1. Introduction
Salvia hispanica, commonly known as chia, is an annual herbaceous plant from the family Lamiaceae. It is native to Mexico and Northern Guatemala and has been a food source in Mesoamerica since at least 3500 BC [
1]. It is a pseudocereal, cultivated for its edible seeds, which are rich in α-linolenic acid [
2]. Chia is commercially grown and consumed in Mexico, Northern Guatemala, Bolivia, Ecuador, Colombia, parts of Australia, and the United States [
3,
4]. Chia has significant value as a cash crop, as chia seeds have gained popularity in recent years due to their nutritional value and health benefits [
5,
6]. However, the seed yield is still very low [
4] and the seed yield is reduced by insects and diseases. To facilitate molecular breeding, DNA markers, including microsatellites, have been identified and used in analysing genetic diversity and relationships in several cultivars from several countries [
4]. The genome [
3] and some transcriptomes [
7] were sequenced. These genomic tools set a solid foundation to accelerate the genetic improvement of important traits, such as yield and resistance to pathogens and insects.
The red spider mite (
Tetranychus neocaledonicus) is a common pest that can affect the production of plants, including S. hispanica [
8]. These mites feed on the plant's leaves, causing discoloration, curling, and even death in severe cases. The effects of red spider mites on chia production can vary depending on the severity of the infestation and the stage of growth of the plants [
9]. In the early stages of growth, red spider mites can cause stunted growth and reduced vigor in chia plants. This can result in lower yields at harvest [
9]. If the infestation is severe, the mites can cause premature defoliation, which can lead to even lower yields or complete crop failure [
8,
9,
10]. In addition to reducing yields, red spider mites can also affect the quality of chia seeds. Several approaches are available to prevent and control red spider mites in plant production, such as crop rotation, biological controls, and the careful use of pesticides [
9]. However, these approaches are tedious and costly. Genetic improvement of pest resistance using conventional selective breeding approaches is long lasting and complicated [
11]. Understanding the molecular mechanisms of the defensive response of plants to pests will help molecular breeding for pest resistance [
12]. Unfortunately, to the best of our knowledge, in S. hispanica, no study has been performed to understand how this plant responds to the infection of red spider mites. Therefore, the purposes of this study were to understand the molecular mechanisms behind the response to the infection of
Tetranychus neocaledonicus by sequencing, assembling, and annotating the transcriptomes of leaves of normal chia and chia infected by red spider mites.
2. Results
2.1. Morphological responses of leaves to insect herbivory
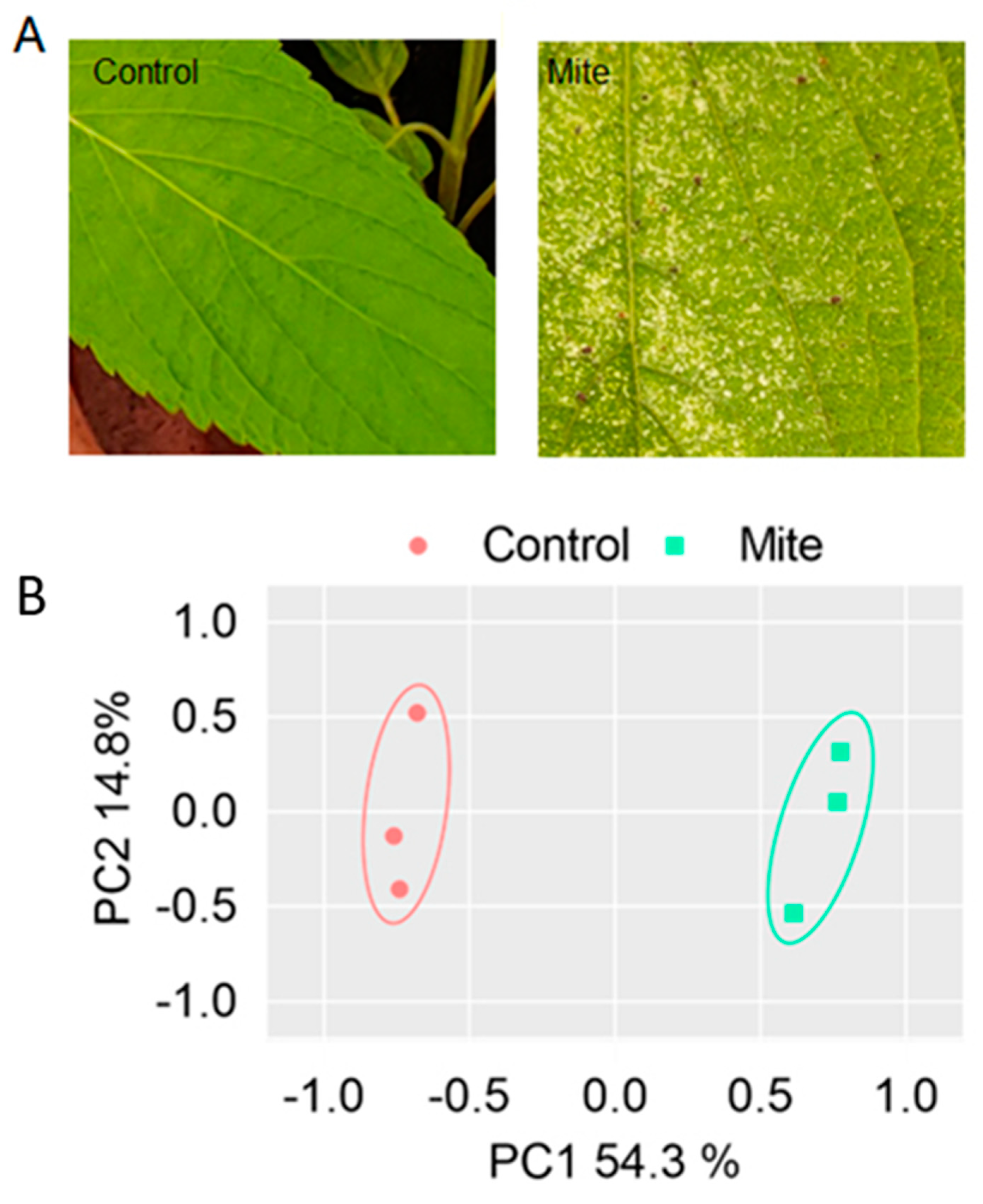
Spider mites were observed feeding on the underside of the leaves of the chia plant. There were obvious and observable morphological changes in the leaves under spider mite attack. In the mite-infested samples, areas of the leaf that had been attacked by the mites were observed to be discoloured, forming yellow or white patches (
Figure 1A). Following continuous feeding by the mites, the leaves appear bleached and drop off.
2.2. Summary of RNA-Seq
The sequencing of six libraries from the control and mite-infested plants produced a total of 129.1 million high quality paired end reads after trimming and filtering. An average of 21.5 million cleaned reads were obtained across all six samples. The controls had higher reads coverage compared to the mite attacked samples (
Supplementary Table S1). Over 13.3 million reads for each library were uniquely aligned to the reference genome. The plants that were attacked by mites showed a slightly lower uniquely mapping rate compared to the controls (86.3% vs 90.9%).
