Submitted:
29 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Crop production and quality can be improved by using algal-bacteria and algae consortiums
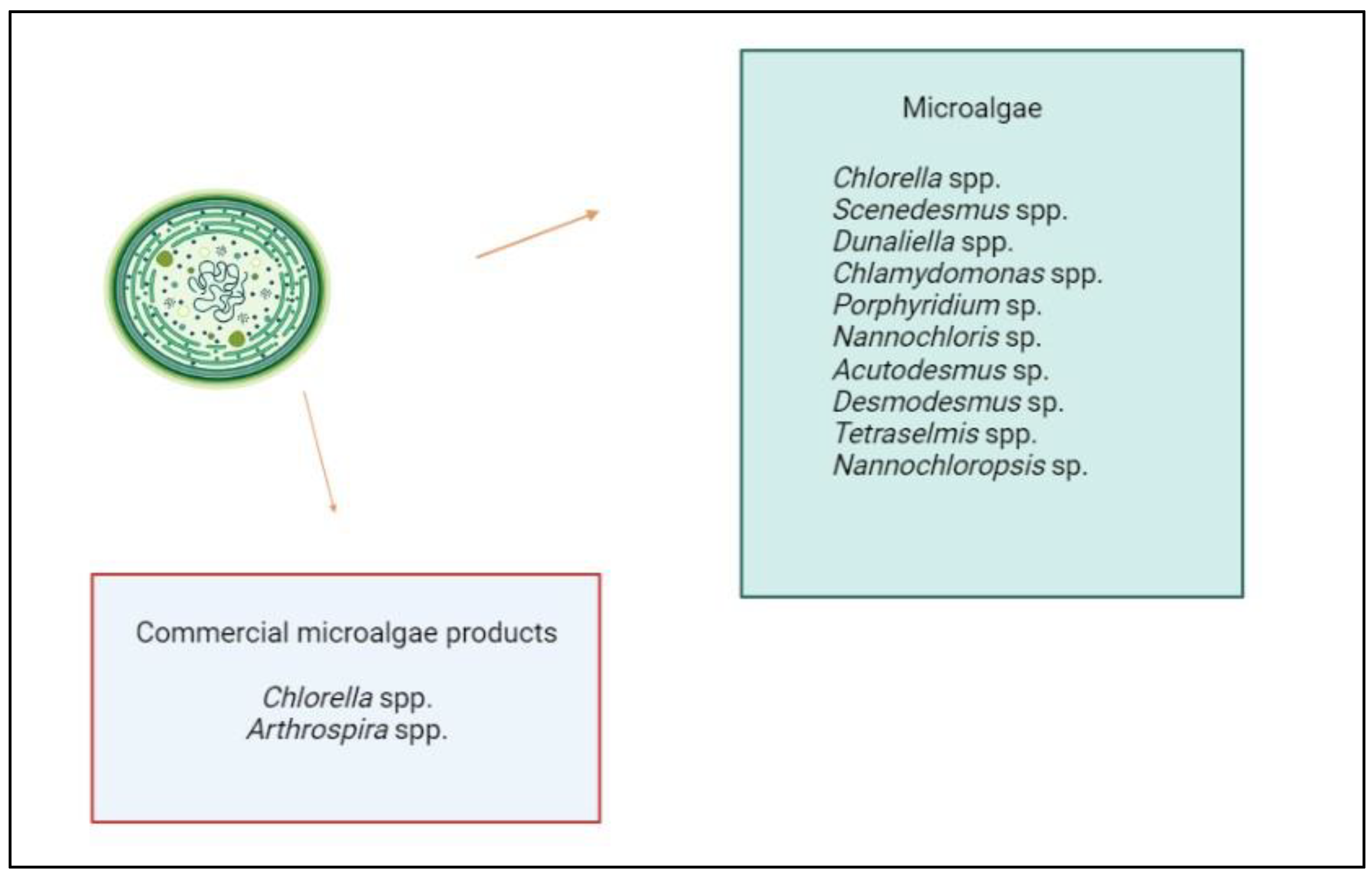
3. Biomass production of microalgae
4. Microalgae as a new source of biostimulants
5. Processes and applications of biostimulating algal biomass
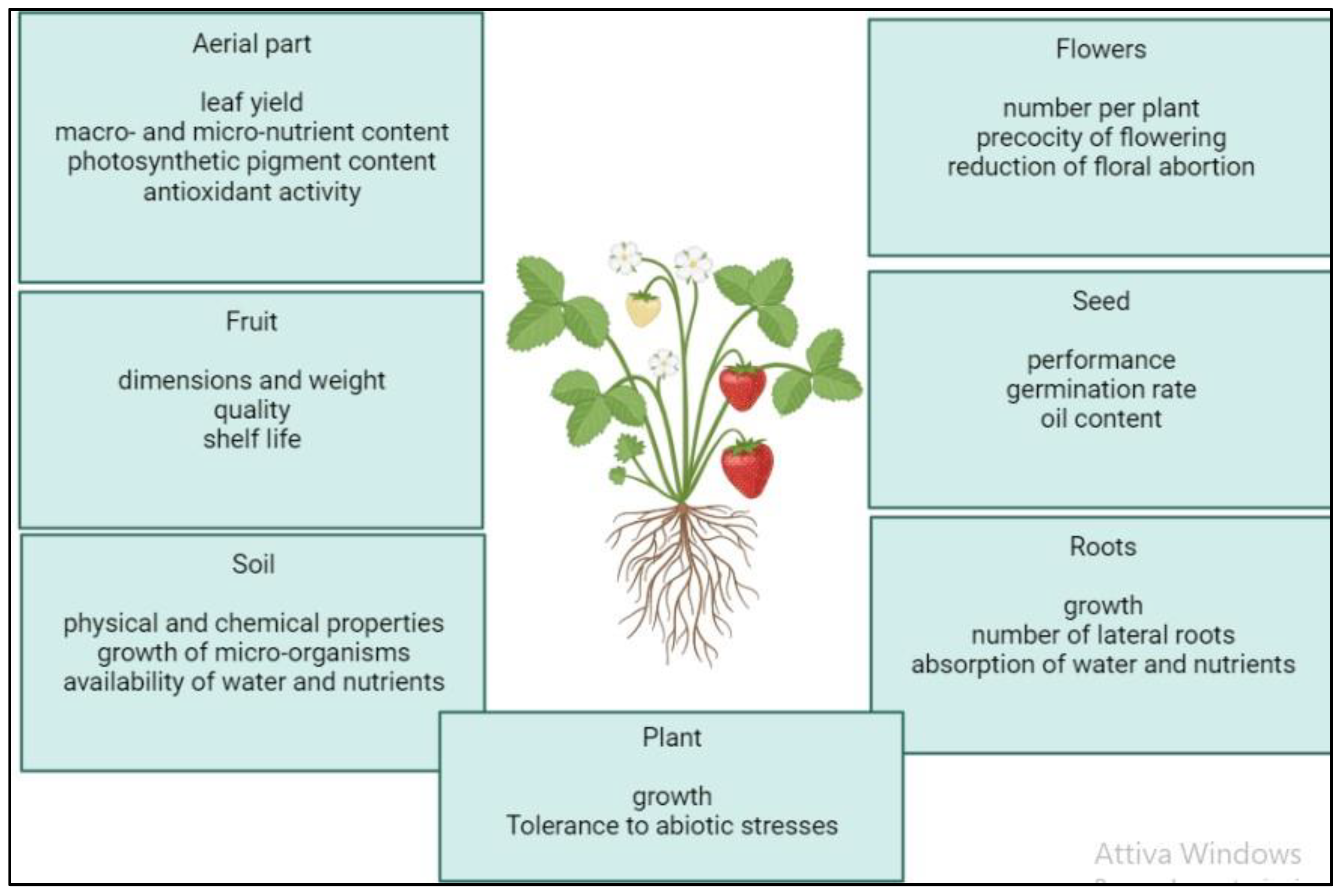
6. Main biostimulating effects of microalgae on the plant
7. Advantages and critical issues in the use of microalgae for biostimulants
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Dmytryk, A.; Chojnacka, K. Algae as fertilizers, biostimulants, and regulators of plant growth. In: Algae Biomass: Characteristics and Applications. Springer International Publishing. 2018, 115–122.
- Mzibra, A.; Aasfar, A.; Benhima, R.; Khouloud, M.; Boulif, R.; Douira, A.; Bamouh, A.; Meftah Kadmiri, I. Biostimulants derived from moroccan seaweeds: seed germination metabolomics and growth promotion of tomato plant. J. Plant Growth Regul. 2020, 1–18. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. (Amsterdam). 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Arnau, L.; Richard, H. Techno-Economic Feasibility Study for the Production of Microalgae Based Plant Biostimulant. KTH Royal Institute of Technology. 2016; pp. 300–365.
