1. Introduction
Worldwide, esophageal cancer is the fifth most common cause of cancer-related death for men and the eighth for women [
1]. Esophageal cancer is relatively rare but still a significant global health concern. According to the World Health Organization, in 2020, there were an estimated 604,000 new cases of esophageal cancer worldwide and 544,000 deaths from the disease. Squamous cell carcinoma of the esophagus is also more common in eastern Asia and parts of Africa. Preoperative chemotherapy for locally advanced esophageal cancer is Japan's current standard of care [
2,
3]. After controlling local and systemic micrometastases with preoperative chemotherapy, surgery has improved radicality and prolonged prognosis [
4]. Still, esophageal cancer surgery is a highly invasive procedure, and depending on the underlying disease and physical status, there are populations in which surgery should be avoided. The complete histopathological response can have been obtained in several cases by recent chemotherapy regimen [
5]. Pathological response and prognosis may correlate, so determining preoperative chemotherapy's efficacy is important [
6].
The nematode
Caenorhabditis elegans (
C. elegans) possesses a sophisticated sense of smell that enables it to detect even slight changes in the concentration of odorants in its natural habitat and exhibit chemotaxis in the direction of bacteria, which serves as its food source. With approximately 1,200 olfactory receptor-like genes (compared to around 800 in dogs),
C. elegans approaches desirable odors while avoiding unpleasant ones [
7]. Hirotsu et al. have shown that
C. elegans is attracted to urine from cancer patients but tends to avoid urine from healthy individuals [
8]. Nematode Nose (N-NOSE) is a novel cancer screening test that utilizes the chemotaxis index of
C. elegans. Although previous studies have compared nematode chemotaxis before and after surgery, no studies have examined whether N-NOSE can be used to determine the efficacy of preoperative or induction chemotherapy in the esophageal cancer patient. Thus, we designed a single-institution, prospective study to confirm the ability of N-NOSE as a tool to determine the efficacy of preoperative or induction chemotherapy for esophageal cancer patients.
2. Patients and methods
Eligibility criteria:
This study was conducted with patients who underwent preoperative or induction chemotherapy and elective esophagectomy for thoracic esophageal cancer in the Department of Gastroenterological Surgery and Pediatric Surgery of the Gifu University, Japan. Patients who were >20 years of age at the time of registration and who had histologically confirmed Stage II-IVb esophageal squamous cell carcinoma were included in the present study. The other inclusion criteria were an Eastern Cooperative Oncology