Submitted:
28 June 2023
Posted:
03 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Introduction
2.2. Preprocessing
2.3. Estimate of correlation
2.3.1. Phase lag index
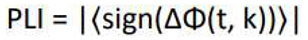
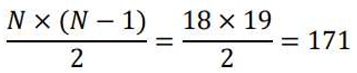
2.3.2. Statistical analysis of data
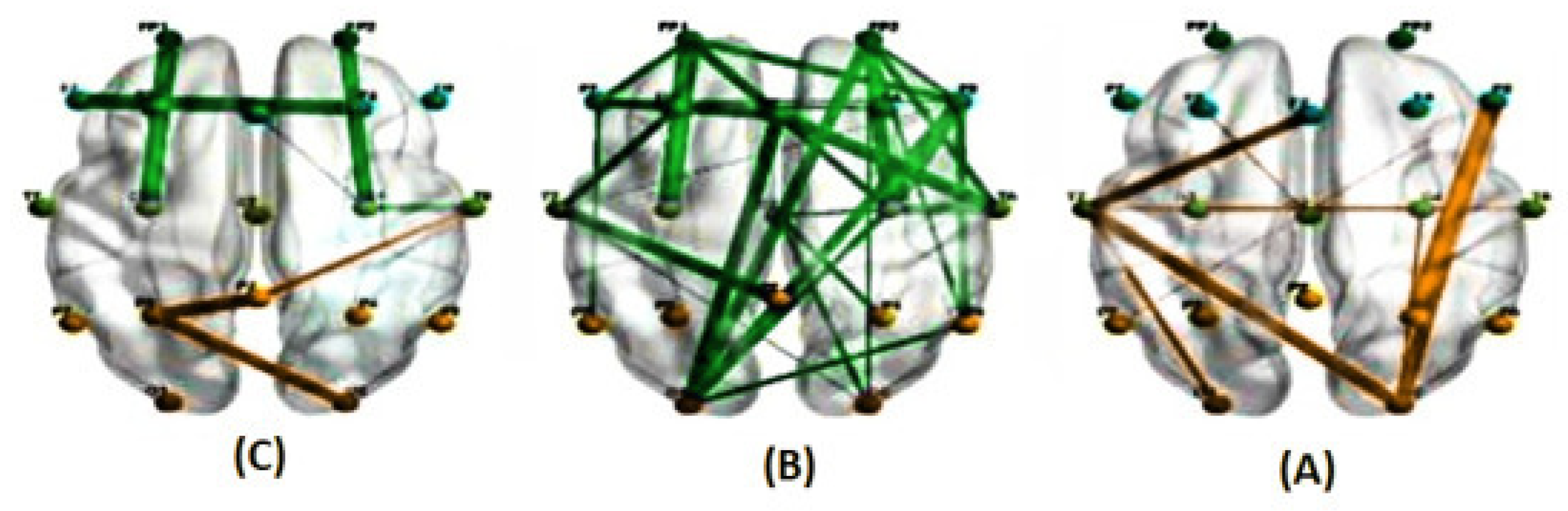
3. Findings and Discussion
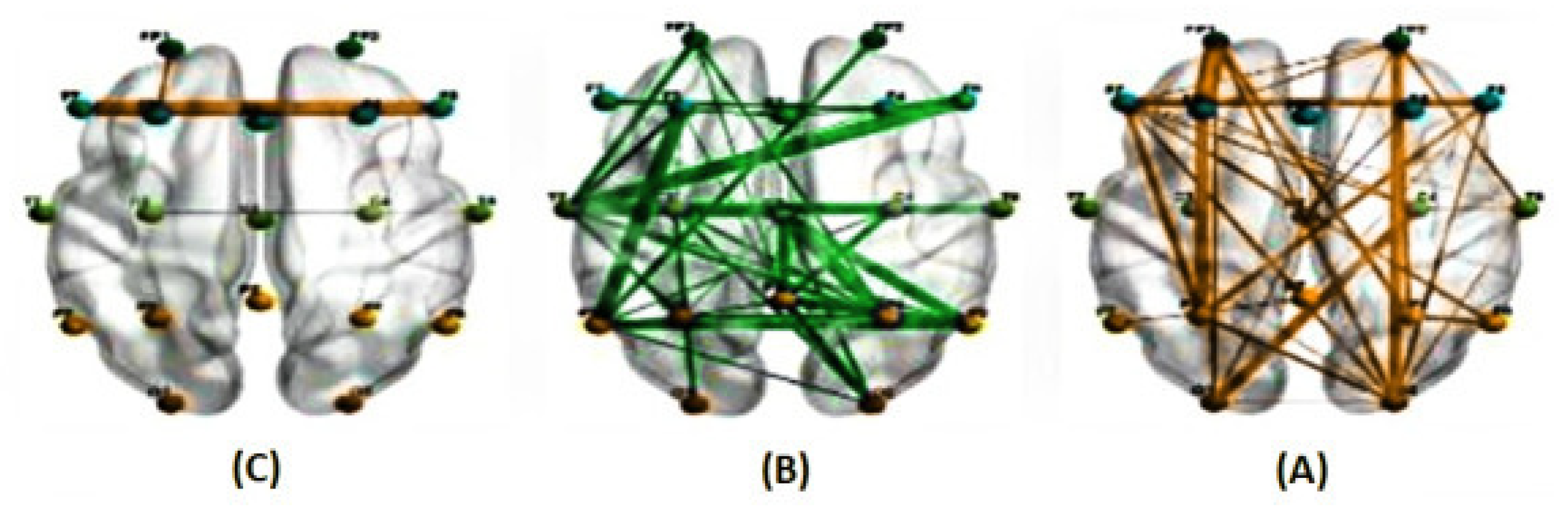
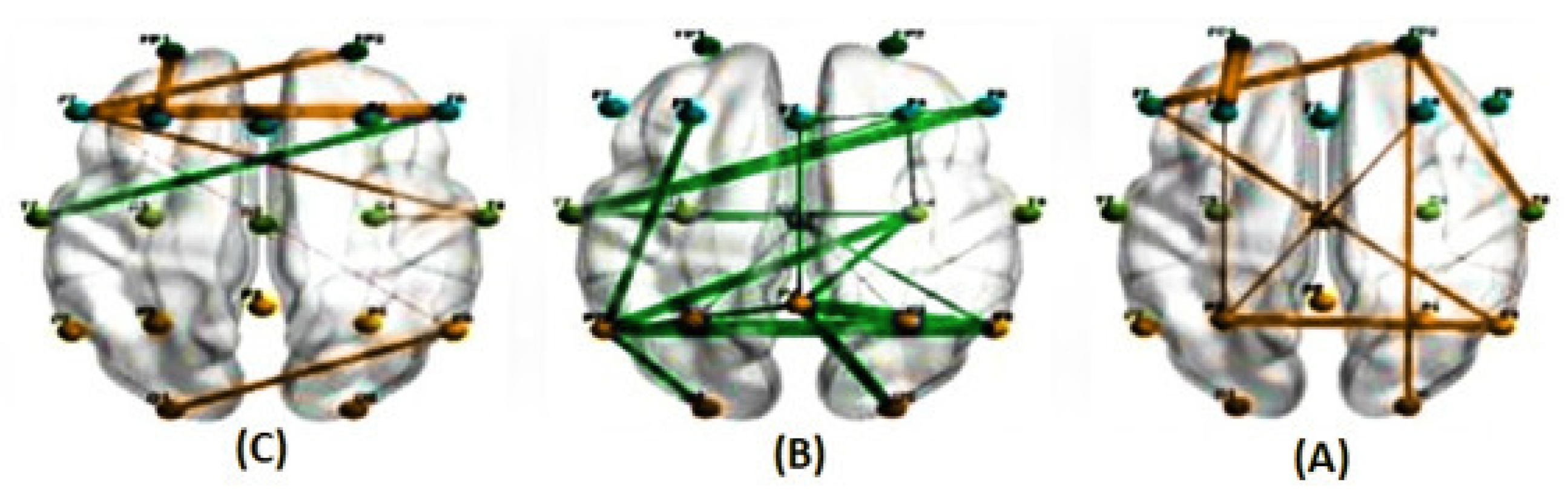
3.1. Comparison of stimulation with clockwise and counterclockwise shapes
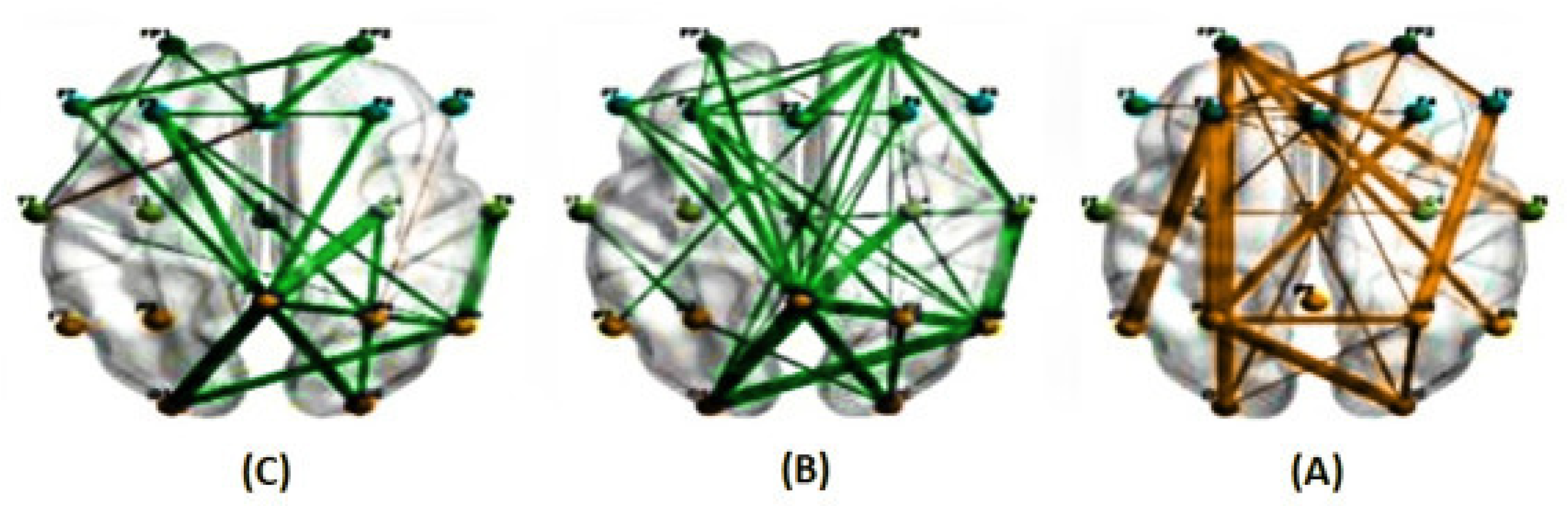
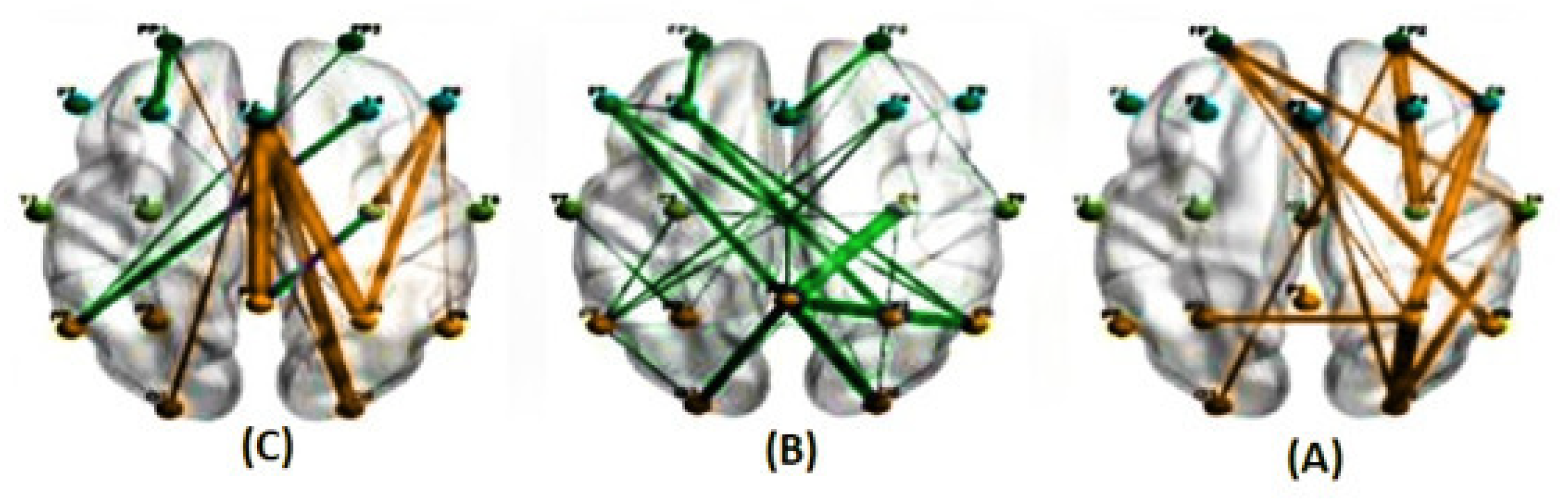
