1. Introduction
Ovarian cancer (OC) has the highest mortality among gynecologic malignancies and is the fifth leading cause of overall cancer death in women in the United States, estimated to contribute 2.08% of new cancer diagnoses and 4.61% of cancer-related fatalities in women in 2023[
1] . Due to atypical/vague symptoms and lack of effective diagnostic methods or screening tests in the early stages, more than 70% of patients with ovarian cancer are not diagnosed until the disease has progressed to an advanced stage [
2,
3]. The conventional treatment for OC patients is tumor debulking surgery in combination with platinum-taxane based chemotherapy [
4]. Although many women achieve remission with standard treatment, the recurrence of cancer and resistance to chemo-therapeutics pose significant challenges for the majority of OC patients [
4,
5]. Thus, novel therapies, including targeted therapy, are in high demand for the treatment of OC, especially for those patients who have relapsed [
6,
7].
Cyclin-dependent kinases (CDKs) are a group of proline-directed serine/threonine protein kinases that regulate cell cycle process by phosphorylating several proteins involved in diverse aspects of cell cycle control [
8]. CDK1 is a stimulator of cell cycle progression and specifically controls the G2/M phase transition of the cell cycle and chromosome segregation, through phosphorylating many key targets involved in nuclear envelope breakdown and progression through mitosis [
9]. The plasticity of CDK1 enables it to adequately compensate for the genetic loss of interphase CDKs, and thus, making CDK1 the only non-redundant cell cycle regulator in the CDK family [
10]. In addition to the control of the cell cycle, CDK1 participates in many biologic processes involved in the growth of cancer cells, including DNA homologous recombination (HR) repair, maintenance of cancer stem cell properties, mitochondrial bioenergetics, and tumor resistance [
8,
11,
12]. Overexpression of CDK1 has been implicated in the intrinsic modulation of altered Wnt, mTOR, and p53/Rb signaling pathways, suggesting that CDK1 is an essential driver of tumorigenesis [
13]. Multi-dimensional bioinformatics analysis from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) datasets confirmed that CDK1 was significantly overexpressed in cancer cells and strongly associated with prognosis in many types of cancer, including OC [
13,
14,
15,
16,
17]. Inhibition of CDK1 expression by small inhibitors, or CDK1 knockdown, reduces cell viability, induces apoptosis, and impairs cell migration in multiple types of cancer cell lines and xenograft mouse models [
18,
19,
20,
21,
22,
23]. Dinaciclib, a small molecule inhibitor of CDK1, CDK2, CDK5, and CDK9, demonstrated well-tolerated and promising efficacy in phase II and III clinical trials in relapsed multiple myeloma and chronic lymphocytic leukemia, but showed low clinical efficacy in solid tumors [
24,
25,
26,
27,
28]. Milciclib, an inhibitor of CDK1, CDK2, CDK4, and CDK5, exhibited clinical activity in combination with gemcitabine in a phase I clinical trial in refractory solid tumors [
29]. Collectively, the evidence supports that targeting CDK1 by small molecule inhibitors deserves further exploration of their therapeutic value for tumors in pre-clinical models and clinical trials [
11].
Due to their significant degree of similarity, most inhibitors targeting CDK1 also inactivate CDK2, which is activate throughout S and G2 phases of the cell cycle. The exception to this is RO-3306, a selective, ATP-competitive, and cell-permeable CDK1 inhibitor that demonstrates potent anti-tumor activity in multiple pre-clinical models [
30,
31,
32]. Given its high selectivity and specificity for CDK1, the current study used RO-3306 to address the anti-tumor potential of CDK1 inhibition in OC cell lines and a transgenic mouse model of OC. Effects of CDK1 inhibition by RO-3306 on cell viability, apoptosis, cell cycle distribution, and invasion were analyzed in OC cells. The effect of RO-3306 on tumor growth in a genetically engineered mouse model of OC (KpB mouse model) under obese and lean conditions was also analyzed. The current study finds that targeting CDK1 by RO-3306 shows anti-proliferative and anti-metastatic effects in OC cells and KpB mouse model.
2. Results
Effect of CDK1 on prognosis in patients with OC.
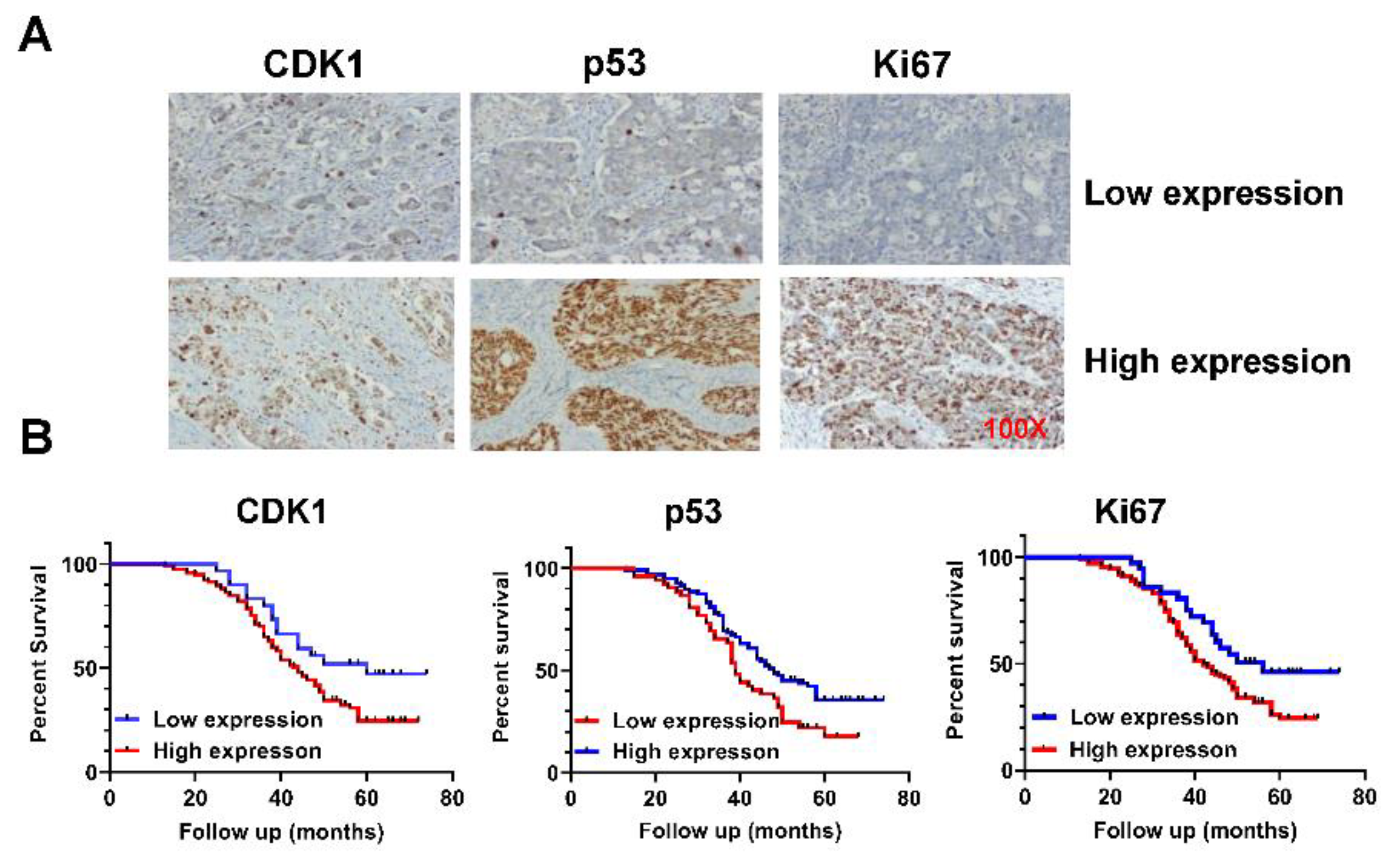
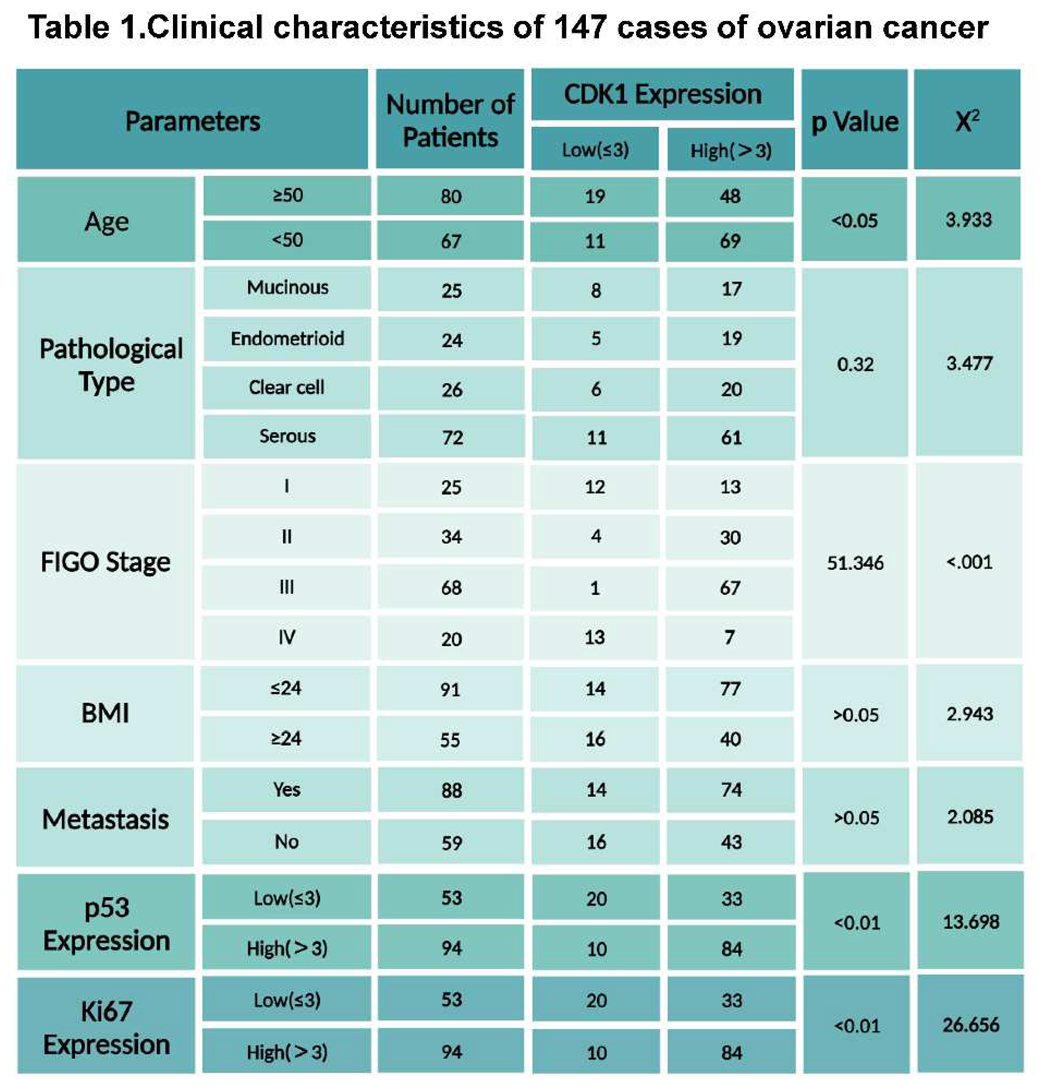
To explore the prognostic values of CDK1 expression in OC, we first examined the expression of CDK1 protein by IHC staining in 147 OC tissues. The median patient age was 51.43 years old, with the youngest patient at 30 years and the oldest patient at 76 years. Of the 147 patients in our group, 72 cases were serous type; 25, 24, and 26 cases were mucinous, endometrioid, and clear types, respectively. Detailed patient clinical information is given in Table 1. All OC samples were stained with CDK1, p53, and Ki-67 antibodies (
Figure 1A). Among the OC samples, the expression of CDK1 was significantly associated with FIGO stage, but there was no relationship between other clinical factors including lymph node metastasis and histological tumor grade. Given the roles of p53 and Ki-67 in carcinogenesis and progression in OC, we further compared the relationship between the expression of CDK1 and p53 and Ki-67. The results showed that the expression of p53 and Ki-67 was positively correlated with the expression of CDK1 in OC tissues. In addition, among 147 patients with OC, 79.59% had high expression of CDK1, and only 20.41% had low CDK1 expression. Kaplan-Meier analysis confirmed that overexpression of Ki-67 and p53 was associated with prognosis, and high expression of CDK1 was significantly associated with poorer survival compared with the low expression group (
Figure 1B, p<0.05). These results suggest that CDK1 is an independent prognostic factor of overall survival in patients with OC.
