Introduction
S-Adenosylmethionine (SAMe) aka adenosylmethionine (AdoMet) is the second-most used enzyme substrate after ATP. It is used by over 200 methyltransferase enzymes to methylate histone and non-histone proteins, nucleic acids (DNA and RNA), phospholipids, hormones, and small molecules1.
SAMe is important because recent research has demonstrated that differential methylation of DNA is the primary driver of the epigenome, which determines which genes are silenced (DNA promoter region is hypermethylated) and which are activated (DNA promoter region is hypomethylated). The folate and methionine cycles are the primary determinants of SAMe status and control of the epigenome via hypomethylation or hypermethylation. MTHFR is the primary enzyme determining the status of these intertwined cycles. MTHFR mutations, common in the general public, play a vital role in the phenotypic expression of many diseases, including LC, CFS, POTS, MCAS, SIBO, and EDS.
Discussion
1. MTHFR and Methylation
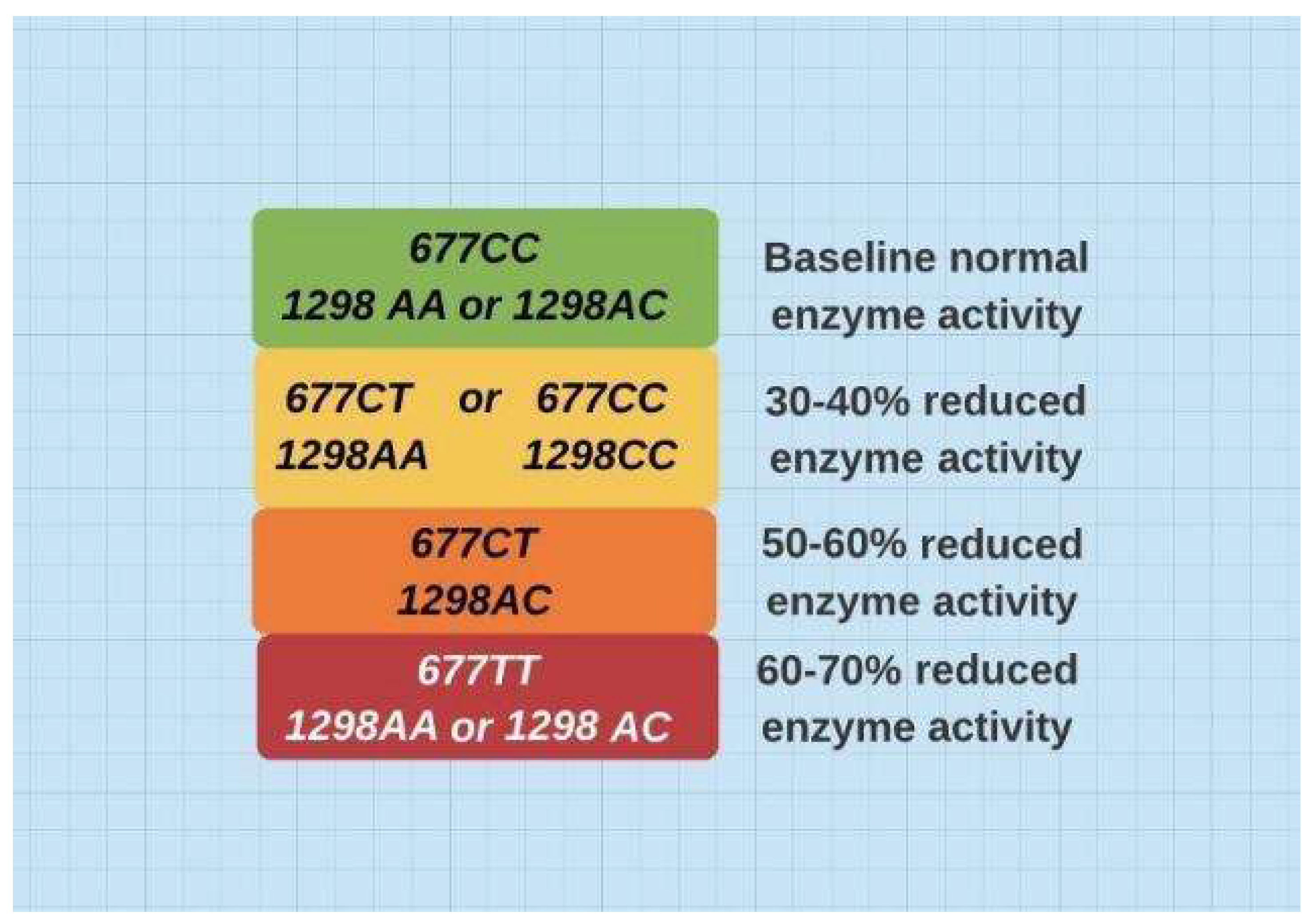
The healthy wild type MTHFR gene is 1298AA/677CC. There are at least 30 variants of MTHFR, but 1298C and/or 677T represent over 90% of the abnormal MTHFR single nucleotide polymorphisms in the general population. Abnormal MTHFRs plague many. According to the NIH
2, about ⅓ of Caucasians/Asians and about 10% of Africans are heterozygous for 677T. About ⅓ of Europeans, ¼ of Asians, and ⅙ of Africans are heterozygous for 1298C
3. Genotype roughly predicts activity of the enzyme (see
Figure 1). The defective gene creates a state of hypomethylation and differential DNA methylation controls the epigenome. A single gene can be promoted or suppressed, depending on the state of methylation.
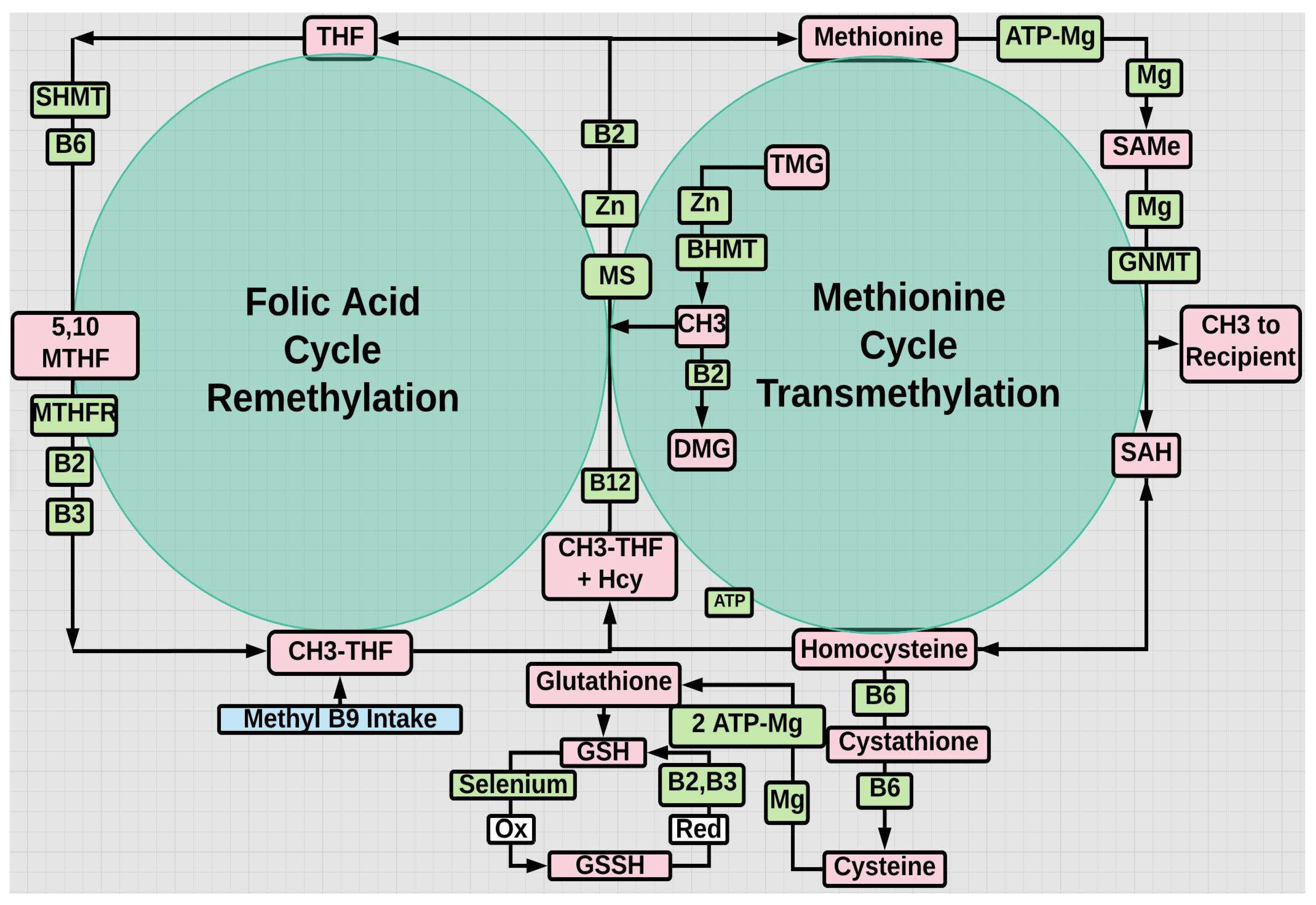
Furthermore, phenotypic expression is greatly influenced by presence or not of required cofactors. Riboflavin (B2), as flavin adenine dinucleotide (FAD) is a required cofactor for MTHFR and methionine synthase
5. But B3,6,9 (folate),12 have significant impact on other enzymes in the two cycles
6 (see
Figure 2).
Those with abnormal MTHFR experience mild to moderate symptoms from Covid-19 and are hypomethylated, whereas those with LC tend to be hypermethylated7. Although hypomethylation is less likely in African-Americans on a genetic MTHFR basis, it is more likely on a vitamin D deficient basis, which increases the risk of hypomethylation8.
