, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.; Farinelli, A.; Billson, F.; Houang, M.; Stern, M. Comparative study of brain magnetic resonance imaging findings in patients with low-tension glaucoma and control subjects. Ophthalmology 1995, 102, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Tomidokoro, A.; Araie, M.; Tomita, G.; Yamagami, J.; Okubo, T.; Masumoto, T. Visual field damage in normal-tension glaucoma patients with or without ischemic changes in cerebral magnetic resonance imaging. Jpn J Ophthalmol 2004, 48, 340–344. [Google Scholar] [CrossRef]
- Rupin, A.; Paysant, J.; Sansilvestri-Morel, P.; Lembrez, N.; Lacoste, J.M.; Cordi, A.; Verbeuren, T.J. Role of NADPH oxidase-mediated superoxide production in the regulation of E-selectin expression by endothelial cells subjected to anoxia/reoxygenation. Cardiovasc Res 2004, 63, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Roth, M.; König, P.; Hofmann, S.; Dony, E.; Goyal, P.; Selbitz, A.C.; Schermuly, R.T.; Ghofrani, H.A.; Kwapiszewska, G.; et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 2007, 101, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kleikers, P.W.; Wingler, K.; Hermans, J.J.; Diebold, I.; Altenhöfer, S.; Radermacher, K.A.; Janssen, B.; Görlach, A.; Schmidt, H.H. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J Mol Med (Berl) 2012, 90, 1391–1406. [Google Scholar] [CrossRef]
- Kietzmann, T.; Görlach, A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol 2005, 16, 474–486. [Google Scholar] [CrossRef]
- Tezel, G.; Wax, M.B. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol 2004, 122, 1348–1356. [Google Scholar] [CrossRef]
- Banasiak, K.J.; Xia, Y.; Haddad, G.G. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol 2000, 62, 215–249. [Google Scholar] [CrossRef]
- Yokota, H.; Narayanan, S.P.; Zhang, W.; Liu, H.; Rojas, M.; Xu, Z.; Lemtalsi, T.; Nagaoka, T.; Yoshida, A.; Brooks, S.E.; et al. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci 2011, 52, 8123–8131. [Google Scholar] [CrossRef]
- Rieger, J.M.; Shah, A.R.; Gidday, J.M. Ischemia-reperfusion injury of retinal endothelium by cyclooxygenase- and xanthine oxidase-derived superoxide. Exp Eye Res 2002, 74, 493–501. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci 2004, 45, 4049–4059. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.-R.; Chae, H.-J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Experimental & Molecular Medicine 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Kroeger, H.; Chiang, W.-C.; Felden, J.; Nguyen, A.; Lin, J.H. ER stress and unfolded protein response in ocular health and disease. The FEBS Journal 2019, 286, 399–412. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Chiang, W.C.; Kurt, T.D.; Sigurdson, C.J.; Lin, J.H. Multiple Mechanisms of Unfolded Protein Response-Induced Cell Death. Am J Pathol 2015, 185, 1800–1808. [Google Scholar] [CrossRef]
- Hurley, D.J.; Normile, C.; Irnaten, M.; O'Brien, C. The Intertwined Roles of Oxidative Stress and Endoplasmic Reticulum Stress in Glaucoma. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Lin, J.H.; Walter, P.; Yen, T.S.B. Endoplasmic Reticulum Stress in Disease Pathogenesis. Annual Review of Pathology: Mechanisms of Disease 2008, 3, 399–425. [Google Scholar] [CrossRef]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 2013, 15, 481–490. [Google Scholar] [CrossRef]
- Hwang, J.; Qi, L. Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem Sci 2018, 43, 593–605. [Google Scholar] [CrossRef]
- Chen, A.C.-H.; Burr, L.; McGuckin, M.A. Oxidative and endoplasmic reticulum stress in respiratory disease. Clinical & Translational Immunology 2018, 7, e1019. [Google Scholar] [CrossRef]
- Kaneko, M.; Niinuma, Y.; Nomura, Y. Activation Signal of Nuclear Factor-κB in Response to Endoplasmic Reticulum Stress is Transduced <i>via</i> IRE1 and Tumor Necrosis Factor Receptor-Associated Factor 2. Biological and Pharmaceutical Bulletin 2003, 26, 931–935. [Google Scholar] [CrossRef]
- Song, B.; Scheuner, D.; Ron, D.; Pennathur, S.; Kaufman, R.J. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 2008, 118, 3378–3389. [Google Scholar] [CrossRef]
- Booth, D.M.; Enyedi, B.; Geiszt, M.; Várnai, P.; Hajnóczky, G. Redox Nanodomains Are Induced by and Control Calcium Signaling at the ER-Mitochondrial Interface. Mol Cell 2016, 63, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Zhai, P.; Shao, D.; Zablocki, D.; Nagarajan, N.; Terada, L.S.; Volpe, M.; Sadoshima, J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ Res 2013, 113, 1253–1264. [Google Scholar] [CrossRef]
- Pahl, H.L.; Baeuerle, P.A. The ER-overload response: activation of NF-κB. Trends in Biochemical Sciences 1997, 22, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Xue, R.; Yang, Y.; Zhang, S.X.; Xiao, H.; Zhu, H.; Li, J.; Chen, G.; Ye, Y.; Yu, M.; et al. Activation of ATF4 triggers trabecular meshwork cell dysfunction and apoptosis in POAG. Aging (Albany NY) 2021, 13, 8628–8642. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Patel, P.D.; Maddineni, P.; Patil, S.; Kiehlbauch, C.; Millar, J.C.; Searby, C.C.; Raghunathan, V.; Sheffield, V.C.; Zode, G.S. ATF4 leads to glaucoma by promoting protein synthesis and ER client protein load. Nature Communications 2020, 11, 5594. [Google Scholar] [CrossRef]
- Peters, J.C.; Bhattacharya, S.; Clark, A.F.; Zode, G.S. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Investigative Ophthalmology & Visual Science 2015, 56, 3860–3868. [Google Scholar] [CrossRef]
- Chai, F.; Yan, H.; Zhao, X.; Li, J.; Pei, C. The role of GRP78 in oxidative stress induced by tunicamycin in trabecular meshwork cells. Acta Biochim Pol 2022, 69, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Doh, S.H.; Kim, J.H.; Lee, K.M.; Park, H.Y.; Park, C.K. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res 2010, 1308, 158–166. [Google Scholar] [CrossRef]
- Marola, O.J.; Syc-Mazurek, S.B.; Libby, R.T. DDIT3 (CHOP) contributes to retinal ganglion cell somal loss but not axonal degeneration in DBA/2J mice. Cell Death Discov 2019, 5, 140. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sato, T.; Ohno-Oishi, M.; Ozawa, M.; Maekawa, S.; Shiga, Y.; Yabana, T.; Yasuda, M.; Himori, N.; Omodaka, K.; et al. CHOP deletion and anti-neuroinflammation treatment with hesperidin synergistically attenuate NMDA retinal injury in mice. Exp Eye Res 2021, 213, 108826. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhang, X.; Xu, X. Nerve Growth Factor Protects Retinal Ganglion Cells Related to Inhibiting Endoplasmic Reticulum Stress by Inhibiting IRE1-JNK-CHOP Signaling Pathway. Ocul Immunol Inflamm 2022, 30, 1341–1346. [Google Scholar] [CrossRef]
- Gao, Z.; Li, M.; Yao, F.; Xia, X.; Duan, T.; Meng, J.; Huang, Y.; He, Y.; Saro, A.; Huang, J. Valdecoxib Protects against Cell Apoptosis Induced by Endoplasmic Reticulum Stress via the Inhibition of PERK-ATF4-CHOP Pathway in Experimental Glaucoma. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim Pol 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Avotri, S.; Eatman, D.; Russell-Randall, K. Effects of Resveratrol on Inflammatory Biomarkers in Glaucomatous Human Trabecular Meshwork Cells. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of Human SIRT1 Activation by Resveratrol*. Journal of Biological Chemistry 2005, 280, 17187–17195. [Google Scholar] [CrossRef]
- Ciccone, L.; Piragine, E.; Brogi, S.; Camodeca, C.; Fucci, R.; Calderone, V.; Nencetti, S.; Martelli, A.; Orlandini, E. Resveratrol-like Compounds as SIRT1 Activators. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Pirhan, D.; Yüksel, N.; Emre, E.; Cengiz, A.; Kürşat Yıldız, D. Riluzole- and Resveratrol-Induced Delay of Retinal Ganglion Cell Death in an Experimental Model of Glaucoma. Curr Eye Res 2016, 41, 59–69. [Google Scholar] [CrossRef]
- Ye, M.J.; Meng, N. Resveratrol acts via the mitogen-activated protein kinase (MAPK) pathway to protect retinal ganglion cells from apoptosis induced by hydrogen peroxide. Bioengineered 2021, 12, 4878–4886. [Google Scholar] [CrossRef]
- Yue, Y.K.; Mo, B.; Zhao, J.; Yu, Y.J.; Liu, L.; Yue, C.L.; Liu, W. Neuroprotective effect of curcumin against oxidative damage in BV-2 microglia and high intraocular pressure animal model. J Ocul Pharmacol Ther 2014, 30, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Buccarello, L.; Dragotto, J.; Hassanzadeh, K.; Maccarone, R.; Corbo, M.; Feligioni, M. Retinal ganglion cell loss in an ex vivo mouse model of optic nerve cut is prevented by curcumin treatment. Cell Death Discov 2021, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Noro, T.; Namekata, K.; Azuchi, Y.; Kimura, A.; Guo, X.; Harada, C.; Nakano, T.; Tsuneoka, H.; Harada, T. Spermidine Ameliorates Neurodegeneration in a Mouse Model of Normal Tension Glaucoma. Invest Ophthalmol Vis Sci 2015, 56, 5012–5019. [Google Scholar] [CrossRef]
- Noro, T.; Namekata, K.; Kimura, A.; Guo, X.; Azuchi, Y.; Harada, C.; Nakano, T.; Tsuneoka, H.; Harada, T. Spermidine promotes retinal ganglion cell survival and optic nerve regeneration in adult mice following optic nerve injury. Cell Death Dis 2015, 6, e1720. [Google Scholar] [CrossRef]
- Liu, X.G.; Wu, S.Q.; Li, P.; Yang, H. Advancement in the chemical analysis and quality control of flavonoid in Ginkgo biloba. J Pharm Biomed Anal 2015, 113, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dong, L.-H.; Zhang, Y.; Liu, Q. A network pharmacology-based strategy for predicting the protective mechanism of Ginkgo biloba on damaged retinal ganglion cells. Chinese Journal of Natural Medicines 2022, 20, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Anne-Marie, B.; Beatriz, V.; Ana Isabel, J.; Covadonga, P. Managing Intraocular Pressure: Innovation in Glaucoma Management. In Glaucoma; Parul, I., Ed.; IntechOpen: Rijeka, 2016; Ch. 6. [Google Scholar] [CrossRef]
- Lee, D.; Shim, M.S.; Kim, K.Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest Ophthalmol Vis Sci 2014, 55, 993–1005. [Google Scholar] [CrossRef]
- Quaranta, L.; Riva, I.; Biagioli, E.; Rulli, E.; Rulli, E.; Poli, D.; Legramandi, L. Evaluating the Effects of an Ophthalmic Solution of Coenzyme Q10 and Vitamin E in Open-Angle Glaucoma Patients: A Study Protocol. Adv Ther 2019, 36, 2506–2514. [Google Scholar] [CrossRef]
- Inman, D.M.; Lambert, W.S.; Calkins, D.J.; Horner, P.J. α-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS One 2013, 8, e65389. [Google Scholar] [CrossRef]
- Sanz-González, S.M.; Raga-Cervera, J.; Aguirre Lipperheide, M.; Zanón-Moreno, V.; Chiner, V.; Ramírez, A.I.; Pinazo-Durán, M.D. Effect of an oral supplementation with a formula containing R-lipoic acid in glaucoma patients. Arch Soc Esp Oftalmol (Engl Ed) 2020, 95, 120–129. [Google Scholar] [CrossRef]
- Kamat, J.P.; Devasagayam, T.P. Nicotinamide (vitamin B3) as an effective antioxidant against oxidative damage in rat brain mitochondria. Redox Rep 1999, 4, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Kim, Y.C.; Park, C.K. Dietary Niacin and Open-Angle Glaucoma: The Korean National Health and Nutrition Examination Survey. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Harder, J.M.; John, S.W.M. Glaucoma as a Metabolic Optic Neuropathy: Making the Case for Nicotinamide Treatment in Glaucoma. J Glaucoma 2017, 26, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin Exp Ophthalmol 2020, 48, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Fan Gaskin, J.C.; Shah, M.H.; Chan, E.C. Oxidative Stress and the Role of NADPH Oxidase in Glaucoma. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Deliyanti, D.; Wilkinson-Berka, J.L. Inhibition of NOX1/4 with GKT137831: a potential novel treatment to attenuate neuroglial cell inflammation in the retina. J Neuroinflammation 2015, 12, 136. [Google Scholar] [CrossRef]
- Dionysopoulou, S.; Wikström, P.; Walum, E.; Thermos, K. Effect of NADPH oxidase inhibitors in an experimental retinal model of excitotoxicity. Exp Eye Res 2020, 200, 108232. [Google Scholar] [CrossRef]
- Goetz, R.K.; Irnaten, M.; O'Brien, C.J. TGF-β induces NOX4 and fibrotic genes in trabecular meshwork cells: role in glaucoma. Investigative Ophthalmology & Visual Science 2019, 60, 3800–3800. [Google Scholar]
- Wan, P.; Su, W.; Zhang, Y.; Li, Z.; Deng, C.; Zhuo, Y. Trimetazidine protects retinal ganglion cells from acute glaucoma via the Nrf2/Ho-1 pathway. Clin Sci (Lond) 2017, 131, 2363–2375. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Kumari, A.; Panwar, A. Astaxanthin: A super antioxidant from microalgae and its therapeutic potential. J Basic Microbiol 2022, 62, 1064–1082. [Google Scholar] [CrossRef] [PubMed]
