1. Introduction
The Corona virus pandemic disease was first recognized in December 2019, in Wuhan, China [
1]. This viral infection has caused hundreds of millions of cases and several million deaths. Therefore, launching new anti-viral drugs to control the pandemic crisis is essential. One of the approved antivirals is molnupiravir (
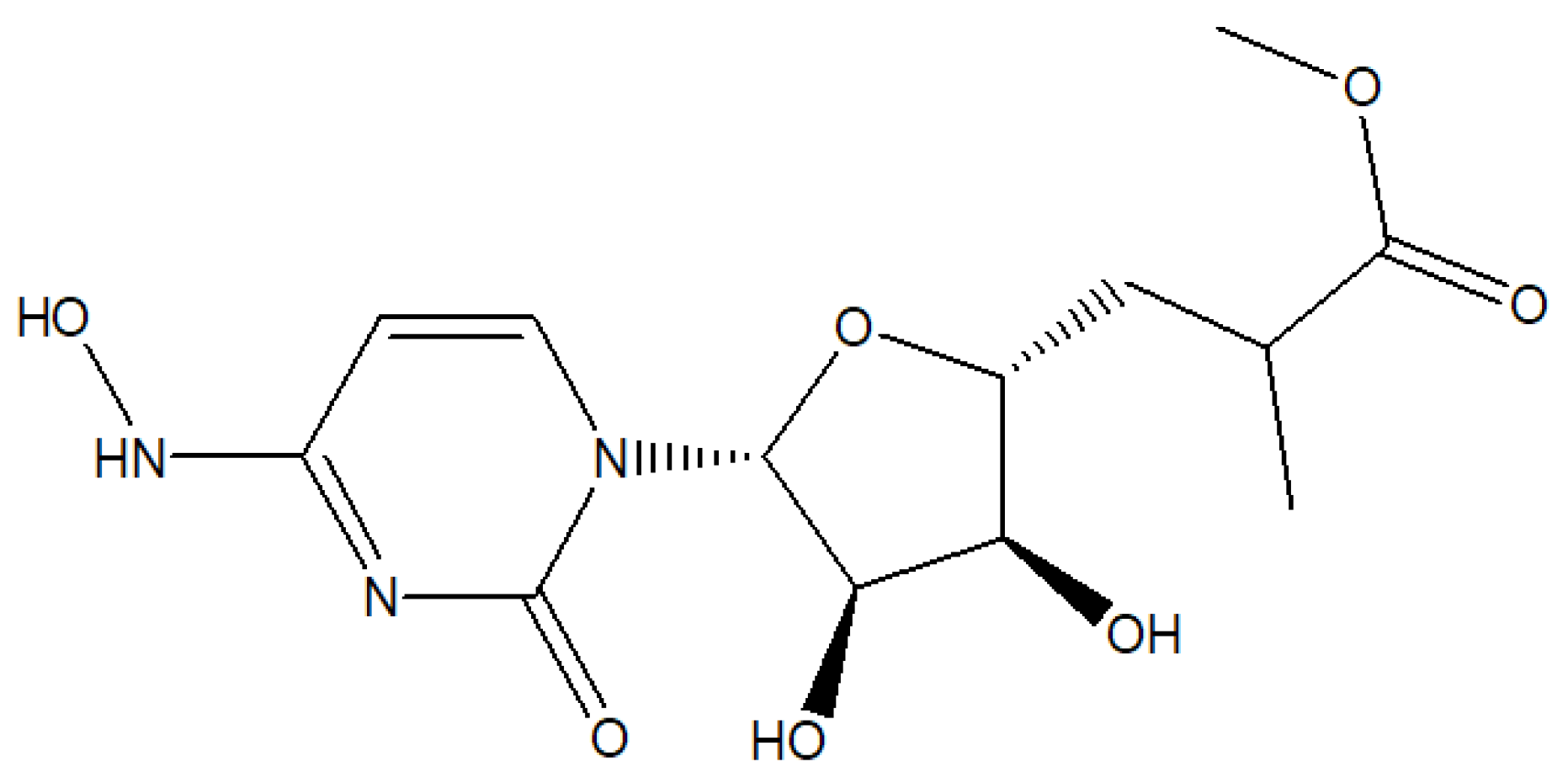
MOV); [(2R,3S,4R,5R)-3,4-dihydroxy-5-[4-(hydroxyamino)-2-oxopyrimidin-1-yl]oxolan-2-yl] methyl 2-methylpropanoate which inhibits RNA replication (
Figure 1). Using of MOV reduced the risk of hospitalization and death in patients with COVID-19. Based on the urgent importance of the studied drug, much effort should be directed for the development of fast, affordable, and reliable methods to measure MOV in different matrices. Different analytical methods have been developed for MOV determination alone or in a mixture, including UV spectrophotometry [
2,
3], fluorimetry [
4] and chromatography [
5,
6,
7]. Although liquid chromatographic approach incorporating mass spectrometric detection was described for the determination of MOV in human plasma and saliva [
8,
9], it is more expensive and, due to its complexity, can only be performed by qualified individuals. In addition, it produces large amounts of wastes in comparison to other new electrochemical techniques. Electrochemical methods surpass spectroscopic and chromatographic approaches for measuring MOV [
10]. A square wave voltammetry method for detecting MOV in pharmaceutical products based on electrooxidation of reduced graphene oxide (rGO) on a glassy carbon electrode has been reported [
11]. The electrochemical behavior and sensitive voltammetric detection of MOV were evaluated using a magnetite nanoparticle modified carbon paste electrode [
12]. On the glassy carbon electrode, a metal-organic framework composited with ethylene glycol treated poly(3,4-ethylene dioxythiophene): Poly(styrene sulfonate) has been characterized as an efficient electrochemical sensor for the detection of MOV [
13].
The electroanalytical technique provides all the advantages required for analysis as higher sensitivity, selectivity, rapidity, simplicity, reliability, and ease of use. Furthermore, the evolution of screen-printing technology has resulted in a potent sensing electrode that is characterized by their high reproducibility, disposable nature, economical, and a wide capacity for adjusting its working surface area [
14,
15]. Thus, the use of electrochemical techniques in conjunction with screen printed electrodes (SPEs) represents an advanced and alternative technique for real time analysis of pharmaceuticals with high sensitivity [
16,
17]. Furthermore, pharmaceutical compounds in a variety of matrices can be easily measured utilizing electroanalytical techniques without the influence from their endogenous components [
18,
19]. Electrochemical sensors are now commonly used instead of traditional analytical tools. These sensors are ideal for ‘in situ’ monitoring of pharmaceutical residues in biomedical, pharmaceutical, and industrial applications because of high sensitivity and selectivity. Moreover, one of the most commonly used nanoparticles in many electrochemical platforms is graphene nanoparticles. As a result, the current work is intended to develop an affordable, simple and sensitive electrochemical sensor with nanoparticle material for sensitive determination of MOV in capsules as well as biological fluids and to improve experimental conditions in order to develop a green voltammetric method. Therefore, preliminary studies were performed to optimize the experimental conditions by studying many factors affecting the performance of the method as electrolyte pH, surfactant concentration, scan rate, surface area of SPE, and the oxidation mechanism of MOV on screen printed reduced graphene oxide electrode (SPrGOE). According to the literature review, the proposed method is the best combination of being rapid, eco-friendly, and economical for determining MOV in various matrices. To the best of our knowledge, voltammetric measurement of MOV based on the electrochemical oxidation properties of the analyte using of a SPrGOE has not been reported.
2. Materials and Methods
2.1. Chemicals and Reagents
The highly pure MOV (>99% purity) used in this study was supplied by National Organization for Drug Control and Research (NODCAR), located in Cairo, Egypt. To facilitate the experimental procedures, several chemicals and reagents of analytical grade were obtained from Sigma-Aldrich (Egypt), including sodium dodecyl sulfate (SDS), Triton X-100, cetyltrimethyl ammonium bromide (CTAB), methanol, carbon, phosphoric acid, acetic acid, boric acid, graphite, paraffin oil, K3[Fe(CN)6], NaOH, and KCl. Bidistilled deionized water was employed throughout the entire experimental process.
For the electrolyte solution, Britton-Robinson (BR) buffer solutions with a pH range of 2 to 12 were prepared by combining appropriate volumes of 0.04 mol/L acetic acid, ortho-phosphoric acid, and boric acid. The solution was then brought to the desired volume using calibrated 500-mL measuring flasks and adjusted to the desired pH using sodium hydroxide. It should be noted that the preparation of the BR buffer solutions followed established protocol [
20]. The pharmaceutical dosage form used in the study was Molupiravir® capsules, specifically Batch no. 2201112. These capsules, containing 200 mg of MOV, were manufactured by Eva Pharmaceutical Company based in Egypt.
2.2. Apparatus
The Autolab Potentiostat-Galvanostat (PGSTAT 30) electrochemical instrument with a three-electrode system and Nova 2.1 software was used to conduct the electrochemical tests. A revolving disc with a diameter of two millimeters (Rotring Co. Ltd, Germany, R505210 N) was applied. All studies were carried out with a three-electrode system; SPrGOE as a working electrode, platinum counter electrode, and silver/silver chloride as a reference electrode. A scanning electron microscope (SEM) at an accelerated voltage of 5 kV (JSM-7610F, JEOL) was used to obtain a high-resolution image of the electrode morphology. The buffer solution was calibrated with standard buffers at room temperature using a digital pH/mV meter (JANEWAY 3510) with a glass combination electrode.
