Submitted:
29 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods and Materials
2.1. Patient Workup
2.2. Treatment
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Treatment
3.2. Treatment Outcomes
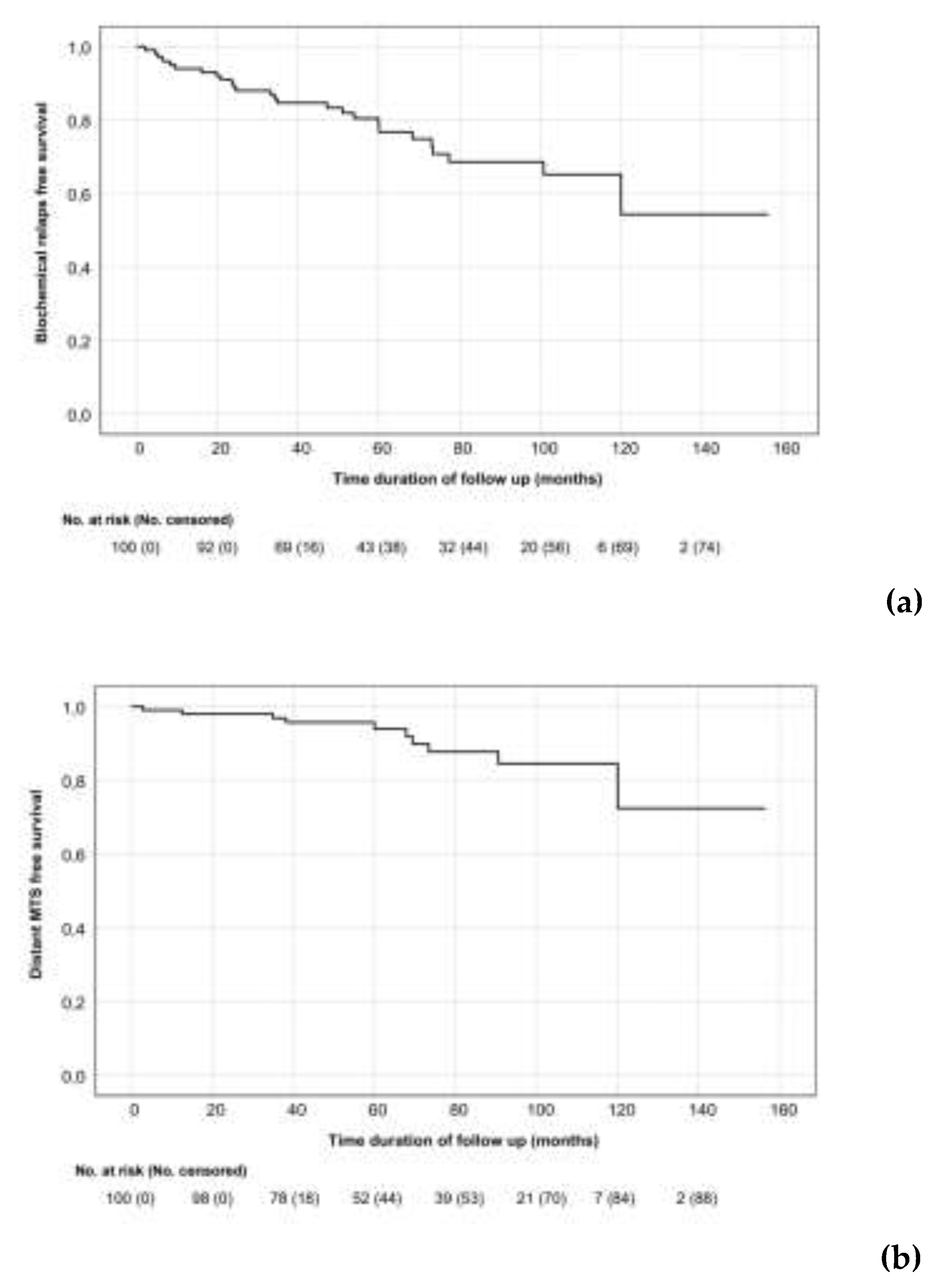
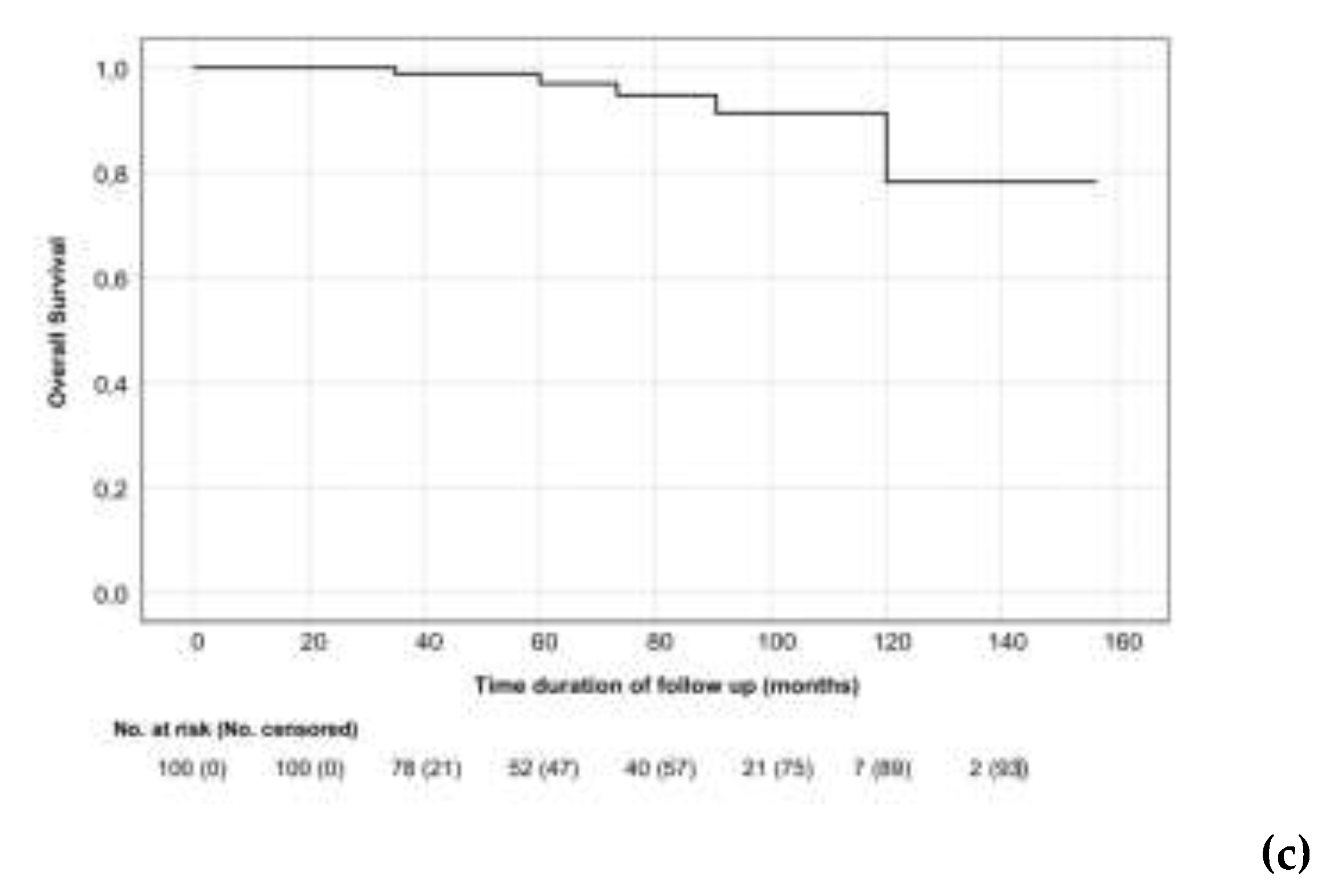

3.3. Toxicity
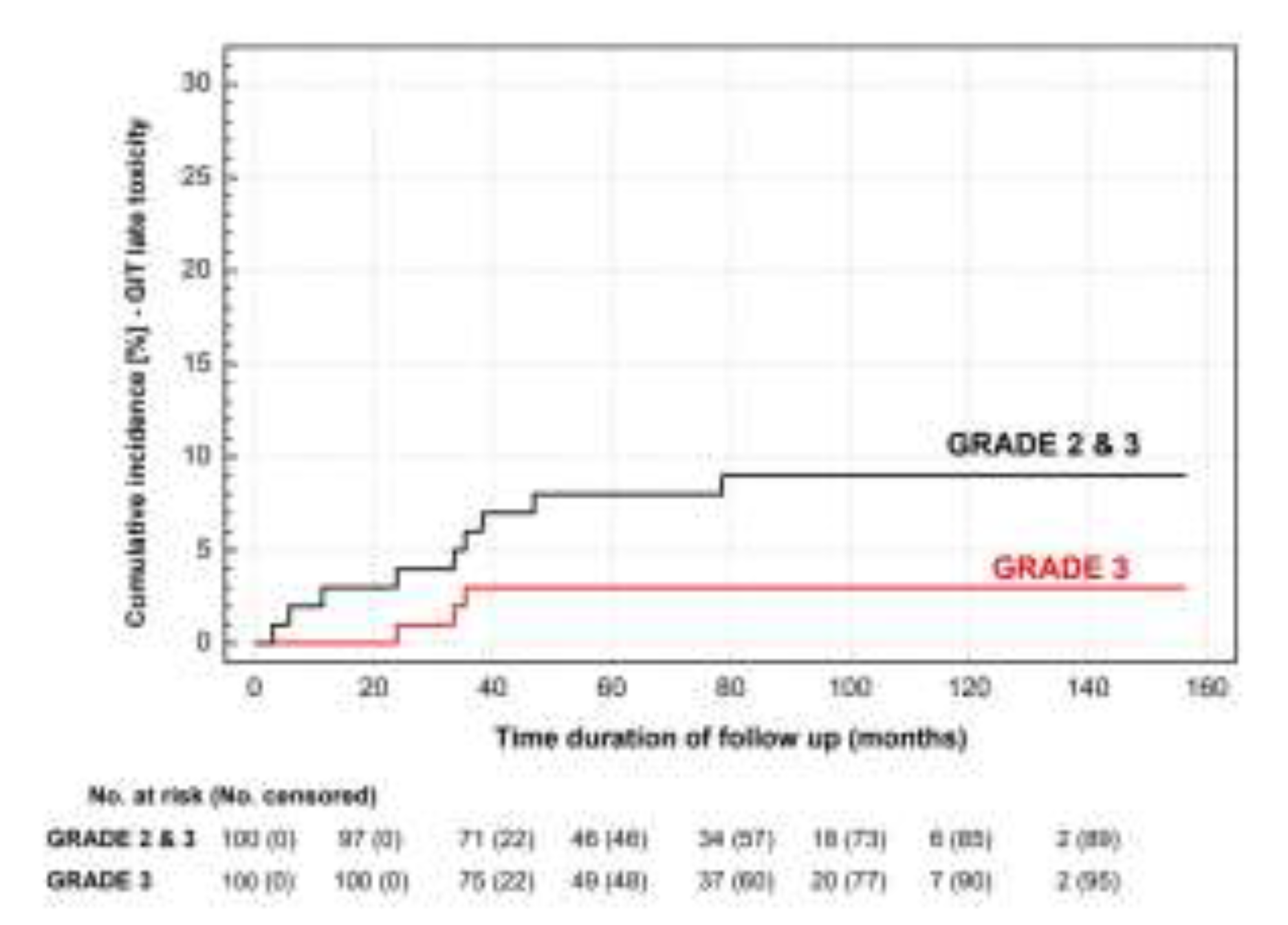
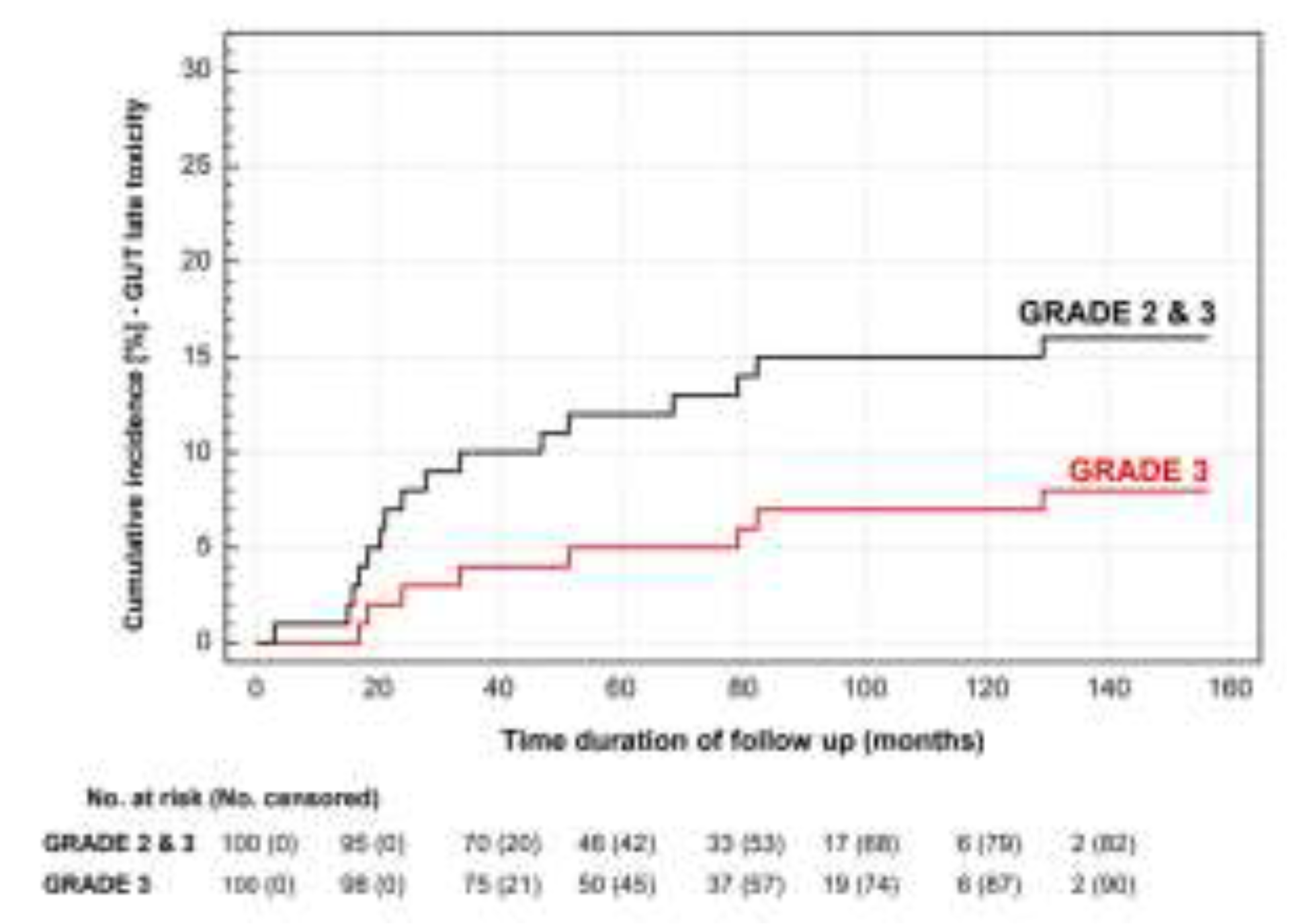
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson IM, Tangen CM, Paradelo J et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term follow up of a randomized clinical trial. J Urol. 2009, 181, 956–62. [CrossRef]
- Bolla M, van Poppel H, Tombal B et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012, 380, 2018–27. [CrossRef]
- Wiegel T, Bartkowiak D, Bottke D et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014, 66, 243–50. [CrossRef]
- Trock BJ, Han M, Freedland SJ et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008, 299, 2760–2769. [CrossRef]
- Cotter SE, Chen MH, Moul JW et al. Salvage radiation in men after prostate-specific antigen failure and the risk of death. Cancer. 2011, 117, 3925–32. [CrossRef]
- Parker CC, Clarke NW, Cook AD et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomized, controlled phase 3 trial. Lancet. 2020, 396, 1413–1421. [CrossRef]
- Sargos P, Chabaud S, Latorzeff I et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [CrossRef]
- Kneebone A, Fraser-Browne C, Duchesne GM et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 1331–1340. [CrossRef]
- Vale CL, Fisher D, Kneebone A et al. ARTISTIC Meta-analysis Group. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020, 396, 1422–1431. [CrossRef]