Group performance status of 0-1; a life expectancy of >12 weeks; and adequate liver, bone marrow, renal, and cardiovascular functions (serum bilirubin ≤1.5 mg/dl; neutrophil count ≥1,500/mm3; serum aspartate aminotransferase and alanine aminotransferase levels ≤ twice the upper limit of normal range; platelet count ≥10×104/mm3; hemoglobin ≥8.0 g/dl; creatinine ≤1.2 mg/dl [or creatinine clearance ≥30 ml/min] and platelet count >10×104 /l. The exclusion criteria were serious concomitant illness, symptomatic infectious disease, severe allergy, peripheral neuropathy, and uncontrolled diabetes mellitus. Patients satisfying the inclusion criteria were registered among consecutive esophageal cancer cases during the period from August 2020 to December 2021.
Treatment strategy and evaluation of chemotherapy:
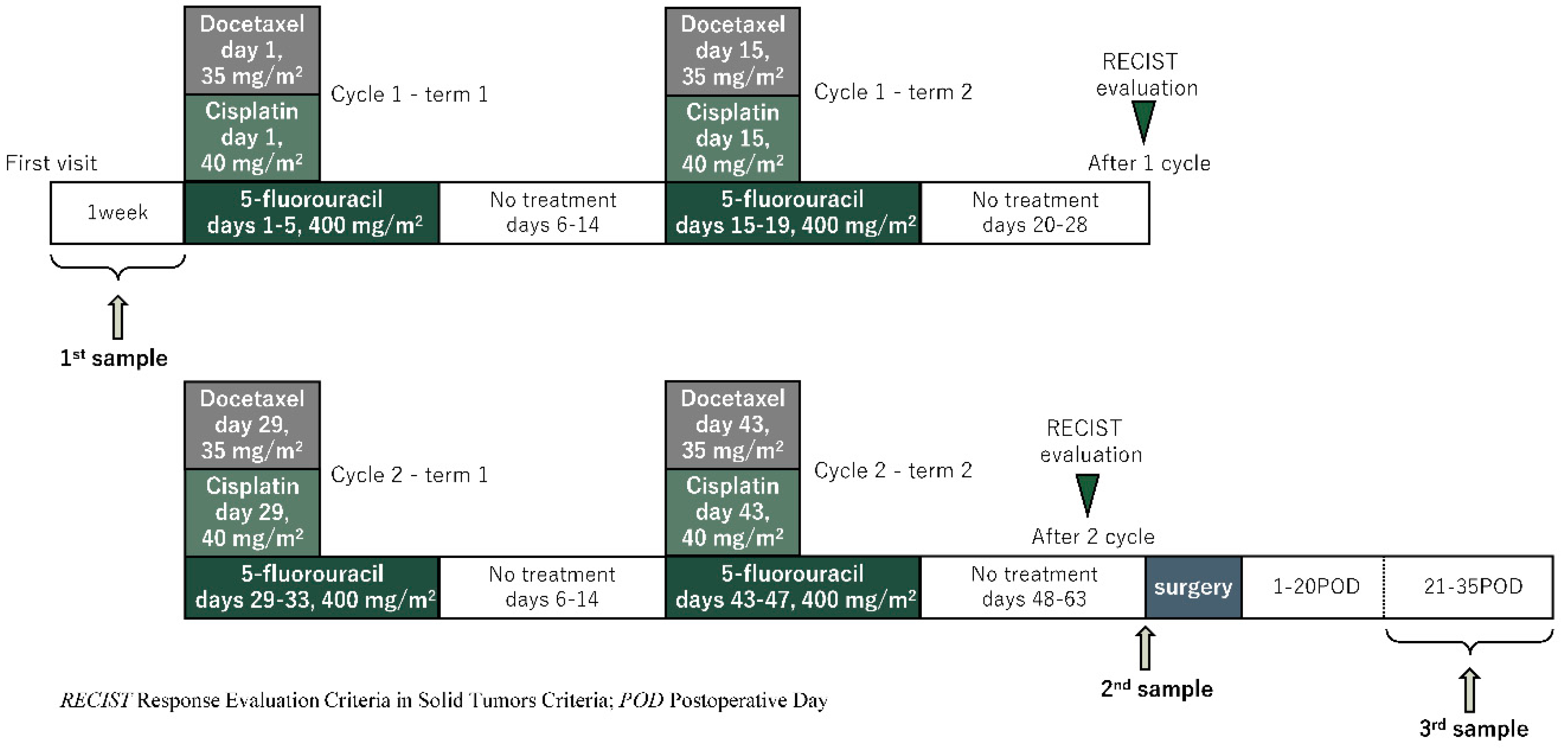
Patients received TXT diluted in 250 mL of normal saline at a dose of 35 mg/m
2. Then, CDDP was prepared in normal saline at a dose of 40 mg/m
2 and administered intravenously (iv) over 2 hours on day 1. 5-FU was prepared in normal saline at a dose of 400 mg/m
2 and administered iv continuously on days 1–5. TXT and CDDP were given on days 1 and 15, and 5-FU was given on days 1–5 and 15–19 of every 28-day cycle (one cycle). All included patients were scheduled to receive two cycles [
5,
9,
10] (
Figure 1). Disease staging was defined according to the International Union Against Cancer TNM classification system, 8th edition [
11]. Measurable lesions were evaluated with computed tomography or magnetic resonance imaging, except for the primary tumor. They were assessed in accordance with the Response Evaluation Criteria in Solid Tumors Criteria (RECIST) version 1.1 [
12].
Study endpoints:
We investigated the predictability of N-NOSE screening for the clinical effects of preoperative chemotherapy for esophageal cancer patients receiving radical surgery.
Measurement method of N-NOSE and index reduction scores:
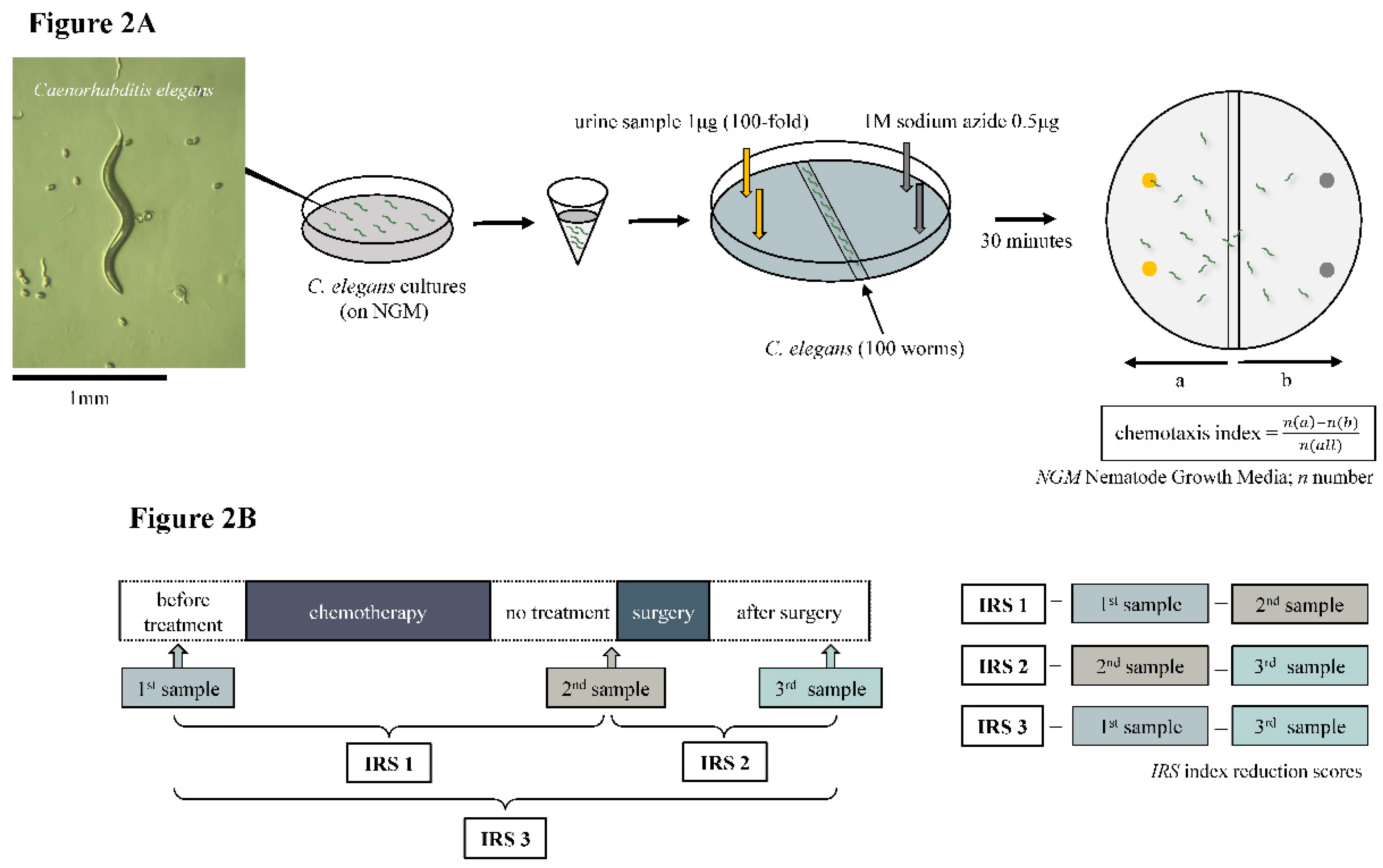
Urine samples were collected from enrolled esophageal cancer patients at three-time points: before the start of chemotherapy, after the end of chemotherapy, and after surgery at each. The first was taken within a week before the start of chemotherapy. The second collection was taken the day before or the morning of surgery to lessen the effect of anticancer drugs on the chemotaxis index. The third was taken 3 to 5 weeks after surgery (
Figure 1). All urine samples were collected from mid-fasting urine. Collected urine samples were frozen at or below -20°C within 2 hours of collection and stored frozen until examined by N-NOSE.
C. elegans (wild-type N2) were cultured with standard methods at 20°C on Nematode Growth Media (NGM) seeded with
Escherichia coli strain NA22 as a food source [
8,
