1. Introduction
Furan carboxamides are furan amides with various pharmacological activities, including antitumor [
1], antifungal [
2], antimicrobial [
3], anticancer [
4], and antidiabetic [
5]. The synthesis of amides commonly using thionyl chloride as coupling agent [
6], which not only produce toxic gas sulfur dioxide but also one of the chemicals that included in the list of Chemical Weapons Conventions (CWC) and the Law of the Republic of Indonesia Number 9 of 2008 concerning the Use of Chemicals and Prohibitions on the Use of Chemicals as Chemical Weapons [
7,
8]. Furan carboxamides have successfully synthesized using a hydroxybenzotriazol coupling reagent [
4], but this material is explosive so the use of it should be avoided [
9]. More environmentally-friendly synthesis of furan carboxamides can be carried out using 1,1’-carbonyldiimidazole (CDI) coupling agent [
10], however, it has low reactivity, especially when compared to similar materials [
11]. Alternately, synthesis of carboxamides by utilizing 2-methyl-6-nitrobenzoic anhydride (MNBA) and 4-dimethylaminopyridine (DMAP) as a coupling agent and a catalyst respectively, showing several advantages, including the reaction can be carried out in one pot at room temperature, and the resulting compounds having high yields and purity [
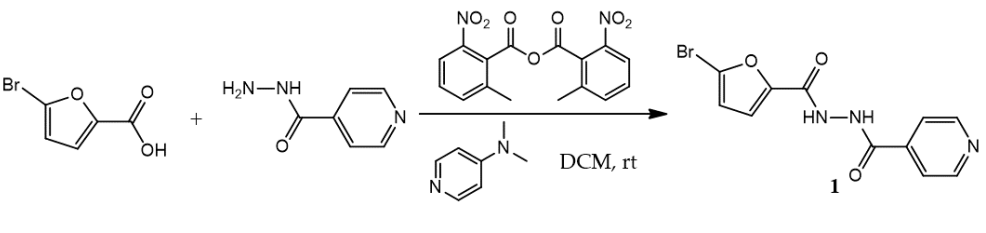
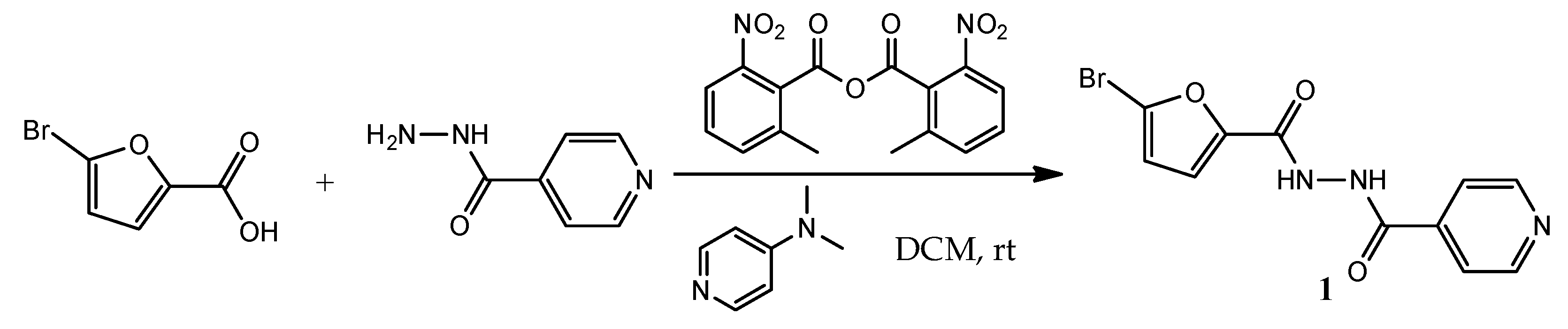
12]. Therefore, this study aimed to utilize MNBA/DMAP for the synthesis of furan carboxamide from furoic acid and, namely
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
(1) with a molecular docking study to understand its molecular behavior against cyclooxygenase-2 (COX-2) protein.
