Submitted:
30 June 2023
Posted:
07 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
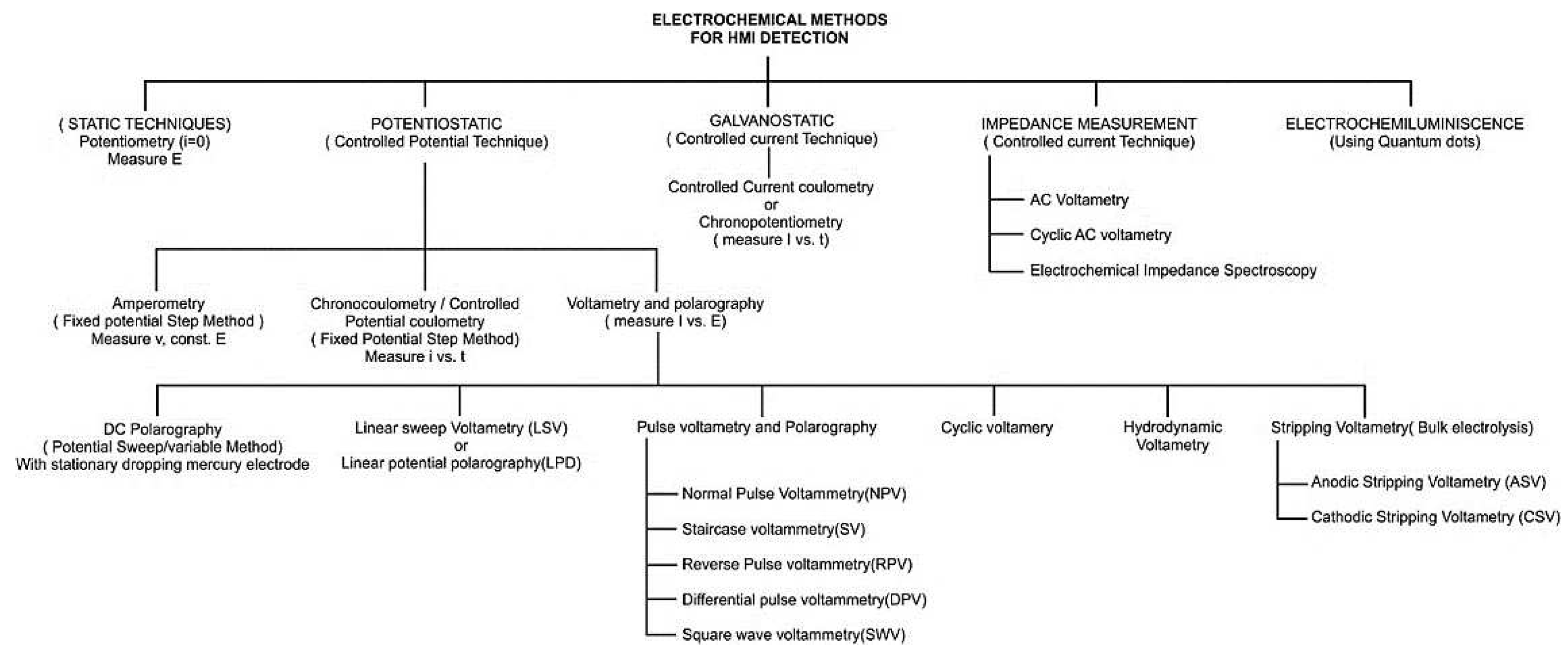
2. Heavy Metal Ion Sensors Modalities: Recent Trends
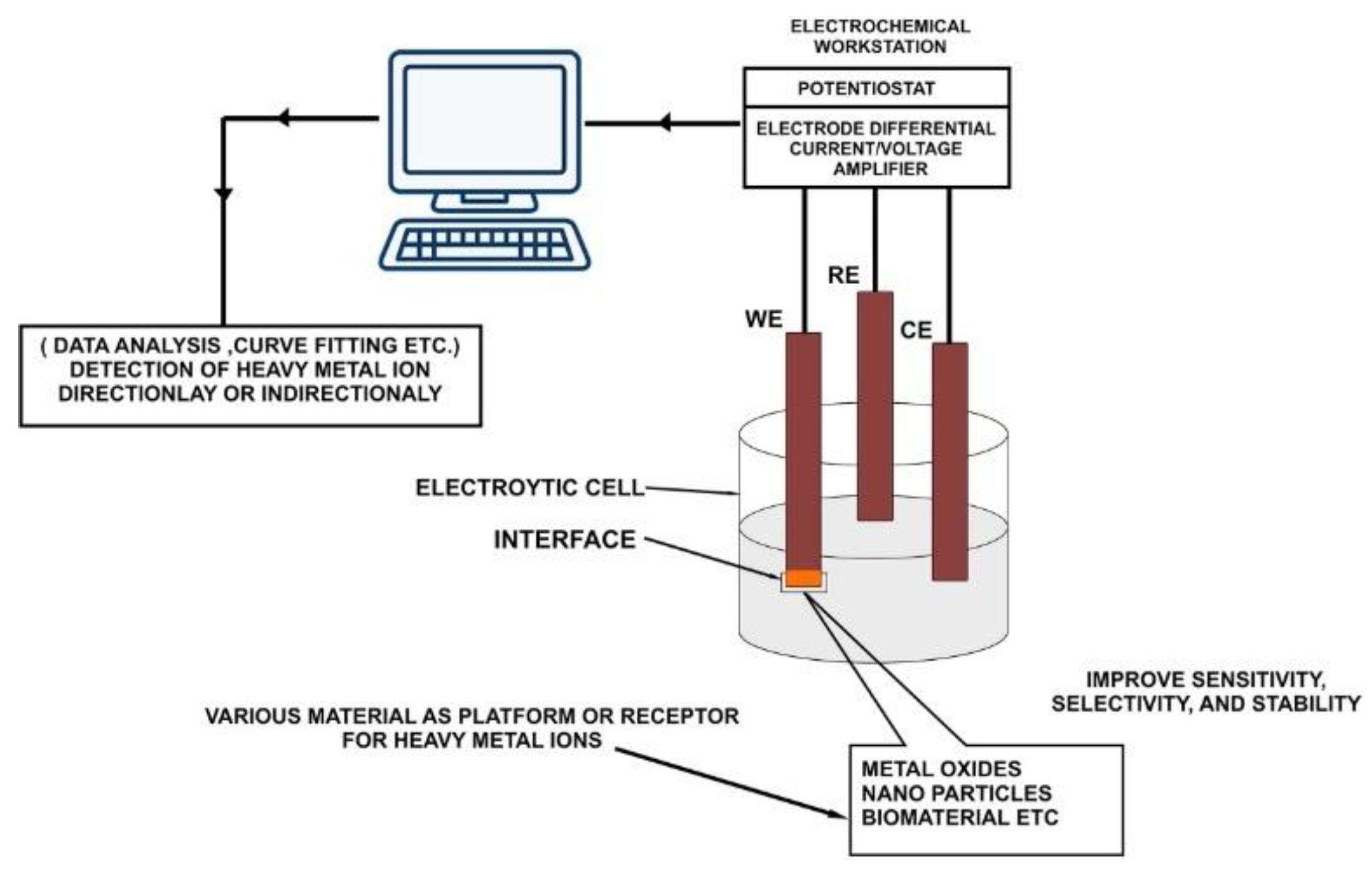
- Excitation signal -> Electrode -> Response function
3. Portable Electrochemical Sensor
- Limited sensitivity: Existing portable devices may not be sensitive enough to detect low concentrations of HMIs accurately.
- Poor selectivity: Portable devices can suffer from poor selectivity, leading to false-positive or false-negative results, particularly in complex samples.
- Limited stability: Some portable devices may have a limited operational lifespan due to the degradation of electrodes or instability of the sensing materials, leading to reduced accuracy and reliability.
- Limited sample handling: Some portable devices may require complex sample preparation steps or may not be suitable for use in the field.
- Advanced electrode materials: For example, nanomaterials can improve the sensitivity and selectivity of portable sensing devices.
- Advanced sensing techniques: Researchers are exploring relevant sensing techniques, such as electrochemical impedance spectroscopy (EIS), to enhance the selectivity and sensitivity of portable sensing devices.
- Microfluidic systems: Integrating microfluidic systems into portable devices can enable better control of sample handling and reduce the need for complex sample preparation.
- Machine learning algorithms: Integrating machine learning algorithms can improve the accuracy and reliability of portable sensing devices by enabling real-time data analysis and pattern recognition.
4. Nanocomposites for the Detection of HMIs
4.1. Metal-Organic Framework (MOF) Based Nanocomposites
- High selectivity: MOFs can be designed with specific ligands to selectively capture certain HMIs, enabling the detection of individual or multiple metal ions in complex samples.
- High sensitivity: MOFs have high surface area and porosity, allowing for efficient adsorption of HMIs and resulting in highly sensitive detection with low detection limits.
- Tunable properties: MOFs have tunable properties, including pore size, surface area, and functionality, which can be tailored to enhance their performance for specific HMIs.
- Fast response time: MOF-based sensors have a fast response time due to the efficient electron transfer properties of MOFs, allowing for real-time detection of HMIs.
- Stability: MOFs are stable in a wide range of chemical and physical conditions, making them suitable for use in harsh environments.
- Cost: The production of MOFs can be costly, particularly for large-scale applications. This can limit their use in some industries.
- Stability: While MOFs are generally stable, some can degrade over time or in certain conditions, which can affect their performance and lifespan as sensors.
- Reproducibility: MOFs can be difficult to synthesize with high reproducibility, making it challenging to ensure consistent performance between different batches of sensors.
- Interference: Other ions or molecules in the sample matrix can compete for adsorption sites on the MOF, leading to false positives or reduced sensitivity for detecting the target HMIs.
- Detection range: MOF-based sensors may have limited detection ranges for specific HMIs, making them less suitable for detecting trace levels of those ions.
- Poor electronics conductance: MOFs have poor electronics conductance, which limits their use for sensor applications.
4.2. Organic Conducting Polymer (OCP) Based Nanocomposite
- Electrical conductivity: Organic conducting polymers are highly conductive, which enables the detection of HMIs through changes in the electrical properties of the polymer upon interaction with the metal ions.
- Electroactive nature: Organic conducting polymers are electroactive and can undergo reversible redox reactions at their surface. This property makes them well-suited for electrochemical sensors, which rely on redox reactions to detect and quantify analytes.
- Sensitivity: Organic conducting polymers have high sensitivity to HMIs, allowing for detection at low concentrations.
- Selectivity: The selectivity of organic conducting polymers for HMIs can be tailored by modifying the polymer structure or incorporating specific ligands or functional groups, enabling the detection of specific metal ions in complex samples.
- Overall, the unique properties of conducting polymers make them well-suited for electrochemical sensors to detect HMIs, with applications in environmental monitoring, food safety, and industrial processes.
- Reproducibility: Organic conducting polymers can be difficult to synthesize with high reproducibility, making it challenging to ensure consistent performance between different batches of sensors.
- Long-term stability: Some organic conducting polymers can undergo degradation over time, affecting their performance and lifespan as sensors.
- Interference: Other ions or molecules in the sample matrix can compete for adsorption sites on the conducting polymer, leading to false positives or reduced sensitivity for detecting the target heavy metal ions.
- Detection range: Organic conducting polymer-based sensors may have limited detection ranges for specific HMIs, making them less suitable for detecting trace levels of those ions.
- Environmental impact: Organic conducting polymers may have environmental impacts due to their non-biodegradable nature, although efforts are being made to develop more sustainable alternatives.
4.3. Carbon Nanotubes Based Nanocomposites
- High sensitivity: Carbon nanotubes have a large surface area and high aspect ratio, allowing efficient HMIs adsorption. This results in highly sensitive detection with low detection limits.
- Selectivity: The surface chemistry of carbon nanotubes can be modified to selectively capture specific HMIs, enabling the detection of individual or multiple metal ions in complex samples.
- Rapid response time: Carbon nanotube-based sensors have a fast response time due to carbon nanotubes' efficient electron transfer properties. This allows for real-time detection of HMIs.
- Durability: Carbon nanotubes are highly durable and can withstand harsh chemical and physical conditions, making them suitable for use in various environmental and industrial settings.
- Low cost: Carbon nanotube-based sensors are relatively inexpensive to produce compared to traditional HMIs detection methods, making them a cost-effective alternative.
4.4. Graphene, Graphene Oxide and Reduced Graphene Oxide-Based Nanocomposites
- High sensitivity: Graphene has an exceptionally high surface area-to-volume ratio, allowing for efficient HMIs adsorption. This results in highly sensitive detection with low detection limits.
- Selectivity: The surface chemistry of graphene can be modified to selectively capture specific HMIs, enabling the detection of individual or multiple metal ions in complex samples.
- Rapid response time: Graphene-based sensors have a fast response time due to graphene's efficient electron transfer properties. This allows for real-time detection of HMIs.
- Stability: Graphene is highly stable and can withstand harsh chemical and physical conditions, making it suitable for use in various environmental and industrial settings.
- Low cost: Graphene-based sensors are relatively inexpensive to produce compared to traditional HMIs detection methods, making them a cost-effective alternative.
4.5. Graphitic Carbon Nitride (g-C3N4) - Based Nanocomposites
- High sensitivity: g-C3N4 has a high surface area and strong adsorption ability, allowing it to capture and detect trace amounts of HMIs in the solution.
- Selectivity: g-C3N4 has a high selectivity towards HMIs due to its surface's unique electronic and chemical properties. This means it can distinguish between HMIs and detect only the specific metal ion(s) of interest.
- Low cost: g-C3N4 is a relatively low-cost material, making it an attractive option for practical applications.
- Stability: g-C3N4 is stable under a wide range of conditions, including high temperatures and harsh chemical environments, making it suitable for real-world applications.
- Environmental friendliness: Unlike many other HMIs detection methods, g-C3N4 does not rely on toxic reagents or generate harmful waste products, making it an environmentally friendly option.
- Poor conductivity: g-C3N4 is an insulating material, which means it has poor electrical conductivity. This limits its usefulness in specific sensor applications that require high conductivity.
- Limited response time: g-C3N4 sensors can have a relatively slow response time compared to other sensing materials, which may limit their use in specific applications that require fast response times.
- Limited stability: While g-C3N4 is generally stable under a wide range of conditions, it can be prone to degradation over time, particularly under certain environmental conditions. This can impact the sensor's performance and longevity.
- Lack of standardization: There is currently a lack of standardized protocols for synthesizing and characterizing g-C3N4, making comparing results between different studies difficult.
- Sensitivity to environmental conditions: g-C3N4 sensors can be sensitive to changes in environmental conditions, such as temperature and humidity, affecting their performance.
4.6. Metal Oxide-Based Nanocomposite
- Sensitivity and selectivity optimization: Metal oxide-based nanocomposites require their sensitivity and selectivity optimization for detecting specific HMIs in complex samples. This requires the development of new functional materials and improved sensing mechanisms.
- Interference from other ions: HMIs detection in complex samples can be complicated by interference from other ions, resulting in false positives or negatives. Researchers will need to develop methods to eliminate or reduce these interferences.
- Stability and reproducibility: The stability and reproducibility of metal oxide-based nanocomposites can be affected by environmental factors, such as pH and temperature, impacting their sensing performance. Researchers will need to develop strategies to enhance the stability and reproducibility of these nanocomposites.
- Environmental impact: The potential environmental impact of metal oxide-based nanocomposites, including their potential release into the environment, is an important consideration that requires careful evaluation.
4.7. Chitosan-Based Nanocomposite
- Sensitivity: Achieving a high level of sensitivity to detect trace amounts of HMIs in water, especially in complex or contaminated samples.
- Stability: Ensuring the nanocomposites remain stable and do not degrade or lose their effectiveness over time or under different environmental conditions.
- Interference: Dealing with potential interference from other substances in the water, such as organic matter, can affect detection accuracy.
- Reproducibility: Ensuring the detection results are consistent and reproducible over time and across different samples.
4.8. Mxene-Based Nanocomposite
4.9. Metal Nanoparticle and Other Material-Based Nanocomposites
- Synthesis of metal nanoparticles and other nanocomposites with controlled properties can be challenging. Researchers must carefully control the nanoparticles' size, shape, and surface chemistry to ensure optimal sensing performance.
- Sensitivity and selectivity: Developing high-sensitivity and selectivity sensors for detecting HMIs can be challenging. Researchers must design nanocomposites that selectively bind to target ions while avoiding interference from other species.
- Stability: The stability of metal nanoparticles and other nanocomposites is important for sensing applications. Researchers must ensure the nanocomposites are stable over time and under different environmental conditions to maintain their sensing performance.
- Reproducibility: The reproducibility of sensing results is crucial for practical applications. Researchers need to ensure that the sensing performance of nanocomposites is consistent across different batches and under different conditions.
5. Summary and Future Prospects
5.1. Summary
5.2. Future Prospects
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
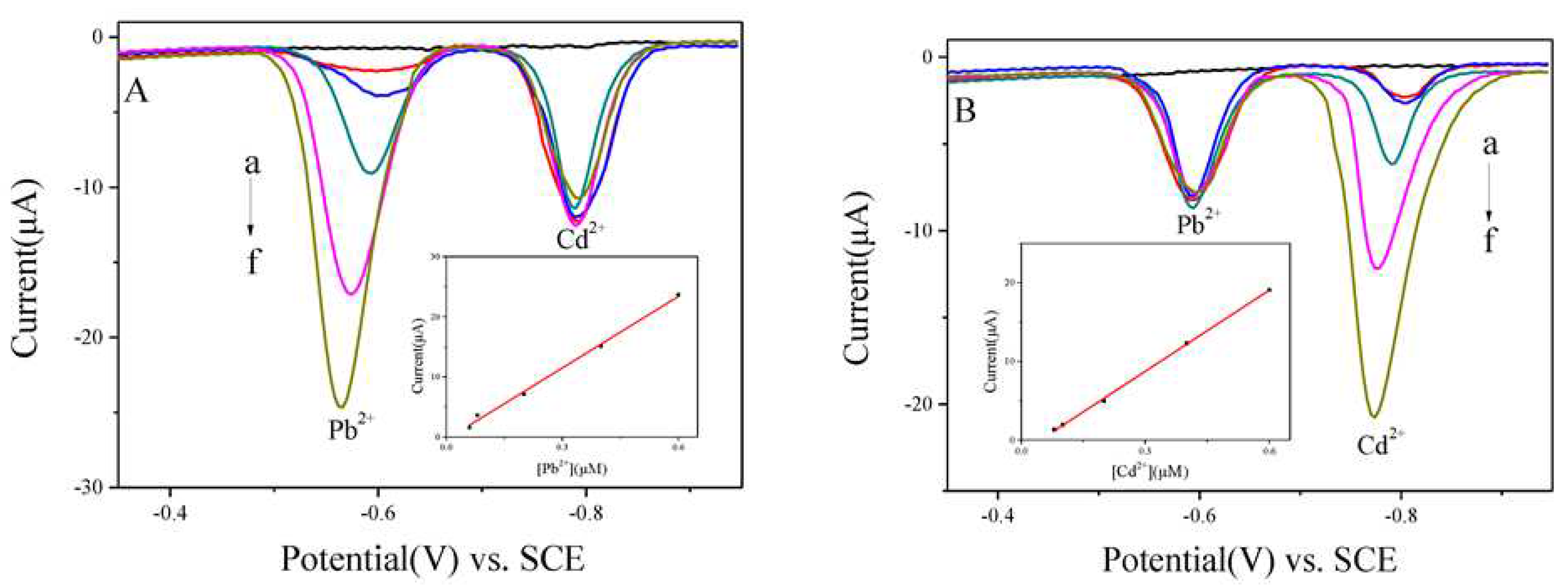
- Sharma, S.K. Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: London, UK, 2014. [Google Scholar] [CrossRef]
- Shadman, S.M.; Daneshi, M.; Shafiei, F.; Azimimehr, M.; Khorasgani, M.R.; Sadeghian, M.; Motaghi, H.; Mehrgardi, M.A. Aptamer-based electrochemical biosensors. In Electrochemical Biosensors; Ensafi, A.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–251. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M. Metals, toxicity and oxidative stress. Current Medic. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Bagal-Kestwal, D.; Karve, M.S.; Kakade, B.; Pillai, V.K. Invertase inhibition based electrochemical sensor for the detection of heavy metal ions in aqueous system: Application of ultra-microelectrode to enhance sucrose biosensor's sensitivity. Biosens. Bioelectron. 2008, 24, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Zularisam, A.; Ismail, A.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—a review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef]
- Vetrimurugan, E.; Brindha, K.; Elango, L.; Ndwandwe, O.M. Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl. Water Sci. 2017, 7, 3267–3280. [Google Scholar] [CrossRef]
- National Primary Drinking Water Regulations, USEPA. 2015.