- Tilki D, Preisser F, Graefen M et al. External Validation of the European Association of Urology Biochemical Recurrence Risk Groups to Predict Metastasis and Mortality After Radical Prostatectomy in a European Cohort. Eur Urol. 2019, 75, 896–900. [CrossRef]
- Fossati N, Karnes RJ, Colicchia M et al. Impact of Early Salvage Radiation Therapy in Patients with Persistently Elevated or Rising Prostate-specific Antigen After Radical Prostatectomy. Eur Urol. 2018, 73, 436–444. [CrossRef]
- Dearnaley D, Syndikus I, Mossop H et al: Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [CrossRef] [PubMed]
- Catton CN, Lukka H, Gu CS et al: Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017, 35, 1884–1890. [CrossRef] [PubMed]
- Lee WR, Dignam JJ, Amin MB et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016, 34, 2325–32. [CrossRef]
- Datta NR, Stutz E, Rogers S et al. Conventional versus hypofractionated radiation therapy for localized or locally advanced prostate cancer: a systematic review and meta-analysis along with therapeutic implications. Int J Radiat Oncol Biol Phys. 2017, 99, 573–589. [CrossRef]
- Mahase S, Nagar H. Hypofractionated postoperative radiotherapy for prostate cancer: is the field ready yet? Eur Urol Open Sci. 2020, 22, 9–16. [CrossRef]
- Michalski JM, Lawton C, El Naqa I et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010, 76, 361–368. [CrossRef]
- Lawton CA, Michalski J, El-Naqa I et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009, 74, 383–7. [CrossRef]
