Submitted:
25 July 2023
Posted:
25 July 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Results
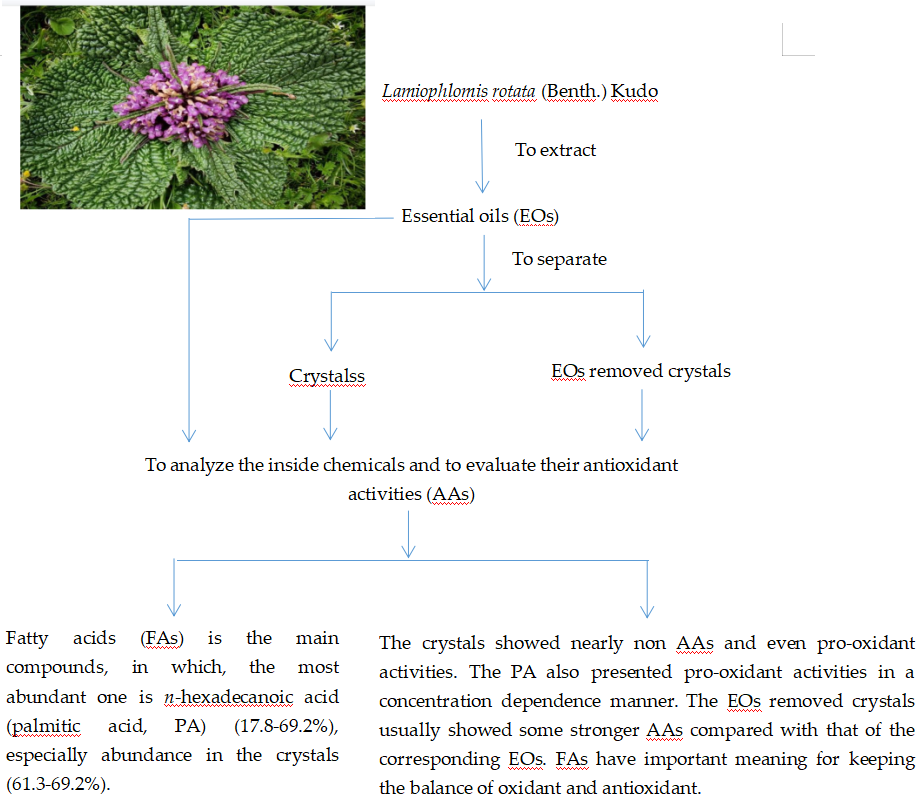
2.1. Chemicals in the EOs of L. ratata
2.2. AAs of these EOs and their representative chemicals
3. Discussion
4. Materials and Methods
4.1. Extraction and Separation
4.2. Sample Preparation
4.3. GC Analyses
4.4. Identification and Quantitation
4.4.1. Identification
4.4.2. Quantitation
4.5. Antioxidant activities
4.5.1. DPPH Assay
4.5.2. ABTS Assay
4.5.3. FRAP Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Chinese materia medica; Shanghai Science and Technology Press: Shanghai, China, 1999; Volume 7, p. 848.
- Pharmacopoeia committee of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume Ⅰ, p. 274. [Google Scholar]
- Yang, Y.C. Flora of Tibetan Medicine; Qinghai people’s Publishing House: Xining, China, 1991; p. 119. ISBN 7225004263. [Google Scholar]
- Zhao, C.X.; Zeng, Y.X.; Wan, M.Z.; Li, R.X.; Liang, Y.Z.; Li, C.Y.; Zeng, Z.D.; Chau, F.T. Comparative analysis of essential oils from eight herbal medicines with pungent flavor and cool nature by GC-MS and chemometric resolution methods. J. Sep. Sci. 2009, 32, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Zhang, G.L.; Li, B.G.; Chen, Y.Z. Phenylpropanoid glycosides from Lamiphlomis rotata. Phytochemistry 1999, 51, 825–828. [Google Scholar] [CrossRef]
- Yue, H.L.; Zhao, X.H.; Wang, Q.L.; Tao, Y.D. Separation and purification of water soluble iridoid glucosides by high speed counter-current chromatography combined with macroporous resin column separation. J. Chromatogr. B. 2013, 936, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, Z.J.; Sun, L.N.; Wang, J.; Tao, X.; Chen, W.S. Iridoid glucosides and a C13-norisoprenoid from Lamiophlomis rotata and their effects on NF-κB activation. Bioorg. Med. Chem. Lett. 2012, 22, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Fan, G.; Yang, R.P.; Luo, W.Z.; Zhou, X.D.; Zhang, Y. Discriminating Lamiophlomis rotata according to geographical origin by 1H-NMR spectroscopy and multivariate analysis. Phytochem. Anal. 2015, 26, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.L.; Chen, Y.L.; Chen, Y.W.; Zhang, Y.; Xiang, L.; Pan, Z. Lamiophlomis rotata identifification via ITS2 barcode and quality evaluation by UPLC-QTOF-MS couple with multivariate analyses. Molecules 2018, 23, 3289. [Google Scholar]
- Jia, Z.P.; Li, M.X.; Zhang, R.X.; Wang, J.H.; Wang, M.; Guo, X.N.; Shen, T. Vitro screening of the effective antitumor components of Herba Lamiophlomis rotata. Med. J. Nation. Defend Force Northwest Chin. 2005, 26, 173–175. [Google Scholar]
- Liu, H.F.; Li, X.; Deng, Y.; Song, X.; Li, H. Study on the chemical constituents of the essential oil from Lamiophlomis rotata. Chin. J. Pharm. Anal. 2006, 26, 1794–1796. [Google Scholar]
