Submitted:
29 June 2023
Posted:
07 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Study design
Literature search
Reconstruction of individual patient data from Kaplan-Meier survival curves
Survival statistics
3. Results
- trial [a]: dasatinib in combination with low-intensity chemotherapy (Rousselot et al [18]);
- trial[b]: nilotinib combined with low-intensity chemotherapy (Rousselot et al [19]);
- trial[c]: dasatinib plus steroids induction followed by dasatinib alone (Chiaretti et al [20]);
- trial[d]: imatinib combined with steroids (Vignetti et al [21]);
- trial[e]: dasatinib induction therapy combined with steroids (Foà et al [22]);
- trial[f]: ponatinib plus prednisone (Martinelli et al [23]);
- trial[g]: dasatinib plus blinatumomab (Chiaretti et al [24]);
- trial[h]: ponatinib plus blinatumomab (Jabbour et al [25]).
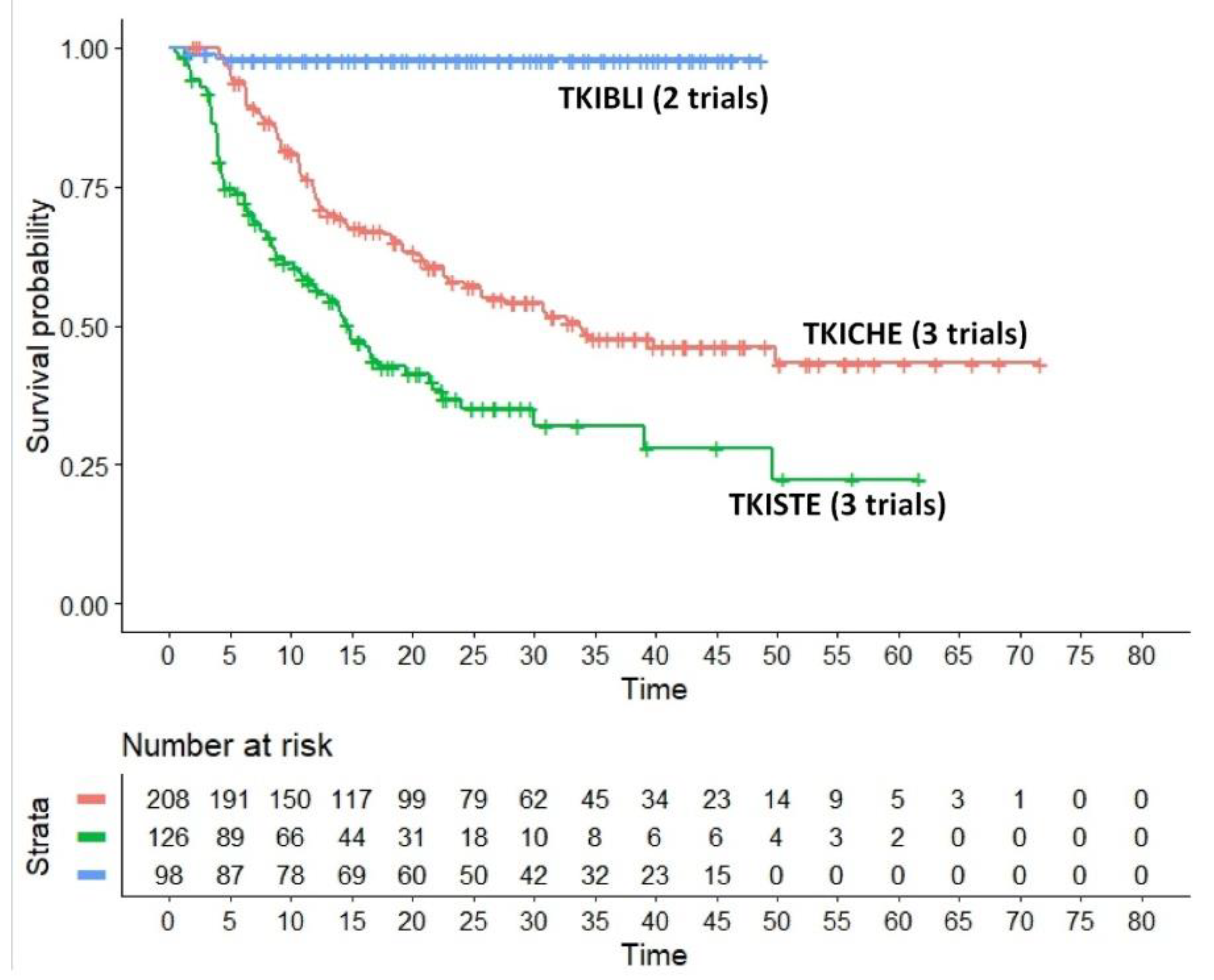
- The regimen denoted as TKICHE (i.e. TKI plus low-intensity chemotherapy), which includes three trials, namely dasatinib plus low-intensity chemotherapy in the trial by Rousselot et al [18], nilotinib plus low-intensity chemotherapy in the GRAAPH-2014 Study by Rousselot et al [19], dasatinib plus steroids induction in the trial by Chiaretti et al [20].
