Submitted:
29 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Data sources and search strategy
Criteria for considering studies for this review
Types of studies
Types of participants
Types of interventions
Types of outcome measures
Data extraction and management
Quality assessment
Overlapping and low-quality reviews
Data synthesis and statistical analysis
Assessment of heterogeneity and subgroup analysis
Sensitivity analysis
Assessment of publication biases
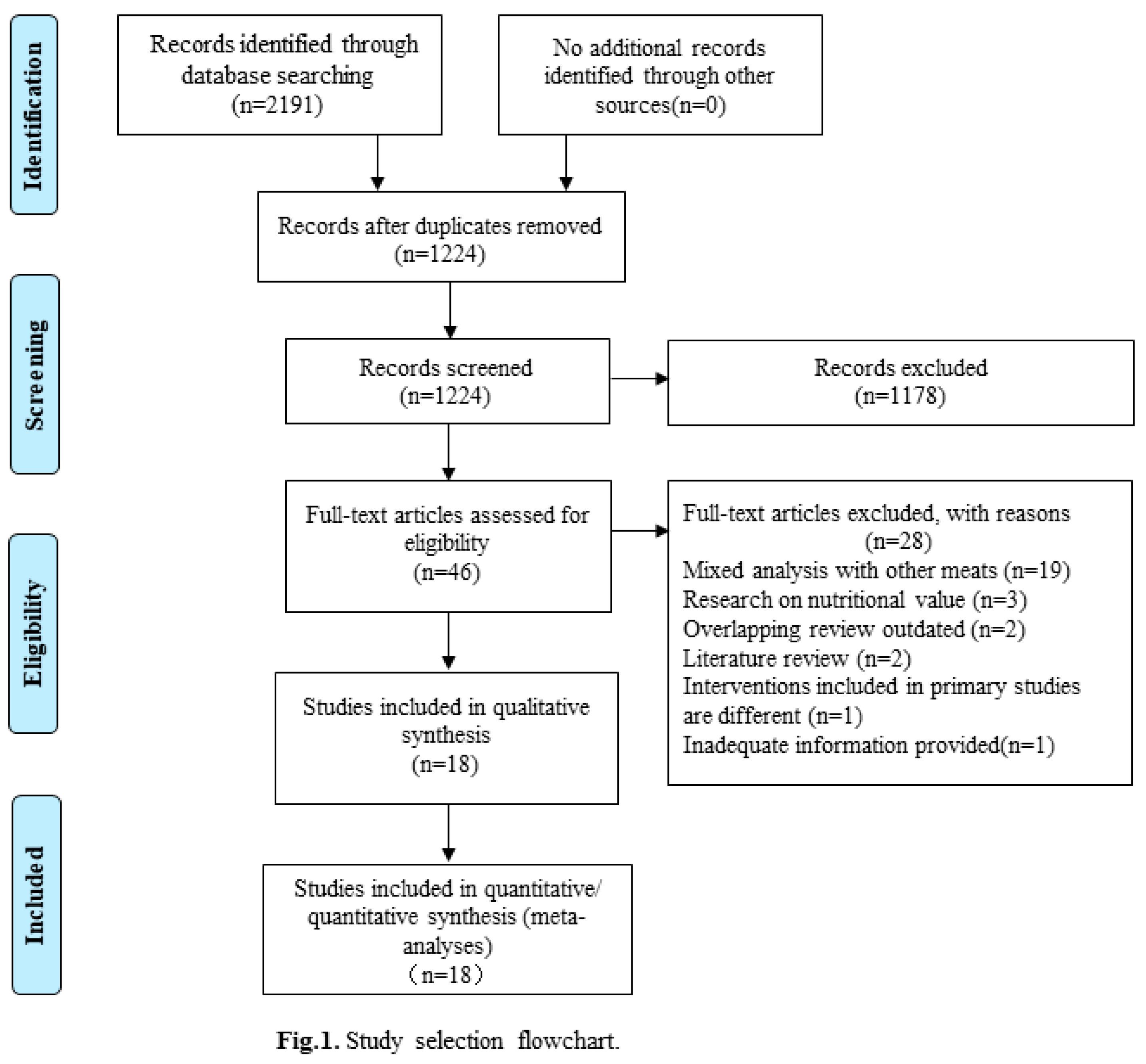
Results
Selection and characteristics of studies
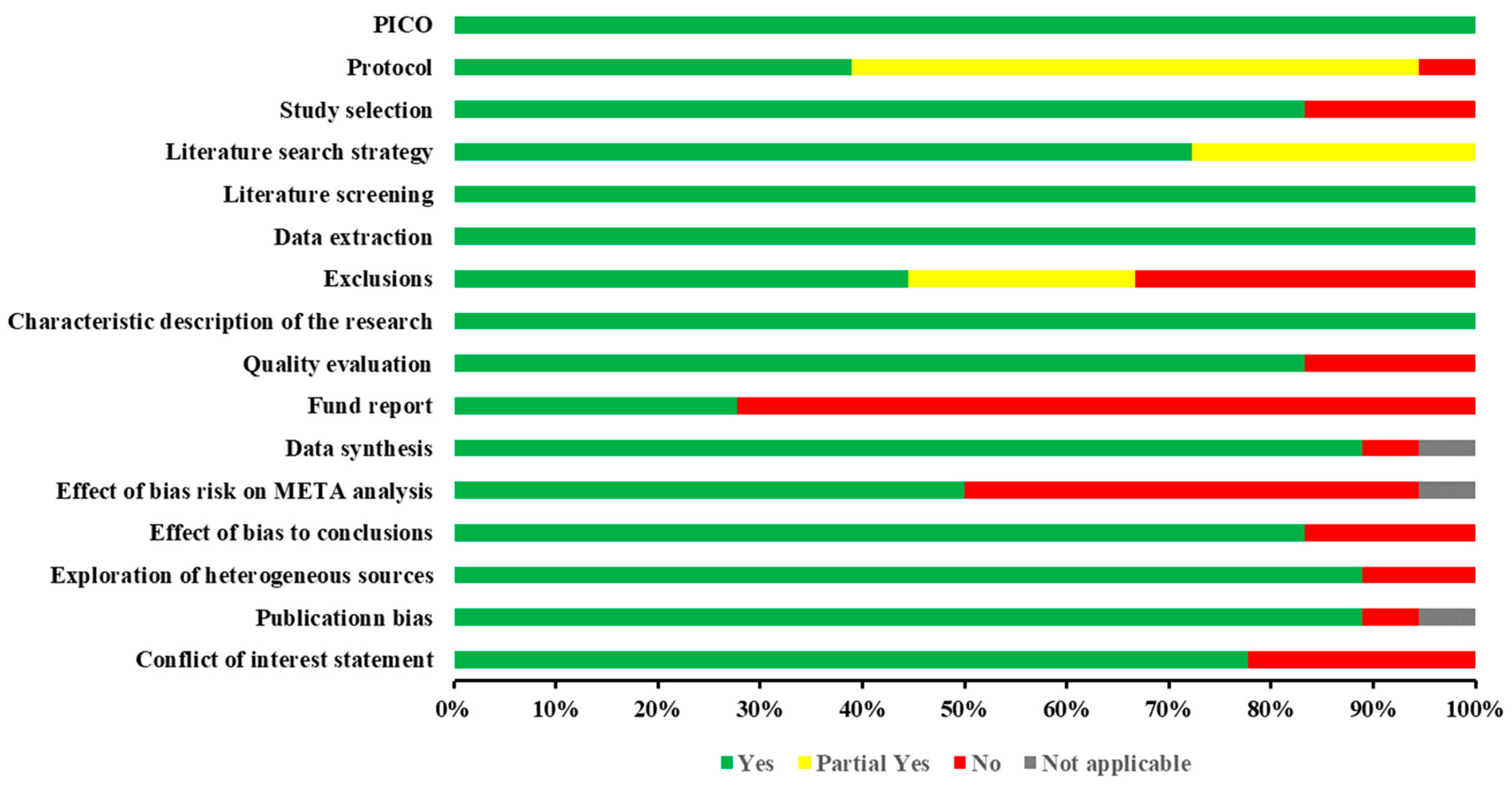
Quality assessment
Overlapping and non-overlapping associations