- Liu, J.; Nan, P.; Wang, L.; Wang, Q.; Tsering, T.; Zhong, Y. Chemical variation in lipophilic composition of Lamiophlomis rotata from the Qinghai-Tibetan plateau. Chem. Nat. Compd. 2006, 42, 525–528. [Google Scholar] [CrossRef]
- Hart, C.M.; Tolson, J.K.; Block, E.R. Fatty acid supplementation protects pulmonary artery endothelial cells from oxidant injury. Am. J. Respir. Cell Mol. BioI. 1990, 3, 479–489. [Google Scholar] [CrossRef]
- Hart, C.M.; Tolson, J.K.; Block, E.R. Supplemental fatty acids alter lipid peroxidation and oxidant injury in endothelial cells. Am. J. Physiol. 1991, 260, L481–L488. [Google Scholar] [CrossRef] [PubMed]
- Dormandy, T.L. Biological rancidification. Lancet 1969, 2, 684–688. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Autor, A.P. The effect of dietary fatty acids on the composition of adult rat lung lipids: relationship to oxygen toxicity. Toxicol. Appl. Pharmacol. 1978, 44, 423–430. [Google Scholar] [CrossRef]
- Kennedy, J.I.; Chandler, D.B.; Fulmer, J.D.; Wert, M.B.; Grizzle, W.E. Dietary fish oil inhibits bleomycin-induced pulmonary fibrosis in the rat. Exp.Lung Res. 1989, 15, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Sosenko, I.R.S.; Innis, S.M.; Frank, L. Polyunsaturated fatty acids and protection of newborn rats from oxygen toxicity. J. Pediatr. 1988, 112, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Sosenko, I.R.S.; Innis, S.M.; Frank, L. Menhaden fish oil, n-3 polyunsaturated fatty acids, and protection of newborn rats from oxygen toxicity. Pediatr. Res. 1989, 25, 399–404. [Google Scholar] [CrossRef]
- Horton, A.A.; Fairhurst, S. Lipid peroxidation and mechanisms of toxicity. CRC Crit. Rev. Toxicol. 1987, 18, 27–79. [Google Scholar] [CrossRef]
- Favre, J.; Yıldırım, C.; Leyen, T.A.; Chen, W.J.Y.; Genugten, R.E.; Golen, L.W.; Garcia-Vallejo, J.J.; Musters, R.; Baggen, J.; Fontijn, R.; Pouw Kraan, T.; Serné, E.; Koolwijk, P.; Diamant, M.; Horrevoets, A.J.G. Palmitic acid increases pro-oxidant adaptor protein p66Shc expression and affects vascularization factors in angiogenic mononuclear cells: Action of resveratrol. Vasc. Pharmacol. 2015, 75, 7–18. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry, ed. 4.1; Allured publishing: Illinois, America, 2017; pp. 1–804. [Google Scholar]
- Wang, J. Alkanes and chemical markers identified in the essential oil from pericarp of Nanfengmiju (Citrus kinokuni Hort. ex Tanaka). J. Mex. Chem. Soc. 2023, 67, 82–93. [Google Scholar] [CrossRef]
- Feng, X.Q. Fatty acids and volatile compounds of meat from Cervus elaphus subspecies hybrid offspring; Gansu agricultural university: Gansu, China, 2008. [Google Scholar]
- Zhao, Q.Y.; Yousaf, L.; Xue, Y.; Shen, Q. Changes in flavor of fragrant rice during storage under different conditions. J. Sci. Food Agric. 2020, 100, 3435–3444. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.D.; Mcfeeters, R.F.; et al. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Usami, A.; Kashima, Y.; Marumoto, S.; Miyazawa, M. Characterization of aroma-active compounds in dry flower of Malva sylvestris L. by GC-MS-O analysis and OAV calculations. J. Oleo Sci. 2013, 62, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S. GC-MS analyses of the essential oils from Ixeris dentate (Thunb.) Nakai and I. stolonifera A. Gray. Korean J. Food Nutr. 2012, 25, 274–283. [Google Scholar] [CrossRef]