2.3. Fabrication of working Electrodes
The carbon paste electrode (CPE) was prepared by hand mixing 0.25 g graphite powder (<20 µm sigma Aldrich Egypt) and 180 μL paraffin oil (0.827-0.890 g/mL at 20 °C with viscosity (110-230 mPa.s)) [
21,
22] and after proper homogenization, the resulted suitable pastes were packed into CPE Body - 2.87 mm ID (surface area Se = 6.75 mm
2). Before each voltammetric measurement, the electrode surface was mechanically regenerated by removing a small amount of paste from the holder and wiping the electrode surface with a piece of clean filter paper. The SPrGOE were fabricated by in-house screen-printing method using carbon ink (Electrodag 421 SS, Acheson) as printing material modified by bulk mixing of the appropriate amount of nanoplatelets graphene powder hydrophobic with lot number MKCC2772 (<50 nm Sigma Aldrich Egypt). A silk-screen printing technique was used to apply carbon ink on the electrode support (inert polyvinyl chloride (PVC) material with a thickness of 2 mm). The printed electrode was dried at room temperature for 20 min and cut into individual sensor strips, and then the equivalent active surface of each tested sensor was fixed by isolating out the appropriate surface area. The electrode was inserted into the batch solution and electric contact was made with a crocodile clamp.
2.4. Preparation of Working Standard Solution
To prepare a solution of MOV with a concentration of 0.1 mg/mL, a precise quantity of 10 mg of the tested pharmaceutical was weighed and transferred into a 100 mL volumetric flask. Then, 5 mL of methanol was added to the flask. The mixture was thoroughly dissolved, and the volume was adjusted by adding more bidistiled water: methanol (1:1) until reaching the mark on the flask, ensuring a final volume of 100 mL.
2.5. General Procedure
Different aliquots of MOV working standard solutions were transferred into a calibrated 10 mL volumetric flask, then adding 0.3 mL of SDS. The prepared mixture was completed to the mark with 0.04 M BR buffer (pH 2) then transferred to the voltammetric vessel cell. The resulting solution was subjected to continuous stirring at a speed of 2000 rpm and room temperature for 10 seconds. After stopping the stirrer, the solution was allowed to rest for 10 seconds before voltammograms were performed using a scan rate of 100 mV/s and a voltage step of 0.95 mV for applying cyclic voltammetry (CV). The scan is performed over a range from +20 to +200 mV, and then derived regression equation was used to calculate the mean concentration of the triple measurements of the samples.
2.6. Preparation of Pharmaceutical Dosage Form
Ten capsules of Molnupiravir® (each labelled to contain 200 mg of MOV) were precisely weighed, and the average weight was calculated. The contents of the capsules were thoroughly mixed, and an appropriate weight equivalent to the content of one capsule was transferred into a 100 mL volumetric flask. The flask was then filled with 50 mL of methanol, the solution was shaken vigorously to ensure complete dissolution, and additional methanol was added until the volume reached the mark on the flask. This resulted in the preparation of MOV stock solution of 2 mg/mL. The stock solution was further diluted in a serial manner to obtain different concentrations. Each diluted solution was added to 10 mL of BR buffer (pH 2). The general procedure mentioned previously was then applied to analyze MOV in these solutions.
2.7. Spiked urine analysis
Free human urine samples collected from healthy volunteers were transferred into dry centrifuge tubes. The tubes were then centrifuged at a speed of 5,000 rpm for 10 minutes at room temperature (22 ± 2 °C). Following centrifugation, the supernatant solution was filtered through a syringe filter to remove any particulate matter. The filtered supernatant (1 mL) was added to a 9 mL portion of 0.04 M BR buffer (pH 2). To establish a concentration, ranging from 250 to 2050 ng/mL, aliquots of working standard solutions of MOV at various concentrations were added to the diluted urine samples. For assessing the analyte concentrations in the spiked urine samples, the general procedure was employed.
2.8. Spiked serum analysis
To prepare human serum samples, a volume of 2 mL of serum was transferred into a dry centrifuge tube. An appropriate volume of MOV standard solution was added to the serum, and the mixture was thoroughly mixed using a forced vortex method. The volume was then adjusted to 5 mL using methanol. The prepared mixtures were further vortexed and centrifuged for 5 minutes at a speed of 5000 rpm. Subsequently, the supernatant was filtered using a 0.45 μm syringe filter. An appropriate aliquot of the filtered supernatant was taken and added to a calibrated measuring flask with a volume of 10 mL. An aliquot of 0.3 mL of 10-4 M SDS was added and the flask was then filled to the marked volume with a 0.04 M BR buffer solution (pH 2). The general procedure was used to determine MOV concentrations in spiked serum samples.
3. Results and discussion
3.1. Characterization of the electrode.
3.1.1. Structural and Surface morphological of working electrodes
The SEM was utilized to perform further characterization of the surface of the prepared electrodes, as depicted in
Figure 2. The substrate exhibited a dense and uniform pattern, demonstrating the controlled features achieved through screen printing with the modified inks. The SEM image of the graphite CPE surface (
Figure 2A) revealed the presence of spherical protrusions as deposits. On the other hand, the multi-walled carbon nanotube (MWCNT) electrode exhibited a surface covered with skeletal sediments, forming cubical caverns, as displayed in
Figure 2B. This surface morphology contributes to an increased active surface area compared to that of the CPE. SEM of SPrGOE (
Figure 2C) revealed flake-like structures with a homogeneous surface that are attributed to rGO sheet stacking, which increases the specific active surface area resulting from the controllable feature of the screen printing with the prepared modifier inks [
23,
24,
25,
26]. The incorporation of rGO inside the complex at a low concentration of 2.5 % wt. is confirmed by the energy-dispersive x-ray spectroscopy (EDX). The hexagonal lattice structure of rGO was confirmed by selected area electron diffraction (SAED) (
Figure 2D), which produced a pattern with bright, visible spots forming a hexagon [
27,
28]. It was observed that the 3D regular porous network formed by the overlapping rGO flakes would result in a large specific area. Consequently, enhancing the electrochemical performance and electro-transfer kinetics based on the large specific site of rGO, allowed the improvement of sensitivity for MOV detection.
3.1.2. Electro characterizations of working electrode
To evaluate the electrochemical behavior and compare the active surface areas of the prepared working electrodes, CV was employed. The voltammetric responses of the working electrodes were investigated in a solution containing 1 mM K
3[Fe(CN)
6] and 0.1 M KCl at various scan rates (
Figure 3). The Randles-Sevcik equation [
29,
30] was utilized to calculate the electroactive surface areas of the examined electrodes as follows:
This equation relates the peak current (ip), concentration (C) the scan rate (ʋ), the number of electrons involved in the redox process (n = 1), the diffusion coefficient (D = 7.6 × 10
−6 cm
2/s), and the electrode area (A). By analyzing the CV data and applying the Randles-Sevcik equation, the electroactive surface area of each electrode was determined and used for further comparisons as listed in
Table 1. These results allowed for a comprehensive assessment of the electrochemical behavior of the electrodes and facilitated a quantitative comparison of their active surface areas which oblivious the optimal surface area at (SPrGOE 2. 5%). Thus, 2.5% SPrGOE-modified electrode was selected as the best sensor owing to its large electro-active area which is the main factor that gives the high responses for MOV determination.
3.1.3. Optimization of pH at SPrGOE
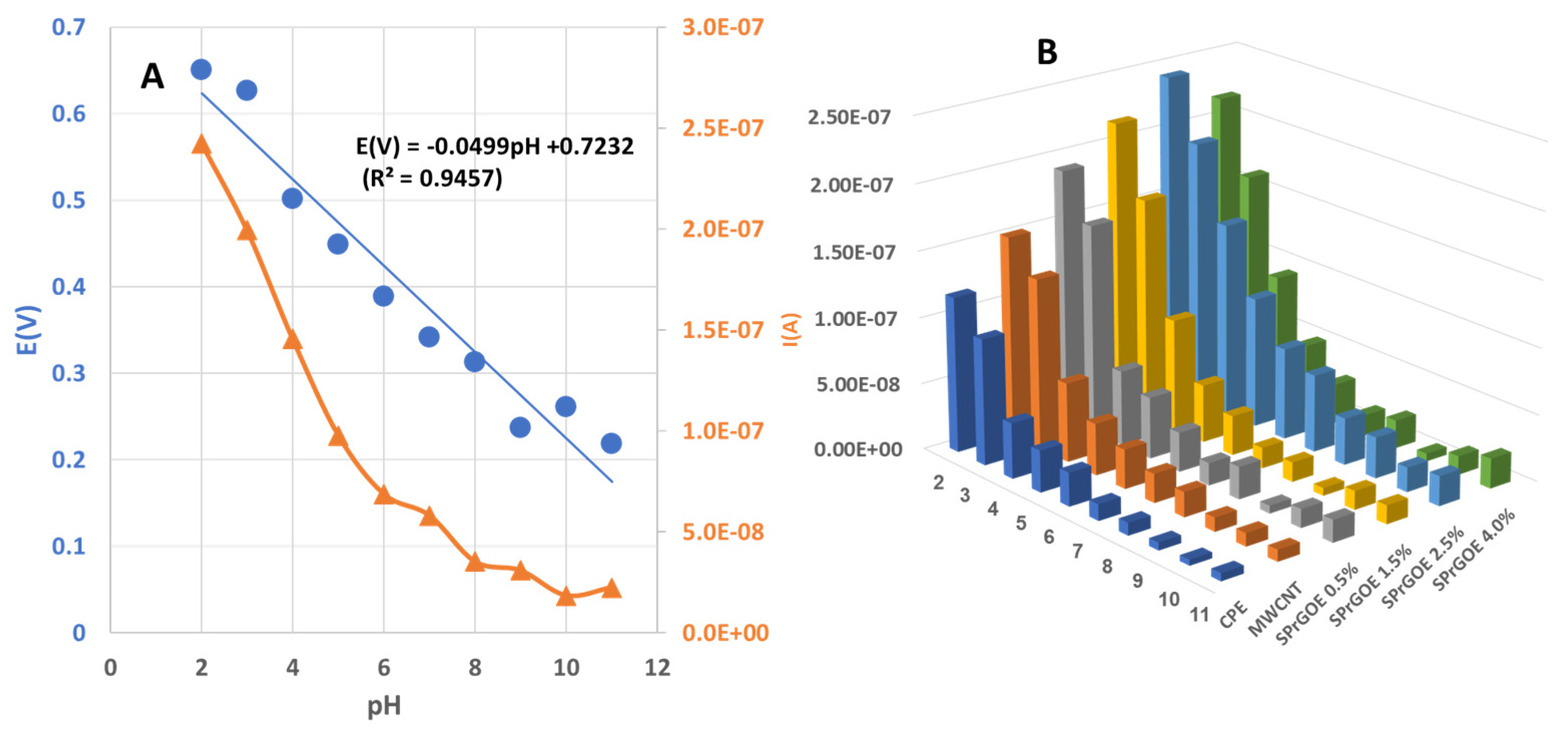
The pH of the supporting electrolyte solution plays a critical role in understanding the electrochemical anodic behavior of the studied drug, making it necessary to optimize this variable and evaluate its impact on the anodic peak potential and current [
31]. Therefore, an investigation was conducted to determine the optimal pH that would yield superior oxidation peak current and potential for MOV measurement. The effect of pH on the electrochemical behavior of MOV was examined, over the range 2-12, using a 2.5% SPrGOE-modified electrode through CV technique in 0.04 M BR buffer solution. As shown in
Figure 4A a linear relationship was observed between the oxidation peak of MOV and the pH of the supporting electrolyte with the movement of oxidation peak potential towards the negative direction and the following equation was achieved:
The oxidation peaks of MOV revealed that the maximum current values for the investigated sensors were observed at pH 2, as shown in
Figure 4B. Based on these results, the optimal pH value selected for the 0.04 M BR buffer solution as an electrolyte was 2.
