1. Introduction
Cadmium (Cd) is a toxic metal pollutant that accumulates in the proximal tubule of kidneys, where it causes tubular cell injury, resulting in cell death, tubular and nephron atrophy, and eventually a reduction in the estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m
2 [
1,
2,
3,
4]. Because diet is an inevitable Cd exposure source, Cd has become one of the environmental toxicants of increasing public health concern worldwide. The Japan total diet study undertaken from 2013 to 2018 reported that rice and its products, green vegetables, cereals, and seeds plus potatoes constituted 38%, 17%, and 11% of total dietary exposure, respectively [
5].
To safeguard against excessive exposure to Cd in the human diet, exposure guidelines, referred to as a tolerable intake level of Cd, was derived by the Joint FAO/WHO Expert Committee on Food Additives and Contaminants (JECFA) [
6]. Of note, the “tolerable” intake level derived was based on the risk assessment model that assumed tubular proteinuria, reflected by an increase in the excretion of the low-molecular-weight protein β2-microglubulin (β
2M, E
β2M) above 300 μg/g creatinine to be an early warning sign of the nephrotoxicity of Cd. Consequently, tubulopathy is the most frequently reported as an adverse effect of Cd exposure. Numerous studies, however, have cast considerable doubt on the utility of E
β2M for such purposes.
This study aimed to examine if exposure to Cd adversely impacts kidney toxicity differently in men and women. To this end, tubular dysfunction and changes in the eGFR were quantified in residents of Cd-polluted and non-polluted regions of Thailand and analyzed in relation to Cd exposure levels. The confounding impact of smoking, diabetes and hypertension were also evaluated. Exposure to Cd was assessed by measurement of blood Cd concentration ([Cd]
b), and urinary Cd excretion (E
Cd). Tubular dysfunction was assessed by E
β2M. Equations developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) were used to compute the estimated GFR (eGFR) [
7].
2. Materials and Methods
2.1. Participants
To obtain a group with a wide range of environmental Cd exposure amenable to dose-effect relationship assessment, we assembled data from 334 women and 114 men who participated in the cross-sectional studies conducted in a high-exposure area of the Mae Sot District, Tak Province [
8] and a low-exposure locality in Pakpoon Municipality of Nakhon-Si-Thammarat Province [
9]. Based on the data from a nationwide survey of Cd levels in soils and food crops [
10], environmental exposure to Cd in Nakhon Si Thammarat were low.
The study protocol for the Mae Sot group was approved by the Institutional Ethical Committees of Chiang Mai University and the Mae Sot Hospital [
8]. The study protocol for the Nakhon Si Thammarat group was approved by the Office of the Human Research Ethics Committee of Walailak University in Thailand [
9].
All participants gave informed consent prior to participation. They had lived at their current addresses for at least 30 years. Exclusion criteria were pregnancy, breast-feeding, a history of metal work, and a hospital record or physician’s diagnosis of an advanced chronic disease. Diabetes was defined as fasting plasma glucose levels ≥126 mg/dL (
https://www.cdc.gov/diabetes/basics/getting-tested.html (accessed on 25 June 2023) or a physician’s prescription of anti-diabetic medications. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg [
11], a physician’s diagnosis, or prescription of anti-hypertensive medications.
2.2. Collection and Analysis of Blood and Urine Samples
Second morning urine samples were collected after overnight fast, and whole blood samples were obtained within 3 h after the urine sampling. Aliquots of urine, whole blood, and plasma were stored at −20 or −80 °C for later analysis. The assay for urine and plasma concentrations of creatinine ([cr]u and [cr]p) was based on the Jaffe reaction. The assay of urinary β2M concentration ([β2M]u) was based on the latex immunoagglutination method (LX test, Eiken 2MGII; Eiken and Shionogi Co., Tokyo, Japan) or the ELISA method (Sino Biological Inc., Wayne, PA, USA).
Urinary Cd concentration ([Cd]
u) was determined using atomic absorption spectrophotometer. Urine standard reference material No. 2670 (National Institute of Standards, Washington, DC, USA) or the reference urine metal control levels 1, 2, and 3 (Lyphocheck, Bio-Rad, Hercules, CA, USA) were used for quality control, analytical accuracy, and precision assurance. was used for quality assurance and control purposes. The limit of detection (LOD) of Cd quantitation was defined as 3 times the standard deviation of blank measurements. When [Cd]
u was below its detection limit (0.1 µg/L), the Cd concentration assigned was the LOD divided by the square root of 2 [
12]
2.3. Estimated Glomerular Filtration Rates (eGFRs)
The GFR is the product of nephron number and mean single nephron GFR, and in theory, the GFR is indicative of nephron function [
13,
14,
15]. In practice, the GFR is estimated from established chronic kidney disease–epidemiology collaboration (CKD-EPI) equations and is reported as eGFR [
15].
Male eGFR = 141 × [plasma creatinine/0.9]Y × 0.993age, where Y = −0.411 if [cr]p ≤ 0.9 mg/dL, and Y = −1.209 if [cr]p > 0.9 mg/dL. Female eGFR = 144 × [plasma creatinine/0.7]Y × 0.993age, where Y = −0.329 if [cr]p ≤ 0.7 mg/dL, and Y = −1.209 if [cr]p > 0.7 mg/dL. CKD stages 1, 2, 3a, 3b, 4, and 5 corresponded to eGFR of 90–119, 60–89, 45–59, 30−44, 15–29, and <15 mL/min/1.73 m2, respectively.
2.4. Normalization of Excretion Rate
Ex was normalized to Ecr as [x]u/[cr]u, where x = Cd or β2M; [x]u = urine concentration of x (mass/volume); and [cr]u = urine creatinine concentration (mg/dL). The ratio [x]u/[cr]u was expressed in μg/g of creatinine.
E
x was normalized to C
cr as E
x/C
cr = [x]
u[cr]
p/[cr]
u, where x = Cd or β
2M; [x]
u = urine concentration of x (mass/volume); [cr]
p = plasma creatinine concentration (mg/dL); and [cr]
u = urine creatinine concentration (mg/dL). E
x/C
cr was expressed as the excretion of x per volume of filtrate [
7].
2.5. Statistical Analysis
Data were analyzed with IBM SPSS Statistics 21 (IBM Inc., New York, NY, USA). The Mann–Whitney U test was used to assess differences in mean values in women and men, and the Pearson chi-squared test was used to assess differences in percentages. The one-sample Kolmogorov–Smirnov test was used to identify departures of continuous variables from a normal distribution, and logarithmic transformation was applied to variables that showed rightward skewing before they were subjected to parametric statistical analysis.
The multivariable logistic regression analysis was used to determine the Prevalence Odds Ratio (POR) for categorical outcomes. Reduced eGFR was assigned when eGFR ≤ 60 mL/min/1.73 m
2. For C
cr-normalized data, tubular dysfunction was defined as (E
β2M/C
cr) × 100 ≥ 300 µg/L of filtrate. For E
cr-normalized data, tubular dysfunction was defined as E
β2M/E
cr ≥ 300 µg/g creatinine [
6]. Univariate analysis of covariance with Bonferroni correction in multiple comparisons was used to obtain covariate-adjusted mean E
Cd/C
cr and mean E
β2M/C
cr. For all tests, p-values ≤ 0.05 were considered to indicate statistical significance.
3. Results
3.1. Descriptive Characteristics of Participants
This cohort consisted of 334 women (mean age 51.5 years) and 114 men (mean age 49.9 years) (
Table 1).