- Choi, H.S. Chemical composition of Cirsium japonicum var. ussurience Kitamura and the quantitative changes of major compounds by the harvesting season. Korean J. Food Nutr. 2016, 29, 327–334. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Wolosiak, R.; Druzynska, B.; Derewiaka, D.; et al. Verification of the conditions for determination of antioxidant activity by ABTS and DPPH assays-A practical approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Wei, R.; Liu, Y. Metformin combined with liraglutide has a synergistic protective effect on palmitic acid-induced oxidative damage of endothelial cells. Chin. J. Diabetes Mellitus 2014, 5. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liang, Y.; Hu, Q.; Zhang, T. Review on gas chromatographic retention index. Chinese J. Anal. Chem. 2005, 33, 715–721. [Google Scholar]
- Zengin, G.; Sarikurkcu, C.; Uyar, P.; et al. Crepis foetida L. subsp. rhoeadifolia (Bieb.) Celak. as a source of multifunctional agents: Cytotoxic and phytochemical evaluation. J. Funct. Foods 2015, 17, 698–708. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
| No. | Compounds | CAS | LRIsb, d | LRIsa | LRIsc | E8 | C8 | RC8 | E9 | C9 | RC9 | E10 | C10 | RC10 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FID | DB-5 | FFAP | FID | DB-5 | FFAP | FID | DB-5 | FFAP | FID | DB-5 | FFAP | FID | DB-5 | FFAP | FID | DB-5 | FFAP | FID | DB-5 | FFAP | FID | DB-5 | FFAP | FID | DB-5 | FFAP | ||||||
| 1 | Hexanal | 66-25-1 | 800, 1083 | - | - | 0.1 | nd | nd | 0.3 | 2 | 0.1 | 0.6 | 3.2 | 0.2 | 0.3 | nd | nd | 0.3 | nd | 0.1 | 0.6 | 7.5 | 0.2 | 0.3 | nd | tr | 0.3 | nd | 0.1 | 0.5 | 4.7 | 0.1 |
| 2 | β-Pinene | 127-91-3 | 970, 1112 | - | 1114 | nd | nd | tr | 0.5 | nd | nd | 1.1 | nd | nd | 4.3 | nd | nd | 1.3 | nd | nd | 2.8 | nd | nd | 4.9 | nd | nd | 1.5 | nd | nd | 2.6 | nd | nd |
| 3 | 1-Octen-3-ol | 3391-86-4 | 980, 1450 | 980 | 1454 | nd | nd | nd | 0.2 | nd | nd | 0.3 | nd | nd | nd | nd | 1.8 | 0.3 | nd | 0.7 | 0.7 | nd | 1.1 | nd | nd | 1.6 | 0.3 | 3.7 | 0.6 | 0.7 | nd | 1.1 |
| 4 | Hexanoic acid (6:0) | 142-62-1 | 990, 1846 | - | 1838 | nd | nd | nd | nd | nd | 0.1 | tr | nd | 0.3 | nd | nd | nd | 0.1 | nd | 0.2 | 0.2 | nd | 0.4 | 0.2 | nd | 0.1 | 0.1 | nd | 0.2 | 0.1 | nd | 0.4 |
| 5 | p-Cymene | 99-87-6 | 1011, 1272 | - | 1272 | 1.4 | nd | 0.2 | 0.3 | nd | 0.3 | 0.3 | nd | 0.1 | 1.2 | nd | nd | 0.1 | nd | tr | 0.3 | nd | tr | 0.4 | nd | 0.1 | 0.2 | nd | nd | 0.3 | nd | tr |
| 6 | Limonene | 138-86-3 | 1030, 1200 | 1026 | 1203 | 12.8 | nd | 3.4 | 4.6 | 36.4 | 3 | 2.3 | 5.8 | 0.8 | 12 | nd | 1.5 | 1.0 | nd | 0.2 | 3.0 | 8.1 | 0.5 | 3.2 | 7.3 | 0.9 | 1.3 | nd | 0.1 | 2 | nd | 0.3 |