2.3. Identification of differential expression transcripts
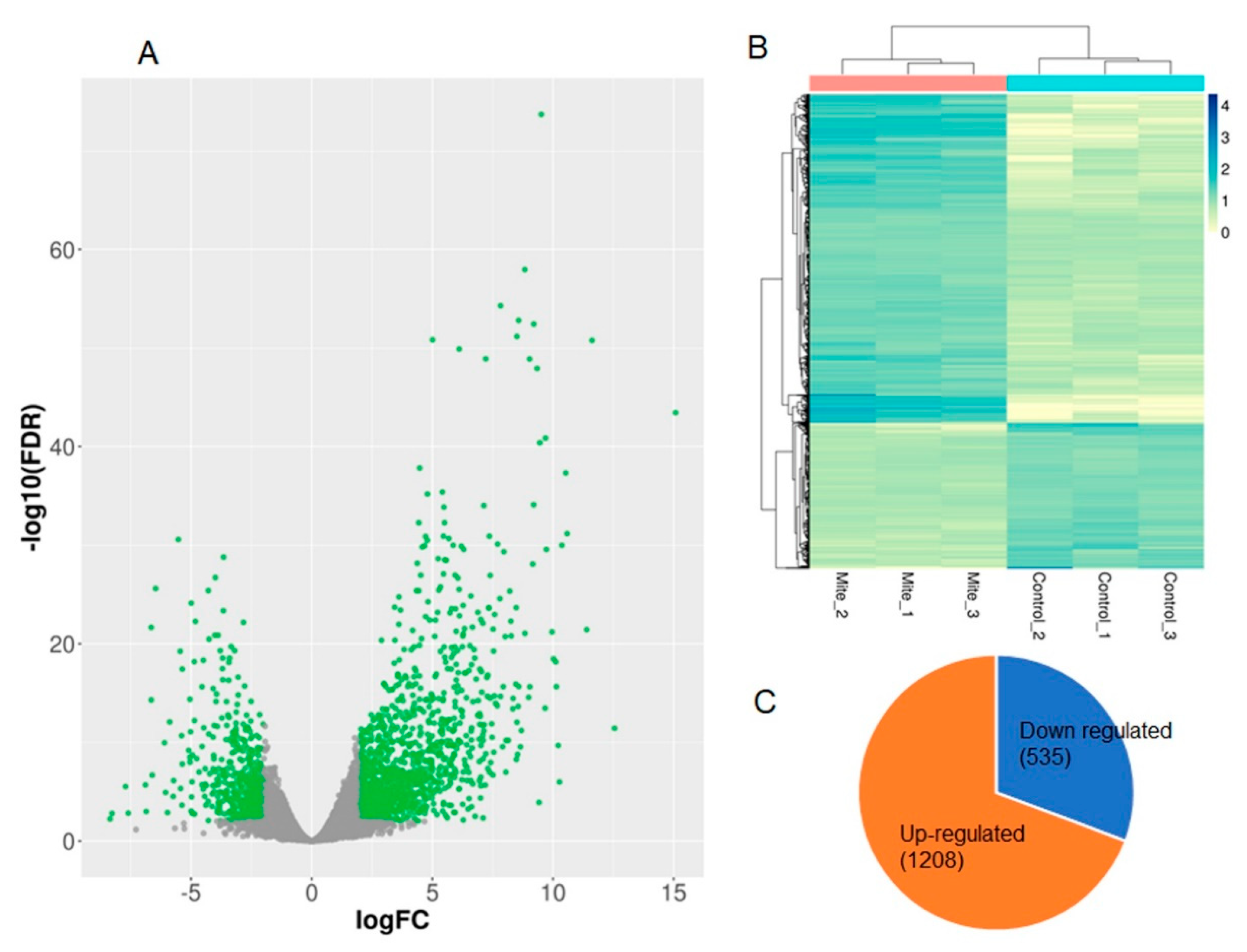
A total of 1743 differentially expressed genes (DEGs) were identified using DESeq2 (
Figure 2A) between the control and mite-infested chia. From these, 1208 (69%) transcripts were up-regulated and 535 (31%) were down-regulated (
Figure 2C). PCA analysis of the genome-wide expression patterns of the protein-coding genes between the controls and mite-infested leaves showed substantial differences in expression profiles (
Figure 1B). Hierarchical clustering analysis also showed that the DEGs clustered according to control and mite-infested groups (
Figure 2B).
2.4. Validation of DEGs using qRT-PCR
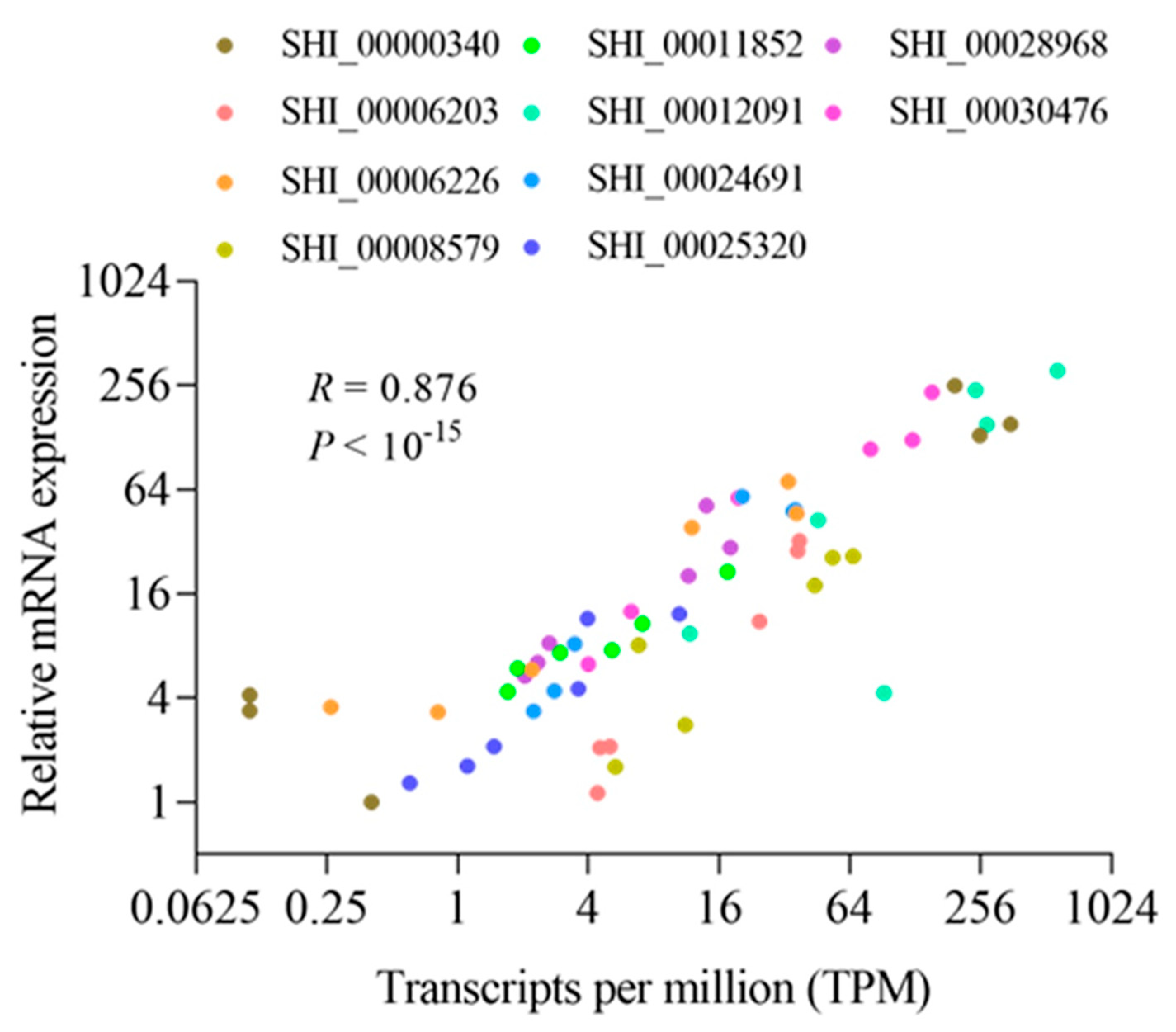
For validation of the expression profile of DEGs identified by RNA-seq, 12 genes were randomly selected, and primers were designed based on the corresponding annotated transcript sequences. The comparison of expression patterns in the RNA-seq and qPCR data (
Figure 3) showed high consistency, with a correlation coefficient of 0.876 (P < 10-15), as examined with Pearson’s correlation test, indicating that the expression profiling of the DEGs determined by RNA-seq is reliable and accurate.