- Cort, A.; Ozturk, N.; Akpinar, D.; Unal, M.; Yucel, G.; Ciftcioglu, A.; Yargicoglu, P.; Aslan, M. Suppressive effect of astaxanthin on retinal injury induced by elevated intraocular pressure. Regul Toxicol Pharmacol 2010, 58, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Dong, Z.; Shinmei, Y.; Murata, M.; Kanda, A.; Noda, K.; Harada, T.; Ishida, S. Cytoprotective Effect of Astaxanthin in a Model of Normal Intraocular Pressure Glaucoma. J Ophthalmol 2020, 2020, 9539681. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Chu, C.; Liu, S. Astaxanthin protects retinal ganglion cells from acute glaucoma via the Nrf2/HO-1 pathway. J Chem Neuroanat 2020, 110, 101876. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xing, Y.; Xiong, L.; Wang, J. Sestrin2 overexpression alleviates hydrogen peroxide-induced apoptosis and oxidative stress in retinal ganglion cells by enhancing Nrf2 activation via Keap1 downregulation. Chem Biol Interact 2020, 324, 109086. [Google Scholar] [CrossRef]
- Li, X.; Leng, Y.; Jiang, Q.; Wang, Z.; Luo, P.; Zhang, C.; Chen, L.; Wang, Y.; Wang, H.; Yue, X.; et al. Eye Drops of Metformin Prevents Fibrosis After Glaucoma Filtration Surgery in Rats via Activating AMPK/Nrf2 Signaling Pathway. Front Pharmacol 2020, 11, 1038. [Google Scholar] [CrossRef]
- Naguib, S.; DeJulius, C.R.; Backstrom, J.R.; Haider, A.A.; Ang, J.M.; Boal, A.M.; Calkins, D.J.; Duvall, C.L.; Rex, T.S. Intraocular Sustained Release of EPO-R76E Mitigates Glaucoma Pathogenesis by Activating the NRF2/ARE Pathway. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Li, G.; Lee, C.; Read, A.T.; Wang, K.; Ha, J.; Kuhn, M.; Navarro, I.; Cui, J.; Young, K.; Gorijavolu, R.; et al. Anti-fibrotic activity of a rho-kinase inhibitor restores outflow function and intraocular pressure homeostasis. Elife 2021, 10. [Google Scholar] [CrossRef]
- Batra, M.; Gupta, S.; Nair, A.B.; Dhanawat, M.; Sandal, S.; Morsy, M.A. Netarsudil: A new ophthalmic drug in the treatment of chronic primary open angle glaucoma and ocular hypertension. Eur J Ophthalmol 2021, 31, 2237–2244. [Google Scholar] [CrossRef]
- Fujimoto, T.; Inoue, T.; Ohira, S.; Awai-Kasaoka, N.; Kameda, T.; Inoue-Mochita, M.; Tanihara, H. Inhibition of Rho Kinase Induces Antioxidative Molecules and Suppresses Reactive Oxidative Species in Trabecular Meshwork Cells. Journal of Ophthalmology 2017, 2017, 7598140. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; Fang, J.; Zhang, Y.; Zhu, W.; Yang, X. Rho-Associated Protein Kinase Inhibitor Treatment Promotes Proliferation and Phagocytosis in Trabecular Meshwork Cells. Front Pharmacol 2020, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Omae, T.; Nakabayashi, S.; Takahashi, K.; Tanner, A.; Yoshida, A. Effect of Rho Kinase Inhibitor Ripasudil (K-115) on Isolated Porcine Retinal Arterioles. J Ocul Pharmacol Ther 2021, 37, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Feillet, F.; Leonard, J.V. Alternative pathway therapy for urea cycle disorders. J Inherit Metab Dis 1998, 21 Suppl 1, 101–111. [Google Scholar] [CrossRef]
- Brown, C.R.; Hong-Brown, L.Q.; Biwersi, J.; Verkman, A.S.; Welch, W.J. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1996, 1, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Ghosh, A.; Jana, A.; Liu, X.; Brahmachari, S.; Gendelman, H.E.; Pahan, K. Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson's disease. PLoS One 2012, 7, e38113. [Google Scholar] [CrossRef]
- Dong, Y.; Li, L.; Xia, T.; Wang, L.; Xiao, L.; Ding, N.; Wu, Y.; Lu, K. Oxidative Stress Can Be Attenuated by 4-PBA Caused by High-Fat or Ammonia Nitrogen in Cultured Spotted Seabass: The Mechanism Is Related to Endoplasmic Reticulum Stress. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Zode, G.S.; Kuehn, M.H.; Nishimura, D.Y.; Searby, C.C.; Mohan, K.; Grozdanic, S.D.; Bugge, K.; Anderson, M.G.; Clark, A.F.; Stone, E.M.; et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest 2011, 121, 3542–3553. [Google Scholar] [CrossRef]
- Maddineni, P.; Kasetti, R.B.; Kodati, B.; Yacoub, S.; Zode, G.S. Sodium 4-Phenylbutyrate Reduces Ocular Hypertension by Degrading Extracellular Matrix Deposition via Activation of MMP9. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Lapchak, P.A. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother 2010, 11, 1753–1763. [Google Scholar] [CrossRef]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid Med Cell Longev 2017, 2017, 9208489. [Google Scholar] [CrossRef]
- Akaiwa, K.; Namekata, K.; Azuchi, Y.; Guo, X.; Kimura, A.; Harada, C.; Mitamura, Y.; Harada, T. Edaravone suppresses retinal ganglion cell death in a mouse model of normal tension glaucoma. Cell Death Dis 2017, 8, e2934. [Google Scholar] [CrossRef] [PubMed]
- Aksar, A.T.; Yuksel, N.; Gok, M.; Cekmen, M.; Caglar, Y. Neuroprotective effect of edaravone in experimental glaucoma model in rats: a immunofluorescence and biochemical analysis. Int J Ophthalmol 2015, 8, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, X.; Zhao, X.; Tong, N.; Gong, Y.; Zhang, W.; Wu, X. Valproic acid regulates antioxidant enzymes and prevents ischemia/reperfusion injury in the rat retina. Curr Eye Res 2012, 37, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Guo, X.; Noro, T.; Harada, C.; Tanaka, K.; Namekata, K.; Harada, T. Valproic acid prevents retinal degeneration in a murine model of normal tension glaucoma. Neurosci Lett 2015, 588, 108–113. [Google Scholar] [CrossRef]
- Tribble, J.R.; Kastanaki, E.; Uslular, A.B.; Rutigliani, C.; Enz, T.J.; Williams, P.A. Valproic Acid Reduces Neuroinflammation to Provide Retinal Ganglion Cell Neuroprotection in the Retina Axotomy Model. Front Cell Dev Biol 2022, 10, 903436. [Google Scholar] [CrossRef]
- Mahalingam, K.; Chaurasia, A.K.; Gowtham, L.; Gupta, S.; Somarajan, B.I.; Velpandian, T.; Sihota, R.; Gupta, V. Therapeutic potential of valproic acid in advanced glaucoma: A pilot study. Indian J Ophthalmol 2018, 66, 1104–1108. [Google Scholar] [CrossRef]
- Harada, C.; Noro, T.; Kimura, A.; Guo, X.; Namekata, K.; Nakano, T.; Harada, T. Suppression of Oxidative Stress as Potential Therapeutic Approach for Normal Tension Glaucoma. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Ozdemir, G.; Tolun, F.I.; Gul, M.; Imrek, S. Retinal Oxidative Stress Induced by Intraocular Hypertension in Rats May be Ameliorated by Brimonidine Treatment and N-acetyl Cysteine Supplementation. Journal of Glaucoma 2009, 18, 662–665. [Google Scholar] [CrossRef]
- Yang, L.; Tan, P.; Zhou, W.; Zhu, X.; Cui, Y.; Zhu, L.; Feng, X.; Qi, H.; Zheng, J.; Gu, P.; et al. N-acetylcysteine protects against hypoxia mimetic-induced autophagy by targeting the HIF-1α pathway in retinal ganglion cells. Cell Mol Neurobiol 2012, 32, 1275–1285. [Google Scholar] [CrossRef]
- Sano, H.; Namekata, K.; Kimura, A.; Shitara, H.; Guo, X.; Harada, C.; Mitamura, Y.; Harada, T. Differential effects of N-acetylcysteine on retinal degeneration in two mouse models of normal tension glaucoma. Cell Death Dis 2019, 10, 75. [Google Scholar] [CrossRef]
- Malagelada, C.; Jin, Z.H.; Jackson-Lewis, V.; Przedborski, S.; Greene, L.A. Rapamycin Protects against Neuron Death in In Vitro andIn Vivo Models of Parkinson's Disease. Journal of Neuroscience 2010, 30, 1166–1175. [Google Scholar] [CrossRef]
- Caccamo, A.; Majumder, S.; Deng, J.J.; Bai, Y.; Thornton, F.B.; Oddo, S. Rapamycin rescues TDP-43 mislocalization and the associated low molecular mass neurofilament instability. Journal of Biological Chemistry 2009, 284, 27416–27424. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Li, Z.; Jia, Y.; Zhuo, Y. Rapamycin is neuroprotective in a rat chronic hypertensive glaucoma model. PLoS One 2014, 9, e99719. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Kwon, Y.W.; Kondo, N.; Bai, J.; Masutani, H.; Nakamura, H.; Fujii, J.; Ohira, A.; Yodoi, J. Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage. J Neurosci 2005, 25, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Shinmei, Y.; Dong, Y.; Inafuku, S.; Fukuhara, J.; Ando, R.; Kitaichi, N.; Kanda, A.; Tanaka, K.; Noda, K.; et al. Effect of geranylgeranylacetone on the protection of retinal ganglion cells in a mouse model of normal tension glaucoma. Heliyon 2016, 2, e00191. [Google Scholar] [CrossRef] [PubMed]
- Zibold, J.; von Livonius, B.; Kolarova, H.; Rudolph, G.; Priglinger, C.S.; Klopstock, T.; Catarino, C.B. Vitamin B12 in Leber hereditary optic neuropathy mutation carriers: a prospective cohort study. Orphanet J Rare Dis 2022, 17, 310. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Turnbull, D.M.; Chinnery, P.F. Leber hereditary optic neuropathy. J Med Genet 2002, 39, 162–169. [Google Scholar] [CrossRef]
- Mashima, Y.; Yamada, K.; Wakakura, M.; Kigasawa, K.; Kudoh, J.; Shimizu, N.; Oguchi, Y. Spectrum of pathogenic mitochondrial DNA mutations and clinical features in Japanese families with Leber's hereditary optic neuropathy. Current Eye Research 1998, 17, 403–408. [Google Scholar] [CrossRef]
- Mackey, D.A.; Oostra, R.J.; Rosenberg, T.; Nikoskelainen, E.; Bronte-Stewart, J.; Poulton, J.; Harding, A.E.; Govan, G.; Bolhuis, P.A.; Norby, S. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet 1996, 59, 481–485. [Google Scholar]
- Macmillan, C.; Johns, T.A.; Fu, K.; Shoubridge, E.A. Predominance of the T14484C Mutation in French-Canadian Families with Leber Hereditary Optic Neuropathy Is Due to a Founder Effect. The American Journal of Human Genetics 2000, 66, 332–335. [Google Scholar] [CrossRef]
- Laberge, A.-M.; Jomphe, M.; Houde, L.; Vézina, H.; Tremblay, M.; Desjardins, B.; Labuda, D.; St-Hilaire, M.; Macmillan, C.; Shoubridge, E.A.; et al. A “Fille du Roy” Introduced the T14484C Leber Hereditary Optic Neuropathy Mutation in French Canadians. The American Journal of Human Genetics 2005, 77, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, C.; Kirkham, T.; Fu, K.; Allison, V.; Andermann, E.; Chitayat, D.; Fortier, D.; Gans, M.; Hare, H.; Quercia, N.; et al. Pedigree analysis of French Canadian families with T14484C Leber's hereditary optic neuropathy. Neurology 1998, 50, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Vignal-Clermont, C. Leber Hereditary Optic Neuropathy: Review of Treatment and Management. Front Neurol 2021, 12, 651639. [Google Scholar] [CrossRef] [PubMed]
- Man, P.Y.W.; Turnbull, D.M.; Chinnery, P.F. Leber hereditary optic neuropathy. Journal of Medical Genetics 2002, 39, 162–169. [Google Scholar] [CrossRef]
- Hudson, G.; Keers, S.; Yu-Wai-Man, P.; Griffiths, P.; Huoponen, K.; Savontaus, M.L.; Nikoskelainen, E.; Zeviani, M.; Carrara, F.; Horvath, R.; et al. Identification of an X-chromosomal locus and haplotype modulating the phenotype of a mitochondrial DNA disorder. Am J Hum Genet 2005, 77, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.P.; Fingert, J.H.; Carelli, V.; Valentino, M.L.; King, T.M.; Daiger, S.P.; Salomao, S.R.; Berezovsky, A.; Belfort, R., Jr.; Braun, T.A.; et al. Evidence for a novel x-linked modifier locus for leber hereditary optic neuropathy. Ophthalmic Genet 2008, 29, 17–24. [Google Scholar] [CrossRef]
- Rabenstein, A.; Catarino, C.B.; Rampeltshammer, V.; Schindler, D.; Gallenmüller, C.; Priglinger, C.; Pogarell, O.; Rüther, T.; Klopstock, T. Smoking and alcohol, health-related quality of life and psychiatric comorbidities in Leber's Hereditary Optic Neuropathy mutation carriers: a prospective cohort study. Orphanet J Rare Dis 2021, 16, 127. [Google Scholar] [CrossRef]
- Pott, J.W.; Wong, K.H. Leber's hereditary optic neuropathy and vitamin B12 deficiency. Graefes Arch Clin Exp Ophthalmol 2006, 244, 1357–1359. [Google Scholar] [CrossRef]
- Chen, B.S.; Holzinger, E.; Taiel, M.; Yu-Wai-Man, P. The Impact of Leber Hereditary Optic Neuropathy on the Quality of Life of Patients and Their Relatives: A Qualitative Study. Journal of Neuro-Ophthalmology 2022, 42, 316–322. [Google Scholar] [CrossRef]
- Gong, J.; Cheung, S.; Fasso-Opie, A.; Galvin, O.; Moniz, L.S.; Earle, D.; Durham, T.; Menzo, J.; Li, N.; Duffy, S.; et al. The Impact of Inherited Retinal Diseases in the United States of America (US) and Canada from a Cost-of-Illness Perspective. Clin Ophthalmol 2021, 15, 2855–2866. [Google Scholar] [CrossRef]
- Amore, G.; Romagnoli, M.; Carbonelli, M.; Barboni, P.; Carelli, V.; La Morgia, C. Therapeutic Options in Hereditary Optic Neuropathies. Drugs 2021, 81, 57–86. [Google Scholar] [CrossRef]