13]. Chemotaxis assays were performed on 9 cm plates containing 10 ml of 2% agar, 5 mM KPO4, 1 mM CaCl2, and 1 mM MgSO4, as previously described [
7,
8,
14]. The nematode chemotaxis analysis was conducted using standard protocols established in previous nematode studies [
8,
15]. To begin, 0.5 μl of 1 M sodium azide was added to two points at both ends of the plates, and then 1 μl of urine sample diluted at 100-fold with ultra-pure water was added to two points. Sodium azide was used to minimize the effects of adaptation and was added before adding the worms to the plate. Only sodium azide was added to the non-urine side of the plate. Therefore, sodium azide was added to four points of the plate (urine side and non-urine side). The worms that showed no chemotaxis behavior to 1 μl of water, which was used as a control. Roughly 100 synchronized young adult worms were collected and washed three times with chemotaxis buffer (0.05% gelatin, 5 mM KPO
4, 1 mM CaCl
2, and 1 mM MgSO
4) placed in the center of the plate. After excess buffer removal, the worms were allowed to roam for 30 minutes. The chemotaxis index was calculated by dividing the number of worms near the urine sample and the number of worms in the region without the sample by the total number of worms [
14,
15]. The chemotaxis index was determined for each urine sample at 100-fold dilution for chemotaxis assay. A positive index (0 to 1) indicates an attraction to the sample, while a negative index (-1 to 0) indicates repulsion to the sample (
Figure 2A). In this study, we focused on the difference in the chemotaxis index. The difference in the chemotaxis index obtained from each therapeutic point was defined as the index reduction score (IRS). In other words, the difference before treatment (1
st sample) and before surgery (2
nd sample) was defined as IRS1, and the difference before surgery (2
nd sample) and after surgery (3
rd sample) was defined as IRS2. The difference before treatment (1
st sample) and after surgery (3
rd sample) was defined as IRS3 and its clinical relevance was examined (
Figure 2B). The Chemotaxis index was judged by several judges who were not informed of any antitumor effect.
Statistical analyses
Statistical analyses, including the Wilcoxon rank-sum test (Mann-Whitney test), One-way ANOVA, and Receiver Operating Characteristic (ROC) analysis to calculate the area under the curve (AUC) values, were conducted using JMP® 14 (SAS Institute, Cary, NC, USA). Statistical significance was defined as a p-value of less than 0.05.
Ethics approval
This study was conducted in accordance with the World Medical Association Declaration of Helsinki. This study protocol was approved by ethics committees of Gifu University School of Medicine (ID: 2022-0107), and written informed consent was obtained from all of the patients before all study-related procedures.
