1. Introduction
Dengue fever/dengue hemorrhagic fever is the most prevalent arboviral disease caused by dengue viruses (DENVs). This virus spreads throughout more than 120 countries in tropical and subtropical regions of the world with a few hundred million dengue infections reported annually [
1]. DENV is a member of the
Flavivirus genus. Its genome is an 11-kbs positive-sense, single-stranded RNA, which encodes 3 structural proteins (capsid (C), pre-membrane (prM) and envelope (E)) and 7 non-structural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, NS5) [
2]. Based on the antigenicity of E protein, DENVs consist of four closely related serotypes (DENV1-4). All serotypes can cause disease that manifests ranging from febrile illness to life-threatening dengue hemorrhagic fever. The specific treatment has not been devised to date, thus, only supportive care is implemented. The mosquito vector control is partially effective. As a result, strategies for developing an effective vaccine have gained more significance. For decades, efforts to develop a safe and effective vaccine to prevent DENV infection have faced several challenges, particularly evaluation of vaccine efficacy and safety to rule out the risks of vaccine-breakthrough infection.
After several decades of research, Dengvaxia is the first licensed dengue vaccine. Unfortunately, Dengvaxia fails to activate uniform protection against all four serotypes of DENVs [
3]. This exacerbates the incidences of hospitalization and severe symptoms upon subsequent natural DENV infection in certain population [
3,
4]. Due to this fact, other platforms of vaccine such as nucleic acid-based and subunit vaccines are on demand. One of the advantages of a subunit vaccine is its adjustable immunogenicity. Thus, balanced immune responses against DENV serotypes can be reached. Unfortunately, the hereditary problem of a subunit vaccine is its low immunogenicity. To overcome this problem, the adjuvant nanodelivery system is used to enhance the immunogenicity of protein immunogens [
5,
6]. Khan R.A. et al revealed that immunization with tetravalent EDIII encapsidated in Poly (lactic-co-glycolic acid) (PLGA) nanoparticles in combination with TLR agonists triggered robust antibody and antigen-specific polyfunctional T cell responses [
7]. Our group recently reported that nanoparticles enhance the immunogenicity of UV-inactivated DENV-2 (UV-DENV2) and NS1
1-279 by at least two mechanisms. First, nanoparticles effectively deliver immunogens into the target cells and control releasing of immunogens within those cells. Secondly, nanoparticles are made from materials that have been shown to exert adjuvant activity [
8,
9]. Beside the advantages mentioned above, this platform of non-replicating vaccine may solve the problem of the immunological interference among vaccine antigens through a dose adjustment. In addition, the heterologous types of immunogens are feasible to be mixed within nanoparticles. This results in a broad range of immune targets [
10,
11,
12,
13]. Thus, a set of adjuvants with delivery system can improve both the breadth and quality of immune response [
14,
15].
The cell-wall skeletons of
Mycobacterium bovis Bacillus Calmette-Guérin (BCG-CWS) have been well recognized as a potent adjuvant for antigen-specific cellular immune response [
16,
17]. The major bioactive components of BCG-CWS are mycolic acids and peptidoglycan attached to arabinogalactan which are toll-like receptor (TLR-2 and TLR-4) agonists [
18,
19]. This adjuvant has been intensively explored in an anti-cancer vaccination [
19,
20]. Beyond anti-tumor enhancing properties, BCG-CWS can strengthen the immunogenicity of the inactivated- and subunit-viral vaccines [
21,
22]. These made BCG-CWS a potent adjuvant of vaccine against not only cancer therapy but also infectious agents.
In the present study, the adjuvant effect of BCG-CWS together with TMC nanodelivery on DENV immunogens was investigated using the murine model. We found that immunization with UV-DENV2 TMC NPs and NS11-279TMC NPs in the presence of BCG-CWS strongly stimulated production of neutralizing antibodies that well activate complement fixation. Moreover, the present study demonstrated that cell-mediated immune responses against DENV-2 and NS11-279 were significantly promoted by BCG-CWS. In conclusion, we revealed, here, that BCG-CWS exerted its adjuvant activities through both arms of immune responses.
2. Materials and Methods
2.1. Cell cultures and viruses
BHK-21 cells, Vero cells and C6/36 cells were grown in Minimum Essential Media (MEM; Gibco;Thermo Fisher Scientific, Inc.) as described [
9].Primary human monocytes derived macrophages (MCs) were generated as described previously [
23]. DENV-1 (strain 16007), DENV-2 (strain 16681), DENV-3 (strain 16562) and DENV-4 (strain 1036) were propagated in C6/36 cells. Viruses were aliquoted and stored at -80°C. The virus titers were quantitated using plaque assay.
2.2. Animals
Adult female BALB/c mice (6–8 weeks old) were purchased from Nomura Siam International (Nomura Siam International Co., Ltd). All animal experiments were performed under ethical guidelines approved by the Faculty of Science, Mahidol University Animal Care and Use Committee (SCMU-ACUC, protocol number: MUSC60-009–359).
2.3. Preparation of BCG cell wall skeleton (BCG-CWS)
BCG-CWS was prepared as previously described [
24]. Briefly,
M. bovis BCG Tokyo 172 were cultivated on Sauton’s medium and were inactivated by autoclaving. The wet mass cells were homogenized in 2% (w/v) Triton X-100 using a VCX 750 ultrasonic processor and cell debris was removed. The cell wall in supernatant was harvested and treated with benzonase (Merck, Darmstadt, Germany) before being washed with 2% (w/v) Triton X-100 and 0.5% (w/v) SDS. The cell wall fraction was subsequently subjected to the deproteinization and delipidation using proteinase E (Merck, Darmstadt, Germany) and the chloroform:methanol extraction, respectively. The purified BCG-CWS was washed with methanol and further dried at 50°C for overnight to obtain a dried mass of BCG-CWS.
2.4. N,N,N-trimethyl chitosan nanoparticles loading dengue antigens (UV-DENV2 TMC NPs and NS11-279TMC NPs)
The UV-DENV2 TMC NPs and NS1
1-279TMC NPs were supplied by Jearanaiwitayakul T
et al. These immunogens were prepared as previously described [
8,
9].
2.5. Immunization study in mouse model
Mice were divided into four groups (3-5 mice/group). The first group was placebo group. The other three groups were immunized with the encapsidated immunogens including UV-DENV2/NS11-279TMC NPs or encapsidated immunogens adjuvanted with 7.5 μg/dose of BCG-CWS (UV-DENV2/NS11-279TMC NPs + BCG-CWS-7.5) or immunogens adjuvanted with 60 μg/dose of BCG-CWS (UV-DENV2/NS11-279TMC NPs + BCG-CWS-60). Mice were administrated at 10 μg/dose of each immunogen through subcutaneous injection (s.c.) with a total volume of 100 μL on day 0, 15 and 30. At two weeks following the last dose, all mice were euthanized. Blood and spleens were harvested for further analysis.
