Submitted:
29 June 2023
Posted:
03 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
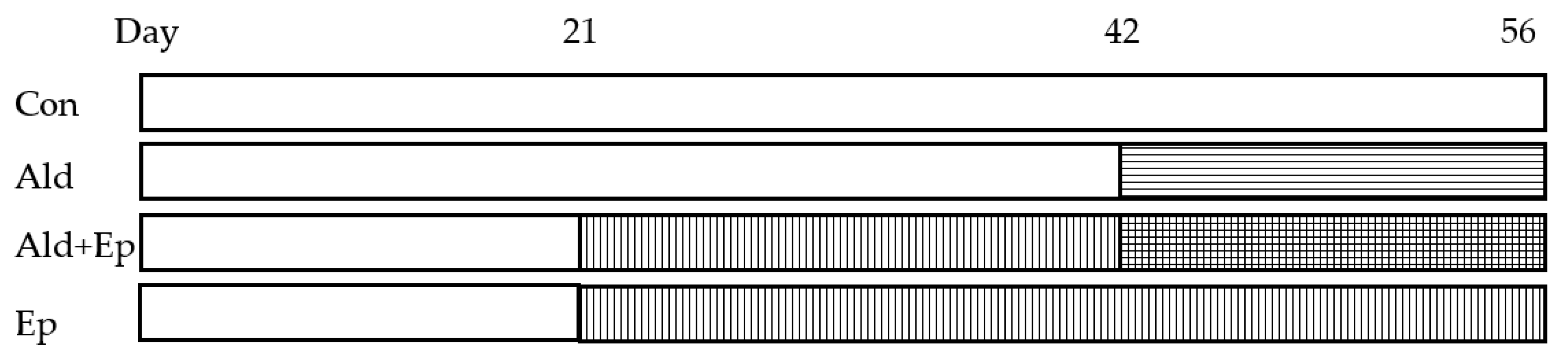
2.1. Treatment of animals
2.2. Organ extraction and sample preparation
2.3. Morphometric analysis
2.4. Western blotting
2.5. Real-time qPCR
2.6. Statistical Analysis
3. Results
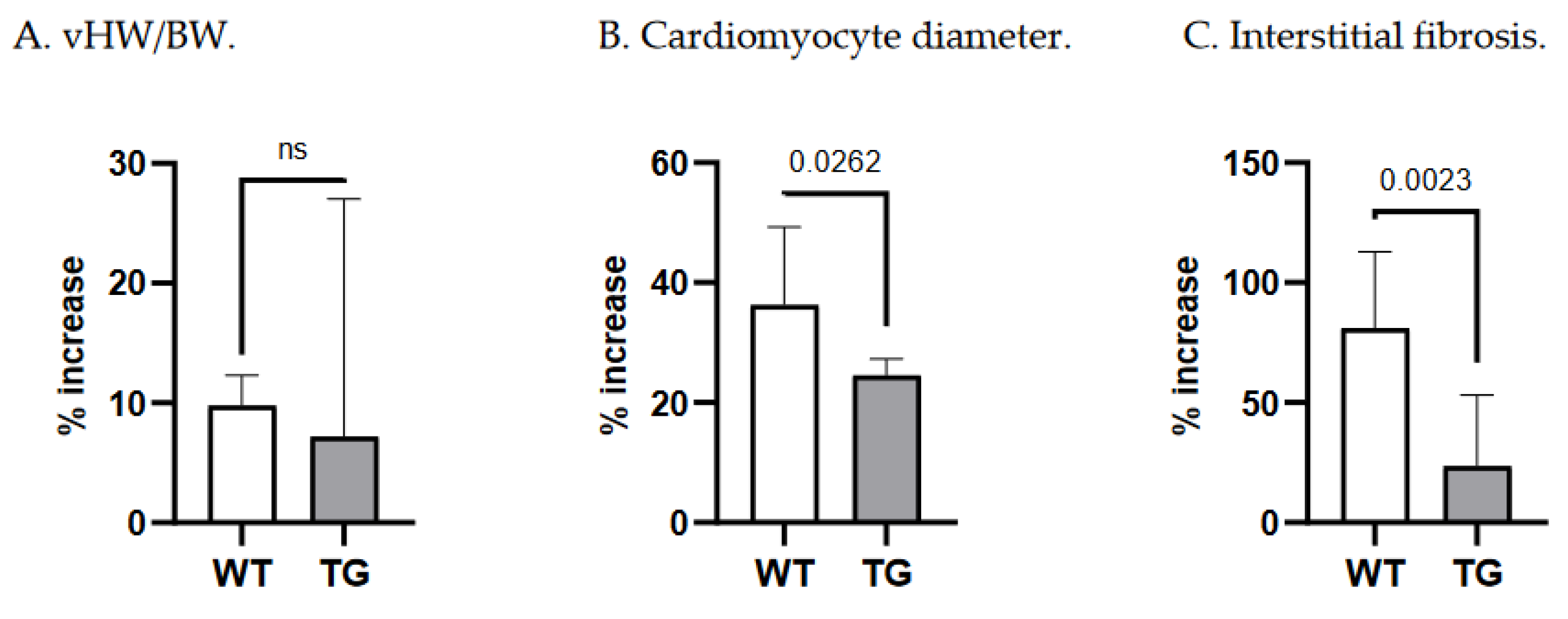
3.1. Baseline characteristics of TGF-β1 mice
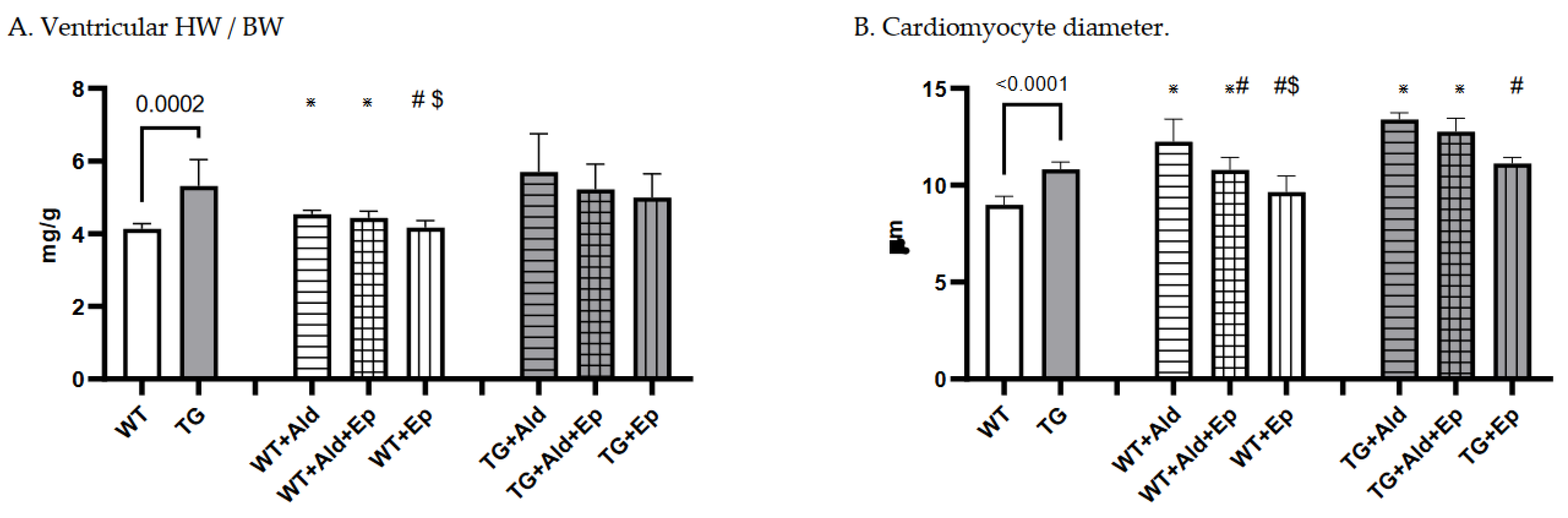
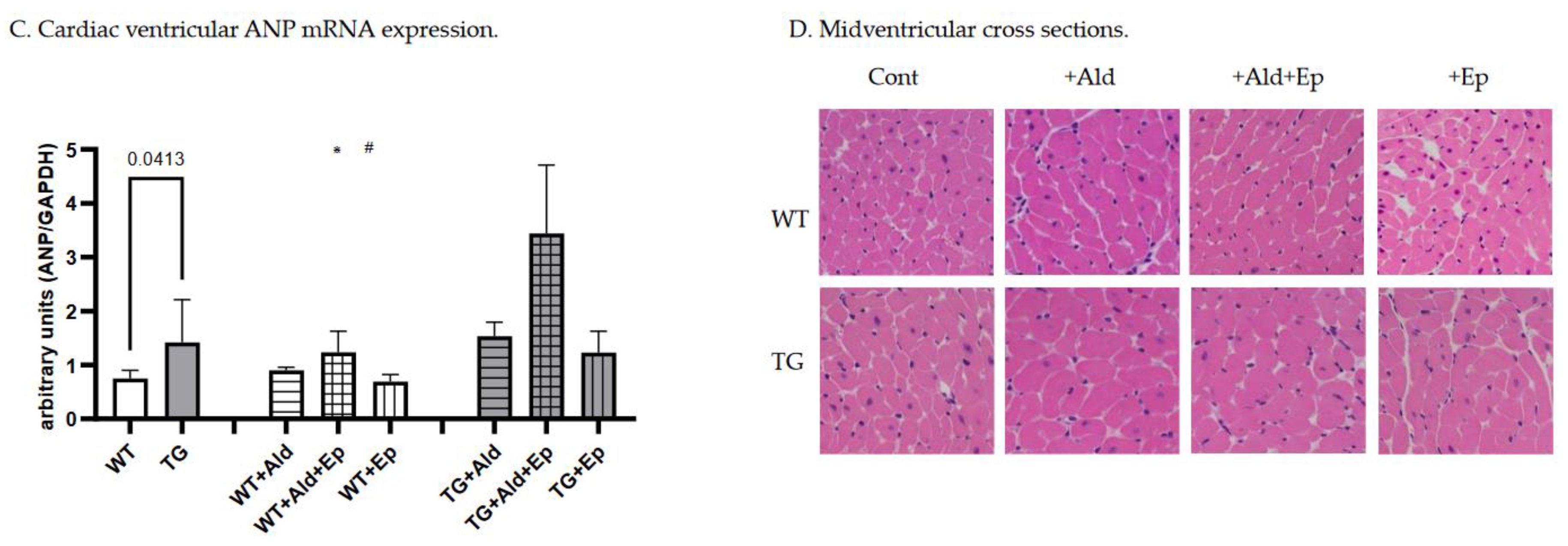
3.2. Effects of Ald and Ep on cardiac hypertrophy
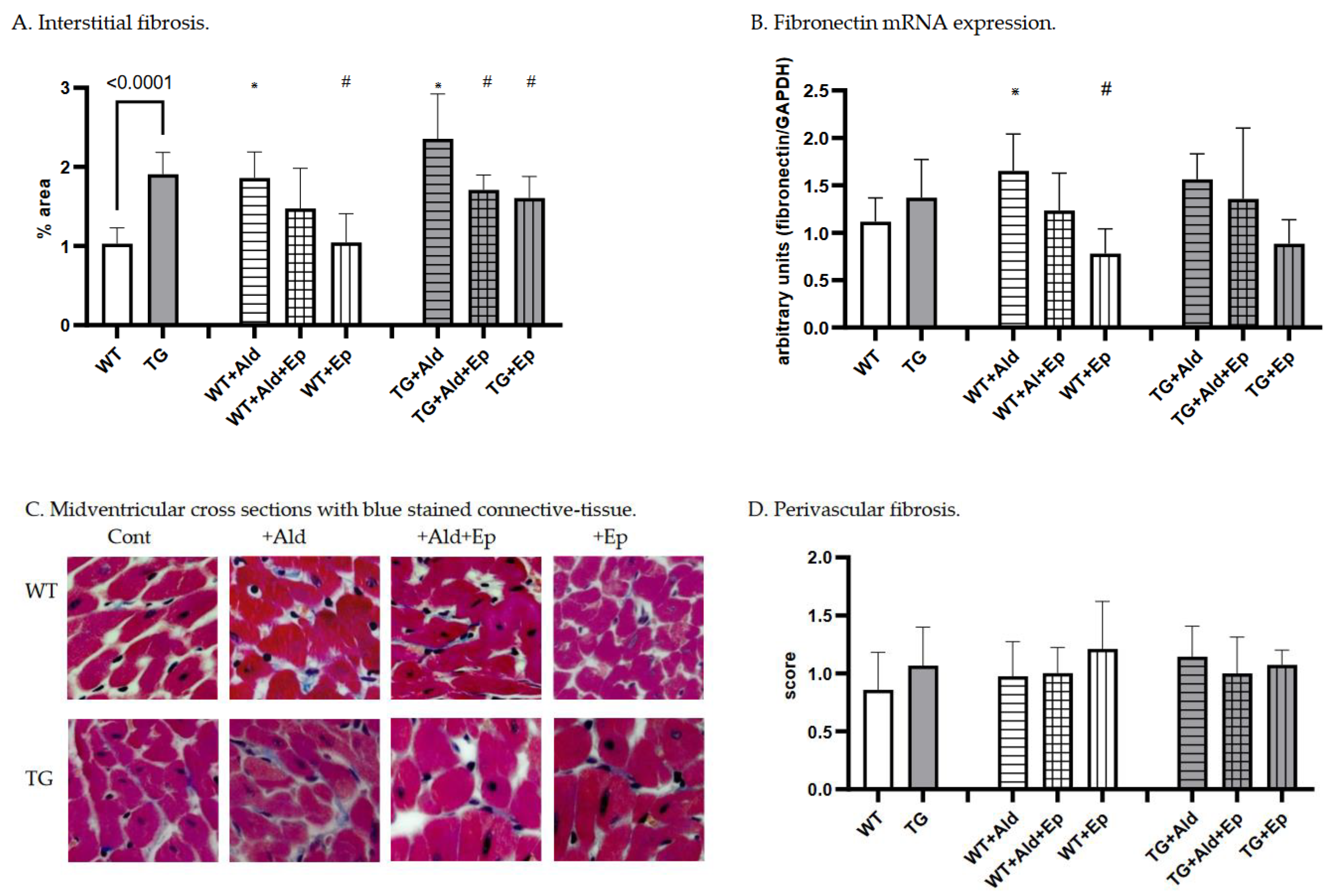
3.3. Effects of Ald and Ep on cardiac fibrosis
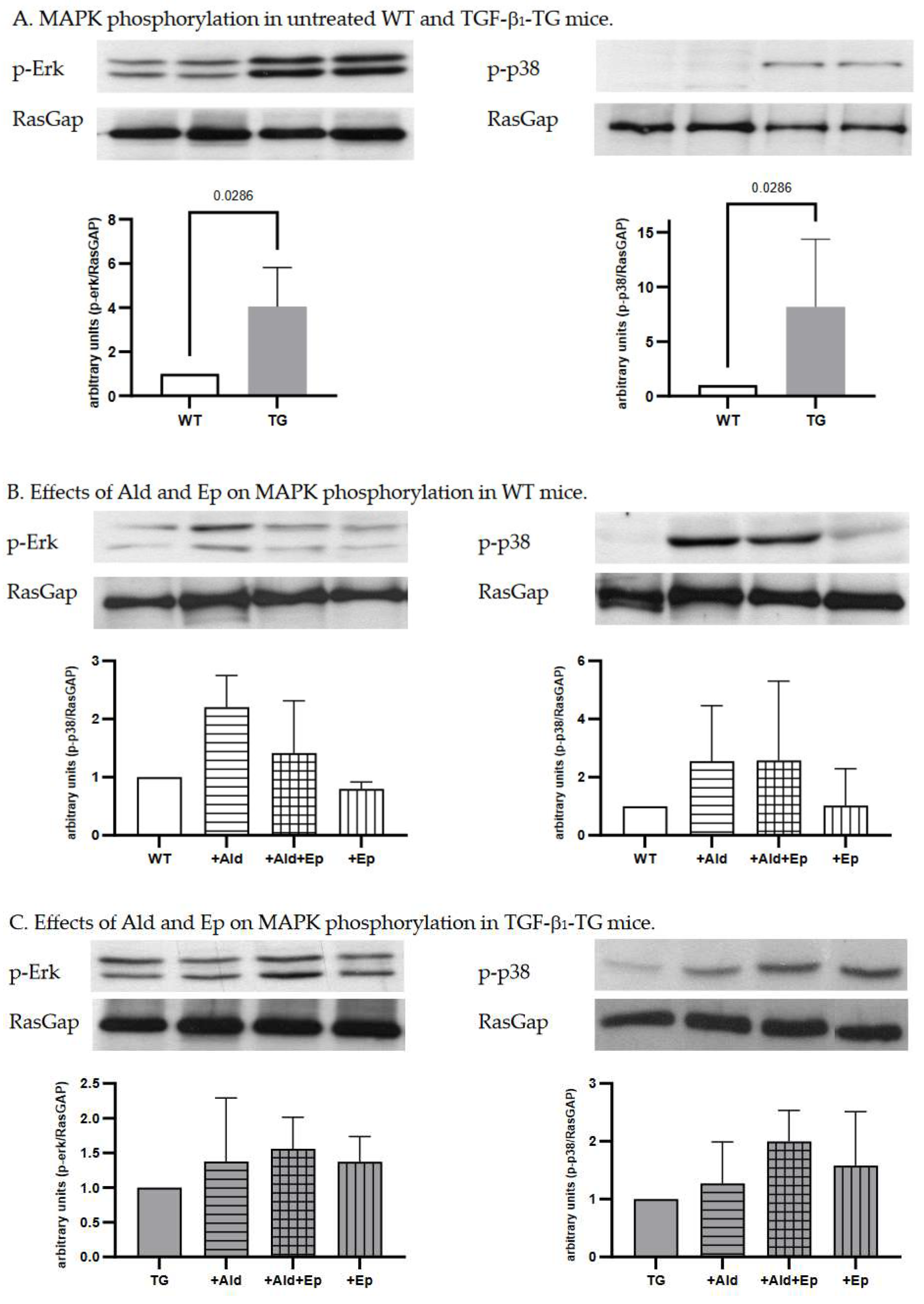
3.4. Effects of Ald and Ep on MAPK-signalling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Buffolo, F.; Tetti, M.; Mulatero, P.; Monticone, S. Aldosterone as a Mediator of Cardiovascular Damage. Hypertension 2022, 79, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Xiao H, Zhang YY. Understanding the role of transforming growth factor-beta signalling in the heart: overview of studies using genetic mouse models. Clin Exp Pharmacol Physiol. 2008, 35, 335–341. [CrossRef]
- Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The Unrecognized Prevalence of Primary Aldosteronism: A Cross-sectional Study. Annals of Internal Medicine. 2020, 173, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Hundemer, G.L.; Nanba, K.; Parksook, W.W.; Brown, J.M. Primary Aldosteronism: State-of-the-Art Review. Am. J. Hypertens. 2022, 35, 967–988. [Google Scholar] [CrossRef]
