1. Introduction
Ascites is a term describing a pathological accumulation of fluid within a peritoneal cavity [
1]. It is a common complication of cirrhosis [
2] but especially in the paediatric population the etiology might be very different in various age groups. What is more, the constitution of the ascitic fluid varies greatly between different etiologies. The literature distinguishes three stages of ascites severity: mild, moderate and large. It also defines uncomplicated and complicated ascites and the latter being broken down further into infected and refractory ascites. The term refractory ascites covers both diuretic-resistant and diuretic-intractable ascites [
3].
Table 1.
Severity of ascites [
3], [
4].
Table 1.
Severity of ascites [
3], [
4].
| Grade 1 (mild) |
Grade 2 (moderate) |
Grade 3 (large) |
| only detectable by ultrasound |
causing moderate symmetrical distension of the abdomen |
causing marked abdominal distension |
Portal hypertension together with sodium and fluid retention are the main elements in the pathophysiology of ascites. Because of that salt restriction and pharmacological therapy consisting of diuretics are key in the management of ascites. Based on the response to the diuretic therapy and the presence of complications, a distinction between uncomplicated and refractory ascites has been made (table 2). Diagnostic paracentesis should be performed in every patient with new onset ascites and in ascitic patients when complications such as spontaneous bacterial peritonitis (SBP) are suspected [
3], [
5].
Table 2.
Division of ascites based on the etiology [
6].
Table 2.
Division of ascites based on the etiology [
6].
| hepatic cirrhotic causes —> ascites as the complication of cirrhosis |
|---|
| hepatic non-cirrhotic causes |
bile duct trauma or perforation |
| congenital hepatic fibrosis |
| cystic fibrosis |
| Budd-chiari syndrome |
| sinusoidal-obstruction syndrome (SOS) |
| non-hepatic non-cirrhotic |
peritoneal infection |
tuberculosis |
| Cytomegalovirus |
| Epstein-Barr Virus |
| intestinal |
Crohn’s disease |
| intestinal atresia |
| meconium ileus |
| eosinophilic enteropathy |
| pancreatic |
acute pancreatitis |
| systemic inflammatory disorders |
systemic lupus erythematosus |
| Henoch- Schonlein purpura |
| cardiac |
heart failure |
| genitourinary disorders |
nephrotic syndrome |
| obstructive uropathy |
| posterior urethral valves |
| bladder rupture |
| ureterocele |
| chylous ascites |
thoracic duct trauma or ligation |
| intestinal lymphangiectasia |
| total parenteral nutrition extravasation |
| malignancy |
lymphoma, Wilms’ tumor,germ cell tumors, neuroblastoma |
| pseudo-ascites |
celiac disease |
| cystic mesothelioma |
| omental cyst |
| ovarian cyst |
| lymphocele |
2. Etiology of ascites.
Paediatric population is unique in its relationship between the most common causes of conditions and the patient’s age. Regardless of age cirrhosis is by far the most common cause of ascites [
2]. Because of that, we can divide all the conditions that result in ascites into three categories: hepatic cirrhotic, hepatic non-cirrhotic and non-hepatic non-cirrhotic.
3. Cirrhotic ascites
When confronted with the patient with ascites the diagnostic process is usually directed to the liver. And rightfully so as liver cirrhosis is by far the most common cause of ascites in both adult and pediatric populations. In adults, cirrhosis is responsible for 85% of ascites with heart failure or malignancy causing together 5% of cases [
6], [
7].
Ascites is a common complication of cirrhosis, especially in younger children and is usually associated with poor prognosis [
8] as it indicates inefficiency of the body’s compensatory mechanisms. The main causes of cirrhosis in neonates and infants are biliary atresia and genetic metabolic diseases. In older children, cirrhosis is most commonly caused by acquired conditions such as chronic viral hepatitis and autoimmune diseases [
8]. With the increasing prevalence of obesity among children and adolescents, as 18% of this population being overweight or obese in 2018 [
9], we must also consider fatty liver disease as the cause of cirrhosis in the paediatric population.
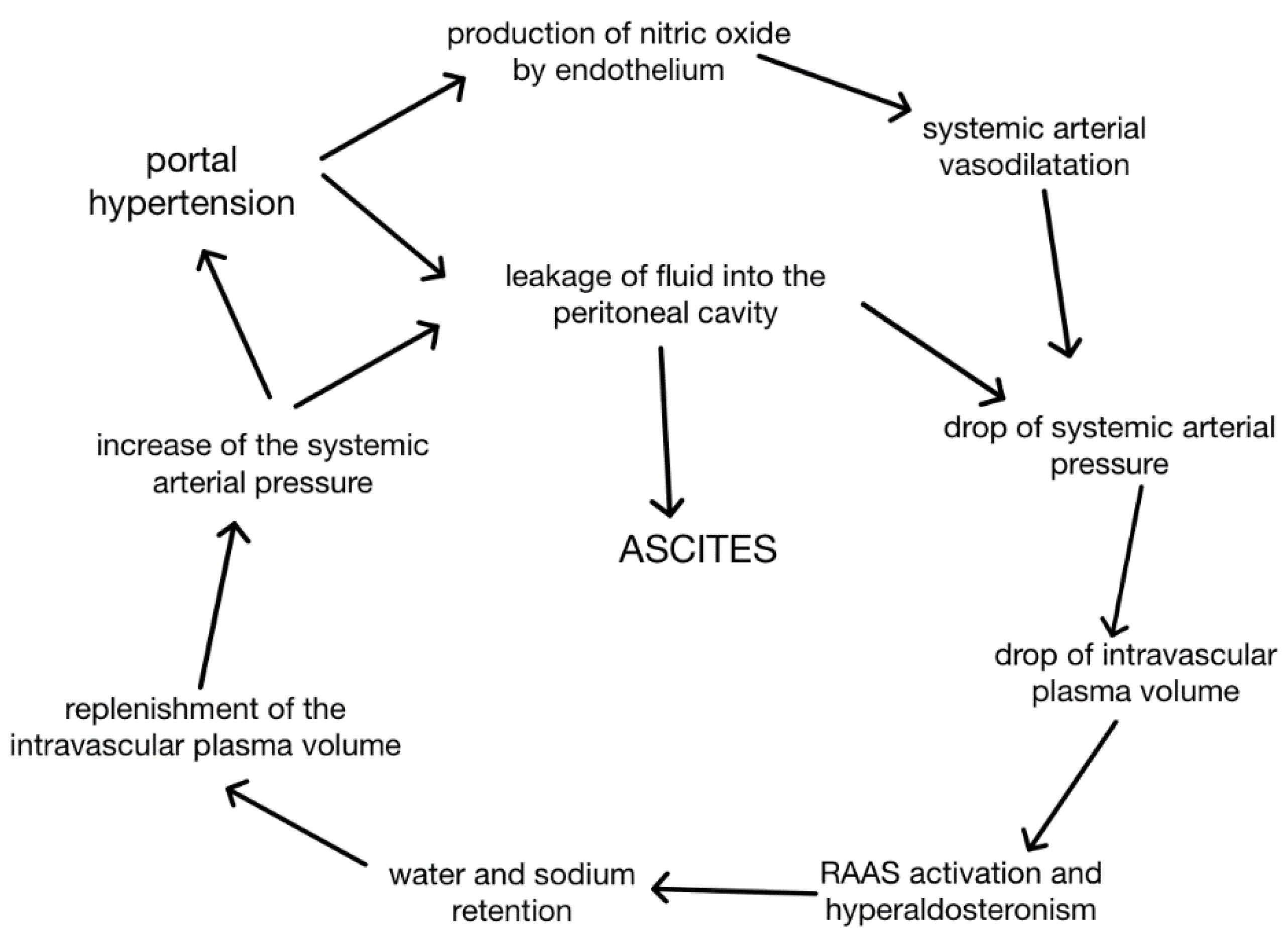
The main mechanisms that contribute to the formation of ascites in cirrhosis are portal hypertension and retention of sodium and water [
3] with subsequent vasodilatation and hyperaldosteronism [
7]. Advancing hepatic fibrosis in cirrhosis causes elevation of intrahepatic resistance and sinusoidal pressure which also correlates with decreased production of NO by the cirrhotic liver, vasoconstriction and further elevation of portal pressure. Nitrous oxide’s ambidextrous role in ascites formation cannot be overlooked as increased portal pressure stimulates its systemic production together with other vasodilatators pushing the body into the state of “effective hypovolaemia” [
10]. It causes splanchnic arterial vasodilatation [
3] and a drop of intravascular plasma volume and systemic arterial pressure. In response, RAAS is activated in order to rebalance blood pressure causing the development of hyperaldosteronism and excessive water and sodium retention. That replenishes intravascular plasma volume and increases systemic blood pressure but also exacerbates portal hypertension as NO production in the liver is still insufficient. That closes the loop of ascites formation that, once started, is difficult to stop. That explains the poor prognosis of cirrhotic patients as ascites formation indicates failure of compensation mechanisms. That is especially significant taking into account that cirrhosis as the disease tends to start with an asymptomatic period [
8] and the diagnosis is often made only after the ascites has already developed.