2.6. Detection of antigen-specific antibodies
The levels of IgG, IgG1 and IgG2a in sera of immunized mice were determined by indirect ELISA as described [
25]. Briefly, purified UV-DENV2 or NS1
1-279 (1 μg/well) were pre-coated for overnight, washed and blocked with 1% BSA (W/V) in PBST. The 2-fold serial diluted sera in blocking solution were then added into each well and incubated for 2 h. After incubation, DENV-2 or NS1
1-279-specific antibodies were detected using 100 μL of HRP-conjugated goat anti-mouse IgG (1:3000 dilution, Invitrogen) and the TMB substrate (Bio-Rad). The reaction was terminated and the absorbance was read at 450 nm. The end-point titer was considered as the reciprocal of the highest dilution which generates an OD value greater than the cut-off value (half of the OD value of the negative control diluted at 1:100) [
25].
For the detection of IgG1 and IgG2a subclasses, HRP-conjugated goat anti-mouse IgG1 or IgG2a antibodies (1:4000, Southern Biotech, USA) were used as probes. The levels of DENV-2 or NS1
1-279-specific IgG1 and IgG2a were reported as OD value as described by Wang R.
et al. [
25].
2.7. Whole virion capture ELISA
Microtiter plates (Corning costar, USA) were coated with DENV immune human serum at dilution of 1:1600, overnight at 4°C. The plates were washed and blocked with 1% BSA (w/v) in PBST before being incubated with UV-DENV2 (1 μg/well). After 2 h of incubation, sera (at 1:2000 dilution) from each mouse were applied. DENV-antibody interaction was detected using HRP-conjugated goat anti-mouse IgG antibody (1:3000 dilution, Invitrogen) and TMB substrate (Bio-Rad). The signals were measured at 450 nm.
2.8. Measurement of neutralizing antibody
The levels of DENV neutralizing antibodies were quantified by plaque reduction neutralization test (PRNT) as described previously [
26]. In brief, heat-inactivated diluted sera were incubated with an equal volume of DENVs containing 50 PFU. The virus-antibody complexes were performed in the presence and in the absence of rabbit complement (1:200, Bio-Rad) before being inoculated onto monolayer of Vero cells. After incubation, the layer of infected cells was overlaid with a plaque medium. The plaque formation was visualized using crystal violet staining. The 50% plaque reduction (PRNT
50) value against virus control was computed by non-linear regression analysis using GraphPad Prism version 7.
2.9. Antibody-dependent complement mediated cytotoxicity assay
The monolayer cultures of DENV-2 infected BHK-21 cells were washed and incubated with heat-inactivated immunized mouse sera at 37°C for 1 h. After treatment, unbound antibodies were removed by washing with PBS. Two hundred microliters of 1:80 diluted rabbit complement (Bio-Rad) was applied and incubated at 37°C for 1 h. After incubation, cultured supernatant was harvested and subjected to lactase dehydrogenase detection using CytoTox 96® Non-Radioactive Cytotoxicity Assay kit (Promega). The percentage of cytotoxicity was computed as described previously [
9].
2.10. Antibody-dependent enhancement of DENV infection in primary human macrophages (MCs)
DENV-1, -3 or -4 at the MOI of 0.1 were incubated with the equal volume of 1:2 diluted heat-inactivated sera. After incubation, the mixtures were inoculated onto the MC cultures and further incubated at 37°C for 1.5 h. Mouse anti-Flavivirus antibody (clone 4G2) at the dilution of 1:1000 was used as a positive control of ADE-infection [
27]. After incubation, inoculated cultures were washed. Cells were further maintained in RPMI 1640 (Gibco) supplemented with 10% FBS at 37°C with a 5% CO
2 atmosphere for 24 h. Culture supernatant was harvested and infectious virus titer was enumerated by plaque assay.
2.11. Stimulation of splenic lymphocytes
Briefly, splenic single cell suspension isolated from spleens of immunized mice (107 cells/well) was cultured and stimulated with purified UV-DENV2 or NS11-279 (10 µg/mL) for 72 h. The mock-stimulated cultures were used as a negative control. For intracellular cytokine staining, Brefeldin A (BioLegend, San Diego, CA, USA) was added to the cultures and cells were harvested, stained with TruStrain FcX (anti-mouse CD16/32 antibody, BioLegend), and double stained with antibodies specific to CD3, CD4, CD8 (BD Biosciences, San Diego, CA, USA) and IFN-γ (BioLegend). The stained cells were subsequently analyzed by flow cytometry.
In parallel, the levels of IFN-γ, IL-2 and IL-4 in supernatant of stimulated cultures were monitored using an ELISA kit according to the manufacturer’s protocol (BioLegend).
2.12. Statistical analysis
The results were presented as mean ± standard deviation (SD). Statistical analysis was conducted using Student’s t-test for comparison between two groups of study. A value of p < 0.05 was considered as statistical significance.
3. Results
3.1. Mice immunized with the BCG-CWS-adjuvanted vaccine elicited strong antibody responses.
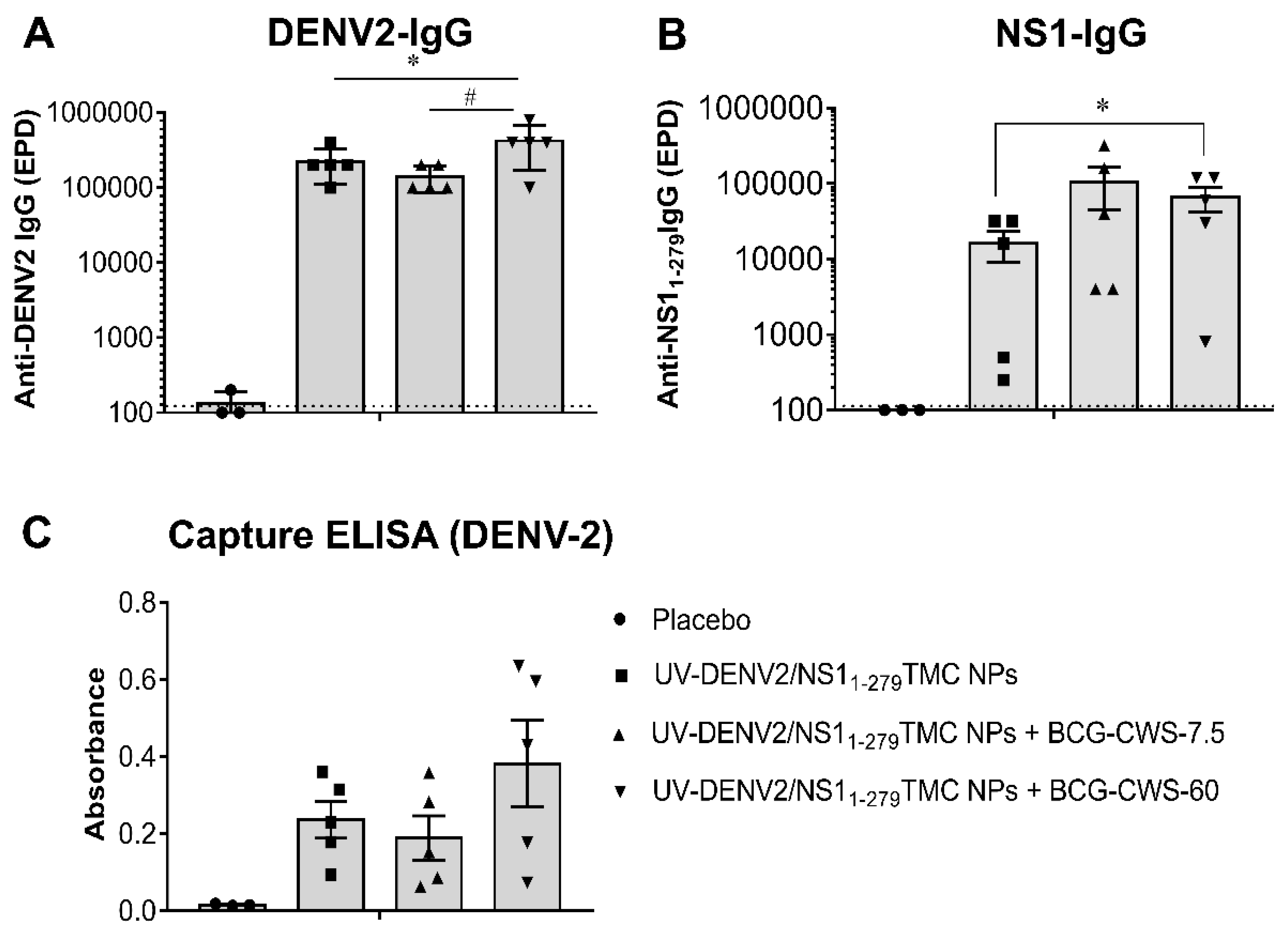
We investigated whether BCG-CWS could enhance the immunogenicity of the tested immunogens in the murine model. BALB/c mice were subcutaneously immunized with three dosages of immunogen as described in Materials and Methods. On day 45 of immunization, blood was harvested and subjected to antibody quantitation using indirect ELISA.