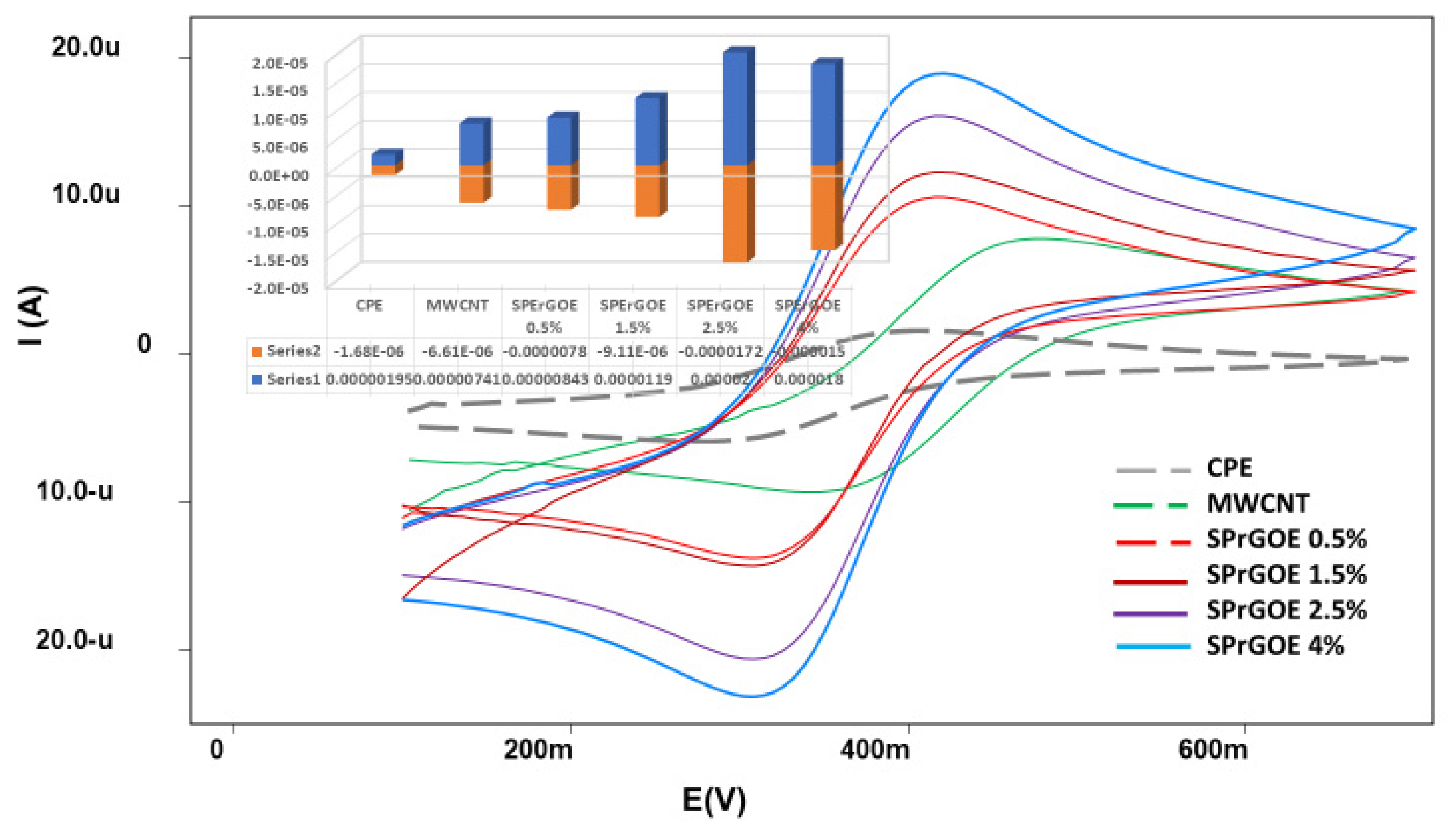
Figure 5 also provides a comparison of the studied electrodes at pH 2. It is evident from this figure that the best current intensity (
Figure 5A) and system performance (
Figure 5B) were achieved when using a 2.5% SPrGOE-modified electrode. The observed enhancement in the electrochemical response, characterized by the highest current values, highlights the significance of the SPrGOE modification for MOV detection. As a result, the use of 2.5% SPrGOE-modified electrode at an optimal pH of 2 provides a more sensitive and reliable analytical platform for MOV measurement.
3.1.4. Effect of scan rate
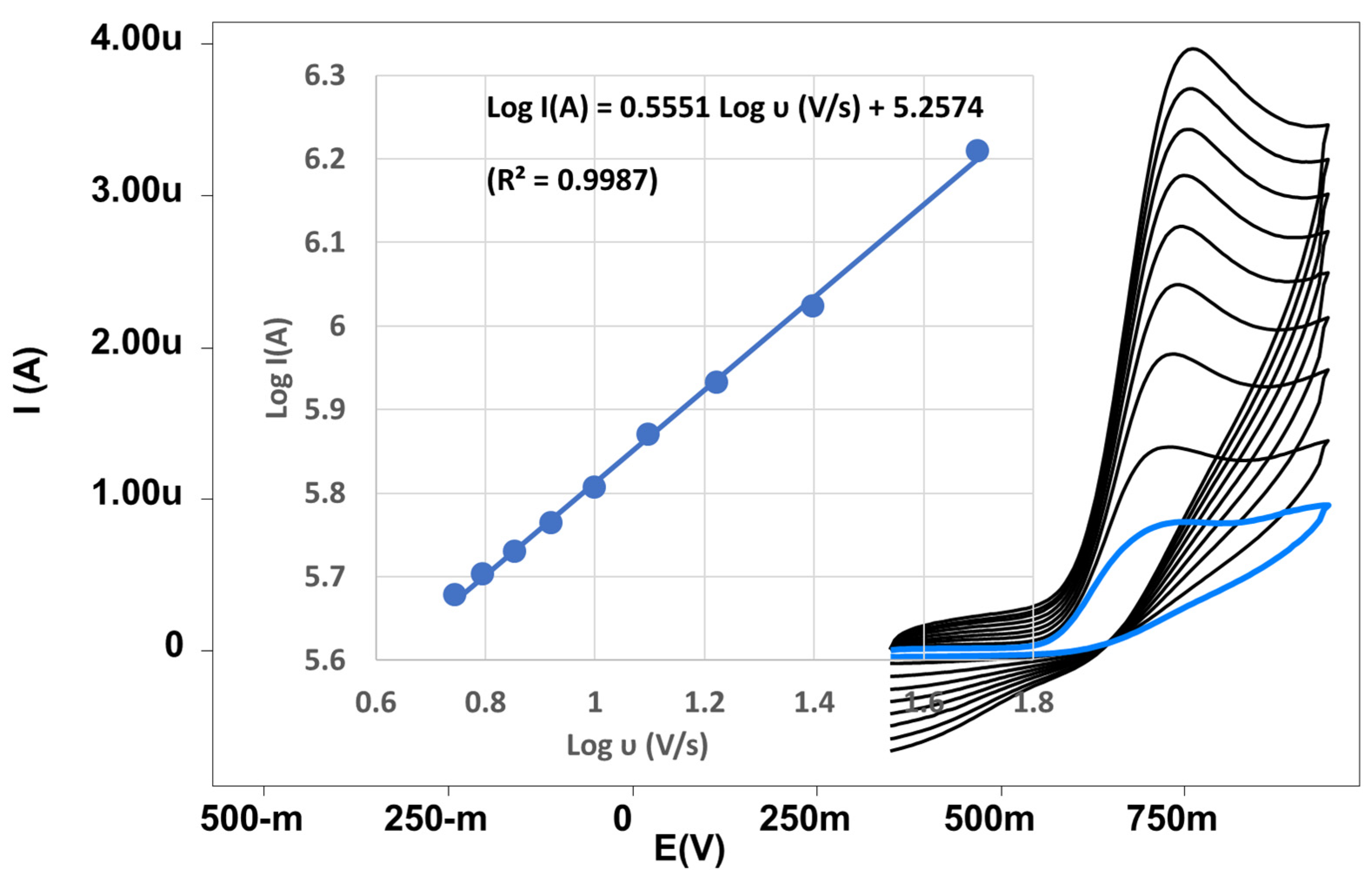
To investigate the effect of scan rate on the oxidation reaction of 500 ng/mL MOV in 0.04 M BR buffer with pH 2, CV studies were conducted using a 2.5% SPrGO-modified electrode. The scan rate was varied from 20 to 200 mV/s within a potential range of 0.6 to 1.6 V. The results revealed that the anodic peak current of the oxidation reaction increased with an increase in the scan rate.
