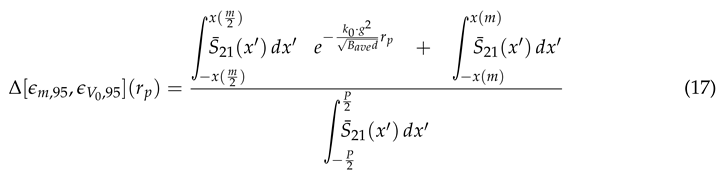2.1. Colloidal Latices
When discussing the different types of particles that exist within solutions that one may apply this technique to, particle size is an important parameter to discuss, as it dictates the types of forces that they may be subjected to. For instance, the number and scope of criterion which qualify a solution as a colloidal system is quite vast, ranging from fog and mist, to milk, to metallic hydroxides, silica gels, and bio-colloids such as blood, bone, muscle, and cells. Interestingly, there exists materials which exhibit all permutations of solid, liquid, and gas combinations that are considered colloidal systems, making them quite a broad set which can be explored. Furthermore, colloidal systems generally contain particles whose sizes range from 1
to 1
; though, the size of particles within the systems can vary dramatically, with some particles much larger than 1
[
7,
8].
One such colloid of interest is latex. Latex particles (latices) are prime candidates for study using this electrophoretic technique via W-band metasurface because of their relatively large particle size, surface charge, ability to sediment, and the amount of research that has been conducted on them. Unfortunately, latex formulations can contain a significant level of volatile organic compounds which can damage the ozone layer, whereas others are linked to greenhouse gasses which contribute to climate change. Latex, therefore, is an important material to study, since it is widely used in architectural coatings, textiles and carpets, various construction materials, paper coatings, inks, gloves (where 135 billion latex gloves were manufactured in 2008 alone), and many other over-the-counter items [
9].
Rubber latex is generally referred to as a stable dispersion of hydrophobic rubber particles. In large part, it owes its stability to the charge carried by each of the particles within the colloidal suspension. Latex contains many charged species, such as ionic surfactants and salts [
9]. The use of surfactants in latex is mainly due to their stabilising properties, and play a vital role in the formation of smooth latex films. In general surfactants are classified by their polar head groups, of which there are four different types. Particularly relevant to this study, cationic surfactants are comprised of positively charged groups such as long chain amines and their salts, quaternary ammonium salts, and polyoxyethylenated long chain amines [
10].
When a colloidal dispersion (such as latices in water) is deposited onto a surface, water evaporation commences and forces the particles to sediment and pack into an often face-centered cubic lattice [
12,
13]. Vertical deposition processes, where the evaporation rate of the solvent is greater than the sedimentation velocity of the particles, is a highly effective method to deposit such colloids. During vertical deposition, convective mass flow and capillarity are the two main driving forces which enable colloidal particles to assemble on a substrate, producing films that can be several microns thick. Finally, it has been shown that patterns on the surface of substrates can be used to control the assembly of colloidal particles, and, most relevant to this study, that they will preferentially attach and assemble in regions of opposite charge [
9].
2.2. Quantifying the Metasurface Regions of Greatest Sensitivity
In this subsection, a technique is presented in which the sensitivity of a metasurface aperture to changes in the ambient environment is mapped out. This technique produces target regions in which statistical analysis of charged particle accumulation will be considered most significant, allowing one to quantify the efficacy of the electrophoretic technique after simulation and experimentation.
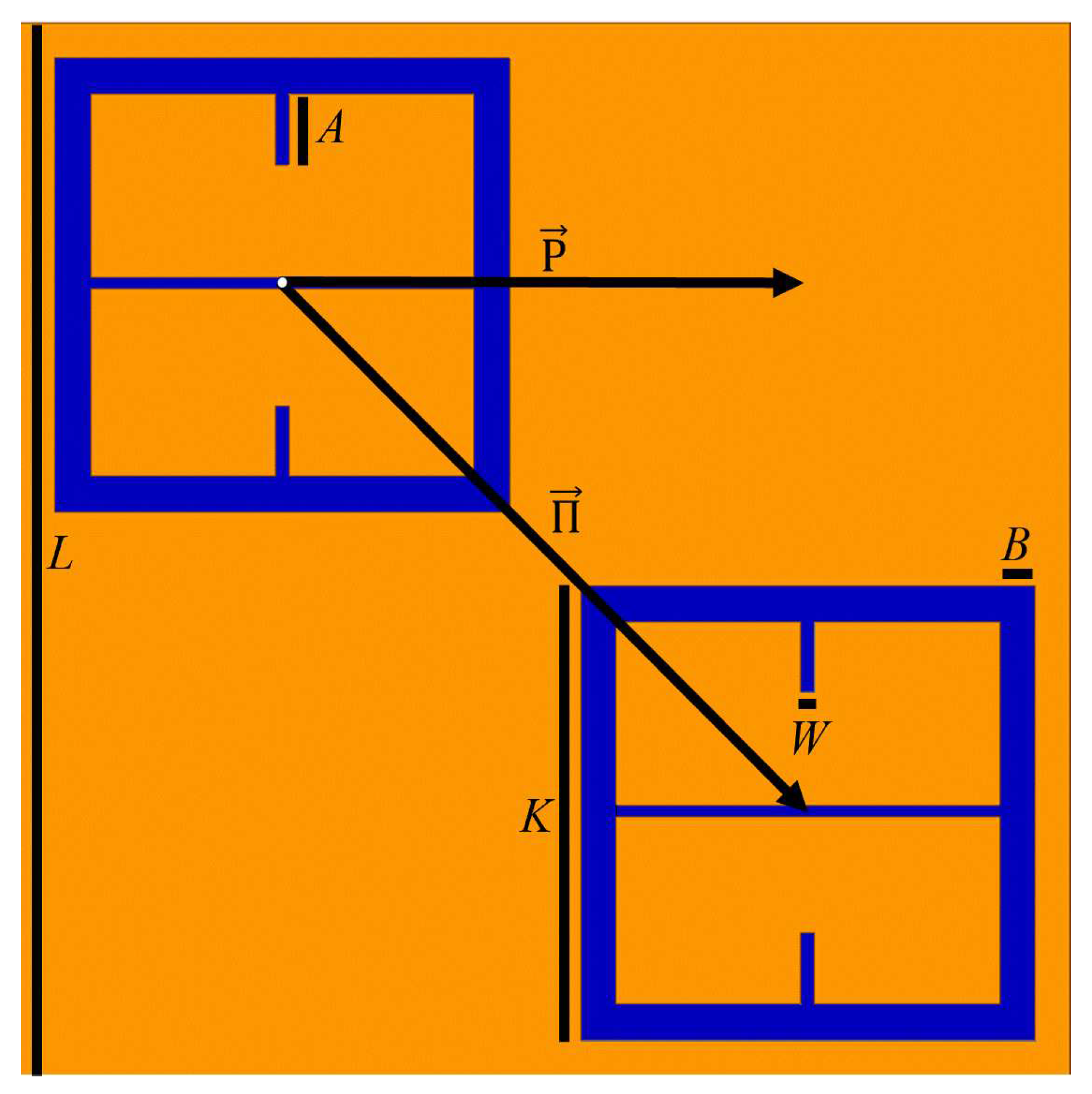
Using HFSS (an electromagnetic simulation software), a 100
thick layer of FR4 (a circuit board material with
and
) with 100
diameter holes in it was digitally place on top of the metasurface, as shown in
Figure 3.
Both holes were independently moved in the
m and
n directions from point
o to point
p, in nine equal steps. The transmittance spectra were simulated after each step from 90-110 GHz (the operational bandwidth of the metasurface). The maximum transmittance amplitude was recorded after each step. Once the transmittance amplitudes for the steps between
o and
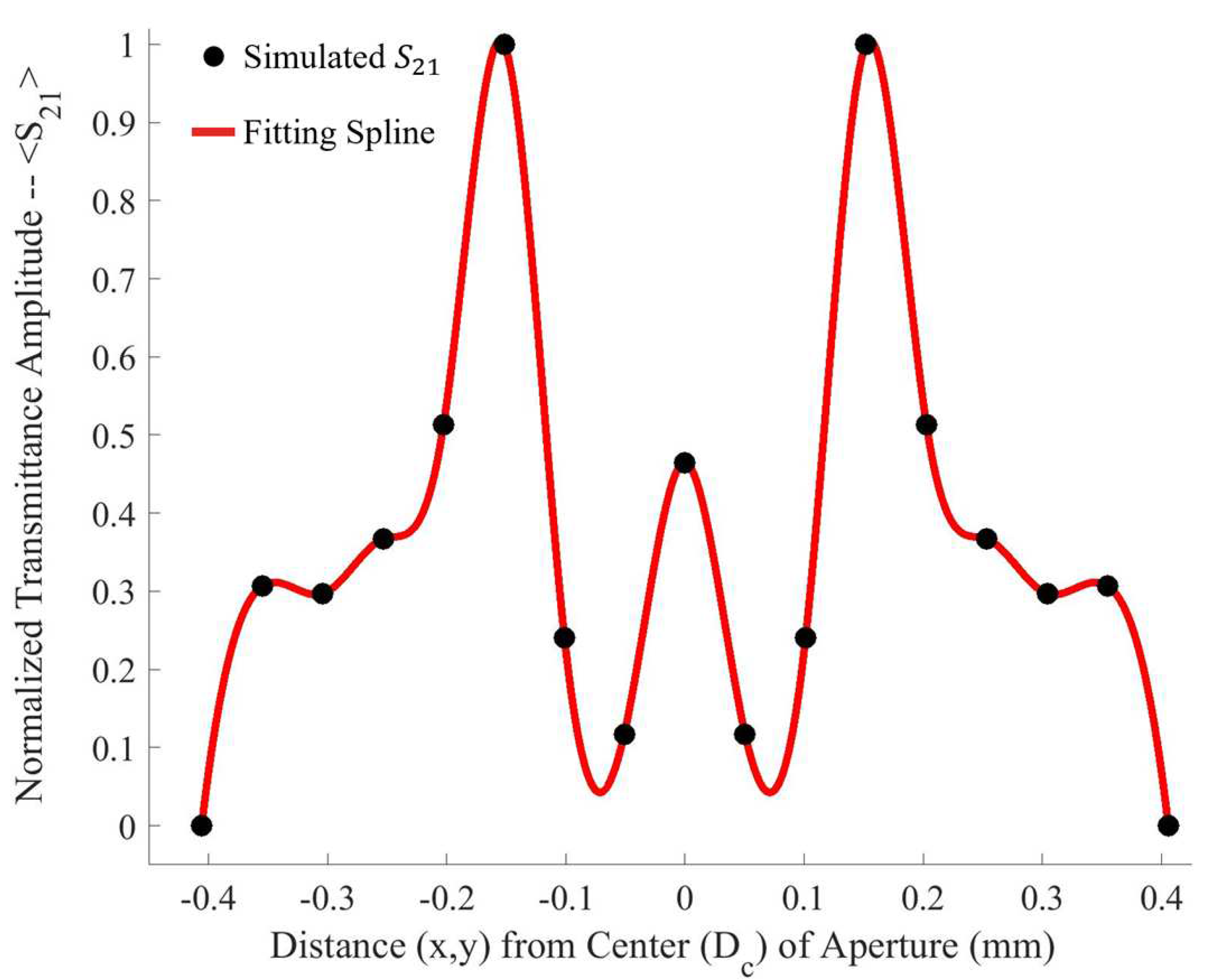
p were recorded- due to the symmetry and periodicity of the metasurface- the values were mirrored across the origin. The resulting average maximum transmittance spectra after each step is presented in
Figure 4.
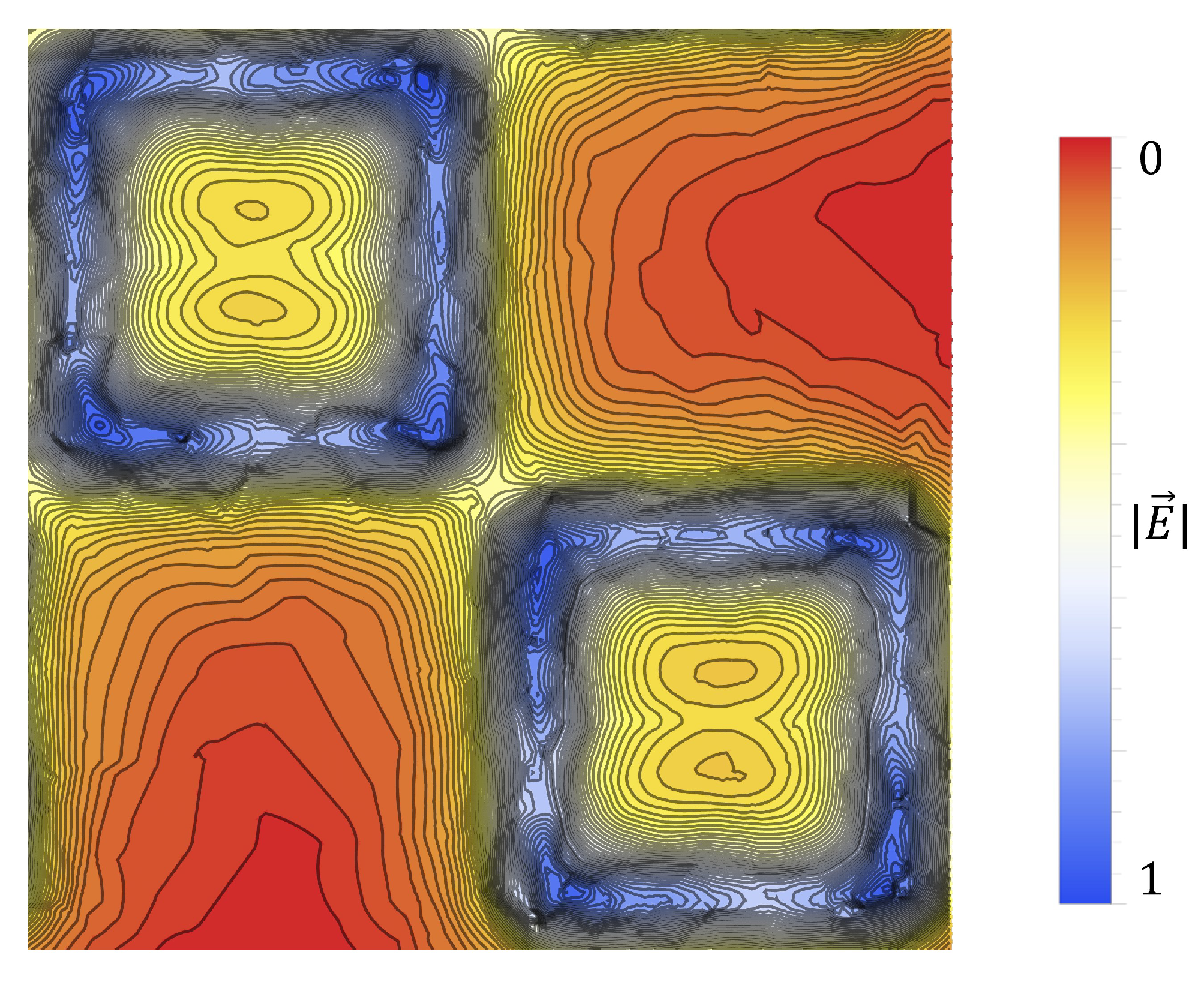
Upon investigation of some of the features of
Figure 4, it is interesting to note the enhanced sensitivity of the metasurface apertures (given by largest transmittance peaks) which correspond to the slits depicted in
Figure 1, labeled
B. This enhanced sensitivity in the region surrounding these slits is due to a massive electric field enhancement across them- a result of the resonant coupling of incident radiation to the metasurface topology, as detailed in [
1]. Furthermore, the sensitivity of the metasurface abruptly drops to its least sensitive position at around 370
from the aperture centers. Upon closer examination, a major portion of the intensity distribution of the normalized transmittance spectra as a function of
(the distance from the center of a metasurface aperture, as seen in
Figure 4) can be very closely modeled using a modified Fraunhofer Diffraction model of a three-slit system [
14]. The parameters of the modified Fraunhofer model are based upon the metasurface geometry, which is presented as Equation (
1).
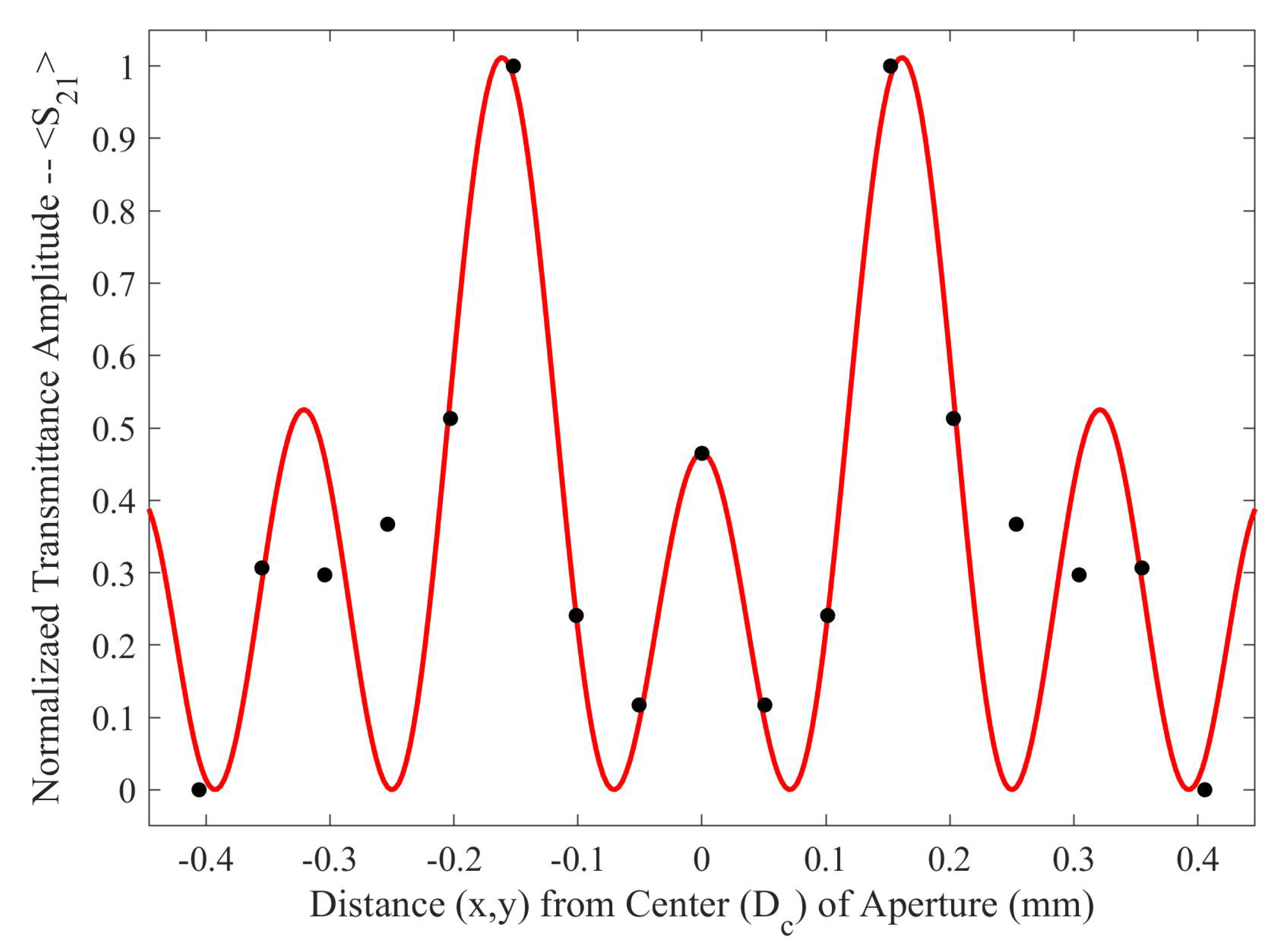
In Equation (
1),
is the normalization factor for the transmittance spectra,
P is the period of the metasurface,
(the wavenumber at the resonant wavelength of the metasurface),
is the average slit width, and
d is the inter-slit spacing. A three slit system is proposed because, in the m-n symmetries of the metasurface apertures, there are three slits of finite size, as seen in
Figure 3. In the case of the m-symmetry, one may still refer to the central discontinuous slit, as a slit (and it seems to work as will be shown below). The full fit of this model to the simulated transmittance data which probes the sensitivity of the metasurface apertures (
Figure 3) is presented in
Figure 5.