Qualitative and quantitative description
Incidence of colon cancer
Incidence of rectal cancer
Incidence of gastric cancer
Preventive factors of breast cancer
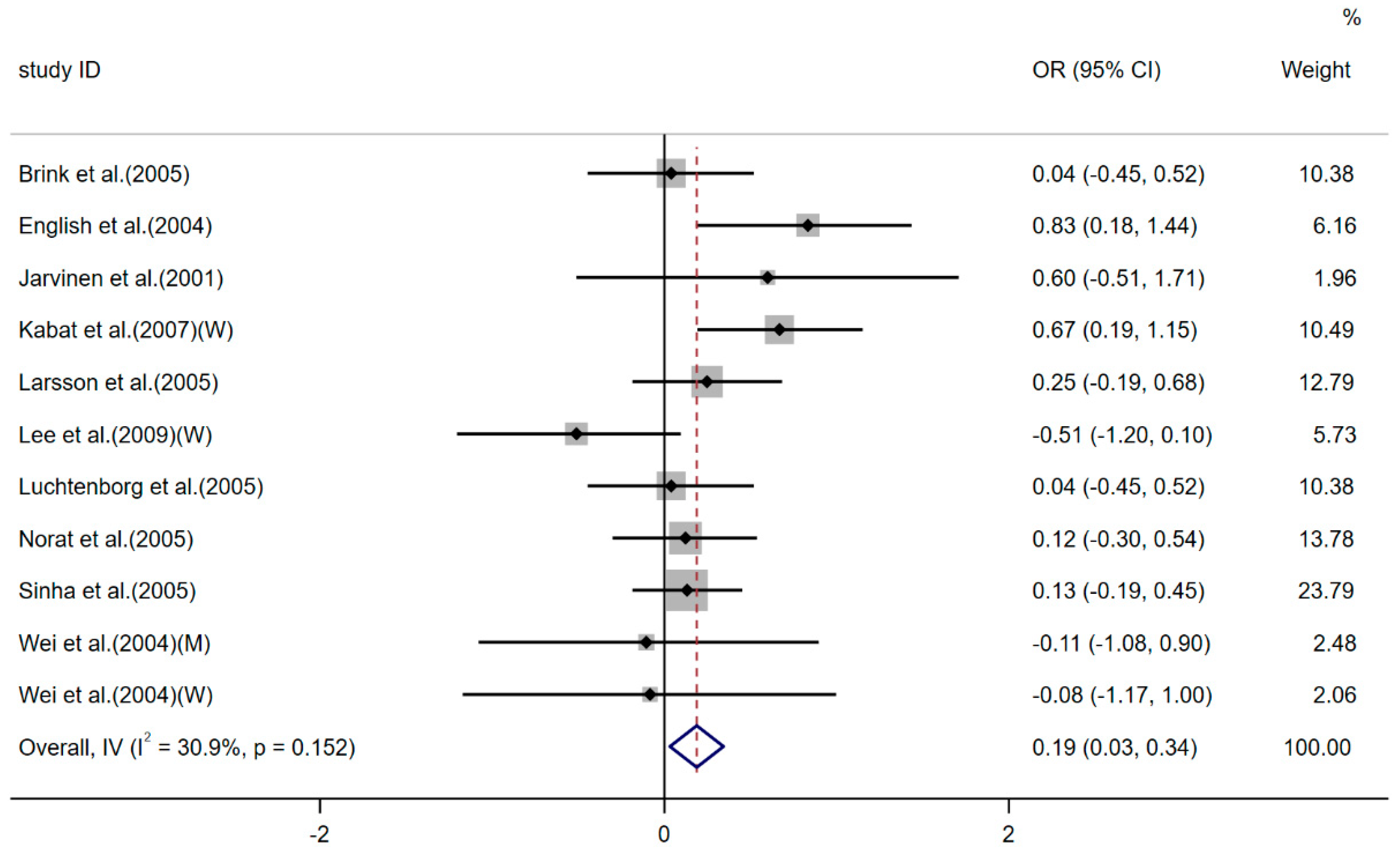
Incidence of non-Hodgkin's lymphoma (NHL)
Risk of Stroke /mortality rate
Change levels of glycemic control and inflammatory biomarkers
Cardiovascular disease outcomes
Changes levels in cardiovascular risk factors
Risk of kidney stones
Change levels of body weight and composition
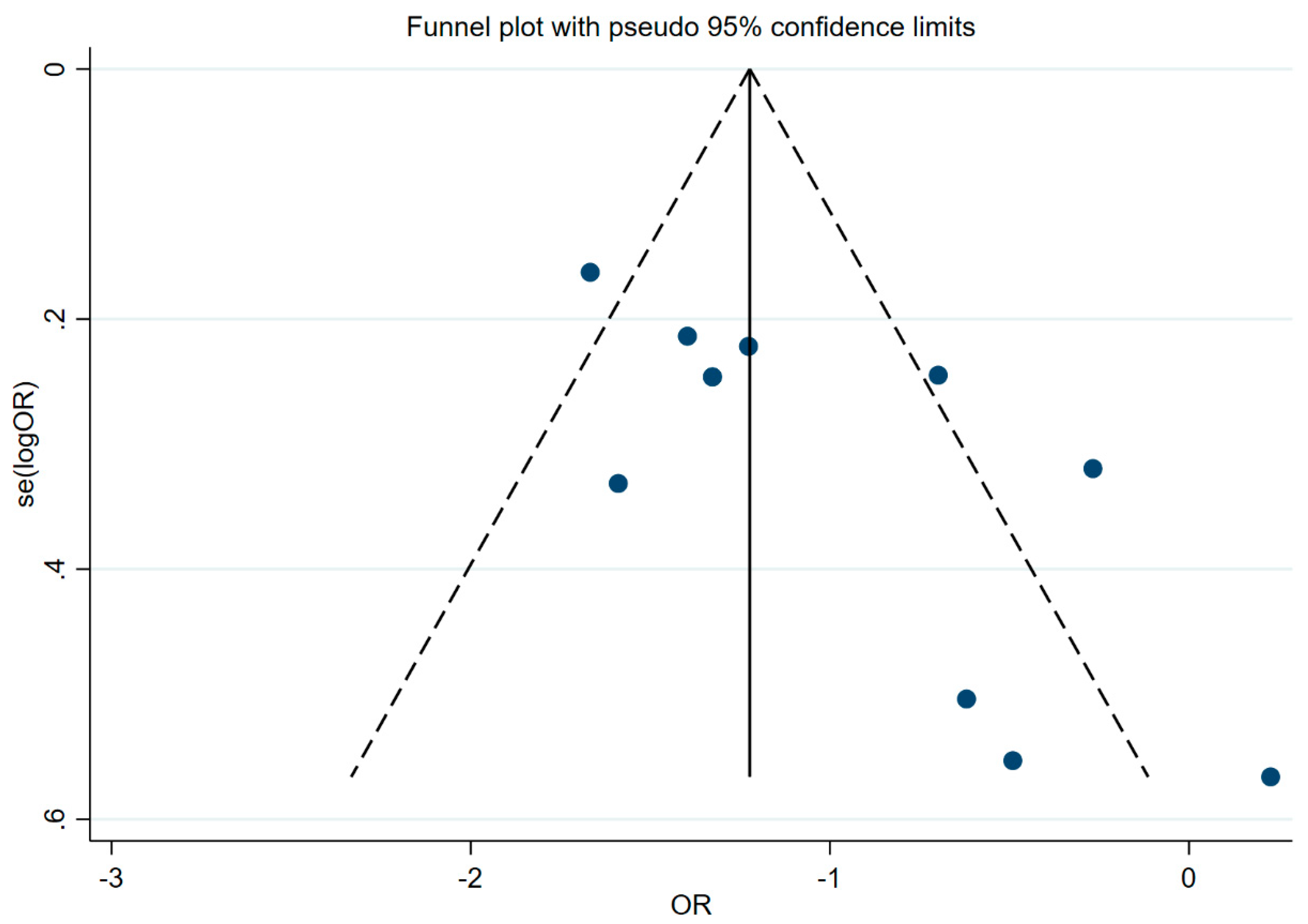
Publication bias
Discussion
Summary of main results
Limitations
Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, D.Z. Red meat; poultry, and egg consumption with the risk of hypertension: a meta-analysis of prospective cohort studies. Journal of human hypertension 2018, 32, 507–517. [Google Scholar] [CrossRef]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A.J.F.C. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms. influencing factors and chemical hazards 2022, 371, 131139. [Google Scholar]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. The Lancet. Oncology 2015, 16, 1599–600. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, J. World agriculture: towards 2015/2030: an FAO perspective. Earthscan 2003.