Figure 6 illustrates the log of the oxidation current (I(A)) plotted against the log of the scan rate (υ), exhibiting a strong linear correlation [
32]. The linear regression equation obtained from the data analysis was log I(A) = 0.5551 log υ (V/s) + 5.2574, with a high correlation coefficient (0.9987). The slope value of the linear regression equation indicates the electro-catalytic action of the 2.5% SPrGOE-modified electrode in the oxidation response of MOV was close to the theoretical value suggesting that the oxidation process follows a diffusion-controlled pathway.
Furthermore, the anodic peak potential is influenced by the scan rate. As the scan rate increases, the peak potential moves towards more positive values, indicating the presence of kinetic limitations in the electrochemical process. This observation supports the understanding that the rate at which the oxidation reaction occurs is influenced by the scan rate. Additionally, a linear relationship was observed between the logarithm of the scan rate and the peak potential, as depicted in
Figure S1. This linear relationship was described by the following regression equation:
and thus, the number of electrons involved in the irreversible redox process of MOV at SPrGOE electrode can be estimated according to Laviron’s theory [
33] for an irreversible process, the following equation exists:
where E
0 is the formal standard potential and
ks is the standard heterogeneous reaction rate constant;
n is the transfer electron number;
α refers to the charge transfer coefficient;
v,
R,
T, and
F have their usual meanings. Since in this study the slope was 0.0482 (
Figure S1), the value of αn was evaluated as 1.224. Likewise, the value of α can be estimated as follow:
Thus, α was determined as 0.62, and the number of electrons transferred in the oxidation process is 1.974.
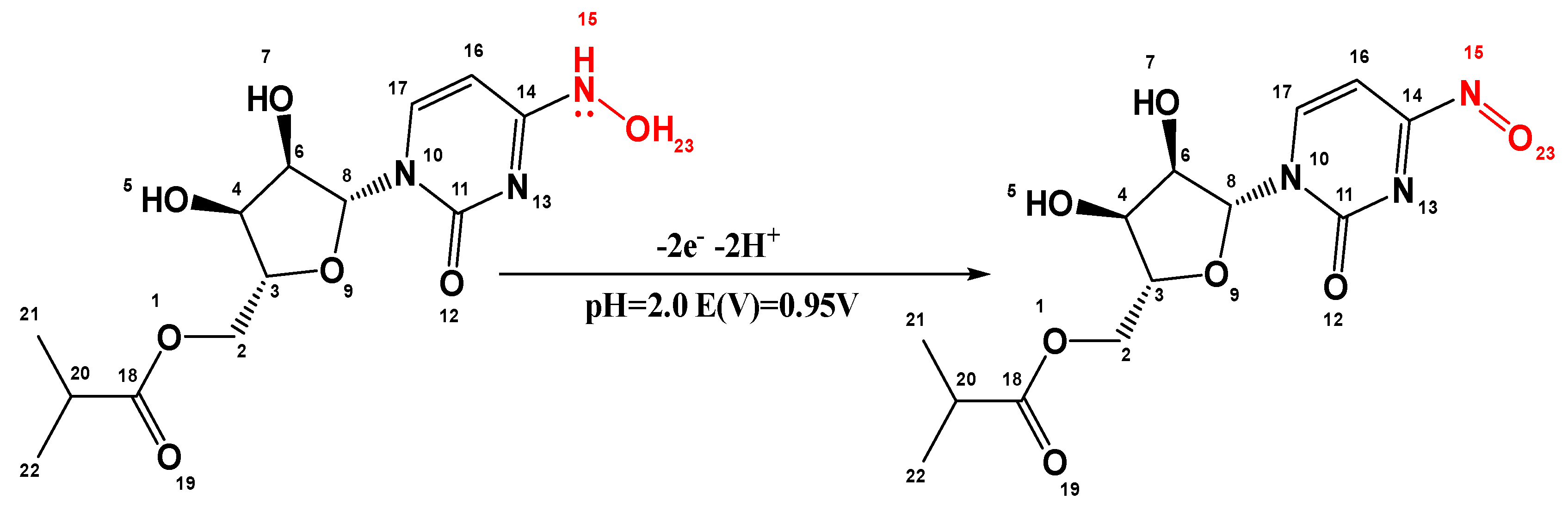
3.1.5. Proposed oxidation mechanism of MOV at SPrGOE (2.5%)
The number of electrons transferred during the oxidation of MOV was found to be 1.974, which is close to 2. This confirms the proposed oxidation mechanism for MOV. A possible electrooxidation mechanism for MOV at the electrode surface is illustrated in
Scheme 1. The terminal aliphatic side chain contains two atoms (N15 and O23) that can donate electrons. This makes it likely that two unlocalized lone pairs of electrons in the N enamine will be lost, along with two protons from the N enamine and O alcohol. This would form a π-bond at E = 0.95V. It is possible that, in the first step, MOV loses one electron to become a cation radical. This is followed by the loss of protons, and in the next step, another electron is transferred to form a π-bond.
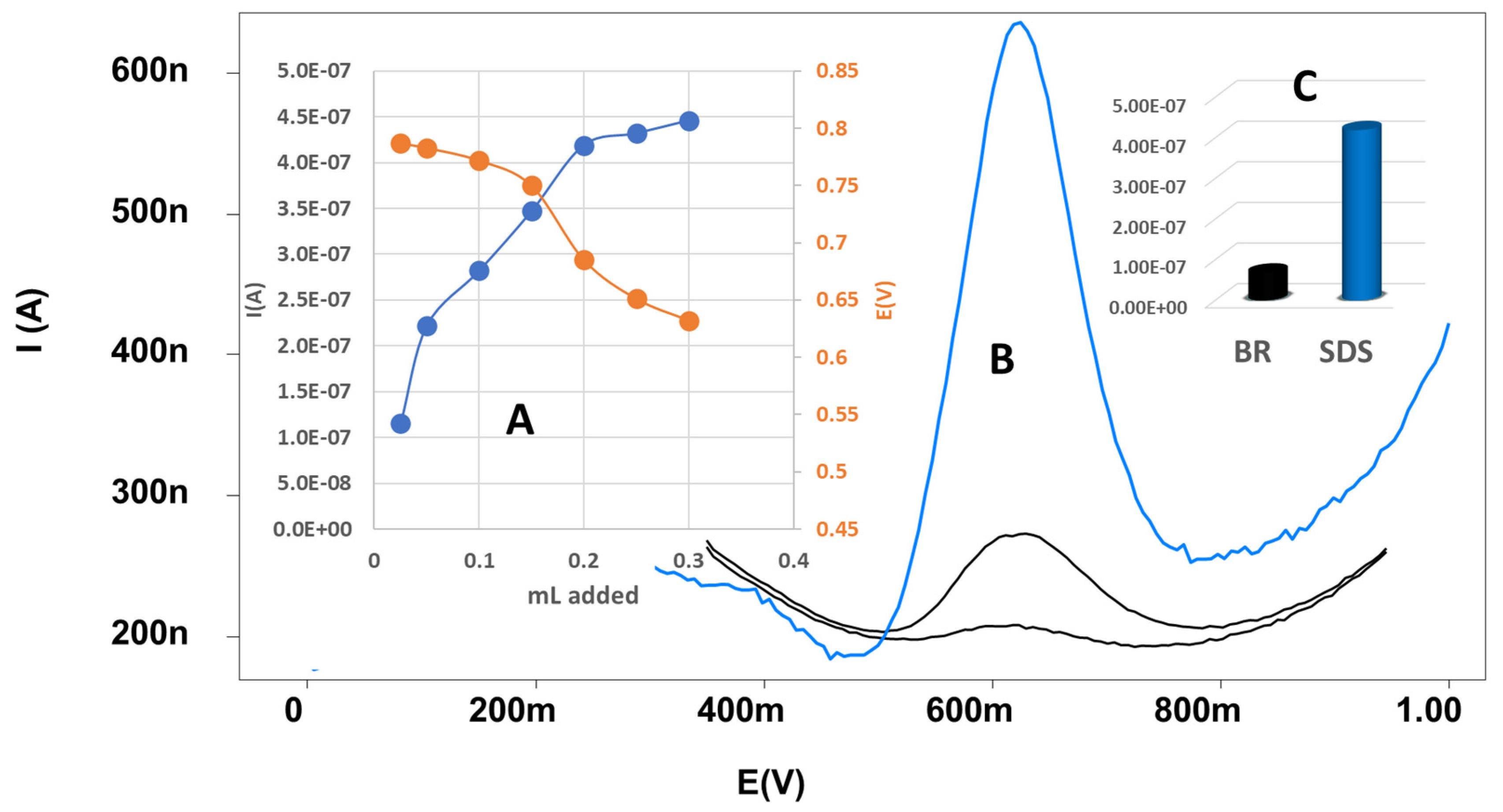
3.1.6. Effect of surfactant concentration
Surfactants, in general, can be utilized to improve the performance of redox reactions in both the bulk solution and on the surface of electrodes by modifying the properties of the electrode surfaces. In this study, several surfactants, including SDS, Triton X-100, and CTAB, were investigated for their potential effects. The study demonstrated that when a surfactant CTAB was used, peak height and peak position were essentially unchanged, while peak height was reduced in the case of Triton X-100. However, the application of SDS resulted in a noticeable negative shift in the peak position, as demonstrated in
Figure 7A and there is enhancement of peak height as displayed in
Figure 7B.