Figure 1A showed that DENV-2 specific IgG responses were induced in all mice immunized with tested immunogens. Significantly, the encapsidated immunogens adjuvanted with 60 µg BCG-CWS stimulated anti-DENV2 IgG titer higher than that of the encapsidated immunogens without and with 7.5 µg BCG-CWS (
Figure 1A). In
Figure 1B, the production of NS1
1-279 specific Ab was monitored. We found that all mice exposed to the immunogens upregulated the anti-NS1
1-279 antibody production. As expected, mice administered with UV-DENV2/NS1
1-279TMC NPs and BCG-CWS adjuvant elicited higher levels of anti-NS1
1-279 antibody than that of mice received immunogens without BCG-CWS (
Figure 1B). Results here demonstrated that BCG-CWS increased the immunogenicity of the encapsidated immunogens.
To determine whether induced DENV-2 specific antibodies could bind to native epitopes of DENV-2 virion, sera harvested on day 45 were subjected to capture ELISA as described in Materials and Methods. As revealed in
Figure 1C, all forms of tested immunogens activated production of the antibodies that bound to native epitopes on DENV-2 virions. Notably, the regimen that contains 60 µg of BCG-CWS adjuvant stimulated the strongest responses. Therefore, the BCG-CWS at 60 µg was chosen for further investigation.
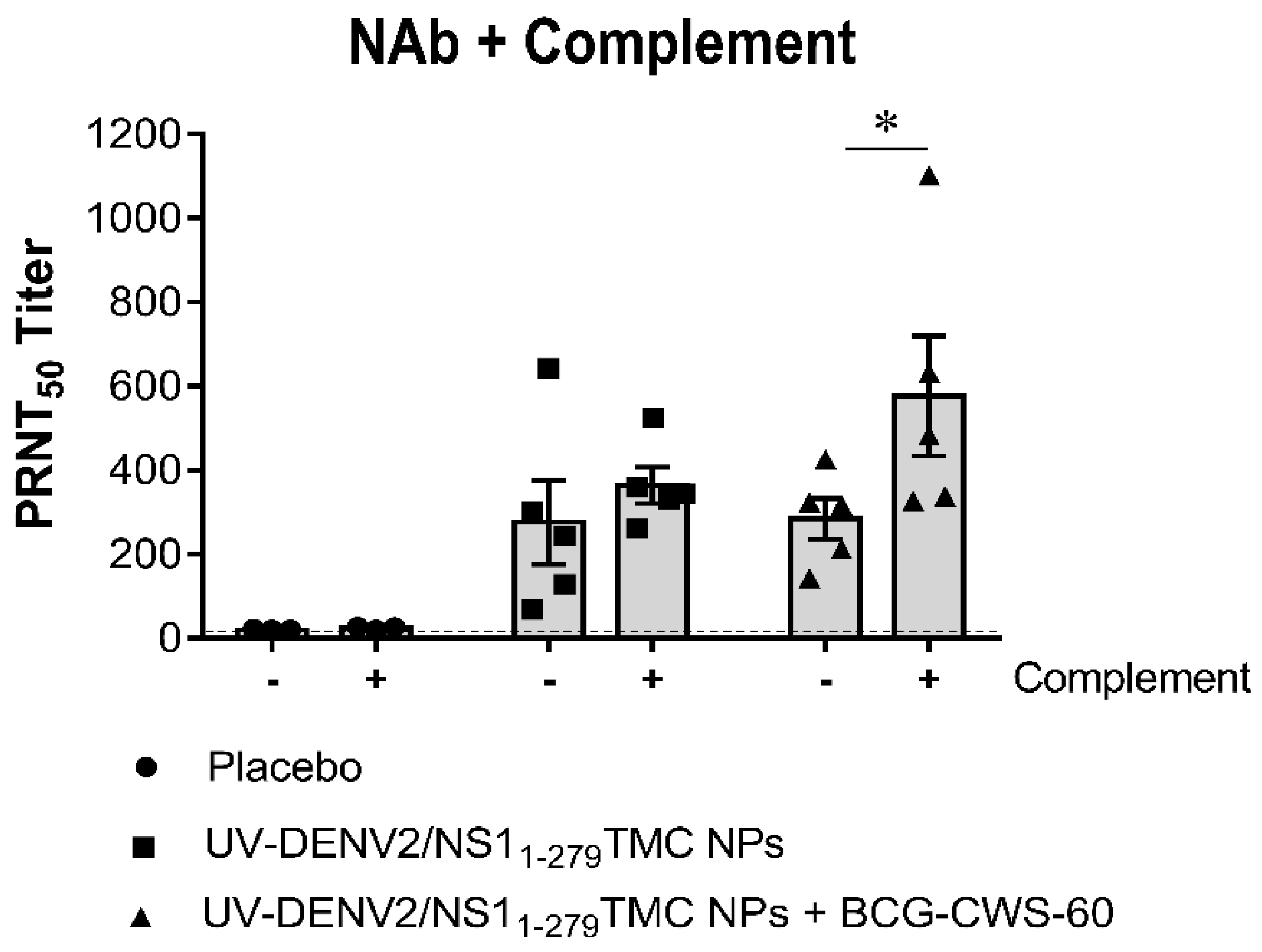
3.2. The BCG-CWS enhances the production of complement-fixing neutralizing antibody.
To determine how potent these antibodies neutralize DENV-2, the PRNT
50 was performed in the presence or the absence of complement. As shown in
Figure 2, the encapsidated immunogens with or without BCG-CWS potently stimulated neutralizing antibody (NAb) activity compared to the control sera. Surprisingly, there was no difference in the levels of neutralizing activities in sera of mice immunized with adjuvanted- or non-adjuvanted immunogens once detected by PRNT
50.