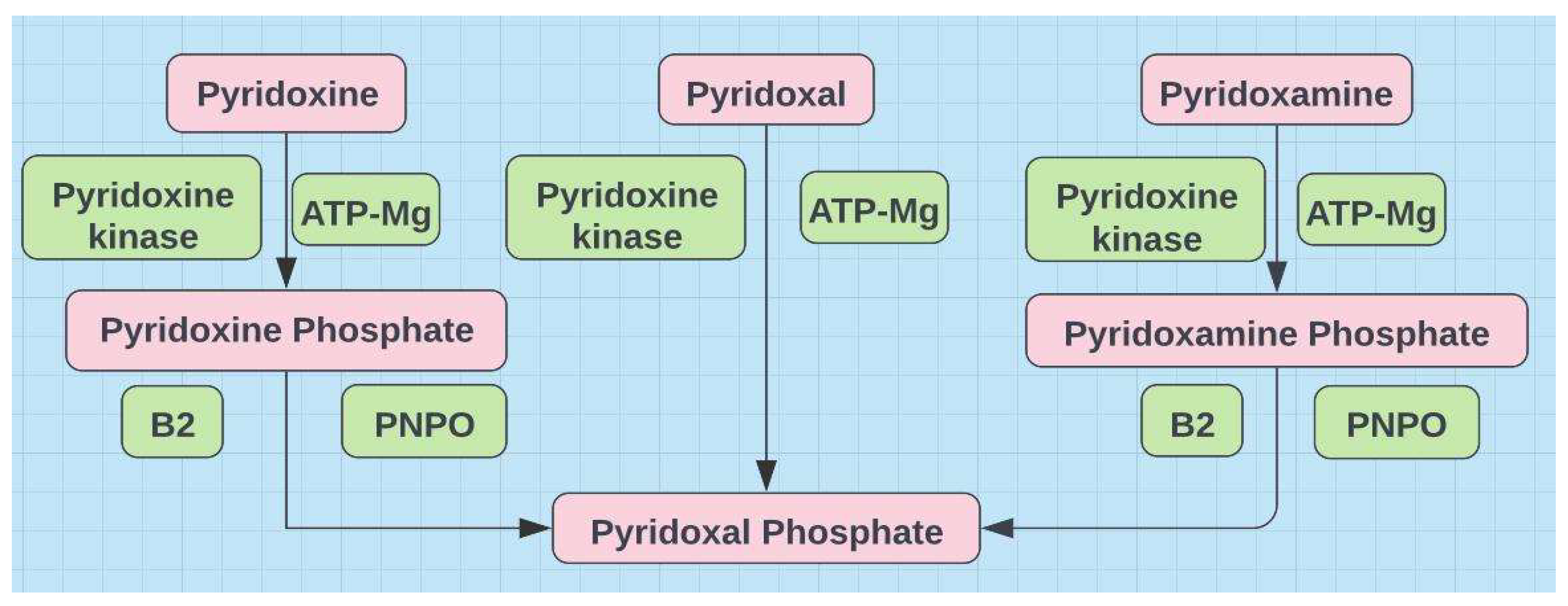
Activation of B6 requires not only ATP-Mg dependent phosphorylation but also riboflavin dependent pyridoxine phosphate oxidase (PNPO), the rate limiting step
9 (see
Figure 3)
SAMe is the universal methyl donor. To produce one molecule of SAMe requires methionine, an ATP-Mg++ complex, methionine adenosyltransferase and an additional Mg++ as cofactor. Furthermore, the enzyme that frees the CH3 group from SAMe, glycine-N-methyltransferase, also requires Mg++ as a cofactor. In fact all methyltransferase activities require magnesium10.
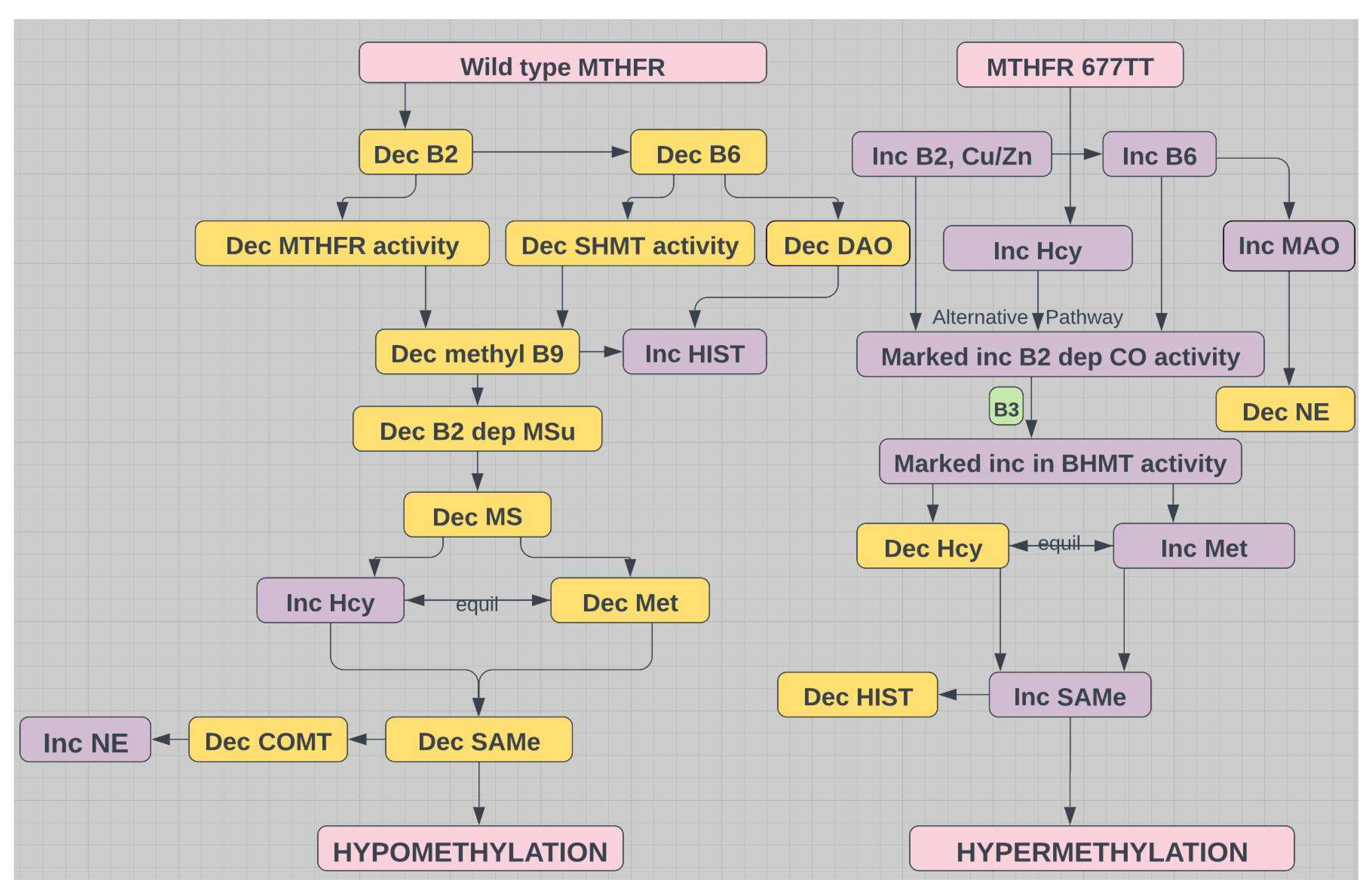
Methylation or transfer of a CH
3 group is a complex topic. There mustn’t be too much or too little and the inputs for either are myriad, e.g., diet, lifestyle, genes. Maintaining a balance between hypomethylation and hypermethylation is precarious
11. The balance is impacted by magnesium status. If deficient, hypomethylation predominates (see
Figure 4). However, 1,25(OH)
2D3 (active form of D) is critical to preventing hypermethylation, as it increases the expression of DNA demethylases that prevent hypermethylation of multiple gene promoter regions
12,13.
2. Vitamins D and B, Magnesium and Zinc
Covid-19 is worse and LC is more frequent in the vitamin D deficient
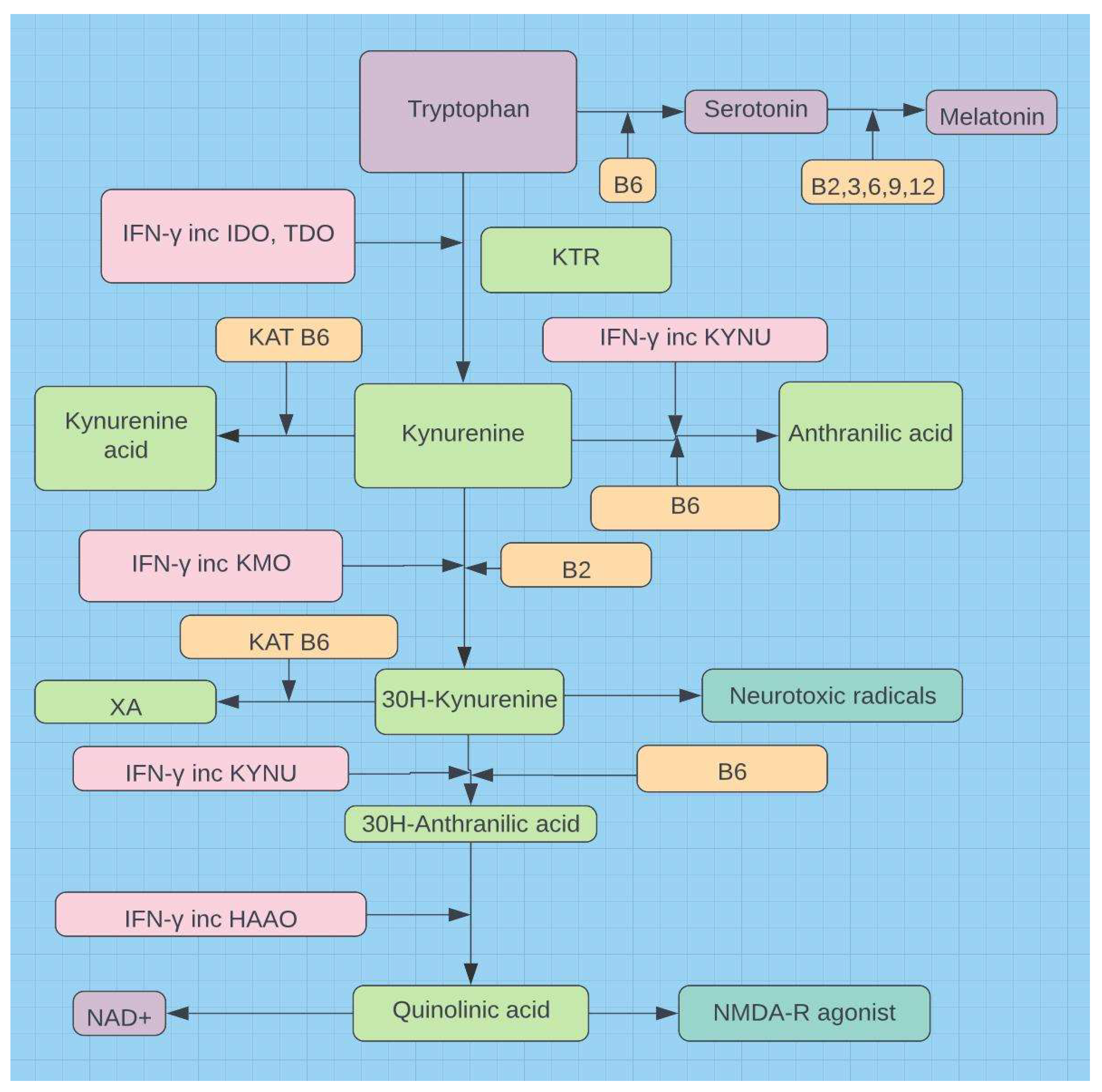
14,15. Vitamin D deficiency is widespread even at the serum level of 30 ng/mL, recommended by the National Academy of Medicine. A much more realistic target is 50 ng/mL, underscored by numerous clinical studies and physiologic reviews demonstrating this via biochemical analysis of the interactions between PTH, calcium, and 25(OH)D3. The latter is the storage form of D and the analyte used to assess adequacy. On the other hand, riboflavin (B2) deficiency is never mentioned in any top ten list of nutrient shortfalls. But its deficiency is only likely to occur after prolonged chronic inflammation. It is probably the first vitamin to be consumed during inflammation and mitochondrial overload, as tryptophan, driven by IFN-ɣ, is stolen from synthesis of serotonin and melatonin and redirected via the kynurenine pathway to produce more NAD
+ (see
Figure 5 and section on Tryptophan).