147 epithelial OC tissues were stained with antibodies to CDK1, p53, and Ki-67 by IHC (A). Kaplan-Meier survival curves show that CDK1 overexpression is significantly associated with poorer survival, compared with OC patients with low CDK1 expression (B, p<0.01).
Effect of RO-3306 on cell proliferation in OC cells.
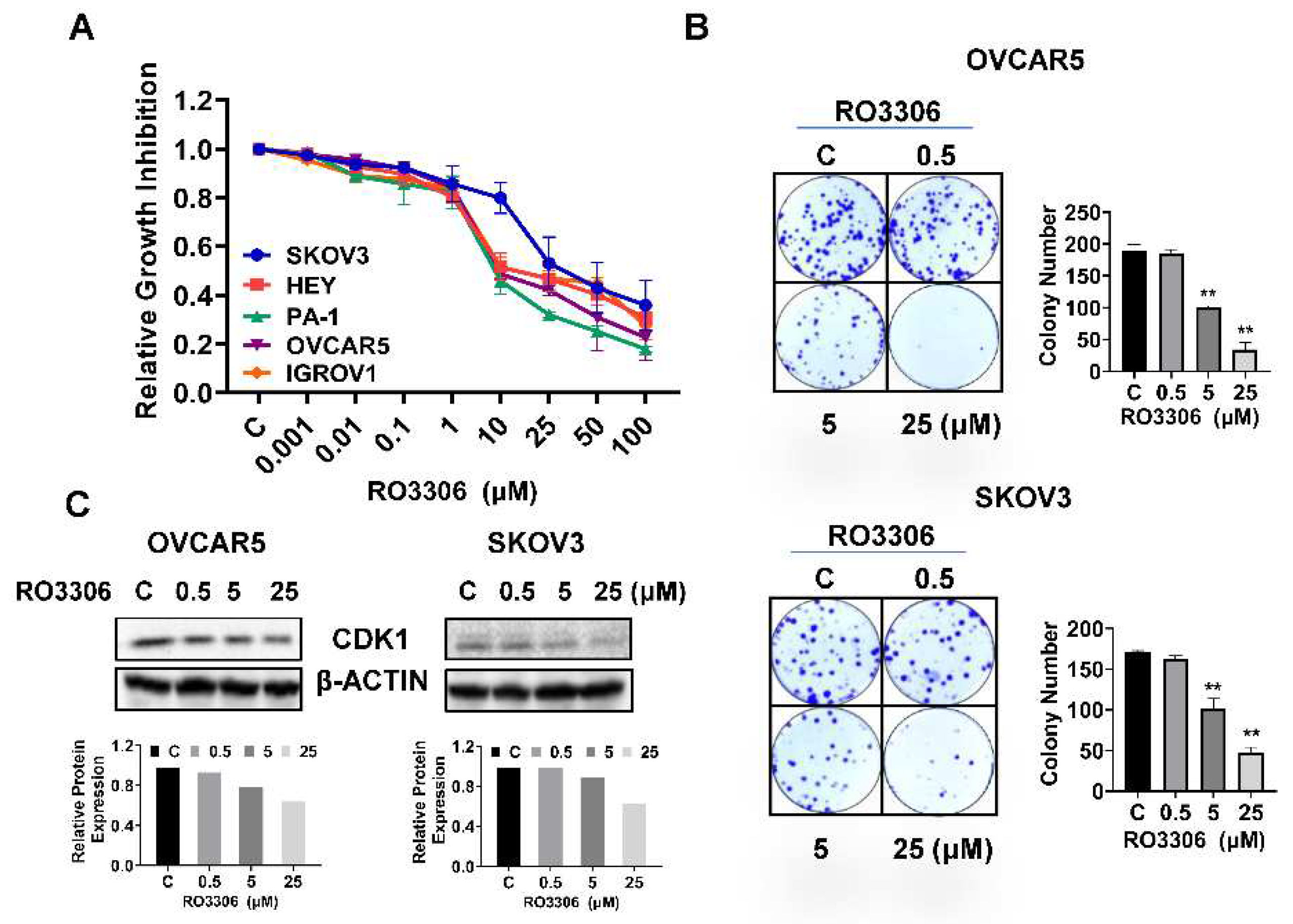
To determine the inhibitory effect of RO-3306 on cell proliferation, five OC cell lines (SKOV3, HEY, PA-1, OVCAR5, and IGROV1) were treated with different concentrations of RO-3306 for 72 hours. Cell proliferation was assessed by MTT assay. RO-3306 significantly inhibited cell proliferation in a dose-dependent manner in all of the OC cell lines. The IC50 values of SKOV3, HEY, PA-1, OVCAR5, and IGROV1 were 16.92, 10.15, 7.24, 8.74, and 13.89 µM, respectively, as shown in
Figure 2A. Next, we selected the OVCAR5 and SKOV3 cell lines to detect further effects of RO-3306 on OC cell growth. Given that colony formation assay is the gold standard for examining the effects of cytotoxic agents on cell growth in cancer cells, the SKOV3 and OVCAR5 cells were treated with RO-3306 at 0.5, 5, and 25 µM doses for 48 hours, and both cell lines were cultured for an additional 12 days. Consistent with the MTT assay, RO-3306 effectively reduced colony formation in a dose-dependent manner in both cell lines. Colony formation in the OVCAR5 and SKOV3 cells was reduced by 66.12% and 52.5%, respectively, after treatment with 25 µM RO-3306 (
Figure 2B). Because RO-3306 is a specific CDK1 inhibitor, western blotting was used to detect the expression of CDK1 in both cell lines after treatment with RO-3306 for 24 hours. As shown in
Figure 2C, different concentrations of RO-3306 significantly reduced the expression of CDK1 in a dose-dependent manner in both cell lines. These results suggest that RO-3306 inhibited cell proliferation in OC cells.
The Hey, PA-1, IGROV1, OVCAR5 and SKOV3 cells were treated with different concentrations of RO-3306 for 72 hours. MTT assay showed that RO-3306 inhibited cell proliferation in a dose-dependent manner in both cell lines (A). Colony assays showed that RO-3306 reduced colony formation in both cell lines after treating cells with RO-3306 for 48 hours and continuing to culture cells for 12 days (B). Western blotting confirmed that RO-3306 decreased the expression of CDK1 in both cell lines after 24 hours of treatment (C). *p<0.05, **p<0.01.
Effect of RO-3306 on cell cycle progression in OC cells.
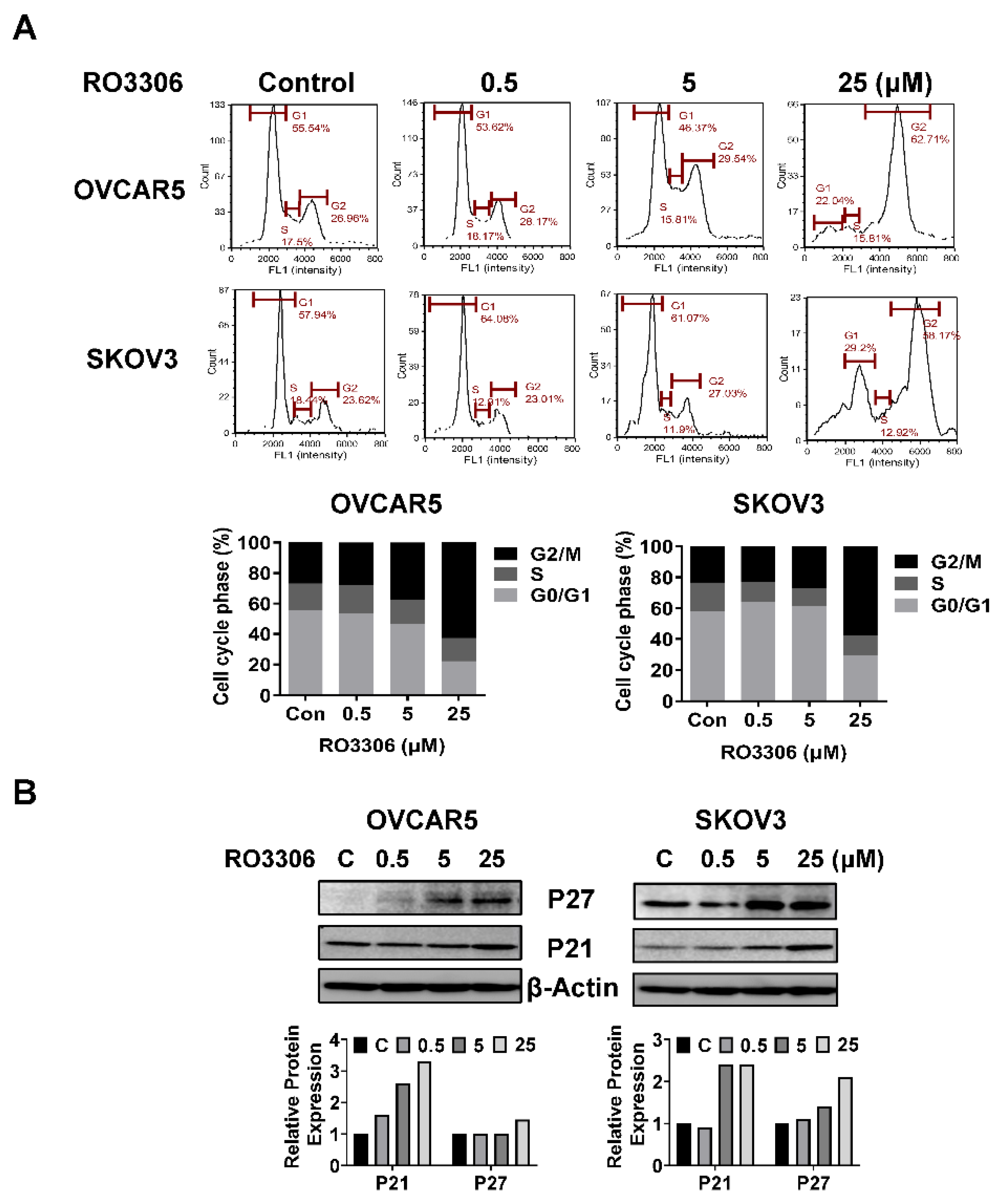
To investigate the underlying mechanism by which RO-3306 inhibited cell growth, the cell cycle profile was analyzed by Cellometer. The OVCAR5 and SKOV3 cells were exposed to different concentrations of RO-3306 for 36 hours. As illustrated in
Figure 3A, RO-3306 treatment resulted in a dose-dependent reduction in the G1 cell cycle phase and increased the G2 phase in the OVCAR5 cells. Treatment of SKOV3 cells with 5 or 25 uM RO-3306 also significantly reduced the fraction of cells in the G1 phase and increased the proportion of cells in G2 phase. The population of G1 phase of OVCAR5 cells and SKOV3 cells reduced from 55.54% to 22.04% and 57.94% to 29.20%, respectively, after 25 µm RO-3306 treatment, as compared with control groups. Furthermore, 25 µM RO-3306 significantly increased accumulation cells in the G2 phase by 35.75% and 34.55% in OVCAR5 and SKOV3 cells, respectively. Western immunoblotting results showed that RO-3306 increased the expression of p21 and p27, which are both strong inhibitors of cell cycle progression (
Figure 3B). These results confirmed that RO-3306 effectively decreased the cell cycle G1 phase in OVCAR5 and SKOV3 cells.