| 7 | γ-Terpinene | 99-85-4 | 1053, 1246 | - | 1247 | 1.6 | nd | 0.1 | 0.5 | nd | 0.2 | 0.5 | nd | nd | 1.3 | nd | nd | 0.8 | nd | nd | 0.8 | nd | nd | 3.2 | nd | nd | 1.4 | nd | nd | 0.7 | nd | nd |
| 8 | cis-Linalool oxide | 5989-33-3 | 1074, 1444 | - | 1441 | nd | nd | nd | 0.9 | nd | nd | 1.2 | nd | nd | 0.5 | nd | nd | 1.1 | nd | 0.6 | 3 | nd | 1.4 | 0.5 | nd | 0.2 | 1.5 | nd | 0.6 | 3.0 | nd | 1.5 |
| 9 | trans-Linalool oxide | 34995-77-2 | 1102, 1452 | - | 1466 | nd | nd | nd | 0.8 | nd | nd | 1.0 | nd | 0.7 | 0.4 | nd | nd | 1.0 | nd | 0.5 | 2.5 | nd | 1.2 | 0.4 | nd | 0.2 | 1.3 | nd | 0.6 | 2.7 | nd | 1.1 |
| 10 | Linalool | 78-70-6 | 1082, 1547 | 1098 | 1552 | 0.2 | nd | 2.4 | 2.2 | 6.6 | 0.7 | 6.8 | 20.9 | 4.0 | 9.1 | nd | 4.2 | 2.9 | 11.7 | 1.1 | 5.5 | 18.0 | 2.1 | 11.0 | 22.3 | 4 | 3.4 | 22.3 | 1.2 | 5.7 | 22.3 | 2 |
| 11 | Caprylic acid (8:0) | 124-07-2 | 1180, 2060 | - | 2053 | nd | nd | nd | nd | nd | 0.1 | nd | nd | 0.3 | nd | nd | nd | nd | nd | 0.1 | nd | nd | 0.2 | nd | nd | 0.1 | nd | nd | 0.1 | nd | nd | 0.2 |
| 12 | α-Terpineol | 98-55-5 | 1189, 1697 | 1185 | 1690 | 71.2 | 100 | 2.8 | 5.4 | 17.5 | 1.1 | 12.8 | 56.5 | 4.7 | 17.8 | 100 | 4.1 | 5.9 | 30.6 | 1.3 | 11.4 | 47.6 | 2 | 14.8 | 70.4 | 3.4 | 4.8 | 27 | 1.2 | 8.5 | 41.4 | 2 |
| 13 | Tridecane | 629-50-5 | 1300, 1300 | - | 1300 | nd | nd | nd | nd | nd | nd | nd | nd | tr | nd | nd | nd | 0.2 | nd | nd | 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 14 | Tetradecane | 629-59-4 | 1400, 1400 | - | 1400 | nd | nd | nd | tr | nd | nd | 0.2 | nd | 0.1 | nd | nd | nd | nd | nd | nd | 0.1 | nd | nd | 0.1 | nd | tr | nd | nd | nd | 0.1 | nd | tr |
| 15 | β-Caryophyllene | 87-44-5 | 1419, 1595 | - | 1583 | 1.5 | nd | 0.1 | 0.2 | nd | 0.1 | 0.3 | nd | 0.1 | 0.6 | nd | nd | 0.2 | nd | tr | 0.4 | nd | tr | 0.4 | nd | 0.1 | 0.2 | nd | tr | 0.4 | nd | tr |
| 16 | Pentadecane | 629-62-9 | 1500, 1500 | - | 1500 | nd | nd | tr | 0.1 | nd | tr | 0.2 | nd | 0.1 | nd | nd | nd | tr | nd | tr | 0.2 | nd | 0.1 | 0.2 | nd | 0.1 | tr | nd | tr | 0.1 | nd | 0.1 |
| 17 | Dodecanoic acid (12:0) | 143-07-7 | 1556, 2498 | - | 2474 | nd | nd | 0.7 | 1.1 | nd | 0.9 | 1.2 | nd | 1.6 | 1 | nd | 1.1 | 1.3 | nd | 1.4 | 2.1 | nd | 2.5 | 0.7 | nd | 1.2 | 1 | nd | 1.3 | 1.7 | nd | 2 |
| 18 | Cedrol | 77-53-2 | 1598, 2116 | - | 2086 | un | nd | tr | un | nd | 0.1 | un | nd | 0.2 | un | nd | nd | un | nd | 0.1 | un | nd | 0.2 | un | nd | 0.1 | un | nd | 0.1 | un | nd | 0.1 |
| 19 | Hexadecane | 544-76-3 | 1600, 1600 | - | 1600 | nd | nd | 0.1 | 0.2 | nd | 0.1 | 0.3 | nd | 0.1 | nd | nd | nd | 0.1 | nd | tr | 0.3 | nd | 0.1 | 0.3 | nd | 0.1 | 0.2 | nd | 0.1 | 0.5 | nd | 0.2 |
| 20 | Heptadecane | 629-78-7 | 1700, 1700 | - | 1700 | nd | nd | 0.1 | 0.1 | nd | 0.1 | 0.2 | nd | 0.2 | nd | nd | nd | tr | nd | 0.1 | 0.2 | nd | 0.2 | 0.1 | nd | 0.1 | 0.1 | nd | 0.1 | 0.7 | nd | 0.3 |