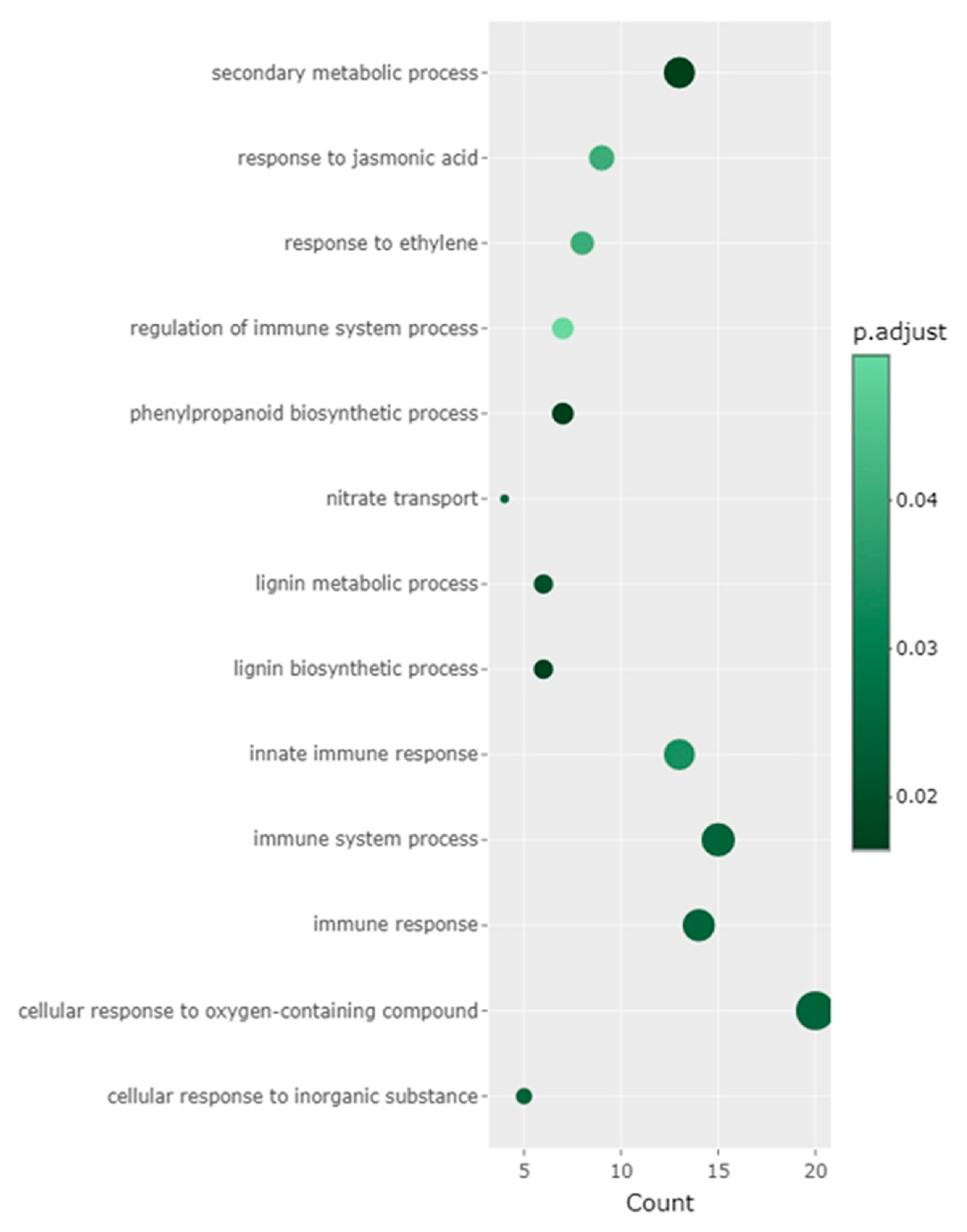
2.5. Overall enriched Gene Ontology (GO) based on all DEGs
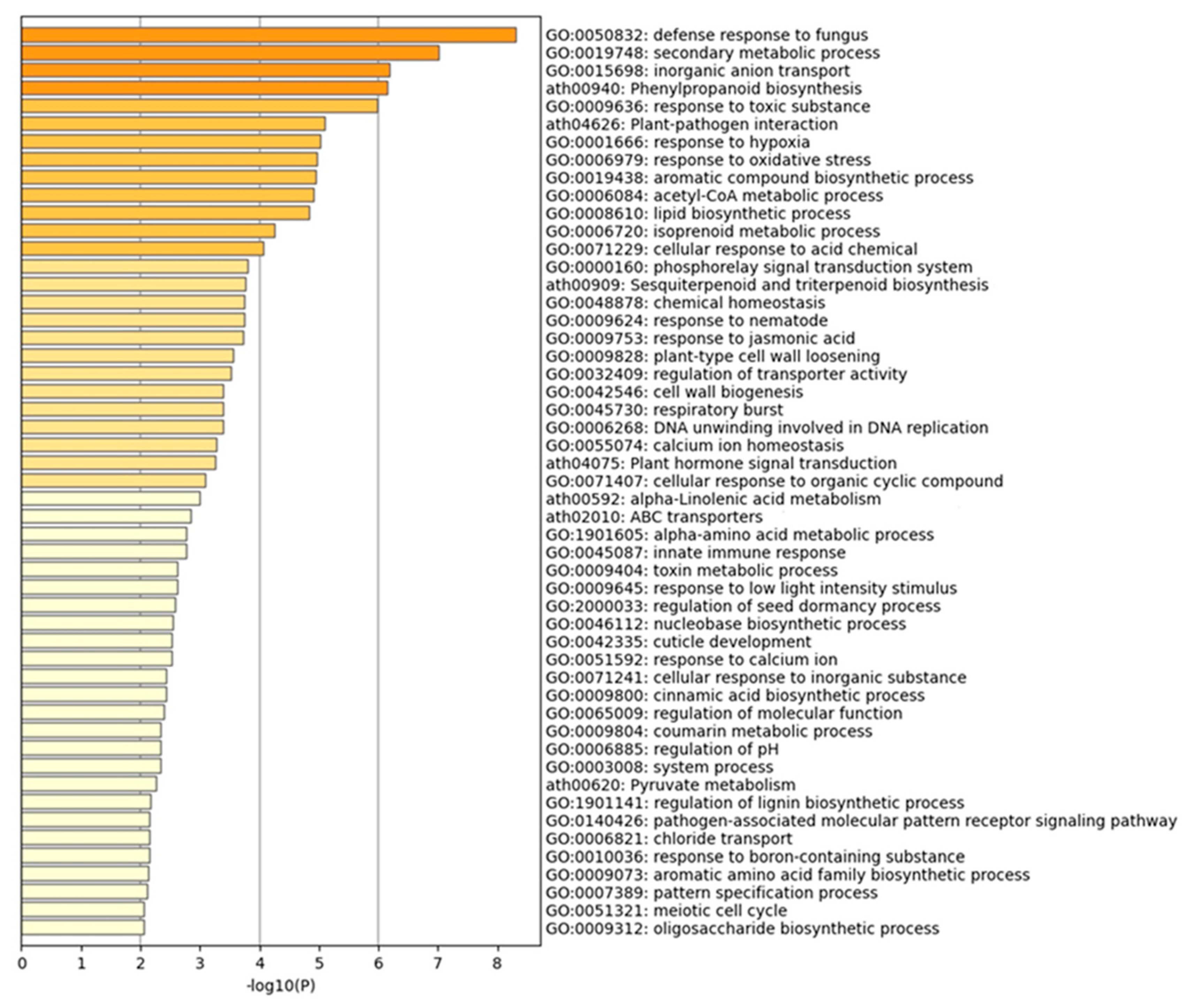
The DEGs between the control and mite-infested transcriptomes were used in enrichment analyses based on the Gene Ontology (GO) terms retrieved against Arabidopsis genome annotations (TAIR10) (
Figure 4). The most enriched GO terms were defense response to fungus (GO:0050832), phenylpropanoid biosysnthesis (ath00940), sesquiterpenoid and triterpenoid biosynthesis (ath00909), response to jasmonic acid (GO:0009753), innate immune response (GO:0045087) which are related to defense response and immunity. GO terms that were related to cell wall biogenesis and functions (e.g., GO:0042546, GO:0009828) were also enriched. Several enrichments related to the production of secondary metabolites such as secondary metabolic process (GO:0019748), isoprenoid metabolic process (GO:0006720) and alpha-Linolenic acid metabolism (ath00592) were also found.