Notes:
n, number of subjects; BMI, body mass index; eGFR, estimated glomerular filtration rate; β
2M, β
2-microglobulin; E
x, excretion of x; cr, creatinine; C
cr, creatinine clearance; Cd, cadmium;
a eGFR, was determined by established CKD-EPI equations [
15];
b reduced eGFR corresponds to eGFR ≤60 mL/min/1.73m
2;
c E
x/E
cr = [x]
u/[cr]
u;
d E
x/C
cr = [x]
u[cr]
p/[cr]
u, where x = Cd or β
2M [
7]. Data for all continuous variables are arithmetic means ± standard deviation (SD). For all tests,
p ≤ 0.05 identifies statistical significance, determined by Pearson chi-square test for % differences and by the Mann–Whitney U-test for mean differences between women and men. #
p = 0.005; *
p = 0.004; **
p = 0.002.
The respective overall percentages of smoking, hypertension, diabetes and reduced eGFR were 31.3%, 48.7% 15.4% and 6.9%. More than half of men (68.4%) smoked cigarettes, while only 18.6% of women did. The % all other ill health conditions in men and women did not differ nor did their mean age.
With the exception for BMI, mean plasma creatinine, mean urine creatinine, and mean blood Cd all were lower in women than men. Mean eGFR, mean urine Cd and mean β2M concentrations in women and men were not statistically different.
For Ccr-normalized data, the mean ECd/Ccr and mean Eβ2M/Ccr in women and men both did not differ statistically. However, there were statistically significant differences in the % of women and men across three Eβ2M/Ccr groups.
For Ecr-normalized data, the mean ECd/Ecr and mean Eβ2M/Ecr were higher in women than men. The % of men across three Eβ2M/Ecr groups differed, but there was no difference in the % of women across Eβ2M/Ecr groups.
3.2. Cadmium Exposure Characterization
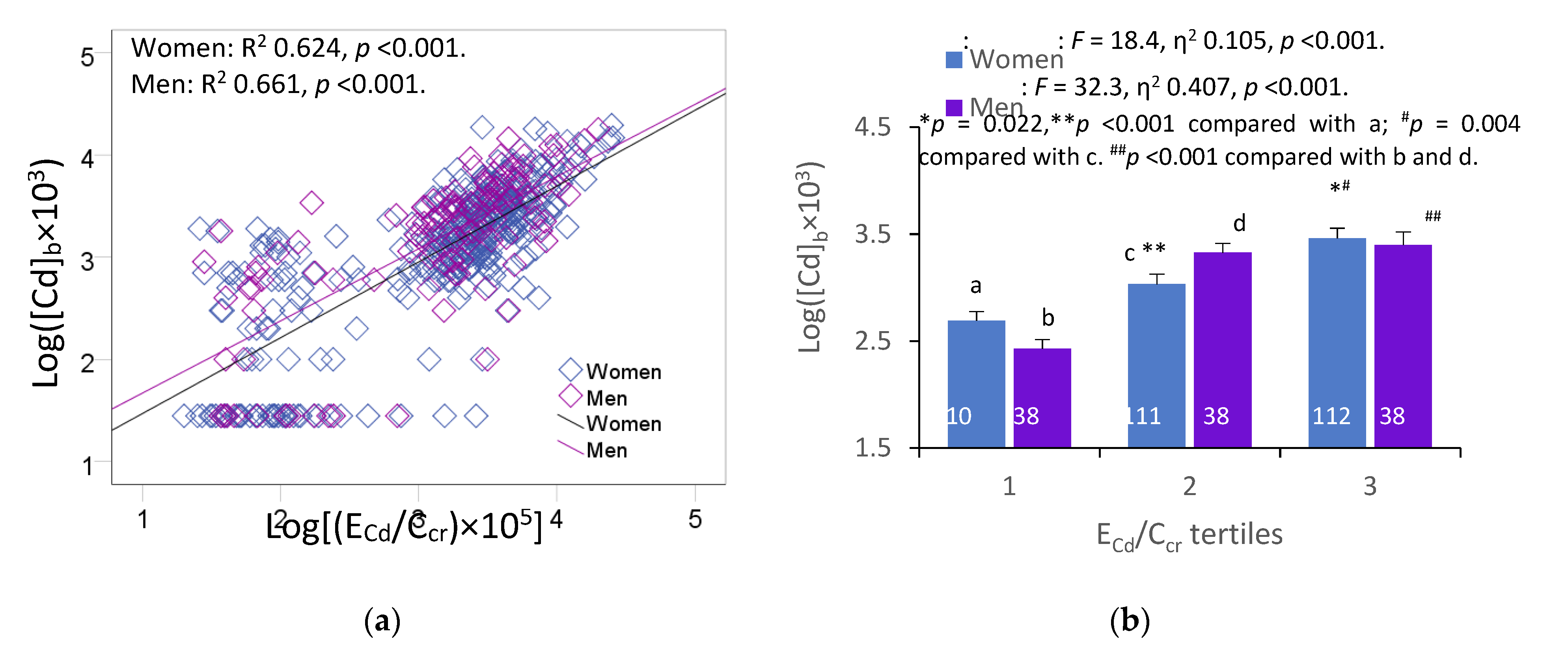
Figure 1 provides scatterplots relating two Cd exposure indicators, namely blood Cd concentration and excretion rate of Cd as E
Cd/C
cr.
A strong positive association between log([Cd]
b×10
3) and [log[(E
Cd/C
cr)×10
5] was evident in both women and men (
Figure 1a). After adjustment for covariates and interactions, Cd body burden ([log[(E
Cd/C
cr) ×10
5] explained a larger proportion of the variation in blood Cd concentrations (log([Cd]
b×10
3) in men (η
2 = 0.407) than in women (η
2 = 0.105) (
Figure 1b).
To further address the variables/factors that may influence blood Cd levels, we conducted multiple regression and univariate analyses of variance that incorporated age, BMI, log[(E
Cd/C
cr) ×10
5], smoking, diabetes and hypertension as independent variables.
Table 2 provides results of these analyses.
β, standardized regression coefficient; adjusted R2, coefficient of determination; DM, diabetes; HTN, hypertension; SMK, smoking; n/a, not applicable. β indicates strength of association of log([Cd]b×103) with independent variables (first column). Adjusted R2 indicates a fractional variation of log([Cd]b×103) explained by all independent variables. Eta square (η2) indicates the fraction of the variability of each dependent variable explained by a corresponding independent variable. p-values ≤ 0.05 indicate a statistically significant contribution of variation of an independent variable to variation of a dependent variable.
In women, higher [Cd]b values were associated strongly with higher ECd/Ccr (β = 0.619), and moderately associated with smoking (β = 0.123), and younger age (β = −0.170). ECd/Ccr, age, and smoking explained respectively 36.7%, 5.7% and 2.8% of the variation of [Cd]b in women, while the interaction between diabetes and hypertension contributed to 1.6% of [Cd]b variability. In men, higher [Cd]b values were associated strongly with higher ECd/Ccr (β = 0.581), moderately with smoking (β = 0.184) and not having diabetes (β = −0.246). ECd/Ccr, smoking and diabetes accounted, respectively for 42%%, 5.5% and 9.5% of the variation of [Cd]b in men, while the interaction between smoking, diabetes and hypertension contributed to 4.2 % of [Cd]b variability.
3.3. Effects of Cadmium Exposure on β2M excretion
We assessed the effects of Cd exposure on Eβ
2M with multiple linear regression and univariate/covariance analysis, where the indicators of Cd exposure ([Cd]
b and E
Cd) were incorporated as the independent variables together with age, BMI, smoking, diabetes, and hypertension (
Table 3).
β, standardized regression coefficient; adjusted R2, coefficient of determination; β indicates strength of association of log[(Eβ2M/Ccr) ×103] with independent variables (first column). Adjusted R2 indicates a fractional variation of log[(Eβ2M/Ccr)×103] explained by all independent variables. For each test, p-values ≤ 0.05 indicate a statistically significant contribution of an independent variable to log[(Eβ2M/Ccr)×103] variability.
In all subjects, Eβ2M/Ccr was associated age (β = 0.137), ECd/Ccr (β = 0.283) and diabetes (β = 0.323). In women, the associations of Eβ2M/Ccr with these three independent variables were evident. In men, Eβ2M/Ccr showed a significant association only with diabetes (β = 0.279).