Figure 7C shows the considerable differences in MOV peak heights at the surface of SPrGOE (2.5%) when SDS is absent (BR alone) or present. Because of SDS’s unique features, it can be self-assembled into micelles, which are small clusters of surfactant molecules with a hydrophobic core and a hydrophilic outer layer. The application of SDS leads to a decrease in the interface between MOV and the electrode surface. This decrease in the interface is evident from the increase in peak height shown in
Figure 7B. By reducing the MOV-electrode surface interaction, SDS facilitates the oxidation process by allowing easier access for the reactants to reach the electrode surface and participate in the redox reaction. Therefore, SDS was chosen to be used as a surfactant and an aliquot of 0.3 mL of 10
-4 M was added to the supporting electrolyte solution [
34,
35].
3.2. Method validation
In the development of a voltammetric method for the determination of MOV, we selected the DPV mode as the peaks are sharper and better defined at lower concentrations of MOV than those produced by CV. Also, low background current by DPV resulted in enhanced resolution. Conducting differential pulse measurements, several parameters can be optimized to improve the accuracy and quality of the results. Selecting pulse amplitude of 100 mV, a pulse width of 20 ms, and a scan rate of 100 mV/s led to superior results, characterized by enhanced sensitivity and well-defined waveforms with narrow peak widths. According to ICH guidelines [
36], the validity of the proposed method was assessed by studying the following parameters: linearity, range, limit of detection (LOD), limit of quantification (LOQ), precision, robustness, and specificity.
3.2.1. Linearity, limit of detection and limit of quantification
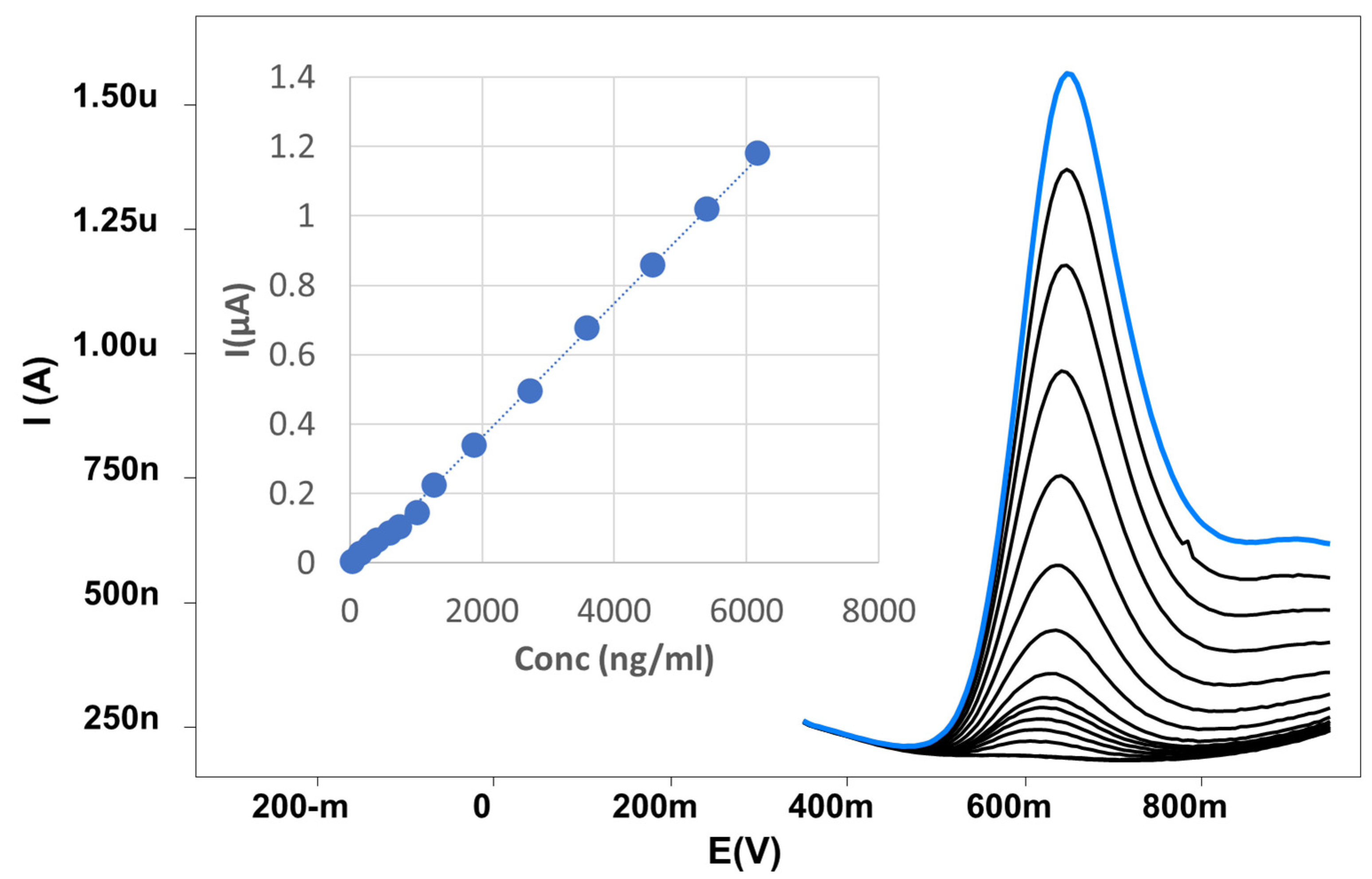
Figure 8 presents the voltammograms, which clearly depict the impact of varying MOV concentrations. It is evident also that lower MOV concentrations yielded distinct and sharper peaks. Notably, using a SPrGOE 2.5%, an increase in MOV concentration led to a significant enhancement in the intensity of the anodic peaks. This enhancement was visually observable, indicating a broad linearity range. The proposed method demonstrates linearity over a concentration range of 50-6017 ng/mL, as confirmed by plotting the peak current against the MOV concentration (
Figure 8).
Table 2 further confirms these experimental results, showing a high degree of linearity with a correlation coefficient of 0.9988. The statistical analysis of the data in
Table 2 revealed low values for the standard deviation (SD), standard error (SE), and relative standard deviation (RSD). These low values indicate that the calibration curve is very linear, with very little scatter in the data points. This is important because it ensures that the method is accurate and reliable over the designated concentration range.
The LOD and LOQ were determined in accordance with the ICH guidelines, utilizing the formulas: LOD = 3.3SD/S and LOQ = 10SD/S, where S represents the slope of the linear calibration curve and SD represents the standard deviation. The LOD of MOV was determined to be 15.98 ng/mL, while the LOQ was found to be 48.43 ng/mL. These values demonstrate the higher sensitivity achieved by the developed method in detecting and quantifying MOV.
3.2.2. Precision
To assess the precision of the proposed method, both intra-day and inter-day precision were evaluated. For intra-day precision (repeatability), three different concentrations of freshly prepared working standard solutions were assayed in triplicate on the same day. On the other hand, for inter-day precision (reproducibility), the same samples were analyzed on three consecutive days. The results obtained from these assessments demonstrated a high level of precision in the developed SPrGOE 2.5% analytical platform. This indicates that the method consistently produces reliable and consistent results. Detailed results can be found in
Table 2, which further confirm the method’s precision and its applicability in ensuring the quality control of MOV.
3.2.3. Specificity
The DPV assay procedure’s specificity for measuring MOV was thoroughly examined in the presence of various excipients, including microcrystalline cellulose, starch, colloidal anhydrous silica, talc, Cl-, SO4-2, Na+, K+, and Mg2+. Different concentrations of these excipients were introduced to a solution containing MOV (at a concentration of 2 µg/mL) and analyzed using the proposed SPrGOE 2.5% modified-electrode. The resulting percentage recoveries obtained from three replicate measurements ranged from 99.1% to 101.2%. These recoveries demonstrated that no significant interferences were detected from the excipients.
3.2.4. Robustness
The robustness of an analytical procedure refers to its ability to withstand small, intentionally introduced variations in method parameters without significant impact. In the case of the proposed method, robustness was demonstrated by the consistent peak current observed despite deliberate minor changes in experimental parameters. The studied variables included; the change in pH (±0.2), and the time considered before each measurement (10s ± 5s). These minor changes that may take place during the experimental operation did not affect the peak current intensity of the studied drug, indicating the reliability of the proposed method.
