1. Introduction
Surgical repair and postoperative management for the full-thickness macular hole (MH) has evolved significantly since Kelly and Wendel first described the benefit of vitrectomy and gas tamponade in 1991 [
1]. The results showed that the anatomical closure rate of the macular hole (MH) after vitrectomy with 1 week of maintaining posturing was 58%. Current MH surgery usually consists of pars plana vitrectomy (PPV) with the removal of all traction around the MH, gas tamponade, and posturing. This has increased the closure rate, resulting in a high success rate [
2,
3]. Tornambe et al. first stated that a facedown posture was not necessary, and other studies have suggested a shortening of the posturing period [
4,
5,
6,
7]. A multicenter randomized controlled study showed that the MH closure rate without facedown posturing was 90% for small idiopathic MHs [
8]. However, there are still concerns that shortening posturing or non-posturing may lead to failure in some eyes [
9]. The uninhibited posture of the facedown position can be a great burden for the elderly, with physical, emotional, and medical risks. Therefore, it is desirable to decrease the amount of time spent in this position. To maximize the success rate of MH closure while minimizing the inconvenience on patients, the facedown posturing should be released after confirming that the MH is closed.
The inability to visualize the macula in gas-filled eyes during early postoperative periods can be one of the most important obstacles for the evaluation of the sealing process in eyes with MH. The advent of optical coherence tomography (OCT) enables comprehensive visualization of the microstructure of the macula by providing high-resolution, cross-sectional tomographic imaging [
10]. If OCT permits early postoperative evaluation of the macula in gas-filled eyes, then it may provide useful clinical information that allows the retinal surgeon to make a clinical decision about when the patient can release the facedown position. Several reports have used spectral domain OCT (SD-OCT) to determine whether the MH was closed in gas-filled eyes [
11,
12,
13]. SD-OCT uses light sources of wavelengths near 800 nm, whereas swept-source OCT (SS-OCT) uses those longer than 1,000 nm. The difference leads SS-OCT to perform significantly better than SD-OCT to visualize the macula in gas-filled eyes [
14].
The purpose of this study was to evaluate the closure of idiopathic full-thickness MHs the day after surgery in minimizing the burden and maximizing outcome. For this purpose, we confirmed the closure of the MH using SS-OCT from the day after MH surgery. Facedown positioning was terminated in cases where MH closure was confirmed using SS-OCT, and facedown positioning was continued in cases where closure was not confirmed. After the gas volume was reduced to less than half of the vitreous cavity, the closure of the MH was reconfirmed using SD-OCT.
2. Patients and Methods
This was a retrospective cross-sectional, single-center study. The Ethics Committee of Akita University Hospital (Akita, Japan) approved the procedures, and the procedures conformed to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants after explaining the nature and possible complications of the study.
2.1. Subjects and Testing Protocol
Patients who had been diagnosed with idiopathic MH between January 2021 and August 2022 at the Department of Ophthalmology of Akita University Hospital were surgically treated for MH. All patients underwent a comprehensive ophthalmic examination, including measurements of best-corrected visual acuity (BCVA), intraocular pressure (IOP), axial length, slit-lamp examination, and fundus examination. Snellen VA values were converted into the logarithm of the minimum angle of resolution (LogMAR) units to generate a linear scale of VA.
Exclusion criteria were a high myopia (axial length ≥27 mm), preexisting macular conditions (e.g., epiretinal membrane (ERM), macular degeneration, vascular occlusive diseases, or diabetic retinopathy), secondary MH, history of vitrectomy, and inability to maintain posturing. Patients were also excluded if their SS-OCT measurements showed poor scan quality [
15] or artifacts (defocus, blink lines, or motion artifacts).