Figure 1.
Pathomechanism of cirrhotic ascites. [
5]
Figure 1.
Pathomechanism of cirrhotic ascites. [
5]
3.1. Treatment
Before applying any treatment non-hepatic reasons of ascites must be ruled out [
11]. These require a catered approach and will be discussed later in this paper together with these causes themselves.
Understanding the mechanism of creation of cirrhotic ascites the main issues that must be targeted are sodium and water retention [
7]. It is also important to remember that ascites is treated mainly symptomatically and proposed ways of treatment do not target the cirrhosis responsible for this condition.
3.1.1. Sodium restriction
In patients with cirrhosis but without ascites there is no evidence that would support sodium restriction as a way of preventing ascites [
3]. Moreover, even in presence of ascites rigorous sodium restriction is not recommended as this diet is difficult to follow, can result in complications and there is not enough research to support its effectiveness in ascites control [
12]. What is recommended in all cirrhotic patients is a moderately salt-restricted diet with no added salt, avoidance of pre-cooked meals and overall daily consumption of sodium being less than 5-6,5 grams [
3]. Other sources restrict it even further, to 1-2 g of sodium per day or 1-2 mEq sodium/kg/day if well tolerated. In exclusively breastfed infants no dietary restrictions or changes are necessary. In formula-fed infants low sodium formula is recommended [
6].
3.1.2. Diuretic treatment
The golden standard of ascites treatment remains symptomatic in a form of diuretics [
3]. Recommendations favor spironolactone for not only promoting negative sodium balance and water excretion but also countering hyperaldosteronism [
3] [
6] as a competitive inhibitor of aldosterone in a distant renal tubule. In infants, dosage is 0.5-1 mg/kg/day and in older children 1-3 mg/kg/day with a maximum of 100mg/day [
7]. It has a long elimination half-life allowing once-a-day dosing [
3] [
6]. Monotherapy is oftentimes semi-effective so spironolactone is frequently used in combination with a loop-diuretic such as furosemide [
3]. It should be noted that spironolactone being the potassium-saving diuretic may cause severe hyperkalemia and loop-diuretics may cause hyponatremia and metabolic alkalosis [
6], especially in patients with pre-existing renal dysfunction. That is why a gradual approach to diuretic therapy is recommended as it helps to minimize the adverse effects of these medications [
7]. All patients should be monitored for adverse effects as almost half of them require dose reduction or discontinuation of treatment with the most severe complications being hepatic encephalopathy and renal function impairment [
3].
Hyponatremia accompanying ascites is associated with a higher percentage of SBP, hepatic encephalopathy and mortality [
3] [
6]. It can be both hypovolemic and hypervolemic. Hypervolemic hyponatremia is more prevalent in cirrhosis and is a result of vasopressin hypersecretion due to effective hypervolemia. It requires a negative water balance as the actual amount of sodium in the body is accurate and serum sodium levels will normalize after the excess water is filtered out. Literature supports moderate fluid restriction [
8] with consumption of 1-1,5l/day in severe cases but more as a way of stopping the progression of hyponatremia rather than resolving it [
3]. It is sodium restriction that prevents further water retention and combined with diuretic treatment resolves the hypervolemia. Hypovolemic hyponatremia is oftentimes a complication of prolonged diuretic therapy causing loss of extracellular fluid combined with dietary sodium restriction.
Uncomplicated ascites in most cases can be managed with sodium restriction and diuretics [
8]. The most efficient and objective way of tracking the progression of ascites treatment in children is by measuring their body weight [
6].
3.1.3. Supplemental albumin
In discussing cirrhotic ascites we must consider other issues linked to the decompensation of liver disease such as hypoalbuminemia which often leads to or exacerbates already existing ascites [
6]. In cases of serum albumin levels <2.5g/dl, intravenous albumin supplementation is recommended with 25% albumin solution dosed at 1g/kg up to three times a day until 2.5g/dl serum albumin concentration is achieved [
7].
3.1.4. Invasive treatment
Large-volume paracentesis is a standard procedure in management of large volume ascites and is especially effective in diuretic-resistant ascites [
3]. It has been proven as a safe method and has become a standard procedure in adults [
7] but is not well studied in children. It can be beneficial as a symptomatic relief in children with rapidly developing ascites. It can cause respiratory distress and consequences of increased abdominal pressure as the abdominal wall does not have time to gradually accommodate to the large volume of ascitic fluid [
7]. There are also discussions about the need for intravenous albumin supplementation as large-volume paracentesis can cause protein depletion.
Peritoneovenous shunting creates connection for the fluid in the peritoneum to return to the vascular space, usually through a one-way pressure sensitive valve to the jugular vein. Complications are frequent and serious what limits the use of this method in the pediatric population to palliative care [
7]. The risk of complications occurring can be decreased by using ultrasound guidance during the procedure [
3].
TIPS’s (transjugular intrahepatic portosystemic shunting) main benefit and purpose is lowering the portal pressure and therefore stopping the cycle of ascites formation. That makes it a commonly used method of preventing complications of portal hypertension [
6]. The complications, however, are frequent and include worsening of liver function [
7]so in the adult population it is mostly used in patients awaiting liver transplant [
6]]. In the pediatric population TIPS use is limited by high cost, limited availability, high risk of complications and small size of the patient.
3.2. Diagnostic process
Ascites can present itself accompanying numerous different conditions and diseases so their correct identification is crucial in order to provide a patient with adequate and thorough care. A visible abdominal distension in children should always raise suspicion of ascites and the diagnostic process begins with taking detailed patient history together with physical examination. Patients should be questioned about weight gain, peripheral edema, risk factors for liver or heart disease and any symptoms that are concerning to the patient or their guardian [
13]. It is also important to remember that abdominal distension can be caused not only by ascites but also by organomegaly, gas accumulation or pathological masses in the abdomen. Therefore altered abdomen percussion in a physical examination may point a physician toward a particular differential diagnosis [
13].
3.2.1. Abdominal ultrasound
Abdominal ultrasound being non-invasive, low-cost and widely accessible is the first-line diagnostic imaging to detect ascites and assess its quantity [
13]. It is also the only way of detecting mild ascites as it does not cause abdominal distension. Because of that it is oftentimes found accidentally during the abdominal ultrasound performed for a completely different reason [
3]. It is possible to initially determine the reason of abdominal distension as it can differentiate between gas, fluid, organomegaly or pathological masses. Moreover, it provides information about signs of portal hypertension or other possible causes of ascites [
13]. It cannot, however, tell if the fluid is collected in a closed space or free-flowing in the peritoneal cavity [
13]. If there is only a small amount of fluid, the patient is obese or there is a question of whether the fluid collected is, in fact, ascites, abdominal CT is decisive [
3].
3.2.2. Diagnostic paracentesis
Abdominal paracentesis is a golden standard and the most important step in the diagnostic process [
13]. It is recommended that every patient with a new-onset ascites undergo this procedure as it allows collection of the ascitic fluid for testing [
3]. It is also recommended if an infection is suspected or the patient’s state worsens [
5]. Fluid analysis should always include cell count and calculation of SAAG [
14]. Based on probable diagnosis, analysis testing for amylase, glucose, cytology, lactate dehydrogenase, lactates, triglycerides and cholesterol should also be considered [
3]. Also the macroscopic appearance of the fluid is significant as it can differentiate the characteristic types of ascites.
Table 3.
Macroscopic appearance of ascetic fluid and its implication on possible etiology [
5].
Table 3.
Macroscopic appearance of ascetic fluid and its implication on possible etiology [
5].
| appearance of ascitic fluid |
type of ascites |
disorder |
| clear or pale |
portal hypertensive |
cirrhosis, congestive heart failure |
| pale yellow or yellow |
urinary ascites |
bladder rupture, nephrotic syndrome |
| bloody or blood stained |
bleeding to the peritoneal cavity or to the pre-existing ascitic fluid |
Henoch-Schonlein purpura, abdominal trauma, malignancy |
| milky, opaque white |
chylous |
cirrhosis, thoracic duct injury, lymphatic malformations |
| cloudy |
peritonitis |
SBP or infectious ascites |
| black |
pancreatic |
acute pancreatitis, pancreatic injury |
| brown or green-brown |
hiperbilirubinemia or biliary |
gallbladder or biliary perforation, bowel perforation |
3.2.3. Ascitic fluid analysis.
SAAG (serum albumin ascites gradient) is currently the best diagnostic tool for determining whether ascites is portal hypertensive or non-portal hypertensive with 97% accuracy [
5]. SAAG = (serum albumin value) - (ascitic fluid albumin value). SAAG has some limitations as it is dependent on serum albumin levels and can be falsely low if serum albumin is low. It may be also falsely high in chylous ascites because of lipids disturbing laboratory albumin counting [
5]. Literature acknowledges these limitations but still recommends the use of SAAG as a marker of portal hypertension in both British and American guidelines [
14].