From the GO enrichment of up-regulated DEGs (Supplementary
Figure 1A), the most enriched GO terms were defense response to fungus (GO:0050832), immune system process (GO:0002376), phenylpropanoid biosynthesis (ath00940) and response to oxidative stress (GO:0006979). The most enriched GO terms that were related to the down-regulated DEGs (Supplementary
Figure 1B) were regulation of molecular function (GO:0065009), tetrapyrrole metabolic process (GO:0033013), photosynthesis - antenna proteins (ath00196) and plant hormone signal transduction (ath04075). These data suggest that plants prioritize defense and immunity over respiration and growth.
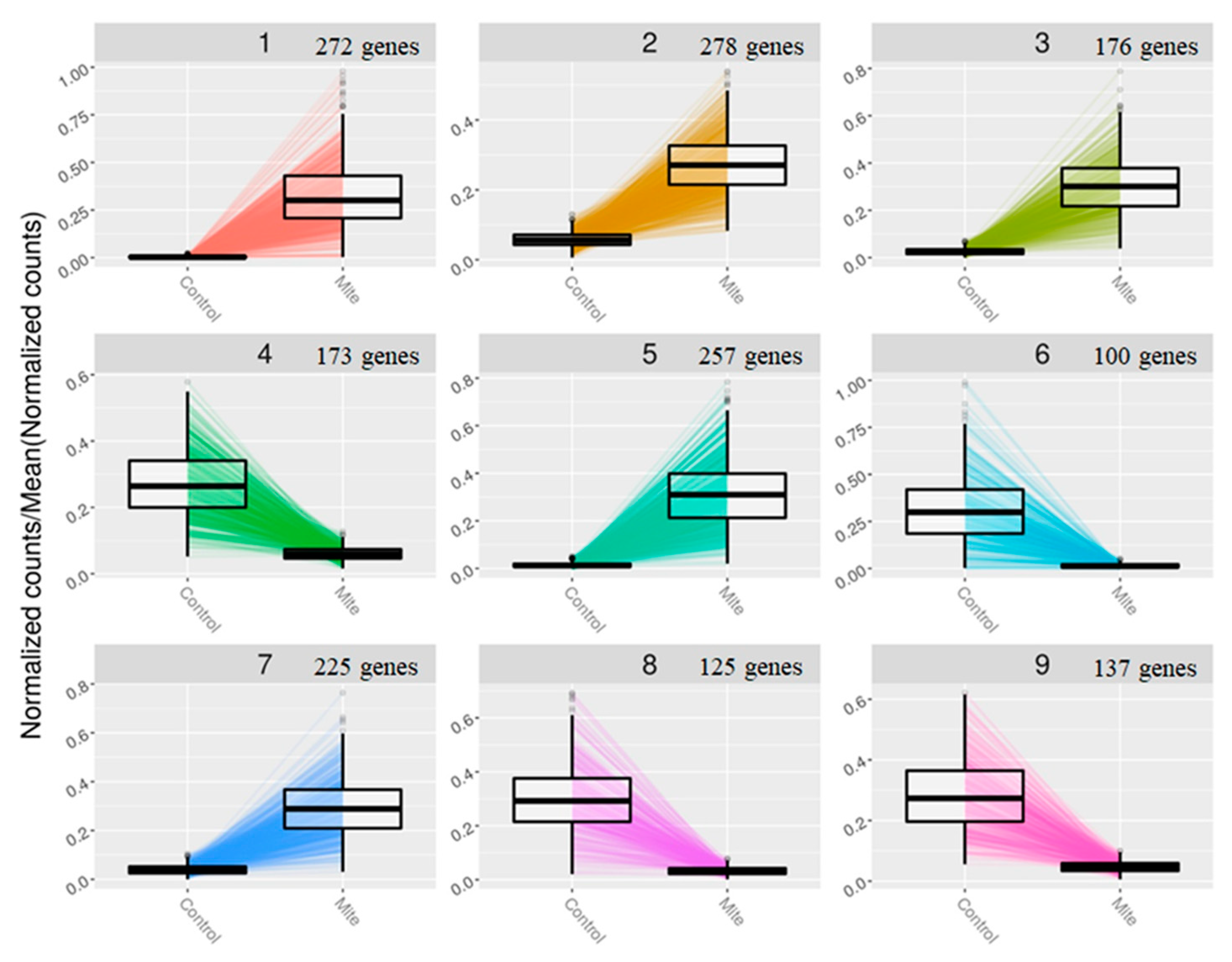
2.6. Construction of gene clusters showing associated expression patterns
The gene expression clusters based on the expression patterns of all the DEGs were constructed (
Figure 5). There were nine clusters in total, each with at least 100 genes. Within each cluster, DEGs showed correlated expression patterns. GO enrichment analysis was performed on clusters 1-3 as significantly enriched GO terms were identified for clusters 1-3, but not for clusters 4-9 (
Figure 6, Supplementary Figure 2A,B). In clusters 1-3, significantly enriched terms represented defense related responses. In gene cluster 1, GO terms such as secondary metabolite processes, response to jasmonic acid, response to ethylene, regulation of immune system processes and phenylpropanoid biosynthesis processes were significantly enriched. In gene cluster 2, only the term jasmonic acid biosynthetic process was significantly enriched while in gene cluster 3, the GO terms secondary metabolic process, phenylpropanoid metabolic process, glycosinolate catabolic process and glucosinolate catabolic process were enriched (Supplementary Figure 2A,B). We focused on the GO term response to jasmonic acid (GO:0009753) in gene cluster 1 and the individual DEGs in this term (
Supplementary Table S3). A majority of the DEGs in this GO term were up-regulated, including those involved in cell wall modification, signalling and transcription factors.
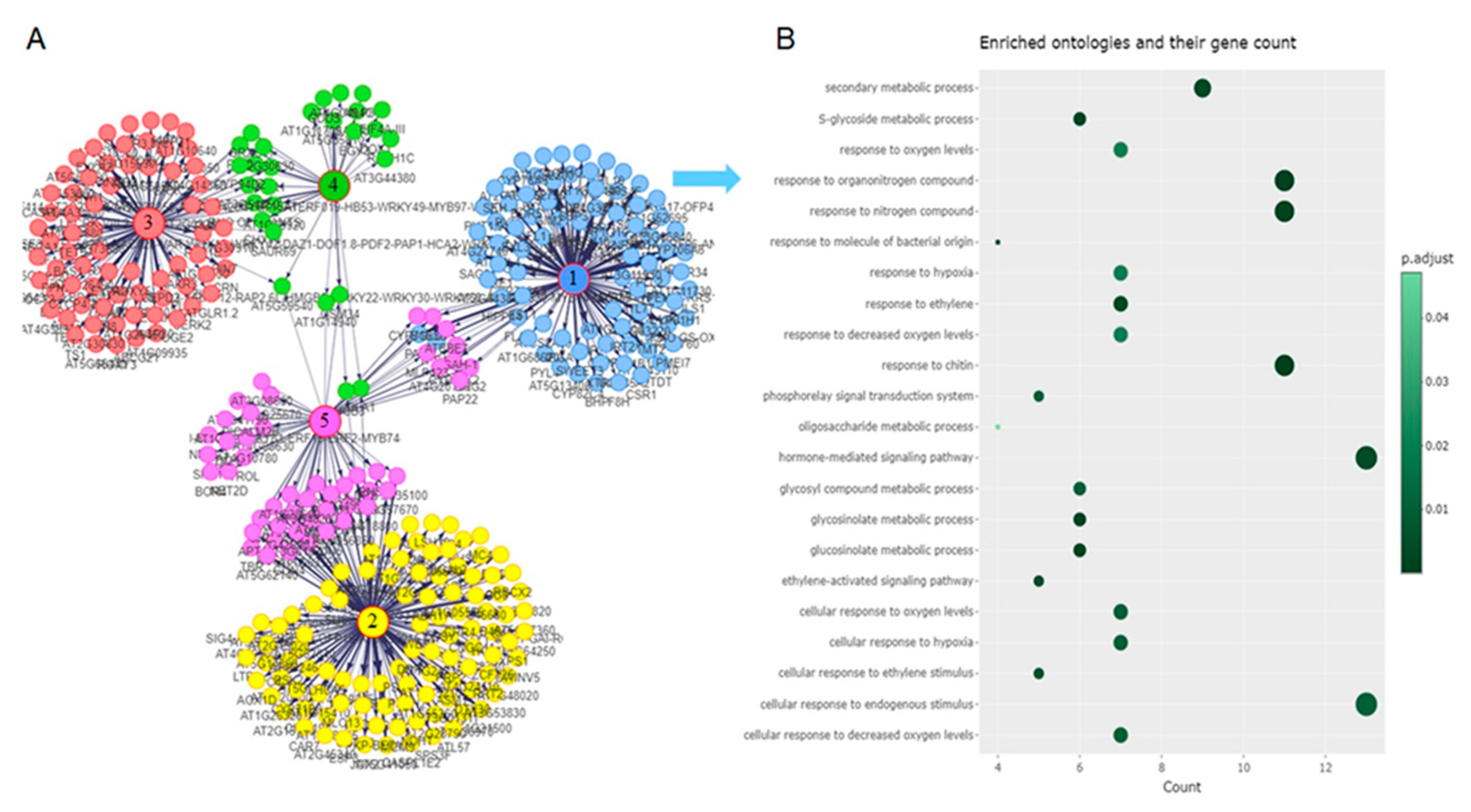
The interactions of the enriched GO terms of clusters 1-3 were investigated using network analysis. Five gene network communities were identified (
Figure 7A). Enrichment analysis was performed on communities 1-3 (
Figure 7B and Supplementary
Figure 3A and 3B) as significantly enriched terms were identified for communities 1-3, but not for communities 4 and 5. In gene network community 1 (
Figure 7B), the genes in the regulatory network was implicated mainly in defense and cellular response to insect herbivory. Gene network community 2 and 3 (Supplementary
Figure 3A and 3B) are involved in plant growth and cell communication. We found that most of the group regulators in each network community were transcription factors (TFs) (
Figure 8B).