Effect of RO-3306 on apoptosis in OC cells.
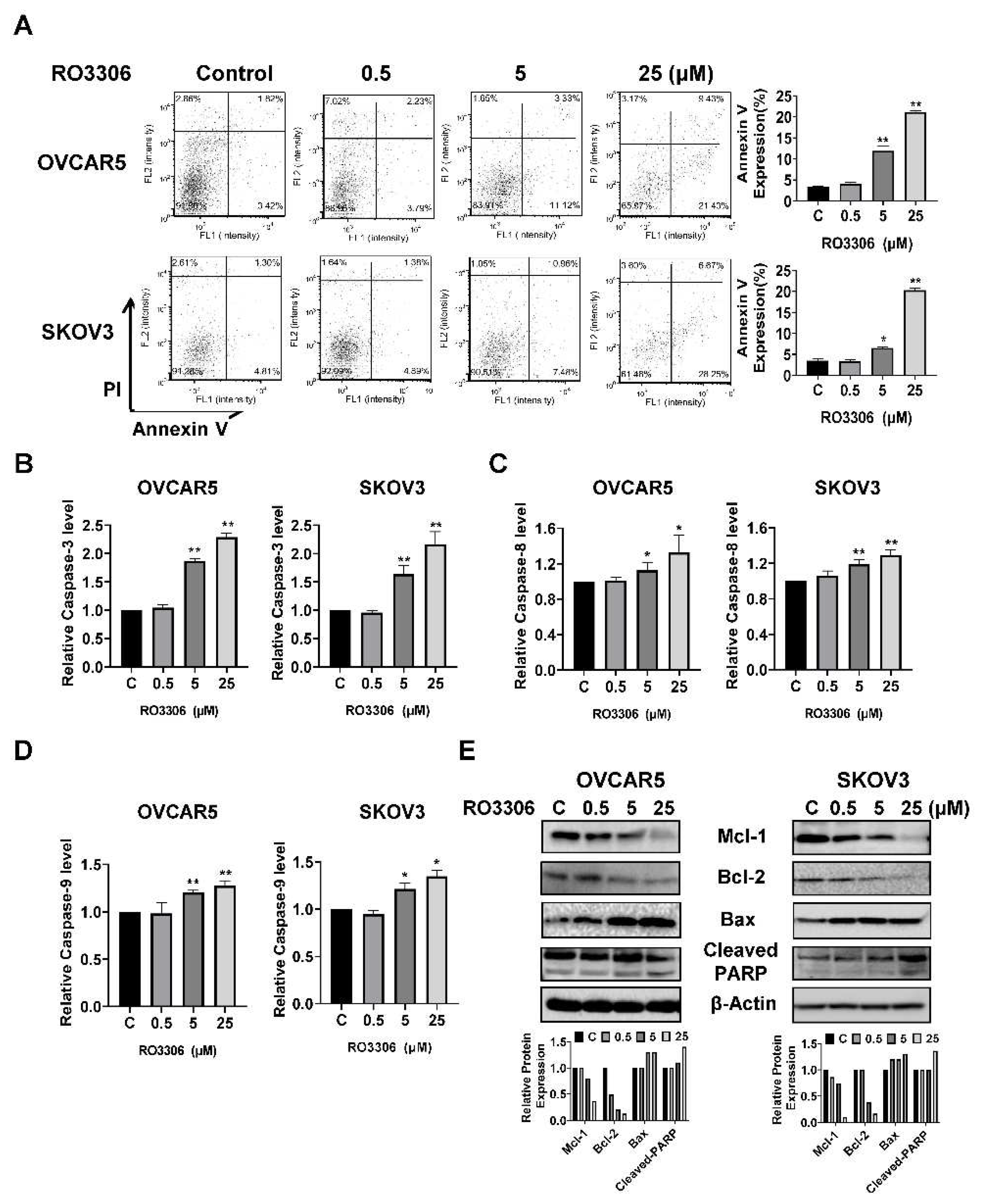
To examine whether RO-3306 induced apoptosis in OC cells, the OVCAR5 and SKOV3 cells were treated with 0.5, 5 and 25 µM RO-3306 for 16 hours, followed by Annexin V staining. RO-3306 increased the percentage of Annexin V positive apoptotic cells in a dose-dependent manner in both cell lines. As shown in
Figure 4A, treatment with 25 µM RO-3306 efficiently increased the population of apoptotic cells by 18.01% in OVCAR5 and 23.44% in SKOV3, respectively, compared with the untreated cells. To explore the effect of RO-3306 on mitochondrial apoptosis pathways, cleaved caspase 3, 8, and 9 activities were detected by ELISA assays in both cell lines. After 14 hours of treatment, RO-3306 enhanced cleaved caspase 3, 8, and 9 activities in a dose-dependent manner in both cell lines. 25 µM RO-3306 treatment increased cleaved caspase 3, 8, and 9 by 1.29, 0.33, and 0.27 times, respectively, in OVCAR5 cells and by 1.16, 0.29, and 0.35 times, respectively, in SKOV3 cells, compared with the control cells (
Figure 4B, C, and D). Western blotting analysis showed that RO-3306 reduced the expression of the anti-apoptotic proteins Mcl-1 and Bcl-2 and induced the pro-apoptotic protein Bax and cleaved PARP expression in both cell lines (
Figure 4E). These results suggest RO-3306 induces apoptosis through extrinsic and intrinsic mitochondrial apoptosis pathways in OC cells.
Treatment with different concentrations of RO-3306 reduced cell cycle G1 phase and caused cell cycle G2 arrest in the OVCAR5 and SKOV3 cell lines. Representative results are shown in A, showing the distribution of the cell cycle following 36 hours of treatment. Histograms showed the results of the cell cycle distribution after RO-3306 treatment. Western blotting analysis demonstrated changes in p21 and p27 expression for both cell lines after 24 hours of treatment. All the experiments performed were repeated at least three times. *p<0.05, **p<0.01.
The OVCAR5 and SKOV3 cells were treated with RO-3306 for 16 hours, followed by Annexin V staining. RO-3306 increased the expression of Annexin V in both cell lines (A). ELISA assays were used to detect cleaved caspase 3, 8, and 9 activities after treatment with RO-3306 for 14 hours. The results showed that RO-3306 (5 or 25 µM) enhanced cleaved caspase 3, 8, and 9 activities (B-D). Treatment of both cell lines with RO-3306 for 24 hours increased the expression of Bax and cleaved PARP and decreased MCL-1 and Bcl-2 expression (E). All the experiments were repeated at least three times. *p<0.05, **p<0.01.
Effect of RO-3306 on cellular stress in OC cells.