Results
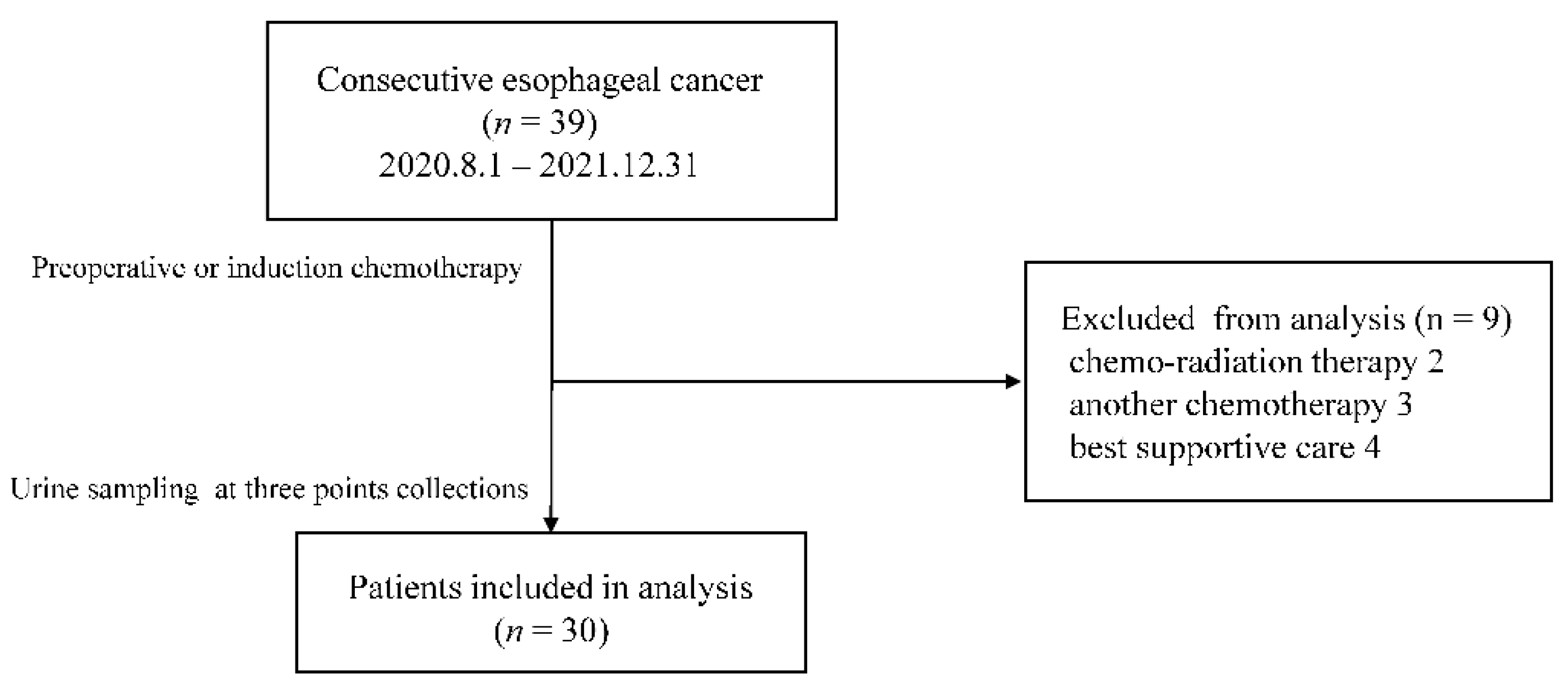
Thirty-nine patients with esophageal cancer were enrolled from August 2020 to December 2021. Preoperative or induction chemotherapy was introduced for all patients on the assumption of radical resection. Nine patients could not undergo surgery due to side effects of chemotherapy, exacerbation of comorbidities, or refusal of treatment. Of the nine patients, two received chemoradiotherapy, three received another chemotherapy, and four were discontinued (
Figure 3). Finally, we analyzed 30 patients. The age, sex, TNM classification, and clinical response are summarized in
Table 1. The median age was 72 years old. Complete response (CR) or partial response (PR) was achieved in 23 cases (76.7%).