- Wolk, A. Potential health hazards of eating red meat. Journal of internal medicine 2017, 281, 106–122. [Google Scholar] [CrossRef]
- Boyle, P.; Boffetta, P.; Autier, P. Diet; nutrition; cancer: public, media and scientific confusion. Annals of oncology : official journal of the European Society for Medical Oncology 2008, 19, 1665–7. [Google Scholar] [CrossRef]
- Johnston, B.C.; Zeraatkar, D.; Han, M.A.; Vernooij, R.W.M.; Valli, C.; El Dib, R.; Marshall, C.; Stover, P.J.; Fairweather-Taitt, S.; Wójcik, G.; Bhatia, F.; de Souza, R.; Brotons, C.; Meerpohl, J.J.; Patel, C.J.; Djulbegovic, B.; Alonso-Coello, P.; Bala, M.M.; Guyatt, G.H. Unprocessed Red Meat and Processed Meat Consumption: Dietary Guideline Recommendations From the Nutritional Recommendations (NutriRECS) Consortium. Annals of internal medicine 2019, 171, 756–764. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Carcinogenicity of consumption of red meat and processed meat: A review of scientific news since the IARC decision. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2017, 105, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Vossen, E.; Hemeryck, L.Y.; Bussche, J.V.; Vanhaecke, L.; De Smet, S. Increased oxidative and nitrosative reactions during digestion could contribute to the association between well-done red meat consumption and colorectal cancer. Food Chem 2015, 187, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Song, M.; Zhang, X.; Pan, A.; Wang, M.; Fuchs, C.S.; Le, N.; Chan, A.T.; Willett, W.C.; Ogino, S.; Giovannucci, E.L.; Wu, K. Processed and Unprocessed Red Meat and Risk of Colorectal Cancer: Analysis by Tumor Location and Modification by Time. PloS one 2015, 10, e0135959. [Google Scholar] [CrossRef]
- Pan, B.; Wu, Y.; Yang, Q.; Ge, L.; Gao, C.; Xun, Y.; Tian, J.; Ding, G. The impact of major dietary patterns on glycemic control, cardiovascular risk factors, and weight loss in patients with type 2 diabetes: A network meta-analysis. Journal of evidence-based medicine 2019, 12, 29–39. [Google Scholar] [CrossRef]
- Vernooij, R.W.M.; Zeraatkar, D.; Han, M.A.; El Dib, R.; Zworth, M.; Milio, K.; Sit, D.; Lee, Y.; Gomaa, H.; Valli, C.; Swierz, M.J.; Chang, Y.; Hanna, S.E.; Brauer, P.M.; Sievenpiper, J.; de Souza, R.; Alonso-Coello, P.; Bala, M.M.; Guyatt, G.H.; Johnston, B.C. Patterns of Red and Processed Meat Consumption and Risk for Cardiometabolic and Cancer Outcomes: A Systematic Review and Meta-analysis of Cohort Studies. Annals of internal medicine 2019, 171, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.E.; Doherty, T.S. Meat Consumption and Health: Food for Thought. Annals of internal medicine 2019, 171, 767–768. [Google Scholar] [CrossRef]
- Hartling, L.; Chisholm, A.; Thomson, D.; Dryden, D.M. A descriptive analysis of overviews of reviews published between 2000 and 2011. PloS one 2012, 7, e49667. [Google Scholar] [CrossRef]
- Becker, L.A.; Oxman, A.D.J.C.H.F.S.R.O.I. Overviews of reviews 2008, 607.
- Khangura, S.; Konnyu, K.; Cushman, R.; Grimshaw, J.; Moher, D.J.S.R. Evidence summaries: the evolution of a rapid review approach. 2012, 1, 1–9. [Google Scholar] [CrossRef]
- Higgins, J.J.W.C.-H.O. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated 11]. The Cochrane Collaboration, (2011). 20 March.
- M J, P.; J E, M.; P M, B.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. International journal of surgery 2021, 88, 105906. [Google Scholar]
- Yan, P.; Yao, L.; Li, H.; Zhang, M.; Xun, Y.; Li, M.; Cai, H.; Lu, C.; Hu, L.; Guo, T.; Liu, R.; Yang, K. The methodological quality of robotic surgical meta-analyses needed to be improved: a cross-sectional study. Journal of clinical epidemiology 2019, 109, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; Henry, D.A. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed.) 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Antoine, S.L.; Mathes, T.; Neugebauer, E.A.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. Journal of clinical epidemiology 2014, 67, 368–75. [Google Scholar] [CrossRef]
- Smith, V.; Devane, D.; Begley, C.M.; Clarke, M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC medical research methodology 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Senn, S.J. Overstating the evidence: double counting in meta-analysis and related problems. BMC medical research methodology 2009, 9, 10. [Google Scholar] [CrossRef]
- Sacks, H.S.; Berrier, J.; Reitman, D.; Ancona-Berk, V.; Chalmers, T.C.J.N.E.J.O.M. Meta-analyses of randomized controlled trials. 1987, 316, 450–455. [Google Scholar] [CrossRef]
- Shea, B.; Dubé, C.; Moher, D.J.S.R.I.H.C.M.-A.I.C. Assessing the quality of reports of systematic reviews: the QUOROM statement compared to other tools. 2001, 122–39. [Google Scholar]
- Bougioukas, K.I.; Liakos, A.; Tsapas, A.; Ntzani, E.; Haidich, A.B. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. Journal of clinical epidemiology 2018, 93, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Pollock, M.; Fernandes, R.M.; Newton, A.S.; Scott, S.D.; Hartling, L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Systematic reviews 2019, 8, 18. [Google Scholar] [CrossRef]