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; Fornasier, F.; Squartini, A.; Nardi, S.; Concheri, G. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L. ). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]
- Kopta, T.; Pavlíkova, M.; Se¸ kara, A.; Pokluda, R.; Marˇsa´lek, B. Effect of bacterial- algal biostimulant on the yield and internal quality of Lettuce (Lactuca sativa L. ) produced for spring and summer crop. Not. Bot. Horti Agrobot. Cluj-Napoca. 2018, 46, 615–621. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy. 2019, 9, 335. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy. 2019, 9, 306. [Google Scholar] [CrossRef]
- Chanda, M. J.; Merghoub, N.; EL Arroussi, H. Microalgae polysaccharides: the new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019; pp. 411–442.
- Chakdar, H.; Jadhav, S.D.; Dhar, D.W.; Pabbi, S. Potential applications of blue green algae. J. Sci. Ind. Res. 2012, 71, 13–20. [Google Scholar]
- Dasgan, H.Y.; Aydoner, G.; Akyol, M. Use of some microorganisms as bio- fertilizers in soilless grown squash for saving chemical nutrients. In: Acta Horticulturae. International Society for Horticultural Science. 2012, 155–162.
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Bansal, R.; Bidyarani, N.; Singh, R.; Shivay, Y.S.; Nain, L.; Ahluwalia, A.S. Wastewater grown microalgal biomass as inoculants for improving micronutrient availability in wheat. Rhizosphere. 2017, 3, 150–159. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Grünewald, C.F.; Lovitt, R. Using microalgae in the circular economy to valorise anaerobic digestate: challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Rumin, J.; Nicolau, E.; Gonçalves de Oliveira Junior, R.; Fuentes-Grünewald, C.; Flynn, K.J.; Picot, L. European commission. Mar. Drugs. 2020, 18, 79. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Bashan, Y. Increased growth of the microalga chlorella vulgariswhen coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef]
- Babu, N.R.; Devaraj, V.R. High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust. J. Crop. Sci. 2008, 2, 40–48. [Google Scholar]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; BuX, F. Microalgae as multi- functional options in modern agriculture: current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Xue, C.; Wang, L.; Wu, T.; Zhang, S.; Tang, T.; Wang, L.; Zhao, Q.; Sun, Y. Characterization of co-cultivation of cyanobacteria on growth, productions of polysaccharides and extracellular proteins, nitrogenase activity, and photosynthetic activity. Appl. Biochem. Biotechnol. 2017, 181, 340–349. [Google Scholar] [CrossRef]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: a critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Hultberg, M.; Carlsson, A.S.; Gustafsson, S. Treatment of drainage solution from hydroponic greenhouse production with microalgae. Bioresour. Technol. 2013, 136, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, X. Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, C.U.; Aoyagi, H.; Uchiyama, H. Photobioreactors for mass cultivation of algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J. Appl. Phycol. 2006, 18, 703–712. [Google Scholar] [CrossRef]
- Pushparaj, B.; Pelosi, E.; Tredici, M.R.; Pinzani, E.; Materassi, R. As integrated culture system for outdoor production of microalgae and cyanobacteria. J. Appl. Phycol. 1997, 9, 113–119. [Google Scholar] [CrossRef]
- Lee, Y.K. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Gong, Y.; Jiang, M. Biodiesel production with microalgae as feedstock: From strains to biodiesel. Biotechnol. Lett. 2011, 33, 1269–1284. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhou, Q. Co-cultivation of Chlorella spp. and tomato in a hydroponic system. Biomass Bioenerg. 2017, 97, 132–138. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Piero, A.R.L.; Giuffrida, F.; Baglieri, A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019, 31, 465–470. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Kaplan, D.; Richmond, A.E.; Dubinsky, Z.; Aaronson, S. Algal nutrition. In Handbook of Microalgal Mass Culture; Richmond, A., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 147–198. [Google Scholar]
- Levasseur, M.; Thompson, P.A.; Harrison, P.J. Physiological acclimation of marine phytoplankton to different nitrogen sources 1. J. Phycol. 1993, 29, 587–595. [Google Scholar] [CrossRef]