The complement-dependent neutralization is recently shown to correlate with protection against DENV infection [
28]. We, thus, compared DENV-2 neutralization in the presence or absence of complement. By using PRNT
50, sera obtained from mice immunized with BCG-CWS-adjuvanted immunogens exerted a higher level of DENV-2 neutralizing activities in the presence of complement than when tested without complement (PRNT
50 titer at 577 ± 142 vs 285 ± 49)(
Figure 2). Results here suggested that BCG-CWS (60 µg)-adjuvanted immunogens strongly activated the production of the complement fixing antibody (CFA) against DENV-2.
Furthermore, serum samples were tested for their ability to cross-neutralize against the heterotypic DENVs using PRNT assay. We observed that all tested regimens induced production of antibodies that neutralized DENV-1,-3 and -4 with a varying degree of cross-neutralization (
Table 1). As expected, mice received encapsidated immunogens with 60 µg BCG-CWS exhibited the strongest cross-reactive NAb responses. The complement-dependent cross-neutralization was also determined using the complement fixing PRNT
50. As expected, we found a significant increase in neutralization in sera of mice received the BCG-CWS-adjuvanted immunogens, when the complement system was implemented (
Table 1). All together, these findings demonstrated that administration with BCG-CWS-adjuvanted DENV-2 nanospheres stimulated a broad neutralizing antibody response.
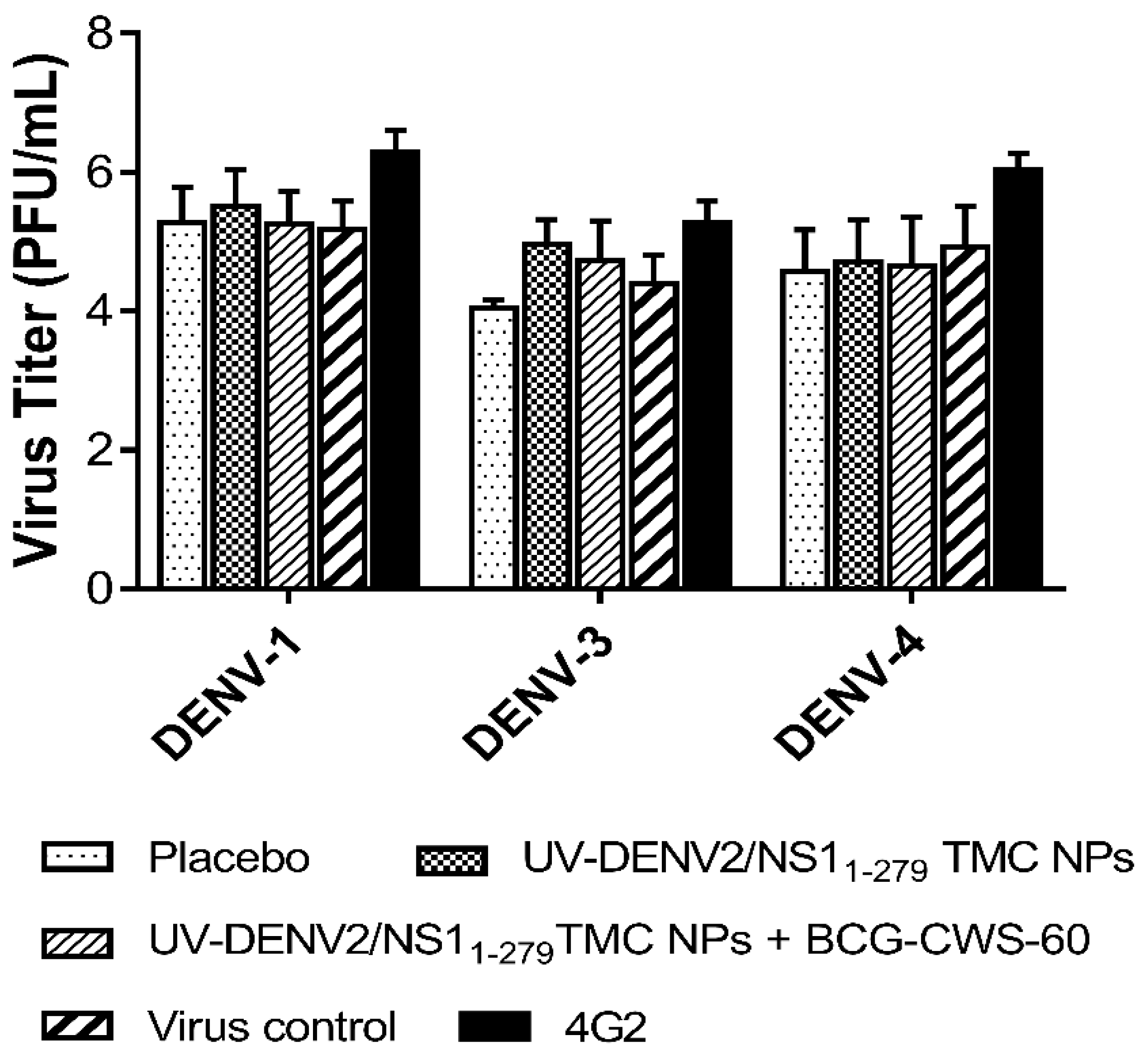
3.3. The antibodies activated by BCG-CWS-adjuvanted vaccine did not possess ADE infection towards heterotypic DENV infection.
Because the BCG-CWS-adjuvanted vaccine induced cross-reactive neutralizing antibodies, we thus investigated whether these antibodies were able to enhance DENV infection in Fcγ receptors (FcγR) expressing cells. An
in vitro antibody-dependent enhancement (ADE) assay was performed using human monocyte-derived macrophages (MCs) as described in Materials and Methods. We found that the tested sera, at 1:2 dilution, did not exert the ADE infection specific to DENV-1, -3 and -4 (
Figure 3). This suggested that immunization with our nanodelivery platform of vaccine may not stimulate the vaccine breakthrough infection.