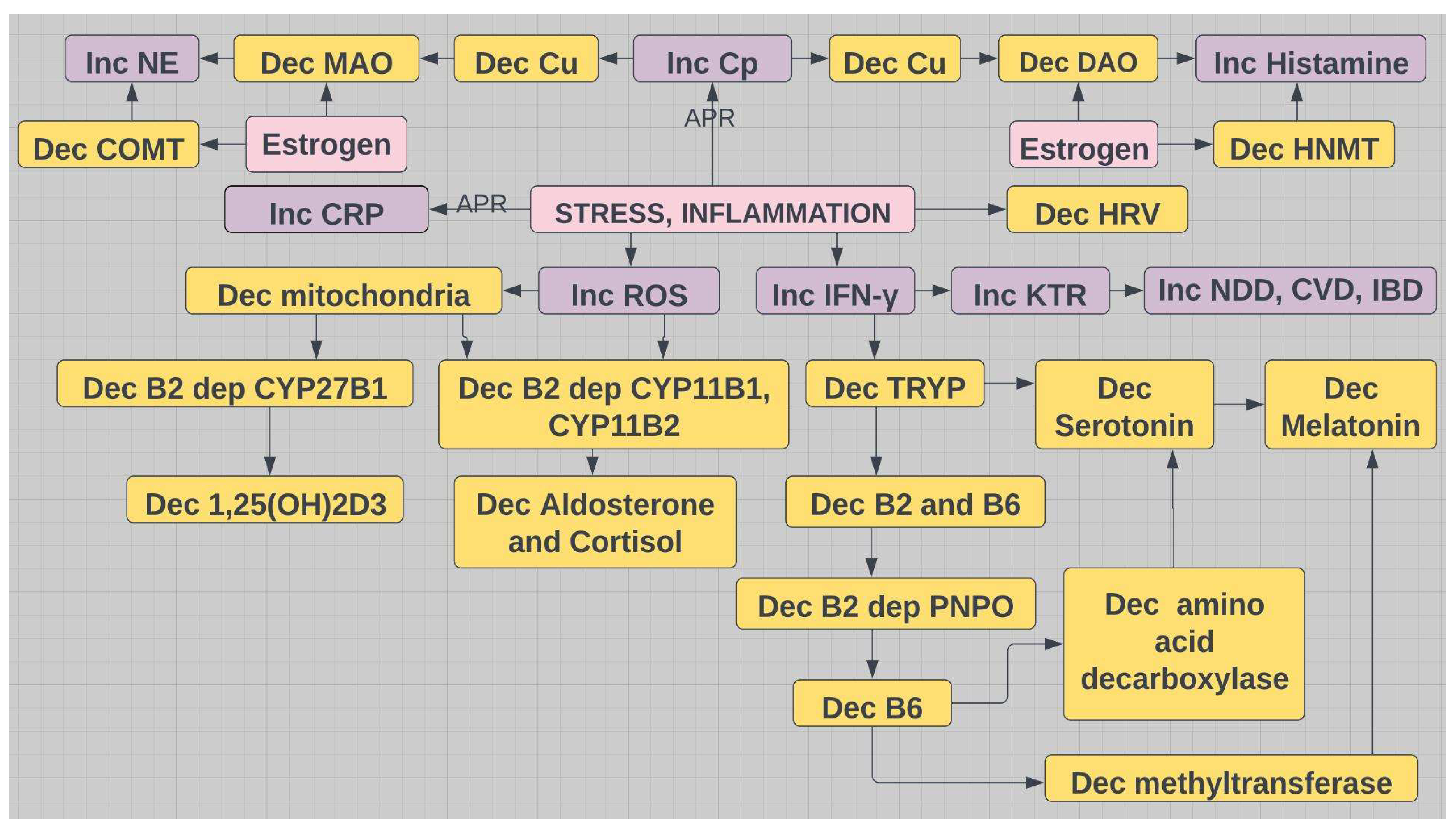
Active forms of both B2 and B6 are easily consumed with detrimental effects on MTHFR. Under such conditions B2 and D become tightly linked. The storage form of D must be hydroxylated to attain active status. CYP27B1 is the only enzyme that can do this, mitochondria are the only organelles that are suitable for the reaction
17,18, and B2 as FAD (in addition to Mg
++) is a required cofactor. Furthermore, one of the most pronounced effects of the active form (1,25(OH)
2D3) is increased synthesis of CYP24A1, which degrades both the active form and the storage form
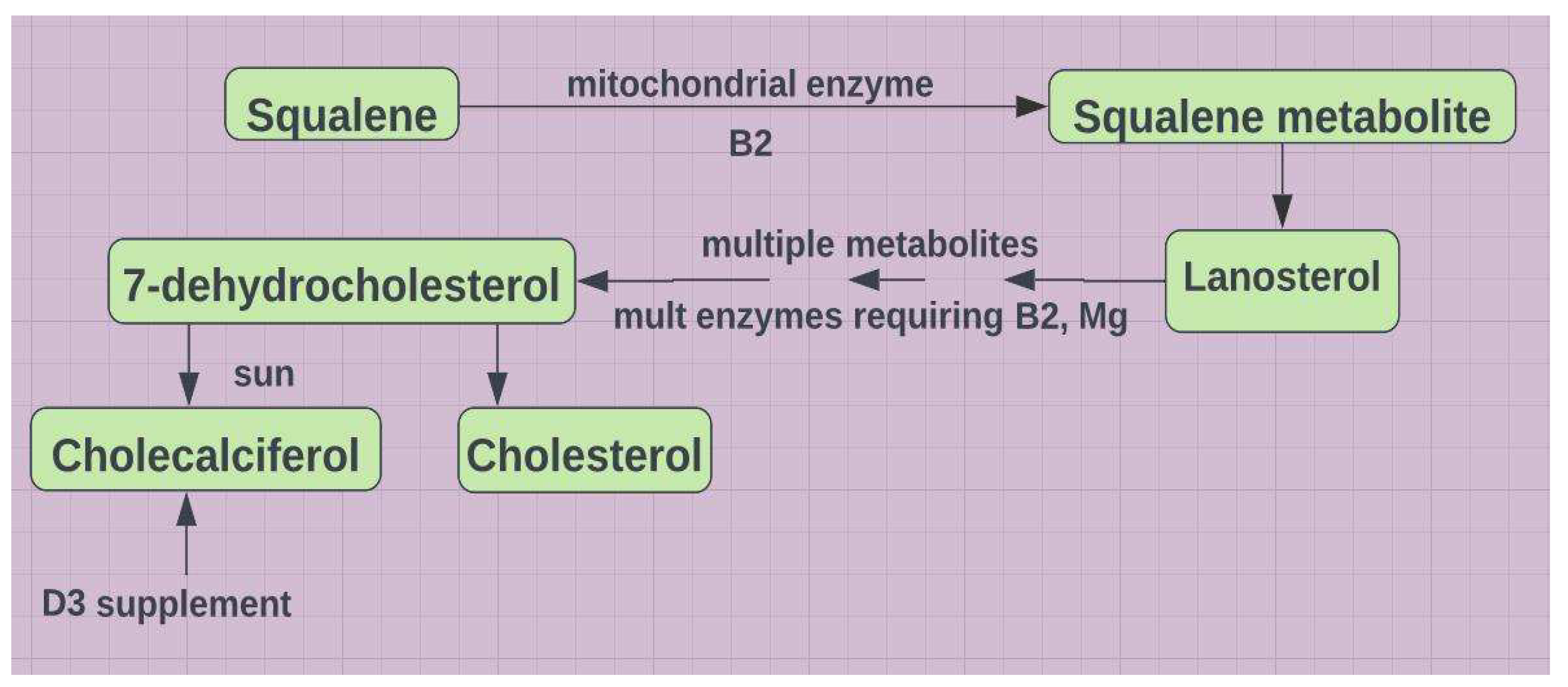
19. Oxidative stress, compromised mitochondria, and riboflavin/magnesium deficiency translate to less of the active form. Accordingly, the storage form of D may overestimate the status of its active form. This overestimate is directly proportional to the degree of mitochondrial dysfunction. Under such conditions perhaps a 50ng/mL target is insufficient. Mitochondrial dysfunction compromises not only synthesis of the active form of vitamin D, but also synthesis of the precursors to D3. Formation of 7-dehydrocholesterol, the precursor to D3 of solar origin, requires intra-mitochondrial flavin dependent enzymes (see
Figure 6).
Stress induces release of norepinephrine and glucocorticoids. Both increase oxidative stress20,21. Aldosterone and cortisol are synthesized in mitochondria. They are both magnesuric. Exercise is good, but cationic sodium lost in sweat is replaced by excreting Mg++ and K+. Dehydration exacerbates this.
B2 is not only a required cofactor for the synthesis of the active form of vitamin D, but also a required cofactor for the synthesis of B6. Therefore all B6 dependent enzymes (see
Figure 3) are B2 dependent, unless B6 is taken as P5P aka PLP. Indeed B2 may be the limiting nutrient for maintaining vitamin B6 status, particularly in individuals with the MTHFR 677TT genotype. MTHFR also requires B3 as cofactor
22. B2 and B6 have an oversized impact on the synthesis of glutathione, the master antioxidant (see
Figure 2). However, the active forms of other B vitamins, especially B6,9,12, figure prominently in the folate and methionine cycles (see
Figure 2). In view of the prevalence of MTHFR problems, they deserve specific attention. Both B9 and B12 must be methylated for activation. Ironically, abnormal MTHFRs only yield hypomethylation, unless Hcy significantly increases and triggers the alternative pathway (see
Figure 2) and hypermethylation (see
Figure 4).
Synthesis of FAD from riboflavin and NAD from niacin requires phosphorylation to reach their activated states. Phosphorylation requires ATP-Mg++. If the supply of ATP from mitochondria or magnesium from diet is marginal, then the activated forms (B1,2,3,6) are compromised.