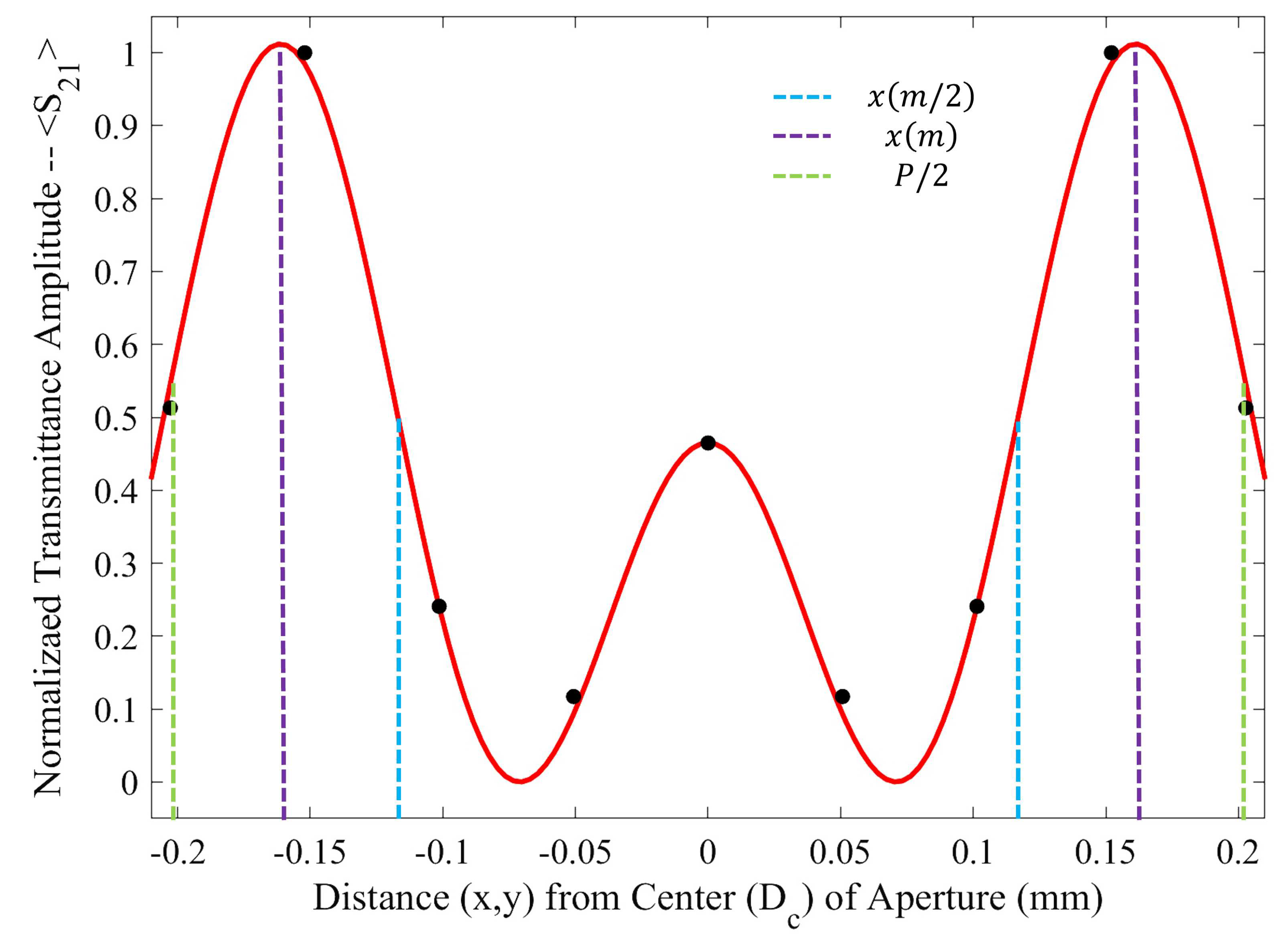
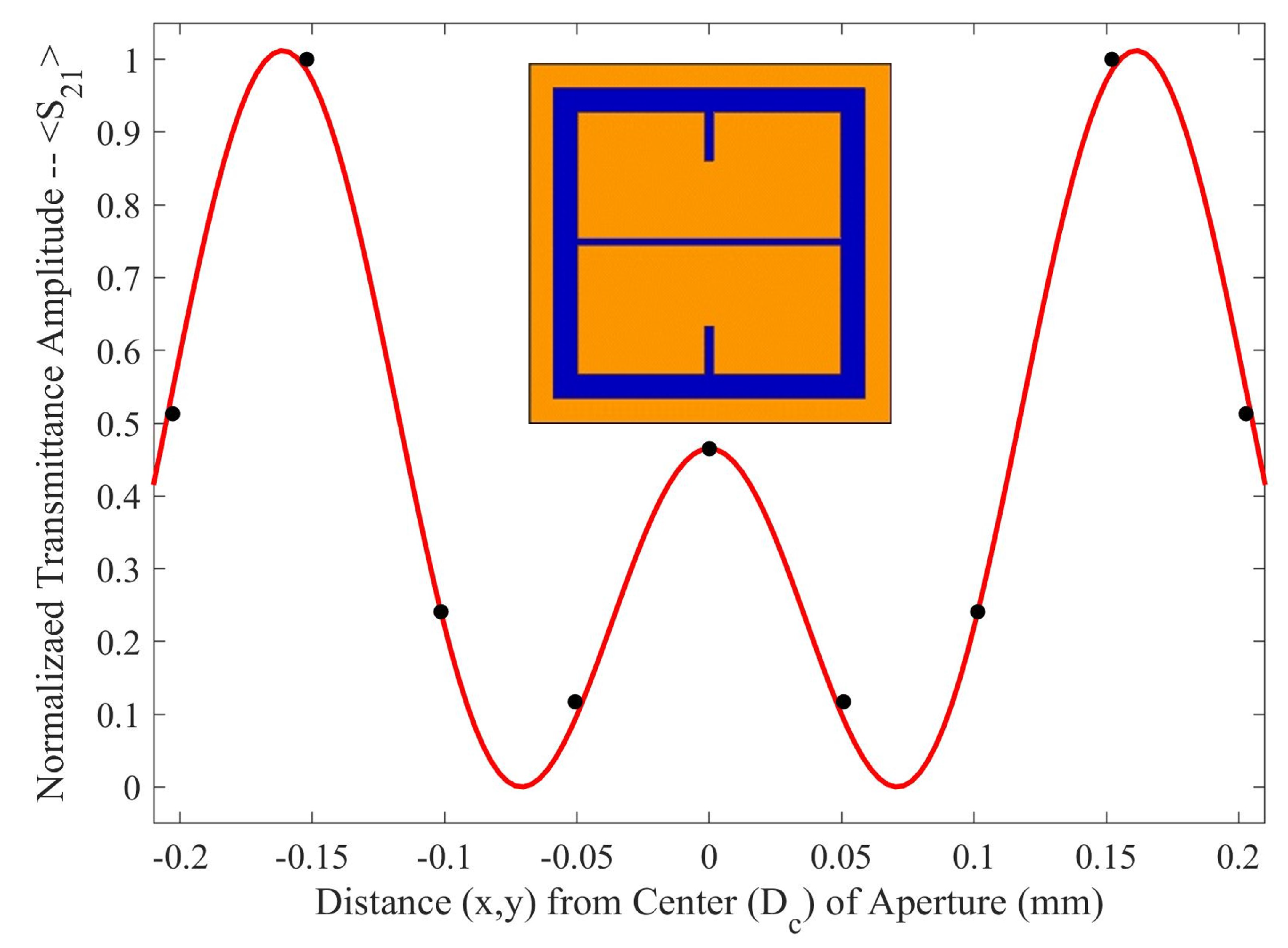
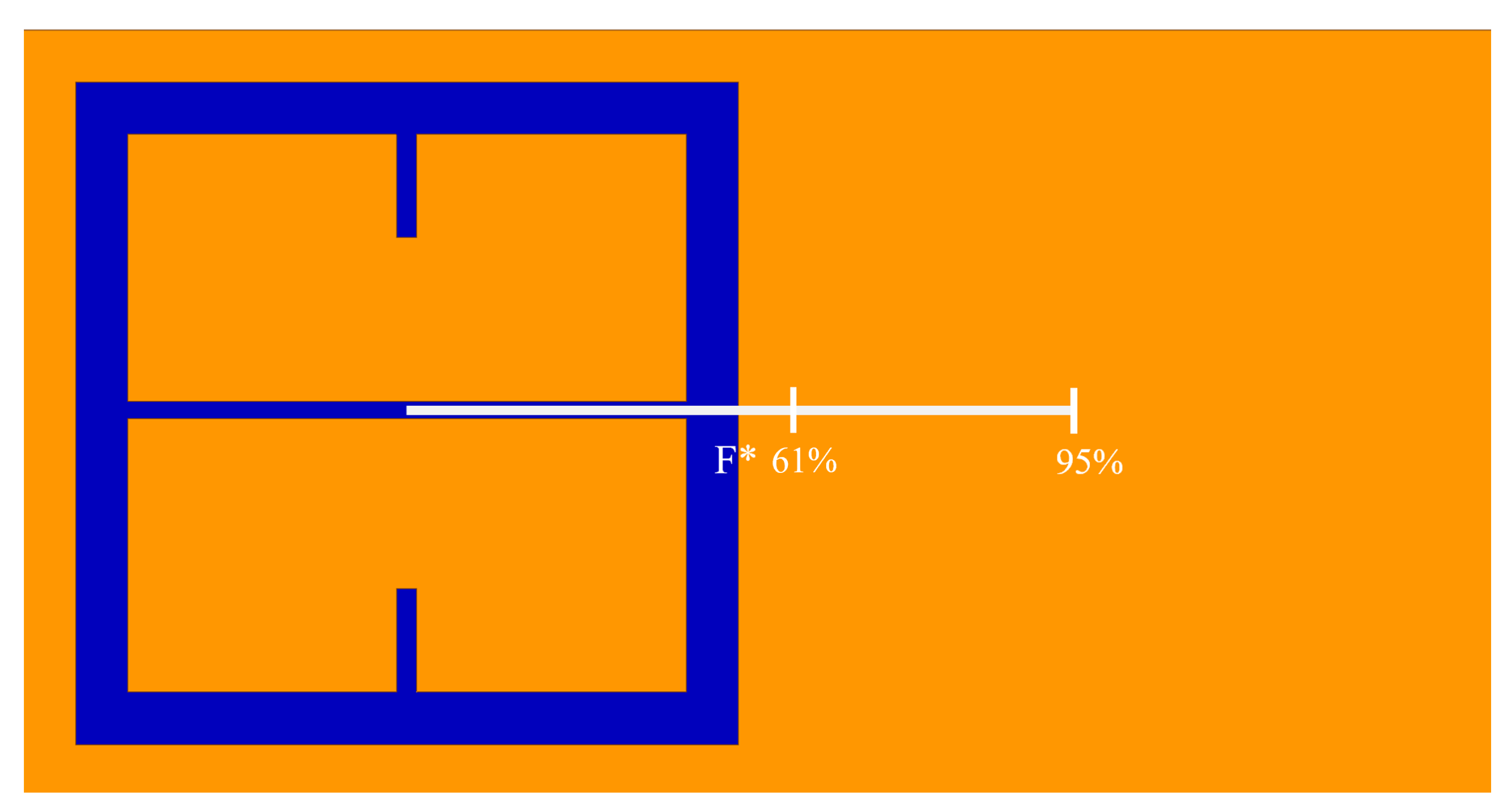
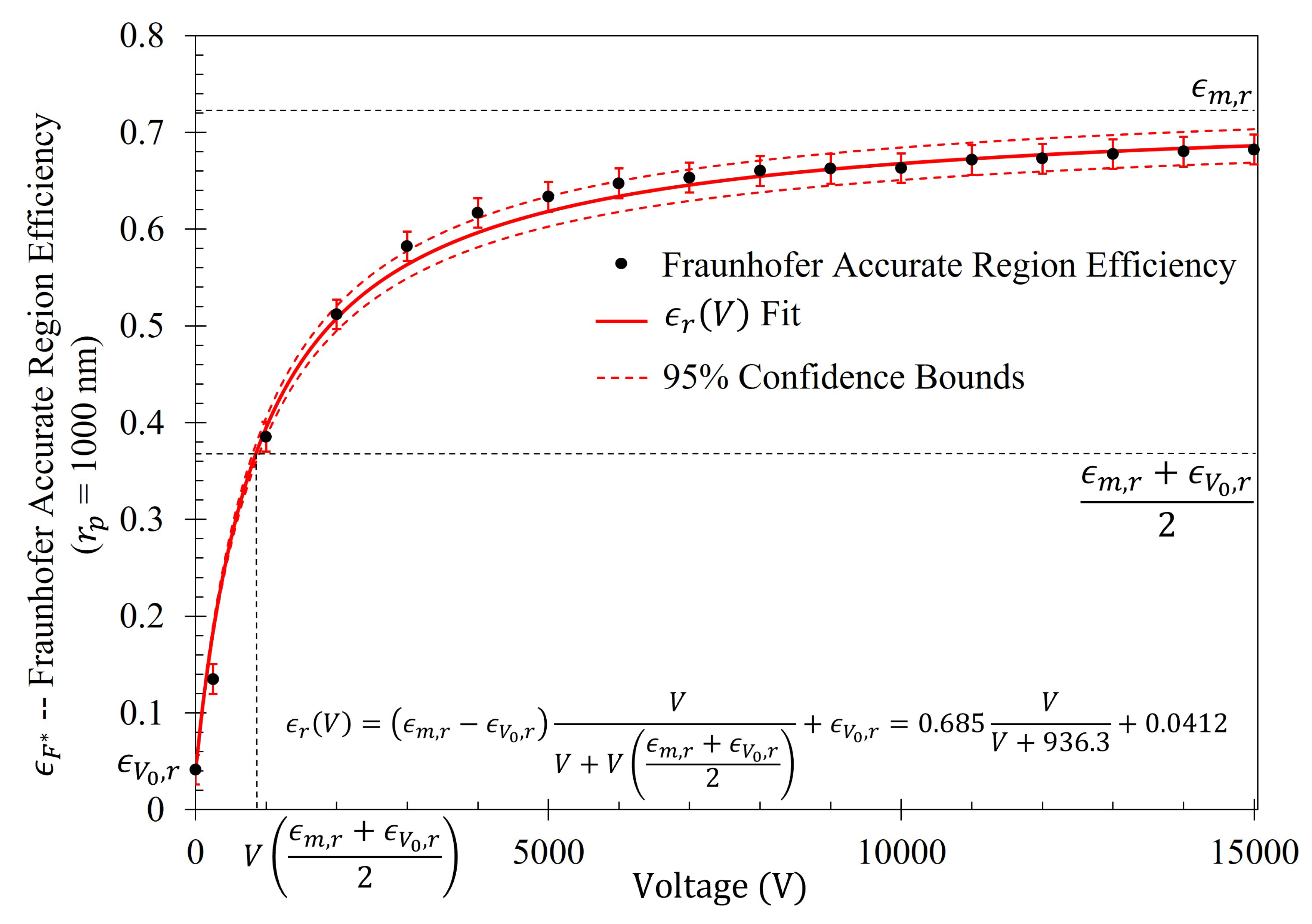
As seen in
Figure 5, there is a region in which the Fraunhofer model is most accurate: a region corresponding to one half of the period,
P, from the center of each metasurface aperture (depicted in
Figure 6). This could be a region of interest when considering how one may quantify the accumulation of charged particles around the metasurface apertures, considering that the metasurface is most sensitive to changes in its ambient environment within this region (evidenced by largest change in transmittance amplitude).