Glucose. Glucose concentration in ascitic fluid corresponds with serum levels as glucose freely passes through membranes. Decreased ascitic glucose levels indicate that it is being metabolized either by bacteria or leukocytes. Literature reports decreased glucose in malignancy and spontaneous peritonitis, and significantly decreased in tuberculous peritonitis. As a marker however, ascitic fluid glucose level has low both sensitivity and specificity but can be used as a supportive test especially if tuberculous ascites is suspected [
14].
Amylase. Amylase is not fully but still highly specific marker of pancreatic ascites. Elevated amylase levels can also result from mesenteric thrombosis, ischaemia and small bowel perforation. The vast majority of cases however, are caused by leakage of amylase-rich pancreatic juice, either from pancreatitis, pancreatic cysts or rupture of pancreatic duct with 90% of patients presenting with these conditions having elevated amylase in the ascitic fluid [
14].
Urea/creatynine. High concentration indicates the presence of urine in the peritoneal cavity [
14]. Detection of an elevated creatinine level in the ascitic fluid is diagnostic for uroascites [
6].
Lipids. High concentration of lipids in the ascitic fluid usually goes together with its white milky appearance and is characteristic of chylous ascites. Triglycerides should be measured and chylomicrons should be present. Chylous ascites is diagnosed if triglyceride concentration in ascitic fluid exceeds 2.25 mmol/l and is higher than serum concentration which is possible without the milky appearance of the fluid [
14].
Cholesterol and lactate dehydrogenase. Literature reports that cholesterol levels are higher in malignancy linked ascites and results from increased vascular permeability together with increased production by neoplastic cells. Similar regularity has been observed with lactate dehydrogenase which levels are significantly raised in malignancy, tuberculous peritonitis and pancreatic ascites [
14]. Cholesterol together with LD are two of the most effective markers to differentiate hepatic from malignant ascites [
14].
Bilirubin. It is not recommended to routinely test ascitic fluid bilirubin concentration. However, if biliary leak is suspected it has been observed that ascitic bilirubin levels >6mg/l are consistent with a bile leak diagnosis [
14].
pH. There is no clear consensus to the use of pH readings as a diagnostic tool. Some studies report lowering of pH being consistent with SBP or tuberculous peritonitis. However, it has not been proven as trustworthy enough and some studies advice against the use of pH readings as they might cause confusion in the diagnostic process [
14].
Adenosine deaminase. AD levels above 20-40U/L are characteristic of tuberculous ascites [
5].
Culture. In suspicion of SBP or infectious ascites it is recommended to run ascitic fluid culture with its main target being accurate antibiotic treatment. As soon as the ascitic fluid is retrieved it should be inoculated into blood culture bottles [
14]. Clinical presentation of culture-positive SBP is very similar to those with culture-negative SBP [
3] as bacterial culture has low sensitivity. In case of culture-negative peritonitis fungal infection should be considered. It is a rare complication with prevalence in urinary ascites and is linked to the high mortality rate of 30% [
3].
Cell count. The white blood cell count is indicative of the infection in the ascitic fluid [
3]. Neutrophil count of >=250 cells/mm3 in the absence of bowel perforation is diagnostic of SBP [
5] [
3].
Cytology. Ascitic fluid cytology is positive in two-thirds of patients with malignant processes in the peritoneum, as malignant cells shed into the fluid [
14]. In the adult population emphasis is placed in peritoneal metastasis of ovarian cancer as 75% of patients already have them at the moment of diagnosis [
15]. Although 100% specific, this marker has only 60% sensitivity and therefore is not recommended in the early stages of the diagnostic process [
14].
Tumour/malignancy markers. When malignancy is suspected it is possible to test ascitic fluid for malignancy markers such as CA-125, CA19-9, CEA or AFP. Looking at them together with other markers such as SAAG, cholesterol and LD raises their diagnostic accuracy as some of them eg. CA-125 can be raised in ascites from any cause [
3].
Table 4.
Correlation between SAAG and mechanism of ascites occurrence [
5], [
14].
Table 4.
Correlation between SAAG and mechanism of ascites occurrence [
5], [
14].
| SAAG >=1.1g/dl (high gradient) |
SAAG <1.1g/dl (low gradient) |
| portal hypertensive |
portal non-hypertensive |
| cirrhosis, congestive heart failure, hepatic metastases (compress intrahepatic veins and cause portal hypertension) |
peritoneal malignancy, tuberculosis, nephrotic syndrome, pancreatic ascites, biliary ascites, chylous ascites |
Table 5.
Characteristic traits of ascitic fluid in particular disorders [
3], [
5], [
14].
Table 5.
Characteristic traits of ascitic fluid in particular disorders [
3], [
5], [
14].
| Parameter |
levels |
explanation |
| glucose |
decreased |
SBP, malignancy, tuberculous peritonitis |
| amylase |
elevated over 1000U/L or 5x serum levels |
Pancreatitis, rupture of pancreatic cyst or duct |
| urea/creatinine |
elevated |
Uroascites |
| lipids (triglycerides) |
elevated (over 2.25 mmol/l) |
Chylous ascites |
| cholesterol and lactate dehydrogenase |
elevated |
malignancy |
| bilirubin |
elevated (>6mg/l or higher than serum levels) |
Biliary ascites |
| adenosine deaminase |
elevated (>20-40 U/l) |
Tuberculous ascites |
| neutrophil count |
count of >=250 cells/mm3 |
SBP |
| cytology |
positive |
malignancy |
| culture |
positive |
SBP |
3.2.4. Probability of diagnosis.
In the diagnostic process it must be taken into account that paediatric population is epidemiologicaly separate from adult population. Most prevalent causes of ascites in adults are completely different from those in children and the alcohol consumption being the main reason for adult ascites is omittable when searching for diagnosis in children [
3]. In the diagnostic process we should consider the age of the patient as in different age groups other causes are more probable than the others [
7].
Table 6.
Most common causes of ascites in age groups [
1], [
7].
Table 6.
Most common causes of ascites in age groups [
1], [
7].
| Fetal ascites |
Neonatal ascites |
Ascites in infants and children |
| Meconium peritonitis, intestinal atresia, cystic fibrosis, CMV, genitourinary disorders |
α1-antitripsin deficiency, biliary atresia, bladder perforation, congenital hepatic fibrosis |
Cirrhosis, eosinophilic enteropathy, viral hepatitis, heart failure |
3.3. Complications
It is important to remember that ascites most commonly is a complication of cirrhosis and it reflects its decompensation. In that case further complications of cirrhosis may occur such as gastrointestinal bleeding and hepatic encephalopathy [
8].
Table 7.
Complication and response of ascites to diuretic treatment [
3] [
4].
Table 7.
Complication and response of ascites to diuretic treatment [
3] [
4].
| |
complicated |
| uncomplicated |
infected ascites |
refractory |
| SBP (spontaneous bacterial peritonitis) |
diuretic-resistant ascites |
diuretic-intractable ascites |
| ascites that is not infected and which is responsive to diuretic treatment |
is refractory to dietary sodium restriction and intensive diuretic treatment |
development of diuretic-induced complications disenables the use of effective diuretic dosages |
3.3.1. Spontaneous bacterial peritonitis (SBP).
Spontaneous bacterial peritonitis is a bacterial infection of ascitic fluid and peritoneum without intestinal perforation or other intraabdominal source of infection [
8]. It is the most common infection in cirrhosis concerning 10-30% of patients [
5] and may further worsen liver function and trigger another complications [
16]. Neutrophil count of >=250 cells/mm3 in the ascitic fluid in the absence of bowel perforation is diagnostic of SBP [
5] [
3]. In children SBP is usually caused by a single species such as Klebsiella spp., E. coli, Enterococcus, Streptococcus pneumonia, and recently, methicillin resistant Staphylococcus aureus [
5]. In bacterial peritonitis secondary to bowel perforation infection is usually caused by multiply species of bacteria simultaneously [
5] [
16].
Empiric antibiotic treatment should be started in all patients with elevated neutrophil count in the ascitic fluid. The antibiotic of choice is cefotaxime as it achieves a good concentration in the ascitic fluid and is effective against the most common etiologies of SBP. Ceftriaxone and co-amoxiclav are also effective and can be used [
5].
There have been discussions about implementing SBP prophylaxis but so far no consensus or recommendations have been formed [
5] [
16].
3.3.2. Refractory ascites.
International Ascites Club defines refractory ascites as “ascites that cannot be mobilized or the early recurrence of which cannot be satisfactorily prevented by medical therapy” which includes diuretic-resistant and diuretic-intractable ascites [
4].
All patients undergoing diuretic therapy should be monitored for adverse effects as almost half of them require lowering the dosage or discontinuing the treatment [
3]. Main reasons for withdrawal of diuretic are muscle cramps, renal failure, hyponatremia, hypokalemia and hyperkaliemia [
16].