- Pollock, M.; Fernandes, R.M.; Newton, A.S.; Scott, S.D.; Hartling, L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Systematic reviews 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G.J.B. Measuring inconsistency in meta-analyses. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Patsopoulos, N.A.; Evangelou, E.J.B. Uncertainty in heterogeneity estimates in meta-analyses. 2007, 335, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M.J.J.O.C.E. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. 2001, 54, 1046–1055. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C.J.B. Bias in meta-analysis detected by a simple. graphical test, 1997, 315, 629–634. [Google Scholar]
- Alexander, D.D.; Weed, D.L.; Cushing, C.A.; Lowe, K.A. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2011, 20, 293–307. [Google Scholar] [CrossRef]
- Smolińska, K.; Paluszkiewicz, P. Risk of colorectal cancer in relation to frequency and total amount of red meat consumption. Systematic review and meta-analysis. Archives of medical science : AMS 2010, 6, 605–10. [Google Scholar] [CrossRef]
- Song, P.; Lu, M.; Yin, Q.; Wu, L.; Zhang, D.; Fu, B.; Wang, B.; Zhao, Q. Red meat consumption and stomach cancer risk: a meta-analysis. Journal of cancer research and clinical oncology 2014, 140, 979–92. [Google Scholar] [CrossRef]
- Poorolajal, J.; Heidarimoghis, F.; Karami, M.; Cheraghi, Z.; Gohari-Ensaf, F.; Shahbazi, F.; Zareie, B.; Ameri, P.; Sahraee, F. Factors for the Primary Prevention of Breast Cancer: A Meta-Analysis of Prospective Cohort Studies. Journal of research in health sciences 2021, 21, e00520. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, H.; Cheraghi, M.; Amoori, N.; Alaf, M. Red meat intake and risk of non-Hodgkin lymphoma: a meta-analysis. Asian Pacific journal of cancer prevention : APJCP 2014, 15, 10421–5. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Wolk, A.; Larsson, S.C. Red meat consumption and risk of stroke: a meta-analysis of prospective studies. Stroke 2012, 43, 2556–60. [Google Scholar] [CrossRef]
- Yang, C.; Pan, L.; Sun, C.; Xi, Y.; Wang, L.; Li, D. Red Meat Consumption and the Risk of Stroke: A Dose-Response Meta-analysis of Prospective Cohort Studies. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 2016, 25, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, G.; Mesquita, G.X.B.; Lima, S.; Silva, D.F.O.; de Azevedo, K.P.M.; Pimenta, I.; de Oliveira, A.; Lyra, C.O.; Martínez, D.G.; Piuvezam, G. Associations of the consumption of unprocessed red meat and processed meat with the incidence of cardiovascular disease and mortality, and the dose-response relationship: A systematic review and meta-analysis of cohort studies. Crit Rev Food Sci Nutr 2022, 1–14. [Google Scholar] [CrossRef]
- O'Connor, L.E.; Kim, J.E.; Clark, C.M.; Zhu, W.; Campbell, W.W. Effects of Total Red Meat Intake on Glycemic Control and Inflammatory Biomarkers: A Meta-Analysis of Randomized Controlled Trials. Advances in nutrition (Bethesda, Md.) 2021, 12, 115–127. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. The American journal of clinical nutrition 2011, 94, 1088–96. [Google Scholar] [CrossRef]
- Zhang, R.; Fu, J.; Moore, J.B.; Stoner, L.; Li, R. Processed and Unprocessed Red Meat Consumption and Risk for Type 2 Diabetes Mellitus: An Updated Meta-Analysis of Cohort Studies. International journal of environmental research and public health 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, D.; Johnston, B.C.; Bartoszko, J.; Cheung, K.; Bala, M.M.; Valli, C.; Rabassa, M.; Sit, D.; Milio, K.; Sadeghirad, B.; Agarwal, A.; Zea, A.M.; Lee, Y.; Han, M.A.; Vernooij, R.W.M.; Alonso-Coello, P.; Guyatt, G.H.; El Dib, R. Effect of Lower Versus Higher Red Meat Intake on Cardiometabolic and Cancer Outcomes: A Systematic Review of Randomized Trials. Annals of internal medicine 2019, 171, 721–731. [Google Scholar] [CrossRef]
- Papier, K.; Knuppel, A.; Syam, N.; Jebb, S.A.; Key, T.J. Meat consumption and risk of ischemic heart disease: A systematic review and meta-analysis. Crit Rev Food Sci Nutr 2023, 63, 426–437. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Satija, A.; Blondin, S.A.; Janiszewski, M.; Emlen, E.; O'Connor, L.E.; Campbell, W.W.; Hu, F.B.; Willett, W.C.; Stampfer, M.J. Meta-Analysis of Randomized Controlled Trials of Red Meat Consumption in Comparison With Various Comparison Diets on Cardiovascular Risk Factors. Circulation 2019, 139, 1828–1845. [Google Scholar] [CrossRef] [PubMed]
- O'Connor, L.E.; Kim, J.E.; Campbell, W.W. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials. The American journal of clinical nutrition 2017, 105, 57–69. [Google Scholar] [CrossRef]
- Asoudeh, F.; Talebi, S.; Jayedi, A.; Marx, W.; Najafi, M.T.; Mohammadi, H. Associations of Total Protein or Animal Protein Intake and Animal Protein Sources with Risk of Kidney Stones: A Systematic Review and Dose-Response Meta-Analysis. Advances in nutrition (Bethesda, Md.) 2022, 13, 821–832. [Google Scholar] [CrossRef]
- Cirone, C.; Cirone, K.D.; Malvankar-Mehta, M.S. Linkage between a plant-based diet and age-related eye diseases: a systematic review and meta-analysis. Nutrition reviews 2023, 81, 428–440. [Google Scholar] [CrossRef]
