1. Introduction
Numerous public health reports, both global and from different regions state that dental caries is among most prevalent chronic diseases in the world. It is estimated that the disease affects around 2.4 bilion people worldwide [
1]. The progression of dental caries is considered to be the result of occurence of four major factors i) cariogenic microorganisms that form a biofilm such as
Streptococcus mutans ii) dietary sugars (mainly glucose, fructose and sucrose), iii) host susceptibility, and iv) time [
2,
3,
4]. It is worth mentioning that the intense increase in caries appearance indicators in recent decades, such as prevalence or decayed-missing-filled index (DMFT index) is mostly related to socioeconomic and dietary factors, in particular, the intake of large amount of processed foods, sweets and sugary drinks in the diet [
5].
Commonly practised methods of caries prevention and treatment include reducing sugar intake, regular fluoride prophylaxis (toothpaste, rinses, dental varnishing), use of other chemotherapeutic agents (e.g. chlorhexidine), and finally, systematic dental check-ups involving, if necessary, treatment of the cavities with fillings that are effective against biofilms [
6]. Despite widespread dental caries prophylaxis and good access to treatment, this disease still poses a serious health, social and financial problem in developed countries [
7]. This situation led to a search for different ways of prevention and treatment. One area of interest covers research on antimicrobial biomolecules and particularly those having antibiofilm properties. This approach aims at limiting drug resistance development, minimising side effects and efficacy only against pathological components of the oral cavity microbiome without interfering with the commensal microbiome [
8].
The biomolecule that meets carriers prevention criteria is lactoperoxidase (LPO), a heme peroxidase that oxidises (pseudo)halogen ions to products with antimicrobial properties at the expense of hydrogen peroxide through halogenation cycle. These products are characterised by high redox potential and ability to impair the biological function of proteins and nucleotides of microorganisms by oxidation of these molecules. Lactoperoxidase is excreted by epithelial cells into multiple biological fluids, including saliva, tears, airway surface fluid, female reproductive tract fluid, milk, among other where the enzyme plays an important role in innate immunity [
9,
10]. The characteristic of the composition of these fluids means that the main substrate for LPO
in vivo is the thiocyanate ion (SCN
-) (LPO unlike myeloperoxidase does not oxidise the chloride ion) which is oxidised to hypothiocyanite (OSCN
-)/hypothiocyanous acid (HOSCN) [
11]. The research conducted over the years found that LPO is characterised by a wider substrate spectrum, and if other (pseudo)halide ions are available, they can also can enter halogenation cycle. Although the antimicrobial action of LPO system where the (pseudo)halide substrates are either SCN
- or I
- is well described with regard to planktonic microorganisms, the effectiveness against microorganisms located in a biofilm structure has not yet been investigated. Furthermore, there is a lack of detailed data on both the antimicrobial and antibiofilm activity of other possible LPO products in particular the newly described systems LPO-selenocyanate (SeCN-) with hyposelenocyanate as the product and LPO-thiocyanate-iodide capable of to cyanogen iodide (ICN) synthesis [
10,
12,
13].
The biofilms formed on the surfaces of teeth
in vivo are multi-species biofilms consisting of commensal and cariogenic microorganisms. Under conditions favorable for the formation of cariogenic biofilms, dysbiosis occurs in the oral cavity, leading to the proliferation of cariogenic microorganisms [
14]. These microorganisms are characterized by efficient metabolism of carbohydrates, resulting in the production of large amounts of organic acids and components of the biofilm matrix, as well as high resistance to acidic environments. Microorganism that meets these criteria is
S. mutans, considered one of the main etiological factors of dental caries and a model organism in in vitro studies on cariogenic biofilms [
15]. In light of the mentioned cariogenic factors of this microorganism, the search for bioactive molecules with anti-caries activity targeted against biofilms should demonstrate the ability to inhibit carbohydrate transport processes into the bacterial cells, inhibit glycolysis, and hinder biofilm biomass growth.
The aim of this study was to assess antibiofilm action of the physiological LPO system and its three modifications utilising non-physiological substrates against the S. mutans biofilm. The evaluation focused on determination of the effect that the LPO systems exhibited on biofilm biomass growth and biofilm carbohydrate metabolism (PTS system activity, glucose and sucrose consumption, intracellular levels of NAD+ and NADH) leading to lactic acid synthesis.
2. Results and discussion
Over the last several years a great deal of research has been published on the antimicrobial activity of LPO. Most of it has focused on the physiological LPO substrate, thiocyanate [
16,
17] and the non-physiological substrate – iodide interactions [
18,
19,
20]. However, in recent years there have been several studies reporting on other possible substrates and products of the LPO system [
13,
21]. In our study in addition to the aforementioned systems, we investigated two other systems: the newly described LPO-selenocyanate with hyposelenocyanite (OSeCN
-) as a product [
13] and the modified physiological system, the LPO-thiocyanate-iodide system, which is characterised by the production of highly toxic cyanogen iodide (ICN) [
12]. Unfortunately, almost all of these previous studies focused on the antimicrobial activity of the LPO system against planktonic cells and not on microorganisms in biofilms. Due to the barrier provided by the extracellular matrix of the biofilm, the greater buffering capacity for antimicrobial compounds, and the biochemical and genetic cooperation between the microorganisms that are part of the consortium attached in biofilms, this structure makes its inhabitants 10 to 1000 times more resistant to antimicrobials than planktonic cells [
22]. In addition, many diseases, including dental caries, do not depend on the presence of planktonic cells, but on the formation of biofilms and successful eradication of biofilms is critical to the control of these diseases.
It should be emphasised that caries development
in vivo is tied to the accumulation of a biofilm and the metabolic activity of numerous species of microorganisms, but
S. mutans is considered to be one of the main etiological factors of this disease. This is linked to its high adhesive properties to hard tooth tissue and its ability to create biofilms, produce organic acids, and thrive in an acidic environment [
23]. For these reasons, the presented research was focused on conducting the experiments on a single-species biofilm. This approach allowed us to eliminate additional factors related to interspecies interaction found in multispecies biofilms that can intervene with the obtained results. Moreover, using single-species biofilms means that experiments can be replicated more easily to verify or extend research [
24,
25].
2.1. System and environmental conditions
In the presented research bovine milk LPO was used due to its high level of similarity (structural and functional) to the human LPO molecule [
10]. A buffered solution of LPO 50 nM was used throughout the study that reflected physiological enzyme concentration found in saliva [
26]. All LPO reactions were performed in phosphate buffered solution (PBS) at pH 7.4 which reflected the environmental conditions of the oral cavity of the healthy person between meals. It should be noted that in the case of LPO-iodide and LPO-thiocyanate-iodide mixture the resulting products are therefore pH dependent. The pH of the oral cavity is not constant and can vary depending on the time of day, the presence of diseases, the time since the last meal and its composition, and finally the metabolic activity of the microorganisms. In the case of the product of the LPO system with SCN
-+I
- as substrates, the formation of highly toxic cyanogen iodide decreases with a decrease in environmental pH [
21]. Considering all of the above, the observed biological effect
in vivo may differ from the effect that was observed in the presented study conducted
in vitro.
In available studies there can be found a two-pronged approach to impact on microorganisms with LPO system [
26,
27]. The first one consists of creating a mixture of substrates and the enzyme, triggering the reaction, and after it reaches a completion, the resulting mixture containing the products is added to the tested microorganisms. The second approach is based on running the reaction in situ, already in the presence of tested microorganisms [
26]. In the present study, the second approach was applied because the physiological LPO system acts
in situ and, in addition, there are reports showing a stronger antimicrobial effect of the LPO system acting
in situ than the system acting
ex situ [
10]. This occurrence may be related to the formation of unstable and reactive intermediates of the LPO system, which are degraded during
ex situ incubation.
In the case of biomass, insoluble polysaccharide mass assay and during lactate/glucose/sucrose kinetic assay, LPO system variants were used in the presence of three different H
2O
2 concentrations (
Figure 1). This approach was taken to evaluate the relationship between H
2O
2 concentration and the observed effect, and, to select the optimal H
2O
2 concentration which would result in the highest LPO system antibiofilm activity but which would not damage host cells.