We next examined the association between the E
Cd/C
cr and E
β2M/C
cr with scatterplots and the covariate-adjusted mean E
β2M in subjects grouped by E
Cd/C
cr tertiles (
Figure 2).
The relationship between E
β2M/C
cr and E
Cd/C
cr was weak and statistically insignificant in all subjects (
Figure 1a), as were in women and men (
Figure 2c). However, with adjustment for covariates that included age and BMI, diabetes, hypertension, and smoking (
Figure 2b), a significant contribution of Cd body burden to the variability of E
β2M became was evident when all subjects were included in an analysis (
F = 4.473, η
2 0.012,
p = 0.021). The E
β2M in subjects of the high E
Cd/C
cr tertile was higher, compared with those of middle and low E
Cd/C
cr tertiles (
Figure 1b). In subgroup analysis (
Figure 1d), a dose-effect relationship of E
Cd and E
β2M was seen in women only (
F = 3.431, η
2 0.021,
p = 0.034).
3.4. Effects of Cadmium Exposure on the Prevalenec Odds of Tubulopathy
Table 4 provides the results of the logistic regression analysis of abnormal E
β2M that incorporated age, BMI, log[(E
Cd/C
cr)×10
5], gender, smoking, diabetes, and hypertension as independent variables.
POR, prevalence odds ratio; CI, confidence interval. The units of (Eβ2M/Ccr) × 100 and log[(ECd/Ccr)×105] are µg/L filtrate; Data were generated from logistic regression analyses relating POR for excessive β2M excretion to seven independent variables (first column). For all tests, p-values ≤ 0.05 indicate a statistically significant association of POR with a given independent variable.
Among seven independent variables, the prevalence odds ratios (POR) for (Eβ2M/Ccr) ×100 ≥ 300–999, and ≥ 1000 µg/L filtrate were increased with age, log[(ECd/Ccr)×105], and diabetes. All other 4 independent variables did not show a significant association with abnormal β2M excretion. For every 10-fold rise of ECd/Ccr the POR for (Eβ2M/Ccr) ×100 of ≥ 300 and ≥ 1000 µg/L were increased by 1.94-fold and 3.34-fold, respectively.
3.5. Effects of Cadmium Exposure on eGFR
Similarly, we assessed the effects of Cd exposure on the estimated glomerular filtration rate (eGFR) by multiple linear regression and logistic regression analyses, where [Cd]
b and E
Cd were incorporated as the independent variables together with age, BMI, smoking, diabetes, and hypertension (
Table 5).
eGFR, estimated glomerular filtration rate; β, standardized regression coefficient; adjusted R2, coefficient of determination; β indicates strength of association of eGFR with independent variables (first column). Adjusted R2 indicates a fractional variation of eGFR explained by all independent variables. For each test, p-values ≤ 0.05 indicate a statistically significant contribution of an independent variable to eGFR variability.
In all subjects, eGFR was inversely associated with age (β = −0.517), ECd (β = −0.148) and diabetes (β = −0.109). In subgroup analysis, inverse associations of eGFR with these three independent variables (age, ECd and diabetes) were seen only in the women group. In men, eGFR was not associated with ECd, but this parameter showed inverse associations with age (β = −0.506) and hypertension (β = −0.212).
In logistic regression of a reduced eGFR (eGFR ≤60 mL/min/1.73m
2), age, BMI, log[(E
Cd/C
cr) ×10
5], gender, smoking, diabetes, and hypertension were incorporated as independent variables (
Table 6).
The POR values for a reduced eGFR were increased with age, log[(ECd/Ccr)×105], and diabetes. For every 10-fold rise of ECd/Ccr, the POR for a reduced eGFR was increased by 3.2-fold. There was a 4.2-fold increase in the POR for a reduced eGFR among the diabetic.
3.6. Inverse Relationship of β2M Excretion and eGFR
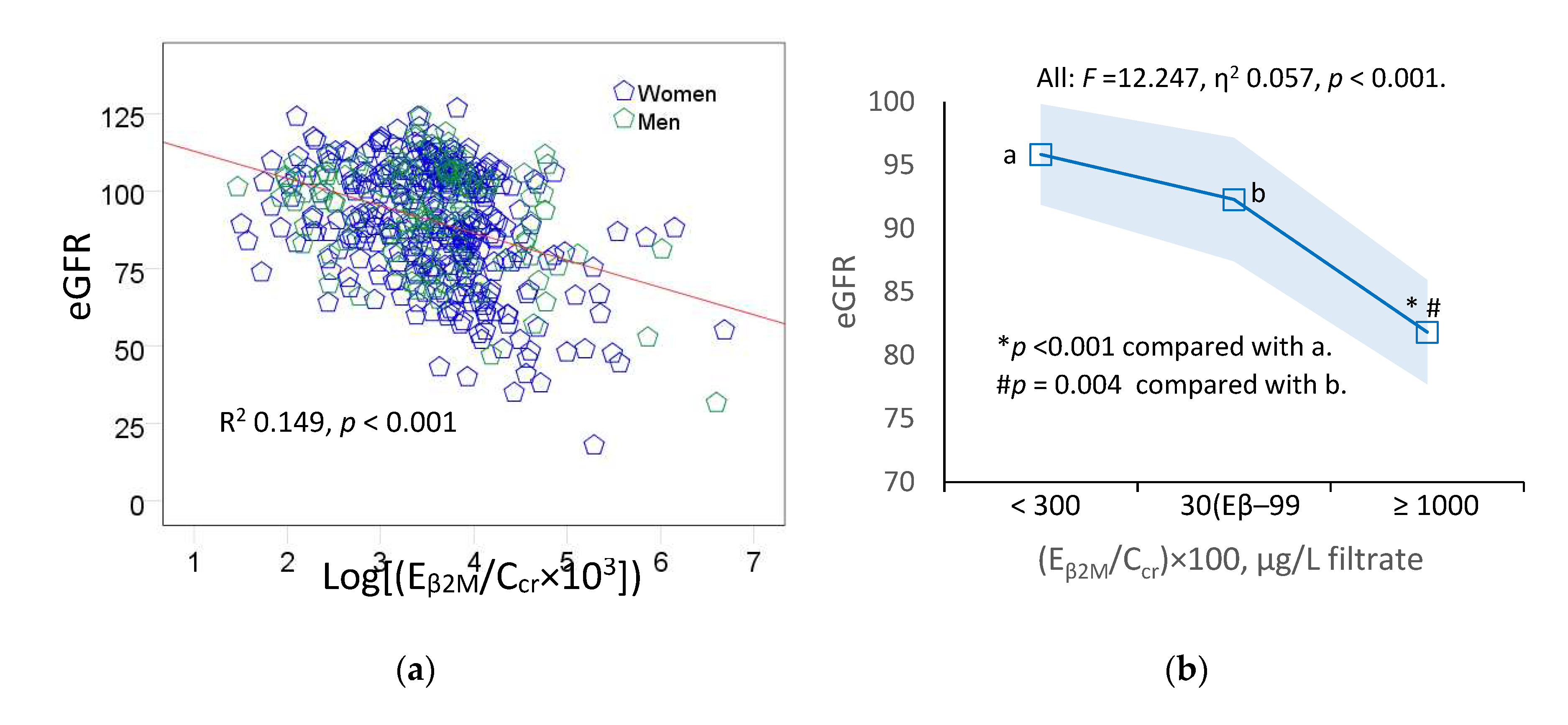
Figure 3 provides scatterplots relating eGFR to E
β2M among study subjects together with covariates-adjusted means of eGFR in women and men.
A statistically significant inverse relationship between eGFR and E
β2M was seen in all subjects (
Figure 3a), as well as in women and men (
Figure 3c). In all subjects (
Figure 3b), eGFR explained 5.7% of the variation in E
β2M/C
cr (
F =12.247,
p < 0.001).