In treatment of refractory ascites Large Volume Paracentesis (LVP) is a first-line option. Albumin infusion is recommended if more than 5 litres of fluid are removed [
16]. Also there are reports of long term albumin administration reducing morbidity and mortality due to refractory ascites [
17] but no recommendations mention it as a routine procedure.
Refractory ascites correlates with poor prognosis and poor survival and a liver transplant should be discussed in all patients. TIPS shows better survival results than LVP and additionally dependence on TIPS is a way of prioritizing patients awaiting a liver transplant. Therefore it should be a first option in severe cirrhosis [
16].
3.3.2. Malnutrition.
Especially in chronic liver disease nutrition status is an important prognostic factor as those patients need 20-80% more calories in comparison to a healthy children. In cirrhotic patients, accompanying nausea and lack of appetite make it difficult for the child to meet their energetic needs. Ascites complicates the situation further as through water retention and organomegaly it makes body weight an undependable indicator of patient’s nutrition status [
8]. Instead, it is recommended to measure triceps skinfold thickness and arm circumference [
18]. In children with liver disease protein should not be restricted unless hyperammonemia occurs [
19]. Lipids should make up 30-35% of all calories consumed. In children with cholestasis deficiency of fat-soluable vitamins often occurs so their levels should be monitored and, if needed, supplementation should be implemented [
20].
4. Other than cirrhosis causes of ascites.
4.1. Hepatic non-cirrhotic causes.
4.1.1. Bile duct trauma or perforation.
Biliary ascites is very rare and results from leakage of bile to the peritoneal cavity [
21]. It can result from gallbladder or bile duct rupture and can be caused by abdominal trauma or happen as a postoperative complication [
22] [
6]. The ascitic fluid typically is opaque and green-brown in colour. The normal range of bilirubin in the ascitic fluid is 0.7 to 0.8 mg/dL. Bilirubin levels of >6 mg/dL indicate the diagnosis of biliary ascites [
23]. Clinically patients present with mild direct hyperbilirubinemia, abdominal distension and in younger children- feeding difficulties [
6]. Biliary ascites can also be caused by a rare spontaneous perforation of the extrahepatic biliary system [
23] or congenital biliary malformations, which are especially prevalent in neonates [
6]. It results in biliary peritonitis as peritoneum is irritated by biliary enzymes and becomes inflamed. It can present as an acute abdomen and is potentially fatal [
23]. Diagnosis is usually made by ultrasound or MR cholangiopancreatography [
6]. Treatment involves drainage, surgical repair or endoscopic stenting [
21].
4.1.2. Congenital hepatic fibrosis.
Congenital hepatic fibrosis (CHF) is a rare genetic disorder considering portobiliary system with an unknown pathogenesis. It often coexists with autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD) or Caroli’s disease. It leads to portal hypertension, splenomegaly, upper gastrointestinal varices, and ascites. Hepatomegaly is the most important symptom prevalent in almost all patients. Diagnosis is confirmed by a liver biopsy [
24].
4.1.3. Cystic fibrosis.
Cystic fibrosis (CF) is caused by mutation in the CFTR gene [
8] and is one of the most commonly diagnosed genetic disorders with a prevalence of 1:3200 in Caucasians [
25]. With new treatment options of the pulmonary manifestations of CF and newborn screening programs life expectancy of the patients has increased. That has increased the role of cystic fibrosis-associated liver disease (CFLD) in morbidity and mortality due to CF. That early complication usually occurs in the first decade of life and concerns up to 25% patients with CF. It can be asymptomatic or present with hepatomegaly, splenomegaly, abnormal liver markers, jaundice, varices, visceral bleeding and ascites [
26].
4.1.4. Budd-Chiari syndrome.
Budd-Chiari syndrome is a rare condition characterised by an obstruction of the hepatic venous outflow tract. It can be either primary due to thrombosis or phlebitis, or secondary caused by compression of the hepatic veins or inferior vena cava [
27]. It is usually caused by hypercoagulable states or myeloproliferative disorders. It results in hepatic congestion, portal hypertension and subsequent hepatic fibrosis. Treatment includes anticoagulation and, in extreme cases, porto-caval shunting [
6].
4.1.5. Sinusoidal obstruction syndrome (SOS).
Also called hepatic veno-occlusive disease, sinusoidal obstruction syndrome is a complication of cytoreductive therapy prior to hematopoietic stem cell transplant [
6] and affects mainly sinusoidal endothelium [
28]. Clinically it presents with jaundice, weight gain, ascites and hepatomegaly. Diagnosis is made based on liver biopsy which shows occlusion of terminal hepatic venules. Treatment includes paracentesis and careful fluid management together with pharmaceuticals such as thrombolytic agents and methylprednisolone. Patients may eventually need a liver transplant [
6].
4.2. Peritoneal infections.
4.2.1. Cytomegalovirus (CMV).
Cytomegalovirus is a herpesvirus that becomes latent for a lifetime after a primary infection [
29]. It plays a significant role in infections and mortality in immunocompromised patients [
30]. Usually the infection is asymptomatic and does not cause severe symptoms. The literature reports cases of CMV-caused ascites in the mechanism of severe hepatitis and following portal hypertension or peritonitis after gut perforation [
30]. The process of ascites creation in CMV induced hepatitis is linked with portal hypertension and has been described in previous paragraphs in this article.
4.2.2. Ebstein-Barr Virus (EBV).
Ebstein-Barr Virus normally causes fever without other symptoms in early childhood or acute infectious mononucleosis in older children and young adults. In immunocompromised patients however, the infection may be unusually serious [
31]. There are a few cases reported of EBV causing hepatitis complicated by ascites [
31]. It can also cause ascites indirectly by producing cholecystitis or nephrotic syndrome [
32]. That is why it is worth considering running EBV testing in children with ascites especially with previous history of infection matching the EBV- infection characteristics.
4.2.3. Tuberculosis.
Tuberculosis in endemic areas can cause in children and infants both congenital and acquired ascites [
6]. It often presents with abdominal pain and fever accompanying abdominal distension. Paracentesis and ascitic fluid analysis confirms the diagnosis [
6] and tuberculous peritonitis is diagnosed with a peritoneal biopsy [
33].
4.3. Intestinal causes.
4.3.1. Crohn’s Disease.
Crohn’s disease is an inflammatory disease of gastrointestinal tract with an increasing prevalence among both children and adults [
7] [
34]. It is suspected that westernized diet is responsible for the increased occurrence in the pediatric population [
35]. It usually presents with diarrhea, abdominal pain, rectal pain, blood in the stool, weight loss, fever and fatique [
34]. Ascites as a primary complication of Crohn’s Disease is extremely rare but is well recognized as a complication of conditions accompanying CD such as portal hypertension, malignancy or protein losing enteropathy [
36]. There are a few mechanism suspected as to formation of primary ascites in CD such as transmural bowel inflammation and lymphatic stasis [
37]. Regardless of the mechanism, literature reports that the presence of ascites in Crohn's disease is associated with increased disease activity and inflammations requiring more aggressive treatment [
38]. Diagnosis is made by exclusion of other causes [
6].
4.3.2. Intestinal atresia.
Bowel obstruction is the most common cause of isolated fetal ascites which in itself is a rare finding [
39]. Intestinal atresia is one of the most prevalent reasons for bowel obstruction in neonates with small bowel obstruction accounting for majority of cases, whereas colonic atresia is quite rare [
40]. Increased pressure in the obstructed bowel may lead to perforation and ascites in the mechanism of meconium peritonitis. Atresia without perforation may also lead to ascites in transducative mechanism [
1]. Treatment of unperforated intestinal atresia is surgical removal of the obstruction [
40]. Later management is dependent on the outcome of the surgery and might involve nutritional treatment and further surgical or medical interventions [
41].
4.3.3. Meconium ileus (MI).
Meconium ileus is one of the earliest manifestations of cystic fibrosis and affect 20% of infants with CF and 80-90% of children with MI have CF [
42]. Due to obstruction and subsequently increased intraintestinal pressure bowel might perforate and cause ascites in the mechanism of meconium peritonitis [
41]. Moreover, the history of meconium ileus in a patient with cystic fibrosis is a risk factor of developing cystic fibrosis-associated liver disease which may also lead to ascites later in life [
26].
4.3.4.Eosinophilic enteropathy.
Eosinophilic enteropathy is a part of Eosinophilic Gastrointestinal Diseases (EGIDs) and affects mostly children under the age of five. It may present with a variety of symptoms depending on which section of intestine is affected. Most common symptoms include nausea, vomiting, abdominal pain, diarrhea, weight loss and abdominal distention. Ascites develops when disease reaches subserosal layer of the intestine [
43]. Eosinophilic enteropathy should be considered in children presenting with ascites and intestinal symptoms without portal hypertension [
6]. Due to its rare occurrence, there is no clear consensus about the best treatment protocol but corticosteroids, mast cell inhibitors and dietary therapy in form of elimination diets are usually applied [
43].