- Carelli, V.; Carbonelli, M.; de Coo, I.F.; Kawasaki, A.; Klopstock, T.; Lagrèze, W.A.; La Morgia, C.; Newman, N.J.; Orssaud, C.; Pott, J.W.R.; et al. International Consensus Statement on the Clinical and Therapeutic Management of Leber Hereditary Optic Neuropathy. J Neuroophthalmol 2017, 37, 371–381. [Google Scholar] [CrossRef]
- Stramkauskaitė, A.; Povilaitytė, I.; Glebauskienė, B.; Liutkevičienė, R. Clinical Overview of Leber Hereditary Optic Neuropathy. Acta Med Litu 2022, 29, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Nikoskelainen, E.; Hoyt, W.F.; Nummelin, K. Ophthalmoscopic findings in Leber's hereditary optic neuropathy. II. The fundus findings in the affected family members. Arch Ophthalmol 1983, 101, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A. Idebenone: A Review in Leber's Hereditary Optic Neuropathy. Drugs 2016, 76, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Polster, B.M. Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier? J Bioenerg Biomembr 2015, 47, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Gueven, N.; Ravishankar, P.; Eri, R.; Rybalka, E. Idebenone: When an antioxidant is not an antioxidant. Redox Biol 2021, 38, 101812. [Google Scholar] [CrossRef]
- Nagai, Y.; Yoshida, K.; Narumi, S.; Tanayama, S.; Nagaoka, A. Brain distribution of idebenone and its effect on local cerebral glucose utilization in rats. Arch Gerontol Geriatr 1989, 8, 257–272. [Google Scholar] [CrossRef]
- Torii, H.; Yoshida, K.; Kobayashi, T.; Tsukamoto, T.; Tanayama, S. Disposition of idebenone (CV-2619), a new cerebral metabolism improving agent, in rats and dogs. J Pharmacobiodyn 1985, 8, 457–467. [Google Scholar] [CrossRef]
- Heitz, F.D.; Erb, M.; Anklin, C.; Robay, D.; Pernet, V.; Gueven, N. Idebenone protects against retinal damage and loss of vision in a mouse model of Leber's hereditary optic neuropathy. PLoS One 2012, 7, e45182. [Google Scholar] [CrossRef]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. Idebenone: Novel Strategies to Improve Its Systemic and Local Efficacy. Nanomaterials (Basel) 2018, 8. [Google Scholar] [CrossRef]
- Giorgio, V.; Schiavone, M.; Galber, C.; Carini, M.; Da Ros, T.; Petronilli, V.; Argenton, F.; Carelli, V.; Acosta Lopez, M.J.; Salviati, L.; et al. The idebenone metabolite QS10 restores electron transfer in complex I and coenzyme Q defects. Biochim Biophys Acta Bioenerg 2018, 1859, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-Y.; Wang, A.-G.; Wei, Y.-H. Leber's hereditary optic neuropathy: A multifactorial disease. Progress in Retinal and Eye Research 2006, 25, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Trounce, I.A.; Jun, A.S.; Allen, J.C.; Wallace, D.C. Functional Analysis of Lymphoblast and Cybrid Mitochondria Containing the 3460, 11778, or 14484 Leber's Hereditary Optic Neuropathy Mitochondrial DNA Mutation*. Journal of Biological Chemistry 2000, 275, 39831–39836. [Google Scholar] [CrossRef] [PubMed]
- Carelli, V.; Rugolo, M.; Sgarbi, G.; Ghelli, A.; Zanna, C.; Baracca, A.; Lenaz, G.; Napoli, E.; Martinuzzi, A.; Solaini, G. Bioenergetics shapes cellular death pathways in Leber's hereditary optic neuropathy: a model of mitochondrial neurodegeneration. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2004, 1658, 172–179. [Google Scholar] [CrossRef]
- Yen, M.Y.; Kao, S.H.; Wang, A.G.; Wei, Y.H. Increased 8-hydroxy-2'-deoxyguanosine in leukocyte DNA in Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci 2004, 45, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Ghelli, A.; Porcelli, A.M.; Zanna, C.; Martinuzzi, A.; Carelli, V.; Rugolo, M. Protection against oxidant-induced apoptosis by exogenous glutathione in Leber hereditary optic neuropathy cybrids. Invest Ophthalmol Vis Sci 2008, 49, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Floreani, M.; Napoli, E.; Martinuzzi, A.; Pantano, G.; De Riva, V.; Trevisan, R.; Bisetto, E.; Valente, L.; Carelli, V.; Dabbeni-Sala, F. Antioxidant defences in cybrids harboring mtDNA mutations associated with Leber's hereditary optic neuropathy. Febs j 2005, 272, 1124–1135. [Google Scholar] [CrossRef]
- Lin, C.S.; Sharpley, M.S.; Fan, W.; Waymire, K.G.; Sadun, A.A.; Carelli, V.; Ross-Cisneros, F.N.; Baciu, P.; Sung, E.; McManus, M.J.; et al. Mouse mtDNA mutant model of Leber hereditary optic neuropathy. Proc Natl Acad Sci U S A 2012, 109, 20065–20070. [Google Scholar] [CrossRef]
- Qi, X.; Sun, L.; Lewin, A.S.; Hauswirth, W.W.; Guy, J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest Ophthalmol Vis Sci 2007, 48, 1–10. [Google Scholar] [CrossRef]
- Porcelli, A.M.; Angelin, A.; Ghelli, A.; Mariani, E.; Martinuzzi, A.; Carelli, V.; Petronilli, V.; Bernardi, P.; Rugolo, M. Respiratory complex I dysfunction due to mitochondrial DNA mutations shifts the voltage threshold for opening of the permeability transition pore toward resting levels. J Biol Chem 2009, 284, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, A.; Moraes, C.T. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem 1999, 274, 16188–16197. [Google Scholar] [CrossRef] [PubMed]
- Battisti, C.; Formichi, P.; Cardaioli, E.; Bianchi, S.; Mangiavacchi, P.; Tripodi, S.A.; Tosi, P.; Federico, A. Cell response to oxidative stress induced apoptosis in patients with Leber's hereditary optic neuropathy. J Neurol Neurosurg Psychiatry 2004, 75, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, G.; Cortopassi, G. Oxidative stress in inherited mitochondrial diseases. Free Radic Biol Med 2015, 88, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ghelli, A.; Zanna, C.; Porcelli, A.M.; Schapira, A.H.; Martinuzzi, A.; Carelli, V.; Rugolo, M. Leber's hereditary optic neuropathy (LHON) pathogenic mutations induce mitochondrial-dependent apoptotic death in transmitochondrial cells incubated with galactose medium. J Biol Chem 2003, 278, 4145–4150. [Google Scholar] [CrossRef]
- Danielson, S.R.; Wong, A.; Carelli, V.; Martinuzzi, A.; Schapira, A.H.; Cortopassi, G.A. Cells bearing mutations causing Leber's hereditary optic neuropathy are sensitized to Fas-Induced apoptosis. J Biol Chem 2002, 277, 5810–5815. [Google Scholar] [CrossRef]
- Ghelli, A.; Zanna, C.; Porcelli, A.M.; Schapira, A.H.V.; Martinuzzi, A.; Carelli, V.; Rugolo, M. Leber's Hereditary Optic Neuropathy (LHON) Pathogenic Mutations Induce Mitochondrial-dependent Apoptotic Death in Transmitochondrial Cells Incubated with Galactose Medium*. Journal of Biological Chemistry 2003, 278, 4145–4150. [Google Scholar] [CrossRef]
- Danielson, S.R.; Wong, A.; Carelli, V.; Martinuzzi, A.; Schapira, A.H.V.; Cortopassi, G.A. Cells Bearing Mutations Causing Leber's Hereditary Optic Neuropathy Are Sensitized to Fas-induced Apoptosis*. Journal of Biological Chemistry 2002, 277, 5810–5815. [Google Scholar] [CrossRef]
- Zanna, C.; Ghelli, A.; Porcelli, A.M.; Martinuzzi, A.; Carelli, V.; Rugolo, M. Caspase-independent death of Leber’s hereditary optic neuropathy cybrids is driven by energetic failure and mediated by AIF and Endonuclease G. Apoptosis 2005, 10, 997–1007. [Google Scholar] [CrossRef]
- Ng, W.S.V.; Trigano, M.; Freeman, T.; Varrichio, C.; Kandaswamy, D.K.; Newland, B.; Brancale, A.; Rozanowska, M.; Votruba, M. New avenues for therapy in mitochondrial optic neuropathies. Therapeutic Advances in Rare Disease 2021, 2, 26330040211029037. [Google Scholar] [CrossRef]
- Enns, G.M.; Kinsman, S.L.; Perlman, S.L.; Spicer, K.M.; Abdenur, J.E.; Cohen, B.H.; Amagata, A.; Barnes, A.; Kheifets, V.; Shrader, W.D.; et al. Initial experience in the treatment of inherited mitochondrial disease with EPI-743. Mol Genet Metab 2012, 105, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Sadun, A.A.; Chicani, C.F.; Ross-Cisneros, F.N.; Barboni, P.; Thoolen, M.; Shrader, W.D.; Kubis, K.; Carelli, V.; Miller, G. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch Neurol 2012, 69, 331–338. [Google Scholar] [CrossRef]
- Chicani, C.; Chu, E.; Ross-Cisneros, F.; Rockwell, S.; Murase, K.; Thoolen, M.; Miller, G.; Sadun, A. Treatment of Leber's hereditary optic neuropathy (LHON): Results using a novel quinone, EPI-743. Investigative Ophthalmology & Visual Science 2013, 54, 4574–4574. [Google Scholar]
- Pitceathly, R.D.S.; Keshavan, N.; Rahman, J.; Rahman, S. Moving towards clinical trials for mitochondrial diseases. J Inherit Metab Dis 2021, 44, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, B.; Ma, J.; Ge, J.; Wang, K. Protective effect of mitochondria-targeted peptide MTP-131 against oxidative stress-induced apoptosis in RGC-5 cells. Mol Med Rep 2017, 15, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.S.; Kim, J.H.; Min, K.N.; Moon, J.A.; Roh, T.C.; Lee, M.J.; Lee, K.W.; Min, J.E.; Lee, Y.M. KL1333, a Novel NAD(+) Modulator, Improves Energy Metabolism and Mitochondrial Dysfunction in MELAS Fibroblasts. Front Neurol 2018, 9, 552. [Google Scholar] [CrossRef]
- Patel, H.R.; Margo, C.E. Pathology of Ischemic Optic Neuropathy. Arch Pathol Lab Med 2017, 141, 162–166. [Google Scholar] [CrossRef]
- Hayreh, S.S. Ischemic optic neuropathy. Prog Retin Eye Res 2009, 28, 34–62. [Google Scholar] [CrossRef]
- Hattenhauer, M.G.; Leavitt, J.A.; Hodge, D.O.; Grill, R.; Gray, D.T. Incidence of Nonarteritic Anteripr Ischemic Optic Neuropathy. American Journal of Ophthalmology 1997, 123, 103–107. [Google Scholar] [CrossRef]
- Chen, T.-W.; Wu, P.-Y.; Wen, Y.-T.; Desai, T.D.; Huang, C.-T.; Liu, P.-K.; Tsai, R.-K. Vitamin B3 Provides Neuroprotection via Antioxidative Stress in a Rat Model of Anterior Ischemic Optic Neuropathy. Antioxidants 2022, 11, 2422. [Google Scholar] [CrossRef]
- Winkler, A.; True, D. Giant Cell Arteritis: 2018 Review. Mo Med 2018, 115, 468–470. [Google Scholar] [PubMed]
- Bilton, E.J.; Mollan, S.P. Giant cell arteritis: reviewing the advancing diagnostics and management. Eye (Lond) 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Smit, E.; Palmer, A.J.; Hewitt, A.W. Projected worldwide disease burden from giant cell arteritis by 2050. J Rheumatol 2015, 42, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Mackie, S.L.; Dejaco, C.; Appenzeller, S.; Camellino, D.; Duftner, C.; Gonzalez-Chiappe, S.; Mahr, A.; Mukhtyar, C.; Reynolds, G.; de Souza, A.W.S.; et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology (Oxford) 2020, 59, e1–e23. [Google Scholar] [CrossRef]
- Danesh-Meyer, H.; Savino, P.J.; Gamble, G.G. Poor prognosis of visual outcome after visual loss from giant cell arteritis. Ophthalmology 2005, 112, 1098–1103. [Google Scholar] [CrossRef]
- Dinkin, M.; Johnson, E. One Giant Step for Giant Cell Arteritis: Updates in Diagnosis and Treatment. Curr Treat Options Neurol 2021, 23, 6. [Google Scholar] [CrossRef]
- Lyons, H.S.; Quick, V.; Sinclair, A.J.; Nagaraju, S.; Mollan, S.P. A new era for giant cell arteritis. Eye 2020, 34, 1013–1026. [Google Scholar] [CrossRef]
- Genovese, M.C.; McKay, J.D.; Nasonov, E.L.; Mysler, E.F.; da Silva, N.A.; Alecock, E.; Woodworth, T.; Gomez-Reino, J.J. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 2008, 58, 2968–2980. [Google Scholar]
- Jones, G.; Sebba, A.; Gu, J.; Lowenstein, M.B.; Calvo, A.; Gomez-Reino, J.J.; Siri, D.A.; Tomšič, M.; Alecock, E.; Woodworth, T. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Annals of the rheumatic diseases 2010, 69, 88–96. [Google Scholar] [CrossRef]
- Hellmich, B.; Águeda, A.F.; Monti, S.; Luqmani, R. Treatment of Giant Cell Arteritis and Takayasu Arteritis—Current and Future. Current Rheumatology Reports 2020, 22, 84. [Google Scholar] [CrossRef]
- Liu, B.; Yu, Y.; Liu, W.; Deng, T.; Xiang, D. Risk Factors for Non-arteritic Anterior Ischemic Optic Neuropathy: A Large Scale Meta-Analysis. Front Med (Lausanne) 2021, 8, 618353. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Podhajsky, P.A.; Zimmerman, B. Nonarteritic Anterior Ischemic Optic Neuropathy: Time of Onset of Visual Loss. American Journal of Ophthalmology 1997, 124, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, B. Visual Field Abnormalities in Nonarteritic Anterior Ischemic Optic Neuropathy: Their Pattern and Prevalence at Initial Examination. Archives of Ophthalmology 2005, 123, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Biousse, V.; Newman, N.J. Ischemic Optic Neuropathies: Current Concepts. Ann Indian Acad Neurol 2022, 25, S54–s58. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefes Arch Clin Exp Ophthalmol 2008, 246, 1029–1046. [Google Scholar] [CrossRef]
- Berry, S.; Lin, W.V.; Sadaka, A.; Lee, A.G. Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye Brain 2017, 9, 23–28. [Google Scholar] [CrossRef]