2. Results and Discussion
2.1. Chemistry
The synthesis of
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
(1) was carried out by utilizing the reaction of 5-bromofuran-2-carboxylic acid and isoniazid with the presence of MNBA and DMAP in dichloromethane at room temperature (
Scheme 1). The crude product was purified by dry-column flash chromatography to afford pure product
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
(1) as a white solid in 83% yield. The
1H NMR spectrum of the product clearly showed two doublet signals at δ 6.84 and 7.32 ppm indicating two furanyl protons, two doublet of doublets signals at δ 7.80 and 8.79 ppm indicating four pyridinyl protons, and two broad singlet signals at δ 10.62 and 10.81 ppm indicating proton signals of the NH groups. The presence of the NH group was confirmed by the IR spectrum which showed a single absorption at a wavenumber of 3169 cm
-1 indicating the presence of a secondary NH group. It is also supported by the
13C NMR spectrum, which exhibited nine signals which correspond to the nine carbon types in the structure of
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
(1). The carbon of the two carbonyl groups gave signals at δ 156.71 and 164.85 ppm; and aromatic carbons signals at δ 114.69, 117.87, 121.84, 126.21, 139.80, 148.41, and 151.05 ppm. The presence of these carbonyl groups was confirmed by the IR spectrum which showed absorption at a wavenumber of 1644 and 1682 cm
-1. The high-resolution mass spectrum further supported that the reaction product as
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
(1) based on the molecular ion peak at
m/z 309,9885 [M+H]
+ in a positive ionization mode.
