Submitted:
01 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
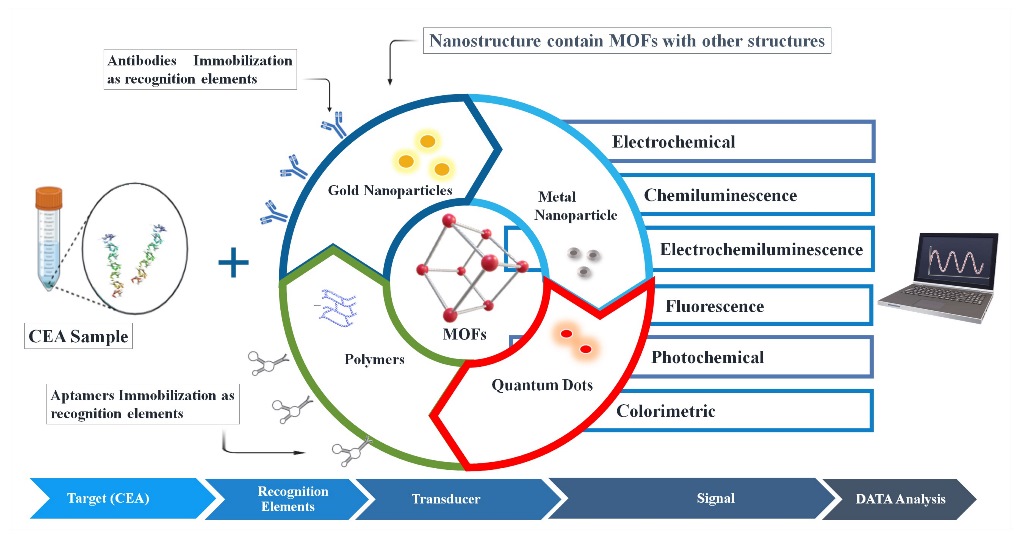
Keywords:
Background
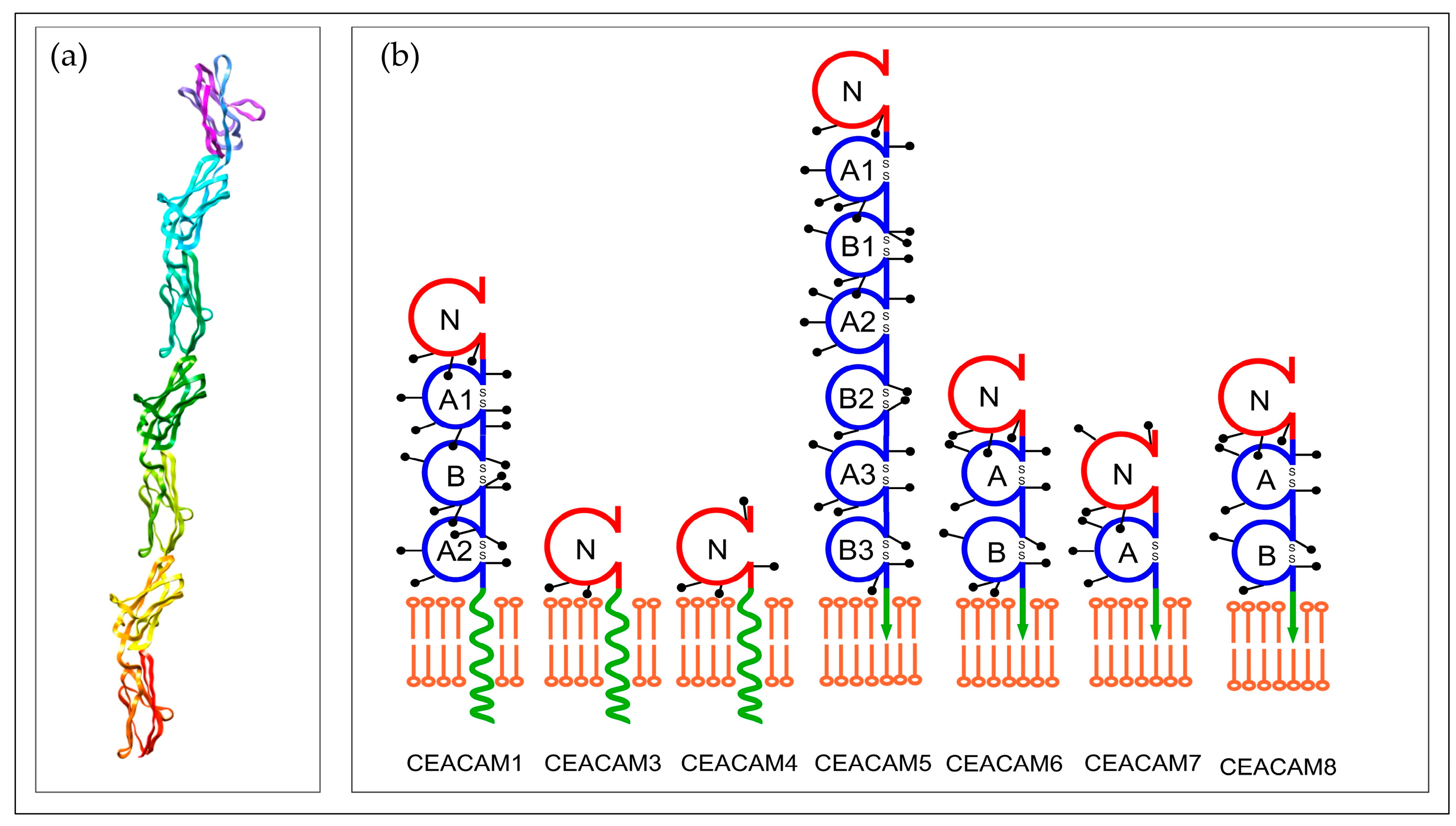
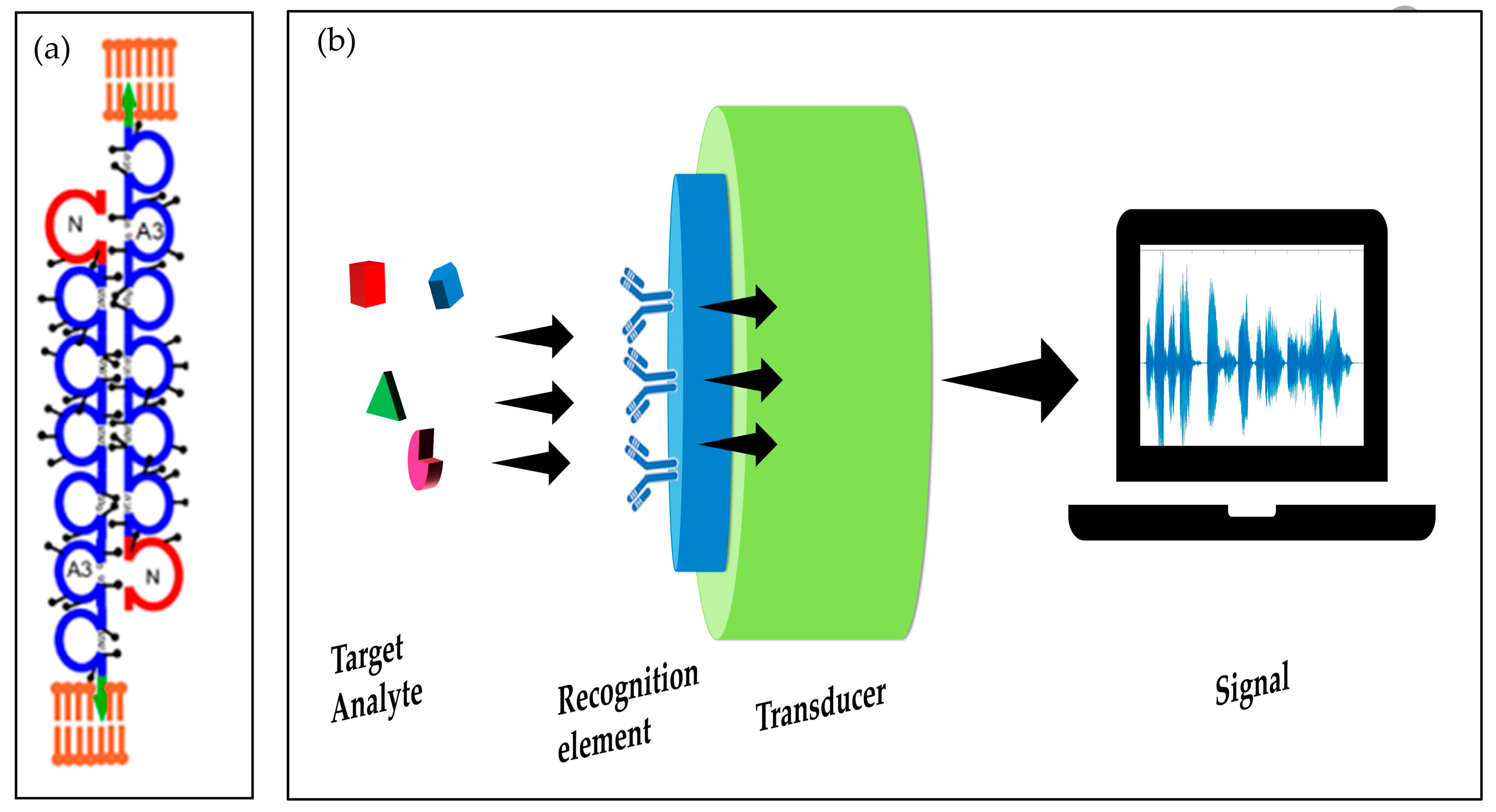
1. Structure and Function of CEA
Detection of CEA biomarker: conventional methods vs biosensors
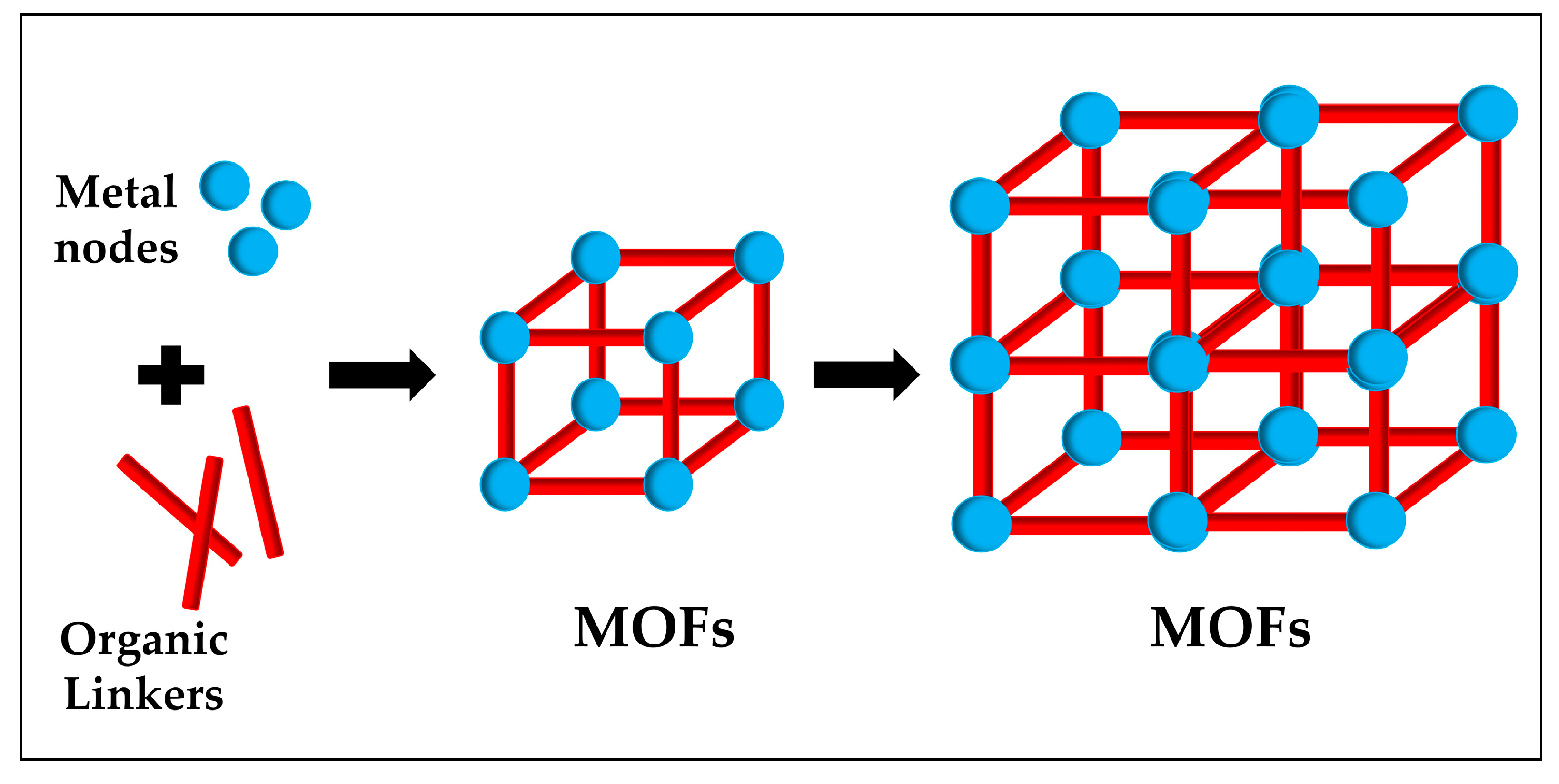
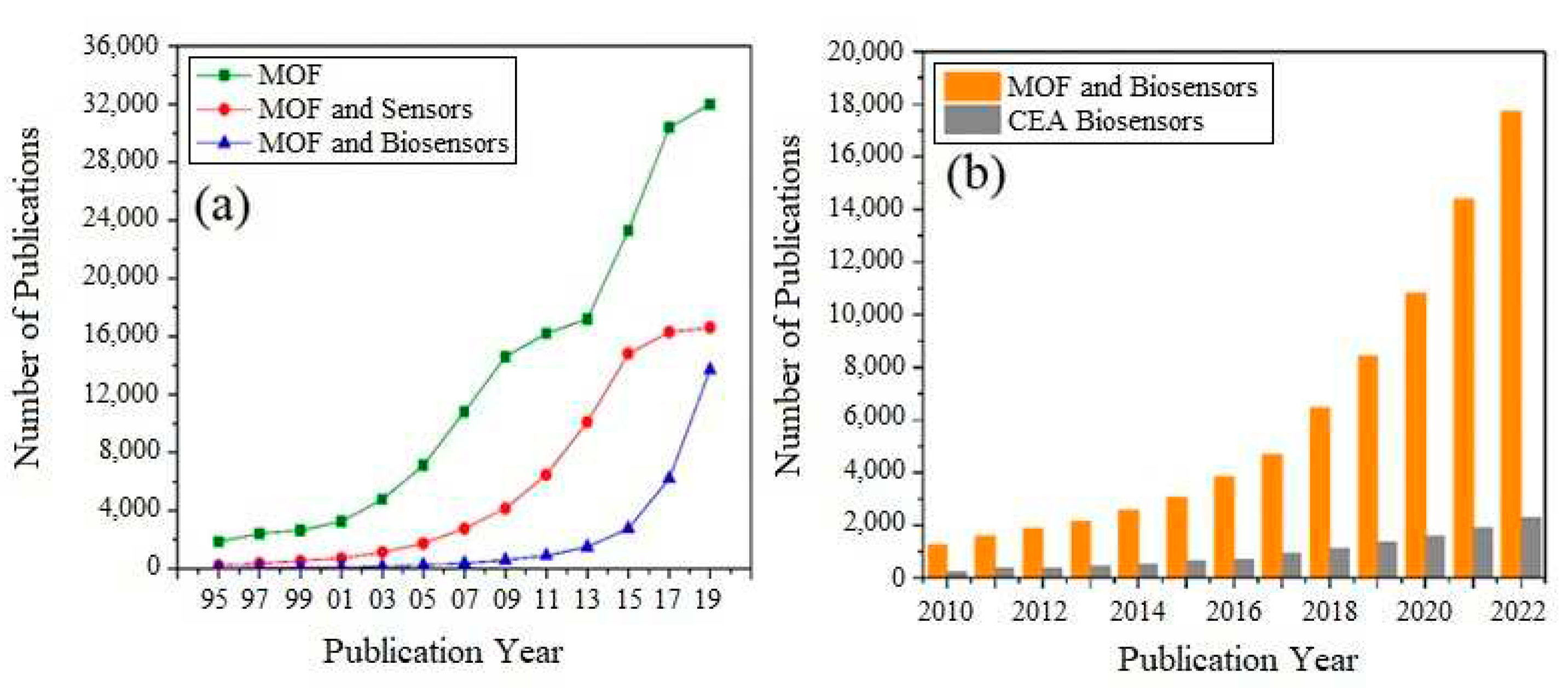
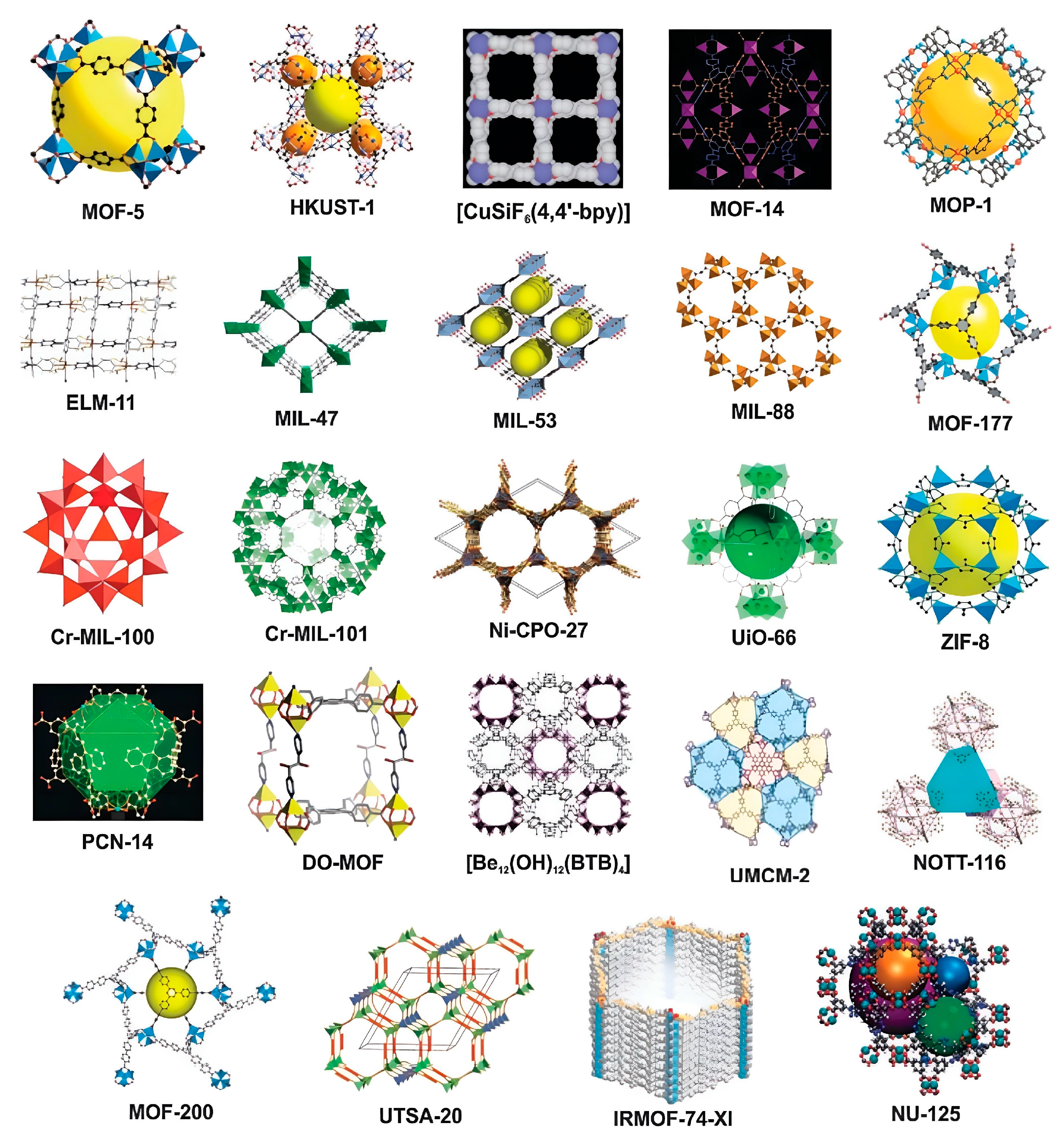
2. Why using MOFs for biosensors
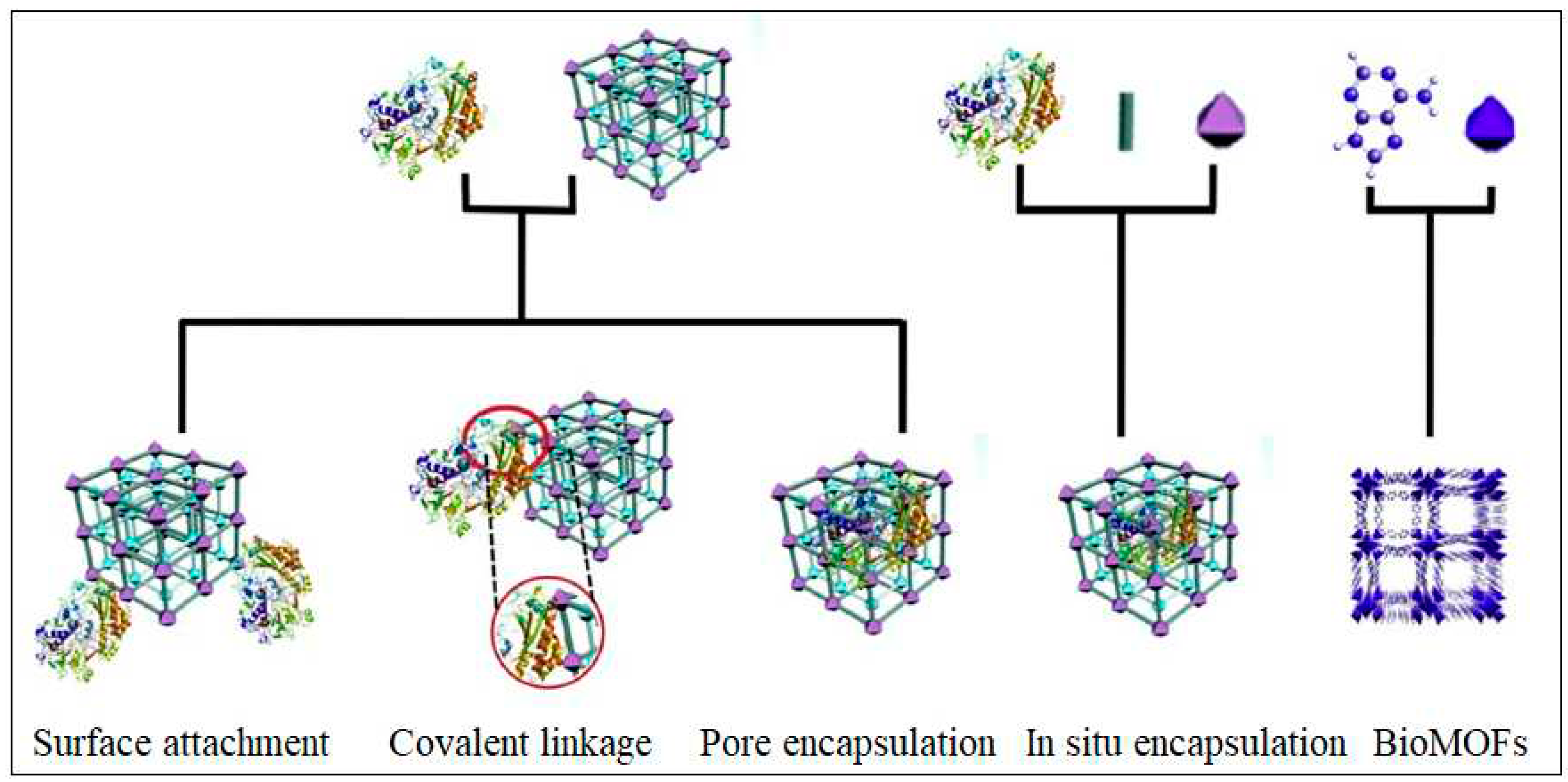
3. Progress in MOF-based biosensors for the detection of CEA
- (a).
- Electrochemical approach
- (b).
- Chemiluminescence Approach
- (c).
- Electrochemiluminescence Approach
- (d).
- Fluorescence Approach
- (e).
- Photoelectrochemical Approach
- (f).
- Colorimetric Approach
Conclusion and Future Perspectives
Acknowledgements
List of Abbreviations
| CEA | Carcinoembryonic antigen |
| MOFs | Metal organic frameworks |
| ELISA | Enzyme-linked immunosorbent assays |
| RIA | Radioimmunoassay |
| IRMA | Immunoradiometric assay |
| RT-PCR | Reverse transcriptase polymerase chain reaction |
| MIPs | Molecular imprinted polymers |
| AuNPs | Gold Nanoparticles |
| CNT | Carbon NanoTubes |
| CV | Cyclic Voltammetry |
| DPV | Differential Pulse Voltammetry |
| SWV | Square Wave Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| GCE | Glass Carbon Electrode |
| AFP | Alpa FetoProtein |
| AgNPs | Silver Nanoparticles |
| LOD | Limit of Detection |
| PDA | PolyDopamine |
| OMC | Ordered Mesoporous Carbon |
| MWCNTs | Multiwall Carbon Nanotubes |
| CL | Chemiluminescence |
| ECL | Electrochemiluminescence |
| GO | Graphene Oxide |
| QDs | Quantum Dots |
| PAD | Paper-based analytical Device |
| PEC | Photoelectrochemical |
| TDN | DNA tetrahedral |
| CDs | Carbon Dots |