3.4. BCG-CWS enhanced the complement-dependent cytolysis of DENV-infected cells.
The structural proteins such as prM, E and NS1 are expressed on the surface of infected cells [
29,
30]. The antibodies targeting these surface antigens may mediate clearance of infected cells via the antibody-dependent complement fixation. We thus evaluated whether sera of mice receiving the BCG-CWS-adjuvanted immunogens could recognize dengue antigens on the surface of infected cells. Flow cytometry analysis showed that BCG-CWS-adjuvanted immune sera efficiently bound to dengue antigens expressed on the surface of DENV-2 infected cells (
Figure S1). Therefore, these infected cells were used as the target of antibody-complement cytotoxic assay. As shown in
Figure 4, sera from mice received the tested immunogens with or without BCG-CWS adjuvant effectively elicited cytotoxic effect specific to DENV-2 infected cells through the complement activation. However, sera from mice received BCG-CWS-adjuvanted immunogens exerted higher cytolytic activities. At the 1:50 dilution of these sera, 16.25 ± 2.79% and 22.06 ± 2.64% cytolysis were induced by BCG-CWS at 7.5 and 60 µg/dose, respectively (
Figure 4). Taken together, our results suggested that BCG-CWS exerted its adjuvant activity through increasing the production of complement fixing antibody.
3.5. Immunization with BCG-CWS-adjuvanted vaccine robustly activated cellular responses specific to both DENV-2 and NS11-279 antigens.
To see whether our platforms of immunogens can activate cellular responses, splenocytes were obtained from immunized mice, cultured and stimulated with either UV-DENV2 or NS1
1-279 protein. The frequencies of total CD4
+ and CD8
+ cells, and functional T cells (IFN-γ
+CD4
+ and IFN-γ
+CD8
+ cells) in the stimulated splenocyte cultures were monitored using flow cytometry.
Figure 5A revealed that all groups of the immunized mice elicited DENV-2 and NS1
1-279 specific total CD4
+ T cell responses at a similar level to that of the placebo group. However, the differences were observed for IFN-γ
+CD4
+ cell quantitation in which DENV-2- and NS1
1-279-specific IFN-γ
+CD4
+ cell responses were strongly induced in response to BCG-CWS-adjuvanted vaccines (
Figure 5C). For CD8
+ cell quantitation, all forms of immunogens increased a remarkable level of total CD8
+ cell responses as well as the IFN-γ
+CD8
+ cells when compared to that of the negative control group (
Figure 5B,D). Surprisingly, among the vaccine formulations, encapsidated immunogens with 7.5 µg BCG-CWS were the most potent activator for DENV-2 and NS1
1-279-specific IFN-γ
+CD8
+cell responses (
Figure 5D). Overall, a significant increase in percentages of activated CD4
+/CD8
+ T cells suggested that combination vaccines containing UV-DENV2/NS1
1-279 TMC NPs and BCG-CWS potently stimulated cellular immunity against DENV-2 and NS1
1-279 antigens.
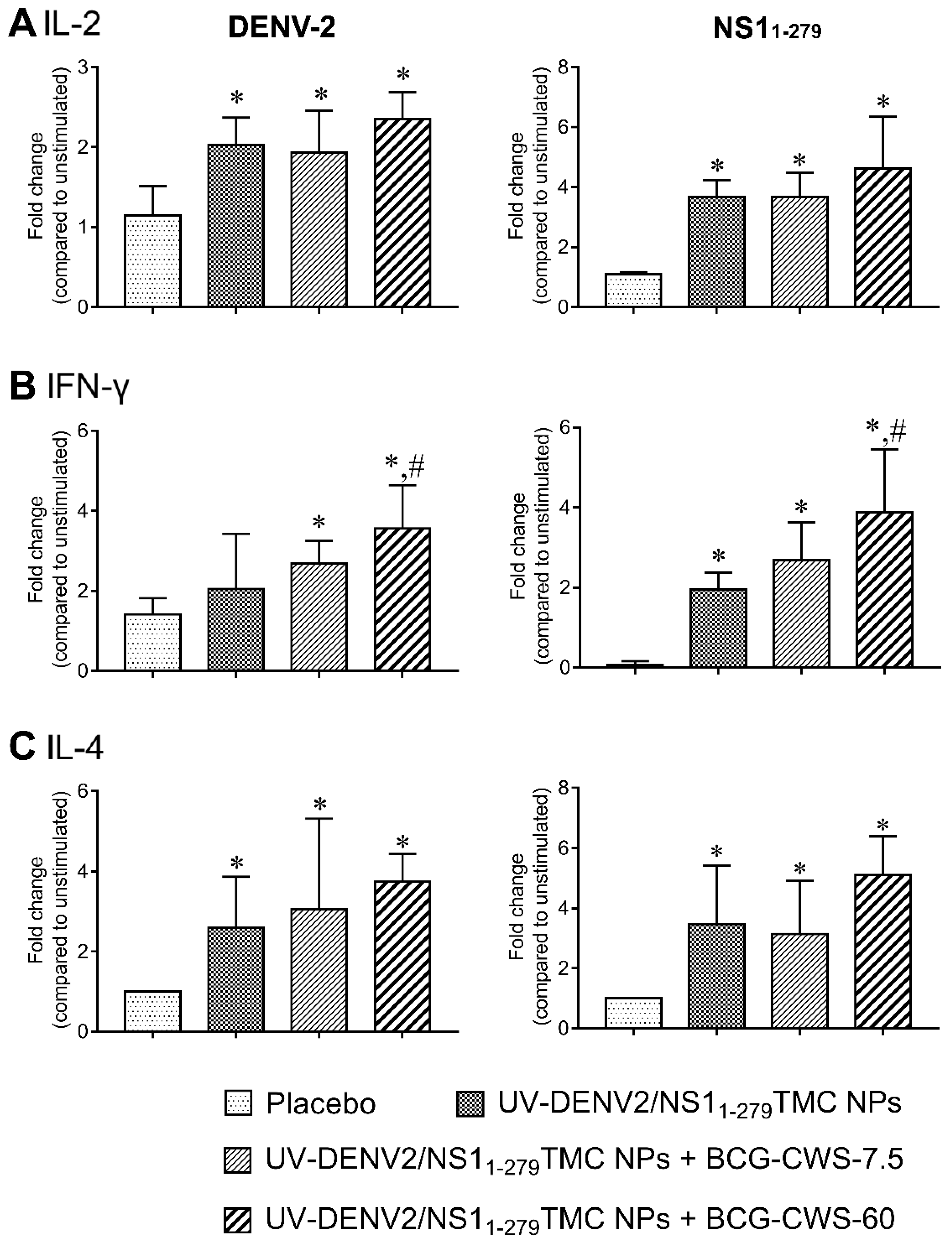
3.6. Combination of UV-DENV2- and NS11-279 nanospheres with BCG-CWS activated mixed Th-1/Th-2 responses.
Th-1 and Th-2 cells play important roles in shaping host defense against pathogens. For example, Th-1 cells mainly secrete IFN-γ, a signature cytokine which activates macrophages and DCs [
31]. On the other hand, IL-4 derived from Th-2 cells stimulates humoral immune response, promotes B cell proliferation and induces antibody production [
32]. We thus investigated the ability of our vaccine platforms to induce Th-1/Th-2 responses. The amounts of secreted cytokines; IL-2 and IFN-γ as Th-1 cytokines and IL-4 as Th-2 cytokine in splenic culture supernatant were determined using ELISA. As shown in
Figure 6A-C, splenocytes isolated from mice received encapsidated immunogens with or without BCG-CWS robustly upregulated production of IL-2, IFN-γ as well as IL-4 in response to DENV-2 or NS1
1-279 stimulation. Importantly, the strongest responses of these cytokines were demonstrated in the culture of splenocytes of mice received BCG-CWS-adjuvanted immunogens (
Figure 6A-C). These results suggest that BCG-CWS synergized with the nanodelivery system to stimulate both Th-1 and Th-2 cytokine responses.