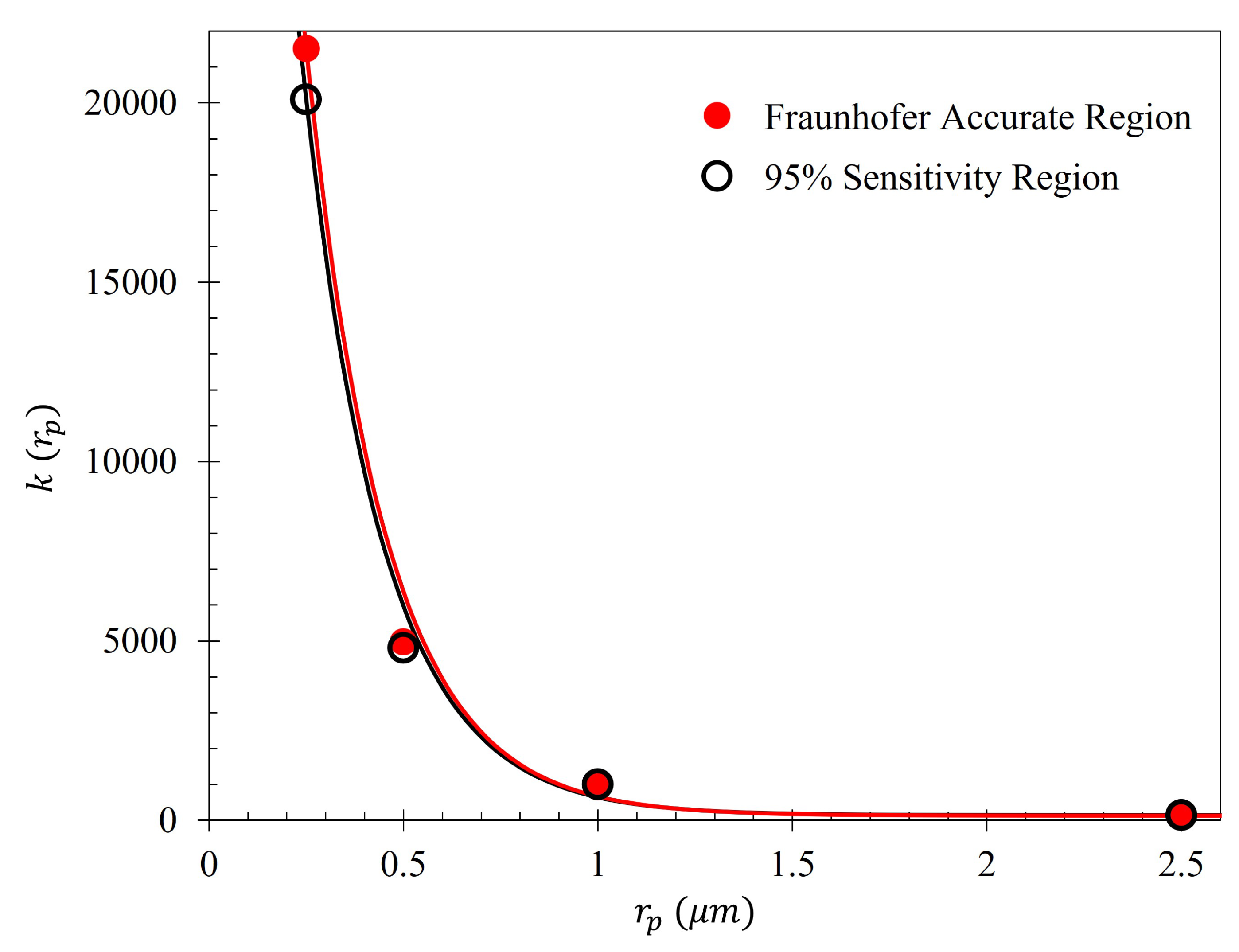
To further quantify the regions of greatest sensitivity, the spline fit, as shown in
Figure 4, was integrated across the data set. If
represents the equation of the spline as a function of distance from the center, the values for
for which the area under the spline contained 95% of the area, and the Fraunhofer region, were found and are presented in
Table 2. The exact regions on the metasurface are depicted for a single aperture in
Figure 7.
2.4. Charged Particles Suspended in an Evaporating Medium while subject to Nonuniform Electric Fields
In simulations and experiments presented herein, charged particles (colloidal latices with net positive surface charge density) are suspended in a nonuniform electric field which emanates from multiple metasurface apertures (see
Figure 9). When an electric field (uniform or nonuniform) is applied to charged particles in suspension, they migrate along the field lines in a predictable manner such that a relative motion between the charged particles and the suspending medium is observed [
17]. The electrostatic force on the charged particles is given as
. Such charged particles in suspension will polarize the suspending solution surrounding it, giving rise to a counter-charged ion cloud called an electric double layer. Since the induced ion cloud is oppositely charged in comparison to the particle, the overall motion of the fluid (suspension medium) will be in the opposite direction of the charged particle’s.
The opposing suspension motion tends to retard the motion of the particle in suspension as an electric force is applied to it, creating a drag-like force called Electrophoretic Retardation (
). However, charged particles within the electrolyte are still able to move with a constant velocity, called the drift velocity (
). In such an instance, the charged particle-double layer volume can be treated as a single particle, with an effective radius of the moving particle including any molecules of water or other solvent that move with it, called Stoke’s Radius (
). The impact that the electric double layer has on the kinematics of the particle are determined by the layer thickness. The thickness of the electric double layer is represented by the Debye length
, and is a characteristic distance from the charged particle in the solution, to a radial distance from the surface normal of the charged particle in which the electric potential decays to approximately
of the total surface potential [
17]. As a charged particle travels through a solution, there is a plane beyond which the oppositely charged ions do not travel along with the particle of interest: the slipping plane, which is defined by the electrical potential at the plane, and the zeta potential, which dictates the amount of repulsion between the surrounding counter-ion layer and the solution. Interestingly, this repulsion is due to the osmotic pressure created between the ionic concentration difference between the double layer and the solution [
18,
19].
However, when taking the Debye-Hückel condition,
(where
r is the Stoke’s Radius), and there is no pressure gradient within the suspension medium, there will be no flow of the suspension medium due to the electric field as there should be no other free charges present within it [
17]. This means that the polarized electric double layer is considered to be very thin, and thus, the Electrophoretic Drag can be treated as negligible. The Debye-Hückel limiting case was considered in this paper, as the surface charge density of latices is considerably small (as will be seen in
Section 2.7). This means that Stoke’s Radius can be treated as the particles radius. Furthermore, since the electric field that the particles are subjected to is non-uniform in this experiment (shown in
Figure 9,
Figure 10 and
Figure 11), the electrostatic force contribution to particulate motion in the Debye-Hückel limit can be expressed as [
17,
20,
25]:
where
q is the charge of the particle, and
E is the electric field strength.
In a nonuniform electric field, an electrically neutral body in suspension will usually move in a direction in which the electric field gradient is the steepest; a phenomenon which was coined Dielectrophoresis by Pohl in 1951 [
21]. The mechanism governing dielectrophoresis depends on the asymmetrical induction and attraction of charge densities within suspensoids, where electric dipoles are induced due to the charge asymmetry [
21,
22]. In simple dielectrophoresis (of an uncharged particle), the motion of the particle is dependent on the dielectric properties of the suspension medium and the particle.
In 1979, Pohl showed that the an effective dipole moment could be utilized in the formulation of the dielectrophoretic force [
23]. For a perfectly insulating spherical particle of radius
r with absolute permittivity
that is suspended in a medium of absolute permittivity
, the dielectrophoretic force can be shown to be:
where
is the Clausis-Mosotti factor. In Equation (
3), it is seen that the dielectrophoretic force is not explicitly dependent on any electrical charge within the medium or particles, but depends on the gradient of the nonuniform electric field and the dielectric properties of the medium and particles [
20,
21]. Upon further observation of Equation (
3), one sees that particles are attracted to regions of stronger electric field when
, and repelled when
, and in general, is not parallel to the electric field [
22,
24].
When a solution is spread across the metasurface, it will naturally evaporate over time; in fact, this is a feature of this metasurface sensing technique that must be considered since the nature of latex sedimentation is highly dependent on evaporation and the properties of the deposition surface [
9]. This phenomenon is not unique to latices or colloidal particles. For instance, in a NaCl solution (with sub-nanometer sized particles) evaporating on a hydrophilic surface, the majority of the salt crystallization during natural evaporation takes place at the liquid-air interface. This results in a ringlike crystal "coffee stain" effect, which is attributed to capillary flow from the center to the edges of the water droplet due to loss of the solvent and "pinning" of the water droplet to imperfections on the substrate surface. However, on a hydrophobic surface, the re-crystallization takes place within the bulk of the water droplet at the liquid-solid interface [
6].
For hydrophobic colloidal particles (such as latices), it has been observed that they will preferentially adsorb onto hydrophobic regions, and in regions of opposite charge. However, it is known that latex films do not dry uniformly. Instead, due to the boundary conditions at a droplets edge, latex particles are laterally transported from the fluid centre to the edge of the droplet where they will be consolidated. This means that, in the case of no external forces but that of evaporation, a high concentration of latices should be observed along the perimeter of a water droplet [
9].
In an ambient environment (
Celsius, and some air flow above the surface), it has been shown that the rate of evaporation of water can be approximated as within
[
27]. In order to test the evaporation rate of the solution as it sat on the metasurface, five single drops of the colloidal solution used in this study were placed onto the metasurface and the time to evaporation measured. The ambient temperature was
Celsius, and the 12V computer fan was turned on within the containment box. The fan was not directly pointing at the water droplets, but only allowed air to circulate within the containment box. It was found that, on average, it took approximately fifteen minutes for the water droplets to evaporate. Each of the droplets was measured to be approximately 3
in radius (radius parallel to the metasurface), with a height of approximately
. Modeling the droplet as half an oblate spheroid [
28], the rate of evaporation for the water in our controlled environment was determined using the following equation:
where
is the equatorial radius (radius of the sphere in the plane parallel to and on the metasurface),
h is the polar radius (the height of the oblate spheroid) [
28],
is the density of water, and
is the approximate average time it took for a droplet to evaporate. Given that
,
, and
s it was found that
.
Therefore, assuming that the evaporation rate of the water remains relatively constant, the change in volume of the water as a function of time can be described by the following equation:
It can be shown that this separable differential equation results in an equation for the volume of the water on a surface as a function of time,
, where
is the initial mass of the water on the surface,
is the initial volume and
is the evaporation rate. Given that the estimated total time it takes to evaporate the water will be
. Solving Equation (
5) produces the following equation:
where A is a normalization constant. Solving for
A while considering that the volume must approach zero at
, one finds
. Keeping the experimentally measured evaporation time and geometry of the droplet of water in mind, the resulting equation is:
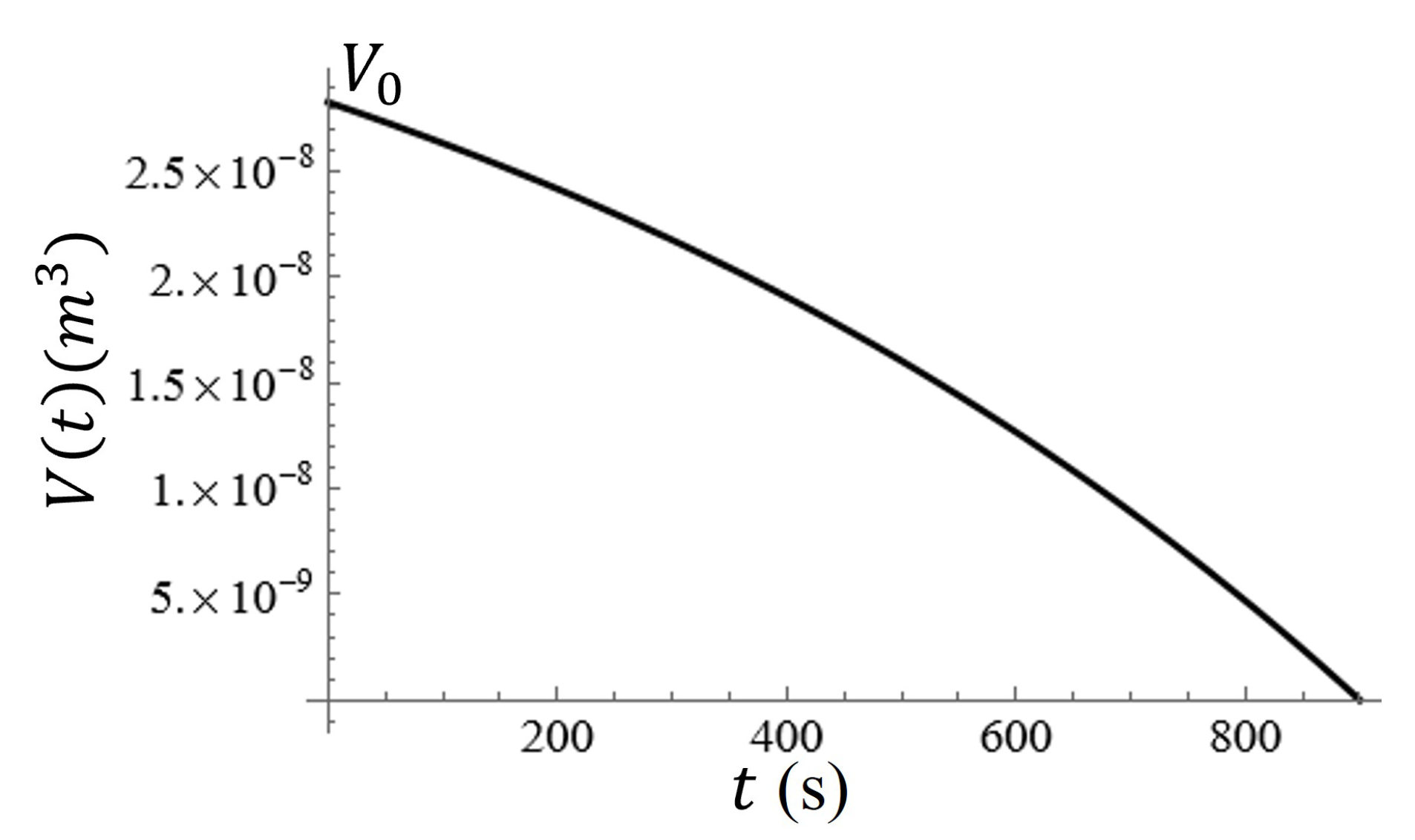
A graph of the volume of the water on the metasurface as a function of time is given in
Figure 12. In
Figure 12, it is clear that the change in volume as a function of time is nonlinear, and therefore, the change in the radius and height of the water droplet should be nonlinear as well. Assuming that the radius of the water spot (approximated as a hemisphere) and its relative height from the surface should both reach zero at
,
, such that in the event that
and
, the radius should decrease at a rate that is two times faster than the height of the droplet at a constant evaporation rate.
Equation (
7) can be re-expressed in terms of the height of the water spot
and the radius of the water spot
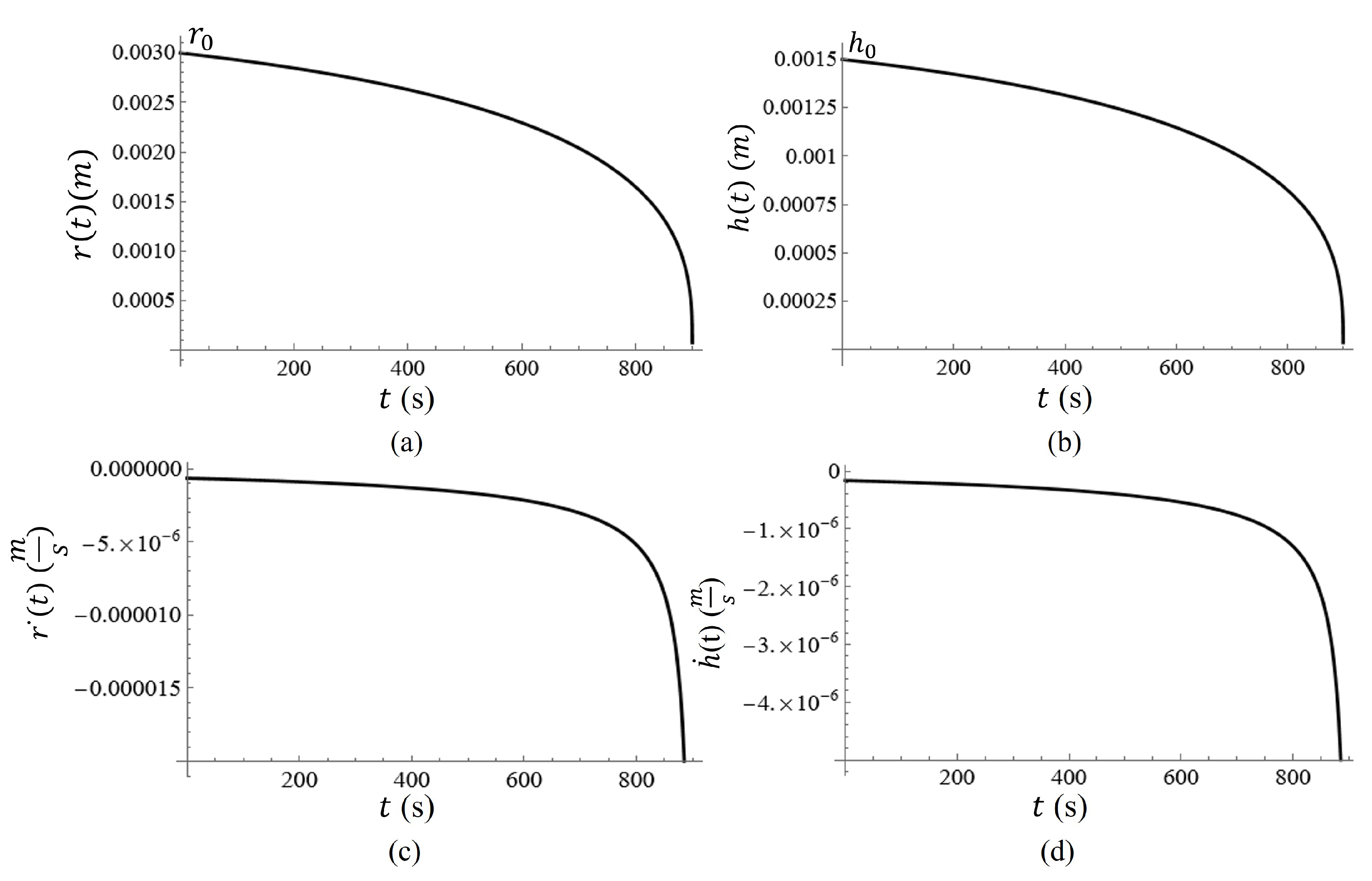
. Then differentiating each with respect to time, the rate at which the height and radius of the droplet can be determined. The graphs of
and
are given in
Figure 13(a,b). This phenomenon subjects the particles to another type of force which accelerates the sedimentation process, thus altering the equation of motion [
29,
30,
31]. The exact rate of change for the radius (
) and height (
) of the water spot are given in
Figure 13c,d, respectively.
During conventional procedures of latex film deposition, it has been shown that evaporation is the driving force behind particle sedimentation. In such film deposition procedures, latex deposition is driven by the directed motion of particular influxing to evaporative regions via the convective mass transfer of water. As was previously described, the dominating sedimentation process takes place at the radial boundary of the water spot on top of the metasurface (due to convective mass transfer), directed in a downward direction at the edge due to the curvature of the water droplet. Since the total evaporation flux of the water must be equal to the bulk water flux to the boundary, it is suggested that the particle velocity due to the bulk water flux,
, can be described as [
29,
30,
31]:
where
is the radial velocity due to evaporation as a function of time, depicted in
Figure 13(c),
is the total surface area of the water spot (half of an oblate spheroid), and
is the surface area of the radial boundary at which a two-dimensional lattice of particles has settled (estimated as the surface area of a cylinder whose height is the particle diameter), and
is particle radius [
29,
30,
31]. The surface area of an oblate spheroid is defined as [
28]:
where
is the equatorial radius (radius of the sphere in the plane parallel to and on the metasurface),
is the polar radius (the height of the oblate spheroid), and
. Solving Equation (
8), one will find that:
It is suggested here that, since the latex particles within the medium are subject to the convective mass transfer of water due to evaporation, using Stoke’s law [
32], the downward force contribution that the evaporative process contributes to particle motion within the solution can be described by:
where
is the particle radius,
is the fluid viscosity,
is the particle velocity due to fluid flux,
g is the acceleration due to gravity,
is the particle density, and
is the fluid density. Furthermore, the contribution from frictional drag due to Stoke’s Law will retard the motion of the particles in directions which are not parallel to the convective mass transfer vectors. This means that, since the majority of the latice deposition takes place at the evaporative front, the force vector due to evaporation (
) contributes to the downward motion, whereas motion parallel to the metasurface is retarded in a manner predicted by Stoke’s Law. The retarding force due to frictional drag, whose vector components are parallel to the metasurface, are given by:
Finally, the net force on a charged particle in suspension subject to an nonuniform electric field during evaporation can be expressed as:
where downward acceleration due to gravity is negligible within the fluid. In the limit that the electric field goes to zero, evaporation will be the only external driving force which causes particle deposition onto the surface.