| 21 | Tetradecanoic acid (14:0) | 544-63-8 | 1748, 2694 | - | 2685 | nd | nd | 3.9 | 5.1 | nd | 5.6 | 3.6 | nd | 6.4 | 1.9 | nd | 2.8 | 3.5 | nd | 4.4 | 3.9 | nd | 5.4 | 2.3 | nd | 3.2 | 4.0 | nd | 5.3 | 4.7 | nd | 6.1 |
| 22 | Octadecane | 593-45-3 | 1800, 1800 | - | 1800 | nd | nd | tr | 0.1 | nd | tr | nd | nd | nd | nd | nd | nd | tr | nd | nd | 0.1 | nd | 0.1 | 0.1 | nd | nd | 0.1 | nd | nd | 0.1 | nd | 0.2 |
| 23 | Hexahydrofarnesyl acetone | 502-69-2 | 1842, 2131 | 1843 | 2119 | nd | nd | 2 | 3.5 | nd | 2.7 | 5 | 7.1 | 6.2 | 2.7 | nd | 2 | 2.5 | nd | 2.3 | 6.4 | nd | 5.6 | 3.7 | nd | 3.0 | 3.8 | 1.9 | 3.5 | 7.9 | 17 | 7 |
| 24 | * Pentadecanoic acid (15:0) | 1002-84-2 | 1823, 2822 | - | 2790 | nd | nd | 0.5 | 0.5 | nd | 0.7 | 0.2 | nd | 0.8 | 0.2 | nd | nd | 0.4 | nd | 0.8 | 0.3 | nd | 0.6 | 0.1 | nd | 0.4 | 0.4 | nd | 0.7 | 0.3 | nd | 0.7 |
| 25 | Nonadecane | 629-92-5 | 1900, 1900 | - | 1900 | nd | nd | 0.1 | nd | nd | tr | nd | nd | 0.1 | nd | nd | nd | 0.1 | nd | nd | 0.3 | nd | nd | nd | nd | nd | nd | nd | tr | nd | nd | 0.1 |
| 26 | Farnesyl acetone | 1117-52-8 | 1919, 2384 | - | 2362 | nd | nd | 0.7 | nd | nd | 0.1 | 0.4 | nd | 0.9 | 0.8 | nd | nd | tr | nd | 0.1 | nd | nd | 0.1 | 0.9 | nd | 0.8 | tr | nd | 0.1 | 0.1 | nd | 0.2 |
| 27 | Methyl hexadecanoate | 112-39-0 | 1926, 2208 | 1924 | 2214 | 0.2 | nd | 1.5 | 1.8 | nd | 1.6 | 2.8 | 6.5 | 4.1 | 2.9 | nd | 2.8 | 2.5 | nd | 3 | 6.7 | 11.1 | 7.1 | 3.8 | nd | 3.9 | 3.6 | 3.3 | 4.1 | 7.8 | 18.4 | 8.3 |
| 28 | 9E-Hexadecenoic acid (16:1, n-7) | 2091-29-4 | 1942, 2954 | - | 2935 | un | nd | 0.9 | un | nd | 0.8 | un | nd | 2.7 | un | nd | nd | un | nd | nd | un | nd | 0.4 | un | nd | 0.3 | un | nd | 0.3 | un | nd | 0.4 |
| 29 | Palmitoleic acid (16:1, n-7) | 373-49-9 | 1951, 2926 | - | 2926 | nd | nd | 1.8 | 0.6 | nd | 1.5 | 1.4 | nd | 4.3 | 0.3 | nd | nd | 0.4 | nd | 0.7 | 0.7 | nd | 1.3 | 0.6 | nd | 0.7 | 0.5 | nd | 0.8 | 0.9 | nd | 1.1 |
| 30 | Dibutyl phthalate | 84-74-2 | 1965, 2680 | - | 2675 | nd | nd | nd | 0.1 | nd | 0.3 | 0.2 | nd | 0.8 | nd | nd | nd | 0.1 | nd | nd | 0.2 | nd | 0.4 | nd | nd | 0.2 | 0.2 | nd | 0.2 | 0.3 | nd | 0.5 |
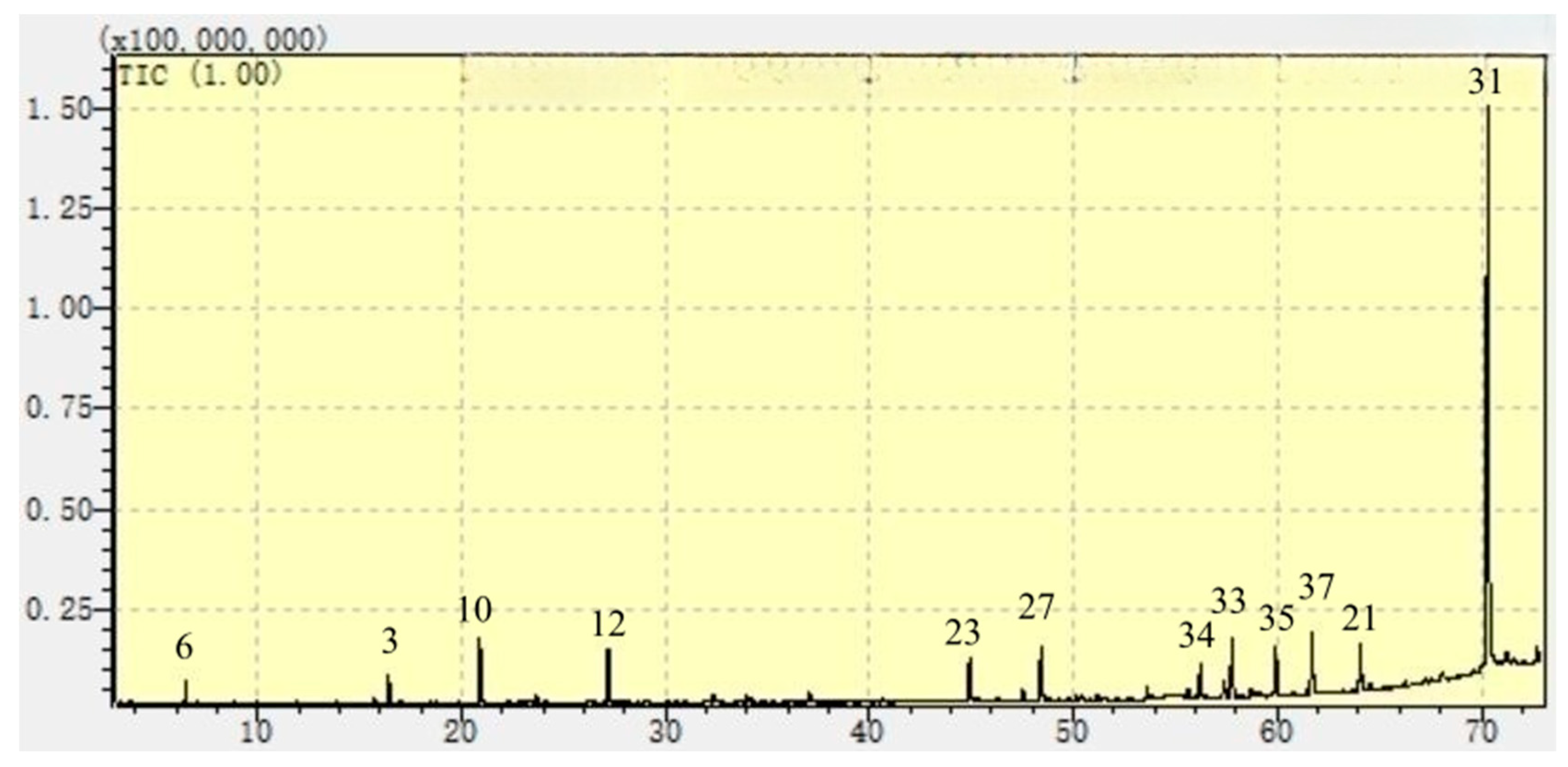
| 31 | n-Hexadecanoic acid (16:0) | 57-10-3 | 1972, 2931 | 1960 | 2894 | 0.9 | nd | 51.9 | 60.3 | 37.5 | 64.1 | 10.0 | nd | 17.8 | 23.8 | nd | 60.8 | 65.0 | 57.7 | 69.2 | 31.9 | 7.7 | 35.7 | 24.2 | nd | 47.1 | 62.1 | 41.8 | 61.3 | 31.7 | 2.1 | 44.8 |
| 32 | Eicosane | 112-95-8 | 2000, 2000 | - | 2000 | 0.1 | nd | tr | 0.1 | nd | tr | 0.3 | nd | 0.1 | nd | nd | nd | nd | nd | nd | 0.1 | nd | 0.1 | nd | nd | nd | 0.1 | nd | tr | 0.2 | nd | 0.1 |
| 33 | Methyl linoleate | 112-63-0 | 2092, 2482 | - | 2485 | un | nd | 1.9 | un | nd | 0.3 | un | nd | 2.8 | un | nd | 4.4 | un | nd | 0.6 | un | nd | 0.9 | un | nd | 4.3 | un | nd | 0.4 | un | nd | 0.6 |