2.2. Surgical Techniques
Standard three-port pars plana vitrectomy (PPV) was performed with 25-gauge (G) instruments. To begin the PPV procedure, a trocar was inserted at an approximate angle of 30° to the limbus. Once the trocar was past the trocar sleeve, the angle was changed to be perpendicular to the retinal surface. After creating the three ports, PPV was performed using the Alcon Constellation system (Alcon Laboratories, Inc., Fort Worth, TX, USA). After completion of the core vitrectomy, a posterior vitreous detachment was created if it was not present. Then, the ILM was peeled from the retina using ILM-peeling forceps (25G ILM forceps MAS25-25; HOYA surgical optics, Gamagori, Aichi, Japan) assisted by triamcinolone acetonide. Fluid-air exchange was performed; then, 20% sulfur hexafluoride (SF6) was injected into the vitreous upon completion of the PPV. After the IOP was adjusted to normal levels, the cannulas were withdrawn, and the sclera was pressed and massaged with an indenter to close the wound. The patients were instructed to maintain a facedown position after surgery. Facedown positioning was terminated in cases where MH closure was confirmed using SS-OCT, and facedown positioning was continued in cases where the closure was not confirmed.
Cataract surgery was performed on all phakic eyes, and a foldable acrylic IOL was implanted into the capsular bag.
2.3. SS-OCT Imaging
A PLEX Elite
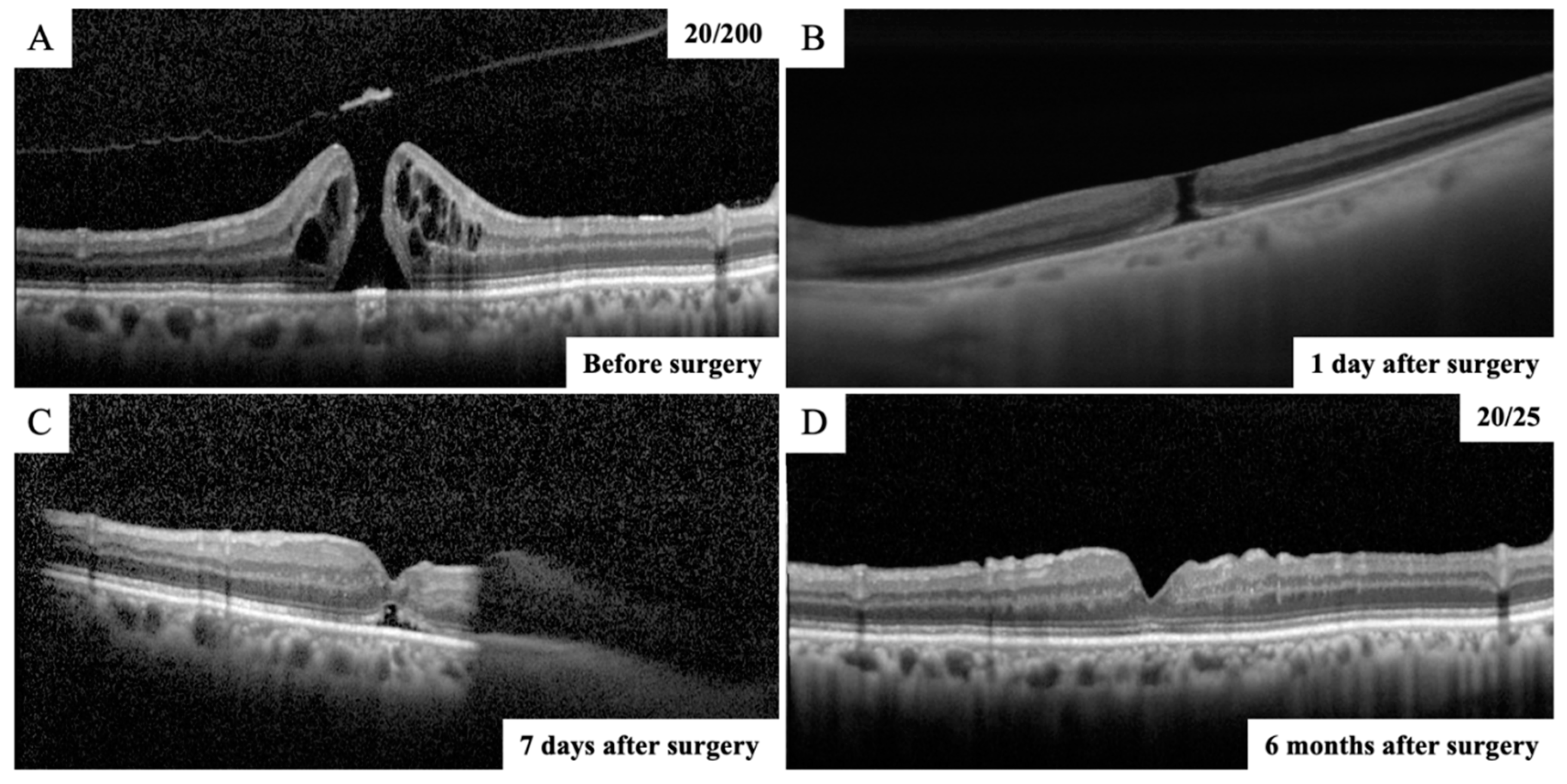
® (Carl Zeiss Meditec, Dublin, CA) is a SS-OCT instrument that uses a swept laser source with a central wavelength from 1040 to 1060 nm (980–1120 nm full bandwidth) and operates at 100,000 A-scans per second. The SS-OCT devices provide line scans. In this study, high-quality 6 mm horizontal line scans were obtained through the fovea and used for analyses. All the OCT scans were performed twice to minimize the possibility of accidental poor performance during OCT examination, and the higher-quality images were used for analyses. The closure of MH was confirmed using the SS-OCT from the next day after surgery (
Figure 1). The patients were divided into two groups, a MH closure group and a non-closure group using SS-OCT examination, depending on whether the MH was closed on the next day after surgery.