The Cu/Zn ratio is also important with regard to the alternative pathway. Because Western plumbing is primarily copper piping, copper is probably more readily ingested than zinc. When elevated, copper is pro-inflammatory. Zinc is generally both an antioxidant and anti-inflammatory
23. These divalent cations compete for absorption. An elevated Cu/Zn is directly proportional to Covid severity
24. But the heavy emphasis on Zn during the pandemic may have sparked a Cu shortfall in some
25 (see
Figure 7 and
Figure 8). It has also been shown that zinc deficiency and zinc excess cause cellular oxidative stress
26.
3. The Tryptophan Connection
Tryptophan is an essential amino acid and is the only precursor for the neurotransmitter serotonin, which is vital to mood, cognition, sleep, gastrointestinal function, and the gut-brain axis. Tryptophan is also the precursor for melatonin (serotonin pathway) and niacin (kynurenine pathway). The kynurenine pathway dominates 95% of tryptophan metabolism (see
Figure 5)
27.
Altered tryptophan metabolism characterizes LC
28 and is a marker for inflammaging
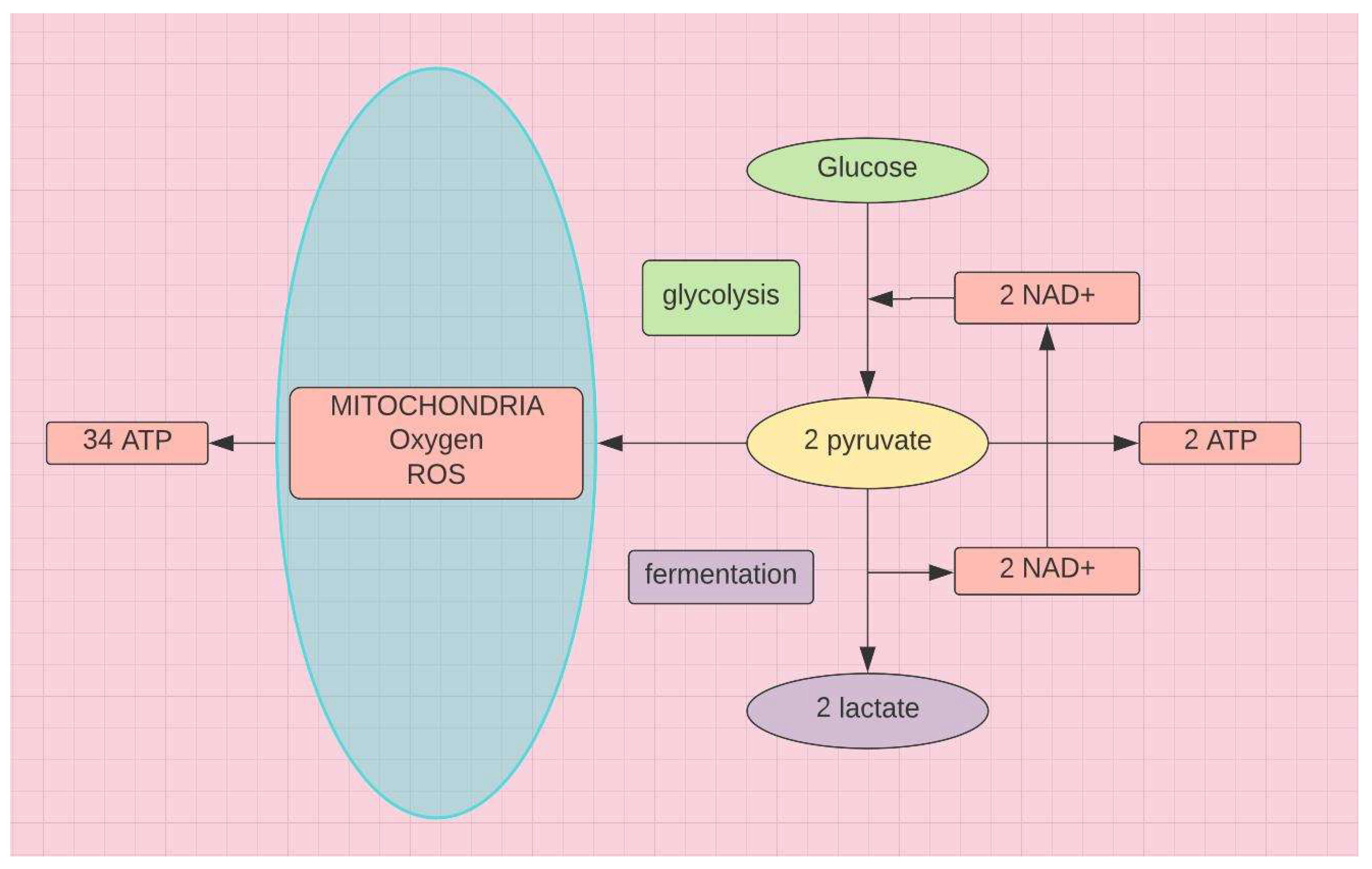
29. Acute or chronic inflammation induced by invading pathogens or other foreign toxins increases demand for energy. Mitochondria go into overdrive. They have the capacity to produce 34 ATPs from each molecule of glucose (see
Figure 9). But this requires oxidative phosphorylation that can generate excessive ROS, if onboard antioxidants are insufficient. Accordingly, to avoid cell lysis due to oxidative stress, mitochondrial activity decreases and the cell pivots to glycolysis and fermentation (see
Figure 9)
During elevated interferon-gamma (IFN-γ) activity, riboflavin and vitamin B6 may be depleted, given the role of these nutrients as cofactors for tryptophan catabolism, a metabolic process that is increased during IFN-γ-mediated immune activity (see
Figure 5)
30. Because FAD serves as a cofactor for MTHFR, riboflavin deficiency secondary to inflammation induced consumption can also contribute to increased homocysteine
31.
In pivoting from oxidative phosphorylation to fermentation to prevent oxidative stress induced cell lysis, ATP production is severely curtailed. Fermentation supplies the increased need for NAD
+, which initiates the tryptophan steal, driven by IFN-ɣ (see
Figure 5). Tryptophan, induced by IFN-γ from T cells and NK cells, is slowly depleted and metabolites increase during chronic immune activation
32. Depletion of tryptophan with increased metabolites (3-hydroxykynurenine and quinolinic acid) (see
Figure 2), increased tryptophan/kynurenine ratio (KTR), and reduced serotonin production contributes to the neurological sequelae of LC.
Females have higher levels of type I IFN (α and β)33. T cell production of IFN-ɣ (IFN type II) is triggered by IFN-α and -β, primary release IFNs. IFN-ɣ is a STAT dependent secondary release IFN34. T cell population decreases with age in males but not females. T cell production of IFN-γ persists in females35. This may in part explain greater longevity, increased frequency of autoimmune disease, and more frequent encounters with chronic fatigue syndromes in females.
4. LC, CFS, POTS, MCAS, SIBO, EDS
4-1. LC, CFS
The symptoms for this alphabet soup of syndromes appear to be linked by oxidative stress. LC and CFS are the poster childs for this. An antioxidant shortfall leads to an excess of mitochondrial reactive oxygen species. If unquenched, cell lysis is threatened. In order to avoid this mitochondria are down regulated and tryptophan is stolen from that earmarked for serotonin/melatonin synthesis and redirected to the synthesis of NAD (B3). Fatigue and brain fog in LC and CFS may in part be due to mitochondrial dysfunction and the neurotoxic metabolites of the kynurenine pathway respectively (see
Figure 5).
The very common MTHFR allele 677T generally results in hypomethylation and may activate genes expressed in EDS, MCAS, and SIBO. Hypermethylation generally characterizes LC7 and MTHFR 677 TT36 and hypomethylation generally characterizes mild to moderate Covid-197 and MTHFR 677T36.
Elevated free Cu is also characteristic of 677TT and hypermethylation36. Zinc deficiency is typically associated with hypomethylation, but may be seen in hypermethylation37, when copper is increased36. This suggests that the hypermethylation in LC may represent those homozygous for the 677T allele. Zn supplements in LC may prove helpful.
Hypermethylation and hypomethylation are demonstrated in CFS38. Although the connection between MTHFR variants and CFS is debated, both LC and CFS are linked by redox imbalance and elevated homocysteine39. Abnormal MTHFR is the most frequent cause of elevated homocysteine. Perhaps 677TT is linked to CFS with hypermethylation and the heterozygous 677T allele is linked to CFS with hypomethylation.
4-2. POTS
The vast majority of POTS exhibits low blood volume and is classified as either low flow or high flow type.
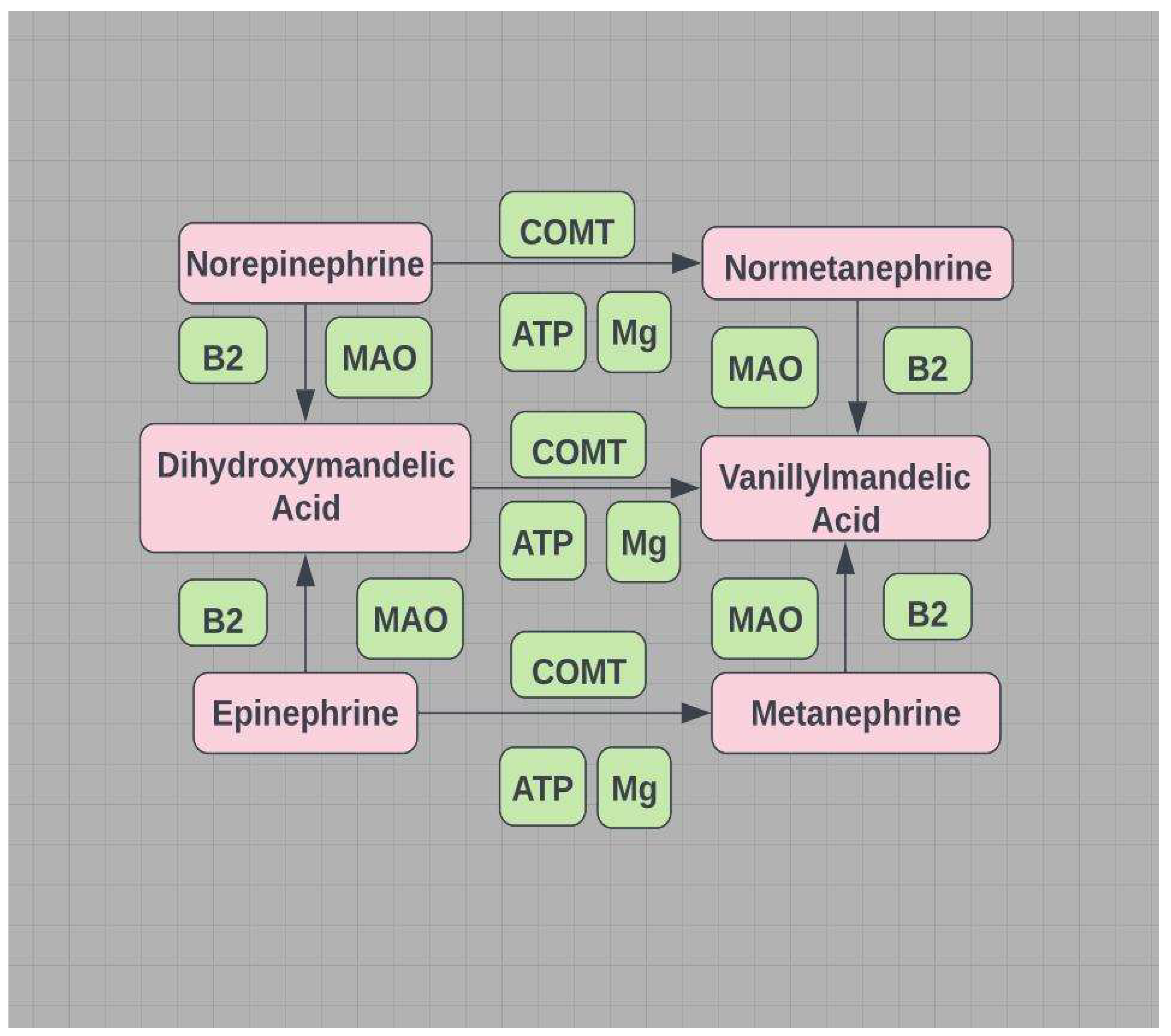
POTS (low flow) - vasoconstriction due to high norepinephrine; catecholamines are primarily degraded by B2,6 dependent MAO (mitochondrial) and Mg
++ dependent COMT (see
Figure 10).