2.2. Molecular Docking Study
Molecular docking was used to investigate the molecular behavior of
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
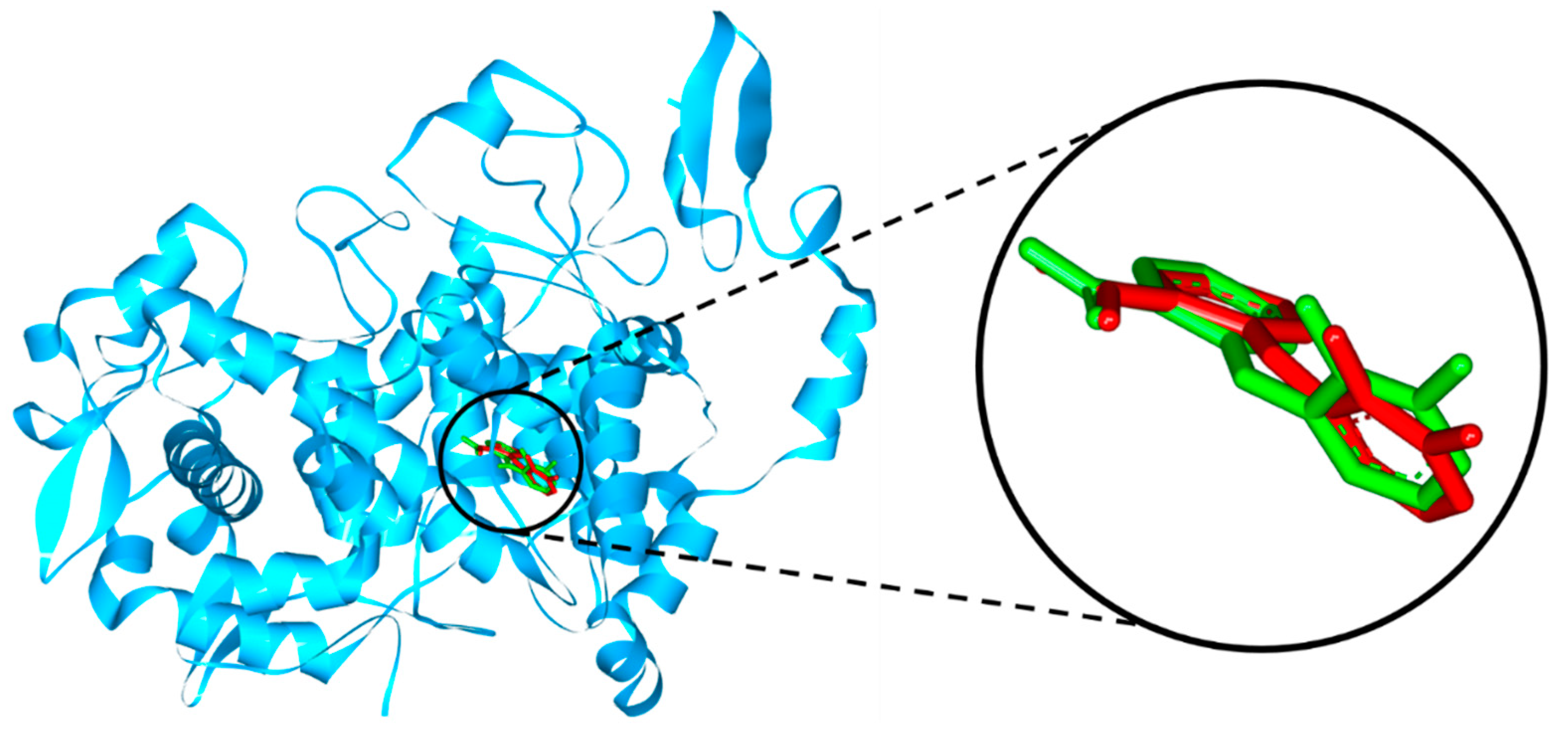
(1) at the COX-2 protein binding site. Human cyclooxygenase-2 complexed with mefenamic acid (PDB ID: 5IKR) was chosen as a molecular target to examine the interaction of compound
(1) with COX-2 protein. Redocking of mefenamic acid, as the native ligand, at the COX-2 binding site resulted in a binding energy of -7.61 kcal/mol and an RMSD value of 0.55 Å (
Figure 1).
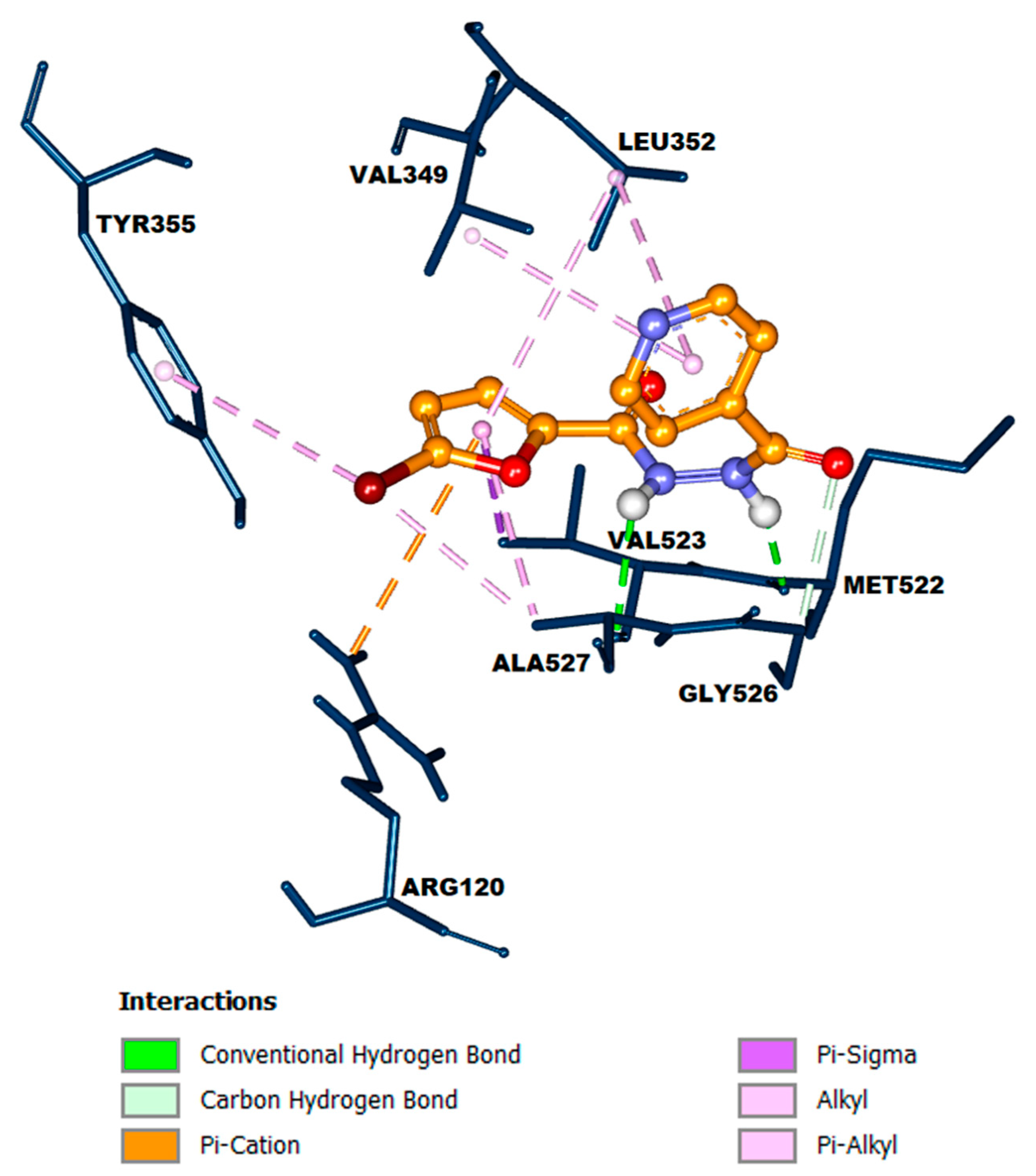
The docking result showed that the title compound
(1) had a binding energy of -7.89 kcal/mol, which was not significantly different from mefenamic acid. Compound
(1) exhibited hydrogen bonding, electrostatic interaction, and hydrophobic interaction (
Figure 2). The pyridine core of compound
(1) generated hydrophobic interactions with Val349 and Leu352 via µ-alkyl interactions. One of the carbonyl groups and two NH groups formed hydrogen bonds with Gly526, Met522, and Ala527, respectively. The furan core formed hydrophobic interactions with Val523, Leu352, and Ala527 in the form of µ-sigma and µ-alkyl interactions. Furthermore, electrostatic interaction was established through µ-cation interaction between the furan core and Arg120. Meanwhile, the bromo atom on the furan moiety formed hydrophobic interactions with Tyr385 and Ala527 in the form of µ-alkyl and alkyl interactions, respectively.
3. Materials and Methods