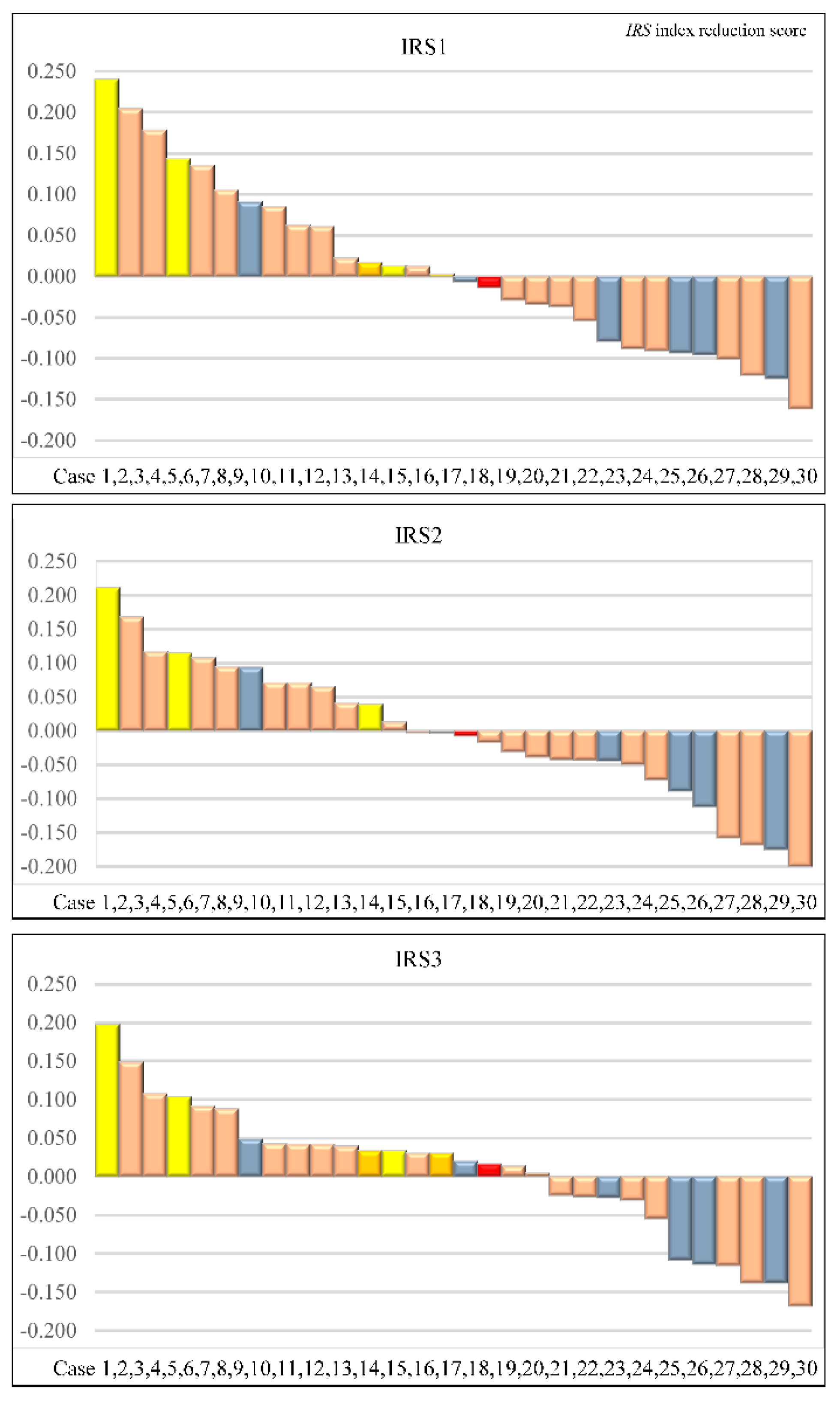
Figure 4 shows the IRS1, IRS2, and IRS3 values and efficacy for each of the 30 cases analyzed.
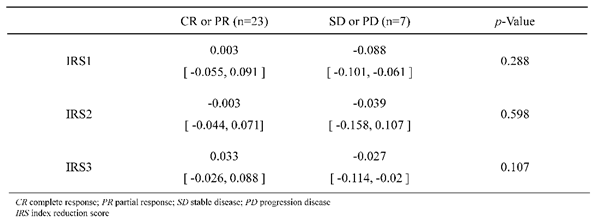
When comparing the response group (CR or PR) and the non-response group (SD or PD), IRS1s were 0.003 [-0.055, 0.091]: median [interquartile range], and -0.088 [-0.101, -0.061], respectively, with a
p-value of 0.288. IRS2s were -0.003 [-0.044, 0.071] and -0.039 [-0.158, 0.107], respectively, with a
p-value of 0.598. IRS3s were 0.033 [-0.026, 0.088] and -0.027 [-0.114, -0.020], respectively, with a
p-value of 0.107 (
Table 2). For the treatment effect for CR, IRS1 was 0.135 [0.003, 0.240], IRS2 was 0.094 [-0.003, 0.167], and IRS3 was 0.103 [0.033, 0.197].