- Katz, D.M.; Trobe, J.D. Is there treatment for nonarteritic anterior ischemic optic neuropathy. Curr Opin Ophthalmol 2015, 26, 458–463. [Google Scholar] [CrossRef]
- Saxena, R.; Singh, D.; Sharma, M.; James, M.; Sharma, P.; Menon, V. Steroids versus No Steroids in Nonarteritic Anterior Ischemic Optic Neuropathy: A Randomized Controlled Trial. Ophthalmology 2018, 125, 1623–1627. [Google Scholar] [CrossRef]
- Goldenberg-Cohen, N.; Dadon-Bar-El, S.; Hasanreisoglu, M.; Avraham-Lubin, B.C.; Dratviman-Storobinsky, O.; Cohen, Y.; Weinberger, D. Possible neuroprotective effect of brimonidine in a mouse model of ischaemic optic neuropathy. Clin Exp Ophthalmol 2009, 37, 718–729. [Google Scholar] [CrossRef]
- Danylkova, N.O.; Alcala, S.R.; Pomeranz, H.D.; McLoon, L.K. Neuroprotective effects of brimonidine treatment in a rodent model of ischemic optic neuropathy. Exp Eye Res 2007, 84, 293–301. [Google Scholar] [CrossRef]
- Aktaş, Z.; Gürelik, G.; Akyürek, N.; Onol, M.; Hasanreisoğlu, B. Neuroprotective effect of topically applied brimonidine tartrate 0.2% in endothelin-1-induced optic nerve ischaemia model. Clin Exp Ophthalmol 2007, 35, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Liao, Y.J.; Goronzy, J.J. The Immunopathology of Giant Cell Arteritis: Diagnostic and Therapeutic Implications. Journal of Neuro-Ophthalmology 2012, 32, 259–265. [Google Scholar] [CrossRef]
- Bilton, E.J.; Mollan, S.P. Giant cell arteritis: reviewing the advancing diagnostics and management. Eye 2023. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Hashimoto, M. Aging-Related Vascular Inflammation: Giant Cell Arteritis and Neurological Disorders. Frontiers in Aging Neuroscience 2022, 14. [Google Scholar] [CrossRef]
- Cid, M.C.; Font, C.; Coll-Vinent, B.; Grau, J.M. Large vessel vasculitides. Current Opinion in Rheumatology 1998, 10. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Zhang, M.; Beard, D.A.; Goldstein, D.R. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circulation Research 2020, 126, 298–314. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circulation Research 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Wang, L.; Ai, Z.; Khoyratty, T.; Zec, K.; Eames, H.L.; van Grinsven, E.; Hudak, A.; Morris, S.; Ahern, D.; Monaco, C.; et al. ROS-producing immature neutrophils in giant cell arteritis are linked to vascular pathologies. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Ianni, A.; Kumari, P.; Tarighi, S.; Argento, F.R.; Fini, E.; Emmi, G.; Bettiol, A.; Braun, T.; Prisco, D.; Fiorillo, C.; et al. An Insight into Giant Cell Arteritis Pathogenesis: Evidence for Oxidative Stress and SIRT1 Downregulation. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Ophir, A.; Berenshtein, E.; Kitrossky, N.; Berman, E.R.; Photiou, S.; Rothman, Z.; Chevion, M. Hydroxyl radical generation in the cat retina during reperfusion following ischemia. Exp Eye Res 1993, 57, 351–357. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Zhang, J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 1996, 27, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.M.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Progress in Retinal and Eye Research 2004, 23, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 2002, 282, C227–C241. [Google Scholar] [CrossRef] [PubMed]
- Aghai, Z.H.; Kumar, S.; Farhath, S.; Kumar, M.A.; Saslow, J.; Nakhla, T.; Eydelman, R.; Strande, L.; Stahl, G.; Hewitt, C.; et al. Dexamethasone Suppresses Expression of Nuclear Factor-kappaB in the Cells of Tracheobronchial Lavage Fluid in Premature Neonates with Respiratory Distress. Pediatric Research 2006, 59, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Birer, S.; Arda, H.; Kilic, D.; Baskol, G. Systemic oxidative stress in non-arteritic anterior ischemic optic neuropathy. Eye (Lond) 2019, 33, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, X.; Wu, X.; Jiang, L.; Ahsan, A.; Ma, S.; Xiao, Z.; Han, F.; Qin, Z.H.; Hu, W.; et al. Somatic autophagy of axonal mitochondria in ischemic neurons. J Cell Biol 2019, 218, 1891–1907. [Google Scholar] [CrossRef]
- Wan, W.; Peng, T.; Jin, X.; Li, Q.; Zhang, F.; Zheng, G.; Lv, Y.; Wan, G.; Zhu, Y. Glutathione-S-Transferase Deletions and Non-arteritic Anterior Ischemic Optic Neuropathy. Molecular Neurobiology 2016, 53, 2361–2367. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Milcarek, B.; Bosley, T.M. GSTM1 and GSTT1 deletion genotypes in various spontaneous optic neuropathies in Arabs. British Journal of Ophthalmology 2009, 93, 1101. [Google Scholar] [CrossRef]
- Bosley, T.M.; Abu-Amero, K.K.; Ozand, P.T. Mitochondrial DNA nucleotide changes in non-arteritic ischemic optic neuropathy. Neurology 2004, 63, 1305–1308. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Zhang, L. Negative regulation of inflammation by SIRT1. Pharmacol Res 2013, 67, 60–67. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-T.; Huang, C.-W.; Liu, C.-P.; Chen, C.-H.; Tu, C.-M.; Hwang, C.-S.; Chen, Y.-H.; Chen, W.-R.; Lin, K.-L.; Ho, Y.-C.; et al. Inhibition of Retinal Ganglion Cell Loss By a Novel ROCK Inhibitor (E212) in Ischemic Optic Nerve Injury Via Antioxidative and Anti-Inflammatory Actions. Investigative Ophthalmology & Visual Science 2021, 62, 21–21. [Google Scholar] [CrossRef]
- Chien, J.Y.; Lin, S.F.; Chou, Y.Y.; Huang, C.F.; Huang, S.P. Protective Effects of Oroxylin A on Retinal Ganglion Cells in Experimental Model of Anterior Ischemic Optic Neuropathy. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.; Nakamura, K.; Guo, X.; Kitaichi, N.; Mitamura, Y.; Yoshida, K.; Ohno, S.; Yoshida, H.; Harada, T. Neuroprotective effect of geranylgeranylacetone against ischemia-induced retinal injury. Mol Vis 2007, 13, 1601–1607. [Google Scholar] [PubMed]
- Zhang, L.; Xue, K.; Fan, P.; Chen, C.; Hu, J.; Huang, J.; Lu, W.; Xu, J.; Xu, S.; Ran, J.; et al. Geranylgeranylacetone-induced heat shock protein70 expression reduces retinal ischemia-reperfusion injury through PI3K/AKT/mTOR signaling. Exp Eye Res 2023, 229, 109416. [Google Scholar] [CrossRef]
- Lin, W.N.; Kapupara, K.; Wen, Y.T.; Chen, Y.H.; Pan, I.H.; Tsai, R.K. Haematococcus pluvialis-Derived Astaxanthin Is a Potential Neuroprotective Agent against Optic Nerve Ischemia. Mar Drugs 2020, 18. [Google Scholar] [CrossRef]
- Ji, K.; Li, Z.; Lei, Y.; Xu, W.; Ouyang, L.; He, T.; Xing, Y. Resveratrol attenuates retinal ganglion cell loss in a mouse model of retinal ischemia reperfusion injury via multiple pathways. Exp Eye Res 2021, 209, 108683. [Google Scholar] [CrossRef]
- Pang, Y.; Qin, M.; Hu, P.; Ji, K.; Xiao, R.; Sun, N.; Pan, X.; Zhang, X. Resveratrol protects retinal ganglion cells against ischemia induced damage by increasing Opa1 expression. Int J Mol Med 2020, 46, 1707–1720. [Google Scholar] [CrossRef]
- Ross, A.G.; Chaqour, B.; McDougald, D.S.; Dine, K.E.; Duong, T.T.; Shindler, R.E.; Yue, J.; Liu, T.; Shindler, K.S. Selective Upregulation of SIRT1 Expression in Retinal Ganglion Cells by AAV-Mediated Gene Delivery Increases Neuronal Cell Survival and Alleviates Axon Demyelination Associated with Optic Neuritis. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Abel, A.; McClelland, C.; Lee, M.S. Critical review: Typical and atypical optic neuritis. Surv Ophthalmol 2019, 64, 770–779. [Google Scholar] [CrossRef]
- Pau, D.; Al Zubidi, N.; Yalamanchili, S.; Plant, G.T.; Lee, A.G. Optic neuritis. Eye (Lond) 2011, 25, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol 2008, 65, 727–732. [CrossRef]
- Ebers, G.C. Optic neuritis and multiple sclerosis. Arch Neurol 1985, 42, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L. Optic Neuritis. Continuum (Minneap Minn) 2019, 25, 1236–1264. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.L.; Narvaez, O.; Altschuler, F.; Calanni, J.S.; González Fleitas, M.F.; Sande, P.H.; Dorfman, D.; Concha, L.; Rosenstein, R.E. Chronobiotic effect of melatonin in experimental optic neuritis. Neuropharmacology 2021, 182, 108401. [Google Scholar] [CrossRef]
- Söderström, M.; Link, H.; Sun, J.B.; Fredrikson, S.; Wang, Z.Y.; Huang, W.X. Autoimmune T cell repertoire in optic neuritis and multiple sclerosis: T cells recognising multiple myelin proteins are accumulated in cerebrospinal fluid. J Neurol Neurosurg Psychiatry 1994, 57, 544–551. [Google Scholar] [CrossRef]
- YOUL, B.D.; TURANO, G.; MILLER, D.H.; TOWELL, A.D.; MACMANUS, D.G.; MOORE, S.G.; JONES, S.J.; BARRETT, G.; KENDALL, B.E.; MOSELEY, I.F.; et al. THE PATHOPHYSIOLOGY OF ACUTE OPTIC NEURITIS: AN ASSOCIATION OF GADOLINIUM LEAKAGE WITH CLINICAL AND ELECTROPHYSIOLOGICAL DEFICITS. Brain 1991, 114, 2437–2450. [Google Scholar] [CrossRef]
- Balcer, L.J. Clinical practice. Optic neuritis. N Engl J Med 2006, 354, 1273–1280. [Google Scholar] [CrossRef]
- Kimura, A.; Namekata, K.; Guo, X.; Noro, T.; Harada, C.; Harada, T. Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis. Oxid Med Cell Longev 2017, 2017, 2817252. [Google Scholar] [CrossRef]
- Trip, S.A.; Schlottmann, P.G.; Jones, S.J.; Altmann, D.R.; Garway-Heath, D.F.; Thompson, A.J.; Plant, G.T.; Miller, D.H. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 2005, 58, 383–391. [Google Scholar] [CrossRef]
- Costello, F.; Coupland, S.; Hodge, W.; Lorello, G.R.; Koroluk, J.; Pan, Y.I.; Freedman, M.S.; Zackon, D.H.; Kardon, R.H. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 2006, 59, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Keltner, J.L.; Johnson, C.A.; Cello, K.E.; Dontchev, M.; Gal, R.L.; Beck, R.W. Visual field profile of optic neuritis: a final follow-up report from the optic neuritis treatment trial from baseline through 15 years. Arch Ophthalmol 2010, 128, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Cleary, P.A.; Anderson, M.M., Jr.; Keltner, J.L.; Shults, W.T.; Kaufman, D.I.; Buckley, E.G.; Corbett, J.J.; Kupersmith, M.J.; Miller, N.R.; et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992, 326, 581–588. [Google Scholar] [CrossRef]
- Gal, R.L.; Vedula, S.S.; Beck, R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev 2015, 2015, Cd001430. [Google Scholar] [CrossRef]
- Tselis, A.; Perumal, J.; Caon, C.; Hreha, S.; Ching, W.; Din, M.; Van Stavern, G.; Khan, O. Treatment of corticosteroid refractory optic neuritis in multiple sclerosis patients with intravenous immunoglobulin. Eur J Neurol 2008, 15, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Roed, H.G.; Langkilde, A.; Sellebjerg, F.; Lauritzen, M.; Bang, P.; Mørup, A.; Frederiksen, J.L. A double-blind, randomized trial of IV immunoglobulin treatment in acute optic neuritis. Neurology 2005, 64, 804–810. [Google Scholar] [CrossRef]
- Noseworthy, J.H.; O'Brien, P.C.; Petterson, T.M.; Weis, J.; Stevens, L.; Peterson, W.K.; Sneve, D.; Cross, S.A.; Leavitt, J.A.; Auger, R.G.; et al. A randomized trial of intravenous immunoglobulin in inflammatory demyelinating optic neuritis. Neurology 2001, 56, 1514–1522. [Google Scholar] [CrossRef]
- Guy, J.; Ellis, E.A.; Hope, G.M.; Rao, N.A. Antioxidant enzyme suppression of demyelination in experimental optic neuritis. Current Eye Research 1989, 8, 467–477. [Google Scholar] [CrossRef]
- Guy, J.; Ellis, E.A.; Rao, N.A. Hydrogen Peroxide Localization in Experimental Optic Neuritis. Archives of Ophthalmology 1990, 108, 1614–1621. [Google Scholar] [CrossRef]
- Qi, X.; Lewin, A.S.; Sun, L.; Hauswirth, W.W.; Guy, J. Suppression of Mitochondrial Oxidative Stress Provides Long-term Neuroprotection in Experimental Optic Neuritis. Investigative Ophthalmology & Visual Science 2007, 48, 681–691. [Google Scholar] [CrossRef]
- Qi, X.; Sun, L.; Lewin, A.S.; Hauswirth, W.W.; Guy, J. Long-term Suppression of Neurodegeneration in Chronic Experimental Optic Neuritis: Antioxidant Gene Therapy. Investigative Ophthalmology & Visual Science 2007, 48, 5360–5370. [Google Scholar] [CrossRef]
- Vural, G.; Gümüşyayla, Ş.; Deniz, O.; Neşelioğlu, S.; Erel, Ö. Relationship between thiol-disulphide homeostasis and visual evoked potentials in patients with multiple sclerosis. Neurological Sciences 2019, 40, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liang, X.M.; Zhang, X.L.; Ling, S.Q.; Yang, T.T.; Li, M.; Peng, F.H. Relationship between serum bilirubin levels and optic neuritis. Chin Med J (Engl) 2013, 126, 3307–3310. [Google Scholar] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid Med Cell Longev 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Plant, G.T.; Sibtain, N.A.; Thomas, D. Hyperacute corticosteroid treatment of optic neuritis at the onset of pain may prevent visual loss: a case series. Mult Scler Int 2011, 2011, 815068. [Google Scholar] [CrossRef]