2.4. SD-OCT Imaging
A Spectralis
® SD-OCT (Heidelberg Engineering, Heidelberg, Germany) was used to obtain all SD-OCT images before surgery and after the gas volume was reduced to less than half of the vitreous cavity. The idiopathic MHs were graded according to the Gass classification [
16]. The images of the retina obtained by SD-OCT B scans through the center of the MH were analyzed. The basal MH size and the minimum MH size were measured in the vertical and horizontal OCT images. The average of two measurements was used for the statistical analyses. After the gas volume was reduced to less than half of the vitreous cavity, the closure of the MH was reconfirmed using the SD-OCT (
Figure 1).
2.5. Statistical Analyses
All statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation. The paired t-test was used to compare the visual acuity between before and after surgery. The unpaired t-test was used to compare the MH size data between the closure and non-closure group. A P value of <0.05 was considered significant.
3. Results
3.1. Clinical Characteristics of the Subjects
In total, 54 eyes of 54 patients with a MH underwent vitrectomy with ILM peeling between January 2021 and August 2022. Of these, 11 eyes were excluded: 6 for ERM, 1 for secondary MH from a rupture of retinal arterial macroaneurysm, 1 for branch retinal vein occlusion, 1 for retinoschisis, and 2 for poor scan quality on the next day after surgery. In the end, 43 eyes of 43 patients (mean age, 69.7 ± 8.6 years) were studied. The demographic and baseline characteristics are summarized in
Table 1.
The number of eyes with MH stage 1 was 4, stage 2 was 12, stage 3 was 15, and stage 4 was 12. The mean axial length was 23.92 ± 1.43 mm. The basal MH size and the minimum MH size were 677.7 ± 318.2 μm and 280.7 ± 165.2 μm, respectively. The preoperative BCVA was 0.69 ± 0.33 logMAR units, and it was significantly improved to 0.24 ± 0.40 logMAR units after surgery (P <0.001). Cataract surgery was performed on all 37 phakic eyes.
3.2. Macular Hole Closure Rate after Surgery
MH closure was confirmed in 40 eyes (93%) on the next day after surgery. The time from surgery to SS-OCT imaging was 24.7 ± 4.6 hours. In the remaining three eyes, MH closure could not be confirmed in the first few postoperative days, and as a result, the closure was confirmed by SD-OCT after the gas volume was reduced to less than half of the vitreous cavity. The MH was closed in 41 eyes (96%) on postoperative Day 7 and in 43 eyes (100%) on postoperative Day 10 (
Figure 2). The MH closure was reconfirmed using SD-OCT after the gas volume was reduced to less than half of the vitreous cavity in eyes in which MH closure was confirmed the day after surgery. All eyes were followed up for 6 months after surgery and it was confirmed that the MH was closed. Thus, all eyes with MH were successfully closed after the initial surgery in this study.