3.3. Applications
3.3.1. Pharmaceutical dosage form
To assess the suitability of the proposed method for analyzing real samples, SPrGOE 2.5% was successfully employed for the determination of MOV in pharmaceutical dosage forms. The outcomes of this analysis are provided in
Table 3. The results demonstrate that the recovery of MOV fell within the range of 101.25% to 98.67%. In order to validate the precision and accuracy of the voltammetric determination of MOV using the proposed electrode, t-test and F-test were also conducted. These statistical tests were performed to ascertain whether there were any significant disparities in the means of analysis between the voltammetric method and the reference technique, HPLC [
37]. The results from these tests revealed no significant differences, affirming the acceptable precision of the voltammetric method in quantifying MOV and indicated a close alignment between the results obtained using the proposed analytical platform and those obtained by the reference HPLC method. Thus, the proposed 2.5 SPrGOE0modified electrode exhibits promise for the reliable analysis of MOV in practical applications. Overall,
Table 3 presents the recovery rates of MOV within the specified range.
3.3.2. Human bio-fluids
The proposed method was employed to determine the concentration of MOV in spiked serum and urine samples. Known quantities of MOV were added to blank serum and urine, and the recoveries were calculated and presented in
Table 4. Notably, no interference from noise peaks or oxidation compounds was observed within the potential range where the analyte’s peak appeared. The obtained recoveries for urine samples ranged from 99.76% to 100.80%, while for serum samples, they ranged from 99.07% to 100.53%. These results provide confidence in the reliability and applicability of the proposed method for the analysis of MOV in both urine and serum matrices. It also ensures accurate and precise measurements without interference from matrix ingredients, affirming the suitability of the method for practical applications in clinical or research settings.
3.4. Stability of the modified electrode
To assess the stability of the SPrGOE 2.5%, CV response of the working electrodes was investigated in a solution containing 1 mM K
3[Fe(CN)
6)] and 0.1 M KCl [
38]. The measurements were recorded weekly over a period of three months under refrigeration. A total of three runs were conducted, and the average RSD was found to be 1.15%. Notably, both the anodic and cathodic peak currents exhibited relatively stable behavior throughout the testing period. The repetitive measurements demonstrated good reproducibility of the 2.5% SPrGOE-modified-electrode and indicated that it was not susceptible to surface-related issues.
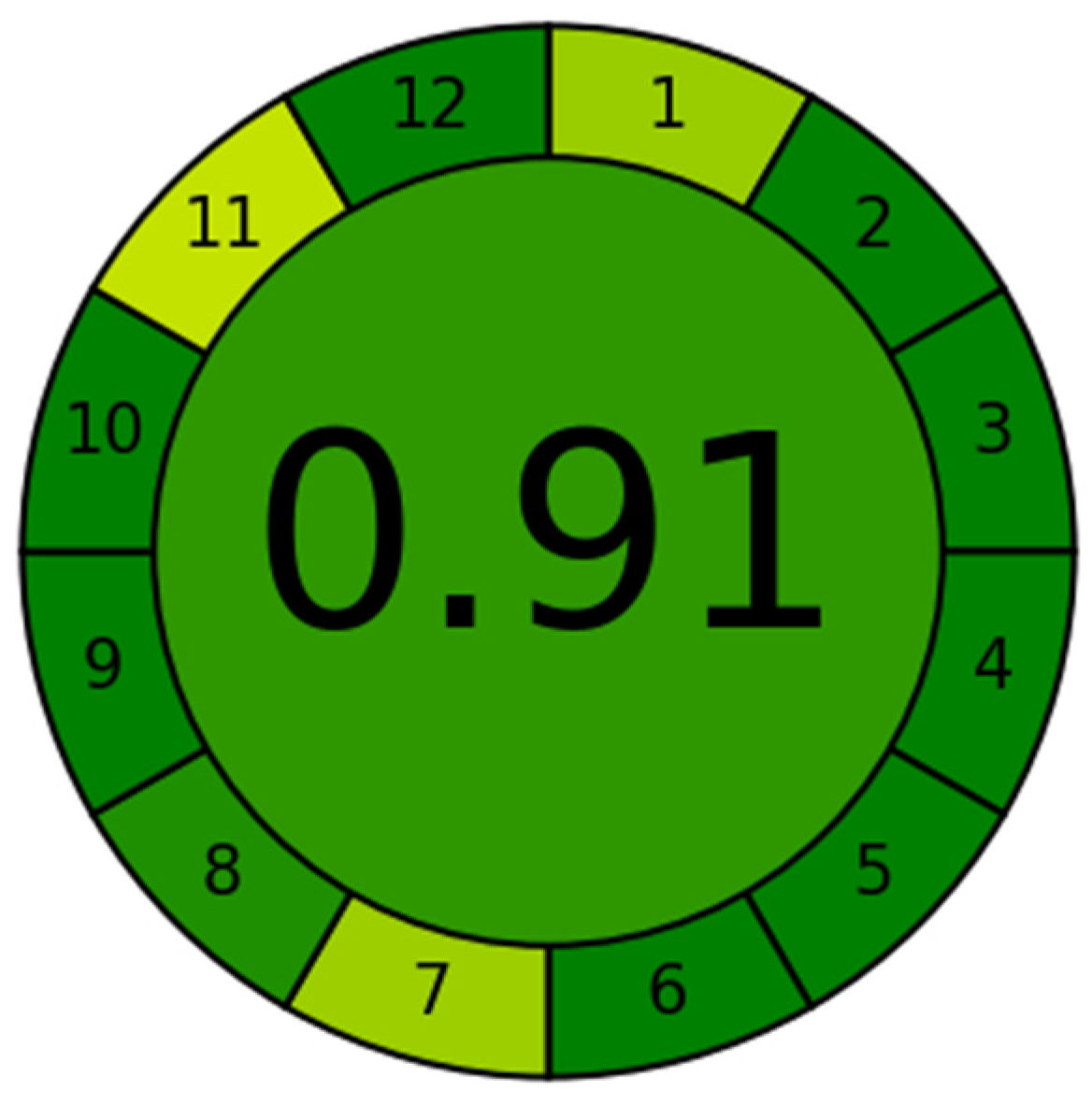
3.5. Assessment of the greenness profile
The AGREE software was used to assess the greenness of the proposed electrochemical technique [
39]. AGREE is a software calculator that evaluates 12 parameters of green analytical aspects, each of which represents one of the green analytical chemistry standards. The results of the assessment are shown in
Figure 9. This figure shows the 12 parameters with different colors, ranging from dark green (low environmental impact) to red (high environmental impact). The majority of the parameters are dark green, with the exception of sections 1, 7, and 11, which are pale green. These three parameters have a higher environmental impact than the others. The proposed electrochemical technique scored 0.91, which is a high score and indicates that the method is relatively green. The high score of the proposed electrochemical technique is encouraging. It suggests that this method has the potential to be a more environmentally friendly alternative to traditional analytical methods.