3.2. Comparison of delays in two predictability and unpredictability conditions
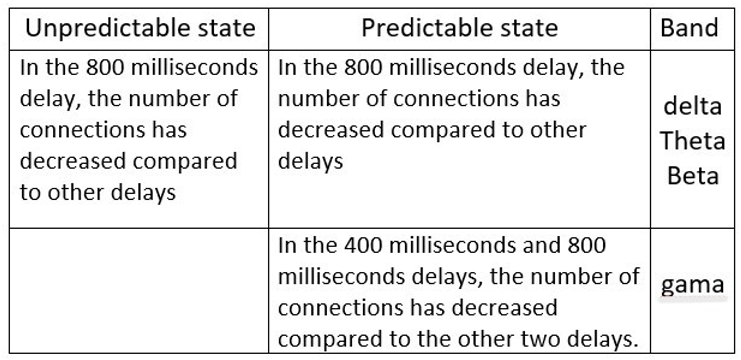
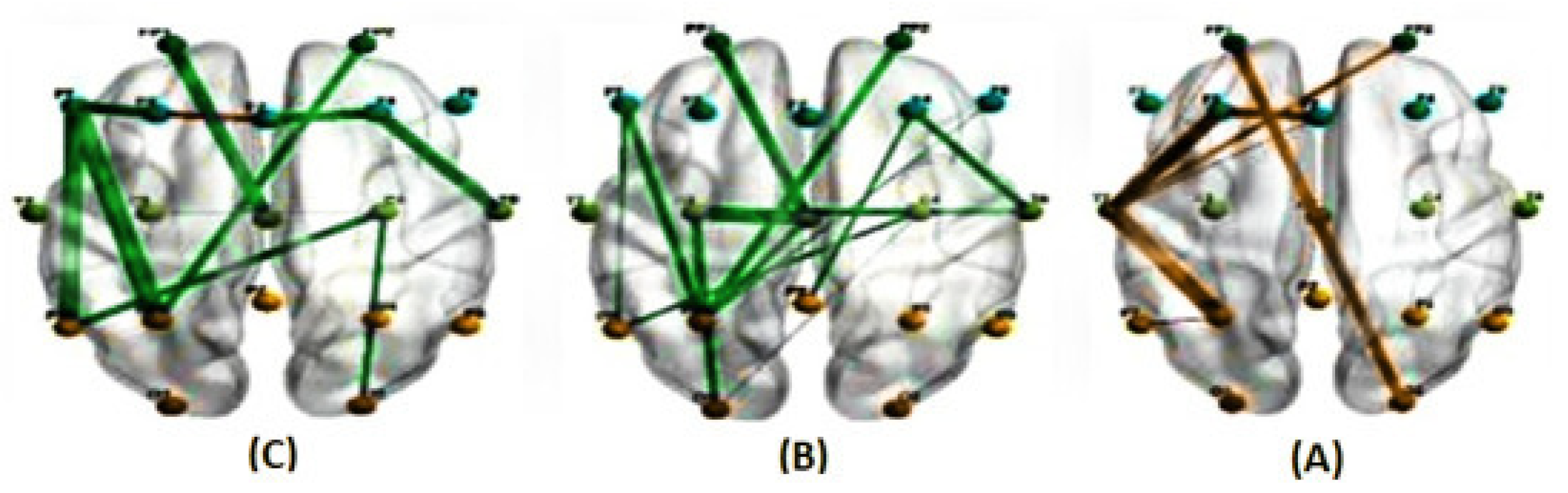
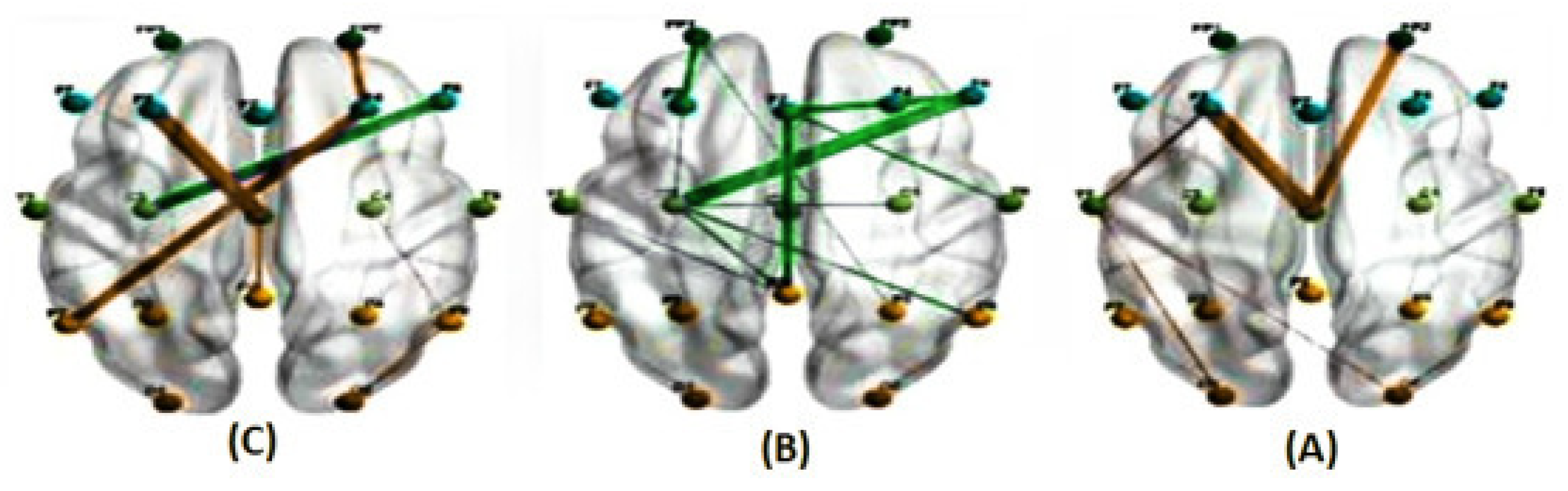
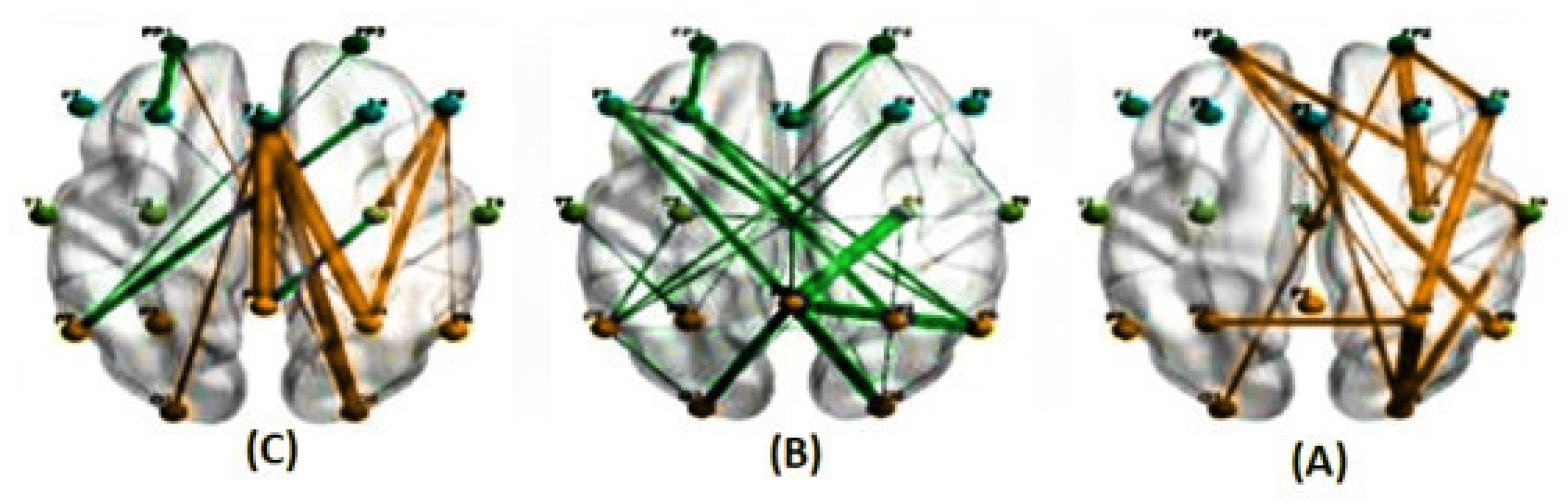
3.3. Comparison of Predictable and Unpredictable Scenarios for Each Delay
4. Conclusions

References
- Yelamanchili, T. Neural correlates of flow, boredom, and anxiety in gaming: An electroencephalogram study; Missouri University of Science and Technology, 2018.
- Mohammad-Rezazadeh, I.; Frohlich, J.; Loo, S.K.; Jeste, S.S. Brain connectivity in autism spectrum disorder. Current opinion in neurology 2016, 29, 137. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.M. Elements of information theory; John Wiley & Sons, 1999. [CrossRef]
- Van Mierlo, P.; Papadopoulou, M.; Carrette, E.; Boon, P.; Vandenberghe, S.; Vonck, K.; Marinazzo, D. Functional brain connectivity from EEG in epilepsy: Seizure prediction and epileptogenic focus localization. Progress in neurobiology 2014, 121, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Vinck, M.; Oostenveld, R.; Van Wingerden, M.; Battaglia, F.; Pennartz, C.M. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Dictionary, M.W. New York: Merriam-Webster. Merriam-Webster. com. Web 2016, 14.
- Minnoye, A.L.; Tajdari, F.; Doubrovski, E.L.; Wu, J.; Kwa, F.; Elkhuizen, W.S.; Huysmans, T.; Song, Y. Personalized product design through digital fabrication. International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. American Society of Mechanical Engineers, 2022, Vol. 86212, p. V002T02A054. [CrossRef]
- Tajdari, F.; Ramezanian, H.; Paydarfar, S.; Lashgari, A.; Maghrebi, S. Flow metering and lane-changing optimal control with ramp-metering saturation. 2022 CPSSI 4th International Symposium on Real-Time and Embedded Systems and Technologies (RTEST). IEEE, 2022, pp. 1–6. [CrossRef]
- Tajdari, F. Online set-point estimation for feedback-based traffic control applications. IEEE Transactions on Intelligent Transportation Systems 2023. [Google Scholar] [CrossRef]
- Tajdari, F.; Roncoli, C. Online set-point estimation for feedback-based traffic control applications. arxiv. arXiv preprint arXiv:2207.13467 2022. arXiv:2207.13467 2022. [CrossRef]
- Tajdari, M.; Tajdari, F.; Pawar, A.; Zhang, J.; Liu, W.K. 2D to 3D volumetric reconstruction of human spine for diagnosis and prognosis of spinal deformities. Conference: 16th US national congress on computational mechanics, 2021.