| 34 | Methyl oleate | 112-62-9 | 2091, 2434 | - | 2439 | 0.2 | nd | 0.9 | 1 | nd | 0.7 | 3.2 | nd | 2.5 | nd | nd | 2.0 | 1.7 | nd | 1.9 | 4.1 | nd | 4.8 | nd | nd | 2.2 | 1.9 | nd | 2.1 | 4.2 | nd | 4.4 |
| 35 | Methyl linolenate | 301-00-8 | 2098, 2571 | - | 2552 | un | nd | 1.8 | un | nd | nd | un | nd | 1.2 | un | nd | 2.8 | un | nd | nd | un | nd | nd | un | nd | 3.4 | un | nd | nd | un | nd | nd |
| 36 | Heneicosane | 629-94-7 | 2100, 2100 | - | 2100 | 0.6 | nd | nd | 1.1 | nd | tr | 3.5 | nd | 0.1 | 5.7 | nd | nd | 1.7 | nd | nd | 4.3 | nd | 0.1 | 6.5 | nd | 0.1 | 2 | nd | tr | 4.3 | nd | 0.1 |
| 37 | Phytol | 150-86-7 | 2104, 2622 | - | 2607 | un | nd | 5.8 | un | nd | 1.5 | un | nd | 7 | un | nd | 2 | un | nd | 0.7 | un | nd | 1.3 | un | nd | 4.4 | un | nd | 1.3 | un | nd | 2 |
| 38 | Unknown-1 | - | -, - | - | 2476 | un | nd | 0.2 | un | nd | 0.3 | un | nd | 2.8 | un | nd | nd | un | nd | 0.2 | un | nd | 0.3 | un | nd | 0.3 | un | nd | 0.2 | un | nd | 0.5 |
| 39 | Methyl stearate | 112-61-8 | 2128, 2418 | - | 2420 | nd | nd | 0.2 | 0.2 | nd | 0.3 | 0.2 | nd | 0.7 | 0.8 | nd | nd | 0.6 | nd | 0.3 | 1.4 | nd | 0.7 | 0.4 | nd | 0.4 | 0.2 | nd | 0.4 | 0.6 | nd | 0.9 |
| 40 | Linoleic acid (18:2, n-6) | 60-33-3 | 2133, 3164 | - | 2884 | un | nd | 7.7 | un | nd | 1.1 | un | nd | 9.7 | un | nd | 2.7 | un | nd | 0.1 | un | nd | 0.7 | un | nd | 5.1 | un | nd | 0.6 | un | nd | nd |
| 41 | Oleic acid (18:1, n-9) | 112-80-1 | 2141, 3173 | - | 2770 | un | nd | 2.9 | un | nd | 2.9 | un | nd | 7 | un | nd | nd | un | nd | 3.3 | un | nd | 10.0 | un | nd | 3.7 | un | nd | 4 | un | nd | nd |
| 42 | Octadecanoic acid (18:0) | 57-11-4 | 2172, 3136 | - | 2700 | un | nd | 1.7 | un | nd | 4.3 | un | nd | nd | un | nd | nd | un | nd | 1.6 | un | nd | 1.5 | un | nd | 0.3 | un | nd | 3.8 | un | nd | nd |
| 43 | Docosane | 629-97-0 | 2200, 2200 | - | 2200 | 1 | nd | 0.1 | 0.2 | nd | 0.1 | 2.8 | nd | 0.1 | nd | nd | nd | 0.1 | nd | nd | 0.2 | nd | 0.2 | 0.2 | nd | nd | 0.1 | nd | 0.1 | 0.4 | nd | 0.1 |
| 44 | Phytol acetate | - | -, - | - | 2512 | un | nd | 0.1 | un | nd | 0.2 | un | nd | 0.6 | un | nd | nd | un | nd | 0.1 | un | nd | 0.4 | un | nd | 0.2 | un | nd | 0.1 | un | nd | 0.2 |
| 45 | Tricosane | 638-67-5 | 2300, 2300 | - | 2300 | 1.5 | nd | 0.2 | 0.3 | nd | 0.2 | 5.1 | nd | 0.5 | 0.3 | nd | nd | 0.3 | nd | 0.2 | 0.6 | nd | 0.6 | 0.3 | nd | 0.2 | 0.3 | nd | 0.2 | 0.8 | nd | 0.4 |
| 46 | Tetracosane | 646-31-1 | 2400, 2400 | - | 2400 | 1.9 | nd | nd | 0.2 | nd | 0.1 | 6.8 | nd | 0.3 | 0.1 | nd | nd | 0.6 | nd | 0.1 | 0.3 | nd | 0.3 | 0.2 | nd | nd | 0.1 | nd | 0.1 | 0.5 | nd | 0.2 |
| 47 | Pentacosane | 629-99-2 | 2500, 2500 | - | 2500 | 1.5 | nd | 0.1 | 0.3 | nd | 0.3 | 6.4 | nd | 0.6 | 0.3 | nd | nd | 0.3 | nd | 0.2 | 0.5 | nd | 0.6 | tr | nd | 0.1 | 0.2 | nd | 0.2 | 0.6 | nd | nd |
| 48 | Methyl 5,6-octadecadienoate | -, - | - | 2515 | un | nd | 0.2 | un | nd | 0.1 | un | nd | 0.4 | un | nd | 0.5 | un | nd | 0.7 | un | nd | 1.4 | un | nd | 0.4 | un | nd | 0.4 | un | nd | 0.9 | |