- Wang, L.; Liu, K.; Tan, X.; Zhou, L.; Zhang, Y.; Liu, X.; Fu, Y.; Qiu, W.; Yang, H. Remedial Effect of Intravenous Cyclophosphamide in Corticosteroid-Refractory Patients in the Acute Phase of Neuromyelitis Optica Spectrum Disorder-Related Optic Neuritis. Front Neurol 2020, 11, 612097. [Google Scholar] [CrossRef]
- Zyla, K.; Larabee, C.M.; Georgescu, C.; Berkley, C.; Reyna, T.; Plafker, S.M. Dimethyl fumarate mitigates optic neuritis. Mol Vis 2019, 25, 446–461. [Google Scholar]
- Alhasani, R.H.; Biswas, L.; Tohari, A.M.; Zhou, X.; Reilly, J.; He, J.F.; Shu, X. Gypenosides protect retinal pigment epithelium cells from oxidative stress. Food Chem Toxicol 2018, 112, 76–85. [Google Scholar] [CrossRef]
- Zhang, H.-K.; Ye, Y.; Li, K.-J.; Zhao, Z.-n.; He, J.-F. Gypenosides Prevent H2O2-Induced Retinal Ganglion Cell Apoptosis by Concurrently Suppressing the Neuronal Oxidative Stress and Inflammatory Response. Journal of Molecular Neuroscience 2020, 70, 618–630. [Google Scholar] [CrossRef]
- Dietrich, M.; Helling, N.; Hilla, A.; Heskamp, A.; Issberner, A.; Hildebrandt, T.; Kohne, Z.; Küry, P.; Berndt, C.; Aktas, O.; et al. Early alpha-lipoic acid therapy protects from degeneration of the inner retinal layers and vision loss in an experimental autoimmune encephalomyelitis-optic neuritis model. J Neuroinflammation 2018, 15, 71. [Google Scholar] [CrossRef]
- Sarezky, D.; Raquib, A.R.; Dunaief, J.L.; Kim, B.J. Tolerability in the elderly population of high-dose alpha lipoic acid: a potential antioxidant therapy for the eye. Clinical Ophthalmology 2016, 10, 1899–1903. [Google Scholar] [CrossRef]
- Huntemann, N.; Rolfes, L.; Pawlitzki, M.; Ruck, T.; Pfeuffer, S.; Wiendl, H.; Meuth, S.G. Failed, Interrupted, or Inconclusive Trials on Neuroprotective and Neuroregenerative Treatment Strategies in Multiple Sclerosis: Update 2015-2020. Drugs 2021, 81, 1031–1063. [Google Scholar] [CrossRef]
- Falardeau, J.; Fryman, A.; Wanchu, R.; Marracci, G.H.; Mass, M.; Wooliscroft, L.; Bourdette, D.N.; Murchison, C.F.; Hills, W.L.; Yadav, V. Oral lipoic acid as a treatment for acute optic neuritis: a blinded, placebo controlled randomized trial. Mult Scler J Exp Transl Clin 2019, 5, 2055217319850193. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, M.; Zhao, S.; Tian, Y.; Zhang, C. Matrine promotes mitochondrial biosynthesis and reduces oxidative stress in experimental optic neuritis. Front Pharmacol 2022, 13, 936632. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Harada, C.; Namekata, K.; Matsuzawa, A.; Camps, M.; Ji, H.; Swinnen, D.; Jorand-Lebrun, C.; Muzerelle, M.; Vitte, P.A.; et al. Regulation of the severity of neuroinflammation and demyelination by TLR-ASK1-p38 pathway. EMBO Mol Med 2010, 2, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Harada, C.; Namekata, K.; Kimura, A.; Mitamura, Y.; Yoshida, H.; Matsumoto, Y.; Harada, T. Spermidine Alleviates Severity of Murine Experimental Autoimmune Encephalomyelitis. Investigative Ophthalmology & Visual Science 2011, 52, 2696–2703. [Google Scholar] [CrossRef]
- Luo, W.; Xu, H.; Xu, L.; Jiang, W.; Chen, C.; Chang, Y.; Liu, C.; Tian, Z.; Qiu, X.; Xie, C.; et al. Remyelination in neuromyelitis optica spectrum disorder is promoted by edaravone through mTORC1 signaling activation. Glia 2023, 71, 284–304. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, H.; Zhai, Q.; Li, H.; Wang, C.; Wang, Y. Traumatic optic neuropathy: a review of current studies. Neurosurg Rev 2022, 45, 1895–1913. [Google Scholar] [CrossRef]
- Sarkies, N. Traumatic optic neuropathy. Eye (Lond) 2004, 18, 1122–1125. [Google Scholar] [CrossRef]
- Atkins, E.J.; Newman, N.J.; Biousse, V. Post-traumatic visual loss. Rev Neurol Dis 2008, 5, 73–81. [Google Scholar]
- Walsh, F.B. Pathological-clinical correlations. I. Indirect trauma to the optic nerves and chiasm. II. Certain cerebral involvements associated with defective blood supply. Invest Ophthalmol 1966, 5, 433–449. [Google Scholar] [PubMed]
- Gross, C.E.; DeKock, J.R.; Panje, W.R.; Hershkowitz, N.; Newman, J. Evidence for orbital deformation that may contribute to monocular blindness following minor frontal head trauma. J Neurosurg 1981, 55, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Singman, E.; McCulley, T.; Wu, C.; Daphalapurkar, N. The Biomechanics of Indirect Traumatic Optic Neuropathy Using a Computational Head Model With a Biofidelic Orbit. Front Neurol 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Ferrigno, L.; Salvo, M.; Bianchi-Marzoli, S.; Boschi, A.; Carta, F. Visual prognosis after indirect traumatic optic neuropathy. J Neurol Neurosurg Psychiatry 2003, 74, 246–248. [Google Scholar] [CrossRef]
- Urolagin, S.B.; Kotrashetti, S.M.; Kale, T.P.; Balihallimath, L.J. Traumatic optic neuropathy after maxillofacial trauma: a review of 8 cases. J Oral Maxillofac Surg 2012, 70, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Steinsapir, K.D.; Goldberg, R.A. Traumatic Optic Neuropathy: An Evolving Understanding. American Journal of Ophthalmology 2011, 151, 928–933.e922. [Google Scholar] [CrossRef]
- Khan, R.S.; Ross, A.G.; Aravand, P.; Dine, K.; Selzer, E.B.; Shindler, K.S. RGC and Vision Loss From Traumatic Optic Neuropathy Induced by Repetitive Closed Head Trauma Is Dependent on Timing and Force of Impact. Transl Vis Sci Technol 2021, 10, 8. [Google Scholar] [CrossRef]
- Ryan, A.K.; Rich, W.; Reilly, M.A. Oxidative stress in the brain and retina after traumatic injury. Front Neurosci 2023, 17, 1021152. [Google Scholar] [CrossRef]
- Bond, W.S.; Rex, T.S. Evidence That Erythropoietin Modulates Neuroinflammation through Differential Action on Neurons, Astrocytes, and Microglia. Front Immunol 2014, 5, 523. [Google Scholar] [CrossRef]
- DeJulius, C.R.; Bernardo-Colón, A.; Naguib, S.; Backstrom, J.R.; Kavanaugh, T.; Gupta, M.K.; Duvall, C.L.; Rex, T.S. Microsphere antioxidant and sustained erythropoietin-R76E release functions cooperate to reduce traumatic optic neuropathy. J Control Release 2021, 329, 762–773. [Google Scholar] [CrossRef]
- Szymanski, C.R.; Chiha, W.; Morellini, N.; Cummins, N.; Bartlett, C.A.; O'Hare Doig, R.L.; Savigni, D.L.; Payne, S.C.; Harvey, A.R.; Dunlop, S.A.; et al. Paranode Abnormalities and Oxidative Stress in Optic Nerve Vulnerable to Secondary Degeneration: Modulation by 670 nm Light Treatment. PLoS One 2013, 8, e66448. [Google Scholar] [CrossRef] [PubMed]
- O'Hare Doig, R.L.; Bartlett, C.A.; Maghzal, G.J.; Lam, M.; Archer, M.; Stocker, R.; Fitzgerald, M. Reactive species and oxidative stress in optic nerve vulnerable to secondary degeneration. Exp Neurol 2014, 261, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Fatteh, N.; El-Sherbiny, N.M.; Naime, M.; Ibrahim, A.S.; El-Sherbini, A.M.; El-Shafey, S.A.; Khan, S.; Fulzele, S.; Gonzales, J.; et al. Potential role of A2A adenosine receptor in traumatic optic neuropathy. J Neuroimmunol 2013, 264, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Colón, A.; Vest, V.; Clark, A.; Cooper, M.L.; Calkins, D.J.; Harrison, F.E.; Rex, T.S. Antioxidants prevent inflammation and preserve the optic projection and visual function in experimental neurotrauma. Cell Death Dis 2018, 9, 1097. [Google Scholar] [CrossRef]
- Wells, J.; Kilburn, M.R.; Shaw, J.A.; Bartlett, C.A.; Harvey, A.R.; Dunlop, S.A.; Fitzgerald, M. Early in vivo changes in calcium ions, oxidative stress markers, and ion channel immunoreactivity following partial injury to the optic nerve. J Neurosci Res 2012, 90, 606–618. [Google Scholar] [CrossRef]
- Cummins, N.; Bartlett, C.A.; Archer, M.; Bartlett, E.; Hemmi, J.M.; Harvey, A.R.; Dunlop, S.A.; Fitzgerald, M. Changes to mitochondrial ultrastructure in optic nerve vulnerable to secondary degeneration in vivo are limited by irradiation at 670 nm. BMC Neurosci 2013, 14, 98. [Google Scholar] [CrossRef]
- Maxwell, W.L.; McCreath, B.J.; Graham, D.I.; Gennarelli, T.A. Cytochemical evidence for redistribution of membrane pump calcium-ATPase and ecto-Ca-ATPase activity, and calcium influx in myelinated nerve fibres of the optic nerve after stretch injury. J Neurocytol 1995, 24, 925–942. [Google Scholar] [CrossRef]
- Cansler, S.M.; Evanson, N.K. Connecting endoplasmic reticulum and oxidative stress to retinal degeneration, TBI, and traumatic optic neuropathy. Journal of Neuroscience Research 2020, 98, 571–574. [Google Scholar] [CrossRef]
- Hu, Y.; Park, K.K.; Yang, L.; Wei, X.; Yang, Q.; Cho, K.S.; Thielen, P.; Lee, A.H.; Cartoni, R.; Glimcher, L.H.; et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron 2012, 73, 445–452. [Google Scholar] [CrossRef]
- Hylin, M.J.; Holden, R.C.; Smith, A.C.; Logsdon, A.F.; Qaiser, R.; Lucke-Wold, B.P. Juvenile Traumatic Brain Injury Results in Cognitive Deficits Associated with Impaired Endoplasmic Reticulum Stress and Early Tauopathy. Dev Neurosci 2018, 40, 175–188. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Turner, R.C.; Logsdon, A.F.; Nguyen, L.; Bailes, J.E.; Lee, J.M.; Robson, M.J.; Omalu, B.I.; Huber, J.D.; Rosen, C.L. Endoplasmic reticulum stress implicated in chronic traumatic encephalopathy. J Neurosurg 2016, 124, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, A.F.; Lucke-Wold, B.P.; Nguyen, L.; Matsumoto, R.R.; Turner, R.C.; Rosen, C.L.; Huber, J.D. Salubrinal reduces oxidative stress, neuroinflammation and impulsive-like behavior in a rodent model of traumatic brain injury. Brain Res 2016, 1643, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Behnammanesh, G.; Wu, Z.; Rong, R.; You, M.; Majid, A.S.A.; Ji, D. Echium amoenum L. Ethanol Extract Protects Retinal Ganglion Cell after Glutamate and Optic Nerve Crush Injury. Dis Markers 2022, 2022, 3631532. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.K.; Le, T.T.; Kim, K.A.; Kim, Y.J.; Lee, W.B.; Jung, S.H. Roots of Lithospermum erythrorhizon promotes retinal cell survival in optic nerve crush-induced retinal degeneration. Exp Eye Res 2021, 203, 108419. [Google Scholar] [CrossRef]
- Li, R.C.; Morris, M.W.; Lee, S.K.; Pouranfar, F.; Wang, Y.; Gozal, D. Neuroglobin protects PC12 cells against oxidative stress. Brain research 2008, 1190, 159–166. [Google Scholar] [CrossRef]
- Watanabe, S.; Takahashi, N.; Uchida, H.; Wakasugi, K. Human neuroglobin functions as an oxidative stress-responsive sensor for neuroprotection. Journal of Biological Chemistry 2012, 287, 30128–30138. [Google Scholar] [CrossRef]
- Sugitani, K.; Koriyama, Y.; Sera, M.; Arai, K.; Ogai, K.; Wakasugi, K. A novel function of neuroglobin for neuroregeneration in mice after optic nerve injury. Biochem Biophys Res Commun 2017, 493, 1254–1259. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.J. Carvedilol Promotes Retinal Ganglion Cell Survival Following Optic Nerve Injury via ASK1-p38 MAPK Pathway. CNS Neurol Disord Drug Targets 2019, 18, 695–704. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine – Scope, Variation, and Limitation. European Journal of Organic Chemistry 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Mei, S.; Xu, X.; Priestley, R.D.; Lu, Y. Polydopamine-based nanoreactors: synthesis and applications in bioscience and energy materials. Chem Sci 2020, 11, 12269–12281. [Google Scholar] [CrossRef]
- Lou, X.; Hu, Y.; Zhang, H.; Liu, J.; Zhao, Y. Polydopamine nanoparticles attenuate retina ganglion cell degeneration and restore visual function after optic nerve injury. J Nanobiotechnology 2021, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Ezoulin, M.J.; Ombetta, J.-E.; Dutertre-Catella, H.; Warnet, J.-M.; Massicot, F. Antioxidative properties of galantamine on neuronal damage induced by hydrogen peroxide in SK–N–SH cells. Neurotoxicology 2008, 29, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Egea, J.; García, A.G.; López, M.G. Synergistic neuroprotective effect of combined low concentrations of galantamine and melatonin against oxidative stress in SH-SY5Y neuroblastoma cells. Journal of pineal research 2010, 49, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Yang, L.; Sameshima, H. Galantamine, an acetylcholinesterase inhibitor, reduces brain damage induced by hypoxia-ischemia in newborn rats. International Journal of Developmental Neuroscience 2014, 37, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Naguib, S.; Bernardo-Colón, A.; Cencer, C.; Gandra, N.; Rex, T.S. Galantamine protects against synaptic, axonal, and vision deficits in experimental neurotrauma. Neurobiol Dis 2020, 134, 104695. [Google Scholar] [CrossRef]