Due to the convective mass flow of the water toward the edges of a water droplet, it should be observed experimentally that the particles should aggregate in clumps near the edge, where a random, smaller distribution of particles is observed over the wider area [
9] without the presence of an externally applied electric field. Conversely, in the presence of an electric field, it should be that the latices are not so highly concentrated along the boundary of a water droplet, but instead, their bulk should become increasingly spread out over a larger surface area and concentrated near metasurface apertures in a manner proportional to the electric field strength they are subjected to.
2.5. Monte Carlo Simulations of Charged Particles Suspended in an Evaporating Medium while subject to Nonuniform Electric Fields
In order to probe the efficacy of the electrophoretic technique which is the focus of this paper, physical parameters were used in order to accurately approximate realistic effects that may be observed in experiment.
Spherical, colloidal latex particles were simulated using this Monte Carlo technique and given radii of r = 250
, 500
, 1000
, and 2500
. This range of radii for various latices is well documented [
29]. From these initial conditions, the mass of each particle (
m) and its net charge (
q) can be readily calculated using existing data.
Table 3 shows the parameters which were used in the Monte Carlo simulations, where m =
is the total mass of each of the colloidal spheres and
q is their net charge. The average surface charge density on a number of latex particles has been previously quantified, and is given as
[
33], the density of industrialized (non-natural) latex is given as approximately 1100
[
34], and the relative permittivity of latex given as
[
35]. Furthermore, since the electric field between the metasurface and ground plate travels through a 400
silicon wafer (
), a
thick sheet of Teflon (
) [
37], and then effects particles that are within water as a suspending medium (
) [
36], the electric field in each of these regions was diminished by the relative permittivity within them, respectively.
In this set of Monte Carlo simulations, particles (whose physical parameters are given in
Table 3) were given an initial position within a
volume centered directly on top of the metasurface region depicted in
Figure 8. One by one, 10,000 particles bearing the physical parameters presented in
Table 3 were exposed to an electric field above the metasurface which was created by a potential difference between the metasurface and the ground plate. Upon using a Monte Carlo technique, it is assumed that the particle concentration within the suspending medium during experimentation will be sufficiently small, such that the particles can be considered non-interacting within the medium. The voltage range explored in these simulations varies from 0 - 15 kV, with simulations of the 250
particles reaching 50kV (why this is so will be later explained). The net force on each of the particles is given by Equation (
13). The efficacy of the technique was quantified using the Fraunhofer Accurate and 95% regions of sensitivity (within the central unit cell shown in
Figure 8) previously quantified in
Section 2.2, and depicted in
Figure 7. The justification to study only the central unit cell was previously given in
Section 2.3.
The gathering efficiency within the Fraunhofer Accurate and 95% Sensitivity Regions is defined as
, where
is the number of particles found in each of the regions of interest, and
the total number of particles studied. The initial gathering efficiency of the metasurface within the Fraunhofer Accurate and 95% Sensitivity Regions was quantified by setting the electric field to zero in the simulation, and determining the number of particles that accumulated within each of the sensitivity regions after it completed. The sedimentation of the particles purely due to evaporation is presented in
Figure A1, and the initial gathering efficiency within these regions presented in
Table 4.
The gathering efficiencies of the metasurface as a function of voltage between the metasurface and grounding plate (
) within this study are well modeled utilizing the equation:
where,
is the maximum efficiency possible within the metasurface region of interest (in the limit that
),
is efficiency within the region of interest when
(given in
Table 4), and
is the voltage at which one observes one-half the sum of the maximum and minimum efficiencies within the region of interest. Furthermore, the shape of the increase in efficiency as a function of voltage (which will be presented in
Section 2.6) suggests that Equation (
14) is a member of a class of rectangular hyperbolic growth models. According to Srinivasan and Rao [
38,
39,
40],
A rectangular hyperbola is a curve that describes a plethora of biological processes including dependence of reaction velocity on substrate concentration in biochemistry, maximum effect of drug concentration in pharmacology, photosynthetic rate/growth rate on light intensity/nutrient level in physiology, microbial growth rates in aqueous environments to the concentration of a limiting nutrient (Monod equation), type II functional response as emphasized by Holling’s disc equation showing the relationship between prey density and prey consumption by predator, several instances in population ecology, Langmuir isotherms and many more.
One such physical process which is extremely similar to the gathering efficiency of the metasurface presented in Equation (
14) is governed by the Michaelis-Menten equation, which is one of the best-known models of enzyme kinetics, given by
, where
is the concentration,
is the maximum reaction rate achievable by the system (in the limit that
), and
is a constant and the concentration at which one achieves half of the maximum reaction rate possible (
) [
40]. In fact, the main difference between Equation (
14) and the Michaelis-Menten equation is that Equation (
14) considers a non-zero initial efficiency. To show that one recovers a Michaelis-Menten-like equation in the event of a starting efficiency of zero, one may take:
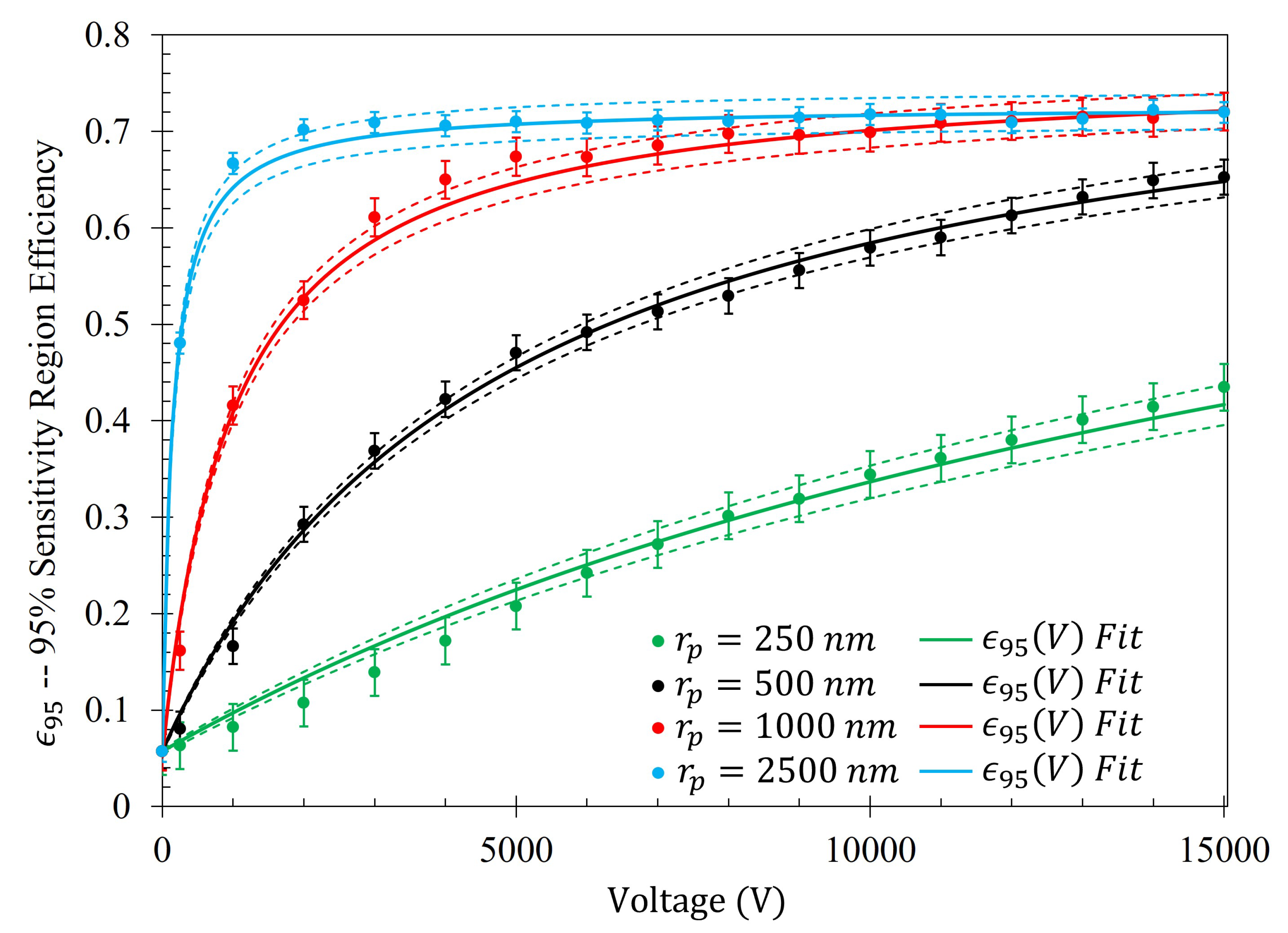
2.6. Gathering Efficiency of = 250 , 500 , 1000 , and 2500 Particles Estimated Via a Monte Carlo Method
The efficiency of the electrophoretic technique was theoretically quantified using the same procedure described at the beginning of
Section 2.5, where the gathering efficiencies of the metasurface as a function of voltage were identically modeled after Equation (
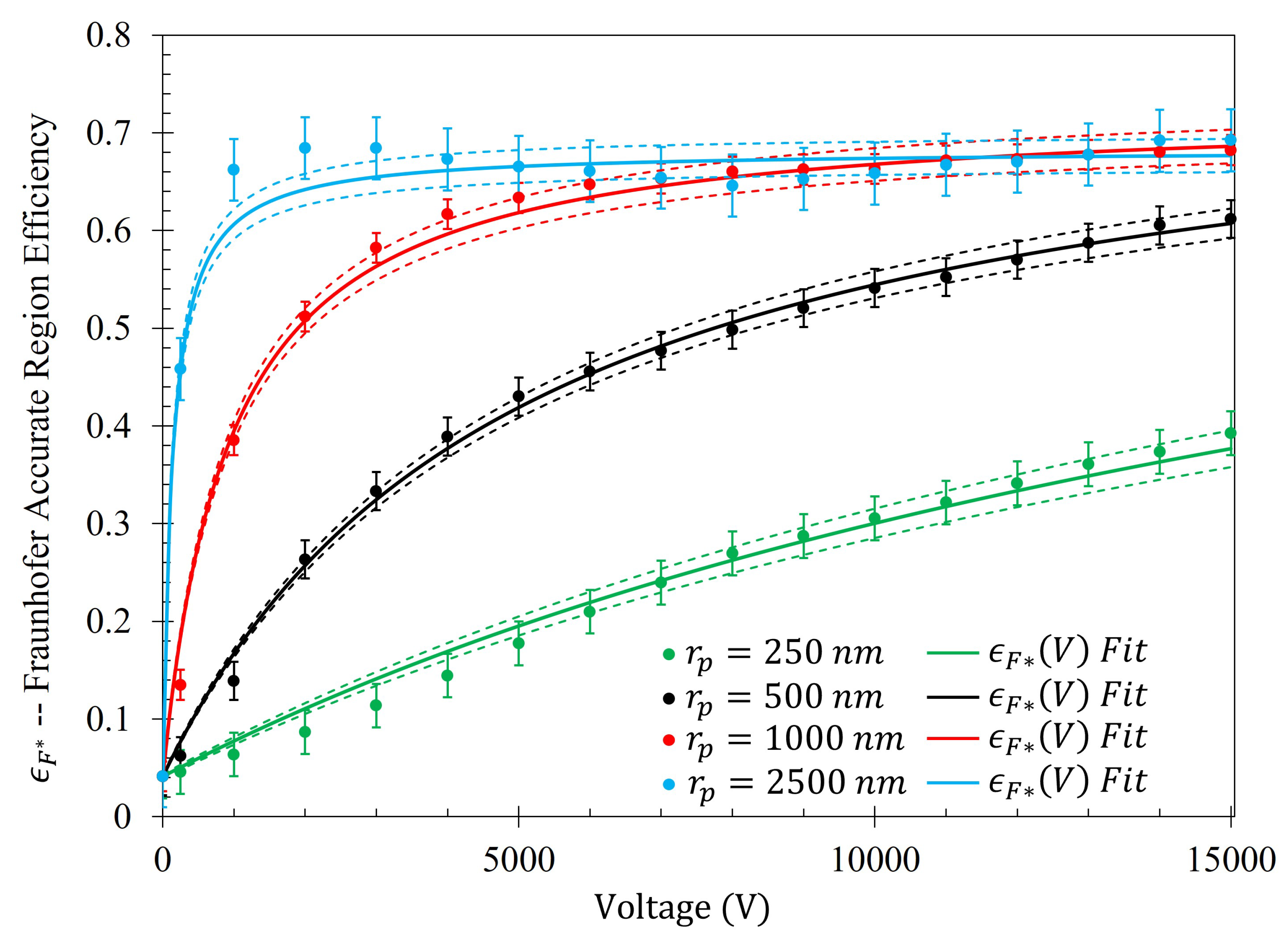
14). The efficiency of the electrophoretic technique (
) as a function of potential difference between the metasurface and the grounding plate within the Fraunhofer (
) and 95% (
) regions of sensitivity for particles of each radii are presented in
Figure 14 and
Figure 15, respectively. Using nonlinear regression, fits taking the form of Equation (
14) for each of the data sets were found. The equations of best fit, and which govern the gathering efficiency as a function of voltage within the Fraunhofer Accurate and 95% Sensitivity Regions, are given in
Table 5. Furthermore,
were determined by taking the limit as
for
, and are also provided in
Table 5.
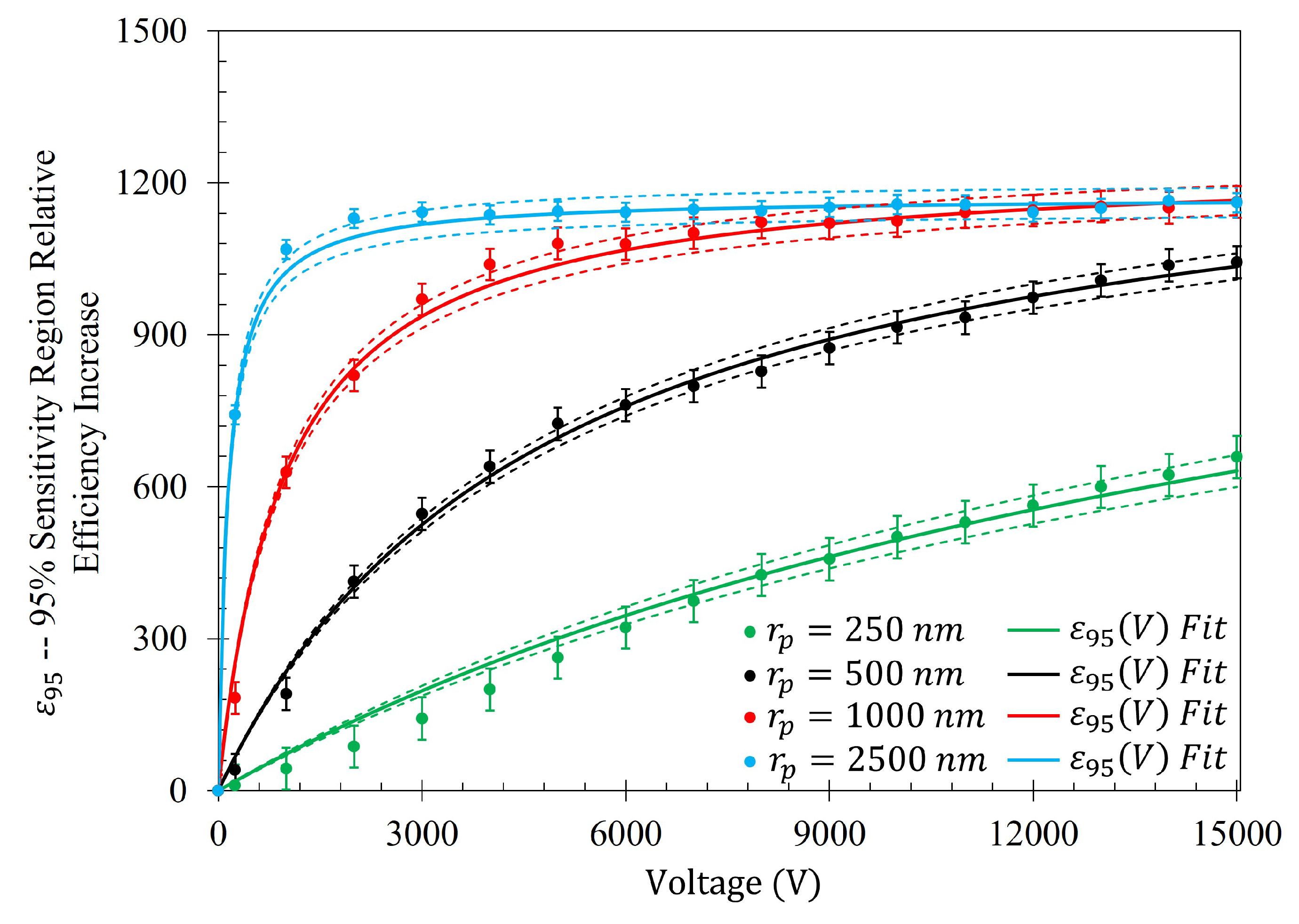
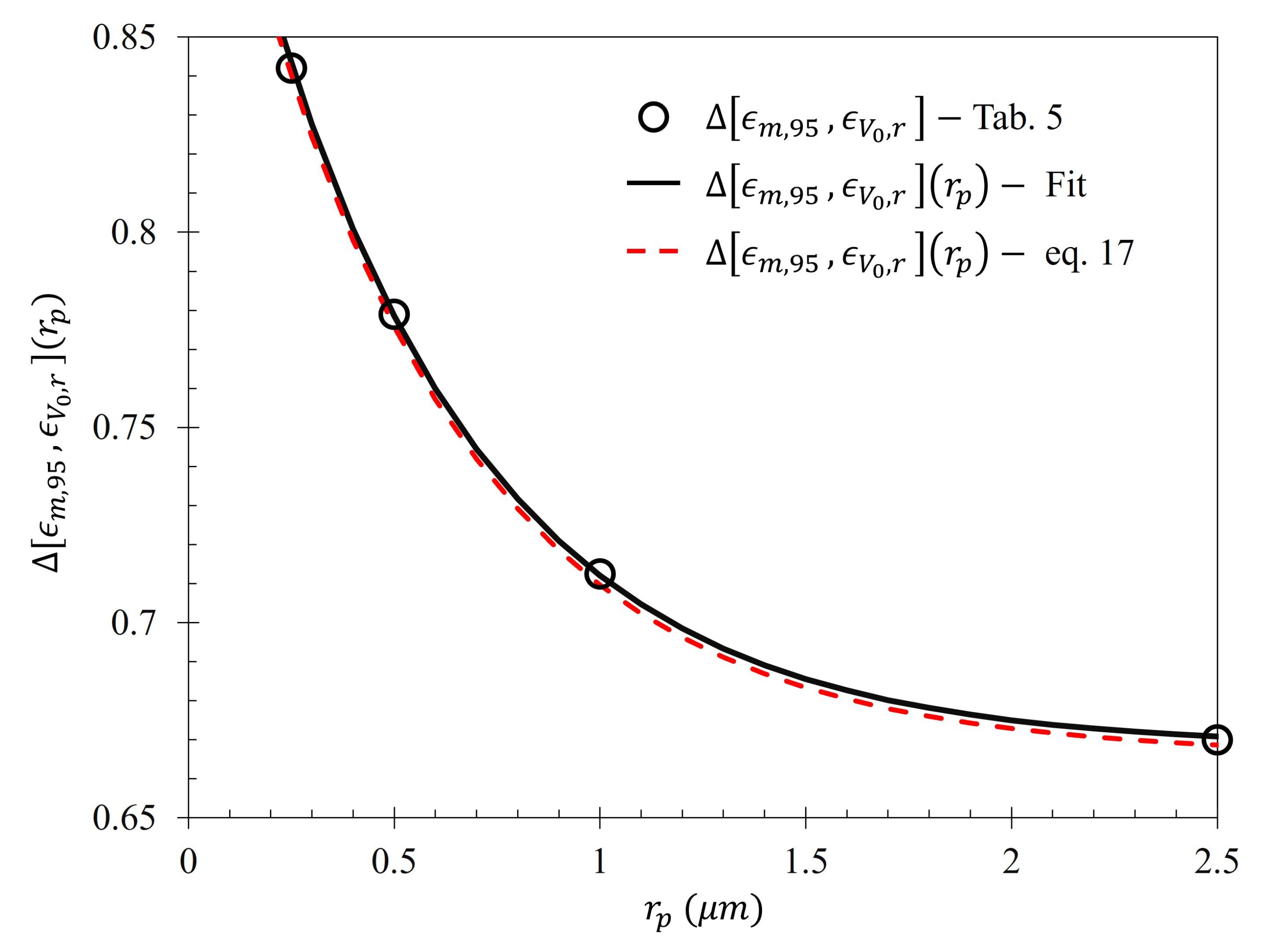
Figure 14, in conjunction with
Table 5, shows that the rate at which each of the particle gathering efficiencies approaches their asymptotic limit is proportional to their radii (and thus, surface charge) within the Fraunhofer Accurate Sensitivity Region. This is largely due to the fact that the surface charge for each of the particles tested differs from one another by an order of magnitude, suggesting that the larger, more highly charged particles should experience a much larger force than their smaller counterparts. In contrast to this observation, the theoretical maximum efficiency achievable for each particle of radius
is inversely proportional to their radius, suggesting that much higher voltages may be necessary to elicit the desired effect (if the net charge of the particles scales in such a nonlinear fashion such as in the case of the latex colloids).