The prediction accuracies of the three IRSs obtained from 30 patients are summarized in
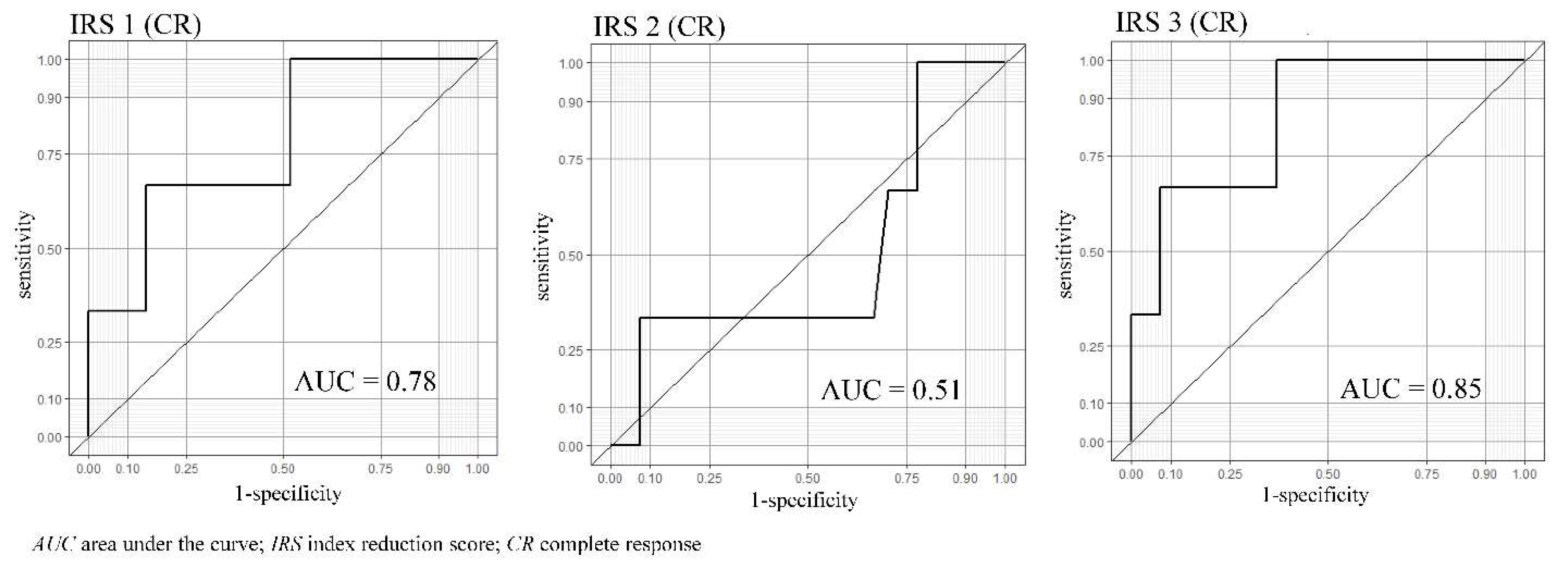
Table 3. For the treatment effect of CR or PR, the three IRSs were IRS1:AUC=0.64 (95% confidence interval (CI): 0.36-0.91), IRS2:AUC=0.57 (95% CI: 0.28-0.86), and IRS3:AUC=0.7 (95% CI: 0.49-0.92), respectively. On the other hand, when the target of the treatment effect was CR only, the three IRSs were IRS1:AUC=0.78 (95% CI: 0.46-1), IRS2:AUC=0.51 (95%CI: 0.06-0.96), and IRS3:AUC=0.85 (95% CI: 0.62-1), respectively (
Figure 5).
Discussion
The chemotaxis response of
C. elegans to urine samples depends on the concentration of odorant [
14] [
8]. These findings are the basis for the N-NOSE multi-cancer primary screening test, which is highly sensitive in detecting cancer at its early stages (85% average sensitivity stage 0-I) [
8,
16,
17,
18,
19,
20,
21,
22]. Thus, N-NOSE, commercially available in Japan since late 2020, serves as a primary screening test for cancer. It is characterized by a minimally invasive and simple test using the patient's urine, yet it has a very high sensitivity and specificity, screening even early-stage cancers. Very few clinical references report the relationship between the N-NOSE chemotaxis index and esophageal cancer. Only one patient in the Hirotsu et al. study [
8], one patient in the Inaba et al. study [
20], and 18 esophageal cancer patients in the Kusumoto et al. study [
22] were included. So, evaluations on N-NOSE, to date, focused solely on esophageal cancer patients are scarce. Moreover, only a few studies have focused on changes in N-NOSE before and after surgery, i.e., before and after tumor resection. Kusumoto et al. performed a clinical study to investigate the usefulness of the N-NOSE as a postoperative tool for monitoring the presence of cancer for both gastric and colorectal cancers. In that study, ROC analysis showed that N-NOSE was more accurate than CEA or CA19-9 in correlating with the removal of cancerous tumors [
22]. The result suggested that the olfactory behaviors of
C. elegans reflected the change in urinary cancer-specific odor caused by surgical removal. Asai et al. also compared the chemotaxis index in 83 patients with pancreatic cancer before and after surgery [
18]. Preoperative urine samples had a significantly higher chemotaxis index than postoperative samples in patients with pancreatic cancer, with an AUC=0.845 (10
-1 dilution of urine) and AUC=0.820 (10
-2 dilution). Moreover, using the changes in preoperative and postoperative chemotaxis index, this method had a higher sensitivity for detecting early pancreatic cancer than existing diagnostic markers such as CEA and CA19-9 [
18]. Ferarri et al. reported that the pattern of urinary chemical components, including volatile organic compounds in patients with cancer, changed by surgical therapy, and it is considered that such changes are detected by
C. elegans [
23].