4.4. Pancreatic causes.
Pancreatic ascites is relatively rare and effects from leakage of peritoneal secretion due to a pancreatic duct injury and treatment usually includes creating a shunt directing the fluid from the peritoneal cavity to the bowel and allowing the tear to heal [
44]. It is more common coexisting with pancreatic cysts and necrosis [
45].
In pediatric population another common cause of pancreatic ascites next to trauma is an infection [
6] and literature reports cases of pancreatitis complicated by ascites [
46]. In that case a systematic inflammatory response may occur [
6]. Paracentesis and elevated lipase and amylase levels in ascitic fluid are pathognomonic to the diagnosis of pancreatic ascites [
3]. Treatment involves addressing the underlying cause whether that is injury or pancreatitis and usually includes bowel rest and total parenteral nutrition [
6]. It is also worth mentioning that pancreatic ascites may be complicated by fluid shifts and peritoneal irritation caused by enzymes contained in the pancreatic juice [
7].
4.5. Systemic inflammatory disorders.
4.5.1. Systemic lupus erythematosus (SLE)
Ascites is not characteristic of systemic lupus erythematosus and extremely uncommon as its standalone presentation. There have been a few cases reported of ascites complicating nephrotic syndrome, congestive heart failure or portal hypertension caused by SLE with fluid features consistent with those mechanisms. It can also occur in the mechanism of sterile peritonitis as serositis happens in 13% of patients with SLE [
47]. The pathomechanism of peritonitis in SLE is unclear but it is probably due to deposition of immune complexes in mesenteric vessels causing inflammation and fluid secretion to the peritoneal cavity. Diagnosis of sterile peritonitis is usually made based on analysis of ascitic fluid showing leukocytosis with no signs of malignancy or infection, especially with already diagnosed SLE [
6]. There are no reports of SLE-associated ascites in pediatric populations but with the tendency of autoimmune diseases to present in progressively younger patients with greater severity, it should be taken into account in differential diagnosis especially in patients with juvenile SLE [
48].
4.5.2. Henoch-Schönlein purpura
Henoch-Schönlein purpura is the most common vasculitis in children which symptoms are caused by IgA accumulation in walls of blood vessels, and mostly affects skin, joints, gastrointestinal system and kidneys [
49] with 50-75% of patients presenting gastrointestinal symptoms [
50]. There have been very few cases reported of chylous ascites in the course of Henoch-Schönlein purpura [
50] [
51], all in adult patients with a long history of the disease with the mechanism of ascites formation remaining unknown. Also hemorrhagic ascites is possible but extremely rare with two cases in adults and one case in a child reported [
52].
4.6. Cardiac ascites.
Heart failure as a reason for ascites is worth mentioning as cardiac disorders are among the three most common causes of ascites in pediatric population [
5]. World Health Organisation (WHO) in its 2009 report recognises congenital malformations, cardiomyopathy and anthracycline toxicity as main reasons for heart failure in children and adolescents also noting the prevalence of malaria and malnutrition as causes of serious anemia contributing to pediatric heart failure in lower income countries [
53]. Some studies also show a large role of rheumatic fever and its complication in the form of rheumatic heart disease in the ethology of HF in children [
54]. Clinical presentation of cardiac ascites is usually consistent with elevated SAAG and high ascites protein levels [
55]. It forms in the mechanism of congestive heart failure, increased blood flow through the liver and passive liver congestion causing increased portal pressure [
7] together with increased permeability of hepatic sinusoids [
3] allowing for leakage of protein rich serum into the peritoneal cavity. It also promotes hepatic lymph formation and causes the ascitic fluid to mimic that of chylous ascites [
7]. Literature reports cases of low-SAAG cardiac ascites [
55] which can be explained by secondary activation of RAAS and water retention due to congestive heart failure decreasing effective arterial blood pressure [
7]. Low gradient ascites is especially prevalent in patients with low serum albumin levels and long history of heart failure [
55] or with accompanying cirrhosis causing fibrosis of hepatic sinusoids and blocking protein from entering the ascitic fluid [
3]. Literature seems to favor aggressive diuretic treatment or therapeutic paracentesis as forms of symptomatic treatment of cardiac ascites [
55].
4.7. Genitourinary causes of ascites.
Urinary ascites or uroascites is observed when leakage of urine causes its collection in the peritoneal cavity. Detection of an elevated creatinine level in the ascitic fluid is diagnostic [
6]. It is responsible for almost 30 % of all cases of neonatal ascites with posterior urethral valves (PUV) being the most prevalent cause [
1]. It may be also caused by the perforation of the bladder with over 75 % of cases being the complication of a difficult umbilical arterial catheterisation [
56]. Cases of spontaneous bladder rupture have also been reported but are quite rare [
57].
4.7.1. Obstructive uropathy.
Obstructive uropathy presents itself primarily in bimodal distribution in infants and the elderly. It also affects males significantly more often than females. In the pediatric population the most frequent causes are posterior urethral valves although it makes up only 4% of all cases. The treatment of obstructive uropathy is based around quickly addressing the obstructive process and relieving the pressure in the bladder [
58]. Literature also reports cases of ureterocele causing ascites in the alike obstructive mechanism [
59].
4.7.2. Posterior urethral valves.
Posterior urethral valves have been classified in 1919 into three types with type I being responsible for 95% of cases [
60]. Type I is described as sail-like folds along the urethra that obstruct the flow of the urine. Thanks to the improvement of ultrasonography 30% of the cases are diagnosed in the neonatal period [
61] and 50-70 % within the first year of life [
60]. The finding of dilated posterior urethra, dilated bladder, dilated ureters and hydronephrosis in a male infant indicate high probability of posterior urethral valves [
61].
The main objection in treatment of posterior urethral valves should be preserving bladder and renal function. It should be achieved by resolving the obstruction and relieving the pressure in the urinary tract [
60]. Usually it takes the form of the simplest solution which is the catheter drainage of the bladder with monitoring of serum electrolytes levels and renal function. It is advised to administer oral antibiotics as prevention of catheter-related urinary tract infections. Endoscopic ablation of the valves is usually performed in children weighing over 2000g after the improvement of their state thanks to the drainage of the bladder. Vesicostomy is sometimes performed in children too small for ablation as the transitional step. Review of the literature does not support any option above the others in the uncomplicated cases however vesicostomy may be especially beneficial in cases of severe reflux as it completely relieves the pressure in the bladder [
60].
4.7.3. Nephrotic syndrome.
Ascites is also frequently observed in children with nephrotic syndrome [
62]. In this setting decreased arterial blood volume leads to RAAS activation which causes sodium and water retention. Moreover the hypoalbuminemia causes low oncotic gradient in hepatic capillaries which together with hypervolemia has a key role in secretion of fluid into the peritoneal cavity [
6]. Because of that mechanism, the constitution of ascitic fluid is more similar to the fluid in cirrhotic ascites than to that of uroascites. There have also been a few reports of nephrotic syndrome causing chylous ascites, usually by obstruction to lymphatics [
62].
4.8. Chylous ascites.
Chylous ascites is a rare occurrence in the pediatric population and can develop in three different ways: lymphatic trauma, malformation or obstruction [
6]. It is described as a leakage of lymph into the peritoneal cavity and usually presents itself as painless abdominal distension [
50]. Diagnosis is made based on clinical presentation and analysis of ascitic fluid [
33]. Ascitic fluid from chylous ascites is very characteristic with its ‘milky’ appearance, high fat concentration, an alkaline pH, high SAAG and a negative culture [
1]. It usually presents with distention of the abdomen that progresses through weeks and months without pain [
33]. The quickly accumulating chylous ascites is a firm indication of its traumatic origin.
4.8.1. Trauma, obstruction or malformation.
Primary chylous ascites is most frequently caused by congenital lymphatic dysplasia or malformations [
63] eg. in Noonan syndrome [
64], but is also the rarest etiology of chylous ascites. Secondary chylous ascites can happen through disruption of the lymphatic channels by injury or obstruction. Traumatic chylous ascites is the most prevalent both in adult and pediatric populations with reportedly 11% of abdominal surgeries in adults being complicated by chylous ascites. In children, it is also a frequent complication after surgical resection of abdominal malignancies [
63]. These are caused by direct injury of lymphatic vessels during the surgical procedures. The indirect injury to the abdomen can also result in disruption of lymphatic channels. The estimation is that 10% of all chylous ascites is caused by child abuse. Trauma causes disruption of the lymphatic vessels and leakage of chyle to the peritoneal cavity [
1]. It is also suspected that traumatic chylous ascites can also be caused in the mechanism of portal hypertension. It is probable that increase in hepatic venous pressure provokes a significant increase in hepatic lymph production. That raises the pressure in the lymphatic channels which endothelium is not adapted to, and may therefore rupture and cause leakage of chyle to the peritoneum [
33]. In compliance with that theory every condition existing with increased hepatic pressure such as right-sided heart failure can cause chylous ascites [
63]. Obstruction to the lymphatic channels usually happens due to increased peritoneal lymphatic pressure. That can happen in fibrosis of the lymph node, either infectious or linked to malignancy. Prolonged increased pressure stimulates collagen accumulation in the walls of the lymphatics increasing the pressure even further [
33].