- Funder JW, Carey RM. Primary Aldosteronism: Where Are We Now? Where to From Here? Hypertension. 2022, 79, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Rosenkranz, S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004, 63, 423–432. [Google Scholar] [CrossRef]
- Frangogiannis, NG. Cardiac fibrosis. Cardiovasc Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Schultz JEJ, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002, 109, 787–796. [Google Scholar] [CrossRef]
- Scaglione R, Argano C, Di Chiara T, Parrinello G, Colomba D, Avellone G, et al. Effect of dual blockade of renin-angiotensin system on TGFbeta1 and left ventricular structure and function in hypertensive patients. J Hum Hypertens. 2007, 21, 307–315. [Google Scholar] [CrossRef]
- Schluter, K.D.; Zhou, X.J.; Piper, H.M. Induction of hypertrophic responsiveness to isoproterenol by TGF-beta in adult rat cardiomyocytes. Am. J. Physiol. Physiol. 1995, 269, C1311–C1316. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, N.; Factor, V.; Nagy, P.; Kopp, J.; Kondaiah, P.; Wakefield, L.; Roberts, A.B.; Sporn, M.B.; Thorgeirsson, S.S. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc. Natl. Acad. Sci. USA 1995, 92, 2572–2576. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Hurta, R.; Kondaiah, P.; Khalil, N.; Turley, E.; Wright, J.; Greenberg, A. Autocrine induction of tumor protease production and invasion by a metallothionein-regulated TGF-beta 1 (Ser223, 225). EMBO J. 1992, 11, 1599–1605. [Google Scholar] [CrossRef]