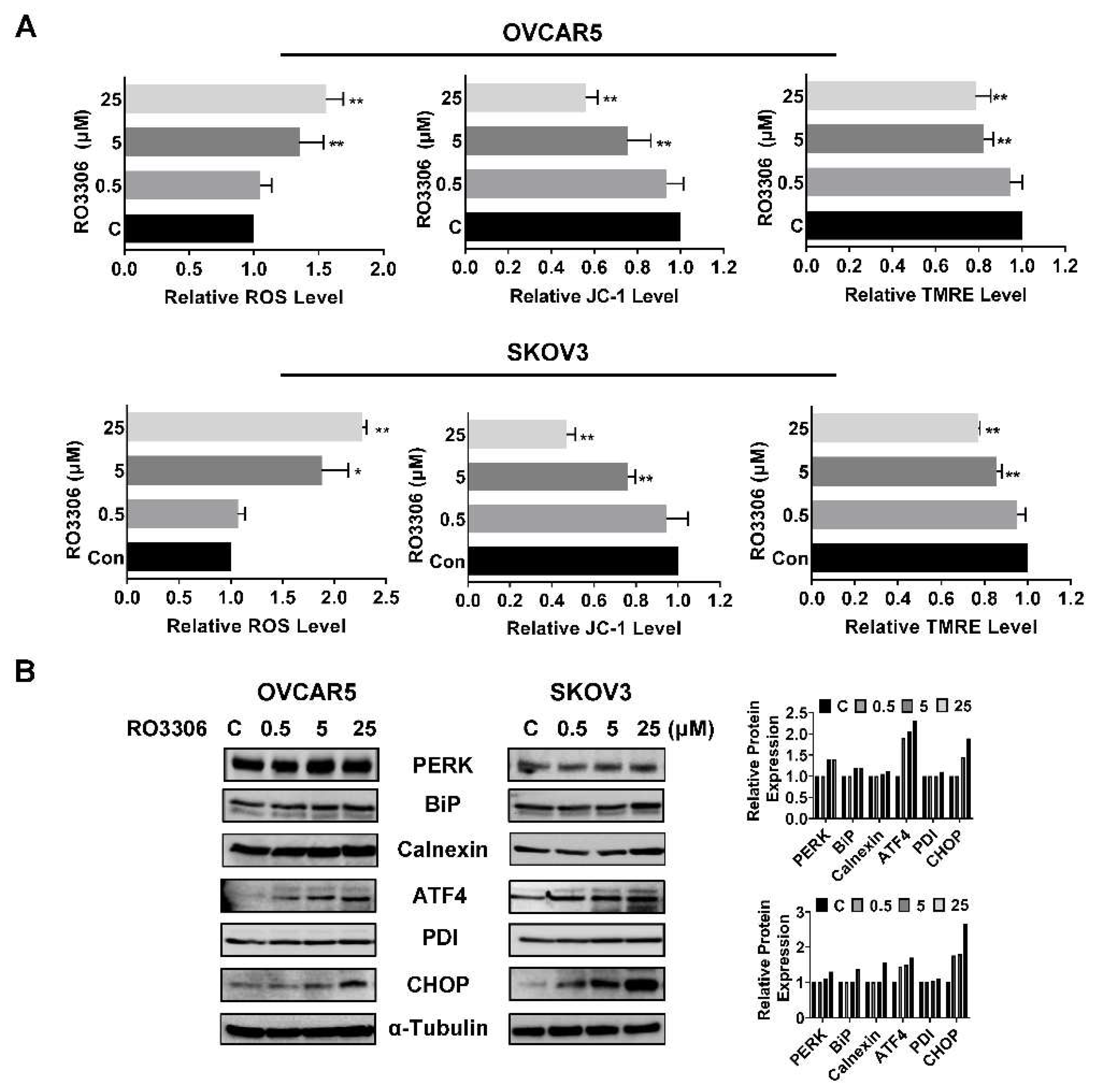
ROS have the potential to cause cellular damage and are known to be sensitive indicators of oxidative stress in cancer cells. We examined the ROS level and mitochondrial potential using the DCFH-DA, JC-1, and TMRE assays. As shown in
Figure 5A, RO-3306 irritated the production of ROS in a dose-dependent manner in both cell lines after 12 hours of treatment. Treatment with 25 µM RO-3306 increased ROS production by 55.63% and 127.61% in OVCAR5 and SKOV3, respectively, compared to the control cells. Treatment of both cell lines with different concentrations of RO-3306 also significantly decreased the level of JC-1 and TMRE in a dose-dependent manner in both cell lines, suggesting that RO-3306 may reduce mitochondrial membrane potential in OC cells. Western blotting results showed that RO-3306 induced the expression of cell stress-related proteins such as PERK, BiP, Calnexin, ATF4, PDI, and CHOP in the OVCAR5 and SKOV3 cell lines after 24 hours of treatment (
Figure 5B). These results demonstrate that cellular stress may also involve the inhibitory effect of RO-3306 on cell proliferation in OC cells.
The OVCAR5 and SKOV3 cells were treated with RO-3306 (0.5, 5 and 25 µM) for 12 hours. Intracellular ROS productions were measured by DCFH-DA assays. JC1 and TMRE assays were used to examine mitochondrial membrane potential. RO-3306 significantly increased cellular ROS production and reduced mitochondrial membrane potential in both cell lines (A). Western blotting analysis showed that treatment with RO-3306 increased the expression of cellular stress related proteins such as PERK, BiP, ATF4, CHOP, Calnexin, and PDI in both cell lines (B). *p<0.05, **p<0.01.
Effect of RO-3306 on tumor growth in the KpB mouse model of OC.
Given that RO-3306 had an anti-proliferative effect
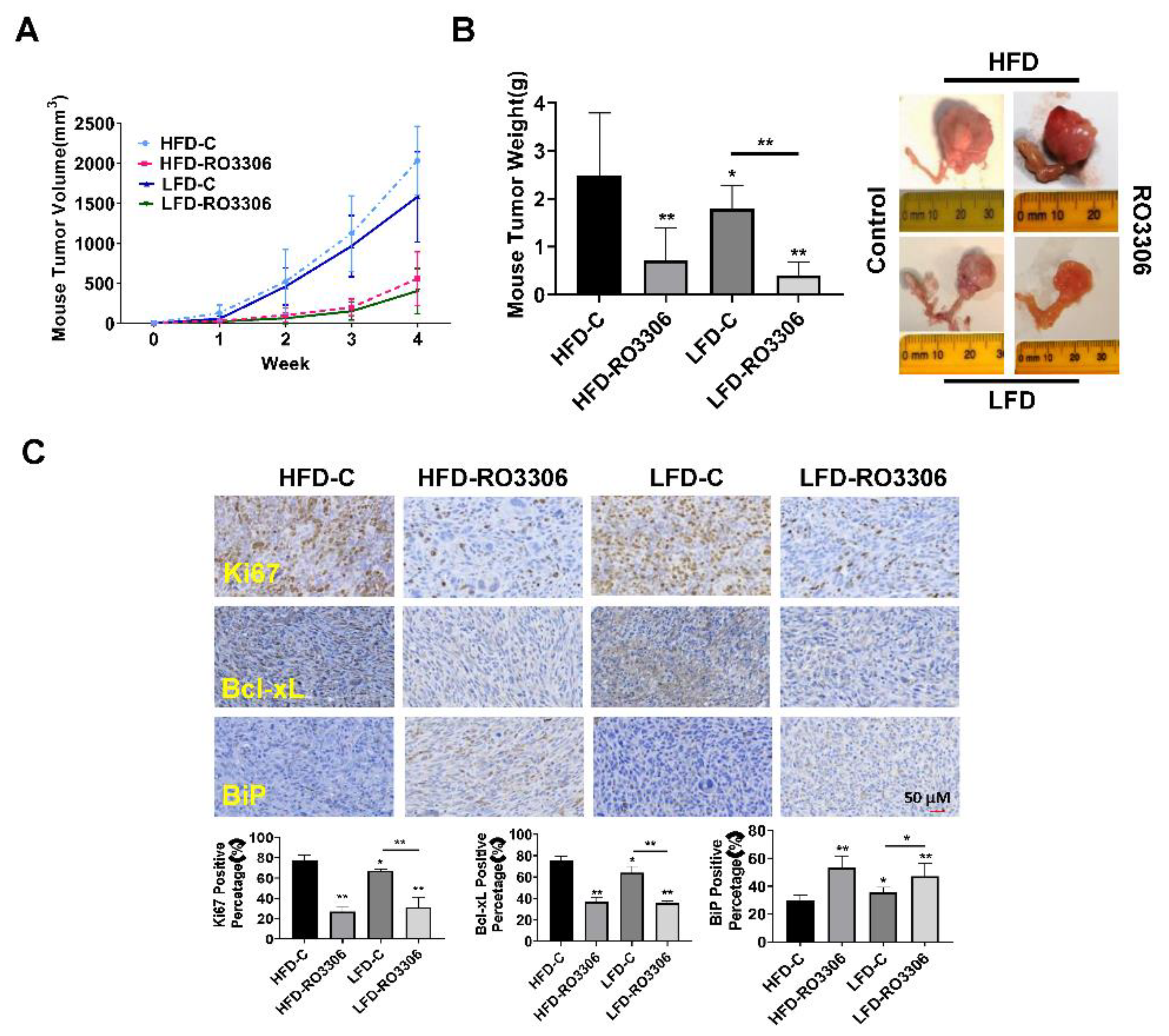
in vitro and obesity is known to increase the risk for ovarian cancer, we determined to detect the effect of RO-3306 on tumor growth in a transgenic mouse model of OC (KpB mouse model) under obese and lean conditions. The KpB mice were fed with HFD or LFD at three weeks of age and were then divided into four groups (15 mice/per group), including HFD (obese), HFD+RO-3306, LFD (lean), and LFD+RO-3306. When ovarian tumors reached approximately 0.1x0.1 cm by palpation, KpB mice were treated with RO-3306 (4 mg/kg, IP, every three days) for four weeks. The initial mean body weight in obese mice at the start of treatment with RO-3306 was 45.6 gm compared to 28.4 gm in lean mice (data not shown, p<0.01). During RO-3306 or placebo treatment period, the mice maintained normal activity and normal body weight, and no deaths were observed. Compared to the lean mice, the KpB mice fed a HFD had a significant increase in tumor volume and tumor weight, suggesting that an obese environment favors ovarian cancer growth. This is consistent with our prior works [
37,
38,
39]. The obese and lean KpB mice treated with RO-3306 had significantly reduced tumor volume and tumor weight compared to the placebo. RO-3306 reduced tumor weight by 71.25% in obese mice and by 73.76% in lean mice, respectively, compared to their controls (
Figure 6A and B). We next detected the protein expression of Ki-67, Bcl-xL, and BiP in the ovarian tumors after RO-3306 treatment by IHC under obese and lean conditions (
Figure 6C). The expression of Ki-67 was significantly reduced by 50.8% and 35.9% in the obese and lean KpB mice treated with RO-3306, respectively, compared to the control mice. RO-3306 reduced Bcl-xL expression in the treated obese mice by 31.44% and by 18.25% in lean mice and increased the expression of Bip from 29.87% to 53.25% in the tumors from the obese group and from 35.56% to 47.13% in the tumors from the lean mice group. These results suggest that RO-3306 inhibits the growth of OC tumors via cellular stress and apoptotic pathways
in vivo.
The KpB mice were fed with HFD (obese) or LFD (lean) at three weeks of age. The obese or lean KpB mice received RO-3306 (4 mg/kg, IP, every three days) for four weeks after the ovarian tumor reached approximately 0.1x0.1 cm in size. RO-3306 treatment significantly decreased tumor volume and tumor weight in obese and lean KpB mice (A and B). Immunohistochemical analysis of ovarian tumors from the RO-3306 and control groups showed downregulation of Ki-67 and Bcl-xL expression, and upregulation of BiP expression under obese and lean conditions (C). *p<0.05, **p<0.01.
Effect of RO-3306 on adhesion and invasion in vitro and in vivo.