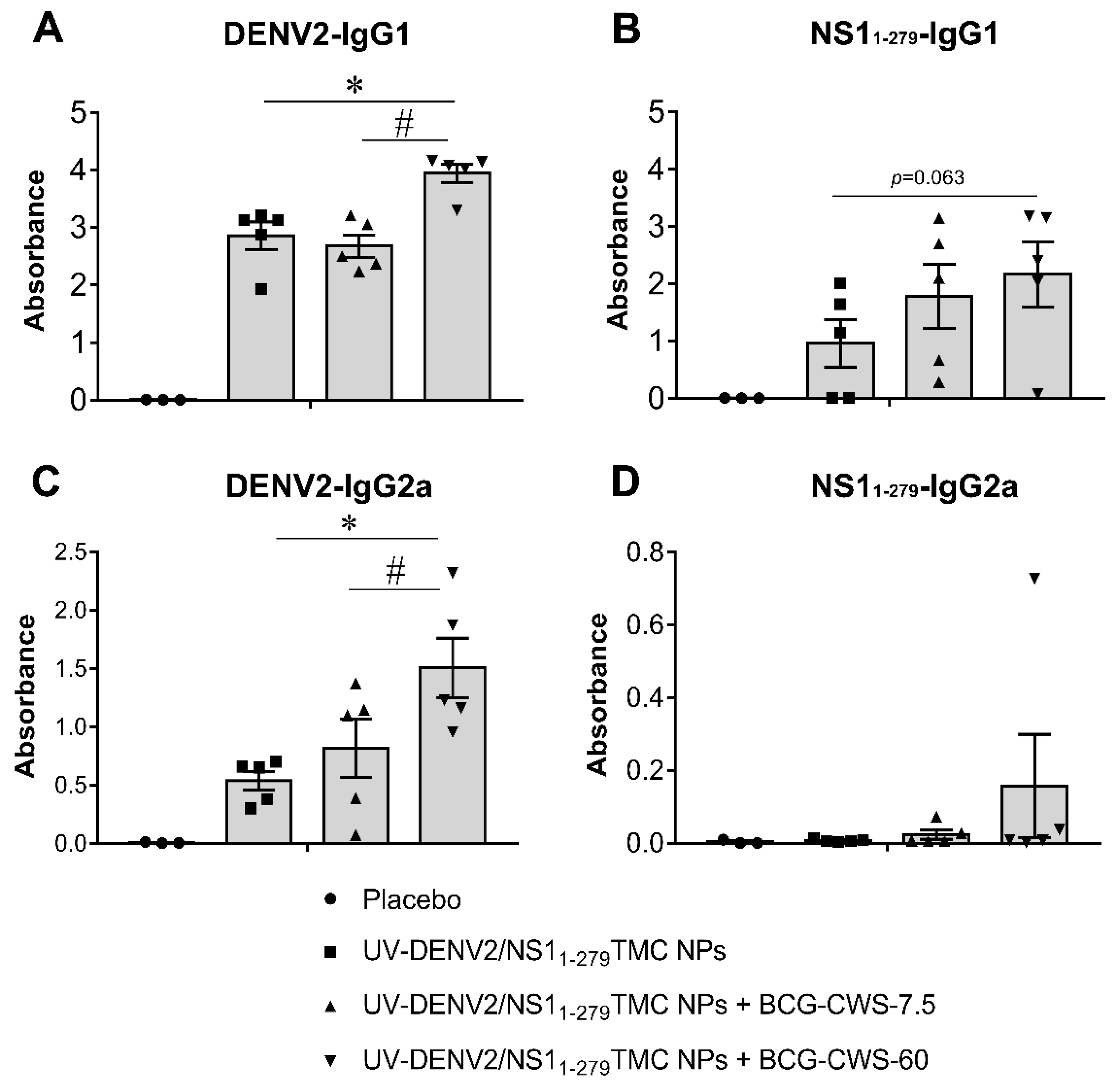
The activation of Th-1/Th-2 immune responses following vaccine administration was further validated using serum IgG2a and IgG1 as the indicative markers of Th-1 and Th-2 skewed responses, respectively. As shown in
Figure 7A, immunization of all tested regimens significantly increased the production of anti-DENV2 IgG1 compared to that of the placebo vaccination. Notably, the greater levels of anti-DENV2 IgG1 production were demonstrated by immunogens administered with BCG-CWS at 60 µg. Similarly, stimulation by immunogens supplemented with 60 µg BCG-CWS also upregulated a robust anti- NS1
1-279 IgG1 response (
Figure 7B).
For IgG2a response, we found that all tested forms of immunogens enhanced anti-DENV-2 IgG2a production compared to that of the control substance (
Figure 7C). As expected, the encapsidated immunogens with 60 µg BCG-CWS induced the highest level of anti-DENV-2 IgG2a than did other forms of immunogens (
Figure 7C). Unexpectedly, encapsidated immunogens alone were not immunogenic to elicit anti-NS1
1-279 IgG2a response, while encapsidated immunogens with BCG-CWS could partially restore anti- NS1
1-279 IgG2a production (
Figure 7D).
4. Discussion
The main desirable characteristic of protective dengue vaccine is the life-long production of protective T and B cell responses against all four serotypes of DENV [
33]. This has shed some light on a multivalent dengue vaccine which is a dose adjustable non-replicating vaccine. In the present study, we developed a bivalent dengue vaccine candidate. This candidate contains UV-inactivated DENV-2 and NS1
1-279 proteins. Immunization with this bivalent vaccine candidate should be able to neutralize virus particles, attenuate dengue toxin and finally eliminate virus-infected cells [
8,
9]. To promote the immunogenicity of our bivalent vaccine, the adjuvant (BCG-CWS) and the delivery system (TMC NPs) were applied. We revealed that the adjuvanticity of BCG-CWS on antibody production was dose dependent. This was supported by BCG-CWS at 60 µg exerted higher adjuvant effect than did BCG-CWS at 7.5 µg. Surprisingly, the adjuvant activity of BCG-CWS on NS1 protein was more pronounced than the activity against UV-DENV2. Importantly, we reported here that BCG-CWS significantly promoted production of the complement fixing antibody.