| 49 | Hexacosane | 630-01-3 | 2600, 2600 | - | 2600 | 1.2 | nd | 0.2 | 0.1 | nd | 0.1 | 5.2 | nd | 0.3 | 0.1 | nd | nd | 0.1 | nd | tr | 0.1 | nd | 0.1 | nd | nd | 0.3 | tr | nd | 0.1 | 0.3 | nd | 0.1 |
| 50 | Heptacosane | 593-49-7 | 2700, 2700 | - | 2700 | nd | nd | 0.2 | nd | nd | 0.4 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.5 | nd | nd | 0.2 | nd | nd | 0.2 | nd | nd | 0.4 |
| 51 | Octacosane | 630-02-4 | 2800, 2800 | - | 2800 | nd | nd | 0.2 | nd | nd | 0.3 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.4 | nd | nd | nd | nd | nd | 0.1 | nd | nd | 0.3 |
| 52 | Unknown-2 | - | 2817 | un | nd | 0.9 | un | nd | 1 | un | nd | 3 | un | nd | nd | un | nd | 0.8 | un | nd | 1.7 | un | nd | 0.8 | un | nd | 0.8 | un | nd | 1.4 | ||
| 53 | Nonacosane | 630-03-5 | 2900, 2900 | - | 2900 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.1 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
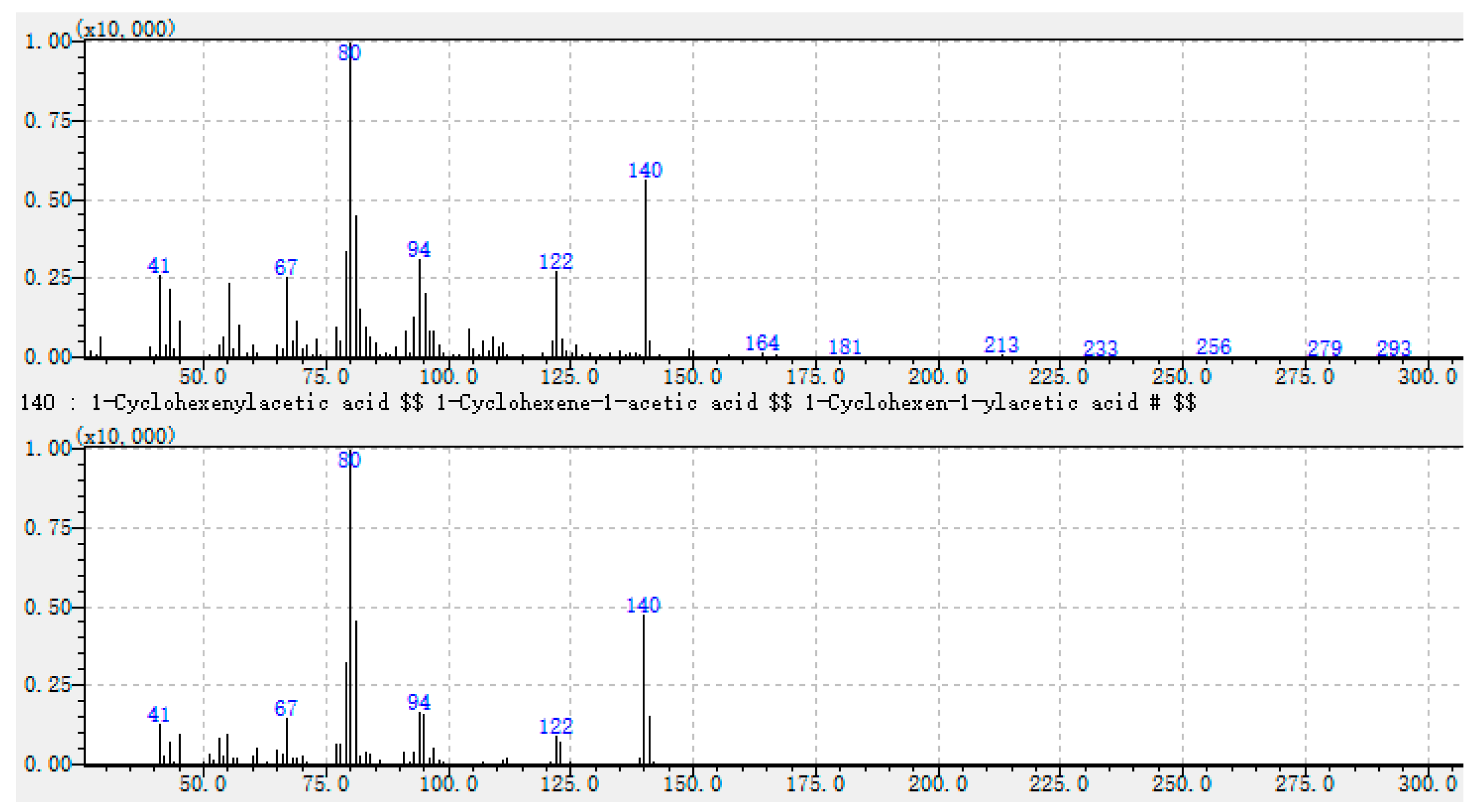
| 54 | Unknown-3 | - | 2952 | un | nd | nd | un | nd | nd | un | nd | nd | un | nd | 4.5 | un | nd | 1 | un | nd | 0.5 | un | nd | nd | un | nd | 0.8 | un | nd | 4.5 | ||
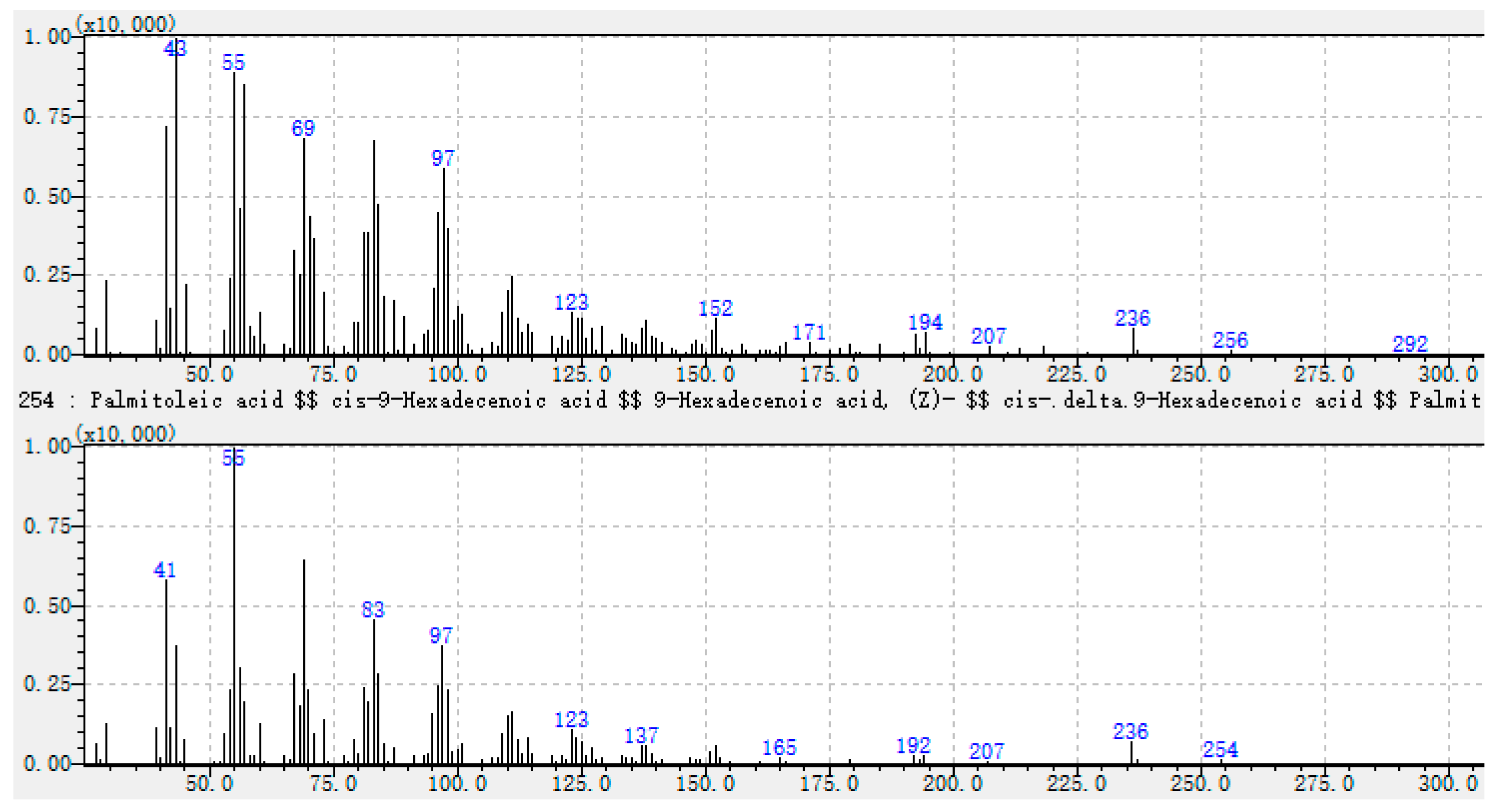
| 55 | Unknown-4 | - | 2975 | un | nd | 1.4 | un | nd | 1.4 | un | nd | 3.1 | un | nd | nd | un | nd | 1.2 | un | nd | 1.9 | un | nd | 1.5 | un | nd | 1.4 | un | nd | 1.9 | ||
| Total (55) | 98.3 | 100 | 100 | 99.6 | 100 | 100 | 98.8 | 100 | 100 | 100 | 100 | 100 | 99.8 | 100 | 100 | 99.9 | 100 | 100 | 97.4 | 100 | 100 | 100 | 100 | 100 | 99.4 | 100 | 100 | |||||
| HMs (4) | 15.8 | 0 | 3.7 | 5.9 | 36.4 | 3.4 | 4.3 | 5.8 | 0.9 | 18.7 | 0.0 | 1.5 | 3.3 | 0 | 0.2 | 7.0 | 8.1 | 0.6 | 11.7 | 7.3 | 1.0 | 4.4 | 0 | 0.1 | 5.6 | 0 | 0.3 | |||||
| AMs (4) | 71.4 | 100 | 5.2 | 9.5 | 24.1 | 1.8 | 21.7 | 77.4 | 9.4 | 27.8 | 100 | 8.3 | 10.9 | 42.3 | 3.5 | 22.4 | 47.6 | 6.7 | 26.8 | 92.7 | 7.8 | 11.1 | 49.4 | 3.6 | 20 | 57.7 | 6.7 | |||||
| HSs (1) | 1.5 | 0 | 0.1 | 0.2 | 0 | 0.1 | 0.3 | 0 | 0.1 | 0.6 | 0 | 0 | 0.2 | 0 | 0 | 0.4 | 0 | 0 | 0.4 | 0 | 0.1 | 0.2 | 0 | 0 | 0.4 | 0 | 0 | |||||
| ASs (1) | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 | 0 | 0.2 | 0 | 0 | 0.1 | 0 | 0 | 0.1 | 0 | 0 | 0.1 | |||||
| ADs (1) | 0.3 | 0 | 5.6 | 1.7 | 0 | 1.2 | 4.2 | 0 | 4.2 | 2.1 | 0 | 2.0 | 0.7 | 0 | 0.5 | 1.3 | 0 | 1.0 | 4.3 | 0 | 4.1 | 1.1 | 0 | 1.1 | 2.1 | 0 | 1.5 | |||||
| Aldehydes & ketones (3) | 0.1 | 0 | 2.7 | 3.7 | 2 | 2.8 | 6 | 10.3 | 7.3 | 3.8 | 0 | 2 | 2.8 | 0 | 2.5 | 7 | 7.5 | 5.9 | 4.9 | 0 | 3.8 | 4.2 | 1.9 | 3.6 | 8.4 | 21.7 | 7.4 | |||||
| FAs (11) | 1 | 0 | 72 | 67.6 | 37.5 | 82.1 | 16.4 | 0 | 50.9 | 27.2 | 0 | 67.4 | 70.7 | 57.7 | 81.8 | 39.1 | 7.7 | 58.7 | 28.1 | 0 | 62.2 | 68.1 | 41.8 | 78.4 | 39.4 | 2.1 | 55.7 | |||||
| LCFAs (9) | 1 | 0 | 72 | 67.6 | 37.5 | 81.9 | 16.4 | 0 | 50.3 | 27.2 | 0 | 67.4 | 70.6 | 57.7 | 81.5 | 38.9 | 7.7 | 58.1 | 27.9 | 0 | 62 | 68 | 41.8 | 78.1 | 39.3 | 2.1 | 55.1 | |||||