The starting materials and reagents used in this study were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Merck (Rahway, NJ, USA) which were used without further purification. Thin layer chromatography was carried out with Merck 0.20 mm precoated silica gel aluminum plates (Kieselgel 60, F254) and was visualized using a 245 nm UV lamp. Dry-column flash chromatography was carried out with Merck 60H. NMR spectra were obtained in DMSO-d6, with a Jeol JNM-ECS400 spectrometer (400 MHz). A high-resolution mass spectrum was recorded on a Thermo Scientific TSQ Vantage Triple State Quadrupole, and an infrared spectrum was obtained on a Thermo Scientific Nicolet iS10 FTIR spectrophotometer.
2.1. Synthesis of N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide (1)
The solution of 5-bromofuran-2-carboxylic acid (0.30 g; 1.62 mmol), MNBA (0.39 g; 1.16 mmol), and DMAP (0.26 g; 2.12 mmol) in dichloromethane (10 mL) was stirred at room temperature for 60 minutes. The solution was added with isoniazid (0.13 g; 0.96 mmol), and stirred further for 5 days (the reaction was monitored by TLC with ethyl acetate:methanol (5:1) as an eluent). The reaction product was evaporated under reduced pressure, and the residue was purified by dry-column flash chromatography with ethyl acetate:
n-hexane (3:1) as eluent to give
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
(1) as a white solid (0.25 g, 83%); mp: 203°C; FTIR (KBr)
vmax (cm
-1): 3168 (N-H), 1682 (C=O amide), 1644 (C=O amide);
1H NMR (DMSO-
d6, 400 MHz): δ 6.84 (1H, d,
J=3.6 Hz, ArH), 7.32 (1H, d,
J=3.6 Hz, ArH), 7.80 (2H, dd,
J=4.4, 1.6 Hz, ArH), 8.79 (2H, dd,
J=4.4, 1.6 Hz, ArH), 10.62 (1H, bs, NH), 10.81 (1H, bs, NH);
13C NMR (DMSO-
d6, 100 MHz): δ 114.69, 117.87, 121.84, 126.21, 139.80, 148.41, 151.05, 156.71, 164.85; HRESIMS
m/z (pos): 309,9885 C
11H
9N
3O
3Br (calcd. 309,9827) (
Supplementary Materials).