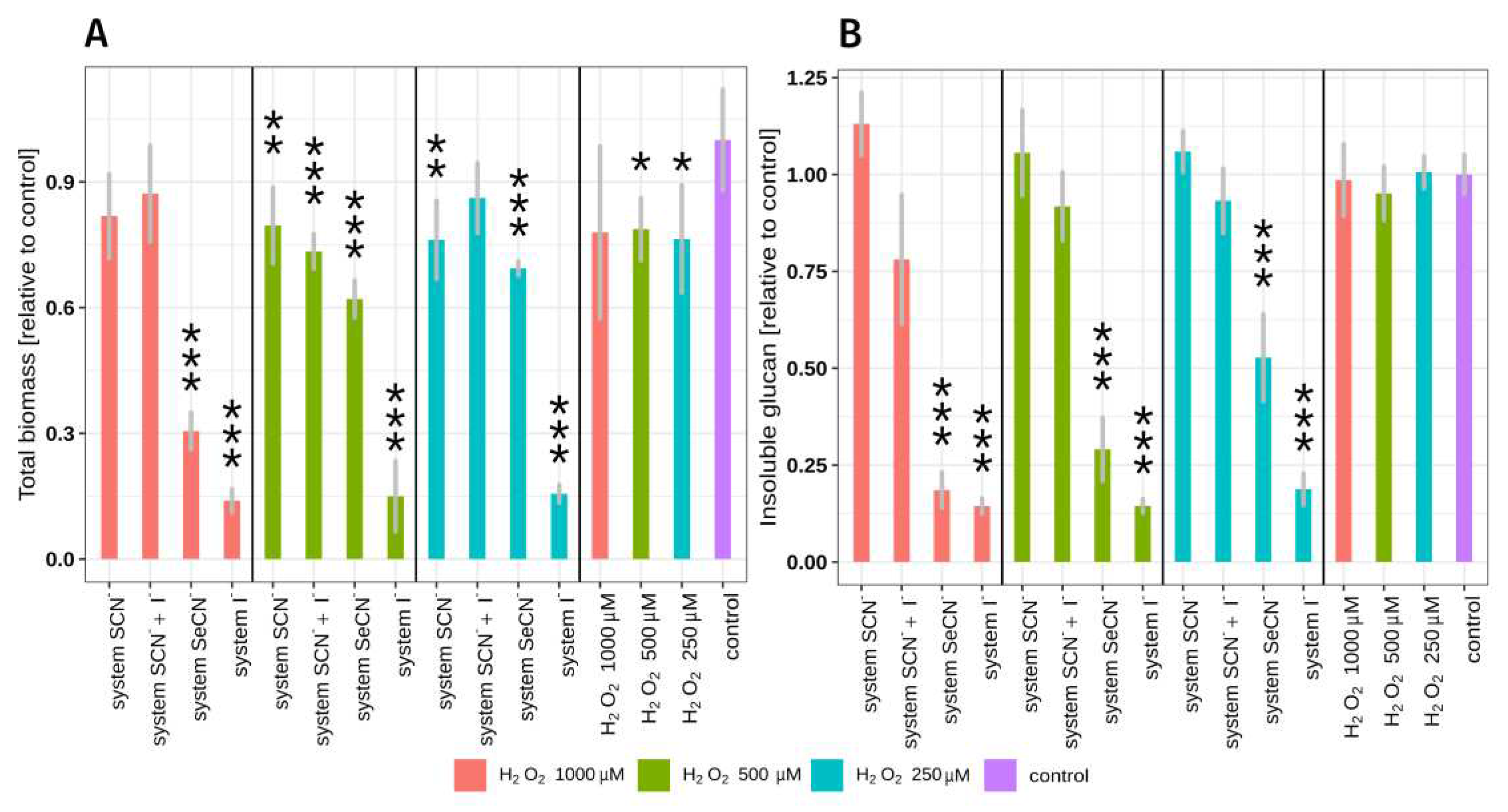
2.2. LPO system influence on total biomass and insoluble polysaccharide mass.
The total biofilm biomass and total biomass of insoluble biofilm polysaccharide after exposure to each of the tested LPO system modifications are shown in
Figure 1 and
Table S1. In this experiments two-hour
S. mutans biofilms (early biofilm formation phase) were exposed to tested systems. The use of early formation phase biofilms made possible to observe any further biofilm biomass increase.
The study has revealed that the rate of reduction in biofilm biomass / insoluble exopolysaccharide mass is dependent on the type of substrate / product of the LPO system and the concentration of hydrogen peroxide and consequently the amount of product of the LPO system. The strongest statistically significant inhibitory effect on biomass growth was observed in the case of LPO system with iodide as substrate, at the highest H
2O
2 (last red column in first segment of
Figure 1B). The second most effective system that significantly decreased biomass and insoluble polysaccharide production contained SeCN
- as a substrate. In the case of physiological LPO system (SCN
- as substrate), no statistically significant reduction in the mass of the insoluble polysaccharide was observed. There was only a slight statistically significant reduction in total biomass in two of three tested concentrations of H
2O
2 (250 μM and 500 μM), respectively. The LPO system with substrate mixture of SCN
- and I
- has not exhibited the ability to significantly reduce biofilm insoluble polysaccharide mass, however, 27% of the total biomass reduction was observed in the sample containing 500 μM H
2O
2.
Hydrogen peroxide alone has no statistically significant effect on total biomass or insoluble polysaccharide mass, and only a slight reduction in total biomass (ca. 12%) was observed at the highest concentration of H
2O
2. It should be noted that the observed inhibitory effect of LPO systems is associated with the production of reactive LPO system product and not with H
2O
2 toxicity. The reason for that is the fact that the reaction mixture combined a small amount of H
2O
2 with high LPO activity, resulting in immediate consumption of all H
2O
2 during the halogenation cycle. The depletion of H
2O
2 occurs in the first few seconds after the start of the reaction which is related to extremely fast reaction of LPO Compound I with (pseduo)halides [
28]. In the H
2O
2 control samples (shown in Fig. 1 and
Table S1), the incubation lasted 15 minutes and the H
2O
2 did not degrade due to the absence of LPO in the mixture causing exposition to H
2O
2 toxic effects.
(Pseudo)halides alone did not influence total biomass and insoluble polysaccharide mass in biofilms tested (Tukey’s test p>0.05) (
Table S1). These observations coincide with other studies on the influence of the LPO system on planktonic bacteria [
29]. It also proves that the observed antibiofilm effect is related to the activity of the LPO (pseudo)halogenation cycle and the production of reactive products.