Treatment is rather conservative especially in cases of traumatic chylous ascites with a known place of rupture [
63]. If obstruction is suspected, abdomen CT should be performed together with cytology of ascitic fluid which in case of positive result has a 97% correlation with presence of peritoneal carcinomas [
33].
4.8.2. Primary intestinal lymphangiectasia (PIL)
There are also cases reported of chylous ascites as a symptom of primary intestinal lymphangiectasia (PIL). Dilated intestinal lacteals cause leakage of lymph into a small bowell. It is usually diagnosed before 3 years of age and usually presents as lower lymph edema but can also lead to anasarca, pericarditis and chylous ascites [
65].
4.8.3. Extravasation of parenteral nutrition.
It is also possible to develop ascites as an adverse reaction to extravasation of parenteral nutrition even though it is rare. In that case ascites should resolve following the removal of the catheter [
66].
4.9. Malignancy.
In the paediatric population malignancy accounts for less than 5% of cases of ascites [
67]. Tumour that is known for its tendency to produce ascites is ovarian cancer [
68]. Presence of ascites in ovarian cancer is connected with a poor prognosis. Ovarian cancer is rare in children, however, with 1,3% being diagnosed in patients below the age of 20 [
69]. There are cases reported of lymphomas causing chylous ascites both in adults [
70]] and in children [
71]. What is worth mentioning is that malignancy is responsible for 17% of asymptomatic chylous ascites [
50]. There are also reports of chylous ascites as a complication Wilms’ tumour or its surgical management [
72] [
73]. There is also a report of Wilms’ tumour causing ascites indirectly by causing sinusoidal obstruction syndrome [
74]]. Other tumours that tend to cause ascites are neuroblastomas and germ cell tumours and they do so in the mechanism of peritoneal metastases [
75].
Abdominal paracentesis is crucial in the diagnostic process of malignancy linked ascites with high levels of cholesterol, lactate dehydrogenase and positive cytology pointing towards malignancy [
14].
5. Pseudo-ascites
As the ascites is described as pathological accumulation of fluid in the peritoneal cavity, any other intraabdominal collection of fluid can be mistaken for ascites [
6].
Clinical presentation in physical examination is oftentimes equal to that of ascites with abdominal distension and altered abdominal percussion. In cases of visible fluid in the abdominal ultrasonography and doubts whether the fluid is in the peritoneal cavity or some other space, abdominal computed tomography is decisive [
13].
5.1. Celiac disease.
Celiac disease may present in a number of different ways and give a variety of symptoms especially if diagnosed late. Children traditionally present with poor appetite, diarrhoea, muscle weakness, abdominal distension and an overall failure to thrive. Inflammation in the intestines may lead to secretion of fluid and small-bowel distension which may give the clinical image that resembles ascites [
76] [
7]. There are also reports of adult or elderly patients with ascites due to severe and previously undiagnosed celiac disease [
77] [
78] [
79].
In children it is possible to diagnose celiac disease without biopsy if strict conditions are met. Therefore, children with clear gastrointestinal symptoms or other symptoms suggestive of celiac disease should be tested for transglutaminase antibodies [
76].
5.2. Intraabdominal cysts.
5.2.1. Omental cysts.
Omental cysts are very rare cystic tumors that originate from the omentum and are mostly benign [
80]. They occur predominantly in children with 75% diagnosed under the age of five [
81], more frequently in girls [
82]. Usually diagnosed through abdominal ultrasonography and CT most cysts present with abdominal mass pain and distension [
80]. A they carry a risk of rupture complicated by acute abdomen, the treatment is complete resection [
81].
5.2.2. Ovarian cysts.
Ovarian cysts are estimated to develop in 5-15% of women in reproductive age [
83]. They can also be congenital diagnosed prenatally, immediately after birth or later in life [
84]. Patients may be asymptomatic or present symptoms due to cyst’s size or rupture and are usually diagnosed through pelvic ultrasonography [
85]. Literature reports a few cases of giant ovarian cysts being misdiagnosed as ascites [
86]. Treatment is usually laparoscopic cystectomy [
83].
5.2.3. Lymphocele.
Lymphocele is usually a postoperative complication when a leakage of lymph collects in a cystic formation. It is usually asymptomatic and self-limiting and therefore does not require surgical treatment [
87]. Spontaneous lymphocele is rare and the treatment depends on size and symptoms with symptomatic lymphocele requiring fine-needle aspiration, catheter draining or surgical approach. With no history of previous injury lymphocele poses a diagnostic challenge and, if large and distending the abdomen, may be misdiagnosed as ascites [
88].
5.2.4. Cystic mesothelioma.
Cystic mesothelioma is a rare benign neoplasm that concerns mainly women of reproductive age and often correlate with endometriosis, ovarian cysts or pelvic inflammatory disease [
89]. There have been 9 cases reported in children [
90] with the most common reasons for admission to hospital being lower abdominal and pelvic pain. There have also been reports of cystic mesothelioma in different localizations than abdomen such as pericardium and chest. In children en block removal through laparoscopy or laparotomy is preferred and frequently used [
91].
6. Summary.
Ascites in children may pose a diagnostic challenge and requires a calm and systematic approach from the physician. Understanding the mechanisms leading to its development and conditions linked to it, will provide the patient with adequate treatment and care.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pediatric Gastrointestinal and liver Disease Elsevier Saunders, fourth edition, 2011, ISBN:9781437707748.
- Hou W Sanyal AJ. Ascites: diagnosis and management. Med Clin North Am. 2009 Jul i 93(4):801-17 vii. [CrossRef] [PubMed]
- Moore KP Aithal GP Guidelines on the management of ascites in cirrhosis Gut 2006 i 55:vi1-vi12.
- Arroyo V Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996 Jan;23(1):164-76. [CrossRef] [PubMed]
- Bavdekar A Thakur N. Ascites in Children. Indian J Pediatr. 2016 Nov i 27278239. 83(11):1334-1340. [CrossRef] [PubMed]
- Lane ER Hsu EK, Murray KF. Management of ascites in children. Expert Rev Gastroenterol Hepatol. 2015 i 26325252. 9(10):1281-92. [CrossRef] [PubMed]
- Giefer MJ Murray KF, Colletti RB. Pathophysiology, diagnosis, and management of pediatric ascites. J Pediatr Gastroenterol Nutr. 2011 May i 21464748. 52(5):503-13. [CrossRef] [PubMed]
- Pinto RB Schneider AC, da Silveira TR. Cirrhosis in children and adolescents: An overview. World J Hepatol. 2015 Mar 27, 25848466 7(3):392-405. [CrossRef]
- https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 30 Arch Med Sci. 2013 Aug i 10.5114/aoms.2013.36904 9(4): 601–607. Published online 2013 Aug 8. [CrossRef]
- Moore CM Van Thiel DH. Cirrhotic ascites review: Pathophysiology, diagnosis and management. World J
Hepatol. 2013 May 27, 23717736 5(5):251-63. [CrossRef]
- Haberl J Zollner G, Fickert P, Stadlbauer V. To salt or not to salt?-That is the question in cirrhosis. Liver Int.
2018 Jul i 29608812. 38(7):1148-1159. [CrossRef] [PubMed]
- Oey RC van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016 Oct i 27762220. 74(8):330-335. [PubMed]
- Tarn AC Lapworth R. Biochemical analysis of ascitic (peritoneal) fluid: what should we measure? Ann Clin
Biochem. 2010 Sep i 20595402. 47(Pt 5):397-407. [CrossRef] [PubMed]
- Anwar A Kasi A. Peritoneal Cancer. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL):
StatPearls Publishing i https://www.ncbi.nlm.nih.gov/books/NBK562138/ 2023 Jan-. Available from:.