The activation of the jasmonic acid signalling pathway results in the synthesis of secondary metabolites, such as glucosinolates [
13]. Glucosinolates are sulfur-containing secondary metabolites found in Brassica and many related plants and likely involved in plant defense [
14]. We focused on the GO term glucosinolate metabolic process (GO:0019760) in gene network community 1 (
Supplementary Table S4). There were 12 DEGs enriched in the GO term. Among them, 9 DEGs were up-regulated. A majority of the up-regulated DEGs were involved in the response to biotic and abiotic stress in the glucosinolate metabolic processes, such as MYB72, MYB73 and PEN3.
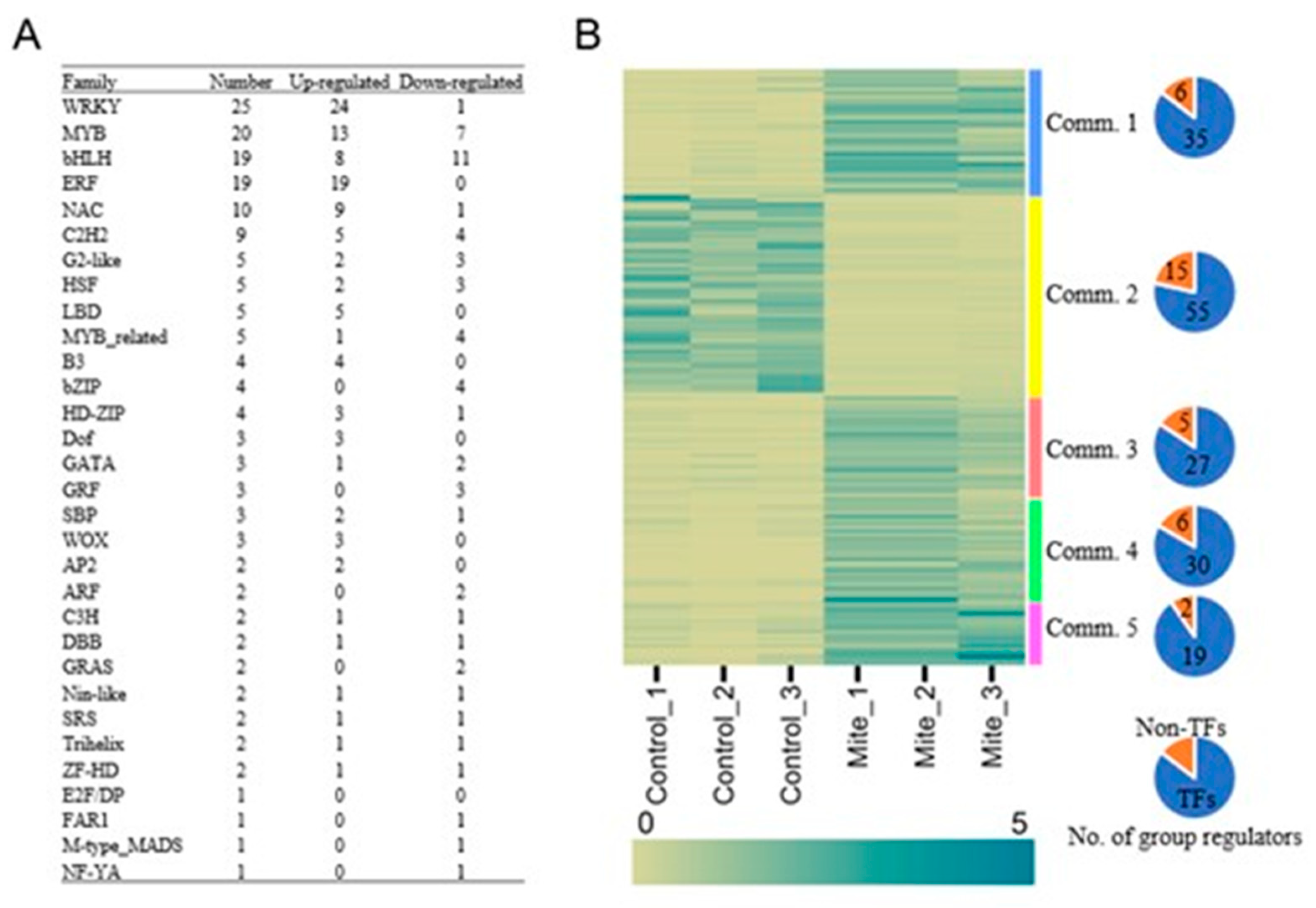
2.7. Transcription factors in spider-mite response
One hundred and seventy-one transcription factors (TFs) were identified based on the Arabidopsis transcription factor database PlantTFDB 5.0 [
15] (
Supplementary Table S5). The differentially expressed TFs were classified into 31 families (
Figure 8A and Supplementary
Figure 4). The major families of differentially expressed TFs were WRKY (25), MYB (20), bHLH (19) and ERF (19). There were 27 TF families that had 10 or fewer genes, including NAC (10), G2-like (5), bZIP (4), GRF (3), ARF (2) and E2F/DP (1). Some of these TFs have been reported to be associated with plant responses to biotic stress. A majority (67%) of the TFs, including WRKYs, MYBs and all of the ERFs, were up-regulated following spider mite infestation.
3. Discussion
In this study, we sequenced transcriptomes of Salvia hispanica leaves in response to spider mite herbivory and determined the DEGs corresponding to the response to insect herbivory. These DEGs and their associated functions were identified to be associated with disease or stress responses to insects.
3.1. DEGs in control and mite infested samples
Transcriptome sequencing can help to understand the mechanisms behind plant-insect interactions by revealing the genes involved in the response to wounding. qPCR was performed and the data was compared to the RNA-seq data. A high consistency was observed in the expression of the genes between the RNA-seq and qPCR data, showing that the RNA-seq data was reliable.