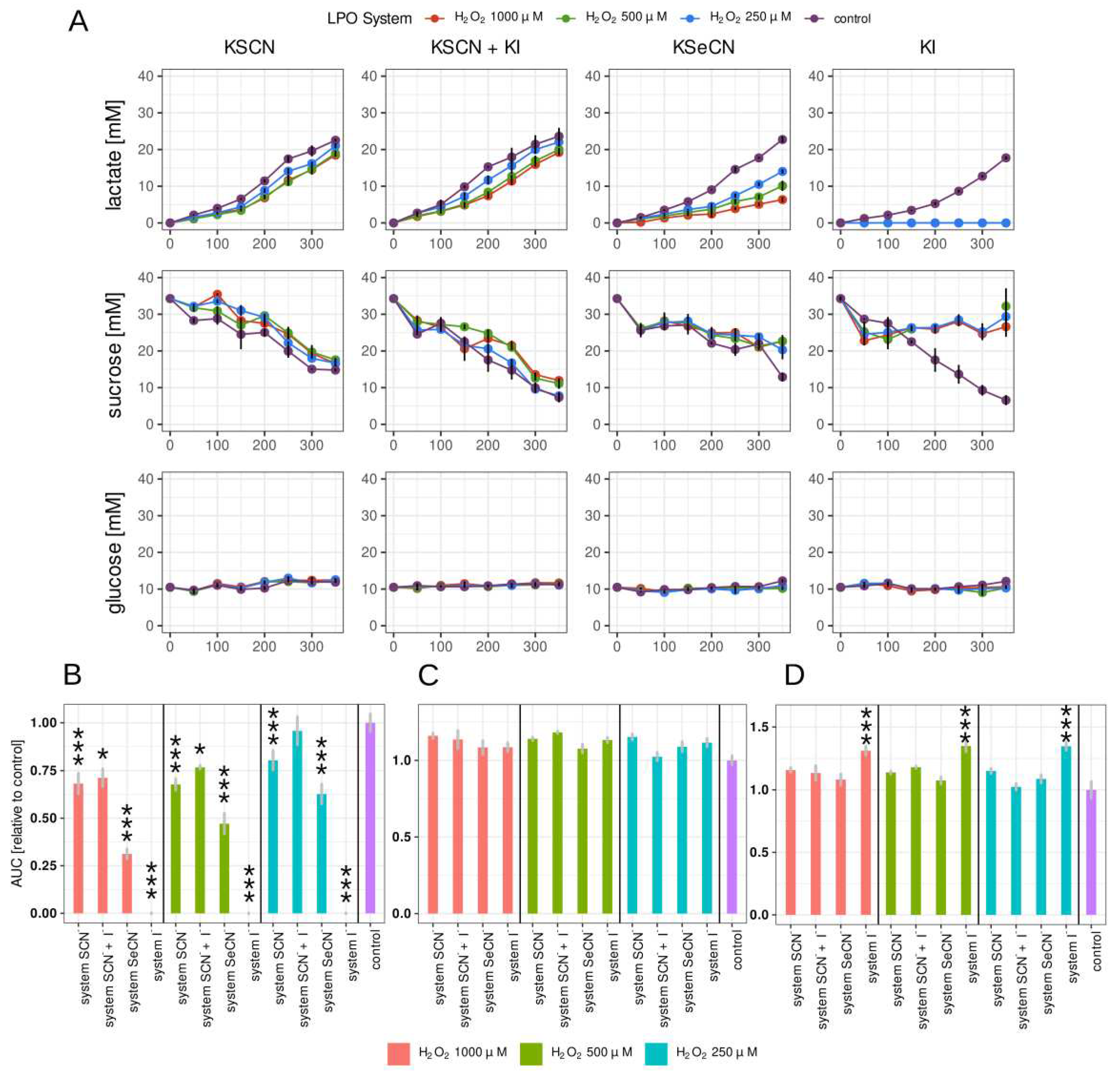
2.3. Lactate, glucose and sucrose metabolism in biofilm
A simple and quick way to measure the effect of compounds action of tested with anticariogenic potential is to investigate parameters related to biofilm carbohydrate metabolism, more specifically lactic acid production and biomass growth. In this study, lactate production, glucose and sucrose consumption were measured with the amperometric method utilising a biosensor (
Figure 2). To our knowledge, this is the first study on microorganisms that involved this measurement technology. This approach provides a fast, semi-automated, accurate measurement suitable for high-capacity analysis for screening.
The kinetic change in concentration of the three compounds was measured.
Figure 2A shows the changes in time in the lactate, glucose and sucrose concentrations in the biofilm growth medium of the biofilm after treating the 24 h biofilms with LPO systems for 15 minutes.
Of all combinations tested, the strongest inhibition of lactate production was observed in the case of the LPO-I
- system (
Figure 2B). There, lactate synthesis was completely inhibited for the duration of the experiment. Promising results were also observed in the sample containing the LPO-SeCN
- system, where the relative AUC decreased significantly in all tested H
2O
2 concentrations. A much smaller but statistically significant reduction in lactate production was observed in the case of the physiological LPO-SCN
- system with a relative decrease in AUC ranging from 32% to 23% for the tested concentrations of H
2O
2. In samples with the LPO-SCN- + I- system, the lactate production rate expressed as relative AUC was statistically significant only for 1000 and 500 μM of H
2O
2.
The results obtained similar to those for the biomass assay point to the LPO-iodide system displaying the strongest potentially anticariogenic action. Surprisingly, the inhibitory effect of the physiological LPO-thiocyanate system was rather weak despite its well-documented bacteriostatic or bactericidal effect on planktonic microorganisms [
10,
29,
30,
31]. Considering the fact that the present study is pioneering in asssess the effect of LPO systems on the biofilms
in vitro, it is not possible to compare the obtained results to previous studies. However, there are the results from clinical trials in which patients received preparations containing glucose oxidase–LPO-thiocyanate system alone [
32] or in combination with other biomolecules such as lactoferrin [
33,
34,
35,
36,
37] and lysozyme [
38]. Various parameters were analysed including volatile sulphur compounds in exhaled air [
36,
39], Colony Forming Unit (CFU) of
S. mutans and
L. acidophilus in saliva [
34], total bacteria count [
34,
38], oral health parameters [
34] and severity of xerostomia [
35]. The results of the referred studies do not conclusively indicate a beneficial effect of preparations enriched with an LPO-thiocyanate system. Although studies conducted by Gudipaneni et al. [
34] and Pinherio et al. [
38] point to a significant decrease in the amount of
S. mutans and total amount of microorganisms in biological material, the studies by Shimizu et al. [
33] and Shin et al. [
36] did not show a statistically significant effect. The differences between the results may be due to the different compositions that the patients received – concentrations as well as the addition of small biomolecules beyond the LPO system. Future clinical trials using the LPO system should consider the possibility of using alternative (pseudo)halide substrates, especially iodide, in addition to the selection of carrier and optimal concentration of the components.
The studies carried out did not show significant inhibitory effect on biomass and insoluble glucan growth or lactate production by the LPO-thiocyanate-iodide system. The product of this system was identified as cyanogen iodide (ICN) by Schlorke et al. [
21] Data on the microbial effects of this product are insufficient. To the best knowledge of the authors, only one paper by Schlorke et al. [
12] has demonstrated the effective bactericidal activity of the this modification of LPO system against
Escherichia coli in planktonic culture. In our study, despite having similar working conditions (LPO 50 nM, SCN
-:I
- ratio 1:2, pH = 7.4) we did not observe a significant effect of the LPO-thiocyanate-iodide system against
S. mutans biofilms. This may be a result of increased resistance of
S. mutans to ICN or impaired penetration of this product into the biofilm. This demonstrates the need for further research on this system. The focus should be on susceptibility testing of individual clinically relevant microbial species and studies of the stability (possible degradation prior to biofilm penetration) and ability of this product to penetrate biological barriers, including the biofilm matrix.
Statistically significant decrease in sucrose consumption by biofilms treated with LPO systems was observed only in the case of LPO-I
- system (
Figure 2C,
Table S2). In samples containing other systems, the decrease is observed at individual time points (
Figure 2 A), but the AUC parameter describing the overall effect was not statistically different from the control sample. It should be noted that statistically significant differences in sucrose consumption may be more difficult to observe due to the greater variability in the results obtained. This is related to indirect measurement of sucrose concentration based on the assessment of glucose concentration in the medium before and after enzymatic hydrolysis of sucrose (see materials and methods section).
Glucose concentration remained constant both in tested samples and in the control. Glucose was a component of the Brain Heart Infusion growth medium (BHI) and could also originate from sucrose hydrolysis. The concentration of glucose could stay constant,despite its uptake by S. mutans, due to the occurrence of an equilibrium state between uptake by bacteria and sucrose hydrolysis.
2.4. Influence of LPO systems on PTS activity
The phosphoenolopuryvate PEP-carbohydrate phosphotransferase (PTS) system is the most important transport system responsible for transmembrane mono- and disaccharide transport in
S. mutans. This system consists of two nonspecific energy coupling enzymes (enzyme I, heat-stable protein) and one carbohydrate-specific multiprotein permease. The carbohydrate transfer by PTS is coupled with its phosphorylation. The phosphate group is first transferred from PEP to permease via enzyme I and a heat-stable protein and then to carbohydrate as it crosses the bacterial membrane [
40].
Our studies are the first to investigate the effect of LPO system on the PTS system and demonstrated that all tested LPO systems can inhibit glucose PTS system (
Figure 3,
Table S3). The strongest inhibitory effect was observed in the case of the LPO-iodide system (85% inhibition compared to the control). The lowest but also relatively high inhibition was observed with the physiological LPO-thiocyanate system (72% inhibition). In the case of the LPO enzyme and (pseudo)halide substrates alone, there were no statistically significant changes in glucose PTS activity (
Table S3).
In the presented experiment, the H
2O
2 concentration was set at 1000 μM because it showed the greatest effect on the other parameters tested. This approach allowed us to examine the mechanism of action of tested LPO systems. This experiment unlike the others in this study was not performed on biofilms, but on planktonic cells. It should be noted that the effect on
S. mutans biofilms could be different (weaker or with different LPO substrate dependence) due to the known higher resistance to antimicrobials and different gene expression in biofilms compared to planktonic cells [
41]. Nevertheless, the experiment was conducted on planktonic cells because the biofilm suspension had a tendency to aggregate during the measurement (due to high content of extracellular polysaccharide), causing false results in the measured absorbance level.