- HR for the comparison of TKIBLI vs TKICHE = 0.042 (95%CI, 0.010 to 0.170);
- HR for the comparison of TKIBLI vs TKISTE = 0.022 (95%CI, 0.005 to 0.091);
- HR for the comparison of TKICHE vs TKISTE = 0.506 (95%CI, 0.370 to 0.692).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Data Sharing Statement
Conflicts of Interest
References
- Chiaretti S: Vitale, A.; Cazzaniga, G.; et al. Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica. 2013, 98, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Kebriaei, P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009, 23, 1043–1063. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, T.; Schwartz, S.; Bartram, C.R.; et al. Patients’ age and BCR–ABL frequency in adult B- precursor ALL: a retrospective analysis from the GMALL study group. Blood. 2008, 112, 918–919. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.V.; Harrison, C.J.; Buck, G.A.; et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/ Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007, 109, 3189–3197. [Google Scholar] [CrossRef]
- Dombret, H.; Gabert, J.; Boiron, J.M.; et al. Outcome of treatment in adults Philadelphia chromosome-positive acute lymphoblastic leukemia-results of the prospective multi-center LALA-94 trial. Blood. 2002, 100, 2357–2366. [Google Scholar] [CrossRef]
- Ottmann, O.G.; Wassmann, B.; Pfeifer, H.; et al. Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Cancer. 2007, 109, 2068–2076. [Google Scholar] [CrossRef]
- Talpaz, M.; Shah, N.P.; Kantarjian, H.; et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006, 354, 2531–2541. [Google Scholar] [CrossRef]
- Bassan, R.; Rossi, G.; Pogliani, E.M.; et al. Chemotherapy-phased imatinib pulses improve long- term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010, 28, 3644–3652. [Google Scholar] [CrossRef]
- Haddad, F.G.; Sawyers, J.; Short, N.J. Treatment de-escalation in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia: the emerging role of chemotherapy-free regimens. Ther Adv Hematol. 2023, 14, 20406207231151294. [Google Scholar] [CrossRef]
- Chiaretti, S.; Vitale, A.; Vignetti, M.; et al. A sequential approach with imatinib, chemotherapy and transplant for adult Ph1 acute lymphoblastic leukemia: final results of the GIMEMA LAL 0904 study. Haematologica. 2016, 101, 1544–1552. [Google Scholar] [CrossRef]
- Jabbour, E.; Haddad, F.G.; Short, N.J.; Kantarjian, H. Treatment of Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia-From Intensive Chemotherapy Combinations to Chemotherapy-Free Regimens: A Review. JAMA Oncol. 2022, 8, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Assi, R.; Kantarjian, H.; Short, N.J.; et al. Safety and Efficacy of Blinatumomab in Combination With a Tyrosine Kinase Inhibitor for the Treatment of Relapsed Philadelphia Chromosome-positive Leukemia. Clin Lymphoma Myeloma Leuk. 2017, 17, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Foà, R.; Bassan, R.; Vitale, A.; et al. Dasatinib-Blinatumomab for Ph+ Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020, 383, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Ossato, A.; Mengato, D.; Chiumente, M.; Messori, A.; Damuzzo, V. Progression-Free and Overall Survival of First-Line Treatments for Advanced Renal Cell Carcinoma: Indirect Comparison of Six Combination Regimens. Cancers (Basel). 2023, 15, 2029. [Google Scholar] [CrossRef] [PubMed]
- Ottmann, O.G.; Pfeifer, H.; Cayuela, J.M.; et al. Nilotinib (Tasigna®) and Low Intensity Chemotherapy for First-Line Treatment of Elderly Patients with BCR-ABL1-Positive Acute Lymphoblastic Leukemia: Final Results of a Prospective Multicenter Trial (EWALL-PH02). Blood 2018, 132 (Suppl. 1), 31. [Google Scholar] [CrossRef]
- Chalandon, Y.; Thomas, X.; Hayette, S.; et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph+ acute lymphoblastic leukemia. Blood 2015, 125, 3711–3719, [published correction appears in Blood. 2015, 126, 1261]. [Google Scholar] [CrossRef]
- Rousselot, P.; Coudé, M.M.; Gokbuget, N.; et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood 2016, 128, 774–782. [Google Scholar] [CrossRef]
- Rousselot, P.; Chalandon, Y.; Chevret, S.; Cayuela, J.M.; Huguet, F.; Chevallier, P.; et al. The Omission of High-Dose Cytarabine during Consolidation Therapy of Ph+ ALL Patients Treated with Nilotinib and Low-Intensity Chemotherapy Results in an Increased Risk of Relapses Despite Non-Inferior Levels of Late BCR-ABL1 MRD Response. First Results of the Randomized Graaph-2014 Study. Blood 2021, 138 (Suppl. 1), 512. [Google Scholar]
- Chiaretti, S.; Ansuinelli, M.; Vitale, A.; et al. A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: final results of the GIMEMA LAL1509 protocol. Haematologica. 2021, 106, 1828–1838. [Google Scholar] [CrossRef]
- Vignetti, M.; Fazi, P.; Cimino, G.; et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007, 109, 3676–3678. [Google Scholar] [PubMed]
- Foà, R.; Vitale, A.; Vignetti, M.; et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011, 118, 6521–6528. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Papayannidis, C.; Piciocchi, A.; et al. INCB84344-201: Ponatinib and steroids in frontline therapy for unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv. 2022, 6, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Bassan, R.; Vitale, A.; et al. Forty Months Update Of The Gimema Lal2116 (D-alba) Protocol And Ancillary Lal2217 Study For Newly Diagnosed Adult Ph+ All [abstract]. EHA Library. 2022 Abstract: P353.