- Rudler M Mallet M, Sultanik P, Bouzbib C, Thabut D. Optimal management of ascites. Liver Int. 2020 Feb,
May 40 Suppl 1:128-135. Erratum in: Liver Int. 2020 i 32077614. 40(5):1247. [CrossRef] [PubMed]
- Di Pascoli M Fasolato S, Piano S, Bolognesi M, Angeli P. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019 i 39(1):98-105. [CrossRef] [PubMed]
- Schneider AC Pinto RB, Silveira TR. Determinação de risco nutricional e desnutrição por antropometria em crianças e adolescentes com cirrose [Nutritional risk and malnutrition determination by anthropometry in cirrhotic children and adolescents]. Arq Gastroenterol. 2007 Oct-Dec;44(4):345-9. Portuguese. [CrossRef] [PubMed]
- Squires RH Ng V, Romero R, Ekong U, Hardikar W, Emre S, Mazariegos GV. Evaluation of the pediatric
patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver
Diseases,. American Society of Transplantation and the North American Society for Pediatric
Gastroenterology, Hepatology and Nutrition. Hepatology 2014; 60: 362-398. [CrossRef]
- Sathe MN Patel AS. Update in pediatrics: focus on fat-soluble vitamins. Nutr Clin Pract. 2010 Aug i
20702838. 25(4):340-6. [CrossRef]
- Blank J., Anderson, E. & Mansoor, A.M. Biliary Ascites. J GEN INTERN MED 37, 3190 (2022). [CrossRef]
- Aug Feliciano DV. Biliary injuries as a result of blunt and penetrating trauma. Surg Clin North Am. 1994,
74(4):897-907 i 8047948. discussion 909-12. [PubMed]
- Huda F Naithani M, Singh SK, Saha S. Ascitic Fluid/Serum Bilirubin Ratio as an aid in Preoperative Diagnosis of Choleperitoneum in a Neglected Case of Spontaneous Common Bile Duct Perforation. Euroasian J Hepato-Gastroenterol 2017 i 7(2):185-187.
- Hasbaoui BE Rifai Z, Saghir S, Ayad A, Lamalmi N, Abilkassem R, Agadr A. Congenital hepatic fibrosis: case report and review of literature. Pan Afr Med J. 2021 Feb 18 i 33995794 38:188. [CrossRef]
- Dickinson KM Collaco JM. Cystic Fibrosis. Pediatr Rev. 2021 Feb, 33526571 42(2):55-67. [CrossRef]
- Palaniappan SK Than NN, Thein AW, Moe S, van Mourik I. Interventions for preventing and managing advanced liver disease in cystic fibrosis. Cochrane Database Syst Rev. 2017 Aug 29 i Da 8(8):CD012056. Update in: Cochrane Database Syst Rev. 2020 Mar 30;3:CD012056. [CrossRef]
- Hitawala AA Gupta V. Budd Chiari Syndrome. 2023 Jan 30. In: StatPearls [Internet]. Treasure Island (FL):
StatPearls Publishing i 32644367 2023 Jan–.
- Valla DC Cazals-Hatem D. Sinusoidal obstruction syndrome. Clin Res Hepatol Gastroenterol. 2016 Sep i
27038846 40(4):378-85. [CrossRef]
- Lu MLR Agarwal A, Sloan J, Kosmin A. Infected ascites: Distinguishing secondary peritonitis from spontaneous bacterial peritonitis in a cirrhotic patient with classic symptoms. IDCases. 2017 Feb 28;8:29-31. [CrossRef]
- Qian JY Bai XY, Feng YL, Zhu WJ, Yao F, Li JN, Yang AM, Li F, Qian JM. Cholestasis, ascites and pancytopenia in an immunocompetent adult with severe cytomegalovirus hepatitis. World J Gastroenterol. 2015 Nov 21;21(43):12505-9. [CrossRef]
- Rajwal S Davison S, Wyatt J, McClean P. Primary Epstein-Barr virus hepatitis complicated by ascites with epstein-barr virus reactivation during primary cytomegalovirus infection. J Pediatr Gastroenterol Nutr. 2003 Jul;37(1):87-90. [CrossRef] [PubMed]
- Rodà D Huici M, Ricart S, Vila J, Fortuny C, Alsina L. Cholecystitis and nephrotic syndrome complicating Epstein-Barr virus primary infection. Paediatr Int Child Health. 2017 Feb;37(1):74-77. [CrossRef] [PubMed]
- Bhardwaj R Vaziri H, Gautam A, Ballesteros E, Karimeddini D, Wu GY. Chylous Ascites: A Review of Pathogenesis, Diagnosis and Treatment. J Clin Transl Hepatol. 2018 Mar 28 i 29577037 6(1):105-113. [CrossRef]
- Veauthier B Hornecker JR. Crohn's Disease: Diagnosis and Management. Am Fam Physician. 2018 Dec 1 i
PMID:30485038 98(11):661-669.
- Verburgt CM Ghiboub M, Benninga MA, de Jonge WJ, Van Limbergen JE. Nutritional Therapy Strategies in Pediatric Crohn's Disease. Nutrients. 2021 Jan 13, PMID: 13(1):212. [CrossRef]
- Kia R White D, Sarkar S. An unusual presentation of fistulating Crohn's disease: Ascites. World J Gastrointest Endosc. 2010 Jan 16, PMID: 2(1):41-3. [CrossRef]
- Paspatis G. A., Kissamitaki, V., Kyriakakis, E., Aretoulaki, D., Giannikaki, E. S., Kokkinaki, M., … Xroniaris,
N. (1999). Ascites associated with the initial presentation of Crohn’s disease. The American Journal of
Gastroenterology. 94(7), 1974–1976. [CrossRef]
- Lee JU Kim YH, Lee SY, Kim KH, Chung CH, Kim KH, Son HJ, Rhee PL, Kim JJ, Rhee JC. Dose ascites mean more severe Crohn's disease? Korean J Gastroenterol. 2004 May i 15156117. 43(5):304-9. PMID:.
- Agrawala G Predanic M, Perni SC, Chasen ST. Isolated fetal ascites caused by bowel perforation due to colonic atresia. J Matern Fetal Neonatal Med. 2005 Apr i 16147839. 17(4):291-4. [CrossRef]
- Aggerwal N Sugandhi N, Kour H, Chakraborty G, Acharya SK, Jadhav A, Bagga D. Total Intestinal Atresia: Revisiting the Pathogenesis of Congenital Atresias. J Indian Assoc Pediatr Surg. 2019. Oct-Dec;24(4):303-306. [CrossRef]
- Osmulikevici O Renji E, Jaffray B, Embleton N. Isolated ascites in a newborn with 'apple peel' jejunal atresia. BMJ Case Rep. 2017 Oct 3, PMID: 2017:bcr2017219781. [CrossRef]
- Parikh NS Ibrahim S, Ahlawat R. Meconium Ileus. [Updated 2023 Mar 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing i Jan-. 2023.
- Koutri E., & Papadopoulou, A. (2018). Eosinophilic Gastrointestinal Diseases in Childhood. Annals of
Nutrition and Metabolism, 73(Suppl. 4), 18–28. [CrossRef] [PubMed]
- Gapp J Hoilat GJ, Chandra S. Pancreatic Ascites. 2022 Aug 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing i PMID:29939628 2023 Jan–.
- Subhani M Sheth A, Palaniyappan N, Sugathan P, Wilkes EA, Aithal GP. Diagnostic accuracy of serum ascites albumin gradient (SAAG) in a contemporary unselected medical cohort. J Int Med Res. 2022 Nov i 10.1177/03000605221140310 50(11):3000605221140310. [CrossRef]
- Bush N., Rana, S.S. Ascites in Acute Pancreatitis: Clinical Implications and Management. Dig Dis Sci 67,
1987–1993 (2022). [CrossRef]
- Aljebreen AM Al-Mogairen SA. Refractory ascites as the first presentation of systemic lupus
erythematosus. Arab J Gastroenterol. 2014 Sep-Dec i PMID:25596976 15(3-4):161-2. [CrossRef]
- Charras A Smith E, Hedrich CM. Systemic Lupus Erythematosus in Children and Young People. Curr Rheumatol Rep. 2021 Feb 10, PMID: 23(3):20. [CrossRef]
- Hetland LE Susrud KS, Lindahl KH, Bygum A. Henoch-Schönlein Purpura: A Literature Review. Acta Derm Venereol. 2017 Nov 15 i PMID:28654132 97(10):1160-1166. [CrossRef]
- Galam P Mundakal JK. Chylous ascites in a case of henoch-schonlein purpura - A case report and literature
review. Ann Afr Med. 2022 Oct-Dec, PMID: 21(4):305-308. [CrossRef]
- Lee TH Lee EY, Cho YS, Yoo B, Moon HB, Lee CK. Concurrent occurrence of chylothorax and chylous ascites in a patient with Henoch-Schönlein purpura. Scand J Rheumatol. 2003 i PMID:15060272 32(6):378-9. [CrossRef]
- Venuta A Bertolani P, Garetti E, Venturelli C, Predieri B, Muttini ED, Compagni E. Hemorrhagic ascites in a child with Henoch-Schönlein purpura. J Pediatr Gastroenterol Nutr. 1999 Sep i PMID:10468007 29(3):358-9. [CrossRef]
- medicine WHO (2008) Cardiac failure in children. 17th expert committee on the selection and use of essential medicines. WHO, Geneva.