- Xie W, Regan MM, Buyse M et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017, 3, 3097–3104. [CrossRef]
- Wong GW, Palazzi-Churas KL, Jarrard DF et al. Salvage hypofractionated radiotherapy for biochemically recurrent prostate cancer after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2008, 70, 449–55. [CrossRef]
- Kruser TJ, Jarrard DF, Graf AK et al. Early hypofractionated salvage radiotherapy for postprostatectomy biochemical recurrence. Cancer. 2011, 117, 2629–36. [CrossRef]
- Lewis SL, Patel P, Song H et al. Image guided hypofractionated postprostatectomy intensity modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2016, 94, 605–11. [CrossRef]
- Tandberg DJ, Oyekunle T, Lee WR et al. Postoperative radiation therapy for prostate cancer: comparison of conventional versus hypofractionated radiation regimens. Int J Radiat Oncol Biol Phys. 2018, 101, 396–405. [CrossRef]
- Picardi C, Perret I, Miralbell R, Zilli T. Hypofractionated radiotherapy for prostate cancer in the postoperative setting: What is the evidence so far? Cancer Treat Rev. 2018, 62, 91–96. [CrossRef]
- Chin S, Fatimilehin A, Walshaw R et al. Ten-year outcomes of moderately hypofractionated salvage postprostatectomy radiation therapy and external validation of a contemporary multivariable nomogram for biochemical failure. Int J Radiat Oncol Biol Phys. 2020, 107, 288–296. [CrossRef]
- Ferrera G, D'Alessandro S, Cuccia F et al. Post-operative hypofractionated radiotherapy for prostate cancer: a mono-institutional analysis of toxicity and clinical outcomes. J Cancer Res Clin Oncol. 2022, 148, 89–95. [CrossRef]