Triclosan is a widely used antimicrobial agent in stomatology. Its mechanism of action is complex and includes the inhibition of glycolysis enzymes and the PTS system [
42]. Unfortunately, at the same time, numerous studies point to multiple side effects of triclosan and possible toxic effects against host cells [
43]. This situation has prompted numerous studies aimed at discovering different substances with similar activity but lower toxicity. Our proposed LPO systems would perfectly meet these requirements. On the one hand, its mechanism of action is similar to that of triclosan: inhibition of glycolytic enzymes [
44,
45] and inhibition of the PTS system (as shown in the present study). On the other hand, it should be noted that the observed toxic effects of triclosan have not been reported for the physiological LPO system. Considering that the LPO system is an element of the antimicrobial response of innate immunity, there are evolutionary mechanisms that protect host cells from its toxicity [
32].
2.5. Influence of LPO systems on biofilm NAD+/NADH
The influence of LPO systems on biofilm NAD+ and NADH levels was evaluated by sweeping via borate complexation capillary electrophoresis. This approach allowed to measure NAD
+ and NADH at very low concentrations (0.5 μg per well/single biofilm). In the present study modified version of the method described by Rageh et al. [
39], originally developed with the aim of urine nucleotides assay was utilized. In this method, the sample is diluted in a low-conductance matrix (molecular grade water) in the absence of borate. In the first step of the analysis, the capillary was filled with the sample to 90% of its total length. When the capillary inlet was placed in the background buffer (25 mM borate buffer; pH=9.25) and the negative voltage was applied, the on-line preconcentration process in the capillary started. At this step, the direction of the electro-osmotic flow is directed toward the capillary input, and the sample matrix is pumped out via the inlet. At the same time, hydroxide and borate (in the form of tetrahydroxyborate) ions start migrating toward the capillary outlet and start sweeping neutral and negatively charged (borate complexation) nucleotides. During the concentration process, the current decreases from -0,5 μA to approximately -55 μA (95% of the current measured in sample-free borate buffer). At this point indicates the end of the concentration process and the removal of almost all of the sample matrix. At this moment, the polarisation was changed and the proper separation process with registration of absorbance at 254 nm was started. The separation process was aided by the addition of the β-cyclodextrin (5 mM in BGE), which has been described by several authors to increase selectivity by increasing mobility differences for nucleotides with the same mass/charge ratio [
39,
46,
47].
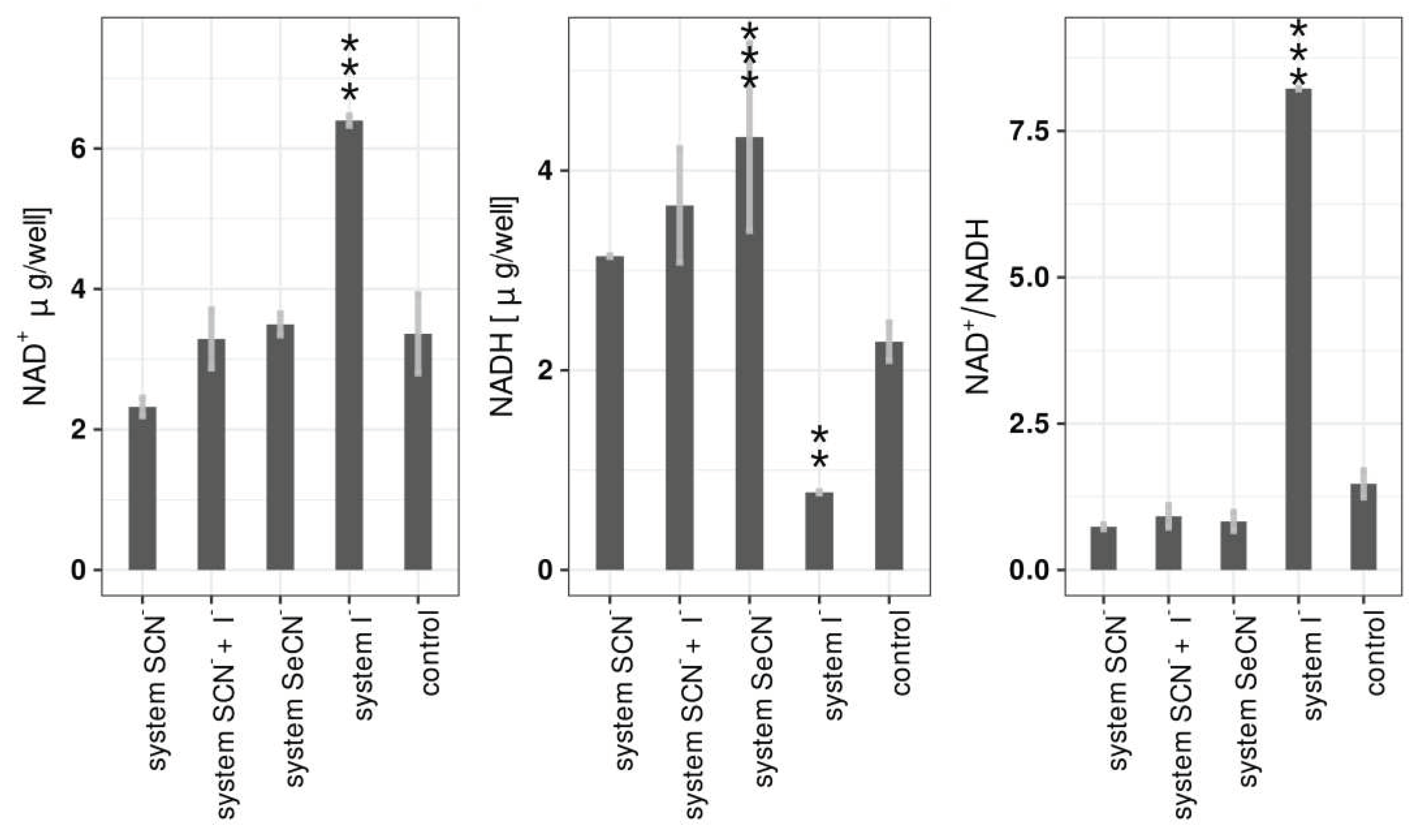
Analysis of NAD
+ and NADH concentrations in biofilms after treatment with LPO systems showed that the most significant changes were observed in the case of the LPO-iodide system. In that case, a statistically significant increase in NAD
+ concentration (6.40 ± 0.07 μg/well vs 3.36 ± 0.56 μg/well in control), a decrease in NADH concentration (0.78 ± 0.01 μg/well vs 2.29 ± 0.19 μg/well in control) and an increase in the NAD+/NADH ratio (8.220 ± 0.012 vs 1.470 ± 0.231) were observed. There were no other statistically significant changes in NAD
+ levels with other LPO systems. The NADH concentration increased slightly but statistically significantly after treatment of with the LPO-thiocyanate-iodide system and LPO-selenocyanate. The (pseudo)halide substrates and hydrogen peroxide alone did not cause statistically significant changes in any of the three parameters after incubation with biofilms (
Table S3).
The NAD+/NADH ratio obtained in the control and the substrate controls (samples not different from the control) was between 1.007 and 1.470 and were close to ratios published in the another study by Baker et al. [
48] where this ratio in the same strain of
S. mutans was on average 1.9 when cultured at pH 7 and 1.3 at pH 5. In this study, biofilms were not cultured in chemostat, therefore with the synthesis of lactic acid, the biofilm environment was acidifying. The absolute concentrations of NAD
+ and NADH cannot be compared with those of other studies because of the large number of factors that influence these parameters (cell density, biofilm growth time, medium composition, atmosphere composition, applied growth surface, etc.) all of which are unique to this research.