References
- Malhotra, B.D.; Kumar, S.; Pandey, C.M.J. J. Phys. Conf. Ser. 2016, 704, 012011. [CrossRef]
- Vinood, P.B.; Victor, P.R. Biomarkers in Disease: Methods, Discoveries and Applications Series. In Textbook of Cancer Epidemiology; Springer: Dordrecht, Netherlands, 2015. [Google Scholar]
- Henry, N.L.; Hayes, D.F. Mol. Oncol. 2012, 6, 140–146. [CrossRef] [PubMed]
- Gold, P.; Freedman, S.O. J. J. Exp. Med. 1965, 122, 467–481. [CrossRef] [PubMed]
- Nan, J.; Li, J.; Li, X.; Guo, G.; Wen, X.; Tian, Y. Biomark. Cancer. 2017, 9. [CrossRef]
- Lee, J.H.; Lee, S.-W. Gastroenterol. Res. Pract., 2017, 2017, 1–11.
- Öbrink, B. Curr. Opin. Cell Biol. 1997, 9, 616–626. [CrossRef]
- Xiang, W.; Lv, Q.; Shi, H.; Xie, B.; Gao, L. Talanta 2020, 214, 120716. [CrossRef]
- Hammarström, S. Semin. Cancer Biol. 1999, 9, 67–81. [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Cancer. Metastasis. Rev. 2013, 32, 643–671. [CrossRef]
- Schwab, M. Encyclopedia of Cancer (Ed.) Encyclopedia of Cancer; (4th ed. 2017 ed.) ; Springer: Berlin, Heidelberg, 2011. [Google Scholar]
- Zheng, C.; Feng, J.; Lu, D.; Wang, P.; Xing, S.; Coll, J.-L.; Yang, D.; Yan, X. PLoS One 2011, 6.
- Li, J.; Li, S.; Yang, C.F. Electroanalysis 2012, 24, 2213–2229. [CrossRef]
- Tang, Z.; Ma, Z. Biosens. Bioelectron. 2017, 98, 100–112. [CrossRef] [PubMed]
- Špringer, T.; Homola, J. Anal. Bioanal.Chem 2012, 404, 2869–2875. [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Liu, Y.; Cui, J.; Liu, H.; Wang, P.; Li, Y.; Chen, L.; Zhao, Z.; Dong, Y. Biosens. Bioelectron. 2017, 90, 31–38. [CrossRef]
- Lai, Y.; Deng, Y.; Yang, G.; Li, S.; Zhang, C.; Liu, X. J. J. Biomed. Nanotechnol. 2018, 14, 1688–1694. [CrossRef] [PubMed]
- Carrasco, S. Biosensors 2018, 8, 92. [CrossRef]