We focused on changes in the chemotaxis index before and after preoperative chemotherapy for esophageal cancer. The novelty of this study lies in its focus on the relationship between the shrinking effect of anticancer drugs and changes in the olfactory behaviors of C. elegans. We used the IRS, which might reflect chemotherapy's biological effect on cancer cells in vivo in the patients after chemotherapy. The results did not show significant AUC for all three IRSs for CR or PR treatment effect. On the other hand, when the treatment effect was limited to CR only, the AUC values were higher in IRS1 and IRS3 (AUC=0.78 and IRS3: AUC=0.85, respectively). The low AUC in the study, including CR and PR cases, may reflect that the nematodes reacted to the residual esophageal cancer in the PR cases. The result may support the screening ability of N-NOSE, which can detect even early-stage cancers. In the group in which CR was obtained, the presence of cancer disappeared on the image, and the AUC of IRS2 was relatively low. Besides, the AUC of IRS1 and IRS3 were relatively high. This result indicates that N-NOSE can accurately determine the response of the therapeutic effect of preoperative chemotherapy, which is confirmed by the fact that the cases achieved CR with preoperative treatment had a low change in C. elegans chemotaxis after surgery (Online Resource 1).
Recently, new methods for determining the presence of biological cancer cells have been developed [
24,
25]. For example, liquid biopsy in rectal cancer has been reported to monitor response to chemoradiation therapy and assess the risk of disease recurrence [
26]. On the other hand, there is also a report that squamous cell carcinoma related antigen, which is a classical tumor marker, does not reflect the disease activity of the esophageal cancer [
27]. Our prospective study using
C. elegans suggested that the non-invasiveness of N-NOSE may be comparable to the usefulness of biological cancer diagnosis, such as liquid biopsy.
This study has several limitations. First, the number of patients enrolled remains insufficient. Second, it is unclear whether the factor that affected the chemotaxis index of the nematodes was simply tumor shrinkage alone. The possibility that the drugs used for chemotherapy or the patient's general condition affected the nematode's movement cannot be ruled out. In addition, since N-NOSE is a tool for predicting the presence of cancer, setting a cut-off value and focusing on the changes in the chemotaxis index requires further investigation.
Conclusion
Index reduction score using N-NOSE screening may reflect the efficacy of chemotherapy for esophageal cancer patients. A large-scale prospective study at multiple centers is desired in the future.
Supplementary Materials
Area of under the curve for complete response or partial response treatment effect and Area of under the curve for complete response only.
Author Contributions
Formal analysis, Takuma Ishihara; Investigation, Hideyuki Hatakeyama and Masayo Morishita; Supervision, Yoshihiro Tanaka, Hiroshi Tsuchiya, Masahiro Fukada, Toshiya Higashi, Itaru Yasufuku, Ryuichi Asai, Jesse Tajima, Shigeru Kiyama, Takaaki Hirotsu, Nobuhisa Matsuhashi and Kazuhiro Yoshida; Validation, Manabu Futamura; Writing – original draft, Yuta Sato; Writing – review & editing, Eric Luccio.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in according to the guidelines of the Declaration of Helsinki and approved by the ethics committees of Gifu University School of Medicine (ID: 2022-0107).
Informed Consent Statement
Informed consent was obtained from all of the patients before all study-related procedures in this study.
Acknowledgments
We thank Dr. Junichi Yamaguchi, Mamie Egashira, Hisayo Noguchi, Kaori Sadasue, Rika Okuda, Kumiko Ishihara, Rie Nakamura, and Nobuaki Kondo (Hirotsu Bio Science Inc., Japan) for their helpful assistance in nematode imaging, N-NOSE data acquisition and statistics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J Clin 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus 2023, 1–30. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: part 2. Esophagus 2023. [Google Scholar] [CrossRef]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012, 19, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yoshida, K.; Yamada, A.; Tanahashi, T.; Okumura, N.; Matsuhashi, N.; Yamaguchi, K.; Miyazaki, T. Phase II trial of biweekly docetaxel, cisplatin, and 5-fluorouracil chemotherapy for advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol 2016, 77, 1143–1152. [Google Scholar] [CrossRef]
- Hipp, J.; Kuvendjiska, J.; Hillebrecht, H.C.; Timme-Bronsert, S.; Fichtner-Feigl, S.; Hoeppner, J.; Diener, M.K. Pathological complete response in multimodal treatment of esophageal cancer: a retrospective cohort study. Dis Esophagus 2022. [Google Scholar] [CrossRef]