- An, R.; Liu, J.; Liu, R. Pork Consumption in Relation to Body Weight and Composition: A Systematic Review and Meta-analysis. American journal of health behavior 2020, 44, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Angua, K.M.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Devall, M.; Dampier, C.H.; Eaton, S.; Ali, M.W.; Díez-Obrero, V.; Moratalla-Navarro, F.; Bryant, J.; Jennelle, L.T.; Moreno, V.; Powell, S.M.; Peters, U.; Casey, G. Novel insights into the molecular mechanisms underlying risk of colorectal cancer from smoking and red/processed meat carcinogens by modeling exposure in normal colon organoids. Oncotarget 2021, 12, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Sinha, R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environmental and molecular mutagenesis 2004, 44, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gamage, S.M.K.; Dissabandara, L.; Lam, A.K.; Gopalan, V. The role of heme iron molecules derived from red and processed meat in the pathogenesis of colorectal carcinoma. Critical reviews in oncology/hematology 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Azadbakht, L.; Esmaillzadeh, A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. The Journal of nutrition 2009, 139, 335–9. [Google Scholar] [CrossRef]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. The American journal of clinical nutrition 2014, 99, 352–60. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Kamat, P.K.; Kalani, A.; Familtseva, A.; Tyagi, S.C. High Methionine Diet Poses Cardiac Threat: A Molecular Insight. Journal of cellular physiology 2016, 231, 1554–61. [Google Scholar] [CrossRef] [PubMed]
- Helmus, D.S.; Thompson, C.L.; Zelenskiy, S.; Tucker, T.C.; Li, L. Red meat-derived heterocyclic amines increase risk of colon cancer: a population-based case-control study. Nutrition and cancer 2013, 65, 1141–50. [Google Scholar] [CrossRef]
- Le Marchand, L.; Hankin, J.H.; Pierce, L.M.; Sinha, R.; Nerurkar, P.V.; Franke, A.A.; Wilkens, L.R.; Kolonel, L.N.; Donlon, T.; Seifried, A.; Custer, L.J.; Lum-Jones, A.; Chang, W. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutation research 2002, 506-507, 205–14. [Google Scholar] [CrossRef]
- Nöthlings, U.; Yamamoto, J.F.; Wilkens, L.R.; Murphy, S.P.; Park, S.Y.; Henderson, B.E.; Kolonel, L.N.; Le Marchand, L. Meat and heterocyclic amine intake, smoking, NAT1 and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009, 18, 2098–106. [Google Scholar]
- Tiemersma, E.W.; Voskuil, D.W.; Bunschoten, A.; Hogendoorn, E.A.; Witteman, B.J.; Nagengast, F.M.; Glatt, H.; Kok, F.J.; Kampman, E. Risk of colorectal adenomas in relation to meat consumption, meat preparation, and genetic susceptibility in a Dutch population. Cancer causes & control : CCC 2004, 15, 225–36. [Google Scholar]
- Gore, F.M.; Bloem, P.J.; Patton, G.C.; Ferguson, J.; Joseph, V.; Coffey, C.; Sawyer, S.M.; Mathers, C.D.J.T.L. Global burden of disease in young people aged 10–24 years: a systematic analysis. 2011, 377, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Patsopoulos, N.A.; Evangelou, E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ (Clinical research ed.) 2007, 335, 914–6. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; Tetzlaff, J.; Deeks, J.J.; Peters, J.; Macaskill, P.; Schwarzer, G.; Duval, S.; Altman, D.G.; Moher, D.; Higgins, J.P. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical research ed.) 2011, 343, d4002. [Google Scholar] [CrossRef]
- Lau, J.; Ioannidis, J.P.; Terrin, N.; Schmid, C.H.; Olkin, I. The case of the misleading funnel plot. BMJ (Clinical research ed.) 2006, 333, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, J.A.O.I.M.P.R.S.A. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009, 151, 264–269. [Google Scholar] [PubMed]
- Herrmann, D.; Sinnett, P.; Holmes, J.; Khan, S.; Koller, C.; Vassar, M. Statistical controversies in clinical research: publication bias evaluations are not routinely conducted in clinical oncology systematic reviews. Annals of oncology : official journal of the European Society for Medical Oncology 2017, 28, 931–937. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. ; Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. International journal of evidence-based healthcare 2015, 13, 132–40. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2009, 181, 488–93. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.J.N. Solutions for a cultivated planet. 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Springmann, M.; Wiebe, K.; Mason-D, D.; Sulser, T.B.; Rayner, M.; Scarborough, P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: a global modelling analysis with country-level detail. The Lancet. Planetary health 2018, 2, e451–e461. [Google Scholar] [CrossRef]
- Recommendations, C.P. 2023. Available online: https://www.aicr.org/resources/media-library/10-cancer-prevention-recommendations/.
- International, W.C.R.F. 2023. Available online: https://www.wcrf.org/diet-activity-and-cancer/cancer-prevention-recommendations/.