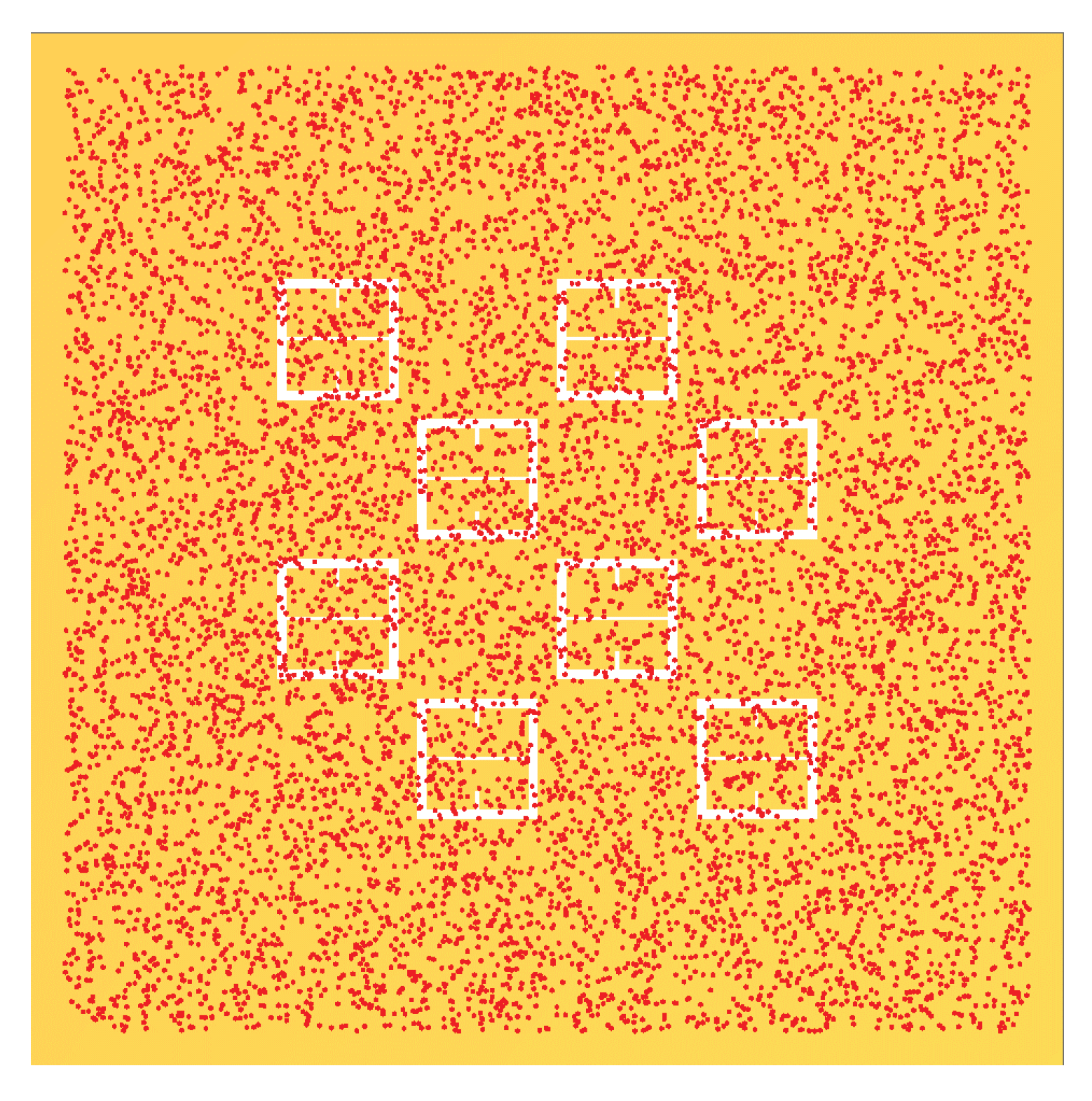
In a similar manner to
Figure 14,
Figure 15 shows the same behavior in the gathering efficiencies as a function of voltage and particle radius within the 95% Sensitivity Region. However, in the case of the 2500
radius particles, the fit equation more accurately depicts the gathering efficiency. This will be an important consideration in further analysis below. In
Figure 14 and
Figure 15, it is known that in the case of the 250
radius particle that the fit curves are accurate because the Monte Carlo simulation was further run for 20 kV, 30 kV, and 50 kV. Within this range, of voltages tested (0-50 kV), the gathering efficiency very closely matches the equations listed in
Table 5 for the Fraunhofer Accurate and 95% Sensitivity regions for the 250
particles, as seen in
Figure A2.
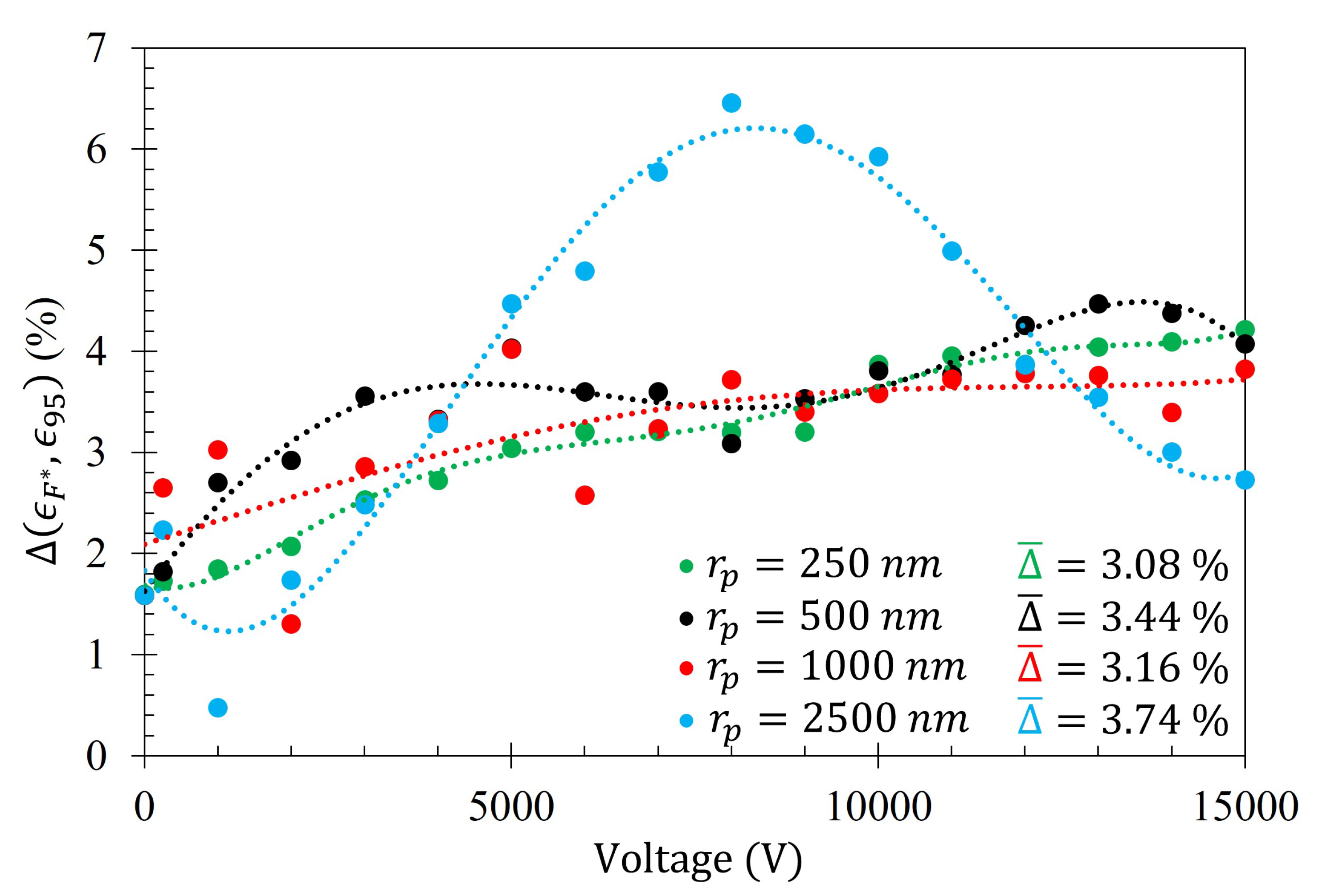
The average difference between the number of particles within the Fraunhofer Accurate and 95% Sensitivity Regions does not exceed 4% for any particle radii tested. This suggests that, of all the particles that settled within the much larger 95% Sensitivity Region, the majority of them are also within the Fraunhofer Accurate sensitivity region. This is an advantageous feature of this technique since, as depicted in
Figure 5, the greatest sensitivity of the metasurface lies within the Fraunhofer Accurate region.
Figure 16 is provided as evidence of the small difference in gathering efficiency between the Fraunhofer Accurate and 95% Sensitivity Regions. Furthermore, as an example of how
,
, and
from Equation (
14) relate to each of the data sets,
Figure 17 is provided.
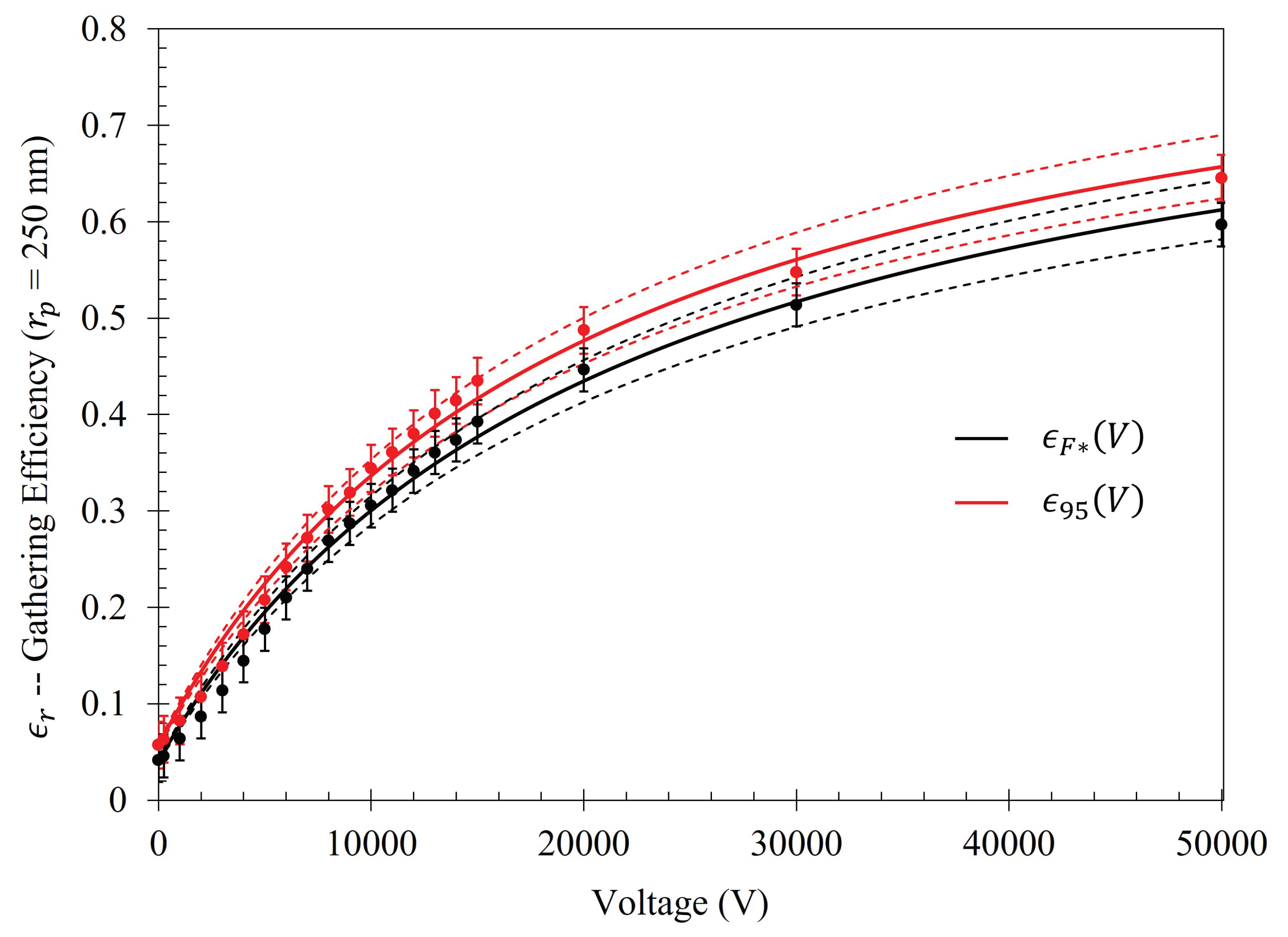
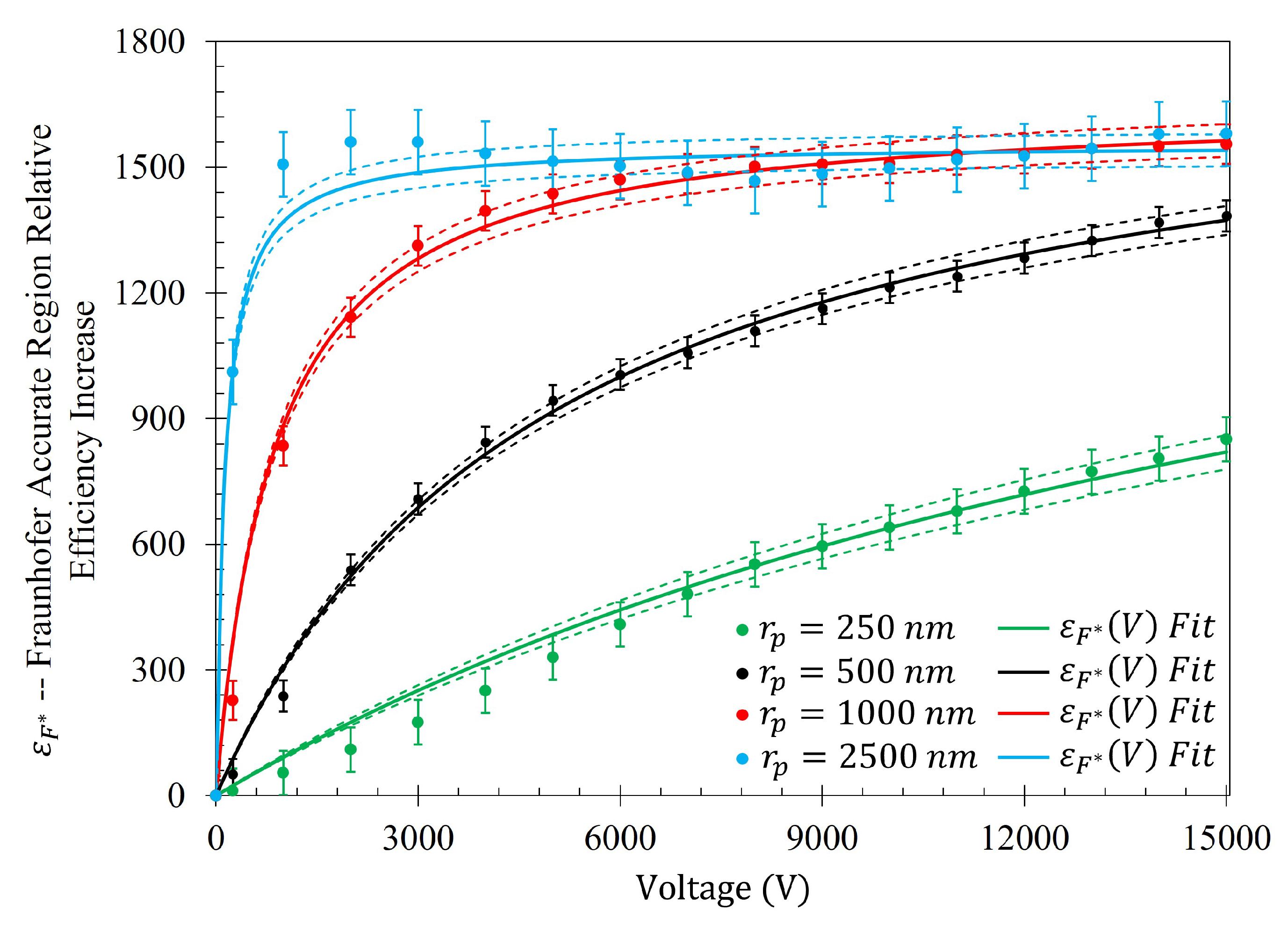
The relative percent increase in gathering efficiency as a function of voltage is given as
. The relative increase in gathering efficiency within the Fraunhofer Accurate and 95% Sensitivity Regions is presented in
Figure A3 and
Figure A4, and their respective equations in
Table A1. Within the Fraunhofer Accurate region, there is approximately a 1000%, 1400%, 1600%, and 1600% increase in the efficacy of the electrophoretic technique at 15 kV when compared to random sedimentation due to evaporation (when V=0) for the
, 500
, 1000
, and 2500
particles, respectively. Within the 95% Sensitivity region, there is approximately a 800%, 1000%, 1200%, and 1200% increase at 15 kV for the
, 500
, 1000
, and 2500
particles, respectively.