- Bargmann, C.I. Chemosensation in C. elegans. WormBook 2006, 1–29. [Google Scholar] [CrossRef]
- Hirotsu, T.; Sonoda, H.; Uozumi, T.; Shinden, Y.; Mimori, K.; Maehara, Y.; Ueda, N.; Hamakawa, M. A highly accurate inclusive cancer screening test using Caenorhabditis elegans scent detection. PloS One 2015, 10, e0118699. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yoshida, K.; Sanada, Y.; Osada, S.; Yamaguchi, K.; Takahashi, T. Biweekly docetaxel, cisplatin, and 5-fluorouracil (DCF) chemotherapy for advanced esophageal squamous cell carcinoma: a phase I dose-escalation study. Cancer Chemother Pharmacol 2010, 66, 1159–1165. [Google Scholar] [CrossRef]
- Sato, Y.; Tanaka, Y.; Imai, T.; Okumura, N.; Matsuhashi, N.; Takahashi, T.; Shimokawa, T.; Yoshida, K. Serum diamine oxidase activity derived from response to chemotherapy affects adverse events and serum amino acid levels. Support Care Cancer 2022, 30, 9369–9377. [Google Scholar] [CrossRef]
- O'Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Pineros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol 2017, 18, 849–851. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirotsu, T.; Tagawa, T.; Oda, S.; Wakabayashi, T.; Iino, Y.; Ishihara, T. Odour concentration-dependent olfactory preference change in C. elegans. Nat Commun 2012, 3, 739. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef]
- Lanza, E.; Di Rocco, M.; Schwartz, S.; Caprini, D.; Milanetti, E.; Ferrarese, G.; Lonardo, M.T.; Pannone, L.; Ruocco, G.; Martinelli, S.; et al. C. elegans-based chemosensation strategy for the early detection of cancer metabolites in urine samples. Scientific Reports 2021, 11, 17133. [Google Scholar] [CrossRef]
- Thompson, M.; Sarabia Feria, N.; Yoshioka, A.; Tu, E.; Civitci, F.; Estes, S.; Wagner, J.T. A Caenorhabditis elegans behavioral assay distinguishes early stage prostate cancer patient urine from controls. Biology Open 2021, 10, bio057398. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Konno, M.; Ozaki, M.; Kawamoto, K.; Chijimatsu, R.; Kondo, N.; Hirotsu, T.; Ishii, H. Scent test using Caenorhabditis elegans to screen for early-stage pancreatic cancer. Oncotarget 2021, 12, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Fujita, A.; Ogawa, T.; Tanisaka, Y.; Mizuide, M.; Kondo, N.; Imaizumi, Y.; Hirotsu, T.; Ryozawa, S. Caenorhabditis elegans as a Diagnostic Aid for Pancreatic Cancer. Pancreas 2021, 50, 673–678. [Google Scholar] [CrossRef]
- Inaba, S.; Shimozono, N.; Yabuki, H.; Enomoto, M.; Morishita, M.; Hirotsu, T.; di Luccio, E. Accuracy evaluation of the C. elegans cancer test (N-NOSE) using a new combined method. Cancer Treatment and Research Communications 2021, 27, 100370. [Google Scholar] [CrossRef]
- Kusumoto, H.; Tashiro, K.; Shimaoka, S.; Tsukasa, K.; Baba, Y.; Furukawa, S.; Furukawa, J.; Niihara, T.; Hirotsu, T.; Uozumi, T. Efficiency of Gastrointestinal Cancer Detection by Nematode-NOSE (N-NOSE). In Vivo (Athens, Greece) 2020, 34, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, H.; Tashiro, K.; Shimaoka, S.; Tsukasa, K.; Baba, Y.; Furukawa, S.; Furukawa, J.; Suenaga, T.; Kitazono, M.; Tanaka, S.; et al. Behavioural Response Alteration in Caenorhabditis elegans to Urine After Surgical Removal of Cancer: Nematode-NOSE (N-NOSE) for Postoperative Evaluation. Biomarkers in Cancer 2019, 11, 1179299X19896551. [Google Scholar] [CrossRef]
- Ferrari, E.; Wittig, A.; Basilico, F.; Rossi, R.; De Palma, A.; Di Silvestre, D.; Sauerwein, W.A.G.; Mauri, P.L. Urinary Proteomics Profiles Are Useful for Detection of Cancer Biomarkers and Changes Induced by Therapeutic Procedures. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Minasian, L.M.; Pinsky, P.; Katki, H.A.; Dickherber, T.; Han, P.K.J.; Harris, L.; Patriotis, C.; Srivastava, S.; Weil, C.J.; Prorok, P.C.; et al. Study design considerations for trials to evaluate multicancer early detection assays for clinical utility. J Natl Cancer Inst 2023, 115, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Brito-Rocha, T.; Constancio, V.; Henrique, R.; Jeronimo, C. Shifting the Cancer Screening Paradigm: The Rising Potential of Blood-Based Multi-Cancer Early Detection Tests. Cells 2023, 12. [Google Scholar] [CrossRef]
- Massihnia, D.; Pizzutilo, E.G.; Amatu, A.; Tosi, F.; Ghezzi, S.; Bencardino, K.; Di Masi, P.; Righetti, E.; Patelli, G.; Scaglione, F.; et al. Liquid biopsy for rectal cancer: A systematic review. Cancer Treat Rev 2019, 79, 101893. [Google Scholar] [CrossRef]
- Lukaszewicz-Zajac, M.; Mroczko, B.; Kozlowski, M.; Niklinski, J.; Laudanski, J.; Szmitkowski, M. Higher importance of interleukin 6 than classic tumor markers (carcinoembryonic antigen and squamous cell cancer antigen) in the diagnosis of esophageal cancer patients. Dis Esophagus 2012, 25, 242–249. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).