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T.J.C.A.C.J.F.C. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. 2012, 62, 30–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sun, Y.; Qi, L.; Zhong, R.; Miao, X. Dietary legume consumption reduces risk of colorectal cancer: evidence from a meta-analysis of cohort studies. Scientific reports 2015, 5, 8797. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, Q.Q. Legume intake and risk of prostate cancer: a meta-analysis of prospective cohort studies. Oncotarget 2017, 8, 44776–44784. [Google Scholar] [CrossRef] [PubMed]
| Study ID | Study design | Number of participants | Study population | Intervention/Exposure | Control | Bias risk assessment tool | Outcomes | Type of metrics | Correction factor | Publication bias test | AMSTAR2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| country/region | age | follow-up | |||||||||||
| Alexander 2011[33] | PCS, NCCS | 25(-) | North America, Europe, Asia | - | - | The highest intake of red meat | Lowest category of red meat intake (include no red meat consumption) | - | Incidence of colorectal cancer | SRRE | total energy, body mass index (BMI), physical activity, alcohol, history of disease, education, income (socioeconomic status) | funnel plot | Critically Low |
| Smolińska 2010[34] | CCS, PCS | 22(-) | Britain, Spain, Switzerland, The Netherlands, Sweden, France, Italy, Australia, Canada | 30-80 age | - | Intake of red meat≥50g/day; Intake of red meat >1 times /day | Eat no more than 50g of red meat a day;Intake of red meat≤1 times/day | - | Incidence of colorectal cancer | RR | - | - | Critically Low |
| Song 2014[35] | CCS, PCS | 18(1,228,327) | Europe, North America, South America, Asia | - | 6.5-18 years | The highest intake of red meat | Lowest category of red meat intake (may include no red meat consumption) | NOS | Incidence of gastric cancer | SRRE | body mass index (BMI), total energy, smoking, vegetable intake | funnel plot, Egger’ stest, Begg’s test | Moderate |
| Jalal 2021[36] | PCS, NCC | 22(2,345,839) | Netherlands,UK,China,France,Italy,USA,Sweden, | 21-90 age | - | The highest intake of red meat | Lowest category of red meat intake (include no red meat consumption) | NOS | breast cancer | RR | Adjusted | Begg’s test, Egger’s test | Low |
| Fallahzadeh 2014[37] | CCS, PCS | 11(27,505) | Europe, USA, Canada | - | - | High intake of red meat (at least 1 serving per week) | Low intake of red meat (less than 1 serving per week) | - | Incidence of non-Hodgkin's lymphoma | OR | age, sex, body mass index (BMI) | funnel plot | Low |
| Kaluza 2012[38] | PCS | 5(329,495) | Europe, USA, Japan | - | - | Increase your red meat intake by one serving a day; One serving equals 100 to 120 g of fresh red meat | - | - | Stroke incidence | RR | sex and age, type and type of red meat consumption, stroke subtype | Egger’ stest | Moderate |
| Yang 2016[39] | PCS | 7(2,079,236) | America, Sweden, China, Japan | 30-83 age | 8-26 years | The highest intake of red meat | Lowest category of red meat intake (include no red meat consumption) | NOS | Stroke risk, stroke mortality | RR | age, smoking, fish, fruits, vegetables, body mass index and history of disease | Egger’ stest, Begg’s test | Critically Low |
| Gidyenne 2022[40] | PCS | 8(612,248) | USA, Japan, Spanish, China, Columbia, Sweden | 29-79 age | 7.6-30 years | The highest intake of red meat | Lowest category of red meat intake (include no red meat consumption) | NOS | the risk of total stroke incidence, (the risk of ischemic stroke incidence, the risk of Hemorrhagic stroke Mortality) | RR | age (in months), calendar time, body mass index (BMI), physical activity, smoking status, alcohol intake | - | Low |
| O'Connor 2020[41] | RCT | 24(1368) | USA, Australia, Iran, Denmark, China, Spain, Canada, Norway, Netherlands | ≥19 age | - | Red meat | Alternatives to red meat | NHLBI | GlycemicControl; Inflammatory Biomarkers | WMD | - | funnel plot, Egger’ stest, Begg's test | HIgh |
| Pan 2011[42] | PCS | 9 (4,033,322) | USA | 20 years old or more | 14 -28 years | Fresh red meat 100g/ day; | blank | - | Incidence of type 2 diabetes | HR | - | funnel plot, Egger’ stest, Begg’s test | Moderate |
| Rui Zhang 2021[43] | PCS | 14(724352) | Europe USA Asia,China,Dutch,Singapore,Spain,Finland,France,Japan,Sweden | 18-90 age | 5-28 years | The highest intake of red meat | Lowest category of red meat intake (include no red meat consumption) | NOS | Incidence of type 2 diabetes | RR | gender, location, follow-up, sample size, case | Egger’s test, Funnel plots | Low |
| Zeraatkar 2019[44] | RCT | 12(48,835) | Australia, France, Brazil, the United States, Israel | 22.4-70.9 age | - | High intake of red meat (at least 1 serving per week) | Low intake of red meat (less than 1 serving per week) | ROB | All-cause mortality, cardiovascular mortality | HR | - | - | High |
| Keren 2021[45] | PCS | 12(34,949) | USA,UK,Japan,China,Denmark | - | - | The highest intake of red meat | Lowest category of red meat intake (include no red meat consumption) | NOS | ischemic heart disease | RR | age, sex, smoking, relative weight, other dietc, location, study center (random effect), wealth index | Funnel plots, Egger’s test | High |
