Submitted:
30 June 2023
Posted:
03 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
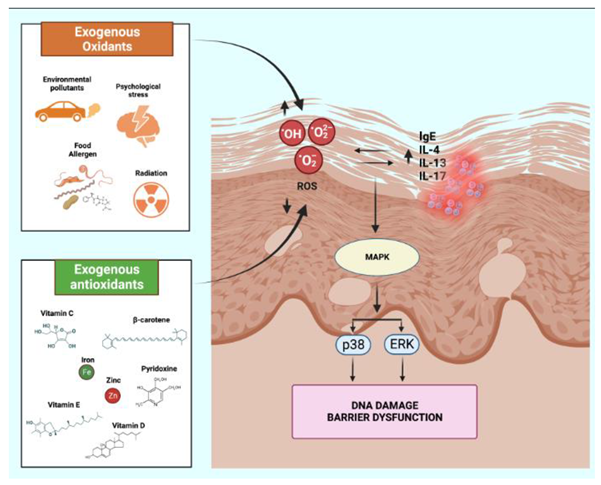
3. Oxidative stress in atopic dermatitis
4. Vitamin D
5. Vitamin E
6. Vitamin C
7. Pyridoxine
8. Melatonin
9. Carotenoids
10. Zinc
11. Iron
| Authors and year | Study design | Evidence |
|---|---|---|
| Vitamin D | ||
| Amestejani et al. 2012 [39] | 60 pts with ADguifenRandomized, double-blind, placebo-controlled: vitamin D group (n=30) and placebo group (n=30) treated for 60 days | The Vitamin D group showed significant improvement in patients with AD in terms of SCORAD and TIS (P<0.05). guifenNo improvement in the placebo group (P<0.05). |
| Sanchez-Armendariz et al. 2018 [40] | 65 pts with ADguifenRandomized, double-blind, placebo-controlled: Vitamin D3 group (n=33) at dose 5000 IU/day or placebo along with baseline therapy (n=32) | Achieving serum levels of 25(OH)D > 20 ng/mL in conjunction with standard therapy is sufficient to achieve a reduction in severity (SCORAD) in pts with AD. |
| Hata et al. 2008 [42] | 28 pts (14 with AD and 14 controls)guifenA single center controlled: received 4000 IU of oral vitamin D3 daily for 3 wk | In patients with AD significant increase in cathelicidin expression, from a median of 3.53 relative copy units (RCU) before supplementation to a median of 23.91 RCU after supplementation (P < 0.01). |
| Javanbakht et al. 2009 [43] | 45 pts with ADguifenRandomized double-blind, placebo controlled: Group P (n = 11): placebo vitamin E and D; Group D (n = 12): 1600 IU vitamin D3 and vitamin E placebo; Group E (n = 11): 600 IU synthetic all-rac-α-tocopherol plus vitamin D placebo; Group DE (n = 11), 1600 IU vitamin D3 plus 600 IU synthetic all-rac-α-tocopherol. Four groups and treated for 60 days | Positive correlation between SCORAD and intensity, objective, subjective and extension (p < 0.001), on the other hand negative association between plasma α-tocopherol and SCORAD, intensity, objective, and extension (p = 0.02). |
| Hata et al. 2013 [44] | 30 pts with AD, 30 non-atopic subjects, and 16 pts with psoriasisguifenA multi-center, placebo-controlled, double-blind study: received cholecalciferol 4000 IU or placebo for 21 days | At baseline, 20% of AD subjects had serum 25OHD levels below 20 ng/mL and low serum 25OHD levels correlated with increased Fitzpatrick Skin Type and elevated BMI, but not AD severity.guifenAt day 21 increased serum Vit D levels, but there were no significant changes in skin cathelicidin, HBD-3, IL-13 or EASI scores. |
| Peroni et al. 2011 [41] | 37 children with AD aged between 8 months and 12 y.guifenObservational study: correlation between Vit D levels and severity | Mean ± SD serum levels of 25(OH)D were significantly higher (P < 0.05) in pts with mild disease (36.9 ± 15.7 ng/mL) compared to those with moderate (27.5 ± 8.3 ng/mL) or severe AD (20.5 ± 5.9 ng/mL). |
| Chiu et al. 2013 [45] | 97 pts with AD aged between 1 to 18 years of age.guifenObservational study | Vitamin D deficiency (25-hydroxyvitamin D <20 ng/mL) was present in 37 subjects (39%), insufficiency (25-hydroxyvitamin D 21-29 ng/mL) in 33 (35%), and sufficiency (25-hydroxyvitamin D ≥30 ng/mL) in 24 (26%).The correlation between 25-hydroxyvitamin D concentration and SCORAD was not significant (r = -0.001; P = .99). |
| Vitamin E | ||
| S-Y Oh et al. 2010 [17] | 180 children with AD and 242 healthy controlsguifenObservational study | Vitamin E levels were found to be inversely associated with AD prevalence |
| Okuda et al. 2010 [53] | 396 Japanese pts with AD aged 10-13 yearsguifenObservational study | Inverse correlation between serum alpha-tocopherol levels and AD prevalence.guifen(OR)s for the third and fourth quartiles of serum α-tocopherol with AD were 0.33 (95% IC: 0.15–0.73) and 0.36 (0.14–0.89), respectively, and the trend was negatively significant (Ptrend=0.048). |
| Lee et al. 2012 [54] | 119 Korean children aged 0-24 months.guifenCross-sectional study | Serum vitamin E levels showed a significantly inverse association with serum total IgE and all specific IgE levels (P < 0.05). |
| Tsoureli-Nikita et al. 2002 [55] | 96 pts with AD aged 10-60 years guifenObservational study | In pts with improving AD in the vitamin E group, there was a 62% decrease in serum IgE levels from baseline while in the placebo group the difference was 34.4%. |
| Pyridoxine | ||
| Koller et al. 1987 [65] | 20 pts with ADguifenA double-blin: patients received 1.5 mg/kg per day. | Improvement in 6 of the 10 pts treated with pyridoxine, only 1 of the placebo-treated pts experienced improvement. |
| Mabin et al. 1995 [63] | 48 pts with AD, of whom 19 received 50 mg/day of pyridoxine hydrochloride and 22 received placebo.guifenDouble-blind, placebo-controlled | There were no significant differences between the two groups. |