| SCFAs (2) | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 | 0 | 0.6 | 0 | 0 | 0 | 0.1 | 0 | 0.3 | 0.2 | 0 | 0.6 | 0.2 | 0 | 0.2 | 0.1 | 0 | 0.3 | 0.1 | 0 | 0.6 | |||||
| SFAs (7) | 0.9 | 0 | 58.7 | 67 | 37.5 | 75.8 | 15 | 0 | 27.2 | 26.9 | 0 | 64.7 | 70.3 | 57.7 | 77.7 | 38.4 | 77 | 46.3 | 27.5 | 0 | 52.4 | 67.6 | 41.8 | 72.7 | 38.5 | 2.1 | 54.2 | |||||
| MUFAs (3) | 0 | 0 | 5.6 | 0.6 | 0 | 5.2 | 1.4 | 0 | 14 | 0.3 | 0 | 0 | 0.4 | 0 | 4 | 0.7 | 0 | 11.7 | 0.6 | 0 | 4.7 | 0.5 | 0 | 5.1 | 0.9 | 0 | 1.5 | |||||
| PUFAs (1) | 0 | 0 | 7.7 | 0 | 0 | 1.1 | 0 | 0 | 9.7 | 0 | 0 | 2.7 | 0 | 0 | 0.1 | 0 | 0 | 0.7 | 0 | 0 | 5.1 | 0 | 0 | 0.6 | 0 | 0 | 0 | |||||
| Esters (8) | 0.4 | 0 | 6.6 | 3.1 | 0 | 3.5 | 6.4 | 6.5 | 13 | 3.7 | 0 | 12.5 | 4.9 | 0 | 6.6 | 12.4 | 11.1 | 15.7 | 4.2 | 0 | 15.0 | 5.9 | 3.3 | 7.8 | 12.9 | 18.4 | 15.7 | |||||
| Phthalate (1) | 0 | 0 | 0 | 0.1 | 0 | 0.3 | 0.2 | 0 | 0.8 | 0 | 0 | 0 | 0.1 | 0 | 0 | 0.2 | 0 | 0.4 | 0 | 0 | 0.2 | 0.2 | 0 | 0.2 | 0.3 | 0 | 0.5 | |||||
| Esters of FAs (7) | 0.4 | 0 | 6.6 | 3 | 0 | 3.2 | 6.2 | 6.5 | 12.2 | 3.7 | 0 | 12.5 | 4.8 | 0 | 6.6 | 12.2 | 11.1 | 15.3 | 4.2 | 0 | 14.8 | 5.7 | 3.3 | 7.6 | 12.6 | 18.4 | 15.2 | |||||
| TOCs (29) | 73.8 | 100 | 92.5 | 91.8 | 63.6 | 91.9 | 66.6 | 94.2 | 87.6 | 79.9 | 100 | 94.0 | 94.3 | 100 | 95.7 | 89.4 | 73.8 | 89.5 | 83.9 | 92.7 | 95 | 94.1 | 100 | 95.4 | 89.1 | 100 | 88.8 | |||||
| n-Alkanes (17) | 7.8 | 0 | 1.3 | 2.8 | 0 | 1.8 | 31 | 0 | 2.6 | 6.5 | 0 | 0 | 3.5 | 0 | 0.7 | 7.4 | 0 | 5.5 | 8 | 0 | 1.2 | 3.2 | 0 | 1.2 | 8.6 | 0 | 2.6 | |||||
| Unknowns (4) | 0 | 0 | 2.5 | 0 | 0 | 2.8 | 0 | 0 | 8.9 | 0 | 0 | 4.5 | 0 | 0 | 3.3 | 0 | 0 | 4.4 | 0 | 0 | 2.6 | 0 | 0 | 3.3 | 0 | 0 | 8.2 | |||||
| Characteristic Ion Peaks (M/W, %) | Compounds |
|---|---|
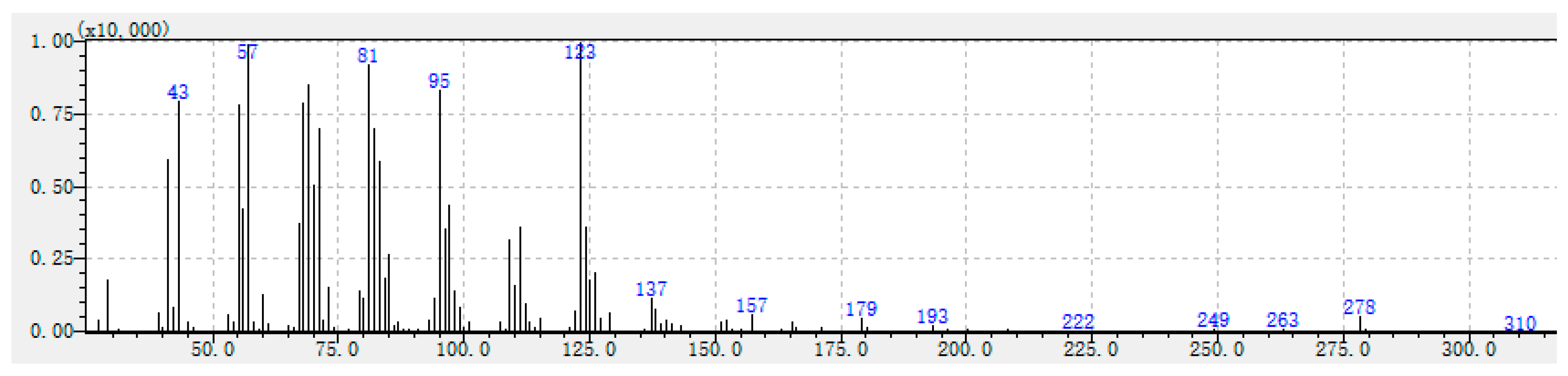
| 123 (100), 57 (97), 81 (90), 43 (81), 69 (81), 95 (80), 68 (77), 55 (76), 82 (68), 278 (6). | Unknown-1 |
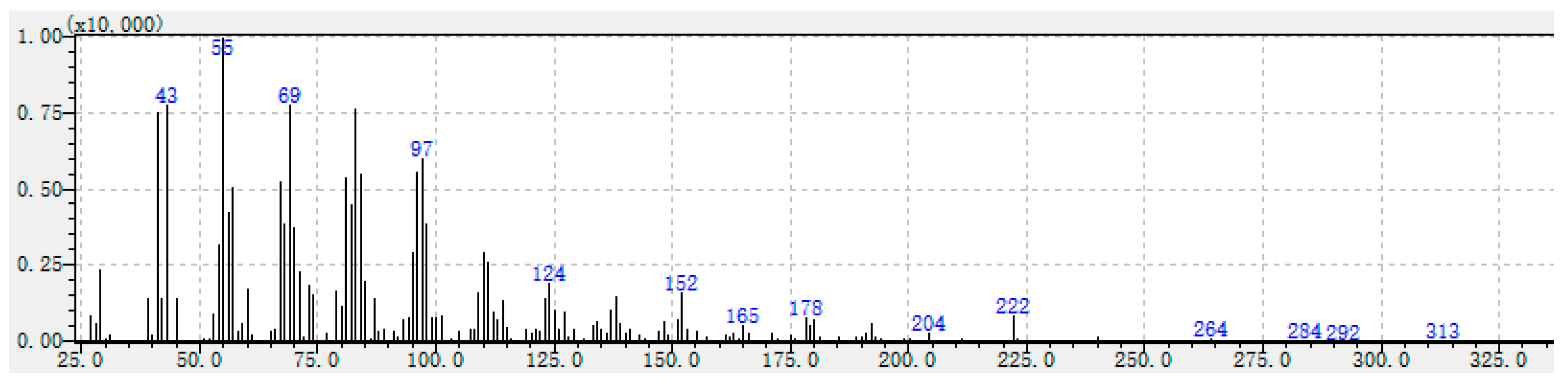
| 55 (100), 41 (77), 69 (76), 43 (74), 83 (73), 97 (59), 57 (57), 96 (56), 84 (56), 222 (11) | Unknown-2 |
| 80 (100), 140 (59), 81 (45), 94 (33), 79 (33), 122 (30), 67 (28), 41 (27), 43 (25), 149 (3). | Unknown-3 |
| 43 (100), 55 (81), 57 (80), 83 (67), 41 (65), 69 (62), 97 (58), 96 (45), 194 (8), 236 (8). | Unknown-4 |
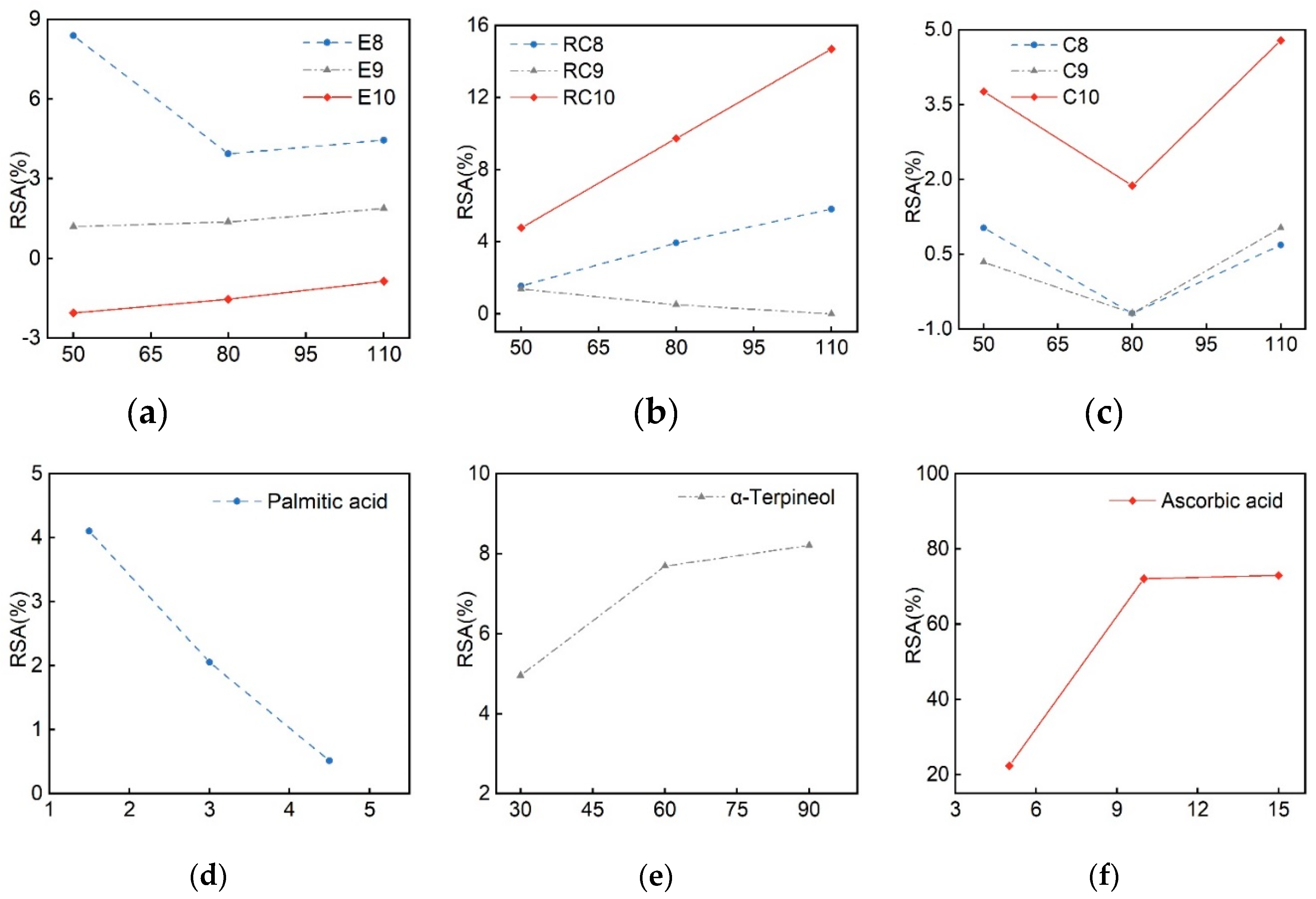
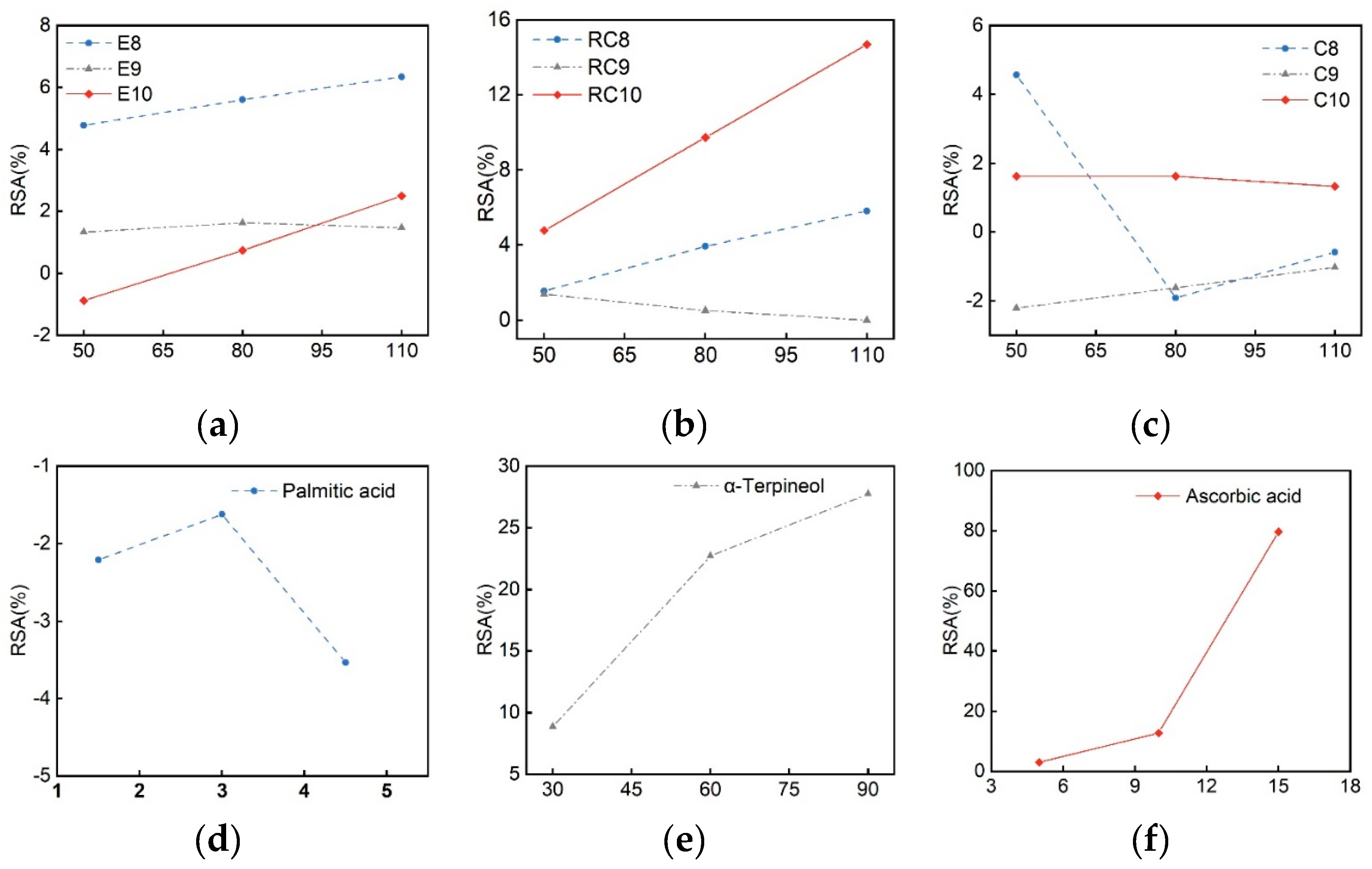
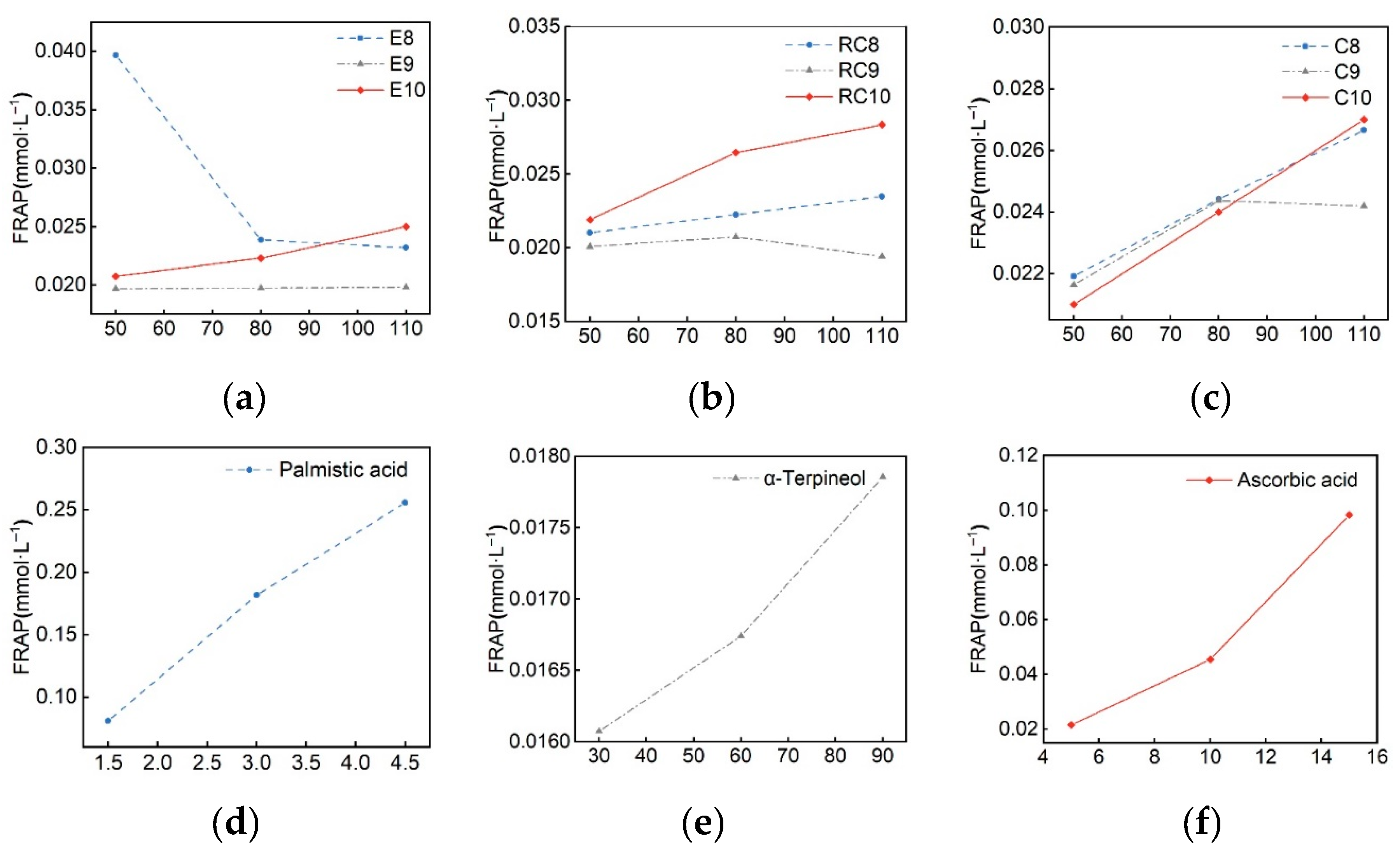
| Samples | IC50 (mg·mL– 1) | Ferric reducing ability (mmol·L– 1) |
|
|---|---|---|---|
| DPPH | ABTS | ||
| E8 | ND | 133.1 | 0.023 |
| E9 | 764.96 | ND | 0.02 |
| E10 | ND | 0.227 | 0.025 |
| RC8 | 0.629 | 0.323 | 0.023 |
| RC9 | ND | 0.541 | 0.019 |
| RC10 | 0.344 | 0.293 | 0.028 |
| C8 | ND | ND | 0.027 |
| C9 | ND | ND | 0.024 |
| C10 | ND | ND | 0.026 |
| Palmitic acid | ND | ND | 0.025 |
| α-Terpineol | 747.9 | 197.65 | 0.018 |
| Ascorbic acid | 0.0077 | 0.0127 | 0.098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