For simplicity, the degree of tubulopathy, assessed with Eβ2M, was graded into three levels where levels 1, 2 and 3 of tubulopathy corresponded to (Eβ2M/Ccr) ×100 <300, 300–999 and ≥ 1000 µg/L filtrate, respectively.
The covariates-adjusted mean eGFR was 14.0 and 10.5 mL/min/1.73m
2 lower in subjects with level 3 tubulopathy, compared to those with tubular dysfunction levels 2 and 1, respectively (
Figure 3b).
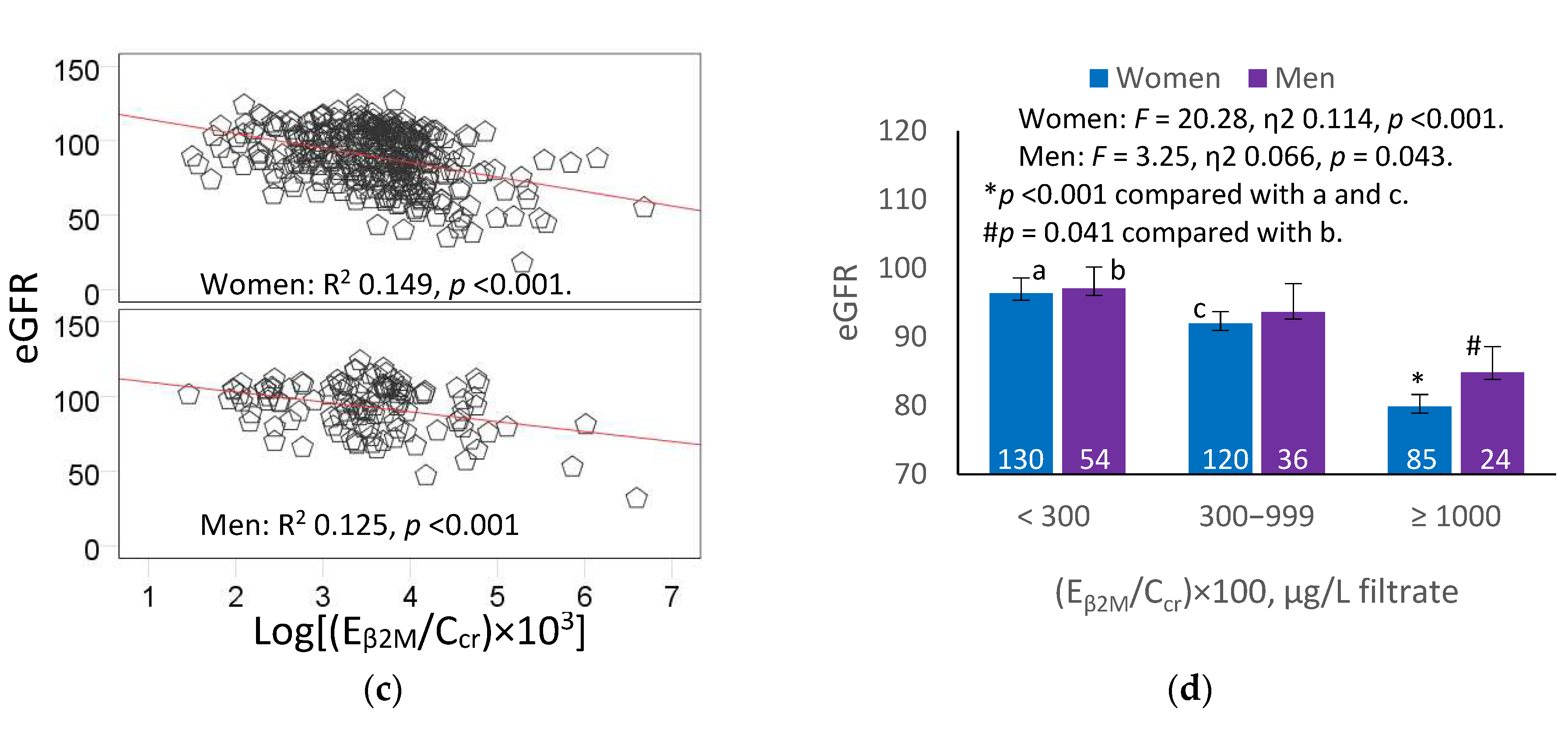
In subgroup analysis (
Figure 3d), the η
2 values indicated a nearly twice larger effect size of eGFR on E
β2M in women (η
2 = 0.114), compared to men (η
2 = 0.066). In women, those with the tubulopathy of level 3 had the covariates-adjusted mean eGFR 16.5 and 12.0 mL/min/1.73 m
2 lower, compared to those with tubulopathy levels 1 and 2, respectively. In men, those with tubulopathy of level 3, had the covariates-adjusted mean eGFR 12.3 mL/min/1.73 m
2 lower, compared to those of tubulopathy level 1. The adjusted mean eGFR values in men of tubulopathy levels 3 and 2 did not differ statistically.
In another logistic regression, a relative contribution of Cd exposure and tubular dysfunction levels to the prevalence of a reduced eGFR was determined. E
Cd was entered as a continuous variable, while E
β2M was categorized into levels 1, 2 and 3, as previously stated.
Table 7 provides results of such analysis.
The POR values for a reduced eGFR rose with age (POR = 1.14), ECd/Ccr (POR = 2.25), tubulopathy level 2 (POR = 8.31), and tubulopathy level 3 (POR = 33.7). All other independent variables, such as diabetes and hypertension, did not show a significant association with the POR for reduced eGFR.
An equivalent logistic regression was conducted using E
cr-normalized E
Cd and E
β2M data (
Table 8).
The POR values for a reduced eGFR increased with age (POR = 1.10), and the levels of tubular dysfunction; Eβ2M/Ecr 300–999 µg/g creatinine (POR = 3.20) and Eβ2M/Ecr ≥100 µg/g creatinine (POR = 19.0). Associations of POR for a reduced eGFR with ECd/Ecr and all other variables were statistically insignificant.
4. Discussion
This study used a cross-sectional analysis of kidney dysfunction, tubular proteinuria and eGFR decline, to determine the differential impact of Cd exposure in men and women. Whereas many previous studies focused primarily on Cd-induced tubulopathy in women, we investigated these health outcomes in both men and women along with confounding risk factors, smoking, diabetes and hypertension. The excretion rate of Cd and β
2M (E
Cd and E
β2M) were normalized to the surrogate measure of GFR, creatinine clearance (C
cr). This C
cr-normalization of E
Cd and E
β2M, as E
Cd/C
cr and E
β2M/C
cr, depict excretion rates per functional nephron, thereby it corrects for differences in the number of functioning nephrons among study subjects [
7]. This C
cr-normalized excretion rate also corrects for urine dilution, but it is unaffected by creatinine excretion (E
cr). Thus, E
Cd/C
cr and E
β2M/C
cr provide an accurate quantification of the kidney burden of Cd and its toxicity to kidney tubular cells.
We selected subjects from two population-based studies, undertaken in an area with endemic Cd contamination in Mae Sot district, Tak province [
8] and a control, non-contamination area in Nakhon-Si-Thammarat province of Thailand [
9,
10]. The Cd content of the paddy soil samples from the Mae Sot district exceeded the standard of 0.15 mg/kg, and rice samples collected from households contained 4 times the amount of the permissible Cd level of 0.1 mg/kg [
16].
4.1. Exposure Levels of Cadmium in Women versus Men
Men and women in this cohort carry the same body burden of Cd, evident from a nearly identical mean (ECd/Ccr) ×100 values of 3.12 vs 3.21 µg/L filtrate. The sources of Cd could be differentiated through an analysis of blood-urine Cd relationships.