- Ferrari, A.G.-M.; Carrington, P.; Rowley-Neale, S.J.; Banks, C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environmental Sci.: Water Research & Technology 2020, 6, 2676–2690. [Google Scholar] [CrossRef]
- Srivastav, A.L. Chemical fertilizers and pesticides: role in groundwater contamination. In Agrochemicals Detection, Treatment and Temediation; Elsevier: 2020; pp. 143-159. [CrossRef]
- Liu, Y.; Deng, Y.; Dong, H.; Liu, K.; He, N. Progress on sensors based on nanomaterials for rapid detection of heavy metal ions. Sci. China Chem. 2017, 60, 329–337. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P. Advances of graphene oxide based nanocomposite materials in the treatment of wastewater containing heavy metal ions and dyes. Current Res. in Green and Sust. Chem. 2022, 100306. [Google Scholar] [CrossRef]
- Huang, H.; Chen, T.; Liu, X.; Ma, H. Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal. Chim. Acta 2014, 852, 45–54. [Google Scholar] [CrossRef]
- Pan, F.; Tong, C.; Wang, Z.; Han, H.; Liu, P.; Pan, D.; Zhu, R. Nanocomposite based on graphene and intercalated covalent organic frameworks with hydrosulphonyl groups for electrochemical determination of heavy metal ions. Microchim. Acta 2021, 188, 295. [Google Scholar] [CrossRef]
- Devaraj, M.; Sasikumar, Y.; Rajendran, S.; Ponce, L.C. Metal organic framework based nanomaterials for electrochemical sensing of toxic heavy metal ions: progress and their prospects. J. Electrochem. Soc. 2021, 168, 037513. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Nga, D.T.N.; Thu, V.T.; Piro, B.; Truong, T.N.P.; Yen, P.T.H.; Le, G.H.; Hung, L.Q.; Vu, T.A.; Ha, V.T.T. Novel nanoscale Yb-MOF used as highly efficient electrode for simultaneous detection of heavy metal ions. J. Mat. Sci. 2021, 56, 8172–8185. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Shen, Y.; Yuan, Y.; Zhang, L.; Zhang, C.; Sun, Y. A ratiometric electrochemical sensor for simultaneous detection of multiple heavy metal ions based on ferrocene-functionalized metal-organic framework. Sens. Actuat. B: Chemical 2020, 310, 127756. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Akanji, S.P.; Ama, O.M.; Ray, S.S.; Osifo, P.O. Metal oxide nanomaterials for electrochemical detection of heavy metals in water. In Nanostructured Metal-Oxide Electrode Materials for Water Purification: Fabrication, Electrochemistry and Applications; Ama, O., Ray, S., Eds.; Springer: Cham, Germany 2020; pp. 113–126. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; Hedau, B.S.; Deshmukh, M.A.; Patil, H.K.; Shirsat, S.M.; Phase, D.M.; Pandey, K.K.; Shirsat, M.D. Selective and sensitive detection of lead Pb (II) ions: Au/SWNT nanocomposite-embedded MOF-199. J. Mat. Sci. 2021, 56, 474–487. [Google Scholar] [CrossRef]
- Sayyad, P.W.; Ingle, N.N.; Al-Gahouari, T.; Mahadik, M.M.; Bodkhe, G.A.; Shirsat, S.M.; Shirsat, M.D. Sensitive and selective detection of Cu2+ and Pb2+ ions using field effect transistor (FET) based on L-Cysteine anchored PEDOT: PSS/rGO composite. Chem. Phys. Lett. 2020, 761, 138056. [Google Scholar] [CrossRef]
- Sayyad, P.W.; Ingle, N.N.; Al-Gahouari, T.; Mahadik, M.M.; Bodkhe, G.A.; Shirsat, S.M.; Shirsat, M.D. Selective Hg 2+ sensor: rGO-blended PEDOT: PSS conducting polymer OFET. Appl. Physics A 2021, 127, 1–10. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; Hedau, B.S.; Deshmukh, M.A.; Patil, H.K.; Shirsat, S.M.; Phase, D.M.; Pandey, K.K.; Shirsat, M.D. Detection of Pb (II): Au nanoparticle incorporated CuBTC MOFs. Frontiers in Chem. 2020, 8, 803. [Google Scholar] [CrossRef] [PubMed]
- Sayyad, P.W.; Shaikh, Z.A.; Ingle, N.N.; Al-Gahouari, T.; Mahadik, M.M.; Bodkhe, G.A.; Shirsat, S.M.; Shirsat, M.D. Simultaneous reduction of graphene oxide (GO) and formation of rGO/Gly-Gly composite for sensitive detection of Cu2+ ions. J. Phys.: Conf. Ser. 2020, 1644, 012001. [Google Scholar] [CrossRef]
- Mahadik, M.; Patil, H.; Bodkhe, G.; Ingle, N.; Sayyad, P.; Al-Gahaouri, T.; Shirsat, S.M.; Shirsat, M. EDTA modified PANI/GO composite based detection of Hg (II) ions. Frontiers in Materials 2020, 7, 81. [Google Scholar] [CrossRef]
- AL-Gahouari, T.; Bodkhe, G.; Sayyad, P.; Ingle, N.; Mahadik, M.; Shirsat, S.M.; Deshmukh, M.; Musahwar, N.; Shirsat, M. Electrochemical sensor: L-cysteine induced selectivity enhancement of electrochemically reduced graphene oxide–multiwalled carbon nanotubes hybrid for detection of lead (Pb 2+) ions. Frontiers in Materials 2020, 7, 68. [Google Scholar] [CrossRef]
- Patil, H.K.; Deshmukh, M.A.; Bodkhe, G.A.; Shirsat, S.M.; Asokan, K.; Shirsat, M.D. Dimethylglyoxime modified swift heavy oxygen ions irradiated polyaniline/single walled carbon nanotubes composite electrode for detection of cobalt ions. Materials Research Express 2018, 5, 065048. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/SWCNTs nanocomposite modified electrode for electrochemical determination of copper (II), lead (II) and mercury (II) ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Patil, H.K.; Bodkhe, G.A.; Yasuzawa, M.; Koinkar, P.; Ramanavicius, A.; Pandey, S.; Shirsat, M.D. EDA modified PANI/SWNTs nanocomposite for determination of Ni (II) metal ions. Coll. Surf. A: Physicochemical and Engineering Aspects 2018, 537, 303–309. [Google Scholar] [CrossRef]
- Deore, K.B.; Patil, S.S.; Narwade, V.N.; Takte, M.A.; Khune, A.S.; Mohammed, H.Y.; Farea, M.; Sayyad, P.W.; Tsai, M.-L.; Shirsat, M.D. Chromium-benzenedicarboxylates metal organic framework for supersensitive and selective electrochemical sensor of Toxic Cd2+, Pb2+, and Hg2+ metal ions: Study of their interactive mechanism. J. Electrochem. Soc. 2023, 170, 046505. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: a review. Environmen. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Rubino, A.; Queirós, R. Electrochemical determination of heavy metal ions applying screen-printed electrodes based sensors. A review on water and environmental samples analysis. Talanta 2023, 7, 100203. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal organic frameworks for electrochemical sensor applications: A review. Environmen. Res. 2022, 204, 112320. [Google Scholar] [CrossRef] [PubMed]
- Nemiwal, M.; Kumar, D. Recent progress on electrochemical sensing strategies as comprehensive point-care method. Chemical Monthly 2021, 152, 1–18. [Google Scholar] [CrossRef]
- Munonde, T.S.; Nomngongo, P.N. Nanocomposites for electrochemical sensors and their applications on the detection of trace metals in environmental water samples. Sensors 2020, 21, 131. [Google Scholar] [CrossRef]
- Buledi, J.A.; Amin, S.; Haider, S.I.; Bhanger, M.I.; Solangi, A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environment. Sci. Pollut. Res. 2021, 28, 58994–59002. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Kalambate, P.K.; Mugo, S.M.; Kamau, P.; Chen, J.; Jain, R. Polymer hydrogel interfaces in electrochemical sensing strategies: A review. TrAC Trends Anal. Chem. 2019, 118, 488–501. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Yuan, B. Polymer-based electrochemical sensing platform for heavy metal ions detection—A critical review. Int. J. Electrochem. Sci 2019, 14, 8760–8771. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: A review on recent advances. Microchim. Acta 2019, 186, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Khosla, A.; Khan, M.; Mishra, P.; Ansari, W.A.; Syed, M.A.; Hahn, Y.-B. Recent advances in nanostructured graphitic carbon nitride as a sensing material for heavy metal ions. J. Electrochem. Soc. 2019, 167, 037519. [Google Scholar] [CrossRef]
- Sawan, S.; Maalouf, R.; Errachid, A.; Jaffrezic-Renault, N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. TrAC Trends Anal. Chem. 2020, 131, 116014. [Google Scholar] [CrossRef]
- Kumunda, C.; Adekunle, A.S.; Mamba, B.B.; Hlongwa, N.W.; Nkambule, T.T. Electrochemical detection of environmental pollutants based on graphene derivatives: A review. Frontiers in Materials 2021, 7, 616787. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Khalilzadeh, M.A.; Jang, H.W.; Venditti, R.A.; Varma, R.S.; Shokouhimehr, M. Recent developments in polymer nanocomposite-based electrochemical sensors for detecting environmental pollutants. Industrial Eng. Chem. Res. 2021, 60, 1112–1136. [Google Scholar] [CrossRef]
- Langari, M.M.; Antxustegi, M.M.; Labidi, J. Nanocellulose-based sensing platforms for heavy metal ions detection: A comprehensive review. Chemosphere 2022, 134823. [Google Scholar] [CrossRef] [PubMed]
- Raju, C.V.; Cho, C.H.; Rani, G.M.; Manju, V.; Umapathi, R.; Huh, Y.S.; Park, J.P. Emerging insights into the use of carbon-based nanomaterials for the electrochemical detection of heavy metal ions. Coordinat. Chem. Rev. 2023, 476, 214920. [Google Scholar] [CrossRef]
- Meng, R.; Zhu, Q.; Long, T.; He, X.; Luo, Z.; Gu, R.; Wang, W.; Xiang, P. The innovative and accurate detection of heavy metals in foods: A critical review on electrochemical sensors. Food Control 2023, 109743. [Google Scholar] [CrossRef]
- Huang, R.; Lv, J.; Chen, J.; Zhu, Y.; Zhu, J.; Wågberg, T.; Hu, G. Three-dimensional porous high boron-nitrogen-doped carbon for the ultrasensitive electrochemical detection of trace heavy metals in food samples. J. Hazard. Mat. 2023, 442, 130020. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, M.; Sturgeon, R.E.; Mester, Z.; Shi, Z.; Galea, R.; Saull, P.; Yang, L. Metal ion-assisted photochemical vapor generation for the determination of lead in environmental samples by multicollector-ICPMS. Anal. Chem. 2015, 87, 4495–4502. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Jr, F.; Krug, F.J.; Lima, É.C. On-line coupling of electrochemical preconcentration in tungsten coil electrothermal atomic absorption spectrometry for determination of lead in natural waters. Spectrochim. Acta Part B: Atomic Spectroscopy 1999, 54, 1155–1166. [Google Scholar] [CrossRef]
- Rahmalan, A.; Abdullah, M.Z.; Sanagi, M.M.; Rashid, M. Determination of heavy metals in air particulate matter by ion chromatography. J. Chromatography A 1996, 739, 233–239. [Google Scholar] [CrossRef]
- Nyholm, L. Electrochemical techniques for lab-on-a-chip applications. Analyst 2005, 130, 599–605. [Google Scholar] [CrossRef]
- Piliarik, M.; Párová, L.; Homola, J. High-throughput SPR sensor for food safety. Biosens. Bioelectron. 2009, 24, 1399–1404. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Han, Y.; Feng, Y.; Zhang, Z.; Zhang, B. Fabrication of a new corrole-based covalent organic framework as a highly efficient and selective chemosensor for heavy metal ions. Chem. Materials 2020, 32, 2532–2540. [Google Scholar] [CrossRef]
- Lv, M.; Zhou, W.; Tavakoli, H.; Bautista, C.; Xia, J.; Wang, Z.; Li, X. Aptamer-functionalized metal-organic frameworks (MOFs) for biosensing. Biosens. Bioelectron. 2021, 176, 112947. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A. Cross-sensitivity evaluation of chemical sensors for electronic tongue: determination of heavy metal ions. Sens. Actuat. B: Chemical 1997, 44, 532–537. [Google Scholar] [CrossRef]
- Falina, S.; Syamsul, M.; Rhaffor, N.A.; Sal Hamid, S.; Mohamed Zain, K.A.; Abd Manaf, A.; Kawarada, H. Ten years progress of electrical detection of heavy metal ions (hmis) using various field-effect transistor (fet) nanosensors: A review. Biosensors 2021, 11, 478. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Ramli, N.H.; Poobalan, H.; Qi Tan, K.; Abdul Razak, K. Recent advancement in disposable electrode modified with nanomaterials for electrochemical heavy metal sensors. Critical Rev. Anal. Chem. 2023, 53, 253–288. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Heidari, G.; Fallah, Z.; Zare, E.N. One-Dimensional Polymeric nanocomposites for heavy metal detection. In One-Dimensional Polymeric Nanocomposites: Synthesis to Emerging Applications, 1st ed.; Gupta, R.K., Nguyen, T.A., Eds.; CRC Press: London, UK, 2023; pp. 1–20. [Google Scholar] [CrossRef]