- Yap, M.H.; Fow, K.L.; Chen, G.Z. Green Energy Environ. 2017, 2, 218–245. [CrossRef]
- Afreen, S.; He, Z.; Xiao, Y.; Zhu, J.J. J. J. Mater. Chem. B 2020, 8, 1338–1349. [CrossRef]
- Anik, Ü.; Timur, S.; Dursun, Z. Mikrochim. Acta 2019, 186, 18–24.
- Asefa, T.; Shi, Y.L. J. J. Mater. Chem 2009, 18, 5604–5614. [CrossRef]
- Zhang, Z.H.; Duan, F.H.; Tian, J.Y.; He, J.Y.; Yang, L.Y.; Zhao, H.; Zhang, S.; Liu, C.S.; He, L.H.; Chen, M.; Chen, D.M.; Du, M. ACS Sens. 2017, 7, 982–989.
- Du, L.; Chen, W.; Zhu, P.; Tian, Y.; Chen, Y.; Wu, C. Biotechnol. J. 2020, 16, 1900424.
- Osman, D.I.; El-Sheikh, S.M.; Sheta, S.M.; Ali, O.L.; Salem, A.M.; Shousha, W.G.; EL-Khamisy, S.F.; Shawky, S.M. Biosens. Bioelectron. 2019, 141, 111451. [CrossRef] [PubMed]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Coord. Chem. Rev. 2019, 384, 90–106.
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; Wang, Q.; Zou, L.; Zhang, Y.; Zhang, L.; Fang, Y.; Li, J.; Zhou, H.C. Adv. Mater. 2018, 30, 1–35.
- Zhang, Y.; Bo, X.; Nsabimana, A.; Han, C.; Li, M.; Guo, L. J. J. Mater. Chem, 2015, 3, 732–738. [CrossRef]
- Zhang, B.; Luo, Y.; Kanyuck, K.; Saenz, N.; Reed, K.; Zavalij, P.; Mowery, J.; Bauchan, G. RSC Adv. 2018, 833059–33064.
- Khan, N.A.; Jhung, S.H. Coord. Chem. Rev 2015, 285, 11–23.
- Campagnol, N.; Souza, E.R.; De Vos, D.E.; Binnemans, K.; Fransaer, J. Chem. Commun. 2014, 50, 12680–12683.
- Masoomi, M.Y.; Morsali, A.; Junk, P.C. CrystEngComm 2015, 17, 686–692. [CrossRef]
- Choi, J.S.; Son, W.J.; Kim, J.; Ahn, W.S. Microporous Mesoporous Mater. 2008, 116, 727–731. [CrossRef]
- Li, M.; Zhang, G.; Boakye, A.; Chai, H.; Qu, L. Front. bioeng. biotechnol. 2021, 9, 1–8.
- Ma, T.; Li, H.; Ma, J.; Cheng, P. Dalton Trans 2020, 49, 17121–17129.
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Environ. Res., 2022, 204, 112320.