| Vitamin C | ||
| Shin et al 2016 [61] | 17 pts with AD. guifenObservational study | 82.4% of them had plasma vitamin C levels below 25 μmol/L, while only 3 pts had levels within the normal range (25-80 μmol/L). |
| Melatonin | ||
| Yung-Sen Chang et al. 2016 [78] | 73 pts with at least 5% skin involvement by AD, aged 1-18 y. guifenMelatonin was administered at a dosage of 3 mg/day or placebo for 4 wk with a washout of 2 wk. | After melatonin treatment, SCORAD was reduced by 9.1 compared with placebo, from 49.1 to 40.2. In addition, the sleep latency interval was reduced by 21.4 minutes after melatonin treatment compared with placebo, but SCORAD improvement was not significantly correlated with change in sleep latency. |
| Devadasan et al. 2020 [79] | 30 AD pts aged 6 to 12 years and 30 healthy patients. guifenObservational study: evaluated serum levels of melatonin, peroxide dismutase, and glutathione reductase. | Melatonin levels were higher in AD subjects than in controls. In contrast, levels of glutathione peroxidase and superoxide dismutase were higher in cases than in controls, but the difference was not statistically significant. |
| Lycopene | ||
| Inoue et al. 2023 [94] | 263 mothers and their children, 40 with AD and 263 non-AD, in 1 y of ageguifenObservational study. | Maternal blood lutein level (OR, 0.002; p = .002), and infant blood lycopene level at 1 y (OR, 0.01; p = .007) were significantly related to AD at 1 year of age. |
| Ruhl et al. 2010 [92] | 122 children, 41 with AD and 81 non-ADguifenObservational study | Lower concentrations of lycopene and non-vitamin A carotenoids were observed in atopic infants, suggesting that lycopene may provide protection against atopy |
| Beta-carotene | ||
| Gromadzinska et al. 2018 [90] | 252 mother-child pairsguifenObservational study | No statistically significant associations between β-carotene, vitam A and the risk AD in children up to 2 y of age. |
| S-Y Oh et al. 2010 [17] | 422 children, 180 with AD and 242 with non-ADguifenObservational study | Reduced AD risk for the highest quintile of b-carotene (OR 0.69, p< 0.0166) |
| Miyake et al., 2010 [93] | 763 mother-child pairs guifenObservational study | b-carotene during pregnancy was significantly associated with a reduced risk of eczema in the offspring between the highest and lowest quartile (OR: 0,41, p<0,01) |
| Zinc | ||
| David et al. 1984 [102] | 65 children with atopic eczema and 79 controls guifenObservational study | Mean serum zinc of the patients with AD, (11.4±2.0 micromol/l) was significantly lower than that of the controls (13.7±2.3 micromol/l, P<oooo1). There was no correlation between serum Zn concentration and eczema severity or height/weight percentile |
| Karabacak et al., 2016 [104] | 67 pts with AD and 49 controlsguifenObservational study | Erythrocyte zinc levels were significantly lower in AD patients than in the control group (p < 0.001), serum zinc levels did not differ between the groups (p = 0.148). In the AD patient group, there was a negative correlation between the SCORAD score and erythrocyte zinc levels (r = –0.791; p < 0.001). |
| Ewing et al, 1991 [105] | 50 children with AD aged 1-16 yearsguifenInterventional study | No significant improvement in disease severity after eight-week zinc sulfate supplementation |
| Kim et al, 2014 [106] | 58 AD pts, 43 controlsguifenInterventional study | Mean zinc level was significantly reduced in AD pts (113.1 μg/g vs. 130.9 μg/g, p=0.012) at baseline. After 8 wk of supplement, hair zinc level increased significantly (p<0.001) and EASI scores, TEWL, and visual analogue scales for pruritus improved (p=0.044, 0.015 and <0,001 respectively) |
| Iron | ||
| Drury et al. 2015 [113] | 207.007 young childrenguifenCase-control study | Children with AD had higher odds of microcytic anemia (p<0.009) |
| Rhew et al. 2020 [112] | 1,468,033 patients guifenInterventional study | Higher prevalence of iron deficiency anemia in patients with atopic dermatitis (OR: 1,40, p<0,001) |
| S-Y Oh et al. 2010 [17]guifen | 422 children, 180 with AD and 242 with non-ADguifenInterventional study | Reduced AD risk for the highest quintile of iron (OR: 0.39, p< 0.01), and after iron supplementation is not associated with AD risk (OR=0,51, p=0,18) |
| Fortes et al. 2018 [114] | 395 mother-child pairsguifenInterventional study | Mothers use of both iron and folic acid supplementation is correlated to a decreased risk of developing AD [OR=0.22; p=0.02] |
12. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. The Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; Bruin-Weller, M.; Eckert, L. Epidemiology of Atopic Dermatitis in Adults: Results from an International Survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Naldi, L.; Parazzini, F.; Gallus, S. Prevalence of Atopic Dermatitis in Italian Schoolchildren: Factors Affecting Its Variation. Acta Derm Venereol 2008, 89, 122–125. [Google Scholar] [CrossRef]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-Based European Guidelines for Treatment of Atopic Eczema (Atopic Dermatitis) in Adults and Children: Part I. Journal of the European Academy of Dermatology and Venereology 2018, 32, 657–682. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis anAsthma. J Clin Cell Immunol 2014, 05. [CrossRef]
- Paller, A.; Jaworski, J.C.; Simpson, E.L.; Boguniewicz, M.; Russell, J.J.; Block, J.K.; Tofte, S.; Dunn, J.D.; Feldman, S.R.; Clark, A.R.; et al. Major Comorbidities of Atopic Dermatitis: Beyond Allergic Disorders. Am J Clin Dermatol 2018, 19, 821–838. [Google Scholar] [CrossRef] [PubMed]
- De Simoni, E.; Rizzetto, G.; Molinelli, E.; Lucarini, G.; Mattioli-Belmonte, M.; Capodaglio, I.; Ferretti, G.; Bacchetti, T.; Offidani, A.; Simonetti, O. Metabolic Comorbidities in Pediatric Atopic Dermatitis: A Narrative Review. Life 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Goteri, G.; Ranaldi, R.; Simonetti, O.; Capretti, R.; Menzo, S.; Stramazzotti, D.; Morichetti, D.; Offidani, A.M.; Rupoli, S.; Leoni, P. Clinicopathological Features of Primary Cutaneous B-Cell Lymphomas from an Academic Regional Hospital in Central Italy: No Evidence of Borrelia Burgdorferi Association. Leuk Lymphoma 2007, 48, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Silvestri, C.; Arzeni, D.; Ganzetti, G.; Castelletti, S.; Simonetti, O.; Cirioni, O.; Kamysz, W.; Kamysz, E.; Spreghini, E.; et al. In Vitro Susceptibility of Dermatophytes to Conventional and Alternative Antifungal Agents. Med Mycol 2009, 47, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Boguniewicz, J.; Boguniewicz, M.; Ong, P.Y. The Infectious Complications of Atopic Dermatitis. Annals of Allergy, Asthma & Immunology 2021, 126, 3–12. [Google Scholar] [CrossRef]
- Faergemann, J. Atopic Dermatitis and Fungi. Clin Microbiol Rev 2002, 15, 545–563. [Google Scholar] [CrossRef]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat Genet 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Irvine, A.D.; McLean, W.H.I.; Leung, D.Y.M. Filaggrin Mutations Associated with Skin and Allergic Diseases. New England Journal of Medicine 2011, 365, 1315–1327. [Google Scholar] [CrossRef]
- Tamari, M.; Hirota, T. Genome-Wide Association Studies of Atopic Dermatitis. J Dermatol 2014, 41, 213–220. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New Insights into Atopic Dermatitis. Journal of Clinical Investigation 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Kim KE, C.D.P.HJ. Air Pollution and Skin Diseases: Adverse Effects of Airborne Particulate Matter on Various Skin Diseases. Life Sci. 2016;152(6):126–134. [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem Photobiol 2017, 93, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Md Jaffri, J. Reactive Oxygen Species and Antioxidant System in Selected Skin Disorders. Malaysian Journal of Medical Sciences 2023, 30, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Wang, S.Q. Recognizing the Impact of Ambient Air Pollution on Skin Health. Journal of the European Academy of Dermatology and Venereology 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Chung, J.; Kim, M.-K.; Kwon, S.O.; Cho, B.-H. Antioxidant Nutrient Intakes and Corresponding Biomarkers Associated with the Risk of Atopic Dermatitis in Young Children. Eur J Clin Nutr 2010, 64, 245–252. [Google Scholar] [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y. Do Regulation of Reactive Oxygen Species Generation in Cell Signaling. Mol Cells 2011, 32, 491–509. [Google Scholar] [CrossRef]
- De Simoni, E.; Rizzetto, G.; Molinelli, E.; Capodaglio, I.; Offidani, A.; Simonetti, O. The Role of Diet in Children with Psoriasis: Emerging Evidence and Current Issues. Nutrients 2023, 15, 1705. [Google Scholar] [CrossRef]
- Bacchetti, T.; Simonetti, O.; Ricotti, F.; Offidani, A.; Ferretti, G. Plasma Oxidation Status and Antioxidant Capacity in Psoriatic Children. Arch Dermatol Res 2020, 312, 33–39. [Google Scholar] [CrossRef]
- Klonowska, J.; Gleń, J.; Nowicki, R.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets. Int J Mol Sci 2018, 19, 3086. [Google Scholar] [CrossRef]