2.2. Molecular Docking Study
The crystal structure of the COX-2 protein was obtained from the Protein Data Bank (PDB ID: 5IKR) and prepared using MGLTools 1.5.6 [
13]. The 3D structure of
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
(1) was generated using the MarvinSketch tool [
14]. The location of the receptor binding site was set using a grid box placed at the position of the native ligand (mefenamic acid) with a centering of x: 38.042; y: 2.131; z: 61.280,
xyz dimensions of 30x30x30, and a spacing of 0.375 Å. The 200 iterations of the Lamarckian genetic algorithm were performed on the Autodock4.2 program in order to obtain the best binding pose of
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide
(1) at the binding site of COX-2 protein [
15]. The complex formed between compound
(1) and COX-2 was analyzed using Biovia Discovery Studio 2020 [
16].
4. Conclusions
N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide (1) was successfully synthesized from the reaction of 5-bromofuran-2-carboxylic acid and isoniazid in the presence of MNBA and DMAP, which obtained a good yield (83%). Molecular docking showed that N'-(5-bromofuran-2-carbonyl)isonicotinohydrazide (1) interacted with the residues of the COX-2 protein through several interactions, including hydrogen bonding, electrostatic interaction, and hydrophobic interaction, and had a good binding affinity with a value of -7.89 kcal/mol. Isonicotinohydrazide has the potential to serve as a lead structure in the development of a COX-2 inhibitor.
Supplementary Materials
The following supporting information can be downloaded online, Figure S1: IR spectrum of the title compound (1); Figure S2: 1H NMR spectrum of the title compound (1); Figure S3: 13C NMR spectrum of the title compound (1); Figure S4: Mass spectrum of the title compound (1).
Author Contributions
Conceptualization, M.S.; methodology, M.S.; software, N.P.A.; validation, E.S., E.Y.R.; formal analysis, E.Y.R.; investigation, E.Y.R.; resources, M.S.; data curation, L.A.; writing—original draft preparation, E.Y.R.; writing—review and editing, M.S. and N.P.A.; visualization, N.P.A.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Institut Teknologi Sepuluh Nopember (Penelitian Keilmuan Dana ITS grant number 1690/PKS/ITS/2023).