A total of 1743 differentially expressed genes were identified between the control and mite-attacked leaves. The majority of genes were up-regulated in the leaves that were attacked by mites. Genes that were involved in cell wall modification, such as those that involved the synthesis and deposition of cell wall components like hemicellulose and pectin; and secondary cell wall biosynthesis, such as the TBL gene family, were up-regulated. The TBL gene family promotes the o-acetylation of cell wall polysaccharides which changes the structure and function of the cell wall and influences the resistance of plants against pathogens and insects [
16,
17]. These structural and biochemical changes in the cell wall could be in response to the spider mite attack [
18], as well as to pathogen invasion [
18,
19] as the cells reinforce their defence. In the spider mite infested plants, several genes that were involved in defense mechanisms, such as pathogen/bacterial resistance genes, were up-regulated. Amongst the down-regulated DEGs, there were many genes involved in plant growth. These include ERL2 and HCA2, which are related to cell division, IAA14 and PIN7 which are involved in root development, as well as genes associated with response to light, such as those encoding photoreceptors like BAS1, PHOT1 and PHOT2. Plant defensive responses are energetically costly [
20], and this down-regulation would allow the plant to conserve energy and redirect its resources towards defense-related responses [
21].
3.2. Pathways involved in the response to the infestation of Tetranychus neocaledonicus
GO enrichment analysis showed that the DEGs that were up-regulated were implicated in response to biotic and abiotic stress, such as defense response to fungus (GO:0050832), immune system response (GO:002376), phenylpropanoid biosynthesis (ath00940), response to jasmonic acid (GO: 0009753) and response to ethylene (GO:0009723).
Jasmonates (JA) and its derivatives are involved in a variety of processes, having functions in growth and development, and in response to environmental stresses [
22,
23,
24]. In particular, the JA signalling pathway has been shown to be activated in response to tissue wounding, especially by insect herbivores [
25,
26,
27].
Studies have shown that jasmonate Zinc finger Inflorescence Meristem (ZIM)-domain (JAZ) proteins are the repressors of JA-signalling. Under normal conditions, relatively high levels of JAZ proteins inhibit the transcription factors that activate the JA-mediated response [
28]. However, during insect attack, the levels of JA increases in the cytoplasm. JA transporters move the cytoplasmic JA into the nucleus, which triggers the interaction of coronatine insensitive1 (COI1) with JAZ proteins and subsequently leads to the degradation of JAZ proteins to relieve the inhibition to plant growth caused by JA signalling. The degradation of JAZ proteins triggers the release of TFs to activate downstream defense responses [
29,
30] such as secondary metabolites, which are repellent to herbivorous insects. In our present study, DEGs involving JAZ10 proteins, and the JA-signalling pathway were up-regulated. The up-regulation of DEGs involving JAZ10 proteins could suggest JAZ10 proteins are likely being degraded to activate the transcriptional genes involved in plant defense, allowing the plant to mount a defence response against the insect pests. Conversely, as JAZ proteins are repressors of JA-signalling, the up-regulation of the DEGs involving JAZ10 could also serve to terminate the defence response soon after initiation [
31]. This might be important in mediating the energy demands of a sustained defence response. The up-regulation of DEGs involving JAZ10 could also suggest that the cells are trying to maintain homeostasis, increasing the levels of JAZ proteins after their degradation during plant defence as a response to wound healing. Spider mites have also been known to induce [
10] and repress [
32] JA response. The up-regulation of DEGs that repress the JA response in our study could be caused by the feeding of spider mites on the chia plant [
33]. Certainly, further studies should be done to determine if spider mites repress the defence response in chia.
JA and its derivatives increase the production of secondary metabolites after attack by herbivores [
34]. Plant secondary metabolites play crucial roles in response to environmental stresses and defence against pathogens, herbivores, and insects [
35,
36]. Previous studies have shown that glucosinolate plays an important role in plant defence responses [
37]. It has been reported that glucosinolates play a role in plant defence responses against insects and pathogens [
14]. Following damage to plant tissue by insect herbivory, glucosinolates are hydrolysed by myrosinases to produce a myriad of hydrolytic compounds like nitriles, epithionitriles, thiocyanates, and/or isothiocyanates to repel insect herbivores [
14,
38]. Here we observed that the majority of DEGs involved in response to biotic and abiotic stress in the GO term glucosinolate metabolic process were up-regulated, e.g., PEN3, MYB72, MYB73 and NAC042. Interestingly, these DEGs are involved in responses to pathogens. Plants are susceptible to invasion and infection by microbial pathogens following tissue wounding [
39]. In our study, the up-regulation of the DEGs in the GO term defense response to fungus (GO:0050832) as well as the glucosinolate metabolic process suggests that the plants were attacked by fungal pathogens following tissue wounding from the insect attack.
3.3. TFs involved in the response to the infestation of Tetranychus neocaledonicus
Transcription factors play diverse roles in plant defense responses to biotic stresses by regulating the activation, modulation, and coordination of defence related gene expression, cross-talk between defence pathways, immune responses, and hormone signalling [
40].
We identified 171 transcription factors that were differentially expressed upon the infestation of spider mites. Among them, many TFs were found in the WRKY, MYB, bHLH and ERF families. This might suggest that WRKYs, MYBs, bHLHs and ERFs play more important roles in the regulation of defence processes compared to other TF gene families. In our study, a majority of the differentially expressed transcription factors triggered by spider mite herbivory were from the WRKY family. The expression levels of many WRKY genes were significantly up-regulated. The WRKY transcription factor family has been associated with responses to biotic and abiotic stress [
41]. WRKY TFs play important roles in plant defence signalling pathways such as jasmonic acid and ethylene pathways [
42] by directly regulating the expression of JA and ethylene responsive genes. WRKY TFs also confer resistance against pathogens as well as regulate the expression of pathogenesis-related genes and secondary metabolite biosynthesis genes [
43]. In our study, most of the up-regulated WRKY TFs were from gene network community 1. The genes in network community 1 are mainly involved in defence. Many of the WRKY TFs that were from gene network community 1 were involved with defence against pathogens. Among them were WRKY18 and WRKY60. WRKY18 and WRKY60 work in a complex with WRKY40 in response to pathogens [
44]. WRKY22, which is up-regulated in our study, has been found to be activated in response to aphid infestation and play a role in both salicylic acid (SA) and jasmonic acid (JA) signalling [
45].
The other TF families that were also similarly affected by spider mite herbivory were MYB (12%), bHLH (11%) and ERF (11%). NAC (6%) and C2H2-type zinc finger (5%) families also responded to spider mite attacks. These TFs are also able to be induced by JA signalling and are involved in the response to biotic and abiotic stressors [
29].
MYB and NAC TFs are large families of TFs that are involved in regulating various processes such as development and stress responses. MYB TFs play a role in the defence response by regulating the biosynthesis of secondary metabolites such as phenylpropanoids [
46] and glucosinolates [
47]. The MYB TF family regulates plant growth and development, cell morphology and pattern building, physiological activity metabolism, primary and secondary metabolic reactions, and responses to environmental stresses [
48]. NAC TFs are important for regulating responses to drought, salt stress, and pathogen attack [
49]. The bHLH TF family plays a role in regulating the genes involved in JA and Ethylene signalling and the biosynthesis of secondary metabolites such as glucosinolates and diterpenoid phytoalexins which confer resistance to herbivores and pathogens [
50]. Ethylene response factors (ERF) TFs are involved in regulating the genes involved in biotic and abiotic stress [
40], and biosynthesis of secondary metabolites such as alkaloids [
50]. They play an important role in defense against necrotrophic pathogens by regulating the expression of genes involved in the biosynthesis of defence metabolites, ethylene signalling pathways, and downstream defence responses [
50,
51].
4. Materials and Methods
4.1. Plant materials
All
Salvia hispanica plants were grown in a greenhouse in Temasek Life Sciences Laboratory as described in previous papers [
3,
4]. At 50 days post germination (dpg), six plants were challenged with ~100 adult red spider mites/plant, while other plants were free of red mites. At the age of 65 dpg, the fifth leaf from the top of each of three control plants and three plants affected by
Tetranychus neocaledonicus was collected, respectively.
4.2. RNA extraction, library preparation, and sequencing
Total RNA was isolated from leaves using the RNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer’s protocol. The integrity and purity of the total RNA were assessed with gel electrophoresis. The concentration of each RNA sample was measured using a NanoDrop (Thermo Fisher Scientific, USA). Total RNA was treated with RNase-free DNase I (Sigma-Aldrich, Singapore) and used for mRNA library construction with the Illumina TruSeq RNA Library Prep Kit v2 (Illumina, USA) in accordance with the manufacturer’s protocol. The prepared libraries were paired-end (2 x 150 bp) sequenced on the Illumina NovaSeq sequencing platform by NovogeneAIT Genomics, Singapore.