A shortfall in the required ATP-Mg
++ compromises MAO/DAO (mitochondrial) and a shortfall in the required cofactor Mg
++ compromises HNMT (see
Figure 8). Estrogen down-regulates COMT/HNMT and MAO/DAO, increasing NE and low flow POTS or increasing histamine and high flow POTS (see Figure11). Gut dysbiosis is often associated with MCAS, SIBO, and EDS and impairs DAO activity
40. Dysbiosis and histamine secreting bacteria and/or impaired DAO may represent an additional incentive for those with MCAS, SIBO, and EDS to favor high flow POTS over low flow POTS (see
Figure 11).
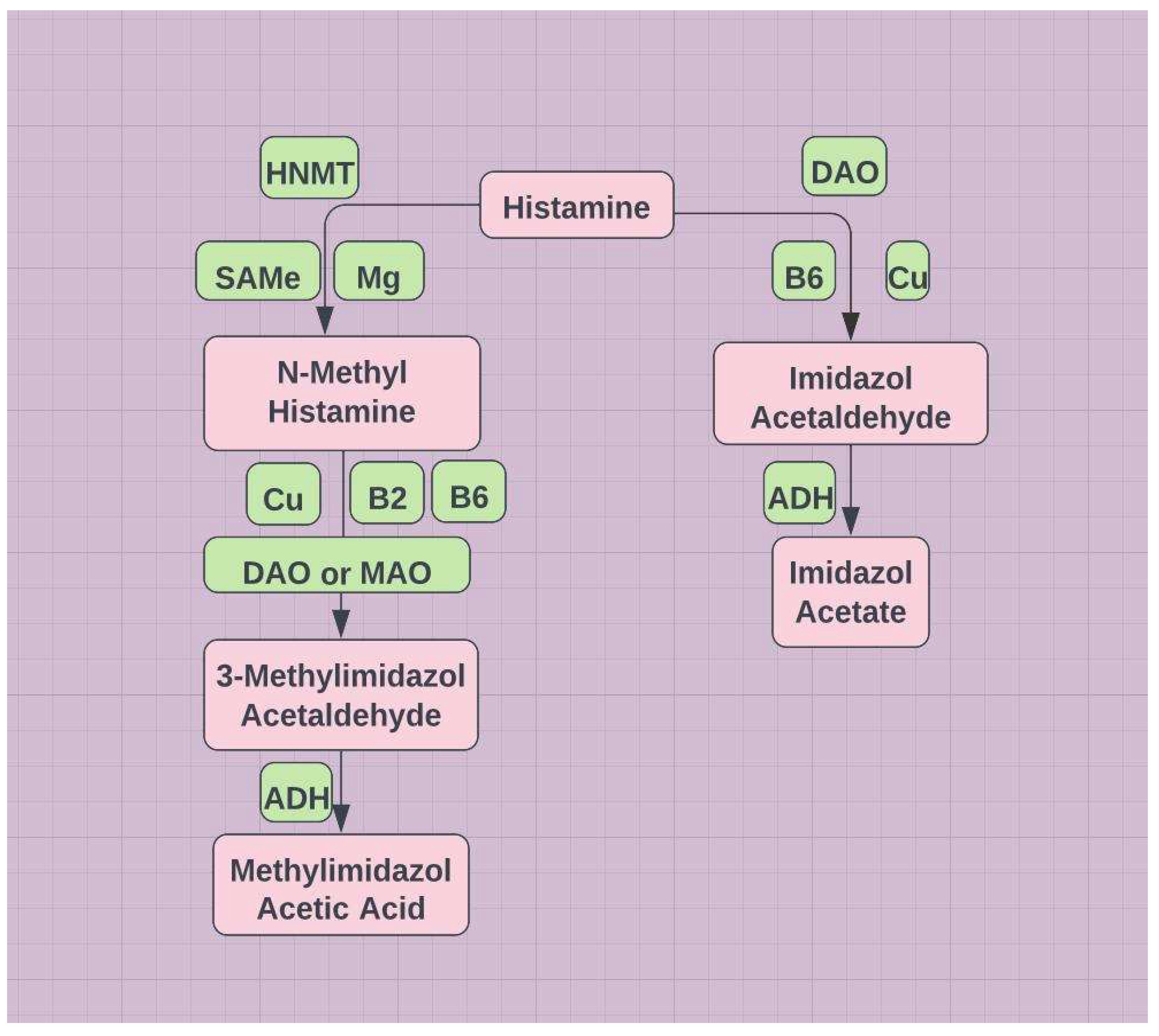
POTS (high flow) - vasodilation due to histamine; histamine is degraded by methylation and involves several mitochondrial based enzymes - DAO and ADH (see
Figure 8). MTHFR variants primarily exhibit hypomethylation and low SAMe.
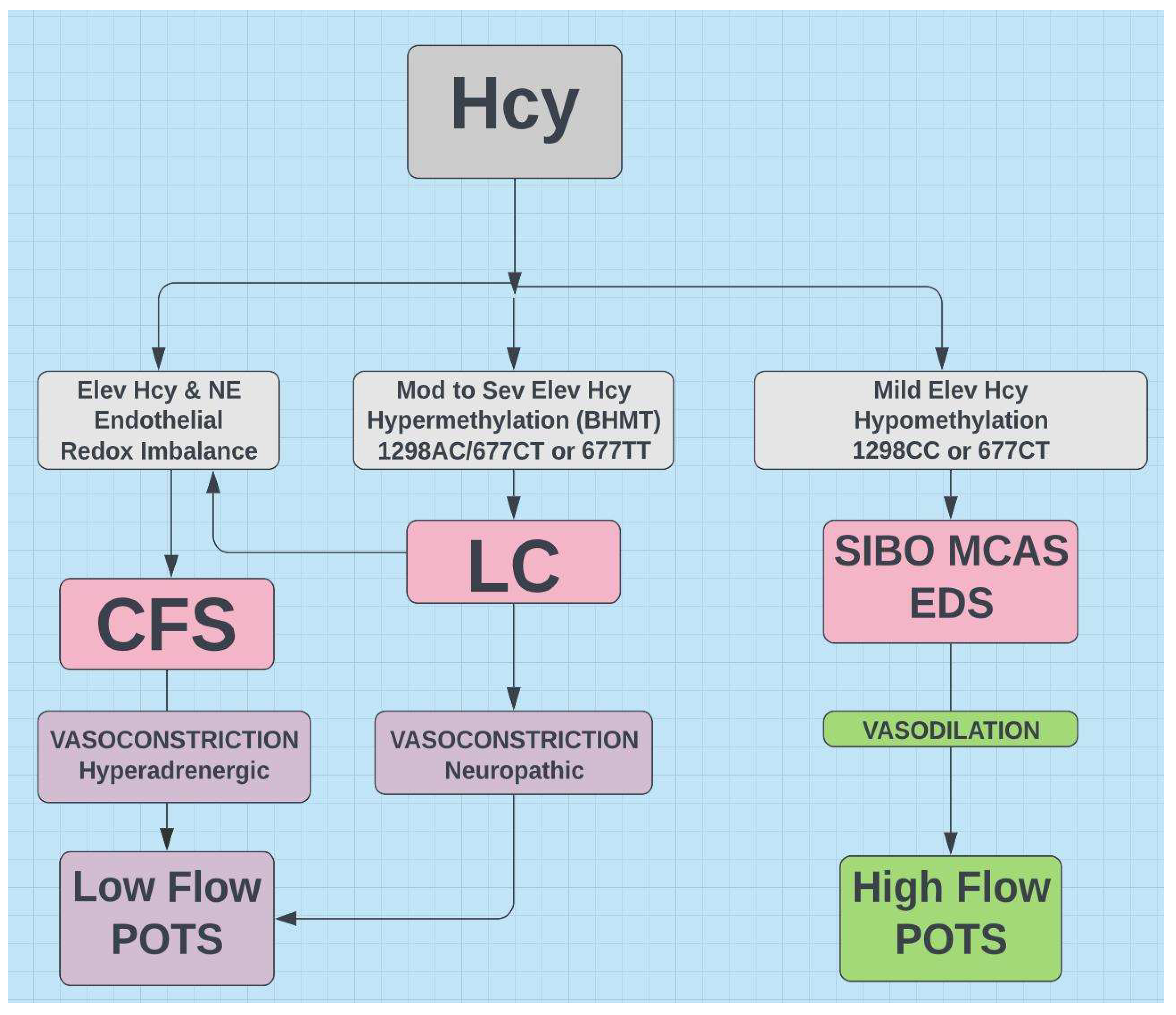
MTHFR 677T and 1298C work in concert to create hypomethylation. However, if Hcy is sufficiently elevated by an abnormal MTHFR, the alternative pathway (BHMT) can be initiated to lower Hcy (see
Figure 2 and
Figure 11). This pathway redirects to hypermethylation, enabling SAMe for MAO/DAO (mitochondrial), and COMT/HNMT to degrade monoamines and histamine respectively.
In summary
Catecholamines are degraded by MAO oxidation or COMT methylation.
Histamine is degraded by DAO oxidation or HNMT methylation
MAO/DAO are the primary determinants of catecholamine and histamine degradation, as both COMT and HNMT require subsequent mitochondrial based metabolism by MAO/DAO and aldehyde dehydrogenase (ADH) (see
Figure 8 and
Figure 10)
Estrogen down regulates COMT/HNMT and MAO/DAO.
Hypomethylation and low Hcy favor high flow POTS, while high Hcy favors low flow POTS (see
Figure 11).