- Viani GA, Gouveia AG, Leite ETT et al. Moderate hypofractionation for salvage radiotherapy (HYPO-SRT) in patients with biochemical recurrence after prostatectomy: A cohort study with meta-analysis. Radiother Oncol. 2022, 171, 7–13. [CrossRef]
- Lewis SL, Patel P, Song H et al. Image guided hypofractionated postprostatectomy intensity modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2016, 94, 605–11. [CrossRef]
- Valero J, Montero A, Hernando O et al. Moderate hypofractionated post-prostatectomy radiation therapy is feasible and well tolerated: experience from a single tertiary cancer centre. Clin Transl Oncol. 2021, 23, 1452–1462. [CrossRef]
- Leite ETT, Ramos CCA, Ribeiro VAB et al. Hypofractionated radiation therapy to the prostate bed with intensity-modulated radiation therapy (IMRT): a phase 2 trial. Int J Radiat Oncol Biol Phys. 2021, 109, 1263–1270. [CrossRef]
- Tilki D, Chen MH, Wu J, et al. Adjuvant versus early salvage radiation therapy for men at high risk for recurrence following radical prostatectomy for prostate cancer and the risk of death. J Clin Oncol. 2021, 39, 2284–2293. [CrossRef]
- King, CR. Adjuvant versus salvage radiotherapy for high-risk prostate cancer patients. Semin Radiat Oncol. 2013, 23, 215–21. [Google Scholar] [CrossRef] [PubMed]
- Fossati N, Karnes RJ, Cozzarini C et al. Assessing the optimal timing for early salvage radiation therapy in patients with prostate-specific antigen rise after radical prostatectomy. Eur Urol. 2016, 69, 728–733. [CrossRef]
- Tendulkar RD, Agrawal S, Gao Tet al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016, 34, 3648–3654. [CrossRef]