There are several possible explanations for the changes observed in the LPO-iodide system. The observed decrease in NADH concentration may be related to the direct oxidation of this molecule by reactive iodide species synthesised by LPO. The products of the lactoperoxidase system generated from thiocyanate, iodide, and bromide have been shown to directly oxidise NADH and NADPH molecules [
49], which is one of the nonspecific defence mechanisms against the toxicity of the LPO system in both host and microbial cells. The lack of intracellular NADH may be a direct reason for the observed total inhibition of lactate synthesis in biofilms treated with the LPO iodide system, due to the role of NADH as a substrate for lactate dehydrogenase (LDH) [
50]. Importantly, unlike the LPO-thiocyanate system, oxidation product of the LPO-iodidte system is not NAD
+, but a number of different degradation products (irreversible loss of the molecule) [
51]. Therefore, the simultaneously observed increase in the concentration of NAD+ must be related to another mechanism, where its increased synthesis takes place. Although the results of the studies on LPO systems affecting the process of de novo NAD
+/NADH synthesis are not available, there are studies investigating the effect of the system on the glycolysis process. Glyceraldehyde-3-phosphate dehydrogenase is an enzyme responsible for the conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate with concomitant reduction of NAD
+ to NADH. Reactive products of the LPO system have been shown to be capable of inhibiting this enzyme, thus stopping the reduction of NAD
+ to NADH, which may explain the observed rise in its concentration due to accumulation [
52]. Another mechanism explaining the increment in the NAD
+/NADH ratio may be an increase in the expression of water-forming NADH oxidase in response to oxidative stress. This enzyme plays a key role in
S. mutans cells by keeping NAD
+ available for glycolysis and protecting it from oxidative stress [
48].
It can be hypothesised that the reason for the increase in NADH concentration in the LPO-selenocyanate system is the inhibitory effect of the product of the LPO system on lactate dehydrogenase (NADH accumulation). To that, it can be added the inability of hyposelenocyanite to reduce the accumulated NADH to NAD or other degradation products, as in the case of the LPO-iodide system. However, this mechanism requires a further in-depth verification beyond the scope of this study.
2.6. Translating results to in vivo conditions, limitations and further research
All scientific studies have certain limitations. The main limitation of the present study is that it was carried out
in vitro, which certainly affects the interpretation of the results obtained. In the environment of living organisms, more specifically in the oral cavity, the number of factors influencing the action of the LPO system is much greater than under
in vitro conditions. First of all, in a living organism there are a large number of defence mechanisms that can inactivate reactive products of the LPO system. These include substances that affect the antioxidant capacity of saliva (small molecule substances and proteins). Second, there is a constant removal of saliva due to its continuous production in the oral cavity environment. It should be noted that in the case of exogenous delivery of the LPO system without the use of carriers that would slow its release, the effect of the system would be limited to only a few minutes after application [
53].
The important factor that also influences the action of the LPO system
in vivo is the method of H
2O
2 delivery, the availability of which is a limiting factor for the lactoperoxidase reaction. In the present study, this substrate was delivered in one portion at the beginning of the experiment in a rapid initial synthesis in the first few seconds of the reaction and sudden rapid depletion of the substrate. Furthermore, subsequent reactions between the generated products and the available H
2O
2 were not unlikely, especially in the case of the LPO iodide system [
9]. H
2O
2 is continuously produced
in vivo by host oxidases and microorganisms, but in small amounts that are immediately scavenged by reactive oxygen species (ROS) defence enzymes and by peroxidases (including LPO) [
54].
The pH of the oral environment also plays an important role in influencing the biological effect of the LPO system
in vivo. Some products of the tested systems (hypothiocyanite, hyposelenocyanite, some of the reactive iodide species) are acidic substances that can undergo electrolytic dissociation. The protonated and deprotonated forms of mentioned products are in equilibrium [
55]. Lowering the environmental pH, shifts the equilibrium towards acidic (protonated) form. Due to the fact that undissociated acidic forms penetrate biological membranes more easily than dissociated forms, high pH will not favour the penetration of dissociated reactive products from LPO systems into microbial cells, thus reducing their effectiveness [
56]. Furthermore, the LPO-iodide system generated different products depending on the environmental pH. These products can be characterised with different potential antibiofilm properties. In an environment with a pH below 6, the only reactive product is I
2. At pH 6 to 9 the LPO-iodide system synthesises HOI, I
2OH, I
2 and I
3- with HOI being the most reactive in this environment. It is worth noting that oral hygiene products are alkaline (pH 7-10), which are important in the future development of LPO system-enriched products [
57]. In view of the above, the effect of the LPO-thiocyanate and LPO-selenocyanate systems in this type of preparation will be weakened by the dissociation of the main product in an alkaline environment. The opposite effect would have been observed for the LPO-iodide system. In an alkaline environment, the predominant product is HOI, which is characterised by a strong reactivity toward thiol groups, thioether groups, amine moieties, and oxidation of NAD(P)H [
51,
58]. This is another favourable argument for further basic and applied research on the LPO-iodide system.
Further investigation of the use of a modified LPO system in the treatment of biofilm-related diseases should include optimising the concentration of each component, particularly H2O2, which is a limiting substrate in the reaction catalysed by LPO. A particularly promising approach for in vivo application appears to be the replacement of H2O2 in substantia with the H2O-generating system, such as glucose oxidase, or lactate oxidase in future oral hygiene products but in this case the selection of the enzyme that will be active in an alkaline environment is crucial. This would extend the period of efficacy of the LPO system by increasing the time that H2O is available and, more generally, solve the problem of the low stability of H2O in solutions, allowing a preparation with greater stability and a longer shelf life to be produced.
Finally, the necessary step will be to determine the lowest concentration of LPO system components that will exhibit a significant
in vitro and
in vivo antibiofilm effect while remaining nontoxic to host cells. In our previous studies on the modifications toxicity of the described in the LPO system against Human Gingival Fibroblasts, we found that the LPO-iodide system is characterised by the highest toxicity and the physiological system (LPO-SCN
-) by the lowest toxicity [
26]. The results showed that modifying the LPO system enhances both its antibiofilm activity and its negative effects on host cells.
There is still a lot of work to be done in the field of combating cariogenic biofilms, and dental caries is still a major clinical, social and financial problem, but we are sure that the results presented are of added value and show the potential that the modified LPO system has in fighting the cariogenic biofilms and, in a further perspective, also biofilms related to different diseases.
3. Materials and Methods
3.1. LPO system setup
In each of the experiments, the LPO concentration was 50 nM, which reflects its concentration in human saliva. Each experiment was preceded by the preparation of a LPO solution where 1 mg of the enzyme was dissolved in 1 ml of PBS followed by measuring absorbance at 412 nm (ε412= 112 000) to determine the concentration.
(Pseudo)halide substrates were added in a concentration of 10 mM, which is about two times higher than that of the physiological LPO substrate (SCN
-) present in human saliva [
59]. This decision was based on the need to use the excess of the substrate over hydrogen peroxide (tenfold excess over the highest tested concentration of H
2O
2).
Three concentrations of hydrogen peroxide (1000, 500, and 250 μM) were used in the present study. The deficiency of H2O2 on the (pseudo)halide substrate played a limiting role in the reaction and additionally controlled the amount of product generated, which was equal in molar amount to the H2O2 provided.
3.2. Biofilm growth
The present study was conducted on
Streptocuccus mutans ATCC 25175 bacterial strain. It was grown in Brain Heart Infusion (BHI) medium (Graso Biotech, Owidz, Poland) for 12h after which it was centrifuged (1200 RPM; 5 min). The remaining bacterial mass was then suspended in 5 ml of BHI and immediately used for the preparation of a suspension of 0.2 McF BHI + 5% sucrose. The suspension was added to 24-well plates (1 ml per well) or 96-well plates (250 μL per well), depending on the experiment. The evaluations of the selected parameters were performed on biofilms in the early biofilm growth phase (2h) and on 24 h old biofilms, depending on the experiment [
5].
3.3. Total biofilm biomass assay
The total biomass of the biofilm was assessed with previously described crystal violet method [
5]. After the planktonic bacteria, the biofilms were stained with 0.1% crystal violet solution for 20 min, after which the excess dye was washed with PBS. The binded dye was extracted with a 33% acetic acid water solution and transferred to a fresh 96-well plate to measure the absorbance at 540 nm. The amount of crystal violet binded by the biofilm (absorbance) was directly proportional to the total biomass of the biofilm.