4-3. SIBO, MCAS
SIBO is characterized by gut dysbiosis and malabsorption of critical nutrients. In general gut microbiota are net consumers of B12 and net producers of folate41. Both B9 and B12 are critical to optimal function of the folate and methionine cycles.
Sulfur reducing bacteria detectable by breath test produce H
2S. This compromises the availability of methionine, one of the nine essential amino acids and the only amino acid of the official 21 that contains sulfur. It is critical to the methionine cycle (see
Figure 2) and methylation.
Histamine intolerance is characterized by gut dysbiosis and a compromised intestinal barrier. This suggests a possible link to high flow POTS often associated with MCAS. POTS42, EDS42, and MCAS43 are all linked to low magnesium. POTS44, EDS45, and MCAS46 are also linked to low vitamin D. The gut microbiome of those with histamine intolerance lacks biodiversity47
Elevated tryptase has been linked to MCAS, EDS, and POTS. Tryptase primarily reflects mast cell function. Up-regulated mast cells produce symptoms that are seen in many conditions that overlap with Long Covid (and magnesium deficiency), including CFS, FM, MCAS, and POTS48. The brain fog of Long Covid resembles that of MCAS49 and may be due to inadequate magnesium50. Histamine is upregulated in magnesium deficiency. Magnesium deficiency not only increases histamine release but also increases mast cells43.
4-4. EDS
EDS is clearly genetic, but is just as clearly epigenetically modifiable. For example, in Covid-19 magnesium intervention in those with Ca:Mg > 2.6, transmembrane serine protease 2 (TMPRSS2) restricted cellular entry by enabling methylation of its gene. This assisted in the prevention of Covid-1951.
It is well known that hypermethylation leads to overall gene silencing and hypomethylation leads to overall gene activation
DNA methylation typically acts to repress gene transcription, i.e., serine protease production was suppressed by Mg++ intervention.
An elevated mixed metalloproteinase-2 (MMP-2) has been implicated in EDS and a connection to elevated folate and MTHFR polymorphisms proposed52. In EDS unmetabolized folate accumulates in the blood., i.e., MMP-2 is hypomethylated and its promoter gene activated in EDS. An abnormal MTHFR is linked to hypomethylation, which tends to activate promoter genes. The authors speculate that methyl folate therapy might improve symptoms by methylating the MMP-2 promoter region. Support for this speculation can be found in ischemic stroke. Hypomethylation of the MMP-2 gene is associated with ischemic stroke53, which is increased in EDS type IV54. Not surprisingly EDS appears to be associated with cognitive decline55. A nutritional shortfall has been implicated in the dysautonomia seen in EDS. Intramuscular magnesium sulfate lessened pain, improved emotional state, and increased energy42. EDS is linked to magnesium deficiency and POTS. Magnesium deficiency in rats leads to a blunted baroreflex and enhanced HR response to stress, e.g., standing from lying56.
The abundance of gastrointestinal issues in EDS suggests possible concomitant histamine intolerance and high flow POTS57
MTHFR and EDS are frequently viewed as genetic and beyond host control. But epigenetics has revealed that there is much that can be controlled. For another example, the APOE epsilon or ϵ4 allele, when homozygous, predicts about 15 times increase in AD. However, Native Americans have an abundance of this allele, but AD is largely absent58.
Their Paleolithic diet provides an excellent Ca:Mg balance. Perhaps this dementia is not primarily genetic but is primarily epigenetic, due to an elevated Ca:Mg and decreased Mg++ absorption. These two cations share the same receptor - CaSR (calcium sensing receptor). DNA methylation occurs via SAMe and requires Mg++. DNA in AD exhibits a characteristic differential methylation pattern.
5. Autoimmunity and Cancer
Hypermethylation can help drive carcinogenesis, autoimmune disease, and neurodegenerative disease59. Hypomethylation can also drive cancer, autoimmune disease60, and perhaps neurodegenerative disease as well61.
The discussed syndromes all exhibit a distinct female preponderance. Autoimmune disease is also more common in females. Many believe that autoimmunity is at the heart of POTS, LC, CFS, and MCAS62.
Possible explanations for the female preponderance include
Estrogen downregulates COMT63 and DAO/MAO64 => increased NE (low flow POTS), histamine (high flow POTS)
Females produce more IFN-ɣ which drives the tryptophan steal
Mast cells secrete not only histamine but also bradykinin; estrogen degrades ACE, which would otherwise degrade bradykinin
Magnesium deficiency is greater in females during their reproductive years
Chronic inflammation, migraines, and pain are prominent features in all these syndromes, even EDS65
Gut dysbiosis and abnormal tryptophan metabolism have been associated with autoimmunity67. Pleiotropic IFN-ɣ, prominent in the tryptophan steal, is tightly linked to autoimmune disease68.
The constant flow of IFN in autoimmune disease depletes both B6 and tryptophan. Perhaps the constant flow of IFN and subsequent demand for B2, the B2-requiring B6, and the B2-requiring MTHFR (required to methylate B9 (folate) and B12) might connect POTS, LC, CFS, and MCAS to reports of autoantibodies to Ang II and beta adrenergic receptors28.
This then poses another link between LC and abnormal MTHFR.
An increased KTR induced by IFN-ɣ provides additional support for the tryptophan connection to autoimmune disease69, neurodegenerative disease, cancer, CVD, and aging16. An elevated KTR responds to ARBs70. Indeed, a post mortem study of brains demonstrated more neuropathology in non-hypertensives, than in hypertensives on ARBs71.
IFN-γ is an anti-inflammatory pleiotropic cytokine and in an environment of chronic inflammation IFN-γ may switch to a pro-inflammatory (autoimmune) role68. Hypomethylation of the IFN-γ promoter gene may mediate this transition in autoimmune disease72. Hcy73 and B2 deficiency74 are associated with increased risk of MS. The latter is also linked to SLE75. MTHFR 677T is associated with MS76 and RA77.
Methylation operates similarly with respect to carcinogenesis. Some cancers are potentiated by hypomethylation, others by hypermethylation. Some, including breast and lung, can be seen under both conditions78. Hypomethylation is linked to hypertension79 and B2 given to hypertensives with 677TT is more effective than anti-hypertensives alone80
6. Therapeutic Considerations
The first goal is to avoid the extremes of hypomethylation and hypermethylation. Optimal levels of vitamin D (at least 50 ng/mL) have the capacity to demethylate and may assist in the search for the Goldilocks state of methylation12,13. Clearly sufficient intake of antioxidants is also in the equation, but many require methylation for activation. Those requiring methylation include melatonin, betaine, choline, cysteine, taurine, CoQ10, carni- tine, creatine, creatinine, and lysine.
Knowing one’s MTHFR status is not necessary, but knowing one’s Hcy level might help motivate.
To avoid hypomethylation, maintain an optimal Ca:Mg of around 2.6. Otherwise calcium crowds out magnesium in the competition for the CaSR sites. Water soluble B vitamins, especially B2,3,6,9,12, must be in their active forms. This means methyl folate, methylcobalamin, and phosphorylated B6. Most supplements contain B6 as pyridoxine. Magnesium absorption is enhanced, possibly doubled when accompanied by P5P during a meal81. The inactive pyridoxine form of B6 competitively inhibits the P5P aka pyridoxal phosphate (PLP). So B6 (pyridoxine) supplementation can produce symptoms of vitamin B6 deficiency82.
Beware of blindly increasing methylating agents to address an elevated Hcy. For example, intake of trimethylglycine aka betaine or methyl folate can trigger cold sores83,84. Choline is often recommended as a methylating agent, as it is the precursor to betaine. But this metabolic process is intramitochondrial. Eating more fruits and vegetables is a healthful dietary change, but food today is less nutritious and supplements are rightfully becoming more popular. Probiotics for improving the gut microbiome and antioxidants to protect mitochondria add security.
It is not necessary to understand all the biochemical intricacies. Common characteristics of the 677T phenotype have been described. An acceptable glimpse of your own status can be gained from self examination and some rudimentary tests. Are you prehypertensive? Are you mildly anemic with macrocytic indices? A concomitant iron deficiency with microcytic indices could cloak this. Are you slightly myopic? Were you a good student? Do you tend toward “hay fever” or migraines? Do you have a family history of neuropsychiatric illness? If an Hcy level within normal limits is revealed, a value near the upper limit of normal should give pause for minor concern, as the MTHFR variants are quite prevalent in the healthy population from which the normal limits are determined. Serum histamine may provide further insight, as histamine is inversely proportional to methylation. In addition a complete blood count (CBC) with a minimally elevated mean corpuscular volume (MCV) or a low normal hematocrit, hemoglobin, and/or red cell count suggest a possible B9 or B12 shortage (active forms require methylation). Stress and/or chronic inflammation compromise mitochondrial function, exacerbate hypomethylation, and increase Hcy. What is your CRP or resting HRV? Keep a balanced Cu:Zn. A diet rich in nuts and seeds should help. What is your 25(OH)D3 level? Try an antihistamine. ARBs may offer relief if all else has failed.
Conclusion
Two MTHFR variant alleles (1298C and 677T) are present in a majority of Americans. They directly impact the folic acid and methionine cycles, the primary determinants of the methylation process. The six listed syndromes may represent related phenotypes triggered by hypomethylation of epigenes due to abnormal MTHFRs.
The extremely common MTHFR variants are the most frequent cause of an elevated Hcy. Hcy is the predominant marker for abnormal methylation. Abnormal methylation plays vital epigenetic and biochemical roles in not only LC, CFS, POTS, MCAS/SIBO, and EDS but also aging, cancer, CVD, IBD, neurodegenerative disease, autoimmune disease, and quality of life.
The preventative benefits of vitamin D are legion and well known, but the intra-mitochondrial site for synthesis of its active form compromises efficacy for the inflammaged. This underscores the prophylactic value of a diet rich in antioxidants, judiciously supplemented. Synthesis of vitamin D is heavily dependent on magnesium. Methyltransferases all require magnesium as cofactor. Vitamin D and magnesium deficiencies are common in these six syndromes. MTHFR and EDS are clearly genetic. We may not be in direct control of the genome, but the epigenome is beckoning.
References
- Fukumoto, K. , et al. “Excess S-adenosylmethionine inhibits methylation via catabolism to adenine” Commun Biol 5, 313 (2022). [CrossRef]
- National Library of Medicine, National Center for Biotechnology Information https://www.ncbi.nlm.nih.gov/snp/rs1801133. 1801.
- National Library of Medicine, National Center for Biotechnology Information https://www.ncbi.nlm.nih.gov/snp/rs1801131. 1801.