The significance of complement fixing antibody in DENV infection is recently shown by Dias AG Jr
et al. [
28]. They demonstrated that the level of complement-fixing neutralizing antibody correlates with protection against DENV-3 infection. This protection is likely mediated by deposition of complement on antibodies targeted DENV E- and NS1 proteins [
28]. The immune complexes induce lysis of virus particles as well as virus-infected cells, leading to enhanced viral elimination [
28]. Our results supported their findings. We showed that BCG-CWS enhanced the activities of DENV particle- and DENV infected cell elimination via complement-dependent antibody neutralization. This suggested that one of the adjuvant effects of BCG-CWS was to promote the production of antibody with potent Fc effector function.
The sub-neutralizing level of anti-DENV antibody has been shown to mediate pathogenesis during subsequent infection with the heterotypic serotypes [
34]. In our present study, mice administered with BCG-CWS-adjuvanted immunogens mounted a strong complement fixing antibody response that reacted to all four serotypes of DENV. Significantly, the mounted antibody did not exert an
in vitro ADE phenomenon. How these induced antibodies restricted ADE phenomenon in our study remains unclear. It was previously shown that C1q which interacted with the Fc region of the antibody could interfere with the FcR-Fc interaction. This interference attenuated the cell signaling through Fc function [
35]. Whether IgG antibody in our model inhibits ADE phenomenon via C1q function awaits further investigation.
BCG-CWS is well demonstrated to have a strong anti-tumor effect by enhancing T cell activation [
16]. After administration, BCG-CWS is efficiently uptaken by DCs and MHC-II
+ cells in the spleen, resulting in induction of antigen-specific cytotoxic T cell (CTL) responses and regression of tumor growth in mice [
16,
36]. These data indicated that BCG-CWS possessed potent immunomodulatory activity on cellular responses. Consistently, we revealed here that co-delivery of BCG-CWS and vaccine candidate led to a strong elicitation of CD4
+ and CD8
+ T cell responses with cytokine production. Because TMC NPs have been proposed to possess an endosomal escape mechanism into cytoplasm, this will further promote cross-presentation of encapsidated immunogens through MHC-I pathway [
37]. An increase in activated CD8
+ cell expansions seen in mice that received BCG-CWS-adjuvanted vaccine implied that TMC may collaborate with BCG-CWS to augment CTL responses. In addition, both Th-1- and Th-2 responses were well elicited by vaccines containing BCG-CWS. This was shown by an upregulation of Th-1/Th-2 cytokine production in splenocyte cultures as well as strong responses of IgG1 and IgG2a. These confirmed the findings that BCG-CWS can enhance the immunogenicity of UV-DENV2 and NS1
1-279 protein.
In conclusion, the adjuvant effects of BCG-CWS were applied to enhance the immunogenicity of combination vaccines containing UV-DENV2- and NS11-279-TMC NPs. We revealed that BCG-CWS stimulated a high magnitude of complement-fixing antibody response against those antigens, resulting in a broad anti-viral immunity towards infectious virus and infected cells. These, therefore, restricted the ADE phenomenon. In addition, BCG-CWS also enhanced the immunogenicities of T cell epitopes presented on the UV-DENV2 and NS1 proteins. Nevertheless, the limitation of this present work is lacking of the challenge study. Therefore, the study evaluating vaccine effectiveness is warranted for further investigation.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: Reactivity of immunized sera to the surface of DENV-2 infected cells.
Author Contributions
Conceptualization, T.J. and S.U.; methodology, T.J., S.W. and S.U.; formal analysis, T.J., S.W. and S.U.; investigation, T.J., S.W., K.L., M.S. and J.L.; resources, S.W., P.M., P.S. and S.U.; data curation, T.J.and S.U.; writing—original draft preparation, T.J.; writing—review and editing, T.J. and S.U.; visualization, T.J. and S.U.; supervision, S.W., P.S. and S.U..; project administration, S.U.; funding acquisition, S.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Vaccine Institute, Thailand (Grant No. 2558.1/7) to Ubol S.
Institutional Review Board Statement
The use of human monocytes from healthy subjects was approved by the ethics committee on Human Rights Related to Human experimentation, Mahidol University, Bangkok, Thailand (protocol: 2016/011.2201) in compliance with the ethical standards of the Declaration of Helsinki. All animal experiments were performed under ethical guidelines approved by the Faculty of Science, Mahidol University Animal Care and Use Committee (SCMU-ACUC, protocol number: MUSC60-009–359).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
The author would like to acknowledge Runglawan Chawengkirttikul, Apamas Sukkaew and Suttikarn Apichirapokey for their technical contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013, 496, 504–7.
- Dwivedi VD, Tripathi IP, Tripathi RC, Bharadwaj S, Mishra SK. Genomics, proteomics and evolution of dengue virus. Briefings in Functional Genomics. 2017, 16, 217–27.
- Thomas SJ, Yoon IK. A review of Dengvaxia®: development to deployment. Hum Vaccin Immunother. 2019, 15, 2295–314. [CrossRef] [PubMed]
- Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med. 2018, 379, 327–40. [CrossRef] [PubMed]
- Nantachit N, Sunintaboon P, Ubol S. EDIII-DENV3 nanospheres drive immature dendritic cells into a mature phenotype in an in vitro model. Microbiol Immunol. 2017, 61, 305–17. [CrossRef]
- Vemireddy S, Madhurantakam PP, Talati MN, Sampath Kumar HM. Cationic pH-Responsive Polycaprolactone Nanoparticles as Intranasal Antigen Delivery System for Potent Humoral and Cellular Immunity against Recombinant Tetravalent Dengue Antigen. ACS applied bio materials. 2019, 2, 4837–46. [CrossRef]
- Khan RA, Ahmed F, Afroz S, Khan N. Tetravalent formulation of polymeric nanoparticle-based vaccine induces a potent immune response against dengue virus. Biomaterials Science. 2022, 10, 2917–28. [CrossRef]
- Jearanaiwitayakul T, Sunintaboon P, Chawengkittikul R, Limthongkul J, Midoeng P, Chaisuwirat P, et al. Whole inactivated dengue virus-loaded trimethyl chitosan nanoparticle-based vaccine: immunogenic properties in ex vivo and in vivo models. Hum Vaccin Immunother. 2021, 17, 2793–807. [CrossRef]
- Jearanaiwitayakul T, Sunintaboon P, Chawengkittikul R, Limthongkul J, Midoeng P, Warit S, et al. Nanodelivery system enhances the immunogenicity of dengue-2 nonstructural protein 1, DENV-2 NS1. Vaccine. 2020, 38, 6814–25. [CrossRef]
- Afchangi A, Jalilvand S, Arashkia A, Latifi T, Farahmand M, Abolghasem Shirazi MM, et al. Co-administration of rotavirus nanospheres VP6 and NSP4 proteins enhanced the anti-NSP4 humoral responses in immunized mice. Microb Pathog. 2022, 163, 105405. [CrossRef]
- Kao YS, Yu CY, Huang HJ, Tien SM, Wang WY, Yang M, et al. Combination of Modified NS1 and NS3 as a Novel Vaccine Strategy against Dengue Virus Infection. J Immunol. 2019, 203, 1909–17. [CrossRef]
- Wang X, Ku Z, Dai W, Chen T, Ye X, Zhang C, et al. A bivalent virus-like particle based vaccine induces a balanced antibody response against both enterovirus 71 and norovirus in mice. Vaccine. 2015, 33, 5779–85. [CrossRef]
- Seesen M, Jearanaiwitayakul T, Limthongkul J, Midoeng P, Sunintaboon P, Ubol S. A bivalent form of nanoparticle-based dengue vaccine stimulated responses that potently eliminate both DENV-2 particles and DENV-2-infected cells. Vaccine. 2023, 41, 1638–48.