- Jose, J.; Prakash, P.; Jeyaprabha, B.; Abraham, R.; Mathew, R.M.; Zacharia, E.S.; Thomas, V.; Thomas, J. Principle, design, strategies, and future perspectives of heavy metal ion detection using carbon nanomaterial-based electrochemical sensors: a review. J. Iranian Chem. Soc. 2023, 20, 775–791. [Google Scholar] [CrossRef]
- Shen, Y.; Gao, X.; Lu, H.-J.; Nie, C.; Wang, J. Electrochemiluminescence-based innovative sensors for monitoring the residual levels of heavy metal ions in environment-related matrices. Coordinat. Chem. Rev. 2023, 476, 214927. [Google Scholar] [CrossRef]
- Tatavarthi, S.S.; Wang, S.-L.; Wang, Y.-L.; Chen, J.-C. Rapid and highly sensitive extended gate FET-based sensors for Arsenite detection using a handheld device. ECS J. Solid State Sci. Technol. 2020, 9, 115014. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Nataraj, N.; Meyyappan, M.; Pal, U. Graphene-based field-effect transistors in biosensing and neural interfacing applications: Recent advances and prospects. Anal. Chem. 2023, 95, 2590–2622. [Google Scholar] [CrossRef]
- Farahmandpour, M.; Kordrostami, Z.; Rajabzadeh, M.; Khalifeh, R. Flexible bio-electronic hybrid metal-oxide channel FET as a glucose sensor. IEEE Transactions on NanoBioscience 2023. [Google Scholar] [CrossRef]
- Zhang, X.; Pu, Z.; Su, X.; Li, C.; Zheng, H.; Li, D. Flexible organic field-effect transistors-based biosensors: progress and perspectives. Anal. Bioanal. Chem. 2023, 415, 1607–1625. [Google Scholar] [CrossRef]
- Jiang, D.; Sheng, K.; Gui, G.; Jiang, H.; Liu, X.; Wang, L. A novel smartphone-based electrochemical cell sensor for evaluating the toxicity of heavy metal ions Cd2+, Hg2+, and Pb2+ in rice. Anal. Bioanal. Chem. 2021, 413, 4277–4287. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Lee, N.Y. Recent progress in smartphone-based techniques for food safety and the detection of heavy metal ions in environmental water. Chemosphere 2021, 275, 130096. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Xiao, M.; Jiang, L.; Yi, C. A smartphone-based quantitative point-of-care testing (POCT) system for simultaneous detection of multiple heavy metal ions. Chem. Eng. J. 2020, 394, 124966. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Liu, F.; Zou, X.; Xu, Y.; Xu, X. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Chang, F.; Han, X.; Ge, C.; Lin, S. Wireless water quality monitoring and spatial mapping with disposable whole-copper electrochemical sensors and a smartphone. Sens. Actuat. B: Chemical 2020, 306, 127557. [Google Scholar] [CrossRef]
- Nemiroski, A.; Christodouleas, D.C.; Hennek, J.W.; Kumar, A.A.; Maxwell, E.J.; Fernández-Abedul, M.T.; Whitesides, G.M. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc. Nat. Acad. Sci. 2014, 111, 11984–11989. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Yu, H.; Tian, L.; Wang, Z. Portable and smart devices for monitoring heavy metal ions integrated with nanomaterials. TrAC Trends in Analytical Chemistry 2018, 98, 190–200. [Google Scholar] [CrossRef]
- Li, Z.; Xu, D.; Zhang, D.; Yamaguchi, Y. A portable instrument for on-site detection of heavy metal ions in water. Anal. Bioan. Chem. 2021, 413, 3471–3477. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, X.; Cheng, C.; Yang, J.; Liu, Z.; Shi, Z.; Zhu, L.; Lu, Y.; Low, S.S.; Liu, Q. Fully integrated battery-free and flexible electrochemical tag for on-demand wireless in situ monitoring of heavy metals. Sens. Actuat.B: Chemical 2020, 310, 127809. [Google Scholar] [CrossRef]
- Muhammad-Aree, S.; Teepoo, S. On-site detection of heavy metals in wastewater using a single paper strip integrated with a smartphone. Anal. Bioanal. Chem. 2020, 412, 1395–1405. [Google Scholar] [CrossRef]
- Fang, X.; Chen, X.; Liu, Y.; Li, Q.; Zeng, Z.; Maiyalagan, T.; Mao, S. Nanocomposites of Zr (IV)-based metal–organic frameworks and reduced graphene oxide for electrochemically sensing ciprofloxacin in water. ACS Appl. Nano Mat. 2019, 2, 2367–2376. [Google Scholar] [CrossRef]
- Dehdashtian, S.; Hashemi, B.; Aeenmehr, A. The application of perlite/cobalt oxide/reduced graphene oxide (PC-rGO)/metal organic framework (MOF) composite as electrode modifier for direct sensing of anticancer drug idarubicin. IEEE Sensors J. 2019, 19, 11739–11745. [Google Scholar] [CrossRef]
- Altass, H.M.; Morad, M.; Khder, A.E.-R.S.; Mannaa, M.A.; Jassas, R.S.; Alsimaree, A.A.; Ahmed, S.A.; Salama, R.S. Enhanced catalytic activity for CO oxidation by highly active Pd nanoparticles supported on reduced graphene oxide/copper metal organic framework. J. Taiwan Inst. Chem. Eng. 2021, 128, 194–208. [Google Scholar] [CrossRef]
- Baghayeri, M.; Ghanei-Motlagh, M.; Tayebee, R.; Fayazi, M.; Narenji, F. Application of graphene/zinc-based metal-organic framework nanocomposite for electrochemical sensing of As (III) in water resources. Anal. Chim. Acta 2020, 1099, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Niu, Q.; Jian, L.; Zhao, W.; Li, Y.; Dong, W.; Zhang, K.; Liang, W.; Yang, C. Synthesis of porphyrinic metal-organic framework/rGO nanocomposite for electrochemical recognition of copper ions in water. J. Organometal. Chem. 2023, 985, 122597. [Google Scholar] [CrossRef]
- Li, D.; Yan, D.; Zhang, X.; Li, J.; Lu, T.; Pan, L. Porous CuO/reduced graphene oxide composites synthesized from metal-organic frameworks as anodes for high-performance sodium-ion batteries. J. Coll. Interface Sci. 2017, 497, 350–358. [Google Scholar] [CrossRef] [PubMed]
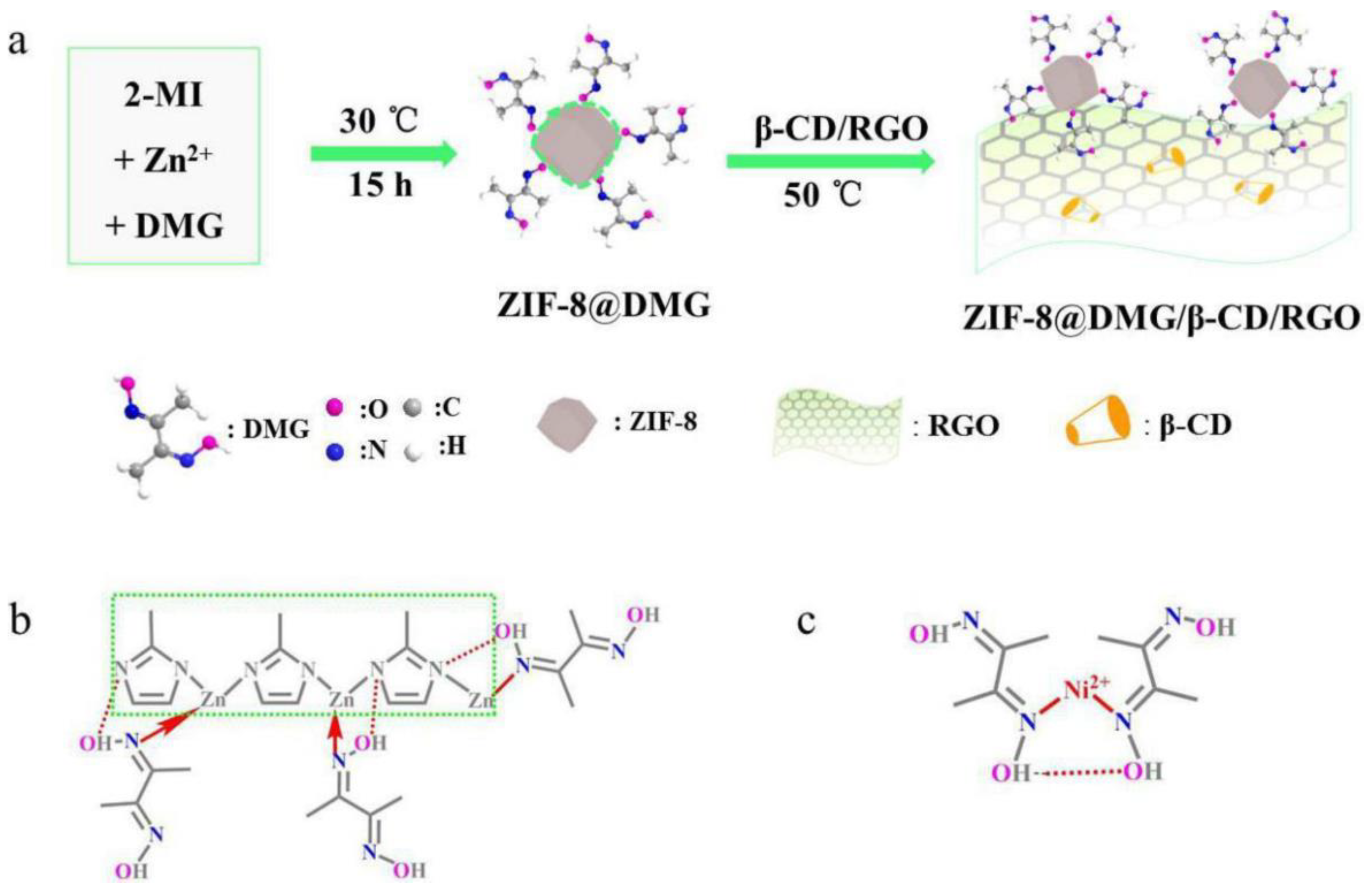
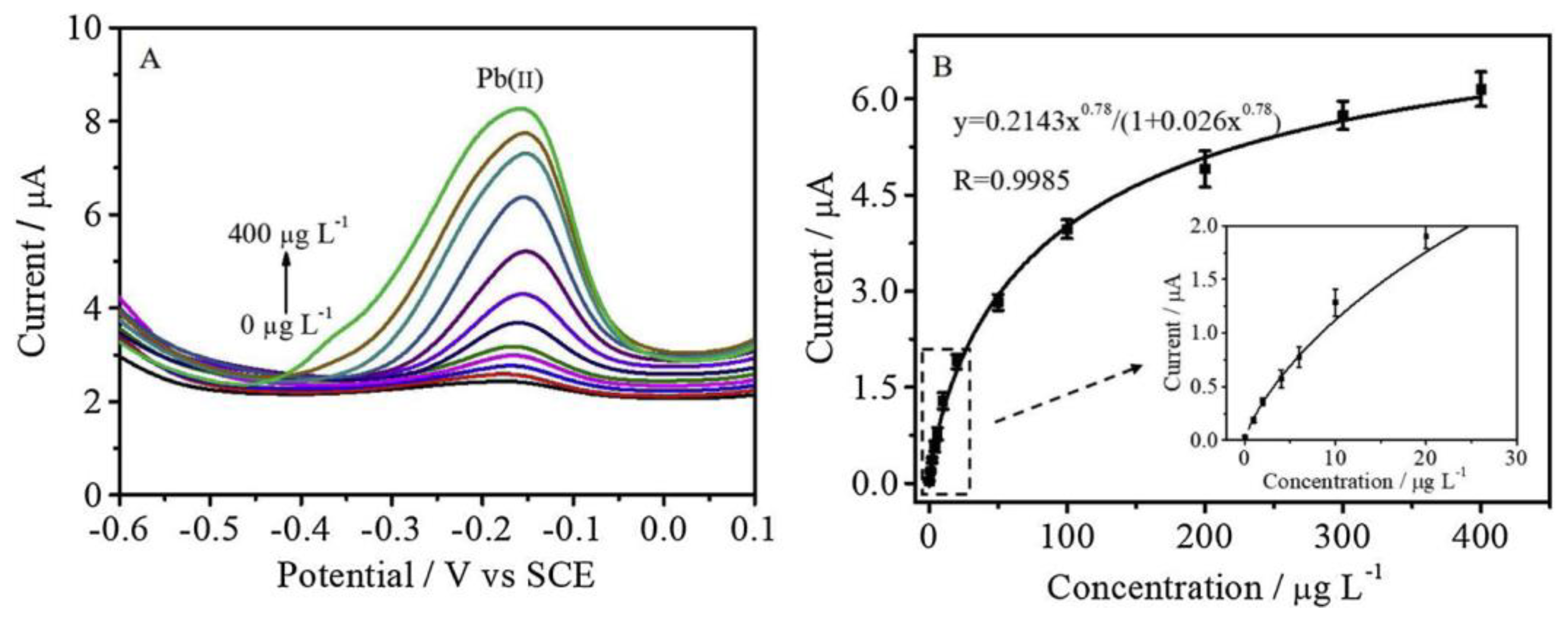
- Cui, X.; Yang, B.; Zhao, S.; Li, X.; Qiao, M.; Mao, R.; Wang, Y.; Zhao, X. Electrochemical sensor based on ZIF-8@ dimethylglyoxime and β-cyclodextrin modified reduced graphene oxide for nickel (II) detection. Sens. Act. B: Chemical 2020, 315, 128091. [Google Scholar] [CrossRef]
- Huo, D.; Zhang, Y.; Li, N.; Ma, W.; Liu, H.; Xu, G.; Li, Z.; Yang, M.; Hou, C. Three-dimensional graphene/amino-functionalized metal–organic framework for simultaneous electrochemical detection of Cd (II), Pb (II), Cu (II), and Hg (II). Anal. Bioanal. Chem. 2022, 414, 1475–1585. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.-T.; Cao, X.-Y.; An, Y.-Y.; Zhang, X.-L.; Yan, J.-Z. Sulfur-bridged Co (II)-thiacalix [4] arene metal–organic framework as an electrochemical sensor for the determination of toxic heavy metals. Inorganic Chem. 2023, 438, 135–639. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, F.; Dong, C.; Li, J.; Zhang, C.; Han, X. UiO-66 based electrochemical sensor for simultaneous detection of Cd (II) and Pb (II). Inorganic Chem. Commun. 2021, 131, 108785. [Google Scholar] [CrossRef]
- Yang, S.; Yang, M.; Yao, X.; Fa, H.; Wang, Y.; Hou, C. A zeolitic imidazolate framework/carbon nanofiber nanocomposite based electrochemical sensor for simultaneous detection of co-existing dihydroxybenzene isomers. Sens. Actuat. B: Chemical 2020, 320, 128294. [Google Scholar] [CrossRef]
- Yang, H.; Peng, C.; Han, J.; Song, Y.; Wang, L. Three-dimensional macroporous Carbon/Zr-2, 5-dimercaptoterephthalic acid metal-organic frameworks nanocomposites for removal and detection of Hg (II). Sens. Actuat. B: Chemical 2020, 320, 128447. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Zhan, Y.; Singh, V.; Van Hung, T.; Nam, N.D. A novel highly efficient and ultrasensitive electrochemical detection of toxic mercury (II) ions in canned tuna fish and tap water based on a copper metal-organic framework. J. Hazardous Mat. 2020, 399, 123042. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Wang, X.; Cui, X.; Wang, F.; Ji, H.; Du, X.; Lu, X. GaOOH-modified metal-organic frameworks UiO-66-NH2: Selective and sensitive sensing four heavy-metal ions in real wastewater by electrochemical method. Talanta 2021, 234, 122679. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, W.; Shen, Z.; Sun, S.; Dai, H.; Ma, H.; Lin, M. Sensitive and selective detection of Pb (II) and Cu (II) using a metal-organic framework/polypyrrole nanocomposite functionalized electrode. Sens. Actuat. B: Chemical 2020, 304, 127286. [Google Scholar] [CrossRef]
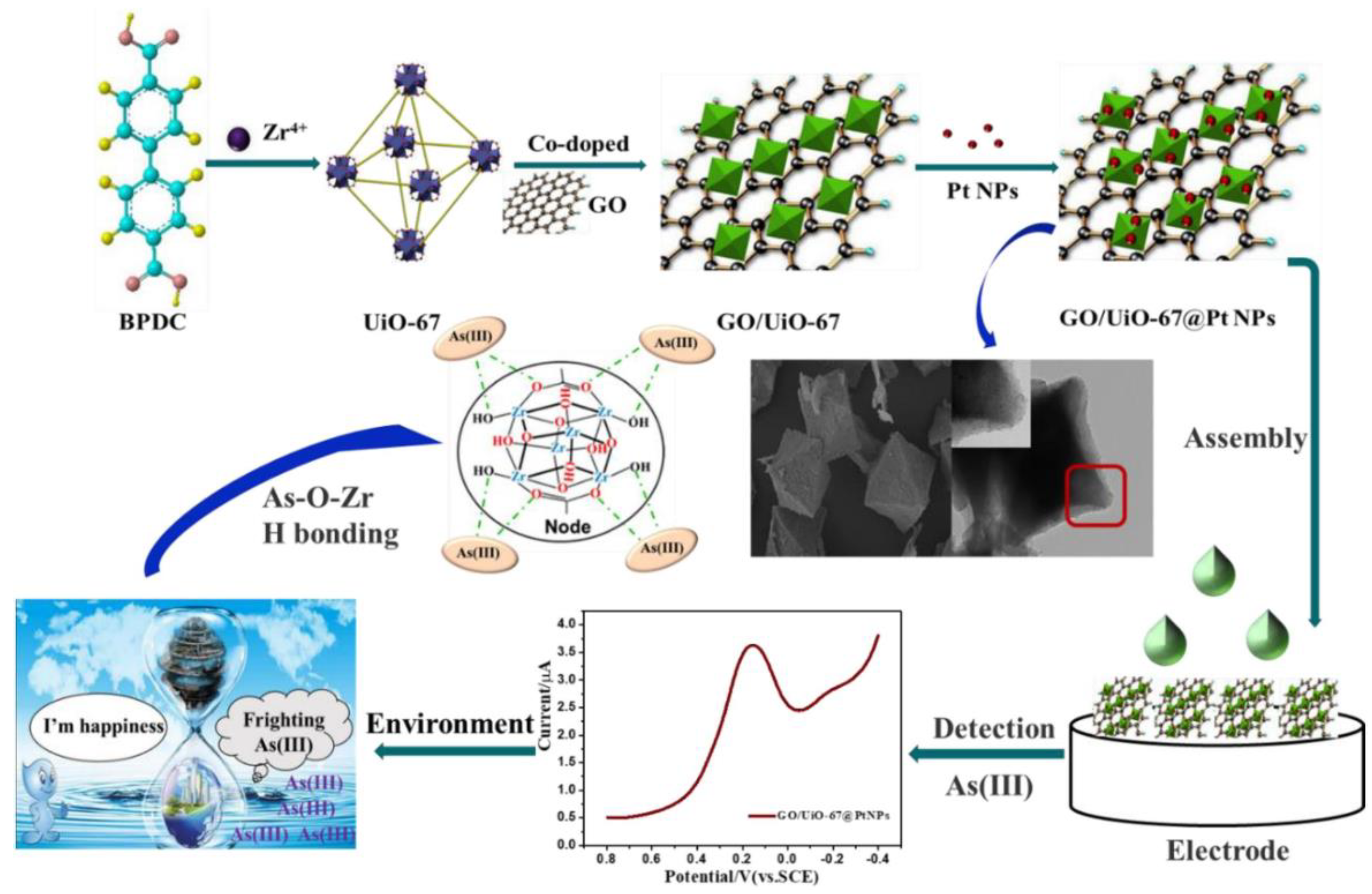
- Ru, J.; Wang, X.; Zhao, J.; Yang, J.; Zhou, Z.; Du, X.; Lu, X. Evaluation and development of GO/UiO-67@ PtNPs nanohybrid-based electrochemical sensor for invisible arsenic (III) in water samples. Microchem. J. 2022, 181, 107765. [Google Scholar] [CrossRef]
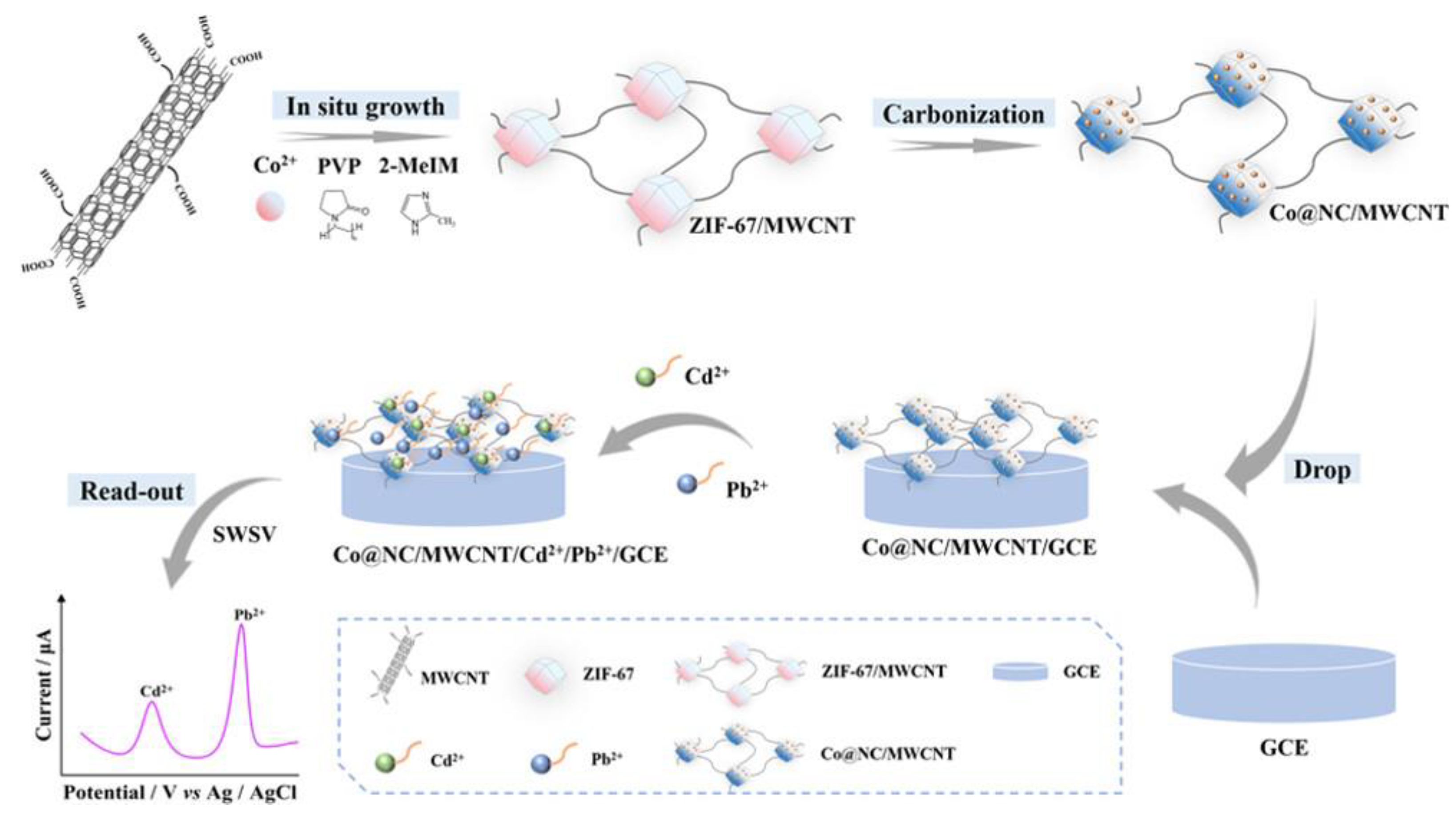
- Zhao, J.; Long, Y.; He, C.; Yang, H.; Zhao, S.; Luo, X.; Huo, D.; Hou, C. Simultaneous Electrochemical detection of Cd2+ and Pb2+ based on an MOF-derived carbon composite linked with multiwalled carbon nanotubes. ACS Sustainable Chem. Eng. 2023, 11, 2160–2171. [Google Scholar] [CrossRef]
- Hanif, F.; Tahir, A.; Akhtar, M.; Waseem, M.; Haider, S.; Aboud, M.F.A.; Shakir, I.; Imran, M.; Warsi, M.F. Ultra-selective detection of Cd2+ and Pb2+ using glycine functionalized reduced graphene oxide/polyaniline nanocomposite electrode. Synthetic Metals 2019, 257, 116185. [Google Scholar] [CrossRef]
- Oularbi, L.; Turmine, M.; El Rhazi, M. Preparation of novel nanocomposite consisting of bismuth particles, polypyrrole and multi-walled carbon nanotubes for simultaneous voltammetric determination of cadmium (II) and lead (II). Synthetic Metals 2019, 253, 1–8. [Google Scholar] [CrossRef]
- Akhtar, M.; Tahir, A.; Zulfiqar, S.; Hanif, F.; Warsi, M.F.; Agboola, P.O.; Shakir, I. Ternary hybrid of polyaniline-alanine-reduced graphene oxide for electrochemical sensing of heavy metal ions. Synthetic Metals 2020, 265, 116410. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Basirun, W.; Alias, Y.; MengWoi, P. Investigating the effectiveness of g-C3N4 on Pt/g-C3N4/polythiophene nanocomposites performance as an electrochemical sensor for Hg2+ detection. J. Environmental Chem. Eng. 2020, 8, 104204. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Alias, Y.; Woi, P.M.; Yousefi, R.; Basirun, W. An electrochemical sensor based on Pt/g-C3N4/polyaniline nanocomposite for detection of Hg2+. Adv. Powder Technol. 2020, 31, 3372–3380. [Google Scholar] [CrossRef]
- Guo, X.; Cui, R.; Huang, H.; Li, Y.; Liu, B.; Wang, J.; Zhao, D.; Dong, J.; Sun, B. Insights into the role of pyrrole doped in three-dimensional graphene aerogels for electrochemical sensing Cd (II). J. Electroanal. Chem. 2020, 871, 114323. [Google Scholar] [CrossRef]
- Katowah, D.F.; Alsulami, Q.A.; Alam, M.; Ismail, S.H.; Asiri, A.M.; Mohamed, G.G.; Rahman, M.M.; Hussein, M.A. The performance of various SWCNT loading into CuO–PMMA nanocomposites towards the detection of Mn2+ ions. J. Inorganic Organometal. Polymers Mat. 2020, 30, 5024–5041. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Shirsat, M.D.; Ramanaviciene, A.; Ramanavicius, A. Composites based on conducting polymers and carbon nanomaterials for heavy metal ion sensing. Critical Rev. Anal. Chem. 2018, 48, 293–304. [Google Scholar] [CrossRef] [PubMed]
- El Rhazi, M.; Majid, S.; Elbasri, M.; Salih, F.E.; Oularbi, L.; Lafdi, K. Recent progress in nanocomposites based on conducting polymer: application as electrochemical sensors. Int. Nano Lett. 2018, 8, 79–99. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polymer-Plastics Technol. Mat. 2021, 60, 504–521. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Patil, H.K.; Bodkhe, G.A.; Yasuzawa, M.; Koinkar, P.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA-modified PANI/SWNTs nanocomposite for differential pulse voltammetry based determination of Cu (II) ions. Sens. Actuat. B: Chemical 2018, 260, 331–338. [Google Scholar] [CrossRef]
- Bashir, S.; Ramesh, S.; Ramesh, K.; Numan, A.; Iqbalc, J. Conducting polymer composites in electrochemical sensors. Conducting Polymer Composites Central West Publishing, Australia 2018, 41-68.