Data Availability Statement
Acknowledgments
The authors acknowledge Institut Teknologi Sepuluh Nopember for funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tikhomirov, A.S.; Lin, C.Y.; Volodina, Y.L.; Dezhenkova, L.G.; Tatarskiy, V. V.; Schols, D.; Shtil, A.A.; Kaur, P.; Chueh, P.J.; Shchekotikhin, A.E. New Antitumor Anthra[2,3-b]Furan-3-Carboxamides: Synthesis and Structure-Activity Relationship. European Journal of Medicinal Chemistry 2018, 148, 128–139. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Liu, Q.; Li, A.; Wang, W.; Gu, W. Design, Synthesis, and Antifungal Activity of Novel Thiophene/Furan-1,3,4-Oxadiazole Carboxamides as Potent Succinate Dehydrogenase Inhibitors. J Agric Food Chem 2021, 69, 13373–13385. [Google Scholar] [CrossRef]
- Zanatta, N.; Alves, S.H.; Coelho, H.S.; Borchhardt, D.M.; Machado, P.; Flores, K.M.; da Silva, F.M.; Spader, T.B.; Santurio, J.M.; Bonacorso, H.G.; Martins, M.A.P. Synthesis, Antimicrobial Activity, and QSAR Studies of Furan-3-Carboxamides. Bioorganic and Medicinal Chemistry 2007, 15, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Xu, L.; Dong, X.; Dong, J.; Yi, X.; Ma, X.; Qiu, N.; Li, J.; Yang, B.; Zhou, Y.; Hu, Y. Design, Synthesis and Biological Evaluation of Pyrazol-Furan Carboxamide Analogues as Novel Akt Kinase Inhibitors. European Journal of Medicinal Chemistry 2016, 117, 47–58. [Google Scholar] [CrossRef]
- He, M.; Li, Y.-J.; Shao, J.; Li, Y.-S.; Cui, Z.-N. Synthesis and Biological Evaluation of 2,5-Disubstituted Furan Derivatives Containing 1,3-Thiazole Moiety as Potential α-Glucosidase Inhibitors. Bioorg Med Chem Lett 2023, 83, 129173. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.; El-Mekabaty, A.; Elattar, K.M. Five-Membered Ring Systems with One Heteroatom: Synthetic Routes, Chemical Reactivity, and Biological Properties of Furan-Carboxamide Analogues. Synthetic Communications 2018, 48, 839–875. [Google Scholar] [CrossRef]
- Narasimhan, B.; Belsare, D.; Pharande, D.; Mourya, V.; Dhake, A. Esters, Amides and Substituted Derivatives of Cinnamic Acid: Synthesis, Antimicrobial Activity and QSAR Investigations. European Journal of Medicinal Chemistry 2004, 39, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, X.; Zhang, H.J.; Li, N.; Xiao, Y.; Zhu, H.L.; Ye, Y.H. Synthesis, Structure, and Biological Assay of Cinnamic Amides as Potential EGFR Kinase Inhibitors. Medicinal Chemistry Research 2013, 22, 986–994. [Google Scholar] [CrossRef]
- Dunetz, J.R.; Magano, J.; Weisenburger, G.A. Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals. Organic Process Research and Development 2016, 20, 140–177. [Google Scholar] [CrossRef]
- Shwetha, B.; Sudhanva, M.S.; Jagadeesha, G.S.; Thimmegowda, N.R.; Hamse, V.K.; Sridhar, B.T.; Thimmaiah, K.N.; Ananda Kumar, C.S.; Shobith, R.; Rangappa, K.S. Furan-2-Carboxamide Derivative, a Novel Microtubule Stabilizing Agent Induces Mitotic Arrest and Potentiates Apoptosis in Cancer Cells. Bioorganic Chemistry 2021, 108, 104586. [Google Scholar] [CrossRef]
- Métro, T.X.; Martinez, J.; Lamaty, F. 1,1′-Carbonyldiimidazole and Mechanochemistry: A Shining Green Combination. ACS Sustainable Chemistry and Engineering 2017, 5, 9599–9602. [Google Scholar] [CrossRef]
- Shiina, I.; Kawakita, Y.I. The Effective Use of Substituted Benzoic Anhydrides for the Synthesis of Carboxamides. Tetrahedron 2004, 60, 4729–4733. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; Isakson, P.C.; Stallings, W.C. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Aijijiyah, N.P.; Wati, F.A.; Rahayu, R.; Srilistiani, A.; Mahzumi, F.; Aulia, T.; Santoso, L.; Pamela, E.; Ramadhani, E.Y.; Ilfahmi, Y.A.; Purnomo, A.S.; Putra, S.R.; Santoso, E.; Ningsih, S.; Firdausi, N.; Santoso, M. Synthesis, α-Glucosidase Inhibitory Activity, and Molecular Docking of Cinnamamides. Med Chem Res 2023, 32, 723–735. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Santoso, M.; Pamela, E.; Ramadhani, E.Y.; Ilfahmi, Y.A.; Aijijiyah, N.P.; Purnomo, A.S.; Putra, S.R. 4-Methoxyphenethyl (E)-3-(o-Tolyl)Acrylate. Molbank 2022, 2022, M1519. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).