- Ulhakim, M.T.; Rezki, M.; Dewi, K.K.; et al. J. Electrochem. Soc. 2020, 167, 136509. [CrossRef]
- Zhang, P.; Huang, H.; Wang, N.; Li, H.; Shen, D.; Ma, H. Mikrochim. Acta 2017, 184, 4037–4045.
- Li, W.; Ma, C.; Song, Y.; Hong, C.; Qiao, X.; Yin, B. Nanotechnology 2020, 31, 185605. [CrossRef]
- Liu, J.; Shang, Y.; Zhu, Q.; Zhang, X.; Zheng, J. Mikrochim. Acta 2019, 186, 509.
- Zhou, X.; Guo, S.; Gao, J.; Zhao, J.; Xue, S.; Xu, W. Biosen. Bioelectron. 2017, 98, 83–90. [CrossRef]
- Guo, C.; Su, F.; Song, Y.; Hu, B.; Wang, M.; He, L.; Peng, D.; Zhang, Z. ACS Appl. Mater. Interfaces 2017, 9, 41188–41199. [CrossRef]
- Li, J.; Liu, L.; Ai, Y.; Liu, Y.; Sun, H.; Liang, Q. ACS Appl. Mater. Interfaces 2020, 12, 5500–5510. [CrossRef] [PubMed]
- Bao, T.; Fu, R.; Wen, W.; Zhang, X.; Wang, S. ACS Appl. Mater. Interfaces 2020, 12, 2087–2094. [CrossRef] [PubMed]
- Hong, F.; Wang, Q.; Wang, W.; et al, J.; et al. J. Electroanal. Chem. 2020, 861, 113956. [CrossRef]
- Zhang, Y.; Zhang, Z.; Rong, S.; Yu, H.; Gao, H.; Ding, P.; Chang, D.; Pan, H. Mikrochim. Acta, 2020, 187, 265.
- Liu, J.; Shang, Y.; Xu, J.; Chen, Y.; Jia, Y.; Zheng, J. J. J. Electroanal. Chem., 2020, 877, 114563. [CrossRef]
- Biswas, S.; Lan, Q.; Li, C.; Xia, X.H. Anal. Chem. 2020, 94, 3013–3019.
- Liu, M.; Lin, Z.; Lin, J. Anal. Chim. Acta, 2010, 670, 1–10. [CrossRef]
- Aboul-Enein, H.Y.; Stefan, R.; van Staden, J.F. Crit. Rev. Anal. Chem. 1999, 29, 323–331. [CrossRef]
- Han, R.; Sun, Y.; Dai, Y.; Gao, D.; Wang, X.; Luo, C. Sens. Actuators B Chem., 2021, 326, 128833. [CrossRef]
- Cao, Y.; Gou, X.; Zhu, J. Anal. Chem. 2020, 92, 431–454.
- Zhou, J.; Li, Y.; Wang, W.; Tan, X.; Lu, Z.; Han, H. Biosens. Bioelectron. 2020, 164, 112332. [CrossRef] [PubMed]