- Bruder-Nascimento, T.; Ferreira, N.S.; Zanotto, C.Z.; Ramalho, F.; Pequeno, I.O.; Olivon, V.C.; Neves, K.B.; Alves-Lopes, R.; Campos, E.; Silva, C.A.A.; et al. NLRP3 Inflammasome Mediates Aldosterone-Induced Vascular Damage. Circulation 2016, 134, 1866–1880. [Google Scholar] [CrossRef] [PubMed]
- Stael, S.; Miller, L.P. ; Fernández-Fernández, D.; Van Breusegem, F. Detection of Damage-Activated Metacaspase Activity by Western Blot in Plants. 2022, 2447, 127–137. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb–prot5439. [Google Scholar] [CrossRef] [PubMed]
- Kakoki, M.; Pochynyuk, O.M.; Hathaway, C.M.; Tomita, H.; Hagaman, J.R.; Kim, H.-S.; Zaika, O.L.; Mamenko, M.; Kayashima, Y.; Matsuki, K.; et al. Primary aldosteronism and impaired natriuresis in mice underexpressing TGFβ1. Proc. Natl. Acad. Sci. 2013, 110, 5600–5605. [Google Scholar] [CrossRef]
- Nishioka, T.; Suzuki, M.; Onishi, K.; Takakura, N.; Inada, H.; Yoshida, T.; Hiroe, M.; Imanaka-Yoshida, K. Eplerenone Attenuates Myocardial Fibrosis in the Angiotensin II-Induced Hypertensive Mouse: Involvement of Tenascin-C Induced by Aldosterone-Mediated Inflammation. J. Cardiovasc. Pharmacol. 2007, 49, 261–268. [Google Scholar] [CrossRef]
- Tsybouleva, N.; Zhang, L.; Chen, S.; Patel, R.; Lutucuta, S.; Nemoto, S.; DeFreitas, G.; Entman, M.; Carabello, B.A.; Roberts, R.; et al. Aldosterone, Through Novel Signaling Proteins, Is a Fundamental Molecular Bridge Between the Genetic Defect and the Cardiac Phenotype of Hypertrophic Cardiomyopathy. Circulation 2004, 109, 1284–1291. [Google Scholar] [CrossRef]