As a graphic example of the gathering efficiency as a function of voltage using this electrophoretic technique,
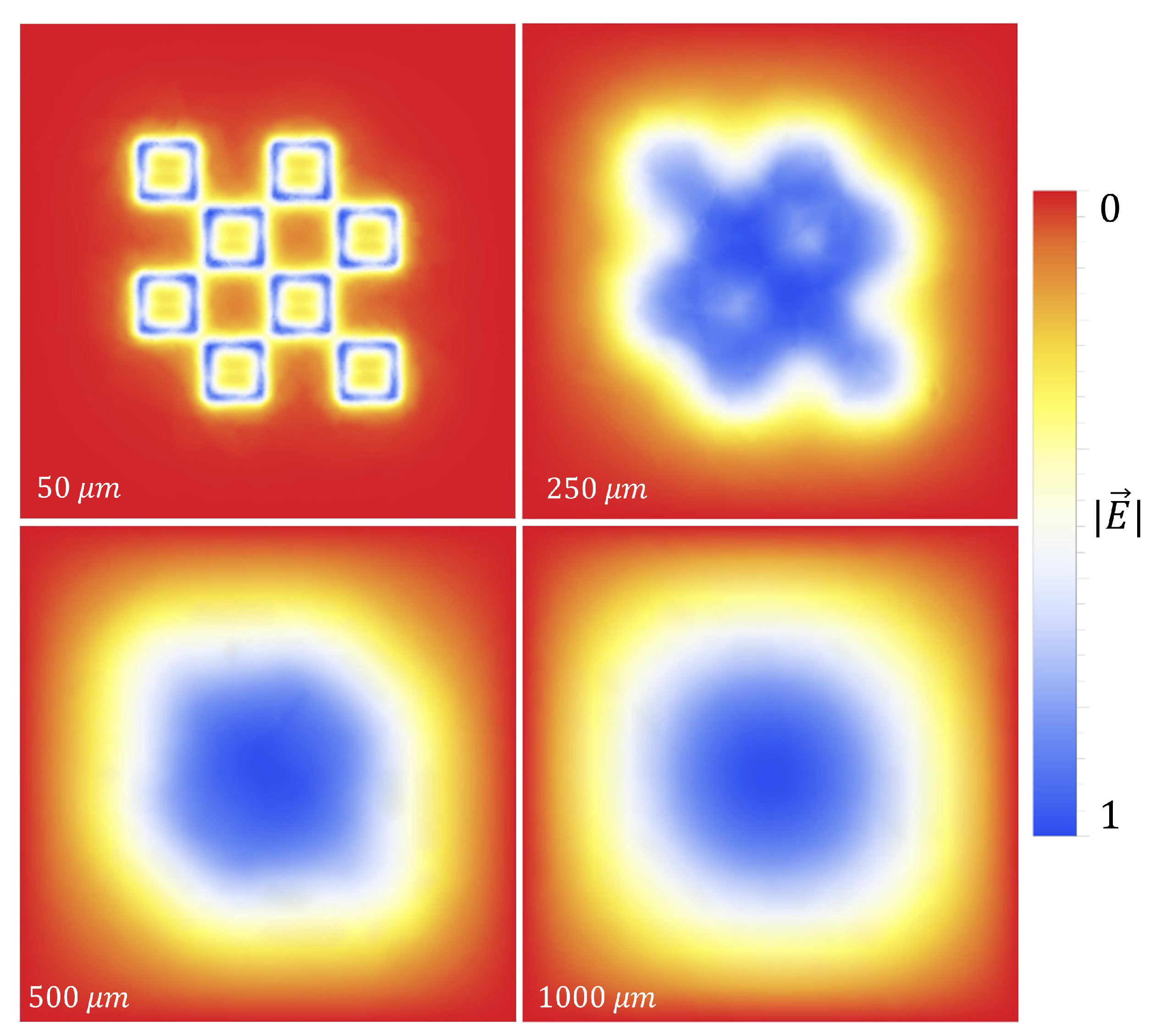
Figure 18 is provided for the reader, which depicts the gathering efficiency within the area presented in
Figure 8 for the 500
radius latices described in
Table 3. As can be seen in
Figure 18, the particles are forced to settle closer to the center of the entire area presented in
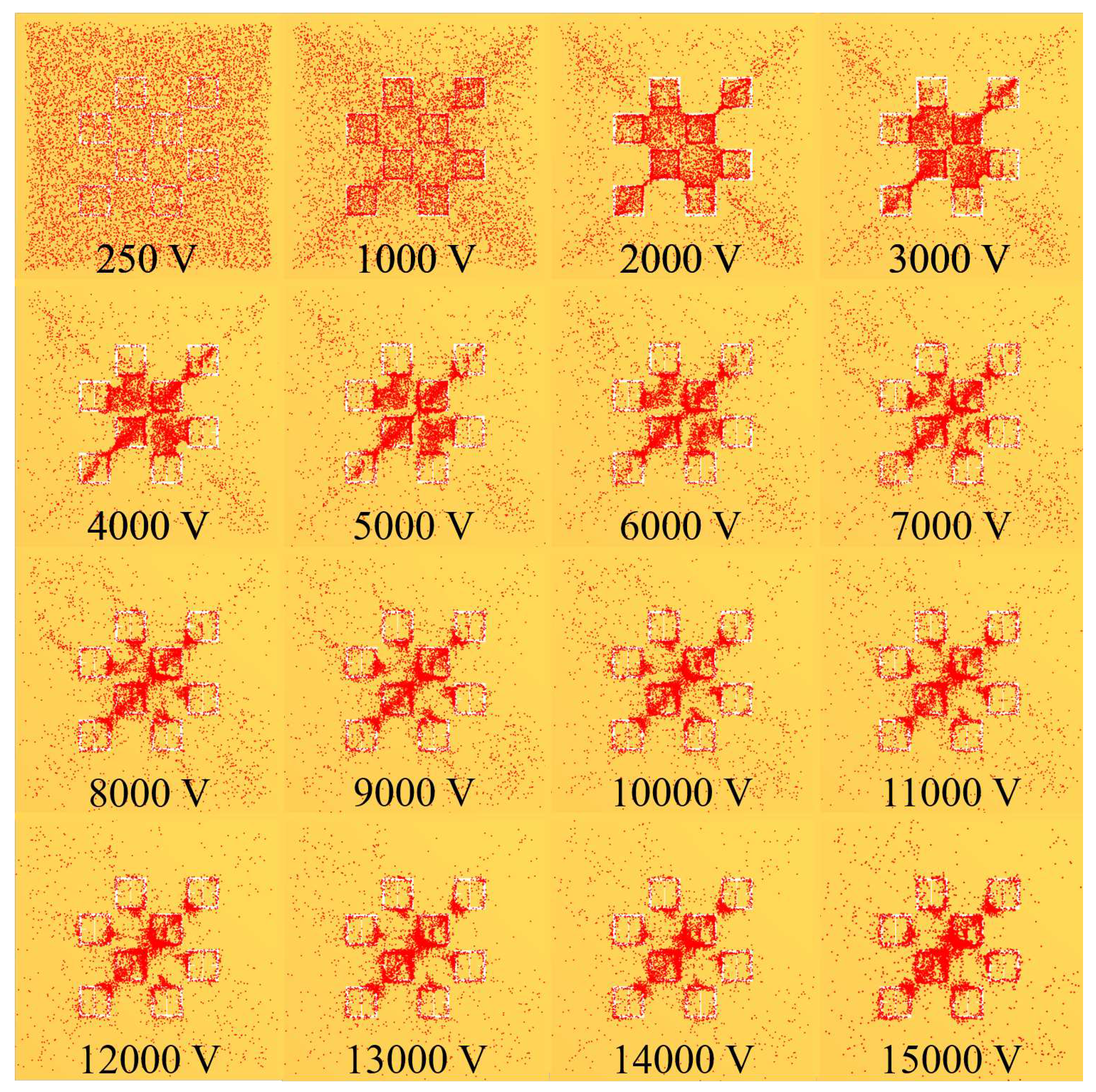
Figure 8 as the voltage increases. Some interesting and typical features observed at many voltages after simulating the electrophoretic technique are presented in
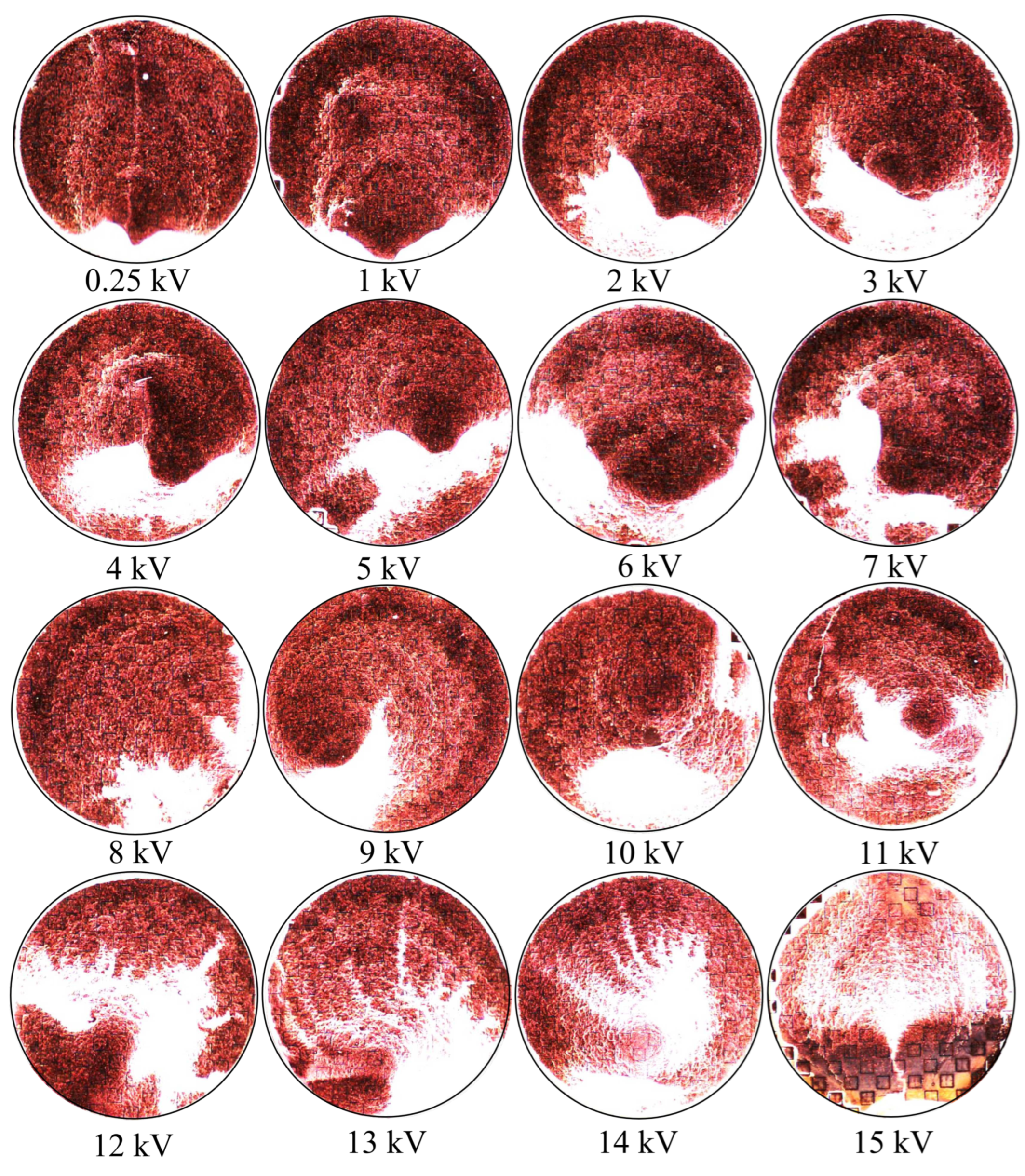
Figure 19. If one looks closely at
Figure 18, they will see similar features at other voltages not depicted in
Figure 19. In
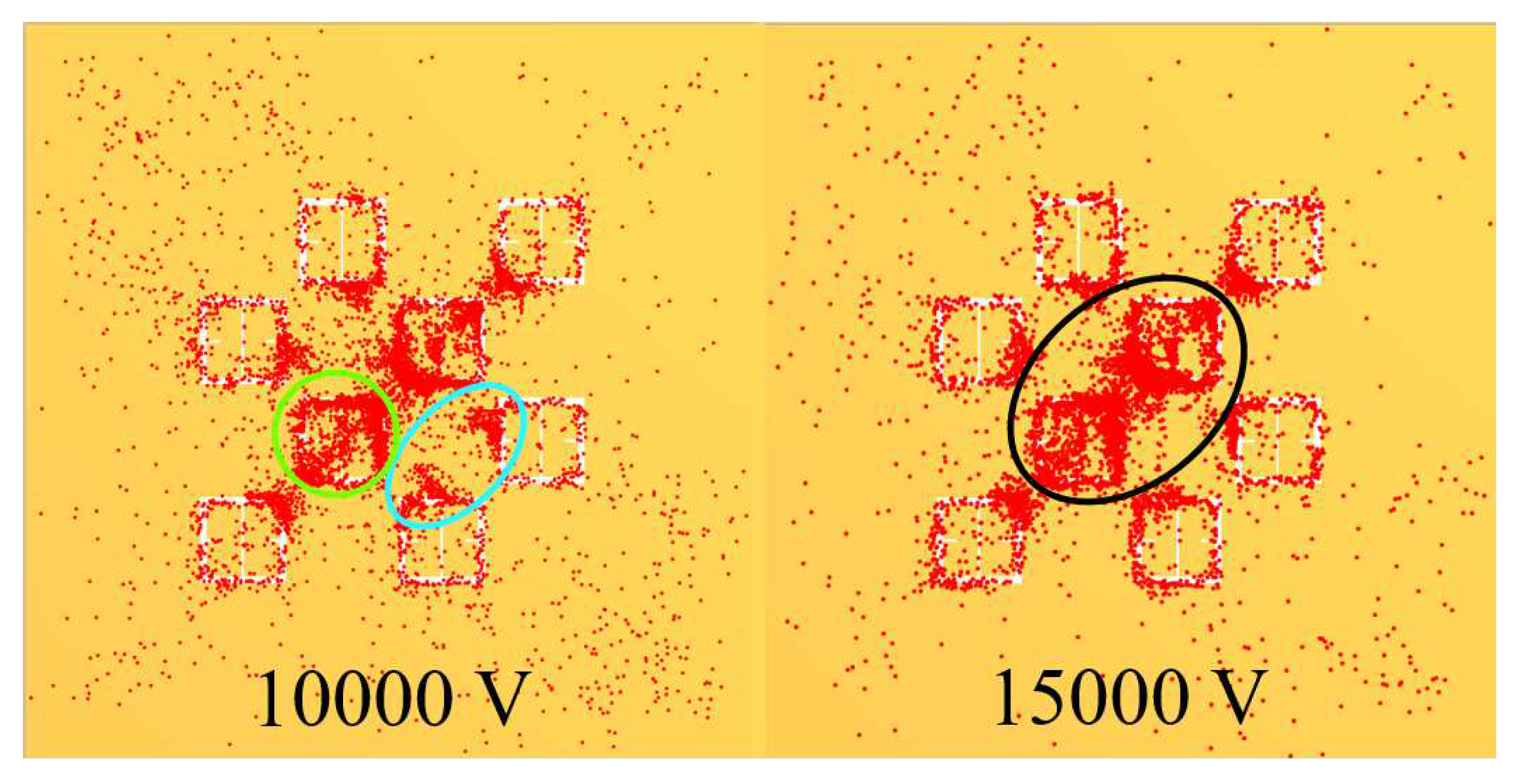
Figure 19, the blue oval in the 10 kV frame shows what appear to be tendril-like extensions from the largest slits in the metasurface aperture. These tendrils appear to be stretching out to meet the center unit cell apertures. The green circle in
Figure 19 encloses a line of particles which runs from the bottom left corner of the central most unit cell, diagonally toward the center of the frame. At the center of the black oval in
Figure 19, the particles have extended from corner to corner of the central most unit cells in the frame. The density of the particles at 15 kV is well represented in
Figure 20.