- van der Put NM, et al. “A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects?” Am J Hum Genet. 1998 62(5):1044-51. [CrossRef]
- McAuley, E. , et al. “Riboflavin status, MTHFR genotype and blood pressure: Current evidence and implications for personalised nutrition” Proceedings of the Nutrition Society, 75(3), 405-414. [CrossRef]
- Moll, S, Varga, EA. “Homocysteine and MTHFR Mutations” Circulation. 2015 Jul 7;132(1):e6-9. [CrossRef]
- 7. Nikesjö, F, et al. “Defining post-acute COVID-19 syndrome (PACS) by an epigenetic biosignature in peripheral blood mononuclear cells”. Clin Epigenetics. 14;14.1 (2022): 172. [CrossRef]
- Zhu H, et al. “Race/Ethnicity-Specific Association of Vitamin D and Global DNA Methylation: Cross-Sectional and Interventional Findings” PLoS One. 2016 6;11(4):e0152849. [CrossRef]
- Marashly ET, Bohlega SA. “Riboflavin Has Neuroprotective Potential: Focus on Parkinson's Disease and Migraine” Front Neurol. (2017) 20;8:333. [CrossRef]
- Plasma Methylation Enzyme and Nutrition Guide, Doctor’s Data, Inc., St. Charles, Illinois https://site-akiajqrf22xmaqzsiz6q.s3.amazonaws.com/DDI+Website/Resource+Guides/78338+DDI+PlasmaMethylationEnzyme+WP_R4.pdf.
- Ehrlich, M. “DNA methylation in cancer: too much, but also too little” Oncogene 21, 5400–5413 (2002). [CrossRef]
- Pereira F, et al. “Vitamin D has wide regulatory effects on histone demethylase genes” Cell Cycle. 2012 Mar 15;11(6):1081-9. [CrossRef]
- Berridge, MJ. Vitamin D deficiency and diabetes. Biochem J. 2017 Mar 24;474(8):1321-1332. [CrossRef]
- Luigi di Filippo et al. “Low Vitamin D Levels Are Associated With Long COVID Syndrome in COVID-19 Survivors” The Journal of Clinical Endocrinology & Metabolism, 2023;, dgad207. 2023. [CrossRef]
- DEWang, Z, et al. “Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis” Clin Endocrinol (Oxf) (2022) Mar;96(3):281-287. [CrossRef]
- Midttun, Ø. et al. “A cross-sectional study of inflammatory markers as determinants of circulating kynurenines in the Lung Cancer Cohort Consortium” Sci Rep 13, 1011 (2023). [CrossRef]
- Pinto, JT, Cooper, AJ. “From cholesterogenesis to steroidogenesis: role of riboflavin and flavoenzymes in the biosynthesis of vitamin D” Adv Nutr. 2014 Mar 1;5(2):144-63. [CrossRef]
- Bikle, DD. “Vitamin D metabolism, mechanism of action, and clinical applications” Chem Biol. 2014 Mar 20;21(3):319-29. [CrossRef]
- Veldurthy, V, et al. “25-Hydroxyvitamin D₃ 24-Hydroxylase: A Key Regulator of 1,25(OH)₂D₃ Catabolism and Calcium Homeostasis” Vitam Horm. 2016;100:137-50. [CrossRef]
- Deo, SH, et al. “Norepinephrine increases NADPH oxidase-derived superoxide in human peripheral blood mononuclear cells via α-adrenergic receptors” Am J Physiol Regul Integr Comp Physiol. 2013;305(10):R1124-32. [CrossRef]
- Flaherty, R.L. , et al. “Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer” Breast Cancer Res 19, 35 (2017). [CrossRef]
- Jarrett, H, et al. “Vitamin B-6 and riboflavin, their metabolic interaction, and relationship with MTHFR genotype in adults aged 18-102 years” Am J Clin Nutr. 2022;116(6):1767-1778. [CrossRef]
- Prasad, AS. “Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health” Front Nutr. 2014 Sep 1;1:14. [CrossRef]
- Ivanova, ID, et al. “Evaluation of zinc, copper, and Cu:Zn ratio in serum, and their implications in the course of COVID-19” J Trace Elem Med Biol. 2022 Feb 8;71:126944. [CrossRef]
- Hackler, J, et al. “Relation of Serum Copper Status to Survival in COVID-19” Nutrients (2021) 13(6):1898. [CrossRef]
- Lee, SR. “Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems” Oxid Med Cell Longev. 2018 Mar 20;2018:9156285. [CrossRef]
- Roth W, et al. “Tryptophan Metabolism and Gut-Brain Homeostasis” International Journal of Molecular Sciences (2021) 22(6):2973. [CrossRef]
- Eroğlu İ, et al. “Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19)” Nutrition (2021) 90:111308. [CrossRef]
- Sorgdrager FJH, et al. “Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target” Front Immunol. (2019) 10:2565. [CrossRef]
- Christensen, MH, et al. “Vitamin B6 status and interferon-γ-mediated immune activation in primary hyperparathyroidism” J Intern Med. 2012 Dec;272(6):583-91. [CrossRef]
-
31.J.W. Miller, Homocysteine in Encyclopedia of Human Nutrition (Third Edition), 2013c.
- Schröcksnadel K, et al. “Monitoring tryptophan metabolism in chronic immune activation” Clin Chim Acta. (2006) 364(1-2):82-90. [CrossRef]
- Pujantell M, Altfeld M. “Consequences of sex differences in Type I IFN responses for the regulation of antiviral immunity” Front Immunol. 2022 Sep 16;13:986840. [CrossRef]
- Chambers, P.W. (2022) Long Covid, Short Magnesium. Open Access Library Journal, 9, e8736. https://www.scirp.org/journal/paperinformation.aspx?paperid=117413. 1174. [Google Scholar]
- Goetzl, EJ, et al. “Gender specificity of altered human immune cytokine profiles in aging” FASEB J. 2010 Sep;24(9):3580-9. [CrossRef]
- Fryar-Williams, S. “Fundamental Role of Methylenetetrahydrofolate Reductase 677 C → T Genotype and Flavin Compounds in Biochemical Phenotypes for Schizophrenia and Schizoaffective Psychosis” Front Psychiatry. 9;7 (2016): 172. [CrossRef]
- 37. Azimi, Z., et al. “Association of zinc level with DNA methylation and its consequences: A systematic review” Heliyon (2022) 8(10):e10815 . [CrossRef]
- de Vega WC, et al. “DNA methylation modifications associated with chronic fatigue syndrome” PLoS One (2014) 9(8):e104757. [CrossRef]
- Paul BD, et al. “Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome” Proc Natl Acad Sci U S A. (2021) 118(34):e2024358118. [CrossRef]
- Sanchez-Perez, S. , et al. “The dietary treatment of histamine intolerance reduces the abundance of some histamine-secreting bacteria of the gut microbiota in histamine intolerant women. A pilot study” Front. Nutr. (2022) Sec. Nutrition and Microbes, v9. [CrossRef]
- Achufusi, TGO, et al. “Small Intestinal Bacterial Overgrowth: Comprehensive Review of Diagnosis, Prevention, and Treatment Methods” Cureus. (2020) 12(6):e8860. [CrossRef]
- Do, T. , et al. “Nutritional Implications of Patients with Dysautonomia and Hypermobility Syndromes” Curr Nutr Rep 10, 324–333 (2021). [CrossRef]
- Takemoto, S, et al. “Magnesium deficiency induces the emergence of mast cells in the liver of rats” J Nutr Sci Vitaminol (Tokyo). 2013;59(6):560-3. [CrossRef]
- Chandralekha, Ashangari, Suleman, A. “Abstract 121: Vitamin D Deficiency Study in Postural Orthostatic Tachycardia Syndrome” Circulation: Cardiovascular Quality and Outcomes (2015) 8:A121. [CrossRef]
- Busch, A. , et al. “Vascular type Ehlers-Danlos syndrome is associated with platelet dysfunction and low vitamin D serum concentration” Orphanet J Rare Dis 11, 111 (2016). [CrossRef]
- Murdaca G, et al. “Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases” Biomedicines (2022) 10(8):1877. [CrossRef]
- Schindler, PC, “Microbial patterns in patients with histamine intolerance” Journal of Physiology and Pharmacology (2018) 69(4):579-93. [CrossRef]
- Theoharides, T. , et al. “Brain “Fog,” Inflammation and Obesity: Key Aspects of Neuropsychiatric Disorders Improved by Luteolin” Frontiers in Neuroscience (2015) 9, Article 225. [CrossRef]
- Weinstock, L.B. , et al. “Mast Cell Activation Syndrome (MCAS) Symptoms Are Prevalent in Long-COVID. International Journal of Infectious Diseases ( 2021) 112, 217–226. [CrossRef]
- Nishio, A. , et al. “Specific Change of Histamine Metabolism in Acute Magnesium-Deficient Young Rats” (1987) Drug-Nutrient Interactions 5, 89-96. https://pubmed.ncbi.nlm.nih.gov/3111814/. 3111. [Google Scholar]
- Fan L, et al. “Magnesium treatment on methylation changes of transmembrane serine protease 2 (TMPRSS2)” Nutrition (2021) 89:111340. [CrossRef]
- Courseault J, et al. “Folate-dependent hypermobility syndrome: A proposed mechanism and diagnosis” Heliyon. (2023) 9(4):e15387. [CrossRef]
- 53. Lin, HF, et al. “Methylation in the Matrix Metalloproteinase-2 Gene is Associated with Cerebral Ischemic Stroke” Journal of Investigative Medicine : the Official Publication of the American Federation for Clinical Research (2017) 65(4):794-799. [CrossRef]
- North KN, et al. “Cerebrovascular complications in Ehlers-Danlos syndrome” Ann Neurol. (1995) 38(6):960-4. [CrossRef]
- Hamonet, Claude et al. “Cognitive and Psychopathological Aspects of Ehlers-Danlos Syndrome - Experience in a Specialized Medical Consultation” (2018) https://gavinpublishers.com/admin/assets/articles_pdf/1525934180article_pdf97661175.pdf.
- Murasato, Y. et al. “Effect of Magnesium Deficiency on Autonomic Circulatory Regulation in Conscious Rats” Hypertension (1999) 34:247-252. https://www.ahajournals.org/doi/pdf/10.1161/01.HYP.34.2.247.