- Kumar, H.; Kumari, N.; Sharma, R. Nanocomposites (conducting polymer and nanoparticles) based electrochemical biosensor for the detection of environment pollutant: Its issues and challenges. Environment. Impact Assess. Rev. 2020, 85, 106438. [Google Scholar] [CrossRef]
- Rashed, M.A.; Ahmed, J.; Faisal, M.; Alsareii, S.; Jalalah, M.; Harraz, F.A. Highly sensitive and selective thiourea electrochemical sensor based on novel silver nanoparticles/chitosan nanocomposite. Coll. Surf. A: Physicochemical and Engineering Aspects 2022, 644, 128879. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alamry, K.A.; Awual, M.R.; Mekky, A.E. Efficient Hg (II) ionic probe development based on one-step synthesized diethyl thieno [2, 3-b] thiophene-2, 5-dicarboxylate (DETTDC2) onto glassy carbon electrode. Microchem. J. 2020, 152, 104291. [Google Scholar] [CrossRef]
- Feng, T.; Chen, K.; Zhong, J.; Cheng, Y.; Zhao, H.; Lan, M. In-situ polymerization of dendritic polyaniline nanofibers network embedded with Ag@SiO2 core-shell nanoparticles for electrochemical determination of trace arsenic (III). Sens. Actuat. B: Chemical 2022, 369, 132265. [Google Scholar] [CrossRef]
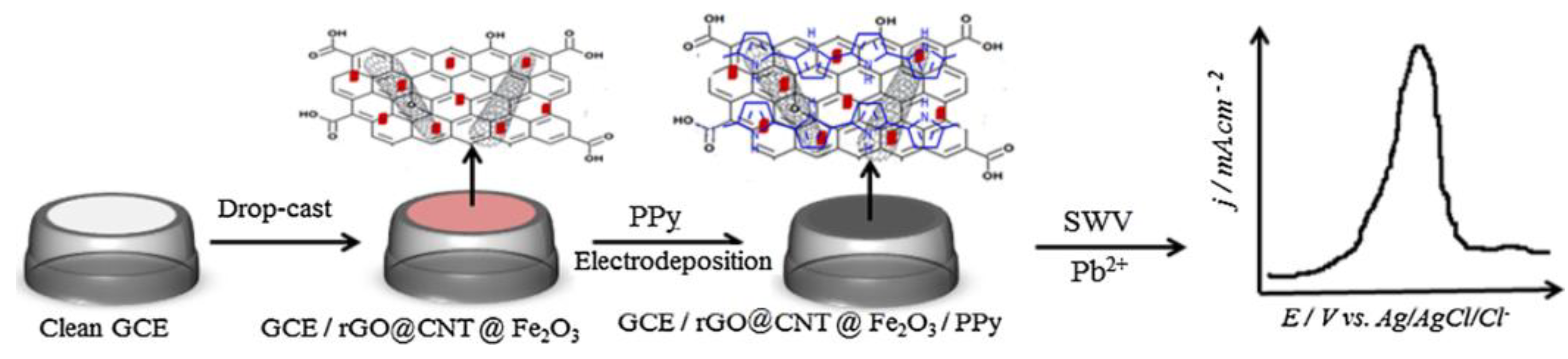
- Fall, B.; Diaw, A.K.; Fall, M.; Sall, M.L.; Lo, M.; Gningue-Sall, D.; Thotiyl, M.O.; Maria, H.J.; Kalarikkal, N.; Thomas, S. Synthesis of highly sensitive rGO@ CNT@Fe2O3/polypyrrole nanocomposite for the electrochemical detection of Pb2+. Materials Today Commun. 2021, 26, 102005. [Google Scholar] [CrossRef]
- Oularbi, L.; Turmine, M.; El Rhazi, M. Electrochemical determination of traces lead ions using a new nanocomposite of polypyrrole/carbon nanofibers. J. Solid State Electrochem. 2017, 21, 3289–3300. [Google Scholar] [CrossRef]
- Seenivasan, R.; Chang, W.-J.; Gunasekaran, S. Highly sensitive detection and removal of lead ions in water using cysteine-functionalized graphene oxide/polypyrrole nanocomposite film electrode. ACS Appl. Mat. Interf. 2015, 7, 15935–15943. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.; Seydou, M.; Bensghaïer, A.; Pires, R.; Gningue-Sall, D.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole-wrapped carbon nanotube composite films coated on diazonium-modified flexible ITO sheets for the electroanalysis of heavy metal ions. Sensors 2020, 20, 580. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Simultaneous determination of lead (II) and cadmium (II) at a glassy carbon electrode modified with GO@Fe3O4@ benzothiazole-2-carboxaldehyde using square wave anodic stripping voltammetry. J. Molec. Liquids 2018, 249, 1125–1132. [Google Scholar] [CrossRef]
- Boonkaew, S.; Chaiyo, S.; Jampasa, S.; Rengpipat, S.; Siangproh, W.; Chailapakul, O. An origami paper-based electrochemical immunoassay for the C-reactive protein using a screen-printed carbon electrode modified with graphene and gold nanoparticles. Microchim. Acta 2019, 186, 1–10. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. Tracking metal ions with polypyrrole thin films adhesively bonded to diazonium-modified flexible ITO electrodes. Environment. Sci. Pollut. Res. 2018, 25, 20012–20022. [Google Scholar] [CrossRef]
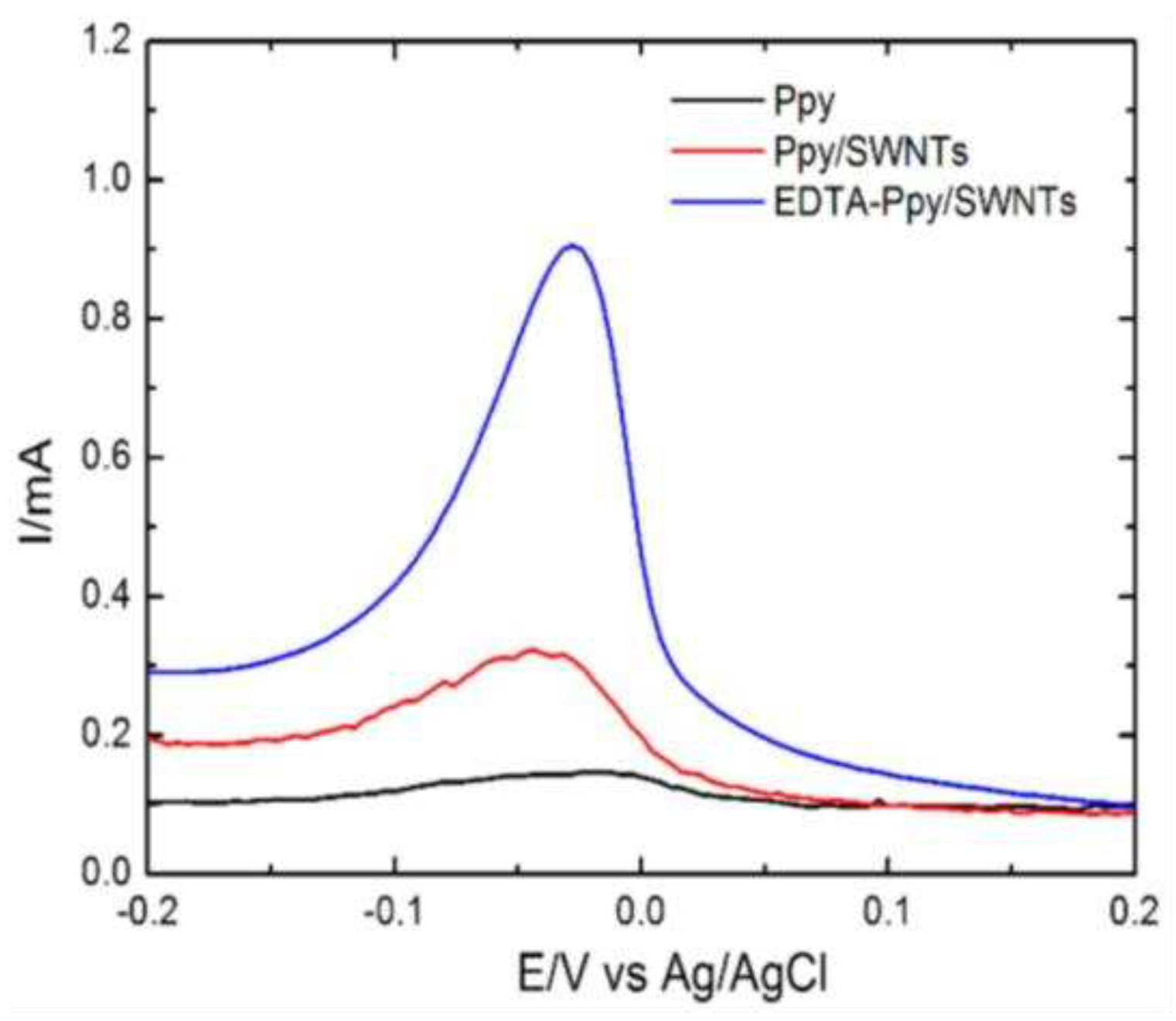
- Deshmukh, M.A.; Bodkhe, G.A.; Shirsat, S.; Ramanavicius, A.; Shirsat, M.D. Nanocomposite platform based on EDTA modified Ppy/SWNTs for the sensing of Pb (II) ions by electrochemical method. Frontiers in chemistry 2018, 6, 451. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jia, M.; Zhang, Z.; Chen, X.; Zhang, Q.; Zhang, W.; Li, P.; Chen, L. Sensitive, selective and simultaneous electrochemical detection of multiple heavy metals in environment and food using a lowcost Fe3O4 nanoparticles/fluorinated multi-walled carbon nanotubes sensor. Ecotoxicol. Environmental Safety 2019, 175, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A sensitive electrochemical sensor for simultaneous voltammetric sensing of cadmium and lead based on Fe3O4/multiwalled carbon nanotube/laser scribed graphene composites functionalized with chitosan modified electrode. Mat. Chem. Phys. 2019, 238, 121877. [Google Scholar] [CrossRef]
- Le Hai, T.; Hung, L.C.; Phuong, T.T.B.; Ha, B.T.T.; Nguyen, B.-S.; Hai, T.D.; Nguyen, V.-H. Multiwall carbon nanotube modified by antimony oxide (Sb2O3/MWCNTs) paste electrode for the simultaneous electrochemical detection of cadmium and lead ions. Microchem. J. 2020, 153, 104456. [Google Scholar] [CrossRef]
- Mariyappan, V.; Manavalan, S.; Chen, S.-M.; Jaysiva, G.; Veerakumar, P.; Keerthi, M. Sr@FeNi-S nanoparticle/carbon nanotube nanocomposite with superior electrocatalytic activity for electrochemical detection of toxic mercury (II). ACS Appl. Electronic Mat. 2020, 2, 1943–1952. [Google Scholar] [CrossRef]
- Katowah, D.F.; Hussein, M.A.; Alam, M.; Ismail, S.H.; Osman, O.; Sobahi, T.; Asiri, A.M.; Ahmed, J.; Rahman, M.M. Designed network of ternary core-shell PPCOT/NiFe2O4/C-SWCNTs nanocomposites. A Selective Fe3+ ionic sensor. J. Alloys and Compounds 2020, 834, 155020. [Google Scholar] [CrossRef]
- Yıldız, C.; Bayraktepe, D.E.; Yazan, Z.; Önal, M. Bismuth nanoparticles decorated on Na-montmorillonite-multiwall carbon nanotube for simultaneous determination of heavy metal ions-electrochemical methods. J. Electroanalyt. Chem. 2022, 910, 116205. [Google Scholar] [CrossRef]
- Yu, L.; Wan, J.-W.; Meng, X.-Z.; Gu, H.-W.; Chen, Y.; Yi, H.-C. A simple electrochemical method for Cd (II) determination in real samples based on carbon nanotubes and metal-organic frameworks. Int. J. Environ. Anal. Chem. 2022, 102, 4757–4767. [Google Scholar] [CrossRef]
- Tan, R.; Jiang, P.; Pan, C.; Pan, J.; Gao, N.; Cai, Z.; Wu, F.; Chang, G.; Xie, A.; He, Y. Core–shell architectured NH2-UiO-66@ ZIF-8/multi-walled carbon nanotubes nanocomposite-based sensitive electrochemical sensor towards simultaneous determination of Pb2+ and Cu2+. Microchim. Acta 2023, 190, 30. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chen, L.; Jiang, R.; Ye, J.; Li, H.; Shi, Y.; Luo, Y.; Wang, G.; Hou, J.; Guo, X. ZnFe2O4 nanoparticles for electrochemical determination of trace Hg (II), Pb (II), Cu (II), and glucose. ACS Appl. Nano Mat. 2021, 4, 4026–4036. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Zhang, Q.; Wang, H.; Zhang, Y.; Cao, W.; Zhang, N.; Du, B.; Wei, Q. Electrochemical aptasensor based on gold modified graphene nanocomposite with different morphologies for ultrasensitive detection of Pb2+. Sens. Actuat. B: Chemical 2019, 288, 325–331. [Google Scholar] [CrossRef]
- Baghayeri, M.; Alinezhad, H.; Fayazi, M.; Tarahomi, M.; Ghanei-Motlagh, R.; Maleki, B. A novel electrochemical sensor based on a glassy carbon electrode modified with dendrimer functionalized magnetic graphene oxide for simultaneous determination of trace Pb (II) and Cd (II). Electrochim. Acta 2019, 312, 80–88. [Google Scholar] [CrossRef]
- Priya, T.; Dhanalakshmi, N.; Karthikeyan, V.; Thinakaran, N. Highly selective simultaneous trace determination of Cd2+ and Pb2+ using porous graphene/carboxymethyl cellulose/fondaparinux nanocomposite modified electrode. J. Electroanal. Chem. 2019, 833, 543–551. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Fang, C.; Ai, L.; Chen, J.; Su, J.; Zhang, Q.; Fu, Q. Facile synthesis of reduced graphene oxide/silver nanoparticles composites and their application for detecting heavy metal ions. J. Alloys Comp. 2019, 787, 683–693. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Abdelfatah, M.M.; El-Khouly, M.E.; El-Mehasseb, I.M.; El-Shaer, A.; Ramadan, M.S.; Masoud, M.S.; El-Kemary, M.A. Magnetite nano-spherical quantum dots decorated graphene oxide nano sheet (GO@ Fe3O4): Electrochemical properties and applications for removal heavy metals, pesticide and solar cell. Appl. Surf. Sci. 2020, 506, 144896. [Google Scholar] [CrossRef]
- Tan, Z.; Wu, W.; Feng, C.; Wu, H.; Zhang, Z. Simultaneous determination of heavy metals by an electrochemical method based on a nanocomposite consisting of fluorinated graphene and gold nanocage. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Das, T.R.; Sharma, P.K. Hydrothermal-assisted green synthesis of Ni/Ag@rGO nanocomposite using Punica granatum juice and electrochemical detection of ascorbic acid. Microchem. J. 2020, 156, 104850. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Sharma, T.S.K.; Ganguly, A. Design strategy of rGO–HNT–AgNPs based hybrid nanocomposite with enhanced performance for electrochemical detection of 4-nitrophenol. Inorganic Chem. Frontiers 2020, 7, 1981–1994. [Google Scholar] [CrossRef]