3.3. Comparison of the MH Size between the Closure Group and the Non-Closure Group on the next Day after Surgery
The basal MH size was 1067.5 ± 155.9 μm in the non-closure group and 648.4 ± 315.5 μm in the closure group, and it was significantly larger in the non-closure group than that in the closure group (p = 0.027) (
Figure 3). The minimum MH size was 467.3 ± 240.1 μm in the non-closure group and 267.2 ± 156.9 μm in the closure group, and it was significantly larger in the non-closure group than that in the closure group (p = 0.043).
4. Discussion
MH closure was confirmed in 40 eyes (93%) using SS-OCT in idiopathic MHs on the next day after surgery. The time from surgery to SS-OCT imaging was 24.7 hours. Despite facedown positioning was terminated in cases where MH closure was confirmed, and there were no cases in which the MH was reopened afterward. Eyes with no closure of the MH on the next day tended to have larger preoperative MH basal and minimum MH size.
With the modifications of the surgical technique, such as ILM peeling, MH has become a treatable disease with a high anatomical success rate reaching more than 90% [
2,
3]. Despite these improvements, there is continued debate over whether postoperative posturing is necessary. Since the immobility arising from prolonged postoperative facedown positioning negatively impacts the quality of life of patients and causes occasional but severe adverse events [
8,
17], there has been an attempt to alleviate patient discomfort by shortening the duration of posturing [
13,
18,
19,
20]. Tornambe et al. first stated that facedown posturing was not necessary [
4,
5,
6,
7]. In addition, a multicenter randomized controlled study showed that the MH closure rate without facedown posturing was 90% for small idiopathic MHs [
8]. Those results indicate that there are still concerns that shortening- or non- posturing might lead to failure in some eye.
Some investigators have utilized postoperative OCT analysis in gas-filled eyes to confirm early closure of the MH and to individualize the period of posturing [
11,
13,
20,
21,
22], Shah et al. proposed the concept of “OCT-guided facedown positioning” [
11]. This method was very effective in reducing positioning time without sacrificing the MH closure rate, since the hole was less likely to reopen after early postoperative filling. In that study, however, SD-OCT was used. The closure of MH was determined by SD-OCT in between 75 and 86 % of cases on postoperative Day 1 [
11,
23]. In our study, 93% of MH eyes were determined to be closed by SS-OCT on postoperative Day 1. It has been reported that SS-OCT performs better than SD-OCT for visualization of the macula in gas-filled eyes [
14]. Although we cannot directly compare our results with previous reports [
11,
23] because of the different backgrounds of the patients, the use of SS-OCT may be one of the reasons for the high number of cases in which closure of the MH at postoperative D1 was confirmed in this study. In addition, SS-OCT may be the better choice for imaging used for OCT-guided facedown positioning in gas-filled eyes.
The MH size was significantly larger in the non-closure group than that in the closure group in our study. In general, the prognosis for closure is correlated with the size of the hole [
24] [
25]. Recently, it has been reported that the MH size is significantly and positively correlated with the time of closure [
13], meaning that the larger the MH size, the longer the time to closure. In the present study, there was also a trend that the MH did not close on postoperative D1 in cases with larger MH sizes; in cases where the MH did not close on postoperative D1, MH closure was confirmed in a maximum of 10 days. However, the MH closure was difficult to confirm when the gas volume was a little more than half of the vitreous cavity; although the MH closure was confirmed at 10 days, it may have actually occurred a little earlier. Since the inverted flap technique was not used in this study, it may have taken longer to close the MH [
26]. In cases with larger MH sizes, preoperative explanation of the possibility of a prolonged period of facedown positioning and the use of the inverted flap technique should be considered.
After confirming closure of the MH by SS-OCT on the next day after surgery, the MH did not reopen, although facedown positioning was terminated after the confirmation. Reopening of the MH after surgery has been reported in between 2% and 10% of cases in the literature [
27]. Ip et al. reported that reopening larger than 400 μm was seen in MHs [
24]. In the present study, the MH size tended to be smaller in patients with MH closure on the day after surgery. As mentioned earlier, it has been reported that the MH size is significantly and positively correlated with the time of closure [
13]. Therefore, once closure of the MH was confirmed the day after the surgery, it was unlikely that the MH would have reopened even if facedown positioning was terminated, since the MH was not large in many of these cases.