- Emmert, H.; Fonfara, M.; Rodriguez, E.; Weidinger, S. NADPH Oxidase Inhibition Rescues Keratinocytes from Elevated Oxidative Stress in a 2D Atopic Dermatitis and Psoriasis Model. Exp Dermatol 2020, 29, 749–758. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.-K. Oxidative Stress in Atopic Dermatitis. Oxid Med Cell Longev 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K. Atopic Dermatitis: New Insight into the Etiology, Pathogenesis, Diagnosis and Novel Treatment Strategies. Immunopharmacol Immunotoxicol 2021, 43, 105–125. [Google Scholar] [CrossRef]
- Borgia, F.; Custurone, P.; Peterle, L.; Pioggia, G.; Gangemi, S. Role of Epithelium-Derived Cytokines in Atopic Dermatitis and Psoriasis: Evidence and Therapeutic Perspectives. Biomolecules 2021, 11, 1843. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in Chronic Inflammatory Skin Disorders. Int J Mol Sci 2021, 22, 12035. [Google Scholar] [CrossRef]
- Simonetti, O.; Lucarini, G.; Rubini, C.; Goteri, G.; Zizzi, A.; Staibano, S.; Campanati, A.; Ganzetti, G.; Di Primio, R.; Offidani, A. Microvessel Density and VEGF, HIF-1α Expression in Primary Oral Melanoma: Correlation with Prognosis. Oral Dis 2013, 19, 620–627. [Google Scholar] [CrossRef]
- Samochocki, Z.; Bogaczewicz, J.; Sysa-Jędrzejowska, A.; McCauliffe, D.P.; Kontny, E.; Wozniacka, A. Expression of Vascular Endothelial Growth Factor and Other Cytokines in Atopic Dermatitis, and Correlation with Clinical Features. Int J Dermatol 2016, 55, e141–e146. [Google Scholar] [CrossRef]
- Hellemans, L.; Corstjens, H.; Neven, A.; Declercq, L.; Maes, D. Antioxidant Enzyme Activity in Human Stratum Corneum Shows Seasonal Variation with an Age-Dependent Recovery. Journal of Investigative Dermatology 2003, 120, 434–439. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and Non-Enzymic Antioxidants in Epidermis and Dermis of Human Skin. Journal of Investigative Dermatology 1994, 102, 122–124. [Google Scholar] [CrossRef]
- Irei, A. V; Takahashi, K.; Le, D.S.N.T.; Ha, P.T.N.; Hung, N.T.K.; Kunii, D.; Sakai, T.; Matoba, T.; Yamamoto, S. Obesity Is Associated with Increased Risk of Allergy in Vietnamese Adolescents. Eur J Clin Nutr 2005, 59, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Ferretti, G.; Salvi, A.; Offidani, A.M.; Bossi, G. Plasma Lipid Changes in Psoriatic Children. Dermatology 1992, 185, 96–100. [Google Scholar] [CrossRef]
- Undurti, A.; Huang, Y.; Lupica, J.A.; Smith, J.D.; DiDonato, J.A.; Hazen, S.L. Modification of High Density Lipoprotein by Myeloperoxidase Generates a Pro-Inflammatory Particle. Journal of Biological Chemistry 2009, 284, 30825–30835. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Nukuna, B.; Brennan, M.-L.; Sun, M.; Goormastic, M.; Settle, M.; Schmitt, D.; Fu, X.; Thomson, L.; Fox, P.L.; et al. Apolipoprotein A-I Is a Selective Target for Myeloperoxidase-Catalyzed Oxidation and Functional Impairment in Subjects with Cardiovascular Disease. Journal of Clinical Investigation 2004, 114, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, T.; Ferretti, G.; Carbone, F.; Ministrini, S.; Montecucco, F.; Jamialahmadi, T.; Sahebkar, A. Dysfunctional High-Density Lipoprotein: The Role of Myeloperoxidase and Paraoxonase-1. Curr Med Chem 2021, 28, 2842–2850. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Toh, R.; Hasokawa, M.; Nakajima, H.; Honjo, T.; Otsui, K.; Mori, K.; Miyamoto-Sasaki, M.; Shinohara, M.; Nishimura, K.; et al. Serum Myeloperoxidase/Paraoxonase 1 Ratio as Potential Indicator of Dysfunctional High-Density Lipoprotein and Risk Stratification in Coronary Artery Disease. Atherosclerosis 2014, 234, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Bacchetti, T.; Ferretti, G.; Molinelli, E.; Rizzetto, G.; Bellachioma, L.; Offidani, A. Oxidative Stress and Alterations of Paraoxonases in Atopic Dermatitis. Antioxidants 2021, 10, 697. [Google Scholar] [CrossRef]
- Trakaki, A.; Marsche, G. High-Density Lipoprotein (HDL) in Allergy and Skin Diseases: Focus on Immunomodulating Functions. Biomedicines 2020, 8, 558. [Google Scholar] [CrossRef]
- Simonetti, O.; Lucarini, G.; Campanati, A.; Goteri, G.; Zizzi, A.; Marconi, B.; Ganzetti, G.; Minardi, D.; Di Primio, R.; Offidani, A. VEGF, Survivin and NOS Overexpression in Psoriatic Skin: Critical Role of Nitric Oxide Synthases. J Dermatol Sci 2009, 54, 205–208. [Google Scholar] [CrossRef]
- Reichrath, J. Vitamin D and the Skin: An Ancient Friend, Revisited. Exp Dermatol 2007, 16, 618–625. [Google Scholar] [CrossRef]
- Kim, G.; Bae, J.-H. Vitamin D and Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrition 2016, 32, 913–920. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in Foods and as Supplements. Prog Biophys Mol Biol 2006, 92, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Morteza Amestejani 1, B.S.S.M.V.A.S.M.K.H.A.S.K.K.N.S.B.G.A.H.B. Vitamin D Supplementation in the Treatment of Atopic Dermatitis: A Clinical Trial Study. J Drugs Dermatol . 2012 Mar;11(3):327-30.