[Cd]
b and E
Cd/C
cr correlated strongly with each other in women (R
2 =0.624) and men (R
2 = 0.661) (
Figure 1a), and the covariate-adjusted means [Cd]
b showed a stepwise increase through E
Cd/C
cr tertiles in both genders. Of note, E
Cd/C
cr explained a larger fraction of the variation in [Cd]
b in men than it did in women (η
2 0.407 vs. 0.105) (
Figure 1b). The variability in [Cd]
b was associated mostly with E
Cd/C
cr in both genders while smoking explained a larger fraction of the [Cd]
b variability among men than among women (5.5% vs 2.8%). This result was expected, given the high % of smokers in the men group (68.4% vs. 18.6%) and the higher mean [Cd]
b in men than that of women (3.25 vs. 4.36 µg/L) In men only, [Cd]
b variation was associated with diabetes.
4.2. The Toxic Manifestation of Cadmium Exposure in Women versus Men
An independent health survey reported that the prevalence of chronic kidney disease (CKD), defined as the eGFR ≤ 60 mL/min/1.73 m
2, among Mae Sot residents was 16.1%, while the prevalence of tubulopathy, referred to as tubular proteinuria was 36.1% [
17]. This reported tubular proteinuria was based on the cut-off value of E
β2M/E
cr at 300 µg/g creatinine [
6], which equates E
β2M/C
cr of 2–3 µg/ L filtrate or (E
β2M/C
cr) ×100 of 200–300 µg/ L filtrate. Notably, the cut-off value for E
β2M/E
cr at 300 µg/g creatinine was used as the critical effect of exposure to Cd in the human diet [
6].
In this cohort, tubular proteinuria affected more than half of women (61.1%) and men (52.6%). One of four women had severe tubular impairment [(Eβ2M/Ccr) ×100 ≥ 1000 µg/L filtrate], whereas one of five men had this abnormality.
In women, E
β2M/C
cr showed a moderate positive association with age (β = 0.131), and an equally strong association with E
Cd/C
cr (β = 0.306) and diabetes (β = 0.349) (
Table 3). In men, E
β2M/C
cr did not show a significant association with age or E
Cd/C
cr, but this tubular defect was associated with diabetes only (β = 0.279). In covariance analysis, the contribution of E
Cd/C
cr to the variability of E
β2M/C
cr in women was demonstrable together with a dose-effect relationship after adjustment of covariates and interactions (
Figure 2d). In contrast, the contribution of E
Cd/C
cr to the variation of E
β2M/C
cr in men was statistically insignificant (
Figure 2d). An association of the marker of tubular dysfunction (E
βM) and diabetes seen in both men and women is in line with the current knowledge that diabetes adversely affects both glomerular (GFR) and tubular function, termed diabetic tubulopathy [
18,
19].
The overall mean eGFR was 90 mL/min/1.73 m
2 and the overall prevalence of a reduced in eGFR in this cohort was 6.9% (
Table 1). The difference in the % of a reduced eGFR in women and men (8.1% vs. 3.5%) did not reach a statistical significance level (
p = 0.097) nor did the difference in mean eGFR in women and men (
p = 0.145). The weaker effect of Cd exposure on eGFR in men, compared to women, remains to be confirmed with sufficiently large sample group of men. However, the regression analysis also indicated gender differences in susceptibility to the nephrotoxicity of Cd (
Table 5). In women, the eGFR was inversely associated with age (β = −0.511), E
Cd/C
cr (β =−0.185) and diabetes (β = −0.128). In comparison, the eGFR in men was not associated with E
Cd/C
cr, while showing an inverse association with age (β = −0.506) and hypertension (β = −0.212). Adverse effects of hypertension and diabetes on the eGFR have been noted in a cross-sectional study of the U.S. general population, where Cd-induced GFR reduction was more pronounced in those who had diabetes and/or hypertension [
20].
We speculate that gender differences in levels of some protective factors, notably body status of nutritionally essential metals such as iron and zinc may contribute to the increased susceptibility to Cd nephrotoxicity seen in women. Similarly, environmental Cd exposure has been linked to a reduction eGFR among participants in various cycles of the U.S. National Health and Nutrition Examination Survey (NHANES) undertaken over 18 years (1999 to 2016) [
20,
21,
22]. Lin et al. (2014) reported that the risk for a reduced eGFR was higher in those with lower serum zinc (OR 3.38), compared to those with similar Cd exposure levels and serum zinc > 74 μg/dL (OR 2.04) [
22].
4.3. Increment of β2M Excretion as GFR Falls
The protein β
2M with the molecular weight of 11,800 Da is filtered freely by the glomeruli, and is reabsorbed almost completely by the kidney’s tubular epithelial cells [
13]. Thus, defective tubular re-absorption of β
2M will result in an enhanced excretion rate of β
2M [
23,
24,
25,
26]. Loss of nephrons also raises the excretion β2M for the following reasons [
23,
26]. When the reabsorption rate of β
2M per nephron remains constant, its excretion will vary directly with its production. If the production and reabsorption per nephron remain constant as nephrons are lost, the excretion of β
2M will rise [
27].
It can thus be expected that the excretion of β
2M will increase, when GFR falls for any causes. Indeed, E
β2M/C
cr was inversely associated with eGFR in both women and men (
Figure 3c) although the causes of their eGFR decreases seemed to be different. In a quantitative analysis (
Figure 3d), the η
2 value describing the effect size of E
β2M/C
cr on eGFR variability among women was 1.7-fold larger (0.114 vs. 0.066). In both women and men, eGFR was lowest in those who had (E
β2M/C
cr) ×100 ≥1000 µg/L filtrate, indicative of severely impaired tubular function.
Of note, the POR for a reduced eGFR was increased by 8.3-fold and 33.7-fold those with (E
β2M/C
cr) ×100 of 300–999, and ≥1000 µg/L filtrate, respectively (
Table 7), compared to those with E
β2M/C
cr) ×100 <300 µg/L filtrate. A substantial loss of functioning nephrons was a likely cause of massive increases in E
β2M/C
cr seen in those who had eGFR below 60 mL/min/1.73 m
2.
4.4. The Pitfall of Adjusting Excretion Rate to Ecr and Implication for Health Risk Estimation
The C
cr-normalized data indicate that women and men shared the same burden of Cd (
Table 1). The data indicate also that the % of women and men across three categories of tubulopathy were all statistically different, thereby linking Cd exposure to the severity of tubulopathy in both genders. The logistic regression data (
Table 7) show that the likelihood of having a reduced eGFR was increased by 8.3-fold and 33.7-fold in those who had (E
β2M/C
cr) ×100 of 300–999 and ≥1000 µg/L filtrate, compared to those with (E
β2M/C
cr) ×100 <300 µg/L filtrate.
The E
cr-normalized data indicated that the mean E
Cd/E
cr in women was statistically higher than that of men (4.26 vs. 3.30 µg/g creatinine). They also indicated that the difference in % of women across the three tubulopathy categories was minuscule, and that the % distribution of men across tubulopathy categories was statistically significant. These data suggest an association of Cd exposure and the severity of tubulopathy in men only. The logistic regression data (
Table 8) show that the likelihood of having a reduced eGFR was increased by 3.2-fold and 19-fold in those who had E
β2M/E
cr of 300–999 and ≥1000 µg/g creatinine, compared to those with E
β2M/E
cr < E
β2M/E
cr.
Previously, E
β2M/E
cr of 100–299, 300–999, and ≥1000 μg/g creatinine were found to be associated with 4.7-, 6.2- and 10.5-fold increases in the prevalence odds of a reduced eGFR [
28]. Similarly, a rise in E
β2M/E
cr to levels not higher than 100 μg/g creatinine was associated with an increased risk of hypertension in the Japanese general population [
29], while a prospective cohort data showed that E
β2M/E
cr was associated with a 79% increase in the likelihood of having a large decline in eGFR (10 mL/min/1.73 m
2) over a five-year period [
30]. Thus, a cut-off value for E
β2M/E
cr above 300 μg/g creatinine does not reflect an early warning sign of the nephrotoxicity of Cd. The utility of this E
β2M/E
cr value as a toxicity criterion to derive a toxicity threshold level for Cd is inappropriate.