- Zuo, L.; Khan, R.S.; Lee, V.; Dine, K.; Wu, W.; Shindler, K.S. SIRT1 promotes RGC survival and delays loss of function following optic nerve crush. Invest Ophthalmol Vis Sci 2013, 54, 5097–5102. [Google Scholar] [CrossRef]
- Mou, Q.; Yao, K.; Ye, M.; Zhao, B.; Hu, Y.; Lou, X.; Li, H.; Zhang, H.; Zhao, Y. Modulation of Sirt1-mTORC1 Pathway in Microglia Attenuates Retinal Ganglion Cell Loss After Optic Nerve Injury. J Inflamm Res 2021, 14, 6857–6869. [Google Scholar] [CrossRef]
- Khong, J.J.; McNab, A.A.; Ebeling, P.R.; Craig, J.E.; Selva, D. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol 2016, 100, 142–150. [Google Scholar] [CrossRef]
- Dolman, P.J. Dysthyroid optic neuropathy: evaluation and management. J Endocrinol Invest 2021, 44, 421–429. [Google Scholar] [CrossRef]
- Giaconi, J.A.; Kazim, M.; Rho, T.; Pfaff, C. CT scan evidence of dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg 2002, 18, 177–182. [Google Scholar] [CrossRef]
- Pelewicz-Sowa, M.; Miśkiewicz, P. Dysthyroid optic neuropathy: emerging treatment strategies. Journal of Endocrinological Investigation 2023, 46, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Khoo, T.K.; Bahn, R.S. Pathogenesis of Graves' ophthalmopathy: the role of autoantibodies. Thyroid 2007, 17, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Pappa, A.; Jackson, P.; Stone, J.; Munro, P.; Fells, P.; Pennock, C.; Lightman, S. An ultrastructural and systemic analysis of glycosaminoglycans in thyroid-associated ophthalmopathy. Eye (Lond) 1998, 12 ( Pt 2) Pt 2, 237–244. [Google Scholar] [CrossRef]
- Bednarek, J.; Wysocki, H.; Sowiński, J. Peripheral parameters of oxidative stress in patients with infiltrative Graves' ophthalmopathy treated with corticosteroids. Immunol Lett 2004, 93, 227–232. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, S.B.; Cheng, C.Y.; Kao, S.C.; Kau, H.C.; Chiou, S.H.; Hsu, W.M.; Wei, Y.H. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves' ophthalmopathy: evidence that oxidative stress has a role in this disorder. Eye (Lond) 2010, 24, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Li, Y.; Ji, Y.S.; Yoon, K.C. Oxidative stress markers in tears of patients with Graves’ orbitopathy and their correlation with clinical activity score. BMC Ophthalmology 2018, 18, 303. [Google Scholar] [CrossRef]
- Tsai, C.C.; Cheng, C.Y.; Liu, C.Y.; Kao, S.C.; Kau, H.C.; Hsu, W.M.; Wei, Y.H. Oxidative stress in patients with Graves' ophthalmopathy: relationship between oxidative DNA damage and clinical evolution. Eye (Lond) 2009, 23, 1725–1730. [Google Scholar] [CrossRef]
- Kau, H.C.; Wu, S.B.; Tsai, C.C.; Liu, C.J.; Wei, Y.H. Cigarette Smoke Extract-Induced Oxidative Stress and Fibrosis-Related Genes Expression in Orbital Fibroblasts from Patients with Graves' Ophthalmopathy. Oxid Med Cell Longev 2016, 2016, 4676289. [Google Scholar] [CrossRef]
- Burch, H.B.; Lahiri, S.; Bahn, R.S.; Barnes, S. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves' ophthalmopathy. Exp Eye Res 1997, 65, 311–316. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, S.B.; Kao, S.C.; Kau, H.C.; Lee, F.L.; Wei, Y.H. The protective effect of antioxidants on orbital fibroblasts from patients with Graves' ophthalmopathy in response to oxidative stress. Mol Vis 2013, 19, 927–934. [Google Scholar]
- Hou, T.Y.; Wu, S.B.; Kau, H.C.; Tsai, C.C. The Role of Oxidative Stress and Therapeutic Potential of Antioxidants in Graves' Ophthalmopathy. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res 2009, 41, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Rotondo Dottore, G.; Leo, M.; Casini, G.; Latrofa, F.; Cestari, L.; Sellari-Franceschini, S.; Nardi, M.; Vitti, P.; Marcocci, C.; Marinò, M. Antioxidant Actions of Selenium in Orbital Fibroblasts: A Basis for the Effects of Selenium in Graves' Orbitopathy. Thyroid 2017, 27, 271–278. [Google Scholar] [CrossRef]
- Kim, B.Y.; Jang, S.Y.; Choi, D.H.; Jung, C.H.; Mok, J.O.; Kim, C.H. Anti-inflammatory and Antioxidant Effects of Selenium on Orbital Fibroblasts of Patients With Graves Ophthalmopathy. Ophthalmic Plast Reconstr Surg 2021, 37, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Kahaly, G.J.; Krassas, G.E.; Bartalena, L.; Prummel, M.; Stahl, M.; Altea, M.A.; Nardi, M.; Pitz, S.; Boboridis, K.; et al. Selenium and the course of mild Graves' orbitopathy. N Engl J Med 2011, 364, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Baldeschi, L.; Boboridis, K.; Eckstein, A.; Kahaly, G.J.; Marcocci, C.; Perros, P.; Salvi, M.; Wiersinga, W.M. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J 2016, 5, 9–26. [Google Scholar] [CrossRef]
- Lanzolla, G.; Marinò, M.; Marcocci, C. Selenium in the Treatment of Graves' Hyperthyroidism and Eye Disease. Front Endocrinol (Lausanne) 2020, 11, 608428. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, H.J.; Chae, M.K.; Lee, S.Y.; Lee, E.J. Cigarette smoke extract-induced adipogenesis in Graves' orbital fibroblasts is inhibited by quercetin via reduction in oxidative stress. J Endocrinol 2013, 216, 145–156. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, H.J.; Choi, S.H.; Chang, E.J.; Lee, S.Y.; Lee, E.J. Quercetin inhibits IL-1β-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves' orbitopathy. PLoS One 2011, 6, e26261. [Google Scholar] [CrossRef] [PubMed]
- Rotondo Dottore, G.; Ionni, I.; Menconi, F.; Casini, G.; Sellari-Franceschini, S.; Nardi, M.; Vitti, P.; Marcocci, C.; Marinò, M. Action of three bioavailable antioxidants in orbital fibroblasts from patients with Graves' orbitopathy (GO): a new frontier for GO treatment? J Endocrinol Invest 2018, 41, 193–201. [Google Scholar] [CrossRef]
- Rotondo Dottore, G.; Ionni, I.; Menconi, F.; Casini, G.; Sellari-Franceschini, S.; Nardi, M.; Vitti, P.; Marcocci, C.; Marinò, M. Antioxidant effects of β-carotene, but not of retinol and vitamin E, in orbital fibroblasts from patients with Graves' orbitopathy (GO). J Endocrinol Invest 2018, 41, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chang, T.C.; Kao, S.C.; Kuo, Y.F.; Chien, L.F. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves' ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh) 1993, 129, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Balazs, C.; Kiss, E.; Vamos, A.; Molnar, I.; Farid, N.R. Beneficial effect of pentoxifylline on thyroid associated ophthalmopathy (TAO)*: a pilot study. J Clin Endocrinol Metab 1997, 82, 1999–2002. [Google Scholar] [CrossRef] [PubMed]
- Finamor, F.E.; Martins, J.R.; Nakanami, D.; Paiva, E.R.; Manso, P.G.; Furlanetto, R.P. Pentoxifylline (PTX)--an alternative treatment in Graves' ophthalmopathy (inactive phase): assessment by a disease specific quality of life questionnaire and by exophthalmometry in a prospective randomized trial. Eur J Ophthalmol 2004, 14, 277–283. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, H.J.; Chae, M.K.; Byun, J.W.; Lee, E.J.; Yoon, J.S. Therapeutic Effect of Resveratrol on Oxidative Stress in Graves' Orbitopathy Orbital Fibroblasts. Invest Ophthalmol Vis Sci 2015, 56, 6352–6361. [Google Scholar] [CrossRef]
- Bartalena, L.; Martino, E.; Marcocci, C.; Bogazzi, F.; Panicucci, M.; Velluzzi, F.; Loviselli, A.; Pinchera, A. More on smoking habits and Graves' ophthalmopathy. J Endocrinol Invest 1989, 12, 733–737. [Google Scholar] [CrossRef]
- Bouzas, E.A.; Karadimas, P.; Mastorakos, G.; Koutras, D.A. Antioxidant agents in the treatment of Graves' ophthalmopathy. Am J Ophthalmol 2000, 129, 618–622. [Google Scholar] [CrossRef]
- Chandran, G.; Sirajudeen, K.N.; Yusoff, N.S.; Swamy, M.; Samarendra, M.S. Effect of the antihypertensive drug enalapril on oxidative stress markers and antioxidant enzymes in kidney of spontaneously hypertensive rat. Oxid Med Cell Longev 2014, 2014, 608512. [Google Scholar] [CrossRef]
- de Cavanagh, E.M.; Inserra, F.; Ferder, L.; Fraga, C.G. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol 2000, 278, R572–R577. [Google Scholar] [CrossRef] [PubMed]
- Botta, R.; Lisi, S.; Marcocci, C.; Sellari-Franceschini, S.; Rocchi, R.; Latrofa, F.; Menconi, F.; Altea, M.A.; Leo, M.; Sisti, E.; et al. Enalapril reduces proliferation and hyaluronic acid release in orbital fibroblasts. Thyroid 2013, 23, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ataabadi, G.; Dabbaghmanesh, M.H.; Owji, N.; Bakhshayeshkaram, M.; Montazeri-Najafabady, N. Clinical Features of Graves' Ophthalmopathy and Impact of Enalapril on the Course of Mild Graves' Ophthalmopathy: A Pilot Study. Endocr Metab Immune Disord Drug Targets 2020, 20, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Rachwani Anil, R.; Rocha-de-Lossada, C.; Zamorano Martín, F.; Santos Ortega, A.; Luque Aranda, G.; España Contreras, M.; Escudero Gómez, J. Infiltrative optic neuropathy as a relapse of acute lymphoblastic leukemia. J Fr Ophtalmol 2021, 44, 293–295. [Google Scholar] [CrossRef]
- Kuan, H.C.; Mustapha, M.; Oli Mohamed, S.; Abdul Aziz, R.A.; Loh, C.K.; Mohammed, F.; Naffi, A.A.; Othman, O.; Nasaruddin, R.A.; Alias, H. Isolated Infiltrative Optic Neuropathy in an Acute Lymphoblastic Leukemia Relapse. Cureus 2022, 14, e25625. [Google Scholar] [CrossRef]
- Myers, K.A.; Nikolic, A.; Romanchuk, K.; Weis, E.; Brundler, M.A.; Lafay-Cousin, L.; Costello, F. Optic neuropathy in the context of leukemia or lymphoma: diagnostic approach to a neuro-oncologic emergency. Neurooncol Pract 2017, 4, 60–66. [Google Scholar] [CrossRef]
- Jain, R.; Trehan, A.; Singh, R.; Srinivasan, R.; Dogra, M.; Bansal, D.; Marwaha, R.K. Unusual sites of relapse in pre-B acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2014, 36, e506–e508. [Google Scholar] [CrossRef]
- Currie, J.N.; Lessell, S.; Lessell, I.M.; Weiss, J.S.; Albert, D.M.; Benson, E.M. Optic neuropathy in chronic lymphocytic leukemia. Arch Ophthalmol 1988, 106, 654–660. [Google Scholar] [CrossRef]
- Takkar, A.; Naheed, D.; Dogra, M.; Goyal, M.K.; Singh, R.; Gupta, N.; Gupta, K.; Mittal, B.R.; Lal, V. Infiltrative Optic Neuropathies: Opening Doors to Sinister Pathologies. Neuroophthalmology 2017, 41, 279–283. [Google Scholar] [CrossRef]
- Takahashi, T.; Oda, Y.; Isayama, Y. Leukemic optic neuropathy. Ophthalmologica 1982, 185, 37–45. [Google Scholar] [CrossRef]
- Soares, M.d.F.; Braga, F.T.; Rocha, A.J.d.; Lederman, H.M. Optic nerve infiltration by acute lymphoblastic leukemia: MRI contribution. Pediatric Radiology 2005, 35, 799–802. [Google Scholar] [CrossRef]
- Vegunta, S.; Patel, B.C. Optic Nerve Coloboma. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL), 2022.
- Saffren, B.D.; Yassin, S.H.; Geddie, B.E.; de Faber, J.; Blieden, L.S.; Bhate, M.; Gamio, S.; Rutar, T.; Levin, A.V. Optic Nerve Aplasia. J Neuroophthalmol 2022, 42, e140–e146. [Google Scholar] [CrossRef] [PubMed]
- Skriapa Manta, A.; Olsson, M.; Ek, U.; Wickström, R.; Teär Fahnehjelm, K. Optic Disc Coloboma in children - prevalence, clinical characteristics and associated morbidity. Acta Ophthalmol 2019, 97, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Amador-Patarroyo, M.J.; Pérez-Rueda, M.A.; Tellez, C.H. Congenital anomalies of the optic nerve. Saudi J Ophthalmol 2015, 29, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Margolin, E.; Blair, K.; Shemesh, A. Toxic and Nutritional Optic Neuropathy. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL), 2022.
- Grzybowski, A.; Zülsdorff, M.; Wilhelm, H.; Tonagel, F. Toxic optic neuropathies: an updated review. Acta Ophthalmol 2015, 93, 402–410. [Google Scholar] [CrossRef]
- Kovač, L.; Volk, M.; Šuštar Habjan, M.; Hawlina, M. Oxidative Stress in Antibiotic Toxic Optic Neuropathy Mimicking Acute LHON in a Patient with Exacerbation of Cystic Fibrosis. Stresses 2023, 3, 387–396. [Google Scholar] [CrossRef]
- Spillane, J.D. Nutritional disorders of the nervous system in the Middle East. Proc R Soc Med 1946, 39, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Epidemic Neuropathy—Cuba, 1991-1994. JAMA 1994, 271, 1154–1156. [CrossRef]
- Plant, G.T.; Dolin, P.; Mohamed, A.A.; Mlingi, N. Confirmation that neither cyanide intoxication nor mutations commonly associated with Leber's Hereditary Optic Neuropathy are implicated in Tanzanian Epidemic Optic Neuropathy. J Neurol Sci 1997, 152, 107–108. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Kobak, J.; Tyczyńska, M.; Dudek, I.; Maani, A.; Teresiński, G.; Buszewicz, G.; Januszewski, J.; Flieger, J. Toxic and Nutritional Optic Neuropathies-An Updated Mini-Review. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef]
- Margolin, E.; Blair, K.; Shemesh, A. Toxic and Nutritional Optic Neuropathy. In StatPearls, StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL), 2023.