- Sánchez-Armendáriz, K.; García-Gil, A.; Romero, C.A.; Contreras-Ruiz, J.; Karam-Orante, M.; Balcazar-Antonio, D.; Domínguez-Cherit, J. Oral Vitamin D3 5000 IU/Day as an Adjuvant in the Treatment of Atopic Dermatitis: A Randomized Control Trial. Int J Dermatol 2018, 57, 1516–1520. [Google Scholar] [CrossRef]
- Peroni, D.G.; Piacentini, G.L.; Cametti, E.; Chinellato, I.; Boner, A.L. Correlation between Serum 25-Hydroxyvitamin D Levels and Severity of Atopic Dermatitis in Children. British Journal of Dermatology 2011, 164, 1078–1082. [Google Scholar] [CrossRef]
- Hata, T.R.; Kotol, P.; Jackson, M.; Nguyen, M.; Paik, A.; Udall, D.; Kanada, K.; Yamasaki, K.; Alexandrescu, D.; Gallo, R.L. Administration of Oral Vitamin D Induces Cathelicidin Production in Atopic Individuals. Journal of Allergy and Clinical Immunology 2008, 122, 829–831. [Google Scholar] [CrossRef]
- Javanbakht, M.H.; Keshavarz, S.A.; Djalali, M.; Siassi, F.; Eshraghian, M.R.; Firooz, A.; Seirafi, H.; Ehsani, A.H.; Chamari, M.; Mirshafiey, A. Randomized Controlled Trial Using Vitamins E and D Supplementation in Atopic Dermatitis. Journal of Dermatological Treatment 2011, 22, 144–150. [Google Scholar] [CrossRef]
- Hata, T.R.; Audish, D.; Kotol, P.; Coda, A.; Kabigting, F.; Miller, J.; Alexandrescu, D.; Boguniewicz, M.; Taylor, P.; Aertker, L.; et al. A Randomized Controlled Double-Blind Investigation of the Effects of Vitamin D Dietary Supplementation in Subjects with Atopic Dermatitis. Journal of the European Academy of Dermatology and Venereology 2014, 28, 781–789. [Google Scholar] [CrossRef]
- Chiu, Y.E.; Havens, P.L.; Siegel, D.H.; Ali, O.; Wang, T.; Holland, K.E.; Galbraith, S.S.; Lyon, V.B.; Drolet, B.A. Serum 25-Hydroxyvitamin D Concentration Does Not Correlate with Atopic Dermatitis Severity. J Am Acad Dermatol 2013, 69, 40–46. [Google Scholar] [CrossRef]
- Mustacich, D.J.; Bruno, R.S.; Traber, M.G. Vitamin E. In; 2007; pp. 1–21.