In summary, adjusting ECd and Eβ2M to Ecr produces an erroneous interpretation of the effect of Cd exposure on the eGFR, while underestimating the severity of Cd-induced tubulopathy. These data calls into question the utility of Eβ2M/Ecr of 300 µg/g creatinine to represent the critical effect of exposure to Cd in the human diet. New health guidance values need to be established for this toxic metal as well as public measures to minimize the Cd contamination of food chains.
Figure 1.
Blood cadmium and urinary cadmium excretion relationship. Scatterplots relate log([Cd]b × 103] to log[(ECd/Ccr) × 105] in women and men (a). Coefficients of determination (R2) and p-values are provided for all scatterplots. Bar graph (b) depicts means log([Cd]b × 103] values in women and men across ECd/Ccr tertiles. All means were obtained by univariate analysis with adjustment for covariates and interactions. For women, respective arithmetic means and standard deviations (SD) for (ECd/Ccr)×100 tertiles 1, 2 and 3 are 0.37 (0.47), 2.34 (0.52) and 6.84 (4.46) µg/L of filtrate. For men, respective arithmetic means and standard deviations (SD) for (ECd/Ccr)×100 tertiles quartiles 1, 2 and 3 are 0.36 (0.42), 2.14 (0.63) and 6.81 (3.78) µg/L of filtrate. For all tests, p-values ≤ 0.05 indicate statistically significant differences.
Figure 1.
Blood cadmium and urinary cadmium excretion relationship. Scatterplots relate log([Cd]b × 103] to log[(ECd/Ccr) × 105] in women and men (a). Coefficients of determination (R2) and p-values are provided for all scatterplots. Bar graph (b) depicts means log([Cd]b × 103] values in women and men across ECd/Ccr tertiles. All means were obtained by univariate analysis with adjustment for covariates and interactions. For women, respective arithmetic means and standard deviations (SD) for (ECd/Ccr)×100 tertiles 1, 2 and 3 are 0.37 (0.47), 2.34 (0.52) and 6.84 (4.46) µg/L of filtrate. For men, respective arithmetic means and standard deviations (SD) for (ECd/Ccr)×100 tertiles quartiles 1, 2 and 3 are 0.36 (0.42), 2.14 (0.63) and 6.81 (3.78) µg/L of filtrate. For all tests, p-values ≤ 0.05 indicate statistically significant differences.
Figure 2.
Dose-effect relationship of β2-microgloubulin and cadmium excretion. Scatterplots relate log[(EβM/Ccr) × 103] to log[(ECd/Ccr) × 105] in all subjects (a), and women and men (c). Coefficients of determination (R2) and p-values are provided for all scatterplots. The color-coded area graph (b) depicts means of log[(Eβ2M/Ccr)×103] across ECd/Ccr tertiles. Shaded areas indicate variability of means. Bar graph (d) depicts mean log[(Eβ2M/Ccr) × 103] in women and men in each ECd/Ccr tertile. The respective numbers of subjects in ECd/Ccr tertiles 1, 2 and 3 are 149, 149 and 150. All means were obtained by univariate analysis with adjustment for covariates and interaction. For women, respective arithmetic means and standard deviations (SD) for (ECd/Ccr)×100 tertiles 1, 2 and 3 are 0.37 (0.47), 2.34 (0.52) and 6.84 (4.46) µg/L of filtrate. For men, respective arithmetic means and standard deviations (SD) for (ECd/Ccr)×100 tertiles quartiles 1, 2 and 3 are 0.36 (0.42), 2.14 (0.63) and 6.81 (3.78) µg/L of filtrate. For all tests, p-values ≤ 0.05 indicate statistically significant differences.
Figure 2.
Dose-effect relationship of β2-microgloubulin and cadmium excretion. Scatterplots relate log[(EβM/Ccr) × 103] to log[(ECd/Ccr) × 105] in all subjects (a), and women and men (c). Coefficients of determination (R2) and p-values are provided for all scatterplots. The color-coded area graph (b) depicts means of log[(Eβ2M/Ccr)×103] across ECd/Ccr tertiles. Shaded areas indicate variability of means. Bar graph (d) depicts mean log[(Eβ2M/Ccr) × 103] in women and men in each ECd/Ccr tertile. The respective numbers of subjects in ECd/Ccr tertiles 1, 2 and 3 are 149, 149 and 150. All means were obtained by univariate analysis with adjustment for covariates and interaction. For women, respective arithmetic means and standard deviations (SD) for (ECd/Ccr)×100 tertiles 1, 2 and 3 are 0.37 (0.47), 2.34 (0.52) and 6.84 (4.46) µg/L of filtrate. For men, respective arithmetic means and standard deviations (SD) for (ECd/Ccr)×100 tertiles quartiles 1, 2 and 3 are 0.36 (0.42), 2.14 (0.63) and 6.81 (3.78) µg/L of filtrate. For all tests, p-values ≤ 0.05 indicate statistically significant differences.
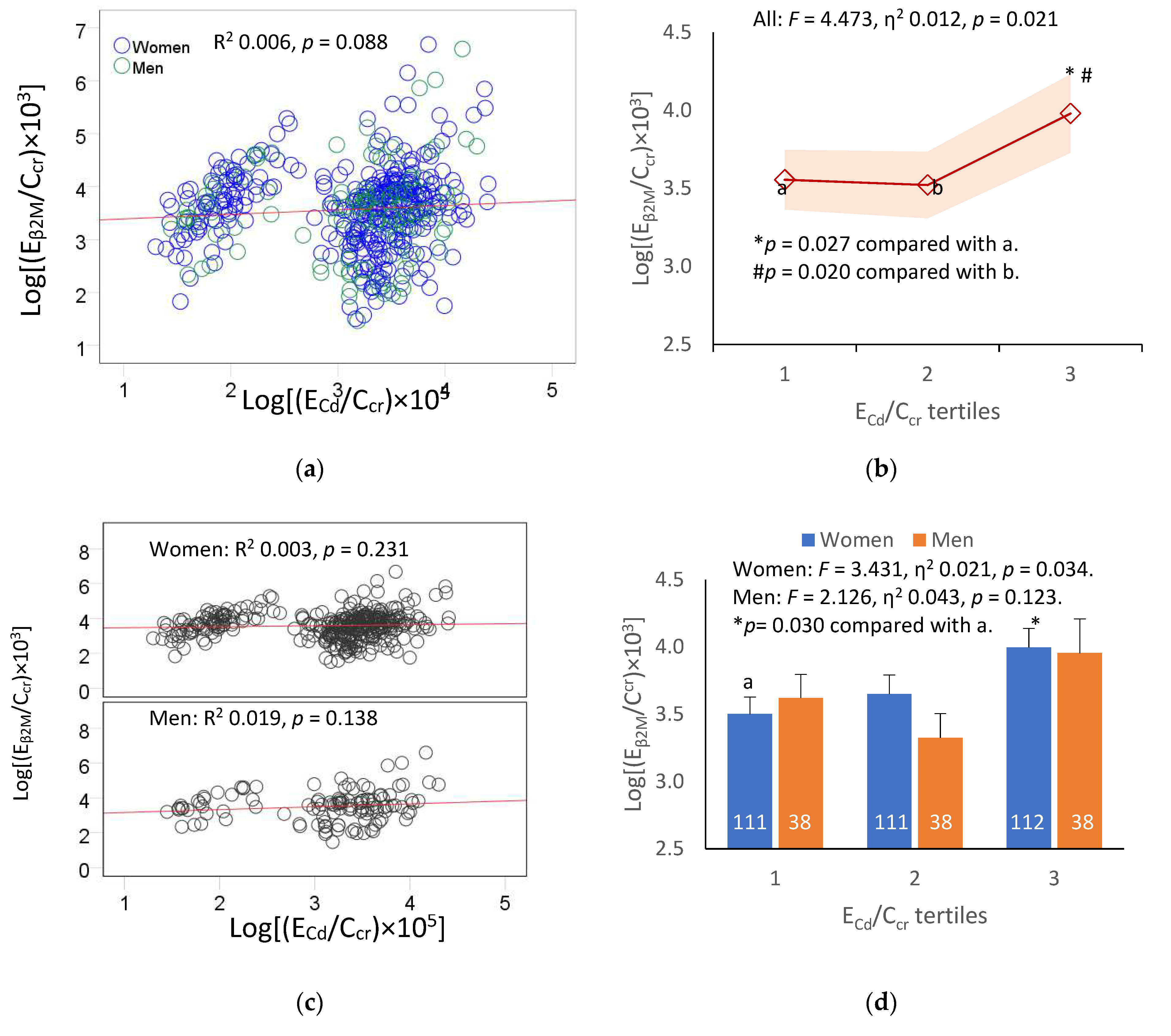
Figure 3.