3.4. Insouble extracellular polysaccharide mass assay
Insoluble extracellular polysaccharide biomass was measured using the anthrone-sulfuric acid method [
60]. The biofilms were washed with PBS and scraped from the bottom of the wells with pipette tips. The biofilm released was then transferred to eppendorf type tube and centrifuged (5500 RCF; 5 min). After the supernatant liquid, the biofilm was washed with PBS and centrifuged three more times. Once the soluble saccharides had been removed from the biofilm matrix, the water-insoluble fraction was dissolved by adding 500 µL of 1 M NaOH folowed by incubation at room temperature for 30 minutes with constant mechanical stirring. Next, the insoluble microorganic and biofilm residue was centrifuged and 20 μL of each of supernatant solution was transferred to a 96-well plate. The mass of the extracted polysaccharide was evaluated using anthrone method by adding 200 μL 0.15% of anthrone solution in 80% (v/v) H
2SO
4. The absorbance at 626 nm was measured after 30 min of incubation at 95 °C. The absorbance was directly proportional to the mass of insoluble polysaccharide found in the biofilm.
3.5. Lactate, glucose and sucrose concentration assay
Lactate and glucose concentrations were measured in biofilm growth medium using a direct bioamperometric method utilising the Biosen C-line analyzer (EKF Diagnostics), which allows to automatically measure lactate and glucose concentrations in the range of 0,5-40 mM. The device was equipped with lactate and glucose enzymatic biosensors for the simultaneous assay of the glucose and lactate concentration from one sample.
24 h biofilms were subjected to tested LPO systems for 15 min and then washed three times with 1 mL of PBS, after which 1 mL of BHI with 1% of sucrose was added to the wells. Lactate, glucose, and sucrose concentrations were measured every 50 min for 350 min (with the first measurement taking place immediately after adding the medium). The measurement was performed by transferring 10 μL of growth medium from each well to 500 μL of System Solution (EKF Diagnostics, Cardiff, United Kindgom) in an Eppendorf type tube. The tubes were inserted into the analyser, and the measurement was performed. The sucrose concentration was determined by transferring 20 μL of the sample to 50 μL solution of invertase from Sacharomyces cerevisiae (Sigma-Aldrich, Schnelldorf, Germany) (1 mg/mL in 0.05 M citrate buffer pH=4.5). The samples were incubated at 55 °C for 20 min which ensured complete sucrose hydrolysis. This was followed by measurement of glucose concentration in digested sample with the same method as described previously (sum of glucose from BHI and glucose after breakdown). The sucrose concentration in each sample was calculated by subtracting the glucose concentration before hydrolysis to from the total glucose concentration (the number of moles of glucose generated from sucrose hydrolysis is equal to the number of moles of sucrose present in the sample). The results of the assay were then calculated applying the correction for volume changes (decrease of total medium volume in wells after each measurement) and expressed as lactate/glucose/sucrose moles for the well.
3.6. PTS system activity assay
The overnight culture of
S. mutans in BHI medium was put in falcon tubes and then were centrifuged and washed with PBS. To obtain log phase cells, a fresh portion of BHI medium was added and the bacteria were growth for 2 h. After that, the cells were again centrifuged and washed with PBS. The suspension of 2 McF was prepared in PBS and 1 mL of that suspension was transferred to an Eppendorf type tubes. To each tube the components of the LPO system were added (full system – LPO + (pseudo)halides + hydrogen peroxide; control samples – only LPO, only (pseudo)halides); the control sample consisting of suspension of
S. mutans without any addition). After 15 minu of incubation, the tubes were centrifuged and the supernatant was decanted. The cells were suspended in PBS with 5 mM Mg
2+ (decryptification buffer) and permeabilized with 50 µL of acetone-toluene (9:1) mixture (9: 1) for 5 min. The activity of PTS was measured based on method described by LeBlanc et al. [
61]. The reagent mixture consisted of 10 mM glucose, 10 mM sodium fluoride, 0.1 mM NADH, 10 U/mL lactic acid dehydrogenase in PBS with 5mM Mg
2+. Measurement was carried out in 96-well plates by adding 75 μL of S. mutans suspension and 200 uL of reagent mixture to each well. The reaction was started by adding phosphoenolpuryvate (PEP) (final concentration 5mM) and the change in NADH concentration was measured for 4 min by detecting the change in absorbance at 340 nm. Simultaneously, the second sample without the addition of PEP was analysed as a correction to the result (subtraction of NADH oxidation without the dependence of PTS activity). Finally, the result was expressed as nanomoles of oxidised NADH per second depending on PEP consumption by the PTS system [
48].
3.7. NAD+/NADH EC assay
The biofilms after treatment with tested LPO systems were washed three times with PBS and one time with molecular grade water. Next, the supernatant liquid was discarded and the biofilms that remained in the 24-well plate were frozen at -20 ° C and then lyophilised for 4 h to eliminate all the residual water from the samples. In each well 600 μL of ice cold 1:1 acetonitrile-water mixture was added and then the biofilm was scraped using a pipette tip. All the released biofilm biomass was transferred to 1.5 mL tube and 0.2 g of glass beads were added. After that, the extraction process of NAD+/NADH was performed for 60 min with 30 s of shaking every 5 minutes. During extraction the samples were incubated at 4 °C. Finally, the samples were centrifuged at 14 000 RCF for 5 min at 4 °C and then the supernatant was collected in a fresh 1.5 mL tube.
The concentrations of NAD+ and NADH in extracts were determined using capillary electrophoresis using a sweeping borate complexation method. Separation was performed using the Prince Technologies CE System equipped with the Bischoff Lambda 1010 UV/VIS detector set to 254 nm. All separations were performed using fused-silica capillaries - 75 μm internal diameter, 65 cm length (effective length 50 cm). Each day of experiment the capilary was rinsed with NaOH 1M (5 min), water (5 min) and buffer (5 min). As background electrolyte (BGE) 25 mM borate buffer (pH=9.25) with 5mM β-cyclodextrin was used. The samples, diluted three times with molecular grade water, were injected into the capillary at a pressure of 200 mbar for 50 s. After that, online sample concentration process was started applying negative voltage (-30 kV) until the end of sweeping process (until current reached 90% of the current measured in the BGE only filled capillary). Separation was started by applying 30 kV of positive voltage. After each measurement, the capillary was rinsed with NaOH 1M, water, and BGE (1 minute for each). Signal detection and qualitative and quantitative analyse were performed using DAx software (Prince Technologies, Emmen, The Netherlands). The concentration of NAD+ and NADH was calculated by comparing the obtained peak areas with the peak areas of the standard solutions.
3.8. Statistical analysis
The R environment v4.3 (The R Foundation for Statistical Computing)was utilised for data analysis. The ggplot2 package was used for plot preparation. Variances homogeneity was determined with the Levene’s test. Statistical significance between groups was evaluated using one-way analysis of variance (ANOVA) with the post hoc Tukey test.
4. Conclusions
The lactoperoxidase system can be an effective agent of natural origin against cariogenic biofilms. This requires some modification, which changes the physiological substrate to an alternative (pseudo)halide substrate, mainly selenocyanite and iodide. The effects of these changes are associated with impaired carbohydrates transport and metabolism, resulting in reduced expression of key virulence factors of Streptococcus mutans, particularly the ability to produce biofilms that include the polysaccharide matrix and lactic acid.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1 - Influence of all tested LPO system modifications on
S. mutans total biofilm biomass and total insoluble polysaccharide mass; Table S2 - Amounts of NAD
+, NADH in the well and NAD
+/NADH ratio after treatment biofilm with all the tested systems (1000 μM H
2O
2); Table S3 - PTS system activity in
S. mutans cells treated with tested LPO system modifications and control sample; Table S4 - Area under kinetic curves (relative to control) of lactate production and glucose/sucrose consumption.
Author Contributions
Conceptualization, M.M.; methodology, M.M and W.K.; software, M.M.; validation, M.M., E.P., S.A.S., and W.K.; formal analysis, M.M and W.K..; investigation, M.M., K.Kl., P.M., W.P. and M.L; resources, M.M.; data curation, M.M. and W.K.; writing—original draft preparation, M.M.; writing—review and editing, M.M., W.K., S.A.S., E.P. and K.Kę.; visualization, M.M.; supervision, W.K.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by National Science Center (grant no. 2021/41/N/NZ7/03315). The support from the Jagiellonian University (program no. U1C/W42/NO/28.10) is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Mr. Andrzej Biedroń from Allmed AB sp z o.o for providing Biosen C-line analyzer which made this study possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Untreated Caries. J Dent Res 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Chenicheri, S.; R, U.; Ramachandran, R.; Thomas, V.; Wood, A. Insight into Oral Biofilm: Primary, Secondary and Residual Caries and Phyto-Challenged Solutions. Open Dent J 2017, 11, 312–333. [Google Scholar] [CrossRef] [PubMed]
- Kozmos, M.; Virant, P.; Rojko, F.; Abram, A.; Rudolf, R.; Raspor, P. Bacterial Adhesion of Streptococcus Mutans to Dental Material Surfaces. 2020, 1–13.