- Yang, Y.; Hu, G.B.; Liang, W.B.; Yao, L.Y.; Huang, W.; Zhang, Y.J.; Zhang, J.L.; Wang, J.M.; Yuan, R.; Xiao, D.R. New J. Chem. 2020, 12, 5932–5941.
- Huang, X.; Deng, X.; Qi, W.; Wu, D. New J. Chem. 2018, 42, 13558–13564.
- Liu, Q.; Yang, Y.; Liu, X.P.; Wei, Y.P.; Mao, C.J.; Chen, J.S.; Niu, H.L.; Song, J.M.; Zhang, S.Y.; Jin, B.K.; Jiang, M. Sens. Actuators. B Chem. 2017, 242, 1073–1078. [CrossRef]
- Huo, Y.; Liu, S.; Gao, Z.; Ning, B.; Wang, Y. Mikrochim. Acta 2021, 4, 1–29.
- Yang, J.; Ni, W.; Ruan, B.; Tsai, L.; Ma, N.; Shi, D.; Jiang, T.; Tsai, F. ECS J. Solid State Sci. Technol. 2021, 10, 056003. [CrossRef]
- Zhao, D.; Wu, Z.; Yu, J.; Wang, H.; Li, Y.; Duan, Y. Chem. Eng. J., 2020, 383, 123230.
- Lv, S.; Tang, Y.; Zhang, K.; Tang, D. Anal. Chem. 2018, 90, 14121–14125.
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. J. J. otochem. Photobiol. C Photochem. Rev., 2015, 24, 43–63. [CrossRef]
- Liu, X.; Zhao, Y.; Li, F. Biosen. Bioelectron. 2021, 173, 112832. [CrossRef]
- Li, Y.; Zhang, X.; Liu, P.; He, M.; Li, C.; Wang, Y. Nanoscale 2021, 13, 9757–9765. [CrossRef] [PubMed]
- Mauriz, E. Sensors 2020, 20, 6214. [CrossRef] [PubMed]
- Sha, L.; Zhu, M.; Lin, F.; Yu, X.; Dong, L.; Wu, L.; Ding, R.; Wu, S.; Xu, J. Gels 2021, 7, 181. [CrossRef] [PubMed]
- Zeng, Y.; Wang, M.; Sun, Z.; Sha, L.; Yanga, J.; Li, G. J. J. Mater. Chem. B, 2022, 10, 450–455. [CrossRef] [PubMed]
- Yan, H.; Jiao, L.; Xu, H.W.W.; Wu, Y.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Sens. Actuators B Chem., 2019, 297, 126760. [CrossRef]
| # | MOFs | Metal used | Organic Ligand | Surface Modifications & added materials | Types of Sensing | LOD/Detection range | Reference |
|---|---|---|---|---|---|---|---|