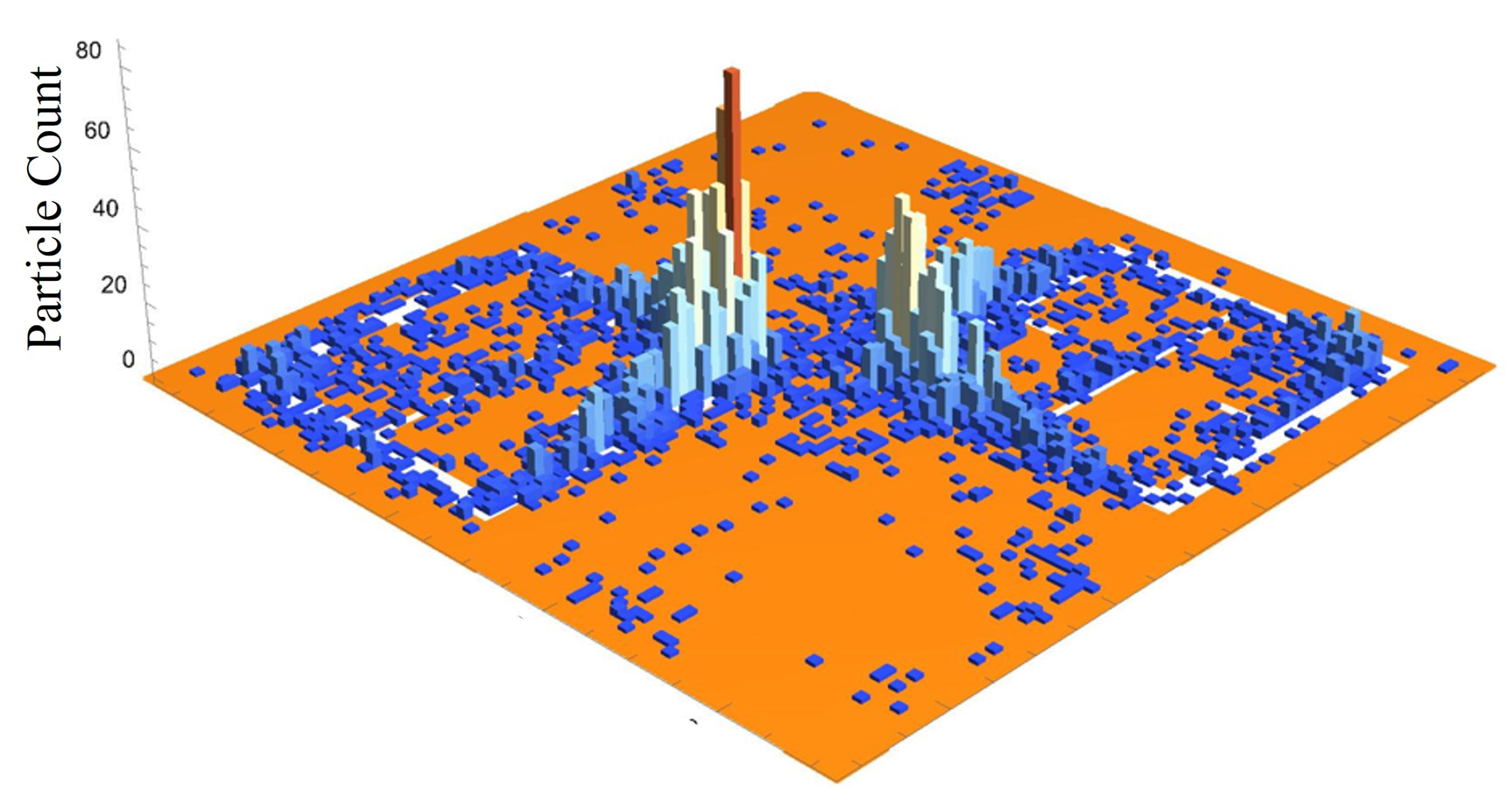
Figure 20 is a three dimensional histogram, focused on the center-most unit cell of the 15 kV frame from
Figure 19. The histogram bins are 60
× 60
(2500 bins total on the surface). According to this histogram, there appears to be a very high concentration of particles at the corners of the metasurface atoms, at the center of the frame. Furthermore, between the corners of the metasurface atoms, a less populated mass extends between them, bridging the gap. In the central slit of each of the metasurface atoms, and along the larger slits at their periphery, it appears that particles have also preferentially gathered in these regions.
Figure 19 and
Figure 20 hint at the types of densities and structures that may be observed experimentally. However, it was suspected that since the particles would like to minimize their potential energy on the surface, instead of stacking upon one another in a never ending fashion during the experiment, they will be forced to spread out as much as the electric field allows them to near the highest density regions depicted in
Figure 20. This may lead to a more evenly distributed "bridge" in the density of the particles between the meta-atom corners.
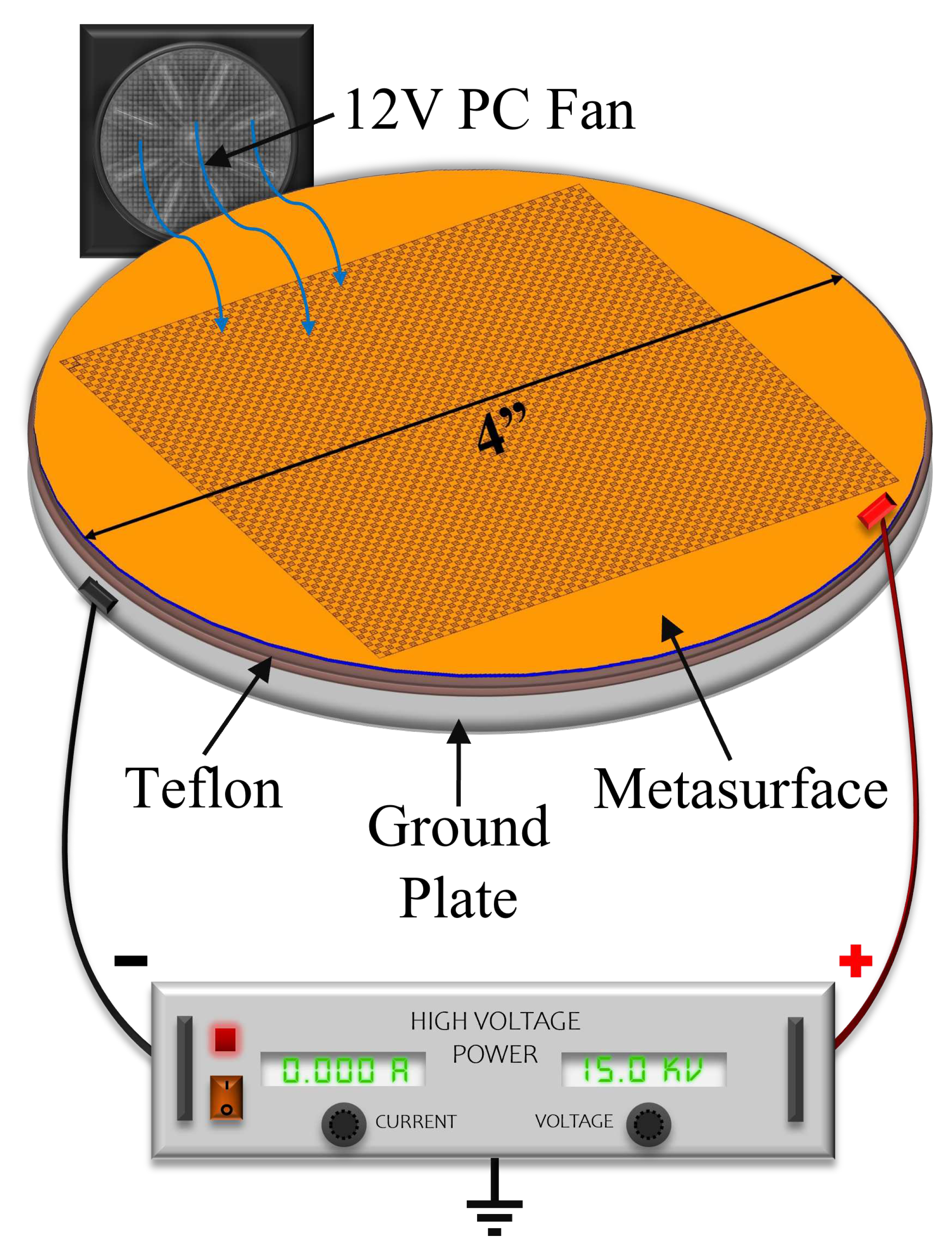
2.7. Experimental Parameters
To test the electrophoretic technique, an experimental apparatus was constructed, whose diagram is presented in
Figure 2, and image is shown in
Figure 21. A Glassman High Voltage power supply was connected to the metasurface and ground plate via high voltage wire. The metasurface, ground plate and fan were encased in an acrylic containment box with a 12V computer fan placed inside to accelerate evaporation (facing at the corner, away from the metasurface).
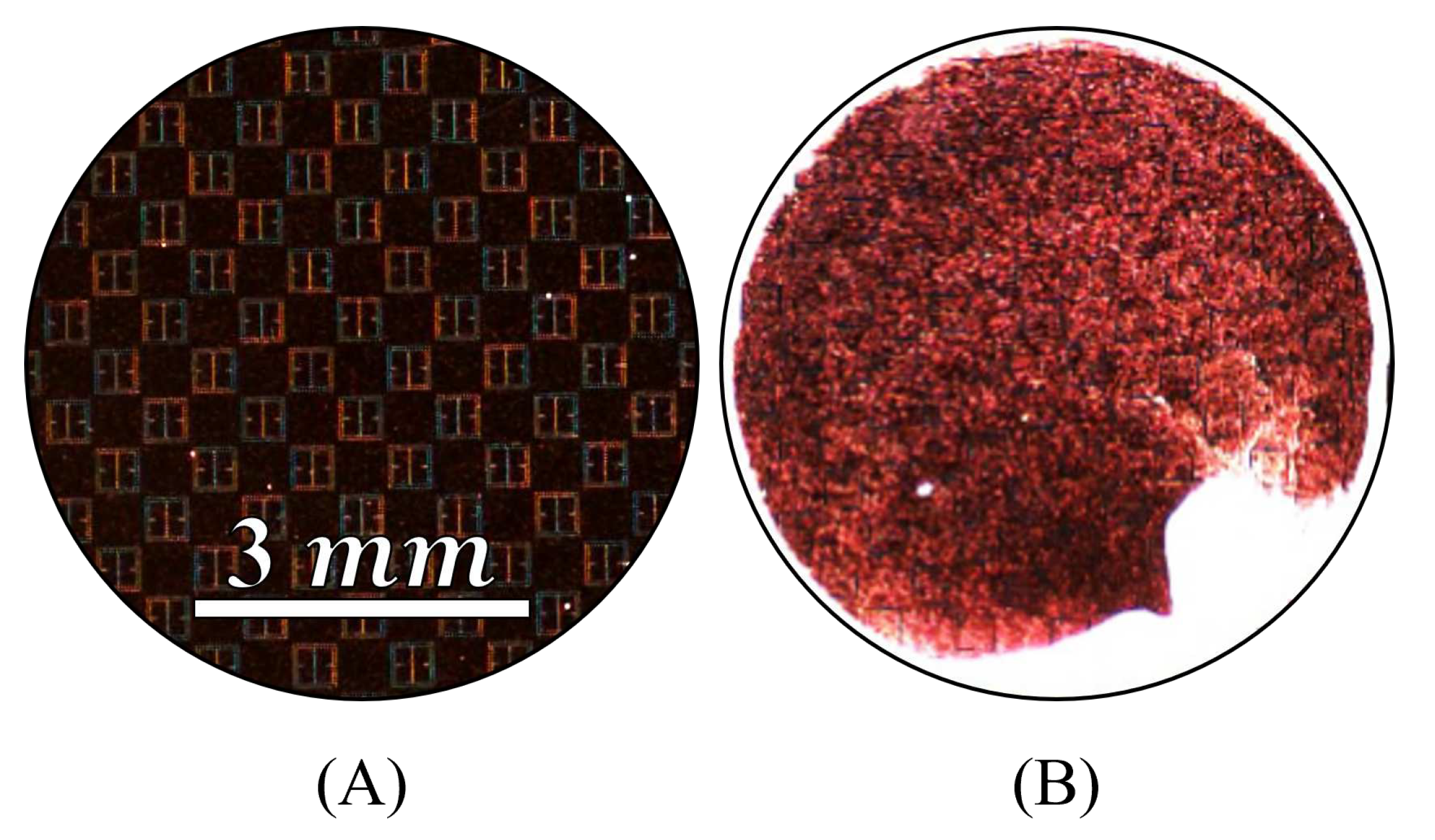
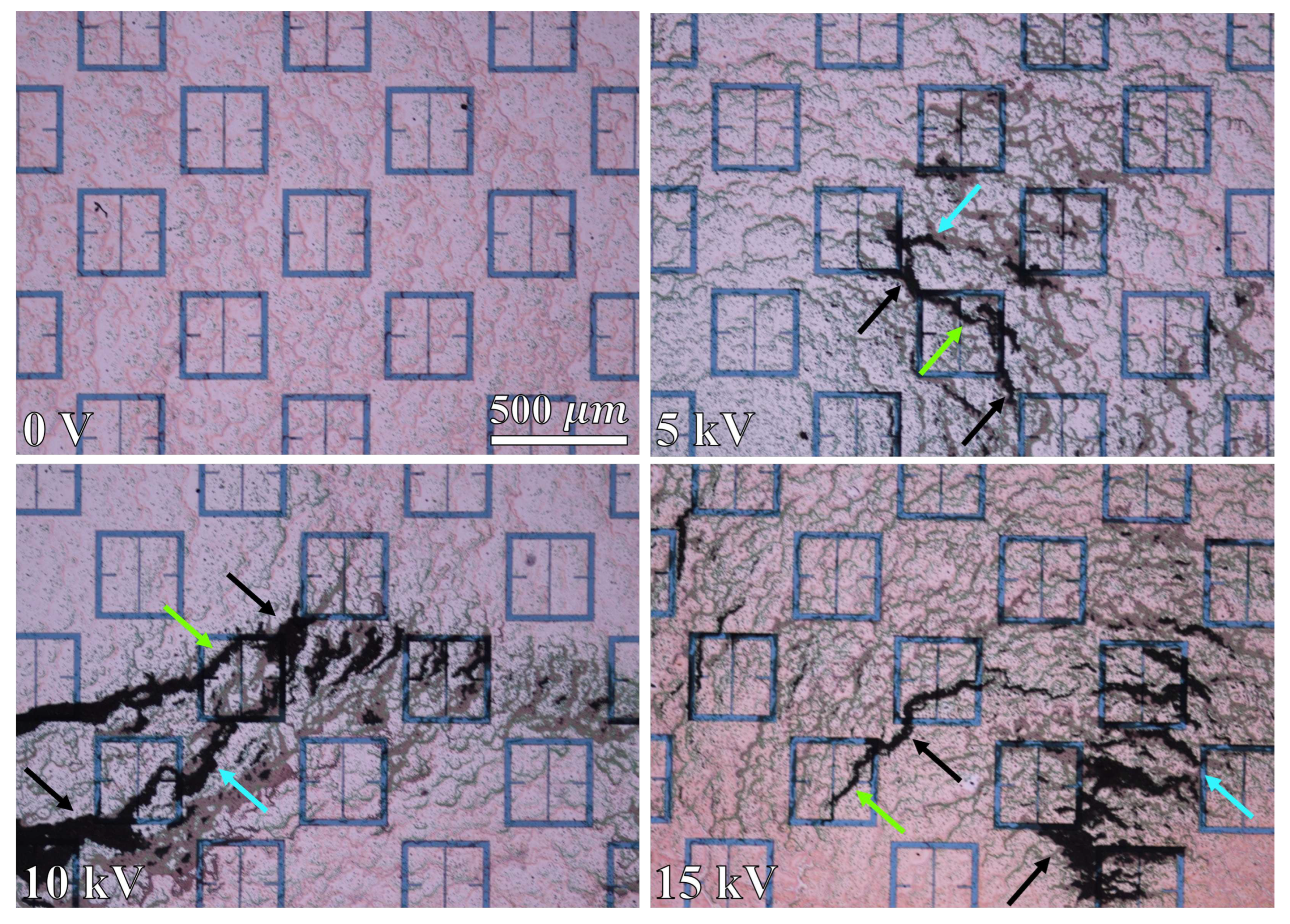
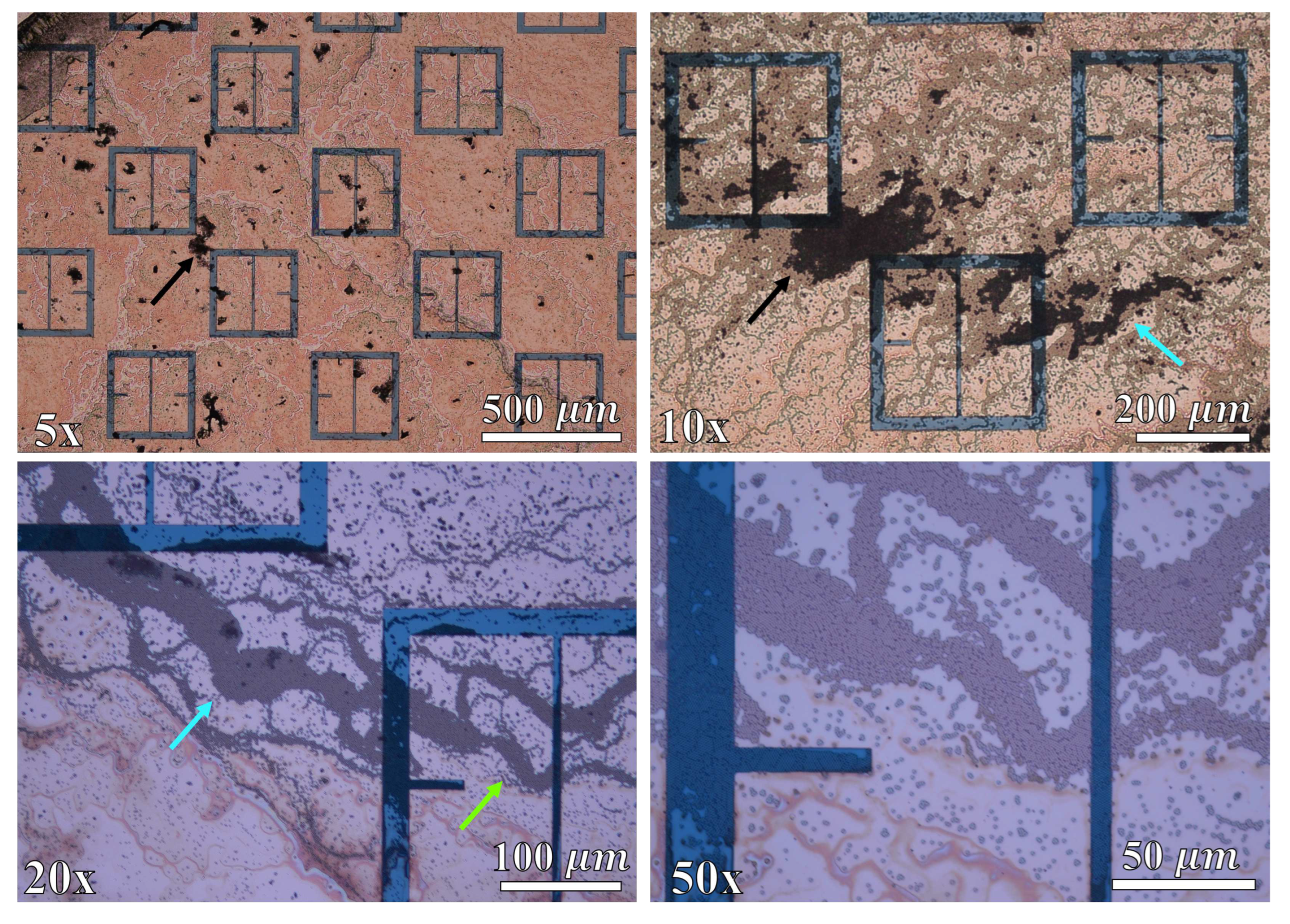
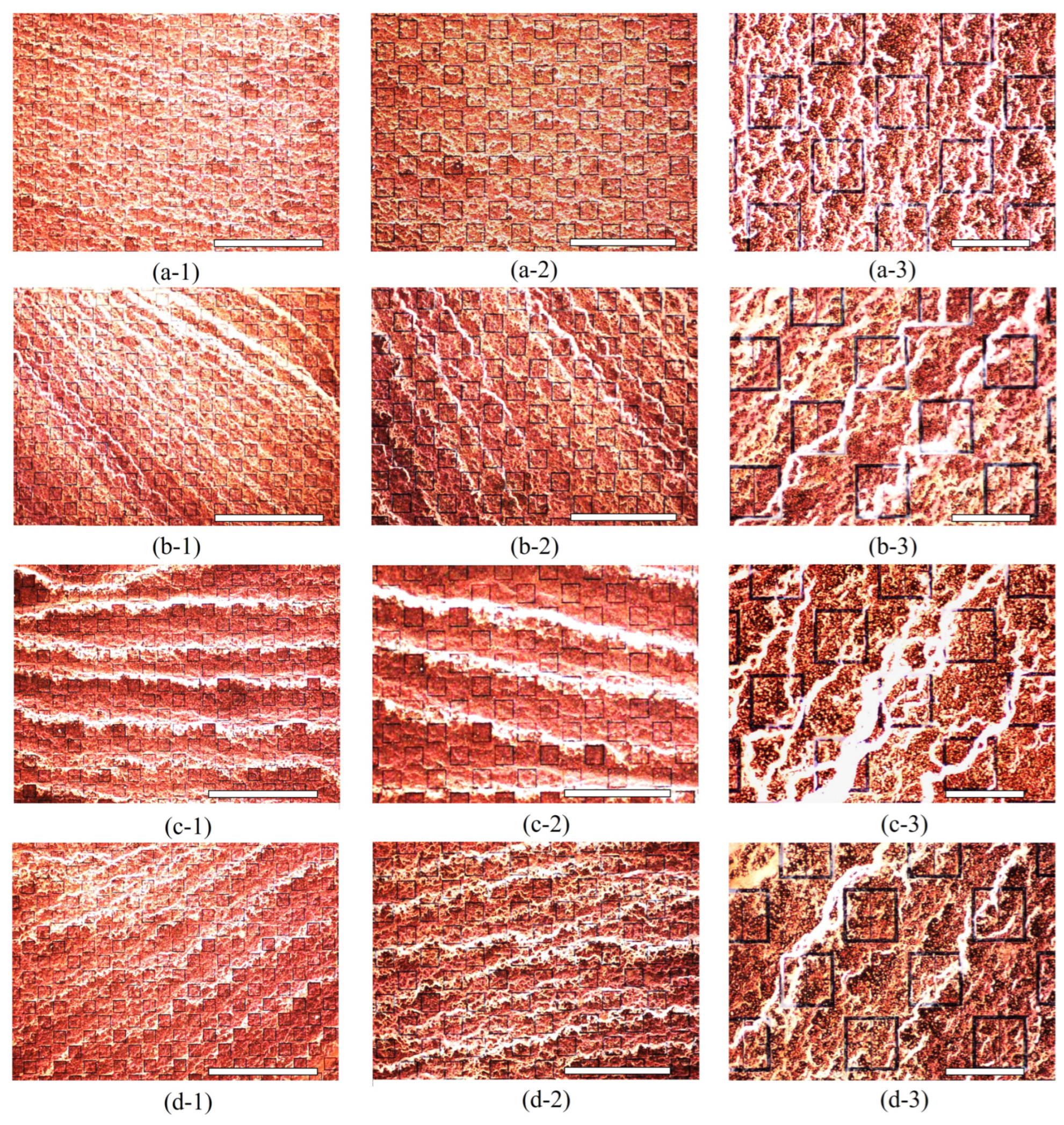
A 15mL solution of 10% by mass, aminated 500 radius latex nanoparticles suspended in distilled water was obtained from MAGSPHERE, INC. The specific gravity of the particles were estimated by MAGSPHERE, INC. to be approximately 1050 . No exact surface charge information was provided by MAGSPHERE, INC., and we lack the ability to precisely measure such properties. 0.5 mL of the 10% latex nanoparticle solution was diluted in 150 mL of distilled water, producing a solution that was approximately 333 parts per million (ppm) latex nanoparticles, by mass. The 333 ppm latex nanoparticle solution was deposited (at room temperature) onto the metasurface a single drop at a time using a serological pipette. After deposition of a single drop, the containment lid was closed, the apparatus turned on, and the droplet left to evaporate. In total, seventeen drops were placed onto the metasurface. Each drop was independently exposed to a successively increasing electric field strength via the potential difference between the metasurface and the ground plate. For instance, the first drop was left to evaporate under 0 V between the metasurface and ground plate, the second drop was left to evaporate under 250 V, and the third through the seventeenth drops under 1 - 15 kV (increasing by 1 kV increments from the third drop up to the seventeenth drop).