- Yu, J.J.; Lee, D.H.; Gallagher, S.P.; Kenney, M.C.; Boisvert, C.J. Mitochondrial Impairment in Antibiotic Induced Toxic Optic Neuropathies. Current Eye Research 2018, 43, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol 2014, 166, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician 2017, 96, 384–389. [Google Scholar] [PubMed]
- Grzybowski, A.; Zülsdorff, M.; Wilhelm, H.; Tonagel, F. Toxic optic neuropathies: an updated review. Acta Ophthalmologica 2015, 93, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, F.; Sanjari, M.S.; Naderi, A.; Pirmarzdashti, N.; Haghighi, A.; Kashkouli, M.B. Erythropoietin in Treatment of Methanol Optic Neuropathy. J Neuroophthalmol 2018, 38, 167–171. [Google Scholar] [CrossRef]
- Pakravan, M.; Esfandiari, H.; Sanjari, N.; Ghahari, E. Erythropoietin as an adjunctive treatment for methanol-induced toxic optic neuropathy. Am J Drug Alcohol Abuse 2016, 42, 633–639. [Google Scholar] [CrossRef]
- Fang, C.E.H.; Guo, L.; Hill, D.; Yap, T.E.; Cordeiro, M.F. Neuroprotective Strategies in Glaucoma - Translation to Clinical Trials. OBM Neurobiology 2020, 04, 062. [Google Scholar] [CrossRef]