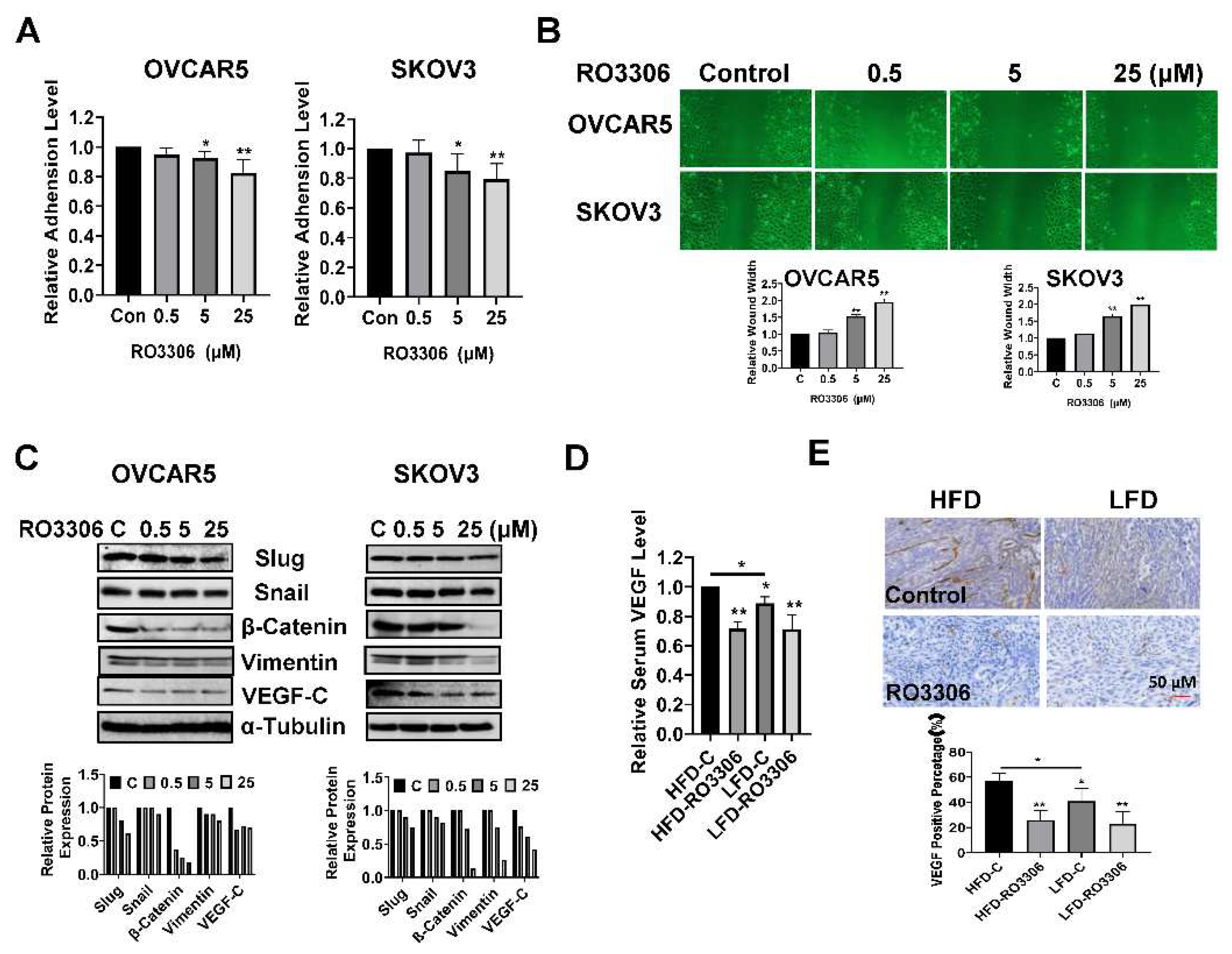
Given that increasing the activity of CDK1 has been found to enhance the ability of tumor cells to invade and migrate as shown in other cancer types, we investigated the effect of RO-3306 on adhesion and invasion in OC cell lines and tumors[
40,
41,
42]. The laminin adhesion assay was employed to analyze the effects of RO-3306 on cell adhesion, after being treated with 0.5, 5 and 25 µM this drug for 1.5 to two hours.
Figure 7A shows that the cell adhesion was decreased by 18.14% and 20.62% in OVCAR5 and SKOV3 cells, respectively, after treatment with 25 µM RO-3306. Wound healing assays were used to investigate the capacity of cell migration. Cell migration was significantly inhibited by 5 µM and 25 µM RO-3306 in both cell lines after 48 hours of treatment (
Figure 7B). Western blotting results showed that different concentrations of RO-3306 significantly reduced the expression of Slug, Snail, β-Catenin, Vimentin, and VEGF-C in both cell lines after 24 hours of treatment (
Figure 7C). In addition, serum VEGF levels were reduced by 29.51% in RO-3306-treated obese mice and 20.47% in lean mice, compared with the placebo control groups (
Figure 7D). IHC results also demonstrated that RO-3306 effectively reduced the expression of VEGF in the ovarian tumors of both obese and lean mice, in comparison to placebo-treated mice (
Figure 7E). Collectively, these results indicate that the effect of RO-3306 on cell invasion may be dependent on the EMT pathway and angiogenesis in OC
.
Treatment of OVCAR5 and SKOV3 cells with RO-3306 for 1.5 to two hours significantly reduced cell adhesion (A). Wound healing assays showed that RO-3306 inhibited cell migratory capacity after 48 hours of treatment in both cell lines (B). Western blotting results indicated that RO-3306 decreased the expression of EMT related proteins, including Slug, Snail, β-Catenin, Vimentin, and VEGF-C in the OVCAR5 and SKOV3 cells (C). Serum VEGF production was decreased by RO-3306 treatment in KpB mice (D). Immunohistochemical analysis confirmed that RO-3306 reduced the expression of VEGF in ovarian tissues in obese and lean KpB mice (E). *p<0.05, **p<0.01
3. Discussion
Previous studies have found that overexpression of CDK1 in OC tissues conferred a significantly worse prognosis in patients with epithelial OC, and knockdown of CDK1 resulted in inhibition of cell proliferation, induction of apoptosis, and distribution of cell cycle phase in OC cells [
23,
36,
43]. By analyzing the genome-wide expression profile of human OC tissues, CDK1 has been identified as one of the most important prognostic factors in OC [
16]. In this study, we also demonstrated that overexpression of CDK1 is associated with poor overall survival in patients with epithelial OC as well as positively correlates with the expression of p53 and Ki-67 in OC tissues. Targeting CDK1 by RO-3306 significantly inhibited cell proliferation, induced apoptosis and cellular stress, and reduced cell invasion in OVCAR5 and SKOV3 cell lines. Importantly, treatment with RO-3306 for four weeks resulted in significant inhibition of tumor growth in the KpB OC mouse model under obese and lean conditions, with a concomitant decrease in the expression of Ki-67, VEGF, and Bcl-xL, and an increase in the expression of BiP. Overall, these results suggest that CDK1 is an ideal target for the treatment of OC.
Emerging evidence supports the important biologic role of CDK1 in controlling the cell cycle in many cancers, and the complexes of CDK1 with cyclin A and cyclin B are sufficient to drive cancer cell cycle progression through the phosphorylation of hundreds of proteins at multiple sites [
13,
44]. Inhibition of CDK1 usually induces cell cycle arrest at the G2/M phase and results in the induction of cell apoptosis [
45]. Although the mechanism by which CDK1 inhibition induces mitochondria-initiated apoptosis is not well understood, several studies have demonstrated that the CDK1/cyclin B complex regulates apoptosis through the stabilization of the mitochondria, modifying several pro-apoptosis proteins and anti-apoptotic proteins, and mediating FOXO1 activity [
12,
46]. Prior studies have found that targeting CDK1 by RO-3306 induced expression of cleaved caspase 3, cleaved-PARP, and BAD in a dose- and time-dependent manner in the OVCA-429 and OVCAR-3 OC cell lines [
43]. We found that RO-3306 induced apoptosis was also associated with both cleaved caspase 3, 8, and 9 activation and decreased expression of Bcl-2 and Mcl-1 in a dose-dependent manner in the OVCAR5 and SKOV3 cells. Additionally, the expression of p21 and p27 was upregulated in both OC cell lines after treatment with RO-3306 for 24 hours. Given that p21 and p27 are checkpoint CDK inhibitors and have both oncosuppressive and oncopromoting properties, we propose that RO-3306 induces mitochondria-initiated apoptosis that is dependent cell cycle regulation [
47,
48,
49].
The CDK1/cyclin B complex effectively regulates mitochondrial dynamics, mitochondrial protein influx, and bioenergetics through the phosphorylation of mitochondrial and Bcl family proteins to sustain metabolic reprogramming in the face of increased energy requirements for cancer cell survival [
12,
50]. Several anti-tumor agents such as dinaciclib, apigenin, LX1570, and matrine were found to induce cellular stress through CDK1 inhibition in cancer cells [
50,
51,
52,
53]. Our results indicate inhibition of CDK1 by RO-3306 significantly increases cellular ROS production and decreases mitochondrial membrane potential, accompanied by changes in cellular stress-related proteins in ovarian cancer cells. Given that RO-3306 reduced the expression of Bcl-2 and Mcl-1, the mechanism by which RO-3306 induces cellular stress may depend on the process of phosphorylation of Bcl family proteins by CDK1 in OC cells.
OC has a unique metastatic pattern in which ovarian cancer cells disseminate directly from the primary tumor site, spread easily into the peritoneal cavity, and invade peritoneal organs, a process regulated by EMT and the integrin-mediated upregulation of matrix metalloproteinases (MMPs) [
54,
55]. Increased CDK1 activity has been linked to an increased ability for cancer cells to invade and migrate[
41,
42]. Meanwhile, knockdown of CDK1 significantly reduced the ability of invasion and migration through regulation of EMT proteins in adrenocortical carcinoma cells and head and neck squamous cell carcinomas (HNSCCs) cells [
56,
57]. Similar results were recently found in OC cells where knockdown of CDK1 reduced cell proliferation, migration, and invasion in CAOV3 cells [
58]. Our results reveal that RO-3306 significantly inhibited cell adhesion, migration, and invasion in a dose-dependent manner in the OVCAR5 and SKOV3 cells. Results of Western blotting confirm that RO-3306 downregulates the expression of EMT-related proteins in OC cells, suggesting that CDK1 is a driver that regulates adhesion and invasion by modulating the EMT process in OC cells. Additionally, we found that RO-3306 reduces the expression of VEGF in OC cells and OC tissues. Given that VEGF promotes the EMT process and CDK1 enhances VEGF mRNA expression in cancer cells [
59,
60,
61], we speculate that inhibition of VEGF by CDK1 may be another pathway to inhibit invasion of OC.
The results of the expression of CDK1 in OC patients show that the expression of CDK1 is upregulated in OC, especially in advanced-stage patients. More importantly, CDK1 upregulation shows a significant positive correlation with the expression of Ki-67 and mutant p53. High expression of Ki-67 has been associated with poor overall survival in OC patients, whereas p53 mutations are the most frequent genetic alternation in OC and a genomic hallmark in advanced/high-grade serous (HGS) OCs [
62,
63,
64,
65]. Overall, these data further support the biological role of CDK1 in tumorigenesis and progression in OC. We are continuing to assess the prognostic value of CDK1, p53, and Ki-67 expression in combination for OC outcomes as part of our ongoing work.