- Bi, C.-C.; Ke, X.-X.; Chen, X.; Weerasooriya, R.; Hong, Z.-Y.; Wang, L.-C.; Wu, Y.-C. Assembling reduced graphene oxide with sulfur/nitrogen-“hooks” for electrochemical determination of Hg (II). Anal. Chim. Acta 2020, 1100, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Fu, H.; Kong, W.; Jiang, R.; Ye, J.; Zhao, Z.; Hou, J.; Sun, K.; Zheng, Y.; Chen, L. Design of NiCo2O4 nanoparticles decorated N, S co-doped reduced graphene oxide composites for electrochemical simultaneous detection of trace multiple heavy metal ions and hydrogen evolution reaction. Chem. Engin. J. 2022, 433, 133854. [Google Scholar] [CrossRef]
- Erçarıkcı, E.; Alanyalıoğlu, M. Dual-functional graphene-based flexible material for membrane filtration and electrochemical sensing of heavy metal ions. IEEE Sens. J. 2020, 21, 2468–2475. [Google Scholar] [CrossRef]
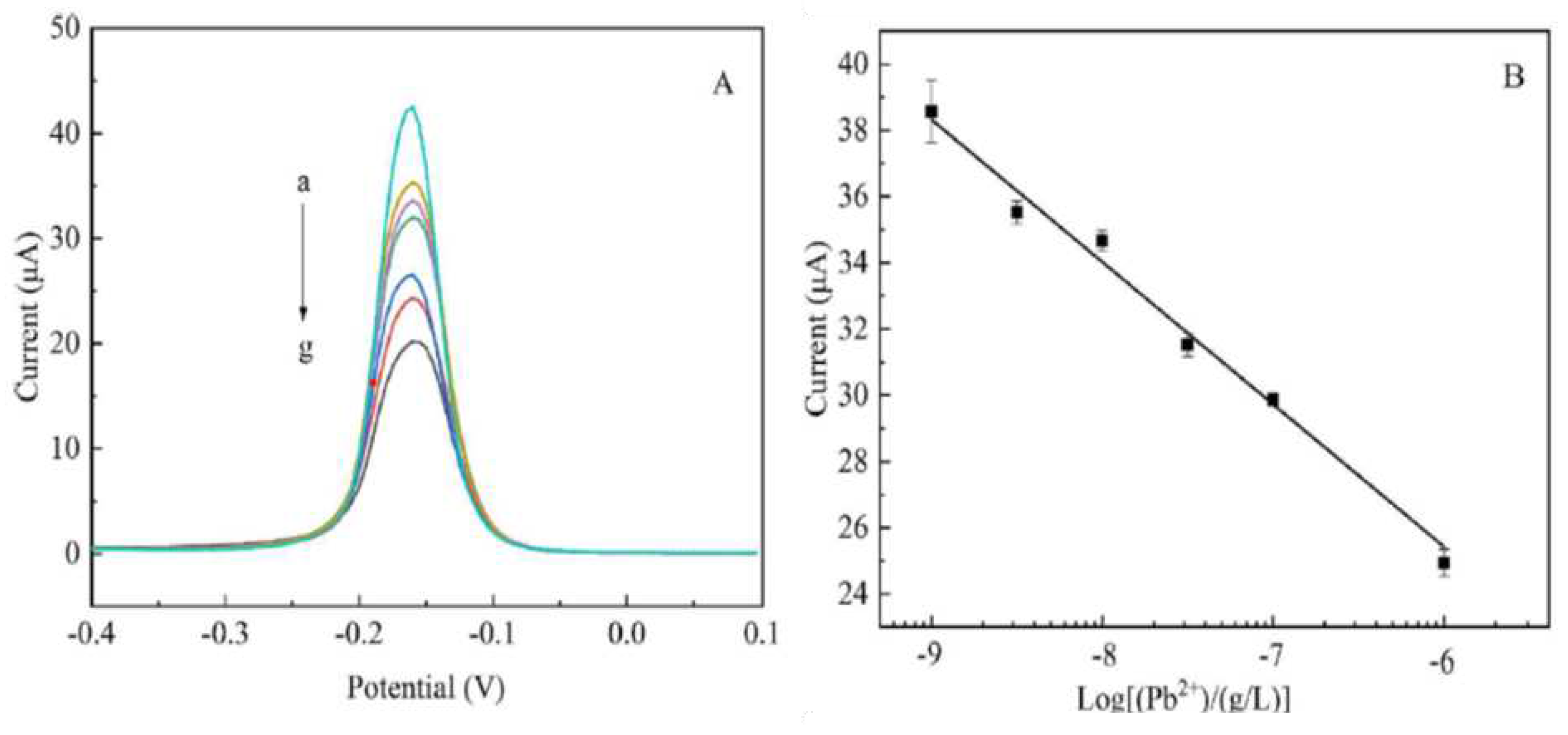
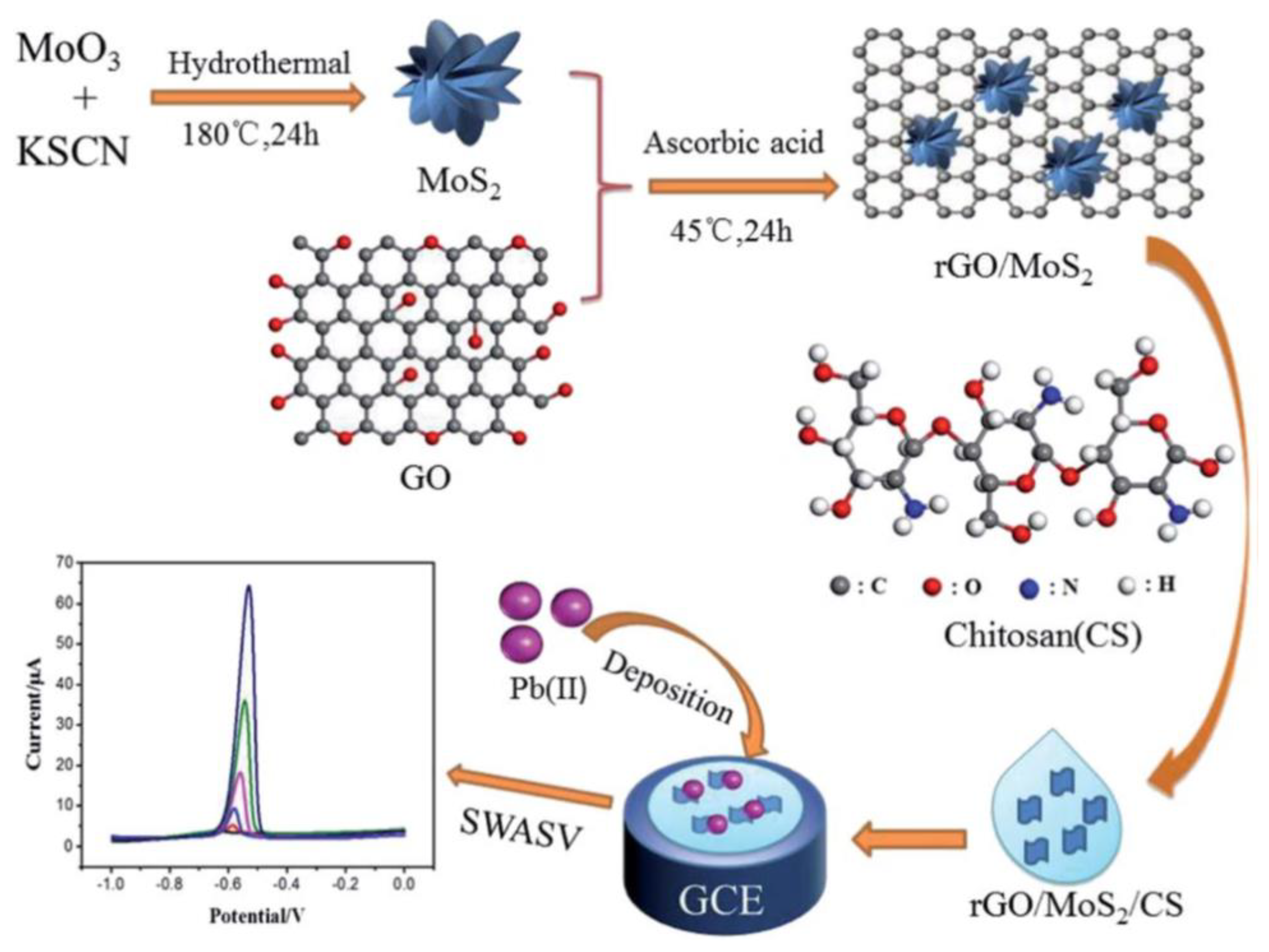
- Guo, C.; Wang, C.; Sun, H.; Dai, D.; Gao, H. A simple electrochemical sensor based on rGO/MoS2/CS modified GCE for highly sensitive detection of Pb (II) in tobacco leaves. Rsc Advances 2021, 11, 29590–29597. [Google Scholar] [CrossRef]
- Bhardiya, S.R.; Asati, A.; Sheshma, H.; Rai, A.; Rai, V.K.; Singh, M. A novel bioconjugated reduced graphene oxide-based nanocomposite for sensitive electrochemical detection of cadmium in water. Sens. Actuat. B: Chemical 2021, 328, 129019. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Fu, H. An electrochemical aptasensor for the sensitive detection of Pb2+ based on a chitosan/reduced graphene oxide/titanium dioxide. Microchem. J. 2022, 174, 106977. [Google Scholar] [CrossRef]
- Kushwah, M.; Yadav, R.; Berlina, A.N.; Gaur, K.; Gaur, M. Development of an ultrasensitive rGO/AuNPs/ssDNA-based electrochemical aptasensor for detection of Pb2+. J. Solid State Electrochem. 2023, 27, 559–574. [Google Scholar] [CrossRef]
- Zheng, H.; Ntuli, L.; Mbanjwa, M.; Palaniyandy, N.; Smith, S.; Modibedi, M.; Land, K.; Mathe, M. The effect of gC3N4 materials on Pb (II) and Cd (II) detection using disposable screen-printed sensors. Electrocatalysis 2019, 10, 149–155. [Google Scholar] [CrossRef]
- Xiao, X.-Y.; Chen, S.-H.; Li, S.-S.; Wang, J.; Zhou, W.-Y.; Huang, X.-J. Synergistic catalysis of N vacancies and∼ 5 nm Au nanoparticles promoted the highly sensitive electrochemical determination of lead (ii) using an Au/N-deficient-C3N4 nanocomposite. Environment. Sci.: Nano 2019, 6, 1895–1908. [Google Scholar] [CrossRef]
- Ramalingam, M.; Ponnusamy, V.K.; Sangilimuthu, S.N. A nanocomposite consisting of porous graphitic carbon nitride nanosheets and oxidized multiwalled carbon nanotubes for simultaneous stripping voltammetric determination of cadmium (II), mercury (II), lead (II) and zinc (II). Microchim. Acta 2019, 186, 1–10. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sivanesan, S.; Panneerselvam, P. Turn-On fluorescence sensor based detection of heavy metal ion using carbon dots@ graphitic-carbon nitride nanocomposite probe. J. Photochem. Photobiol. A: Chemistry 2020, 389, 112204. [Google Scholar] [CrossRef]
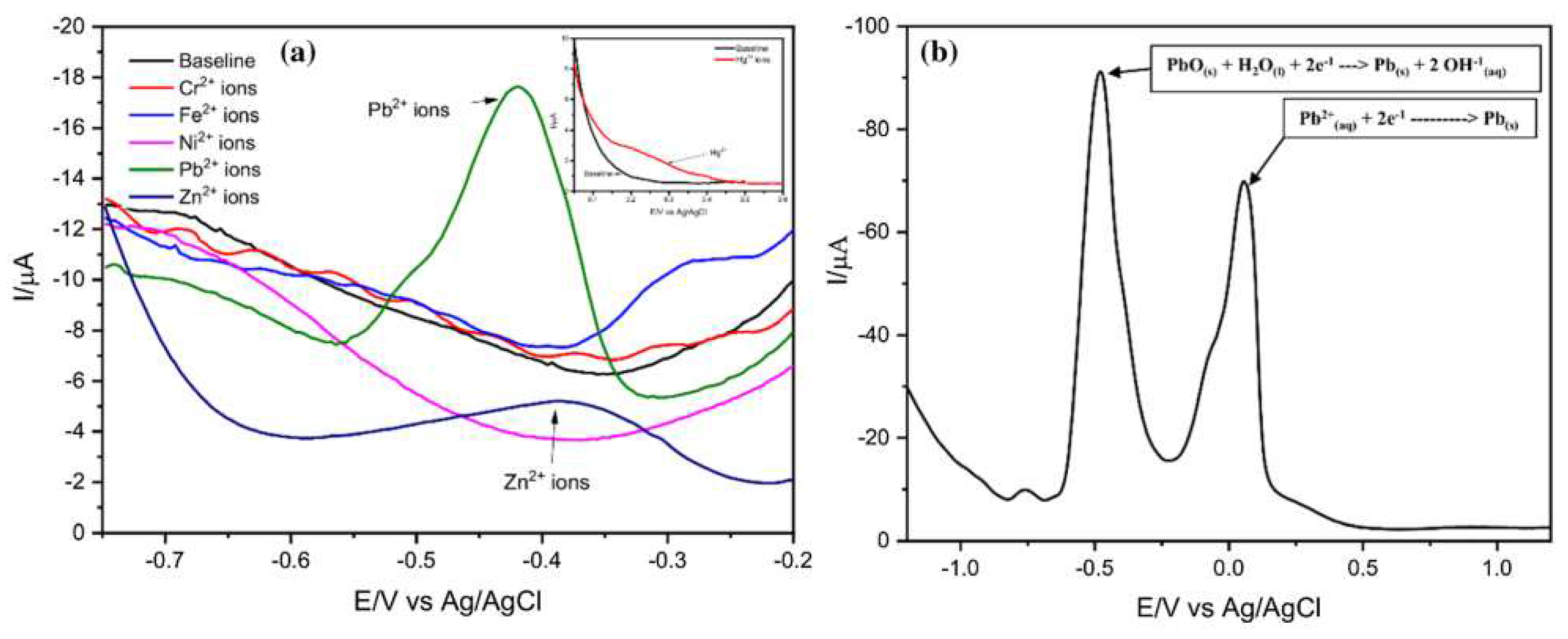
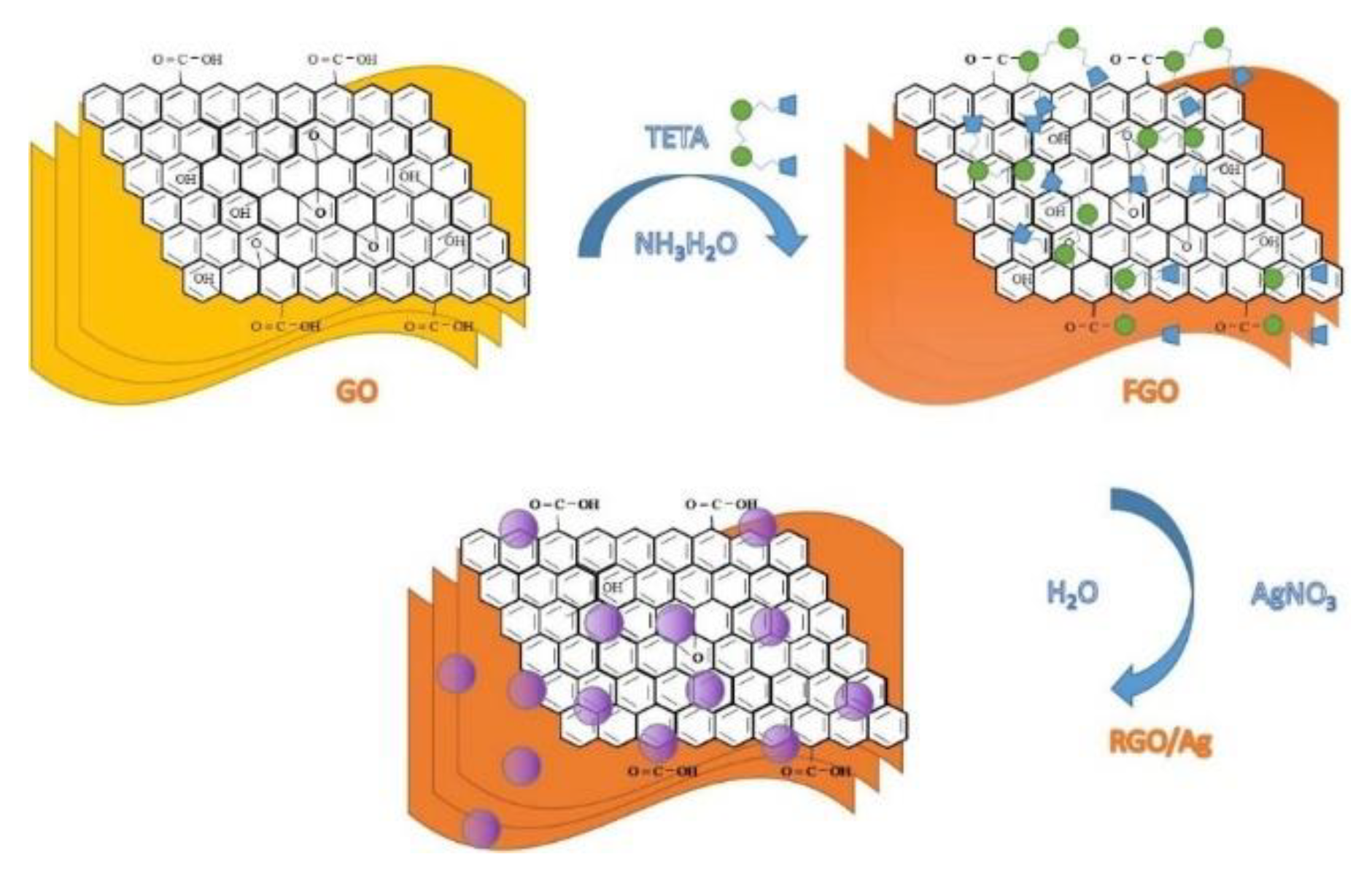
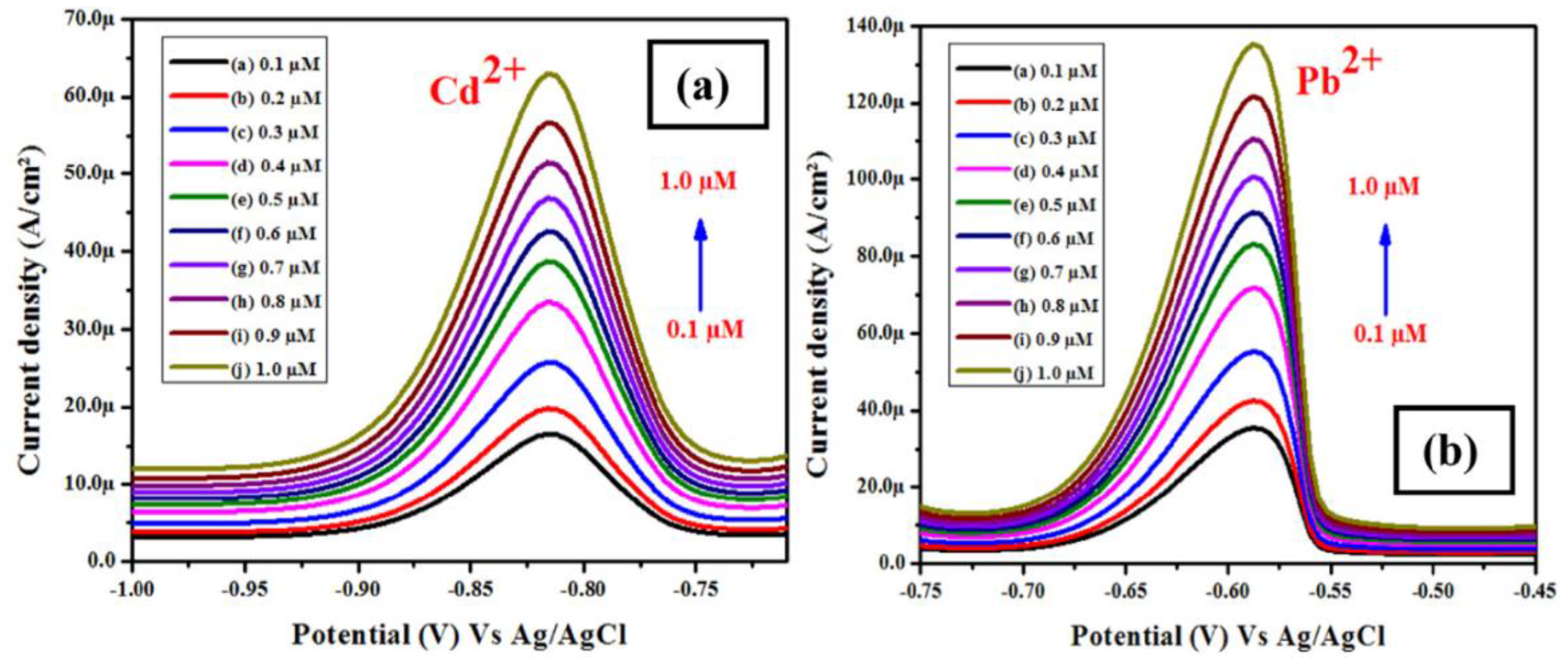
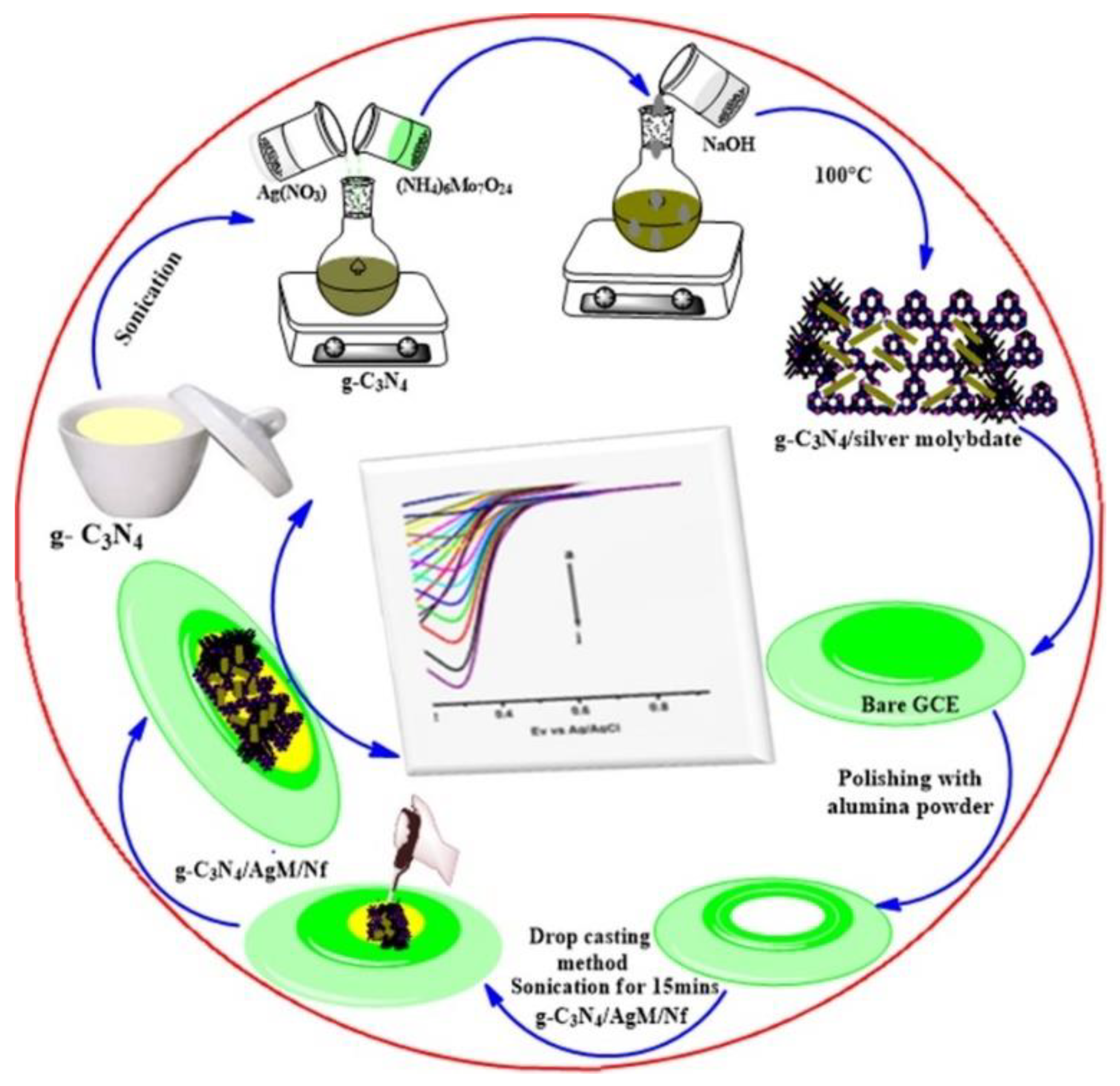
- Karthika, A.; Nikhil, S.; Suganthi, A.; Rajarajan, M. A facile sonochemical approach based on graphene carbon nitride doped silver molybdate immobilized nafion for selective and sensitive electrochemical detection of chromium (VI) in real sample. Adv. Powder Technol. 2020, 31, 1879–1890. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Li, Z.; Zhai, C.-Y.; Wang, J.-F.; Zeng, L.-X.; Zhu, M.-S. Plasmonic photo-assisted electrochemical sensor for detection of trace lead ions based on Au anchored on two-dimensional gC3N4/graphene nanosheets. Rare Metals 2021, 40, 1727–1737. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Zhai, C.; Zeng, L.; Zhu, M. Photo-assisted simultaneous electrochemical detection of multiple heavy metal ions with a metal-free carbon black anchored graphitic carbon nitride sensor. Anal. Chim. Acta 2021, 1183, 338951. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, Y.; Yu, Z.; Lu, L.; Wang, X. Simultaneous determination of Cd2+ and Pb2+ by an electrochemical sensor based on Fe3O4/Bi2O3/C3N4 nanocomposites. Talanta 2021, 3, 100024. [Google Scholar] [CrossRef]
- Eswaran, M.; Tsai, P.-C.; Wu, M.-T.; Ponnusamy, V.K. Novel nano-engineered environmental sensor based on polymelamine/graphitic-carbon nitride nanohybrid material for sensitive and simultaneous monitoring of toxic heavy metals. J. Hazardous Mat. 2021, 418, 126267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nie, Z.; Li, X.; Zhao, Y.; Wang, H. Highly sensitive and selective electrochemical sensor based on porous graphitic carbon nitride/CoMn2O4 nanocomposite toward heavy metal ions. Sens. Actuat. B: Chemical 2021, 346, 130539. [Google Scholar] [CrossRef]
- Hassanpoor, S.; Rouhi, N. Electrochemical sensor for determination of trace amounts of cadmium (II) in environmental water samples based on MnO2/RGO nanocomposite. Int. J. Environmental Anal. Chem. 2021, 101, 513–532. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, P.-H.; Yang, M.; Huang, X.-J. Highly sensitive electrochemical detection of Pb (II) based on excellent adsorption and surface Ni (II)/Ni (III) cycle of porous flower-like NiO/rGO nanocomposite. Sens. Actuat. B: Chemical 2019, 292, 136–147. [Google Scholar] [CrossRef]
- Vajedi, F.; Dehghani, H. The characterization of TiO2-reduced graphene oxide nanocomposites and their performance in electrochemical determination for removing heavy metals ions of cadmium (II), lead (II) and copper (II). Mat. Sci. Engineering: B 2019, 243, 189–198. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Li, Y.; Zeng, T.; Wan, Q.; Yang, N. Morphology-controlled electrochemical sensing of environmental Cd2+ and Pb2+ ions on expanded graphite supported CeO2 nanomaterials. Anal. Chim. Acta 2020, 1126, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Koshki, M.-S.; Baghayeri, M.; Fayazi, M. Application of sepiolite/FeS2 nanocomposite for highly selective detection of mercury (II) based on stripping voltammetric analysis. J. Food Measurement and Characterization 2021, 15, 5318–5325. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.; Li, C.-Y.; Xie, X.-Y.; Wei, Y.-Y.; Shen, W.; Wang, J.-P.; Yang, M. Highly sensitive and selective electrochemical detection of Pb (II) in serum via an α-Fe2O3/NiO heterostructure: Evidence from theoretical calculations and adsorption investigation. Sens. Actuat. B: Chemical 2021, 344, 130295. [Google Scholar] [CrossRef]
- Cheng, X.-L.; Xu, Q.-Q.; Li, S.-S.; Li, J.; Zhou, Y.; Zhang, Y.; Li, S. Oxygen vacancy enhanced Co3O4/ZnO nanocomposite with small sized and loose structure for sensitive electroanalysis of Hg (II) in subsidence area water. Sens. Actuat. B: Chemical 2021, 326, 128967. [Google Scholar] [CrossRef]
- Buica, G.-O.; Stoian, A.B.; Manole, C.; Demetrescu, I.; Pirvu, C. Zr/ZrO2 nanotube electrode for detection of heavy metal ions. Electrochem. Commun. 2020, 110, 106614. [Google Scholar] [CrossRef]
- Jin, W.; Fu, Y.; Hu, M.; Wang, S.; Liu, Z. Highly efficient SnS-decorated Bi2O3 nanosheets for simultaneous electrochemical detection and removal of Cd (II) and Pb (II). J. Electroanalyt. Chem. 2020, 856, 113744. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, J.-J.; Xie, F.; Wei, Y.; Yang, M. Ruthenium-loaded cerium dioxide nanocomposites with rich oxygen vacancies promoted the highly sensitive electrochemical detection of Hg (II). Sens. Actuat. B: Chemical 2020, 320, 128355. [Google Scholar] [CrossRef]
- Krishnan, S.; Chatterjee, S.; Solanki, A.; Guha, N.; Singh, M.K.; Gupta, A.K.; Rai, D.K. Aminotetrazole-functionalized SiO2 coated MgO nanoparticle composites for removal of acid fuchsin dye and detection of heavy metal ions. ACS Appl. Nano Mat. 2020, 3, 11203–11216. [Google Scholar] [CrossRef]
- Maleki, B.; Baghayeri, M.; Ghanei-Motlagh, M.; Zonoz, F.M.; Amiri, A.; Hajizadeh, F.; Hosseinifar, A.; Esmaeilnezhad, E. Polyamidoamine dendrimer functionalized iron oxide nanoparticles for simultaneous electrochemical detection of Pb2+ and Cd2+ ions in environmental waters. Measurement 2019, 140, 81–88. [Google Scholar] [CrossRef]
- Karthika, A.; Raja, V.R.; Karuppasamy, P.; Suganthi, A.; Rajarajan, M. Electrochemical behaviour and voltammetric determination of mercury (II) ion in cupric oxide/poly vinyl alcohol nanocomposite modified glassy carbon electrode. Microchem. J. 2019, 145, 737–744. [Google Scholar] [CrossRef]
- Singh, S.; Pankaj, A.; Mishra, S.; Tewari, K.; Singh, S.P. Cerium oxide-catalyzed chemical vapor deposition grown carbon nanofibers for electrochemical detection of Pb (II) and Cu (II). J. Environmental Chem. Eng. 2019, 7, 103250. [Google Scholar] [CrossRef]