| Guasch-Ferré 2019[46] | RCT | 36(1,803) | Europe, United States | 18 years old or more | - | Red meat | A combination of alternatives to red meat | NHLBI | Total blood cholesterol levels, LDL cholesterol levels, HDL cholesterol levels, triglyceride levels, etc | WMD | - | funnel plot, Egger’ stest, Begg's test | High |
| O'Connor 2017[47] | RCT | 24(-) | USA | 19 years old or more | - | Total red meat≥0.5 servings (35 grams or 1.25 ounces) per day | Eat less than 0.5 servings (35 grams or 1.25 ounces) of red meat per day | ROB | Total blood cholesterol levels,LDL cholesterol levels, HDL cholesterol levels, triglyceride levels, etc | WMD | age, sex, body mass index (BMI), duration of intervention, total energy | - | Moderate |
| Farzaneh Asoudeh 2022[48] | POS | 5(251,742) | UK, USA | 20-75 age | 6-26 years | The highest intake of red meat | Lowest category of red meat intake (include no red meat consumption) | NOS | risk of kidney stones | RR | body mass index (BMI), alcohol consumption, smoking | Begg’s test, Egger’s test | High |
| Cristina 2022[49] | CCS | 8(62438) | Australia and New Zealand;United Kingdom;Iceland;Netherlands;United States | 10-70 age | - | Red meat | Low intake of red meat (less than 1 serving per week) | NOS | age-related eye disease | OR | - | funnel plot | Low |
| An 2020[50] | RCT, PCS | 12(41478) | USA, Australia, Netherlands, Denmark, Canada, China, Belgium, Germany Poland | 18-70 age | - | Pork meat | Other foods (such as chicken) | NIHSS, ROB | Body weight, Lean mass, Body fat percentage, Fat mass, Overweight, Obesity | β, OR | - | Egger’ stest, Begg's test | Low |
| Study ID | Human health outcomes | Type of metrics | Summary effect size (95%Cl) | Model | P value | I2(P value) | Egger's P value | Statistically significant |
|---|---|---|---|---|---|---|---|---|
| Smolińska 2010[34] | Incidence of colon cancer | RR | 1.21 (1.07, 1.37) | Random | - | - | - | Yes |
| Song 2014[35] | Incidence of gastric cancer | SRREs | 1.37(1.18, 1.59) | Random | P < 0.05 | 67.6% (0.001) | 0.52 | Yes |
| Jalal 2021[36] | Effect of red meat intake on breast cancer | RR | 1.05(1.00, 1.11) | Random | P = 0.03 | 52% | 0.022 | No |
| Fallahzadeh 2014[37] | the risk of non-Hodgkin’s lymphoma | OR | 1.10(1.02, 1.19) | Random | P = 0.01 | 59.4% (0.001) | Publication bias based on funnel plot was not significant | Yes |
| the subtype of diffuse large B-cell lymphoma | OR | 1.20(1.04, 2.37) | Random | P = 0.05 | 74.9% (0.001) | - | Yes | |
| Kaluza 2012[38] | Relative risks of total incident stroke and stroke mortality | RR | 1.11(1.03, 1.20) | Random | - | 0%(0.65) | 0.76 | Yes |
| O'Connor 2020[41] | blood glucose concentration | WMD | 0.040(-0.049, 0.129) | Random | P > 0.05 | 68% | P > 0.05 | No |
| blood insulin concentration | WMD | -0.710(-6.582, 5.162) | Random | P > 0.05 | 28% | P > 0.05 | No | |
| HOMA-IR | WMD | 0.11(-0.072, 0.293) | Random | P > 0.05 | 64% | P > 0.05 | No | |
| C-reactive protein | WMD | 2.424(-1.460, 6.309) | Random | P > 0.05 | 27% | P > 0.05 | No | |
| Pan 2011[42] | Incidence of type 2 diabetes | RR | 1.19(1.04, 1.37) | Random | P < 0.001 | 93.3% (0.001) | 0.35 | Yes |
| Zeraatkar[44] | all-cause mortality | HR | 0.99(0.95, 1.03) | Random | P > 0.05 | - | - | No |
| cardiovascular mortality rate | HR | 0.98(0.91, 1.06) | Random | P > 0.05 | - | - | No | |
| cardiovascular disease | HR | 0.99(0.94, 1.05) | Random | P > 0.06 | - | - | No | |
| Keren 2021[45] | ischemic heart disease | RR | 1.09(1.06, 1.12) | Random | NA | 41.3% (0.04) | 0.7 | Yes |
| Guasch-Ferré 2019[46] | total cholesterol | WMD | 0.264(0.144, 0.383) | Random | P < 0.001 | - | P > 0.05 | Yes |
| triglyceride concentrations | WMD | -0.181(-0.349, -0.013) | Random | P = 0.035 | - | P > 0.05 | Yes | |
| LDL cholesterol | WMD | 0.198(0.065, 0.330) | Random | P = 0.003 | - | P > 0.05 | Yes | |
| HDL cholesterol | WMD | -0.065(-0.109, -0.020) | Random | P = 0.004 | - | P > 0.05 | Yes | |
| Farzaneh Asoudeh 2022[48] | risk of kidney stones | RR | 1.02(1.91, 1.15) | Random | NA | 81.1% (0.00) | 0.01 | No |
| Cristina 2022[49] | age-related eye disease | OR | 1.41(1.07, 1.86) | Random | NA | 83.8% (0.000) | Publication bias based on funnel plot was not significant | Yes |
| An 2020[50] | Overweight | OR | 0.89(0.48, 1.64) | Random | P > 0.05 | 64% | - | No |
| Obesity | OR | 1.06(0.30, 3.71) | Random | P > 0.05 | 81% | - | No | |
| In experimental studies that did not limit total daily energy intake | ||||||||
| Body weight | β | -0.86(-1.55, -0.17) | Random | P < 0.05 | 0% | - | Yes | |
| Body fat percentage | β | -0.77(-1.43, -0.11) | Random | P < 0.05 | 90.40% | - | Yes | |
| Lean mass | β | 1.79(-1.74, 5.32) | Random | P > 0.05 | 98.40% | - | No | |
| In experimental studies that restricted energy intake | ||||||||
| Body weight | β | -5.56(-10.59, -0.55) | Random | P < 0.05 | 98.70% | - | Yes | |
| Lean mass | β | -1.50(-1.62, -1.39) | Random | P < 0.05 | 0% | - | Yes | |
| Fat mass | β | -6.6(-6.79, -6.42) | Random | P < 0.05 | 0% | - | Yes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