In conclusion, CDK1 expression and function are clearly implicated in the carcinogenesis and prognosis of OC. Targeting CDK1 by RO-3306 inhibited cell proliferation, induced cellular stress and apoptosis, reduced invasive capacity, and reduced tumor growth in OC cells and a transgenic mouse model of OC. Our results provide biological evidence for future clinical trials of CDK1 inhibitors in OC.
4. Materials and Methods
Patients and OC samples
A total of 147 patients with epithelial OC admitted to Chongqing University Cancer Hospital were registered in this study between 2017 and 2021. The protocol for this study was approved by the Ethics Committee of Chongqing University Cancer Hospital. Written informed consent was obtained from each patient. Experienced pathologists in the Department of Pathology of Chongqing University Cancer Hospital established pathological diagnoses on H&E staining slides based on World Health Organization (WHO) standards. The International Federation of Gynecology and Obstetrics (FIGO) staging system was used to determine a patient’s clinical stage. The clinical and pathological characteristics of the patients are shown in Table 1. Immunohistochemistry (IHC) staining for CDK1, p53, and Ki67 was performed at the Department of Pathology of Chongqing University Cancer Hospital.
Cell culture and reagents
Five OC cell lines, PA-1, Hey, IGROV1, OVCAR5 and SKOV3, were used in this study. OVCAR5, Hey and IGROV1 were cultured in RPMI supplemented with 5 or 10% fetal bovine serum (FBS). PA-1 cells were cultured in DMEM medium with 10% FBS, and SKOV3 cells were maintained in McCoy's 5A medium with 10% FBS. All media were supplemented with 2 mM L-glutamine, 100U/ml penicillin, and 100 ug/ml streptomycin. Cells were cultured in an incubator under 5% CO2 at 37°C. RO-3306 was purchased from Sigma (Cat# SML0569, St. Louis, MO). All antibodies were purchased from Cell Signaling Technology (Beverly, MA) and Abclonal Science (Woburn, MA). All other chemicals were purchased from Thermo Fisher Scientific (Waltham, MA), Sigma, and MedChemExpress (Monmouth Junction, NJ).
Cell proliferation assay
The OVCAR5 and SKOV3 cells were cultured overnight in 96-well plates with a concentration of 4,000 cells/well. The cells were then treated with various concentrations of RO-3306 for 72 hours. 5 ul of 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium Bromide (MTT, 5 mg/ml) were added into each well, followed by incubation for 1 to 1.5 hours. MTT absorbance was detected by a microplate reader (Tecan, Morrisville, NC) at a wavelength of 562nm after mixing 100 µL dimethyl sulfoxide (DMSO) into each well. The effect of RO-3306 on cell proliferation was calculated as a percentage of control, and the IC50 was calculated using the AAT Bioquest IC50 calculator (Pleasanton, CA). Each experiment was performed at least three times for consistency.
Colony assays
The OVCAR5 and SKOV3 cells were plated in 6-well plates at the density of 400 cells/well. After 24 hours of growth, the cells were treated with 0.5, 5, and 5 µM of RO-3306 or vehicle for 48 hours. The cells were continuously cultured for 12 days with fresh medium changes every three or four days. Cells were stabilized with methanol and stained with 0.5% crystal violet buffer for 30 minutes. The clones were imaged and quantified using Image J software (National Institutes of Health, Bethesda, MD).
Cell cycle assay
The OVCAR5 and SKOV3 cells were grown in 6-well plates overnight and then treated with different concentrations of RO-3306 for 36 hours. Cells were harvested with trypsin, and the cell pellets were resuspended in ice-cold 90% methanol for 30 minutes. The methanol was removed, and the cells were resuspended in 100 µl RNase A solution for 30 minutes followed by incubation with propidium iodide (2 mg/ml) at room temperature in dark conditions for 20 minutes. The profile of cell cycle progression was measured by Cellometer (Nexcelom, Lawrence, MA). FCS4 express software (Molecular Devices, Sunnyvale, CA) was used to analyze the distribution of the cell cycle. The experiment was repeated at least three times for consistency.
Annexin V assay
The OVCAR5 and SKOV3 cells were treated with various concentrations of RO-3306 for 16 hours in 6-well plates. Cells were harvested with 0.25% trypsin and rinsed three times in cold PBS. Cells were then suspended in 100 µl binding buffer containing Annexin V-FITC and 0.5 μl of propidium iodide for 15 minutes. The proportion of Annexin V-labeled cells was quantified by Cellometer, and the results were analyzed by FCS4 express software. The experiments were repeated three times for consistency.
Cleaved caspase 3, 8, and 9 ELISA assays
The OVCAR5 and SKOV3 cells were seeded in 6-well plates at a concentration of 2.5x105 cells/well for 24 hours and then treated with different concentrations of RO-3306 for 14 hours. After washing each well with cold PBS, 150-180 µl of 1X caspase lysis buffer was added to each well, and the concentration of protein in each well was detected by a BCA kit (Thermo Fisher). An equal amount of protein from each well and reaction buffer with caspase 3, 8, and 9 substrates was added to a new black 96-well plate and incubated at 37°C for 15 minutes. The fluorescence intensity for cleaved caspase 3 (Ex/Em=400/505), cleaved caspase 8 (Ex/Em=376/482), and cleaved caspase 9 (Ex/Em=341/441) was recorded using a Tecan microplate reader. These assays were repeated three times for consistency.
Reactive oxygen species (ROS) assay
5.0×103 cells/well of OVCAR5 and SKOV3 cells were cultured in 96-well plates overnight, followed by exposure to varying doses of RO-3306 for 12 hours. Then, 15µM DCFH-DA was added into each well, and the plate was incubated at 37°C for 30 minutes. The ROS production was determined by detecting the fluorescence intensity at Ex/Em=485/530nm using a Tecan microplate reader. All experiments were performed at least three times for consistency.
Mitochondrial membrane potential assays
Mitochondrial membrane potential was analyzed with specific fluorescent probes for JC-1 and TMRE (AAT Bioquest, Sunnyvale, CA). Both cells were seeded in 96-well plates overnight and then treated with different concentrations of RO-3306 for 14 hours. Cells were then treated with 2 µM JC-1 or 800 µM TMRE for 30 minutes at 37°C. The levels of fluorescent probes were measured by a Tecan plate reader. The wavelength of fluorescence intensity for JC-1 was Ex/Em=480/590 nm (red) and Ex/Em=535/590 nm (green) and was Ex/Em=549/575 nm for the TMRE assay. Each experiment was repeated three times for consistency.
Adhesion assay
96-well plates were pre-coated with 100 μl laminin-1 (10 ug/ml) and incubated at 37°C for one hour. The plate was rinsed two times with cold PBS, then 200 μl of blocking buffer was added into each well for 45 to 60 minutes at 37°C. 1.5x104 cells of OVCAR5 and SKOV3 and varying concentrations of RO-3306 were added into each well, followed by incubation at 37°C for 1.5 to two hours. The plate was washed with PBS to remove nonadherent cells, then 100 µl of 5% glutaraldehyde was added to fix cells for 30 minutes. 100 µl of 0.1% crystal violet was added to each well for 30 minutes. The cells were then washed repeatedly with water, and 100 µl of 10% acetic acid was added to each well to solubilize the dye. The absorbance was measured at a wavelength of 562 nm using a Tecan microplate reader. Each experiment was repeated three times for consistency.
Wound healing assay
The OVCAR5 and SKOV3 cells were plated at 4.0×105 cells/well in 6-well plates and cultured overnight. A 200 µl pipette tip was used to draw straight lines in each well, and the cells were treated with 0.5, 5 and 25 µM RO-3306 for 48 hours. Photos were taken at 24 and 48 hours after scratching, and the width of the wound was measured with Image J software (National Institutes of Health, Bethesda, MD). The experiment was repeated three times for consistency.
Western immunoblotting
The OVCAR5 and SKOV3 cells were treated with varying concentrations of RO-3306 for six to 36 hours and then the total protein was harvested with radioimmunoprecipitation assay buffer (RIPA buffer, Thermo Fisher). The protein concentration was measured by BCA assay. Equal amounts of protein were electrophoresed by 10-12% acrylamide gel and transferred onto a PVDF membrane. The membranes were incubated with primary antibody (1:1000) overnight at 4°C and then washed three times with tris-buffered saline-tween 20 (TBS-T) buffer. The appropriate secondary antibody was incubated with membranes for one hour at room temperature. Proteins were visualized using SuperSignal West Pico Substrate (Thermo Scientific) by the ChemiDoc image system (Bio Rad, Hercules, CA). Each experiment was repeated at least twice for consistency.
KpB transgenic mouse model of OC
The KpB (K18-gT
+/− 121 ;p53
fl/fl;Brca1
fl/fl) transgenic mouse model of high-grade serous ovarian cancer was used in this study as previously described [
33,
34]. The animal protocol was approved by the University of North Carolina-Chapel Hill Institutional Animal Care and Use Committee (IACUC, protocol #21-229). Sixty KpB mice were housed on a 12-hour light, 12-hour dark cycle, with free access to food and water. Given that obesity is a risk factor for the development of ovarian cancer potentially via CDK signaling, we evaluated the efficacy of RO-3306 in both obese and lean mice [
35]. To imitate diet-induced obesity (DIO), one-half of the KpB female mice were fed a high fat diet (HFD, 60% calories from fat), and the other one-half were fed a low-fat diet (LFD, 10% calories from fat, Research Diets) at three weeks of age. At six to eight weeks old, all mice were injected with 5 µl recombinant adenovirus Ad5- CMV-Cre (2.5 x10
10 P.F.U, Transfer Vector Core, University of Iowa) into the left ovarian bursa cavity. The mice kept feeding with HFD and LFD chow and were examined weekly for the appearance of ovarian tumors by abdominal palpation. Once tumors had reached an average size of 0.1x0.1 cm in diameter by palpation, the mice were assigned into four groups: HFD control, LFD control, HFD+RO-3306, and LFD+RO-3306 (N=15 mice/group). KpB mice were treated for four weeks with RO-3306 (4mg/kg, IP, every three days) when the ovarian tumor reached approximately 0.1x0.1 cm. All animals were checked daily for any signs of toxicity and weighed weekly during the treatment. Tumor size was monitored using palpation twice a week until the tumor grew to a size suitable for caliper measurements. All mice were sacrificed by CO
2 asphyxiation after four weeks of treatment. Ovarian tumors and serum were collected. One-half of the tumor tissues were frozen at -80°C, and the remaining tissues and serum were embedded in paraffin blocks. Blood serum was also stored in -80°C. Ovarian tumor volumes were calculated as follows: (width2 × length)/2.
Serum VEGF assay
VEGF productions of mice serum were detected via VEGF ELISA Kit (#MMV00, R&D Systems, Minneapolis, MN). Each sample from the HFD control, LFD control, HFD+RO-3306, and LFD+RO-3306 groups was measured in duplicate. VEGF levels were measured at 570 nm using a Tecan plate reader.