- Danesi, E.D.G.; Rangel-Yagui, C.O.; de Carvalho, J.C.M.; Sato, S. An investigation of effect of replacing nitrate by urea in the growth and production of chlorophyll by Spirulina platensis. Biomass Bioenerg. 2002, 23, 261–269. [Google Scholar] [CrossRef]
- Colla, L.M.; Reinehr, C.O.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Czerpak, R.; Bajguz, A.; Piotrowska, A.; Dobrogowska, R.; Matejczyk, W.; Wieslawski, W. Biochemical activity of di- and polyamines in the green alga Chlorella vulgaris Beijerinck (Chlorophyceae). Acta Soc. Bot. Pol. 2003, 72, 19–24. [Google Scholar] [CrossRef]
- Hourmant, A.; Mereau, N.; Penot, M.; Cann, C.; Caroff, J. Influence of polyamines on growth and metabolism of Dunaliella primolecta. Acta Bot. Neerl. 1994, 43, 129–136. [Google Scholar] [CrossRef]
- Czerpak, R.; Krotke, A.; Mical, A. Comparison of stimulatory effect of auxins and cytokinins on protein, saccharides and chlorophylls content in Chlorella pyrenoidosa Chick. Pol. Arch. Hydrobiol. 1999, 46, 71–82. [Google Scholar]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P. , Corrado, G., Chiaiese, P., Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro- and microalgal biostimulants. Biology. 2020, 9, 253. [Google Scholar] [CrossRef]
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant biostimulants from cyanobacteria: an emerging strategy to improve yields and sustainability in agriculture. Plants. 2021, 10, 643. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Biazzi, E.; Parati, K. , Carminati, D., Carminati, E., Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy. 2019, 9, 192. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? J.Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R. , Sbabou, L.; El Arroussi, H. Microalgae polysaccharides biostiluating effect on tomato plants: growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: a critical review of microalgae as renewable resources for agriculture. Algal Research. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant applications in low input horticultural cultivation systemes. Italus hortus. 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; Lo Piero, A.R.; Baglieri, A. Biostimulant effect and biochemical response in lettuce seedlings treated with a Scenedesmus quadricauda extract. Plants. 2020, 9, 123. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Manjunath, M.; Kanchan, A.; Rajan, K.; Venkatachalam, S.; Prasanna, R.; Ramakrishnan, B.; Hossain, F.; Nain, L.; Shivay, Y.S.; Rai, A.B. Beneficial cyanobacteria and eubacteria synergistically enhance bioavailability of soil nutrients and yield of okra. Heliyon. 2016, 2, e00066. [Google Scholar] [CrossRef]
- Adessi, A.; de Carvalho, R.C.; De Philippis, R.; Branquinho, C.; da Silva, J.M. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar]
- Plaza, B.M.; Gomez-Serrano, C.; Acien-Fernandez, F.G.; Jimenez-Becker, S. Effetc of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp. ) on Petunia x hybrida growth. Environ. Boil. Fishes. 2018, 30, 2359–2365. [Google Scholar]
- Navarro, Q.R.; de Oliveira Correa, D.; Behling, A.; Noseda, M.D.; Amano, E.; Mamoru Suzuki, R.; Lopes Fortes Ribas, L. Efficient use of biomass and extract of the microalga Desmodesmus subspicatus (Scenedesmaceae) in asymbotic seed germination and seedling development of the orchid Cattleya warneri. J. Appl. Phycol. 2021, 2, 134–141. [Google Scholar]
- Mutale-Joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; El Arroussi, H. Screening of microalgae liquid extracts for their biostimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic plant biostimulants and fruit quality- A review. Agronomy. 2020, 10, 98. [Google Scholar] [CrossRef]
- Shariatmadari, Z.; Riahi, H.; Abdi, M.; Hashtroudi, M.S.; Ghassempour, A.R. Impact of cyanobacterial ectracts on the growth and oil content of the medicinal plant Menthas piperita L. Environ. Boil. Fishes. 2015, 27, 2279–2287. [Google Scholar]
- Geries, L.S.M.; Elsadany, A.Y. Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen fixing endophyte Pseudomonas stutzeri. Arch. Microbiol. 2021, 203, 169–181. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci hortic. 2015, 196, 38–48. [Google Scholar] [CrossRef]
- Panda, D.; Pramanik, K.; Nayak, B.R. Use of seaweed extracts as plant growth regulators for sustainable agriculture. Int. J. Bioresource Stress Manag. 2012, 3, 404–411. [Google Scholar]