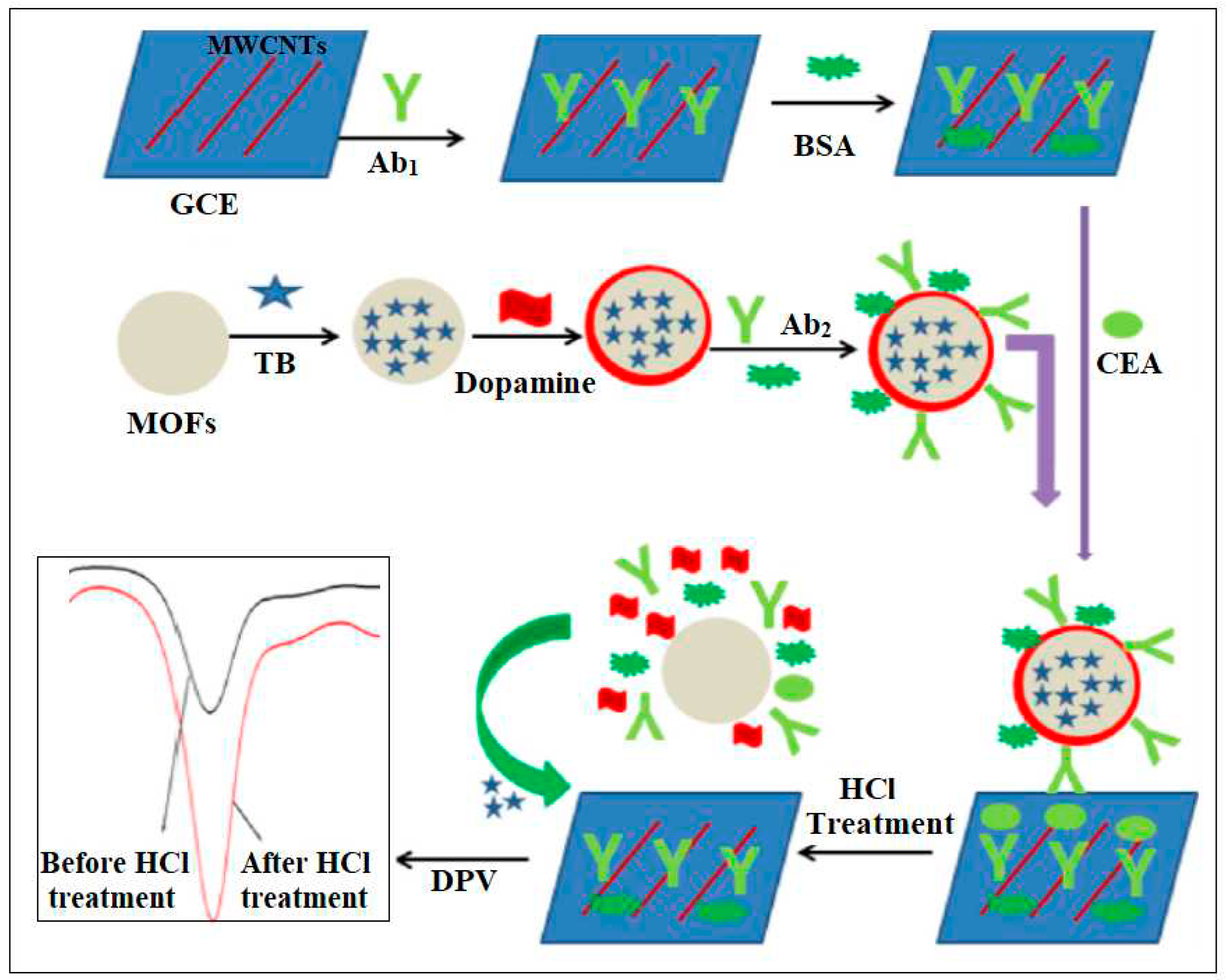
| 1 | Pd/Cd-MOFs | Pd/Cd | 2- aminoterephthalic acid | Immobilization of labels for secondary anti-CEA and secondary anti-AFP antibody | Electrochemical | 0.03 pg/mL and 0.1 pg/mL | [38] |
| 2 | Ce-MOF | Ce | 1,3,5-benzenetricarboxylic acid | hyaluronic acid was coated on the surface of Ce-MoF, which loaded with silver nanoparticles (AgNPs) and horseradish peroxidase | Electrochemical | 0.2 pg / mL | [39] |
| 3 | Ag-MOF | Ag | Terephthalic acid | Ag-MOF was dopped by gold nanoparticles and labelled by anti-CEA | Electrochemical | 8.0 fg/mL | [40] |
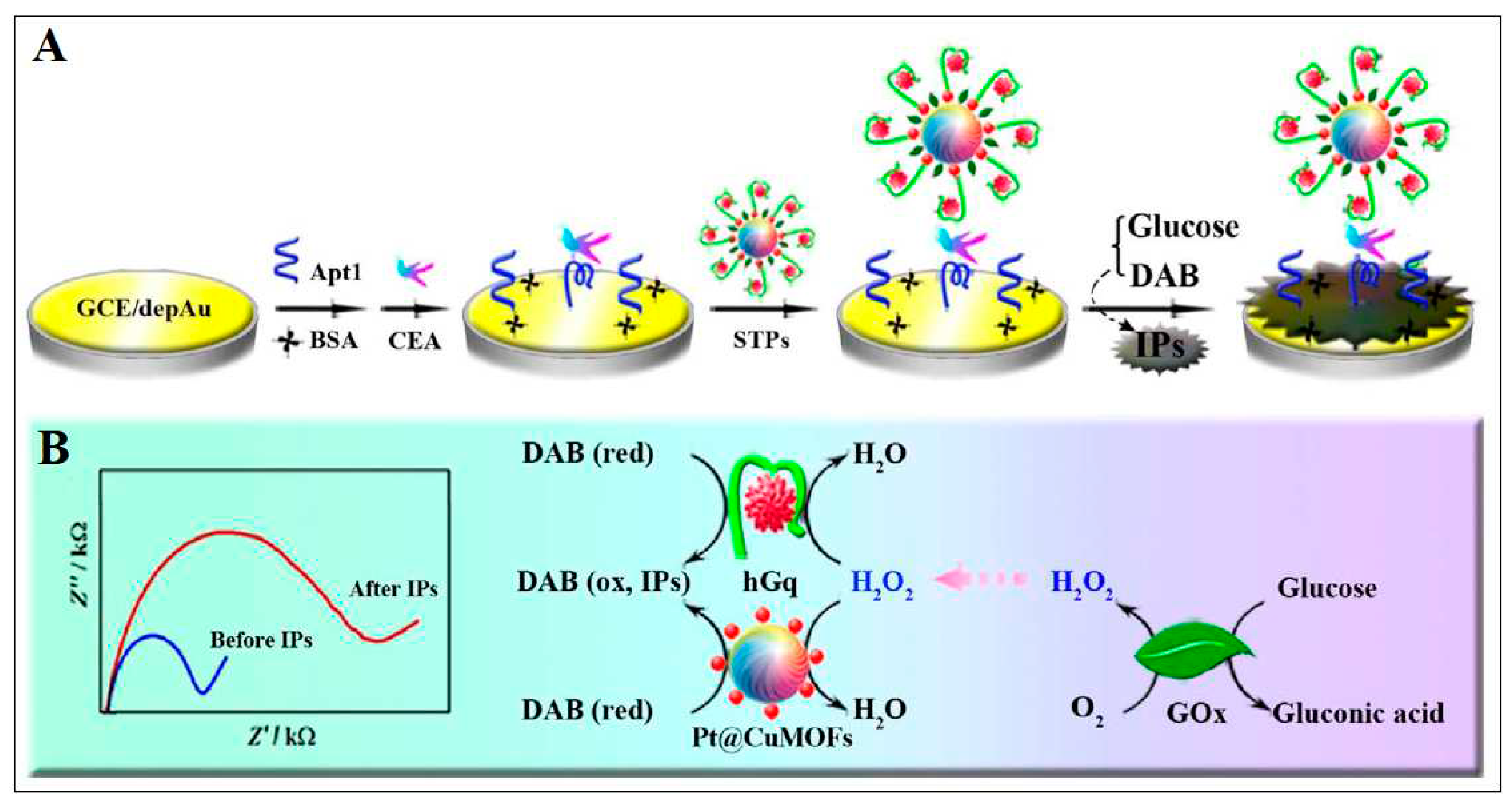
| 4 | Cu-MOF | Cu | 2-amino terephthalic acid | Platinum nanoparticles (PtNPs) was linked to Cu-MOF then, CEA aptamer loaded onto Pt@CuMOFs, finally, this was bound with hemin to form hemin@G-quadruplex (hGq) with mimicking peroxidase activity | Electrochemical | 0.023 pg /mL |
[41] |
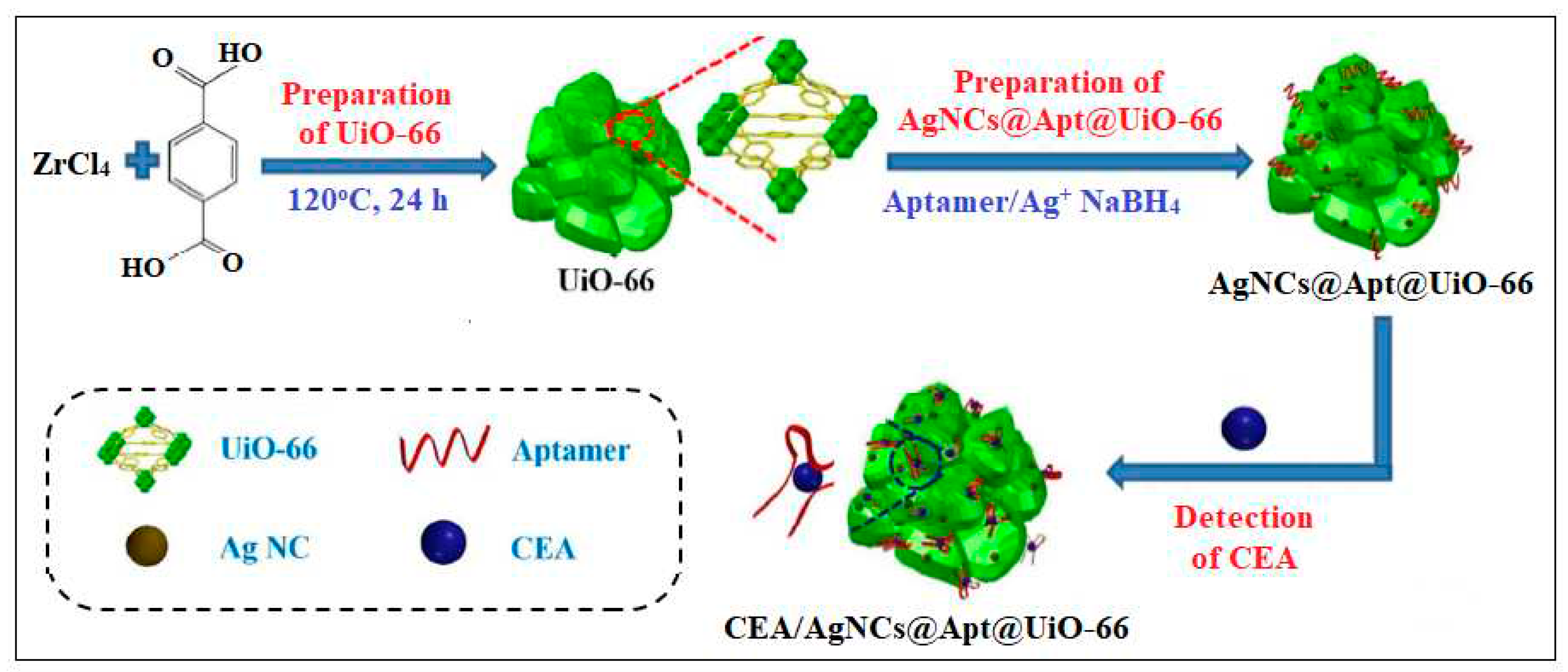
| 5 | UiO-66 | Zr | 2-Aminoterephthalic acid | MOF embedded with silver nanoclusters (AgNCs) using the carcinoembryonic antigen (CEA)-targeted aptamer as template | A. Electrochemical 1-Impedance |
8.88 Pg/mL |
[42] |
| 2-Differential pulse voltammetry | 4.93 pg/mL |
||||||
| B. SPR | 0.3 ng/mL | ||||||
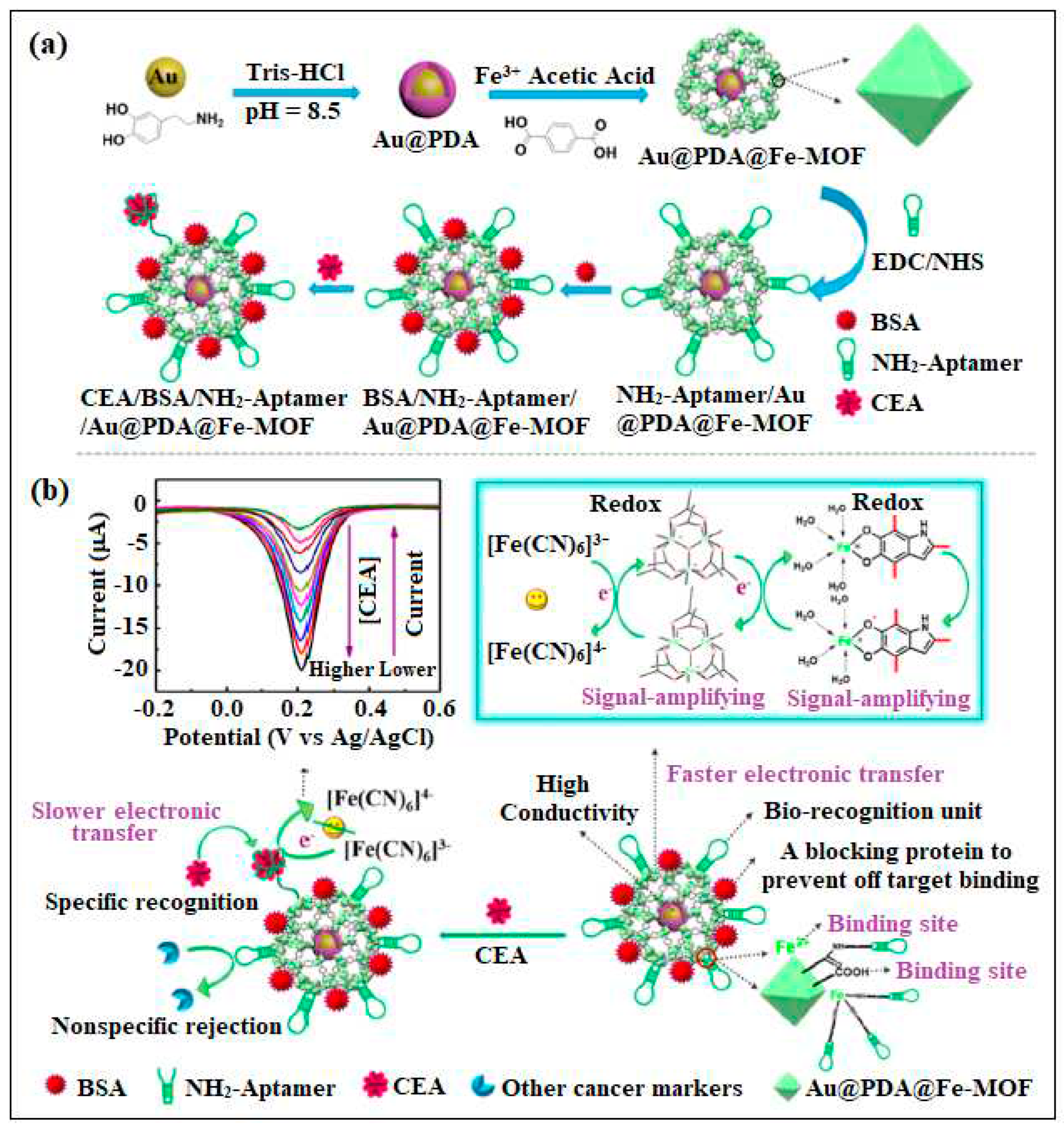
| 6 | Fe-MOF | Fe | 1, 4-dicarboxybenzene | self-polymerized dopamine-decorated AuNPs was loaded on Fe-MOF and attached to CEA aptamer | Electrochemical | 0.33 fg /mL | [43] |
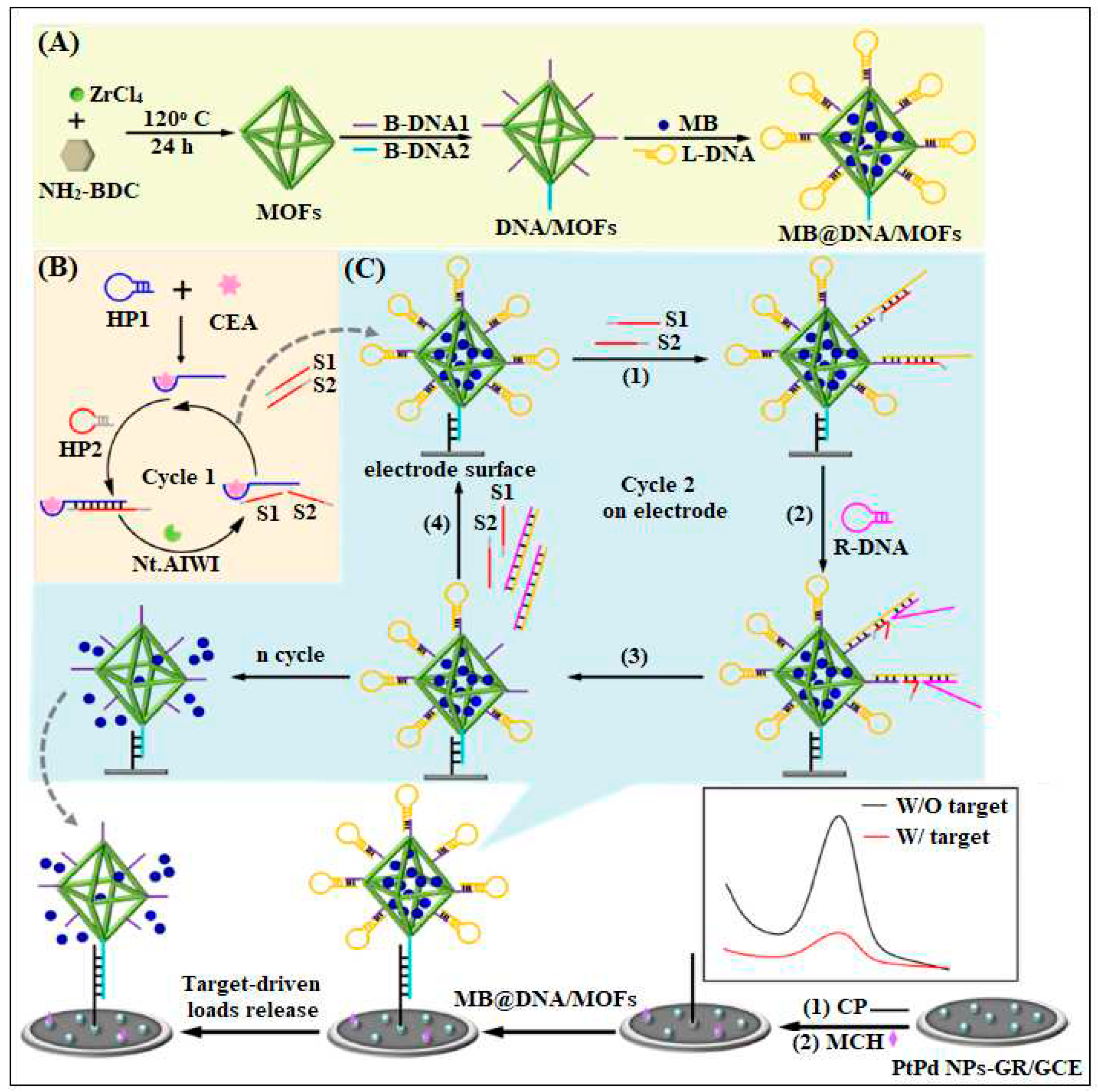
| 7 | UiO-66-NH2 | Zr | 2-Aminoterephthalic acid | By using MOF as nanocarrier of electroactive molecules (methylene blue, MB) and functionalized by the assembled DNA | Electrochemical | 16 fg/mL | [44] |