- Wubben, T.J.; Besirli, C.G.; Johnson, M.W.; Zacks, D.N. Retinal Neuroprotection: Overcoming the Translational Roadblocks. American Journal of Ophthalmology 2018, 192, xv–xxii. [Google Scholar] [CrossRef]
- Almasieh, M.; Levin, L.A. Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annual Review of Vision Science 2017, 3, 91–120. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef]
- Normando, E.M.; Yap, T.E.; Maddison, J.; Miodragovic, S.; Bonetti, P.; Almonte, M.; Mohammad, N.G.; Ameen, S.; Crawley, L.; Ahmed, F.; et al. A CNN-aided method to predict glaucoma progression using DARC (Detection of Apoptosing Retinal Cells). Expert Review of Molecular Diagnostics 2020, 20, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Mehta, C. Adaptive Designs for Clinical Trials. New England Journal of Medicine 2016, 375, 65–74. [Google Scholar] [CrossRef] [PubMed]
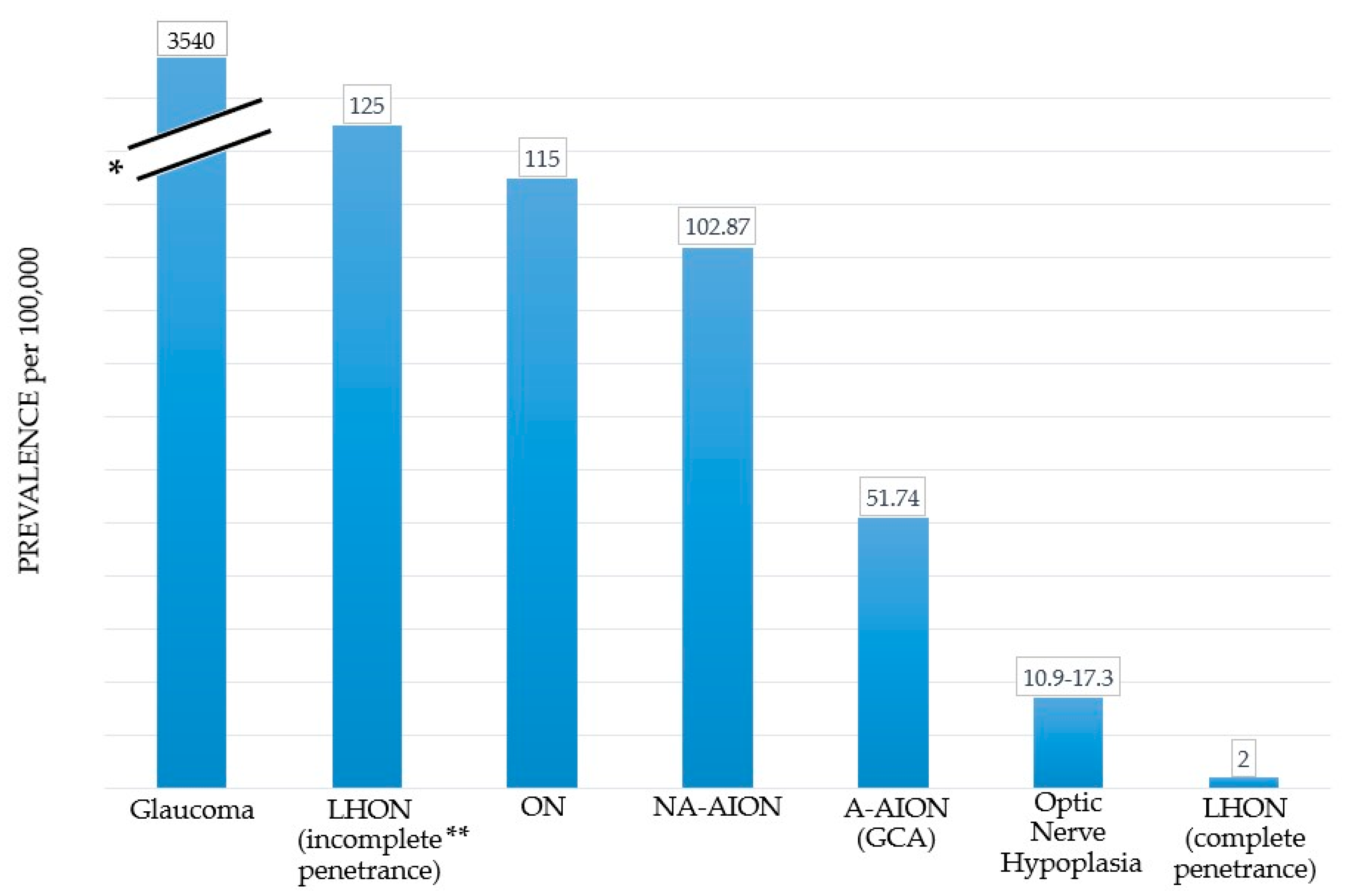
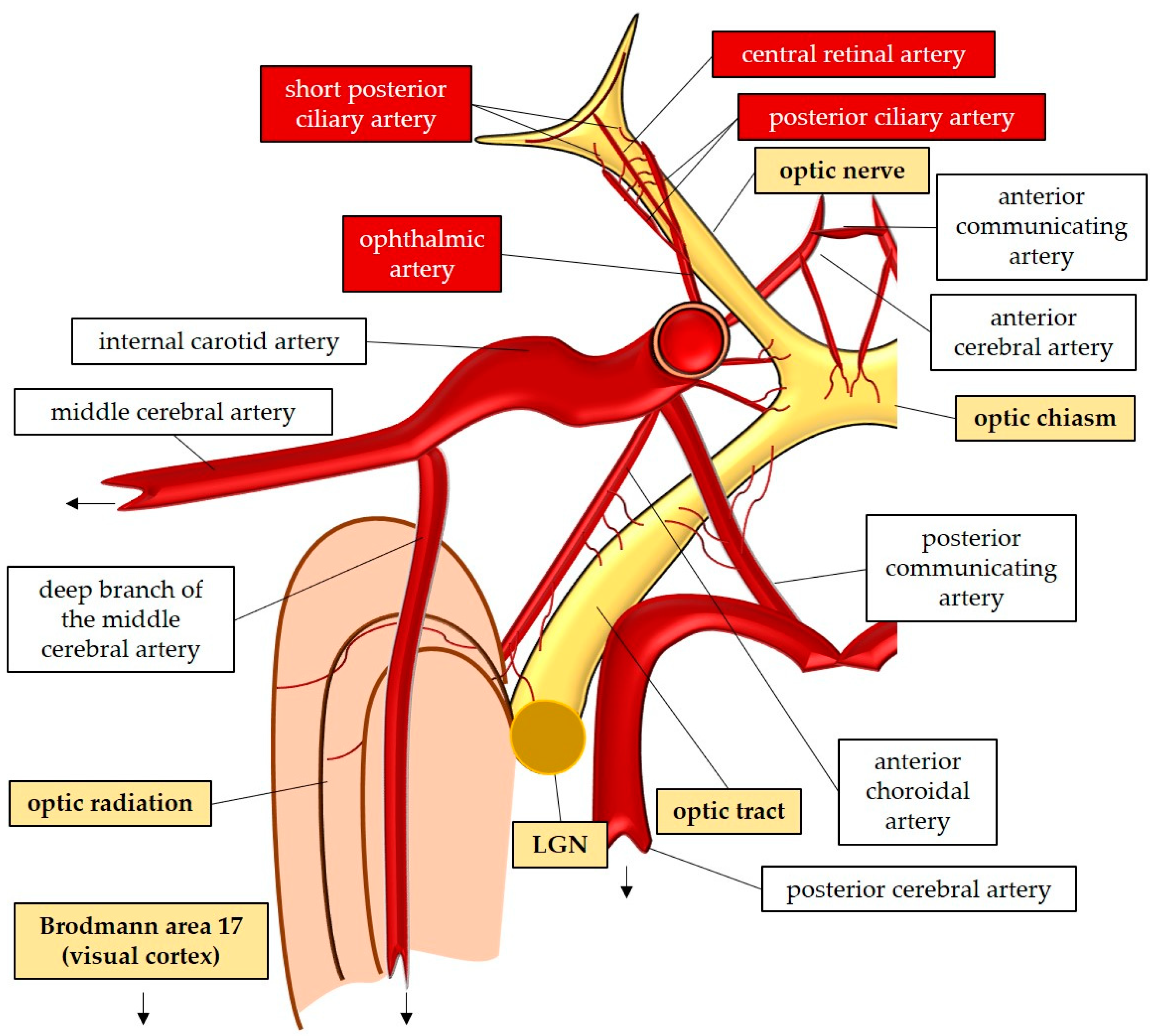
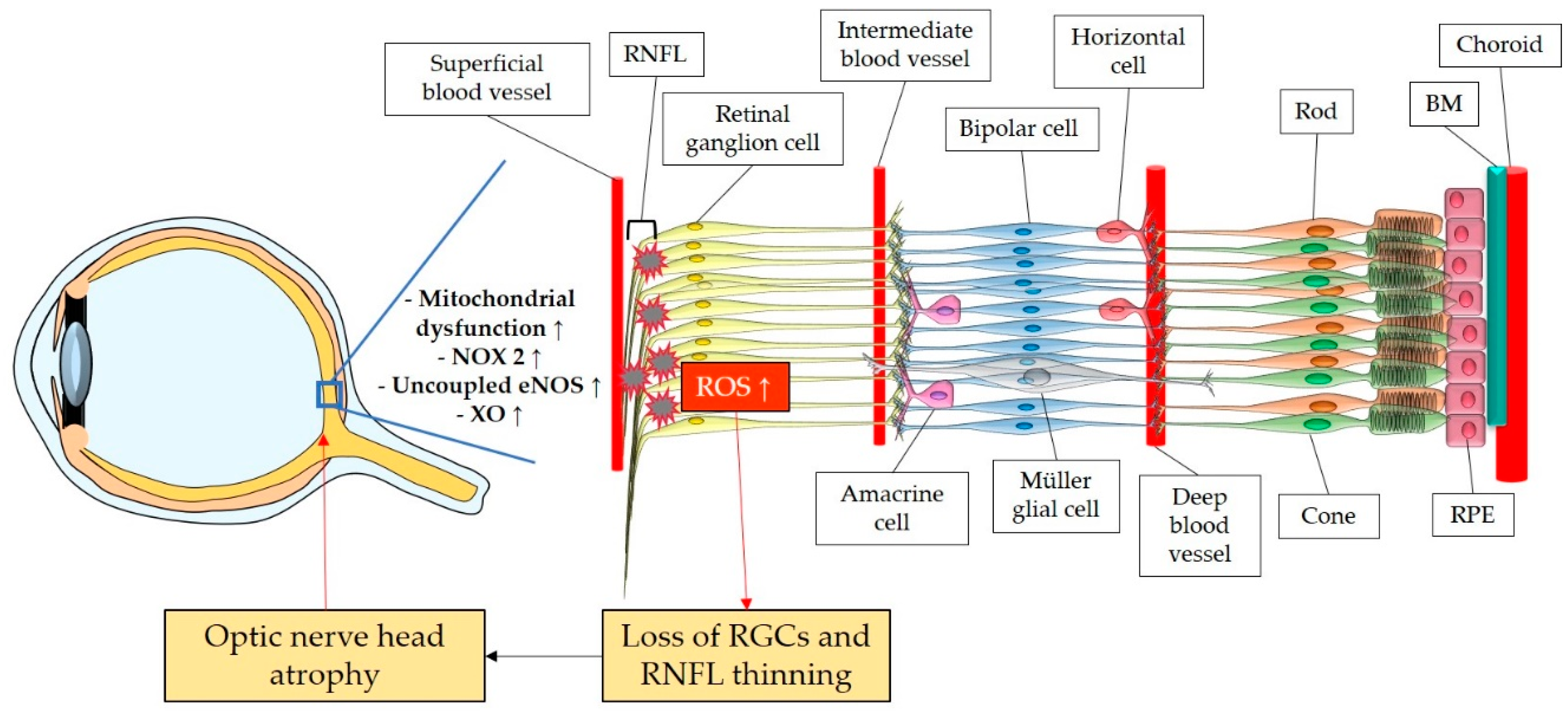
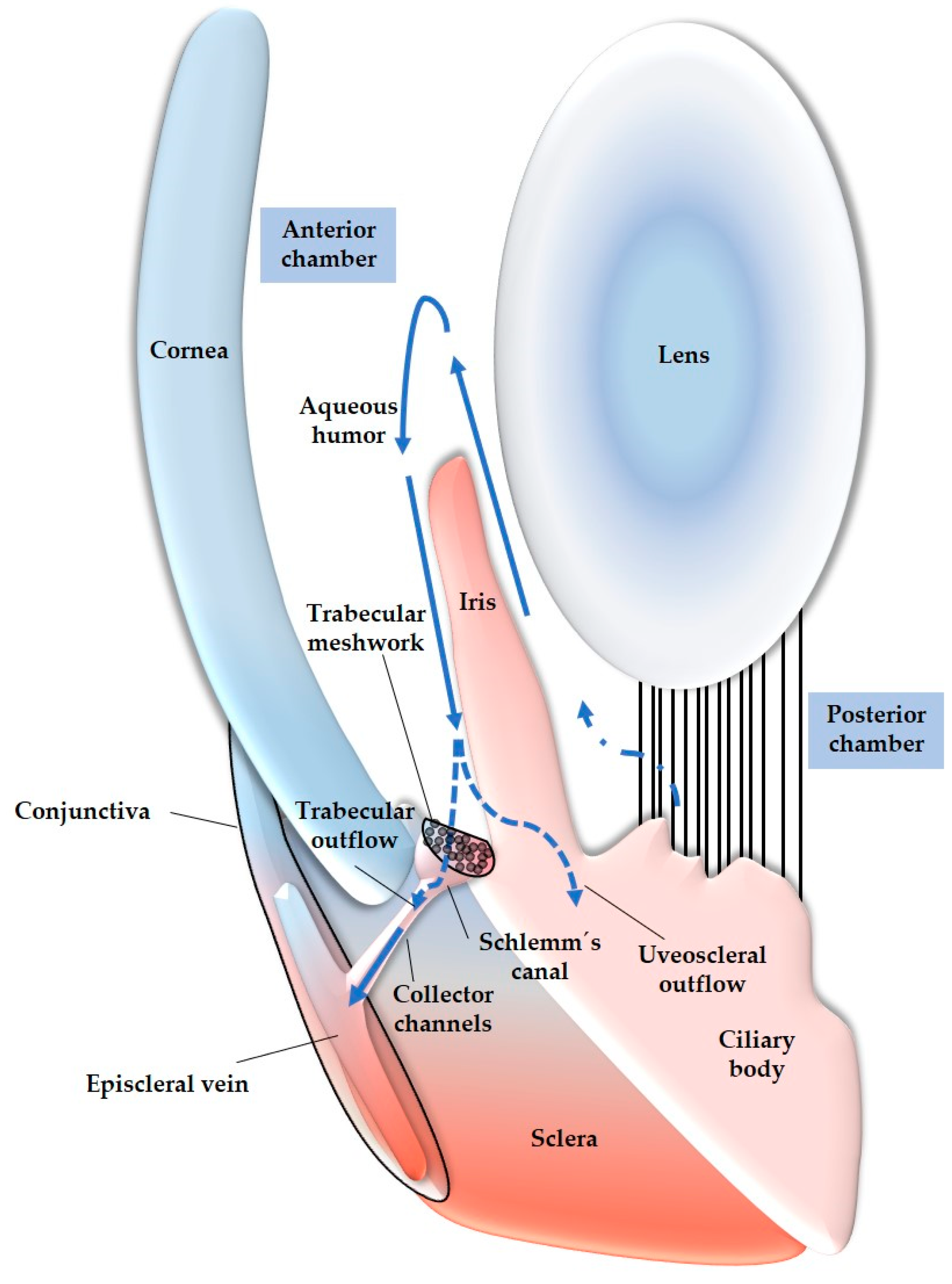
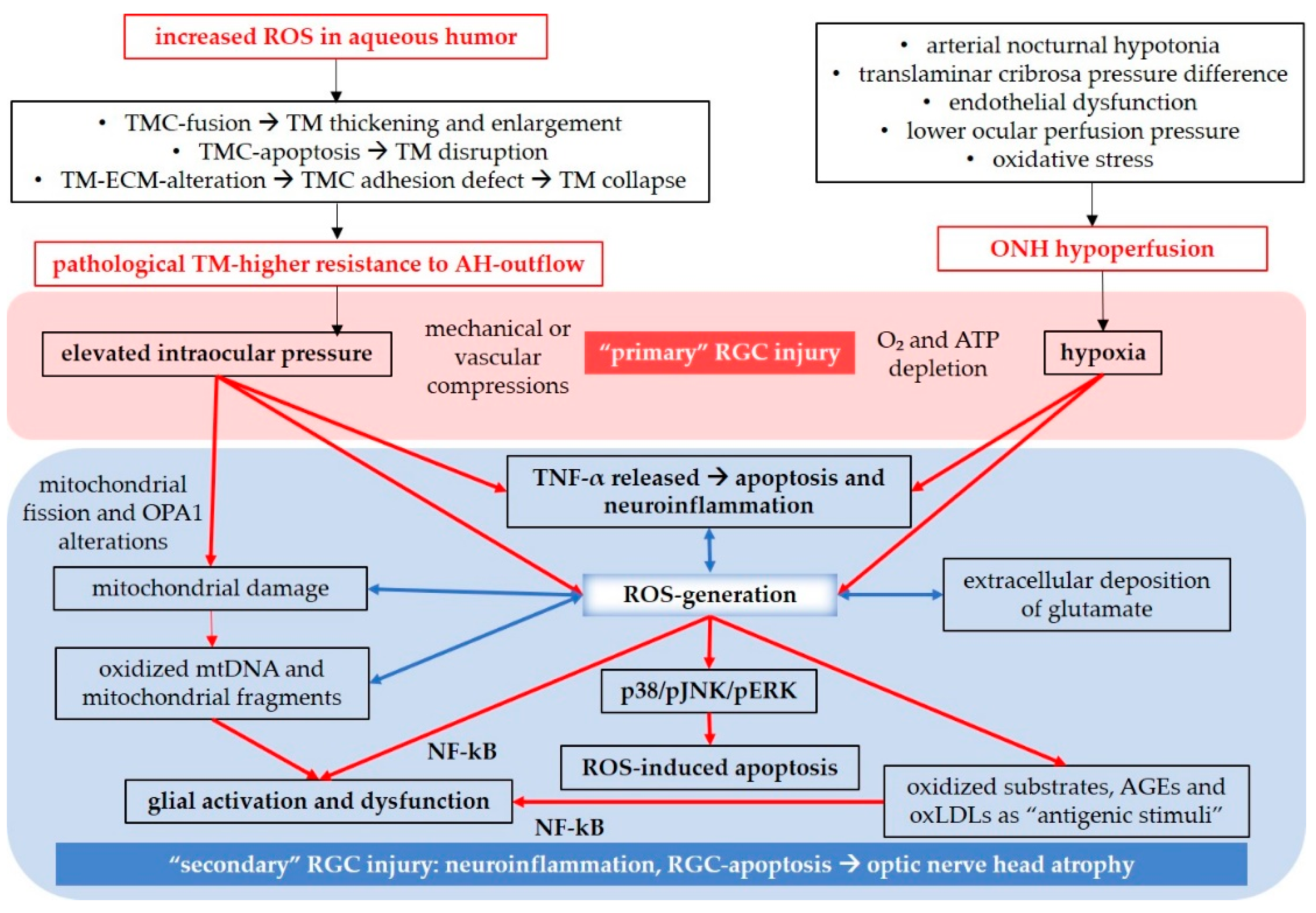
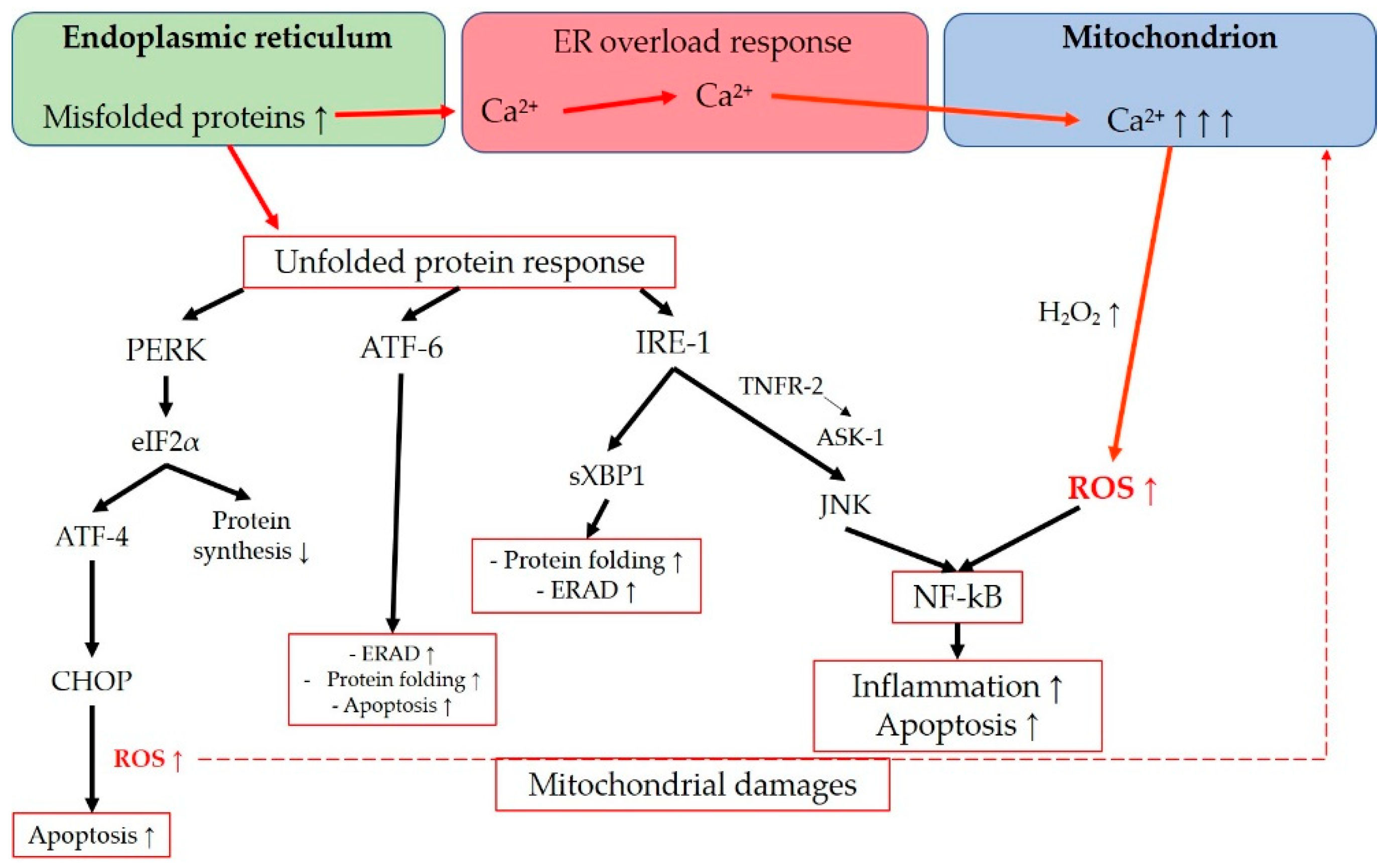
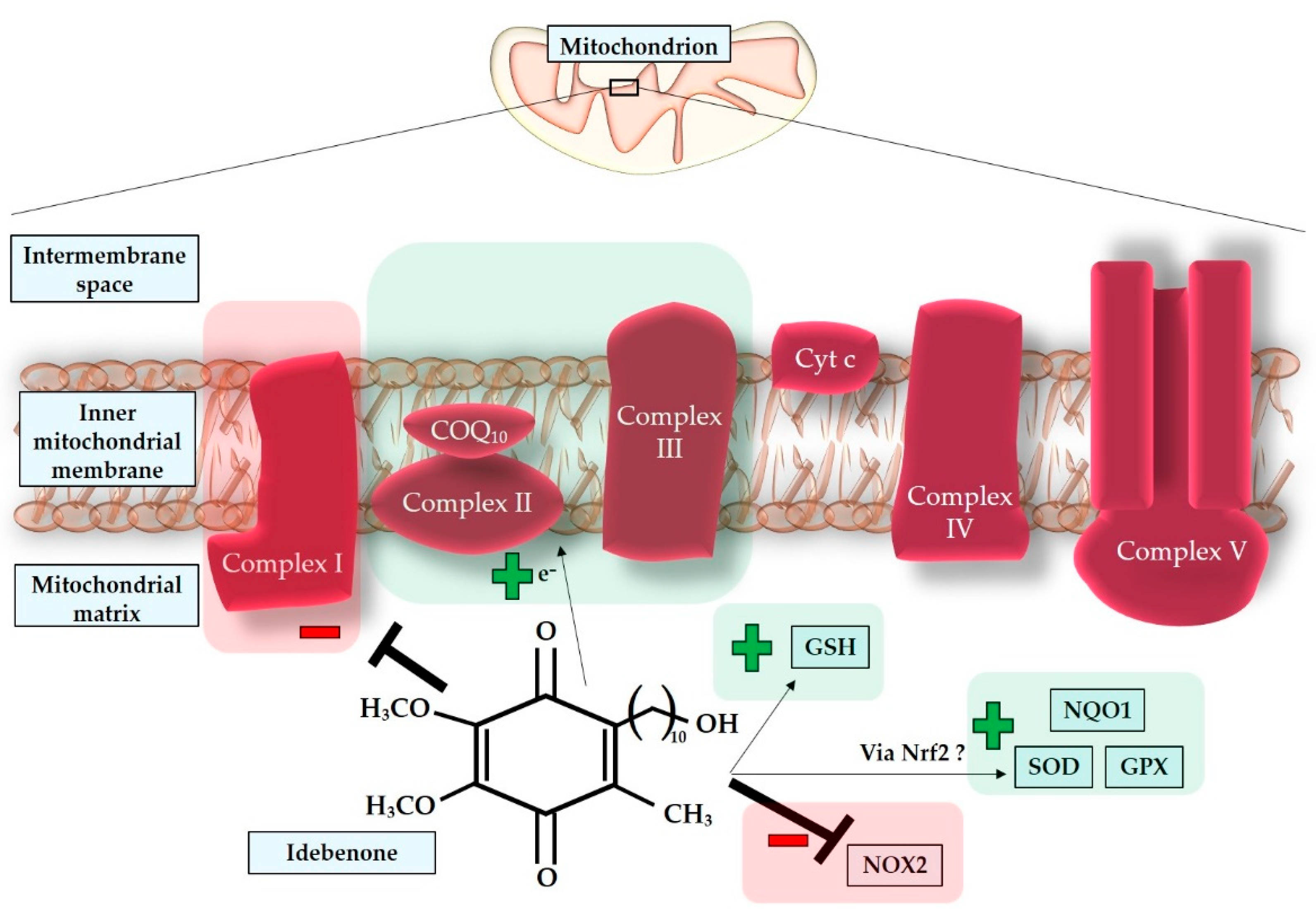
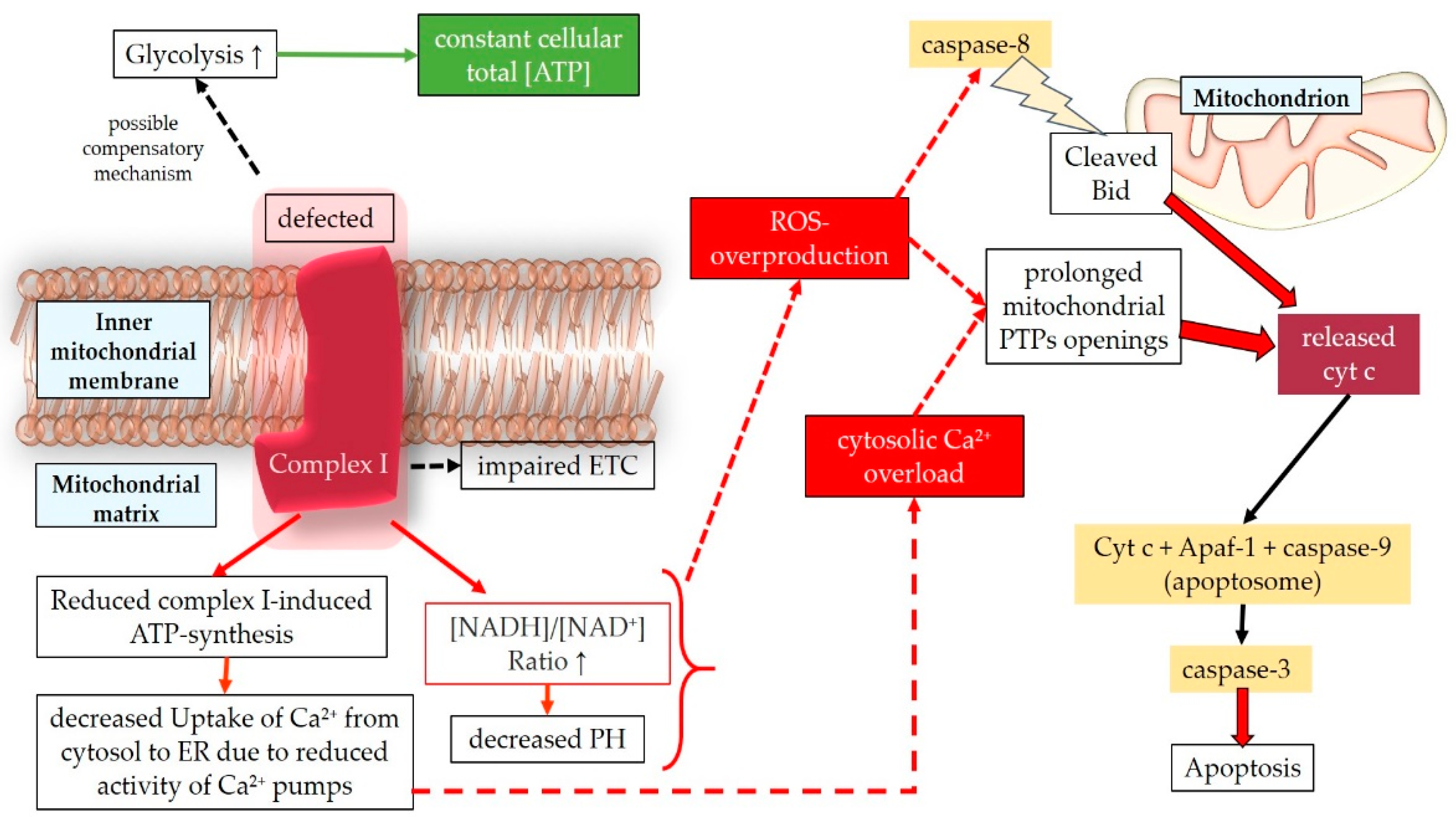
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