- Han JS, Choi BS, Yang CW, Kim YS. Aldosterone-induced TGF-beta1 expression is regulated by mitogen-activated protein kinases and activator protein-1 in mesangial cells. J Korean Med Sci. 2009, 24, S195–203. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Takeda, Y.; Karashima, S.; Kometani, M.; Aono, D.; Demura, M.; Higashitani, T.; Konishi, S.; Yoneda, T.; Takeda, Y. Impact of mineralocorticoid receptor blockade with direct renin inhibition in angiotensin II-dependent hypertensive mice. Hypertens. Res. 2020, 43, 1099–1104. [Google Scholar] [CrossRef]
- Zhang Y, Shao L, Ma A, Guan G, Wang J, Wang Y, et al. Telmisartan delays myocardial fibrosis in rats with hypertensive left ventricular hypertrophy by TGF-β1/Smad signal pathway. Hypertens Res. 2014, 37, 43–9. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-F.; Yang, S.-F.; Chu, H.-J.; Ueng, K.-C. Cross-talk between mineralocorticoid receptor/angiotensin II type 1 receptor and mitogen-activated protein kinase pathways underlies aldosterone-induced atrial fibrotic responses in HL-1 cardiomyocytes. Int. J. Cardiol. 2013, 169, 17–28. [Google Scholar] [CrossRef]
- Matsuki, K.; Hathaway, C.K.; Chang, A.S.; Smithies, O.; Kakoki, M. Transforming growth factor beta1 and aldosterone. Curr. Opin. Nephrol. Hypertens. 2015, 24, 139–144. [Google Scholar] [CrossRef]
- Khan, R. Examining Potential Therapies Targeting Myocardial Fibrosis through the Inhibition of Transforming Growth Factor-Beta 1. Cardiology 2007, 108, 368–380. [Google Scholar] [CrossRef]
- Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol. 2002, 283, H1253–1262. [Google Scholar] [CrossRef]
- Seeland U, Haeuseler C, Hinrichs R, Rosenkranz S, Pfitzner T, Scharffetter-Kochanek K, et al. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. 2002, 32, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Seeland U, Schäffer A, Selejan S, Hohl M, Reil JC, Müller P, et al. Effects of AT1- and beta-adrenergic receptor antagonists on TGF-beta1-induced fibrosis in transgenic mice. Eur J Clin Invest. 2009, 39, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Nakajima H, Nakajima HO, Salcher O, Dittiè AS, Dembowsky K, Jing S, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000, 86, 571–579. [Google Scholar] [CrossRef]
- Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol. 2000, 32, 187–195. [Google Scholar] [CrossRef]
- Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002, 106, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Burch ML, Yang SNY, Ballinger ML, Getachew R, Osman N, Little PJ. TGF-beta stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad2. Cell Mol Life Sci. 2010, 67, 2077–2790. [Google Scholar] [CrossRef]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006, 70, 1992–2003. [Google Scholar] [CrossRef]
- Muslin, A.J. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin. Sci. 2008, 115, 203–218. [Google Scholar] [CrossRef]
- Zhang, D.; Gaussin, V.; Taffet, G.E.; Belaguli, N.S.; Yamada, M.; Schwartz, R.J.; Michael, L.H.; Overbeek, P.A.; Schneider, M.D. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat. Med. 2000, 6, 556–563. [Google Scholar] [CrossRef]