- Jabbour, E.; Short, N.J.; Jain, N.; Huang, X.; Montalban-Bravo, G.; Banerjee, P.; et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial. Lancet Haematol. 2023, 10, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Boissel, N.; Chevallier, P.; et al. Complete Hematologic and Molecular Response in Adult Patients With Relapsed/Refractory Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia Following Treatment With Blinatumomab: Results From a Phase II, Single-Arm, Multicenter Study [published correction appears in J Clin Oncol. 2017, 35, 2722] J Clin Oncol. 2017, 35, 1795–1802. [published correction appears in J Clin Oncol. 2017, 35, 2856]. 35.
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016, 30, 1648–1671. [Google Scholar] [CrossRef]
- Messori, A.; Rivano, M.; Mengato, D.; Cancanelli, L.; Di Spazio, L.; Chiumente, M. A preliminary estimate of survival gain and cost-effectiveness of CAR-T in adult patients with acute lymphoblastic leukaemia. Leukemia & Lymphoma 2021 Dec 31:1-4. https://www.tandfonline.com/eprint/TWPDKPRVUQV6YKXDHKDX/full?target=10.1080/10428194.2021. 2022. [Google Scholar]
- Messori, A.; Chiumente, M.; Mengato, D. CAR T-cells in large B-cell lymphoma: analysis of overall survival based on reconstructed patient-level data. Clin Ther 2022 Dec 8:S0149-2918(22)00377-0.
- Messori, A.; Damuzzo, V.; Leonardi, L.; Agnoletto, L.; Chiumente, M.; Mengato, D. CAR-T Treatment: Determining the Survival Gain in Patients With Relapsed or Refractory Diffuse Large B-cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2020, 20, 490–491. [Google Scholar] [CrossRef]
- Messori, A. Long-term progression-free survival in patients with chronic lymphocytic leukaemia treated with novel agents: an analysis based on indirect comparisons. Eur J Haematol. 2023, 110, 60–66. [Google Scholar] [CrossRef]
| First Author |
Year | Trial | Treatment | Inclusion criteria | Events | N° of patients | Notes | |
|---|---|---|---|---|---|---|---|---|
| TKICHE | Chaladon et al [17] | 2015 | GRAAPH-2005 study | Imatinib combined with low-intensity chemotherapy | Patients aged 18 to 59 years with newly diagnosed Ph1 and/or BCRABL1–positive ALL were eligible | 65 | 135 | Excluded from our analysis because only induction was not intensive |
| Rousselot et al [18] | 2016 | EWALL-PH-01 international study |
Dasatinib in combination with low-intensity chemotherapy | Patients aged 55 years or older were eligible if they had newly diagnosed Ph1 and/or BCR-ABL ALL | 40 | 71 | Included | |
| Rousselot et al [19] | 2021 | Graaph-2014 Study | Nilotinib combined with low-intensity chemotherapy | Ph-Positive ALL patients aged 18-60 years old were randomized | 23 | 79 | Included | |
| Chiaretti et al [20] | 2020 | GIMEMA LAL1509 | Dasatinib plus steroids induction followed by dasatinib alone | Adult Ph+ ALL patients (18-60 years). | 13 | 58 | Included | |
| TKISTE | Vignetti et al [21] | 2007 | GIMEMA LAL0201-B | Imatinib combined with steroids | Patients with a diagnosis of ALL who were older than 60 years were eligible if they carried either the Ph chromosome or the BCR-ABL molecular translocation |
16 | 29 | Included |
| Foà et al [22] | 2011 | GIMEMA LAL1205 | Dasatinib induction therapy combined with steroids | Patients 18 years of age or older (with no upper age limit) were eligible if they had been diagnosed with Ph/BCR-ABLALL | 23 | 53 | Included | |
| Martinelli et al [23] | 2022 | GIMEMA LAL 1811 | Ponatinib plus prednisone | Patients had new-onset Ph+ ALL, and were ≥ 60 years or were ≥ 18 years but unfit for a program of intensive chemotherapy and SCT | 34 | 44 | Included | |
| TKIBLI | Chiaretti et al [24] | 2022 | GIMEMA LAL2117 | Dasatinib + blinatumomab |
Ph-Positive ALL Patients, median trial age of participants was 54 years ( 24-82; no upper age limit) |
9 | 58 | Included |
| Jabbour et al [25] | 2023 | NCT03263572 | Ponatinib + blinatumomab |
Patients with newly diagnosed, relapsed/refractory Ph+ ALL or CML in lymphoid blast phase |
2 | 40 | Included |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