4.3. Bioinformatic analyses of RNA-seq data
The program process_shortreads in the Stacks package [
52] was used to demultiplex the raw reads, filter adapters, and clean up low quality reads. The cleaned reads were mapped to the reference genome of chia [
3], using default parameters, with the program STAR [
53]. The expression patterns of annotated genes were analysed using only uniquely mapped reads. The expression level of each annotated gene was counted with the program HTSeq-count [
54] based on the information of the annotated gene features in the genome annotation file [
3]. The program DESeq2 [
55] was used to normalize the relative expression of transcripts and identify differentially expressed genes (DEGs) across samples.
Transcripts with a count per million (CPM) mapped reads of >1 were retained for further analysis. Transcripts with a fold change (FC) value of > 2 or < − 2 and with a significance value of 0.01 after application of the Benjamini–Hochberg false discovery rate (FDR) were considered differentially expressed genes.
4.4. Validation of RNA-seq data using qRT-PCR
Total RNA samples from control and mite-infested samples were digested with DNase I recombinant RNase-free (Roche, Basel, Switzerland) and reverse transcribed with M-MLV Reverse Transcriptase (Promega, Madison, USA) following the manufacturer’s protocol. A total of 12 primers targeting 12 genes (Table 6) that were differentially expressed between the control and mite-infested transcriptomes were designed according to the coding sequences obtained from the annotated reference genome [
3] using the program Primer3 [
56]. Quantitative PCR (qPCR) was carried out on the BioRad CFX96 (Bio-Rad Laboratories, Hercules, USA) using SYBR® Green as a fluorescent dye. qPCR reactions were carried out in triplicate, each 20 µl reaction volume containing 2.5 µl of 5x diluted cDNA, 0.4 µl (4 µlM) of each primer, 10 µl of 2x master mix from KAPA SYBR® FAST qPCR kits (Life Technologies, Carlsbad, CA, United States), and 6.7 µl of sterile water according to the manufacturer’s instructions. Raw data was converted to cycle threshold (Ct) values using software provided by BioRad (Bio-Rad Laboratories, Hercules, USA). The 2−ΔΔCT method was used to quantify the expression level using the glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) as the housekeeping gene to normalize the relative expression of the genes according to our previous study [
57]. The fold change of each gene was the ratio of the relative expression of the mite-infested leaf to that of the control leaf. The correlation between transcriptome sequencing (transcripts per million), and qPCR data set (relative mRNA expression) was assessed by Pearson’s correlation coefficient.
4.5. Functional annotation of DEGs
Gene Ontology (GO) accessions were retrieved for each DEG against the annotation of Arabidopsis using the program Blast2GO [
58]. The samples were clustered based on the relative expression of DEGs, using both principal component analysis (PCA) and heatmaps with the program ClustVis [
59] to investigate the overall expression patterns between the two groups. The program Metascape [
60] was used to carry out gene ontology enrichment analysis and to study protein-protein interaction signalling pathways with Arabidopsis as a reference. Regulatory network analysis was conducted using the program DIANE (Dashboard for the Inference and Analysis of Networks from Expression data) with default parameters and using Arabidopsis as reference [
61].
5. Conclusions
We have sequenced and annotated the leaf transcriptomes of chia subjected to spider mite herbivory. Plants have a wide arsenal of defensive responses and phytochemicals to deter many herbivores and pathogens, including jasmonic acid signalling and glucosinolates. Understanding the balance between jasmonic acid and salicylic acid signalling pathways could lead to strategies to boost plant defence without compromising growth and development. We showed that TFs played an important role in the Salvia hispanica responses to spider mite herbivory. WRKY, MYB, bHLH, ERF and NAC TF genes were the most involved in the responses. As the demand for sustainable agriculture grows, understanding of plant defence responses to pests and pathogens could aid in developing environmentally friendly strategies that reduce the use of chemical pesticides. Identification of crucial genes and pathways involved in nonhost defence can provide valuable insights for developing plant varieties that are more resistant to pests.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary
Figure 1,
Figure 2,
Figure 3 and
Figure 4. Figure S1 Gene ontology (GO) analysis of differentially expressed genes (DEGs) that are up-regulated and down-regulated between mite-feeding and controls of chia leaf transcriptomes at the significance level of
P < 0.05. Figure S2 Gene ontology (GO) analysis of differentially expressed genes (DEGs) in gene expression cluster 2 (A) and 3 (B). GO terms at the significance level of 0.05 are indicated. Figure S3 Gene ontology (GO) analysis of differentially expressed genes (DEGs) in gene regulation network community 2 (A) and 3 (B). GO terms at the significance level of 0.05 are indicated. Figure S4 Proportion of the number of differentially expressed transcription factors of each transcription family out of the total differentially expressed transcription factors.
Author Contributions
Conceptualization, G.H.Y.; methodology, M.L. and LW.; software, G.H.Y.; validation, M.L., L.W. and G.H.Y.; formal analysis, L.W.; investigation, M.L. and L.W.; resources, G.H.Y..; data curation, M.L. and L.W.; writing—original draft preparation, M.L., L.W. and G.H.Y.; writing—review and editing, G.H.Y.; visualization, L.W.; supervision, G.H.Y.; project administration, G.H.Y.; funding acquisition, G.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the internal fund of the Temasek Life Sciences Laboratory (Cost center 5020), Singapore. The APC was funded by the Temasek Life Sciences Laboratory.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be available upon reasonable request.
Acknowledgments
We would like to thank our institute for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coates, W.; Ayerza, R. , Production potential of chia in northwestern Argentina. Ind. Crops Prod. 1996, (3), 229–233. [Google Scholar]
- Ayerza, R.; Coates, W. , An ω-3 fatty acid enriched chia diet: Influence on egg fatty acid composition, cholesterol and oil content. Can. J. Anim. Sci. 1999, (1), 53–58. [Google Scholar] [CrossRef]
- Wang, L.; Lee, M.; Sun, F.; Song, Z.; Yang, Z.; Yue, G.H. A chromosome-level genome assembly of chia provides insights into high omega-3 content and coat color variation of its seeds. Plant Communications 2022, (4), 100326. [Google Scholar] [CrossRef]
- Yue, G. H.; Lai, C. C.; Lee, M.; Wang, L.; Song, Z. J. Developing first microsatellites and analysing genetic diversity in six chia (Salvia hispanica L.) cultivars. Genet. Resour. Crop Evol. 2022, 69, 1303–1312. [Google Scholar] [CrossRef]
- Jamshidi, A.; Amato, M.; Ahmadi, A.; Bochicchio, R.; Rossi, R. Chia (Salvia hispanica L.) as a novel forage and feed source: A review. Ital. J. Agron. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Kaur, S.; Bains, K. Chia (Salvia hispanica L.) – a rediscovered ancient grain, from Aztecs to food laboratories. Food Sci. Nutr. 2019, 50, 463–479. [Google Scholar]
- Peláez, P.; Orona-Tamayo, D.; Montes-Hernández, S.; Valverde, M. E.; Paredes-López, O.; Cibrián-Jaramillo, A. Comparative transcriptome analysis of cultivated and wild seeds of Salvia hispanica (chia). Sci. Rep. 2019, 9, 9761. [Google Scholar] [CrossRef]
- Rachana, R. R.; Manjunatha, M. J.; Devi, S. G.; Naik, M. , Seasonal incidence of red spider mite Tetranychus neocaledonicus Andre and its natural enemies. Karnataka J. Agric. Sci. 2009, 22, 213–214. [Google Scholar]
- Oliveira Briozo, M. E.; Silva, J. F.; Barros Ferraz, J. C.; Ramalho Silva, P. R.; Da Silva Melo, J. W.; De França, S. M. Biology and life table of Tetranychus neocaledonicus André (1933) (Acari: Tetranychidae) in different hosts. Syst. Appl. Acarol. 2023, 28, 497–507. [Google Scholar]
- Kant, M. R.; Ament, K.; Sabelis, M. W.; Haring, M. A.; Schuurink, R. C. Differential Timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004, 135, 483–495. [Google Scholar] [CrossRef]