- Vieira, A.R. Genetics and Caries: Prospects. Braz Oral Res 2012, 26, 7–9. [Google Scholar] [CrossRef]
- Staszczyk, M.; Jurczak, A.; Magacz, M.; Kościelniak, D.; Gregorczyk-Maga, I.; Jamka-Kasprzyk, M.; Kępisty, M.; Kołodziej, I.; Kukurba-Setkowicz, M.; Krzyściak, W. Effect of Polyols and Selected Dental Materials on the Ability to Create a Cariogenic Biofilm–On Children Caries-Associated Streptococcus Mutans Isolates. Int J Environ Res Public Health 2020, 17, 3720. [Google Scholar] [CrossRef]
- Lee, Y. Diagnosis and Prevention Strategies for Dental Caries. J Lifestyle Med 2013, 3, 107–109. [Google Scholar] [PubMed]
- Costa, S.M.; Martins, C.C.; Bonfim, M. de L.C.; Zina, L.G.; Paiva, S.M.; Pordeus, I.A.; Abreu, M.H.N.G. A Systematic Review of Socioeconomic Indicators and Dental Caries in Adults. Int J Environ Res Public Health 2012, 9, 3540–3574. [CrossRef]
- Luiz, M.T.; di Filippo, L.D.; Dutra, J.A.P.; Viegas, J.S.R.; Silvestre, A.L.P.; Anselmi, C.; Duarte, J.L.; Calixto, G.M.F.; Chorilli, M. New Technological Approaches for Dental Caries Treatment: From Liquid Crystalline Systems to Nanocarriers. Pharmaceutics 2023, 15, 762. [Google Scholar] [CrossRef] [PubMed]
- Bafort, F.; Parisi, O.; Perraudin, J.-P.; Jijakli, M.H. Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review. Enzyme Res 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Reiter, B. The Lactoperoxidase-Thiocyanate-Hydrogen Peroxide Antibacterium System. In; 2008; pp. 285–294.
- Schlorke, D.; Atosuo, J.; Flemmig, J.; Lilius, E.M.; Arnhold, J. Impact of Cyanogen Iodide in Killing of Escherichia Coli by the Lactoperoxidase-Hydrogen Peroxide-(Pseudo)Halide System. Free Radic Res 2016, 50, 1287–1295. [Google Scholar] [CrossRef]
- Day, B.J.; Bratcher, P.E.; Chandler, J.D.; Kilgore, M.B.; Min, E.; LiPuma, J.J.; Hondal, R.J.; Nichols, D.P. The Thiocyanate Analog Selenocyanate Is a More Potent Antimicrobial Pro-Drug That Also Is Selectively Detoxified by the Host. Free Radic Biol Med 2020, 146, 324–332. [Google Scholar] [CrossRef]
- Zheng, H.; Xie, T.; Li, S.; Qiao, X.; Lu, Y.; Feng, Y. Analysis of Oral Microbial Dysbiosis Associated with Early Childhood Caries. BMC Oral Health 2021, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The Virulence of Streptococcus Mutans and the Ability to Form Biofilms. European Journal of Clinical Microbiology & Infectious Diseases 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Cawley, A.; Golding, S.; Goulsbra, A.; Hoptroff, M.; Kumaran, S.; Marriott, R. Microbiology Insights into Boosting Salivary Defences through the Use of Enzymes and Proteins. J Dent 2019, 80, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.R.; Boon, N.; Bernaerts, K.; Slomka, V.; Verspecht, T.; Quirynen, M.; Teughels, W. Clinical Concentrations of Peroxidases Cause Dysbiosis in in Vitro Oral Biofilms. J Periodontal Res 2018, 53, 457–466. [Google Scholar] [CrossRef]
- Smith, M.L.; Sharma, S.; Singh, T.P. Iodide Supplementation of the Anti-Viral Duox-Lactoperoxidase Activity May Prevent Some SARS-CoV-2 Infections. Eur J Clin Nutr 2022, 76, 629–630. [Google Scholar] [CrossRef]
- Yousefi, M.; Nematollahi, A.; Shadnoush, M.; Mortazavian, A.M.; Khorshidian, N. Antimicrobial Activity of Films and Coatings Containing Lactoperoxidase System: A Review. Front Nutr 2022, 9. [Google Scholar] [CrossRef]
- Thomas, E.L.; Aune, T.M. Susceptibility of Escherichia Coli to Bactericidal Action of Lactoperoxidase, Peroxide, and Iodide or Thiocyanate. Antimicrob Agents Chemother 1978, 13, 261–265. [Google Scholar] [CrossRef]
- Schlorke, D.; Flemmig, J.; Birkemeyer, C.; Arnhold, J. Formation of Cyanogen Iodide by Lactoperoxidase. J Inorg Biochem 2016, 154, 35–41. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob Resist Infect Control 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Chen, L.; Ren, Z.; Zhou, X.; Zeng, J.; Zou, J.; Li, Y. Inhibition of Streptococcus Mutans Biofilm Formation, Extracellular Polysaccharide Production, and Virulence by an Oxazole Derivative. Appl Microbiol Biotechnol 2016, 100, 857–867. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The Virulence of Streptococcus Mutans and the Ability to Form Biofilms. European Journal of Clinical Microbiology & Infectious Diseases 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Krzyściak, W.; Kościelniak, D.; Papież, M.; Vyhouskaya, P.; Zagórska-Świeży, K.; Kołodziej, I.; Bystrowska, B.; Jurczak, A. Effect of a Lactobacillus Salivarius Probiotic on a Double-Species Streptococcus Mutans and Candida Albicans Caries Biofilm. Nutrients 2017, 9, 1242. [Google Scholar] [CrossRef]
- Magacz, M.; Papież, M.; Kościelniak, D.; Jurczak, A.; Kędziora, K.; Pamuła, E.; Krzyściak, W. Safety Assessment of the Modified Lactoperoxidase System—In Vitro Studies on Human Gingival Fibroblasts. Int J Mol Sci 2023, 24, 2640. [Google Scholar] [CrossRef]
- Al-Baarri, A.N.; Damayanti, N.T.; Legowo, A.M.; Tekiner, İ.H.; Hayakawa, S. Enhanced Antibacterial Activity of Lactoperoxidase–Thiocyanate–Hydrogen Peroxide System in Reduced-Lactose Milk Whey. Int J Food Sci 2019, 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Furtmüller, P.G.; Jantschko, W.; Regelsberger, G.; Jakopitsch, C.; Arnhold, J.; Obinger, C. Reaction of Lactoperoxidase Compound I with Halides and Thiocyanate. Biochemistry 2002, 41, 11895–11900. [Google Scholar] [CrossRef] [PubMed]
- Lumikari, M.; Soukka, T.; Nurmio, S.; Tenovuo, J. Inhibition of the Growth of Streptococcus Mutans, Streptococcus Sobrinus and Lactobacillus Casei by Oral Peroxidase Systems in Human Saliva. Arch Oral Biol 1991, 36, 155–160. [Google Scholar] [CrossRef]
- SOUKKA, T.; LUMIKARI, M.; TENOVUO, J. Combined Inhibitory Effect of Lactoferrin and Lactoperoxidase System on the Viability of Streptococcus Mutans, Serotype c. Eur J Oral Sci 1991, 99, 390–396. [Google Scholar] [CrossRef]
- Thomas, E.L.; Pera, K.A.; Smith, K.W.; Chwang, A.K. Inhibition of Streptococcus Mutans by the Lactoperoxidase Antimicrobial System. Infect Immun 1983, 39, 767–778. [Google Scholar] [CrossRef]
- Lenander-Lumikari, M.; Tenovuo, J.; Mikola, H. Effects of a Lactoperoxidase System-Containing Toothpaste on Levels of Hypothiocyanite and Bacteria in Saliva. Caries Res 1993, 27, 285–291. [Google Scholar] [CrossRef]
- Shimizu, E.; Kobayashi, T.; Wakabayashi, H.; Yamauchi, K.; Iwatsuki, K.; Yoshie, H. Effects of Orally Administered Lactoferrin and Lactoperoxidase-Containing Tablets on Clinical and Bacteriological Profiles in Chronic Periodontitis Patients. Int J Dent 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Gudipaneni, R.K. Short Term Comparative Evaluation of Antimicrobial Efficacy of Tooth Paste Containing Lactoferrin, Lysozyme, Lactoperoxidase in Children with Severe Early Childhood Caries: A Clinical Study. JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH 2014. [Google Scholar] [CrossRef]