- Schnedl WJ, Enko D. “Histamine Intolerance Originates in the Gut” Nutrients. 2021; 13(4):1262. [CrossRef]
- Suchy-Dicey, A, et Al. “APOE genotype, hippocampus, and cognitive markers of Alzheimer's disease in American Indians: Data from the Strong Heart Study” Alzheimer's Dement. 2022; 18: 2518– 2526. [CrossRef]
- Ehrlich, M. “DNA hypermethylation in disease: mechanisms and clinical relevance” Epigenetics. 2019 14(12):1141-1163. [CrossRef]
- Wilson AS, et Al. “DNA hypomethylation and human diseases” Biochim Biophys Acta. 2007 Jan;1775(1):138-62. [CrossRef]
- D'Alessandro A, et Al. “The role of PIMT in Alzheimer's disease pathogenesis: A novel hypothesis“ Alzheimers Dement. 2023 May 8. [CrossRef]
- Xu Y, Chen G. “Mast cell and autoimmune diseases” Mediators Inflamm. 2015:246126. [CrossRef]
- Jiang, H, et al. “Human catechol-O-methyltransferase down-regulation by estradiol” Neuropharmacology. 2003 Dec;45(7):1011-8. [CrossRef]
- Klaiber EL, et al. “Effects of estrogen therapy on plasma MAO activity and EEG driving responses of depressed women” Am J Psychiatry. 1972 Jun;128(12):1492-8. [CrossRef]
- Caliogna L, et al. “Biomarkers for Ehlers-Danlos Syndromes: There Is a Role?” International Journal of Molecular Sciences. 2021; 22(18):10149. [CrossRef]
- Rainero, I, et al. “Targeting MTHFR for the treatment of migraines” Expert Opin Ther Targets. 2019 Jan;23(1):29-37. [CrossRef]
- Brown J, et al. “Intestinal Dysbiosis and Tryptophan Metabolism in Autoimmunity” Front Immunol. 2020 Aug 4;11:1741. [CrossRef]
- Lees, JR. “Interferon gamma in autoimmunity: A complicated player on a complex stage” Cytokine. (2015) 74(1):18-26. [CrossRef]
- Opitz, C.A. , et al. “Tryptophan degradation in autoimmune diseases” Cell. Mol. Life Sci. 64, 2542 (2007). [CrossRef]
- Wu M-H, et al. “Kynurenine/Tryptophan Ratio Predicts Angiotensin Receptor Blocker Responsiveness in Patients with Diabetic Kidney Disease” Diagnostics. 2020; 10(4):207. [CrossRef]
- 71. Hoffman, LB, et al. “Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons”Neurology. 72.20 (2009): 1720-6. [CrossRef]
- Funes SC, et al. “Contribution of Dysregulated DNA Methylation to Autoimmunity” International Journal of Molecular Sciences. 2021; 22(21):11892. [CrossRef]
- Mititelu RR, et al. “Homocysteine as a Predictor Tool in Multiple Sclerosis” Discoveries 2021, 9(3): e135. [CrossRef]
- Naghashpour M, et al. “Update on riboflavin and multiple sclerosis: a systematic review” Iran J Basic Med Sci. 2017 Sep;20(9):958-966. [CrossRef]
- Sam, N.B. , Zhang, Q., et al. “Serum/plasma homocysteine levels in patients with systemic lupus erythematosus: a systematic review and meta-analysis” Clin Rheumatol 39, 1725–1736 (2020). [CrossRef]
- Naghibalhossaini F, Ehyakonandeh H, et al. “Association Between MTHFR Genetic Variants and Multiple Sclerosis in a Southern Iranian Population” Int J Mol Cell Med. 2015 4(2):87-93. http://www.ncbi.nlm.nih. 4499.
- Bagheri-Hosseinabadi, Z., Imani, D., et al. “MTHFR gene polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis based on 16 studies” Clin Rheumatol 39, 2267–2279 (2020) . [CrossRef]
- Hasan, T. , Arora, R., et al. “Disturbed homocysteine metabolism is associated with cancer” Exp Mol Med 51, 1–13 (2019). [CrossRef]
- Gonzalez-Jaramillo, V., Portilla-Fernandez, E., et al. “The role of DNA methylation and histone modifications in blood pressure: a systematic review” J Hum Hypertens 33, 703–715 (2019) . [CrossRef]
- Wilson CP, et al. “Blood pressure in treated hypertensive individuals with the MTHFR 677TT genotype is responsive to intervention with riboflavin: findings of a targeted randomized trial” Hypertension. 2013 61(6):1302-8. [CrossRef]
- Boylan LM, Spallholz JE. “In vitro evidence for a relationship between magnesium and vitamin B-6” Magnes Res. 1990 Jun;3(2):79-85 https://pubmed.ncbi.nlm.nih.gov/2133627/. 2133.
- Vrolijk MF, et al. “The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function” Toxicol In Vitro. 2017 Oct;44:206-212. [CrossRef]
- Huang J, et al. “Trimethylation of histone H3 lysine 4 by Set1 in the lytic infection of human herpes simplex virus 1” J Virol. 2006 Jun;80(12):5740-6. [CrossRef]
- Jacquemont B, Huppert J. “Inhibition of viral RNA methylation in herpes simplex virus type 1-infected cells by 5' S-isobutyl-adenosine" J Virol. 1977 22(1):160-7. [CrossRef]
Figure 1.
Various combinations of these two SNPs reflect predictable enzyme activity. 677T (heterozygous) and 677TT (homozygous) are the prime determinants of reduced activity4.
Figure 1.
Various combinations of these two SNPs reflect predictable enzyme activity. 677T (heterozygous) and 677TT (homozygous) are the prime determinants of reduced activity4.
Figure 2.
THF=tetrahydrofolate; TMG=trimethylglycine aka betaine; MS=methionine synthase; DMG=dimethylglycine; BHMT=betaine homocysteine methyltransferase; GNMT=glycine-N-methyltransferase; SHMT=serine hydroxymethyltransferase; SAH=S-adenosylhomocysteine; enzymes and cofactors are green; substrates are pink.
Figure 2.
THF=tetrahydrofolate; TMG=trimethylglycine aka betaine; MS=methionine synthase; DMG=dimethylglycine; BHMT=betaine homocysteine methyltransferase; GNMT=glycine-N-methyltransferase; SHMT=serine hydroxymethyltransferase; SAH=S-adenosylhomocysteine; enzymes and cofactors are green; substrates are pink.
Figure 3.
Most B vitamin supplements contain B6 as pyridoxine that ironically can compete with pyridoxal-5-phosphate (P5P) aka pyridoxal phosphate (PLP) for receptor sites and lead to symptoms of B6 deficiency. B2 sufficiency may be the most important indicator of B6 sufficiency.
Figure 3.
Most B vitamin supplements contain B6 as pyridoxine that ironically can compete with pyridoxal-5-phosphate (P5P) aka pyridoxal phosphate (PLP) for receptor sites and lead to symptoms of B6 deficiency. B2 sufficiency may be the most important indicator of B6 sufficiency.
Figure 4.
Comparison of methylation states for wild type MTHFR with low B2 versus abnormal MTHFR with elevated B2; MTHFR=methylenetetrahydrofolate reductase; HIST=histamine; NE=norepinephrine; COMT=catechol-O-methyltransferase; SHMT=serine hydroxymethyl transferase; Hcy=homocysteine; CO=choline oxidase; BHMT=betaine hydroxymethyl transferase; MSu=methionine sulfoxidase; MS=methionine synthase; SAMe=S-adenosyl methionine.
Figure 4.
Comparison of methylation states for wild type MTHFR with low B2 versus abnormal MTHFR with elevated B2; MTHFR=methylenetetrahydrofolate reductase; HIST=histamine; NE=norepinephrine; COMT=catechol-O-methyltransferase; SHMT=serine hydroxymethyl transferase; Hcy=homocysteine; CO=choline oxidase; BHMT=betaine hydroxymethyl transferase; MSu=methionine sulfoxidase; MS=methionine synthase; SAMe=S-adenosyl methionine.
Figure 5.
Demonstrates the goal (NAD) behind the tryptophan steal. IDO=indoleamine-2,3-dioxygenase-1, TDO=tryptophan 2,3-dioxygenase, KTR=kynurenine tryptophan ratio, KAT=kynurenine aminotransferases, KYNU=kynureninase (mitochondrial), KMO=kynurenine 3-monooxygenase, XA=xanthurenic acid, HAAO=hydroxyanthranilic acid dioxygenase16.
Figure 5.
Demonstrates the goal (NAD) behind the tryptophan steal. IDO=indoleamine-2,3-dioxygenase-1, TDO=tryptophan 2,3-dioxygenase, KTR=kynurenine tryptophan ratio, KAT=kynurenine aminotransferases, KYNU=kynureninase (mitochondrial), KMO=kynurenine 3-monooxygenase, XA=xanthurenic acid, HAAO=hydroxyanthranilic acid dioxygenase16.
Figure 6.
Solar exposure in the face of a B2 shortfall or mitochondrial compromise promises little benefit.
Figure 6.
Solar exposure in the face of a B2 shortfall or mitochondrial compromise promises little benefit.
Figure 7.
The interdependencies of vitamins B2, B6, D and the imbalance caused by inflammation. Both CYP enzymes are mitochondria based. All CYP enzymes require Mg++. All methyltransferases require Mg++. APR=acute phase reactant; MAO=monoamine oxidase; DAO=diamine oxidase; Cp=ceruloplasmin; CRP=C-reactive protein; HRV=heart rate variability; KTR=kynurenine tryptophan ratio; NDD=neurodegenerative disease; CVD=cardiovascular disease; IBD=inflammatory bowel disease; TRYP=tryptophan; PNPO=pyridoxine phosphate oxidase; yellow indicates decrease; purple indicates increase.
Figure 7.
The interdependencies of vitamins B2, B6, D and the imbalance caused by inflammation. Both CYP enzymes are mitochondria based. All CYP enzymes require Mg++. All methyltransferases require Mg++. APR=acute phase reactant; MAO=monoamine oxidase; DAO=diamine oxidase; Cp=ceruloplasmin; CRP=C-reactive protein; HRV=heart rate variability; KTR=kynurenine tryptophan ratio; NDD=neurodegenerative disease; CVD=cardiovascular disease; IBD=inflammatory bowel disease; TRYP=tryptophan; PNPO=pyridoxine phosphate oxidase; yellow indicates decrease; purple indicates increase.
Figure 8.
DAO=diamond oxidase; HNMT=histamine-N-methyltransferase; ADH=aldehyde dehydrogenase (mitochondrial).
Figure 8.
DAO=diamond oxidase; HNMT=histamine-N-methyltransferase; ADH=aldehyde dehydrogenase (mitochondrial).
Figure 9.
Demonstrates the switch from glycolysis to fermentation for energy thru NAD+ during mitochondrial dysfunction.
Figure 9.
Demonstrates the switch from glycolysis to fermentation for energy thru NAD+ during mitochondrial dysfunction.
Figure 10.
COMT=catecholamines-O-methyltransferase. MAO= monoamine oxidase (mitochondrial).
Figure 10.
COMT=catecholamines-O-methyltransferase. MAO= monoamine oxidase (mitochondrial).
Figure 11.
Proposed flow chart indicating hypothetical relationships for LC, CFS, SIBO, MCAS, EDS, POTS, and MTHFR with Hcy as common denominator. BHMT=betaine- homocysteine methyltransferase and represents the alternative pathway (see
Figure 2). NE=norepinephrine.
Figure 11.
Proposed flow chart indicating hypothetical relationships for LC, CFS, SIBO, MCAS, EDS, POTS, and MTHFR with Hcy as common denominator. BHMT=betaine- homocysteine methyltransferase and represents the alternative pathway (see
Figure 2). NE=norepinephrine.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).