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in Human Skin: Organ-Specific Physiology and Considerations for Its Use in Dermatology. Mol Aspects Med 2007, 28, 646–667. [Google Scholar] [CrossRef]
- Parish, W.E.; Read, J.; Paterson, S.E. Changes in Basal Cell Mitosis and Transepidermal Water Loss in Skin Cultures Treated with Vitamins C and E. Exp Dermatol 2005, 14, 684–691. [Google Scholar] [CrossRef]
- De Pascale, M.C.; Bassi, A.M.; Patrone, V.; Villacorta, L.; Azzi, A.; Zingg, J.-M. Increased Expression of Transglutaminase-1 and PPARγ after Vitamin E Treatment in Human Keratinocytes. Arch Biochem Biophys 2006, 447, 97–106. [Google Scholar] [CrossRef]
- Kato, E.; Takahashi, N. Improvement by Sodium Dl-α-Tocopheryl-6-O-Phosphate Treatment of Moisture-Retaining Ability in Stratum Corneum through Increased Ceramide Levels. Bioorg Med Chem 2012, 20, 3837–3842. [Google Scholar] [CrossRef] [PubMed]
- HAYASHI, D.; SUGAYA, H.; OHKOSHI, T.; SEKIZAWA, K.; TAKATSU, H.; SHINKAI, T.; URANO, S. Vitamin E Improves Biochemical Indices Associated with Symptoms of Atopic Dermatitis-Like Inflammation in NC/Nga Mice. J Nutr Sci Vitaminol (Tokyo) 2012, 58, 161–168. [Google Scholar] [CrossRef]
- Plevnik Kapun, A.; Salobir, J.; Levart, A.; Tavčar Kalcher, G.; Nemec Svete, A.; Kotnik, T. Vitamin E Supplementation in Canine Atopic Dermatitis: Improvement of Clinical Signs and Effects on Oxidative Stress Markers. Veterinary Record 2014, 175, 560–560. [Google Scholar] [CrossRef]
- Okuda, M.; Bando, N.; Terao, J.; Sasaki, S.; Sugiyama, S.; Kunitsugu, I.; Hobara, T. Association of Serum Carotenoids and Tocopherols with Atopic Diseases in Japanese Children and Adolescents. Pediatric Allergy and Immunology 2010, 21, e705–e710. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ahn, K.; Paik, H.Y.; Chung, S.-J. Serum Immunoglobulin E (IgE) Levels and Dietary Intake of Korean Infants and Young Children with Atopic Dermatitis. Nutr Res Pract 2012, 6, 429. [Google Scholar] [CrossRef] [PubMed]
- Tsoureli-Nikita, E.; Hercogova, J.; Lotti, T.; Menchini, G. Evaluation of Dietary Intake of Vitamin E in the Treatment of Atopic Dermatitis: A Study of the Clinical Course and Evaluation of the Immunoglobulin E Serum Levels. Int J Dermatol 2002, 41, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Mh Javanbakht 1, S.K.A.M.M.D.F.S.M.E.A.F.H.S.A.E.M.C. The Effects of Vitamins e and d Supplementation on Erythrocyte Superoxide Dismutase and Catalase in Atopic Dermatitis. Iran J Public Health . 2010;39(1):57-63. Epub 2010 Mar 31.
- Nwaru, B.I.; Erkkola, M.; Ahonen, S.; Kaila, M.; Kronberg-Kippilä, C.; Ilonen, J.; Simell, O.; Knip, M.; Veijola, R.; Virtanen, S.M. Intake of Antioxidants during Pregnancy and the Risk of Allergies and Asthma in the Offspring. Eur J Clin Nutr 2011, 65, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front Physiol 2018, 9. [CrossRef]
- Leveque, N.; Robin, S.; Muret, P.; Mac-Mary, S.; Makki, S.; Humbert, P. High Iron and Low Ascorbic Acid Concentrations in the Dermis of Atopic Dermatitis Patients. Dermatology 2003, 207, 261–264. [Google Scholar] [CrossRef]
- Assier, H.; Wolkenstein, P.; Grille, C.; Chosidow, O. Contact Dermatitis Caused by Ascorbyl Tetraisopalmitate in a Cream Used for the Management of Atopic Dermatitis. Contact Dermatitis 2014, 71, 60–61. [Google Scholar] [CrossRef]
- Shin, J.; Kim, Y.J.; Kwon, O.; Kim, N.-I.; Cho, Y. Associations among Plasma Vitamin C, Epidermal Ceramide and Clinical Severity of Atopic Dermatitis. Nutr Res Pract 2016, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Stach, K.; Stach, W.; Augoff, K. Vitamin B6 in Health and Disease. Nutrients 2021, 13, 3229. [Google Scholar] [CrossRef] [PubMed]
- Mabin, D.C.; Hollis, S.; Lockwood, J.; David, T.J. Pyridoxine in Atopic Dermatitis. British Journal of Dermatology 2006, 133, 764–767. [Google Scholar] [CrossRef]
- Merrill, A.H.; Henderson, J.M. Diseases Associated with Defects in Vitamin B6 Metabolism or Utilization. Annu Rev Nutr 1987, 7, 137–156. [Google Scholar] [CrossRef]
- Koller DY, P.C.J.R. Pyridoxine HCI Improves Atopic Eczema Dermatitis: Changes of IL-1 Beta, IL-2, ACTH and Cortisol in Plasma. . Clin Exp Allergy 1992;22:126.