- Shaddy RE George AT, Jaecklin T, Lochlainn EN, Thakur L, Agrawal R, Solar-Yohay S, Chen F, Rossano JW, Severin T, Burch M. Systematic Literature Review on the Incidence and Prevalence of Heart Failure in Children and Adolescents. Pediatr Cardiol. 2018 Mar;39(3):415-436. [CrossRef]
- Trongtorsak A Kittipibul V, Antala D, Meng Q, Puwanant S. Heart Failure-Related Ascites With Low Serum-Ascites Albumin Gradient: Diagnostic Clues From Triphasic Abdominal Computed Tomography. Cureus. 2022 Jan 14 i P 14(1):e21251. [CrossRef]
- Oei J Garvey PA, Rosenberg AR. The diagnosis and management of neonatal urinary ascites. J Paediatr Child Health. 2001 Oct i PMID:11885721 37(5):513-5. [CrossRef]
- DeFoor W Tackett L, Minevich E, Wacksman J, Sheldon C. Risk factors for spontaneous bladder perforation after augmentation cystoplasty. Urology. 2003 Oct i PMID:14550454 62(4):737-41. [CrossRef]
- Rishor-Olney CR Hinson MR. Obstructive Uropathy. 2022 Aug 22. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing i PMID:32644347 2022 Jan–.
- Kozlova D Gilboa Y, Sade-Zalts C, Gielchinsky Y, Shteingart S, Kidron D. Fetal Urinoma Due to Circulatory Disorders in an Umbilical Artery: Case Report. Pediatr Dev Pathol. 2021 Jul-Aug i PMI 24(4):383-387. [CrossRef] [PubMed]
- Hodges SJ Patel B, McLorie G, Atala A. Posterior urethral valves. ScientificWorldJournal. 2009 Oct 14,
19838598 9:1119-26. [CrossRef]
- Nov-Dec Basak D. Evolution of Management of Posterior Urethral Valve. J Indian Assoc Pediatr Surg. 2021
i inni.
- Ghosh S Vasudev AS, Srivastava RN. Chylous Ascites in Nephrotic Syndrome. Indian Pediatr. 2020 Nov 15 i PMID:33231184 57(11):1081.
- Moussa AM Maybody M, Santos E, Gonzalez-Aguirre AJ. Intranodal Lymphangiography and Lymphatic Embolization for Management of Iatrogenic Chylous Ascites in Children. Lymphat Res Biol. 2021 Dec i 19(6):531-538. [CrossRef]
- Linglart L Gelb BD. Congenital heart defects in Noonan syndrome: Diagnosis, management, and treatment.
Am J Med Genet C Semin Med Genet. 2020 Mar, PMID: 184(1):73-80. [CrossRef]
- Vignes S Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis. 2008
Feb 22, PMID: 3:5. [CrossRef]
- Kotinatot S Shankar S, Ba'Ath ME, Almaazmi MM. Unexplained abdominal distention in a neonate: culprit femoral central venous line extravasation. BMJ Case Rep. 2019 Dec 3 i 12(12):e232537. [CrossRef]
- Runyon BA. Ascites and bacterial peritonitis. In: Sleisenger MH Fordtran JS, editors. Gastrointestinal Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia: Saunders Elsevier i 2010.
- Ford CE Werner B, Hacker NF, Warton K. The untapped potential of ascites in ovarian cancer research and
treatment. Br J Cancer. 2020 Jul, 32382112 123(1):9-16. [CrossRef]
- Baert T Storme N, Van Nieuwenhuysen E, Uyttebroeck A, Van Damme N, Vergote I, Coosemans A. Ovarian cancer in children and adolescents: A rare disease that needs more attention. Maturitas. 2016 Jun i M 88:3-8. [CrossRef]
- Oosterbosch L Leloup A, Verstraeten P, Jordens P. Chylothorax and chylous ascites due to malignant lymphoma. Acta Clin Belg. 1995 i PMID:7725834 50(1):20-4. [CrossRef]
- Zhang YT Zhong XD, Gao YL, Chang J. Evaluation of IL-2 and Dexamethasone intracavitary injection on the management of malignant effusion in children with solid tumors or lymphoma. BMC Cancer. 2021 Dec 6 i 21(1):1302. [CrossRef]
- Jayabose S Kogan S, Berezin S, Slim M, San Filippo JA, Godine L, Beneck D, Tugal O, Sunkara S. Combined occurrence of chyloperitoneum and chylothorax after surgery and chemotherapy for Wilms' tumor. Cancer. 1989 Nov 1 i 64(9):1790-5. [CrossRef] [PubMed]
- Weiser AC Lindgren BW, Ritchey ML, Franco I. Chylous ascites following surgical treatment for wilms tumor. J Urol. 2003 Oct, 2):1667-9 170(4 Pt i PMID:14501687 discussion 1669. [CrossRef]
- Totadri S Trehan A, Bansal D, Jain R. Sinusoidal Obstruction Syndrome during Treatment for Wilms' Tumor: A Life-threatening Complication. Indian J Med Paediatr Oncol. 2017 Oct-Dec i 38(4):447-451. [CrossRef]
- Chung CJ Bui V, Fordham LA, Hill J, Bulas D. Malignant intraperitoneal neoplasms of childhood. Pediatr Radiol. 1998 May i PMID:9569270 28(5):317-21. [CrossRef]
- Al-Toma A Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019. Jun;7(5):583-613. [CrossRef]
- Meena DS Kumar D, Bohra GK, Choudhary S. Hypoalbuminemia and generalized edema as an atypical presentation of celiac disease. J Family Med Prim Care. 2020 Feb 28 i 9(2):1206-1208. [CrossRef]
- Kravchuk S Mishchanchuk V, Kozyk M, Strubchevska K. A Clinical Case of a Celiac Crisis in an Adult With Type 1 Diabetes and Neurological Symptoms. Cureus. 2023 Mar 2 i 15(3):e35696. [CrossRef]
- Jameshorani M Pourshams A, Sadeghi A, Saffar H, Malekzadeh R. Celiac Crisis in a Young Woman: Raising Awareness of a Life-Threatening Condition. Middle East J Dig Dis. 2019 Oct i 11(4):230-233. [CrossRef]
- Nam SH Kim DY, Kim SC, Kim IK. The surgical experience for retroperitoneal, mesenteric and omental cyst in children. J Korean Surg Soc. 2012 Aug i 83(2):102-6. [CrossRef]
- Robbins KJ Antiel RM, Shakhsheer BA. Omental cyst: a case report and review of the literature. Ann Pediatr Surg. 2021 i 17(1):62. [CrossRef]
- Gupta RK Sah S, Sah PL, Shah BP. Congenital omental cyst. BMJ Case Rep. 2012 Aug 2 i 2012:bcr2012006643. [CrossRef]
- Mansouri G Safinataj M, Shahesmaeili A, Allahqoli L, Salehiniya H, Alkatout I. Effect of laparoscopic cystectomy on ovarian reserve in patients with ovarian cyst. Front Endocrinol (Lausanne). 2022 Aug 30;13:964229. [CrossRef]
- Bucuri C Mihu D, Malutan A, Oprea V, Berceanu C, Nati I, Rada M, Ormindean C, Blaga L, Ciortea R. Fetal
Ovarian Cyst-A Scoping Review of the Data from the Last 10 Years. Medicina (Kaunas). 2023 Jan 17 i doi:
59(2):186. [CrossRef]
- Idris S Daud S, Ahmad Sani N, Tee Mei Li S. A Case of Twisted Ovarian Cyst in a Young Patient and Review of the Literature. Am J Case Rep. 2021 Nov 17 i 22:e933438. [CrossRef]
- Olasinde A Ogunlaja O, Olasinde YT, Mobolaji-Ojibara MU, Adelaja-Ojulari N. Giant Ovarian Cyst in a Term Pregnancy Simulating a Massive Ascites: A Case Report. Cureus. 2022 Dec 31 i 36726894 14(12):e33199. [CrossRef]
- Lv S Wang Q, Zhao W, Han L, Wang Q, Batchu N, Ulain Q, Zou J, Sun C, Du J, Song Q, Li Q. A review of the postoperative lymphatic leakage. Oncotarget. 2017 Apr 20 i 8(40):69062-69075. [CrossRef]
- Góes Junior AM Jeha SA. Idiopathic lymphocele: a possible diagnosis for infraclavicular masses. Case Rep Surg. 2012 i 2012:593028. [CrossRef]
- O'Connor DB Beddy D, Aremu MA. Benign cystic mesothelioma of the appendix presenting in a woman: a case report. J Med Case Rep. 2010 Dec 3, 21129176 4:394. [CrossRef]
- Tuncer AA Narcı A, Dilek FH, Embleton DB, Çetinkurşun S. Benign Cystic Mesothelioma in a Child: Case Report and Review of the Literature. Balkan Med J. 2016 Mar i 33(2):232-4. [CrossRef]
- Tuncer AA Narcı A, Dilek FH, Embleton DB, Çetinkurşun S. Benign Cystic Mesothelioma in a Child: Case Report and Review of the Literature. Balkan Med J. 2016 Mar i 27403396 33(2):232-4. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).