- Tajdari, F. Adaptive time-delay estimation and control of optimized Stewart robot. Journal of Vibration and Control 2022, p. 10775463221137141. [CrossRef]
- Tajdari, M.; Tajdari, F.; Shirzadian, P.; Pawar, A.; Wardak, M.; Saha, S.; Park, C.; Huysmans, T.; Song, Y.; Zhang, Y.J.; others. Next-generation prognosis framework for pediatric spinal deformities using bio-informed deep learning networks. Engineering with Computers 2022, 38, 4061–4084. [Google Scholar] [CrossRef]
- Tajdari, F. ; others. Optimal and adaptive controller design for motorway traffic with connected and automated vehicles. 2023. [Google Scholar]
- Tajdari, F.; Huysmans, T.; Song, Y. Non-rigid registration via intelligent adaptive feedback control. IEEE transactions on visualization and computer graphics 2023. [Google Scholar] [CrossRef]
- Tajdari, F.; Roncoli, C.; Papageorgiou, M. Feedback-based ramp metering and lane-changing control with connected and automated vehicles. IEEE Transactions on Intelligent Transportation Systems 2020, 23, 939–951. [Google Scholar] [CrossRef]
- Tajdari, F.; Khodabakhshi, E.; Kabganian, M.; Golgouneh, A. Switching controller design to swing-up a two-link underactuated robot. 2017 IEEE 4th International Conference on Knowledge-Based Engineering and Innovation (KBEI). IEEE, 2017, pp. 0595–0599. [CrossRef]
- Tajdari, F.; Roncoli, C. Adaptive traffic control at motorway bottlenecks with time-varying fundamental diagram. IFAC-PapersOnLine 2021, 54, 271–277. [Google Scholar] [CrossRef]
- Tajdari, F.; Kabganian, M.; Khodabakhshi, E.; Golgouneh, A. Design, implementation and control of a two-link fully-actuated robot capable of online identification of unknown dynamical parameters using adaptive sliding mode controller. 2017 Artificial Intelligence and Robotics (IRANOPEN). IEEE, 2017, pp. 91–96. [CrossRef]
- Tajdari, F.; Toulkani, N.E.; Zhilakzadeh, N. Semi-real evaluation, and adaptive control of a 6dof surgical robot. 2020 11th Power Electronics, Drive Systems, and Technologies Conference (PEDSTC). IEEE, 2020, pp. 1–6. [CrossRef]
- Tajdari, F.; Roncoli, C.; Bekiaris-Liberis, N.; Papageorgiou, M. Integrated ramp metering and lane-changing feedback control at motorway bottlenecks. 2019 18th European Control Conference (ECC). IEEE, 2019, pp. 3179–3184. [CrossRef]
- Khakzand, M.; Babaei, S. A framework for designing the open spaces in the children’s educational centers based on the (Seven Cs) with emphasis on pProve the learning. Technology of Education Journal (TEJ) 2016, 10, 305–318. [Google Scholar]
- Gallistel, C.R.; Gibbon, J. Time, rate, and conditioning. Psychological review 2000, 107, 289. [Google Scholar] [CrossRef] [PubMed]
- Pasquereau, B.; Turner, R.S. Dopamine neurons encode errors in predicting movement trigger occurrence. Journal of neurophysiology 2015, 113, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Cravo, A.M.; Rohenkohl, G.; Wyart, V.; Nobre, A.C. Temporal expectation enhances contrast sensitivity by phase entrainment of low-frequency oscillations in visual cortex. Journal of Neuroscience 2013, 33, 4002–4010. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.; Karmos, G.; Mehta, A.D.; Ulbert, I.; Schroeder, C.E. Entrainment of neuronal oscillations as a mechanism of attentional selection. science 2008, 320, 110–113. [Google Scholar] [CrossRef]
- Rohenkohl, G.; Gould, I.C.; Pessoa, J.; Nobre, A.C. Combining spatial and temporal expectations to improve visual perception. Journal of vision 2014, 14, 8–8. [Google Scholar] [CrossRef]
- Ghaffari, A.; Khodayari, A.; Kamali, A.; Tajdari, F.; Hosseinkhani, N. New fuzzy solution for determining anticipation and evaluation behavior during car-following maneuvers. Proceedings of the Institution of Mechanical Engineers, Part D: Journal of automobile engineering 2018, 232, 936–945. [Google Scholar] [CrossRef]
- Khodayari, A.; Ghaffari, A.; Kamali, A.; Tajdari, F. A new model of car following behavior based on lane change effects using anticipation and evaluation idea. Iranian Journal of Mechanical Engineering Transactions of the ISME 2015, 16, 26–38. [Google Scholar]
- Rad, N.F.; Yousefi-Koma, A.; Tajdari, F.; Ayati, M. Design of a novel three degrees of freedom ankle prosthesis inspired by human anatomy. 2016 4th International Conference on Robotics and Mechatronics (ICROM). IEEE, 2016, pp. 428–432. [CrossRef]