- Romero-Becerra, R.; Santamans, A.M.; Folgueira, C.; Sabio, G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int. J. Mol. Sci. 2020, 21, 7412. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Vitacolonna, A.; Bonzano, A.; Comoglio, P.; Crepaldi, T. ERK: A Key Player in the Pathophysiology of Cardiac Hypertrophy. Int. J. Mol. Sci. 2019, 20, 2164. [Google Scholar] [CrossRef]
- Duangrat, R.; Parichatikanond, W.; Morales, N.P.; Pinthong, D.; Mangmool, S. Sustained AT1R stimulation induces upregulation of growth factors in human cardiac fibroblasts via Gαq/TGF-β/ERK signaling that influences myocyte hypertrophy. Eur. J. Pharmacol. 2022, 937, 175384. [Google Scholar] [CrossRef]
- Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000, 19, 6341–6350. [Google Scholar] [CrossRef]
- Yoshida, K.; Kim-Mitsuyama, S.; Wake, R.; Izumiya, Y.; Izumi, Y.; Yukimura, T.; Ueda, M.; Yoshiyama, M.; Iwao, H. Excess Aldosterone under Normal Salt Diet Induces Cardiac Hypertrophy and Infiltration via Oxidative Stress. Hypertens. Res. 2005, 28, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Kobayashi, N.; Yoshida, K.; Ohno, T.; Matsuoka, H. Cardioprotective Mechanisms of Spironolactone Associated with the Angiotensin-Converting Enzyme/Epidermal Growth Factor Receptor/Extracellular Signal-Regulated Kinases, NAD(P)H Oxidase/Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1, and Rho-Kinase Pathways in Aldosterone/Salt-Induced