This study has some limitations. First, this was a retrospective study with a relatively small sample size. Second, although the MH closure was confirmed daily after surgery, the MH closure was difficult to confirm when the gas volume was a little more than half of the vitreous cavity. Perhaps it would be more accurate to check the time of MH closure by taking frequent SS-OCT images, e.g., every 6 hours after surgery. However, this is a retrospective study, and it is not usual to take images frequently. Third, the inverted flap technique was not used for the treatment of MH in this study. It has been reported the inverted flap technique requires a shorter time and achieves higher success rates in closing the MH. The present method might be more effective because the MH closure may be obtained more quickly and reliably using the technique. Further prospective studies on a larger number of eyes with more frequent imaging to examine the closure time of the MH after surgery are required to confirm our method.
5. Conclusions
Closure was confirmed in 93% of eyes with MH using SS-OCT on the next day after surgery, and facedown positioning was terminated in cases where MH closure was confirmed using SS-OCT. In conclusion, the present method is useful in that it shortens the period of facedown positioning without causing the recurrence of MH, which is a great burden for the elderly.
Author Contributions
The design and conduct of the study (M.S., T.I.); collection of data (M.S.); management, analysis, and interpretation of data (M.S., T.I.); and preparation, review, and approval of the manuscript (M.S., T.I.).
Funding
This work was supported Grant-in-Aid for Scientific Research C (T.I., Number 20K09802) from JSPS KAKENHI (
http://www.jsps.go.jp/).
Institutional Review Board Statement
The study protocol conformed to the tenets of the Declaration of Helsinki for research involving human subjects. The protocol was approved by the Institutional Review Board of Akita University Graduate School of Medicine, and it conformed to the principles of Good Clinical Practice and the Helsinki Guidelines.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript
| OCT |
optical coherence tomography |
| SD |
spectral domain |
| BCVA |
best-corrected visual acuity |
| IOP |
intraocular pressure |
| LogMAR |
logarithm of the minimum angle of resolution |
| SS |
swept-source |
References
- Kelly, N.E.; Wendel, R.T. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991, 109, 654-659. [CrossRef]
- Wendel, R.T.; Patel, A.C.; Kelly, N.E.; Salzano, T.C.; Wells, J.W.; Novack, G.D. Vitreous surgery for macular holes. Ophthalmology. 1993, 100, 1671-1676. [CrossRef]
- Brooks, H.L., Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000, 107, 1939-48; discussion 1948-1949. [CrossRef]
- Tornambe, P.E.; Poliner, L.S.; Grote, K. Macular hole surgery without face-down positioning. A pilot study. Retina. 1997, 17, 179-185. [CrossRef]
- Dhawahir-Scala, F.E.; Maino, A.; Saha, K.; Mokashi, A.A.; McLauchlan, R.; Charles, S. To posture or not to posture after macular hole surgery. Retina. 2008, 28, 60-65. [CrossRef]
- Wickens, J.C.; Shah, G.K. Outcomes of macular hole surgery and shortened face down positioning. Retina. 2006, 26, 902-904. [CrossRef]
- Mittra, R.A.; Kim, J.E.; Han, D.P.; Pollack, J.S. Sustained postoperative face-down positioning is unnecessary for successful macular hole surgery. Br J Ophthalmol. 2009, 93, 664-666. [CrossRef]
- Tadayoni, R.; Vicaut, E.; Devin, F.; Creuzot-Garcher, C.; Berrod, J.P.; Le Mer, Y.; Korobelnik, J.F.; Aout, M.; Massin, P.; Gaudric, A. A randomized controlled trial of alleviated positioning after small macular hole surgery. Ophthalmology. 2011, 118, 150-155. [CrossRef]
- Krohn, J. Duration of face-down positioning after macular hole surgery: a comparison between 1 week and 3 days. Acta Ophthalmol Scand. 2005, 83, 289-292. [CrossRef]