- Mak RH, Hunt D, Efstathiou JA et al. Acute and late urinary toxicity following radiation in men with an intact prostate gland or after a radical prostatectomy: A secondary analysis of RTOG 94-08 and 96-01. Urol Oncol. 2016, 34, 430–e1. [CrossRef]
- Katayama S, Striecker T, Kessel K et al. Hypofractionated IMRT of the prostate bed after radical prostatectomy: acute toxicity in the PRIAMOS-1 trial. Int J Radiat Oncol Biol Phys. 2014, 90, 926–33. [CrossRef]
- Fersino S, Tebano U, Mazzola R et al. Moderate hypofractionated postprostatectomy volumetric modulated arc therapy with daily image guidance (VMAT-IGRT): a mono-institutional report on feasibility and acute toxicity. Clin Genitourin Cancer. 2017, 15, e667–e673. [CrossRef]
- Ghadjar P, Hayoz S, Bernhard J et al. ; Swiss Group for Clinical Cancer Research (SAKK). Dose-intensified Versus Conventional-dose Salvage Radiotherapy for Biochemically Recurrent Prostate Cancer After Prostatectomy: The SAKK 09/10 Randomized Phase 3 Trial. Eur Urol. 2021, 80, 306–315. [CrossRef]
- Parker CC, Clarke N, Cook A et al. Duration of androgen deprivation therapy (ADT) with post-operative radiotherapy (RT) for prostate cancer: First results of the RADICALS-HD trial. Ann Oncol. 2022, 33, S1427. [CrossRef]
- Pollack A, Karrison TG, Balogh AG et al. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet. 2022, 399, 1886–1901. [CrossRef]
- Parker C, Clarke N, Logue J et al. ; RADICALS Trial Management Group. RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery). Clin Oncol (R Coll Radiol). 2007, 19, 167–71. [CrossRef]