Since it was suspected that the particles could not be considered non-interacting at a concentration of 333 ppm (based upon visual inspection of the droplet area after evaporation), a second test of the experiment was conducted in order to more accurately test the Monte Carlo model presented in
Section 2.5. To do so, 0.25 mL of the 10%, 500
radius latex nanoparticle solution was diluted in 200 mL of distilled water, producing a solution that was 125 ppm latex nanoparticles by mass. After the 333 ppm and 125 ppm droplets evaporated, the resulting particle distributions were imaged via an inspection scope and a high powered microscope. The resulting particle distributions were then compared to the morphological results obtained via the Monte Carlo simulations.
After inspecting the efficacy of the electrophoretic technique using the 333 ppm and 125 ppm solution, the metasurface was thoroughly cleansed of latices using pure Acetone and Class 100 Cleanroom Wipes (confirmed under microscope and inspection scope), and the entirety of the sensing surface covered with 5 mL of the 333 ppm solution. As observed in [
1], the metasurface utilized in this study has a resonant wavelength of approximately 2.8 mm, and may be capable of detecting inhomogeneous powder layers on its surface which are effectively smaller than 20
thick. However, to be sure that we would have the greatest chance of detecting a change in the resonant spectra of the metasurface in the presence of the 500
radius latex nanoparticles at W-band, the 333 ppm solution was used. First, 5 mL of solution was deposited on the entire sensing area of the metasurface and left to evaporate without any electric field applied to it. Using the experimental apparatus depicted in
Figure A5 [
1], the transmittance spectra of the metasurface measured between 90 - 110 GHz (with a frequency resolution of 16 MHz) was then recorded using a Network Analyzer and set of W-band lenses and horns, following the same measurement procedure stated in [
1] :
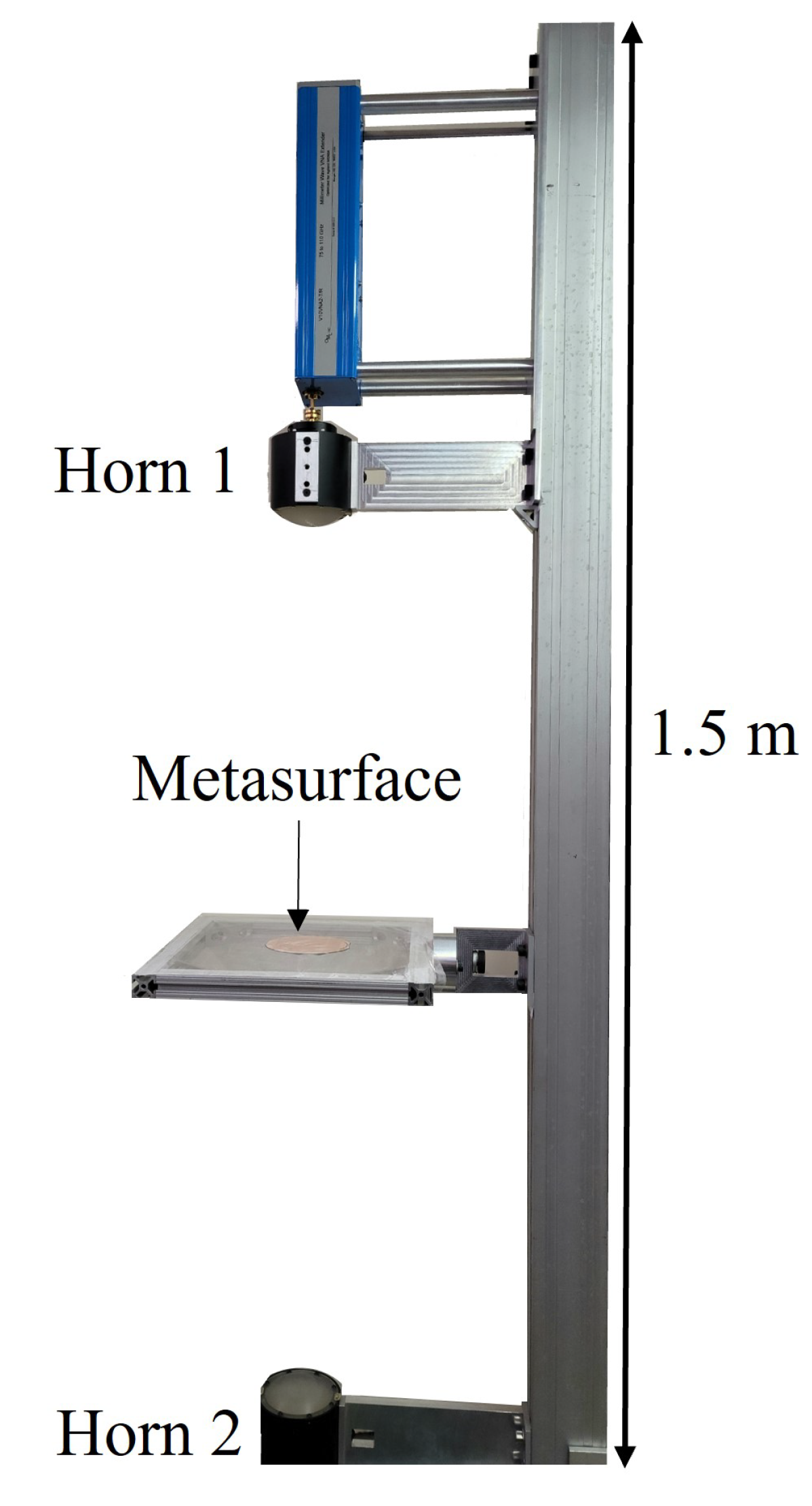
The Network Analyzer was calibrated using a response calibration technique. Only transmittance data, denoted in this study as (the total transmitted power detected by Horn 2 as it leaves Horn 1), was considered. The horns were erected approximately 80 cm apart. The Gaussian beam produced through the focusing lenses at each horn had a FWHM of approximately two inches in diameter in the focal plane and was centered on the metasurface (4-inch diameter). The measurement window was isolated from the ambient environment and the effects of noise were removed from the calibrated data by applying time-domain gating of the space outside of the sample area. Furthermore, the optical bench that the apparatus was erected on, and various metallic surfaces in the area, were covered in mm-wave absorbing material to further reduce error in the measurements.
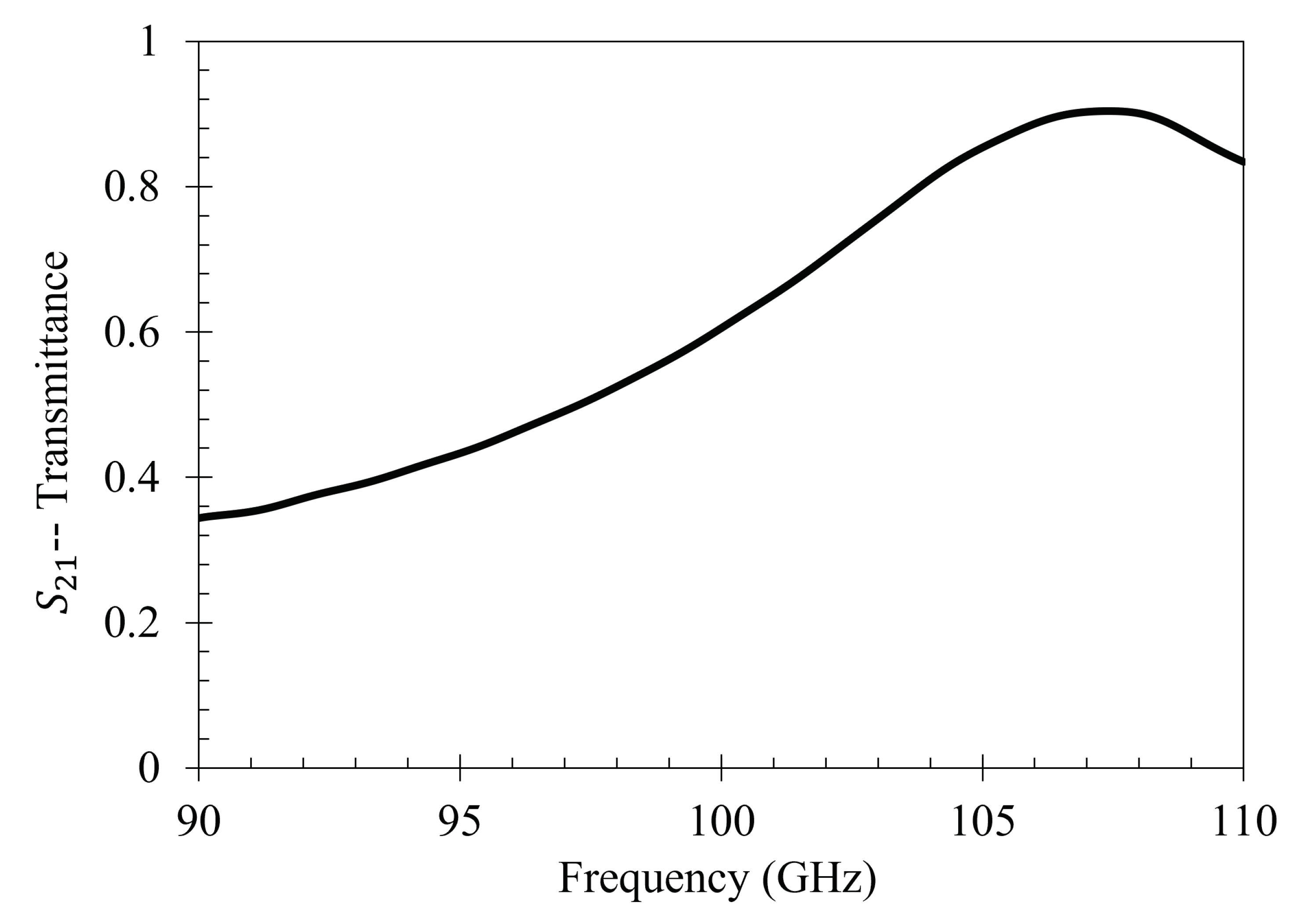
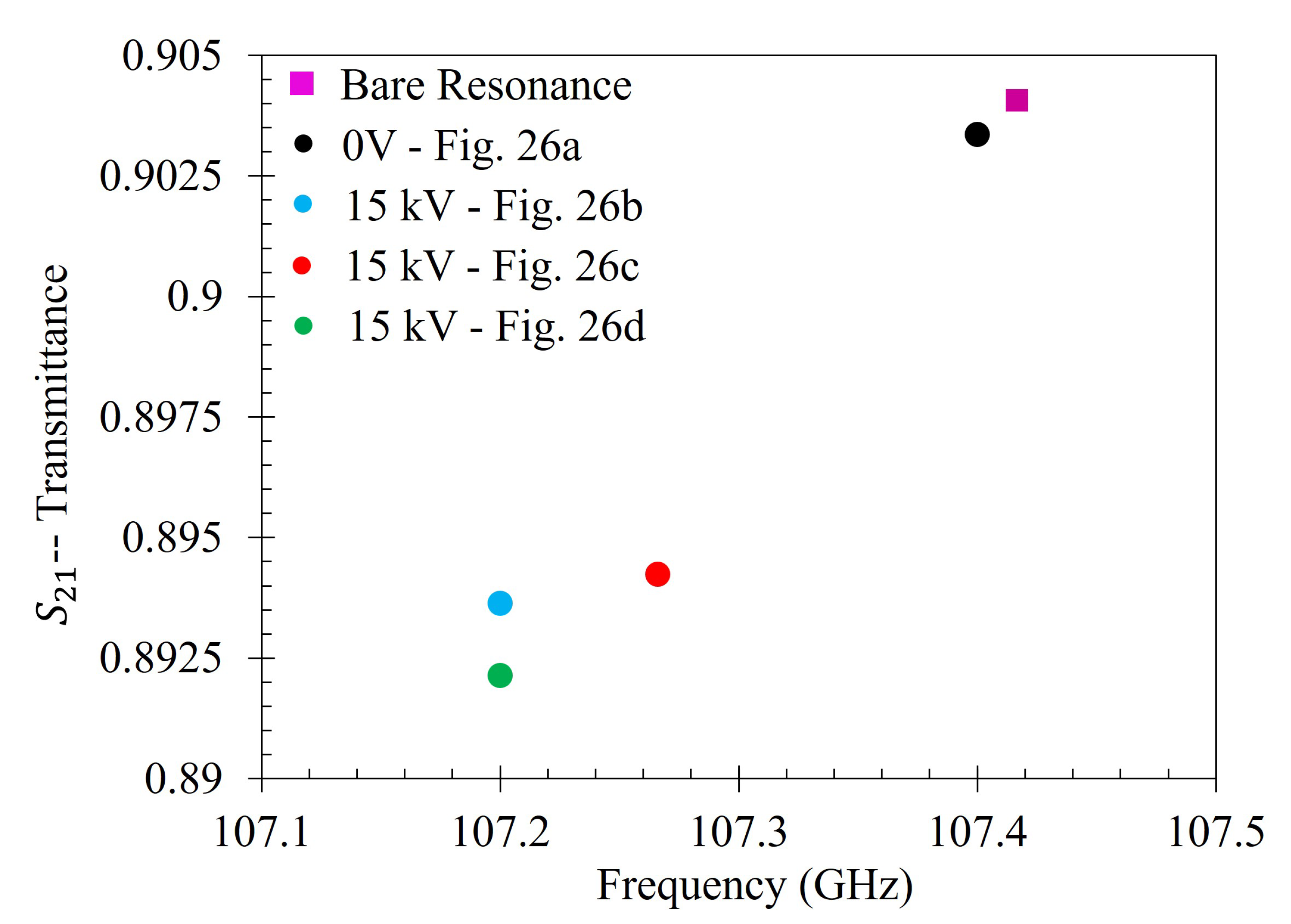
Then the metasurface was thoroughly cleaned again, and the 333 ppm solution was reapplied to the entire sensing area of the metasurface. It was then left to dry under 15 kV between the metasurface and the ground plate. After evaporation, the transmittance spectrum of 333 ppm solution which dried under 15 kV was measured. The 15 kV 5 mL spot test was conducted three separate times. The spectra of the 0 V and the three 15 kV latex films was then compared to the spectra of the bare metasurface. The experimentally measured transmittance spectrum of the bare metasurface is presented in
Figure 22.