- Choudhary, A.; Raje, R.; Datta, S.; Sultana, R.; Ontagodi, T. Conventional and molecular approaches towards genetic improvement in pigeonpea for insects resistance. Am. J. Plant Sci. 2013, 4, 372–385. [Google Scholar] [CrossRef]
- Ashra, H.; Nair, S. Review: Trait plasticity during plant-insect interactions: From molecular mechanisms to impact on community dynamics. Plant Sci. 2022, 317, 111188. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, N.; Reichelt, M.; van de Mortel, J. E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J. J. A.; Dicke, M.; Pineda, A. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, (11), 1786. [CrossRef]
- Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Jin, J.; Gao, G. PlantRegMap: charting functional regulatory maps in plants. Nucleic Acids Res. 2019, 48, D1104–D1113. [Google Scholar] [CrossRef]
- Sun, A.; Yu, B.; Zhang, Q.; Peng, Y.; Yang, J.; Sun, Y.; Qin, P.; Jia, T.; Smeekens, S.; Teng, S. MYC2-Activated trichome birefringence-like37 acetylates cell walls and enhances herbivore resistance. Plant Physiol. 2020, 184, 1083–1096. [Google Scholar] [CrossRef]
- Yuan, Y.; Teng, Q.; Zhong, R.; Ye, Z.-H. TBL3 and TBL31, Two Arabidopsis DUF231 Domain proteins, are required for 3- o -monoacetylation of xylan. Plant Cell Physiol. 2015, 57, 35–45. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.-X.; Chen, R.; He, S. Y. , Challenging battles of plants with phloem-feeding insects and prokaryotic pathogens. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 23390–23397. [Google Scholar] [CrossRef]
- Molina, A.; Miedes, E.; Bacete, L.; Rodríguez, T.; Mélida, H.; Denancé, N.; Sánchez-Vallet, A.; Rivière, M.-P.; López, G.; Freydier, A.; Barlet, X.; Pattathil, S.; Hahn, M.; Goffner, D. , Arabidopsis cell wall composition determines disease resistance specificity and fitness. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2010243118. [Google Scholar]
- Züst, T.; Agrawal, A. A. Trade-Offs Between Plant Growth and defense against insect herbivory: an emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef]
- Arena, G. D.; Ramos-González, P. L.; Rogerio, L. A.; Ribeiro-Alves, M.; Casteel, C. L.; Freitas-Astúa, J.; Machado, M. A. , Making a better home: modulation of plant defensive response by brevipalpus mites. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M. S.; Javed, R.; Siddiqui, M. H.; Hasanuzzaman, M. Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021, 40, 1513–1541. [Google Scholar]
- Guo, Q.; Major, I. T.; Howe, G. A. Resolution of growth–defense conflict: mechanistic insights from jasmonate signaling. Curr. Opin. Plant Biol. 2018, 44, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sözen, C.; Schenk, S. T.; Boudsocq, M.; Chardin, C.; Almeida-Trapp, M.; Krapp, A.; Hirt, H.; Mithöfer, A.; Colcombet, J. Wounding and insect feeding trigger two independent MAPK pathways with distinct regulation and kinetics. Plant Cell 2020, 32, 1988–2003. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant Responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar]
- Zhang, G.; Zhao, F.; Chen, L.; Pan, Y.; Sun, L.; Bao, N.; Zhang, T.; Cui, C.-X.; Qiu, Z.; Zhang, Y.; Yang, L.; Xu, L. Jasmonate-mediated wound signalling promotes plant regeneration. Nature Plants 2019, 5, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Browse, J.; Wager, A. Social network: JAZ protein interactions expand our knowledge of jasmonate signaling. Front. Plant Sci. 2012, 3, 41. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Melotto, M.; Mecey, C.; Niu, Y.; Chung, H. S.; Katsir, L.; Yao, J.; Zeng, W.; Thines, B.; Staswick, P.; Browse, J.; Howe, G. A.; He, S. Y. A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J. 2008, 55, 979–988. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S. Y.; Howe, G. A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [PubMed]
- Villarroel, C. A.; Jonckheere, W.; Alba, J. M.; Glas, J. J.; Dermauw, W.; Haring, M. A.; Van Leeuwen, T.; Schuurink, R. C.; Kant, M. R. Salivary proteins of spider mites suppress defenses in Nicotiana benthamiana and promote mite reproduction. Plant J. 2016, 86, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Melotto, M.; Yao, J.; He, S. Y. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 2017, 68, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, D.-D.; Fang, X.; Chen, X.-Y.; Mao, Y.-B. Plant specialized metabolism regulated by jasmonate signaling. Plant Cell Physiol. 2019, 60, 2638–2647. [Google Scholar] [CrossRef]
- Divekar, P. A.; Narayana, S.; Divekar, B. A.; Kumar, R.; Gadratagi, B. G.; Ray, A.; Singh, A. K.; Rani, V.; Singh, V.; Singh, A. K.; Kumar, A.; Singh, R. P.; Meena, R. S.; Behera, T. K. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Savatin, D. V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: the defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef]
- Shroff, R.; Vergara, F.; Muck, A.; Svatoš, A.; Gershenzon, J. , Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 6196–6201. [Google Scholar]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The Cellular and subcellular organization of the glucosinolate–myrosinase system against herbivores and pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef]
- Gossner, M. M.; Beenken, L.; Arend, K.; Begerow, D.; Peršoh, D. Insect herbivory facilitates the establishment of an invasive plant pathogen. ISME Commun. 2021, 1, 6. [Google Scholar] [CrossRef]
- Singh, K. B.; Foley, R. C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef]
- Agarwal, P.; Reddy, M. P.; Chikara, J. WRKY: its structure, evolutionary relationship, DNA-binding selectivity, role in stress tolerance and development of plants. Mol. Biol.Reports 2011, 38, 3883–3896. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Somssich, I. E. Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 2007, 10, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, C.; Fan, B.; Chen, Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 2006, 18, 1310–1326. [Google Scholar] [CrossRef]
- Kloth, K. J.; Wiegers, G. L.; Busscher-Lange, J.; van Haarst, J. C.; Kruijer, W.; Bouwmeester, H. J.; Dicke, M.; Jongsma, M. A. AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. J. Exp. Bot. 2016, 67, 3383–3396. [Google Scholar] [CrossRef]
- Liu, J.; Osbourn, A.; Ma, P. , MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef]
- Seo, M.-S.; Kim, J. S. Understanding of MYB transcription factors involved in glucosinolate biosynthesis in Brassicaceae. Molecules 2017, 22, 1549. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, P.; Yadav, N. R.; Yadav, R. C. MYB transcription factor genes as regulators for plant responses: an overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Sharoni, A. M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Fron. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef]
- Meraj, T. A.; Fu, J.; Raza, M. A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Kim, N. Y.; Jang, Y. J.; Park, O. K. AP2/ERF family transcription factors ORA59 and RAP2.3 Interact in the nucleus and function together in ethylene responses. Front. Plant Sci. 2018, 9, 1675. [Google Scholar] [CrossRef]
- Catchen, J.; Hohenlohe, P. A.; Bassham, S.; Amores, A.; Cresko, W. A. Stacks: an analysis tool set for population genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C. A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T. R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2012, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P. T.; Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 2014, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M. I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B. C.; Remm, M.; Rozen, S. G. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Li, Y.; Chia, J. M.; Bartfai, R.; Christoffels, A.; Yue, G. H.; Ding, K.; Ho, M. Y.; Hill, J. A.; Stupka, E.; Orban, L. Comparative analysis of the testis and ovary transcriptomes in zebrafish by combining experimental and computational tools. Comp. Funct. Genomics 2004, 5, 403–18. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J. M.; Terol, J.; Williams, T. D.; Nagaraj, S. H.; Nueda, M. J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.; Tanaseichuk, O.; Benner, C. Chanda, SK Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun 2019, 10, 1523. [Google Scholar] [CrossRef]
- Cassan, O.; Lèbre, S.; Martin, A. Inferring and analyzing gene regulatory networks from multi-factorial expression data: a complete and interactive suite. BMC Genom. 2021, 22, 387. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).