- Buyyounouski MK, Pugh SL, Chen RC et al. Primary endpoint analysis of a randomized phase III trial hypofractionated versus conventional post-prostatectomy radiotherapy NRG oncology GU003. Int J Radiat Oncol Biol Phys. 2021, 111, S2–S3. [CrossRef]
| Characteristics | n = 100 | |
|---|---|---|
| Age, years | Median Range |
64 47 - 77 |
| Initial PSA [ng/ml] | Median Range |
9.8 1 - 91 |
| pT, n (%) | pT2 pT3a pT3b pTX |
48 (48) 31 (31) 19 (19) 2 (2) |
| ECE, n (%) | No Yes NA |
53 (53) 44 (44) 3 (3) |
| SVI, n (%) | No Yes NA |
78 (78) 19 (19) 3 (3) |
| ISUP Grade group (Gleason score), n (%) |
1 (≤6) 2 (3+4) 3 (4+3) 4 (8) 5 (9-10) NA |
27 (27) 41 (41) 15 (15) 7 (7) 9 (9) 1 (1) |
| Surgical margin, n (%) | R0 R1 NA |
27 (27) 60 (60) 13 (13) |
| pN, n (%) | pN0 pN1 pNX |
29 (29) 8 (8) 63 (63) |
| rPSA [ng/ml], n (%) | <0.2 0.2 – 0.5 0.5 – 2.0 ≥2.0 |
25 (25) 33 (33) 29 (29) 13 (13) |
| RT indication, n (%) | Adjuvant Early salvage Salvage |
19 (19) 46 (46) 35 (35) |
| Independent variable | 5-year bRFS (%) | p value |
|---|---|---|
| Extracapsular extension Yes No |
80 79.5 |
0.69 |
| Resection margin status R1 R0 |
80.5 72.8 |
0.40 |
| ADT administration Yes No |
70.4 85.7 |
0.11 |
| Volume irradiated Prostate bed only Prostate bed and pelvic nodes |
78 NA |
0.75 |
| ISUP grade group 1+2+3 4+5 |
82.5 54.6 |
0.079 |
| Seminal vesicle invasion Yes No |
56.8 82.6 |
≤ 0.01 |
| Recurrent PSA [ng/ml] <0.2 0.2 – 0.5 0.5 – 2.0 ≥2.0 |
95.2 79.3 67.3 68.4 |
0.002 |
| Treatment indication Adjuvant or early salvage Salvage |
84.6 67.6 |
0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