4. Conclusions
In this study, a novel disposable SPrGOE 2.5% analytical platform has been applied to determine MOV in pure, dosage forms, and biological fluid using CV. MOV detection at SPrGOE 2.5% offers a precise technique, where the voltammetric oxidation behavior of the analyte appears to be a single anodic peak. Employing SDS would result in increased surface area and greater stability in aqueous solutions. Because of its simplicity, time-saving, and real-time analytical properties, the proposed approach was adopted as an ecologically friendly alternative method for MOV measuring. The MOR recoveries from urine and serum were ranged from 99.76% to 100.80% and 99.07% to 100.53%, respectively, indicating method’s reliability and appropriateness for the measurement of the tested analyte in bio-fluid matrices. Because the modified SPrGOE sensor has remarkable features such as low detection limit, high sensitivity and selectivity, and quick response, it could replace existing expensive techniques for measuring MOV in various matrices.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
A.N.: sample analysis, investigation, validation, and writing—original draft; H.A.M.H.: conceptualization, methodology and results interpretation; R.A.: conceptualization and supervision; R.M.A.: investigation, sample analysis and English editing; A.S.: investigation, results interpretation, validation and writing—original draft; S.E.: conceptualization and supervision; N.A.: methodology, sample analysis; results interpretation, validation and writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohan, B.; Vinod, N. COVID-19: An Insight into SARS-CoV2 Pandemic Originated at Wuhan City in Hubei Province of China. J. Infect. Dis. Epidemiol. 2020, 6, 146. [Google Scholar] [CrossRef]
- Abdelazim, A.H.; Abourehab, M.A.S.; AbdElhalim, L.M.; Almrasy, A.A.; Ramzy, S. Green Adherent Spectrophotometric Determination of Molnupiravir Based on Computational Calculations; Application to a Recently FDA-Approved Pharmaceutical Dosage Form. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121911. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.R.; Abolmagd, E.; Nour, I.M.; Badrawy, M.; Hasan, M.A. Earth-Friendly-Assessed Silver-Nanoparticles Spectrophotometric Method for Rapid and Sensitive Analysis of Molnupiravir, an FDA-Approved Candidate for COVID-19: Application on Pharmaceutical Formulation and Dissolution Test. BMC Chem. 2023, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salman, B.I.; Hara, M.A.; El Deeb, S.; Ibrahim, A.E.; Saraya, R.E.; Ali, M.F.B. Zinc(II) Complexation Strategy for Ultra-Sensitive Fluorimetric Estimation of Molnupiravir: Applications and Greenness Evaluation. Arch. Pharm. (Weinheim). 2023, 356, e2300005. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, Y.A.; El Deeb, S.; Ibrahim, A.E.; Al-Harrasi, A.; Sayed, R.A. Two Green Micellar HPLC and Mathematically Assisted UV Spectroscopic Methods for the Simultaneous Determination of Molnupiravir and Favipiravir as a Novel Combined COVID-19 Antiviral Regimen. Molecules 2022, 27, 2330. [Google Scholar] [CrossRef] [PubMed]
- Reçber, T.; Timur, S.S.; Kablan, S.E.; Yalçın, F.; Karabulut, T.C.; Gürsoy, R.N.; Eroğlu, H.; Kır, S.; Nemutlu, E. A Stability Indicating RP-HPLC Method for Determination of the COVID-19 Drug Molnupiravir Applied Using Nanoformulations in Permeability Studies. J. Pharm. Biomed. Anal. 2022, 214, 114693. [Google Scholar] [CrossRef]
- Saraya, R.E.; ElDeeb, S.; Salman, B.I.; Ibrahim, A.E. Highly Sensitive High-Performance Thin-Layer Chromatography Method for the Simultaneous Determination of Molnupiravir, Favipiravir, and Ritonavir in Pure Forms and Pharmaceutical Formulations. J. Sep. Sci. 2022, 45, 2582–2590. [Google Scholar] [CrossRef]
- Amara, A.; Penchala, S.D.; Else, L.; Hale, C.; FitzGerald, R.; Walker, L.; Lyons, R.; Fletcher, T.; Khoo, S. The Development and Validation of a Novel LC-MS/MS Method for the Simultaneous Quantification of Molnupiravir and Its Metabolite ß-d-N4-Hydroxycytidine in Human Plasma and Saliva. J. Pharm. Biomed. Anal. 2021, 206, 114356. [Google Scholar] [CrossRef]
- Parsons, T.L.; Kryszak, L.A.; Marzinke, M.A. Development and Validation of Assays for the Quantification of β-D-N4-Hydroxycytidine in Human Plasma and β-D-N4-Hydroxycytidine-Triphosphate in Peripheral Blood Mononuclear Cell Lysates. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1182, 122921. [Google Scholar] [CrossRef]
- Gupta, V.K.; Karimi-Maleh, H.; Sadegh, R. Simultaneous Determination of Hydroxylamine, Phenol and Sulfite in Water and Waste Water Samples Using a Voltammetric Nanosensor. Int. J. Electrochem. Sci. 2015, 10, 303–316. [Google Scholar] [CrossRef]
- Kablan, S.E.; Reçber, T.; Tezel, G.; Timur, S.S.; Karabulut, C.; Karabulut, T.C.; Eroğlu, H.; Kır, S.; Nemutlu, E. Voltammetric Sensor for COVID-19 Drug Molnupiravir on Modified Glassy Carbon Electrode with Electrochemically Reduced Graphene Oxide. J. Electroanal. Chem. 2022, 920, 116579. [Google Scholar] [CrossRef]
- Vural, K.; Karakaya, S.; Dilgin, D.G.; Gökçel, H.İ.; Dilgin, Y. Voltammetric Determination of Molnupiravir Used in Treatment of the COVID-19 at Magnetite Nanoparticle Modified Carbon Paste Electrode. Microchem. J. 2023, 184, 108195. [Google Scholar] [CrossRef] [PubMed]
- Erk, N.; Bouali, W.; Mehmandoust, M.; Soylak, M. An Electrochemical Sensor for Molnupiravir Based on a Metal-Organic Framework Composited with Poly(3,4-Ethylene Dioxythiophene): Poly(Styrene Sulfonate). ChemistrySelect 2022, 7, e202203325. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.T.; Li, D.W.; Long, Y.T. Recent Developments and Applications of Screen-Printed Electrodes in Environmental Assays-A Review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-Printed Electrodes Modified with Metal Nanoparticles for Small Molecule Sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Torralba-Cadena, L.; Espriu-Gascon, A.; Casas, I.; Bastos-Arrieta, J.; Florido, A. Strategies for Surface Modification with Ag-Shaped Nanoparticles: Electrocatalytic Enhancement of Screen-Printed Electrodes for the Detection of Heavy Metals. Sensors (Switzerland) 2019, 19, 4249. [Google Scholar] [CrossRef]
- Meier, J.; Schiøtz, J.; Liu, P.; Nørskov, J.K.; Stimming, U. Nano-Scale Effects in Electrochemistry. Chem. Phys. Lett. 2004, 390, 440–444. [Google Scholar] [CrossRef]
- Li, C.M.; Hu, W. Electroanalysis in Micro- and Nano-Scales. J. Electroanal. Chem. 2013, 688, 20–31. [Google Scholar] [CrossRef]
- Jirasirichote, A.; Punrat, E.; Suea-Ngam, A.; Chailapakul, O.; Chuanuwatanakul, S. Voltammetric Detection of Carbofuran Determination Using Screen-Printed Carbon Electrodes Modified with Gold Nanoparticles and Graphene Oxide. Talanta 2017, 175, 331–337. [Google Scholar] [CrossRef]
- Fernández, C.M.; Martin, V.C. Preparation d’un Tampon Universel de Force Ionique 0,3 M. Talanta 1977, 24, 747–748. [Google Scholar] [CrossRef]
- Rizk, M.; Taha, E.A.; El-Alamin, M.M.A.; Hendawy, H.A.M.; Sayed, Y.M. Highly Sensitive Carbon Based Sensors Using Zinc Oxide Nanoparticles Immobilized Multiwalled Carbon Nanotubes for Simultaneous Determination of Desvenlafaxine Succinate and Clonazepam. J. Electrochem. Soc. 2018, 165, H333–H341. [Google Scholar] [CrossRef]
- Hendawy, H.A.M.; Salem, W.M.; Abd-Elmonem, M.S.; Khaled, E. Nanomaterial-Based Carbon Paste Electrodes for Voltammetric Determination of Naproxen in Presence of Its Degradation Products. J. Anal. Methods Chem. 2019, 2019, 5381031. [Google Scholar] [CrossRef]
- Jahani, P.M.; Beitollahi, H.; Tajik, S. Surface Amplification of Graphite Screen Printed Electrode Using Reduced Graphene Oxide/Polypyrrole Nanotubes Nanocomposite; a Powerful Electrochemical Strategy for Determination of Sulfite in Food Samples. Food Chem. Toxicol. 2022, 167, 113274. [Google Scholar] [CrossRef] [PubMed]
- Er, E. A Portable Electrochemical Platform Based on Graphene Nanosheets by Metal Intercalation Engineering for Anticancer Drug Pemetrexed Sensing. FlatChem 2022, 33, 100353. [Google Scholar] [CrossRef]
- Hasanpour, M.; Pardakhty, A.; Tajik, S. The Development of Disposable Electrochemical Sensor Based on MoSe2-RGO Nanocomposite Modified Screen Printed Carbon Electrode for Amitriptyline Determination in the Presence of Carbamazepine, Application in Biological and Water Samples. Chemosphere 2022, 308, 136336. [Google Scholar] [CrossRef]
- Barthasarathy, P.R.; Ahmed, N.A.; Salim, W.W.A.W. Reduced Graphene Oxide on Screen-Printed Carbon Electrodes as Biosensor for Escherichia Coli O157:H7 Detection. Proceedings 2020, 60, 13. [Google Scholar]
- Wu, Y.; Zhang, T.; Su, L.; Wu, X. Electrodeposited rGO/AuNP/MnO2 Nanocomposite-Modified Screen-Printed Carbon Electrode for Sensitive Electrochemical Sensing of Arsenic(III) in Water. Biosensors 2023, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Dinani, H.S.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Ebrahimi, S.A.S.; Shayeh, J.S.; Ghorbani, M. Fabrication of Au/Fe3O4/RGO Based Aptasensor for Measurement of MiRNA-128, a Biomarker for Acute Lymphoblastic Leukemia (ALL). Eng. Life Sci. 2022, 22, 519–534. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Eloul, S.; Tschulik, K.; Compton, R.G. Planar Diffusion to Macro Disc Electrodes—What Electrode Size Is Required for the Cottrell and Randles-Sevcik Equations to Apply Quantitatively? J. Solid State Electrochem. 2014, 18, 3251–3257. [Google Scholar] [CrossRef]
- Al Alamein, A.M.A.; Hendawy, H.A.M.; Elabd, N.O. Voltammetric Determination of Dicyclomine Hydrochloride by Carbon Paste Electrode Modified with Iron (III) Oxide Nanoparticles and Activated Glassy Carbon Electrode in Pharmaceutical Dosage Form, Human Plasma and Urine. Int. J. Electrochem. Sci. 2018, 13, 7989–8005. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; El-Sayyad, G.S.; Ashour, A.H.; El-Batal, A.I.; Abd-Elmonem, M.S.; Hendawy, H.A.M.; Abdel-Khalek, E.K.; Labib, S.; Abdeltwab, E.; El-Okr, M.M. Synthesis and Characterization of Metals-Substituted Cobalt Ferrite [Mx Co (1-x) Fe2O4; (M= Zn, Cu and Mn; x= 0 and 0.5)] Nanoparticles as Antimicrobial Agents and Sensors for Anagrelide Determination in Biological Samples. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, H.A.M.; Youssif, R.M.; Salama, N.N.; Fayed, A.S.; Salem, M.Y. Challenge Approach of an Inexpensive Electrochemical Sensor for Rapid Selective Determination of Two Non-Classical β-Lactams in Presence of Different Degradants and Interference Substances. Electroanalysis 2017, 29, 2708–2718. [Google Scholar] [CrossRef]
- Laviron, E. Surface Linear Potential Sweep Voltammetry. Equation of the Peaks for a Reversible Reaction When Interactions between the Adsorbed Molecules Are Taken into Account. J. Electroanal. Chem. 1974, 52, 395–402. [Google Scholar] [CrossRef]
- Fekry, A.M.; Shehata, M.; Azab, S.M.; Walcarius, A. Voltammetric Detection of Caffeine in Pharmacological and Beverages Samples Based on Simple Nano- Co (II, III) Oxide Modified Carbon Paste Electrode in Aqueous and Micellar Media. Sensors Actuators B Chem. 2020, 302, 127172. [Google Scholar] [CrossRef]
- Boltia, S.A.; Kandeel, N.H.; Hegazy, M.A.; Hendawy, H.A. Analytical Eco-Scale Determination of Vortioxetine using Advanced Electrochemical Platform for Screen-Printed Disposable Multi-Walled Carbon Nanotube Electrode in Presence of an Anionic Surfactant. New J. Chem. 2023, 47, 11015–11029. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH: Q2). Guideline on Validation of Analytical Procedures: Text and Methodology 2005.