An inverse relationship of eGFR with β2-microgloubulin excretion. Scatterplots relate eGFR to log[(EβM/Ccr) × 103] in all subjects (a), and women and men (c). Coefficients of determination (R2) and p-values are provided for all scatterplots. The color-coded area graph (b) depicts means of eGFR across three Eβ2M/Ccr ranges. Shaded areas indicate variability of means. Bar graph (d) depicts means of eGFR in women and men in each Eβ2M/Ccr range. The respective numbers of subjects in (Eβ2M/Ccr)×100 <300, 300−999 and ≥1000 µg/L of filtrate are 184, 156 and 109. All means were obtained by univariate analysis with adjustment for covariates and interaction. For all tests, p-values ≤ 0.05 indicate statistically significant differences.
Figure 3.
An inverse relationship of eGFR with β2-microgloubulin excretion. Scatterplots relate eGFR to log[(EβM/Ccr) × 103] in all subjects (a), and women and men (c). Coefficients of determination (R2) and p-values are provided for all scatterplots. The color-coded area graph (b) depicts means of eGFR across three Eβ2M/Ccr ranges. Shaded areas indicate variability of means. Bar graph (d) depicts means of eGFR in women and men in each Eβ2M/Ccr range. The respective numbers of subjects in (Eβ2M/Ccr)×100 <300, 300−999 and ≥1000 µg/L of filtrate are 184, 156 and 109. All means were obtained by univariate analysis with adjustment for covariates and interaction. For all tests, p-values ≤ 0.05 indicate statistically significant differences.
Table 1.
Characteristics of Study Subjects.
Table 1.
Characteristics of Study Subjects.
| Parameters |
All Subjects, n = 448 |
Women, n = 334 |
Men, n = 114 |
p |
| Age, years |
51.1 ± 8.6 |
51.5 ± 9.0 |
49.9 ± 7.2 |
0.344 |
| BMI, kg/m2
|
24.8 ± 4.0 |
25.2 ± 4.0 |
23.7 ± 3.6 |
<0.001 |
| Smoking, % |
31.3 |
18.6 |
68.4 |
<0.001 |
| Hypertension, % |
48.7 |
50.6 |
43.0 |
0.160 |
| Diabetes, % |
15.4 |
16.2 |
0.442 |
0.442 |
| eGFR a, mL/min/1.73 m2
|
90 ± 18 |
89 ± 19 |
93 ± 16 |
0.145 |
| Reduced eGFR b, % |
6.9 |
8.1 |
3.5 |
0.097 |
| Plasma creatinine, mg/dL |
0.82 ± 0.22 |
0.78 ± 0.21 |
0.95 ± 0.21 |
<0.001 |
| Urine creatinine, mg/dL |
114 ± 74 |
108 ± 74 |
132 ± 72 |
<0.001 |
| Blood Cd, µg/L |
2.75 ± 3.19 |
2.58 ± 3.10 |
3.25 ± 3.41 |
0.038 |
| Urine Cd, µg/L |
4.22 ± 5.67 |
4.36 ± 6.14 |
3.82 ± 4.01 |
0.875 |
| Urine β2M, µg/L |
3122 ± 18836 |
2596 ± 17238 |
4665 ± 22903 |
0.544 |
| Normalized to Ccr (Ex/Ccr) c
|
|
|
|
|
| (ECd/Ccr) ×100, µg/L filtrate |
3.19 ± 3.72 |
3.21 ± 3.79 |
3.12 ± 3.55 |
0.639 |
| (Eβ2M/Ccr) ×100, µg/L filtrate |
3839 ± 30422 |
3078 ± 26986 |
6072 ± 38837 |
0.212 |
| (Eβ2M/Ccr) ×100, µg/L filtrate, % |
|
|
|
|
| < 300 |
41.1 |
38.9 |
47.4 |
|
| 300−999 |
34.8 |
35.9 |
31.6 |
|
| ≥ 1000 |
24.1 |
25.1 # |
21.1 ** |
|
| Normalized to Ecr (Ex/Ecr) d
|
|
|
|
|
| ECd/Ecr, µg/g creatinine |
4.02 ± 4.41 |
4.26 ± 4.62 |
3.30 ± 3.68 |
0.028 |
| Eβ2M/Ecr, µg/g creatinine |
3220 ± 21847 |
3005 ± 22812 |
3850 ± 18815 |
0.017 |
| Eβ2M/Ecr, µg/g creatinine, % |
|
|
|
|
| < 300 |
35.5 |
32.3 |
45.6 |
|
| 300−999 |
34.6 |
34.7 |
34.2 |
|
| ≥ 1000 |
29.7 |
32.9 |
20.2 * |
|
Table 2.
Determinants of blood cadmium concentration with cadmium excretion and other variables.
Table 2.
Determinants of blood cadmium concentration with cadmium excretion and other variables.
Independent
Variables/Factors |
Log([Cd]b ×103), µg/L |
| Women, n = 334 |
Men, n = 114 |
| |
β |
η2
|
p |
p |
η2
|
p |
| Age, years |
−0.170 |
0.057 |
<0.001 |
−0.004 |
1×10−6
|
0.946 |
| BMI, kg/m2
|
−0.019 |
0.001 |
0.586 |
−0.079 |
0.022 |
0.180 |
| Log[(ECd/Ccr)×105], µg/L filtrate |
0.619 |
0.367 |
<0.001 |
0.581 |
0.420 |
<0.001 |
| Smoking |
0.123 |
0.028 |
0.001 |
0.184 |
0.055 |
0.002 |
| Diabetes |
-0.053 |
0.010 |
0.182 |
−0.246 |
0.095 |
<0.001 |
| Hypertension |
0.048 |
0.007 |
0.162 |
−0.061 |
0.004 |
0.287 |
| DM× HTN |
n/a |
0.016 |
0.023 |
n/a |
n/a |
n/a |
| SMK × DM × HTN |
n/a |
n/a |
n/a |
n/a |
0.042 |
0.036 |
| Adjusted R2
|
0.624 |
n/a |
<0.001 |
0.661 |
n/a |
<0.001 |
Table 3.
Associations of β2-mcirogloubulin excretion with cadmium exposure measures.
Table 3.
Associations of β2-mcirogloubulin excretion with cadmium exposure measures.