- Fujimoto, J.G. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003, 21, 1361-1367. [CrossRef]
- Shah, S.P.; Manjunath, V.; Rogers, A.H.; Baumal, C.R.; Reichel, E.; Duker, J.S. Optical coherence tomography-guided facedown positioning for macular hole surgery. Retina. 2013, 33, 356-362. [CrossRef]
- Yamashita, T.; Yamashita, T.; Kawano, H.; Sonoda, Y.; Yamakiri, K.; Sakamoto, T. Early imaging of macular hole closure: a diagnostic technique and its quality for gas-filled eyes with spectral domain optical coherence tomography. Ophthalmologica. 2013, 229, 43-49. [CrossRef]
- Yamashita, T.; Sakamoto, T.; Yamashita, T.; Sonoda, S.; Yamakiri, K.; Otsuka, H.; Hisatomi, T.; Imaki, H.; Ishibashi, T.; Dugel, P.U. Individualized, spectral domain-optical coherence tomography-guided facedown posturing after macular hole surgery: minimizing treatment burden and maximizing outcome. Retina. 2014, 34, 1367-1375. [CrossRef]
- Ahn, S.J.; Park, S.H.; Lee, B.R. VISUALIZATION OF THE MACULA IN GAS-FILLED EYES: Spectral Domain Optical Coherence Tomography Versus Swept-Source Optical Coherence Tomography. Retina. 2018, 38, 480-489. [CrossRef]
- Shoji, T.; Yoshikawa, Y.; Kanno, J.; Ishii, H.; Ibuki, H.; Ozaki, K.; Kimura, I.; Shinoda, K. Reproducibility of Macular Vessel Density Calculations Via Imaging With Two Different Swept-Source Optical Coherence Tomography Angiography Systems. Transl Vis Sci Technol. 2018, 7, 31. [CrossRef]
- Gass, J.D. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995, 119, 752-759. [CrossRef]
- Au Eong, K.G.; Beatty, S.; Thomas, W.; Sen, V.; Turner, G.S. Pulmonary embolism following head positioning for retinal reattachment surgery in a young patient with factor V leiden mutation. Arch Ophthalmol. 2000, 118, 1300-1301.
- Nadal, J.; Delas, B.; Piñero, A. Vitrectomy without face-down posturing for idiopathic macular holes. Retina. 2012, 32, 918-921. [CrossRef]
- Guillaubey, A.; Malvitte, L.; Lafontaine, P.O.; Jay, N.; Hubert, I.; Bron, A.; Berrod, J.P.; Creuzot-Garcher, C. Comparison of face-down and seated position after idiopathic macular hole surgery: a randomized clinical trial. Am J Ophthalmol. 2008, 146, 128-134. [CrossRef]
- Chow, D.R.; Chaudhary, K.M. Optical coherence tomography-based positioning regimen for macular hole surgery. Retina. 2015, 35, 899-907. [CrossRef]
- Masuyama, K.; Yamakiri, K.; Arimura, N.; Sonoda, Y.; Doi, N.; Sakamoto, T. Posturing time after macular hole surgery modified by optical coherence tomography images: a pilot study. Am J Ophthalmol. 2009, 147, 481-488.e2. [CrossRef]
- Eckardt, C.; Eckert, T.; Eckardt, U.; Porkert, U.; Gesser, C. Macular hole surgery with air tamponade and optical coherence tomography-based duration of face-down positioning. Retina. 2008, 28, 1087-1096. [CrossRef]
- Ehlers, J.P.; Uchida, A.; Srivastava, S.K.; Hu, M. Predictive Model for Macular Hole Closure Speed: Insights From Intraoperative Optical Coherence Tomography. Transl Vis Sci Technol. 2019, 8, 18. [CrossRef]
- Ip, M.S.; Baker, B.J.; Duker, J.S.; Reichel, E.; Baumal, C.R.; Gangnon, R.; Puliafito, C.A. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002, 120, 29-35. [CrossRef]
- Duker, J.S.; Kaiser, P.K.; Binder, S.; de Smet, M.D.; Gaudric, A.; Reichel, E.; Sadda, S.R.; Sebag, J.; Spaide, R.F.; Stalmans, P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013, 120, 2611-2619. [CrossRef]
- Michalewska, Z.; Michalewski, J.; Adelman, R.A.; Nawrocki, J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010, 117, 2018-2025. [CrossRef]
- Benson, W.E.; Cruickshanks, K.C.; Fong, D.S.; Williams, G.A.; Bloome, M.A.; Frambach, D.A.; Kreiger, A.E.; Murphy, R.P. Surgical management of macular holes: a report by the American Academy of Ophthalmology. Ophthalmology. 2001, 108, 1328-1335. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).