| 8 | Pd-MOF | Pd | 2- amino-1,4-benzenedicarboxylic acid (H2N-BDC) | dendritic (Hybridization chain reaction) HCR-triggered DNA nano structure was labeled with Pb-MOF | Electrochemical | 0.333 pg /mL | [45] |
| 9 | Zif-8 | Zn | Methyl Imidazole | It is based on the use of Au NPs modified ZIF-8 and ordered mesoporous carbon (OMC) | Electrochemical | 1.3 pg/mL | [46] |
| 10 | Cu-MOF | Cu | Terephthalic acid | Toluidine blue (TB) loaded mesoporous Cu-MOFs with polydopamine (PDA) coating were employed as a signal probe | Electrochemical | 3.0 fg/mL | [47] |
| 11 | Zr- MOF | Zr | 4′,4‴,4′′′′- nitrilotris[1,1′-biphenyl]-4-carboxylic acid (H3NBB) | Aptamers of CEA, thrombin, and kanamycin were separately immobilized on the MOF |
Electrochemical | 0.40 pg/ mL 0.21pg/mL 0.37pg/mL |
[23] |
| 12 | Sm-MOF | Sm | trimesic acid (TMA), meso-tetra(4-carboxyphenyl)porphine (TCPP), and 1,3,6,8-tetra(4-carboxylphenyl) pyrene(TBPy) | Anti-CEA immobalization | Electrochemical | SmTMA, SmTBPy, and SmTCPP MOF-based immunosensors are determined to be 0.001, 0.05, and 0.01 U /mL, respectively |
[48] |
| 13 | MIL-88B | Fe | 2-aminoterephthalic acid | Hemin modified Mil-88B Immobilization of CEA aptamer |
CL | 1.5 × 10-3 ng/mL | [51] |
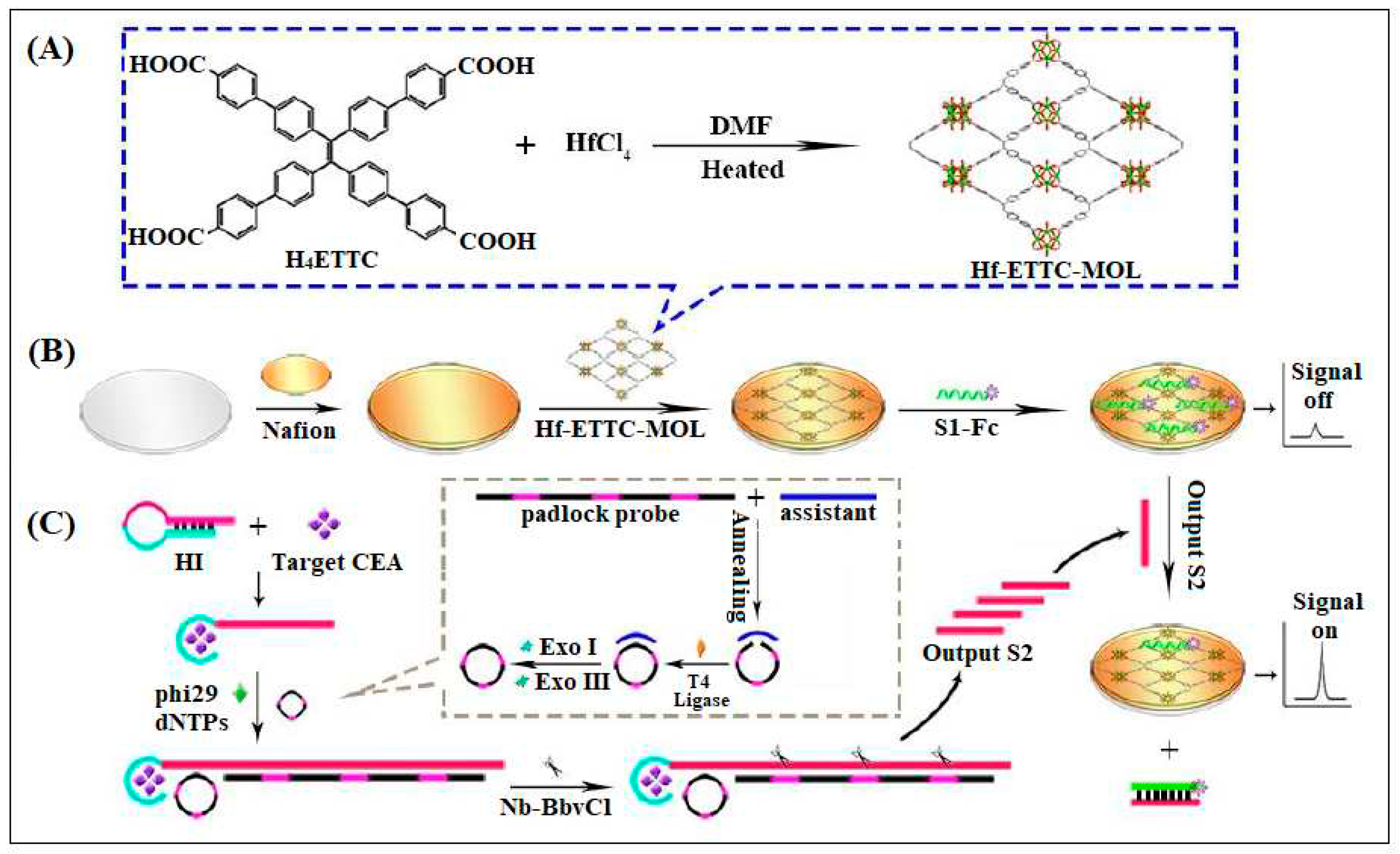
| 14 | Hf-ETTC-MOL | Hf | H4ETTC (H4ETTC = 4',4''',4''''',4'''''''-(ethene-1,1,2,2-tetrayl)tetrakis(([1,1'-biphenyl]-4-carboxylic acid))) | two-dimensional (2D) ultrathin metal-organic layer (MOL) was used as a platform for CEA detection | ECL | 0.63 fg/mL | [54] |
| 15 | Zif-8 | Zn | Methyl imidazole | ZIF-8 and graphene oxide (GO) to form a ZIF-8@GO composite Then, the in situ growth of AuNPs due to the p–p interaction between AuNPs and Zif-8@GO take place |