- Wang, L.; Lei, T.; Ren, Z.; Jiang, X.; Yang, X.; Bai, H.; Wang, S. Fe3O4@ PDA@MnO2 core-shell nanocomposites for sensitive electrochemical detection of trace Pb (II) in water. J. Electroanal. Chem. 2020, 864, 114065. [Google Scholar] [CrossRef]
- Bakhsh, E.M.; Khan, S.B.; Asiri, A.M.; Shah, A. Zn/Fe nanocomposite based efficient electrochemical sensor for the simultaneous detection of metal ions. Physica E: Low-dimensional Systems and Nanostructures 2021, 130, 114671. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Zhang, G.; Wang, S.; Li, K.; Wu, J.; Wan, X.; Liu, Y.; Li, Q. Low-cost voltammetric sensors for robust determination of toxic Cd (II) and Pb (II) in environment and food based on shuttle-like α-Fe2O3 nanoparticles decorated β-Bi2O3 microspheres. Microchem. J. 2022, 179, 107515. [Google Scholar] [CrossRef]
- Padmalaya, G.; Vardhan, K.H.; Kumar, P.S.; Ali, M.A.; Chen, T.-W. A disposable modified screen-printed electrode using egg white/ZnO rice structured composite as practical tool electrochemical sensor for formaldehyde detection and its comparative electrochemical study with Chitosan/ZnO nanocomposite. Chemosphere 2022, 288, 132560. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Pathak, P.; Wang, X.; Rodriguez, K.L.; Cho, H.J.; Lee, W.H. A novel bismuth-chitosan nanocomposite sensor for simultaneous detection of Pb (II), Cd (II) and Zn (II) in wastewater. Micromachines 2019, 10, 511. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, Z.; Song, R.; Li, Z.; Chen, C. An ion-imprinted sensor based on chitosan-graphene oxide composite polymer modified glassy carbon electrode for environmental sensing application. Electrochim. Acta 2019, 317, 93–101. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Dai, X.; Zhang, Z.; Ding, F.; Li, S. An ultrasensitive electrochemical platform based on imprinted chitosan/gold nanoparticles/graphene nanocomposite for sensing cadmium (II) ions. Microchem. J. 2020, 155, 104710. [Google Scholar] [CrossRef]
- Nguyen, L.D.; Doan, T.C.D.; Huynh, T.M.; Nguyen, V.N.P.; Dinh, H.H.; Dang, D.M.T.; Dang, C.M. An electrochemical sensor based on polyvinyl alcohol/chitosan-thermally reduced graphene composite modified glassy carbon electrode for sensitive voltammetric detection of lead. Sens. Actuat. B: Chemical 2021, 345, 130443. [Google Scholar] [CrossRef]
- He, Y.; Ma, L.; Zhou, L.; Liu, G.; Jiang, Y.; Gao, J. Preparation and application of bismuth/MXene nano-composite as electrochemical sensor for heavy metal ions detection. Nanomaterials 2020, 10, 866. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, B.; Li, L.; Wu, L.; Chen, S.; Huang, L.; Yang, J.; Liang, S.; Xiao, K.; Hu, J. A micromilled microgrid sensor with delaminated MXene-bismuth nanocomposite assembly for simultaneous electrochemical detection of lead (II), cadmium (II) and zinc (II). Microchim. Acta 2019, 186, 1–7. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Wen, Y.; Bahrami, A.; Karaman, C.; Zare, N.; Karimi-Maleh, H.; Vasseghian, Y. Magnetic-MXene-based nanocomposites for water and wastewater treatment: A review. J. Water Process Eng. 2022, 47, 102696. [Google Scholar] [CrossRef]
- Rhouati, A.; Berkani, M.; Vasseghian, Y.; Golzadeh, N. MXene-based electrochemical sensors for detection of environmental pollutants: A comprehensive review. Chemosphere 2022, 291, 132921. [Google Scholar] [CrossRef]
- Dhillon, A.; Singh, N.; Nair, M.; Kumar, D. Analytical methods to determine and sense heavy metal pollutants using MXene and MXene-based composites: Mechanistic prophecy into sensing properties. Chemosphere 2022, 135166. [Google Scholar] [CrossRef]
- Zhang, X.; An, D.; Bi, Z.; Shan, W.; Zhu, B.; Zhou, L.; Yu, L.; Zhang, H.; Xia, S.; Qiu, M. Ti3C2-MXene@ N-doped carbon heterostructure-based electrochemical sensor for simultaneous detection of heavy metals. J. Electroan. Chem. 2022, 911, 116239. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, P.; Liang, Y.; Ma, Y.; Liu, Y.; Zhao, J.; Hou, J.; Hou, C.; Huo, D. A sensitive electrochemical sensor based on 3D porous melamine-doped rGO/MXene composite aerogel for the detection of heavy metal ions in the environment. Talanta 2023, 124294. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.-S.; Kim, S.-Y. Electrochemical sensing interfaces based on novel 2D-MXenes for monitoring environmental hazardous toxic compounds: A concise review. J. Industrial and Engineering Chem. 2022. [Google Scholar] [CrossRef]
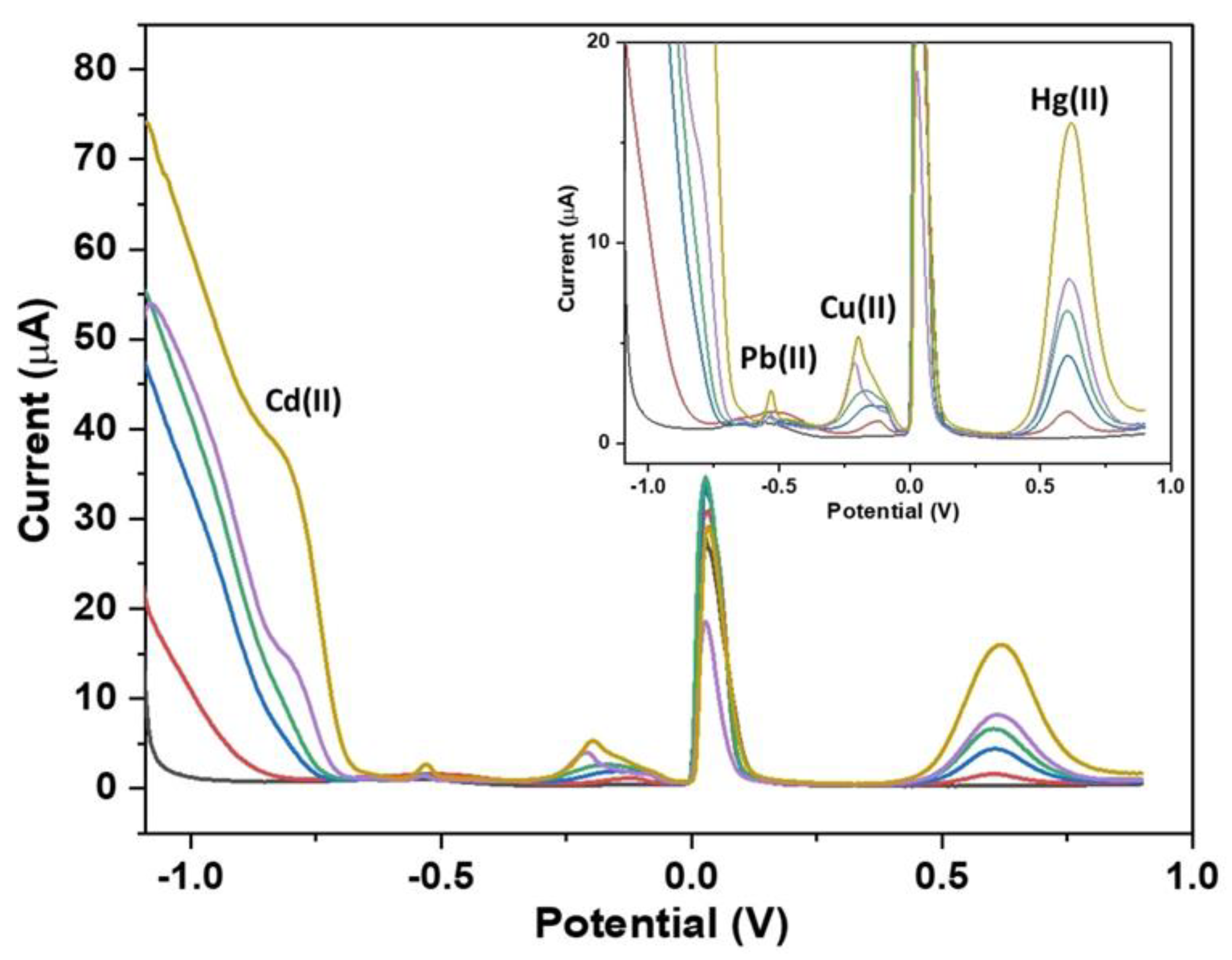
- Zhu, X.; Liu, B.; Hou, H.; Huang, Z.; Zeinu, K.M.; Huang, L.; Yuan, X.; Guo, D.; Hu, J.; Yang, J. Alkaline intercalation of Ti3C2 MXene for simultaneous electrochemical detection of Cd (II), Pb (II), Cu (II) and Hg (II). Electrochim. Acta 2017, 248, 46–57. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Z.; Long, R.; Li, X.; Shao, L.; Zeng, H.; Zeng, G.; Zuo, Y. Self-assembling 2D/2D (MXene/LDH) materials achieve ultra-high adsorption of heavy metals Ni2+ through terminal group modification. Separ. Purific. Technol. 2020, 253, 117525. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Ponraj, J.; Mahmoud, K.A. Sensitive electrochemical detection of l-cysteine based on a highly stable Pd@ Ti3C2Tx(MXene) nanocomposite modified glassy carbon electrode. Anal. Meth. 2019, 11, 3851–3856. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Singhal, R.K.; Saha, S.; Kailasa, S.K. Ultra-small two dimensional MXene nanosheets for selective and sensitive fluorescence detection of Ag+ and Mn2+ ions. Coll. Surf. A: Physicochemical and Engineering Aspects 2019, 565, 70–77. [Google Scholar] [CrossRef]
- Naseri, M.; Mohammadniaei, M.; Ghosh, K.; Sarkar, S.; Sankar, R.; Mukherjee, S.; Pal, S.; Ansari Dezfouli, E.; Halder, A.; Qiao, J. A Robust electrochemical sensor based on butterfly-shaped silver nanostructure for concurrent quantification of heavy metals in water samples. Electroanalysis 2023, 35, e202200114. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Park, J.; Yu, Y.; Kumari, M.A.; Chandrasekaran, S.; Kim, H.-S.; Choi, M.Y. Fabrication strategies and surface tuning of hierarchical gold nanostructures for electrochemical detection and removal of toxic pollutants. J. Hazardous Mat. 2021, 420, 126648. [Google Scholar] [CrossRef]
- Malakootian, M.; Hamzeh, S.; Mahmoudi-Moghaddam, H. A new electrochemical sensor for simultaneous determination of Cd (II) and Pb (II) using FeNi3/CuS/BiOCl: RSM optimization. Microchem. J. 2020, 158, 105194. [Google Scholar] [CrossRef]
- Pathak, P.; Hwang, J.-H.; Li, R.H.; Rodriguez, K.L.; Rex, M.M.; Lee, W.H.; Cho, H.J. Flexible copper-biopolymer nanocomposite sensors for trace level lead detection in water. Sens. Actuat. B: Chemical 2021, 344, 130263. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Ma, L.; Zhou, L.; Jiang, Y.; Gao, J. Synthesis of bismuth nanoparticle-loaded cobalt ferrite for electrochemical detection of heavy metal ions. RSC Adv. 2020, 10, 27697–27705. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Zhou, Y.; Zhao, S.; Dong, C.; Shuang, S. Carbon-supported X-manganate (XNi, Zn, and Cu) nanocomposites for sensitive electrochemical detection of trace heavy metal ions. J. Hazardous Mat. 2022, 435, 129036. [Google Scholar] [CrossRef] [PubMed]
- Qureashi, A.; Pandith, A.H.; Bashir, A.; Manzoor, T.; Malik, L.A.; Sheikh, F.A. Citrate coated magnetite: A complete magneto dielectric, electrochemical and DFT study for detection and removal of heavy metal ions. Surf. Interf. 2021, 23, 101004. [Google Scholar] [CrossRef]
- Teodoro, K.B.; Shimizu, F.M.; Scagion, V.P.; Correa, D.S. Ternary nanocomposites based on cellulose nanowhiskers, silver nanoparticles and electrospun nanofibers: Use in an electronic tongue for heavy metal detection. Sens. Actuat. B: Chemical 2019, 290, 387–395. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, G.; Pan, J.; Pan, Y.; Wang, S.; Zhai, H. A nanocomposite consisting of ionic liquid-functionalized layered Mg (II)/Al (III) double hydroxides for simultaneous electrochemical determination of cadmium (II), copper (II), mercury (II) and lead (II). Microchim. Acta 2019, 186, 1–7. [Google Scholar] [CrossRef]
- Padmalaya, G.; Sreeja, B.; Dinesh Kumar, P.; Radha, S.; Poornima, V.; Arivanandan, M.; Shrestha, S.; Uma, T. A facile synthesis of cellulose acetate functionalized zinc oxide nanocomposite for electrochemical sensing of cadmium ions. J. Inorganic and Organometallic Polymers and Materials 2019, 29, 989–999. [Google Scholar] [CrossRef]
- Mourya, A.; Sinha, S.K.; Mazumdar, B. Glassy carbon electrode modified with blast furnace slag for electrochemical investigation of Cu2+ and Pb2+ metal ions. Microchem. J. 2019, 147, 707–716. [Google Scholar] [CrossRef]