- Bindu, M.; Gandla, K.; Vemireddy, S.; Samuel, S.; Praharsha, Y. A Validated Stability Indicating RP-HPLC Method for the Determination of Molnupiravir in Pharmaceutical Dosage Form. World J. Adv. Res. Rev. 2022, 15, 580–590. [Google Scholar] [CrossRef]
- Lin, K.C.; Chen, S.M. Reversible Cyclic Voltammetry of the NADH/NAD+ Redox System on Hybrid Poly(Luminol)/FAD Film Modified Electrodes. J. Electroanal. Chem. 2006, 589, 52–59. [Google Scholar] [CrossRef]
- Soliman, S.S.; Sedik, G.A.; Elghobashy, M.R.; Zaazaa, H.E.; Saad, A.S. Greenness Assessment Profile of a QbD Screen-Printed Sensor for Real-Time Monitoring of Sodium Valproate. Microchem. J. 2022, 182. [Google Scholar] [CrossRef]
Figure 1.
The chemical structure of MOV.
Figure 1.
The chemical structure of MOV.
Figure 2.
(A) SEM image of selected area of CPE. (B) SEM image of MWCNT, (C) SEM image of SPrGOE (2.5%), and (D) EDX of SPrGOE (2.5%).
Figure 2.
(A) SEM image of selected area of CPE. (B) SEM image of MWCNT, (C) SEM image of SPrGOE (2.5%), and (D) EDX of SPrGOE (2.5%).
Figure 3.
Cyclic voltammograms of studied working different electrodes in 1 mM K3[Fe(CN)6] and 0.1 M KCl solution at a scan rate of 50 mV/s.
Figure 3.
Cyclic voltammograms of studied working different electrodes in 1 mM K3[Fe(CN)6] and 0.1 M KCl solution at a scan rate of 50 mV/s.
Figure 4.
(A) Dependence of the oxidation potential and current peak height of 500 ng/mL MOV in 0.04 M BR electrolyte solution with different pH values at SPrGOE (2.5%) using CV at scan rate 100 mV/s. (B) comparison of the current peak height of 500 ng/mL MOV in BR electrolyte solution (0.04 M) at different pH values using different sensors at scan rate 100 mV/s.
Figure 4.
(A) Dependence of the oxidation potential and current peak height of 500 ng/mL MOV in 0.04 M BR electrolyte solution with different pH values at SPrGOE (2.5%) using CV at scan rate 100 mV/s. (B) comparison of the current peak height of 500 ng/mL MOV in BR electrolyte solution (0.04 M) at different pH values using different sensors at scan rate 100 mV/s.
Figure 5.
(A) The current intensity of cyclic voltammograms at pH 2 employing different sensors. (B) Cycle voltammograms of 500 ng/mL MOV in 0.04 M BR electrolyte solution (pH 2) using different sensors at scan rate 100 mV/s.
Figure 5.
(A) The current intensity of cyclic voltammograms at pH 2 employing different sensors. (B) Cycle voltammograms of 500 ng/mL MOV in 0.04 M BR electrolyte solution (pH 2) using different sensors at scan rate 100 mV/s.
Figure 6.
Cyclic voltammograms for 500 ng/ml MOV in 0.04 M BR electrolyte solution (pH 2) at different consecutive scan rates at SPrGOE 2.5%. The insight figure is the linear relationship between log (V/s) and log I(A).
Figure 6.
Cyclic voltammograms for 500 ng/ml MOV in 0.04 M BR electrolyte solution (pH 2) at different consecutive scan rates at SPrGOE 2.5%. The insight figure is the linear relationship between log (V/s) and log I(A).
Scheme 1.
Proposed oxidation mechanism of MOV at (SPrGOE 2.5%).
Scheme 1.
Proposed oxidation mechanism of MOV at (SPrGOE 2.5%).
Figure 7.
(A) Effect of SDS concentration on the peak potential and anodic current. (B) DPV of MOV in 0.04 M BR at the surface of CPE and SPrGOE (2.5%). (C) Peak potential at the surface of SPrGOE 2.5% in the absence and presence of SDS.
Figure 7.
(A) Effect of SDS concentration on the peak potential and anodic current. (B) DPV of MOV in 0.04 M BR at the surface of CPE and SPrGOE (2.5%). (C) Peak potential at the surface of SPrGOE 2.5% in the absence and presence of SDS.
Figure 8.
Calibration curve of MOV using DPV method at SPrGOE in 0.04 M BR electrolyte with pH at 2.
Figure 8.
Calibration curve of MOV using DPV method at SPrGOE in 0.04 M BR electrolyte with pH at 2.
Figure 9.
Analytical greenness metric for evaluation of the proposed method greenness.
Figure 9.
Analytical greenness metric for evaluation of the proposed method greenness.
Table 1.
Comparison of active surface area and redox characteristic peaks of different studied electrodes.
Table 1.
Comparison of active surface area and redox characteristic peaks of different studied electrodes.
| Electrode |
ΔE(V) |
Ipa/Ipc(A) |
Active Area (cm2) |
| CPE |
0.089 |
0.86 |
0.035 |
| MWCNT |
0.089 |
0.833 |
0.061 |
| SPrGOE (0.5%) |
0.089 |
0.765 |
0.055 |
| SPrGOE (1.5%) |
0.09 |
0.925 |
0.076 |
| SPrGOE (2.5%) |
0.083 |
0.992 |
0.083 |
| SPrGOE (4.0%) |
0.081 |
0.862 |
0.086 |
Table 2.
Characteristic parameters of the calibration line (n = 3) for the quantitative determinations MOV at SPrGOE (2.5%) by using the proposed voltammetric technique.
Table 2.
Characteristic parameters of the calibration line (n = 3) for the quantitative determinations MOV at SPrGOE (2.5%) by using the proposed voltammetric technique.
| Parameters |
MOV |
| E(mV) |
641 |
| pH |
2.0 |
| Linearity range (ng/mL) |
50-6017 |
| Intercept |
-0.0200 |
| Slope |
0.0010 |
| Multiple R |
0.9994 |
| R Square |
0.9989 |
| Standard Error |
0.01 |
| RSD |
1.42 |
| LOD (ng/mL) |
15.98 |
| LOQ (ng/mL) |
48.43 |
| Repeatability of the peak current (RSD %) |
0.86 |
| Reproducibility of the peak current (RSD %) |
1.23 |
| Repeatability of the peak potential (RSD %) |
0.95 |
| Reproducibility of the peak potential (RSD %) |
1.12 |
Table 3.
Application of the proposed method for the determination of MOV in pharmaceutical dosage form and statistical comparative study between the proposed method and reported method.
Table 3.
Application of the proposed method for the determination of MOV in pharmaceutical dosage form and statistical comparative study between the proposed method and reported method.
| MOV (Batch no. 2201112) |
Added (ng/mL) |
Found (ng/mL) |
Bias (%) |
Developed method Recovery (%) * |
Reported method [38] Recovery (%) * |
| |
150 |
148 |
1.33 |
98.67 |
100.12 |
| |
450 |
455 |
-1.11 |
101.11 |
98.97 |
| |
800 |
810 |
-1.25 |
101.25 |
100.18 |
| |
2400 |
2408 |
-0.33 |
100.33 |
99.95 |
| Mean |
|
|
|
100.34 |
99.80 |
| Variance |
|
|
|
1.408 |
0.319 |
| F test |
4.41 |
| t test |
0.81 |
| F tabulated a
|
(9.28) |
| t tabulated a
|
(2.45) |
Table 4.
Analytical data of spiked human urine and serum of MOV on a 2.5% SPrGOE-modified electrode.
Table 4.
Analytical data of spiked human urine and serum of MOV on a 2.5% SPrGOE-modified electrode.
| MOV |
Spiked (ng/ml) |
Found (ng/ml) |
Bias% |
Recovery (%) |
RSD (%) |
| Human urine |
250 |
252 |
-0.80 |
100.80 |
0.84 |
| 900 |
907 |
-0.78 |
100.78 |
1.05 |
| 1500 |
1507 |
-0.47 |
100.47 |
0.95 |
| 2050 |
2045 |
0.24 |
99.76 |
1.11 |
| Human serum |
380 |
382 |
-0.53 |
100.53 |
1.24 |
| 750 |
743 |
0.93 |
99.07 |
0.98 |
| 1800 |
1792 |
0.44 |
99.56 |
0.78 |
| 2500 |
2490 |
0.40 |
99.60 |
1.33 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).