- De Philippis, R.; Colica, G.; Micheletti, E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbial. Biotechnol. 2011, 92, 697–708. [Google Scholar] [CrossRef]
- Arora, M.; Kaushik, A.; Rani, N. Kaushik, C.P. Effect of cyanobacterial exopolysaccharides on salt stress alleviation and seed germination J. Environ. Biol. 2010, 31, 701–704. [Google Scholar]
- Seifikalhor, M.; Hassani, S.B.; Aliniaeifard, S. Seed priming by cyanobacteria (Spirulina platensis ) and Salep Gum enhances tolerance of Maize plant against Cadmium toxicity. J. Plant Growth Regul. 2019, 39, 1009–1021. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Basladynska, S. , Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mogor, A.F.; Ordog, V.; Pace Pereira Lima, G.; Molnar, Z.; Mogor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Tuhy, L. Samoraj, M.; Witkowska, Z.; Chojnacka, K. Biofortification of maize with micronutrients by Spirulina. OpenChem. 2015, 13, 1119–1126. [Google Scholar]
- Dias, G.A.; Rocha, R.H.C.; Araujo, J.L.; de Lima, J.F.; Guedes, W.A. Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Spirulina platensis) treatments. Semina: Ciencias Agrarias, Londrina. 2016, 6, 3893–3902. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertiliser results in tomatoes with increased carotenoid and sugar levels. J.Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Ertani, A.; Sambo, P.; Nicoletto, C.; Santagata, S.; Schiavon, M.; Nardi, S. The use of organic biostimulants in hot pepper plants to help low input sustainable agriculture. Chem. Biol. Technol. Agric. 2015, 2, 11. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-shady, A.M.; El-Nagar, M.M.F. Cyanobacterial Arthrospira (Spirulina platensis) as safener against harmaful effects of fusilade herbicide on faba bean plant. Rend. Fis. Acc. Lincei. 2016, 27, 455–462. [Google Scholar] [CrossRef]
- Shalaby, T.A.; El-Ramady, H. Effect of foliar application of biostimulants on growth, yield, components, and storability of garlic (Allium sativum L.). Aust. J. Crop. Sci. 2014, 8, 271. [Google Scholar]
- Gemin, L.G.; Mogor, A.F.; De Oliveira Amatussi, J.; Mogor, G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Horticult. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Tredici, M.R. Mass production of microalgae: Photobioreactors. In: A. Richmond (Ed.), Handbook of Microalgal Culture: Biotechnology and Applied Phycology. 2004; pp. 178–214.
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N.; Neish, I.C.; Hurtado, A.Q.; Msuya, F.E.; Krishnan, M.; Narayanakumar, R.; Kronen, M.; Robledo, D.; Gasca-Leyva, E.; Fraga, J. The economics of Kappaphycus seaweed cultivation in developing countries: a comparative analysis of farming systems. Aquac. Econ. Manag. 2015, 19, 251–277. [Google Scholar] [CrossRef]
- Fortser, J.; Radulovich, R. Seaweed and food security. Seaweed sustainability: Food and Non-Food applications, Elsevier. 2015; pp. 289–313.

| Species | Genera of microalgae | Effects | Ref. |
|---|---|---|---|
| Lettuce (Lactuca sativa L.) | Chlorella, Scenedesmus quadricauda, Spirulina platensis | Improved productivity, antioxidant capacity, carotenoid content, increased dry matter, chlorophyll and protein in seedlings | [68] |
| Mais (Zea mays L.) | Spirulina platensis | Increased production of caryopses and micro-nutrients | [69] |
| Aubergine (Solanum melongena L.) | Spirulina platensis | Increased vegetative growth and fruit production | [70] |
| Tomato (Solanum lycopersicum L.) | Acutodesmus dimorphus, Chlorella vulgaris, Scenedesmus quadricauda, Nannochloropsis oculata | Increased seed germination, crop biomass, root development and dry matter. Increased sugar and carotenoid content in the fruit | [58,71] |
| Pepper (Capsicum annuum L.) | Spirulina platensis, Dunaliella salina | Plant growth stimulation and salt stress mitigation in seed germination | [72] |
| Cucumber (Cucumis sativus L.) | Spirulina platensis | Improving fresh weight | [68] |
| Fava (Vicia faba L.) | Spirulina platensis | Improved protein and amino acid levels of roots and sprouts | [73] |
| Garlic (Allium sativum L.) | Arthrospira fusiformis | Increasing plant height | [74] |
| Onion (Allium cepa L.) | Spirulina platensis, Scenedesmus subspicatus | Increased production, photosynthetic pigments, root development and sugar and protein content | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