Independent
Variables/Factors |
Log[(Eβ2M/Ccr) ×103], µg/ L filtrate |
| All subjects |
Women |
Men |
| β |
p |
β |
p |
β |
p |
| Age, years |
0.137 |
0.013 |
0.131 |
0.041 |
0.128 |
0.238 |
| BMI, kg/m2
|
−0.089 |
0.065 |
−0.102 |
0.062 |
−0.043 |
0.664 |
| Log([Cd]b×103), µg/L filtrate |
−0.016 |
0.824 |
−0.083 |
0.328 |
0.217 |
0.180 |
| Log[(ECd/Ccr)×105], µg/L filtrate |
0.283 |
<0.001 |
0.306 |
<0.001 |
0.175 |
0.247 |
| Gender (male) |
0.052 |
0.318 |
− |
− |
− |
− |
| Smoking |
0.063 |
0.255 |
0.093 |
0.094 |
−0.065 |
0.526 |
| Diabetes |
0.323 |
<0.001 |
0.349 |
<0.001 |
0.279 |
0.017 |
| Hypertension |
0.015 |
0.745 |
−0.023 |
0.660 |
0.142 |
0.139 |
| Adjusted R2
|
0.105 |
<0.001 |
0.125 |
<0.001 |
0.059 |
0.060 |
Table 4.
Prevalence odds for excessive excretion of β2M in relation to cadmium excretion and other variables.
Table 4.
Prevalence odds for excessive excretion of β2M in relation to cadmium excretion and other variables.
| Independent variables/factors |
Number of Subjects |
(Eβ2M/Ccr) ×100 ≥ 300 µg/L |
(Eβ2M/Ccr) × 100 ≥ 1000 µg/L |
| POR (95% CI) |
p |
POR (95% CI) |
p |
| Age, years |
448 |
1.036 (1.007, 1.067) |
0.016 |
1.062 (1.027, 1.098) |
<0.001 |
| BMI, kg/m2
|
448 |
0.971 (0.919, 1.025) |
0.284 |
0.958 (0.896, 1.023) |
0.203 |
| Log[(ECd/Ccr)×105], µg/L filtrate |
448 |
1.940 (1.344, 2.802) |
<0.001 |
3.343 (2.036, 5.488) |
<0.001 |
| Gender (male) |
114 |
1.406 (0.835, 2.367) |
0.200 |
1.299 (0.687, 2.458) |
0.421 |
| Smoking |
140 |
1.067 (0.645, 1.765) |
0.801 |
1.388 (0.763, 2.522) |
0.282 |
| Diabetes |
69 |
5.294 (2.526, 11.09) |
<0.001 |
11.52 (5.004, 26.50) |
<0.001 |
| Hypertension |
218 |
1.066 (0.714, 1.592) |
0.753 |
1.535 (0.942, 2.501) |
0.085 |
Table 5.
Associations of eGFR with cadmium exposure measures and other variables.
Table 5.
Associations of eGFR with cadmium exposure measures and other variables.
Independent.
Variables/Factors |
eGFR, mL/min/1.73m2
|
| All subjects |
Women |
Men |
| β |
p |
β |
p |
β |
p |
| Age, years |
−0.517 |
<0.001 |
−0.511 |
<0.001 |
−0.506 |
<0.001 |
| BMI, kg/m2
|
−0.064 |
0.136 |
−0.048 |
0.327 |
−0.149 |
0.095 |
| Log([Cd]b×103), µg/L filtrate |
0.053 |
0.420 |
0.102 |
0.182 |
−0.153 |
0.291 |
| Log[(ECd/Ccr)×105], µg/L filtrate |
−0.148 |
0.026 |
−0.185 |
0.018 |
0.011 |
0.933 |
| Gender (male) |
−0.001 |
0.977 |
− |
− |
− |
− |
| Smoking |
0.025 |
0.610 |
0.018 |
0.717 |
0.071 |
0.438 |
| Diabetes |
−0.109 |
0.023 |
−0.128 |
0.021 |
−0.055 |
0.593 |
| Hypertension |
−0.079 |
0.055 |
−0.050 |
0.295 |
−0.212 |
0.014 |
| Adjusted R2
|
0.279 |
<0.001 |
0.281 |
<0.001 |
0.249 |
<0.001 |
Table 6.
Prevalence odds for a reduced eGFR in relation to cadmium excretion and other variables.
Table 6.
Prevalence odds for a reduced eGFR in relation to cadmium excretion and other variables.
Independent Variables/
Factors |
Reduced eGFR a
|
| β Coefficients |
POR |
95% CI |
p |
| (SE) |
|
Lower |
Upper |
|
| Age, years |
0.136 (0.027) |
1.146 |
1.086 |
1.209 |
<0.001 |
| BMI, kg/m2
|
0.020 (0.051) |
1.021 |
0.923 |
1.128 |
0.688 |
| Log[(ECd/Ccr)×105], µg/L filtrate |
1.154 (0.358) |
3.172 |
1.572 |
6.402 |
0.001 |
| Gender (male) |
−0.542 (0.649) |
0.582 |
0.163 |
2.075 |
0.404 |
| Smoking |
−0.228 (0.583) |
0.796 |
0.254 |
2.493 |
0.695 |
| Diabetes |
1.439 (0.524) |
4.217 |
1.510 |
11.78 |
0.006 |
| Hypertension |
0.115 (0.427) |
1.122 |
0.486 |
2.591 |
0.787 |
Table 7.
Prevalence odds of a reduced eGFR in relation to cadmium and β2M excretion rates normalized to Ccr.
Table 7.
Prevalence odds of a reduced eGFR in relation to cadmium and β2M excretion rates normalized to Ccr.
Independent Variables/
Factors |
Reduced eGFR a
|
| Number of subjects |
POR |
95% CI |
p |
| Lower |
Upper |
| Age, years |
448 |
1.140 |
1.072 |
1.211 |
<0.001 |
| BMI, kg/m2
|
448 |
1.066 |
0.950 |
1.198 |
0.278 |
| Log[(ECd/Ccr) ×105], µg/L filtrate |
448 |
2.251 |
1.043 |
4.858 |
0.039 |
| Gender (male) |
114 |
0.674 |
0.176 |
2.583 |
0.565 |
| Smoking |
140 |
0.885 |
0.270 |
2.899 |
0.840 |
| Diabetes |
69 |
1.216 |
0.394 |
3.753 |
0.734 |
| Hypertension |
218 |
1.199 |
0.487 |
2.957 |
0.693 |
| (Eβ2M/Ccr) ×100, µg/L filtrate |
|
|
|
|
|
| < 300 |
184 |
Referent |
|
|
|
| 300–999 |
156 |
8.310 |
2.655 |
26.01 |
<0.001 |
| ≥ 1000 |
108 |
33.731 |
4.193 |
271.3 |
0.001 |
Table 8.
Prevalence odds of a reduced eGFR in relation to cadmium and β2M excretion rates normalized to Ecr.
Table 8.
Prevalence odds of a reduced eGFR in relation to cadmium and β2M excretion rates normalized to Ecr.
Independent Variables/
Factors |
Reduced eGFR a
|
| Number of subjects |
POR |
95% CI |
p |
| Lower |
Upper |
| Age, years |
448 |
1.101 |
1.045 |
1.160 |
<0.001 |
| BMI, kg/m2
|
448 |
1.032 |
0.929 |
1.147 |
0.555 |
| Log[(ECd/Ecr) ×103], µg/g creatinine |
448 |
1.278 |
0.630 |
2.593 |
0.497 |
| Gender (male) |
114 |
0.671 |
0.181 |
2.484 |
0.550 |
| Smoking |
140 |
0.762 |
0.240 |
2.418 |
0.644 |
| Diabetes |
69 |
1.614 |
0.581 |
4.484 |
0.359 |
| Hypertension |
218 |
1.041 |
0.449 |
2.415 |
0.926 |
| (Eβ2M/Ccr)×100, µg/g creatinine |
|
|
|
|
|
| <300 |
160 |
|
|
|
|
| 300–999 |
155 |
3.204 |
1.226 |
8.375 |
0.018 |
| ≥1000 |
133 |
19.042 |
2.387 |
151.907 |
0.005 |