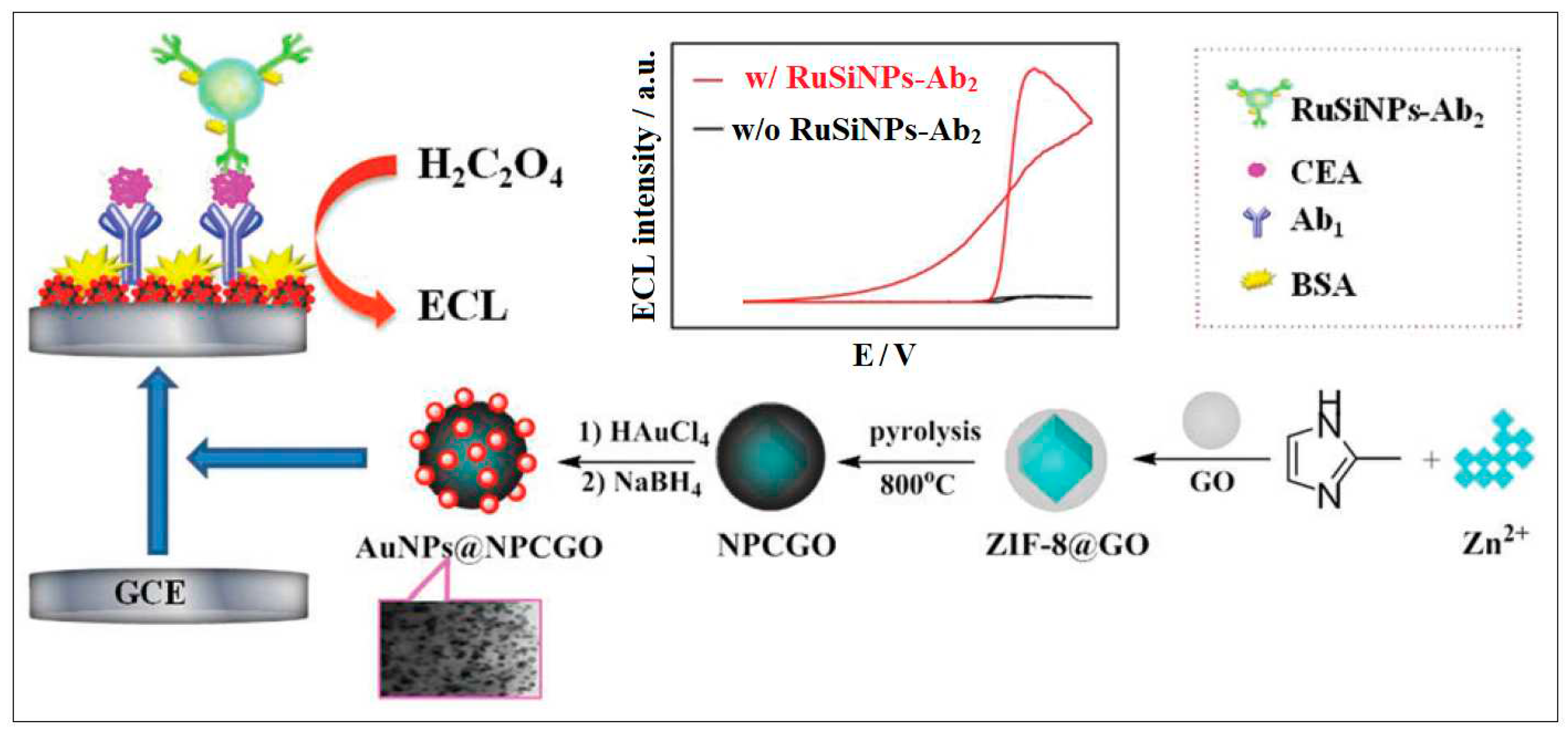
ECL | 0.003 ng/mL | [55] |
| 16 | MIL-101 | Cr | Terephthalic acid | prepared MIL-101-CdSe nanocomposites antibodies for CEA was linked |
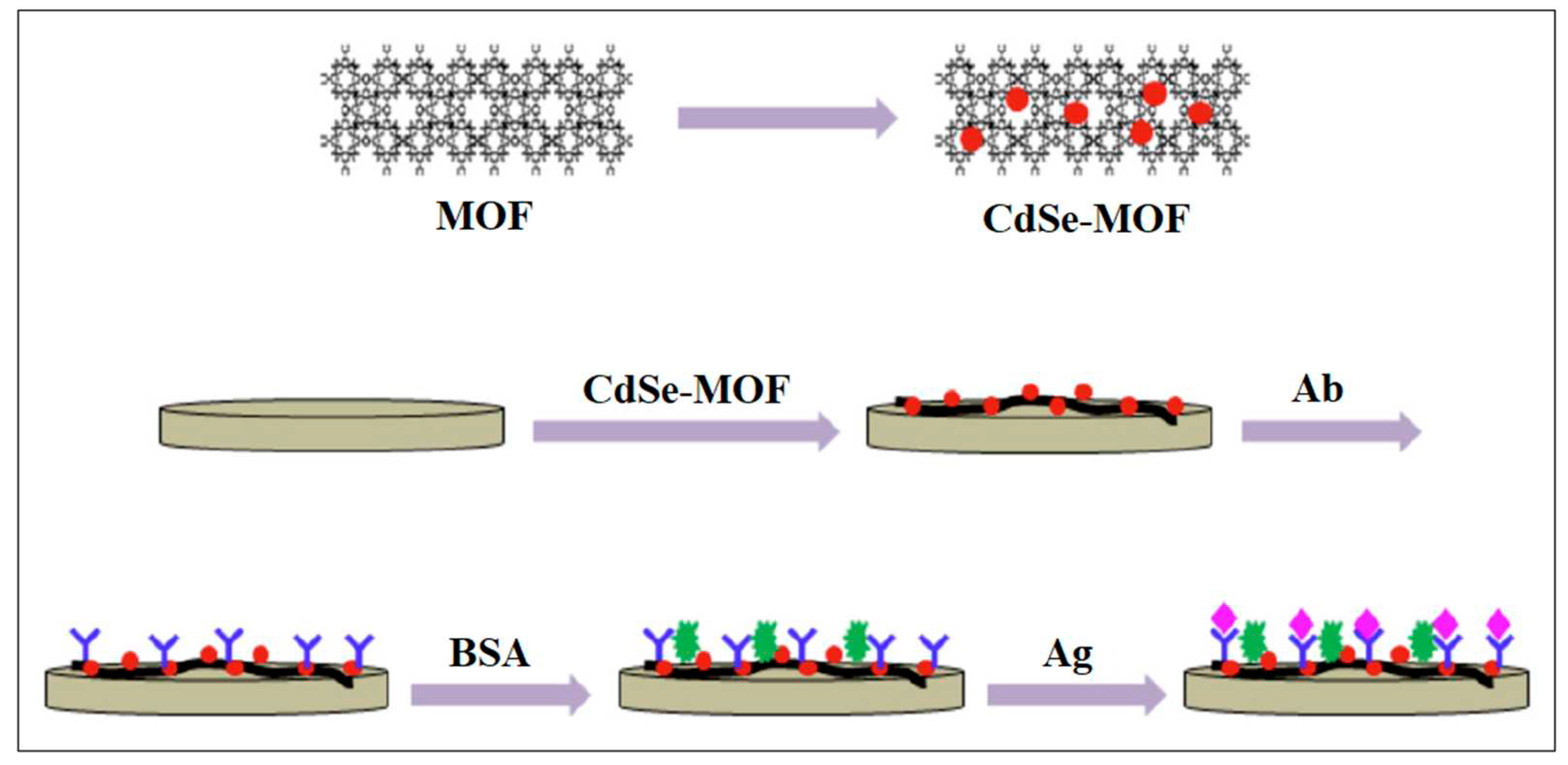
ECL | 0.33 fg /mL | [56] |
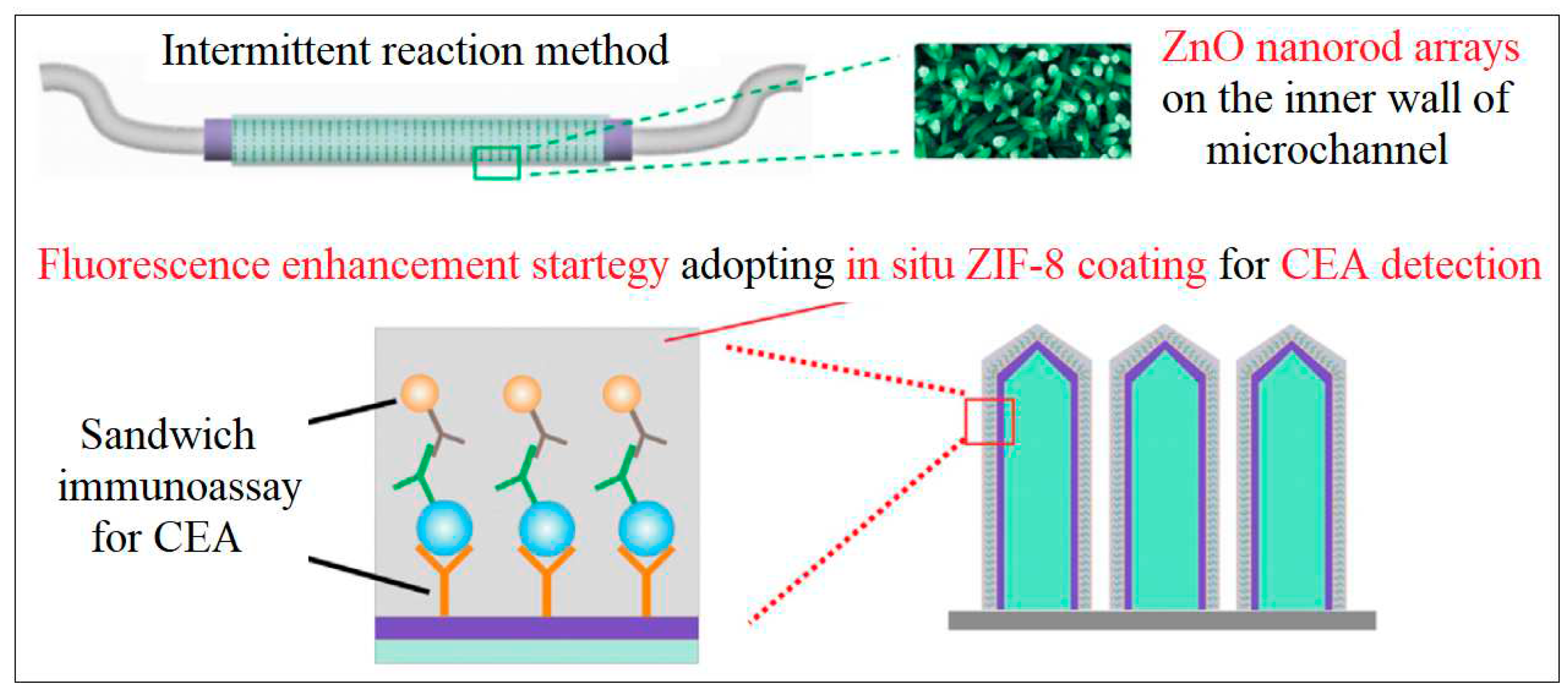
| 17 | Zif-8 | Zn | Methyl Imidazole | formation of sandwich immunoassay by using Zif-8 coated with the ZnO/PAA (Polyacrylic acid) nanorod arrays | Fluorescence | 0.01 pg /mL | [59] |
| 18 | NH2-MIL-125(Ti) | Ti | 2- amino-1,4-benzenedicarboxylic acid (H2N-BDC) | Gold nanoparticles heavily functionalized with glutamate dehydrogenase (GDH) and secondary antibody were used for generation of wet NH3 | Fluorescence | 0.041 ng /mL | [60] |
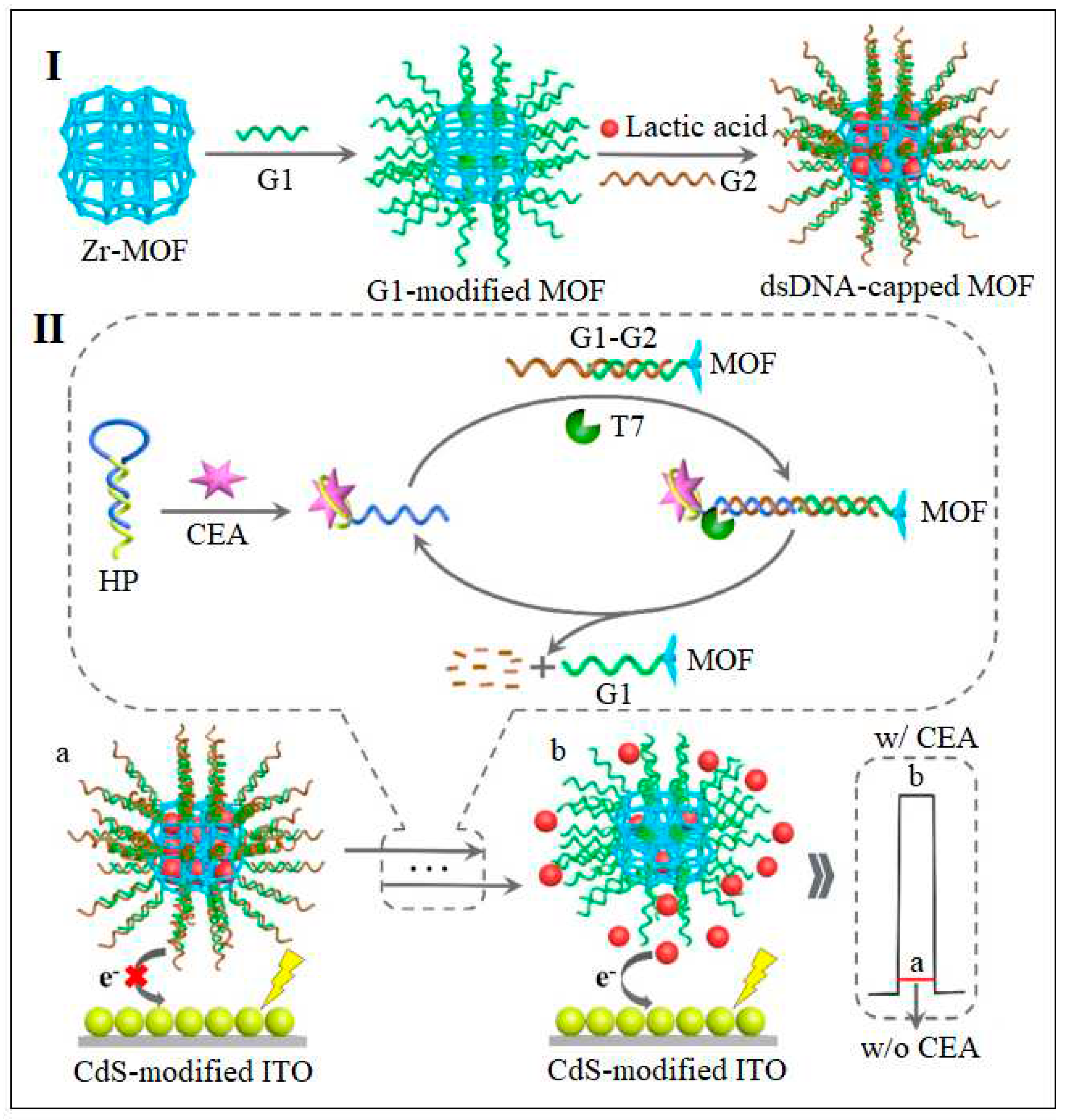
| 19 | UiO-66-NH2 | Zr | 2-Aminoterephthalic acid | MOF Loaded with lactic acid and attached to dsDNA | PEC | 0.36 fg /mL | [62] |
| 20 | Yb-MOF | Yb | 1,1′-(1,5-dihydropyrene-2,7-diyl)bis(3-(4-carboxybenzyl)-1H-imidazol-3-ium) bromide [DDPDBCBIm(Br)2] ionic liquid | Combined with Gold Nanoparticles | PEC | 0.005-15 ng/mL | [63] |
| 21 | PCN-222 | Zr and Fe-based MOF | meso-tetra (4-carboxyphenyl) porphine ferric chloride (Fe-TCPP) | CEA Aptamed was immobilized on the PCN-222 | Colorimetric | 3.3 pg/mL | [65] |
| 22 | Cu-TCPP | Cu-MOF | TCPP | Gold Nanoparticles immobilization and aptamer | Colorimetric | 1pg/mL to 1000 ng/mL | [66] |
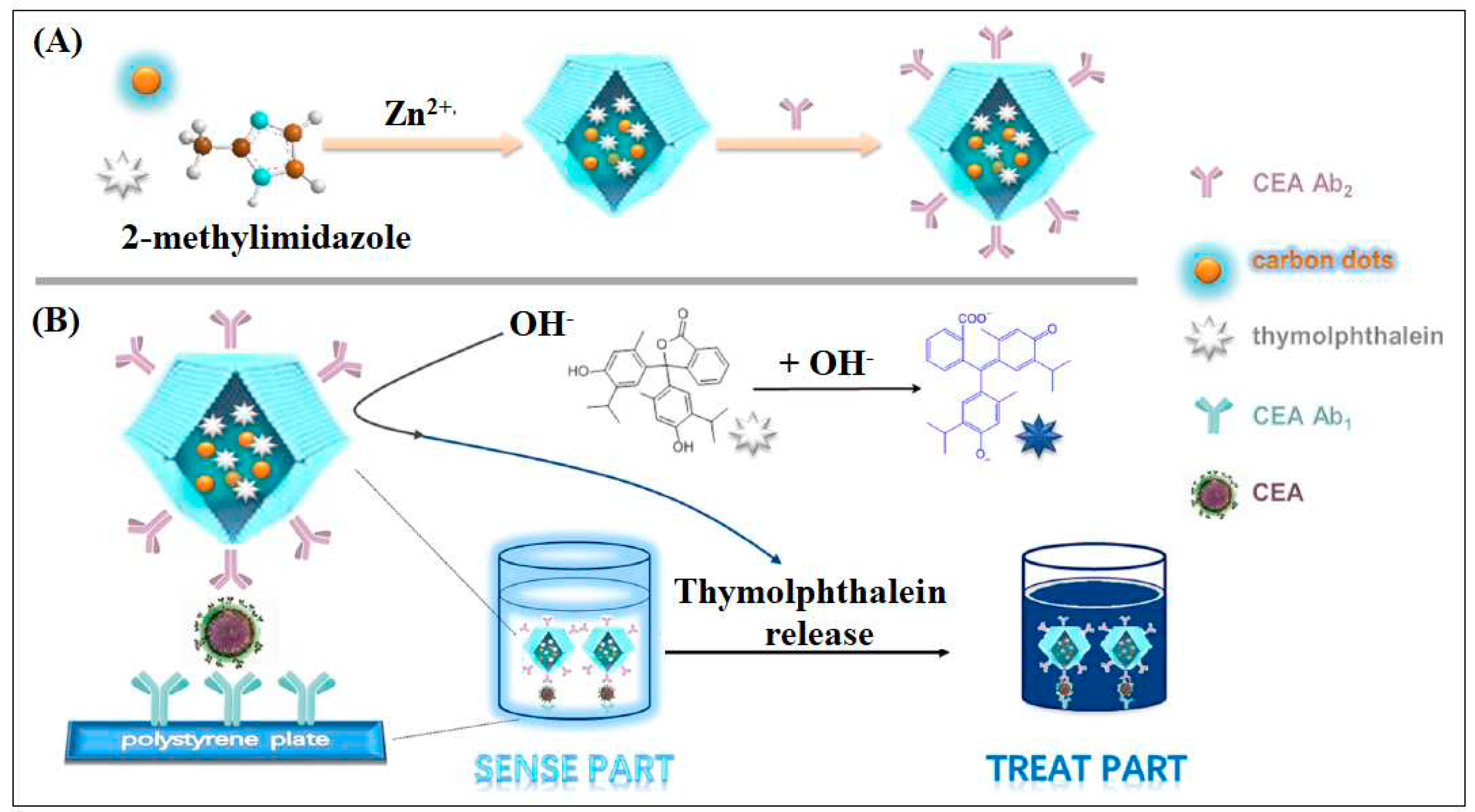
| 23 | Zif-8 | Zn | Methyl Imidazole | ZIF-8 used as the carrier to deliver the tracer agent carbon dots (CDs) and the “drug” thymolphthalein (TP) | ELIZA | 10 pg/mL | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