Hypertensive Rats. Hypertens. Res. 2005, 28, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Rudolph, A.E.; Bond, B.R.; Rocha, R.; Blomme, E.A.; Goellner, J.J.; Funder, J.W.; McMahon, E.G.; K, T.; R, W.; et al. Transgenic Model of Aldosterone-Driven Cardiac Hypertrophy and Heart Failure. Circ. Res. 2003, 93, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction wtih the renin-angiotension system. Am J Hypertens. 2004, 17, 597–603. [Google Scholar] [CrossRef]
- Johar, S.; Cave, A.C.; Narayanapanicker, A.; Grieve, D.J.; Shah, A.M. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006, 20, 1546–1548. [Google Scholar] [CrossRef]
- Brilla, C.G. Aldosterone and myocardial fibrosis in heart failure. Herz 2000, 25, 299–306. [Google Scholar] [CrossRef]
- Tsukamoto, O.; Minamino, T.; Sanada, S.; Okada, K.-I.; Hirata, A.; Fujita, M.; Shintani, Y.; Yulin, L.; Asano, Y.; Takashima, S.; et al. The Antagonism of Aldosterone Receptor Prevents the Development of Hypertensive Heart Failure Induced by Chronic Inhibition of Nitric Oxide Synthesis in Rats. Cardiovasc. Drugs Ther. 2006, 20, 93–102. [Google Scholar] [CrossRef]
- Okoshi, M.P.; Yan, X.; Okoshi, K.; Nakayama, M.; Schuldt, A.J.; O'Connell, T.D.; Simpson, P.C.; Lorell, B.H. Aldosterone directly stimulates cardiac myocyte hypertrophy. J. Card. Fail. 2004, 10, 511–518. [Google Scholar] [CrossRef]
- Nehme, J.; Mercier, N.; Labat, C.; Benetos, A.; E Safar, M.; Delcayre, C.; Lacolley, P. Differences Between Cardiac and Arterial Fibrosis and Stiffness in Aldosterone-Salt Rats: Effect of Eplerenone. J. Renin-Angiotensin-Aldosterone Syst. 2006, 7, 31–39. [Google Scholar] [CrossRef]