- Rad, N.F.; Ayati, M.; Basaeri, H.; Yousefi-Koma, A.; Tajdari, F.; Jokar, M. Hysteresis modeling for a shape memory alloy actuator using adaptive neuro-fuzzy inference system. 2015 3Rd RSI international conference on robotics and mechatronics (ICROM). IEEE, 2015, pp. 320–324. [CrossRef]
- Janssen, P.; Shadlen, M.N. A representation of the hazard rate of elapsed time in macaque area LIP. Nature neuroscience 2005, 8, 234–241. [Google Scholar] [CrossRef]
- Jazayeri, M.; Shadlen, M.N. Temporal context calibrates interval timing. Nature neuroscience 2010, 13, 1020–1026. [Google Scholar] [CrossRef]
- Darlington, T.K.; Wager-Smith, K.; Ceriani, M.F.; Staknis, D.; Gekakis, N.; Steeves, T.D.; Weitz, C.J.; Takahashi, J.S.; Kay, S.A. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 1998, 280, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Nobre, A.C. Perceiving the passage of time: neural possibilities. Annals of the New York Academy of Sciences 2014, 1326, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Perrett, S.P. Temporal discrimination in the cerebellar cortex during conditioned eyelid responses. Experimental brain research 1998, 121, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Cognitive signals in the primate motor thalamus predict saccade timing. Journal of Neuroscience 2007, 27, 12109–12118. [Google Scholar] [CrossRef]
- Leon, M.I.; Shadlen, M.N. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron 2003, 38, 317–327. [Google Scholar] [CrossRef]
- Brody, C.D.; Hernández, A.; Zainos, A.; Romo, R. Timing and neural encoding of somatosensory parametric working memory in macaque prefrontal cortex. Cerebral cortex 2003, 13, 1196–1207. [Google Scholar] [CrossRef]
- Genovesio, A.; Tsujimoto, S.; Wise, S.P. Neuronal activity related to elapsed time in prefrontal cortex. Journal of Neurophysiology 2006, 95, 3281–3285. [Google Scholar] [CrossRef]
- Genovesio, A.; Tsujimoto, S.; Wise, S.P. Feature-and order-based timing representations in the frontal cortex. Neuron 2009, 63, 254–266. [Google Scholar] [CrossRef]
- Oshio, K.i.; Chiba, A.; Inase, M. Temporal filtering by prefrontal neurons in duration discrimination. European Journal of Neuroscience 2008, 28, 2333–2343. [Google Scholar] [CrossRef]
- Lebedev, M.A.; O’Doherty, J.E.; Nicolelis, M.A. Decoding of temporal intervals from cortical ensemble activity. Journal of neurophysiology 2008, 99, 166–186. [Google Scholar] [CrossRef]
- Harrington, D.L.; Haaland, K.Y.; Knight, R.T. Cortical networks underlying mechanisms of time perception. Journal of Neuroscience 1998, 18, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Matell, M.S.; Meck, W.H.; Lustig, C. Not “just” a coincidence: Frontal-striatal interactions in working memory and interval timing. Memory 2005, 13, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Toosi, T.; K. Tousi, E.; Esteky, H. Learning temporal context shapes prestimulus alpha oscillations and improves visual discrimination performance. Journal of Neurophysiology 2017, 118, 771–777. [Google Scholar] [CrossRef]
- Tajdari, M.; Pawar, A.; Li, H.; Tajdari, F.; Maqsood, A.; Cleary, E.; Saha, S.; Zhang, Y.J.; Sarwark, J.F.; Liu, W.K. Image-based modelling for adolescent idiopathic scoliosis: mechanistic machine learning analysis and prediction. Computer methods in applied mechanics and engineering 2021, 374, 113590. [Google Scholar] [CrossRef]
- Tajdari, F.; Kabganian, M.; Rad, N.F.; Khodabakhshi, E. Robust control of a 3-dof parallel cable robot using an adaptive neuro-fuzzy inference system. 2017 Artificial Intelligence and Robotics (IRANOPEN). IEEE, 2017, pp. 97–101. [CrossRef]
- Tajdari, F.; Toulkani, N.E.; Zhilakzadeh, N. Intelligent optimal feed-back torque control of a 6dof surgical rotary robot. 2020 11th Power Electronics, Drive Systems, and Technologies Conference (PEDSTC). IEEE, 2020, pp. 1–6. [CrossRef]
- Tajdari, F.; Ghaffari, A.; Khodayari, A.; Kamali, A.; Zhilakzadeh, N.; Ebrahimi, N. Fuzzy control of anticipation and evaluation behaviour in real traffic flow. 2019 7th International Conference on Robotics and Mechatronics (ICRoM). IEEE, 2019, pp. 248–253. [CrossRef]