- Dirix, P.; Nuyts, S.; Vander Poorten, V.; Delaere, P.; Van den Bogaert, W. Efficacy of the BioXtra Dry Mouth Care System in the Treatment of Radiotherapy-Induced Xerostomia. Supportive Care in Cancer 2007, 15, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Yaegaki, K.; Murata, T.; Ii, H.; Tanaka, T.; Aoyama, I.; Yamauchi, K.; Toida, T.; Iwatsuki, K. Effects of a Composition Containing Lactoferrin and Lactoperoxidase on Oral Malodor and Salivary Bacteria: A Randomized, Double-Blind, Crossover, Placebo-Controlled Clinical Trial. Clin Oral Investig 2011, 15, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Shimizu, E.; Wakabayashi, H.; Yamauchi, K.; Abe, F. A Randomized, Double-Blind, Crossover, Placebo-Controlled Clinical Trial to Assess Effects of the Single Ingestion of a Tablet Containing Lactoferrin, Lactoperoxidase, and Glucose Oxidase on Oral Malodor. BMC Oral Health 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.L.; Azenha, G.R.; Araujo, G.S.A.; Puppin Rontani, R.M. Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate and Lysozyme, Lactoferrin, and Lactoperoxidase in Reducing Streptococcus Mutans Counts in Dentinal Caries. Gen Dent 2017, 65, 47–50. [Google Scholar] [PubMed]
- Rageh, A.H.; Kaltz, A.; Pyell, U. Determination of Urinary Nucleosides via Borate Complexation Capillary Electrophoresis Combined with Dynamic PH Junction-Sweeping-Large Volume Sample Stacking as Three Sequential Steps for Their on-Line Enrichment. Anal Bioanal Chem 2014, 406, 5877–5895. [Google Scholar] [CrossRef]
- Kawada-Matsuo, M.; Oogai, Y.; Komatsuzawa, H. Sugar Allocation to Metabolic Pathways Is Tightly Regulated and Affects the Virulence of Streptococcus Mutans. Genes (Basel) 2016, 8, 11. [Google Scholar] [CrossRef]
- Burne, R.A.; Chen, Y.-Y.M.; Penders, J.E.C. Analysis of Gene Expression in Streptococcus Mutans in Biofilms in Vitro. Adv Dent Res 1997, 11, 100–109. [Google Scholar] [CrossRef]
- Phan, T.-N.; Marquis, R.E. Triclosan Inhibition of Membrane Enzymes and Glycolysis of Streptococcus Mutans in Suspensions and Biofilms. Can J Microbiol 2006, 52, 977–983. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Pellegrino, M.; Saturnino, C.; Longo, P.; Aquaro, S. Triclosan: A Small Molecule with Controversial Roles. Antibiotics 2022, 11, 735. [Google Scholar] [CrossRef]
- Adamson, M.; Pruitt, K.M. Lactoperoxidase-Catalyzed Inactivation of Hexokinase. Biochimica et Biophysica Acta (BBA) - Enzymology 1981, 658, 238–247. [Google Scholar] [CrossRef]
- Oram, J.D.; Reiter, B. The Inhibition of Streptococci by Lactoperoxidase, Thiocyanate and Hydrogen Peroxide. The Effect of the Inhibitory System on Susceptible and Resistant Strains of Group N Streptococci. Biochem J 1966, 100, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Capillary Electrophoretic Separation of Mono- and Dinucleotides of Adenosine Using Cyclodextrin Solutions with MgCl2 Additive. J Chromatogr A 1998, 802, 167–177. [Google Scholar] [CrossRef]
- Markuszewski, M.J.; Britz-McKibbin, P.; Terabe, S.; Matsuda, K.; Nishioka, T. Determination of Pyridine and Adenine Nucleotide Metabolites in Bacillus Subtilis Cell Extract by Sweeping Borate Complexation Capillary Electrophoresis. J Chromatogr A 2003, 989, 293–301. [Google Scholar] [CrossRef]
- Baker, J.L.; Derr, A.M.; Faustoferri, R.C.; Quivey, R.G. Loss of NADH Oxidase Activity in Streptococcus Mutans Leads to Rex-Mediated Overcompensation in NAD+ Regeneration by Lactate Dehydrogenase. J Bacteriol 2015, 197, 3645–3657. [Google Scholar] [CrossRef]
- McC. Hogg, D.; Jago, G.R. The Oxidation of Reduced Nicotinamide Nucleotides by Hydrogen Peroxide in the Presence of Lactoperoxidase and Thiocyanate, Iodide or Bromide. Biochemical Journal 1970, 117, 791–797. [CrossRef]
- Ma, Q.; Pan, Y.; Chen, Y.; Yu, S.; Huang, J.; Liu, Y.; Gong, T.; Zhang, Q.; Sun, Q.; Zou, J.; et al. Acetylation of Lactate Dehydrogenase Negatively Regulates the Acidogenicity of Streptococcus Mutans. mBio 2022, 13. [Google Scholar] [CrossRef]
- Prütz, W.A.; Kissner, R.; Koppenol, W.H.; Rüegger, H. On the Irreversible Destruction of Reduced Nicotinamide Nucleotides by Hypohalous Acids. Arch Biochem Biophys 2000, 380, 181–191. [Google Scholar] [CrossRef]
- Carlsson, J.; Iwami, Y.; Yamada, T. Hydrogen Peroxide Excretion by Oral Streptococci and Effect of Lactoperoxidase-Thiocyanate-Hydrogen Peroxide. Infect Immun 1983, 40, 70–80. [Google Scholar] [CrossRef]
- Watanabe, S. Salivary Clearance from Different Regions of the Mouth in Children. Caries Res 1992, 26, 423–427. [Google Scholar] [CrossRef]
- Geiszt, M.; Witta, J.; Baffi, J.; Lekstrom, K.; Leto, T.L. Dual Oxidases Represent Novel Hydrogen Peroxide Sources Supporting Mucosal Surface Host Defense. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2003, 17, 1502–1504. [Google Scholar] [CrossRef]
- Thomas, E.L. Lactoperoxidase-Catalyzed Oxidation of Thiocyanate: Equilibriums between Oxidized Forms of Thiocyanate. Biochemistry 1981, 20, 3273–3280. [Google Scholar] [CrossRef] [PubMed]
- Kalmár, J.; Woldegiorgis, K.L.; Biri, B.; Ashby, M.T. Mechanism of Decomposition of the Human Defense Factor Hypothiocyanite Near Physiological PH. J Am Chem Soc 2011, 133, 19911–19921. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Balsandorj, Z.; Hao, Z.; Pan, L. High-Precision Measurement of PH in the Full Toothpaste Using NMR Chemical Shift. Journal of Magnetic Resonance 2020, 317, 106771. [Google Scholar] [CrossRef]
- VIRION, A.; MICHOT, J.L.; DEME, D.; POMMIER, J. NADPH Oxidation Catalyzed by the Peroxidase/H2O2 System. Iodide-Mediated Oxidation of NADPH to Iodinated NADP. Eur J Biochem 1985, 148, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Luepker, R. V; Pechacek, T.F.; Murray, D.M.; Johnson, C.A.; Hund, F.; Jacobs, D.R. Saliva Thiocyanate: A Chemical Indicator of Cigarette Smoking in Adolescents. Am J Public Health 1981, 71, 1320–1324. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Emam, T.; Raafat, M.M. Hindering of Cariogenic Streptococcus Mutans Biofilm by Fatty Acid Array Derived from an Endophytic Arthrographis Kalrae Strain. Biomolecules 2020, 10, 811. [Google Scholar] [CrossRef]
- LeBlanc, D.J.; Crow, V.L.; Lee, L.N.; Garon, C.F. Influence of the Lactose Plasmid on the Metabolism of Galactose by Streptococcus Lactis. J Bacteriol 1979, 137, 878–884. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).