| WT | TG |
p |
WT+Ald | WT+Ald+Ep |
WT+Ep |
p (WT) |
TG+Ald | TG+Ald+Ep |
TG+Ep | p (TG) |
||
| Ventricular HW /BW |
n | 8 | 14 |
<10-3 |
7 | 7 | 8 |
<10-4 |
9 | 9 | 9 |
0.31 |
| mg/g | 4.1±0.1 | 5.3±0.7 | 4.5±0.1* | 4.4±0.2* | 4.2±0.2#$ | 5.7±1.1 | 5.2±0.7 | 5±0.6 | ||||
| Cardiomyocyte diameter | n | 8 | 9 |
<10-4 |
7 | 7 | 8 |
<10-4 |
9 | 9 | 9 |
<10-4 |
| µm | 8.8(0.8) | 10.9(0.3) | 12.3±1.2* | 10.8±0.6*# | 9.6±0.8#$ | 13.4(0.5)* | 12.8(1.1)* | 11(0.5)# | ||||
| ANP/GAPDH mRNA |
n | 7 | 14 |
0.041 |
6 | 7 | 6 |
10-3 |
7 | 7 | 6 |
<10-4 |
| - | 0.8±0.2 | 1.4±0.8 | 0.9±0.06 | 1.2±0.4* | 0.7±0.1$ | 1.5±0.3 | 3.4±1.3*# | 1.2±0.4$ | ||||
| Interstitial fibrosis |
n | 8 | 9 |
<10-4 |
7 | 7 | 8 |
<10-3 |
9 | 9 | 10 |
<10-3 |
| % | 1±0.1 | 1.9±0.1 | 1.9±0.1* | 1.5±0.2 | 1.05±0.1# | 2.4±0.2* | 1.7±0.1# | 1.61±0.1# | ||||
| Perivascular fibrosis |
n | 8 | 9 |
0.21 |
7 | 6 | 6 |
0.27 |
9 | 9 | 10 |
0.73 |
| score | 0.9±0.3 | 1.1±0.3 | 1±0.3 | 1±0.22 | 1.2± 0.4 | 1.2±0.26 | 1±0.3 | 1.1±0.1 | ||||
| FBN/GAPDH mRNA |
n | 7 | 15 |
0.15 |
7 | 7 | 7 |
<10-3 |
7 | 9 | 7 |
0.06 |
| - | 1.1±0.3 | 1.4±0.4 | 1.7±0.4* | 1.2±0.4 | 0.8±0.3# | 1.6±0.3 | 1.4±0.8 | 0.9±0.3 | ||||
| Kidney W /BW |
n | 8 | 14 |
0.15 |
7 | 10 | 8 |
<10-4 |
9 | 9 | 9 |
<10-4 |
| mg/g | 11.9±0.3 | 12.8±1.6 | 14.9±1.6* | 13.7±1.2* | 11.4±1.5#$ | 15.9±1.5* | 13±0.8# | 12.3±1.8# | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