IHC for ovarian tumors of KpB mice
The ovarian tumor slides (4 μm) were incubated in protein block solution (Dako) for one hour and then primary antibodies (VEGF, Ki67, Bcl-xL, and BiP) were probed overnight at 4°C. The slides were then washed with TBS-T and incubated with secondary antibodies at room temperature for one hour. Each slide was further processed by ABC-Staining Kits (Vector Labs, Burlingame, CA) and hematoxylin. All slides were scanned by Motic (Feasterville, PA) and analyzed by ImagePro software (Vista, CA). The degree of staining was scored according to the percentage of positive tumor cells: 0 (negative and <5% cells), 1 (5-25%), 2 (25-50%), 3 (50-75%), and 4 (>75%). The immunostaining intensity of each tumor slide was evaluated as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). Scores less than or equal to 3 were considered the low expression of CDK1; scores greater than 3 were considered strong positive CDK1 overexpression when the staining score and intensity score were added together [
36].
Statistical analysis
All data were reported as mean ± SD from three independent assays. Both Student’s t-test and one-way ANOVA tests were used in this study. GraphPad Prism 8 (La Jolla, CA) statistical software was employed to calculate the comparisons. All tests were two-sided with p < 0.05 considered significant.
Author Contributions
YH, YF, ZZ, XZ, WS, HS and YY performed experiments in vitro. YH, YF, YY and WS conducted animal experiments. YH and CZ analyzed IHC results in OC patients. KT, AS, SRP, LW, XS, BD and YH participated in analyzing and interpreting the data. CZ and VB wrote the manuscript. MJE, CZ and VB designed experiments and provided financial support. All authors read and approved the final manuscript.
Funding
This research received no funding
Institutional Review Board Statement
The animal protocol was approved by the University of North Carolina-Chapel Hill Institutional Animal Care and Use Committee (IACUC, protocol #21-229)
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Acknowledgments
We thank Dr. Rui Chen for reviewing the IHC slides.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R. L.; Miller, K. D.; Wagle, N. S.; Jemal, A. , Cancer statistics, 2023. CA Cancer J Clin 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Penny, S. M. , Ovarian Cancer: An Overview. Radiol Technol 2020, 91, 561–575. [Google Scholar] [PubMed]
- Rauh-Hain, J. A.; Krivak, T. C.; Del Carmen, M. G.; Olawaiye, A. B. , Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol 2011, 4, 15–21. [Google Scholar]
- Sambasivan, S. , Epithelial ovarian cancer: Review article. Cancer Treat Res Commun 2022, 100629. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ray, C. M.; Spira, A. I.; Trepel, J. B.; Carter, C. A.; Cottrill, H. M. , A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol 2017, 34, 103. [Google Scholar] [CrossRef] [PubMed]
- Mogos, R. A.; Popovici, R.; Tanase, A. E.; Calistru, T.; Popovici, P.; Grigore, M.; Carauleanu, A. , New approaches in ovarian cancer based on genetics and carcinogenesis hypotheses (Review). Exp Ther Med 2022, 23, 423. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, A. S.; Dumitrescu, E. A.; Komporaly, I. A.; Mihăilă, R. I.; Lungulescu, C. V.; Stănculeanu, D. L. , New Targeted Therapies and Combinations of Treatments for Cervical, Endometrial, and Ovarian Cancers: A Year in Review. Curr Oncol 2022, 29, 2835–2847. [Google Scholar] [CrossRef]
- Enserink, J. M.; Chymkowitch, P. , Cell Cycle-Dependent Transcription: The Cyclin Dependent Kinase Cdk1 Is a Direct Regulator of Basal Transcription Machineries. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Chohan, T. A.; Qayyum, A.; Rehman, K.; Tariq, M.; Akash, M. S. H. , An insight into the emerging role of cyclin-dependent kinase inhibitors as potential therapeutic agents for the treatment of advanced cancers. Biomed Pharmacother 2018, 107, 1326–1341. [Google Scholar] [CrossRef]
- Vassilev, L. T.; Tovar, C.; Chen, S.; Knezevic, D.; Zhao, X.; Sun, H.; Heimbrook, D. C.; Chen, L. , Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci U S A 2006, 103, 10660–10665. [Google Scholar] [CrossRef]
- Wijnen, R.; Pecoraro, C.; Carbone, D.; Fiuji, H.; Avan, A.; Peters, G. J.; Giovannetti, E.; Diana, P. , Cyclin Dependent Kinase-1 (CDK-1) Inhibition as a Novel Therapeutic Strategy against Pancreatic Ductal Adenocarcinoma (PDAC). Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wang, S.; Jiang, N.; Li, J. J. , Cyclin B1/CDK1-regulated mitochondrial bioenergetics in cell cycle progression and tumor resistance. Cancer letters 2019, 443, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sofi, S.; Mehraj, U.; Qayoom, H.; Aisha, S.; Almilaibary, A.; Alkhanani, M.; Mir, M. A. , Targeting cyclin-dependent kinase 1 (CDK1) in cancer: molecular docking and dynamic simulations of potential CDK1 inhibitors. Medical oncology 2022, 39, 022–01748. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Guo, X.; Yuan, Q.; Nian, S.; Kang, P.; Xu, Z.; Li, L.; Ye, Y. , Systematic Pan-Cancer Analysis Identifies CDK1 as an Immunological and Prognostic Biomarker. J Oncol 2022, 2022, 8115474. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yuan, Q.; Liu, Y.; Zeng, Y.; Ke, D.; Dai, X.; Shuai, Y.; Hu, J.; Shi, H. , Identification of key molecular markers in epithelial ovarian cancer by integrated bioinformatics analysis. Taiwan J Obstet Gynecol 2021, 60, 983–994. [Google Scholar] [CrossRef]
- Dong, C.; Tian, X.; He, F.; Zhang, J.; Cui, X.; He, Q.; Si, P.; Shen, Y. , Integrative analysis of key candidate genes and signaling pathways in ovarian cancer by bioinformatics. Journal of ovarian research 2021, 14, 021–00837. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Zhu, Y.; Fang, X.; Zhu, M.; Wang, M.; Liu, S.; Jiang, X.; Zheng, J.; Zhang, X.; Chen, P. , Prognostic Biomarker Identification Through Integrating the Gene Signatures of Hepatocellular Carcinoma Properties. EBioMedicine 2017, 19, 18–30. [Google Scholar] [CrossRef]
- Yamamura, M.; Sato, Y.; Takahashi, K.; Sasaki, M.; Harada, K. , The cyclin-dependent kinase pathway involving CDK1 is a potential therapeutic target for cholangiocarcinoma. Oncol Rep 2020, 43, 306–317. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Ma, Y.; Kuang, J.; Liang, J.; Yuan, Y. , NUCKS1 Promotes Proliferation, Invasion and Migration of Non-Small Cell Lung Cancer by Upregulating CDK1 Expression. Cancer Manag Res 2020, 12, 13311–13323. [Google Scholar] [CrossRef]
- Lu, X.; Pang, Y.; Cao, H.; Liu, X.; Tu, L.; Shen, Y.; Jia, X.; Lee, J. C.; Wang, Y. , Integrated Screens Identify CDK1 as a Therapeutic Target in Advanced Gastrointestinal Stromal Tumors. Cancer Res 2021, 81, 2481–2494. [Google Scholar] [CrossRef]
- Yang, Y.; Dai, Y.; Yang, X.; Wu, S.; Wang, Y. , DNMT3A Mutation-Induced CDK1 Overexpression Promotes Leukemogenesis by Modulating the Interaction between EZH2 and DNMT3A. Biomolecules 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, W.; Fu, Z.; Xie, H.; Guo, Z.; Liu, D.; Ge, J.; Zhong, S.; Liu, L.; Liu, J.; Zhu, D. , Selected using bioinformatics and molecular docking analyses, PHA-793887 is effective against osteosarcoma. Aging (Albany NY) 2021, 13, 16425–16444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shi, H.; Ren, F.; Zhang, M.; Ji, P.; Wang, W.; Liu, C. , The aberrant upstream pathway regulations of CDK1 protein were implicated in the proliferation and apoptosis of ovarian cancer cells. Journal of ovarian research 2017, 10, 017–0356. [Google Scholar] [CrossRef]
- Ghia, P.; Scarfò, L.; Perez, S.; Pathiraja, K.; Derosier, M.; Small, K.; McCrary Sisk, C.; Patton, N. , Efficacy and safety of dinaciclib vs ofatumumab in patients with relapsed/refractory chronic lymphocytic leukemia. Blood 2017, 129, 1876–1878. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. K.; LaPlant, B.; Chng, W. J.; Zonder, J.; Callander, N.; Fonseca, R.; Fruth, B.; Roy, V.; Erlichman, C.; Stewart, A. K. , Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015, 125, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J. J.; Small, K. A.; Kirschmeier, P.; Zhang, D.; Zhu, Y.; Jou, Y. M.; Statkevich, P.; Yao, S. L.; Bannerji, R. , A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med 2013, 11, 259. [Google Scholar] [CrossRef]
- Murphy, A. G.; Zahurak, M.; Shah, M.; Weekes, C. D.; Hansen, A.; Siu, L. L.; Spreafico, A.; LoConte, N.; Anders, N. M.; Miles, T.; Rudek, M. A.; Doyle, L. A.; Nelkin, B.; Maitra, A.; Azad, N. S. , A Phase I Study of Dinaciclib in Combination With MK-2206 in Patients With Advanced Pancreatic Cancer. Clin Transl Sci 2020, 13, 1178–1188. [Google Scholar] [CrossRef]
- Mita, M. M.; Mita, A. C.; Moseley, J. L.; Poon, J.; Small, K. A.; Jou, Y. M.; Kirschmeier, P.; Zhang, D.; Zhu, Y.; Statkevich, P.; Sankhala, K. K.; Sarantopoulos, J.; Cleary, J. M.; Chirieac, L. R.; Rodig, S. J.; Bannerji, R.; Shapiro, G. I. , Phase 1 safety, pharmacokinetic and pharmacodynamic study of the cyclin-dependent kinase inhibitor dinaciclib administered every three weeks in patients with advanced malignancies. Br J Cancer 2017, 117, 1258–1268. [Google Scholar] [CrossRef]
- Aspeslagh, S.; Shailubhai, K.; Bahleda, R.; Gazzah, A.; Varga, A.; Hollebecque, A.; Massard, C.; Spreafico, A.; Reni, M.; Soria, J. C. , Phase I dose-escalation study of milciclib in combination with gemcitabine in patients with refractory solid tumors. Cancer Chemother Pharmacol 2017, 79, 1257–1265. [Google Scholar] [CrossRef]