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int J Biochem Cell Biol 2006, 38, 313–316. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Salpietro, C.; Arrigo, T.; Barberi, I.; Reiter, R.; Gitto, E. Melatonin and Atopy: Role in Atopic Dermatitis and Asthma. Int J Mol Sci 2014, 15, 13482–13493. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The Cutaneous Serotoninergic/Melatoninergic System: Securing a Place under the Sun. The FASEB Journal 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of Antioxidant Enzymes: A Significant Role for Melatonin. J Pineal Res 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int J Mol Sci 2022, 23, 1238. [Google Scholar] [CrossRef]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.-X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective Effects of Melatonin in Reducing Oxidative Stress and in Preserving the Fluidity of Biological Membranes: A Review. J Pineal Res 2014, 56, 225–237. [Google Scholar] [CrossRef]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin Enhances Antioxidative Enzyme Gene Expression (CAT, GPx, SOD), Prevents Their UVR-Induced Depletion, and Protects against the Formation of DNA Damage (8-Hydroxy-2’-Deoxyguanosine) in Ex Vivo Human Skin. J Pineal Res 2013, 54, 303–312. [Google Scholar] [CrossRef]
- Srinivasan, V.; Maestroni, G.; Cardinali, D.; Esquifino, A.; Perumal, S.P.; Miller, S. Melatonin, Immune Function and Aging. Immunity & Ageing 2005, 2, 17. [Google Scholar] [CrossRef]
- WITHYACHUMNARNKUL, B.; NONAKA, K.O.; SANTANA, C.; ATTIA, A.M.; REITER, R.J. Interferon-γ Modulates Melatonin Production in Rat Pineal Glands in Organ Culture. J Interferon Res 1990, 10, 403–411. [Google Scholar] [CrossRef]
- M Majewska 1, K.Z.M.Z.M.S. Influence of Melatonin and Its Precursor L-Tryptophan on Th1 Dependent Contact Hypersensitivity. J Physiol Pharmacol . 2007 Dec;58 Suppl 6:125-32.
- Muñoz-Hoyos, A.; Espín-Quirantes, C.; Molina-Carballo, A.; Uberos, J.; Contreras-Chova, F.; Narbona-López, E.; Gutiérrez-Salmerón, M.J. Neuroendocrine and Circadian Aspects (Melatonin and β-Endorphin) of Atopic Dermatitis in the Child. Pediatric Allergy and Immunology 2007, 18, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Tsai, C.-C.; Yang, P.-Y.; Tang, C.-Y.; Chiang, B.-L. Topical Melatonin Exerts Immunomodulatory Effect and Improves Dermatitis Severity in a Mouse Model of Atopic Dermatitis. Int J Mol Sci 2022, 23, 1373. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Lin, M.-H.; Lee, J.-H.; Lee, P.-L.; Dai, Y.-S.; Chu, K.-H.; Sun, C.; Lin, Y.-T.; Wang, L.-C.; Yu, H.-H.; et al. Melatonin Supplementation for Children With Atopic Dermatitis and Sleep Disturbance. JAMA Pediatr 2016, 170, 35. [Google Scholar] [CrossRef]
- Devadasan, S.; Sarkar, R.; Barman, K.; Kaushik, S. Role of Serum Melatonin and Oxidative Stress in Childhood Atopic Dermatitis: A Prospective Study. Indian Dermatol Online J 2020, 11, 925. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Haag, S.F.; Taskoparan, B.; Darvin, M.E.; Groth, N.; Lademann, J.; Sterry, W.; Meinke, M.C. Determination of the Antioxidative Capacity of the Skin in Vivo Using Resonance Raman and Electron Paramagnetic Resonance Spectroscopy. Exp Dermatol 2011, 20, 483–487. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol Nutr Food Res 2019, 63, 1801045. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol Aspects Med 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch Biochem Biophys 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Nakazawa, M.; Takahashi, K.; Aihara, M.; Minami, M.; Hirasawa, T.; Ikezawa, Z. Effects of Zinc Deficient Diet on Development of Atopic Dermatitis-like Eruptions in DS-Nh Mice. J Dermatol Sci 2008, 50, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kake, T.; Hasegawa, S.; Imai, M. Effects of Post-Administration of β-Carotene on Diet-Induced Atopic Dermatitis in Hairless Mice. J Oleo Sci 2019, 68, 793–802. [Google Scholar] [CrossRef]
- Kake, T.; Imai, M.; Takahashi, N. Effects of Β-carotene on Oxazolone-induced Atopic Dermatitis in Hairless Mice. Exp Dermatol 2019, 28, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Gromadzinska, J.; Polanska, K.; Kozlowska, L.; Mikolajewska, K.; Stelmach, I.; Jerzynska, J.; Stelmach, W.; Grzesiak, M.; Hanke, W.; Wasowicz, W. Vitamins A and E during Pregnancy and Allergy Symptoms in an Early Childhood—Lack of Association with Tobacco Smoke Exposure. Int J Environ Res Public Health 2018, 15, 1245. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, H.; Li, T. Comorbidity of Vitamin A and Vitamin D Deficiency Exacerbates the Severity of Atopic Dermatitis in Children. Dermatology 2019, 235, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R.; Taner, C.; Schweigert, F.J.; Wahn, U.; Grüber, C. Serum Carotenoids and Atopy among Children of Different Ethnic Origin Living in Germany. Pediatric Allergy and Immunology 2010, 21, 1072–1075. [Google Scholar] [CrossRef]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of Vegetables, Fruit, and Antioxidants during Pregnancy and Wheeze and Eczema in Infants. Allergy 2010, 65, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Yamamoto, Y.; Suzuki, S.; Ochiai, S.; Eguchi, A.; Nakano, T.; Yamaide, F.; Hasegawa, S.; Kojima, H.; Mori, C.; et al. Maternal and Infant Serum Carotenoids Are Associated with Infantile Atopic Dermatitis Development. Allergy 2023. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int J Mol Sci 2017, 18, 2285. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical, Immunological, Anti-Inflammatory and Antioxidant Roles of Zinc. Exp Gerontol 2008, 43, 370–377. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and Immunity: An Essential Interrelation. Arch Biochem Biophys 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and Immunity: An Essential Interrelation. Arch Biochem Biophys 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Dajee, M.; Muchamuel, T.; Schryver, B.; Oo, A.; Alleman-Sposeto, J.; De Vry, C.G.; Prasad, S.; Ruhrmund, D.; Shyamsundar, R.; Mutnick, D.; et al. Blockade of Experimental Atopic Dermatitis via Topical NF-ΚB Decoy Oligonucleotide. Journal of Investigative Dermatology 2006, 126, 1792–1803. [Google Scholar] [CrossRef]
- Altobelli, G.G.; Van Noorden, S.; Balato, A.; Cimini, V. Copper/Zinc Superoxide Dismutase in Human Skin: Current Knowledge. Front Med (Lausanne) 2020, 7. [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and Skin Disorders. Nutrients 2018, 10, 199. [Google Scholar] [CrossRef]
- DAVID, T.J.; WELLS, F.E.; SHARPE, T.C.; GIBBS, A.C.C. Low Serum Zinc in Children with Atopic Eczema. British Journal of Dermatology 1984, 111, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Maywald, M.; Rink, L. Zinc Homeostasis and Immunosenescence. Journal of Trace Elements in Medicine and Biology 2015, 29, 24–30. [Google Scholar] [CrossRef]
- Karabacak, E.; Aydin, E.; Kutlu, A.; Ozcan, O.; Muftuoglu, T.; Gunes, A.; Dogan, B.; Ozturk, S. Erythrocyte Zinc Level in Patients with Atopic Dermatitis and Its Relation to SCORAD Index. Advances in Dermatology and Allergology 2016, 5, 349–352. [Google Scholar] [CrossRef]
- C I Ewing 1, A.C.G.C.A.T.J.D. Failure of Oral Zinc Supplementation in Atopic Eczema. Eur J Clin Nutr . 1991 Oct;45(10):507-10.
- Kim, J.; Yoo, S.; Jeong, M.; Ko, J.; Ro, Y. Hair Zinc Levels and the Efficacy of Oral Zinc Supplementation in Patients with Atopic Dermatitis. Acta Dermato Venereologica 2014, 94, 558–562. [Google Scholar] [CrossRef]
- Zhang, C. Essential Functions of Iron-Requiring Proteins in DNA Replication, Repair and Cell Cycle Control. Protein Cell 2014, 5, 750–760. [Google Scholar] [CrossRef]
- Oluwole, O.; Arinola, O.G.; Adu, M.D.; Adepoju, A.; Adedokun, B.O.; Olopade, O.I.; Olopade, C.O. Relationships between Plasma Micronutrients, Serum IgE, and Skin Test Reactivity and Asthma among School Children in Rural Southwest Nigeria. J Biomark 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Brouwer, I.D.; Nguyen, K.C.; Burema, J.; Kok, F.J. The Effect of Iron Fortification and De-Worming on Anaemia and Iron Status of Vietnamese Schoolchildren. British Journal of Nutrition 2007, 97, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Jason, J.; Archibald, L.K.; Nwanyanwu, O.C.; Bell, M.; Jensen, R.J.; Gunter, E.; Buchanan, I.; Larned, J.; Kazembe, P.N.; Dobbie, H.; et al. The Effects of Iron Deficiency on Lymphocyte Cytokine Production and Activation: Preservation of Hepatic Iron but Not at All Cost. Clin Exp Immunol 2002, 126, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Nyakeriga, A.M.; Williams, T.N.; Marsh, K.; Wambua, S.; Perlmann, H.; Perlmann, P.; Grandien, A.; Troye-Blomberg, M. Cytokine MRNA Expression and Iron Status in Children Living in a Malaria Endemic Area. Scand J Immunol 2005, 61, 370–375. [Google Scholar] [CrossRef]
- Rhew, K.; Brown, J.D.; Oh, J.M. Atopic Disease and Anemia in Korean Patients: Cross-Sectional Study with Propensity Score Analysis. Int J Environ Res Public Health 2020, 17, 1978. [Google Scholar] [CrossRef] [PubMed]
- Drury, K.E.; Schaeffer, M.; Silverberg, J.I. Association Between Atopic Disease and Anemia in US Children. JAMA Pediatr 2016, 170, 29. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Mannooranparampil, T.J.; Di Lallo, D. Pre-Natal Folic Acid and Iron Supplementation and Atopic Dermatitis in the First 6 Years of Life. Arch Dermatol Res 2019, 311, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Dom, S.; Droste, J.H.J.; Sariachvili, M.A.; Hagendorens, M.M.; Oostveen, E.; Bridts, C.H.; Stevens, W.J.; Wieringa, M.H.; Weyler, J.J. Pre- and Post-Natal Exposure to Antibiotics and the Development of Eczema, Recurrent Wheezing and Atopic Sensitization in Children up to the Age of 4 Years. Clinical & Experimental Allergy 2010, 40, 1378–1387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