- Shang, J.; Zhao, M.; Qu, H.; Li, H.; Gao, R.; Chen, S. New application of pn junction in electrochemical detection: The detection of heavy metal ions. J. Electroanal. Chem. 2019, 855, 113624. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Cheng, N.; Xie, Y.; Huang, K.; Xu, W. Dual-recognition aptazyme-driven DNA nanomachine for two-in-one electrochemical detection of pesticides and heavy metal ions. Sens. Actuat. B: Chemical 2020, 321, 128598. [Google Scholar] [CrossRef]
- Xin, X.; Hu, N.; Ma, Y.; Wang, Y.; Hou, L.; Zhang, H.; Han, Z. Polyoxometalate-based crystalline materials as a highly sensitive electrochemical sensor for detecting trace Cr (VI). Dalton Transactions 2020, 49, 4570–4577. [Google Scholar] [CrossRef]
- Cao, L.; Kang, Z.-W.; Ding, Q.; Zhang, X.; Lin, H.; Lin, M.; Yang, D.-P. Rapid pyrolysis of Cu2+-polluted eggshell membrane into a functional Cu2+-Cu+/biochar for ultrasensitive electrochemical detection of nitrite in water. Sci. Total Environment 2020, 723, 138008. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Z.; Zhang, C.; Wu, X.; Liu, L.; Guo, C.; Li, C.M. Highly stable branched cationic polymer-functionalized black phosphorus electrochemical sensor for fast and direct ultratrace detection of copper ion. J. Coll. Interface Sci. 2021, 603, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, P.; Liu, S.; Lv, Y.; Zhang, D. Catalyst-free synthesis of phenolic-resin-based carbon nanospheres for simultaneous electrochemical detection of Cu (II) and Hg (II). Diamond and Related Materials 2021, 111, 108170. [Google Scholar] [CrossRef]
- Hajjaoui, H.; Soufi, A.; Boumya, W.; Abdennouri, M.; Barka, N. Polyaniline/nanomaterial composites for the removal of heavy metals by adsorption: A Review. J. Compos. Sci. 2021, 5, 233. [Google Scholar] [CrossRef]
- Yadav, R.; Kushwah, V.; Gaur, M.; Bhadauria, S.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B. Electrochemical aptamer biosensor for As3+ based on apta deep trapped Ag-Au alloy nanoparticles-impregnated glassy carbon electrode. Int. J. Environment. Anal. Chem. 2020, 100, 623–634. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Y.; Wang, H.; Li, X.; Xu, Y.; Qiu, J. Recent aptamer-based biosensor for Cd2+ detection. Biosensors 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
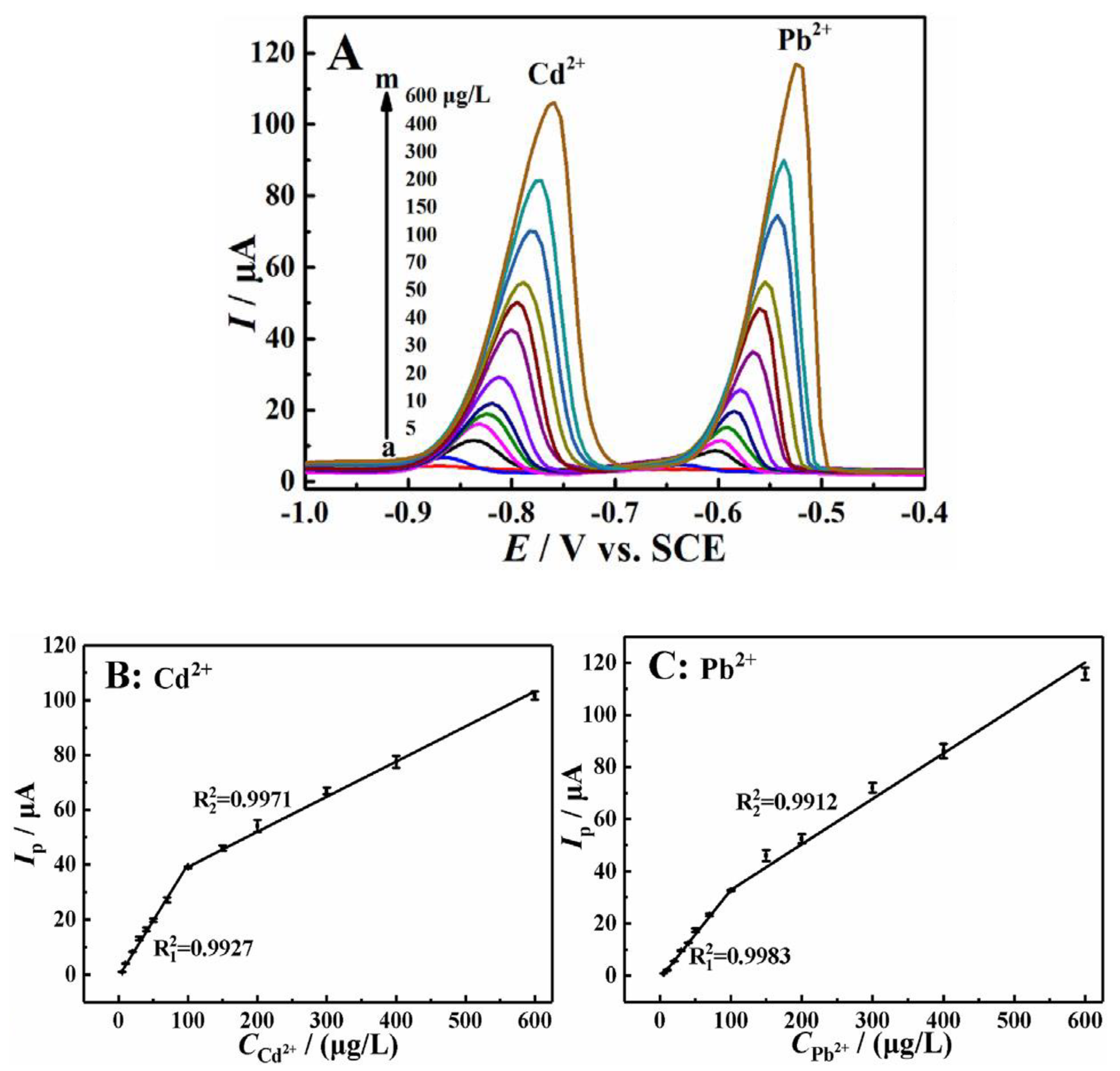
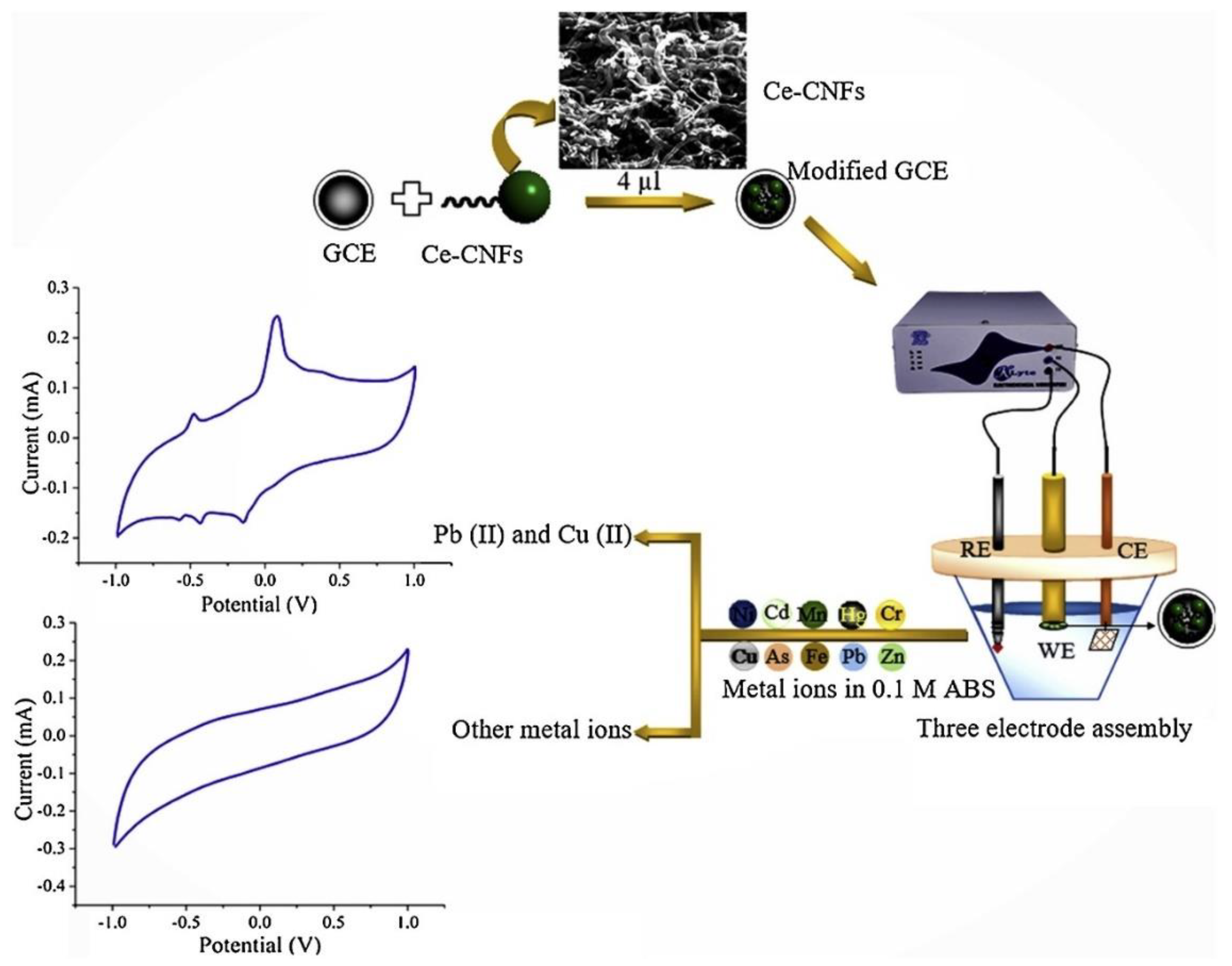
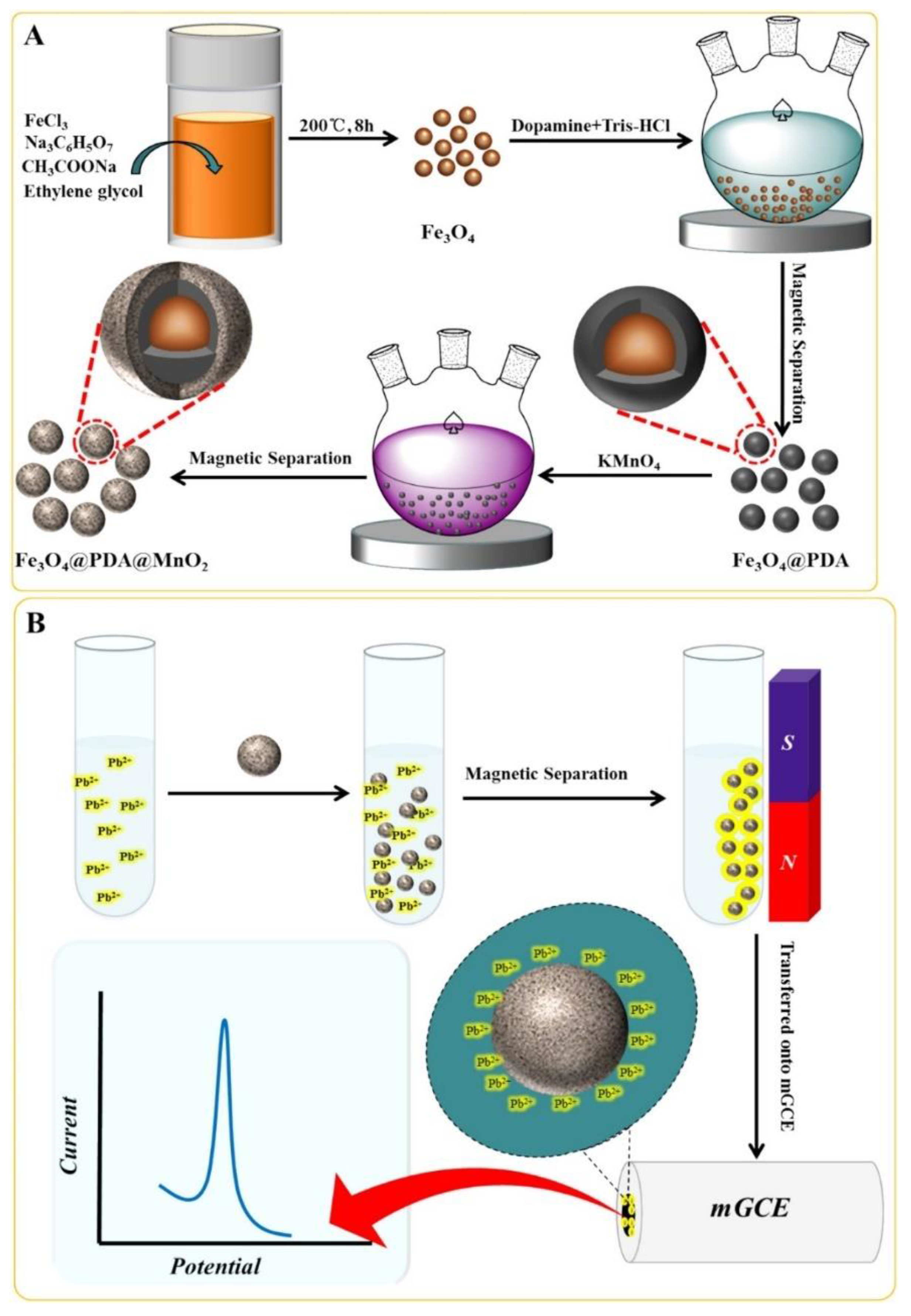
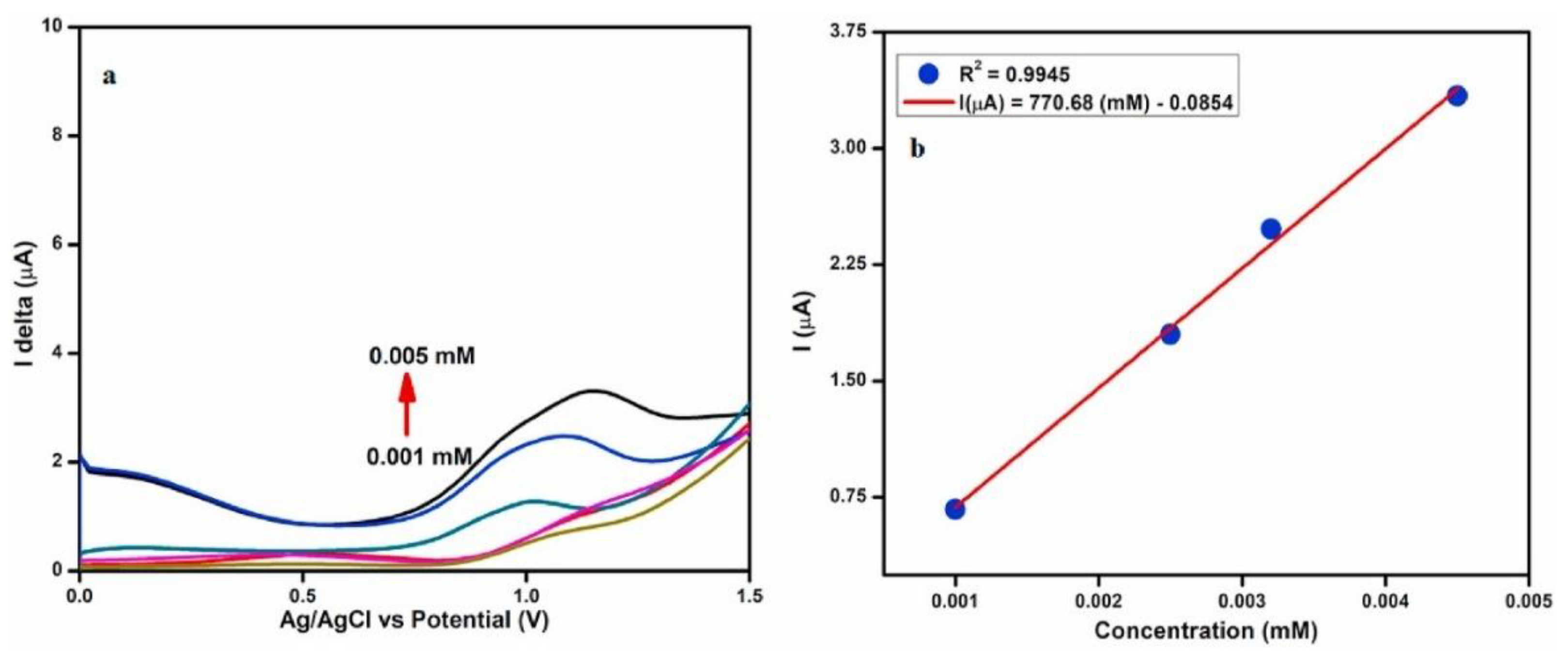
| Heavy metal | MCL (µg/L) | |
|---|---|---|
| Antimony | Sb | 6 |
| Arsenic | As | 10 |
| Cadmium | Cd | 5 |
| Chromium | Cr | 100 |
| Copper | Cu | 1.3 |
| Lead | Pb | 15 |
| Mercury | Hg | 2 |
| Nickel | Ni | 100 |
| Selenium | Se | 50 |
| Electrode | Method | Deposition potential | Deposition time (s) | Linear range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|---|---|
| PPy/CNFs/CPE | SWASV | -1.2 V vs. SCE | 600 | 0.2–130 | 0.05 | [109] |
| Fe2O3/G/Bi-GCE | DPASV | -1.2 V vs. Ag/AgCl | 300 | 1–100 | 0.07 | [17] |
| rGO/PPy-SPE | DPASV | -1.2 V vs. Ag/AgCl | 600 | 1.4–28; | 0.07 | [110] |
| 28–280; | ||||||
| 280–14,000 | ||||||
| PPy/CNT/NH2-ITO | DPV | 600 | 0.01 – 0.3 | 2.9 × 10-3 | [111] | |
| Bi/PPy/MWCNT/CPE | SWASV | 1.2 V vs. SCE | 240 | 0.11–120 | 0.099 | [93] |
| GO@Fe3O4@2-CBT | SWASV | 1.2 V vs. Ag/AgCl | 180 | 3×10-4–0.072; 0.072– 0.43 |
10-4 | [112] |
| PA/PPy/ZIF-8@ZIF-67 | DPV | – | – | 0.02-200; 200–600 |
2.9 × 10-4 | [113] |
| ITO/AP/PPy | DPASV | -1.2 V vs. SCE | 200 | 0.01- 0.25 | 9.9 × 10-4 | [114] |
| rGO@CNT@Fe2O3/ PPy/GCE | SWASV | -1.3 V vs. Ag/AgCl | 300 | 0.02– 26 | 1 × 10-4 | [108] |
| HMIs | Nanocomposite | Method of detection | LOD | Stability, repeteability, reproducibility | Linear range | Ref. |
|---|---|---|---|---|---|---|
| Hg(II) | Sucrose sensor using platinum ultra-microelectrode | CV | 5 × 10−10 M | 57 days, No, Yes | (5 - 12.5)×10−10 M | [4] |
| Pb(II) Cd(II) |
Graphene-MWCNTs | DPASV | 0.2 µg/L | No | 0.5–30 µg/L | [12] |
| Cd(II) Pb(II) Cu(II) Hg(II) |
Hydrosulphonyl functional COF (COF-SH) | SWV | Cd: 0.3 µg/L Pb: 0.2 µg/L Cu: 0.2 µg/L Hg: 1.1 µg/L |
Yes, Yes, No | Cd: 1–1000 µg/L Pb: 1–800 µg/L Cu: 1–800 µg/L Hg: 5-1000 µg/L |
[13] |
| Cd(II) Pb(II) |
Yb-MOF | DPASV | Cd: 3.0 ppb Pb: 1.6 ppb |
Yes, Yes,Yes | -- | [15] |
| Cd(II) Pb(II) Cu(II) |
trGNO/Fc-NH2-UiO-66 | DPASV | Cd: 8.5 nM Pb: 0.6 nM Cu: 0.8 nM |
20 days, No, Yes | Cd: 0.01–2 μM Pb: 0.001–0.1 µM Cu: 0.001–0.1 μM |
[16] |
| Zn(II) Cd(II), Pb(II) |
Fe2O3/G/Bi | DPASV | Zn: 0.11 µg/L Cd: 0.08 µg/L Pb: 0.07 µg/L |
Yes, Yes, No | 1–100 µg/L | [17] |
| Pb(II) | Au/SWNTs@MOF-199 | DPV | 25 pM | No, Yes, Yes | 0.1 mM–1 pM | [19] |
| Cu(II) Pb(II) |
PEDOT:PSS/rGO | FET | Cu: 0.33 µg/L Pb: 2.36 µg/L |
No | 1–60 µg/L | [20] |
| Cd(II) Hg(II) Pb(II) |
rGO/MoS2 | DPV | 5 μM | No, No, No |
5 – 160 μM | [65] |
| Cd(II), Cu(II), Hg(II), Pb(II) | rGO/SMOF/PEI modified SPCEs | DPV | Cd: 0.296 μM Cu: 0.055 μM Hg: 0.351 μM Pb: 0.025 μM | – | Cd: 0.50–15.0 μM, Cu: 0.50–13.0 μM, Hg: 1.0–5.0 μM Pb: 0.50–13.0 μM |
[67] |
| Cd(II), Pb(II) and Hg(II) | nano-Au-modified electrode | ICP-MS | Cd: 1.1 μg/L Pb: 1.0 μg/L Hg: 1.2 μg/L |
– | 0-200 μg/L | [68] |
| Pb(II) | nano-Cu WE | SWV | 45 nM | – | 0.5–1 μM | [69] |
| Zn(II), Cu(II), Hg(II), Pb(II) | DEP chips | DPV | Pb: 2.2 µg/L Hg: 2.5 µg/L Cu: 15.5 µg/L Zn: 10 µg/L |
– | Pb: 10–500 ppb Hg: 25–1000ppb Cu: 25–500 ppb Zn: 10–300 ppb |
[72] |
| Cd(II), Pb(II) | gold/bismuth film | (SWASV) | 2.20 μg/L | Yes | 0–300 μg/L | [73] |
| Zn, Cr, Cu, Pb, Mn | Colorimetric paper strip | ICP-OES | Zn: 0.63 mg/L Cr: 0.07 mg/L Cu: 0.17 mg/L Pb: 0.03 mg/L Mn: 0.11 mg/L |
7 days, Yes, No |
– | [74] |
| As(III) | GO/MOF | DPASV | 0.06 ppb | No, No, Yes | 0.2–25 ppb | [78] |
| Cu(II) | Porphyrinic MOF/rGO nanocomposite | DPV | 1.5 μM | 21 days, No, No | 5–150 μM | [79] |
| Ni(II) | ZIF-8@DMG/β-CD/RGO | DPASV | 0.005 μM | – | 0.01–1.0 μM | [81] |
| Cd(II), Hg(II), Cu(II), and Pb(II) | UiO-66-NH2/SPCE | DPV | Cd: 10.90 fM, Pb: 5.98 fM Cu: 2.89 fM Hg: 3.1 fM |
– | 0.01–0.35 pM | [82] |
| Cd(II), Pb(II), Cu(II), Hg(II) | Co-TIC4R-I | SWASV | Cd: 17 nM Pb: 8 nM Cu: 16 nM Hg: 7 nM |
Yes | Cd: 0.10–17.00 μM Pb: 0.05–16.00 μM Cu: 0.05–10.00 μM Hg: 0.80–15.00 μM |
[83] |
| Cd(II) Pb(II) |
CUiO-66/Bi/GCE | SWASV | Cd: 1.16 µg/L Pb: 1.14 µg/L |
Yes | Cd: 10–50 µg/L Pb: 10 – 50 µg/L |
[84] |
| Hg(II) | Zr-DMBD MOFs/3D-KSC | SWASV | 0.05 μM | Yes | 0.25–3.5 μM | [86] |
| Hg(II) | Cu-MOF | DPV | 0.063 nM | Yes | 0.1–50 nM | [87] |
| Cd(II), Pb(II), Cu(II) , Hg(II) | GaOOH-UiO-MOFs | DPV | Cd: 0.016 μM Pb: 0.028 μM Cu: 0.006 μM Hg: 0.019 μM |
Yes | Cd: 0.35–1.60 μMPb: 0.55–2.50 μMCu: 0.30–1.40 μMHg: 0.10–0.45 μM | [88] |
| Pb (II) Cu (II) |
NH2-MIL-53(Al)/PPy | DPV | Pb: 0.315 µg/L Cu: 0.244 µg/L |
Yes | 1–400 µg/L | [89] |
| As(III) | GO/UiO-67@PtNPs | SWASV | 0.48 nM | Yes | 2.7–33.4 nM | [90] |
| Cd(II) Pb(II) |
Co@NC/MWCNT | SWASV | Cd: 4.5 nM Pb: 4.9 nM |
No | 0.12–2.5 μM | [91] |
| Pb(II) Cd(II) |
Gly/rGO/PANI | SWASV | Pb: 0.07 nM Cd: 0.072 nM |
Yes | Pb: 0–1.0 μM Cd: 0 –1.0 μM |
[92] |
| Cd(II) Pb(II) |
Bi-PPy/MWCNT/CPE | SWASV | Cd: 0.157 μg/L Pb: 0.099 μg/L |
Yes | Cd: 0.16–120 μg/L Pb: 0.11–120 μg/L |
[93] |
| Cd(II), Pb(II), Cu(II) | rGO/Ala/PANI | SWASV | Cd: 0.03 nM Pb: 0.063 nM Cu: 0.045 nM |
Yes | 80 pM–100 nM | [94] |
| Hg(II) | Pt/g-C3N4/PTh NCs | DPV | 0.009 nM | No | 1–500 nM | [95] |
| Hg(II) | Pt/g-C3N4 /PANI NCs | DPV | 0.014 nM | No | 1–500 nM | [96] |
| Cd(II) | 3DGO-Py10 | SWASV | 3.6 μg/L | Yes | 5–400 μg/L | [97] |
| Mn(II) | PMMA–SWCNT NCs/GCE | DPV | 92.67 ± 4.63 pM | 7 days | 0.1 nM – 0.01 mM | [98] |
| Cu(II) | EDTA-PANI/SWNTs | DPV | 1.4 μM | Yes | 0–2 nM | [102] |
| Pb(II) and Cu(II) | NH2-UiO-66@ZIF-8/ /multi-walled carbon nanotubes | DPV | Pb: 1 nM Cu: 10 nM |
21 days, Yes, Yes | Pb: 0–80 mM Cu: 0–50 mM |
[123] |
| Hg(II), Pb(II), Cu(II) | ZnFe2O4 | DPASV | Hg: 1.61 nM Pb:7.38 nM Cu:12.03 nM |
7 days, No, Yes | 0.1–1 mM | [124] |
| Pb(II) | Gold modified graphene | Amperometric | 1.67 pM | 14 days, No, Yes | 1 nM–1 mM |
[125] |
| Pb(II), Cd(II) | GO-Fe3O4-PAMAM | SWASV | Pb: 130 ng/L Cd: 70 ng /L | 3 weeks, Yes, Yes |
Pb: 0.4–120 μg/L Cd: 0.2–140 μg/L |
[126] |
| Cd(II), Pb(II) | porous graphene/carboxymethyl cellulose/fondaparinux | SWASV | Cd: 0.28 nM Pb: 0.17 nM |
20 days, No, Yes | 2 – 20 nM | [127] |
| Cu(II), Cd(II), Hg(II) | reduced graphene oxide/silver | DPV | Cu: 10−15 M Cd: 10−21 M Hg: 10−29 M | –, No, No | – | [128] |
| Zn(II), Cd(II), Pb(II), Cu(II), Hg(II) | FGP/AuNC | SWASV | Zn: 0.08 μg/L Cd: 0.09 μg/L Pb: 0.05 μg/L Cu: 0.19 μg/L Hg: 0.01 μg/L | 30 days, Yes, Yes | Zn: 6–7000 μg/L Cd: 4–6000 μg/L Pb: 6–5000 μg/L Cu: 4–4000 μg/L Hg: 6–5000 μg/L |
[130] |
| Hg(II) | SN-rGO | SWASV | 8.93 nM | twelve successive cycles, No, Yes | 0.4–12 000 nM | [133] |
| Cd(II), Cu(II), Hg(II) | NCO/N, S-rGO | DPASV | Cd: 123 nM Cu: 14.4 nM Hg: 67 nM |
– | – | [134] |