- Chatzikleanthous D, O’Hagan DT, Adamo R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol Pharm. 2021, 18, 2867–88. [CrossRef] [PubMed]
- Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021, 594, 253–8. [CrossRef] [PubMed]
- Murata, M. Activation of Toll-like receptor 2 by a novel preparation of cell wall skeleton from Mycobacterium bovis BCG Tokyo (SMP-105) sufficiently enhances immune responses against tumors. Cancer Sci. 2008, 99, 1435–40. [Google Scholar] [CrossRef]
- Whang YM, Yoon DH, Hwang GY, Yoon H, Park SI, Choi YW, et al. Liposome-Encapsulated Bacillus Calmette-Guérin Cell Wall Skeleton Enhances Antitumor Efficiency for Bladder Cancer In Vitro and In Vivo via Induction of AMP-Activated Protein Kinase. Cancers (Basel). 2020, 12.
- Uenishi Y, Okada T, Okabe S, Sunagawa M. Study on the cell wall skeleton derived from Mycobacterium bovis BCG Tokyo 172 (SMP-105): establishment of preparation and analytical methods. Chem Pharm Bull (Tokyo). 2007, 55, 843–52. [CrossRef]
- Masuda H, Nakamura T, Noma Y, Harashima H. Application of BCG-CWS as a Systemic Adjuvant by Using Nanoparticulation Technology. Mol Pharm. 2018, 15, 5762–71. [CrossRef] [PubMed]
- Shibata T, Takata E, Sakamoto J, Shioya A, Yamada S, Takakura M, et al. A retrospective study of immunotherapy using the cell wall skeleton of Mycobacterium bovis Bacillus Calmette-Guérin (BCG-CWS) for cervical cancer. Medicine (Baltimore). 2022, 101, e32481. [CrossRef] [PubMed]
- Kim KH, Lee YT, Park Y, Ko EJ, Jung YJ, Kim YJ, et al. BCG Cell Wall Skeleton As a Vaccine Adjuvant Protects Both Infant and Old-Aged Mice from Influenza Virus Infection. Biomedicines. 2021, 9.
- Koike Y, Yoo YC, Mitobe M, Oka T, Okuma K, Tono-oka S, et al. Enhancing activity of mycobacterial cell-derived adjuvants on immunogenicity of recombinant human hepatitis B virus vaccine. Vaccine. 1998, 16, 1982–9. [CrossRef] [PubMed]
- Seesen M, Jearanaiwitayakul T, Limthongkul J, Sunintaboon P, Ubol S. Mice immunized with trimethyl chitosan nanoparticles containing DENV-2 envelope domain III elicit neutralizing antibodies with undetectable antibody-dependent enhancement activity. J Gen Virol. 2022, 103.
- Paik TH, Lee JS, Kim KH, Yang CS, Jo EK, Song CH. Mycobacterial cell-wall skeleton as a universal vaccine vehicle for antigen conjugation. Vaccine. 2010, 28, 7873–80. [CrossRef] [PubMed]
- Wang R, Zheng X, Sun J, Feng K, Gao N, Fan D, et al. Vaccination With a Single Consensus Envelope Protein Ectodomain Sequence Administered in a Heterologous Regimen Induces Tetravalent Immune Responses and Protection Against Dengue Viruses in Mice. Front Microbiol. 2019, 10, 1113. [CrossRef]
- Hunsawong T, Sunintaboon P, Warit S, Thaisomboonsuk B, Jarman RG, Yoon IK, et al. A novel dengue virus serotype-2 nanovaccine induces robust humoral and cell-mediated immunity in mice. Vaccine. 2015, 33, 1702–10. [CrossRef] [PubMed]
- Flipse J, Diosa-Toro MA, Hoornweg TE, van de Pol DP, Urcuqui-Inchima S, Smit JM. Antibody-Dependent Enhancement of Dengue Virus Infection in Primary Human Macrophages; Balancing Higher Fusion against Antiviral Responses. Sci Rep. 2016, 6, 29201. [CrossRef]
- Dias AG, Jr., Atyeo C, Loos C, Montoya M, Roy V, Bos S, et al. Antibody Fc characteristics and effector functions correlate with protection from symptomatic dengue virus type 3 infection. Sci Transl Med. 2022, 14, eabm3151. [CrossRef]
- Sun P, Morrison BJ, Beckett CG, Liang Z, Nagabhushana N, Li A, et al. NK cell degranulation as a marker for measuring antibody-dependent cytotoxicity in neutralizing and non-neutralizing human sera from dengue patients. J Immunol Methods. 2017, 441, 24–30. [CrossRef]
- Chen HR, Lai YC, Yeh TM. Dengue virus non-structural protein 1: a pathogenic factor, therapeutic target, and vaccine candidate. J Biomed Sci. 2018, 25, 58. [CrossRef]
- Lee AJ, Ashkar AA. The Dual Nature of Type I and Type II Interferons. Front Immunol. 2018, 9, 2061. [CrossRef] [PubMed]
- Kubo, M. The role of IL-4 derived from follicular helper T (TFH) cells and type 2 helper T (TH2) cells. Int Immunol. 2021, 33, 717–22. [Google Scholar] [CrossRef]
- Izmirly AM, Alturki SO, Alturki SO, Connors J, Haddad EK. Challenges in Dengue Vaccines Development: Pre-existing Infections and Cross-Reactivity. Front Immunol. 2020, 11, 1055. [CrossRef] [PubMed]
- Chan CYY, Low JZH, Gan ES, Ong EZ, Zhang SL, Tan HC, et al. Antibody-Dependent Dengue Virus Entry Modulates Cell Intrinsic Responses for Enhanced Infection. mSphere. 2019, 4.
- Mehlhop E, Ansarah-Sobrinho C, Johnson S, Engle M, Fremont DH, Pierson TC, et al. Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass-specific manner. Cell Host Microbe. 2007, 2, 417–26. [CrossRef]
- Masuda H, Nakamura T, Harashima H. Distribution of BCG-CWS-Loaded Nanoparticles in the Spleen After Intravenous Injection Affects Cytotoxic T Lymphocyte Activity. J Pharm Sci. 2020, 109, 1943–50. [CrossRef]
- Henao MC, Ocasion C, Puentes PR, González-Melo C, Quezada V, Cifuentes J, et al. Translocating Peptides of Biomedical Interest Obtained from the Spike (S) Glycoprotein of the SARS-CoV-2. Membranes (Basel). 2022, 12.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).