- Ying, X.; Che, X.; Wang, J.; Zou, G.; Yu, Q.; Zhang, X. , CDK1 serves as a novel therapeutic target for endometrioid endometrial cancer. J Cancer 2021, 12, 2206–2215. [Google Scholar] [CrossRef]
- Voce, D. J.; Bernal, G. M.; Cahill, K. E.; Wu, L.; Mansour, N.; Crawley, C. D.; Campbell, P. S.; Arina, A.; Weichselbaum, R. R.; Yamini, B. , CDK1 is up-regulated by temozolomide in an NF-κB dependent manner in glioblastoma. Sci Rep 2021, 11, 5665. [Google Scholar] [CrossRef] [PubMed]
- Swadi, R. R.; Sampat, K.; Herrmann, A.; Losty, P. D.; See, V.; Moss, D. J. , CDK inhibitors reduce cell proliferation and reverse hypoxia-induced metastasis of neuroblastoma tumours in a chick embryo model. Sci Rep 2019, 9, 9136. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Zhou, C.; Zhong, Y.; Kuan, P. F.; Fan, C.; Sampey, B. P.; Difurio, M.; Bae-Jump, V. L. , Obesity increases tumor aggressiveness in a genetically engineered mouse model of serous ovarian cancer. Gynecol Oncol 2014, 133, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Szabova, L.; Yin, C.; Bupp, S.; Guerin, T. M.; Schlomer, J. J.; Householder, D. B.; Baran, M. L.; Yi, M.; Song, Y.; Sun, W.; McDunn, J. E.; Martin, P. L.; Van Dyke, T.; Difilippantonio, S. , Perturbation of Rb, p53, and Brca1 or Brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res 2012, 72, 4141–4153. [Google Scholar] [CrossRef] [PubMed]
- Benot-Dominguez, R.; Cimini, A.; Barone, D.; Giordano, A.; Pentimalli, F. , The Emerging Role of Cyclin-Dependent Kinase Inhibitors in Treating Diet-Induced Obesity: New Opportunities for Breast and Ovarian Cancers? Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Xi, Q.; Huang, M.; Wang, Y.; Zhong, J.; Liu, R.; Xu, G.; Jiang, L.; Wang, J.; Fang, Z.; Yang, S. , The expression of CDK1 is associated with proliferation and can be a prognostic factor in epithelial ovarian cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2015, 36, 4939–4948. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Fang, Z.; Pierce, S. R.; West, L.; Staley, A.; Tucker, K.; Yin, Y.; Sun, W.; Kong, W.; Prabhu, V.; Allen, J. E.; Zhou, C.; Bae-Jump, V. L. , Anti-Tumor and Anti-Invasive Effects of ONC201 on Ovarian Cancer Cells and a Transgenic Mouse Model of Serous Ovarian Cancer. Frontiers in oncology 2022, 12, 789450. [Google Scholar] [CrossRef]
- Xu, G.; Kong, W.; Fang, Z.; Fan, Y.; Yin, Y.; Sullivan, S. A.; Tran, A. Q.; Clark, L. H.; Sun, W.; Hao, T.; Zhao, L.; Zhou, C.; Bae-Jump, V. L. , Asparagus officinalis Exhibits Anti-Tumorigenic and Anti-Metastatic Effects in Ovarian Cancer. Frontiers in oncology 2021, 11, 688461. [Google Scholar] [CrossRef]
- Han, J.; Wysham, W. Z.; Zhong, Y.; Guo, H.; Zhang, L.; Malloy, K. M.; Dickens, H. K.; Huh, G.; Lee, D.; Makowski, L.; Zhou, C.; Bae-Jump, V. L. , Increased efficacy of metformin corresponds to differential metabolic effects in the ovarian tumors from obese versus lean mice. Oncotarget 2017, 8, 110965–110982. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M. J.; Xia, L.; Zhang, H. , The biological function of m6A methyltransferase KIAA1429 and its role in human disease. PeerJ 2022, 10, e14334. [Google Scholar] [CrossRef]
- Xie, R.; Li, B.; Jia, L.; Li, Y. , Identification of Core Genes and Pathways in Melanoma Metastasis via Bioinformatics Analysis. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, H.; Li, D.; Jiang, C.; Zhao, H.; Teng, Y. , Identification of novel biomarkers in breast cancer via integrated bioinformatics analysis and experimental validation. Bioengineered 2021, 12, 12431–12446. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cho, H.; Shin, H. Y.; Chung, J. Y.; Kang, E. S.; Lee, E. J.; Kim, J. H. , Accumulation of cytoplasmic Cdk1 is associated with cancer growth and survival rate in epithelial ovarian cancer. Oncotarget 2016, 7, 49481–49497. [Google Scholar] [CrossRef] [PubMed]
- Huis In 't Veld, P. J.; Wohlgemuth, S.; Koerner, C.; Müller, F.; Janning, P.; Musacchio, A. , Reconstitution and use of highly active human CDK1:Cyclin-B:CKS1 complexes. Protein Sci 2022, 31, 528–537. [Google Scholar] [CrossRef]
- Swadi, R. R.; Sampat, K.; Herrmann, A.; Losty, P. D.; See, V.; Moss, D. J. , CDK inhibitors reduce cell proliferation and reverse hypoxia-induced metastasis of neuroblastoma tumours in a chick embryo model. Sci Rep 2019, 9, 019–45571. [Google Scholar] [CrossRef]
- Kojima, K.; Shimanuki, M.; Shikami, M.; Andreeff, M.; Nakakuma, H. , Cyclin-dependent kinase 1 inhibitor RO-3306 enhances p53-mediated Bax activation and mitochondrial apoptosis in AML. Cancer science 2009, 100, 1128–1136. [Google Scholar] [CrossRef]
- Blagosklonny, M. V. , Are p27 and p21 cytoplasmic oncoproteins? Cell cycle 2002, 1, 391–393. [Google Scholar] [CrossRef]
- Mitrea, D. M.; Yoon, M. K.; Ou, L.; Kriwacki, R. W. , Disorder-function relationships for the cell cycle regulatory proteins p21 and p27. Biol Chem 2012, 393, 259–274. [Google Scholar] [CrossRef]
- Georgakilas, A. G.; Martin, O. A.; Bonner, W. M. , p21: A Two-Faced Genome Guardian. Trends Mol Med 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Chang, J. G.; Tien, N.; Chang, Y. C.; Lin, M. L.; Chen, S. S. , Oxidative Stress-Induced Unscheduled CDK1-Cyclin B1 Activity Impairs ER-Mitochondria-Mediated Bioenergetic Metabolism. Cells 2021, 10. [Google Scholar] [CrossRef]
- Nguyen, T. K.; Grant, S. , Dinaciclib (SCH727965) inhibits the unfolded protein response through a CDK1- and 5-dependent mechanism. Molecular cancer therapeutics 2014, 13, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Hu, S.; Wang, W.; Li, Y.; Zhi, W.; Lu, S.; Shi, Q.; Wang, Y.; Yang, Y. , Matrine inhibits prostate cancer via activation of the unfolded protein response/endoplasmic reticulum stress signaling and reversal of epithelial to mesenchymal transition. Molecular medicine reports 2018, 18, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, T.; Lu, Y.; Lv, Y.; Du, Y.; Wu, J.; Ma, R.; Xu, C.; Feng, J. , VLX1570 regulates the proliferation and apoptosis of human lung cancer cells through modulating ER stress and the AKT pathway. Journal of cellular and molecular medicine 2022, 26, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V. H. L.; Yue, C.; Du, K. Y.; Salem, M.; O'Brien, J.; Peng, C. , The Role of microRNAs in Epithelial Ovarian Cancer Metastasis. International journal of molecular sciences 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, D.; Shepherd, T. G. , Molecular and cellular mechanisms controlling integrin-mediated cell adhesion and tumor progression in ovarian cancer metastasis: a review. Clin Exp Metastasis 2022, 39, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yang, Y.; Li, W.; Zheng, X.; Liu, J.; Li, S.; Yang, H.; Zhang, Y.; Ge, B.; Zhang, S.; Fu, W.; Dong, D.; Du, G.; Wang, J. , CDK1 serves as a therapeutic target of adrenocortical carcinoma via regulating epithelial-mesenchymal transition, G2/M phase transition, and PANoptosis. Journal of translational medicine 2022, 20, 022–03641. [Google Scholar] [CrossRef]
- Chen, H.; Hu, K.; Xie, Y.; Qi, Y.; Li, W.; He, Y.; Fan, S.; Liu, W.; Li, C. , CDK1 Promotes Epithelial-Mesenchymal Transition and Migration of Head and Neck Squamous Carcinoma Cells by Repressing ∆Np63α-Mediated Transcriptional Regulation. International journal of molecular sciences 2022, 23. [Google Scholar] [CrossRef]
- Chen, S.; Wu, W.; Li, Q. H.; Xie, B. M.; Shen, F.; Du, Y. P.; Zong, Z. H.; Wang, L. L.; Wei, X. Q.; Zhao, Y. , Circ-NOLC1 promotes epithelial ovarian cancer tumorigenesis and progression by binding ESRP1 and modulating CDK1 and RhoA expression. Cell Death Discov 2021, 7, 020–00381. [Google Scholar] [CrossRef]
- Hubbi, M. E.; Gilkes, D. M.; Hu, H.; Kshitiz; Ahmed, I. ; Semenza, G. L., Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor 1α to promote cell-cycle progression. Proc Natl Acad Sci U S A 2014, 111, E3325-34. [Google Scholar] [CrossRef]
- Fantozzi, A.; Gruber, D. C.; Pisarsky, L.; Heck, C.; Kunita, A.; Yilmaz, M.; Meyer-Schaller, N.; Cornille, K.; Hopfer, U.; Bentires-Alj, M.; Christofori, G. , VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res 2014, 74, 1566–1575. [Google Scholar] [CrossRef]
- Chen, L.; Lin, G.; Chen, K.; Liang, R.; Wan, F.; Zhang, C.; Tian, G.; Zhu, X. , VEGF promotes migration and invasion by regulating EMT and MMPs in nasopharyngeal carcinoma. J Cancer 2020, 11, 7291–7301. [Google Scholar] [CrossRef]
- Garziera, M.; Montico, M.; Bidoli, E.; Scalone, S.; Sorio, R.; Giorda, G.; Lucia, E.; Toffoli, G. , Prognostic Role of Serum Antibody Immunity to p53 Oncogenic Protein in Ovarian Cancer: A Systematic Review and a Meta-Analysis. PLoS One 2015, 10, e0140351. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A. A.; Etemadmoghadam, D.; Temple, J.; Lynch, A. G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; Bowtell, D.; Brenton, J. D. , Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol 2010, 221, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, K.; No, J. H.; Jeon, Y. T.; Jeon, H. W.; Kim, Y. B. , Prognostic value of biomarkers related to drug resistance in patients with advanced epithelial ovarian cancer. Anticancer Res 2012, 32, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, M. T.; Kumar, S.; Dasari, P. , The expression of immunohistochemical markers estrogen receptor, progesterone receptor, Her-2-neu, p53 and Ki-67 in epithelial ovarian tumors and its correlation with clinicopathologic variables. Indian J Pathol Microbiol 2012, 55, 33–37. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).