- Tajdari, F.; Huysmans, T.; Yang, Y.; Song, Y. Feature preserving non-rigid iterative weighted closest point and semi-curvature registration. IEEE Transactions on Image Processing 2022, 31, 1841–1856. [Google Scholar] [CrossRef]
- Tajdari, F.; Ebrahimi Toulkani, N. Implementation and intelligent gain tuning feedback–based optimal torque control of a rotary parallel robot. Journal of Vibration and Control 2022, 28, 2678–2695. [Google Scholar] [CrossRef]
- Tajdari, F.; Tajdari, M.; Rezaei, A. Discrete time delay feedback control of stewart platform with intelligent optimizer weight tuner. 2021 IEEE International Conference on Robotics and Automation (ICRA). IEEE, 2021, pp. 12701–12707. [CrossRef]
- Tajdari, F.; Toulkani, N.E.; Nourimand, M. Intelligent architecture for car-following behaviour observing lane-changer: Modeling and control. 2020 10th International Conference on Computer and Knowledge Engineering (ICCKE). IEEE, 2020, pp. 579–584. [CrossRef]
- Tajdari, F.; Golgouneh, A.; Ghaffari, A.; Khodayari, A.; Kamali, A.; Hosseinkhani, N. Simultaneous intelligent anticipation and control of follower vehicle observing exiting lane changer. IEEE Transactions on Vehicular Technology 2021, 70, 8567–8577. [Google Scholar] [CrossRef]
- Rosenblum, M.G.; Pikovsky, A.S.; Kurths, J. Phase synchronization of chaotic oscillators. Physical review letters 1996, 76, 1804. [Google Scholar] [CrossRef]
- Tajdari, F.; Eijck, C.; Kwa, F.; Versteegh, C.; Huysmans, T.; Song, Y. Optimal position of cameras design in a 4D foot scanner. International design engineering technical conferences and computers and information in engineering conference. American Society of Mechanical Engineers, 2022, Vol. 86212, p. V002T02A044. [CrossRef]
- Tajdari, F.; Kwa, F.; Versteegh, C.; Huysmans, T.; Song, Y. Dynamic 3d mesh reconstruction based on nonrigid iterative closest-farthest points registration. International design engineering technical conferences and computers and information in engineering conference. American Society of Mechanical Engineers, 2022, Vol. 86212, p. V002T02A051. [CrossRef]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Human brain mapping 2007, 28, 1178–1193. [Google Scholar] [CrossRef]
- Van Diessen, E.; Numan, T.; Van Dellen, E.; Van Der Kooi, A.; Boersma, M.; Hofman, D.; Van Lutterveld, R.; Van Dijk, B.; Van Straaten, E.; Hillebrand, A.; others. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clinical Neurophysiology 2015, 126, 1468–1481. [Google Scholar] [CrossRef]
- Heesen, P. Adaptive step up tests for the false discovery rate (FDR) under independence and dependence. PhD thesis, Düsseldorf, Heinrich-Heine-Universität, Diss., 2014, 2015.
- Beste, C.; Dinse, H.R. Learning without training. Current biology 2013, 23, R489–R499. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.J.; Ivry, R.B. Duration selectivity in right parietal cortex reflects the subjective experience of time. Journal of Neuroscience 2020, 40, 7749–7758. [Google Scholar] [CrossRef]
- Fontes, R.M.; Marinho, V.; Carvalho, V.; Rocha, K.; Magalhães, F.; Moura, I.; Ribeiro, P.; Velasques, B.; Cagy, M.; Gupta, D.S.; others. Time estimation exposure modifies cognitive aspects and cortical activity of attention deficit hyperactivity disorder adults. International Journal of Neuroscience 2020, 130, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Mioni, G.; Grondin, S.; Bardi, L.; Stablum, F. Understanding time perception through non-invasive brain stimulation techniques: A review of studies. Behavioural brain research 2020, 377, 112232. [Google Scholar] [CrossRef]
- Ghaderi, A.H.; Moradkhani, S.; Haghighatfard, A.; Akrami, F.; Khayyer, Z.; Balcı, F. Time estimation and beta segregation: An EEG study and graph theoretical approach. PLoS One 2018, 13, e0195380. [Google Scholar] [CrossRef]
- Rivera-Tello, S.; Romo-Vázquez, R.; Ramos-Loyo, J. Correlation of EEG Brain Waves in a Time Perception Task. VIII Latin American Conference on Biomedical Engineering and XLII National Conference on Biomedical Engineering: Proceedings of CLAIB-CNIB 2019, October 2-5, 2019, Cancún, México. Springer, 2020, pp. 79–84. [CrossRef]
- Jazayeri, M.; Shadlen, M.N. A neural mechanism for sensing and reproducing a time interval. Current Biology 2015, 25, 2599–2609. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