| Cd(II), Pb(II), Cu(II), Hg(II) | rGO/ZnO-NPs-EDTA | SWV | Cd: 5.6 μM Pb: 6.8 μM Cu: 2.5 μM Hg: 10 μM |
–, No, Yes | Cd: 18.5–500 μM Pb: 22.4–700 μM Cu: 8.3–200 μM Hg: 3.3–300 μM |
[135] |
| Pb(II) | rGO/MoS2/CS | SWASV | 0.0016 μM | 15 days, No, Yes | 0.005–0.05–2.0 μM | [136] |
| Cd (II) | SnO2@BCG | DPV | 1 × 10−4 ppm | – | 0.001−0.4 ppm | 30 Days, No, Yes |
| Pb(II) | CS/rGO/TiO2 | DPV | 0.33 ng /L | Yes, Yes, Yes | 1 ng–1000 ng/L | [138] |
| Pb(II) | rGO/AuNPs/ssDNA | CV | 1.52 nM | 30 days, No, Yes | 5–50 nM | [139] |
| Pb(II), Cd(II) | Bi/g-C3N4 | SWASV | Cd: 21.8 μg /L Pb: 10.4 μg /L | No, No, No | Cd: 30 –120 μg/L Pb: 30 – 110 μg/L |
[140] |
| Pb(II) | Au/N-deficient-C3N4 | SWASV | 0.029 μM | Yes, No, Yes | 0.2–0.8 μM | [141] |
| Cd(II), Hg(II), Pb(II), Zn(II) | g-C3N4/O-MWCNTs | DPSV | Hg: 0.04 ng/L Pb: 0.008 ng/L Cd: 0.03 ng/L Zn: 0.06 ng/L |
Yes, No, Yes | Hg: 4.8 – 93.0 ng/L Pb: 6.5 – 110 ng/L Cd: 4.25 – 79.0 ng/L Zn: 4.2 – 202.0 ng/L |
[142] |
| Cr (VI), Cu (II), Pb(II) | carbon dots@ graphitic-carbon nitride(CDs@g-C3N4) nanocomposite | Fluorescence | Cr: 0.54 nM Cu: 0.18 nM Pb: 0.2 nM |
– | Cr: 0 –10 nM Cu: 0 –10 nM Pb: 0 – 50 nM |
[143] |
| Cr(VI) | graphene carbon nitride doped silver molybdate immobilized nafion (g-C3N4/AgM/Nf | CV | 0.0016 μM | 15 days Yes, Yes | 10 – 100 μM | [144] |
| Cd(II), Pb(II), Hg(II) | metal-free g-C3N4/carbon black (CB) composite | DPASV | Cd: 2.1 nM Pb: 0.26 nM Hg: 0.22 nM |
–, No, Yes | Cd: 0–700 nM Pb: 0–300 nM Hg: 0–500 nM |
[145] |
| Cd(II), Pb(II), Hg(II) | metal-free g-C3N4/carbon black composite | DPASV | Cd: 2.1 nM Pb: 0.26 nM Hg: 0.22 nM |
–, No, Yes | Cd: 0–700 nM Pb: 0–300 nM Hg: 0–500 nM |
[146] |
| Cd(II), Pb(II) | Fe3O4/Bi2O3/C3N4/GCE | SWASV | Cd: 3 nM Pb: 1 nM |
15 days, Yes, Yes | 0 – 3 µM | [147] |
| Pb(II), Cd(II) | M/g-C3N4/ASPE | DPV | Pb: 0.008 µM Cd: 0.02 µM | 7 days, No,Yes | 0.1 – 1.0 μM | [148] |
| Cd(II), Pb(II) | pg-C3N4/CoMn2O4 | SWASV | Cd: 0.021 μM Pb: 0.014 μM | excellent stability, Yes, Yes | Cd: 0.5–7.0 μM Pb: 0.2–4.4 μM |
[149] |
| Cd(II) | MnO2/rGO | DPASV | 1.12 μg/L | 14 days | 4.0 – 130 μg/L | [150] |
| Pb(II), Cd(II), Cu(II), Hg(II) | NiO/rGO | SWASV | 0.01 μM | –, No, Yes | - | [151] |
| Cd(II), Pb(II) | r-CeO2/EG composite | DPV | Cd: 0.39 μg/L Pb: 0.21 μg/L |
–, No, No | 0–100 μg/L | [153] |
| Hg(II) | sepiolite/pyrite (Sep/FeS2) | SWASV | 4.12 nM | good reproducibility | 10 – 120 nM | [154] |
| Pb(II) | α-Fe2O3/NiO heterostructure | SWASV | 0.02 μM | 30 days , No, good reproducibility | 0.05 – 0.9 μM | [155] |
| Hg(II) | Co3O4/ZnO | SWASV | 0.3 µM | 20 days, No, No | 0 – 2.1 µM | [156] |
| Pb(II), Cu(II), Hg(II) | Zr/ZrO2 | DPV | Pb: 0.8 nM Cu: 0.5 nM Hg: 0.4 nM |
– | Pb: 0.8 nM – 10 µM Cu: 0.5 nM – 2 µM Hg: 0.4 nM –10 µM |
[157] |
| Cd(II), Pb(II) | SnS-Bi2O3 | SWASV | Cd: 1.50 nM Pb: 1.40 nM |
–, No, No | 0 – 1 µM | [158] |
| Hg(II) | Ru/CeO2 | SWASV | 0.019 μM | –, No, No | 0 – 0.9 µM | [159] |
| Pb(II), Hg(II) | MgO–SiO2 | SWASV | Pb: 0.019 μM Hg: 0.041 μM | – | – | [160] |
| Pb(II), Cd(II) | Fe3O4@G2-PAD | SWASV | Pb: 0.17 µg/L Cd: 0.21 µg/L | – | 0.5 – 80 µg/L | [161] |
| Hg(II) | CuO/PVA | DPV | 0.42 nM | –, No, Yes, | 10–70 μM | [162] |
| Pb(II), Cu(II) |
Cerium oxide | CV, DPV | Pb: 0.6 ppb Cu: 0.3 ppb |
No, Yes, No | Pb: 0.6 – 12 ppb Cu: 0.3 –- 10 ppb |
[163] |
| Pb(II) | Fe3O4@ PDA@ MnO2 | DPV | 0.03 μg/L | 28 days, Yes, No | 0.1 – 150 μg/L | [164] |
| Cd(II), Pb(II), Cu(II) |
Zn/Fe nanocomposite | CV | Cd: 0.14 mg/L Pb: 0.07 mg/L Cu: 0.04 mg/L |
No, No, Yes | 0 – 16.5 mg/L | [165] |
| Cd(II), Pb(II) | Fe2O3/Bi2O3 | SWASV | Cd: 0.56 nM Pb: 0.36 nM |
21 days, Yes, Yes | 0.002 – 4 μM | [166] |
| Zn(II), Cd(II), Pb(II) |
Bi/Chitosan | SWASV | Zn: 0.1 ppb Cd: 0.1 ppb Pb: 0.2 ppb |
No, Yes, Yes | Zn: 1 – 5 ppb Cd: 1 – 5 ppb Pb: 1 – 10 ppb |
[168] |
| Cu(II) | Chitosan/GO | DPASV | 0.15 μM | No, Yes, Yes | 0.5 – 100 μM | [169] |
| Cd(II) | Chitosan/Au/Graphene | DPV | 0.162 nM | 30 days, Yes, No | 0.1 – 0.9 μM | [170] |
| Pb(II) | PVA/Chitosan/rGO | SWASV | 0.05 ppb | No, Yes, Yes | 1 – 50 ppb | [171] |
| Pb(II), Cd(II) | Bi/MXene | SWASV | Pb: 10.8 nM, Cd: 12.4 nM |
42 days, Yes, Yes | Pb: 0.06 – 0.6 μM Cd: 0.08 – 0.8 μM |
[172] |
| Pb(II), Cd(II), Zn(II) |
Bi/MXene | SWASV | Pb: 0.2 μg/L Cd: 0.4 μg/L Zn: 0.5 μg/L |
14 days, Yes, Yes | 1 – 20 μg/L | [173] |
| Cd(II), Pb(II) | Ti3C2 MXene/ Carbon heterostructure | SWASV | Cd: 2.55 nM, Pb: 1.10 nM |
7 days, Yes, Yes | Cd: 0.1– 8 μM, Pb: 0.25 – 2 μM |
[177] |
| Zn(II), Cd(II), Pb(II) |
Melamine/rGO/MXene aerogel | DPASV | Zn: 0.48 μg/L Cd: 0.45 μg/L Pb: 0.29 μg/L |
No, Yes, Yes | 3–900 μg/L | [178] |
| Cd(II), Pb(II), Cu(II),Hg(II) |
Alk- Ti3C2 | SWASV | Cd: 0.098 μM Pb: 0.041 μM Cu: 0.032 μM Hg: 0.130 μM |
21 Days, No, Yes | Cd: 0.1 – 1 μM, Pb: 0.1 – 0.55 μM Cu: 0.1 – 1.4 μM Hg: 0 – 1.9 μM |
[180] |
| Cd(II), Pb(II), Cu(II), Hg(II) | AgNs/SPCE | DPSV | Cd: 0.4 ppb Pb: 2.5 ppb Cu: 7.3 ppb Hg: 0.7 ppb |
–, No, Yes | Cd: 5 – 300 ppb Pb: 5 – 300 ppb Cu: 50 – 500 ppb Hg: 5 –100 ppb |
[184] |
| Cd(II), Pb(II) | FeNi3/ CuS/ BiOC | SWASV | Cd: 0.4 µg/L Pb: 0.1 µg/L |
30 days, Yes, Yes | Cd: 1 – 150.0 µg/L Pb: 0.5 – 120.0 µg/L |
[186] |
| Pb(II) | Cu-chitosan nanocomposite | SWSAV | 0.72 ppb | – | 0 – 60 ppb | [187] |
| Pb(II), Cd(II) | BiNPs/CoFe2O4 | SWASV | Pb: 7.3 nM Cd: 8.2 nM |
42 days, No, Yes | Pb: 0.06 – 0.6 µM Cd: 0.08 – 0.8 µM |
[188] |
| Pb(II), Hg(II) | Ni NMO - GR | SWASV | Pb: 0.050 µM Hg: 0.027 µM |
– | Pb: 1.4 – 7.7 µM Hg: 0.7 – 607 µM |
[189] |
| Pb(II) | Ternary nanocomposites CNW,CNW:Ag, AgNPs |
EIS | 10 nM | – | 10 nM – 1 mM | [191] |
| Cd(II), Cu(II), Hg(II), Pb(II) | Mg(II)/Al(II) | SWASV | Cd: 250 ng/L Cu: 25 ng/L Hg: 250 ng/L Pb: 16 ng/L |
–, Yes, Yes | Cd: 0.5 – 0.20 µg/L Cu: 0.05 – 0.20 µg/L Hg: 0.5 – 20 µg/L Pb: 0.05 – 0.20 µg/L |
[192] |
| Cd(II) | CA functionalized ZnO | CV, SWV | 0.41 µM | 30 days, No, No |
0.1 – 0.50 µM | [193] |
| Pb(II), Cu(II) | BFS | DPV | Pb: 0.084 µM Cu: 0.44 µM |
– | 0–80 µM | [194] |
| Cu(II) | Ni/NiO/MoO3/Chitosan | DPV | 5.69 nM | – | 0 – 25 µM | [195] |
| Pb(II) | Aptazyme driven DNA | DPV | 0.034 nM | 28 days | 0 – 0.5 µM | [196] |
| Cr(VI) | polyoxometalates | DPV | 0.174 µM | – | 2 – 2.61 mM | [197] |
| Cu(II) | Eggshell membrane | DPV | 0.63 µM | – | 1 – 300 µM | [198] |
| Cu(II) | PIE/BP | SWASV | 0.02 µM | – | 0.25 – 177 µM | [199] |
| Cu(II), Hg(II) | CSs | DPASV | 405 nM | – | 12.5 nM | [200] |
| As(III) | Ag, Au alloy NPs | DPV | 0.003 µg/L | –, Yes, Yes | 0.01 – 10 µg/L | [201] |
| Pb(II) | Ag, Au alloy NPs | CV, DPV | 0.3 ng/L | 20 days, No, Yes |
0.01 – 10 µg/L | [202] |
| Hg(II) | DETTDC2 | Amperometric | 12.80 ± 0.64 pM |
Yes | 0.1 nM – 0.01 M | [106] |
| As(III) | Ag@SiO2/PANI NFs | SWASV | 0.013 μg/L | Yes | 0.1 – 100 μg/L | [107] |
| Pb(II) | rGO@CNT@Fe2O3/GCE | SWASV | 0.1 nM | Yes | 0.02 – 0.26 μM | [108] |
| Pb(II) | PPy/CNFs/CPE | SWASV | 0.05 μg/L | Yes | 0.2 – 130 μg/L | [109] |
| Pb(II) | sGO/PPy-SPE | DPASV | 0.07 ppb | Yes | 1.4−28 ppb | [110] |
| Pb(II) | PPy/CNT/NH2-ITO | DPV | 2.9 nM | No | 10 nM – 0.1 µM | [111] |
| Cd(II), Pb(II) | GO@Fe3O4@2-CBT | SWASV | Cd: 0.03 µg/L Pb: 0.02 µg/L | Yes | 0.08 – 90 µg/L | [112] |
| Pb(II), Cu(II), Cd(II) | ITO-AP-PPy-ABS | DPV | PB: 11.1 nM Cu: 8.95 nM Cd: 0.99 nM |
Yes | – | [114] |
| Pb(II | DTA-Ppy/SWNTs | DPV | 0.07 μM | Yes | 0.15 – 800 μM | [115] |
| Cd(II), Pb(II), Cu(II), Hg(II) | Fe3O4/F-MWCNTs | SWASV | Cd: 0.05 nM Pb: 0.08 nM Cu: 0.02 nM Hg: 0.05 nM |
Yes | Cd: 0.5–30.0 μM Pb: 0.5–30.0 μM Cu: 0.5–30.0 μM Hg: 0.5–20.0 μM |
[116] |
| Cd(II), Pb(II) | Fe3O4/MWCNTs/LSG/CS/GCE | SWASV | Cd: 0.1 μg/L Pb: 0.07 μg/L |
No | 1 –200 μg/L | [117] |
| Cd(II), Pb(II) | Sb2O3/MWCNTs | LSASV | Cd: 11.23 ppb Pb: 2.68 ppB |
– | Cd: 80–150 ppb Pb: 5–35 ppb |
[118] |
| Hg(II) | Sr@FeNi-S/SWCNTs | DPV | 0.52 nM | Yes | 0.05–279 μM | [119] |
| Fe3+ | PPCOT/NF/C-SWCNT | Amperometric | 97.08 ± 4.85 pM | Yes | 0.1 nM – 0.01 mM. | [120] |
| Zn(II), Cd(II), Pb(II), Cu(II), | BiNP/MWCNT-NNaM/PGE | SWASV | Zn: 0.707 µM Cd: 0.097 µM Pb: 0.008 µM Cu: 0.157 µM | – | Zn: 2.36–40; 40–180 µM Cd: 0.32–2; 2–240 µM, Pb: 0.03–5; 5–80 µM Cu: 0.52–10; 10–40 µM |
[121] |
| Cd(II) | CNTs-UiO-66-NH2/GCE | DPV | 0.2 μM | Yes | 0.3 – 150 μM | [122] |
| Cd(II), Pb(II) | MOF-derived BCN material. | SWASV | Cd: 0.41 μg/L Pb: 0.93 μg/L |
good repeatability, stability, and specificity |
Cd: 1–150 μg/L Pb: 2–150 μg/L |
[46] |
| Pb(II) | transition metal ion assisted PVG | PVG | 0.005 ng/g | – | 0.005 – 100 ng/g | [47] |
| Pb(II) | Polytetrafluoroethylene (PTFE) | AAS | 0.2 µg/L | – | 0.0–8.0 µg/L | [48] |
| As(III)) | 5,10,15,20-tetrakis (4-methoxyphenyl) porphyrinatocobalt(II) (TMOPP-Co) | FET | >10-10 M | – | 0.1 nM – 0.1 mM | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





